
Leica DM4000 B
Leica DM4000 M
Leica DM5000 B
Operating Manual
1

Published 2003 by:
Leica Microsystems Wetzlar GmbH
Ernst-Leitz-Straße
D-35578 Wetzlar (Germany)
Responsible for contents:
Katja Peter, Karin Schwab
Marketing CM, Compound Microscopy, Product Management
In case of questions, please contact:
2
Phone +49(0)6441-292261
Fax +49(0)6441-292255
E-mail: MQM-Hotline@leica-microsystems.com

Leica DM4000 B
Leica DM4000 M
Leica DM5000 B
Operating Manual
3

Copyrights
Copyrights
All rights to this documentation are held by Leica
Microsystems Wetzlar GmbH. Reproduction of
text or illustrations (in whole or in part) by print,
photocopy, microfilm or other methods (including electronic systems) is not allowed without
express written permission from Leica
Microsystems Wetzlar GmbH.
The term "Windows" can be used in the following
text without further identification. It is a
registered trademark of the Microsoft
Corporation. Otherwise, no inference with
regard to the free usability of product names
may be drawn from the use of those names.
The instructions contained in the following documentation reflect state-of-the-art techno-logy
and knowledge standards. We have compiled
the texts and illustrations as accurately as
possible. Nevertheless, no liability of any kind
may be assumed for the accuracy of this manual’s contents. Still, we are always grateful for
comments and suggestions regarding potential
mistakes within this documentation.
The information in this manual is subject to modification at any time and without notification.
4

Contents
Contents
1. Important Notes about this Manual ..... 7
2. Safety Notes .............................................. 8
2.1. General Safety Notes ............................... 8
2.2. Electrical Safety ........................................ 8
3. Overview of the Instrument .................... 10
4. Unpacking the Microscope .................... 14
5. Assembling the Microscope .................. 16
5.1 Stage ........................................................... 17
5.2 Condenser .................................................. 18
5.3 Tube and Eyepieces ................................. 19
5.4 Objectives .................................................. 19
5.5 Light Sources for the
Transmitted Light Axis ............................. 20
5.6 Light Sources for the
Incident Light Axis .................................... 21
5.7 Equipping the
Incident Light filter turret ........................ 26
5.8 Polarizer and Analyzer ............................. 27
5.9 DIC Prisms .................................................. 28
5.10 Optional Accessories ............................... 29
5.11 Connection to the Power Supply............ 30
5.12 Connection to the
CTR5000 Electronics Box ......................... 30
6. Startup ........................................................ 31
6.1 Functional Principle.................................. 31
6.2 Switching on the Microscope ................ 34
6.3 The Display
(Leica DM4000 B/DM4000 M) ................. 35
6.4 The Function Keys .................................... 36
6.5 Köhler Illumination .................................... 37
6.6. Checking Phase Contrast Rings ............. 39
6.7 Adjusting the Light Sources .................... 40
7. Operation ................................................... 46
7.1 Switching on the Microscope ................ 46
7.2 Stages and Specimen Displacement .... 46
7.3 Focusing ..................................................... 47
7.4 Tubes...........................................................
48
7.5 Eyepieces ................................................... 49
7.6 Objectives .................................................. 50
7.7 Magnification Changer ............................ 51
7.8 Light Sources ............................................. 52
7.9 Aperture Diaphragm and
Field Diaphragm ........................................ 52
8. Imaging Procedure for
Leica DM4000 B/Leica DM5000 B ......... 53
8.1 Transmitted Light ...................................... 53
8.1.1 Bright Field ...................................... 53
8.1.2 Phase Contrast ............................... 53
8.1.3 Dark Field......................................... 54
8.1.4 Polarization ..................................... 55
8.1.5 Differential
Interference Contrast .................... 56
5

Contents
8.2 Fluorescence ............................................. 57
9. Imaging Procedure for
Leica DM4000 M ....................................... 58
9.1 Incident Light ............................................. 58
9.1.1 Bright Field ...................................... 58
9.1.2 Dark Field......................................... 58
9.1.3 Polarization ..................................... 59
9.1.4 Interference Contrast .................... 60
9.2 Transmitted Light ...................................... 60
9.2.1 Bright Field ...................................... 60
10. Trouble Shooting ...................................... 61
11. Care of the Microscope ........................... 64
11.1 Dust Cover .................................................. 64
11.2 Cleaning ...................................................... 64
11.3 Handling Acids and Bases ...................... 65
12. Essential
Wear and Spare Parts ............................. 66
13. Abbreviations and Pictograms .............. 67
14. Index ........................................................... 68
15. EU Declaration of Conformity ................ 69
6
1. Important Notes about this Manual
1. Important Notes about this Manual
Caution!
This operating manual is an essential component of the microscope, and must be read
carefully before the microscope is put into
operation or used.
Text symbols and their meanings:
(1.2)
→ p. 20
!
This operating manual contains important instructions and information for the operational
safety and maintenance of the microscope and
accessories. Therefore, it must be kept and
taken care of.
Numbers in parentheses, such as "(1.2)", correspond to illustrations (in the example, Figure 1,
Item 2).
Numbers with pointer arrows (for example
→ p.20), point to a certain page of this manual.
Special safety instructions are indicated
with the triangle symbol shown here, and
have a gray background.
Caution! The microscope and accessories can
be damaged when operated incorrectly.
Explanatory note.
*
Item not contained in all configurations.
7
2. Safety Notes
2. Safety Notes
2.1 General Safety Notes
This safety class 1 device is constructed and
tested in accordance with EN 61010-1/IEC 1010-1,
safety regulations for electrical measuring, control, and laboratory devices.
Caution!
In order to maintain this condition and to ensure safe operation, the user must follow the
instructions and warnings contained in this
operating manual.
Caution!
The devices and accessories described in
this operating manual have been tested for
safety and potential hazards.
The responsible Leica affiliate or the main
plant in Wetzlar must be consulted whenever the device is altered, modified or used
in conjunction with non-Leica components
that are outside of the scope of this manual.
Unauthorized alterations to the device or
noncompliant use shall void all rights to any
warranty claims!
2.2 Electrical Safety
General specifications
Leica CTR5000 electronics box (for DM5000 B)
For indoor use only.
Supply voltage:
Frequency:
Power input:
Fuses:
Ambient temperature:
Relative humidity:
Overvoltage category:
Pollution degree:
Microscope
For indoor use only.
Supply voltage:
Frequency:
Power input:
DM4000
DM5000
Fuses:
DM4000
DM5000
Ambient temperature:
Relative humidity:
Overvoltage category:
Pollution degree:
90-250 V~
50-60 Hz
max. 290 VA
T6,3 A
(IEC 60127-2/3)
15-35°C
max. 80% to 30°C
II
2
90-250 V~
50-60 Hz
max. 180 VA
max. 290 VA
T6,3 A
(IEC 60127-2/3)
See CTR5000
15-35°C
max. 80% to 30°C
II
2
8

Supply unit ebq 100
For indoor use only.
Supply voltage:
Frequency:
Power input:
Fuses:
Ambient temperature:
Relative humidity:
Overvoltage category:
Pollution degree:
(see enclosed manual)
Caution!
90-250 V~
50-60 Hz
max. 155 VA
2xT2A (IEC 127)
15-35°C
max. 80% to 30°C
II
2
2. Safety Notes
Caution!
Never use any fuses as replacements other
than those of the types and the current ratings listed here. Using patched fuses or
bridging the fuse holder is not permitted.
Caution!
The microscope’s electrical accessory components are not protected against water.
Water can cause electric shock.
The power plug may only be plugged into an
outlet equipped with a grounding contact.
Do not interfere with the grounding function
by using an extension cord without a ground
wire. Any interruption of the ground wire inside or outside of the device, or release of
the ground wire connection, can cause the
device to become hazardous. Intentional
ground interruption is not permitted!
Caution!
Through connection to the grounding connection, ancillary equipment with its own
and/or extra power supply may be brought to
the same ground wire potential. For
connections without a ground connector,
Leica Service must be consulted.
Caution!
Protect the microscope from excessive temperature fluctuations. Such fluctuations can
lead to the accumulation of condensation,
which can damage the electrical and optical
components.
Ambient temperature: 15-35°C.
Caution!
Before exchanging the fuses or lamps, be
absolutely certain to switch-off the main
power switch and remove the power cable.
9
3. Overview of the Instrument
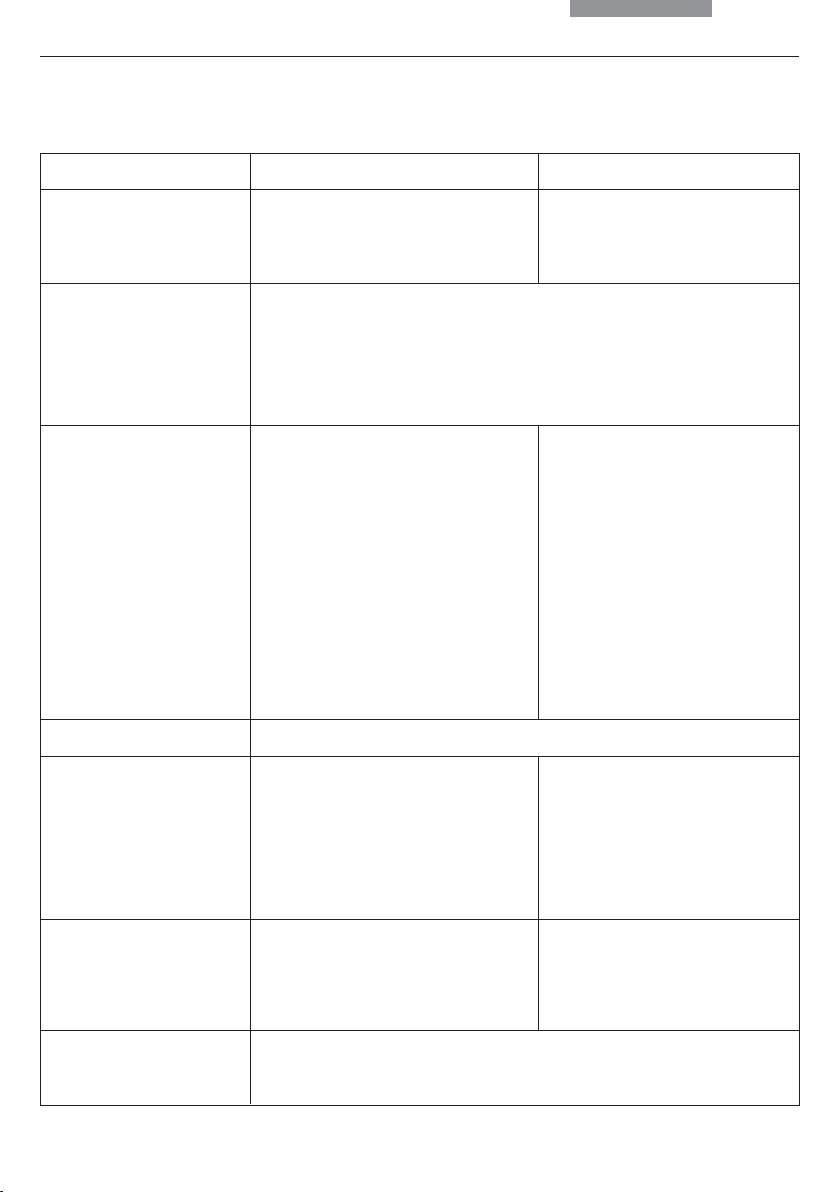
3. Overview of the Instrument
Specification
Imaging Procedure
Transmitted Light Axis
Incident Light Axis
Z Pinion
Leica DM4000 B / DM5000 B
• transmitted light: BF, DF, PH,
Pol (DM5000 B also ICT)
• incident light: fluorescence
• automatic Illumination Manager
(motorized aperture diaphragm and field diaphragm,
motorized intensity control)
• automatic Constant Color Intensity Control (CCIC)
• motorized shutter
• integrated into the stand
• motorized 5x filter turret
(DM5000 B 8x optional)
• with FIM (Fluorescence
Intensity Managemer) for decreasing light intensity in 5
stages
• mechanical “Booster Lens”
for increasing fluorescence
intensity
• motorized shutter
• manual
Leica DM4000 M
• transmitted BF, DF, PH
light: ICT, Pol
• incident light: BF, DF, ICR, Pol
• integrated into the stand
• motorized 4x filter turret
• automatic Illumination
Manager
• motorized shutter
Objective nosepiece
X/Y Stage
Tube
10
• manual
• absolute coded
• 6x with M25 thread
(DM5000 B: 7x; mot. DIC
objective prism turret with 4
positions optional)
• manual
• replaceable specimen stage
• coaxial pinion length: 155 mm
• manual or motorized
• optionally with two camera outputs
• manual
• absolute encoded
• 6x with M32 thread
• slot for DIC prisms
and Pol compensators
(optional)
• manual
• replaceable specimen stage
• coaxial pinion length: 140 mm
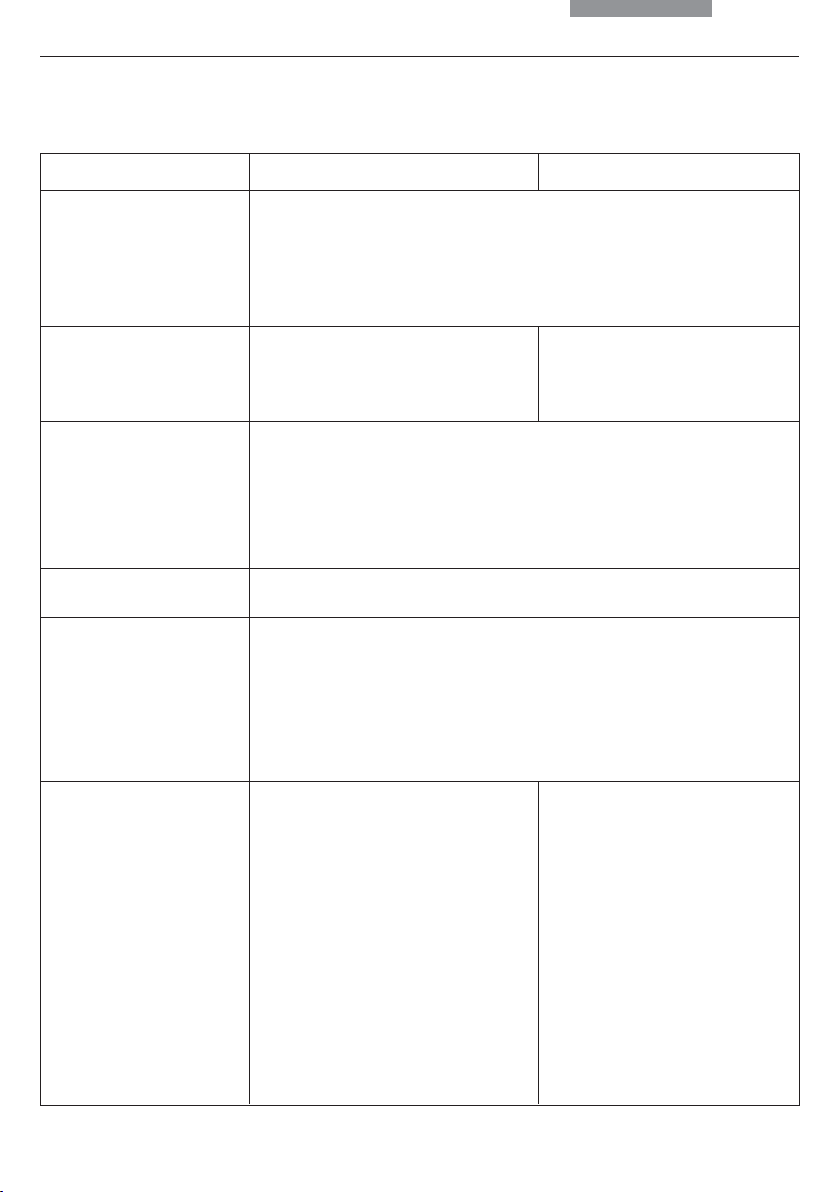
3. Overview of the Instrument
Specification
Condenser
Magnification Changer
Control Panels
Computer Interface
Software Tools
Leica DM4000 B / DM5000 B
• motorized condenser head
• motorized condenser turret for light rings,
DF stop, DIC prisms
• optional polarizer integrated and motorized
• automatic Köhler Illumination
• manual
• absolute coded
• 1x; 1.25x; 1.6x
• operating buttons for all motorized microscope functions
• additional variable function keys
• focusing knobs
• LC display
• DM5000 B with LeicaScreen (touchscreen)
• RS232C
• Leica DMControl for Windows
• with plugins for:
• customitsation
• DM Operation
(remote control)
• basic Image Viewer
Leica DM4000 M
• manual
• absolute coded
• 1x; 1.5x; 2x
TM
2000, XP, NT;
CTR5000
Electronics Box
For Leica DM5000 B only:
Separate control unit with
power supply for 100W halogen
lamp
see p. 8 (electrical safety)
11
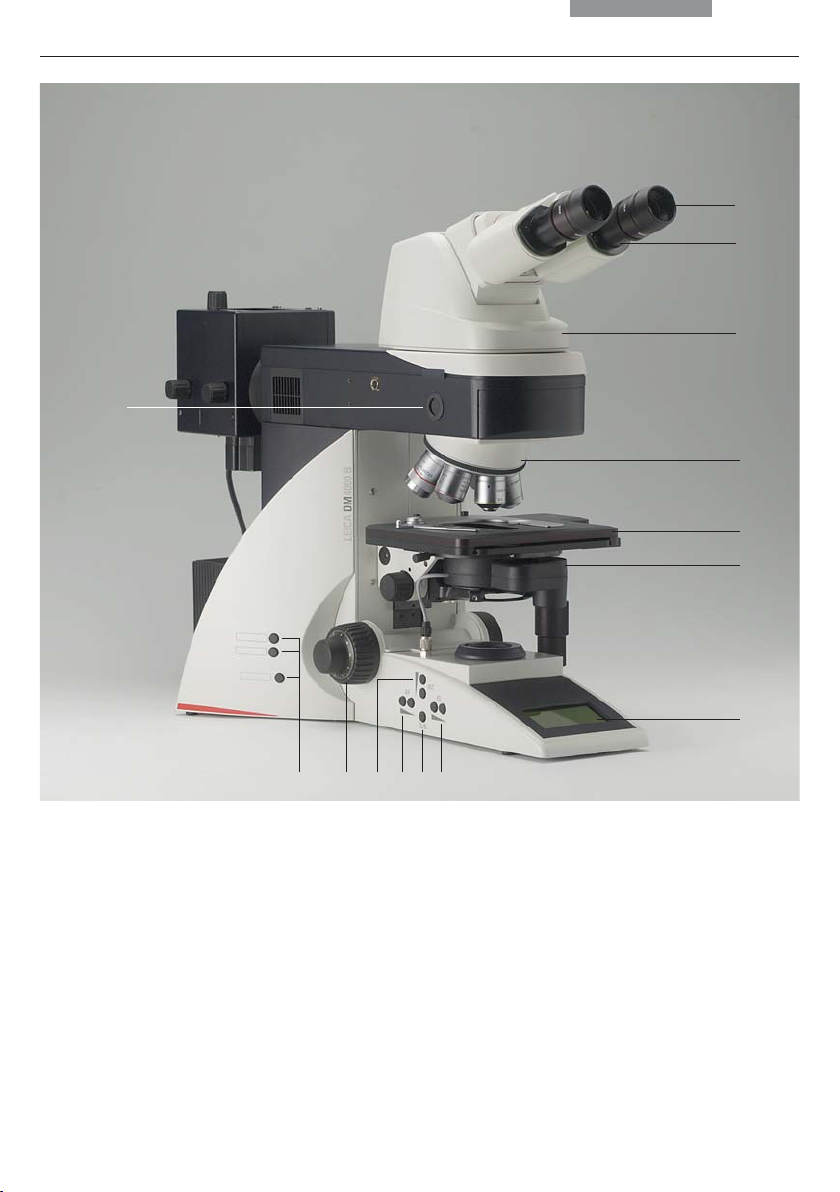
3. Overview of the Instrument
14
1
2
3
4
5
6
8910111213
Fig. 1 Leica DM4000 M left side of the stand with AET22 advanced ergotube
1 Eyepiece
2 Eyepiece tube
3 Tube
4 Objective nosepiece with objectives
5 Specimen stage with specimen holder
6 Condenser
7 LC display
8 Function keys field diaphragm
9 Transmitted light/incident light switch
10 Function keys aperture diaphragm
11 Function keys: Light intensity
12 Focus dial with coarse and fine adjustment
13 Variable function keys (factory pre-assigned)
14 Lamp adjustment window
12
7

22
3. Overview of the Instrument
15
16
21 20 19 18 17
Fig. 2 Leica DM4000 B right side of the stand with Advanced Ergotube AET22
15 Lamp housing for incident light
16 Lamp housing for transmitted light
17 Transmitted light filter, optional
18 Transmitted light filter, optional
19 Variable function keys (factory pre-assigned)
20 X/Y coaxial drive, height adjustable
21 Focus fine adjustment
22 Motorized filter cube exchanger
13
4. Unpacking the Microscope
4. Unpacking the Microscope
The device is delivered in two boxes.
The stand box contains the following compo-
nents:
• Stand with integrated incident light axis and
objective nosepiece
• Specimen stage with stage bracket
• Power cable and PC connecting cable
• CD with Leica software package
• Instructions and list of microscope default
settings (“Identification Sheet”)
The system box contains the microscope acces-
sories:
• Tube
• Eyepieces
• Objectives
The external ebq 100 supply unit* is delivered in
separate packaging.
For the Leica DM5000 B microscope:
The CTR5000 electronics box is also delivered in
separate packaging.
First, carefully remove all components from the
transportation and packaging materials.
Note:
Avoid touching the lens surfaces of the
objectives. If fingerprints do appear on the glass
surfaces, remove them with a soft leather or
linen cloth. Even small traces of finger
perspiration can damage the surfaces of optical
surfaces in a short time. See the chapter, "Care
of the microscope" →
structions.
Caution!
p. 64, for additional in-
• Condenser
• Lamp housings with accessories
• Fitting tool
• Depending on configuration, additional microscope accessories such as filter cubes, etc.
14
Do not yet connect the microscope and peripherals to the power supply at this point!

4. Unpacking the Microscope
Installation location
Work with the microscope should be performed
in a dust-free room, which is free of oil vapors
and other chemical vapors, as well as extreme
humidity. At the workplace, large temperature
fluctuations, direct sunlight and vibrations
should be avoided. These conditions can distort
measurements and micrographic images.
Allowable ambient conditions
Temperature 15-35°C
Relative humidity maximum 80% up to 30°C
Microscopes in warm and warm-damp climatic
zones require special care in order to prevent
the build up of fungus.
See the chapter, "Care of the microscope" →
for additional instructions.
Caution:
Electrical components must be assembled at
least 10 cm from the wall and away from
flammable substances.
p. 64,
Transport
For shipping or transporting the microscope
and its accessory components, the original
packaging should be used.
As a precaution to prevent damage from vibrations, the following components should be disassembled and packaged separately:
• Unscrew the objectives.
• Remove the condenser.
• Remove the stage.
• Remove the lamp housings.
• Disassemble the burner of 106 z lamp housing.
• Remove all moving or loose parts.
15

5. Assembly
5. Assembling the Microscope
The microscope components are logically assembled in this order:
• Stage
• Condenser
• Tube
• Eyepieces
• Objectives
• Light sources
• Filter cubes/reflectors*
Only a few commonly used screwdrivers and
keys are necessary for assembly, which are included in the delivery package.
When using intermediate systems and optical
accessories, the sequence may vary.
In this case, read Chapter,
"5.10 Optional accessories" → p. 29
16

5. Assembly
5.1 Stage
Caution:
!
Before assembling the stage, make sure no objectives are installed!
• Place the specimen holder on the stage and
fasten it with the two screws (3.1).
• Using the condenser height adjuster (3.2), turn
the condenser holder completely upwards, i.e.
as close to the stage as possible.
• Loosen the stage clamp (3.3) slightly.
Fig. 3 Mechanical object stage
1 Locking screws for specimen holder
2 Condenser height adjuster
3 Stage clamp
• From above, set the stage clamp onto the
dovetail guide (4.2) and push the stage downwards until the upper end of the dovetail guide
is tightly fastened to the upper end of the
stage clamp.
• Firmly tighten the stage clamp (4.1).
Note:
For thicker specimens (Leica DM4000 M) the
stage can be set to a correspondingly lower
level.
Fig. 4 Assembling the stage
1 Stage clamp
2 Dovetail guide
1
23
1
2
17

5. Assembly
5.2 Condenser
• Using the condenser height adjuster (5.4), turn
the condenser holder (5.1) completely downwards.
• Unscrew the clamping screw for the condenser (5.3) far enough so that the condenser
can be inserted from the front.
• From the front, insert the condenser into the
condenser holder as far as it will go. On the
underside of the condenser, there is an orientation pin (6.1), which must be located in the
guiding notch (7.1).
• Pull the condenser’s clamping screw (5.3) so
that the condenser is locked in place.
• Connect the condenser over the connection
(8.1) with the stand.
Note:
The condenser must be centered before using
the microscope.
Köhler illumination p. 37.
→
Fig. 6
Underside of condenser
1 Orientation pin
Fig. 7 Condenser holder
1 Guiding notch
1
1
Fig. 5 Condenser holder
1 Condenser holder
2 Condenser centering
3 Clamping screw for condenser
4 Condenser height adjuster
1
23 4
18
Fig. 8 Condenser connector
1 Condenser cable socket
1
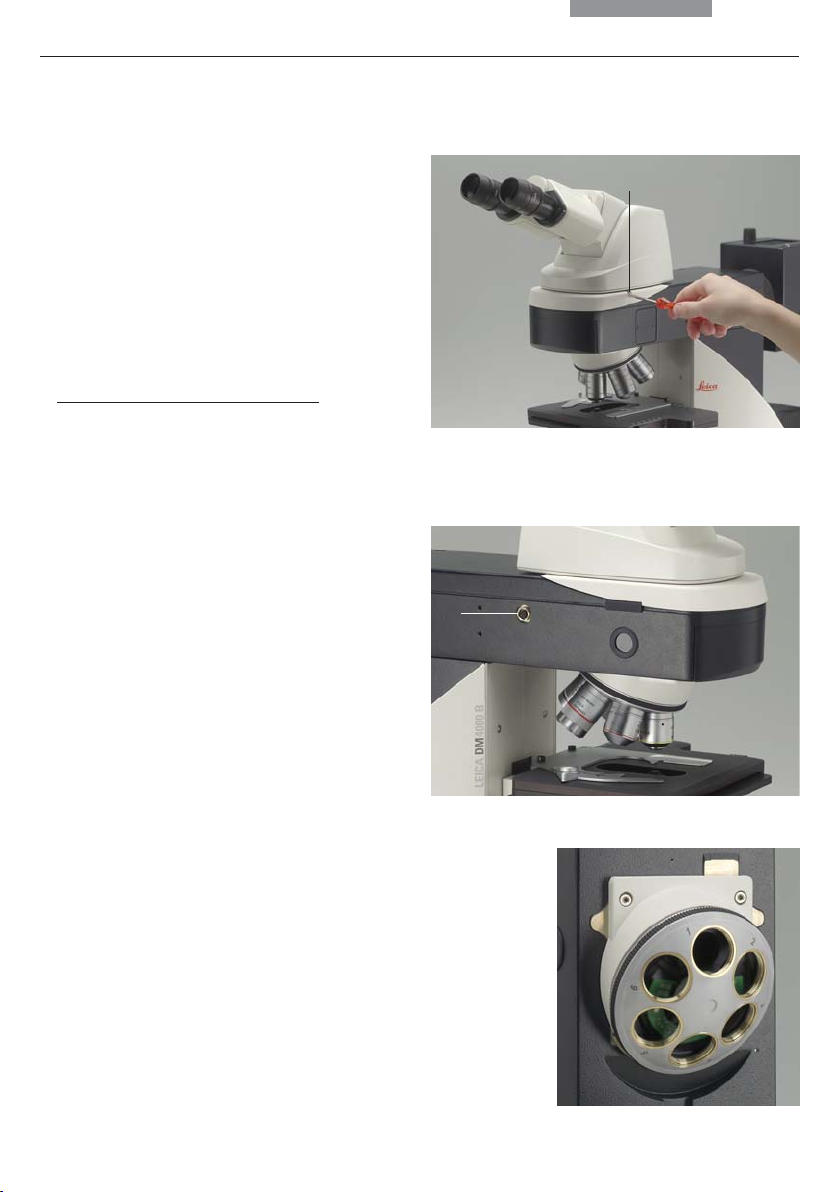
5. Assembly
5.3 Tube and Eyepieces
The tube is mounted to the stand either directly or
with the use of intermediate modules. It is fastened
in place with the side clamping screw (9.1).
• Loosen the clamping screw (9.1).
• Insert the tube in the circular receptacle
(dovetail ring).
• Retighten the clamping screw (9.1).
Only for the MBDT motorized tube:
•
Connect the tube to the stand with the connector socket (10.1).
• The eyepieces are inserted into the eyepiece
tubes on the tube.
6.4 Objectives
The receptacles on the objective turrets are
numbered (Fig. 11). The individual objectives
have already pre-assigned positions at the
factory according to their configuration.
A list of the exact objective positions is provided
in shipment. (“Identification Sheet”)
Fig. 9 Fastening the tube
1 Clamping screw
1
Fig. 10 Motorized tube connection
1 Connector socket
1
Caution:
!
Cover unoccupied threads on the turret with
dust protector caps!
Fig. 11
Objective turret
with labeled
objective
receptacles
19

5. Assembly
5.5 Light Sources for the Transmitted Light Axis
Caution:
Be sure that the lamp housing is disconnected from the power supply. Unplug the
power plug and the power supply during assembly.
107/2 Lamp Housing
This lamp housing is used with a 12V 100W halogen lamp, which is already mounted.
In case the lamp has to be removed:
• Remove the fastener screw on the housing
(Fig. 12).
• Remove the housing by pulling it upwards.
• Remove the lamp
• Insert the new 12V 100W lamp (13.1) with dust
cover straight into the socket until it stops. Be
sure that the lamp is inserted straight.
• Remove the lamp’s dust cover.
Caution:
Do not remove the lamp’s dust cover until
you have installed the lamp. Avoid
fingerprints on the lamp.
Fig. 12
Lamp housing 107/2
Releasing the
fastening screw
Fig. 13
Lamp housing 107/2,
opened
1 Mount with
halogen lamp
2 Collector
1
2
Fig. 14 Rear side of stand
1 Incident light lamp housing receptacle
2 Transmitted light lamp housing receptacle
3 12 V 100 W connection for transmitted light (symbol: )
4 12 V 100 W connection for incident light (symbol: )
• Replace the housing and fasten it in place using the fastening screw.
• Place the lamp housing in the transmitted light
lamp housing receptacle (14.2) and fasten it
with the clamping screw on the side.
• Connect the lamp housing to the power
supplyfor transmitted light (symbol: ) (14.3).
20
1
2
34

5. Assembly
5.6 Light Sources for the Incident Light Axis
Caution:
During assembly, always unplug the power
supply unit of the 106 z lamp housing from its
socket.
Never touch the glass parts of the burner
with bare hands.
Never look directly into the beam path (blinding hazard).
During assembly work on xenon burners, always wear the supplied protective gloves and
face protection (Fig. 15) (risk of explosion).
106 z lamp housing
Fig. 15
Protective gloves and mask
This lamp housing is used with a 12V 100W halogen lamp or various gas discharge lamps.
Inserting the 12V 100W halogen lamp into the
106 z lamp housing
• Unscrew the fastening screws of the cover
and lift up the cover (16.1).
• Unscrew the fastening screws of the lamp
mount (16.8) and pull out the mount (Fig. 17).
Fig. 16 106 z lamp housing (on the side, open)
1 Cover raised
2 Collector
3 12 V 100 W lamp or
gas discharge lamp in mount
4 Reflector (mirror)
5, 6, 7 Adjusting screw for x-y reflector
8 Fastening screw for lamp mount
9 Socket for contact plug
1
2
4
5
3
6
7
898
21

5. Assembly
• Insert the lamp with the dust cover straight
into the socket until it stops.
• Remove the dust cover.
• Reinsert the lamp mount and retighten the fastening screw (16.8).
Caution:
Do not remove the lamp’s dust cover until after you have installed the lamp. Be certain to
avoid getting fingerprints on the lamp.
• Close the lamp housing and retighten the fastening screws.
Fig. 17 Lamp mount with 12 V 100 W halogen lamp
• Place the lamp housing in the incident light
lamp housing receptacle (18.1) and fasten it
with the clamping screw on the side.
• Connect the lamp housing to the power supply
for incident light (symbol ) (18.4).
Fig. 18 Rear side of stand
1 Incident light lamp housing receptacle
2 Transmitted light lamp housing receptacle
3 12 V 100 W connection for transmitted light (symbol: )
4 12 V 100 W connection for incident light (symbol: )
22
1
2
34

5. Assembly
Inserting the gas discharge lamps (Hg and Xe)
into the 106z lamp housing
Hg and Xe lamps are powered by the separate
ebq 100 supply unit.
Read the separate instruction manual provided
with this supply unit.
The following gas discharge lamps may be used
and require different lamp mounts (Fig. 19):
Type Typical bulb life*
50 W high-pressure mercury burner (alternating current) 100 hrs.
100 W high-pressure mercury burner (direct current, stabilized/not stabilized 200 hrs.
100 W high-pressure mercury burner (direct current, stabilized/not stabilized, type 103 W/2) 300 hrs.
75 W High-pressure xenon burner (direct current, stabilized) 400 hrs.
* Please regard the data sheets of the burners.
23

5. Assembly
• To open the 106 z lamp housing, unscrew the
fastening screws on the cover.
Caution:
• Remove the transport anchorage (red plastic
rod in place of the burner) in the lamp mount.
To do so, remove the lower clamp (19.1). Pull
up the cooling element (19.3) and turn it to the
side. Detach the lower clamp system (19.2)
and remove the transport anchorage.
• Install the burner in mirror image fashion.
Fig. 19 a-d Lamp mounts for gas discharge lamps
1 Upper clamping system, 2 Lower clamping system, 3 Cooling element
4 Nipple of the mercury 50 burner, 5 Dust cover of the mercury 75 burner
Hg 50
1
4
a
3
2
Hg 50 burner:
After installation, the labeling must be
If a glass melt nipple is present (19a.4), position it by turning the burner so that the nipple
does not come in the way of the beam path
later, but instead is positioned
Xe 75 burner:
Remove the burner’s dust cover (19b.5) after
you have installed the burner.
Xe 75
upright.
sideways.
b
3
1
5
2
24
Hg 100
1
2
c
3
Hg 100
Stab.
d
3
1
2

• Insert the lamp mount, with the burner installed, into the lamp housing and tighten it
with the screws (20.8).
5. Assembly
• Put the lid down again. Plug in the contact
plug as far as it goes and retighten the
screws.
• Place the lamp housing in the incident light
lamp housing receptacle (21.1) and fasten it
with the clamping screw on the side.
• Connect the lamp housing to the power supply
(22.1).
Fig. 21 Rear side of stand
1 Incident light lamp housing receptacle
2 Transmitted light lamp housing receptacle
3 12 V 100 W connection for transmitted light (symbol: )
4 12 V 100 W connection for incident light (symbol: )
Fig. 20 106 z lamp housing (on the side, open)
1 Cover raised
2 Collector
3 12 V 100 W lamp or
gas discharge lamp in mount
4 Reflector (mirror)
5, 6, 7 Adjusting screw for x-y reflector
8 Fastening screw for lamp mount
9 Socket for contact plug
1
2
3
898
Fig. 22 Rear side of the ebq 100 supply unit
1 Lamp connection
4
5
6
7
1
1
2
34
25

5. Assembly
5.7 Equipping the Incident Light filter turret
The receptacles on the turret are numbered.
According to your equipment, the individual filter
and/or reflector cubes have already preassigned positions. A list is provided along with
your shipment (“Identification Sheet”).
Insert the filter and reflector cubes in the following manner:
• Equip the incident light turret only when the
microscope is switched off.
• Remove the face plate from the upper part of
the microscope (Fig. 25). Turn the turret in any
direction until the locking pin engages.
• Insert the filter or reflector cube into the
mounting in front of you according to the
identification sheet provided.
To do so, place the filter or reflector cube on
the right side and press it to the left into the
mounting (Fig. 26).
Fig. 23 Filter cube
front side
Fig. 25 Removing the front panel
1 Filter receptacle
2 Retention pin
3 Front panel
Fig. 24 Filter cube
back side
1
2
3
• Push the retention pin (25.2) and continue to
turn the filter turret until you reach the next
locking position.
• Again make sure that the turret engages
(retention pin unlocks) and insert the next
filter and/or reflector cube as described
above.
• When all filters and reflector cubes have been
inserted, close the front cover plate again.
5.8 Polarizer and Analyzer
26
Fig. 26 Inserting the filter or reflector cubes
1 Mounting
1
1

5. Assembly
ICT/P transmitted light polarizer
• Using the left clamping screw, fasten the ICT/P
transmitted light polarizer to the underside of
the condenser holder (Fig. 27).
• Make sure that the red index point on the front
of the polarizer is aligned with 0.
• If necessary, insert the compensators (λ- and
λ/4 plates) into the polarizer’s receptacle
(Fig. 28).
Fig. 27 Assembly of the ICT/P transmitted light polarizer
1 Clamping screw
1
Fig. 28 Inserting the compensators
Incident light polarizers:
R/P polarizer, rotating polarizer
L/ICR, R/ICR polarizer
• Remove the plug cap on the right side of the
incident light axis (Fig. 29).
• Insert the polarizer into the receptacle until it
latches in place.
Motorized polarizer
• A motorized polarizer is already installed and
ready for operation in the DIC condenser.
Transmitted light and incident light analyzer
Fig. 29 Inserting the polarizer
1 The plug cap is replaced with the polarizer.
1
27

5. Assembly
• Remove the plug cap on the left side of the
stand.
• Insert the polarizer into the receptacle until it
latches in place (Fig. 30).
Motorized analyzer
• Insert the analyzer cube as described in section 5.7 "Equipping the Incident Light filter
turret" → p. 26, in the corresponding position
on the filter turret. See the list provided
(“identification Sheet”) for the correct
position.
6.9 DIC Prisms
• Insert the objective prism into the tube slot
(Fig. 31.1). The code letter must match the
code letter on the objective.
• With the microscope Leica DM5000 B the DIC
objective prisms are already mounted in the
DIC turret above the objective revolving
nosepiece(Fig. 68).
5.10 Optional Accessories
Fig. 30 Inserting the analyzer
1 The plug cap is replaced with the analyzer.
1
28
Fig. 31 Inserting the objective prism slide
1 Objective prism slide
1

5. Assembly
Ergomodule
For raising the eye level of the tube opening, the
ergomodule may be used.
It is fastened in place with the side clamping
screw.
Mirror Housing
• Place the mirror housing directly onto the
lamp housing receptacle on the back of the
stand and attach it using the side clamping
screw.
• Place the lamp housing onto the mirror housing and fasten it using the corresponding
clamping screw on the side.
Booster Lens / Excitation Manager
• Remove the clamping ring from the filter slide.
• Insert the Booster Lens or Excitation
Manager.
• Push the cover back.
• Insert the clamping ring.
• Insert the filter slide into the front receptacle
on the right side of the stand (32.1, 33.1).
• Using two filter sliders, the Excitation
Manager can be inserted into the back
receptacle.
5.11 Connection to the Power Supply
Fig. 32
1 Insert of Booster Lens
Fig. 33
1 Insert of Excitation Manager
1
1
29

5. Assembly
After completing the assembly work, connect
the stand to the power supply using the power
cable (Fig. 34.2).
5.12 Connection to the CTR5000 Electronics Box
Fig. 34 Rear side of stand Leica DM4000 B/M
1 Power switch
2 Power supply
1
2
Fig. 35 Rear side of electronics box CTR5000
1 Microscope connection
2 Power supply
Only for the Leica DM5000 B:
• Connect the microscope (36.1) to the
"Microscope" jack (35.1) on the rear of the
electronics box. Use the cable with the 25-pin
plug.
• Connect the electronics box to the power supply using the power cable (35.2).
Fig. 36 Rear side of stand Leica DM5000 B
1 Connection to the CTR5000 electronics box
30
1
1
2
2

6. Startup
6. Startup
6.1 Functional Principle
The microscope’s most important functions may be easily accessed using function keys.
• The microscope may be switched between various contrast processes by pressing a single button.
• The microscope recognizes the objective chosen and the respective contrast process. Therefore, the values for intensity (INT), aperture diaphragm (AP) and field diaphragm (FD) are always set correctly.
• The values for INT, AP and FD can be changed individually. This overwrites the previous
setting. Actual settings are stored automatically.
• The specifications for INT, AP and FD always relate to the currently activated light axis (transmitted light or incident light).
• In addition to the preset function keys for INT, AP and FD, there are also variable function keys.
Variable function keys:
• These function keys are assigned logical functions before delivery(see “Identification Sheet”)
• These functions can be reprogrammed according to your individual wishes.
The microscope can be reset to the default functions programmed at the factory:
• When the microscope is switched off, press all 3 variable function keys on the left stand
section.
• Switch on the stand.
• Hold the keys pressed down until initialization is complete.
• The standard information is shown in the display.
• Switch off the instrument and switch it on again. The settings are stored now.
6.2 Switching on the Microscope
• First, swivel the objective with the least magnification into position.
Note: (Reset-Function)
31

6. Startup
Possible Assignments for the Function Keys
For Leica DM4000 B/DM5000 B:
Function key Meaning
BF Bright field (Transmitted light)
PH Phase contrast (Transmitted light)
ICT Interference contrast (Transmitted light)
DF Dark field (Transmitted light)
POL Polarization (Transmitted light)
CHANGE TL Switch through all transmitted light processes
|
INT ↑ Increase brightness (transmitted light)
INT ↓ Reduce brightness (transmitted light)
AP ↑ Open aperture diaphragm (transmitted light)
AP ↓ Close aperture diaphragm (transmitted light)
FD ↑ Open field diaphragm (transmitted light)
FD ↓ Close field diaphragm (transmitted light)
SHUTTER TL Open/close transmitted light shutter
FLUO Fluorescence (last filter cube)
CUBE 1 Select fluorescence cube at position 1
CHANGE CUBE Switch through fluorescence cubes in clockwise fashion
CHANGE CUBE Switch through fluorescence cubes in counterclockwise fashion
|
|
SHUTTER FLUO Open/close fluorescence shutter
INT FLUO ↑ Increase brightness (fluorescence)
INT FLUO ↓ Reduce brightness (fluorescence)
FD FLUO ↑ Open field diaphragm (fluorescence)
FD FLUO ↓ Open field diaphragm (fluorescence)
COMBI Combination mode
|
(PH / fluorescence or ICT / fluorescence)
CHANGE COMBI Switch through all combination modes
|
32

For Leica DM4000 M:
Function key Meaning
BF Bright field (Incident light)
ICR Interference contrast (Incident light)
DF Dark field (Incident light)
POL Polarization (Incident light)
CHANGE RL Switch through all incident light processes
INT ↑ Increase brightness (incident light)
INT ↓ Reduce brightness (incident light)
AP ↑ Open aperture diaphragm (incident light)
AP ↓ Close aperture diaphragm (incident light)
FD ↑ Open field diaphragm (incident light)
FD ↓ Close field diaphragm (incident light)
SHUTTER RL Open/close incident light shutter
FLUO Fluorescence (last filter cube)
CUBE 1 Select fluorescence cube at position 1
CHANGE FLUO Switch through fluorescence cubes
|
6. Startup
FOCUS FINDER Select smallest field diaphragm and switch back to original field
diaphragm by pressing the key again
BF TL Bright field (Transmitted light )
INT ↑ Increase brightness (transmitted light)
INT ↓ Reduce brightness (transmitted light)
AP ↑ Open aperture diaphragm (transmitted light)
AP ↓ Close aperture diaphragm (transmitted light)
FD ↑ Open field diaphragm (transmitted light)
FD ↓ Close field diaphragm (transmitted light)
COMBI Combination process (BF and BF TL)
|
33

6. Startup
• Switch-on the microscope at the power
switch (34.1,36.1). All motorized microscope
components first undergo an initialization
phase.
After initialization is complete, the display on the
stand shows the current microscope setting (Fig.
37).
The microscopic components such as diaphragms, condenser, light and phase rings are
already pre-centered in the factory. However,
re-centering may be necessary due to transportation and assembly.
Before proceeding with the necessary steps,
first familiarize yourself with the stand’s display
and control panel.
Caution:
After turning on the gas discharge lamps, the
burner must be immediately adjusted. Therefore, do not turn on the power supply unit
yet. First, work in transmitted light in order to
familiarize yourself with the microscope’s
controls.
6.3 The Display (Leica DM4000 B/DM4000 M)
34
Fig. 37 Display after initialization

6. Startup
The display shows the current microscope settings. The display depends on the microscope’s
configuration. In the first column, corresponding
pictograms indicate the type of information: contrast method, magnification, light intensity, diaphragms, light splitting for photo tubes.
Please see the abbreviation index for a list of ab-
breviations and pictograms used →
Contrast Method
In the first row, you find an indication of the active light axis (transmitted light or incident light)
of the current contrast method and the current
filter cube.
The shutter status is displayed for the
transmitted light or incident light shutter:
Transmitted light shutter open
↑
Transmitted light shutter closed
↑
Incident light shutter open
↓
Incident light shutter closed
p. 67.
↓
The actual brightness setting is graphically depicted by a beam. Additionally, the light intensity
is indicated in 20 (coarse adjustment) or in 255
(fine adjustment) increments →
The values for the field diaphragm (FD) and the
aperture diaphragm (AP) are indicated numerically. The field diaphragm may be either round or
rectangular. Accordingly, the FD designation is
set in parentheses or in brackets: (FD) or [FD].
When using a digital camera, rectangular field
diaphragms are recommended.
If a motorized tube is used, the light splitting
between ocular (Eye) and photo output (Docu) is
indicated in %.
Light Intensity
Diaphragms
Note:
Beam splitting
p. 52.
+
Magnification
The current objective magnification, sometimes
followed by the re-magnification of the magnification changer, appears along with the total
magnification:
Σ = Objective x Re-magnification x Eyepiece
6.4 The Function Keys
Note:
The display may flash after the initialization
phase or even during microscopy session. This
always occurs when the contrast method
selected can not be performed with the actual
microscopic settings. For example, an objective
may be swiveled in that is not suited to the
contrast method chosen.
Then check your settings.
35

6. Startup
There is a row of function keys both on the right
and left side of the stand. Some of these keys
are defined, and some of them are variable. The
variable function keys have various meanings
depending on the microscope configuration.
Defined Function Keys on the left side of the
stand
The TL/IL key (38.1) switches between incident
light and transmitted light. The last contrast
method used is restored.
The INT (38.3) keys adjust the light intensity indi-
vidually. Settings can be made either in large or
small increments. Pushing both INT buttons at
the same time switches between coarse and
fine setting. The display indicator changes
accordingly →
p. 52.
The AP (38.4) keys for the aperture diaphragm
and FD (38.2) for the field diaphragm are used
to set each diaphragm. The optimal values are
automatically preset when selecting the contrast method.
Variable function keys:
A factory preset is performed which fits your
microscope configuration. The function keys are
labeled accordingly, and a separate description
of the key occupation accompanies the
microscope (“Identification Sheet”).
Abbreations are listed on p.32f.
6.5 Köhler Illumination
For each objective, optimal values for the aperture diaphragm and the field diaphragm are already set. The condenser is also already
adjusted in the factory.
Fig. 38 Defined Function Keys
1 Transmitted light/incident light
2 Field diaphragm
3 Light Intensity
4 Aperture diaphragm
2
36
3
4
1

6. Startup
However, depending on how the condenser is
disassembled and reassembled, it may be necessary to re-adjust the condenser in some
cases. Therefore, check the condenser
centering.
The following procedure is provided for the
transmitted light-bright field illumination.
• Select an objective with moderate
magnification (10x-40x).
• Activate the transmitted light axis by pushing
the TL/IL button (38.1). "TL" appears in the first
line of the display.
• Choose "bright field" as the contrast method by
pressing the BF (one of the variable function
keys, behind the focus dials).
"TL BF" appears in the first line of the display.
• Insert the specimen in the stage’s specimen
holder (39.3).
• Focus on the specimen. The focus wheel on
the left side of the stage allows focus adjustment in large and small increments. On the
right side of the stage, there is also a focus
wheel for fine focus adjustment.
• Set the light intensity using the INT keys (38.3).
• Close the field diaphragm with the FD function
key (38.2) until the edge of the diaphragm appears in the specimen plane.
• Using the condenser height adjuster (39.4), adjust the condenser until the edge of the field
diaphragm appears in sharp relief.
• If the image does not appear in the middle of
the field of view (41c), the condenser must be
moved into the middle of the field of view with
the help of the two leveling screws (40.1).
Fig. 39 Stage with specimen holder
1 Object motion (X direction)
2 Object motion (Y direction)
3 Specimen holder
4 Condenser height adjuster
3
2
1
4
37

6. Startup
• Open the field diaphragm just enough for it to
disappear from the field of view (41d).
Caution:
Do not adjust the aperture diaphragm. The aperture diaphragm is already set optimally for each
objective.
6.6. Checking Phase Contrast Rings
Fig. 40 Condenser centering
1 Centering bolts
11
38
Fig. 41 Köhler Illumination
a Field diaphragm not focused, not centered
b Field diaphragm focused, but not centered
c Field diaphragm focused and centered
Diameter is too small, however
d Field diameter (light) = Field diameter (view)
(Köhler Illumination)
A
CD
B

If your microscope is equipped for the use of
phase contrast, the light rings that fit the objectives are built into the condenser.
The light rings are already leveled in the factory.
However, the leveling should be rechecked.
6. Startup
• In the place of an eyepiece, insert the focusing telescope (Fig. 42) into the observation
tube.
• Swivel in the phase contrast objective with
the least magnification.
Note:
Every objective is assigned its own light ring in
the condenser disc. Therefore, a check must be
performed for each objective. When swiveling in
a suitable objective for phase contrast, the corresponding light ring is set automatically.
• Press the BF (Bright Field) button (one of the
variable function keys, behind the focus dials).
Fig. 42 Focusing telescope
1 Adjustable eyelens
2 Clamping ring for fixing the focus position
• Focus the ring structure (43a) by slightly loosening the clamping ring (42.2) and moving the
eyelens (42.1).
• Retighten the clamping ring.
• Press the PH (Phase Contrast) button. The ring
diaphragm in the condenser is pivoted in.
• If the light ring and the phase ring are not
shown as arranged in Fig. 43c, the light ring
must be leveled.
Fig. 43 Phase contrast centering procedure
PH=phase contrast ring, LR=light ring
a Condenser in bright field (BF) position
b Condenser in phase contrast (PH) position
Light ring (LR) not centered
c Light ring and phase ring centered
1
AB C
2
PH LR
39

6. Startup
• Insert the centering key through the corresponding openings (44.1) in the condenser
holder.
• Turn the centering screws until the dark ring
(phase ring in the objective) is congruent with
the slightly narrower bright ring (light ring in
condenser) (43 c).
• Repeat the process for all other phase contrast objectives.
• Remove the centering keys after the centering
procedure.
Note:
During change of objectives the centering keys
must not remain in the openings of the
condenser.
6.7 Adjusting the Light Sources
Transmitted Light Axis (TL) with 107/2 Lamp
housing
Fig. 44 Light ring centering
1 Clamping screw
1
40

6. Startup
The 107/2 lamp housing with 12 V 100 W halogen
lamp has a defined presetting. The lamp need
not to be centered.
Incident light axis (IL) with 106 z lamp housing
• When a supply unit is used, it is turned on first.
• Activate the incident light axis using the TL/IL
function key. FLUO (Leica DM4000 B/ DM5000 B)
or IL (Leica DM4000 M) appears in the display.
• Insert the reflector for lamp adjustment
(Fig. 45) into the filter turret in place of a filter
cube. (See →
p. 26).
Note the name of the exchanged filter cube.
• Turn the reflector into the light path.
The reflector has reached the correct position
when the name of the exchanged filter cube is
shown in the upper right of the display.
Caution:
Never look directly into the light path!
When switching to the BF or Smith reflectors,
there is a danger of being glared!
For the 106z lamp housing, the direct filament image (for halogen lamps) or direct arc image (for gas
discharge lamps), and its mirror image are focused
separately and adjusted to each other.
On the left side of the microscope, there is an
adjustment window (1.14, p. 12) for mapping the
light source.
While observing the light source in the adjustment window, the lamp is adjusted as follows:
Centering the 12 V 100 W Halogen Lamp
Fig. 46 106 z lamp housing
1 Lamp height adjustment
2,4 Mirror image height and side adjustment
3 Focusing the reflector
5 Lamp side adjustment
6 Collector (focusing of the lamp image)
Fig. 45 Reflector cube for lamp adjustment
(similar to illustration)
516
2
3
4
41

6. Startup
• In the adjustment window, you see the direct
filament image and the mirror image, which in
most cases are shifted together.
• Focus the direct filament image with the collector (46.6).
• Use the adjusting buttons on the rear side of
the lamp housing (46.2, 46.4) to pivot the lamp
filament’s mirror image to the side or completely out of the beam path. The lamp filament’s focused image remains visible (Fig. 47).
• Adjust the direct filament image using the adjusting knobs (46.1) and (46.5) so that the
centering surface is halfway covered (Fig. 48).
• Then pivot the lamp filament’s mirror image
with the adjusting knobs (46.2 and 4), and
focus it using the reflector (46.3).
• Align the mirror image symmetrically to the filament image (Fig. 49). To do so, use the adjusting knobs (46.2) and (46.4) again.
Fig. 47 Direct lamp filament image focused,
but not centered
(in reality, the image is less focused)
Fig. 48 Direct lamp filament image in target position
(in reality, the image is less focused)
• Defocus the image with the collector head
(46.6) until the filament image and mirror image are no longer recognizable and the image
is uniformly illuminated.
• Exchange the reflector cube for lamp
adjustment for the original filter cube.
Note:
Turn off the microscope before exchanging
the reflector cube.
Centering the Hg 50 W mercury lamp
• In the adjustment window, you see the direct
arc image and the mirror image, which in most
cases are shifted together.
42
Fig. 49 Direct lamp filament image and mirror image in
target position
(in reality, the image is less focused)

• Focus the direct image with the collector
(46.6).
• Use the adjusting buttons on the rear side of
the lamp housing (46.2,46.4) to pivot the arc’s
mirror image to the side or completely out of
the beam path. The lamp filament’s focused
image remains visible (Fig. 50).
• Use the adjusting buttons (46.1) and (46.5) to
place the direct arc image right or left on an
imaginary center line of the centering plane
(Fig. 51).
6. Startup
Fig. 50 Direct arc image focused but decentered
(in reality, the image is less focused)
• Then pivot the arc’s mirror image with the adjusting knobs (46.2 and 4) and focus it using
the reflector (46.3).
• Use the adjusting knobs (46.2 and 4) to orient
the mirror image symmetrically to the direct
image (Fig. 52).
• Defocus the image with the collector knob
(46.6) until the arc image and mirror image are
no longer recognizable and the image is
uniformly illuminated.
• Exchange the reflector cube for lamp
adjustment for the original filter cube.
Centering the Hg 100 W and Xe 75 W
mercury lamps
• In the adjustment window, you see the direct
arc image and the mirror image, which in most
cases are shifted together.
Fig. 51 Direct arc image in target position
(in reality, the image is less focused)
Fig. 52 Direct arc image and mirror image in target
position (in reality, the image is less focused)
43

6. Startup
• Focus the direct image with the collector
(46.6).
• Use the adjusting buttons to pivot the arc’s
mirror image on the rear side of the lamp
housing (46.2,46.4) to the side or completely
out of the beam path. The arc’s focused image remains visible (Fig. 53).
• Use the adjusting buttons (46.1 and 5) to place
the direct arc image in the middle of the
centering plane, whereby the bright tip of the
arc, the focal spot, should lie slightly outside
the center (Fig. 54).
Fig. 53 Direct arc image focused but not centered
(in reality, the image is less focused)
• Then pivot the arc’s mirror image with the adjusting knobs (46.2) and (46.4) and focus it using the reflector (46.3).
• Use the adjusting knobs (46.2 and 4) to orient
the mirror image symmetrically to the direct
image (Fig. 55).
The V-shaped irradiation of the direct image
and mirror image arcs can be superimposed.
Caution:
The bright tips of the arcs, the focal spots, must
never be projected onto each other, as this results in a danger of explosion by overheating.
Fig. 54 Direct arc image in target position
(in reality, the image is less focused)
Fig. 55 Direct arc image and mirror image in target
position (in reality, the image is less focused)
44

In older lamps, the structure of the arc is no
longer clearly recognizable. The image is
then more like that of a HG 50 lamp. The image and mirror image can no longer be superimposed exactly. In this case, align both
images.
• Using the collector, defocus the image with
the knob (46.6) until the arc image and mirror
image are no longer recognizable and the image is uniformly illuminated.
• Exchange the reflector cube for lamp
adjustment for the original filter cube.
Note:
Turn off the microscope before exchanging
the reflector cube.
6. Startup
45

7. Operation
7. Operation
7.1 Switching on the Microscope
When using a gas discharge lamp, the ebq 100
external supply unit must be turned on
separately (56.1).
Then switch-on the microscope at the power
switch.
All motorized microscope components first undergo an initialization phase.
After the initialization is complete, the display on
the stand (Fig. 57) shows the current microscope
setting.
Fig. 56 Front view of the ebq 100 supply unit
1 Power switch
2 Lamp status
7.2 Stages and Specimen Displacement
Lengthening the coaxial pinion
• For lengthening, pull the lower grip (58.2)
downwards. Repeat with the upper grip (58.1).
Torque adjustment
The torque is already optimally set at the factory,
however, it can be individually adjusted using
two knurled rings (58.3, 58.4).
1 2
Fig. 57 Display after initialization
46
Fig. 58 Revolving object stage
1 Object motion (Y direction)
2 Object motion (X direction)
3 Torque adjustment (Y direction)
4 Torque adjustment (X direction)
5 Focus dial for fine focusing
1
2
3
5
4

7. Operation
Rotating the Stage
The swiveling range of the rotating stages is
0°- 110°.
• In order to revolve the stage, loosen the fastening screw (59.1).
• Bring the table into the desired position.
• Retighten the fastening screw.
7.3 Focusing
There is a focus dial on the left side of the stage for
coarse and fine focus adjustment (Fig. 59).
On the right side of the stand, there is also a
focus dial, which is used exclusively for fine
focusing (58.4).
The special design of this dial makes it possible
to simultaneously grasp the coaxial drive with
your hand while operating the fine drive with
one finger.
Fig. 59 Revolving object stage
1 Clamping screw
2 Fine focusing
3 Coarse focusing
1
23
47

7. Operation
7.4 Tubes
Note:
Close any unused tube openings, as otherwise
stray light can interfere with observation.
Note:
Make sure that the connector cable is plugged in
on the MBDT25+ motorized tube (60.1).
Adjusting the Viewing Distance
• Adjust the viewing distance of the eyepieces so that a congruent total image is seen
(Fig. 60).
Adjusting the Viewing Angle
• For the AET22 and EDT22 ergotubes, the viewing angle can be adjusted by tilting the binocular viewer in the range of 5° - 32° (Fig. 61).
Adjusting the Eyepiece Extension to the Arm
Length
• With the AET22 tube, the eyepieces can be
extended up to 30 mm (Fig. 61).
Fig. 60 Tube setting
↔↔
↔
Personal eyebase settings
↔↔
1 Motorized tube connection
1
48
↔↔
↔
↔↔
Fig. 61 With AET22 tube individual adjustments

7. Operation
Beam Splitting in Photo Tubes
EDT22 tube:
The beam splitting between the observation and
documentation outputs has a definite presetting
(50:50).
BDT25+ tube:
The beam splitting is set manually by pulling out
a control bar.
Control Bar Observation Photo
VIS 100 % 0 %
50/50 150 % 50 %
PHOTO 110 % 100 %
MBDT25+ tube:
This tube is similar to the documentation tube
BDT25+, but it is motorized.
The control positions are selected using a variable function key on the stand.
HC L 2TU tube:
The beam splitting is set manually by pulling out
a control bar.
Control Bar Observation Photo
VIS 100 % 0 %
PHOTO 110 % 100 %
Fig. 62 BDT25+ tube with digital camera
1 Control bar
7.5 Eyepieces
Note:
The eyepiece’s aperture protector must be
removed,or at least folded back, during
microscopy while wearing eyeglasses.
Eyeglasses with multifocal lenses (bifocals and
smooth view glasses) must be removed while
operating the microscope.
• For the adjustable tubes with documentation
output, choose the 100% position.
Eyepieces with Inlaid Reticle
• Focus the reticle by adjusting the eyelens.
• Focus on the object through this eyepiece.
• Then, close that eye and focus on the
specimen by adjusting only the second
ocular.
Correction for Vision Problems
• With your right eye, look through the right
eyepiece and bring the specimen into sharp
focus.
• Then, with your left eye, view the same speci-
men and rotate the left eyepiece tube until
the object is brought into sharp focus. Do not
use the focus dial.
1
49

7. Operation
7.6 Objectives
• Start with a small level of magnification. Then
switch to the next higher objective.
The objective must be moved manually into the
light path. Be sure that the nosepiece turret
locks into place.
The objective’s position in the turret is factoryset and must be adhered to while screwing in
the objectives (see Objective Assembly → p. 19)
When you rotate the objective into position, the
microscope
• the selected contrast method
• the optimal settings for field and aperture
diaphragm
• the optimal condenser setting
The objective magnification and the total magni-
fication appear in the display → p. 35.
automatically recognizes:
• For immersion objectives use the appropriate
immersion medium.
OIL: only use optical immersion oil
according to DIN/ISO standards.
Cleaning → p. 65.
W: Water immersion.
IMM: Universal objective for water, glycerol,
oil immersion.
Caution!
Follow safety instructions for immersion oil!
50

7. Operation
For lockable immersion objectives:
• Lock these by pushing the front part upwards
until it stops (approx. 2 mm).
• Then, after a gentle turning motion to the right,
the objective is locked (Fig. 64).
For objectives with corrective mounts:
• Turn the knurl to adjust the objective to the
thickness of the cover glass.
7.7 Magnification Changer
Optionally, a coded magnification changer can
be used, which is manually operated.
On the knurled ring, the following magnification
factors can be set:
B Stand M Stand
1x 1x
1.25x 1.5x
1.6x 2x
The selected factor is indicated in the display
and included in the total magnification.
Fig. 63 Immersion objective (released)
↔↔
↔↔
↔
Fig. 64 Immersion objective (locked)
↔↔
↔↔
↔
51

7. Operation
7.8 Light Sources
• The brightness is set using the function keys
(65.5). Then, the INT function keys are assigned to the currently active axis for transmitted light (TL) or incident light (IL).
• For TL and IL:
Settings can be made either in large or small
increments. Pushing both INT buttons
simultaneously switches between coarse and
fine setting. The display indicator changes accordingly.
0-20
Coarse adjustment:
======
0-255
Fine adjustment:
----------
• For Fluo:
The brightness is set in 5 fixed steps (FIM):
100% / 55% / 35% / 20% / 10%
7.9 Aperture Diaphragm and Field Diaphragm
Both diaphragms are already factory-set to the
optimum setting for the current objective.
• The AP (65.2) keys for the aperture diaphragm
and the FD keys (65.4) for the field diaphragm
may be used to change each diaphragm’s setting at any time.
Then, the function keys are assigned to the
currently active axis for transmitted light (TL)
or incident light (IL).
Caution:
When doing so, old values are overwritten and
the new values are stored!
Caution:
While using PH or DF the aperture diaphragm is
completely opened and locked.
Fig. 645 Control panel
1 Variable function keys
2 Aperture diaphragm
3 Transmitted light/incident light
4 Field diaphragm
5 Light intensity
12345
52

8. Imaging Procedure for Leica DM4000 B/DM5000 B
8. Imaging Procedure
for Leica DM4000 B/ Leica DM5000 B
8.1 Transmitted Light
8.1.1 Bright Field (TL)
• Switch to the transmitted light axis (TL) by
pushing the TL/IL button.
8.1.2 Phase Contrast
• Switch to the transmitted light axis (TL) by
pushing the TL/IL button.
• Select the BF (bright field) contrast method.
Do so by pressing the BF variable key.
Alternatively: Press the CHANGE TL
variable key.
(For key occupation please see “Identification
Sheet”.)
The display indicates BF.
• Insert a transmitted light specimen.
• Rotate an appropriate objective into place.
• Bring the image into focus using the focus dial
and set the brightness using the INT function
key.
|
• Select the PH contrast (phase contrast) method.
Do so by pressing the PH variable key.
Alternatively: Press the CHANGE TL
variable key.
(For key occupation please see “Identification
Sheet”.)
The display indicates PH.
• Insert a transmitted light specimen.
• Rotate an appropriate objective into place.
Objectives that are suitable for phase contrast
are engraved with PH.
• Bring the image into focus using the focus dial
and set the brightness using the INT function
key.
|
53

8. Imaging Procedure for Leica DM4000 B/DM5000 B
8.1.3 Dark Field (TL)
Notes:
• The microscope automatically selects the
correct light ring in the condenser.
• When selecting the phase contrast method,
the aperture diaphragm is opened completely
and may not be adjusted. To avoid errors in
operation, the function keys for setting the aperture diaphragm (AP) are locked.
• Switch to the transmitted light axis (TL) by
pushing the TL/IL button.
• Select the DF (dark field) contrast method.
Do so by pressing the DF variable key.
Alternatively: Press the CHANGE TL
variable key.
(For key occupation please see “Identification
Sheet”.)
The display indicates DF.
The dark field ring (dark field stop) is set automatically.
• Insert a transmitted light specimen.
• Rotate an appropriate objective into place.
• Bring the image into focus using the focus dial
and set the brightness using the INT function
key.
Notes:
|
54
• The maximum objective aperture which may
be used for dark field is 0.75. All objectives
with greater aperture are automatically
blocked for this procedure ("DF" flashes in the
display).
• The microscope automatically selects the
correct light ring in the condenser.
• When selecting the dark field method, the
aperture diaphragm is opened completely and
may not be adjusted. To avoid errors in operation, the function keys for setting the aperture
diaphragm (AP) are locked.

8. Imaging Procedure for Leica DM4000 B/DM5000 B
8.1.4 Polarization (TL)
• Switch to the transmitted light axis (TL) by
pushing the TL/IL button.
• Select the POL (polarization) contrast method.
Do so by pressing the POL variable key.
Alternatively: Press the CHANGE TL
|
variable key.
(For key occupation please see “Identification
Sheet”.)
The display indicates POL.
Mechanical procedure:
• Turn the polarizer on the underside of the
condenser in the light path (Fig. 66). Make sure
that the red index point on the front of the
polarizer is aligned with 0.
• Insert the analyzer into the left side of the
stand (67.1).
• Bring the polarizer and analyzer into cross position until they reach maximum darkness.
• Insert a specimen and rotate a suitable objective into place.
Motorized procedure:
• After selecting the POL contrast method, the
condenser automatically switches to the position of the polarizer. The analyzer cube is also
automatically brought into the light path.
Combined procedure:
• For the Leica DM4000 B and Leica DM5000 B
microscopes, it is possible to combine
mechanical and motorized components.
Fig. 66 Swivel in polarizer
1 Polarizer
1
Fig. 67 Insert analyzer
1 Analyzer
1
55

8. Imaging Procedure for Leica DM4000 B/DM5000 B
8.1.5 Differential Interference Contrast (TL)
(only for DM5000 B)
• Switch to the transmitted light axis (TL) by
pushing the TL/IL button.
• Insert a specimen and rotate a suitable objective into place.
• Select the DIC contrast method.
Do so by pressing the DIC variable key.
Alternatively: Press the CHANGE TL
|
variable key.
(For key occupation please see “Identification
Sheet”.)
The display indicates ICT.
• The polarizer located in the condenser and the
fitting condenser prism are automatically
brought into the light path. The corresponding
objective prism and the analyzer cube are also
positioned automatically.
• For fine adjustment use the knurled ring above
the objective nose piece (Fig. 68).
Alternatively:
• Manually rotate the polarizer on the underside
of the condenser into the light path (Fig. 66).
• Likewise, manually insert the analyzer into the
left side of the stand (Fig. 67).
Objective and coindenser prisms are
automatically moved into the light path as
well.
• Fine adjustment is possible using the knurled
ring above the objective nosepiece.
56
Fig. 68 Objective prism slide
1 Knurled wheel for fine adjusting
1

8. Imaging Procedure for Leica DM4000 B/DM5000 B
8.2 Fluorescence
• Switch to the fluorescent light axis (FLUO) by
pushing the TL/IL button.
• Insert a specimen and rotate a suitable objective into place.
• The current fluorescence cube is indicated on
the display.
• Closing the incident light shutter protects your
specimen from fading.
Do so by pressing the SHUTTER variable key.
(For key occupation please see “Identification
Sheet”.)
The display indicates the symbol: ↓
• Selecting the fluorescence filter cube:
Press the variable keys
Cube or Cube
|
|
• The fluorescence intensity can be increased
using the Booster Lens on the right side of the
stand (Fig. 69).
• For multifluorescence, use of a Excitation
Manager is recommended. The Excitation
Manager is inserted into the right side of the
stand up to the last stop (Fig. 70).
• Using Booster Lens
and Excitation Manager,
the Excitation Manager can be inserted into
the back receptacle.
Fig. 70 Inserting the Excitation ManagerFig. 69 Inserting the Booster Lens
57

9. Imaging Procedure for Leica DM4000 M
9. Imaging procedure
for Leica DM4000 M
9.1 Incident Light
9.1.1 Bright Field
• Switch to the incident light axis (IL) by pushing
the TL/IL button.
• Select the BF (bright field) contrast method.
Do so by pressing the BF variable key.
Alternatively: Press the CHANGE RL
variable key.
(For key occupation please see “Identification
Sheet”.)
The display indicates BF.
• Insert a specimen.
• Rotate an appropriate objective into place.
• Bring the image into focus using the focus dial
and set the brightness using the INT function
key.
|
9.1.2 Dark Field
• Switch to the incident light axis (IL) by pushing
the TL/IL button.
• Select the DF (dark field) contrast method.
Do so by pressing the DF variable key.
Alternatively: Press the CHANGE RL
variable key.
(For key occupation please see “Identification
Sheet”.)
The display indicates DF.
The DF reflector is turned into the beam
path.
• Insert a specimen.
• Rotate an appropriate objective into place.
• Bring the image into focus using the focus dial
and set the brightness using the INT function
key.
Notes:
|
58
• The maximum objective aperture which may
be used for dark field is 0.75. All objectives
with greater aperture are automatically
blocked for this procedure ("DF" flashes in the
display).
• When selecting the dark field method, the
aperture diaphragm is opened completely and
may not be adjusted. To avoid errors in operation, the function keys for setting the aperture
diaphragm (AP) are locked.

9. Imaging Procedure for Leica DM4000 M
9.1.3 Polarization
• Switch to the incident light axis (IL) by pushing
the TL/IL button.
• Select the POL (polarization) contrast method.
Do so by pressing the POL variable key.
Alternatively: Press the CHANGE RL
|
variable key.
(For key occupation please see “Identification
Sheet”.)
The display indicates POL.
Automatic procedure:
• The ICR filter cube is automatically brought
into the light path.
Mechanical procedure:
• Rotate the appropriate polarizer (71.3) and the
IC/P analyzer (72.1) on the stand manually into
the light path. Also bring the polarizer and
analyzer into cross position until they reach
maximum darkness.
• Insert a specimen and rotate a suitable objective into place.
Fig. 71 Objective prism slide
1 Knurled wheel for fine focusing
2 Prism slot with inserted objective prism slide
3 Insert polarizer
1
Fig. 72
1 Insert analyzer
1
3
2
59

9. Imaging Procedure for Leica DM4000 M
9.1.4 Interference Contrast
• Switch to the incident light axis (IL) by pushing
the TL/IL button.
• Insert a specimen and rotate a suitable objective into place.
• Select the DIC contrast method.
Do so by pressing the DIC variable key.
Alternatively: Press the CHANGE RL
variable key.
(For key occupation please see “Identification
Sheet”.)
The display indicates ICR.
• The ICR filter cube (containing polarizer and
analyzer) is automatically brought into the light
path on the incident light axis. Insert the objective prism slide into the prism slot (71.2).
Alternatively:
• Rotate the ICR polarizer (71.3) and the IC/P
analyzer (72.1) on the stand manually into the
light path.
|
9.2 Transmitted Light
9.2.1 Bright Field
• Switch to the transmitted light axis by pushing
the TL/IL button.
• Select the BF (bright field) contrast method.
Do so by pressing the BF variable key.
Alternatively: Press the CHANGE RL
variable key.
(For key occupation please see “Identification
Sheet”.)
The display indicates BF.
• Insert a transmitted light specimen.
• Rotate an appropriate objective into place.
• Use the focus dial to bring the image into focus and set the brightness using the INT function key.
|
• Insert the objective prism slide into the prism
slot (71.2).
• For fine adjustment, rotate the knurled screw
(71.1) on the objective prism slide.
60

10. Trouble Shooting
10. Trouble Shooting
Problem
Stand
The microscope does not respond.
Illumination
The image is completely dark.
Cause/Remedy
Make sure that voltage is impressed.
Make sure that the microscope is connected
to the power supply.
Check the cable connections.
Inform service technician to change the fuses.
Open the shutter (→
Check the connection of the lamp houses to
p. 35).
the microscope.
Transmitted axis:
Incident (Fluo) axis:
Make sure that the lamps are connected to the
power supply.
Inform service technician to change the fuses
of the ebq 100.
The image is unevenly or not uniformly illuminated.
The illumination "flickers."
The lamp does not illuminate immediately upon
being switched on.
Remove all unneeded filters from the light
path.
Center the lamp (→
Replace the old lamp (→
Be sure that there is no loose connection at
p. 41ff).
p. 20ff).
the power supply.
Replace the old lamp (→
The ebq 100 must be switched-on repeatedly.
Hot Hg lamps should cool down before
p. 20ff).
switching on again.
61

10. Trouble Shooting
Problem
Bright Field
The specimen can not be brought into focus.
Dark Field
No definite DF contrast is possible.
The image is unevenly or not uniformly illuminated.
Cause/Remedy
Use the correct immersion medium.
Lay the specimen with the cover glass to-
wards the top.
Make sure that the cover glass thickness is
correct and that is conform to the indication
on the objective.
Check the condenser centering.
Be sure that a DF objective is being used.
The objective aperture setting is too high
(maximum 0.75). If necessary, reduce the objective aperture using the iris diaphragm on
the objective.
Check the condenser centering.
The magnification is too weak. Use a higher
magnification.
Undesirable stray light
Phase contrast
No phase contrast is possible.
62
Clean the specimen and neighboring lenses
p. 65).
(→
The specimen is too thick.
The refraction index of embedding material
and object is identical.
The cover glass is not placed evenly.
Therefore, check the centering of the light
rings (→
p. 39).

10. Trouble Shooting
Problem
Polarization
No polarization contrast is possible.
Fluorescence
The image is completely dark (no fluorescence).
The fluorescence is too weak.
Display
The display flashes.
Cause/Remedy
Bring the polarizer and analyzer into cross po-
sition until they reach maximum darkness
(without specimen) (→
Open the shutter (→
Select the incident light axis (IL) (→
Check the antigen-antibody combination.
Insert the Booster Lens (→
Center the lamp (→
Insert a new lamp (→
Rotate an appropriate objective for the con-
p. 55, 59).
p. 57).
p. 41ff).
p. 20f).
p. 29).
p. 36).
trast method into the light path.
FAIL! appears.
Check insertion of objectives, cubes, etc.
63

11. Care of the Microscope
11. Care of the Microscope
Caution!
Unplug the power supply before performing
cleaning and maintenance work!
Protect electrical components from moisture!
Microscopes in warm and warm-damp climatic
zones require special care in order to prevent
fungus contamination.
The microscope should be cleaned after each
use, and the microscope optics should be kept
strictly clean.
11.1 Dust Cover
Note:
To protect against dust, cover the microscope and
accessories with the dust cover after each use.
Caution!
Let lamps cool down before covering the
stand with a dust cover. The dust cover is
not heat-resistant. In addition condensation
water may occur.
11.2 Cleaning
Caution:
Residual fiber and dust can create unwanted
!
background fluorescence.
Cleaning Coated Parts
Dust and loose dirt particles can be removed
with a soft brush or lint-free cotton cloth.
Clinging dirt can be cleaned with all
commercially available water solutions, benzine
or alcohol.
For cleaning coated parts, use a linen or leather
cloth that is moistened with one of these substances.
Caution:
Acetone, xylene or nitro-containing thinner
!
can harm the microscope and thus may not
be used.
Test cleaning solutions of unknown composition
first on a less visible area of the unit. Be sure
that coated or plastic surfaces do not become
matted or etched.
Cleaning the Stage
Remove light-colored spots on the stage by rubbing with paraffin oil or acid-free Vaseline.
64

11. Care of the Microscope
Cleaning Glass Surfaces
Remove dust on glass surfaces with a fine, dry
and fat-free hair brush, by blowing with a blow
bag or vacuum suction.
Carefully remove stubborn dirt on glass surfaces
with a clean cloth moistened with distilled
water. If the dirt still can not be removed, use
pure alcohol, chloroform or benzine.
Cleaning Objectives
Caution!
The objective may not be unscrewed during
cleaning. If damage appears on inner surfaces, the objectives must be sent to your
Leica subsidiary for repair. We also advise
against cleaning the inside surfaces of the
eyepieces.
The front lenses of objectives are cleaned as described under "Cleaning Glass Surfaces". The
upper lens is cleaned by being blown off with a
pneumatic pump.
Removing Immersion Oil
Caution!
Follow safety instructions for immersion oil!
First, wipe off the immersion oil with a clean cotton cloth, and then re-wipe the surface several
times with ethyl alcohol.
11.3 Handling Acids and Bases
For examinations using acids or other aggressive chemicals, particular caution must be
taken.
!
Caution:
Be absolutely certain to prevent the optics
and mechanical parts from coming these
chemicals.
65

12. Wear and Spare Parts
12. Essential Wear and Spare Parts
Order No.
Material No. Name Used for
Replacement Lamp
500 974 Halogen lamp 12 V 100 W 107/2 lamp housing
500 137 High-pressure mercury burner 50 W 106 z lamp housing
500 138 High-pressure mercury burner 100 W 106 z lamp housing
500 321 High-pressure mercury burner 100 W 106 z lamp housing
500 139 High-pressure xenon burner 75 W 106 z lamp housing
Screw cap for unused objective receptacles
020-422.570-000 Screw cap M 25 Objective turret
Replacement eyecup (diaphragm protection) for HC PLAN eyepiece
021-500.017-005 HC PLAN eyecup 10x/25 eyepiece
021-264.520-018 HC PLAN eyecup 10x/22 eyepiece
021-264.520-018 HC PLAN eyecup 10x/20 eyepiece
Immersion Oil conforming to DIN/ISO standards, fluorescence-free
513 787 110 ml OIL and IMM objectives
513 522 100 ml and oil condenser heads
513 788 500 ml
(103 W/2)
66

13. Abbreviations and Pictograms
13. Abbreviations and Pictograms
Contrast method
+
Magnification
↑
↑
↓
↓
AET Advanced Ergo Tube
AP Aperture diaphragm
BF Brifght field
COMBI Combination contrast method
CUBE Filter cube
DF Darkfield
DIC Differentiated interference contrast
FD Field diaphragm
FLUO Fluo axis (incident light)
ICR Interference contrast reflected light
ICT Interference contrast transmitted light
IL Incident light (axis)
INT Intensity
MBDT Motorized Basic Documentation Tube
PH Phase contrast
POL Polarization
TL Transmitted light (axis)
Light intensity/diaphragms
Beam splitting
Transmitted light shutter open
Transmitted light shutter closed
Incident light shutter open
Incident light shutter closed
67

14. Index
14. Index
Adjusting the light sources 41
Allowable ambient conditions 15
Ambient temperature 8, 9
Ambient conditions 15
Analyzer 28, 55, 56, 59
Analyzer cube 55, 56
Aperture diaphragm 12, 35, 38, 52
Beam splitting 49
Bright field 53, 58
Booster Lens 29, 57
Cleaning 64
Cleaning objectives 65
Coaxial pinion 46
Condenser connector 18
Condenser 11, 12, 18
Condenser centering 38
Condenser height adjuster 17, 18
Condenser holder 18
Connection to power supply 30
Dark field 54, 58
Dark field stop 54
Defined function keys 36
DICprisms 28
Differentiated interference contrast 56
Display 12, 35
DMControl 11, 14
Electrical safety 8
Electronics box Leica CTR5000 8, 30
Ergomodule 29
Excitation Manager 10, 29, 57
Eyepiece 12, 49
Field diaphragm 12, 35, 52
Filter block exchanger 13
Filter cube 26
Filter cube ICR 59, 60
FIM (Fluo Intensity Manager) 10, 52
Fluorescence 57
Fluorescence intensity 57
Focus finder 33
Focusing 37, 47
Focusing telescope 39
Function keys 12, 31, 36
Gas discharge lamps 23, 24
Halogen lamp 21, 22, 42
Hg 50 burner 24
ICT/P transmitted light polarizator 27
Identification Sheet 19, 26, 28, 53
Imaging procedure10, 53, 58
Immersion oil 50, 65
Incident light axis 10
Incident light nosepiece disc 26
Incident light polarizators 27
Incident light shutter 35
Initialization 34
Interference contrast 60
Intermediate systems 16
Köhler illumination 18, 37
Lamp housing 13
Lamp housing 106 z 21, 41
Lamp housing 107/2 20, 41
Lamp housing receptacle 20, 22, 25
Light intensity 35
Light sources 41, 52
Light sources (incident light axis) 21
Light sources (transmitted light axis) 20
Magnification changer 11, 51
Mercury lamp Hg 50 W 43
Mercury lamp Hg 100W /Xe 75W 44
Mirror housing 29
Motorized analyzer 28
Motorized polarizer 27
Objective aperture 54
Objective prism slide 60
Objectives 19, 50
Objective turret 10, 12
Object stage 17, 46
Phase contrast 39, 53
Phase contrast rings (checking) 39
Polarization 55, 59
Polarizer 55, 56, 59
Polarizer L/ICR 27
Polarizer R/ICR 27
Polarizer R/P 27
Prism slot 60
Reflector cube 26
Reflector cube for lamp adjustment 41
Reset function 31
Retention pin 26
Rotating polarizer 27
Shutter 57
Software Leica DMControl 11, 14
Specimen holder 17
Supply unit ebq 100 9, 25, 46
Switch transmitted/incident light 12
Touchscreen 11
Transmitted light and
incident light analyzer 28
Transmitted light axis 10
Transmitted light filter 13
Transmitted light shutter 35
Variable function keys 12, 13, 31, 36
Vision problems 49
Xe 75 burner 24
68

15. EU Declaration of Conformity
15. EU Declaration of Conformity
69
 Loading...
Loading...