Page 1
Instruction Manual
SPECIFICATIONSSPECIFICATIONS
SPECIFICATIONS
SPECIFICATIONSSPECIFICATIONS
INSTRUCTIONSINSTRUCTIONS
INSTRUCTIONS
INSTRUCTIONSINSTRUCTIONS
HI 38000
Sulfate
Test Kit
www.hannainst.com
Dear Customer,
Thank you for choosing a Hanna Product.
Please read the instructions carefully before using the
chemical test kit. It will provide you with the necessary
information for correct use of the kit.
Remove the chemical test kit from the packing material and
examine it carefully to make sure that no damage has
occurred during shipping. If there is any noticeable damage, notify your Dealer or the nearest Hanna office
immediately.
Each kit is supplied with:
HI 38000A-0 Sulfate Reagent, packets (100 pcs);
•
HI 38000B-0 Sulfate Reagent
•
Complexing Agent
•
• 1 glass test tube (50 mL);
• 1 plastic vessel (50 mL);
1 plastic pipette (3 mL);
•
1 spoon.
•
Note: Any damaged or defective item must be returned in
its original packing materials.
, 1 bottle with dropper (10 mL);
, 1 bottle (53 g);
Range 20 to 100 mg/L (ppm) as Sulfate
Smallest Increment 5 mg/L from 20 to 30 mg/L
10 mg/L from 30 to 100 mg/L
Analysis Method Turbidimetric
Sample Size 50
Number of Tests 100
Case Dimensions 275x57x78 mm (10.8x2.2x3.1")
Shipping Weight 290 g (10.2 oz.)
SIGNIFICANCE AND USESIGNIFICANCE AND USE
SIGNIFICANCE AND USE
SIGNIFICANCE AND USESIGNIFICANCE AND USE
Sulfate is largely present in natural waters in a wide range
of concentrations. It is not toxic but has to be kept below a
certain threshold to prevent it from creating an unpleasant
taste in water. The concentrations are particularly higher
close to mine run-off water. Sulfate is extensively used as a
nutrient in agriculture.
The procedure for determining sulfate is a modification of
the Barium Sulfate Turbidimetric Method.
Note:
mg/L is equivalent to ppm (parts per million).
CHEMICAL REACTIONCHEMICAL REACTION
CHEMICAL REACTION
CHEMICAL REACTIONCHEMICAL REACTION
Sulfate is precipitated as barium sulfate by reaction with
barium chloride in acidic medium. The turbidity is proportional to the concentration of sulfate:
2-
SO
+ Ba2+ → BaSO
4
ISTR38000 10/99 PRINTED IN ITALY
mL
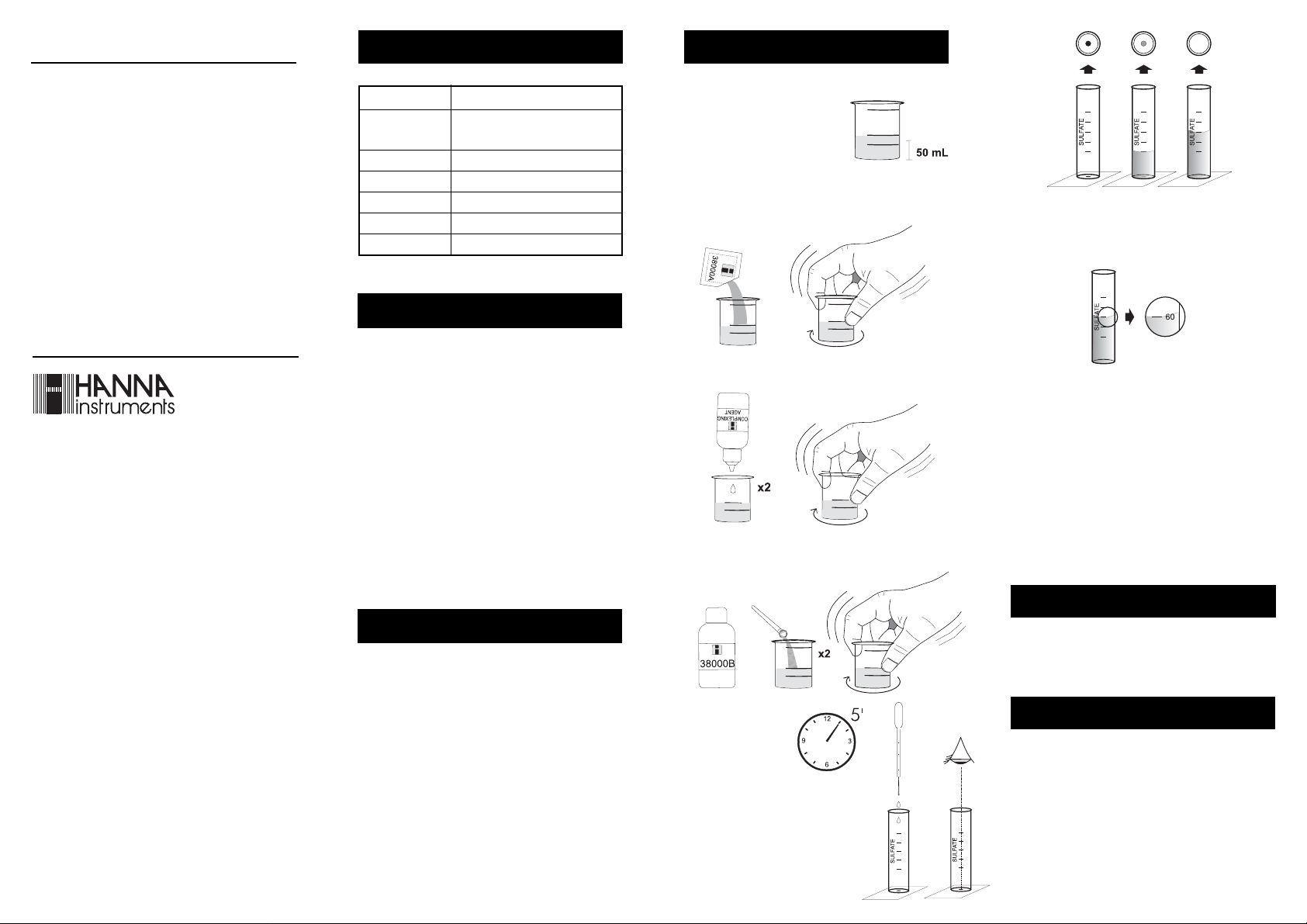
READ THE ENTIRE INSTRUCTIONS BEFORE USING THE KIT
• Fill the plastic vessel with 50
mL of the sample, up to the
mark.
• Add 1 packet of HI 38000A-0 Sulfate Reagent and
swirl gently to dissolve.
• Read the concentration in mg/L (ppm) of Sulfate, in
correspondence of the level of the liquid in the test tube.
• Add 2 drops of Complexing Agent and swirl to mix.
Note: In the case the spot on the bottom disappears with
the liquid level under the 100 ppm mark , the
sulfate concentration is higher than 100 ppm; in the
case the spot disappears with the liquid level above
the 20 ppm mark, the sulfate concentration is lower
than 20 ppm.
Note: To measure Sulfate in the 100 to 10000 ppm range,
• Add two spoons of HI 38000B-0 reagent and swirl
gently to mix.
• Wait for 5 minutes
to allow reaction
4
to occur.
• Keep the test tube on a white
surface and look from the top at the
black spot on the bottom. Use the
plastic pipette to fill the tube with
the reacted sample until the black
spot has completely disappeared.
use the HI 38001 Sulfate Low and High Range Test
Kit.
REFERENCESREFERENCES
REFERENCES
REFERENCESREFERENCES
Adaptation of the Barium Sulfate Turbidimetric Method.
HEALTH AND SAFETYHEALTH AND SAFETY
HEALTH AND SAFETY
HEALTH AND SAFETYHEALTH AND SAFETY
The chemicals contained in this kit may be hazardous if
improperly handled. Read Health and Safety Data Sheet
before performing this test.
 Loading...
Loading...