Page 1

Prodigy Advance
128cm (51”)
Tailored for advanced skeletal and metabolic
health assessment
Optimal health depends on accurate diagnosis
and preventive treatment. That’s why so many
professionals around the globe rely on Prodigy™
for comprehensive body composition analysis,
including bone mineral density (BMD) and lean
and fat tissue mass.
Prodigy delivers reliable dual-energy X-ray
absorptiometry (DXA) with excellent precision and
extremely low radiation dose. Prodigy efficiently
streamlines patient care and practice workflow.
You can trust Prodigy to help ensure the vitality
of your patients and your practice.
Scanner dimensions
Full-size bed:
262cm (103”) 201cm (79”) 109cm (43”)
Compact bed:
25cm (10”)
US/CALA version - enCORE v16 - Some features may not be available in all markets.
63cm (25”)
67cm (26”)
Page 2
2.4m (8')
2.3m (7.5')
Prodigy Advance specifications (nominal)
Software applications and features:
Clinical applications:
• AP spine
• Femur
• DualFemur
• Forearm/supine forearm
• Total body BMD
• Dual-energy Vertebral
Assessment (DVA)
(lateral and AP)
• Fracture risk assessment tool:
®2
FRAX
• Total and regional body
composition
1
• Advanced body composition
(data visualization, trending
& reporting tools)
1
• Advanced Hip Assessment
(AHA) with hip strength
analysis
• Pediatric spine/femur/
Complete Pediatric
Hand/supine hand
Small animal total body
• CoreScan™ (visceral fat
quantification)
1
2
1
• Orthopedic hip
• Orthopedic knee
Workflow:
• Previous scan image
comparison
• OneVision
• Automatic metal detection
• Image preview
• OneScan measurement
• SmartScan
• QuickView measurement
(10sec)
Analysis & reporting:
• Custom region of interest
analysis
• Composer reporting tools
• Custom reference creation
• ScanCheck
• Practice management tools
Connectivity:
• HIPAA secure view
• SQL server
• DICOM interface
• HL7 interface
TeleDensitometry (e-mail, fax)
Multi-User DataBase access
(MUDB) (1-3 users)
Multi-User DataBase access
(MUDB) (1-10 users)
Scanner table specifications:
Scanner size (full-size bed) ..............262(W) x 109(D) x 128(H)cm
Scanner size (compact bed) .............201(W) x 109(D) x 128(H)cm
Scanner weight (full-size bed) ....................... 272kg (599lbs)
Scanner weight (compact bed) ...................... 254kg (559lbs)
Patient table top height (adjustable) ....................>63cm (25”)
Maximum patient weight supported .................159kg (350 Ibs)
Drive system ............. stepper motor with reinforced drive belts
Active scan area (full-size bed) ...................197.5cm x 60.0cm
Active scan area (compact bed) ..................135.2cm x 59.5cm
Start position indicator ........cross laser light (class II, <1mW power)
Pad .........................................washable patient mat
Attenuation of patient support table....................<0.7mm AL
Communication cable............................7.62m (25ft) serial
Scanner leakage current ........meets IEC 60601-1 safety standard
Detector specifications:
Detector..............................LYSO x-ray counting detector
Computer specifications:
Non-US customers will need to verify that the computer is certified
to local requirements. The computer must meet the minimum
requirements that follow:
• 1.2 GHz Intel™ Celeron™
or 2.69 GHz AMD Athlon™
processor
• Windows® 7 Professional
(32-bit, 64-bit)
• 2 GB RAM
• 160 GB hard disk
• DVD-R drive
• 17” SVGA monitor with at
least 1024 x 768 32-bit color
• External hard drive
(data archive location)
• Internet Explorer® version 8.0
• Windows-compatible printer
• 1 Serial port required
• Adobe™ Acrobat™ reader
• Serial Port: Onboard RS-232
115K baud DB-9 or StarTech
PCI serial adapter PCI1S950DV
(GE Healthcare P/N LU44192)
OPTIONAL
3
(103” x 43” x 51”)
(79” x 43” x 51”)
Environmental specifications
Power ..................100-120 VAC 50/60Hz 20A dedicated circuit
220-240 VAC 50/60Hz 10A dedicated circuit
Consumption .........................Idling 40VA, Scanning 450VA
Distortion...................sinusodal waveform, less than 5% THD
Humidity ...............................20%-80% non-condensing
Room temperature ..........................18°C-27°C (65°F-81°F)
Scanner heat output ....... idling 150 BTU/hr, scanning 1500BTU/hr
Console heat output ...........approx. 400BTU/hr with 17” monitor
Ventilation.................all cooling vents must remain unblocked
Dust, fumes, debris ..........install system in clean, ventilated area
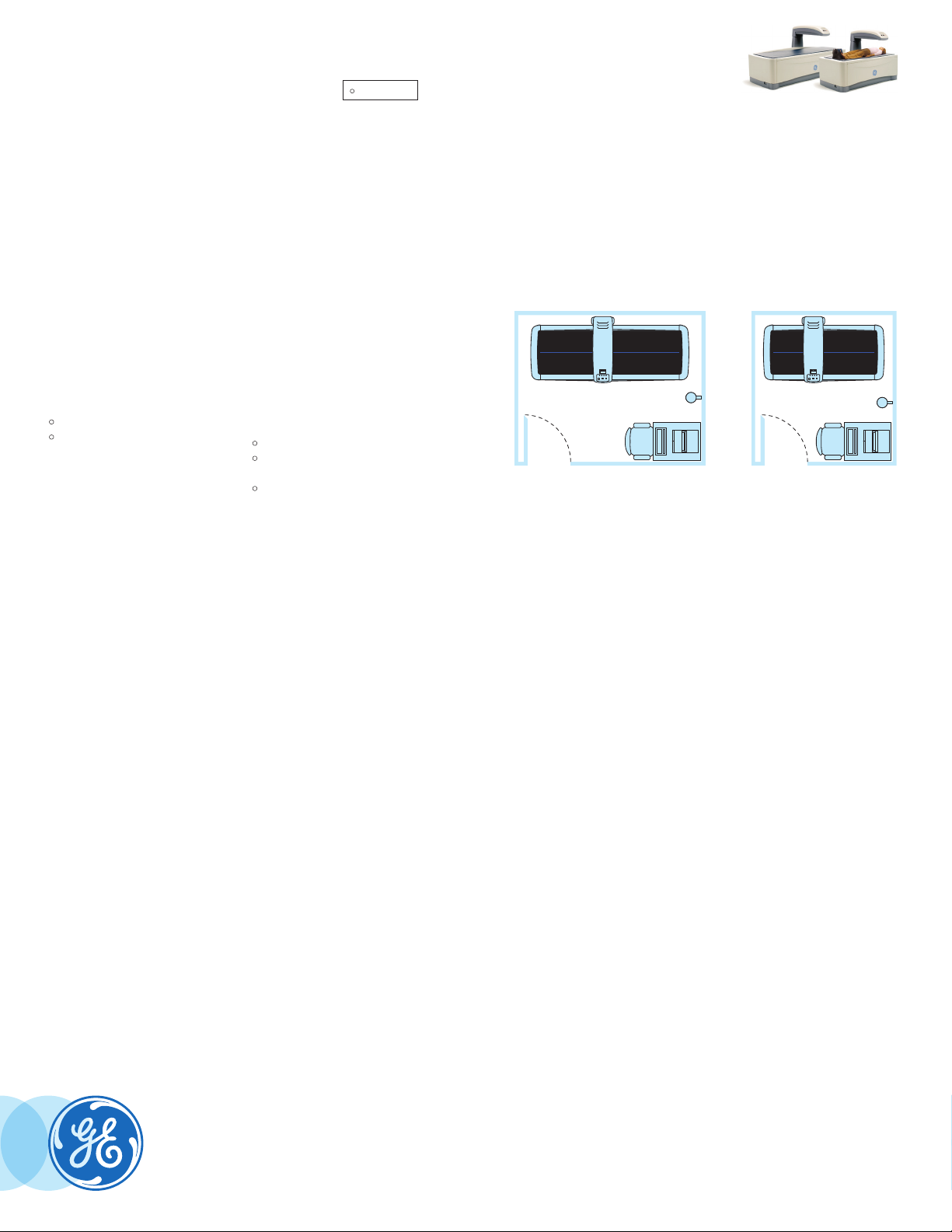
Minimum room dimensions
5
Full-size bed: Compact bed:
2.4m (8')
4
3.0m (10')
About GE Healthcare
GE Healthcare provides transformational medical technologies and services that
are shaping a new age of patient care. Our broad expertise in medical imaging
and information technologies, medical diagnostics, patient monitoring systems,
drug discovery, biopharmaceutical manufacturing technologies, performance
improvement and performance solutions services help our customers to deliver
better care to more people around the world at a lower cost. In addition, we
partner with healthcare leaders, striving to leverage the global policy change
necessary to implement a successful shift to sustainable healthcare systems.
Our “healthymagination” vision for the future invites the world to join us on our
journey as we continuously develop innovations focused on reducing costs,
increasing access and improving quality around the world. Headquartered in
the United Kingdom, GE Healthcare is a unit of General Electric Company (NYSE:
GE). Worldwide, GE Healthcare employees are committed to serving healthcare
professionals and their patients in more than 100 countries. For more information
about GE Healthcare, visit our website at www.gehealthcare.com.
1. Full-size table only.
2. Laboratory animals only
3. Standard in US, may be optional in other regions.
4. Additional hardware may be required for fax capabilities.
5. A small room kit with isolation transformer may be required. Please refer to local regulations.
© 2015 General Electric Company – All rights reserved.
GE, GE monogram, Prodigy and CoreScan are trademarks
of General Electric Company or one of its subsidiaries.
FRAX is a registered trademark of the World Health
Organization Collaborating Centre for Metabolic Bone
Diseases, University of Sheffield, UK. DICOM is a trademark
of National Electrical Manufacturers Association. Windows
and Internet Explorer are registered trademarks of
Microsoft Corporation. Intel and Celeron are trademarks of
Intel Corporation. Athlon is a trademark of Advanced Micro
Devices, Inc. Adobe and Acrobat are trademarks of Adobe
Systems Incorporated. All other trademarks, service marks,
company names and product names are the property of
their respective owners.
General Electric Company reserves the right to make
changes in specifications and features shown herein, or
discontinue the product described at any time without
notice or obligation. Contact your GE representative for
the most current information.
GE Healthcare, a division of General Electric Company
Indications for use: The Prodigy series bone densitometer
provides an estimate of bone mineral density and fat
and lean tissue mass. The values can then be compared
to a reference population at the sole discretion of the
physician.
CAUTION: Federal Law restricts this device to sale by or on
the order of a physician.
Lunar Product Division Americas
GE Healthcare Lunar
Global Headquarters
P.O. Box 7550
Madison, WI 53707-7550
T: +1-800-535-7339
F: +1-608-223-2482
Lunar Product Division Europe
GE Healthcare Europe
Buc Headquarters
283 Rue de la Minière
78533 BUC Cèdex, France
T: +33-800-90-87-19
Asia & Pacific
GE Healthcare Lunar
3/F GE China Technology Park
No. 1 Hua Tuo Road
Shanghai 201203, China
T: +86-21-38777888 (Ext. 60128 or 60480)
F: +86-21-38777451
Lunar Product Division EAGM
(ME, Africa, Russia CIS, Turkey CA)
GE Healthcare
Building #18
Dubai Internet City
P.O. Box 74594
Dubai, United Arab Emirates
T: +971-4-429-6218
F: +971-4-429-6218
US/CALA version - enCORE v16 - Some features may not be available in all markets.
JB20792XXi
 Loading...
Loading...