Page 1

Technical
Publications
Direction 5116269-100
Rev. 2
GE Medical Systems
LOGIQ 7/LOGIQ 7 Pro Advanced Reference
Manual
R4.2.0/R4.2.0Pro
Copyright© 2004, 2005 By General Electric Co.
Operating Documentation
Page 2
Regulatory Requirement
This product complies with regulatory requirements of the following European
Directive 93/42/EEC concerning medical devices
This manual is a reference for the LOGIQ 7/LOGIQ 7 Pro. It applies to all versions of the
SW R4.2.0/R4.2.0Pro and later software for the LOGIQ 7/LOGIQ 7 Pro ultrasound
systems.
GE Medical Systems
GE Medical Systems: Telex 3797371
P.O. Box 414, Milwaukee, Wisconsin 53201 U.S.A.
(Asia, Pacific, Latin America, North America)
GE Medical Systems
283, rue de la Miniere,78533 Buc Cedex
France
Page 3
Revision History
Table i-1: Reason for Change
REV DATE REASON FOR CHANGE
Rev. 1 Aug. 30th, 2004 Initial Release
Rev. 2 February 25, 2005 R4.2.0/R4.2.0Pro Release
List of Effective Pages
Table i-2: List of Effective Pages
REVISION
PAGE NUMBER
Title Page Rev. 2 Chapter 2 Rev. 2
Revision History Rev. 2 Chapter 3 Rev. 2
Table of Contents Rev. 2 Index Rev. 2
Chapter 1 Rev. 2
NUMBER PAGE NUMBER
REVISION
NUMBER
Please verify that you are using the latest revision of this document.
Information pertaining to this document is maintained on GPC (GE
Medical Systems Global Product Configuration). If you need to know the
latest revision, contact your distributor, local GE Sales Representative or
in the USA call the GE Ultrasound Clinical Answer Center at
1 800 682 5327 or 1 262 524 5698.
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual i-1
Direction 5116269-100 Rev. 2
Page 4

This page intentionally left blank.
i-2 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
Page 5

Chapter 1 — Acoustic Output
Bioeffects
Concerns Surrounding the Use of Diagnostic Ultrasound - - - - - - - - - 1-2
Acoustic Output Data
Maximum output summary- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 1-3
Chapter 2 — Measurement Formulas
Cardiac Measurement
Cardiac measurement abbreviation list- - - - - - - - - - - - - - - - - - - - - - 2-2
Measurement Formulas
Formulas–Generic - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-14
Formulas-Urology - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-15
Formulas-Abdominal/Small Parts- - - - - - - - - - - - - - - - - - - - - - - - - 2-16
Formulas–OB- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-17
Formulas–GYN - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-21
Formulas–Vascular- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-23
Formulas-Cardiac - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-24
Table of Contents
Table of Contents
Chapter 3 — OB Tables
OB Tables
ASUM - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-2
Berkowitz- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-8
Bertagnoli - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-9
Brenner - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-9
Campbell - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-10
Chitty - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-10
Eriksen - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-16
Erik-Nes - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-17
Goldstein - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-19
Hadlock - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-20
Hansmann - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-27
Hellman - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-35
Hill - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-35
Hohler - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-36
Jeanty - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-36
JSUM - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-48
Kurtz - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-52
Mayden - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-53
Mercer - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-54
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual TOC-1
Direction 5116269-100 Rev. 2
Page 6

Merz - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-55
Moore - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-65
Nelson - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-65
Osaka - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-66
Paris - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-70
Rempen - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-73
Robinson - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-78
Tokyo - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-78
Tokyo Shinozuka - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-82
Williams - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-88
Yarkoni - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-88
TOC-2 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
Page 7

Chapter 1
Acoustic Output
Provides Acoustic Output information and tables, as
well as possible bioeffects and prudent use.
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual 1-1
Direction 5116269-100 Rev. 2
Page 8

Acoustic Output
Bioeffects
Concerns Surrounding the Use of Diagnostic Ultrasound
During a diagnostic ultrasound examination, high frequency
sound penetrates and interacts with tissue in and around the
area of anatomy to be imaged. Only a small portion of this sound
energy is reflected back to the probe for use in constructing the
image while the remainder is dissipated within the tissue. The
interaction of sound energy with tissue at sufficiently high levels
can produce biological effects (aka bioeffects) of either a
mechanical or thermal nature. Although the generation of
bioeffect is intentional with therapeutic ultrasound, it is generally
undesired in diagnostic applications and may be harmful in
some conditions.
NOTE: The American Institute of Ultrasound in Medicine has published
a document entitled "Medical Ultrasound Safety". This three
part document covers Bioeffects and Biophysics, Prudent Use
and Implementing ALARA.
Ultrasound users should read the AIUM to become more familiar
with Ultrasound safety.
NOTE: Only for U.S.A. : A copy of this document is included as part of
the documentation package (Document 2163920-100).
To contact the AIUM concerning their publications:
• In the USA, by telephone at 1-800-638-5352.
• To write them, use the following address:
AIUM
14750 Sweitzer Lane
Suite 100
Laurel, MD, USA 20707-5906
1-2 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
Page 9
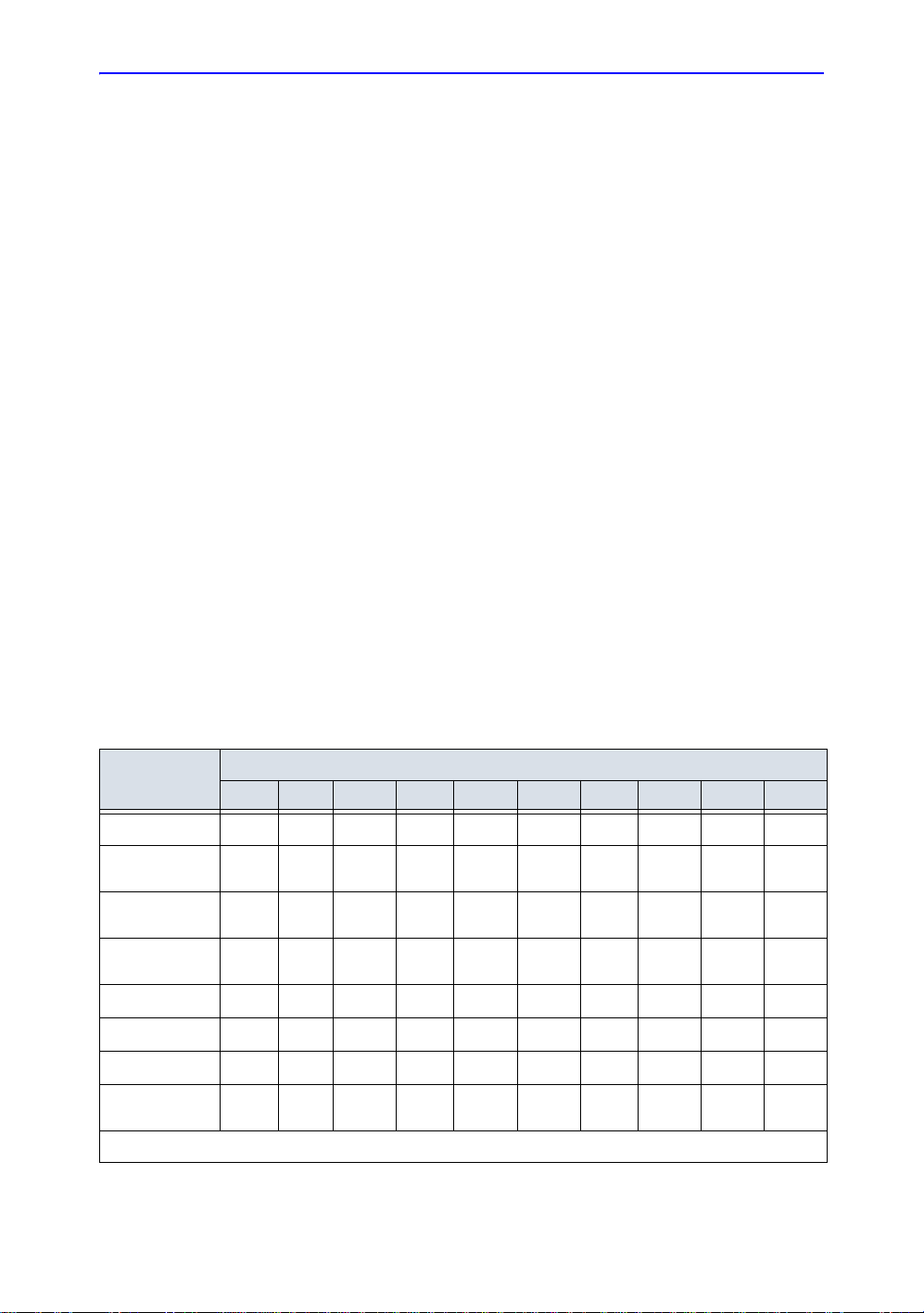
Maximum output summary
The following tables list the typical maimum acoustic output
levels achievalbe with LOGIQ 7/LOGIQ 7 Pro for all probes and
operational modes. It is intended that this is information be
useful in making ALARA decisions and selecting the most
appropriate probe for the application . In ac co rd an ce with US
FDA Guidelines, the overall maximum acoustic SPTA intensity
for LOGIQ 7/LOGIQ 7 Pro is limited to 720 mW/cm
limited to 1.9. Modes for which TI does not exceed 0.1 are
indicated by <0.1.
• The accuracy of acoustic output table of this manual : +0%/-
50%
• Accuracy of TI and MI is ±50%.
Acoustic Output Data
Acoustic Output Data
2
and MI is
Operating Mode
B-mode
M-mode
(inc.B-mode)
Color Flow (inc.
B-mode)
Pulsed Doppler
(inc.B-mode)
B-Flow n/a
Contrast n/a
CW Doppler n/a n/a
Other
(specify)
*LOGIQ 7 Only
3C 3.5C 3.5CS 5C E8C BE9C 8C M7C* 7L 10L
YES YES YES YES <1 <1 <1 YES <1 YES
YES YES YES YES <1 <1 <1 YES <1 YES
YES YES YES YES YES YES YES YES YES YES
YES YES YES YES YES YES YES YES YES YES
n/a n/a
Table 1-1 shows all of the probes’ operating modes. The
following pages contain probe acoustic outpu t tables for those
noted in Table 1-1.
Table 1-1: Summary Table
Transducer Modell
YES YES
YES YES
n/a
n/a
n/a n/a
n/a n/a
n/a n/a
n/a n/a
n/a n/a
n/a n/a
n/a n/a
n/a n/a
n/a
n/a
n/a
n/a
YES YES
YES YES
n/a n/a
n/a n/a
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual 1-3
Direction 5116269-100 Rev. 2
Page 10

Acoustic Output
Tab le 1- 2: Summary Table
Transducer Modell
Operating Mode
B-mode
M-mode
(inc.B-mode)
Color Flow (inc. B-
mode)
Pulsed Doppler
(inc.B-mode)
B-Flow
Contrast
CW Doppler
Other
(specify)
*LOGIQ 7 Only
M12L i12L T739 3S 7S 10S 6T M3S* P2D P6D
YES YES YES YES YES YES YES <1
YES YES YES YES <1 YES YES <1
YES YES YES YES YES YES YES YES
YES YES YES YES YES <1 YES YES
YES
n/a
n/a
n/a
n/a
n/a
n/a
n/a
YES
n/a
n/a YES YES YES YES YES YES
n/a
n/a
YES
n/a
n/a
n/a
n/a
n/a
n/a
n/a
n/a n/a
n/a YES
n/a n/a
n/a n/a
n/a n/a
n/a n/a
n/a n/a
n/a n/a
n/a n/a
<1
n/a n/a
1-4 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
Page 11
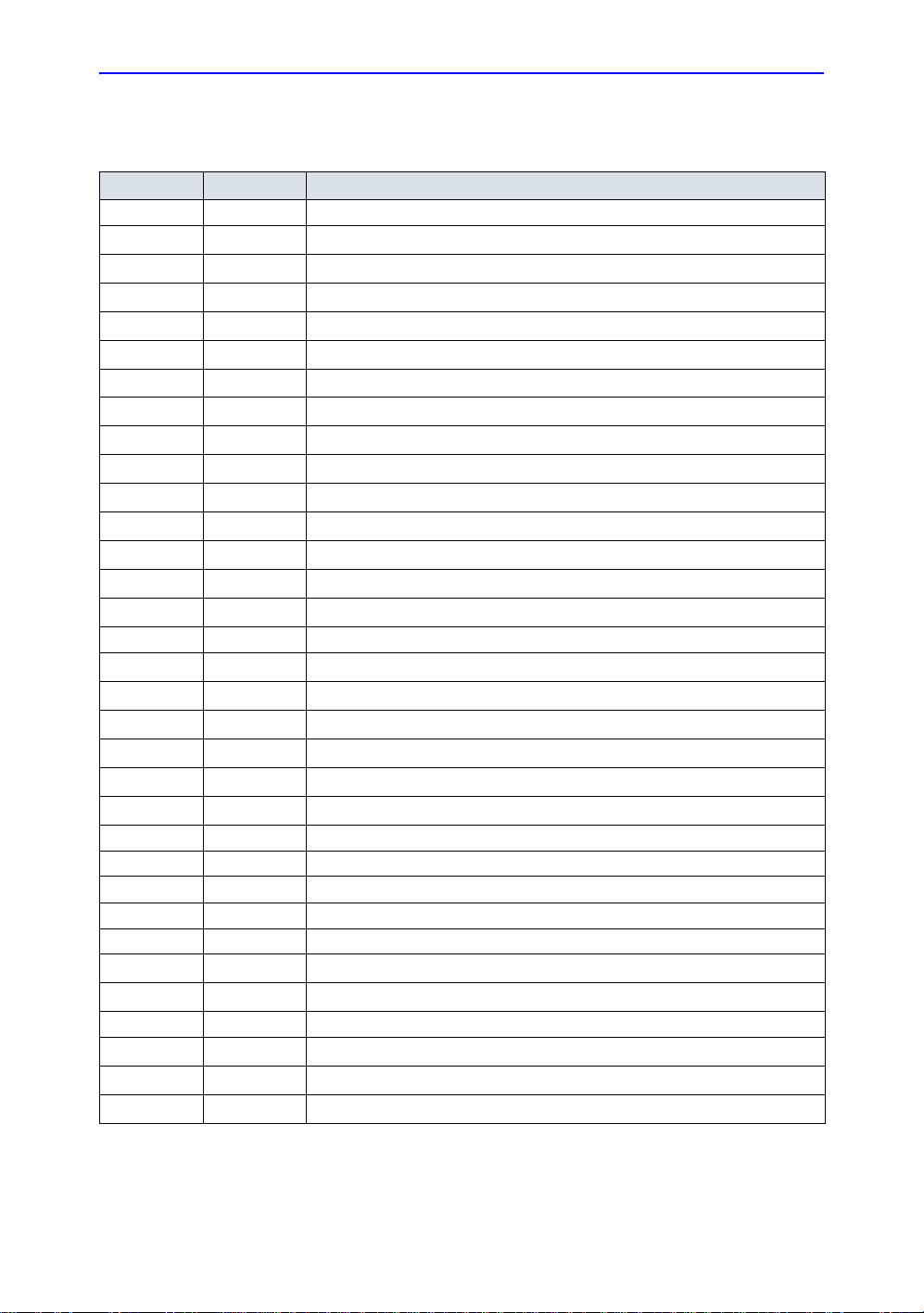
Acoustic Output Data
List of Acoustic Output Parameters
Table 1-3: List of Acoustic Output Parameters
FDA IEC Meaning—IEC 60601-2-37 / FDA & NEMA UD2, UD3
a α Acoustic Attenuation Coefficient / Derating factor (usually 0.3 dB/cm-MHz)
A
aprt
D
eq
d
-6
d
eq
f
c
I
pa
I
pa.3
PII I
PII
.3
I
TA
(Z) I
I
TA.3
I
(Z) I
SPTA
I
(Z) I
SPTA.3
A
arpt
C
MI
D
eq
d
-6
d
eq
ƒ
awf
I
pa
I
pa,α
pi
I
pi,α
Ita(z) Temporal-Average Intensity
(z) Attenuated Temporal-Average Intensity / (at depth z)
ta,α
(z) Spatial-Peak Temporal-Average Intensity
zpta
(z) Attenuated Spatial-Peak Temporal-Average Intensity
zpta,α
MI MI Mechanical Index
W
o
W
(Z) P
.3
W
o1
PII p
p
r
p
r.3
P Output Power / Time average acoustic power at the source
α
P
1
i
p
r
p
rα
PRF prr Pulse Repetition Rate / Pulse repetition frequency
TI TI Thermal Index / (same)
TIB TIB Bone Thermal Index / (same)
TIC TIC Cranial-Bone Thermal Index / (same)
TIS TIS Soft-Tissue Thermal Index / (same)
PD t
x
-12,y-12
d
X, Y -12 dB Output Beam Dimensions / (same)
Z Z Distance from the Source to a Specified Point / (same)
Z
sp
Z
bp
Z
sp
Z
b
Z
bp
Z
s
-12db Output Beam Area / Active aperture area
Normalizing Coefficient
Equivalent Aperture Diameter / (same)
Pulse Beam Width / Beam diameter at –6 dB
Equivalent Beam Diameter
Acoustic Working Frequency / Center frequency
Pulse-Average Intensity
Attenuated Pulse-Average Intensity
Pulse-Intensity Integral
Attenuated Pulse-Intensity Integral
Attenuated Output Power / Time average acoustic power derated to depth z
Bounded Output Power / Power emitted from the central 1cm of aperture
Pulse Pressure Squared Integral / Pulse intensity integral
Peak-Rarefactional Acoustic Pressure / (same)
Attenuated Peak-Rarefactional Acoustic Pressure / (same)
Pulse Duration / (same)
Depth for TIB / Depth at which the relevant index is maximum
Break-Point Depth / (same)
Depth for TIS / Depth at whcih the relevant index is maximum
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual 1-5
Direction 5116269-100 Rev. 2
Page 12
Acoustic Output
3C Probe
Table 1-4: Transducer Mode: 3C Operating Mode: B-Mode
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.5 1.4 - - - 2.7
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 2.4
r.3
(mW) 144.7 - -144.7
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) -
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 3.3
deq(zb) deq(zsp) (cm) -
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 2.6 2.6 - - - 2.6
X(cm) 1.4 - - - 1.4
Y(cm)
PD (µsec) 1.0
1.0 - - - 1.0
prr PRF (Hz) 2927.4
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FLX(cm) 3.5 - - 3.5
I
at max. MI I
pa,α
PA.3
(MPa) 3.2
max
(cm) -
max
FL
(cm) 7.0 - - 7.0
Y
2
@MI
max
(W/cm
447.4
)
Frequency (MHz) T4.0 T4.0 - - - T4.0
Image Depth (cm) 24.0 10.0 - - - 10.0
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
1-6 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
Page 13
3C Probe (continued)
Table 1-5: Transducer Mode: 3C Operating Mode: M-Mode (inc.B-mode)
Acoustic Output Data
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.5 1.4 - <1 1.6 2.4
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 2.4
r.3
(mW) 144.7 - 33.5 126.1
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) #
zs z1(cm) #
zbp zbp(cm) #
zb zsp(cm) 3.3
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 3.3
deq(zb) deq(zsp) (cm) 0.4
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 2.6 2.6 - # 2.6 2.6
X(cm) 1.4 - # 1.4 1.4
Y(cm)
PD (µsec) 1.0
1.0 - # 1.0 1.0
prr PRF (Hz) 2927.4
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FLX(cm) 3.5 - # 3.5
I
at max. MI I
pa,α
PA.3
(MPa) 3.2
max
(cm) 0.4
max
FL
(cm) 7.0 - # 7.0
Y
2
@MI
max
(W/cm
448.9
)
Frequency (MHz) T4.0 T4.0 - # T4.0 T4.0
Image Depth (cm) 24.0 10.0 - # 24.0 24.0
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual 1-7
Direction 5116269-100 Rev. 2
Page 14
Acoustic Output
3C Probe (continued)
Table 1-6: Transducer Mode: 3C Operating Mode: CF-Mode (inch.M-mode)
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
non-
scan
Global Maximum: Index Value 1.6 1.4 1.1 - 3.2 2.5
IEC FDA Units
pra p
P W
min of [P
α
(zs, I
(MPa) 1.9
r.3
o
)] [(W
ta,α(zs
.3(Z1),ITA.3
(mW) 144.7 90.3 90.3 152.8
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) 3.3
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 3.3
deq(zb) deq(zsp) (cm) 0.4
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 2.6 2.6 2.5 - 2.6 2.6
X(cm) 1.4 0.9 - 1.4 1.9
Y(cm)
PD (µsec) 1.0
1.0 1.0 - 1.0 1.0
prr PRF (Hz) 635.2
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
(MPa) 3.3
max
(cm) 0.4
max
(cm) 3.5 2.2 - 4.8
X
FL
(cm) 7.0 7.0 - 3.5
Y
2
@MI
max
(W/cm
447.7
)
Frequency (MHz) D2.5 D2.5 D2.5 - D2.5 D2.5
Image Depth (cm) 3.5 4.8 3.5 - 3.5 4.8
Vel Scale (kHz) 1.80 4.90 1.1 - 1.07 4.90
Penet On On On - On On
ROI 60deg 5deg - - - 5deg
Operating Control
Conditions
PS 14 14 16.0 - 16 14
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
1-8 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
Page 15
Acoustic Output Data
3C Probe (continued)
Table 1-7: Transducer Mode: 3C Operating Mode: PWD-Mode (inch.B-mode)
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.6 1.4 1.1 - 3.2 2.2
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 2.5
r.3
(mW) 144.7 90.3 90.3 103.9
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) 2.6
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 3.3
deq(zb) deq(zsp) (cm) 0.5
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 2.6 2.6 2.5 - 2.5 2.5
X(cm) 1.4 0.9 - 0.9 0.9
Y(cm)
PD (µsec) 1.0
1.0 1.0 - 1.0 1.0
prr PRF (Hz) 1796.0
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
(MPa) 3.3
max
(cm) 0.5
max
(cm) 3.5 2.2 - 2.2
X
FL
(cm) 7.0 7.0 - 7.0
Y
2
@MI
max
(W/cm
447.4
)
Frequency (MHz) D2.5 T4.0 D2.5 - D2.5 D2.5
Image Depth (cm) 3.5 10.0 2.2 - 2.2 2.2
Vel Scale (kHz) 0.64 - 1.23 - 1.23 1.23
SV 3 - 6 - 6 6
Penet on - on - on on
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual 1-9
Direction 5116269-100 Rev. 2
Page 16
Acoustic Output
3.5C Probe
Table 1-8: Transducer Mode: 3.5C Operating Mode: B-Mode
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.5 <1 - - - 2.3
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 2.4
r.3
(mW) #- -216.5
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) -
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 3.6
deq(zb) deq(zsp) (cm) -
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 2.4 # - - - 2.0
X(cm) #- - -3.3
Y(cm)
PD (µsec) 0.7
#- - -1.3
prr PRF (Hz) 2383.2
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FLX(cm) #- - 4.8
I
at max. MI I
pa,α
PA.3
(MPa) 3.2
max
(cm) -
max
FL
(cm) #- - 6.6
Y
2
@MI
max
(W/cm
288.4
)
Frequency (MHz) T5.0 # - - - T4.0
Image Depth (cm) 30.0 # - - - 8.0
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
1-10 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
Page 17
3.5C Probe (continued)
Table 1-9: Transducer Mode: 3.5C Operating Mode: M-Mode (inc.B-mode)
Acoustic Output Data
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.5 < 1 - <1 1.0 2.0
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 2.4
r.3
(mW) #- 34.8 186.2
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) #
zs z1(cm) #
zbp zbp(cm) #
zb zsp(cm) 3.6
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 3.6
deq(zb) deq(zsp) (cm) 0.6
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 2.4 # - # 2.4 2.0
X(cm) #- #2.43.3
Y(cm)
PD (µsec) 0.7
#- #1.31.3
prr PRF (Hz) 2383.2
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FLX(cm) #- # 4.8
I
at max. MI I
pa,α
PA.3
(MPa) 3.2
max
(cm) 0.6
max
FL
(cm) #- # 6.6
Y
2
@MI
max
(W/cm
288.4
)
Frequency (MHz) T5.0 # - # T5.0 T5.0
Image Depth (cm) 30.0 # - # 30.0 30.0
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual 1-11
Direction 5116269-100 Rev. 2
Page 18
Acoustic Output
3.5C Probe (continued)
Table 1-10: Transducer Mode: 3.5C Operating Mode: CF-Mode (inch.M-mode)
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
non-
scan
Global Maximum: Index Value 1.7 <1 - <1 2.1 2.3
IEC FDA Units
pra p
P W
min of [P
α
(zs, I
(MPa) 1.8
r.3
o
)] [(W
ta,α(zs
.3(Z1),ITA.3
(mW) #- 31.5 214.2
(z1)]) #
zs z1(cm) #
zbp zbp(cm) #
zb zsp(cm) 5.0
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 5.0
deq(zb) deq(zsp) (cm) 0.5
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 3.1 # - # 3.1 2.0
X(cm) #- #3.33.3
Y(cm)
PD (µsec) 0.8
#- #1.31.3
prr PRF (Hz) 635.2
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
(MPa) 4.5
max
(cm) 0.5
max
(cm) #- # 4.8
X
FL
(cm) #- # 6.6
Y
2
@MI
max
(W/cm
452.8
)
Frequency (MHz) D3.3 # - # D3.3 D2.0
Image Depth (cm) 4.8 # - # 4.8 4.8
Vel Scale (kHz) 1.80 # - # 1.23 7.69
Penet Off # - # Off On
ROI 70deg # - # - 5deg
Operating Control
Conditions
PS 12 # - # 16 12
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
1-12 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
Page 19
Acoustic Output Data
3.5C Probe (continued)
Table 1-11: Transducer Mode: 3.5C Operating Mode: PWD-Mode (inch.B-mode)
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.7 <1 - <1 2.1 <1
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 3.0
r.3
(mW) #- 31.5 #
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) #
zs z1(cm) #
zbp zbp(cm) #
zb zsp(cm) 3.7
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 5.0
deq(zb) deq(zsp) (cm) 0.3
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 3.1 # - # 2.0 #
X(cm) #- #2.4#
Y(cm)
PD (µsec) 0.8
#- #1.3#
prr PRF (Hz) 1796.0
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
(MPa) 4.5
max
(cm) 0.3
max
(cm) #- # #
X
FL
(cm) #- # #
Y
2
@MI
max
(W/cm
452.3
)
Frequency (MHz) D3.3 # - # D2.0 #
Image Depth (cm) 4.8 # - # 3.5 #
Vel Scale (kHz) 0.64 - - # 1.23 #
SV 1 - - # 4 #
Penet Off - - # On #
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual 1-13
Direction 5116269-100 Rev. 2
Page 20
Acoustic Output
3.5C Probe (continued)
Table 1-12: Transducer Mode: 3.5C Operating Mode: Contrast
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.5 2.0 - - - 1.5
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 2.7
r.3
(mW) 141.8 - - 141.8
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) -
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 3.5
deq(zb) deq(zsp) (cm) -
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 3.2 3.2 - - - 3.2
X(cm) 3.3 - - - 3.3
Y(cm)
PD (µsec) 0.4
1.3 - - - 1.3
prr PRF (Hz) 2383.2
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
Frequency (MHz) CHA
(MPa) 4.1
max
(cm) -
max
(cm) 4.8 - - 4.8
X
FL
(cm) 6.6 - - 6.6
Y
2
@MI
max
(W/cm
337.0
)
3.0
CHA
3.0
---CHA
3.0
Image Depth (cm) 30.0 8.0 - - - 8.0
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
1-14 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
Page 21
3.5C Probe (continued)
Table 1-13: Transducer Mode: 3.5C Operating Mode: B-Flow
Acoustic Output Data
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.7 <1 - <1 2.1 1.8
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 2.8
r.3
(mW) #- 31.5 162.4
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) #
zs z1(cm) #
zbp zbp(cm) #
zb zsp(cm) 3.7
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 2.5
deq(zb) deq(zsp) (cm) 0.3
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 2.7 # - # 2.0 2.0
X(cm) #- #2.42.5
Y(cm)
PD (µsec) 1.9
#- #1.31.3
prr PRF (Hz) 300.0
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
(MPa) 3.4
max
(cm) 03
max
(cm) #- # 5.8
X
FL
(cm) #- # 6.6
Y
2
@MI
max
(W/cm
173.6
)
Frequency (MHz) BF3.3 # - # D2.0 T4.0
Image Depth (cm) 25.0 # - # 3.5 8.0
Vel Scale (kHz) - # - # 1.23 SV - # - # 4 Penet - # - # On -
Operating Control
Conditions
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual 1-15
Direction 5116269-100 Rev. 2
Page 22
Acoustic Output
3.5CS Probe
Table 1-14: Transducer Mode: 3.5CS Operating Mode: B-Mode
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.2 <1 - - - 2.6
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 1.9
r.3
(mW) #- -189.1
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) -
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 3.2
deq(zb) deq(zsp) (cm) -
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 2.5 # - - - 2.0
X(cm) #- - -2.0
Y(cm)
PD (µsec) 0.8
#- - -1.3
prr PRF (Hz) 2290.0
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FLX(cm) #- - 4.8
I
at max. MI I
pa,α
PA.3
(MPa) 2.6
max
(cm) -
max
FL
(cm) #- - 6.6
Y
2
@MI
max
(W/cm
144.6
)
Frequency (MHz) T5.0 # - - - T4.0
Image Depth (cm) 30.0 # - - - 8.0
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
1-16 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
Page 23
Acoustic Output Data
3.5CS Probe (continued)
Table 1-15: Transducer Mode: 3.5CS Operating Mode: M-Mode (inc.B-mode)
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.2 <1 - <1 <1 2.3
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 1.9
r.3
(mW) #- #164.3
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) #
zs z1(cm) #
zbp zbp(cm) #
zb zsp(cm) #
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 3.2
deq(zb) deq(zsp) (cm) #
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 2.5 # - # # 2.0
X(cm) #- ##2.0
Y(cm)
PD (µsec) 0.8
#- ##1.3
prr PRF (Hz) 2290.0
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FLX(cm) #- # 4.8
I
at max. MI I
pa,α
PA.3
(MPa) 2.6
max
(cm) #
max
FL
(cm) #- # 6.6
Y
2
@MI
max
(W/cm
144.6
)
Frequency (MHz) T5.0 # - # # T5.0
Image Depth (cm) 30.0 # - # # 30.0
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual 1-17
Direction 5116269-100 Rev. 2
Page 24
Acoustic Output
3.5CS Probe (continued)
Table 1-16: Transducer Mode: 3.5CS Operating Mode: CF-Mode (inch.M-mode)
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
non-
scan
Global Maximum: Index Value 1.2 <1 - <1 1.6 1.7
IEC FDA Units
pra p
P W
min of [P
α
(zs, I
(MPa) 1.9
r.3
o
)] [(W
ta,α(zs
.3(Z1),ITA.3
(mW) #- 50.1 119.6
(z1)]) #
zs z1(cm) #
zbp zbp(cm) #
zb zsp(cm) 4.3
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 3.2
deq(zb) deq(zsp) (cm) 0.6
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 2.5 # - # 3.3 2.0
X(cm) #- #2.02.0
Y(cm)
PD (µsec) 0.8
#- #1.31.3
prr PRF (Hz) 2290.0
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
(MPa) 2.6
max
(cm) 0.6
max
(cm) #- # 4.8
X
FL
(cm) #- # 6.6
Y
2
@MI
max
(W/cm
144.6
)
Frequency (MHz) D3.3 # - # D3.3 D2.0
Image Depth (cm) 4.8 # - # 4.8 4.8
Vel Scale (kHz) 1.80 # - # 1.23 7.69
Penet Off # - # Off On
ROI 70deg # - # - 5deg
Operating Control
Conditions
PS 12 # - # 16 12
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
1-18 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
Page 25
Acoustic Output Data
3.5CS Probe (continued)
Table 1-17: Transducer Mode: 3.5CS Operating Mode: PWD-Mode (inch.B-mo de)
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.2 <1 - <1 1.6 1.3
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 1.9
r.3
(mW) #- 50.1 84.8
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) #
zs z1(cm) #
zbp zbp(cm) #
zb zsp(cm) 3.4
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 3.2
deq(zb) deq(zsp) (cm) 0.7
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 2.5 # - # 2.0 2.0
X(cm) #- #1.41.4
Y(cm)
PD (µsec) 0.8
#- #1.31.3
prr PRF (Hz) 2290.0
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
(MPa) 2.6
max
(cm) 0.7
max
(cm) #- # 4.8
X
FL
(cm) #- # 6.6
Y
2
@MI
max
(W/cm
144.6
)
Frequency (MHz) D3.3 # - # D2.0 D2.0
Image Depth (cm) 4.8 # - # 3.5 3.5
Vel Scale (kHz) 0.64 - - # 1.23 1.23
SV 1 - - # 4 4
Penet Off - - # On On
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual 1-19
Direction 5116269-100 Rev. 2
Page 26
Acoustic Output
3.5CS Probe (continued)
Table 1-18: Transducer Mode: 3.5CS Operating Mode: Contrast
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.5 1.3 - - - 2.4
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 2.2
r.3
(mW) 193.5 - - 193.5
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) -
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 4.5
deq(zb) deq(zsp) (cm) -
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 2.1 2.1 - - - 2.1
X(cm) 2.5 - - - 2.5
Y(cm)
PD (µsec) 1.1
1.3 - - - 1.3
prr PRF (Hz) 2288.9
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
Frequency (MHz) CHA
(MPa) 3.2
max
(cm) -
max
(cm) 5.1 - - 5.1
X
FL
(cm) 6.6 - - 6.6
Y
2
@MI
max
(W/cm
288.3
)
3.0
CHA
3.0
---CHA
3.0
Image Depth (cm) 30.0 6.0 - - - 6.0
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
1-20 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
Page 27
3.5CS Probe (continued)
Tab le 1-19: Transducer Mode: 3.5CS Operating Mode: B-Flow
Acoustic Output Data
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.7 <1 - <1 1.6 1.1
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 2.8
r.3
(mW) #- 50.1 81.3
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) #
zs z1(cm) #
zbp zbp(cm) #
zb zsp(cm) 3.4
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 4.5
deq(zb) deq(zsp) (cm) 0.7
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 2.7 # - # 2.0 2.7
X(cm) #- #1.42.0
Y(cm)
PD (µsec) 1.9
#- #1.31.3
prr PRF (Hz) 2285.2
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
(MPa) 3.9
max
(cm) 0.7
max
(cm) #- # 4.5
X
FL
(cm) #- # 6.6
Y
2
@MI
max
(W/cm
282.4
)
Frequency (MHz) BF3.3 # - # D2.0 BF3.3
Image Depth (cm) 30.0 # - # 3.5 5.0
Vel Scale (kHz) - # - # 1.23 SV - # - # 4 Penet - # - # On -
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual 1-21
Direction 5116269-100 Rev. 2
Page 28
Acoustic Output
5C Probe
Table 1-20: Transducer Mode: 5C Operating Mode: B-Mode
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.3 1.2 - - - 1.6
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 2.4
r.3
(mW) 76.9 - -76.9
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) -
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 2.9
deq(zb) deq(zsp) (cm) -
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 3.5 3.5 - - - 3.5
X(cm) 1.3 - - - 1.3
Y(cm)
PD (µsec) 0.5
0.9 - - - 0.9
prr PRF (Hz) 2927.4
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FLX(cm) 4.5 - - 4.5
I
at max. MI I
pa,α
PA.3
(MPa) 3.1
max
(cm) -
max
FL
(cm) 6.0 - - 6.0
Y
2
@MI
max
(W/cm
645.8
)
Frequency (MHz) 4.0 4.0 - - - 4.0
Image Depth (cm) 24.0 15.0 - - - 15.0
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
1-22 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
Page 29
5C Probe (continued)
Table 1-21: Transducer Mode: 5C Operating Mode: M-Mode (inc.B-mode)
Acoustic Output Data
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.3 1.2 <1 - <1 1.4
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 2.4
r.3
(mW) 76.0 # #66.7
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) #
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 2.9
deq(zb) deq(zsp) (cm) #
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 3.5 3.5 # - # 3.5
X(cm) 1.3 # - # 1.3
Y(cm)
PD (µsec) 0.5
0.9 # - # 0.9
prr PRF (Hz) 2927.4
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FLX(cm) 4.5 # - 4.5
I
at max. MI I
pa,α
PA.3
(MPa) 3.1
max
(cm) #
max
FL
(cm) 6.0 # - 6.0
Y
2
@MI
max
(W/cm
645.8
)
Frequency (MHz) 4.0 4.0 # - # 4.0
Image Depth (cm) 24.0 15.0 # - # 24.0
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual 1-23
Direction 5116269-100 Rev. 2
Page 30
Acoustic Output
5C Probe (continued)
Table 1-22: Transducer Mode: 5C Operating Mode: CF-Mode (inch.M-mode)
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
non-
scan
Global Maximum: Index Value 1.8 1.2 - <1 1.6 1.2
IEC FDA Units
pra p
P W
min of [P
α
(zs, I
(MPa) 3.2
r.3
o
)] [(W
ta,α(zs
.3(Z1),ITA.3
(mW) 76.9 - 37.8 51.4
(z1)]) #
zs z1(cm) #
zbp zbp(cm) #
zb zsp(cm) 3.1
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 3.3
deq(zb) deq(zsp) (cm) 0.6
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 3.9 3.5 - # 3.9 4.0
X(cm) 1.3 - # 1.4 0.9
Y(cm)
PD (µsec) 0.6
0.9 - # 0.9 0.9
prr PRF (Hz) 635.3
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
(MPa) 4.8
max
(cm) 0.6
max
(cm) 4.5 - # 2.1
X
FL
(cm) 6.0 - # 6.0
Y
2
@MI
max
(W/cm
460.1
)
Frequency (MHz) D4.0 D4.0 - # D4.0 D4.0
Image Depth (cm) 3.3 2.1 - # 3.3 2.1
Vel Scale (kHz) 1.80 8.53 - # 2.13 8.53
Penet On On - # On On
ROI 60deg 10deg - # - 10deg
Operating Control
Conditions
PS 14 14 - # 16 14
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
1-24 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
Page 31

Acoustic Output Data
5C Probe (continued)
Table 1-23: Transducer Mode: 5C Operating Mode: PWD-Mode (inch.B-mode)
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.8 1.2 - <1 1.6 <1
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 3.2
r.3
(mW) 76.9 - 37.8 #
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) #
zs z1(cm) #
zbp zbp(cm) #
zb zsp(cm) 3.4
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 3.3
deq(zb) deq(zsp) (cm) 0.7
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 3.9 3.5 - # 4.0 #
X(cm) 1.3 - # 4.0 #
Y(cm)
PD (µsec) 0.6
0.9 - # 0.9 #
prr PRF (Hz) 635.3
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
(MPa) 4.8
max
(cm) 0.4
max
(cm) 4.5 - # #
X
FL
(cm) 6.0 - # #
Y
2
@MI
max
(W/cm
460.1
)
Frequency (MHz) D4.0 4.0 - # D4.0 #
Image Depth (cm) 3.3 15.0 - # 3.3 #
Vel Scale (kHz) 0.64 - - # 0.68 #
SV 1 - - # 3 #
Penet On - - # On #
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual 1-25
Direction 5116269-100 Rev. 2
Page 32

Acoustic Output
8C Probe
Table 1-24: Transducer Mode: 8C Operating Mode: B-Mode
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.3 <1 - - - <1
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 2.6
r.3
(mW) #- -#
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) -
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 1.8
deq(zb) deq(zsp) (cm) -
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 4.2 # - - - #
X(cm) #- - -#
Y(cm)
PD (µsec) 0.5
#- - -#
prr PRF (Hz) 5376.3
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FLX(cm) #- - #
I
at max. MI I
pa,α
PA.3
(MPa) 3.4
max
(cm) -
max
FL
(cm) #- - #
Y
2
@MI
max
(W/cm
303.2
)
Frequency (MHz) T8.0 # - - - #
Image Depth (cm) 12.0 # - - - #
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
1-26 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
Page 33

8C Probe (continued)
Table 1-25: Transducer Mode: 8C Operating Mode: M-Mode (inc.B-mode)
Acoustic Output Data
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.3 <1 <1 - <1 <1
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 2.6
r.3
(mW) ## ##
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) #
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 1.8
deq(zb) deq(zsp) (cm) #
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 4.2 # # - # #
X(cm) ## - ##
Y(cm)
PD (µsec) 0.5
## - ##
prr PRF (Hz) 5376.3
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FLX(cm) ## - #
I
at max. MI I
pa,α
PA.3
(MPa) 3.4
max
(cm) #
max
FL
(cm) ## - #
Y
2
@MI
max
(W/cm
303.2
)
Frequency (MHz) T8.0 # # - # #
Image Depth (cm) 12.0 # # - # #
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual 1-27
Direction 5116269-100 Rev. 2
Page 34

Acoustic Output
8C Probe (continued)
Table 1-26: Transducer Mode: 8C Operating Mode: CF-Mode (inch.M-mode)
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
non-
scan
Global Maximum: Index Value 1.4 <1 <1 - 1.5 1.3
IEC FDA Units
pra p
P W
min of [P
α
(zs, I
(MPa) 3.1
r.3
o
)] [(W
ta,α(zs
.3(Z1),ITA.3
(mW) ## 26.7 32.2
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) 1.9
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 1.9
deq(zb) deq(zsp) (cm) 0.4
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 4.7 # # - 4.7 4.7
X(cm) ## -0.60.6
Y(cm)
PD (µsec) 0.6
## -0.50.5
prr PRF (Hz) 635.2
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
(MPa) 4.1
max
(cm) 0.4
max
(cm) ## - 2.4
X
FL
(cm) ## - 2.7
Y
2
@MI
max
(W/cm
399.2
)
Frequency (MHz) D5.0 # # - D5.0 D5.0
Image Depth (cm) 2.4 # # - 2.4 2.4
Vel Scale (kHz) 0.64 # # - 1.92 3.84
Penet Off # # - Off Off
ROI 130deg # # - - 5deg
Operating Control
Conditions
PS 14 # # - 16 14
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
1-28 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
Page 35

Acoustic Output Data
8C Probe (continued)
Table 1-27: Transducer Mode: 8C Operating Mode: PWD-Mode (inch.B-mode)
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.4 <1 <1 - 1.5 1.1
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 3.1
r.3
(mW) ## 26.7 28.1
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) 2.0
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 1.9
deq(zb) deq(zsp) (cm) 0.7
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 4.7 # # - 4.9 4.9
X(cm) ## -0.60.6
Y(cm)
PD (µsec) 0.6
## -0.50.5
prr PRF (Hz) 635.2
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
(MPa) 4.1
max
(cm) 0.4
max
(cm) ## - 2.4
X
FL
(cm) ## - 2.7
Y
2
@MI
max
(W/cm
399.2
)
Frequency (MHz) D5.0 # # - D5.0 D5.0
Image Depth (cm) 2.4 # # - 2.4 2.4
Vel Scale (kHz) 0.64 - # - 1.23 1.23
SV 1 - # - 2 2
Penet Off - # - Off Off
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual 1-29
Direction 5116269-100 Rev. 2
Page 36

Acoustic Output
E8C Probe
Table 1-28: Transducer Mode: E8C Operating Mode: B-Mode
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.5 <1 - - - <1
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 3.0
r.3
(mW) #- -#
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) -
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 1.5
deq(zb) deq(zsp) (cm) -
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 4.1 # - - - #
X(cm) #- - -#
Y(cm)
PD (µsec) 0.5
#- - -#
prr PRF (Hz) 5379.2
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FLX(cm) #- - #
I
at max. MI I
pa,α
PA.3
(MPa) 3.7
max
(cm) -
max
FL
(cm) #- - #
Y
2
@MI
max
(W/cm
469.8
)
Frequency (MHz) T8.0 # - - - #
Image Depth (cm) 12.0 # - - - #
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
1-30 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
Page 37

Acoustic Output Data
E8C Probe (continued)
Tab le 1-29: Transducer Mode: E8C Operating Mode: M-Mode (inc.B-mode)
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.5 <1 <1 - <1 <1
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 3.0
r.3
(mW) ## ##
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) #
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 1.5
deq(zb) deq(zsp) (cm) #
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 4.1 # # - # #
X(cm) ## - ##
Y(cm)
PD (µsec) 0.5
## - ##
prr PRF (Hz) 5379.2
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FLX(cm) ## - #
I
at max. MI I
pa,α
PA.3
(MPa) 3.7
max
(cm) #
max
FL
(cm) ## - #
Y
2
@MI
max
(W/cm
469.8
)
Frequency (MHz) T8.0 # # - # #
Image Depth (cm) 12.0 # # - # #
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual 1-31
Direction 5116269-100 Rev. 2
Page 38

Acoustic Output
E8C Probe (continued)
Table 1-30: Transducer Mode: E8C Operating Mode: CF-Mode (inch.M-mode)
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
non-
scan
Global Maximum: Index Value 1.5 <1 <1 - 2.4 1.6
IEC FDA Units
pra p
P W
min of [P
α
(zs, I
(MPa) 3.0
r.3
o
)] [(W
ta,α(zs
.3(Z1),ITA.3
(mW) ## 32.0 40.4
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) 1.7
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 1.5
deq(zb) deq(zsp) (cm) 0.4
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 4.1 # # - 4.8 4.8
X(cm) ## -0.60.6
Y(cm)
PD (µsec) 0.5
## -0.50.5
prr PRF (Hz) 800.0
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
(MPa) 3.7
max
(cm) 0.4
max
(cm) ## - 2.4
X
FL
(cm) ## - 2.7
Y
2
@MI
max
(W/cm
469.8
)
Frequency (MHz) D5.0 # # - D5.0 D5.0
Image Depth (cm) 2.4 # # - 2.4 2.4
Vel Scale (kHz) 0.64 # # - 1.92 3.84
Penet Off # # - Off Off
ROI 130deg # # - - 5deg
Operating Control
Conditions
PS 14 # # - 16 14
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
1-32 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
Page 39

Acoustic Output Data
E8C Probe (continued)
Tab le 1-31: Transducer Mode: E8C Operating Mode: PWD-Mode (inch.B-mode)
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.5 <1 <1 - 2.4 1.1
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 3.0
r.3
(mW) ## 32.0 34.1
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) 1.8
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 1.5
deq(zb) deq(zsp) (cm) 0.3
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 4.1 # # - 5.0 5.0
X(cm) ## -1.31.3
Y(cm)
PD (µsec) 0.5
## -0.50.5
prr PRF (Hz) 5379.2
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
(MPa) 3.7
max
(cm) 0.3
max
(cm) ## - 2.4
X
FL
(cm) ## - 2.7
Y
2
@MI
max
(W/cm
469.8
)
Frequency (MHz) D5.0 # # - D5.0 D5.0
Image Depth (cm) 2.4 # # - 2.4 2.4
Vel Scale (kHz) 0.64 - # - 1.23 1.23
SV 1 - # - 2 2
Penet Off - # - Off Off
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual 1-33
Direction 5116269-100 Rev. 2
Page 40

Acoustic Output
BE9C Probe
Table Addendum-2: Transducer Model: BE9C Operating Mode: CF-Mode (inch.M-mode)
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
non-
scan
Global Maximum: Index Value 1.3 <1 <1 - 1.6 <1
IEC FDA Units
pra p
P W
min of [P
α
(zs, I
(MPa) 2.8
r.3
o
)] [(W
ta,α(zs
.3(Z1),ITA.3
(mW) ## 18.0 #
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) 1.7
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 1.8
deq(zb) deq(zsp) (cm) 0.2
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 4.9 # # - 4.9 #
X(cm) ## -1.1#
Y(cm)
PD (µsec) 0.5
## -0.5#
prr PRF (Hz) 700.3
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
(MPa) 3.8
max
(cm) 0.2
max
(cm) ## - #
X
FL
(cm) ## - #
Y
2
@MI
max
(W/cm
396.7
)
Frequency (MHz) D5.0 # # - D5.0 #
Image Depth (cm) 1.5 # # - 1.5 #
Vel Scale (kHz) 0.30 # # - 2.82 #
Penet On # # - On #
ROI Max # # - - #
Operating Control
Conditions
PS 8 # # - 16 #
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
1-34 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
Page 41

Acoustic Output Data
(continued)
Addendumï-3: Transducer Model: BE9C Operating Mode: PWD-Mode (inch.B-mode)
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.3 <1 <1 - 1.6 <1
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 2.8
r.3
(mW) ## 18.0 #
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) 2.0
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 1.8
deq(zb) deq(zsp) (cm) 0.2
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 4.9 # # - 5.0 #
X(cm) ## -0.6#
Y(cm)
PD (µsec) 0.5
## -0.5#
prr PRF (Hz) 700.3
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
(MPa) 3.8
max
(cm) 0.2
max
(cm) ## - #
X
FL
(cm) ## - #
Y
2
@MI
max
(W/cm
396.7
)
Frequency (MHz) D5.0 # # - D5.0 #
Image Depth (cm) 2.2 # # - 2.2 #
Vel Scale (kHz) 0.70 - # - 0.70 #
SV 1 - # - 5 #
Penet On - # - On #
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual 1-35
Direction 5116269-100 Rev. 2
Page 42

Acoustic Output
M7C Probe (LOGIQ 7 Only)
Table 1-1: Transducer Mode: M7C Operating Mode: B-Mode
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.7 1.6 - - - 2.3
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 3.4
r.3
(mW) 116.6 - -116.6
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) -
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 2.2
deq(zb) deq(zsp) (cm) -
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 3.3 3.5 - - - 3.5
X(cm) 1.4 - - - 1.4
Y(cm)
PD (µsec) 0.8
0.9 - - - 0.9
prr PRF (Hz) 3012.0
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FLX(cm) 2.2 - - 2.2
I
at max. MI I
pa,α
PA.3
(MPa) 4.1
max
(cm) -
max
FL
(cm) 11.5 - - 11.5
Y
2
@MI
max
(W/cm
472.6
)
Frequency (MHz) 6.0 CE6.0 - - - CE
6.0
Image Depth (cm) 24.0 9.0 - - - 9.0
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
1-36 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
Page 43

M7C Probe (LOGIQ 7 Only) (continued) (LOGIQ 7 Only)
Table 1-2: Transducer Mode: M7C Operating Mode: M-Mode (inc.B-mode)
Acoustic Output Data
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.7 < 1 - <1 1.3 1.0
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 3.4
r.3
(mW) #- 20.3 75.9
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) #
zs z1(cm) #
zbp zbp(cm) #
zb zsp(cm) 2.2
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 2.2
deq(zb) deq(zsp) (cm) 0.5
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 3.3 # - # 3.3 3.4
X(cm) #- #1.43.1
Y(cm)
PD (µsec) 0.8
#- #0.90.9
prr PRF (Hz) 3012.0
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FLX(cm) #- # 4.8
I
at max. MI I
pa,α
PA.3
(MPa) 4.1
max
(cm) 0.5
max
FL
(cm) #- # 11.5
Y
2
@MI
max
(W/cm
472.6
)
Frequency (MHz) 6.0 # - # 6.0 6.0
Image Depth (cm) 24.0 # - # 24.0 24.0
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual 1-37
Direction 5116269-100 Rev. 2
Page 44

Acoustic Output
M7C Probe (LOGIQ 7 Only) (continued) (LOGIQ 7 Only)
Table 1-3: Transducer Mode: M7C Operating Mode: CF-Mode (inch.M-mode)
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
non-
scan
Global Maximum: Index Value 1.7 1.1 - <1 2.2 1.2
IEC FDA Units
pra p
P W
min of [P
α
(zs, I
(MPa) 3.4
r.3
o
)] [(W
ta,α(zs
.3(Z1),ITA.3
(mW) 78.9 - 54.2 78.9
(z1)]) #
zs z1(cm) #
zbp zbp(cm) #
zb zsp(cm) 3.4
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 3.4
deq(zb) deq(zsp) (cm) 0.3
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 3.7 3.9 - # 3.9 3.9
X(cm) 2.3 - # 2.3 2.3
Y(cm)
PD (µsec) 0.4
0.9 - # 0.9 0.9
prr PRF (Hz) 635.2
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
(MPa) 5.1
max
(cm) 0.4
max
(cm) 3.5 - # 3.5
X
FL
(cm) 11.5 - # 11.5
Y
2
@MI
max
(W/cm
)
1425.0
Frequency (MHz) D4.0 D4.0 - # D4.0 D4.0
Image Depth (cm) 3.5 3.5 - # 3.5 3.5
Vel Scale (kHz) 1.80 7.69 - # 1.23 7.69
Penet On On - # On On
ROI 60deg 5deg - # - 5deg
Operating Control
Conditions
PS 12 12 - # 16 12
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
1-38 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
Page 45

Acoustic Output Data
M7C Probe (LOGIQ 7 Only) (continued) (LOGIQ 7 Only)
Table 1-4: Transducer Mode: M7C Operating Mode: PWD-Mode (inch.B-mode)
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.7 1.1 - <1 2.2 <1
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 3.4
r.3
(mW) 78.9 - 54.2 #
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) #
zs z1(cm) #
zbp zbp(cm) #
zb zsp(cm) 3.4
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 3.4
deq(zb) deq(zsp) (cm) 0.4
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 3.7 3.9 - # 4.0 #
X(cm) 2.3 - # 2.3 #
Y(cm)
PD (µsec) 0.4
0.9 - # 0.9 #
prr PRF (Hz) 635.2
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
(MPa) 5.1
max
(cm) 0.4
max
(cm) 3.5 - # #
X
FL
(cm) 11.5 - # #
Y
2
@MI
max
(W/cm
)
1425.0
Frequency (MHz) D4.0 6.0 - # D4.0 #
Image Depth (cm) 3.5 8.0 - # 3.5 #
Vel Scale (kHz) 0.64 - - # 0.70 #
SV 1 - - # 3 #
Penet On - - # On #
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual 1-39
Direction 5116269-100 Rev. 2
Page 46

Acoustic Output
7L Probe
Table 1-5: Transducer Mode: 7L Operating Mode: B-Mode
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.7 <1 - - <1
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 3/3
r.3
(mW) #- -#
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) -
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 2.1
deq(zb) deq(zsp) (cm) -
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 3.5 # - - - #
X(cm) #- - -#
Y(cm)
PD (µsec) 0.6
#- - -#
prr PRF (Hz) 4717.0
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FLX(cm) #- - #
I
at max. MI I
pa,α
PA.3
(MPa) 4.2
max
(cm) -
max
FL
(cm) #- - #
Y
2
@MI
max
(W/cm
902.5
)
Frequency (MHz) T6.0 # - - - #
Image Depth (cm) 14.0 # - - - #
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
1-40 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
Page 47

7L Probe (continued)
Table 1-6: Transducer Mode: 7L Operating Mode: M-Mode (inc.B-mode)
Acoustic Output Data
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
Global Maximum: Index Value 1.7 <1
<1
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 3.3
r.3
(mW) ## ##
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) #
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 2.1
deq(zb) deq(zsp) (cm) #
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 3.5 # # - # #
X(cm) ## - ##
Y(cm)
PD (µsec) 0.6
## - ##
prr PRF (Hz) 4717.0
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FLX(cm) ## - #
I
at max. MI I
pa,α
PA.3
(MPa) 4.2
max
(cm) #
max
FL
(cm) ## - #
Y
2
@MI
max
(W/cm
902.5
)
Frequency (MHz) T6.0 # # - # #
Image Depth (cm) 14.0 # # - # #
-<1<1
TIC
nonscan
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual 1-41
Direction 5116269-100 Rev. 2
Page 48

Acoustic Output
7L Probe (continued)
Table 1-7: Transducer Mode: 7L Operating Mode: CF-Mode (inch.M-mode)
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
Global Maximum: Index Value 1.7 1.1
<1
IEC FDA Units
pra p
P W
min of [P
α
(zs, I
(MPa) 3.3
r.3
o
)] [(W
ta,α(zs
.3(Z1),ITA.3
(mW) 58.3 # 38.1 58.3
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) 1.7
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 2.1
deq(zb) deq(zsp) (cm) 0.2
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 3.5 4.0 # -
X(cm) 0.6 # - 0.6 0.6
Y(cm)
PD (µsec) 0.6
0.6 # - 0.6 0.6
prr PRF (Hz) 4717.0
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
(MPa) 4.2
max
(cm) 0.2
max
(cm) 2.2 # - 2.2
X
FL
(cm) 3.0 # - 3.0
Y
2
@MI
max
(W/cm
902.5
)
Frequency (MHz) D4.0 D4.0 # - D4.0 D4.0
Image Depth (cm) 2.2 2.2 # - 2.2 2.2
Vel Scale (kHz) 1.80 7.69 # - 1.80 7.69
Penet On On # - On On
ROI 40mm 5mm # - - 5mm
Operating Control
Conditions
PS 14 14 # - 16 14
-2.22.1
non-
scan
4.0
TIC
4.0
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
1-42 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
Page 49

7L Probe (continued)
Table 1-8: Transducer Mode: 7LOperating Mode: B-Flow
Acoustic Output Data
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.7 1.3 <1 - 2.2 1.2
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 3.3
r.3
(mW) 44.3 # 38.1 39.7
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) 2.3
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 2.1
deq(zb) deq(zsp) (cm) -
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 3.5 4.0 # - 4.0 4.0
X(cm) 1.9 # - 0.8 0.8
Y(cm)
PD (µsec) 0.6
0.6 # - 0.6 0.6
prr PRF (Hz) 4717.0
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
(MPa) 4.2
max
(cm) 0.3
max
(cm) 3.2 # - 3.0
X
FL
(cm) 3.0 # - 3.0
Y
2
@MI
max
(W/cm
902.5
)
Frequency (MHz) BF4.0 BF4.0 # - D4.0 #
Image Depth (cm) 14.0 4.0 # - 3.0 #
Vel Scale (kHz) - - # - 1.60 SV - - # - 2 Penet - - # - On -
Operating Control
Conditions
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual 1-43
Direction 5116269-100 Rev. 2
Page 50

Acoustic Output
7L Probe (continued)
Table 1-9: Transducer Mode: 7L Operating Mode: Contrast
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.5 1.5 - - - 1.7
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 3.0
r.3
(mW) 82.4 - - 82.4
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) -
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 2.0
deq(zb) deq(zsp) (cm) -
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 3.9 3.9 - - - 3.9
X(cm) 1.9 - - - 1.9
Y(cm)
PD (µsec) 0.5
0.6 - - - 0.6
prr PRF (Hz) 4721.4
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
Frequency (MHz) CHA
(MPa) 3.9
max
(cm) -
max
(cm) 3.6 - - 3.6
X
FL
(cm) 3.0 - - 3.0
Y
2
@MI
max
(W/cm
486.6
)
4.0
CHA
4.0
---CHA
4.0
Image Depth (cm) 14.0 3.6 - - - 3.6
Operating Control
Conditions
1-44 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
Page 51

Acoustic Output Data
7L Probe (continued)
Table 1-10: Transducer Mode: 7L Operating Mode: PWD-Mode (inch.B-mode)
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
Global Maximum: Index Value 1.7 1.3
<1
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 3.3
r.3
(mW) 44.3 # 38.1 43.2
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) 2.3
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 2.1
deq(zb) deq(zsp) (cm) 0.3
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 3.5 4.0 # -
X(cm) 1.9 # - 0.8 0.8
Y(cm)
PD (µsec) 0.6
0.6 # - 0.6 0.6
prr PRF (Hz) 4717,0
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
(MPa) 4.2
max
(cm) 0.3
max
(cm) 3.2 # - 3.0
X
FL
(cm) 3.0 # - 3.0
Y
2
@MI
max
(W/cm
902.5
)
Frequency (MHz) D4.0 BF4.0 # - D4.0 D4.0
Image Depth (cm) 3.0 4.0 # - 3.0 3.0
Vel Scale (kHz) 0.64 - # - 2 2
SV 1 - # - 2 2
Penet On - # - On On
Operating Control
Conditions
-2.21.5
nonscan
4.0
TIC
4.0
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual 1-45
Direction 5116269-100 Rev. 2
Page 52

Acoustic Output
10L Probe
Table 1-11: Transducer Mode: 10L Operating Mode: B-Mode
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.6 <1 - - - 2.2
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 3.4
r.3
(mW) #- -115.1
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) -
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 2.3
deq(zb) deq(zsp) (cm) -
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 4.5 # - - - 4.6
X(cm) #- - -2.2
Y(cm)
PD (µsec) 0.4
#- - -0.6
prr PRF (Hz) 6807.4
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FLX(cm) #- - 2.2
I
at max. MI I
pa,α
PA.3
(MPa) 5.1
max
(cm) -
max
FL
(cm) #- - 2.5
Y
2
@MI
max
(W/cm
)
1357.2
Frequency (MHz) T8.0 # - - - T8.0
Image Depth (cm) 10.0 # - - - 4.0
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
1-46 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
Page 53

10L Probe (continued)
Tab l e 1- 12 : Transducer Mode: 10 L Operating Mode: M-Mode (inc.B-mode)
Acoustic Output Data
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.7 <1 - <1 <1 2.1
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 3.8
r.3
(mW) #- #108.0
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) #
zs z1(cm) #
zbp zbp(cm) #
zb zsp(cm) #
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 2.3
deq(zb) deq(zsp) (cm) #
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 4.5 # - # # 4.6
X(cm) #- ##2.2
Y(cm)
PD (µsec) 0.4
#- ##0.6
prr PRF (Hz) 800.0
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FLX(cm) #- # 2.2
I
at max. MI I
pa,α
PA.3
(MPa) 5.3
max
(cm) #
max
FL
(cm) #- # 2.5
Y
2
@MI
max
(W/cm
)
1370.9
Frequency (MHz) T8.0 # - # # T8.0
Image Depth (cm) 10.0 # - # # 10.0
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual 1-47
Direction 5116269-100 Rev. 2
Page 54

Acoustic Output
10L Probe (continued)
Table 1-13: Transducer Mode: 10L Operating Mode: CF-Mode (inch.M-mode)
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
Global Maximum: Index Value 1.8 5.0
<1
IEC FDA Units
pra p
P W
min of [P
α
(zs, I
(MPa) 3.9
r.3
o
)] [(W
ta,α(zs
.3(Z1),ITA.3
(mW) 172.0 # 32.5 172.0
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) 1.9
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 1.9
deq(zb) deq(zsp) (cm) 0.8
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 4.9 5.1 # - 4.9 5.1
X(cm) 1.2 # - 0.9 1.2
Y(cm)
PD (µsec) 0.5
0.6 # - 0.6 0.6
prr PRF (Hz) 635.2
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
(MPa) 5.3
max
(cm) 0.8
max
(cm) 3.0 # - 3.0
X
FL
(cm) 2.5 # - 2.5
Y
2
@MI
max
(W/cm
)
1183.5
Frequency (MHz) D5.0 D5.0 # - D5.0 D5.0
Image Depth (cm) 1.4 0.6 # - 1.4 0.6
Vel Scale (kHz) 1.80 19.61 # - 1.80 19.61
Penet On On # - On On
ROI (mm) 24.9 5 # - - 5
Operating Control
Conditions
PS 14 14 # - 16 14
-2.34.4
TIC
non-
scan
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
1-48 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
Page 55

Acoustic Output Data
10L Probe (continued)
Tab l e 1- 14 : Transducer Mode: 10 L Operating Mode: PWD-Mode (inch.B-mode)
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
Global Maximum: Index Value 1.8 5.0
<1
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 3.9
r.3
(mW) 172.0 # 32.5 67.3
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) 2.4
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 1.9
deq(zb) deq(zsp) (cm) 0.3
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 4.9 5.1 # - 5.0 5.1
X(cm) 1.2 # - 0.9 1.2
Y(cm)
PD (µsec) 0.5
0.6 # - 0.6 0.6
prr PRF (Hz) 635.2
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
(MPa) 5.3
max
(cm) 0.3
max
(cm) 3.0 # - 3.0
X
FL
(cm) 2.5 # - 2.5
Y
2
@MI
max
(W/cm
)
1183.5
Frequency (MHz) D5.0 T8.0 # - D5.0 D5.0
Image Depth (cm) 2.8 4.0 # - 2.8 2.8
Vel Scale (kHz) 0.64 - # - 2.60 2.60
SV 1 - # - 3 3
Penet On - # - On On
Operating Control
Conditions
-2.31.8
TIC
nonscan
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual 1-49
Direction 5116269-100 Rev. 2
Page 56

Acoustic Output
10L Probe (continued)
Table 1-15: Transducer Mode: 10L Operating Mode: B-Flow
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.6 2.2 <1 - 2.3 4.3
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 3.4
r.3
(mW) 207.2 # 32.5 207.2
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) 2.4
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 3.1
deq(zb) deq(zsp) (cm) 0.3
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 4.5 4.5 # - 5.0 4.5
X(cm) 1.9 # - 0.9 1.9
Y(cm)
PD (µsec) 1.2
0.6 # - 0.6 0.6
prr PRF (Hz) 4717.0
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
(MPa) 5.5
max
(cm) 0.3
max
(cm) 3.8 # - 3.8
X
FL
(cm) 2.5 # - 2.5
Y
2
@MI
max
(W/cm
722.1
)
Frequency (MHz) BF6.0 BF6.0 # - D5.0 BF6.0
Image Depth (cm) 14.0 14.0 # - 2.8 14.0
Vel Scale (kHz) - - # - 2.60 SV - - # - 3 Penet - - # - On -
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
1-50 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
Page 57

10L Probe (continued)
Table 1-16: Transducer Mode: 10L Operating Mode: Contrast
Acoustic Output Data
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.6 3.3 - - - 2.3
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 3.5
r.3
(mW) 132.3 - - 132.3
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) -
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 2.0
deq(zb) deq(zsp) (cm) -
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 4.9 4.2 - - - 4.2
X(cm) 2.6 - - - 2.6
Y(cm)
PD (µsec) 0.4
0.6 - - - 0.6
prr PRF (Hz) 4755.1
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
Frequency (MHz) CHA
(MPa) 4.9
max
(cm) -
max
(cm) 2.6 - - 2.6
X
FL
(cm) 2.5 - - 2.5
Y
2
@MI
max
(W/cm
548.1
)
8.0
CHA
8.0
---CHA
8.0
Image Depth (cm) 14.0 2.6 - - - 2.6
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual 1-51
Direction 5116269-100 Rev. 2
Page 58

Acoustic Output
i12L Probe
Table 1-17: Transducer Mode: i12L Operating Mode: B-Mode
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.3 2.2 - - - 1.7
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 3.2
r.3
(mW) 74.8 - -74.8
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) -
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 1.4
deq(zb) deq(zsp) (cm) -
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 6.3 6.3 - - - 6.3
X(cm) 1.4 - - - 1.4
Y(cm)
PD (µsec) 0.2
0.7 - - - 0.7
prr PRF (Hz) 6257.8
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FLX(cm) 1.4 - - 1.4
I
at max. MI I
pa,α
PA.3
(MPa) 4.4
max
(cm) -
max
FL
(cm) 1.3 - - 1.3
Y
2
@MI
max
(W/cm
579.4
)
Frequency (MHz) 6.0 6.0 - - - 6.0
Image Depth (cm) 10.0 4.0 - - - 4.0
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
1-52 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
Page 59

Acoustic Output Data
i12L Probe (continued)
Table 1-18: Transducer Mode: i12L Operating Mode: M-Mode (inc.B-mode)
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.3 2.2 <1 - <1 1.6
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 3.2
r.3
(mW) 74.8 # #69.8
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) #
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 1.4
deq(zb) deq(zsp) (cm) #
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 6.3 6.3 # - # 6.3
X(cm) 1.4 # - # 1.4
Y(cm)
PD (µsec) 0.2
0.7 # - # 0.2
prr PRF (Hz) 6257.8
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FLX(cm) 1.4 # - 1.4
I
at max. MI I
pa,α
PA.3
(MPa) 4.4
max
(cm) #
max
FL
(cm) 1.3 # - 1.3
Y
2
@MI
max
(W/cm
579.4
)
Frequency (MHz) 6.0 6.0 # - # 6.0
Image Depth (cm) 10.0 4.0 # - # 10.0
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual 1-53
Direction 5116269-100 Rev. 2
Page 60

Acoustic Output
i12L Probe (continued)
Table 1-19: Transducer Mode: i12L Operating Mode: CF-Mode (inch.M-mode)
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
non-
scan
Global Maximum: Index Value 1.4 2.2 1.4 - 2.5 2.7
IEC FDA Units
pra p
P W
min of [P
α
(zs, I
(MPa) 2.7
r.3
o
)] [(W
ta,α(zs
.3(Z1),ITA.3
(mW) 74.8 56.7 56.7 56.2
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) 1.2
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 1.2
deq(zb) deq(zsp) (cm) 0.2
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 6.5 6.3 6.5 - 6.5 6.8
X(cm) 1.4 0.6 - 0.6 0.3
Y(cm)
PD (µsec) 0.4
0.7 0.7 - 0.7 0.7
prr PRF (Hz) 635.2
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
(MPa) 4.7
max
(cm) 0.2
max
(cm) 1.4 1.4 - 0.6
X
FL
(cm) 1.3 1.3 - 1.3
Y
2
@MI
max
(W/cm
)
1252.9
Frequency (MHz) D6.6 D6.6 D6.6 - D6.6 D6.6
Image Depth (cm) 1.4 0.6 1.4 - 1.4 0.6
Vel Scale (kHz) 1.80 19.61 1.86 - 1.86 19.61
Penet On On On - On On
ROI (mm) 24.9 5 - - - 5
Operating Control
Conditions
PS 14 14 16 - 16 14
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
1-54 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
Page 61

Acoustic Output Data
i12L Probe (continued)
Table 1-20: Transducer Mode: i12L Operating Mode: PWD-Mode (inch.B-mode)
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.4 2.2 1.4 - 2.5 1.9
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 3.6
r.3
(mW) 74.8 56.7 56.7 56.6
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) 1.9
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 1.2
deq(zb) deq(zsp) (cm) 0.4
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 6.5 6.3 5.3 - 5.3 5.3
X(cm) 1.4 0.9 - 0.9 0.9
Y(cm)
PD (µsec) 0.4
0.7 0.7 - 0.7 0.7
prr PRF (Hz) 1796.0
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
(MPa) 4.7
max
(cm) 0.4
max
(cm) 1.4 2.3 - 2.2
X
FL
(cm) 1.3 1.3 - 1.3
Y
2
@MI
max
(W/cm
)
1252.2
Frequency (MHz) D6.6 6.0 D5.0 - D5.0 D5.0
Image Depth (cm) 1.4 4.0 2.2 - 2.2 2.2
Vel Scale (kHz) 0.64 - 6.25 - 6.25 6.25
SV 1 - 1 - 1 1
Penet On - On - On On
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual 1-55
Direction 5116269-100 Rev. 2
Page 62

Acoustic Output
M12L Probe
Table 1-21: Transducer Mode: M12L Operating Mode: B-Mode
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.4 <1 - - - 1.9
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 3.7
r.3
(mW) #- -24.3
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) -
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 0.7
deq(zb) deq(zsp) (cm) -
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 6.4 # - - - 5.3
X(cm) #- - -0.6
Y(cm)
PD (µsec) 0.3
#- - -0.2
prr PRF (Hz) 6131.2
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FLX(cm) #- - 0.8
I
at max. MI I
pa,α
PA.3
(MPa) 4.3
max
(cm) -
max
FL
(cm) #- - 1.5
Y
2
@MI
max
(W/cm
476.4
)
Frequency (MHz) T10.0 # - - - T10.0
Image Depth (cm) 10.0 # - - - 4.0
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
1-56 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
Page 63

Acoustic Output Data
M12L Probe (continued)
Table 1-22: Transducer Mode: M12L Operating Mode: M-Mode (inc.B-mode)
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.4 <1 <1 - <1 1.7
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 3.7
r.3
(mW) ## #22.6
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) #
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 0.7
deq(zb) deq(zsp) (cm) #
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 6.4 # # - # 5.3
X(cm) ## - #0.6
Y(cm)
PD (µsec) 0.3
## - #2.2
prr PRF (Hz) 6131.2
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FLX(cm) ## - 0.8
I
at max. MI I
pa,α
PA.3
(MPa) 4.3
max
(cm) #
max
FL
(cm) ## - 1.5
Y
2
@MI
max
(W/cm
476.4
)
Frequency (MHz) T10.0 # # - # T10.0
Image Depth (cm) 10.0 # # - # 10.0
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual 1-57
Direction 5116269-100 Rev. 2
Page 64

Acoustic Output
M12L Probe (continued)
Table 1-23: Transducer Mode: M12L Operating Mode: CF-Mode (inch.M-mode)
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
Global Maximum: Index Value 1.4 2.1
<1
IEC FDA Units
pra p
P W
min of [P
α
(zs, I
(MPa) 3.7
r.3
o
)] [(W
ta,α(zs
.3(Z1),ITA.3
(mW) 69.5 # 16.5 69.5
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) 0.7
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 0.7
deq(zb) deq(zsp) (cm) 0.2
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 6.4 6.5 # - 5.1 6.5
X(cm) 0.6 # - 0.6 0.6
Y(cm)
PD (µsec) 0.3
0.2 # - 0.2 0.2
prr PRF (Hz) 6131.2
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
(MPa) 4.3
max
(cm) 0.2
max
(cm) 0.8 # - 0.8
X
FL
(cm) 1.5 # - 1.5
Y
2
@MI
max
(W/cm
476.4
)
Frequency (MHz) D5.0 D6.6 # - D5.0 D6.6
Image Depth (cm) 0.8 0.8 # - 0.8 0.8
Vel Scale (kHz) 1.80 19.61 # - 19.61 0.8
Penet On Off # - On Off
ROI (mm) 10 10 # - - 10
Operating Control
Conditions
PS 10 12 # - 16 12
-1.65.3
TIC
non-
scan
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
1-58 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
Page 65

Acoustic Output Data
M12L Probe (continued)
Table 1-24: Transducer Mode: M12L Operating Mode: PWD-Mode (inch.B-mode)
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
Global Maximum: Index Value 1.5 2.9
<1
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 3.4
r.3
(mW) 92.1 # 16.5 29.7
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) 1.5
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 2.5
deq(zb) deq(zsp) (cm) 0.2
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 5.0 6.5 # - 5.0 6.5
X(cm) 1.8 # - 0.5 0.6
Y(cm)
PD (µsec) 1.1
0.6 # - 0.2 0.2
prr PRF (Hz) 6192.0
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
(MPa) 5.3
max
(cm) 0.2
max
(cm) 2.4 # - 0.8
X
FL
(cm) 2.1 # - 1.5
Y
2
@MI
max
(W/cm
370.3
)
Frequency (MHz) D5.0 BF6.0 # - D5.0 D5.0
Image Depth (cm) 1.8 3.0 # - 1.8 1.8
Vel Scale (kHz) 0.70 - # - 3.90 3.90
SV 1 - # - 2 2
Penet On - # - On On
Operating Control
Conditions
-1.62.3
TIC
nonscan
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual 1-59
Direction 5116269-100 Rev. 2
Page 66

Acoustic Output
M12L Probe (continued)
Tab l e 1- 25 : Transducer Mode: M1 2L Operating Mode: B-Flow
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.5 2.9 <1 - 1.6 2.0
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 3.4
r.3
(mW) 92.1 # 16.5 92.1
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) 1.5
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 2.5
deq(zb) deq(zsp) (cm) 0.2
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 5.0 6.5 # - 5.0 6.5
X(cm) 1.8 # - 0.5 1.8
Y(cm)
PD (µsec) 1.1
0.6 # - 0.2 0.6
prr PRF (Hz) 6192.0
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
(MPa) 5.3
max
(cm) 0.2
max
(cm) 2.4 # - 2.4
X
FL
(cm) 2.1 # - 2.1
Y
2
@MI
max
(W/cm
370.3
)
Frequency (MHz) BF6.0 BF6.0 # - D5.0 BF6.0
Image Depth (cm) 10.0 3.0 # - 1.8 3.0
Vel Scale (kHz) - - # - 3.90 SV - - # - 2 Penet - - # - On -
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
1-60 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
Page 67

T739 Probe
Acoustic Output Data
Table 1-26: Transducer Model: T739 Operating Mode: B-Mode
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.6 <1 - - - 1.2
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 3.4
r.3
(mW) #- -60.3
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) -
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 2.4
deq(zb) deq(zsp) (cm) -
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 4.5 # - - - 4.5
X(cm) #- - -2.2
Y(cm)
PD (µsec) 0.4
#- - -0.6
prr PRF (Hz) 6317.1
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FLX(cm) #- - 2.2
I
at max. MI I
pa,α
PA.3
(MPa) 4.5
max
(cm) -
max
FL
(cm) #- - 2.5
Y
2
@MI
max
(W/cm
554.6
)
Frequency (MHz) T8.0 # - - - T8.0
Image Depth (cm) 10.0 # - - - 2.2
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual 1-61
Direction 5116269-100 Rev. 2
Page 68

Acoustic Output
T739 Probe (continued)
Table 1-27: Transducer Model: T739 Operating Mode: M-Mode (inc.B-mode)
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.6 <1 - <1 <1 1.1
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 3.4
r.3
(mW) #- #56.6
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) #
zs z1(cm) #
zbp zbp(cm) #
zb zsp(cm) #
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 2.4
deq(zb) deq(zsp) (cm) #
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 4.5 # - # # 4.5
X(cm) #- ##2.2
Y(cm)
PD (µsec) 0.4
#- ##0.6
prr PRF (Hz) 6317.1
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FLX(cm) #- # 2.2
I
at max. MI I
pa,α
PA.3
(MPa) 4.5
max
(cm) #
max
FL
(cm) #- # 2.5
Y
2
@MI
max
(W/cm
554.6
)
Frequency (MHz) T8.0 # - # # T8.0
Image Depth (cm) 10.0 # - # # 10.0
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
1-62 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
Page 69

Acoustic Output Data
T739 Probe (continued)
Table 1-28: Transducer Model: T739 Operating Mode: CF-Mode (inch.M-mode)
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
non-
scan
Global Maximum: Index Value 1.8 2.1 <1 - 1.8 2.4
IEC FDA Units
pra p
P W
min of [P
α
(zs, I
(MPa) 3.9
r.3
o
)] [(W
ta,α(zs
.3(Z1),ITA.3
(mW) 91.9 # 25.0 91.9
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) 1.9
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 2.0
deq(zb) deq(zsp) (cm) 0.8
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 4.9 4.9 # - 4.9 4.9
X(cm) 1.2 # - 0.9 1.2
Y(cm)
PD (µsec) 0.8
0.6 # - 0.6 0.6
prr PRF (Hz) 700.3
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
(MPa) 5.2
max
(cm) 0.8
max
(cm) 3.0 # - 3.0
X
FL
(cm) 2.5 # - 2.5
Y
2
@MI
max
(W/cm
518.7
)
Frequency (MHz) D5.0 D5.0 # - D5.0 D5.0
Image Depth (cm) 2.2 3.8 # - 2.2 3.8
Vel Scale (kHz) 1.70 1.50 # - 1.80 1.50
Penet On On # - On On
ROI (mm) 24.9 5 # - - 5
Operating Control
Conditions
PS 14 14 # - 16 14
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual 1-63
Direction 5116269-100 Rev. 2
Page 70

Acoustic Output
T739 Probe (continued)
Tab le 1-29: Transducer Model: T739 Operating Mode: PWD-Mode (inch.B-mode)
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.8 2.2 <1 - 1.8 1.1
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 3.9
r.3
(mW) 98.7 # 25.0 41.7
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) 2.4
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 2.0
deq(zb) deq(zsp) (cm) 0.3
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 4.9 4.5 # - 5.0 4.9
X(cm) 2.6 # - 0.9 1.2
Y(cm)
PD (µsec) 0.6
0.6 # - 0.6 0.6
prr PRF (Hz) 700.3
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
(MPa) 5.2
max
(cm) 0.3
max
(cm) 3.8 # - 3.0
X
FL
(cm) 2.5 # - 2.5
Y
2
@MI
max
(W/cm
518.7
)
Frequency (MHz) D5.0 BF6.0 # - D5.0 D5.0
Image Depth (cm) 2.8 2.2 # - 2.8 2.8
Vel Scale (kHz) 0.70 - # - 2.60 2.60
SV 1 - # - 3 3
Penet On - # - On On
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
1-64 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
Page 71

T739 Probe (continued)
Tab le 1-30: Transducer Model: T739 Operating Mode: B-Flow
Acoustic Output Data
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.8 2.2 <1 - 1.8 1.8
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 3.9
r.3
(mW) 98.7 # 25.0 98.7
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) 2.4
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 2.0
deq(zb) deq(zsp) (cm) 0.3
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 4.9 4.5 # - 5.0 4.5
X(cm) 2.6 # - 0.9 2.6
Y(cm)
PD (µsec) 0.6
0.6 # - 0.6 0.6
prr PRF (Hz) 700.3
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
(MPa) 5.2
max
(cm) 0.3
max
(cm) 3.8 # - 3.8
X
FL
(cm) 2.5 # - 2.5
Y
2
@MI
max
(W/cm
518.7
)
Frequency (MHz) D5.0 BF6.0 # - D5.0 BF6.0
Image Depth (cm) 2.8 3.8 # - 2.8 3.8
Vel Scale (kHz) 0.70 - - - 2.60 SV 1 - - - 3 Penet On - - - On -
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual 1-65
Direction 5116269-100 Rev. 2
Page 72

Acoustic Output
3S Probe
Table 1-31: Transducer Mode: 3S Operating Mode: B-Mode
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.4 <1 - - - 1.3
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 2.0
r.3
(mW) #- -89.9
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) -
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 3.3
deq(zb) deq(zsp) (cm) -
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 1.9 # - - - 1.8
X(cm) #- - -1.8
Y(cm)
PD (µsec) 1.1
#- - -1.3
prr PRF (Hz) 2383.2
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FLX(cm) #- - 4.8
I
at max. MI I
pa,α
PA.3
(MPa) 2.4
max
(cm) -
max
FL
(cm) #- - 8.0
Y
2
@MI
max
(W/cm
146.4
)
Frequency (MHz) T3.0 # - - - T3.0
Image Depth (cm) 30.0 # - - - 30.0
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
1-66 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
Page 73

3S Probe (continued)
Tab le 1-32: Transducer Mode: 3S Operating Mode: M-Mode (inc.B-mode)
Acoustic Output Data
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.4 <1 - <1 <1 1.1
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 2.0
r.3
(mW) #- #77.7
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) #
zs z1(cm) #
zbp zbp(cm) #
zb zsp(cm) #
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 3.3
deq(zb) deq(zsp) (cm) #
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 1.9 # - # # 1.8
X(cm) #- ##1.8
Y(cm)
PD (µsec) 1.1
#- ##1.3
prr PRF (Hz) 800.0
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FLX(cm) #- # 4.8
I
at max. MI I
pa,α
PA.3
(MPa) 2.4
max
(cm) #
max
FL
(cm) #- # 8.0
Y
2
@MI
max
(W/cm
146.4
)
Frequency (MHz) T3.0 # - # # T3.0
Image Depth (cm) 30.0 # - # # 30.0
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual 1-67
Direction 5116269-100 Rev. 2
Page 74

Acoustic Output
3S Probe (continued)
Table 1-33: Transducer Mode: 3S Operating Mode: CF-Mode (inch.M-mode)
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
non-
scan
Global Maximum: Index Value 1.7 <1 - <1 4.3 1.7
IEC FDA Units
pra p
P W
min of [P
α
(zs, I
(MPa) 2.4
r.3
o
)] [(W
ta,α(zs
.3(Z1),ITA.3
(mW) #- 107.5 105.0
(z1)]) #
zs z1(cm) #
zbp zbp(cm) #
zb zsp(cm) 3.0
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 2.6
deq(zb) deq(zsp) (cm) 1.1
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 1.9 # - # 2.0 2.0
X(cm) #- #0.91.4
Y(cm)
PD (µsec) 1.4
#- #1.31.3
prr PRF (Hz) 1796.0
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
(MPa) 2.8
max
(cm) 1.1
max
(cm) #- # 3.5
X
FL
(cm) #- # 8.0
Y
2
@MI
max
(W/cm
382.5
)
Frequency (MHz) D2.0 # - # D2.0 D2.0
Image Depth (cm) 2.2 # - # 2.2 3.5
Vel Scale (kHz) 1.80 # - # 1.07 4.73
Penet Off # - # Off Off
ROI 60deg # - # - 10deg
Operating Control
Conditions
PS 14 # - # 16 14
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
1-68 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
Page 75

Acoustic Output Data
3S Probe (continued)
Tab le 1-34: Transducer Mode: 3S Operating Mode: PWD-Mode (inch.B-mode)
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.7 <1 - <1 2.1 <1
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 2.4
r.3
(mW) #- 44.9 #
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) #
zs z1(cm) #
zbp zbp(cm) #
zb zsp(cm) 4.5
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 2.6
deq(zb) deq(zsp) (cm) 0.4
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 1.9 # - # 2.0 #
X(cm) #- #1.8#
Y(cm)
PD (µsec) 1.4
#- #1.3#
prr PRF (Hz) 1796.0
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
(MPa) 2.8
max
(cm) 0.4
max
(cm) #- # #
X
FL
(cm) #- # #
Y
2
@MI
max
(W/cm
382.5
)
Frequency (MHz) D2.0 # - # D2.0 #
Image Depth (cm) 2.2 # - # 4.8 #
Vel Scale (kHz) 0.64 - - # 0.64 #
SV 3 - - # 4 #
Penet Off - - # Off #
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual 1-69
Direction 5116269-100 Rev. 2
Page 76

Acoustic Output
3S Probe (continued)
Tab le 1-35: Transducer Mode: 3S Operating Mode: Contrast
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.6 <1 - - - 1.2
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 2.0
r.3
(mW) #- - 84.6
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) -
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 4.1
deq(zb) deq(zsp) (cm) -
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 1.6 # - - - 1.6
X(cm) #- - -1.7
Y(cm)
PD (µsec) 1.4
#- - -1.3
prr PRF (Hz) 0.3
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
Frequency (MHz) TAD
(MPa) 2.4
max
(cm) -
max
(cm) #- - 5.8
X
FL
(cm) #- - 8.0
Y
2
@MI
max
(W/cm
186.4
)
-- - -PI3.0
1.53
Image Depth (cm) 30.0 - - - - 30.0
Vel Scale (kHz) 0.3 - - - - ROI 90deg - - - - -
Operating Control
Conditions
SV 4 - - - - -
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
1-70 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
Page 77

3S Probe (continued)
Table 1-36: Transducer Mode: 3S Operating Mode: CWD-Mode
Acoustic Output Data
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.7 <1 - <1 4.3 <1
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 2.4
r.3
(mW) #- 107.5 #
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) #
zs z1(cm) #
zbp zbp(cm) #
zb zsp(cm) 3.0
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 2.6
deq(zb) deq(zsp) (cm) 1.1
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 1.9 # - # 2.0 #
X(cm) #- #0.9#
Y(cm)
PD (µsec) 1.4
#- #1.3#
prr PRF (Hz) 1796.0
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
(MPa) 2.8
max
(cm) 1.1
max
(cm) #- # #
X
FL
(cm) #- # #
Y
2
@MI
max
(W/cm
582.8
)
Frequency (MHz) T3.6 # - # CW2.0 #
Image Depth (cm) 30.0 # - # 3.5 #
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual 1-71
Direction 5116269-100 Rev. 2
Page 78

Acoustic Output
7S Probe
Table 1-37: Transducer Mode: 7S Operating Mode: B-Mode
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.3 <1 - - 1.0
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 2.6
r.3
(mW) ## #42.4
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) #
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 2.6
deq(zb) deq(zsp) (cm) -
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 3.9 # - - - 4.0
X(cm) #- - -1.3
Y(cm)
PD (µsec) 0.4
#- - -0.6
prr PRF (Hz) 3377.2
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FLX(cm) #- - 3.0
I
at max. MI I
pa,α
PA.3
(MPa) 3.7
max
(cm) -
max
FL
(cm) #- - 3.5
Y
2
@MI
max
(W/cm
364.1
)
Frequency (MHz) 4.0 # - - - 4.0
Image Depth (cm) 20.0 # - - - 4.0
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
1-72 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
Page 79

7S Probe (continued)
Tab le 1-38: Transducer Mode: 7S Operating Mode: M-Mode (inc.B-mode)
Acoustic Output Data
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
Global Maximum: Index Value 1.3 <1
<1
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 2.6
r.3
(mW) ## ##
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) #
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 2.6
deq(zb) deq(zsp) (cm) #
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 3.9 # # - # #
X(cm) ## - ##
Y(cm)
PD (µsec) 0.4
## - ##
prr PRF (Hz) 3377.2
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FLX(cm) ## - #
I
at max. MI I
pa,α
PA.3
(MPa) 3.7
max
(cm) #
max
FL
(cm) ## - #
Y
2
@MI
max
(W/cm
364.1
)
Frequency (MHz) 4.0 # # - # #
Image Depth (cm) 20.0 # # - # #
-<1<1
TIC
nonscan
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual 1-73
Direction 5116269-100 Rev. 2
Page 80

Acoustic Output
7S Probe (continued)
Table 1-39: Transducer Mode: 7S Operating Mode: CF-Mode (inch.M-mode)
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
Global Maximum: Index Value 1.7 1.5
<1
IEC FDA Units
pra p
P W
min of [P
α
(zs, I
(MPa) 3.5
r.3
o
)] [(W
ta,α(zs
.3(Z1),ITA.3
(mW) 75.5 # 29.4 75.5
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) 2.9
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 2.7
deq(zb) deq(zsp) (cm) 0.3
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 4.0 4.0 # -
X(cm) 1.4 # - 1.4 1.4
Y(cm)
PD (µsec) 0.8
0.6 # - 0.6 0.6
prr PRF (Hz) 635.2
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
(MPa) 4.8
max
(cm) 0.3
max
(cm) 3.0 # - 3.0
X
FL
(cm) 3.5 # - 3.5
Y
2
@MI
max
(W/cm
475.3
)
Frequency (MHz) D4.0 D4.0 # - D4.0 D4.0
Image Depth (cm) 2.7 3.0 # - 2.7 3.0
Vel Scale (kHz) 0.30 8.50 # - 1.80 8.50
Penet On On # - On On
ROI 90deg 10deg # - - 10deg
Operating Control
Conditions
PS 8 8 # - 16 8
-2.31.8
non-
scan
4.0
TIC
4.0
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
1-74 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
Page 81

Acoustic Output Data
7S Probe (continued)
Tab le 1-40: Transducer Mode: 7S Operating Mode: PWD-Mode (inch.B-mode)
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
Global Maximum: Index Value 1.7 1.5
<1
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 3.5
r.3
(mW) 75.5 # 29.4 41.0
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) 2.9
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 2.7
deq(zb) deq(zsp) (cm) 0.3
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 4.0 4.0 # -
X(cm) 1.4 # - 1.4 1.0
Y(cm)
PD (µsec) 0.8
0.6 # - 0.6 0.6
prr PRF (Hz) 635.2
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
(MPa) 4.8
max
(cm) 0.3
max
(cm) 3.0 # - 3.0
X
FL
(cm) 3.5 # - 3.5
Y
2
@MI
max
(W/cm
475.3
)
Frequency (MHz) D4.0 0.0 # - D4.0 D4.0
Image Depth (cm) 3.0 0.0 # - 3.0 3.0
Vel Scale (kHz) 0.64 - # - 1.60 1.60
SV 1 - # - 4 4
Penet On - # - On On
Operating Control
Conditions
-1.91.1
nonscan
4.0
TIC
4.0
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual 1-75
Direction 5116269-100 Rev. 2
Page 82

Acoustic Output
7S Probe (continued)
Table 1-41: Transducer Mode: 7S Operating Mode: CWD-Mode
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.5 1.5 <1 - 2.3 1.5
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 3.0
r.3
(mW) 75.5 # 38.7 47.9
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) 2.6
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 2.9
deq(zb) deq(zsp) (cm) 0.3
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 4.0 4.0 # - 4.0 4.0
X(cm) 1.4 # - 0.6 0.6
Y(cm)
PD (µsec) 0.8
0.6 # - 0.6 0.6
prr PRF (Hz) 300.0
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
(MPa) 4.4
max
(cm) 0.3
max
(cm) 3.0 # - 3.9
X
FL
(cm) 3.5 # - 35.0
Y
2
@MI
max
(W/cm
424.5
)
Frequency (MHz) 4.0 D4.0 # - CW4.0 CW4.0
Image Depth (cm) 20.0 3.0 # - 3.9 3.9
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
1-76 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
Page 83

10S Probe
Acoustic Output Data
Table 1-42: Transducer Mode: 10S Operating Mode: B-Mode
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.7 <1 - - - 1.2
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 3.6
r.3
(mW) #- -33.3
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) -
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 1.9
deq(zb) deq(zsp) (cm) -
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 4.9 # - - - 4.7
X(cm) #- - -0.9
Y(cm)
PD (µsec) 0.3
#- - -0.4
prr PRF (Hz) 5379.2
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FLX(cm) #- - 2.2
I
at max. MI I
pa,α
PA.3
(MPa) 4.8
max
(cm) -
max
FL
(cm) #- - 2.8
Y
2
@MI
max
(W/cm
582.8
)
Frequency (MHz) 8.0 # - - - 8.0
Image Depth (cm) 12.0 # - - - 12.0
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual 1-77
Direction 5116269-100 Rev. 2
Page 84

Acoustic Output
10S Probe (continued)
Table 1-43: Transducer Mode: 10S Operating Mode: M-Mode (inc.B-mode)
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
Global Maximum: Index Value 1.7 <1
<1
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 3.6
r.3
(mW) ## #28.6
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) #
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 1.9
deq(zb) deq(zsp) (cm) #
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 4.9 # # - # 4.7
X(cm) ## - #0.9
Y(cm)
PD (µsec) 0.3
## - #0.4
prr PRF (Hz) 5379.2
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FLX(cm) ## - 2.2
I
at max. MI I
pa,α
PA.3
(MPa) 4.8
max
(cm) #
max
FL
(cm) ## - 2.8
Y
2
@MI
max
(W/cm
582.8
)
Frequency (MHz) 8.0 # # - # 8.0
Image Depth (cm) 12.0 # # - # 12.0
-<11.0
TIC
nonscan
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
1-78 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
Page 85

Acoustic Output Data
10S Probe (continued)
Table 1-44: Transducer Mode: 10S Operating Mode: CF-Mode (inch.M-mode)
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
Global Maximum: Index Value 1.7 <1
<1
IEC FDA Units
pra p
P W
min of [P
α
(zs, I
(MPa) 3.6
r.3
o
)] [(W
ta,α(zs
.3(Z1),ITA.3
(mW) ## 23.3 #
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) 1.5
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 1.9
deq(zb) deq(zsp) (cm) 0.3
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 4.7 # # - 5.0 #
X(cm) ## -0.3#
Y(cm)
PD (µsec) 0.3
## -0.4#
prr PRF (Hz) 5379.2
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
(MPa) 4.8
max
(cm) 0.3
max
(cm) ## - #
X
FL
(cm) ## - #
Y
2
@MI
max
(W/cm
582.8
)
Frequency (MHz) D5.0 # # - D5.0 #
Image Depth (cm) 2.2 # # - 2.2 #
Vel Scale (kHz) 1.80 # # - 1.41 #
Penet On # # - On #
ROI 60deg # # - - #
Operating Control
Conditions
PS 14 # # - 16 #
-1.7<1
TIC
non-
scan
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual 1-79
Direction 5116269-100 Rev. 2
Page 86

Acoustic Output
10S Probe (continued)
Table 1-45: Transducer Mode: 10S Operating Mode: PWD-Mode (inch.B-mode)
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
Global Maximum: Index Value 1.7 <1
<1
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 3.6
r.3
(mW) ## ##
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) #
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 1.9
deq(zb) deq(zsp) (cm) #
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 4.9 # # - # #
X(cm) ## - ##
Y(cm)
PD (µsec) 0.3
## - ##
prr PRF (Hz) 5379.2
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
(MPa) 4.8
max
(cm) #
max
(cm) ## - #
X
FL
(cm) ## - #
Y
2
@MI
max
(W/cm
582.8
)
Frequency (MHz) D5.0 # # - # #
Image Depth (cm) 2.2 # # - # #
Vel Scale (kHz) 0.64 - # - # #
SV 1 - # - # #
Penet On - # - # #
Operating Control
Conditions
-<1<1
TIC
nonscan
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
1-80 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
Page 87

10S Probe (continued)
Table 1-46: Transducer Mode: 10S Operating Mode: CWD-Mode
Acoustic Output Data
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.7 <1 <1 - 1.7 <1
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 3.6
r.3
(mW) ## 23.3 #
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) 1.5
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 1.9
deq(zb) deq(zsp) (cm) 0.3
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 4.9 # # - 5.0 #
X(cm) ## -0.3#
Y(cm)
PD (µsec) 0.3
## -0.4#
prr PRF (Hz) 5379.2
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
(MPa) 4.8
max
(cm) 0.3
max
(cm) ## - #
X
FL
(cm) ## - #
Y
2
@MI
max
(W/cm
582.8
)
Frequency (MHz) 8.0 # # - CW5.0 #
Image Depth (cm) 12.0 # # - 2.8 #
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual 1-81
Direction 5116269-100 Rev. 2
Page 88

Acoustic Output
6T Probe
Table 1-47: Transducer Mode: 6T Operating Mode: B-Mode
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.4 1.2 - - - 1.2
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 2.7
r.3
(mW) 47.4 - -47.7
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) -
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 1.3
deq(zb) deq(zsp) (cm) -
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 3.4 3.4 - - - 3.4
X(cm) 0.8 - - - 0.8
Y(cm)
PD (µsec) 0.6
0.9 - - - 0.9
prr PRF (Hz) 3470.0
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FLX(cm) 3.9 - - 3.9
I
at max. MI I
pa,α
PA.3
(MPa) 3.2
max
(cm) -
max
FL
(cm) 5.6 - - 5.6
Y
2
@MI
max
(W/cm
398.1
)
Frequency (MHz) T6.0 T6.0 - - - T6.0
Image Depth (cm) 20.0 20.0 - - - 20.0
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
1-82 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
Page 89

6T Probe (continued)
Table 1-48: Transducer Mode: 6T Operating Mode: M-Mode (inc.B-mode)
Acoustic Output Data
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
Global Maximum: Index Value 1.6 1.2
<1
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 3.0
r.3
(mW) 47.7 - -42.0
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) #
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 1.3
deq(zb) deq(zsp) (cm) #
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 3.4 3.4 # - # 3.4
X(cm) 0.8 # - # 0.8
Y(cm)
PD (µsec) 0.6
0.9 # - # 0.9
prr PRF (Hz) 800.0
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FLX(cm) 3.9 # - 3.9
I
at max. MI I
pa,α
PA.3
(MPa) 3.6
max
(cm) #
max
FL
(cm) 5.6 # - 5.6
Y
2
@MI
max
(W/cm
490.3
)
Frequency (MHz) T6.0 T6.0 # - # T6.0
Image Depth (cm) 20.0 20.0 # - # 20.0
-<11.1
TIC
nonscan
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual 1-83
Direction 5116269-100 Rev. 2
Page 90

Acoustic Output
6T Probe (continued)
Table 1-49: Transducer Mode: 6T Operating Mode: CF-Mode (inch.M-mode)
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
non-
scan
Global Maximum: Index Value 1.7 1.2 1.1 - 4.5 1.2
IEC FDA Units
pra p
P W
min of [P
α
(zs, I
(MPa) 3.4
r.3
o
)] [(W
ta,α(zs
.3(Z1),ITA.3
(mW) 47.7 58.3 58.3 45.2
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) 1.2
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 1.0
deq(zb) deq(zsp) (cm) 0.8
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 3.9 3.4 4.0 -
X(cm) 0.8 0.4 - 0.4 0.8
Y(cm)
PD (µsec) 0.7
4.0
3.4
0.9 0.9 - 0.9 0.9
prr PRF (Hz) 1000.0
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
(MPa) 3.9
max
(cm) 0.8
max
(cm) 3.9 1.4 - 3.9
X
FL
(cm) 5.6 5.6 - 5.6
Y
2
@MI
max
(W/cm
460.8
)
Frequency (MHz) D4.0 D4.0 D4.0 - D4.0 D4.0
Image Depth (cm) 2.2 1.7 2.2 - 2.2 1.7
Vel Scale (kHz) 1.90 5.30 2.13 - 2.13 5.30
Penet On On On - On On
ROI Max Min - - - Min
Operating Control
Conditions
PS 8 8 16 - 16 8
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
1-84 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
Page 91

Acoustic Output Data
6T Probe (continued)
Table 1-50: Transducer Mode: 6T Operating Mode: PWD-Mode (inch.B-mode)
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
Global Maximum: Index Value 1.7 1.2
<1
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 3.4
r.3
(mW) 47.7 # 42.7 44.0
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) 1.4
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 1.0
deq(zb) deq(zsp) (cm) 0.4
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 3.9 3.4 # - 4.0 4.0
X(cm) 0.8 # - 0.8 0.8
Y(cm)
PD (µsec) 0.7
0.9 # - 0.9 0.9
prr PRF (Hz) 1000.0
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
(MPa) 3.9
max
(cm) 0.4
max
(cm) 3.9 # - 1.7
X
FL
(cm) 5.6 # - 5.6
Y
2
@MI
max
(W/cm
460.8
)
Frequency (MHz) D4.0 T6.0 # - D4.0 D4.0
Image Depth (cm) 1.1 20.0 # - 1.7 1.7
Vel Scale (kHz) 1.00 - # - 2.10 2.10
SV 1 - # - 2 2
Penet On - # - On On
Operating Control
Conditions
-2.81.1
TIC
nonscan
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual 1-85
Direction 5116269-100 Rev. 2
Page 92

Acoustic Output
6T Probe (continued)
Table 1-51: Transducer Mode: 6T Operating Mode: CWD-Mode
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.7 1.2 1.1 - 4.5 <1
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 3.3
r.3
(mW) 47.7 58.3 58.3 #
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) 1.2
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 1.4
deq(zb) deq(zsp) (cm) 0.8
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 3.9 3.4 4.0 - 4.0 #
X(cm) 0.8 0.4 - 0.4 #
Y(cm)
PD (µsec) 0.7
0.9 0.9 - 0.9 #
prr PRF (Hz) 1860.0
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
(MPa) 3.7
max
(cm) 0.8
max
(cm) 3.9 1.4 - #
X
FL
(cm) 5.6 5.6 - #
Y
2
@MI
max
(W/cm
533.3
)
Frequency (MHz) D4.0 T6.0 CW4.0 - CW4.0 #
Image Depth (cm) 2.2 20.0 1.4 - 1.4 #
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
1-86 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
Page 93

M3S Probe (LOGIQ 7 Only)
Table 1-52: Transducer Mode: M3S Operating Mode: B-Mode
Acoustic Output Data
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.7 <1 - - - <1
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 2.3
r.3
(mW) #- -#
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) -
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 2.1
deq(zb) deq(zsp) (cm) -
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 1.9 # - - - #
X(cm) #- - -#
Y(cm)
PD (µsec) 0.8
#- - -#
prr PRF (Hz) 2350.0
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FLX(cm) #- - #
I
at max. MI I
pa,α
PA.3
(MPa) 2.4
max
(cm) -
max
FL
(cm) #- - #
Y
2
@MI
max
(W/cm
268.2
)
Frequency (MHz) T4.0 # - - - #
Image Depth (cm) 19.0 # - - - #
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual 1-87
Direction 5116269-100 Rev. 2
Page 94

Acoustic Output
M3S Probe (LOGIQ 7 Only) (continued) (LOGIQ 7 Only)
Table 1-53: Transducer Mode: M3S Operating Mode: M-Mode (inc.B-mode)
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.7 <1 - <1 <1 <1
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 2.3
r.3
(mW) #- ##
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) #
zs z1(cm) #
zbp zbp(cm) #
zb zsp(cm) #
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 2.1
deq(zb) deq(zsp) (cm) #
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 1.9 # - # # #
X(cm) #- ###
Y(cm)
PD (µsec) 0.8
#- ###
prr PRF (Hz) 800.0
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FLX(cm) #- # #
I
at max. MI I
pa,α
PA.3
(MPa) 2.4
max
(cm) #
max
FL
(cm) #- # #
Y
2
@MI
max
(W/cm
268.2
)
Frequency (MHz) T4.0 # - # # #
Image Depth (cm) 19.0 # - # # #
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
1-88 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
Page 95

Acoustic Output Data
M3S Probe (LOGIQ 7 Only) (continued) (LOGIQ 7 Only)
Table 1-54: Transducer Mode: M3S Operating Mode: CF-Mode (inch.M-mode)
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
non-
scan
Global Maximum: Index Value 1.7 <1 - <1 3.9 1.5
IEC FDA Units
pra p
P W
min of [P
α
(zs, I
(MPa) 2.7
r.3
o
)] [(W
ta,α(zs
.3(Z1),ITA.3
(mW) #- 86.0 94.7
(z1)]) #
zs z1(cm) #
zbp zbp(cm) #
zb zsp(cm) 3.5
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 5.0
deq(zb) deq(zsp) (cm) 0.4
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 2.3 # - # 2.0 2.0
X(cm) #- #1.12.2
Y(cm)
PD (µsec) 0.8
#- #1.31.3
prr PRF (Hz) 635.2
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
(MPa) 3.8
max
(cm) 0.4
max
(cm) #- # 5.8
X
FL
(cm) #- # 5.8
Y
2
@MI
max
(W/cm
374.3
)
Frequency (MHz) D2.0 # - # D2.0 D2.0
Image Depth (cm) 5.8 # - # 5.8 5.8
Vel Scale (kHz) 2.29 # - # 1.41 4.13
Penet On # - # On On
ROI 90deg # - # - 10deg
Operating Control
Conditions
PS 10 # - # 16 10
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual 1-89
Direction 5116269-100 Rev. 2
Page 96

Acoustic Output
M3S Probe (LOGIQ 7 Only) (continued) (LOGIQ 7 Only)
Table 1-55: Transducer Mode: M3S Operating Mode: PWD-Mode (inch.B-mode)
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.7 <1 - <1 3.3 1.5
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 2.7
r.3
(mW) #- 94.7 94.7
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) #
zs z1(cm) #
zbp zbp(cm) #
zb zsp(cm) 5.1
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 5.0
deq(zb) deq(zsp) (cm) 0.5
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 2.3 # - # 2.5 2.5
X(cm) #- #1.51.5
Y(cm)
PD (µsec) 0.8
#- #1.31.3
prr PRF (Hz) 635.2
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
(MPa) 3.8
max
(cm) 0.5
max
(cm) #- # 6.1
X
FL
(cm) #- # 5.8
Y
2
@MI
max
(W/cm
374.3
)
Frequency (MHz) D2.5 # - # D2.5 D2.5
Image Depth (cm) 7.4 # - # 6.1 6.1
Vel Scale (kHz) 0.64 - - # 1.00 1.00
SV 1 - - # 6 6
Penet Off - - # Off Off
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
1-90 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
Page 97

M3S Probe (LOGIQ 7 Only) (continued) (LOGIQ 7 Only)
Table 1-56: Transducer Mode: M3S Operating Mode: Contrast
Acoustic Output Data
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.6 <1 - - - 1.5
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 2.3
r.3
(mW) #- - 90.5
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) -
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 4.2
deq(zb) deq(zsp) (cm) -
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 2.0 # - - - 1.9
X(cm) #- - -1.4
Y(cm)
PD (µsec) 0.7
#- - -1.3
prr PRF (Hz) 2350.0
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
Frequency (MHz) CHA
(MPa) 3.0
max
(cm) -
max
(cm) #- - 4.5
X
FL
(cm) #- - 5.8
Y
2
@MI
max
(W/cm
392.9
)
-- - -CHA
2.0
2.0
Image Depth (cm) 30.0 - - - - 14.0
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual 1-91
Direction 5116269-100 Rev. 2
Page 98

Acoustic Output
M3S Probe (LOGIQ 7 Only) (continued) (LOGIQ 7 Only)
Table 1-57: Transducer Mode: M3SOperating Mode: CWD-Mode
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value 1.7 <1 - <1 3.9 <1
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) 2.3
r.3
(mW) #- 86.0 #
.3(Z1),ITA.3
ta,α(zs
o
)] [(W
(z1)]) #
zs z1(cm) #
zbp zbp(cm) #
zb zsp(cm) 3.5
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) 2.1
deq(zb) deq(zsp) (cm) 0.4
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) 1.9 # - # 2.0 #
X(cm) #- #1.1#
Y(cm)
PD (µsec) 0.8
#- #1.3#
prr PRF (Hz) 2350.0
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
(MPa) 2.4
max
(cm) 0.4
max
(cm) #- # #
X
FL
(cm) #- # #
Y
2
@MI
max
(W/cm
268.2
)
Frequency (MHz) T4.0 # - # CW2.0 #
Image Depth (cm) 19.0 # - # 5.4 #
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
1-92 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
Page 99

P2D Probe
Acoustic Output Data
Table 1-58: Transducer Mode: P2D Operating Mode: CWD-Mode
Index Label MI
scan
TIS TIB
non-scan
Aaprt< Aaprt>1
TIC
nonscan
Global Maximum: Index Value <1 <1 <1 - 2.2 <1
IEC FDA Units
pra p
P W
min of [P
(zs, I
α
(MPa) #
r.3
o
)] [(W
ta,α(zs
.3(Z1),ITA.3
(mW) ## 53.5 #
(z1)]) -
zs z1(cm) zbp zbp(cm) zb zsp(cm) 2.9
Assoc Acoustic Paramete
z at max. Ipi,α z
sp
(cm) #
deq(zb) deq(zsp) (cm) 0.9
ƒ
awf
Dim of A
t
d
aprt
f
c
(MHz) # # # - 2.0 #
X(cm) ## - -#
Y(cm)
PD (µsec) #
## - -#
prr PRF (Hz) #
pr at max. Ipi pr@PII
deq at max. Ipi deq@PII
Other infromation
Focal Length FL
I
at max. MI I
pa,α
PA.3
(MPa) #
max
(cm) 0.4
max
(cm) ## - #
X
FL
(cm) ## - #
Y
2
@MI
max
(W/cm
#
)
Frequency (MHz) # # # - CW2.0 #
Operating Control
Conditions
NOTE:
a. This index is not required for this operation mode; see section 4.1.3.1 of the
Output Display Standard(NEMA UD-3)
b. This probe is not intended for transcranial or neonatal cephalic uses.
c. This formulation for TIS is less than that for an alternate formulation in this
mode.
# No data are reported for this operating condition since the global maximum
index value is not reported for the reason listed.
LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual 1-93
Direction 5116269-100 Rev. 2
Page 100

Acoustic Output
1-94 LOGIQ 7/LOGIQ 7 Pro Advanced Reference Manual
Direction 5116269-100 Rev. 2
 Loading...
Loading...