Page 1

Site Specifications
Page 2
Introduction
Intended Use
Intended Use
For In Vitro Diagnostic use only. The VITROS® ECi/ECiQ
Immunodiagnostic System perform random access, batch, and STAT
immunodiagnostic assays on human fluid specimens. The system uses
chemiluminescence detection technology to provide accurate and reliable
results for heterogeneous assays. All reactions for a single quantitative,
semi-quantitative, and qualitative measurement take place within a coated
well.
Note: The availability of the ECi/ECiQ Immunodiagnostic Assays listed
and illustrated in this Guide are subject to regulatory registration,
licensing, clearance, or approval.
Installation and Site Specifications
Although equipment service representatives uncrate and install the
VITROS ECi/ECiQ Immunodiagnostic System at the laboratory site, the
site needs to be prepared according to specifications.
This section describes general requirements for installing the ECi/ECiQ
System at your laboratory, including physical and environmental
requirements.
Physical Dimensions
The following sections provide general component dimensions and a site
drawing that illustrates setup and space requirements.
Dimensions
Table 1-1 provides the physical dimensions of the system, the printer, and
the printer stand.
Printer:
Printer:
Epson LX/
System
Width 111.8 cm (44 in.) 36.6 cm (14.4 in.) 41.1 cm (16.3 in.) 39.9 cm (15.7 in.) 61 cm (24 in.)
Depth 73.7 cm (29 in.) 27.5 cm (10.8 in.) 36.8 cm (14.4 in.) 34.5 cm (13.6 in.) 73.2 cm (28.8 in.)
Height 130.2 cm (51.25 in.),
top cover down
179.1 cm (70.5 in.),
top cover up
Wei ght 366 kg (807 lbs.) 4.4 kg (9.8 lbs.) 7.5 kg (16.5 lbs.) 7.3 kg (16 lbs.) 10.9 kg (24 lbs.)
*Standard printer for the system. Printers are ordered in accordance with regional specifications.
Model 300*
16.0 cm (6.3 in.) 16.0 cm (6.3 in.) 20.3 cm (8 in.) 71.1 cm (28 in.)
Printer:
Epson LQ/
Model 570e
Texas Instruments
Omni 800
Model 830e Printer Stand
Table 1-1. Physical Dimensions
Operator’s Guide Part No. 7B6090
1-4 VITROS ECi/ECiQ Immunodiagnostic System Version 2.0
Page 3
Introduction
Installation and Site Specifications
Site Drawing
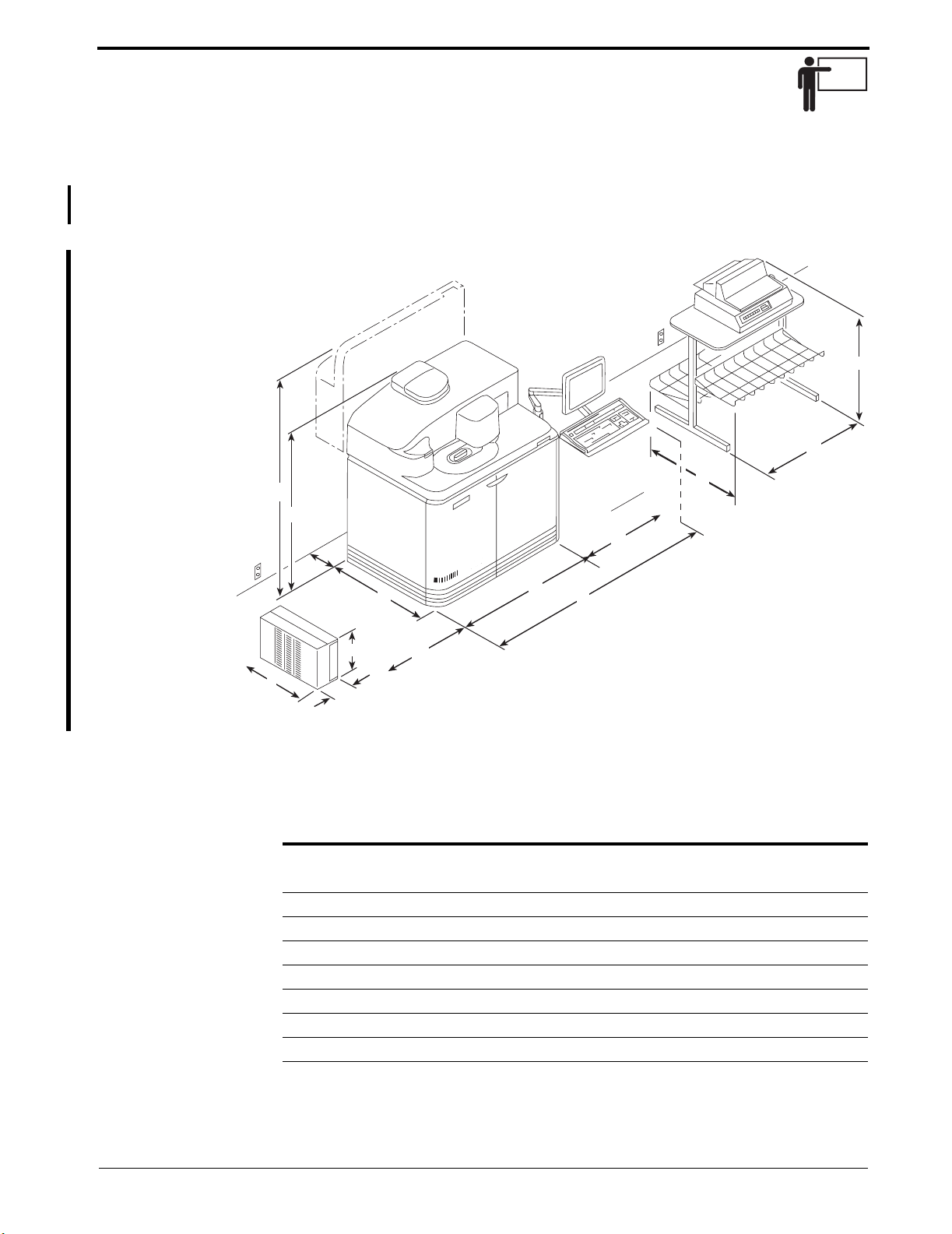
The site drawing in figure 1-1 provides the physical dimensions of the
system. The system weighs approximately 800 pounds (366 kilograms).
M
N
D
C
F
E
K
I
J
Figure 1-1. Physical Dimensions
G
A
H
B
L
The system and printer dimensions are described below:
Reference Dimension
System Dimensions:
A 111.8 cm (44 in.)
B 162.6 cm (64 in.)
C 130.2 cm (51.25 in.)
D 179.1 cm (70.5 in.)
E 73.7 cm (29 in.)
F 61 cm (24 in.)
G 59.7 cm (23.5 in.) - Left side door clearance
H 66 cm (26 in.)
Part No. 7B6090 Operator’s Guide
Version 2.0 VITROS ECi/ECiQ Immunodiagnostic System
1-5
Page 4

Introduction
Installation and Site Specifications
Reference Dimension
UPS Dimensions:
I 49.4 cm (19.4 in.)
J 43.2 cm (17.0 in.)
K 8.9 cm (3.5 in.)
Printer Stand for Epson LQ/Model 570e and Epson LX/Model 300* Printer Dimensions:
L 73.2 cm (28.8 in.)
M 61 cm (24 in.)
N 87.1 cm (34.3 in.)
Printer Stand with Texas Instruments Omni 800/Model 830e Printer Dimensions:
L 73.2 cm (28.8 in.)
M 61 cm (24 in.)
N 91.4 cm (36 in.)
*Standard printer for the system. Printers are ordered in accordance with regional specifications.
Safety Requirements
Power Requirements
The system meets all requirements for bearing the CE marking and safety
standards UL 3101-1, CSA C22.2 No. 1010.1, and IEC 1010-1.
System
The system requires a dedicated, single phase, AC power line and one of
the following:
Maximum
Line Voltage Frequency
North America 120 volt AC 50–60 Hz 12 amps <1.5 KVA
Continental
Europe
200–240 volt AC 50–60 Hz 6 amps <1.5 KVA
Current Draw Input Power
The system’s power cord should be plugged into a 3-wire, grounded
electrical outlet (receptacle type). The power cord should be 1.83 meters (6
ft.) in length and detachable.
• North America: IEC 320 to NEMA 5-20R
• Continental Europe: IEC 320 to CEE7 “Shuko”
Operator’s Guide Part No. 7B6090
1-6 VITROS ECi/ECiQ Immunodiagnostic System Version 2.0
Page 5

Introduction
Installation and Site Specifications
e-Connectivity™
e-Connectivity™ requires a Virtual Private Network (VPN) and Modem.
The VPN and Modem require two electrical outlets and non-dedicated
power lines can be used. They can also be plugged into a single power strip
which can share the same receptacle as the ECi/ECiQ System. Power cords
are supplied with the VPN and Modem.
Line Voltage and Frequency for VPN and Modem used world wide:
VPN Modem
Line Voltage: 100 - 240 volt AC Line Voltage: 100 - 240 volt AC
Frequency: 47/63 Hz Frequency: 50/60 Hz
Refer to Chapter 1, “Introduction” for detailed information on the VPN and
Modem.
Refer to Chapter 2, “System Overview” for detailed information on
e-Connectivity.
Printer
Line Voltage and Frequency for printers used in North America:
Epson LX/Model 300* Epson LQ/Model 570e Texas Instruments Omni 800/Model 830e
Line Voltage: 99-132 volt AC Line Voltage: 99-132 volt AC Line Voltage: 120 volt AC
Frequency: 50/60 Hz Frequency: 50/60 Hz Frequency: 50/60 Hz
*Standard printer for the system. Printers are ordered in accordance with regional specifications.
Electrical Outlet (Receptacle Type)
The electrical outlet (receptacle type) for the system is as follows:
• North America: NEMA 5-20R
• Continental Europe: CEE7 “Shuko”
Electrical Outlet (Receptacle type) for Printers used in North America:
Epson LX/Model 300* Epson LQ/Model 570e Texas Instruments Omni 800/Model 830e
NEMA 5-15R NEMA 5-15R NEMA 5-15R
*Standard printer for the system. Printers are ordered in accordance with regional specifications.
Part No. 7B6090 Operator’s Guide
Version 2.0 VITROS ECi/ECiQ Immunodiagnostic System
1-7
Page 6

Introduction
Installation and Site Specifications
Uninterruptible Power Supply
If you experience frequent power fluctuations, you may want to use an
uninterruptible power supply.
Contact your technical support representative for more information.
Entrance to Room
The entrance door opening should be a minimum of 76.2 cm (30 in.) wide.
Telephone Recommendations
You may also find it convenient to have a telephone near the system to
communicate with technical support personnel during troubleshooting
sessions.
A dedicated analog telephone line is required for e-Connectivity™ which
provides a secure connection between your system and Ortho-Clinical
Diagnostics. The modem installed on your system for e-Connectivity is
connected to the dedicated analog telephone line.
Refer to Chapter 1, “Introduction” for detailed information on the VPN and
Modem.
Refer to Chapter 2, “System Overview” for detailed information on
e-Connectivity.
Operator’s Guide Part No. 7B6090
1-8 VITROS ECi/ECiQ Immunodiagnostic System Version 2.0
Page 7

Environmental Specifications
The environmental limits for normal operation of the system are defined
below and shown in figure 1-2.
• BTU Output: 4100 BTUs per hour
• Operating Temperature: 15–30°C (59–86°F)
• Site Relative Humidity: 15–75% RH noncondensing
• Altitude: -0.1524/2.439 km (-500/8000 ft.)
Temperature
Introduction
Installation and Site Specifications
30.0°C
(86°F)
26.0°C
(78°F)
System Relocation
15.0°C
(59°F)
15% 50% 75%
Relative Humidity
Figure 1-2. Environmental Requirements
The system is mounted on casters to facilitate its relocation within the
laboratory. The new site must meet the same space, electrical, and
environmental requirements as specified for the original site.
Part No. 7B6090 Operator’s Guide
Version 2.0 VITROS ECi/ECiQ Immunodiagnostic System
1-9
 Loading...
Loading...