Page 1

Jaundice Meter
Konica Minolta/Dräger Medical
WARNING:
For a full understanding of the
performance characteristics of
this equipment, the user should
carefully read this manual
before operating.
Model JM-103
Operating Instructions
Page 2

Page 3

PROPRIETARY AND CONFIDENTIAL DRAFT 9 Nov 04
Table of Contents
Section 1: Symbol Definition and Intended Use
Symbol Definition . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 - 1
Intended Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 - 3
Section 2: Introduction, Features, and Specifications
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2 - 1
Measuring Point . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2 - 1
Explanation of the Test . . . . . . . . . . . . . . . . . . . . . . . . . . . .2 - 2
Features. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2 - 5
Controls, Indicators, and Connections . . . . . . . . . . . . . . . .2 - 5
Display . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2 - 7
Standard Features . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2 - 8
Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2 - 9
Standard Features . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2 - 9
Regulations, Standards, and Codes . . . . . . . . . . . . . . . . . .2 - 10
Device Classification. . . . . . . . . . . . . . . . . . . . . . . . . . . . .2 - 10
Electromagnetic Compatibility (EMC) Guidance and
Manufacturer’s Declarations . . . . . . . . . . . . . . . . . . . . . . .2 - 11
Section 3: Precautions and Safety Tips
Precautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3 - 1
Electromagnetic Compatibility Precautions . . . . . . . . . . . .3 - 3
Safety Tips . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3 - 4
Warning and Caution Labels . . . . . . . . . . . . . . . . . . . . . . . . . . .3 - 5
Section 4: Installation and Assembly
Installation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4 - 1
Charging the Battery . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4 - 1
Selecting the Unit of Measurement. . . . . . . . . . . . . . . . . . .4 - 3
Operational Checkout of the Jaundice Meter . . . . . . . . . . .4 - 4
Section 5: Instructions for Use
Instructions for Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5 - 1
Taking Measurements . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5 - 1
Jaundice Meter (Model JM-103) User Manual (usr070) Page i
Page 4

Setting the Number of Average Measurements . . . . . . . . . 5 - 5
Taking Average Measurements . . . . . . . . . . . . . . . . . . . . . 5 - 6
Section 6: Cleaning, Maintenance, Replacement Parts, and Storage and
Handling
Cleaning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6 - 1
Steam Cleaning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6 - 1
Cleaning Difficult to Access Areas . . . . . . . . . . . . . . . . . . 6 - 1
Disinfecting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6 - 2
Maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6 - 2
Replacement Parts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6 - 2
Storage and Handling . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6 - 3
Disposal. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6 - 3
Section 7: Troubleshooting
Service Calls . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7 - 1
Error Messages . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7 - 1
Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7 - 2
Appendix A: Clinical Performance Summary
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A - 1
Study Design . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A - 1
Performance Data. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A - 2
Appendix B: Doctors’ Office Data
Study Design . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . B - 1
Performance Data. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . B - 3
Conclusion . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . B - 8
Appendix C: Medical and Scientific References on Transcutaneous
Bilirubinometry (1979 - 1997)
Page ii Jaundice Meter (Model JM-103) User Manual (usr070)
Page 5

DRAFT 18 May 2005
Section 1
Symbol Definition
and Intended Use
Symbol Definition
This manual contains different typefaces and icons designed to improve
readability and increase understanding of its content. Note the following
examples:
• Standard text—used for regular information.
• Boldface text—emphasizes a word or phrase.
• NOTE:—sets apart special information or important instruction
clarification.
• The symbol below highlights a WARNING or CAUTION:
Warning and Caution
– A WARNING identifies situations or actions that may affect
patient or user safety. Disregarding a warning could result in
patient or user injury.
– A CAUTION points out special procedures or precautions that
personnel must follow to avoid equipment damage.
• The symbol below highlights a type BF applied part:
Type BF Applied Part
– The instrument provides a specified degree of protection
against electric shock, particularly the leakage current and
reliability of the protective ground connection with an F-type
applied part. An F-type applied part indicates an applied part
isolated from all other parts of the instrument to such a degree
that the patient leakage current allowable in a single-fault
condition is not exceeded when a voltage equal to 1.1 times the
highest-rated mains voltage is applied between the applied part
and ground.
Jaundice Meter (Model JM-103) User Manual (usr070) Page 1 - 1
Page 6
DRAFT 18 May 2005
• The symbol below highlights an ELECTRICAL SHOCK HAZARD
WARNING:
Electrical Shock Hazard Warning
• The symbol below indicates INPUT RATING:
Input Rating Symbol
• The symbol below indicates that the product uses a
RECHARGEABLE BATTERY:
Rechargeable Battery Symbol
• The symbol below indicates RESET:
RESET Button Symbol
• The symbol below, when applied to the device, indicates:
ATTENTION: Consult Accompanying Documents
Page 1 - 2 Jaundice Meter (Model JM-103) User Manual (usr070)
Page 7
DRAFT 18 May 2005
Intended Use
CAUTION:
Magnetic Resonance Imaging (MRI) procedures interfere with
Jaundice Meter operation. Inaccurate readings could occur.
CAUTION:
Do not use a mobile telephone when using the Jaundice Meter. A
measurement error could occur.
Intended Use of the Jaundice Meter
The Jaundice Meter (JM-103) is a non-invasive transcutaneous
bilirubinometer. It measures yellowness of subcutaneous tissue in
newborn infants.
The device is intended for use in hospitals or doctors’ offices under a
physician’s supervision or at their direction to assist clinicians in
monitoring of newborn infants. The device is not intended as a
standalone for diagnosis of hyperbilirubinemia. It is to be used in
conjunction with other clinical signs and laboratory measurements.
Newborn infants whose Jaundice Meter (JM-103) test results are
indicative of hyperbilirubinemia should be evaluated by their
physician(s) for appropriate patient management. Specific neonatal
patient bilirubin levels should be confirmed by other methods, such as
serum bilirubin, prior to treatment determinations.
The Jaundice Meter (JM-103) is not intended for home use.
Limitations (Doctors’ Office Use)
Use only on infants up to 14 days of age.
For doctors’ office application, use only the sternum location when
taking measurements.
Please be aware, performance in doctors’ offices may vary from
performance in hospitals.
Precocious Jaundice
Do not use this device on infants with precocious jaundice. If there is a
possibility that the infant is suffering from precocious jaundice, as a
result of an incompatible blood type or hemolytic jaundice, it is
recommended that the total serum bilirubin be measured.
Jaundice Meter (Model JM-103) User Manual (usr070) Page 1 - 3
Page 8

DRAFT 18 May 2005
Intended Use of the User Manual
This manual provides instructions for installation, use, operator
maintenance, and troubleshooting of the Jaundice Meter (JM-103).
Konica Minolta/Dräger Medical cannot be responsible for the
performance of the Jaundice Meter if the user does not operate the unit
in accordance with the instructions, fails to follow maintenance
recommendations, or makes any repairs with unauthorized components.
Only qualified service personnel should perform repair.
Page 1 - 4 Jaundice Meter (Model JM-103) User Manual (usr070)
Page 9

DRAFT 20 June 2005
Section 2
Introduction, Features,
and Specifications
Introduction
To prevent kernicterus in newborn infants, it is very important to detect
jaundice in its early stages. The Jaundice Meter (JM-103) is a noninvasive transcutaneous bilirubinometer. This hand-held device allows a
quick, non-invasive estimate of bilirubin concentration, to be used as an
aid for the management of jaundice in newborn infants. The
measurements are taken automatically when you place the instrument’s
measuring probe against the measuring site of the infant and press it
gently; the measured value is then displayed.
Measuring Point
Measurements must be taken only on the infant’s forehead (at hospital
sites only) or sternum (at hospital sites or physicians’ offices) where a
sufficient amount of blood is circulated. A possibility exists that the
bilirubin in the subcutaneous tissue may measure low for areas with
minimal blood flow or areas in which the subcutaneous tissue is subject
to keratinization.
Although correlation with serum bilirubin was observed for both
forehead and sternum measurements, the clinical studies performed with
the Jaundice Meter (JM-103) show consistently better results with
measurements taken at the sternum versus the forehead. There is a
possibility that this difference may be more pronounced for infants that
have been exposed to sunlight, such as infants seen at doctors’ offices.
Only sternum measurements were evaluated during the studies
conducted at doctors’ offices; correlation of forehead measurements
with serum bilirubin has not been evaluated, and the device is not
intended for forehead measurements at doctors’ offices. Therefore, use
the sternum location when taking measurements at doctors’ offices.
SPECIFICATIONS
Phototherapy
WARNING:
Do not use the Jaundice Meter after initiation of phototherapy or
after an exchange transfusion as results may be inaccurate under
these conditions. Patient injury could occur.
Jaundice Meter (Model JM-103) User Manual (usr070) Page 2 - 1
Page 10
DRAFT 20 June 2005
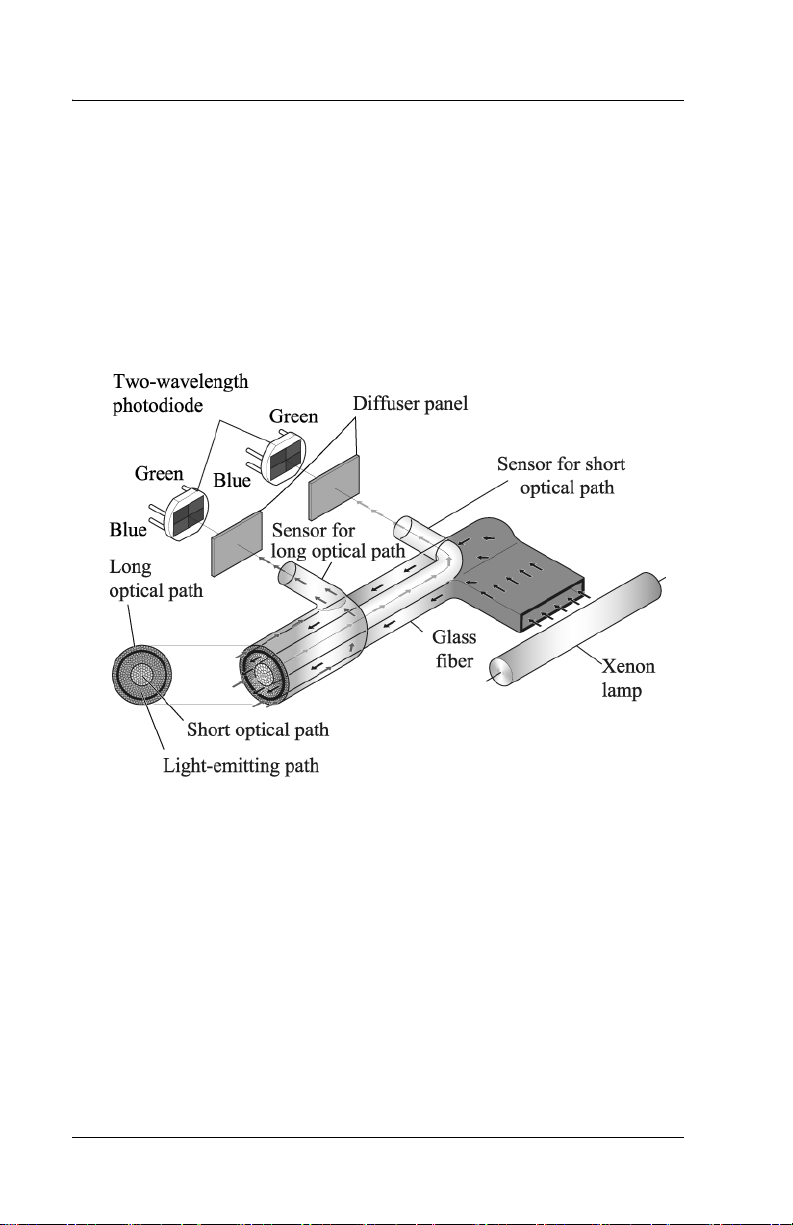
Explanation of the Test
Measuring Principle
The Jaundice Meter determines the yellowness of an infant’s
subcutaneous tissue by measuring the difference in the optical densities
for light in the blue (450 nm) and green (550 nm) wavelength regions.
The measuring probe has two optical paths. This method allows for a
more precise measurement of yellowness in an infant’s subcutaneous
tissue by minimizing the influences of the melanin pigment and the skin
maturity.
When the measuring probe is pressed against the forehead or sternum of
the infant, the built-in xenon lamp flashes. The light from the xenon
lamp passes through the glass fiber and illuminates the skin. The light
scatters and is absorbed in the skin and subcutaneous tissue repeatedly,
and then finally returns to the sensor side of the glass fiber. Of the light
that returns, the part scattered from the shallow areas of the
subcutaneous tissue passes through the inner core, or short-optical path,
of the fiber. The part scattered from the deep areas of the subcutaneous
tissue passes through the outer core, or long-optical path, and then
reaches its corresponding photodiode.
Page 2 - 2 Jaundice Meter (Model JM-103) User Manual (usr070)
Page 11
DRAFT 20 June 2005
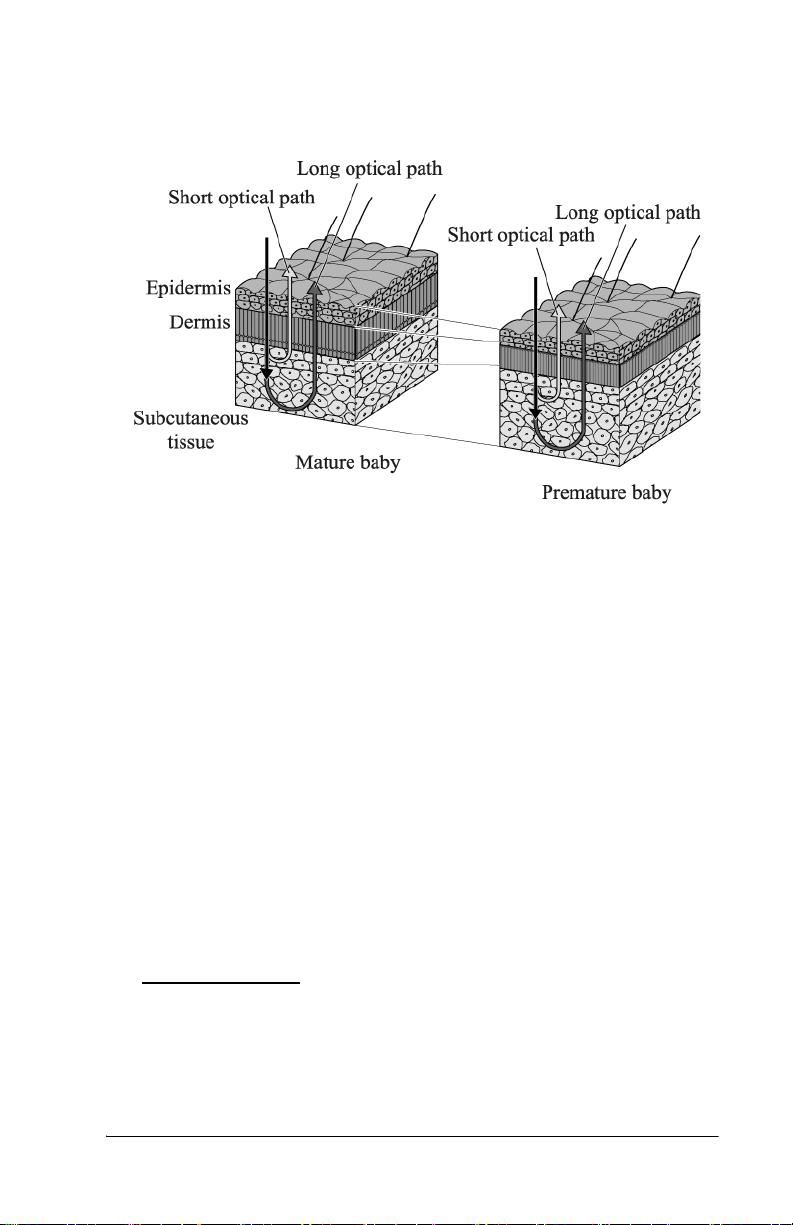
By calculating the difference in the optical densities, the parts that are
common to the epidermis and dermis are deducted, and as a result, the
difference in the optical densities between the two wavelength regions
can be obtained for the subcutaneous tissue only. Since the optical
density difference shows a linear correlation with the total serum
bilirubin concentration, it is converted to the estimated bilirubin
concentration and is indicated digitally.
The Jaundice Meter (JM-103) device software uses a correlation
coefficient to convert the measurement difference from the dual optical
path to an estimated bilirubin concentration. The calculation formula
used includes the correlation coefficients α and γ. These coefficients
were determined in pre-clinical testing. The equation used is as follows:
J
= α(L-S) + γ
sample
Where L and S are the long and short optical path measurements.
Use of the Device
Patient Population
The Jaundice Meter (JM-103) is indicated for use in neonatal patients
born >35 weeks gestation who have not undergone transfusion or
phototherapy treatment.
SPECIFICATIONS
Jaundice Meter (Model JM-103) User Manual (usr070) Page 2 - 3
Page 12

DRAFT 20 June 2005
Averaging of Measurements
Averaging measurements may allow for more precise results. Assess the
advantages of using average measurements at your facility. There was
no significant difference between the averaged measurements and the
single measurement approaches in the largest study for sternum
measurements. The mean of three measurements showed the highest
degree of correlation (r=0.965), however, the difference was minimal
with a single measurement (r=0.963).
Averaging was not evaluated in the physicians’ office study.
Action Levels
Each facility should determine their own action levels based on studies
of performance of the device on their population. Appropriate action
levels may vary depending on performance of the device, such as
precision or correlation with serum bilirubin, in the hands of the user.
Some factors that could affect performance of the device or appropriate
action levels include skin color, age, or measurement site. The bias
relative to serum bilirubin differs between hospital versus physicians’
office sites (see Appendixes A and B). Different action levels may be
appropriate for hospital versus physicians’ offices. Careful selection of
action levels should be made so that false negatives do not prevent
appropriate follow up measures.
Calibration
There is no user calibration of this device. The system does include a
checker that measures the intensity of light from the device to ensure the
light output is acceptable for proper use.
Processing of Measured Values
The Jaundice Meter (JM-103) determines the yellowness of the
subcutaneous tissue by measuring the difference in the optical densities
for light in the blue and green wavelength regions. The optical density
difference has been shown to have a linear correlation with serum
bilirubin concentration. The device computes an estimated bilirubin
concentration based on this linear correlation and provides the value on
the display.
Page 2 - 4 Jaundice Meter (Model JM-103) User Manual (usr070)
Page 13
DRAFT 20 June 2005
Features
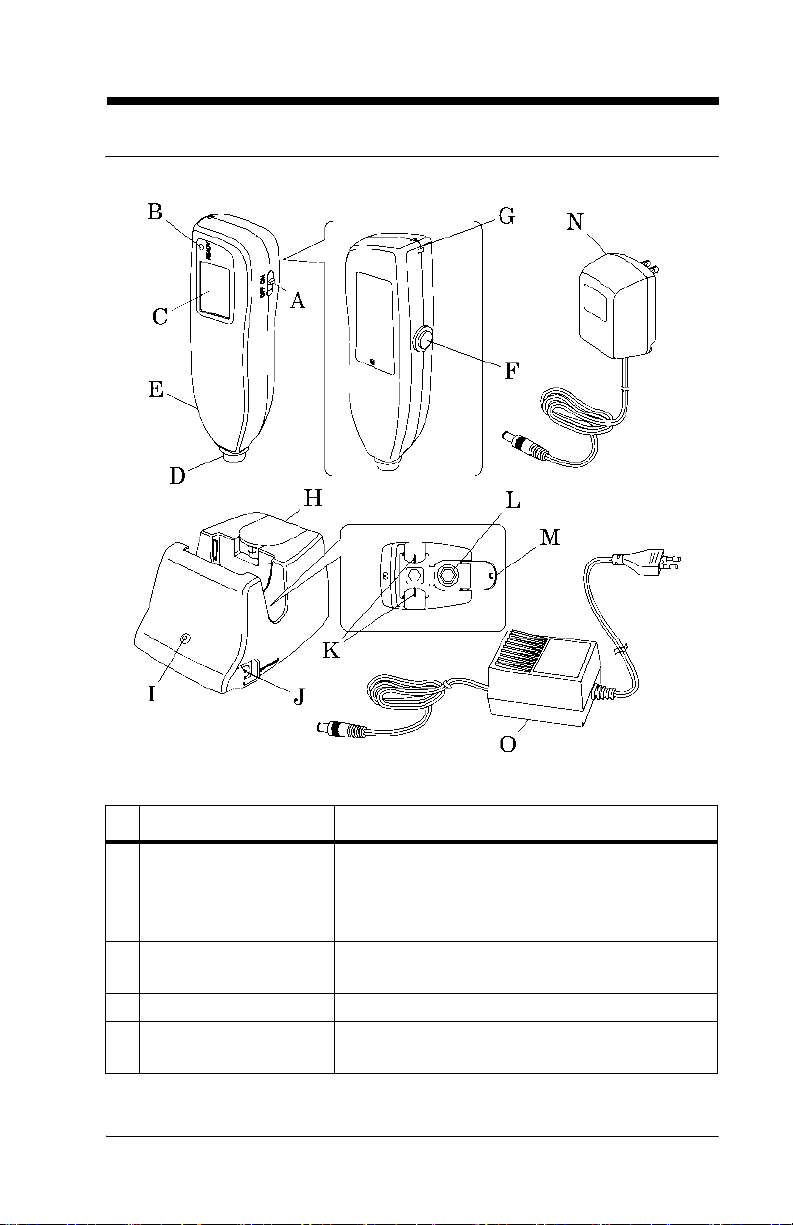
Controls, Indicators, and Connections
SPECIFICATIONS
Controls, Indicators, and Connections
Name Function
A Power switch Turns the Jaundice Meter on and off.
When used with the Reset button, the device
switches to Check Mode and changes the unit
of measurement.
B Ready lamp Illuminates to indicate that the Jaundice Meter
is ready for the next measurement.
C Display Displays the measured value.
D Measuring probe Takes the measurement when pressed against
the measuring point.
Jaundice Meter (Model JM-103) User Manual (usr070) Page 2 - 5
Page 14

DRAFT 20 June 2005
Name Function
E Charger section Connect the charger unit to the charger sec-
tion.
F Reset button Deletes the currently displayed measured
value and prepares for the next measurement.
When used with the Power switch, the device
switches to Check Mode and changes the unit
of measurement.
G Strap attachment area Is where the strap attaches.
H Checker cover Open this checker cover to check the Jaundice
Meter.
I Charger lamp Illuminates to indicate that the Jaundice Meter
is charging.
J DC jack Connect the AC adapter to this jack.
K Charger jack Connect the main body to this jack.
L Checker Checks for the intensity of light output by tak-
ing measurements in Check Mode.
M Standard checker
values
N DC plug Connect the charger’s DC jack to this.
O DC Plug (interna-
tional)
For reference.
Connect the charger’s DC jack to this.
Page 2 - 6 Jaundice Meter (Model JM-103) User Manual (usr070)
Page 15
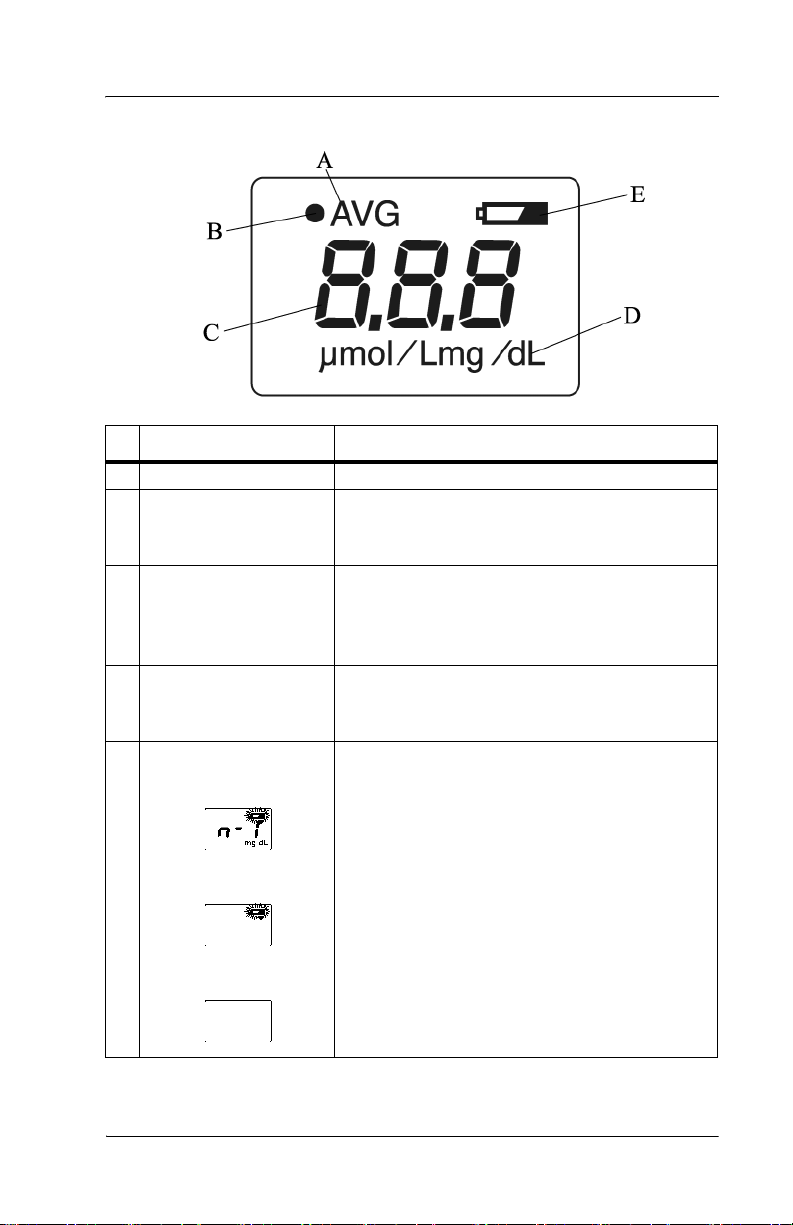
DRAFT 20 June 2005
Display
Name Function
A AVG Illuminates during averaging measurement.
B Optical path indicator
(•)
C Value Displays the measured value.
D Unit of measurement Displays the unit of measurement in either
E Battery indicator When the battery power is low, the battery
When verifying light output with the checker,
(•)illuminates when the L-value appears and
extinguishes when the S-value appears.
NOTE: When the measured value is greater
than 20 mg/dl, the display shows “---” and the
physician should be contacted.
milligrams per deciliter (mg/dL) or micromoles of solute per liter (µmol/L)
indicator blinks. Charge the battery as soon as
possible (see “Charging the Battery” on page
4-1).
If only the battery indicator lights, the battery
has run out. Go to “Charging the Battery” on
page 4-1.
If the power is on and the display is blank, the
battery is completely exhausted. Go to
“Charging the Battery” on page 4-1.
SPECIFICATIONS
Jaundice Meter (Model JM-103) User Manual (usr070) Page 2 - 7
Page 16

DRAFT 20 June 2005
Standard Features
• Jaundice Meter (JM-103)
• Charger unit (Model JM-A30) with a checker
• AC adapter (Model JM-A31)
• Carrying case and wrist strap
Page 2 - 8 Jaundice Meter (Model JM-103) User Manual (usr070)
Page 17
DRAFT 20 June 2005
Specifications
Standard Features
Feature Dimension
Model name JM-103
Measuring method Determines the yellowness of the
subcutaneous tissue by using two
optical paths to measure the optical
density difference at two wavelengths
Measurement range 0.0 mg/dL to 20 mg/dL or 0 µmol/L
to 340 µmol/L
Clinical Data Standard Error of
Estimate (SEE) *
Light source Pulse xenon arc lamp
Light source life 150000 measurements
Sensors Silicon photodiodes
Power source 2.4 V, Special Ni-MH battery
Protection type and level Internally-powered instrument, BF-
Minimum number of
measurements when fully charged
Operating temperature range 10°C (50°F) to 40°C (104°F)
Operating relative humidity range 30% to 95% non-condensing
Storage temperature range -10°C (14°F) to 50°C (122°F)
Storage relative humidity range 30% to 95% non-condensing
Dimensions 48 mm (1.9") wide x 15.5 cm (6.1")
Weight, including battery 150 g (5.3 oz)
AC adapter input for North America 120V, 50/60 Hz, 10 W
AC adapter input for
international use (old)
AC adapter input for
international use (new)
AC adapter output 9V, 500 mA, 4.5W
± 1.5 mg/dL or ± 25.5 µmol/L
type
400 single measurements
high x 33 mm (1.3") deep
200V-240V, 50/60 Hz, 12.5VA
100V-240V, 50/60 Hz, 0.14-0.07A
SPECIFICATIONS
*The standard deviation shown above is based on the average of the
Jaundice Meter (Model JM-103) User Manual (usr070) Page 2 - 9
Page 18

DRAFT 20 June 2005
clinical data available. On average, 66% of results fall within this range,
and the remainder fall outside this range. This value can be affected by
variables such as age, skin color, and preformance of the device in the
hands of the user. Refer to Appendixes A and B for a detailed
description of results by clinical site, measurement location, and patient
demographics. The SEE shown above are based on the clinical data
available and can be affected by variables such as infant developmental
age, ethnicity, etc. Therefore, we recommend that the JM-103 be used in
conjunction with other clinical signs and laboratory measurements.
"Specific Bilirubin Measurement" should be confirmed by other
methods such as laboratory blood serum analysis.
Regulations, Standards, and Codes
In North America
With respect to electrical shock, fire, and
mechanical hazards only, this instrument complies
with UL 60601-1 and CAN/CSA C22.2 No. 601.1.
In Europe, this instrument complies with EN60601-1, EN60601-1-2,
and EN ISO13485, and EN ISO14971.
Directive 2002/96/EC of the European Parliament and of the Council of
2003-01-27 on Waste Electrical and Electronic Equipment (WEEE)
Annex IV, prEN 50419 applies.
Device Classification
The Jaundice Meter (JM-103) meets the requirements for the following
classifications:
• Protection against electrical shock: Internally powered
• Type of applied part: BF
• Degree of protection against harmful ingress of water: Not applicable
• Not suitable for use in the presence of flammable anesthetic mixture
with air or oxygen or nitrous oxide.
• Mode of operation of equipment: Continuous while in use (IEC
60601-1)
Page 2 - 10 Jaundice Meter (Model JM-103) User Manual (usr070)
Page 19

DRAFT 20 June 2005
Electromagnetic Compatibility (EMC) Guidance and Manufacturer’s Declarations
Guidance and Manufacturer’s Declaration—Electromagnetic
Emissions
The Jaundice Meter (JM-103) is intended for use in the electromagnetic environment specified below. The customer or user of the unit
should ensure that the unit is used in such an environment.
Emissions Test Compliance
Radio frequency
Group 1 The Jaundice Meter (JM-103)
(RF) emissions
—CISPR 11
Electromagnetic
Environment—Guidance
uses RF energy only for its internal function. Therefore, its RF
emissions are very low and are
not likely to cause interference
with nearby electronic equipment.
RF emissions—
CISPR 11
Harmonic Emissions—IEC
61000-3-2
Voltage fluctuations/ flicker
Class B The Jaundice Meter (JM-103) is
suitable for use in all establish-
Class A
ments, including domestic and
those directly connected to the
public low-voltage power supply
Complies
network that supplies buildings
used for domestic purposes.
emissions—IEC
61000-3-3
SPECIFICATIONS
Jaundice Meter (Model JM-103) User Manual (usr070) Page 2 - 11
Page 20
DRAFT 20 June 2005
Guidance and Manufacturer’s Declaration—Electromagnetic
Immunity
The Jaundice Meter (JM-103) is intended for use in the electromagnetic environment specified below. The customer or user of the unit
should ensure that the unit is used in such an environment.
Immunity
Test
Electrostatic
discharge
(ESD)—
IEC 610004-2
Electrical
fast transient/burst
—IEC
61000-4-4
Surge—IEC
61000-4-5
IEC 60601
Test L e v e l
± 6 kV contact
± 8 kV air
± 2 kV for
power supply lines
± 1 kV
differential
mode
Compliance
Level
± 6 kV contact
± 8 kV air
± 2 kV for
power supply lines
± 1 kV
differential
mode
Electromagnetic
Environment—
Guidance
The floors should be
wood, concrete, or
ceramic tile. If floors are
covered with synthetic
material, the relative
humidity should be at
least 30%.
Mains power quality
should be that of a typical
commercial or hospital
environment.
Mains power quality
should be that of a typical
commercial or hospital
environment.
Page 2 - 12 Jaundice Meter (Model JM-103) User Manual (usr070)
Page 21
DRAFT 20 June 2005
Guidance and Manufacturer’s Declaration—Electromagnetic
Immunity
The Jaundice Meter (JM-103) is intended for use in the electromagnetic environment specified below. The customer or user of the unit
should ensure that the unit is used in such an environment.
Immunity
Test
Voltage
dips, short
interruptions, and
voltage variations on
power supply input
lines—IEC
61000-4-11
Power frequency
(50/60 Hz)
magnetic
field—IEC
61000-4-8
IEC 60601
Test Level
< 5% U
95% dip in
) for 0.5
U
T
cycles
40% U
(60% dip in
U
) for 5
T
cycles
70% U
(30% dip in
U
) for 25
T
cycles
< 5% U
95% dip in
U
) for 5
T
seconds
(>
T
T
T
(>
T
Compliance
Level
< 5% U
T
(>
95% dip in
) for 0.5
U
T
cycles
40% U
T
(60% dip in
U
) for 5
T
cycles
70% U
T
(30% dip in
U
) for 25
T
cycles
< 5% U
T
(>
95% dip in
U
) for 5
T
seconds
3 A/m 3 A/m The power frequency
Electromagnetic
Environment—
Guidance
Mains power quality
should be that of a typical
commercial or hospital
environment. If the user
of the unit requires continued operation during
power mains interruptions, it is recommended
that the unit be powered
from an uninterruptable
power supply or battery.
magnetic fields should be
at levels characteristic of
a typical location in a typical commercial or hospital environment.
SPECIFICATIONS
NOTE:
U
is the AC mains voltage prior to the application of the test
T
level.
Jaundice Meter (Model JM-103) User Manual (usr070) Page 2 - 13
Page 22
DRAFT 20 June 2005
Guidance and Manufacturer’s Declaration—Electromagnetic
Immunity
The Jaundice Meter (JM-103) is intended for use in the electromagnetic environment specified below. The customer or user of the unit
should ensure that the unit is used in such an environment.
Immunity
Test
Conducted
RF—IEC
61000-4-6
Radiated
RF—IEC
61000-4-3
IEC
60601
Test
Level
3 Vrms
150
kHz to
80
MHz
3 V/m 3 V/m
80
MHz
to 2.5
GHz
Compli
ance
Level
Electromagnetic Environment—
Guidance
Recommended Separation Distance
3 Vrms Portable and mobile RF communica-
tion equipment should be used no
closer to any part of the Jaundice Meter
(JM-103), including cables, than the
recommended separation distance calculated from the equation applicable to
the frequency of the transmitter.
Recommended Separation Distance
d 1.2 P=
d 2.3 P=
80 MHz to 800 MHz
800 MHz to 2.5 GHz
where P is the maximum output power
rating of the transmitter in watts (W)
according to the transmitter manufacturer and d is the recommended separation distance in meters (m).
Field strengths from fixed RF transmitters, as determined by an electromag-
netic site survey
the compliance level in each frequency
b
range.
a
, should be less than
Interference may occur in the vicinity
of equipment marked with the following symbol:
Page 2 - 14 Jaundice Meter (Model JM-103) User Manual (usr070)
Page 23
DRAFT 20 June 2005
Guidance and Manufacturer’s Declaration—Electromagnetic
Immunity
The Jaundice Meter (JM-103) is intended for use in the electromagnetic environment specified below. The customer or user of the unit
should ensure that the unit is used in such an environment.
IEC
Immunity
Test
60601
Test
Level
NOTE:
At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE:
These guidelines may not apply in all situations. Electromagnetic
propagation is affected by absorption and reflection from
structures, objects, and people.
a. Field strengths from fixed transmitters, such as base stations for radio,
cellular/cordless telephones, land-mobile radios, amateur radio, AM and FM
radio broadcast, and TV broadcast cannot be predicted theoretically with
accuracy. To assess the electromagnetic environment due to fixed-RF
transmitters, an electromagnetic site survey should be considered. If the
measured field strength in the location in which the unit is used exceeds the
applicable RF compliance level, observe the unit to verify normal operation.
If abnormal performance is observed, additional measures may be necessary,
such as reorienting or relocating the unit.
b. Over the frequency range 150 kHz to 80 MHz, field strengths should be < 3
V/m.
Compli
ance
Level
Electromagnetic Environment—
Guidance
Recommended Separation Distance
SPECIFICATIONS
Jaundice Meter (Model JM-103) User Manual (usr070) Page 2 - 15
Page 24

NOTES:
DRAFT 20 June 2005
Page 2 - 16 Jaundice Meter (Model JM-103) User Manual (usr070)
Page 25

DRAFT 18 May 2005
Section 3
Precautions and Safety Tips
Precautions
WARNING:
Do not use the instrument in areas where flammable or
combustible gases, such as anesthetic gases, are present. Doing
so could result in a fire. Personal injury or equipment damage
could occur.
WARNING:
If the instrument, the charger unit, or the AC adapter are
damaged, or if smoke or an odd smell occurs, do not use the
instrument, the charger unit, or the AC adapter. In such situations,
immediately turn off the instrument, unplug the AC adapter from
its power source, and contact the nearest authorized service
facility. Failure to do so could result in fire, personal injury, or
equipment damage.
WARNING:
Do not use the Jaundice Meter after initiation of phototherapy or
after an exchange transfusion as results may be inaccurate under
these conditions. Patient injury could occur.
SHOCK HAZARD:
Always plug the instrument into an AC outlet of the correctly rated
voltage and frequency. Failure to do so could result in fire,
personal injury, or equipment damage.
SHOCK HAZARD:
Do not disassemble or modify the instrument, the charger unit, or
the AC adapter. Fire, personal injury, or equipment damage could
occur.
CAUTION:
Do not place the instrument on an unstable or sloping surface.
The instrument or charger unit could drop or overturn. Equipment
damage could occur.
Jaundice Meter (Model JM-103) User Manual (usr070) Page 3 - 1
Page 26

DRAFT 18 May 2005
CAUTION:
Do not use the instrument in direct sunlight. Equipment damage
could occur.
CAUTION:
The Jaundice Meter is a precision instrument. When using it, do
not drop it, expose it to shocks or strong vibrations, or place
heavy objects on it. Equipment damage could occur.
CAUTION:
Do not allow blood or other liquids to come in contact with the
instrument. Should blood or other liquids come in contact with the
instrument, immediately clean the instrument (see “Cleaning” on
page 6-1). Failure to do so could result in equipment damage.
CAUTION:
The instrument has a built-in, non-user-replaceable battery. Do
not disassemble the instrument to replace the battery. To replace
the battery, contact your dealer or authorized service center.
Failure to do so could result in equipment damage.
Page 3 - 2 Jaundice Meter (Model JM-103) User Manual (usr070)
Page 27

DRAFT 18 May 2005
Electromagnetic Compatibility Precautions
General information on electromagnetic compatibility (EMC)
according to the international EMC standard IEC 60601-12: 2001
Pins of connectors identified with the ESD warning
symbol shall not be touched and not be connected
unless ESD precautionary procedures are used. Such
precautionary procedures may include antistatic
clothing and shoes, the touch of a ground stud before
and during connecting the pins or the use of electrically
isolating and antistatic gloves. All staff involved in the
above shall receive instruction in these procedures.
NOTE:
Portable and mobile RF communications equipment can affect medical
electrical equipment.
NOTE:
Medical electrical equipment needs special precautions regarding
electromagnetic compatibility (EMC) and needs to be installed and put
into service according to the EMC information provided in the technical
documentation available from Dräger Service upon request.
Jaundice Meter (Model JM-103) User Manual (usr070) Page 3 - 3
Page 28

DRAFT 18 May 2005
Safety Tips
WARNING:
This instrument emits intense light to take its measurements.
Take measurements only at the forehead or sternum (hospital
application). Doctors’ office use should be performed at the
sternum only. Do not press the measuring probe when it is
directed toward the infant’s or caregiver’s eyes. Damage to the
eyes could occur.
WARNING:
Before use, clean the measuring probe by wiping it with medicinal
alcohol. Failure to do so could result in the spread of infection or
infant injury.
WARNING:
The charger unit (JM-A30) and the AC adapter (JM-A31) are
solely designed for use with the Jaundice Meter (JM-103). Use
them only when charging the instrument. Using them to charge
other equipment could result in personal injury or equipment
damage.
WARNING:
Only facility-authorized personnel should troubleshoot the
Jaundice Meter. Troubleshooting by unauthorized personnel
could result in personal injury or equipment damage.
WARNING:
Follow the product manufacturer’s instructions. Failure to do so
could result in personal injury or equipment damage.
WARNING:
This product has been validated with the accessories and options
listed in this manual and found to comply with all relevant safety
and performance requirements applicable to the device. It is
therefore the responsibility of that person or organization who
makes an unauthorized modification, or incorporates an
unapproved attachment to the device, to ensure that the system
still complies with those requirements. [IHA036]
Page 3 - 4 Jaundice Meter (Model JM-103) User Manual (usr070)
Page 29

DRAFT 18 May 2005
SHOCK HAZARD:
Do not plug or unplug the AC power cord’s plug with wet hands.
Personal injury or equipment damage could occur.
SHOCK HAZARD:
Before cleaning, maintenance, or parts replacement, unplug the
charger unit from its power source. Failure to do so could result in
personal injury or equipment damage.
SHOCK HAZARD:
Do not expose the unit to excessive moisture that would allow for
liquid pooling. Personal injury or equipment damage could occur.
CAUTION:
Do not use harsh cleansers/detergents, such as scouring pads
and heavy duty grease removers, or solvents, such as toluene,
xylene, and acetone. Equipment damage could occur.
Warning and Caution Labels
Jaundice Meter (Model JM-103) User Manual (usr070) Page 3 - 5
Page 30

NOTES:
DRAFT 18 May 2005
Page 3 - 6 Jaundice Meter (Model JM-103) User Manual (usr070)
Page 31

DRAFT 20 June 2005
Section 4
Installation and Assembly
Installation
Before using the instrument, charge and inspect the instrument.
Charging the Battery
When using the instrument for the first time, ensure that it is fully
charged. To maintain a full charge at all times, place the instrument on
the charger unit when it is not being used for measurements. When the
battery power is low, the Battery display blinks.
If the Jaundice Meter is left uncharged for a long period of time, the
power of the battery diminishes; ensure that it is charged prior to use. To
charge the Jaundice Meter, perform the following:
WARNING:
The charger unit (Model JM-A30) and the AC adapter (Model JMA31) are solely designed for use with the Jaundice Meter (JM-
103). Use them only when charging the instrument. Using them
to charge other equipment could result in personal injury or
equipment damage.
1. Plug the AC adapter into the DC jack
of the charger unit. Use only the
charger unit and AC adapter supplied
with the Jaundice Meter.
NOTE:
The shape of the AC adapter varies
according to region.
NOTE:
120V model shown.
Jaundice Meter (Model JM-103) User Manual (usr070) Page 4 - 1
Page 32

DRAFT 20 June 2005
SHOCK HAZARD:
Do not plug or unplug the AC power cord’s plug with wet hands.
Personal injury or equipment damage could occur.
2. Plug the AC adapter’s plug into an AC outlet. Never do so with wet
hands.
CAUTION:
Unit must be placed in charger as shown, or damage to charging
contacts could occur.
3. Place the Jaundice Meter on the
charger unit so that its display faces
you. When the Jaundice Meter is set
on the charger unit properly, the
Charger lamp lights up.
NOTE:
With a fully charged battery, approximately
400 measurements can be taken.
4. Allow approximately 32 hours for
charging to complete.
CAUTION:
The instrument has a built-in, non-user-replaceable battery. Do
not disassemble the instrument to replace the battery. To replace
the battery, contact your dealer or authorized service center.
Failure to do so could result in equipment damage.
5. To replace the battery, contact your dealer or an authorized service
facility.
Page 4 - 2 Jaundice Meter (Model JM-103) User Manual (usr070)
Page 33

DRAFT 20 June 2005
Selecting the Unit of Measurement
1. Hold down the Reset button, and turn
on the Power switch.Do not release the
Reset button.
2. While continuing to press the Reset
button, allow approximately 15
seconds for the unit of measurement to
switch from mg/dL to µmol/L, or vice
versa.
3. Visually ensure that the unit’s display
has changed.
4. Release the Reset button when unit
displays an appropriate unit of
measurement. The Ready lamp lights
up, indicating that the instrument is
ready to take a measurement.
5. To change the unit of measurement
once more, turn off the Power switch,
and repeat step 1.
Jaundice Meter (Model JM-103) User Manual (usr070) Page 4 - 3
Page 34

DRAFT 20 June 2005
Operational Checkout of the Jaundice Meter
Do not touch the checker’s surface in the charging base with your
fingers. If the checker gets dirty, wipe it with a soft cloth dampened with
alcohol, and then wipe it with a dry cloth. Although the JM-103 device
can be damamged as stated on pages 3-1 and 3-4, we recommend the
unit always be verified to be operating properly by following the steps
as stated in sections 4 and 5. Always remove the unit from service if
there are concerns regarding the unit’s performance, and immediately
contact your Dräger Medical representative.
For quality control purposes, periodically compare the JM-103 to serum
bilirubin results. This checks that the instrument maintains consistent
performance over time and that the operators are using the instrument
properly.
Using the checker supplied with the charger unit, check the instrument
to verify that the meter light output is within range for both long and
short wavelengths of light. The labeling on the inside cover of the
checker will state the acceptance ranges for both long and short
wavelengths. The procedure to verify is as follows:
1. Hold the Reset button down, and set
the Power switch to the On position.
NOTE:
If the Reset button is held down for longer
than 15 seconds, the unit of measurement
switches.
2. After CHE appears on the display
window, immediately release the
Reset button. If the Reset button is
held down for longer than 15 seconds,
switch the unit of measurement back to
its previous setting (see “Selecting the
Unit of Measurement” on page 4-3).
3. Visually confirm that CHE appears in
the display and that the Ready lamp
illuminates.
Page 4 - 4 Jaundice Meter (Model JM-103) User Manual (usr070)
Page 35

DRAFT 20 June 2005
4. Open the cover of the checker. Use
only the checker supplied with the
Jaundice Meter (JM-103).
5. Place the measuring probe
perpendicular to the checker, and push
down gently until a click sounds.
6. If the measuring probe contacts the
checker at an angle, place it
perpendicular, and take the
measurement again.
NOTE:
The display interchanges between the Lvalue, the measured value of the longoptical path, and the S-value, the measured
value of the short-optical path. When the Lvalue is displayed, “•” appears in the upper
left-hand corner of the display.
7. Confirm the measured value. If both
the L-value and the S-value are within
± 1.0 of the reference values indicated
on the checker cover, the unit is
acceptable for use.
8. If the measured value exceeds ± 1.0 of
the reference value, perform the
following:
Jaundice Meter (Model JM-103) User Manual (usr070) Page 4 - 5
Page 36

DRAFT 20 June 2005
a. Clean both the checker and the measuring probe.
b. Place the measuring probe perpendicular to the checker, and
push down gently until a click sounds.
c. If the measured value still exceeds ± 1.0 of the reference value,
contact the nearest authorized service facility, and take the unit
out of service.
9. Close the cover of the checker.
10. Set the Power switch to the Off position.
Page 4 - 6 Jaundice Meter (Model JM-103) User Manual (usr070)
Page 37

DRAFT 20 June 2005
Section 5
Instructions for Use
Instructions for Use
Taking Measurements
1. Remove the Jaundice Meter from the
charger unit.
NOTE:
Check the light output of the device at least
once each shift (refer to “Operational
Checkout of the Jaundice Meter” on page
4-4). Proper light output is one factor that
affects meter performance. Light output must
be within the range shown on the inside cover of the checker to obtain
reliable measurements.
CAUTION:
Unit must be placed in charger as shown, or damage to charging
contacts could occur.
WARNING:
Before use, clean the measuring probe
by wiping it with medicinal alcohol.
Failure to do so could result in the
spread of infection or infant injury.
2. Using medicinal alcohol, clean the
measuring probe.
Jaundice Meter (Model JM-103) User Manual (usr070) Page 5 - 1
Page 38

DRAFT 20 June 2005
3. Set the Power switch to the On
position. The measured value for a
single measurement, n-1, appears on the
display.
NOTE:
The JM-103 is a screening device for
Bilirubin in the tissues and should never
be used as a stand-alone measurement.
Always contact the physician whenever
the JM-103 measurements present a concern. The physician will
make the determination regarding the laboratory blood serum
TSB, as well as other clinical considerations, as these are
appropriate measurement for Bilirubin concentration as stated in
the indications for use.
4. Visually check that the Ready lamp
illuminates.
5. If the battery indicator blinks, charge the
battery (see “Charging the Battery” on
page 4-1).
WARNING:
This instrument emits intense light to take its measurements.
Take measurements only at the forehead or sternum (hospital
application). Doctors’ office use should be performed at the
sternum only. Do not press the measuring probe when it is
directed toward the infant’s or caregiver’s eyes. Damage to the
eyes could occur.
6. Perform the following:
a. Place the measuring probe
vertically against the infant’s
forehead (at hospital sites only) or
sternum (at hospital sites or
physicians’ offices). Avoid any
bruises or discolored areas of the
skin.
Page 5 - 2 Jaundice Meter (Model JM-103) User Manual (usr070)
Page 39

DRAFT 20 June 2005
NOTE:
Only use the sternum location for JM-103
measurements when the device is used in a
doctor’s office.
b. Push the measuring probe gently
until a click sounds. The
instrument’s xenon lamp flashes
momentarily, and the measured
value appears on the display.
c. If the measured value is outside the
measurement range of 20 mg/dL or
340 µmol/L, the display shows “---”
and user should contact the
physician.
NOTE:
If the instrument is not operated for more than 60 seconds, the backlight
on the display goes out.
7. To take another measurement, press
the Reset button, and continue from step 4.
8. To stop measuring, perform the following:
a. Set the Power switch to the Off position.
b. Using medicinal alcohol, clean the measuring probe.
c. Place the Jaundice Meter on the charger unit. When the
Jaundice Meter is not in use, keep it on the charger unit.
Jaundice Meter (Model JM-103) User Manual (usr070) Page 5 - 3
Page 40

DRAFT 20 June 2005
NOTE:
If the caregiver has any concerns regarding the values being presented
by the JM 103 for Bilirubin concentration, the physician should be
notified immediately for a determination as to whether supportive
laboratory blood serum analysis is required or infant retest is
appropriate.
Page 5 - 4 Jaundice Meter (Model JM-103) User Manual (usr070)
Page 41

DRAFT 20 June 2005
Setting the Number of Average Measurements
1. Set the Power switch to On or press
the Reset button to prepare the
instrument for measurement.
• n-1, n-2, and so on (up to n-5)
will appear.
2. Press the Reset button for 5 s. The number of average
measurements will switch as follows:
3. Release the Reset button when the required number of average
measurements is displayed.
•If n-2 through n-5 is selected,
AV G appears in the upper left
corner of the display.
• The selected number of average
measurements will be recorded.
Jaundice Meter (Model JM-103) User Manual (usr070) Page 5 - 5
Page 42

DRAFT 20 June 2005
Taking Average Measurements
1. Set the number of average measurements needed.
2. Ensure that the Ready lamp is
illuminated once n-5 appears.
NOTE:
n-5 is used in the steps below as an
example. The number of measurements
you require and set is the number that
should appear in the display.
NOTE:
Each measurement must be taken
individually by the user. The first measurement is complete when the
measuring probe is pressed against the patient and the unit clicks. The
probe must then be lifted from the patient and reapplied for the total
number of measurements selected (in this example 5 measurements).
WARNING:
This instrument emits intense light to take its measurements.
Take measurements only at the forehead or sternum (hospital
application). Doctors’ office use should be performed at the
sternum only. Do not press the measuring probe when it is
directed toward the infant’s or caregiver’s eyes. Damage to the
eyes could occur.
NOTE:
It is recommended that all of the measurements taken for averaging be
taken from the same measuring point, forehead (at hospital sites only) or
sternum (at hospital sites or physicians’ offices).
3. Place the measuring probe vertically
on the measuring point, and then apply
gentle pressure until the probe clicks.
• The measurement will be taken,
and the number of remaining
measurements will be displayed.
Page 5 - 6 Jaundice Meter (Model JM-103) User Manual (usr070)
Page 43

DRAFT 20 June 2005
4. While ensuring that the Ready lamp is illuminated, repeat the
measuring until the number of remaining measurements is 0.
• When the remaining number of
measurements is completed, the
average of the measured values
appears in the display.
• If the instrument is left without
the preset number of
measurements taken, the setting
will be canceled without the
measurement value being
displayed. To set the average
measurements again, press the Reset button and reset the
number of average measurements needed.
• To change the number of average measurements, refer to
“Setting the Number of Average Measurements” on page 5-5.
Jaundice Meter (Model JM-103) User Manual (usr070) Page 5 - 7
Page 44

NOTES:
DRAFT 20 June 2005
Page 5 - 8 Jaundice Meter (Model JM-103) User Manual (usr070)
Page 45

DRAFT 18 May 2005
Section 6
Cleaning, Maintenance,
Replacement Parts, and
Storage and Handling
Cleaning
WARNING:
Follow the product manufacturer’s instructions. Failure to do so
could result in personal injury or equipment damage.
SHOCK HAZARD:
Before cleaning, maintenance, or parts replacement, unplug the
charger unit from its power source. Failure to do so could result in
personal injury or equipment damage.
SHOCK HAZARD:
Do not expose the unit to excessive moisture that would allow for
liquid pooling. Personal injury or equipment damage could occur.
CAUTION:
Do not use harsh cleansers/detergents, such as scouring pads
and heavy duty grease removers, or solvents, such as toluene,
xylene, and acetone. Equipment damage could occur.
If there is no visible soilage with possible body fluids, we recommend
that you clean the unit with alcohol or a medical instrument detergent
and warm wet cloth or gauze sponge. If disinfection is desired, you may
use a disinfectant as explained in “Disinfecting” on page 6-2. Do not
submerge unit in water or hold under running water to rinse.
Steam Cleaning
Do not use any steam cleaning device on the unit. Do not autoclave the
unit. Excessive moisture can damage mechanisms or electronics in this
unit.
Cleaning Difficult to Access Areas
Do not attempt to disassemble the reader or base for cleaning. Wipe
exterior surfaces only. To remove difficult spots or stains, we
Jaundice Meter (Model JM-103) User Manual (usr070) Page 6 - 1
Page 46

DRAFT 18 May 2005
recommend that you use a soft bristle brush and alcohol or a medical
instrument detergent. To loosen heavy, dried-on soil, you may first need
to saturate the spot with a damp gauze sponge or cloth. Disinfecting is
preferable in cases of contamination (visual).
Disinfecting
When there is visible soilage and between patients, we recommend that
you disinfect the unit with a tuberculocidal disinfectant, such as
Kleenaseptic®-b. For customers in the US, the disinfectant should be
registered with the Environmental Protection Agency.
Dilute the disinfectant according to the manufacturer’s instructions.
Maintenance
There are no user serviceable parts and no maintenance or calibration
required. Service is only required if the unit ceases to function as
intended or fails the checker reading (see “Operational Checkout of the
Jaundice Meter” on page 4-4).
NOTE:
For disposal of consummable materials,see “Disposal” on page 6-3.
Replacement Parts
CAUTION:
The instrument has a built-in, non-user-replaceable battery. Do
not disassemble the instrument to replace the battery. To replace
the battery, contact your dealer or authorized service center.
Failure to do so could result in equipment damage.
There are no user-replaceable parts. To replace the battery, contact your
dealer or an authorized service facility.
Page 6 - 2 Jaundice Meter (Model JM-103) User Manual (usr070)
Page 47

DRAFT 18 May 2005
Storage and Handling
When storing the instrument, pay attention to the following conditions:
• Store the instrument at a temperature range of -10°C (14°F) to 50°C
(122°F), and at a non-condensing relative humidity range of 30% to
95%.
• Keep the instrument dry.
• Do not store the instrument in locations that may have an adverse
effect on its performance, such as:
– Direct sunlight—do not store near windows.
– Extreme dust—do not store in closets or bins where dust or lint
can gather.
– Air having salinity or sulphur content.
– Strong magnetic fields—do not store near MRI or other
imaging equipment, and do not store near operating rooms.
• Do not subject the instrument to severe vibration or impact.
• Do not store the instrument in locations where chemicals are stored
or where solvent gases may be emitted.
• To ensure no problems will exist the next time the main body and
charger are used, thoroughly clean the main body and charger with
alcohol, and always store them together.
Disposal
This device is subject to EU Directive 2002/96/EC (WEEE). It is not
registered for use in private households, and may not be disposed of at
municipal collection points for waste electrical and electronic
equipment.
Dräger Medical has authorized a firm to dispose of this device in the
proper manner. For more detailed information, please contact your local
Dräger Medical organization. (Alternatively: further information can be
obtained from our national Dräger Medical organization.)
Jaundice Meter (Model JM-103) User Manual (usr070) Page 6 - 3
Page 48

NOTES:
DRAFT 18 May 2005
Page 6 - 4 Jaundice Meter (Model JM-103) User Manual (usr070)
Page 49

DRAFT 18 May 2005
Section 7
Troubleshooting
Service Calls
When you call technical support about your unit, be prepared to give the
serial number from the product identification label. When you give the
serial number, the technical support representative can identify your unit
and give you the information you need more quickly.
Error Messages
For warnings that may appear on the display window, refer to the table
below.
Error Messages
War ning Cause So lutio n
Er1 The measured value is
abnormal. In the case of an
averaging measurement, the
measurement fluctuation is
excessively large.
Er2
through
Er6
A measurement error may
have occurred during an averaging measurement, or the
hardware is not functioning
properly.
Place the measuring probe
perpendicular to the infant’s
forehead (at hospital sites
only) or sternum (at hospital
sites or physicians’ offices),
and take the measurement
again. If Er1 still appears,
contact the nearest authorized
service facility.
Set the Power switch to the
Off position, and then return
it to the On position. If the
warning still appears, contact
the nearest authorized service
facility.
Jaundice Meter (Model JM-103) User Manual (usr070) Page 7 - 1
Page 50

DRAFT 18 May 2005
Troubleshooting
WARNING:
Only facility-authorized personnel should troubleshoot the
Jaundice Meter. Troubleshooting by unauthorized personnel
could result in personal injury or equipment damage.
If an abnormality occurs with the Jaundice Meter, perform the
following:
1. Refer to the table below, and take the necessary action given.
2. If the abnormality still appears, set the Power switch to the Off
position, and then return it to the On position.
3. If the abnormality still continues, contact the nearest authorized
service facility, and take the unit out of service.
Troubleshooting
Symptom Possible Cause Action
The display is blank
when the Power switch
is in the On position.
The display suddenly
goes blank during a
measurement.
The Charger lamp
does not illuminate
when the Jaundice
Meter is placed on the
charger unit.
It is impossible to take
measurements.
The batteries are
exhausted.
The batteries are
exhausted.
The charger unit and
the AC adapter are not
plugged into an AC
outlet correctly.
The Jaundice Meter is
not placed in the
charger unit correctly.
The Ready lamp is not
illuminated.
The batteries are
exhausted.
Charge the battery (see
“Charging the Battery”
on page 4-1).
Charge the battery (see
“Charging the Battery”
on page 4-1).
Plug the charger unit
and the AC adapter into
an appropriate power
source correctly.
Place the Jaundice
Meter perpendicular to
the measuring point in
the charger unit.
Before taking a
measurement, ensure
that the Ready lamp is
illuminated.
Charge the battery (see
“Charging the Battery”
on page 4-1).
Page 7 - 2 Jaundice Meter (Model JM-103) User Manual (usr070)
Page 51

DRAFT 20 June 2005
Appendix A
Clinical Performance Summary
Introduction
The Jaundice Meter (JM-103) has been the subject of clinical studies in
Japan and the United States. The following is a summary review of two
clinical studies in the US and a later study in the doctor’s office setting.
Because this device performs measurement through the use of light it is
non-invasive and painless for the infant. The objective of the clinical
studies was to confirm that the device measurement, displayed in units
of estimated bilirubin concentration, does correlate with the serum
bilirubin concentration sufficiently well to warrant its use as a screening
tool.
The data in the following sections is provided to demonstrate results of
the JM-103 clinical studies, in comparison to total serum bilirubin. The
JM-103 reports values over a range of 0-20 mg/dL estimated bilirubin.
Appendix A pages A2 - A18 illustrate results of hospital site studies.
Appendix B pages B3 - B8 illustrate results of physicians’ office site
studies.
The following is a summary of the study protocols:
Study Design
Selection Criteria
The patient selection criteria used for the studies included infants less
than 30 days old and weighing greater than 1000 grams. Although the
selection criteria was established to be “less then 30 days of age,” the
infants in the hospital studies would be expected to be primarily NICU
and newborn infants unless their medical condition required a longer
duration of care. The test was performed on infants who were
determined by their physician to require a serum bilirubin test. A
tabulation of weight distribution is provided in graph 22 Infant Weight
Distribution. Error plots by weight are provided in graphs 23 Clinical
Study Site A Birthweight Error Plot, Forehead and 24 Clinical Study
Site A Birthweight Error Plot, Sternum.
Jaundice Meter (JM-103) User Manual (usr070) Page A - 1
Page 52

DRAFT 20 June 2005
Demographics of Patient Population
All patients meeting the above criteria were included in the study. There
was significant effort to ensure that there was sufficient representation
of all skin pigmentation to verify that the JM 103 could be used across
all populations with consistent results. The demographics of the patient
population included Caucasian, African-American, and other.
Sample Size
The total number of infants in the sample populations are shown on the
graphs of the trials presented on pages A-3 through A-15. The hospital
trials studies 613 patients. The data for the doctors’ office study is in
Appendix B and encompassed 201 infants.
Measurement Selection
One study followed a protocol where three measurements were taken,
each measurement was recorded and then the three measurements were
averaged. The second study took one measurement only. Estimated
bilirubin measurements taken during the studies ranged from 1.1 to 20.8
mg/dL. In the doctor’s office setting no measurement averaging was
performed.
Body Sites Tested
In the hospital setting the measurements were taken on the forehead and
sternum each time the measurements were taken for a given patient. In
the doctor’s office setting all measurements were taken at the sternum.
Number of Hospital Sites
There were two hospital sites that participated in the hospital study.
Hospital study site A with 513 patients studied. Clinical study site B
with 100 patients studied.
Performance Data
The data in the following sections, as well as Appendix B, is provided to
demonstrate the performance of the JM 103. This series of graphs show
the correlation of the estimated bilirubin concentration taken noninvasively with the JM 103 to the actual serum bilirubin concentration
measured from a blood sample taken from the patient (TSB) as
explained in Sections 4 and 5. The device operates over a range of 0.0 –
Page A - 2 Jaundice Meter (JM-103) User Manual (usr070)
Page 53

DRAFT 20 June 2005
20.0 mg/dL (0 – 340 µmol/L). The data include graphs in the form of xy plots where x is the total serum bilirubin concentration measured and
y is the JM 103 estimated bilirubin measurement. Refer to the graphics
results provided in this appendix for the results of studies at hospital
sites. To address device performance in doctors’ office setting, a study
of 201 infants was performed. Results showed similar performance at
doctors’ offices as shown in Appendix B. The data for the doctor’s
office setting contained no forehead measurements as all measurements
were taken at the sternum. This data correlated well with prior sternum
data (refer to page A-12). The serum bilirubin measurements were taken
using direct spectrophotometry in Clinical Study A and specifically with
the Beakman-Colter Synchron LX-20by in Hospital Study B. Both
systems were used int he doctors’ office study as well.
Graph 1 – Hospital Study Site A All Patients, Forehead
Jaundice Meter (JM-103) User Manual (usr070) Page A - 3
Page 54

DRAFT 20 June 2005
Graph 2 – ospital Study Site A All Patients, Sternum
Graph 3 – Hospital Study Site A African-American Patients, Forehead
Page A - 4 Jaundice Meter (JM-103) User Manual (usr070)
Page 55

DRAFT 20 June 2005
Graph 4 – Hospital Study Site A African-American Patients, Sternum
Graph 5 – Hospital Study Site A Caucasian Patients, Forehead
Jaundice Meter (JM-103) User Manual (usr070) Page A - 5
Page 56

DRAFT 20 June 2005
Graph 6 – Hospital Study Site A Causasian Patients, Sternum
Graph 7 – Hospital Study Site A Other Patients, Forehead
Page A - 6 Jaundice Meter (JM-103) User Manual (usr070)
Page 57

DRAFT 20 June 2005
Graph 8 – Hospital Study Site A Other Patients, Sternum
Graph 9 – Hospital Study Site B All Patients, Forehead
Jaundice Meter (JM-103) User Manual (usr070) Page A - 7
Page 58

DRAFT 20 June 2005
Graph 10 – Hospital Study Site B All Patients, Sternum
Graph 11– Hospital Study Site B African-American Patients, Forehead
Page A - 8 Jaundice Meter (JM-103) User Manual (usr070)
Page 59

DRAFT 20 June 2005
Graph 12 – Hospital Study Site B African-American Patients, Sternum
Graph 13 – Hospital Study Site B Caucasian Patients, Forehead
Jaundice Meter (JM-103) User Manual (usr070) Page A - 9
Page 60

DRAFT 20 June 2005
Graph 14 – Hospital Study Site B Causasian Patients, Sternum
Graph 15 – Hospital Study Site B Other Patients, Forehead
Page A - 10 Jaundice Meter (JM-103) User Manual (usr070)
Page 61

DRAFT 20 June 2005
Graph 16 – Hospital Study Site B Other Patients, Sternum
Jaundice Meter (JM-103) User Manual (usr070) Page A - 11
Page 62

DRAFT 20 June 2005
Table 1: Summary Table of Slope, Intercept, Standard
Deviation, and Correlation Coefficients for Each Graph
Study Site / Patient
Population
SITE A Forehead All
(n=513)
SITE A Sternum All
(n=513)
SITE A Forehead African American (n=65)
SITE A Sternum African
American (n=65)
SITE A Forehead Caucasian (n=399)
SITE A Sternum Caucasian (n=399)
SITE A Forehead Other
(n=49)
SITE A Sternum Other
(n=49)
SITE B Forehead All
(n=100)
SITE B Sternum All
(n=100)
SITE B Forehead African American (n=48)
SITE B Sternum African American (n=48)
SITE B Forehead Caucasian (n=35)
SITE B Sternum Caucasian (n=35)
SITE B Forehead Other
(17)
SITE B Sternum Other
(17)
Correlation
Slope Intercept
1.05 -0.35 0.914 2.19
1.07 -0.74 0.946 1.02
1.15 -0.5 0.908 1.59
1.11 -0.4 0.908 1.55
1.01 -0.1 0.916 1.2
1.04 -0.6 0.956 0.88
1.06 -0.5 0.941 1.04
1.10 -1.0 0.977 0.65
1.07 -0.00 0.84 2.14
1.16 -0.43 0.89 1.85
1.40 +0.46 0.84 2.27
1.21 -0.17 0.89 1.9
1.10 -1.04 0.87 1.72
1.22 -1.69 0.88 1.81
1.03 -0.56 0.94 1.49
1.03 0.65 0.97 0.94
Coefficient
(r)
Standard
Deviation
(RMSE)
Page A - 12 Jaundice Meter (JM-103) User Manual (usr070)
Page 63

DRAFT 20 June 2005
Graph 17 – Hospital Study Site A Error Plot, Forehead
Graph 18 – Hospital Study Site A Error Plot, Sternum
Jaundice Meter (JM-103) User Manual (usr070) Page A - 13
Page 64

DRAFT 20 June 2005
Graph 19 – Hospital Study Site B Error Plot, Forehead
Graph 20 – Hospital Study Site B Error Plot, Sternum
Page A - 14 Jaundice Meter (JM-103) User Manual (usr070)
Page 65

DRAFT 20 June 2005
Graph 21 – Infant Weight Distribution
Infant Weight
(grams)
Hospital Study A Hospital Study B
Up to 999 6 2
1000 - 1499 15 0
1500 - 1999 23 13
2000 - 2499 37 23
2500 - 2999 68 19
3000 - and up 337 43
Unknown 27 0
Total 513 100
Graph 22 – Hospital Study Site A Birthweight Error Plot, Forehead
Jaundice Meter (JM-103) User Manual (usr070) Page A - 15
Page 66

DRAFT 20 June 2005
Graph 23 – Hospital Study Site A Birthweight Error Plot, Sternum
Reproducibility
Reproducibility of the light output of the device was tested daily using
the checker. The checker determines the intensity of the light output of
the device. The accuracy of the device is determined by how well the
detectors in the unit measure the returning light. The results of the
device testing using the checker show that the device produced output
within the required range over the course of both hospital studies.
Reproducibility testing in the patient population can be derived from the
data taken in Hospital study site A. The reproducibility data is based on
467 babies. Three independent measurements were taken at each site,
forehead and sternum. Each measurement was recorded, the mean and
standard deviation computed and recorded. The average standard
deviation for forehead and sternum measurements was 0.3 for both.
Page A - 16 Jaundice Meter (JM-103) User Manual (usr070)
Page 67

DRAFT 20 June 2005
Table 2: Reproducibility Data
Mean
Estimated
Bilirubin
Measure
ment
Body Site
Deviation
Range
Deviation
Mean
Deviation
Median
Range
(mg/dL)
Sternum 0.0 - 2.2 0.3 0.3 0.8 - 18.5
Forehead 0.0 - 2.6 0.3 0.2 0.1 - 19.5
Conclusion
What the data presented shows is that the JM 103 estimated bilirubin
concentration measurement correlates to the serum bilirubin
measurements. This data supports the use of this non-invasive device
along with other clinical indicators as an aid in the management of
jaundice in the neonatal patient population.
Jaundice Meter (JM-103) User Manual (usr070) Page A - 17
Page 68

NOTES:
DRAFT 20 June 2005
Page A - 18 Jaundice Meter (JM-103) User Manual (usr070)
Page 69

DRAFT 20 June 2005
Appendix B
Doctors’ Office Data
Study Design
Studies were performed at 2 doctors’ office sites comparing JM-103
Total Calculated Bilirubin (TcB) versus laboratory measured total serum
bilirubin (TSB).
Selection Criteria
The ages of the infants in the study ranged from approximately 24 hours
to 7-10 days, with a mean of 3 days (at site 1) and 5 days (at site 2). The
test was performed on infants who were determined by their physician
to require a serum bilirubin test.
Demographics of Patient Population
All patients meeting the above criteria were included in the study. The
majority of babies were Caucasian or no skin tone noted (n=167) with a
small number of reported ethnicity defined infants (n=34). The
demographics of the patient population included Caucasian, AfricanAmerican, and other.
Jaundice Meter (JM-103) User Manual (usr070) Page B - 1
Page 70

DRAFT 20 June 2005
The doctor's office study was composed of the following ethnic groups:
TcB Range Qty Age
Caucasian 2.3-19.5 167
African
American
Mid-Eastern 8.8 - 16.0 5
Indian 6.6- 17 5
Hispanic 7.6 1
Asian 4.8-12.6 10
NOTE:
Doctor’s Office #1: 83.5% Caucasian and 16.5% darker skin tone
Doctor’s Office #2: 75% Caucasian and 25% darker skin tone
Exclusion Criteria
Infants requiring Exchange Transfusion or Phototherapy Initiated were
not allowed in the study.
Sample Size
The doctor’s office use trial studied 201 patients.
4.9-17.3 13
97
≤72 hrs & 70≥72hrs
6
≤72 hrs & 7≥72hrs
4
≤72 hrs & 1≥72hrs
4
≤72 hrs & 1≥72hrs
3
≤72 hrs & 7≥72hrs
Measurement Selection
Estimated bilirubin measurements taken during the studies ranged from
2.3 to 19.5 mg/dL. No "Averaging of Readings" was used with the JM-
103.
Body Sites Tested
All infants were measured at the sternum location ONLY .
Number of Doctors’ Office Sites
For the doctor’s office study, two sites were chosen for application of
the device. Sample size was 201 patients.
Page B - 2 Jaundice Meter (JM-103) User Manual (usr070)
Page 71

DRAFT 20 June 2005
Performance Data
The data in the following sections is provided to demonstrate results of
the JM-103 clinical studies, in comparison to total serum bilirubin. The
JM-103 reports values over a range of 0-20 mg/dL estimated bilirubin.
Although the Doctor's Office Study showed correlation of JM-103 to the
TSB laboratory values, the relationship (bias) of the JM-103 to the TSB
values was somewhat different than previously reported for hospital
sites (as previously shown in Appendix A). This may be related to the
ages of the infants in the doctor's office study and the difference in
individual development. Therefore, please be aware that results at
Doctors offices may differ from results at hospitals and may have more
variability.
The reported data shows that 27% of the JM-103 measurements were
higher than the Laboratory TSB and 71% were lower (2% were matched
sets). Of the readings falling below the TSB value, 87% were within 3
Mg/dL of the TSB value. Those readings that reported above the TSB
value 94% were within 3 Mg/dL of the reported TSB value. The largest
error was a single 5.2 Mg/dL reading. The serum bilirubin
measurements were taken using direct spectrophotometry and the
Beakman-Colter Synchron LX-20by.
See Regression Analysis and infant age graphs below for data at the
individual sites. A regression analysis is also provided on the total of
201 infants.
Jaundice Meter (JM-103) User Manual (usr070) Page B - 3
Page 72

DRAFT 20 June 2005
Graph 1 – Infant Age, Doctors’ Office #1
Graph 2 – Infant Age, Doctors’ Office #2
Page B - 4 Jaundice Meter (JM-103) User Manual (usr070)
Page 73

DRAFT 20 June 2005
Graph 3 – Regression Analysis, Doctors’ Office #1
JM-103 Regression Analysis
30
25
20
15
10
5
JM-103 Reading TcB Mg/dl
0
0 5 10 15 20 25 30
Doctor's office No.1
-5
Serum Bilirubin TSB (mg/dl)
Regression Line Upper Limit Low er Limit Data Points
Regression Analysis Statistics Sample Size (n) = 133.
Y = -0.636 + 0.977 X
Root Mean Square Error (MSE) = 1.572
R-Square = 0.804
95% Confidence Interval (CI) for the Intercept = (-1.526, 0.254)
95% CI for the Slope = (0.894, 1.061)
Jaundice Meter (JM-103) User Manual (usr070) Page B - 5
Page 74

DRAFT 20 June 2005
Graph 4 – Regression Analysis, Doctors’ Office #2
JM-103 Regression Analysis
25
20
15
10
5
JM-103 Reading TcB Mg/dl
0
0 5 10 15 20 25 30
-5
Doctor's Office No.2
Serum Bilirubin TSB (mg/dl)
Regression Line Upper Limit Low er Limit Data Points
Regression Analysis Statistics Sample Size = 68
Y = 0.646 +0.859 X
Root MSE = 1.475
R-Square = 0.769
95% CI for the Intercept = (-0.846, 2.138)
95% CI for the Slope = (0.743, 0.974)
Page B - 6 Jaundice Meter (JM-103) User Manual (usr070)
Page 75

DRAFT 20 June 2005
Graph 5 – Regression Analysis, Doctors’ Office Setting, 2 Locations
JM-103 Regression Analysis
30
25
20
15
10
5
JM-103 Measurement Mg/dl
0
0 5 10 15 20 25 30
Doctor's office setting - 2 locations
-5
Serum Bilirubin TSB (mg/dl)
Regression Line Upper Limit Low er Limit Data Points
Regression Analysis Statistics Sample Size = 201
Y = 0.233 +0.934 X
Root MSE = 1.54
R-Square = 0.899
95% CI for the Intercept = (-0.233, 0.37)
95% CI for the Slope = (0.934, 0.032)
Jaundice Meter (JM-103) User Manual (usr070) Page B - 7
Page 76

DRAFT 20 June 2005
Conclusion
What the data presented shows is that the JM 103 estimated bilirubin
concentration measurement correlates to the serum bilirubin
measurements. This data supports the use of this non-invasive device
along with other clinical indicators as a aid in the management of
jaundice in the neonatal patient population.
Page B - 8 Jaundice Meter (JM-103) User Manual (usr070)
Page 77

DRAFT 9 Nov 2004
Appendix C
Medical and Scientific References on
Transcutaneous Bilirubinometry (1979 - 1997)
1. Amato M, et al;
Transcutaneous bilirubin determination: correlation in white premature infants weighing less than 1500 gm.
Schweiz Med Wochenschr, 1985 Jul 9.
2. Amato M, et al;
Assessment of neonatal jaundice in low birth weight infants comparing transcutaneous, capillary and arterial bilirubin levels.
Eur J Pediatr, 1990 Nov.
3. Amato M
Transcutaneous, capillary, and arterial bilirubin levels.
J. Pediatr. 125(2), 332, 1994
4. Amit Y, et al;
Effect of skinfold thickness on transcutaneous bilirubin measurements.
Biol Neonate, 1993.
5. Arimichi J, et al;
Reliability and future application of a transcutaneous bilirubinometer.
Josanpu Zasshi, 1984 Apr.
6. Berget M, et al;
Transcutaneous bilirubinometry in newborn infants.
Tidsskr Nor Laegeforen, 1984 May 20
7. Bhat V, et al;
Correlation of transcutaneous bilirubinometry with serum bilirubin
in south Indian neonates.
Indian J Med Res, 1987 Jul.
8. Bhutta ZA, et al;
Transcutaneous bilirubinometry in Pakistani newborns: a preliminary report.
JPMA J Pak Med Assoc, 1991 Jul.
Jaundice Meter (JM-103) User Manual (usr070) Page C - 1
Page 78

DRAFT 9 Nov 2004
9. Boo Nem Yun, et al;
Transcutaneous bilirubinometry in Malay, Chinese and Indian term
neonates.
Med J Malaysia, 1984 Mar.
10. Bourchier D, et al;
Transcutaneous bilirubinometry: 22 months experience at Waikato
Women's Hospital.
N Z Med J, 1987 Sep 23.
11. Brown A; et al;
Transcutaneous bilirubinometry in infants: influence of race and
phototherapy.
Pediatr Res, 1981, 15:653
12. Brown LP, et al;
Transcutaneous bilirubinometer: intermeter reliability.
J Perinatol, 1990 Jun.
13. Brown LP, et al;
Transcutaneous bilirubinometer: an instrument for clinical
research.
Nurs Res, 1990 Jul-Aug.
14. Brown LP, et al;
Incidence and pattern of jaundice in healthy breast-fed infants during the first month of life.
Nurs Res, 1993 Mar-Apr.
15. Brucker MC, et al;
Neonatal jaundice in the home: assessment with a noninvasive
device.
J Obstet Gynecol Neonatal Nurs, 1987 Sep-Oct.
16. Cassady G
Transcutaneous monitoring in the newborn infant.
J Pediatr, 1983 Dec.
17. Chen ZL, et al;
Clinical use of transcutaneous bilirubinometry.
Chung Hua I Hsueh Tsa Chih, 1986 Jan.
18. Christo GG, et al;
Transcutaneous bilirubinometry in newborns.
Indian Pediatr, 1988 Nov.
Page C - 2 Jaundice Meter (JM-103) User Manual (usr070)
Page 79

DRAFT 9 Nov 2004
19. Corchia C, et al;
Comment on transcutaneous bilirubin device of Yamanouchi
[letter]
Pediatrics, 1981 Mar.
20. Dai J, et al;
Clinical impact of transcutaneous bilirubinometry as an adjunctive
screen for hyperbilirubinemia
Clin Biochem 29 (6): 581-586, Dec 1996
21. Dai J, et al;
Transcutaneous bilirubinometry: its role in the assessment of neonatal jaundice
Clin Biochem 30 (1): 1-9, Feb 1997
22. Derksen-Samsom JF, et al;
The reliability of transcutaneous bilirubin measurement: a clinical
study with statistical data and literature review.
Tijdschr Kindergeneeskd, 1984 Oct.
23. Dominguez Ortega F, et al;
Transcutaneous bilirubinometry: correlation of the reading site
obtained with spectrophotometry and diazoreaction technique.
An Esp Pediatr, 1993 Nov.
24. Douville P, et al;
Diagnostic value of sequential readings with the Minolta transcutaneous bilirubinometer in normal and low-birthweight infants
[letter]
Clin Chem, 1983 Apr, 29:4, 740-1.
25. Fabris C, et al;
Evaluation of transcutaneous bilirubinometry in newborn infants.
Minerva Pediatr, 1984 Jun 15.
26. Fisher B, et al;
The cephalocaudal progression of jaundice in newborns in relation
to the transfer of bilirubin from plasma to skin.
Early Hum Dev, 1990 Apr, 22:1, 23-8
27. Foged N, et al;
Transcutaneous bilirubinometry. A non-invasive method of measuring physiological jaundice.
Ugeskr Laeger, 1983 Sep. 19.
Jaundice Meter (JM-103) User Manual (usr070) Page C - 3
Page 80

DRAFT 9 Nov 2004
28. Fok TF, et al;
Transcutaneous bilirubinometer: its use in Chinese term infants and
the effect of haematocrit and phototherapy on the TcB index.
Aust Paediatr J, 1986 May.
29. Galletto P, et al;
Supervision of neonatal jaundice by use of the transcutaneous
bilirubinometer.
Minerva Pediatr, 1983 Jan 31.
30. Goldman SL, et al;
Jaundice meter: evaluation of new guidelines.
J Pediatr, 1982 Aug.
31. Grande R, et al;
Physiological variations in the pigmentation of newborn infants.
Hum Biol, 1994 Jun.
32. Hanneman R; et al;
Determination of serum bilirubin by skin reflectance: effect of pigmentation
Pediatr Res 13 (12): 1326-1329 (Dec 1979)
33. Hanneman R; et al;
Evaluation of the Minolta bilirubin meter as a screening device in
white and black infants.
Pediatrics, 1982, 69, 107-109
34. Hegyi T, et al;
Transcutaneous bilirubinometry. I. Correlations in term infants.
J Pediatr, 1981 Mar.
35. Hegyi T, et al;
Transcutaneous bilirubinometry. The cephalocaudal progression of
dermal icterus.
Am J Dis Child, 1981 Jun.
36. Hegyi T
Transcutaneous bilirubinometry: a new light on an old subject.
Pediatrics, 1982 Jan.
37. Hegyi T, et al;
Transcutaneous bilirubinometry II. Dermal bilirubin kinetics during
phototherapy.
Pediatr Res, 1983 Nov.
Page C - 4 Jaundice Meter (JM-103) User Manual (usr070)
Page 81

DRAFT 9 Nov 2004
38. Hegyi T
Transcutaneous bilirubinometry in the newborn infant: state of the
art.
J Clin Monit, 1986 Jan.
39. Hegyi T, et al;
Transcutaneous bilirubinometry. III. Dermal bilirubin kinetics
under green and blue light phototherapy.
Am J Dis Child, 1986 Oct.
40. Heick C, et al;
Transcutaneous bilirubin determination in the newborn infant. Recommendations of the Swiss Neonatology Group.
Helv Paediatr Acta, 1982.
41. Hodr R, et al;
Transcutaneous measurement of the severity of icterus and bilirubinemia in normal neonates.
Cesk Pediatr, 1990 Nov.
42. Hung WT, et al;
Diagnosis of atretic prolonged obstructive jaundice; technetium
99m hepatolite excretion study.
J Pediatr Surg, 1990 Jul.
43. Karrar Z, et al;
Transcutaneous bilirubin measurements in Saudi infants: the use of
the jaundice meter to identify significant jaundice.
Ann Trop Paediatr, 1989 Mar.
44. Kenny M, et al;
Transcutaneous bilirubin monitoring of newborns.
Ann N Y Acad Sci, 1984.
45. Keshishjan E.S., et al;
Diagnostics and control of newborn hyperbilirubinemia with use
noninvasive transcutaneous photometric analyzer "Bilitest" of a
type AHP-02.
The methodical recommendations. Russia ministry of public health.
1992 Feb.
46. Keshishjan E.S., et al;
Transcutaneous bilirubinometry method at newborn jaundice diagnostics and control.
The Russian bulletin perinatology and pediatrics, V38, 5, 1993
Jaundice Meter (JM-103) User Manual (usr070) Page C - 5
Page 82

DRAFT 9 Nov 2004
47. Keshishjan E.S., et al;
Use of the method of transcutaneous bilirubinometry in hyperbilirubinemia in the newborn.
Clinical laboratory diagnostics. 5, 1994 Moscow, Medicine
48. Kivlahan C, et al;
The natural history of neonatal jaundice.
Pediatrics, 1984 Sep.
49. Knudsen A.
Prediction of the development of neonatal jaundice by increased
umbilical cord blood bilirubin.
Acta Pediatr Scand 1989;78:217-21.
50. Knudsen A, et al;
Skin colour and bilirubin.
Arch Dis Child 1989;64:605-9.
51. Knudsen A, et al;
Maternal bilirubin, cord bilirubin, and placenta function at delivery
and the development of jaundice in mature newborns.
Acta Obstet Gynecol Scand 1989:68:719-24.
52. Knudsen A.
The cephalocaudal progression of jaundice in newborns in relation
to the transfer of bilirubin from plasma to skin.
Early Human Development 1990;22:23-28.
53. Knudsen A.
Measurement of the yellow colour of the skin as a test of hyperbilirubinemia in mature newborns.
Acta Pediatr Scand 1990;79:1175-81.
54. Knudsen A.
Maternal smoking and the serum bilirubin concentration in the first
three days of life.
Eur J Obstet Gynecol Reprod Bid 1991; 40:123-2 7.
55. Knudsen A.
The influence of the reserve albumin concentration and pH on the
cephalocaudal progression of jaundice in newborns.
Early Human Development 1991;25:37-41.
56. Knudsen A.
The influence of the basal yellow colour of the skin at birth on later
jaundice meter readings in mature newborn infants.
Acta Pediatr Scand 1992;81:494-97.
Page C - 6 Jaundice Meter (JM-103) User Manual (usr070)
Page 83

57. Knudsen A.
Prediction of later hyperbilirubinemia by measurement of skin
colour at first postnatal day and cord bilirubin.
Dan Med Bull 1992;39:193-6.
58. Knudsen A
Predicting the need for phototherapy in healthy mature neonates
using transcutaneous bilirubinometry on the first postnatal day.
Biol Neonate, 1995.
59. Knudsen A, et al;
Transcutaneous evaluation of icterus in healthy mature newborn
infants. A substitution for blood tests.
Ugeskr Laeger, 1995 Mar 13.
60. Knudsen A, et al;
Transcutaneous bilirubinometry in neonatal intensive care units.
Arch Dis Child Fetal Neonatal Ed, 1996 Jul.
61. Kumar A
Micro-invasive management of neonatal bilirubinemia.
Indian Pediatr, 1992 Sep.
62. Kumar A, et al;
Transcutaneous bilirubinometry in the management of bilirubinemia in term neonates.
Indian J Med Res, 1994 May.
63. Laeeq A, et al;
Transcutaneous bilirubinometry: clinical application.
JPMA J Pak Med Assoc, 1993 Feb.
64. Lebrun F, et al;
Clinical evaluation of transcutaneous bilirubinometry.
Ann Pediatr (Paris), 1982 Sep.
65. Lin YJ, et al;
The clinical application of transcutaneous bilirubinometry in fullterm Chinese infants.
Acta Paediatr Sin, 1993 Mar-Apr.
66. Linder N, et al;
Noninvasive determination of neonatal hyperbilirubinemia: standardization for variation in skin color.
Am J Perinatol, 1994 May.
DRAFT 9 Nov 2004
Jaundice Meter (JM-103) User Manual (usr070) Page C - 7
Page 84

DRAFT 9 Nov 2004
67. Loong EP, et al;
Changes in neonatal transcutaneous bilirubinometer index following intravenous fluid and oxytocin infusion during labour.
Asia Oceania J Obstet Gynaecol, 1988 Dec.
68. Maisels MJ, et al;
Transcutaneous bilirubin measurements in full-term infants.
Pediatrics, 1982 Sep.
69. Maisels MJ, et al;
Transcutaneous bilirubin measurements: variation in meter
response.
Pediatrics, 1983 Mar.
70. Maisels MJ, et al;
Transcutaneous bilirubinometry decreases the need for serum
bilirubin measurements and saves money
Pediatrics 99 (4): 599-601, Apr 1997
71. Moscicka A, et al;
Usefulness of transcutaneous measurements of bilirubin in infants
with jaundice.
Ginekol Pol, 1994 Jun.
72. Myara A, et al;
Early changes in cutaneous bilirubin and serum bilirubin isomers
during intensive phototherapy of jaundiced neonates with blue and
green light
Biol Neonate 71 (2): 75-82, 1997
73. Onks D, et al;
Effect of melanin, oxyhemoglobin and bilirubin on transcutaneous
bilirubinometry.
Acta Paediatr, 1993 Jan.
74. Pallas Alonso C, et al;
Transcutaneous bilirubin measurement in neonates.
An Esp Pediatr, 1993 Jan.
75. Palmer DC, et al;
Transcutaneous bilirubinometry: use in Australia.
Aust Paediatr J, 1982 Dec.
76. Palmer DC; et al;
Jaundice: A 10 year review of 41,000 live born infants.
Australian Pediatrric Journal, 1983, 19(2),86-89
Page C - 8 Jaundice Meter (JM-103) User Manual (usr070)
Page 85

DRAFT 9 Nov 2004
77. Peng DJ, et al;
A study of the effect of pathologic factors on the TcBM readings.
Hua Hsi I Ko Ta Hsueh Hsueh Pao, 1989 Mar.
78. Romo-Pinales J, et al;
Preliminary study in Mexico on the use of the transcutaneous bilirubinometer.
Bol Med Hosp Infant Mex, 1984 Oct.
79. Ruchala PL, et al;
Validating assessment of neonatal jaundice with transcutaneous
bilirubin measurement.
Neonatal Netw, 1996 Jun.
80. Salmeron Escobar FJ
Differential diagnosis of jaundice.
Rev Esp Enferm Dig, 1991 Jan.
81. Schubiger G, et al;
Transcutaneous determination of bilirubin in the neonatal department: an analysis of its uses.
Helv Paediatr Acta, 1986 Aug.
82. Schumacher RE, et al;
Transcutaneous bilirubinometry: a comparison of old and new
methods.
Pediatrics, 1985 Jul.
83. Schumacher RE
Noninvasive measurements of bilirubin in the newborn.
Clin Perinatol, 1990 Jun.
84. Serrao PA, et al;
Significance of anti-A and anti-B isohemagglutinins in cord blood
of ABO incompatible newborn infants: correlation with hyperbilirubinemia
J Perinatol, 1989 Jun.
85. Sharma JN, et al;
Transcutaneous bilirubinometry in newborns.
Indian Pediatr, 1988 Aug.
86. Sheridan-Pereira M, et al;
Transcutaneous bilirubinometry: an evaluation.
Arch Dis Child, 1982 Sep.
Jaundice Meter (JM-103) User Manual (usr070) Page C - 9
Page 86

DRAFT 9 Nov 2004
87. Smith DW, et al;
Use of noninvasive tests to predict significant jaundice in full-term
infants: preliminary studies.
Pediatrics, 1985 Feb.
88. Strange M, et al;
Neonatal transcutaneous bilirubinometry.
Clin erinatol, 1985 Feb.
89. Suckling RJ, et al;
Transcutaneous bilirubinometry as a screening tool for neonatal
jaundice.
Scott Med J, 1995 Feb.
90. Taha SA, et al;
Transcutaneous bilirubin measurement in evaluating neonatal jaundice among Saudi newborns.
Ann Trop Paediatr, 1984 Dec.
91. Tan KL
Transcutaneous bilirubinometry in Chinese and Malay neonates.
Ann Acad Med Singapore, 1985 Oct.
92. Tan KL, et al;
Transcutaneous bilirubinometry in preterm very low birthweight
infants.
Acta Paediatr Scand, 1988 Nov.
93. Tan KL, et al;
Efficacy of phototherapy for hyperbilirubinaemia in infants with
the respiratory distress syndrome
J Paediatr Child Health 31 (2): 127-129, Apr 1995
94. Tan KL, et al;
Transcutaneous bilirubinometry in Chinese, Malay and Indian
infants
Acta Paediatr 85 (8): 986-990, Aug 1996
95. Tsai LT, et al;
Clinical evaluation of transcutaneous jaundice meter in full-term
newborns.
Acta Paediatr Sin, 1988 Nov-Dec.
96. Tudehope DI, et al;
Non-invasive method of measuring bilirubin levels in newborn
infants.
Med J Aust, 1982 Feb 20.
Page C - 10 Jaundice Meter (JM-103) User Manual (usr070)
Page 87

DRAFT 9 Nov 2004
97. Tudehope DI, et al;
Multiple site readings from a transcutaneous bilirubinometer.
Aust Paediatr J, 1982 Jun.
98. Uchida K, et al;
Fundamental studies on transcutaneous bilirubinometry in newborn
infants using an organ scanning spectrophotometer.
Nippon Sanka Fujinka Gakkai Zasshi, 1988 Feb.
99. Utchajkin V.F., et al;
Clinical importance of noninvasive bilirubinemia monitoring by
children viral hepatitis.
2-nd Symposiums "NoninvasiveMethods of Diagnostics". The thesis
of the reports. Moscow. 1995 Oct. 30 - Nov. 2.
100. Vaisman S, et al;
Clinical trial of a transcutaneous bilirubinometer.
Rev Chil Pediatr, 1983 Mar-Apr.
101. Veleminsky M
The significance of transcutaneous measurement of "bilirubin" in
neonates.
Cesk Pediatr, 1992 Nov.
102. Vocel J, et al;
Transcutaneous bilirubinometry using the Minolta/Air-Shields in
neonates.
Cesk Pediatr, 1985 Nov.
103. Vocel J, et al;
Transcutaneous bilirubinometry using the Minolta/Air-Shields
apparatus in neonates with a low birthweight.
Cesk Pediatr, 1987 Jan.
104. Vocel J, et al;
Transcutaneous bilirubinometry in neonates with hyperbilirubinemia treated with phototherapy.
Cesk Pediatr, 1988 Dec.
105. Weiss WJ
A completely implanted left ventricular assist device. Chronic in
vivo testing.
ASAIO J 39(3), M427-M432, 1993
Jaundice Meter (JM-103) User Manual (usr070) Page C - 11
Page 88

DRAFT 9 Nov 2004
106. Yamanouchi I, et al;
Transcutaneous bilirubinometry: preliminary studies of noninvasive transcutaneous bilirubin meter in the Okayama National Hospital.
Pediatrics, 1980 Feb.
107. Yamauchi Y, et al;
Transcutaneous bilirubinometry. Effect of irradiation on the skin
bilirubin index.
Biol Neonate, 1988.
108. Yamauchi Y, et al;
Transcutaneous bilirubinometry. Evaluation of accuracy and reliability in a large population.
Acta Paediatr Scand, 1988 Nov.
109. Yamauchi Y, et al;
Transcutaneous bilirubinometry: bilirubin kinetics of the skin and
serum during and after phototherapy.
Biol Neonate, 1989.
110. Yamauchi Y, et al;
Transcutaneous bilirubinometry: serum bilirubin measurement
using transcutaneous bilirubinometer (TcB). A preliminary study.
Biol Neonate, 1989.
111. Yamauchi Y, et al;
Transcutaneous bilirubinometry in normal Japanese infants.
Acta Paediatr Jpn, 1989 Feb.
112. Yamauchi Y, et al;
Transcutaneous bilirubinometry. Interinstrumental variability of
TcB instruments.
Acta Paediatr Scand, 1989 Nov.
113. Yamauchi Y, et al;
Clinical application of transcutaneous bilirubin measurement. Early
prediction of hyperbilirubinemia.
Acta Paediatr Scand, 1990 Apr.
114. Yamauchi Y, et al;
Transcutaneous bilirubinometry: effect of postnatal age.
Acta Paediatr Jpn, 1991 Oct.
115. Yamauchi Y, et al;
Transcutaneous bilirubinometry: variability of TcB measurements
on the forehead with crying.
Acta Paediatr Jpn, 1991 Oct.
Page C - 12 Jaundice Meter (JM-103) User Manual (usr070)
Page 89

DRAFT 9 Nov 2004
116. Yamauchi Y, et al;
Factors affecting transcutaneous bilirubin measurement: effect of
daylight.
Acta Paediatr Jpn, 1991 Oct.
117. Yip WC, et al;
Transcutaneous bilirubinometry [letter]
Acta Paediatr Scand, 1983 Mar.
Jaundice Meter (JM-103) User Manual (usr070) Page C - 13
Page 90

NOTES:
DRAFT 9 Nov 2004
Page C - 14 Jaundice Meter (JM-103) User Manual (usr070)
Page 91

PROPRIETARY AND CONFIDENTIAL DRAFT 5/14/04
Page 92

Manufactured by: Distributed by: EC Representative:
Konica Minolta Sensing,
Inc.
3-91, Daisennishimachi
Sakai-ku, Sakai, Osaka
590-8551 Japan
Part number Revisions Date
usr070 (MU01380) rg October 2006
Draeger Medical Systems, Inc.
3135 Quarry Road
Telford, PA 18969 USA
USA and Canada Cus-
tomer Support:
(800) 437-2437
http://www.draegermedical.com
Technical Support:
(800) 437-2437
Fax: (215) 721-5782
Konica Minolta Sensing
Europe B.V.
Edisonbaan 14-E
3439MN Nieuwegein,
the Netherlands
Tel: +31 30 248 1166
Fax: +31 30 248 1282
Draeger Medical Systems, Inc. reserves the right to make changes without
notice in design, specifications, and models. The only warranty Draeger Medical Systems, Inc. makes is the express written warranty extended on the sale
or rental of its products.
The information in this manual is confidential and may not be disclosed to
third parties without the prior written consent of Draeger Medical Systems,
Inc.
© 2006 by Draeger Medical Systems, Inc. ALL RIGHTS RESERVED.
 Loading...
Loading...