Page 1

USER MANUAL
Jaundice Meter
(JM-103)
From Konica Minolta and
Hill-Rom Air-Shields
Page 2

Jaundi ce Meter (Mode l JM - 1 03) User Manual (usr 07 0r b )
Page 3

Table of Contents
Section 1: Symbol Definition and Intended Use
Symbol Definition . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 - 1
Intended Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 - 3
Section 2: Introduction, Features, and Specifications
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2 - 1
Measuring Point . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2 - 1
Explanation of the Test . . . . . . . . . . . . . . . . . . . . . . . . . . . .2 - 3
Features. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2 - 6
Controls, Indica tors, and C on n e ctions . . . . . . . . . . . . . . . .2 - 6
Display . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2 - 8
Standard Features . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2 - 9
Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2 -10
Standard Features . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2 - 10
Regulations, Standards, and Codes . . . . . . . . . . . . . . . . . .2 - 11
Section 3: Precautions and Safety Tips
Precautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3 - 1
Safety Tips . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3 - 3
Warning and Caution Labels . . . . . . . . . . . . . . . . . . . . . . . . . . .3 - 4
Section 4: Installation and Assembly
Installation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4 - 1
Charging the Battery . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4 - 1
Selecting the Unit of Measurement . . . . . . . . . . . . . . . . . . .4 - 3
Operational Chec kout of the Jaundice Meter . . . . . . . . . . .4 - 4
Section 5: Instructions f or Use
Instructions for Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5 - 1
Taking Measurements . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5 - 1
Setting the Number of Average Measurements . . . . . . . . .5 - 4
Taking Average Measurements. . . . . . . . . . . . . . . . . . . . . .5 - 5
Jaundice Meter (Model JM-103) User Manual (usr070rb) Page i
Page 4

Section 6: Cleaning, Maintenance, and Re placement Parts
Cleaning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6 - 1
Steam Cleaning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6 - 1
Cleaning Hard to Clean Spots . . . . . . . . . . . . . . . . . . . . . . 6 - 1
Disinfecting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6 - 2
Maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6 - 2
Replacement Parts. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6 - 2
Section 7: Troubleshooting
Service Calls . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7 - 1
Error Messages . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7 - 1
Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7 - 2
Section 8: Storage and Handling
Storage and Handling. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8 - 1
Section 9: Warranty
Warranty. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .9 - 1
Appendix A: Clinical Performance Summary
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A - 1
Selection Criteria . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A - 1
Performance Data. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A - 2
Error Plots . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A - 12
Conclusion . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A - 16
Appendix B: Medical and Scientific References on Transcutaneous
Bilirubinom etr y (1979 - 1997)
Page ii Jaundice Meter (Model JM-103) User Manual (usr070rb)
Page 5
Section 1
Symbol Definition
and Intended Use
Symbol Definition
This manual contains different typefaces and icons des igned to improve
readabili ty and i ncrease unde rsta nding of it s cont ent. No te the foll owing
examples:
• Standard text—used for regular information.
• Boldface text—emphasize s a word or phrase.
• NOTE:—sets apart s p ec ial info r mation o r import an t instruc tion
clarification.
• The symbol below highlights a WARNING or CAUTION:
Warning and Caution
– A WARNIN G identif ies situations o r actions th at may affect
patient or user safety . Disregarding a warning could result in
patient or user injury.
– A CAUTION points out s pecial procedures or precautions that
personnel must follow to avoid equipment damage.
• The symbol below highlights a type BF applied part:
T ype BF Appli ed Part
– The instrument provi des a specified degree of protec tion
against electric shock, particularly the lea kage current and
reliability of the protective ground connection with an F-type
applied part . An F-type applied part indic ates an applied part
isolated fr om all other parts of the instrument to such a degree
that the p at ient leak ag e cur r e n t al lo w able in a sin g l e- fa u l t
condition is no t exce eded whe n a volta ge equa l t o 1.1 ti mes the
highest-rate d main s volta ge is applie d be tween t he appli ed part
and ground.
Jaundice Meter (Model JM-103) User Manual (usr070rb) Page 1 - 1
Page 6
• The symbol below highlights an ELECTRICAL SHOCK HAZARD
WARNING:
Electrical Shock Hazard Warning
• The symbol below indicates INPUT RATING:
Input Rating Symbol
• The symbol below ind icates that the product uses a
RECHARGEABLE BATTERY:
Rechargeable Battery Symbol
• The symbol below ind icates RESET:
RESET Button Symbol
• The symbol below, when applied to the device, indicates:
ATTENTION: Consult Accompanying Documents
Page 1 - 2 Jaundice Meter (Model JM-103) User Manual (usr070rb)
Page 7

Intended Use
Indications for the Use of the Jaundice Meter (JM-103)
The Jaundice Meter (JM-103) is a non-invasive transcutaneous
bilirubi nom eter. It measures yellowness of subcutaneous tissue in
newborn infants and displays a measured value which has been shown
to correlate with serum bilirubin. The device is for use in the hospital to
assist clinicians in monitoring the status of newborn infants for the
development of hyperbilirubinemia. The device is not intended as a
standalone for diagnosis of hyperbilirubinemia. It is to be used in
conjunction with other clinical s igns and laboratory measurements.
Newborn infants whose Jaundice Meter (JM-103) test results are
indicative of hyperbilirubinemia are evaluated by their doctor(s) for
appropriate patient management. Bilirubin levels should be confi r me d
by other methods, such as se rum bilirubin, prior to treatment
determinations.
Intended Use of the User Manual
This manual provides instructions for installation, use, operator
maintenance , and troubleshooting of the Jau ndice Meter (JM-103) from
Konica Minolta and Hill-Rom Air-S hields. Konica Minolta/Hill-Rom
Air-Shields cannot be responsi ble for the performance of the Jaun dice
Meter if the user does not oper ate the unit in accordance with the
instr uc tions, f ails to fol l ow m ainten ance rec ommen dation s , or makes
any repairs with unauthorized compon ents. Only qualified se rvice
personnel shoul d perform repair . Service information is available
through your local distributor or Konica Minolta/Hill-Rom Air-Shields.
Jaundice Meter (Model JM-103) User Manual (usr070rb) Page 1 - 3
Page 8

NOTES:
Page 1 - 4 Jaundice Meter (Model JM-103) User Manual (usr070rb)
Page 9

Section 2
Introduction, Features,
and Specifications
Introduction
To prevent kernict erus in newborn infants, it is very im portant to detect
jaundice in i ts earl y st ages. The Jaundice Meter (JM-103) is a noninvasive tra nscut aneous bi li rubi nometer. Th is hand-he ld d evice allows a
quick, non-invasive estimate of bilirubin concent r ation, to be used as an
aid for the managemen t of jaundice in newborn infant s. The
measur ements are taken au to matically when you place the in strument ’s
measuring probe against the forehea d or sternum of the inf ant and press
it gently; the measured value is then displayed.
The Jaundice Meter (JM-103) measures the yellowness of the
subcutaneous tissue in infants through the detection of the optical
density di fference at two wavelength s. It displays a measured value that
has been sho wn to correla te wit h ser um bili rubi n. The devic e is inten ded
for use in the h ospital to assist clinicians in monit or ing the sta tus of
infants for the deve lopment of hyperbil irubinemia. The device is not
intended a s a standalone for diagnosis of hyperbilirubinemia. It is to be
used in c onjunction with other clinical signs and laboratory
measurements. Infants whose Jaundice Meter (JM-103) test results are
indicative of hyperbiliru binemia are evalu ate d by their doctor(s) for
appropriate patient manag em ent. Bilirubin levels should be confirmed
by other methods (e.g. serum bilirubin) prior to treatment
determinations.
SPECIFICATIONS
Measuring Point
Measurements must be taken only on the infant’s forehead or sternum
where a suf fici ent am ount of b lood is ci rcula ted. A po ssibil it y exis ts th at
the bilirubin in the subcutaneous tissue may measure low for are as with
minimal blood flow or areas in which the subcutaneous tissue is subject
to keratiniz ation.
The measuring points for this device include the forehead and the
sternum of the infant. Although c orrelation with serum bilirubin was
observed for both forehe ad and ster num measurements, the clinical
studies performed with the Jaundice Meter (JM-103) show consis tently
better results with measurement s ta ken at the sternum versus the
forehead.
Jaundice Meter (Model JM-103) User Manual (usr070rb) Page 2 - 1
Page 10

Precocious Jaundice
Do not use this device on infants with precocious jaundice. If the re is a
possibility that the infant is suffering from precocious jaundice, as a
result of an incompatible blood type or hemolytic jaundice, it is
recommended that the total serum bilirubin be measured.
Phototherapy
WARNING:
Do not use the Jaundice Meter after initiation of phototherapy or
after an exchange transfusion. Patient injury could occur.
Page 2 - 2 Jaundice Meter (Model JM-103) User Manual (usr070rb)
Page 11
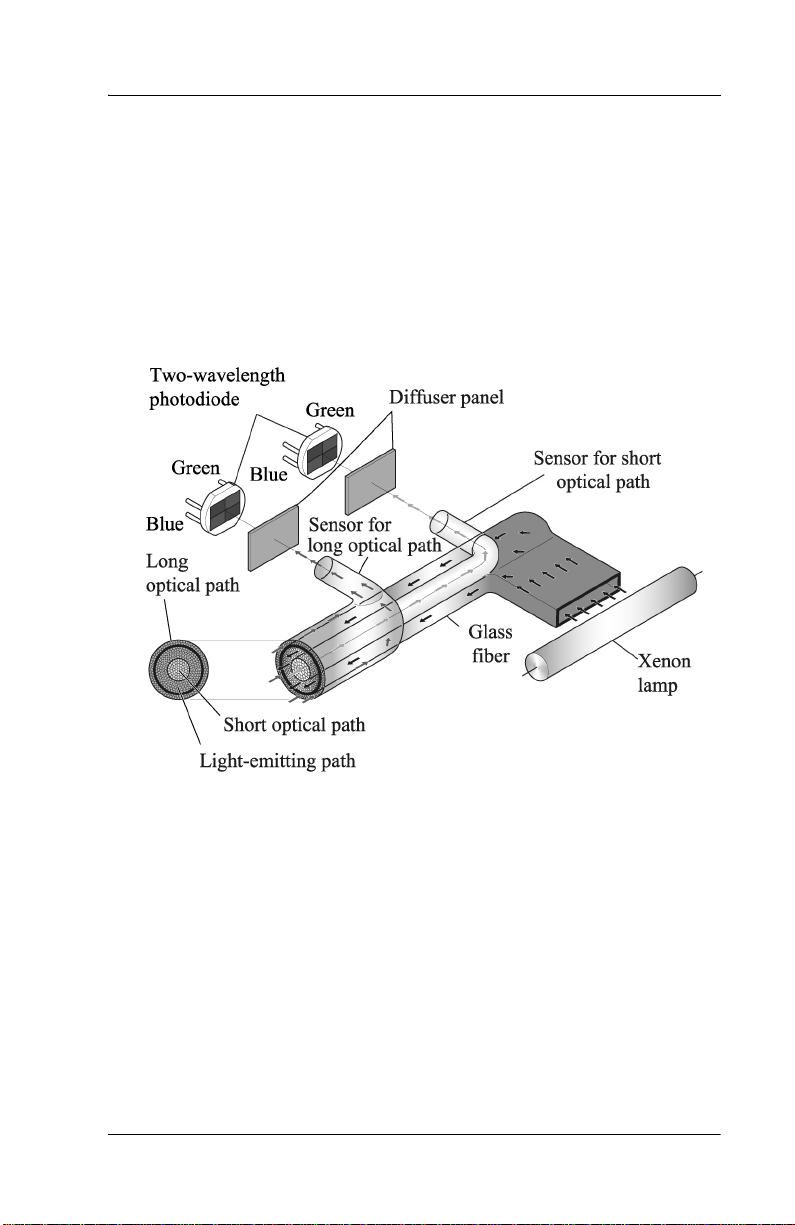
Explanation of the Test
Measurin g Principle
The Jaundice Meter determines the yellowness of an infant’s
subcutaneous tissue by measuring the difference in the optical densities
for light in the blue (450 nm) and green (550 nm) wavele ngth regions.
The measuring probe has two optical paths. This method allows for a
more precise measurement of yellowness in an infant’s subcutaneous
tissue by minimiz ing the infl uence s of the melan in pigment and th e skin
maturity.
SPECIFICATIONS
When the measuring probe is pressed against the forehead or sternum of
the infant, the built-in xenon lamp flashes. The light fr om th e xenon
lamp passes thr ough the glass fiber and illuminates the skin. The light
scatters an d is ab so r b ed in th e sk in an d su b cu taneous tissue rep e at ed l y,
and then finall y returns to the sensor side of the glass fiber. Of the light
that returns, the part scattered from the shallow areas of the
subcutaneous tissue passes through the inner core, or short-optical path,
of the fiber. The part scattered from the deep areas of the subcutaneous
tissue passes through the outer core, or long-optical pa th, and then
reaches it s corresponding photodiode.
Jaundice Meter (Model JM-103) User Manual (usr070rb) Page 2 - 3
Page 12
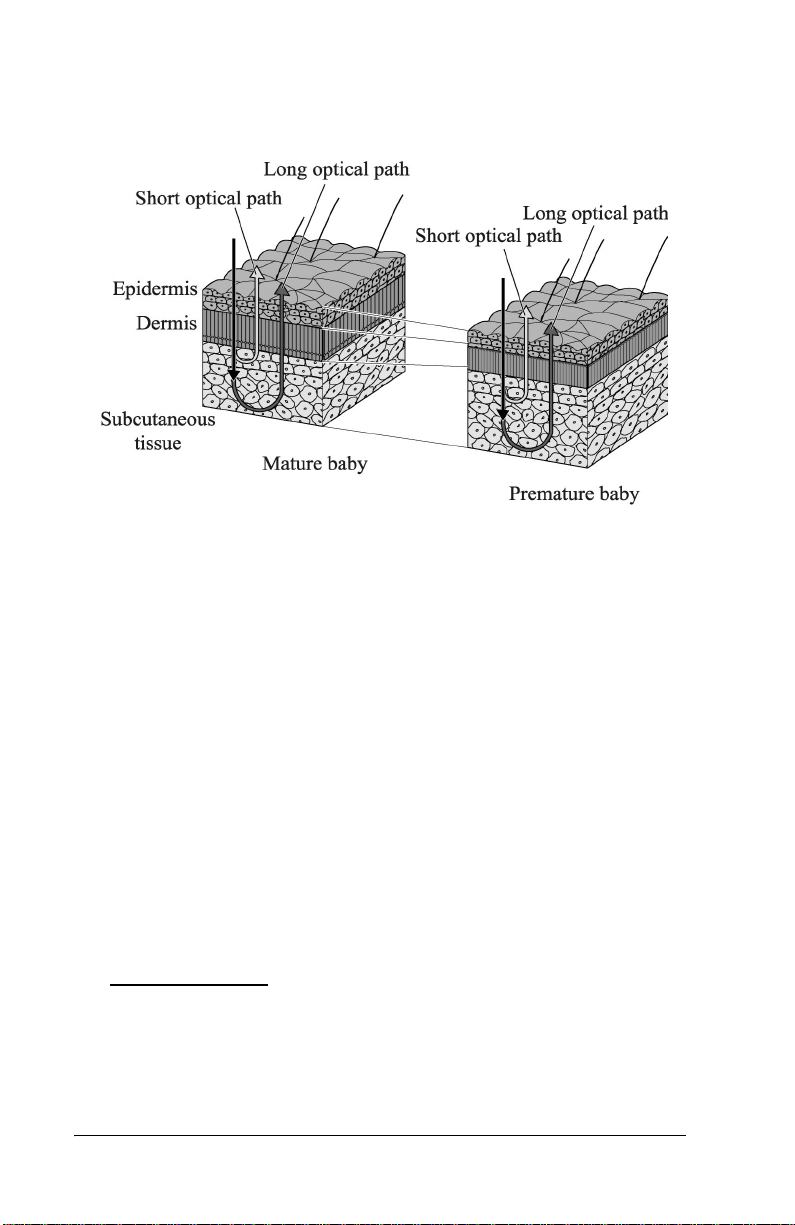
By calculating the difference in the optical densities, the pa rts that are
common to the epidermis and dermis are d educted, and as a re sult, the
differe nce in the optical densities between the two wavelength re gions
can be obtained for the subcutaneous tissue only. Since the optical
density difference shows a linear correlation with the total serum
bilirubin concentration, it is converted to the estimated bilirubin
concentration and is indicated digitally.
The Jaundice Meter (JM-103) de vice software uses a correlation
coeff icient to convert the measurement difference from the dual optical
path to an estimated bilirubin concentration. The calculation formula
used includes the correlation coefficients α and γ. These coefficients
were determined in pre-clinical testing. The equation used is as follows:
J
= α(L-S) + γ
sample
Where L and S are the long and short optical path measurements.
Use of the Device
Patient Population
The Jaundice Meter (JM-103) is indicated for use in neonatal patients
born >35 weeks gestati on who have not undergone transfusion or
photother apy treatment.
Page 2 - 4 Jaundice Meter (Model JM-103) User Manual (usr070rb)
Page 13
Averaging of Measurem ents
There was no significant difference between the averaged measurements
and the single measurement approaches in the largest study for sternum
measurements. The mean of three measure ments showed the highes t
degree of correlation (r=0.965), however, the difference was minimal
with a single measurement (r=0.963).
The advantages of using average measurements should be assessed at
each facility.
Action Levels
Each facility should deter mi ne their own action levels based on studies
of performance of the device on their population. Appropriate a ction
levels may vary depending on performance of the device, such as
precision or correlation with serum bilirubin, in the hands of the user.
Some factors that could affect performance of the device or appropriate
action levels include skin color, age, or measurement site. Careful
selection of ac tion levels should be made so that false negat ives do not
prevent appropriate follow up measures.
Calibration
There is no user calibration of this device. The system doe s include a
checker that me asure s the int ensi ty of l ight fro m the de vice to ensure t he
light output is within ran ge.
Processing of Measured Values
The Jaundice Meter (JM-103) determines the yellowne ss of the
subcutaneous tissue by measuring the difference in the optical densities
for light in the blue and green wavelengt h regions. The optical density
diffe rence has been shown to have a linear correlation with serum
bilirubi n concentration. T he device computes an estimated bilirubin
concentra tion based on this linear correlation and provides the val ue on
the display. The correla tion coefficient used in this computation was
derived from clinical data.
SPECIFICATIONS
Jaundice Meter (Model JM-103) User Manual (usr070rb) Page 2 - 5
Page 14
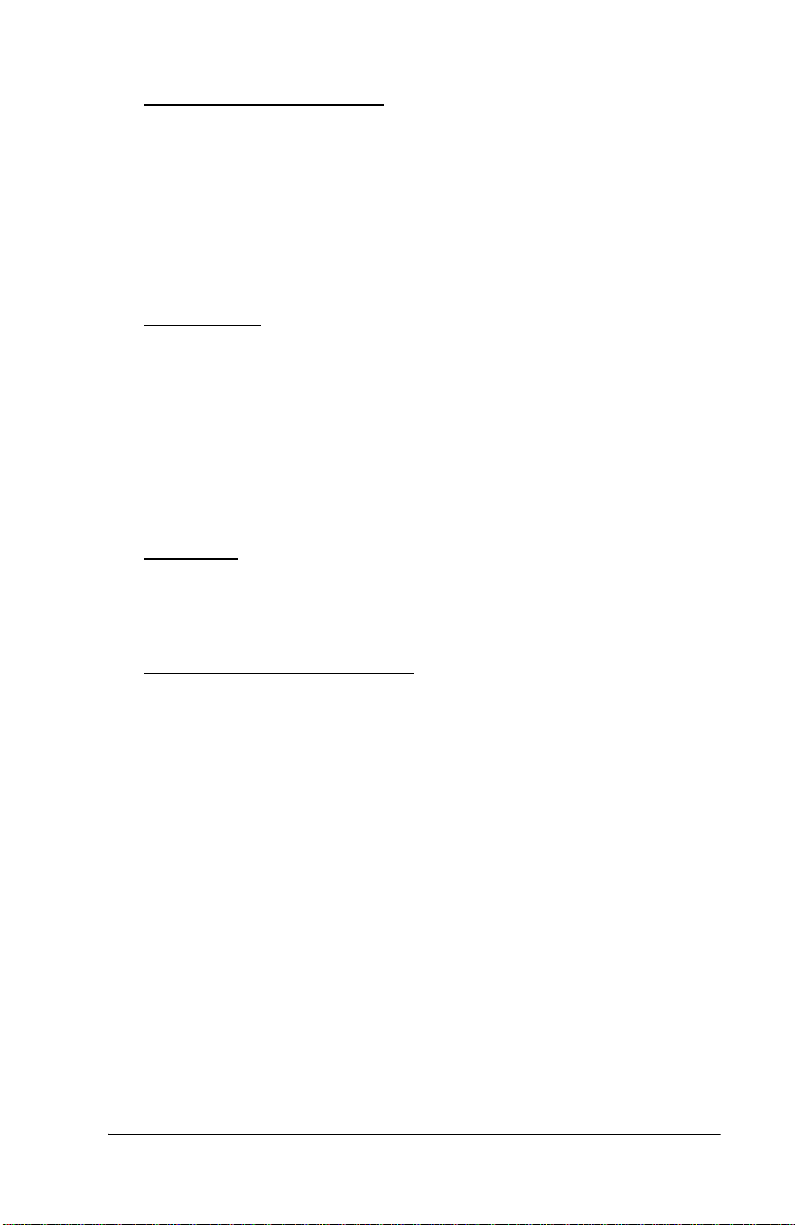
Features
Controls, Indicators, and Connections
Controls, Indicators, and Connect ions
Name Function
A Power switch Turns the Jaundice Meter on and off.
When used with the Reset button, the device
switches to Check Mode and changes the unit
of measurement.
B Ready lamp Illuminates to indicate that the J aundice Meter
is ready for the next measurement.
C Display Displays the measured value.
D Measuring prob e Takes the measurement when pres s ed against
the measuring point.
Page 2 - 6 Jaundice Meter (Model JM-103) User Manual (usr070rb)
Page 15

Name Function
E Charger section Connect the cha rger unit to the charger sec-
tion.
F Reset button Deletes the currently displayed measured
value and prepares for the next measurement.
When used with t he Power switch, the device
switches to Chec k Mo de and chang es the un it
of measurement.
G Str ap attachment area Is where the s trap attaches.
H Checker cover Open this c hecke r cove r to c heck the Ja undice
Meter.
I Charger lamp Illuminates t o indicate that the Jaundice Met er
is chargi n g.
J DC jack Connect the AC adapter to this jack.
K Charger jack Connect the main body to this jack.
L Checker Checks for the intensity of light output by tak-
ing measurements in Check Mode.
M Standard checker
For reference.
values
N DC plug Connect the charger’s DC jack to this.
O DC Plug (interna-
Connect the charger’s DC jack to this.
tional)
SPECIFICATIONS
Jaundice Meter (Model JM-103) User Manual (usr070rb) Page 2 - 7
Page 16
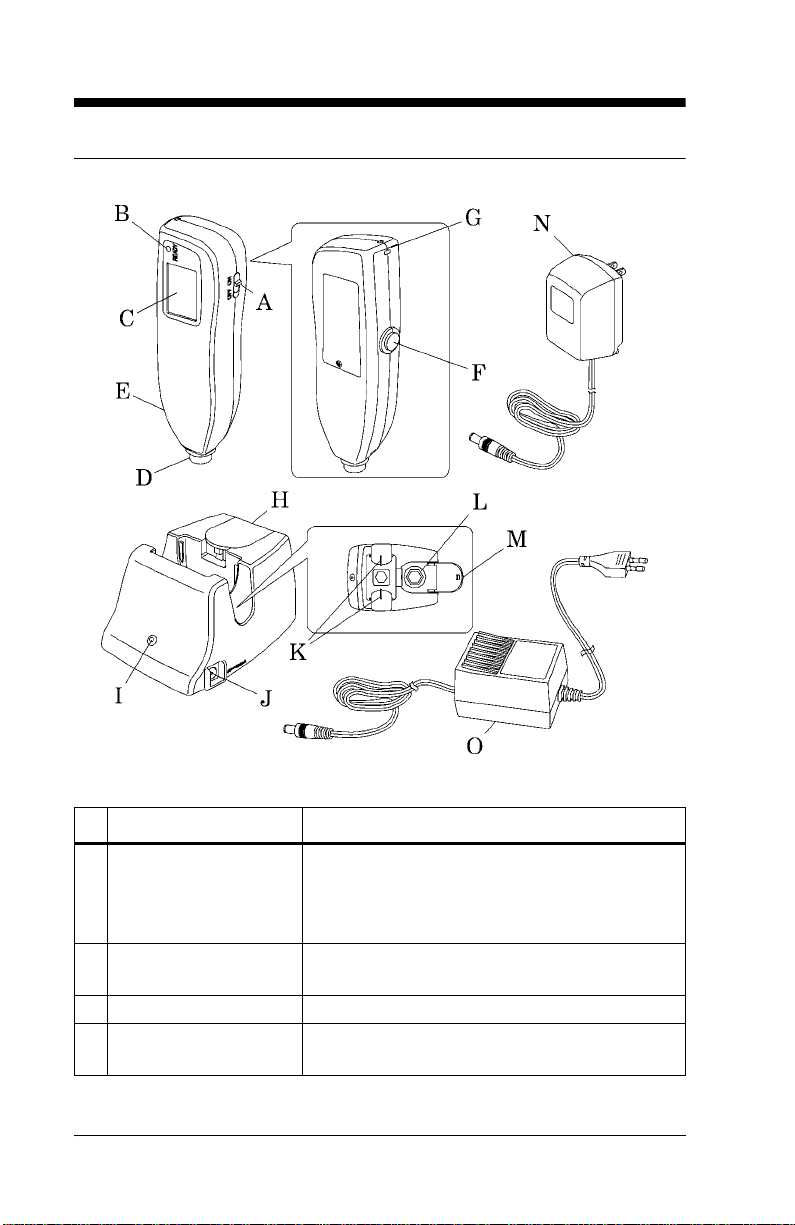
Display
Name Function
A AVG Illuminates duri ng averaging measurement .
B Optical path indicator
(•)
C Value Displays the measured value.
D Unit of measur em ent Displays the unit of measurement in either
E Battery indicator When the battery power is low, the battery
When performing inspections with the
checker, • illuminates when the L-value
appears and extingui shes when the S-value
appears.
NOTE: When the measured value is greater
than 20 mg/dl, the displ ay shows “---” an d the
physician should be contacted.
milligrams per deciliter (mg/dL) or mi cromoles of solute per liter (µmol/L)
indicator blin ks. Ch ar ge the bat tery a s soo n as
possible (see “Charging the Battery” on page
4-1).
If only the battery indicato r lights, the battery
has run out. Go to “Charging the Battery” on
page 4-1.
If the power is on and the displ ay is blank, the
battery is completely exhausted. Go to
“Charging the Battery” on page 4-1.
Page 2 - 8 Jaundice Meter (Model JM-103) User Manual (usr070rb)
Page 17

Standard Features
• Jaundice Meter (JM-103)
• Charger unit (Model JM-A30) with a checker
• AC adapter (Model JM-A31)
SPECIFICATIONS
Jaundice Meter (Model JM-103) User Manual (usr070rb) Page 2 - 9
Page 18
Specificat ions
Standard Features
Feature Dimension
Model name JM-103
Measuring method Determines the yellowness of the
subcutaneous tissue by using two
optical paths to measure the optical
density difference at two wavelengths
Measurement range 0.0 mg/dL to 20 mg/dL or 0 µmol/L
to 340 µmol/L
RMSE
∗
Light source Pulse xen on arc lamp
Light source life 150000 measurements
Sensors Silicon phot odiodes
Power source Special Ni-MH ba ttery
Protectio n type and level Internally-powered instrument, BF-
Minimum number of
measur ements when fully charged
Operating temperature range 10°C (50°F) to 40°C (104°F)
Operating rela tive humidity range 30% to 95%
Storage temperature range -10°C (14°F) to 50°C (122°F)
Storage rela tive humidity range 30% to 95%
Dimensions 48 mm (1.9") wide x 15.5 cm (6.1")
Weight, including battery 150 g (5.3 oz)
AC adapter input for North America 120V, 50/60 Hz, 10 W
AC adapter input for
International use
AC adapter output 9V, 500 mA
± 1.5 mg/dL or ± 25.5 µmol/L
type
400 single measurements
high x 33 mm (1.3") deep
200V-240V, 50/60 Hz, 12.5VA
* This accuracy is based on the aggregate results from both clinical
study groups (n=613). Refer to Appendix A for a detai led descri ption of
results by clinical site, measurement loca tion, and patient
demographics.
Page 2 - 10 Jaundice Meter (Model JM-103) User Manual (usr070rb)
Page 19

Regulations, St a ndar ds, and Codes
In No rth Am e r ica
With respect to electrical shock, fire, and mechanical
hazards only, this instrument complies with UL
2601-1 and CAN/CSA C22.2 No. 601.1.
In Europe, this instr ument complies with EN60 601-1, EN60601-1-2,
and EN ISO13485, and EN ISO14971.
SPECIFICATIONS
Jaundice Meter (Model JM-103) User Manual (usr070rb) Page 2 - 11
Page 20

NOTES:
Page 2 - 12 Jaundice Meter (Model JM-103) User Manual (usr070rb)
Page 21

Section 3
Precautions and Safety Tips
Precautions
WARNING:
Do not use the instrument in areas where flammable or
combustible gases, such as anesthetic gases, are present. Doing
so could result in a fire. Personal injury or equipment damage
could occur.
WARNING:
If the instrument, the charger unit, or the AC adapter are
damaged, or if smoke or an odd smell occurs, do not use the
instrument, the charger unit, or the AC adapter. In such situations,
immediately turn off the instrument, unplug the AC adapter from
its power source, and contact the nearest authorized service
facility. Failure to do so could resul t in fire, personal injury, or
equipment damage.
SHOCK HAZARD:
Always plug the instrument into an AC outlet of the correctly rated
voltage and frequency. Fai lure to do so could result in fire,
personal injury, or equipment damage.
SHOCK HAZARD:
Do not disassemble or modify the instrument, the charger unit, or
the AC adapter. Fire, personal injury, or equipment damage could
occur.
CAUTION:
Do not use a mobile telephone when using the Jaundice Meter. A
measurement error could occur.
CAUTION:
The Jaundice Meter interferes with Magnetic Resonance Imaging
(MRI) procedures. Inaccurate readings could occur.
Jaundice Meter (Model JM-103) User Manual (usr070rb) Page 3 - 1
Page 22

CAUTION:
Do not place the instrument on an unstable or sloping surface.
The instrument or charger unit could drop or overturn. Equipment
damage could occur.
CAUTION:
The Jaundice Meter is a precision instrument. When using it, do
not drop it, expose it to shocks or strong vibrations, or place
heavy objects on it. Equipment damage could occur.
CAUTION:
Do not allow blood or other liquids to come in contact with the
instrument. Should blood or other liquids come in contact with the
instrument, immediately clean the instrument (see “Cleaning” on
page 6-1). Failure to do so could result in equipment damage.
CAUTION:
Do not use the instrument in direct sunlight. Equipment damage
could occur.
CAUTION:
The instrument has a built-in, non-replaceable battery. Do not
disassemble the instrument to replace the battery. To replace the
battery , contact your dealer or authorized Hill-Rom service center.
Failure to do so could result in equipment damage.
Page 3 - 2 Jaundice Meter (Model JM-103) User Manual (usr070rb)
Page 23

Safety Tips
WARNING:
This instrument emits intense light to take its measurements.
Take measurements only at the forehead or sternum, and do not
press the measuring probe when it is directed toward the eyes.
Damage to the eyes could occur.
WARNING:
Before use, clean the measuring probe by wiping it with medicinal
alcohol. Failure to do so could result in the spread of infection or
infant inju r y.
WARNING:
The charger unit (JM-A30) and the AC adapter (JM-A31) are
solely designed for use with the Jaundice Meter (JM-103). Use
them only when chargin g the instrumen t. Using them to charge
other equipment could result in personal injury or equipment
damage.
WARNING:
Only facility-authorized personnel should troubleshoot the
Jaundice Meter. Troubl es hooting by unaut horized personnel
could result in personal injury or equipment damage.
WARNING:
Follow the product manufacturer’s instructions. Failure to do so
could result in personal injury or equipment damage.
WARNING:
Do not use the Jaundice Meter after initiation of phototherapy or
after an exchange transfusion. Patient injury could occur.
SHOCK HAZARD:
Do not plug or unplug the AC power cord’s plug with wet hands.
Personal injury or equipment damage could occur.
Jaundice Meter (Model JM-103) User Manual (usr070rb) Page 3 - 3
Page 24

SHOCK HAZARD:
Before cleaning, maintenance, or parts replacement, unplug the
charger unit from its power source. Failure to do so could result in
personal injury or equipment damage.
SHOCK HAZARD:
Do not expose the unit to excessive moisture that would allow for
liquid pooling. Personal injury or equipment damage could occur.
CAUTION:
Do not use harsh cleansers/detergents, such as scouring pads
and heavy duty grease removers, or solvents, such as toluene,
xylene, and acetone. Equipment damage could occur.
Warning and Caution Labels
Page 3 - 4 Jaundice Meter (Model JM-103) User Manual (usr070rb)
Page 25

Section 4
Installation and Assembly
Installation
Before using th e ins trument, charge and inspect the instrument.
Charging the Battery
When using the instrument for the first time, ensure that it is fully
charged. To maintain a full charge at all times, place the instrument on
the charger unit when it is not being used for measurements. When the
battery power is low, the Battery display blinks.
If the Jaundice Met er is left uncharged for a long period of time, the
power of the battery dimini shes; e nsure t hat it i s charge d pri or to use . To
charge the Jaundice Meter, perform the following:
WARNING:
The charger unit (Model JM-A30) and the AC adapter (Model JMA31) are solely designed for use with the Jaundice Meter (JM-
103). Use them only when charging the instrument. Using them
to charge other equipment could result in personal injury or
equipment damage.
1. Plug the AC adapter into the DC jack
of the c harger u n it. Use only the
charger unit and AC adapter supplied
with the Jaundi ce Meter.
NOTE:
The shape of the AC adapter varies
according to region.
NOTE:
120V model shown.
Jaundice Meter (Model JM-103) User Manual (usr070rb) Page 4 - 1
Page 26

SHOCK HAZARD:
Do not plug or unplug the AC power cord’s plug with wet hands.
Personal injury or equipment damage could occur.
2. Plug the AC ad apter’s plug into an AC out let. Never do so with wet
hands.
3. Place the Jaundic e Meter on the
charger unit so that its displa y faces
you. When the Jaundice Meter is set
on the charger unit properly, the
Charger lamp lights up.
NOTE:
With a fu lly char ge d batte ry, approxi mately
400 measurements can be taken. W ith six
hours worth of char ging, approximately
100 measurements can be taken.
4. Allow approximately 32 hours for charging to complete.
CAUTION:
The instrument has a built-in, non-replaceable battery. Do not
disassemble the instrument to replace the battery. To replace the
battery , contact your dealer or authorized Hill-Rom service center.
Failure to do so could result in equipment damage.
5. To replac e the battery, contact your dealer or an authoriz ed service
facility.
Page 4 - 2 Jaundice Meter (Model JM-103) User Manual (usr070rb)
Page 27

Selecting the Unit of Measurement
1. Hold down the Reset button, and turn
on the Power switch.Do not release the
Reset button.
2. While continuing to press the Reset
button, allow approximately 15
seconds for the unit of m easurement to
switc h f ro m mg/dL to µmol/L, or vice
versa.
3. Visually ensure that the unit’s display
has changed.
4. Releas e th e Reset button. The Ready
lamp lights up, indicating that the
instrument is rea dy to take a
measurement.
5. To change the unit of measurement
once more, turn off the Power switch,
and repeat step 1.
Jaundice Meter (Model JM-103) User Manual (usr070rb) Page 4 - 3
Page 28

Operational Checkout of the Jaundice Meter
Do not touch the checker’s surface with your fing ers . If the checker gets
dirty, wipe it with a soft cloth dampened with water, and then wipe it
with a dry cloth.
Using the checker supplied with the charger unit, check the instrument
to ascertain that the meter lig ht output is within range. Check the
instrument once or more each da y it is us ed . To inspect the Jau n di c e
Meter, perform the following:
1. Hold the Reset button down, and set
the Power switch to the On position.
NOTE:
If the Reset button is held down for longer
than 15 seconds, the unit of measurement
switches.
2. After CHE appears on the display
window, immediately release the
Reset button. If the Reset button is
held down for longer than 15 seconds,
switch the un it of measure ment back to
its previ ous setting (see “Selecting the
Unit of Measurement” on page 4-3).
3. Visually confirm that CHE appea rs in
the display and that the Ready la mp
illuminates.
4. Open the co v er of th e che ck er. Use
only the checker su pplied with the
Jaundice Meter (JM-103).
Page 4 - 4 Jaundice Meter (Model JM-103) User Manual (usr070rb)
Page 29

5. Place the measuring probe
perpendicul ar to the checker, and push
it gently until a click soun ds .
6. If the measuring probe contacts the
checker at an angle, place it
perpendicular, and take the
measur ement again.
NOTE:
The display interchanges between the Lvalue, the measured value of the longoptical path, and the S-value, the measured
value of the short-optical path. When the Lvalue is displayed, “•” ap pears in the upper
left-hand corner of the displa y.
7. Confirm the measured value. If both
the L-va lu e an d th e S- v al ue are with in
± 1.0 of the reference values indicated
on the che ck er, the values ar e
acceptable.
8. If the measured value exceeds ± 1.0 of
the reference value , per f orm the
following:
a. Clean both the chec ker and the
measuring probe.
b. Place the measuring probe perpendicular to the checker, and
push it gently until a c lick sounds.
c. If the measured value still exceeds ± 1.0 of the reference value,
contact the nearest authorized service facility.
9. Close the cover of the checker.
10. Set the Power switch to the Off position.
Jaundice Meter (Model JM-103) User Manual (usr070rb) Page 4 - 5
Page 30

NOTES:
Page 4 - 6 Jaundice Meter (Model JM-103) User Manual (usr070rb)
Page 31

Instructio ns for Use
Taking Measurements
1. Remove the Jaundice Meter from the
charger unit.
WARNING:
Before use, clean the measuring probe
by wiping it with medicinal alcohol.
Failure to do so could result in the
spread of infection or infant injury.
2. Using medicinal alcohol, clean the
measuring probe.
3. Set the Power switch to the On position.
The measured value for a sin g le
measurement, n-1, appears on the
display.
Section 5
Instructions for Use
4. Visually check that the Ready lamp
illuminates.
5. If the batt ery in dicat or bl ink s, c har ge t he
battery (see “Charging the Battery” on
page 4- 1).
Jaundice Meter (Model JM-103) User Manual (usr070rb) Page 5 - 1
Page 32

WARNING:
This instrument emits intense light to take its measurements.
Take measurements only at the forehead or sternum, and do not
press the measuring probe when it is directed to the eyes.
Damage to the eyes could occur.
6. Perform the following:
a. Place the measuring probe
vertically against the infant’s
forehead or sternum. Avoid any
bruises or disc olored areas of the
skin.
b. Push the measuring probe gently
until a click sounds. The
instrument’s xenon lamp flashes
momentarily, and the mea sured
value appear s on the display.
• If the measured value is outside the
measurement range of 20 mg/dL or
340 µmol/L, the display shows “--” and user should cont ac t the
physician.
NOTE:
If the instrument is not operate d for mor e
than 60 seconds, the backlight on the display
goes out.
7. To take another measurement, press
the Reset button, and continue from step 4.
Page 5 - 2 Jaundice Meter (Model JM-103) User Manual (usr070rb)
Page 33

8. To stop measuring, perform the following:
a. Set the Power switch to the Off position.
b. Using medicinal alcohol, clean the measuring probe .
c. Place the Jaundice Meter on the charger unit. When the
Jaundice Meter is not in use, keep it on the charger unit.
Jaundice Meter (Model JM-103) User Manual (usr070rb) Page 5 - 3
Page 34

Setting th e N u mber of Average Measurements
1. Set the Power switch to On or press
the Reset button to prepare the
instrument for measurement.
• n-1, n-2, and so on (up to n-5)
will appear.
2. Press the Reset button for 5 s. The number of average
measur e m en t s will switc h as f o ll o w s:
3. Release the Reset button when the required number of avera ge
measurements is displayed.
• If n-2 through n-5 is selected,
AVG appears in the upper left
corner of th e display.
• The selected number of average
measurements will be recorded.
Page 5 - 4 Jaundice Meter (Model JM-103) User Manual (usr070rb)
Page 35

Taking Average Measurements
1. Set the numb er o f average measurements ne eded.
2. Ensur e th at the Ready lamp is
illum i n at ed once n-5 appears.
NOTE:
n-5 is use d in the steps below as an
example. The number of measurements you
require and set is the number that should
appear in the display.
NOTE:
Each measurement must be take n
individually by the user. The first measurement is complete when the
measuring probe is pressed agains t the patient and the unit clicks. The
probe must then be li fted from the patient and reapplied for the total
number of measurements selected (in this exampl e 5 measurements).
WARNING:
This instrument emits intense light to take its measurements.
Take measurements only at the forehead or sternum, and do not
press the measuring probe when it is directed toward the eyes.
Damage to the eyes could occur.
NOTE:
It is recommended that all of the measurem ents taken for averagi ng be
taken from the same measuring point, forehead or s ternum.
3. Place the measuring probe vertic ally
on the measuring p oint, and then apply
gentle pressure until the probe clicks.
• The measurement will be taken,
and the number of remaini ng
measurements will be displayed.
Jaundice Meter (Model JM-103) User Manual (usr070rb) Page 5 - 5
Page 36

4. While ensu r ing tha t th e Ready lamp is illuminated, repeat the
measuring until the number of remaining measurements is 0.
• When the remaining number of
measurements is completed, the
average of the measured values
appears in the display.
• If the instrument is left without
the preset number of
measurements taken, the setting
will be canceled without the
measurement value being
displayed. To set the average
measurements again, press the Reset button and reset the
number of average measurements needed.
• To change the number of average measurements, refer to
“Setting the Number of Average Measurements” on page 5-4.
Page 5 - 6 Jaundice Meter (Model JM-103) User Manual (usr070rb)
Page 37

Section 6
Cleaning, Maintenance,
and Replacement Parts
Cleaning
WARNING:
Follow the product manufacturer’s instructions. Failure to do so
could result in personal injury or equipment damage.
SHOCK HAZARD:
Before cleaning, maintenance, or parts replacement, unplug the
charger unit from its power source. Failure to do so could result in
personal injury or equipment damage.
SHOCK HAZARD:
Do not expose the unit to excessive moisture that would allow for
liquid pooling. Personal injury or equipment damage could occur.
CAUTION:
Do not use harsh cleansers/detergents, such as scouring pads
and heavy duty grease removers, or solvents, such as toluene,
xylene, and acetone. Equipment damage could occur.
If there is no visible soila ge with possible body fluids, we recommend
that you clean the uni t with a mild detergent and warm water. If
disinfec tion is desired, you may use a combination cleanser/disinfectant
as explained in “Disinfecting” on page 6-2.
Steam Clea n ing
Do not use any steam cleaning device on the unit . Do not autoclave the
unit. Excessive mo isture can damage mechanism s in this unit.
Cleaning Hard to Clean Spots
T o re move dif fic ult spots or s tai ns, we rec ommend th at you us e stan dard
household clea nsers and a soft bristle brush. To loosen heavy, dried-on
soil, you may first need to saturate the spot.
Jaundice Meter (Model JM-103) User Manual (usr070rb) Page 6 - 1
Page 38

Disinfecting
When there is visible soilage and bet ween patients, we rec ommend that
you disinfect the unit with a tuberc ulocidal disinfectant, such as
Kleenaseptic B®. For customers in the US, the d is infectant should be
register ed with the Environmental Protection Agency.
Dilute the d isinfectant according to the manufacturer’s instructions, if
necessary.
Maintenance
There are no user serviceable parts and no maintenance or calibration
required. Serv ic e is o nly re qu i r ed if the un i t ce ases to fun ct i on as
intended or fail s the checker reading (see “Operational Checkout of the
Jaundice Mete r” on page 4-4).
Replacement Parts
CAUTION:
The instrument has a built-in, non-replaceable battery. Do not
disassemble the instrument to replace the battery. To replace the
battery , contact your dealer or authorized Hill-Rom service center.
Failure to do so could result in equipment damage.
There are no user-replaceable parts. To replace the battery, contact your
dealer or an authorized service facility.
Page 6 - 2 Jaundice Meter (Model JM-103) User Manual (usr070rb)
Page 39

Section 7
Troubleshooting
Service Calls
When you call Hill-Rom about your unit, be prepared to give the serial
number from the product identification label. When you give the seria l
number , the Hill -Rom re presenta ti ve can identi fy your unit and give you
the information you need more quickly.
Error Messages
For warnings that may appear on the display window, refer to the table
below.
Error Messages
Warning Cause Solution
Er1 The measured value is
abnormal. In the case of an
averaging measurement, the
measurement fluctuation is
excessively large.
Er2
through
Er6
A measurement error may
have occurred during an averaging me as u r em en t , or th e
hardware is not functioning
properly.
Place the measuring probe
perpendicular to the infant’s
forehead or sternum, a nd take
the measurement again. If
Er1 still appears, contact the
nearest author i zed s er v i ce
facility.
Set the Power switch to the
Off position, and then return
it to the On position. If the
warning stil l appears, contact
the near est a uth oriz ed se rvic e
facility.
Jaundice Meter (Model JM-103) User Manual (usr070rb) Page 7 - 1
Page 40

Troubleshooting
WARNING:
Only facility-authorized personnel should troublesh oot the
Jaundice Meter. Troubleshooting by unauthorized personnel
could result in personal injury or equipment damage.
If an abnormality occurs with the Jaundic e Met er, perform the
following:
1. Refer to the table below, and take the necessary action giv en.
2. If the ab no r m a lity st il l ap p ears, se t the Power sw it ch to th e Off
position, and then return it to the On position.
3. If the abnormality still continues, contact th e nea rest authorized
service facility.
Troubleshooting
Symptom Possible Cause Action
The display is bla nk
when the Power switch
is in the On position.
The display suddenly
goes blank during a
measurement.
The Charger lamp
does not illuminate
when the Jaundice
Meter is placed on the
charger unit.
It is impossible to take
measurements.
The batteries are
exhausted.
The batteries are
exhausted.
The charger unit and
the AC ada p ter are not
plugged into an AC
outlet correctly.
The Jaundice Meter is
not plac ed in th e
charger unit corre ctly.
The Ready lamp is not
illuminated.
The batteries are
exhausted.
Charge the battery (see
“Char ging the Battery”
on page 4-1).
Charge the battery (see
“Char ging the Battery”
on page 4-1).
Plug the cha rger unit
and the AC adapter into
an appropriate power
source correctly.
Place the Jaundice
Meter perpendic ular to
the measuring point in
the charger unit.
Before taking a
measur em en t , en s ur e
that th e Ready lam p is
illuminated.
Charge the battery (see
“Char ging the Battery”
on page 4-1).
Page 7 - 2 Jaundice Meter (Model JM-103) User Manual (usr070rb)
Page 41

Section 8
Storage and Handling
Storage and Handling
When storing the instrument, pay attention to the following conditions:
• Store the instrument at a temperature range of -10°C (14°F) to 50°C
(122°F), and at a non-condensing relative humidity range of 30% to
95%.
• Keep the instrument dry.
• Do not store the inst rument in locations tha t may have an adverse
effect on its perform ance, such as locatio ns subject to the f o llowing:
– High atmospheric pressure
– Direct sunlight—do not store near windows.
– Extreme dust—do n ot stor e in close ts or bi ns where dust or l int
can gath er.
– Air having sali nity or sulphur content
– Strong magnetism—do not store near MRI or other imaging
equipment, a nd do not store near operating rooms.
• Do not subject the instrument to vibration or impact.
• Do not s to re th e instrum e n t in lo cations where chem ic als are stor e d
or where g as may be emit ted.
• To ensure no problems will exist the next tim e the main body and
charger are used, thoroughly clean the main body and char ger, and
then store them together.
Jaundice Meter (Model JM-103) User Manual (usr070rb) Page 8 - 1
Page 42

NOTES:
Page 8 - 2 Jaundice Meter (Model JM-103) User Manual (usr070rb)
Page 43

Section 9
Warranty
Warranty
HILL-ROM COMPANY, INC.
LIMITED WARRANTY
Hill-Rom Company, Inc. (Hill-Rom) has a long tradition of providing superior products and service to our
customers. Our goal is “Total Customer Satisfaction”. In that spirit, Hill-Rom is proud to offer the following
warranty.
GENERAL WARRANTY (APPLICABLE UNLESS A SPECIFIC WARRANTY IS
LISTED)
Hill-Rom warrants to the original purchaser that its products and replacement parts shall be free from defects
in material and workmanship for a period of one (1) year from date of delivery. Hill-Rom’s obligation under
this warranty is expressly limited to supplying replacement parts and/or service for, or replacing, at its option,
any product which is, in the sole discretion of Hill-Rom, found to be defective. In addition to the foregoing
one year warranty, Hill-Rom warrants to the original purchaser that the frame and welds on its products will
be free from structural defects for the life of the product. Any product upgrade or modification initiated by
Hill-Rom does not affect the original product warranty.
SPECIFIC WARRANTIES
MAT TRESS WARRANTIES
Hill-Rom warrants to the original purchaser that its mattress product shall be free from defects in material and
workmanship for a period of two (2) years from date of delivery. However, electro mechanical mattress
components (compress ors, valves, pri nted circ uit boards, hoses , and couplers) are covered by the general one
(1) year warranty.
EXPENDABLES WARRANTIES
A sixty (60) day limited warranty from date of delivery applies to expendable parts such as cushions,
coverlets, software diskettes, locator badge batteries, dome light incandes cent bulbs, overhead fluorescent
tubes, heating elements, temperature probes, filter sheets, and microspheres. This warranty is limited to
replacement of the parts covered.
TO OBTAIN PARTS AND SERVICE
In the United States and Canada, call Hill-Rom Technical Support Department at (800) 523-5756, Monday
through Friday. Outside the United States and Canada, call your authorized Hill-Rom Distributor. In order to
expedite service, we re quest you furnish the following information: customer identificat ion number, product
model number, serial number, and description of problem. A qualified specialist will provide, via telephone
(United States and Canada), or F AX (Outside the United States and Canada), troubleshooting assistance for
facility personnel and provide necessary parts to make repairs. If troubleshooting determines the need for onsite technical service, a qualified service representative will be dispatched. Replacement of non-technical
items will be the responsibility of the customer. If requested by Hill-Rom, products or parts for which a
warranty claim is made shall be returned prepaid to Hill-Rom’s factory.
OUT OF WARRANT Y EXCHANGE POLICY
After the expiration of the original warranty, upon request, Hill-Rom will ship as a replacement, components
such as selected: motors and printed circuit boards, for like units returned to Hill-Rom by the original
purchaser at a substantial savings. Please call Hill-Rom Technical Support Department for current pricing.
PARTS AV AILABILITY POLICY
Hill-Rom will offer parts for new and remanufactured products for ten (10) years from date of sale; for
communi cations products for five (5) yea r s from date of sale.
Note: Some original component parts and assemblies may not be available; functional equivalents may be
substituted.
Jaundice Meter (Model JM-103) User Manual (usr070rb) Page 9 - 1
Page 44

THE FOREGOING WARRANT IES ARE EXCLUSIVE AND IN LIEU OF ALL OTHER EXPRESS
WARRANTIES AND IMPLIED WARRANTIES, INCLUDING BUT NOT LIMITED TO, THE
IMPLIED WARRANTIES OF MERCHANTABILITY AND FITNESS OF PURPOSE. HILL-ROM’S
OBLIGATION UNDER THESE WARRANTIES SHALL NOT INCLUDE ANY LIABILITY FOR
LOSS OF PROFITS, DIRECT, INDIRECT OR CONSEQUENTIAL DAMAGES OR DELAYS. Some
states, provinces, or countries do not allow the exclusion or limitation of incidental or consequential damages,
so the above exclusion or limitation may not apply. Any improper or negligent use, any alterations or repairs
not in accordance with Hill-Rom’s manuals or performed by others in such manner as in Hill-Rom’s sole
judgment affects the product materially and adversely, shall void these warranties. These warranties do not
cover failures due to misuse, abuse, neglect, or lack of routine maintenance. No employee or representative of
Hill-Rom is authorized to change these warranties in any way or grant any other warranty unless in writing
and signed by a Hill-Rom officer. These warranties provide specific legal rights; but, there may be other
available rights, which vary from state to state, province to province, or country to country.
Revise d October 1, 2003
Hill-Rom Company, Inc., 1069 State Route 46 E, Batesville, IN 47006-9167
Page 9 - 2 Jaundice Meter (Model JM-103) User Manual (usr070rb)
Page 45

Appendix A
Clinical Performance Summary
Introduction
The Jaundice Meter (JM-103) has been the subject of three clinical
studies. One study in Japan, the other two studies in the United States.
The following information is a summary of results from the two US
studies.
The purpose of the JM 103 is to measure the yellownes s of
subcutaneous tissue in the neonate. This measurement can be correlated
to serum bilirubin concentration and is therefore an aid in the
management of neona tal jaundice. Since this device performs
measurement through the use of light it is non-invasive and painless for
the infa nt. The ob je ctive of th e clinica l stu dies was to confir m th a t th e
device measurement, di splayed in units of estimated bilirubin
concentration, does correlate with the serum bilirubin concentration
sufficiently well to warrant its use as a screening tool.
The following is a su mmary of the study protoc ols:
Selectio n Criteri a
The patient selection crite ria used for the study included infants less
than 30 days old and weighing grea ter than 1000 grams. The test was
performed on i nfants who were determine d by their physician to requir e
a serum bilirubin test. A tabulation of weight distribution is provided in
grap h 2 2 Infant Weight Distribution. Error plot s by w e i ght are pr o v ided
in graphs 23 Clini cal S tu dy Site A Bir thwei ght Error Plot, Forehead and
24 Clinical Study Site A Birthweight Error Plot, Sternum.
Demographics of Patient Population
All patie nts meet ing th e ab ov e cri te ria were inc lu ded in th e study. There
was significant eff o rt to ensure that there was sufficient representation
of all skin pigmentation to verify that the JM 103 could be used across
all populations with consistent results. The demographics of the patient
population included Caucasian, African-American, and other.
Jaund ice Meter (JM-103) User Manual (usr0 70rb) Page A - 1
Page 46

Sample Size
The total number of infants in the sample population was 613.
Measurement Selection
One study followed a protocol where three measurements were take n,
each measurement was recorded and then the three measurements we re
averaged. The sec ond study took one measurement only. Estimated
bilirubin meas urement s take n during th e stud ies range d from 1.1 t o 20.8
mg/dL.
Body Sites Tested
In both studies the meas urements were taken on the forehe ad and
sternum each time the measurements were take n for a give n patient.
Number of Clinical Sites
There were two clinical sites that participated in the study. Clinical
study site A with 513 patients studied. Clinical study site B with 100
patients studied.
Performance Data
The following data is provided to demonstrate the performance of the
JM 103 in the clinica l use environment. This series of graphs show the
correlatio n of the estima ted bilirub in concentr ation take n non-invasivel y
with the JM 103 to the actual serum bilirubin concentration measured
from a blood sample taken from the pa tient (TSB). The device operates
over a range of 0.0 – 20.0 mg/dL (0 – 340 µmol/L). The data in clude
graphs in the form of x- y plots where x is the total serum bilirubin
concentration measured and y is the JM 103 estimated bilirubin
measurement. Forehea d and s ternum were not combined because the
data would be too difficult to distinguish. The serum bilirubin
measurements were take n using direct spectrophotometry in Clini ca l
Study A a nd specifically with the Bea kman-Colter Synchron LX-20by
in Clinical Study B.
Page A - 2 Jaundice Meter (JM-103) User Manual (usr070rb)
Page 47

Graph 1 – Clinical Study Site A All Patients, Forehead
Graph 2 – Clinical Study Site A All Patients, Sternum
Jaund ice Meter (JM-103) User Manual (usr0 70rb) Page A - 3
Page 48

Graph 3 – Clinical Study Site A African-American Patients, Forehead
Graph 4 – Clinical Study Site A African-American Patients, Sternum
Page A - 4 Jaundice Meter (JM-103) User Manual (usr070rb)
Page 49

Graph 5 – Clinical Study Site A Caucasian Patient s, Forehead
Graph 6 – Clinical Study Site A Causasian Patients, Sternum
Jaund ice Meter (JM-103) User Manual (usr0 70rb) Page A - 5
Page 50

Graph 7 – Clinical Study Site A Other Patients, Forehead
Graph 8 – Clinical Study Site A Other Patients, Sternum
Page A - 6 Jaundice Meter (JM-103) User Manual (usr070rb)
Page 51

Graph 9 – Clinical Study Site B All Patients, Forehead
Graph 10 – Clinical Study Si te B All Pati ents, Sternum
Jaund ice Meter (JM-103) User Manual (usr0 70rb) Page A - 7
Page 52

Graph 1 1– Clinical Study Site B Afri can-American Patients, Forehead
Graph 12 – Clinical Study Site B African-American Patients, Sternum
Page A - 8 Jaundice Meter (JM-103) User Manual (usr070rb)
Page 53

Graph 13 – Clinical Study Site B Caucasian Patients, Forehead
Graph 14 – Cli nical Study Site B Causasian Patients, Sternum
Jaund ice Meter (JM-103) User Manual (usr0 70rb) Page A - 9
Page 54

Graph 15 – Clinical Study Site B Other Patients, Forehead
Graph 16 – Clinical Study Site B Other Pati ents, Sternum
Page A - 10 Jaundice Meter (JM-103) User Manual (usr070rb)
Page 55

Graph 17 – Summary Table of Slope, Intercept, Standard Deviation, and
Correlation Coeffi cients for Each Graph
Study site / patient
population
SITE A Forehead All
(n=513)
SITE A Sternum All
(n=513)
SITE A Forehead
African American (n=65 )
SITE A Sternum African
American (n=6 5 )
SITE A Forehead
Caucasian (n=399)
SITE A Sternum
Caucasian (n=399)
SITE A Forehead Other
(n=49 )
SITE A Sternum Other
(n=49 )
SITE B Forehead All
(n=100)
SITE B Sternum All
(n=100)
SITE B Forehead
African American (n=48 )
SITE B Sternum African
American (n=4 8 )
SITE B Forehead
Caucasian (n=35)
SITE B Sternum
Caucasian (n=35)
SITE B Forehead Other
(17)
SITE B Sternum Other
(17)
Slope Intercept Correlation
Coefficient
(r)
Standard
Deviation
(RM SE)
1.05 -0.35 0.914 1.29
1.07 -0.74 0.946 1.02
1.15 -0.5 0.908 1.59
1.11 -0.4 0.908 1.55
1.01 -0.1 0.916 1.2
1.04 -0.6 0.956 0.88
1.06 -0.5 0.941 1.04
1.10 -1.0 0.977 0.65
1.07 -0 .00 0.84 2.14
1.16 -0 .43 0.89 1.85
1.40 +0.46 0.84 2.27
1.21 -0 .17 0.89 1.9
1.10 -1 .04 0.87 1.72
1.22 -1 .69 0.88 1.81
1.03 -0 .56 0.94 1.49
1.03 0.65 0.97 0.94
Jaund ice Meter (JM-103) User Manual (usr0 70rb) Page A - 11
Page 56

Graph 18 – Clinical Study Sit e A Error Plot, Forehead
Graph 19 – Clinical Study Site A Error Plot, Sternum
Page A - 12 Jaundice Meter (JM-103) User Manual (usr070rb)
Page 57

Graph 20 – Clinical Study Sit e B Error Plot, Forehead
Graph 21 – Clinical Study Site B Error Plot, Sternum
Jaund ice Meter (JM-103) User Manual (usr0 70rb) Page A - 13
Page 58

Graph 22 – Infant Weight Dist ri bution
Infant Weight
(grams)
Clinic a l Study A Clinic a l Study B
Up to 999 6 2
1000 - 1499 15 0
1500 - 1999 23 13
2000 - 2499 37 23
2500 - 2999 68 19
3000 - and up 337 43
Unknown 27 0
Tota l 513 100
Graph 23 – Clinical Study Site A Birthwei ght Error Plot, Forehead
Page A - 14 Jaundice Meter (JM-103) User Manual (usr070rb)
Page 59

Graph 24 – Clinical Study Site A Birthweight Error Plot, Sternum
Reproducibility
Reproducibility of the li ght output of the devi ce was tested daily using
the checker. The checker determines the intensity of the light outpu t of
the device. The accuracy of th e device is determ ine d by how well the
detectors in the unit measure the returning light. The results of the
device testing using the checker show that the device produced output
within the required range over the course of both clinical studies.
Reproducibi lity testin g in the pati ent popul ation can be derived from the
data taken in Clinical s tudy site A. The reproducibili ty da ta is based on
467 babies. Three indepe ndent measurements were taken at each sit e,
forehead and sternum. Each measurement was recorded, the mean and
standard deviation co mputed and recorded. The average standard
deviation for forehe ad an d sternum measurements was 0.3 for both.
Jaund ice Meter (JM-103) User Manual (usr0 70rb) Page A - 15
Page 60

Table 1: Reproducibility Data
Mean
Estimated
Bilirubin
Measure
ment
Body Site
Deviation
Range
Deviation
Mean
Deviation
Median
Range
(mg/dL)
Sternum 0.0 - 2.2 0.3 0.3 0.8 - 18.5
Forehead 0.0 - 2.6 0.3 0.2 0.1 - 19.5
Conclusion
What the data presented sh ows is that the JM 103 estimated bilirubin
concentration measurement correlates to the serum bilirubin
measurements. This data supports the use of this non-invasive device
along with other clinica l ind ic ato rs as a aid in the managem ent of
jaundice in the neonat al pati ent popula tion.
Page A - 16 Jaundice Meter (JM-103) User Manual (usr070rb)
Page 61

Appendix B
Medical and Scientific References on
Transcutaneous Bilirubinometry (1979 - 1997)
1. Amato M, et al;
Transc utaneo us bilirubin de termination : correlati on in white premature infants weighing less than 1500 gm.
Schweiz Med Wochenschr, 1985 Jul 9.
2. Amato M, et al;
Assessment of neonatal jaundice in low birth weight infants comparing transcutaneous, capillary and arterial bilirubin levels.
Eur J Pediatr, 1990 Nov.
3. Amato M
Transcutaneous, capil la ry, and arterial bilirubin levels.
J. Pediatr. 125(2), 332, 1994
4. Amit Y, et al;
Effect of skinf old th ic kness on transcutaneous bilirubin measurements.
Biol Neonate, 1993.
5. Arimichi J, et al;
Reliability and future application of a transcutaneous bilirubinometer.
Josanpu Zasshi, 1984 Apr.
6. Berget M, et al;
Transcut aneous bilirubinometry in newborn infants.
Tidsskr Nor Laegeforen, 1984 May 20
7. Bhat V, et al ;
Correlation of transcutaneous bilirubinome try with serum bilirubin
in south Indian neonates.
Indian J Med Res, 1987 Jul.
8. Bhut ta ZA, et al;
Transcut aneous bilirubinometry in Pakistani newborns: a preliminary report.
JPMA J Pak Med Assoc, 1991 Jul.
Jaund ice Meter (JM-103) User Manual (usr0 70rb) Page B - 1
Page 62

9. Boo Nem Yu n, et al;
Transcutaneous bilirubi nom et ry in Malay, Chinese and Indian term
neonates.
Med J Malaysia, 1984 Mar.
10. Bourchier D, et al;
Transcut aneous bilirubinometry: 22 months experience at Waikato
W omen's Hospital.
N Z Med J, 1987 Sep 23.
11 . B ro wn A; et al;
Transcutaneous bilirubinometry in infants: influence of race and
phototherapy.
Pediatr Res, 1981 , 15:653
12. Brown LP, et al;
Tra n scutaneous bilirubinometer: intermeter reliab ility.
J Perinatol, 1990 Jun.
13. Brown LP, et al;
Transcutaneous bilirubinometer: an instrument for clinical
research.
Nurs Res, 1990 Jul-A ug.
14. Brown LP, et al;
Incidence and patter n of jaun dic e in heal thy breast-fed infants during the first month of life.
Nurs Res, 1993 Mar - Apr .
15. Brucker MC, et al;
Neonatal jaundi ce in the home: assessment with a noninvasive
device.
J Obstet Gynecol Neonatal Nurs, 1987 Sep-Oct.
16. Cassady G
Transcutaneous mo nitoring in the ne w born in fant .
J Pediatr, 1983 Dec.
17. Chen ZL, et al;
Clinical use of transcutaneous bilirubinometry.
Chung Hua I Hsueh Tsa Chih, 1986 Jan.
18. Christo GG, et al;
Transcutaneous bilirubinometry in newborns.
Indian Pediatr, 1988 Nov.
Page B - 2 Jaundice Meter (JM-103) User Manual (usr070rb)
Page 63

19. Corch ia C, et al;
Comment on transcut aneous bilirubin device of Yamanouchi
[letter]
Pediatrics, 1981 Mar.
20. Dai J, et al;
Clinical impact of transcut aneous bilirubinometry as an adjunctive
screen for hyperbiliru bine mi a
Clin Biochem 29 (6): 581-586, Dec 1996
21. Dai J, et al;
Transcutaneous bilirubinometry: its role in the assess ment of neonatal jaundice
Clin Biochem 30 (1): 1-9, Feb 1997
22. Derksen-Samsom JF, et al;
The reliability of trans cut aneous bilirubin measu rem ent : a clinical
study with statisti ca l dat a and literature review.
Tijdschr Kindergeneeskd, 1984 Oct.
23. Dominguez Ortega F , et al;
Transcutaneous bilirubinometry: correlation of the reading site
obtained with spectrophotometry and diaz oreaction technique.
An Esp Pediatr, 1993 Nov.
24. Douville P, et al;
Diagnostic value of sequential readings with the Minolta transcutaneous bilirubi nometer in normal and low-birthweight infants
[letter]
Clin Chem, 1983 Apr, 29:4, 740-1.
25. Fabr i s C , et al;
Evaluation of transcutaneous bilirubinometry in newborn infants.
Minerva Pediatr, 1984 Jun 15.
26. Fisher B, et al;
The cephalocaudal progressi on of jaundice in newborns in relation
to the transfer of bilirub in from plasma to ski n.
Early Hum Dev, 1990 Apr, 22:1, 23-8
27. Foged N, et al;
Transcutaneous bil irubinometry. A non-invasive method of measuring physiologica l jaundice.
Ugeskr Laeger, 1983 Sep. 19.
Jaund ice Meter (JM-103) User Manual (usr0 70rb) Page B - 3
Page 64

28. Fok TF, et al;
Transcu taneou s bilirubi nometer: i ts use in Chines e term infants and
the effect of haema toc rit and phototherapy on the TcB index.
Aust Paediatr J, 1986 May.
29. Galletto P, et al;
Supervision of neonat al jaun dice by use of the transcutaneous
bilirubinometer.
Minerva Pediatr, 1983 Jan 31.
30. Goldman SL, et al;
Jaundice meter: evaluation of new guidelines.
J Pediatr, 1982 Aug.
31. Grande R, et al;
Physiological va ria ti ons in the pigm ent at ion of ne w born infants.
Hum Biol, 1994 Jun.
32. Hanneman R; et al;
Determination of serum bi lirubin by skin reflectance : ef fect of pigmentation
Pediatr Res 13 (12): 1326-1329 (Dec 1979)
33. Hanneman R; et al;
Evaluation of the Minolta bil irubin meter as a screening device in
white and black infants.
Pediatrics, 1982, 69, 107-109
34. Hegyi T, et al;
Tra n scutan eo us bilirubinometr y. I. Corre lations in term infants.
J Pediatr, 1981 Mar.
35. Hegyi T, et al;
Transcutaneous bilirubinometry. The cephalocaudal progression of
derma l icterus .
Am J Dis Child, 1981 Jun.
36. Hegyi T
Transcutaneous bilirubinometry: a new light on an old subje ct .
Pediatrics, 1982 Jan.
37. Hegyi T, et al;
Transcu taneous bilirubin ometry II. Dermal bil irubin kinetics duri ng
phototherapy.
Pediatr Res, 1983 Nov.
Page B - 4 Jaundice Meter (JM-103) User Manual (usr070rb)
Page 65

38. Hegyi T
Transcutaneous bilirubinometry in the newborn infant : sta te of th e
art.
J Clin Monit, 1986 Jan.
39. Hegyi T, et al;
Transcutaneous bilirubinometry. III. Dermal bilirubin kinetics
under green and blue light phototherapy.
Am J Dis Child, 1986 Oct.
40. Heick C, et al;
Transcut aneous bilirubin determi nation in the newborn infant. Recommendations of the Swiss Neonatology Group.
Helv Paediatr Acta, 1982.
41. Hodr R , et al;
Transcutaneous measuremen t of the severi ty of icte rus and bil irubinemia in normal neon at es.
Cesk Pediatr, 1990 Nov.
42. Hung WT, et al;
Diagnosis of atreti c prol onged obstructive jaundice; technetium
99m hepatolite excr etion study.
J Pediatr Surg, 1990 Jul.
43. Karr ar Z, et al;
Transcutaneous bilirubin measurements i n Saudi infants: the use of
the jaundice meter to identify significant jaun dic e.
Ann Trop Paediatr, 1989 Mar.
44. Kenny M, et al;
Transcutaneous bilirubin monitoring of newborns .
Ann N Y Acad Sci, 1984.
45. Keshishjan E.S., et al;
Diagnostics and control of newborn hype rbilirubinemia with use
noninvasive trans cutaneous photometric analyzer "Bilitest" of a
type AHP-02.
The methodical r e commendat ion s. Russia mini s try of public health.
1992 Feb.
46. Keshishjan E.S., et al;
Transcutaneous bilirubinometry method at newborn jaundice diagnost ics and c o ntrol.
The Russian bulletin perinatology and pediatrics, V38, 5, 1993
Jaund ice Meter (JM-103) User Manual (usr0 70rb) Page B - 5
Page 66

47. Keshishjan E.S., et al;
Use of the method of transcutaneous bilirubinometry in hyperbilirubinemia in the newbo rn.
Clinical laboratory diagnostics. 5, 1994 Moscow, Medicine
48. Kivlahan C, et al;
The natural history of neonat al jaun dice.
Pediatrics, 1984 Sep.
49. Knudsen A.
Prediction of the deve lopm en t of neonatal jaundice by increa s ed
umbilical cord blood bil irubin.
Acta Pediatr Scand 1989;78:217-21.
50. Knudsen A, et al;
Skin colour and bilirubin.
Arch Dis Child 1989;64:605-9.
51. Knudsen A, et al;
Maternal bili rubin , c ord bilirubin, and placenta function at delivery
and the development of jaundice in mature newborns.
Acta Obstet Gynecol Scand 1989:68:719-24.
52. Knudsen A.
The cephalocaudal pr ogre s si on of ja undic e in newborns in relation
to the transfer of bilirubin from plasma to skin.
Early Human Development 1990;22:23-28.
53. Knudsen A.
Measurement of the yellow colour of the skin as a test of hyperbilirubinemia in mature ne wborns .
Acta Pediatr Scand 1990;79:1175-81.
54. Knudsen A.
Maternal smokin g and the serum bilirubin concentrati on in the first
three days of life.
Eur J Obstet Gynecol Reprod Bid 1991; 40:123-2 7.
55. Knudsen A.
The influence of the reserv e album in con ce ntra tion and pH on the
cephalocaudal progression of jaundice in newborns.
Early Human Development 1991;25:37-41.
56. Knudsen A.
The influence of the basal yellow colour of the skin at birth on l at er
jaundice meter readings in mature newborn infants.
Acta Pediatr Scand 1992;81:494-97.
Page B - 6 Jaundice Meter (JM-103) User Manual (usr070rb)
Page 67

57. Knudsen A.
Prediction of later hyperbilirubinemi a by measure ment of s kin
colour at first postnata l day and cor d bili rubin.
Dan Med Bull 1992;39:1 93-6.
58. Knudsen A
Predicting the need for phototherapy in healthy mature neonates
using transcutan eous bilirubinometry on th e first postnatal day.
Biol Neonate, 1995.
59. Knudsen A, et al;
Transcutaneous evaluation of icterus in healthy matu re newb orn
infants. A substitution for blood tests.
Ugeskr Laeger, 1995 Mar 13.
60. Knudsen A, et al;
Transcutaneous bilirubinometry in neonatal intensive care units.
Arc h Dis Child Fetal Neonat al Ed, 1996 Jul.
61. Kuma r A
Micro-invasive management of neonatal bilirubinemia.
Indian Pediatr, 1992 Sep.
62. Kuma r A , et al;
Transcut aneous bilirubinometry in the management of bilirubinemia in te rm neonates.
Indian J Med Res, 1994 May.
63. Laeeq A, et al;
Transcutaneous bilirubinometry: clinical applic at ion.
JPMA J Pak Med Assoc, 1993 Feb.
64. Leb ru n F, et al;
Clinical evaluation of transcutaneous bilirubinometry.
Ann Pediatr (Pari s), 1982 Sep.
65. Lin YJ, et al;
The clinical applica ti on of tra ns cutaneous bilirubinom etry in fullterm Chinese infants.
Acta Paediatr Sin, 1993 Mar-Apr.
66. Linder N, et al;
Noninvasive det ermination of neonatal hyperbilirubinemia: s ta ndardization for variation in skin color.
Am J Perinatol, 1994 May.
Jaund ice Meter (JM-103) User Manual (usr0 70rb) Page B - 7
Page 68

67. Loong EP, et al;
Changes in neonatal t ranscutaneous bilirubinometer index follow ing intravenous flu id and oxytoc in in fus ion during labour.
Asia Oceania J Obstet Gynaecol, 1988 Dec.
68. Maisels MJ, et al;
Transcutaneous bilirubin measurements in full-term infants.
Pediatrics, 1982 Sep.
69. Maisels MJ, et al;
Tra n scutaneous bilirubin measurements: variation in me ter
response.
Pediatrics, 1983 Mar.
70. Maisels MJ, et al;
Transcutaneous bilirubinometry decreases the need for serum
bilirubin measurements and saves money
Pediatrics 99 (4): 599-601, Apr 1997
71. Moscicka A, et al;
Usefulness of transc uta neous measurements of bili rubin in infants
with jaundice.
Ginekol Pol, 1994 Jun.
72. Myara A, et al;
Early changes in cutane ous bili rubin and serum bilirubin isom ers
during intensive phototherapy of jaundiced neonates with blue and
green light
Biol Neonate 71 (2): 75-82, 1997
73. Onks D, et al;
Effect of melanin, oxyhemoglobin and bili rubin on transcutaneous
bilirubinometry.
Acta Paediatr, 1993 Ja n.
74. Pallas Alonso C, et al;
Transcutaneous bilirubin measurement in neonates.
An Esp Pediatr, 1993 Jan.
75. Palmer DC, et al;
Transcutaneous bilirubinometry: use in Australia.
Aust Paediatr J, 1982 Dec.
76. Palmer DC; et al;
Jaundice: A 10 year review of 41,000 live born infa nts.
Australian Pediatrri c Journal, 1983, 19(2),86-8 9
Page B - 8 Jaundice Meter (JM-103) User Manual (usr070rb)
Page 69

77. Peng DJ, et al;
A study of the effect of pathologic factors on the TcBM readings.
Hua Hsi I Ko Ta Hsueh Hsueh Pao, 1989 Mar.
78. Romo - P inales J, et al;
Preliminary s tudy in Mexico on the use of the transcutaneous bilirubinometer.
Bol Med Hosp Infant Mex, 1984 Oct.
79. Ruchala PL, et al;
Validating assessme nt of neonatal jaundic e with transcutaneous
bilirubin measure ment.
Neonatal Ne tw, 1996 Jun.
80. Salmeron Escobar FJ
Differential diagnosis of jaundi ce .
Rev Esp Enferm Dig, 1991 Jan.
81. Schubiger G, et al;
Transcut aneous determination of bilirubin in the neonatal department: an analysis of its uses.
Helv Paediatr Acta, 1986 Aug.
82. Schumacher RE, et al;
Transcutaneous bilirubinometry: a comparison of old and new
methods.
Pediatrics, 1985 Jul.
83. Schumacher RE
Noninvasive measurements of bilirubin in the newborn.
Clin Perinatol, 1990 Jun.
84. Serrao PA, et al;
Significance of ant i-A an d anti -B isohemagglutinins in cord blood
of ABO incompatible newborn in fant s : correlation with hyperbilirubinemia
J Perinatol, 1989 Jun.
85. Sharma JN, et al;
Transcutaneous bilirubinometry in newborns.
Indian Pediatr, 1988 Aug.
86. S heridan-Perei r a M, et al;
Transcutaneous bilirubinometry: an evaluation.
Arch Dis Child, 1982 Sep.
Jaund ice Meter (JM-103) User Manual (usr0 70rb) Page B - 9
Page 70

87. Smith DW, et al;
Use of noninvasive tests to pre dic t significant jaundice in full-term
infants: preliminary studies.
Pediatrics, 1985 Feb.
88. Str an ge M, et al ;
Neonatal transcutaneous bilirubinometry.
Clin erinat ol, 1985 Feb.
89. Suckling RJ, et al;
Transcutaneous bilirubinometry as a screening tool for neonat al
jaundice.
Scott Med J, 1995 Feb.
90. Taha SA, et al;
Transcutaneous biliru bin mea su r ement in evaluating neonat al jaun dice among Saudi newborns.
Ann Trop Paediatr, 1984 Dec .
91. Tan KL
Tra n scutan eo us bilirubinome tr y in Chinese and Malay neonate s.
Ann Acad Med Singapore, 1985 Oct.
92. Tan KL, et al;
Transcutaneous bilirubinometry in preterm very low birthwe ight
infants.
Acta Paediatr Scand, 1988 Nov.
93. Tan KL, et al;
Efficacy of phototherapy for hyperbilirubinaemia in infants with
the respiratory distre ss syndrome
J Paediatr Child Health 31 (2): 127-129, Apr 1995
94. Tan KL, et al;
Transcutaneous bilirubinometry in Chinese, Malay and Indian
infants
Acta Paediatr 85 (8): 986-990, Aug 1996
95. Tsai LT, et al;
Clinical evaluat ion of tra ns cu ta neous jaundice meter in full-te r m
newborns.
Acta Paediatr Sin, 1988 Nov-Dec.
96. Tudehope DI, et al;
Non-invasive method of measuring bilirubin leve ls in ne wb orn
infants.
Med J Aust, 1982 Feb 20.
Page B - 10 Jaundice Meter (JM-103) User Manual (usr070rb)
Page 71

97. Tude hope DI, et al;
Multiple site readings from a transcutaneous bilirubinometer.
Aust Paediatr J, 1982 Jun.
98. Uchida K, et al;
Fundamental studi es on trans cutan eous bilir ubinometry in newborn
infants using an organ scanning spectrophotometer.
Nippon Sanka Fujinka Gakkai Zasshi, 1988 Feb.
99. Utchajkin V.F., et al;
Clinical importance of noninvasive bilirubinemia monitoring by
children viral hepat iti s .
2-nd Symposi ums "No ninvas iveMet hods of Dia gnosti cs" . The t hesis
of the reports. Moscow. 1995 Oct. 30 - Nov. 2.
100. Vaisman S, et al;
Clinical trial of a trans cut ane ous bilirubinometer.
Rev Chil Pediatr, 1983 Mar-Apr.
101. Veleminsky M
The significance of transcutaneous measurement of "bilirubi n" in
neonates.
Cesk Pediatr, 1992 Nov.
102. Vocel J, et al;
Transcut aneous bilirubinometry using the Minolta/Air-Shields in
neonates.
Cesk Pediatr, 1985 Nov.
103. Vocel J, et al;
Transcutaneous bilirubinometry using the Minolta/ Air-Shields
apparatus in neonates with a low birthweight.
Cesk Pediatr, 1987 Jan.
104. Vocel J, et al;
Transcutaneous bilirubinometry in neonates with hyperbilirubinemia treated with photot he rapy.
Cesk Pediatr, 1988 Dec.
105. Weiss WJ
A completely implante d left ventricular assist de vic e. Chronic in
vivo testing.
ASAIO J 39(3), M427-M432, 1993
Jaund ice Meter (JM-103) User Manual (usr0 70rb) Page B - 11
Page 72

106. Yamanouchi I, et al;
Transcutaneous bilirubinometry: prelimina ry s tudi es of noninvasive transcutaneous bilirubin meter in the Okayama N ational Hospital.
Pediatrics, 1980 Feb.
107. Yamauchi Y, et al;
Transcutaneous bilirubino me try. Effect of irradiation on the sk in
bilirubin index.
Biol Neonate, 1988.
108. Yamauchi Y, et al;
Transcuta ne o us bilirubi n ometry. Evaluatio n of accuracy and re li ability in a large popul at ion.
Acta Paediatr Scand, 1988 Nov.
109. Yamauchi Y, et al;
Transcutaneous bilirubinometry: bilirubin kine tics of the skin and
serum during and after phot othe rapy.
Biol Neonate, 1989.
110. Yamauchi Y, et al;
Transcut aneous bilirubinometry: serum bilirubin measurement
using transcutane ous bil irubinometer (TcB). A prelim ina ry study.
Biol Neonate, 1989.
111. Yamauchi Y, et al;
Transcutaneous bilirubino me try in norma l Japa nese infants.
Acta Paediatr Jpn, 1989 Feb.
112. Yamauchi Y, et al;
Tra n scutan eo us bilirubinometr y. Interin strumental varia b ility of
TcB instruments.
Acta Paediatr Scand, 1989 Nov.
113. Yamauchi Y, et al;
Clinical ap plicatio n o f transc utane ous bili rubin m easure ment. E arly
prediction of hyperbilirubinemia.
Acta Paediatr Scand, 1990 Apr.
114. Yamauchi Y, et al;
Transcutaneous bilirubinometry: eff ect of postnatal ag e.
Acta Paediatr Jpn, 1991 Oct.
115. Yamauchi Y, et al;
Transcutaneous bilirubinometry: variability of TcB measurements
on the forehead with crying.
Acta Paediatr Jpn, 1991 Oct.
Page B - 12 Jaundice Meter (JM-103) User Manual (usr070rb)
Page 73

1 16. Yamauchi Y, et al;
Factors affecting transcutaneous bilirubin measurement : ef fect of
daylight.
Acta Paediatr Jpn, 1991 Oct.
1 17. Yip WC, et al;
Transcutaneous bilirubin ome try [letter]
Acta Paediatr Scand, 1983 Mar.
Jaund ice Meter (JM-103) User Manual (usr0 70rb) Page B - 13
Page 74

NOTES:
Page B - 14 Jaundice Meter (JM-103) User Manual (usr070rb)
Page 75

Jaundi ce Me t er ( Mo d el JM - 1 03 ) User M an ual (usr07 0)
Page 76

Manufactured by:
Konica Minolta Sensing, Inc.
3-91, Daisennishimachi
Sakai, Osaka 590-8551 Japan
Authorized Representative:
Hill-Rom Europe
Director Quality and Regulatory Affairs
Les Rabelais - Paris Nord II
22 Avenue des Nations
BP 50436 -VILLE PINTE
95 944 - Rois sy CDG CEDEX
France
tel. +01 49 89 40 20
fax +01 49 89 40 40
Distributed by:
330 Jacksonvill e Road, Hatboro, PA 19040 USA
USA: (800) 523-5756
International: Contact your distributor.
Website: www.hill-rom.com
Part number Revisions Date
usr070 rb October, 2003
Hill-Rom reserves the right to make changes wit hout notice in design,
specifications, and models. The only warranty Hill-Rom makes is the express
written warrant y extended on the sale or r ental of its products.
The informati on in th is manual is confidential and may not be disclosed to
third partie s without the prior writt en consent of Hill-Rom.
© 2003 by Hill-Rom Services, Inc. ALL RIGHTS RESERVED.
 Loading...
Loading...