Alaris Asena PK User manual

Asena® PK Syringe Pump
Directions for Use
ENGLISH
1000PB01489 Issue 4
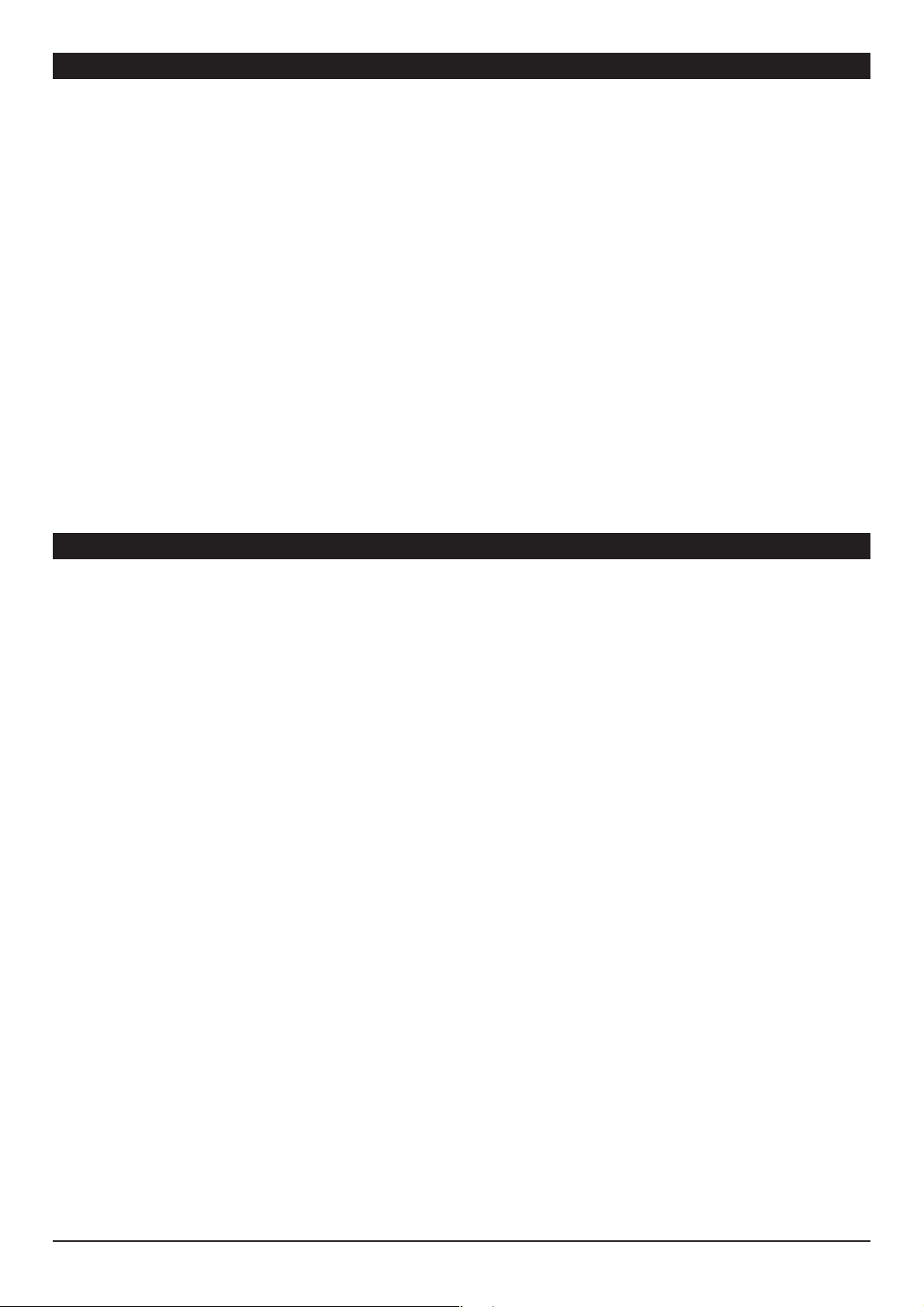
Contents
Page
Introduction 2
TCI Overview 3
Creating a Data Set 5
Features of the Asena® PK Syringe Pump 6
Controls and Indicators 7
Operating Precautions 8
Getting Started 9
Basic Features 12
Operations During Use 13
Alarms and Warnings 15
Configured Options 16
Specifications 20
Compatible Accessories 21
Maintenance 23
Occlusion Pressure Limits 24
IrDA/RS232/Nursecall Specification 25
Trumpet & Start-up Curves 26
Profiles from TCI Mode 27
Service Contacts 30
Warranty 31
Patent, Copyright and Trademarks 32
Introduction
The Asena® PK Syringe Pump provides the user with an infusion tool for the administration of drugs for anaesthesia. The embedded software within the
pump is loaded with three compartment pharmacokinetic predictive models and has 4 modes of operation:
1) Continuous infusion (ml/h)
2) Total Intravenous Anaesthesia (TIVA) mode.
In this mode the user is able to select the infusion rate and administer bolus doses as required. The pharmacokinetic model is used to estimate the
plasma and effect site concentration
3) Plasma target-controlled infusion (TCI).
In this mode the user selects the desired (target) plasma drug concentration, and the pharmacokinetic model is used to calculate the infusion rates
required to achieve that concentration. A graphic display shows the trajectory of the estimated plasma and effect site drug concentration over time.
4) Effect Site target-controlled infusion (TCI).
In this mode the user sets the desired effect site target concentration and the pharmacodynamic model is used to calculate the infusion rates required
to achieve that concentration. A graphic display shows the trajectory of the estimated effect site and plasma concentration over time.
The Asena® PK Syringe Pump has a user friendly interface that displays the infusion rate, the total drug dose delivered, and the estimated plasma and effectsite concentrations to enable the user to follow the drug prescription information from the relevant country.
The Asena® PK Syringe Pump is compatible with a wide range of standard single use, 3 piece Luer-lock syringes. It accepts syringe sizes from 5ml to 50ml.
Specifications are available in the relevant section.
Use of the Asena® PK Syringe Pump DOES NOT limit the responsibility of the anaesthetist for drugs administration. It is important that users operating
the Asena® PK Syringe Pump are fully aware of the available literature for any model used in association with a drug and that they refer to the prescribed
information for rate and dosing limits. Pharmacokinetic and Pharmacodynamic Interactions among anaesthetic drugs are known, but are not taken into
account in the calculation of the plasma and effect site concentrations.
The user should be appropriately trained in the use of the pump and should follow the recommendations of this Direction For Use (DFU).
In particular, the user must be aware that starting the pump in a TCI mode will result in the automatic infusion of a pre-calculated bolus dose followed by
an infusion to achieve the selected target concentration. The initial parameter calculations are displayed on screen prior to starting the infusion. It is thus
essential that the user verifies that the patient characteristics and the selected infusion rate or target concentration conform with the drug prescribing
information of the relevant country.
ALARIS Medical Systems has verified the accuracy of the mathematical model implementation as well as pump delivery accuracy - (specification and
accuracy of pump - delivery are available in Profiles from TCI Mode, pages 27-29).
Different drugs are associated with dedicated models – each model consists of a set of standard pharmacokinetic parameters which can be selected and
used by the embedded 3 compartment model used in the Asena® PK Syringe Pump (where use of that drug in TCI mode is authorised);
Diprivan from ASTRA-ZENECA is the only recommended Propofol formulation to be used in TCI mode as per prescribing information. This pump includes the
“Marsh” model for the calculation of the Diprivan infusion rates, and plasma and effect-site concentrations.
When Remifentanil and Sufentanil are used in TCI mode, – the “Minto” and “Gepts” models respectively – are used to calculate the required infusion rates.
1000PB01489 Iss. 4 2/32
TCI Overview
The dose-response relationship can be divided into three parts: the relationship betwe en administered dose and plasma concentration (the pharmacokinetic
phase), the relationship between ef fect organ concentration and clinical effect (the pharmacodynamic phase) and the coupling between pharmacokinetics
and dynamics. The ultimate goal when administering a particular dose of a drug is to obtain the desired clinical effect, for which a specific therapeutic
concentration of the drug at the site of action (the receptor) is necessary.
Fig. 1: Schematic representation of the pharmacokinetic and dynamic p rocesses de termining the relati onship be tween ad ministere d dose and
resulting e ffect i ntensity of a drug. Pha rmacokin etic factors such as dis tributio n, metabo lism, and /or excreti on determ ine the relationshi p
betwee n drug dose and drug- concentration in the plasma and bi o-pha se (effe ct-site). I n the bio-phase the drug intera cts with th e receptor
resulting i n the pharmacolog ical effect.
Until recently, when intravenous anaesthetic agents were used for induction or maintenance of anaesthesia, they were administered either manually
(by hand) or by simple infusion pumps (the anaesthetist calculated the infusion according to the body weight of the patient). Inline measurement of
concentrations is not possible, and the polyexponential equations required to predict the concentrations requires vast computer processing power. Based
on the pioneering work of Kruger-Thiemer2 and Schwilden et al.3, the TCI concept was developed during the 1980’s and early 1990’s, as advances in computer
technology made inline predictions of drug concentrations feasible.
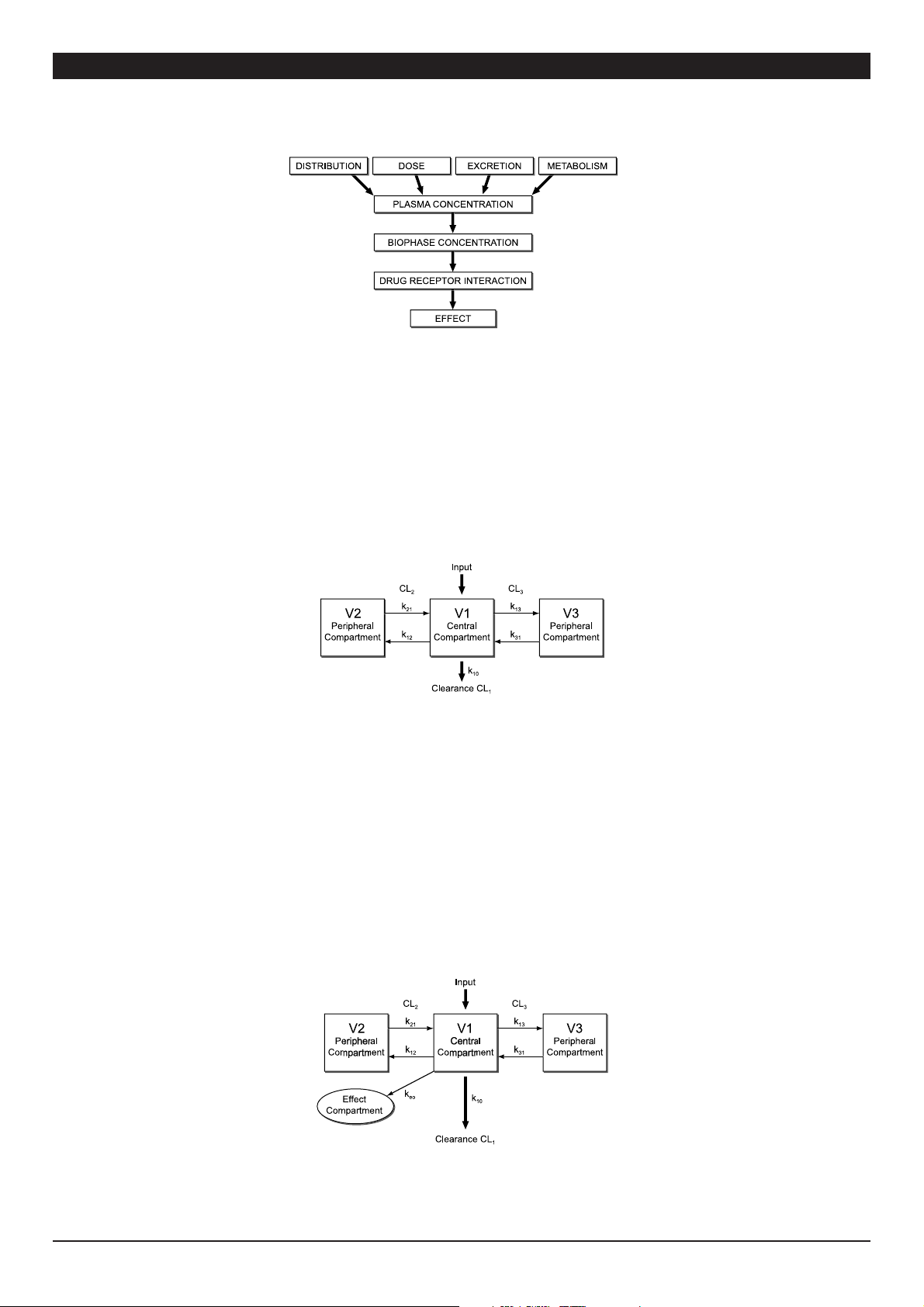
The pharmacokinetic behaviour of most anaesthetic drugs can be described mathematically with a 3-compartment model: usually a central compartment
(V1), a vessel-rich compartment (V2) and a vessel-poor compartment (V3) are described. Transfer of drug between different compartments (distribution) is
described by rate constants (k12, k21, k31 and k13) or clearances. Drug metabolism is described by the rate constant k10 (Fig. 2). The aim of TCI techniques is to use
pharmacokinetic modelling to calculate the infusion rates required to achieve a desired plasma concentration. Thus, instead of specifying an infusion rate,
the user specifies a “target” concentration, based on clinical judgement. When a concentration in the plasma compartment is targeted, this is called “openloop plasma targeted TCI”. When a certain concentration at the effect compartment is targeted, then this is called “open-loop effect-site targeted TCI”.
1
Fig. 2: Schem atic representatio n of the three compar tment model used fo r target-controll ed infusions.
For anaesthetic agents the effect-site (or bio-phase) is not the plasma4 but the brain, where concentrations cannot be directly measured. Until the early
1990’s it was considered that blood-brain equilibration was virtually instantaneous. Early TCI systems were thus all plasma-targeted. For many drugs the
relationship between plasma concentration and clinical effect was described, usually in terms of the Cp50 or Cp95 (the concentrations required to elicit a
specified clinical effect in 50 or 95% of patients respectively). For an example see Ausems et al.5
During the 1990’s it was increasingly appreciated that af ter a change in plasma concentration there is a temporal delay in equilibration between the plasma
and effect-site concentrations. The clinical effect changes in parallel with the effect-site concentration, and so for most drugs the rate of drug transfer into
and from the site of action can be characterized by the time-course of drug effect
6,7
. This means that the effect can be transferred to concentrations, thereby
resulting in a quantitative approach. The concentration at the site of action is called “the effect-site concentration” and the corresponding compar tment8
(see Fig. 3) is called “the effect-site compartment”. Because the actual amount of drug entering the brain is very small, the effect-site compartment can be
regarded as having no volume, the rate constant k1e can be ignored and the rate constant keo can be used to describe the rate of equilibration between the
plasma and effect-site compartments.
Knowledge of the k
for various agents has made targeting of the effect-site possible. With effect-site targeting the TCI system first calculates the necessary
eo
plasma concentration profile required to achieve the effect-site target as rapidly as possible, and then calculates the infusion rates required to achieve that
plasma concentration profile (Fig 3). Effect Site vs Plasma Concentration will generate a larger induction dose followed by a pause in the infusion to allow
plasma to equilibrate with effect site concentration.
Fig. 3: Schematic representation of the concentration-effect relationship.
1000PB01489 Iss. 4 3/32
TCI Overview (continued)
TCI infusion pumps can provide optimal control of anaesthesia when the three elements mentioned above have been accurately modelled and described.
Firstly, the model that controls the pump has to work accurately (The models used in the Asena® PK Syringe Pump are well-validated and accepted).
Secondly, the pharmacokinetic parameter set of a particular drug used by the computer model should match the pharmacokinetics of the patient (it should
be remembered that the models described in the literature are based on “population” data, and apply to an “average” patient. They do not take account of
the inter-patient pharmacokinetic variability). Thirdly, the pharmacodynamics of the administered drug should be well understood to enable the user to
select the plasma or ef fect-site concentration needed for the required effect (with most anaesthetic agents there is broad inter-patient pharmacodynamic
variability, and so the user needs to match knowledge of the general population pharmacodynamic data with careful observation of the individual patient
to ascertain that individual’s sensitivity to the drug, to enable titration to effect if necessary).
Note: Specific model parameters are available in the “TCI Overview” section or directly on the pump via the information key when selecting drugs.
Users should refer to the drug- prescribing information to verify that TCI mode is authorised in their respective countries.
References :
1. Danhof M: D oes variability explain (all) variability in drug ef fect s ?, Topics in ph armaceutical science. Ed ited by Bre imer DD, Crommeli n DJA, Midha K K. Noordwijk , Amsterdam Med . Press BV, 1989, pp
573-586
2. Kruge r-Theime r E: Continuous intravenous infusio n and multicompar tment accumulat ion. Eur J Pharmacol 1968; 4: 317-324
3. Schwilden H: A ge neral method for c alculating the do sage scheme in lin ear pharmacoki netics. Eur J Clin Pha rmacol 1981; 20: 379-86
4. Shafer S L: Towards optimal intravenous dosing strategi es. Seminars in An esthesia 1993; 12: 222-234
5. Ausems ME , Hug CC, Jr., Stanski DR , Burm AG: Plasma concentrati ons of alfentanil r equired to supplement nit rous oxide anesth esia for general surgery. Anesthesiology 1986; 65: 362-73
6. Schnid er TW, Minto CF, Stanski DR: The ef fect compartment conce pt in pharmacody namic modellin g. Anaesthetic Ph armacology Rev iew 1994; 2: 204-213
7. Shafer SL: Principles of pharmacokinetics and pharmacodynamics., Principles and practice of anesthesiology. 2nd Edition. Edited by Longnecker D E, Tinke r JH, Mor gan GE. New York, Mosby-Year Book ,
1998, pp 1159- 1210
8. Shafer SL, Greg g KM: Al gorith ms to rapidly achie ve and maintain stable dru g concentrations at the site of drug effect wi th a computer-controlled i nfusio n pump. J Pharmacok inet Bio pharm 1992; 20 :
147- 69
TCI Precautions
When first starting the infusion the pharmacokinetic / pharmacodynamic models within the Asena® PK Syringe Pump are reset to zero.
Therefore, for any reason, if the pump is switched off during the surgical procedure all current pharmacokinetic / pharmacodynamic model
information will be lost. Under such circumstances switching the pump off and on and restarting the infusion whilst the patient contains a
significant residual drug dose could result in an over-infusion and, therefore, the pump should not be restarted in TCI mode.
Pharmacokinetic models in Asena® PK Syringe Pump and their parameters
Drug: Diprivan Model: Marsh (weight adjusted)
Age Limit: 16 years upwards
Unit of Plasma Concentration: µg/ml
Max. Plasma Concentration: 15 µg/ml
Vc = 0.228 x mass (litres x kg-1)
k10 = 0.119 min
k12 = 0.112 min
k13 = 0.0419 min
k21 = 0.055 min
k31 = 0.0033 min
Keo = 0.26 min
Reference from the literature: Marsh et al.: Brit J Anaesth 1991, 67, 41-48
Drug : Remifentanil Model: Minto
Age Limit: 12 years upwards
Unit of Plasma Concentration: ng/ml
Max. Plasma concentration: 20 ng/ml
Vc = 5.1 - 0.0201 x (age-40) + 0.072 x (lbm-55)
V2 = 9.82 - 0.0811 x (age-40) + 0.108 x (lbm-55)
V3 = 5.42
CL1 = 2.6 - 0.0162 x (age - 40) + 0.0191 x (lbm - 55)
CL
= 2.05 - 0.0301 x (age - 40)
2
= 0.076 - 0.00113 x (age - 40)
CL
3
k10 = Cl1 / Vc
k12 = Cl2 / Vc
k13 = Cl3 / Vc
k
= Cl2 / V2
21
= Cl3 / V3
k
31
= 0.595 - 0.007 x (age - 40)
k
eo
Reference from the literature : Minto et al.: Anesthesiology 1997, 86, 10 - 33
Drug : Sufentanil Model: Gepts (not weight adjusted)
Age Limit: 12 years upwards
Unit of Plasma Concentration: ng/ml
Max. Plasma concentration: 2 ng/ml
Vc = 14.3 l
= 0.0645 min
k
10
k12 = 0.1086 min
k13 = 0.0229 min
k21 = 0.0245 min
k31 = 0.0013 min
Reference from the literature : Gepts et al.: Anesthesiology 1995, 83, 1194-1204
Additional : k
-1
-1
-1
-1
-1
-1
-1
-1
-1
-1
-1
calculated with time to peak effect 5.6 min (keo = 0.17559 min-1) (reference: Shafer et al Anesthesiology. 1991 Jan;74(1):53-63)
eo
1000PB01489 Iss. 4 4/32

Creating a Data Set
To fully utilise the Asena® PK Syringe Pump a Data Set will need to be developed, reviewed, approved, released, uploaded and verified
according to the following process. Refer to the Asena® PK Editor Software Directions for Use (1000CH00016) for further details and
operating precautions.
1. Create Master Lists (Using Asena® PK Editor Software)
Master Drugs* A list of drug names and standard concentrations. These may be for TIVA use or may have an associated
PK/PD model for TCI use.
Asena® PK Syringe Library Configure syringes enabled for use.
2. Create Profile (Using Asena® PK Editor Software)
Drug Library* Drugs and concentrations for this profile with defaults, minimum & maximum limits and targets and
occlusion level.
Configuration** Instrument configuration settings and general options.
3. Review, Approve and Release (Using Asena® PK Editor Software)
Review and Approve Entire Data Set Report to be printed, reviewed and signed as proof of approval by an authorised person
according to Hospital protocol. Signed printout to be kept safe for use during verification procedure.
Release Data Set status to be promoted to Released (password is required).
4. Upload Data Set to Asena® PK Syringe Pump (Using Asena® PK Editor Transfer Tool)
Data Set transfers should only be performed by qualified technical personnel.
5. Verify Data Set Upload
First or Individual Instrument Verification
On completion of upload record CRC number shown on the Asena® PK Syringe Pump.
Download the Data Set from the pump using the Asena® PK Verification Tool.
Compare Data Set downloaded with the approved signed Data Set printout. Reviewer should sign the
printout and also record the CRC number on the printout as record.
Subsequent Instruments Verification
On subsequent uploads of the Data Set compare CRC number on the instrument with CRC number
recorded on First Instrument Verification.
* Note: Drug parameters have to be in accordance to local regulation and prescribed information.
** See important note in Configured Options section.
1000PB01489 Iss. 4 5/32
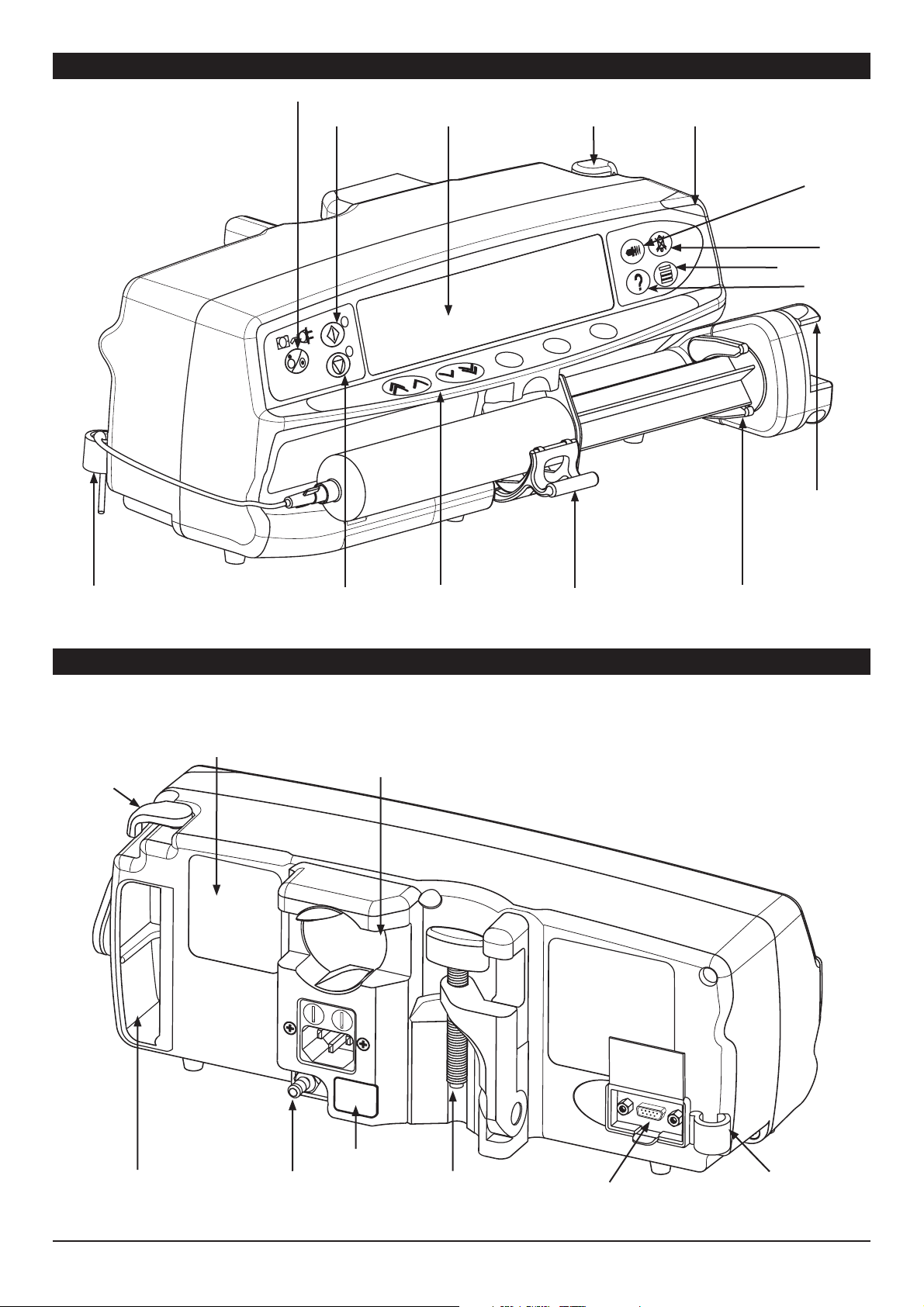
Features of the Asena® PK Syringe Pump - Front View
ON/OFF
RUN
Display
Release lever for
MDI
High visibility
Alarm Indicator
PURGE/
BOLUS
MUTE
PRESSURE
OPTION
Finger
Grips
Extension
set hook
Release
lever for
MDI
Features of the Asena® PK Syringe Pump - Rear View
Rating Plate (see
Symbol Definitions for
an explanation of the
symbols used)
HOLD
Medical Device
Interface (MDI)
- rotating cam to
lock on to horizontal
rectangular bars.
Shelf for
chevron keys
and softkeys
Syringe Clamp
Positive Plunger
Grippers
Carrying
Handle
IR Comms port
Functional
Earth
Folded Pole
Clamp
1000PB01489 Iss. 4 6/32
RS232
Connector
(optional)
Extension set
hook
Controls and Indicators
!
"
ON/OFF - Press once to switch the pump ON.
Press and hold down for 3 seconds to switch the
pump OFF.
RUN button - Press to start the infusion. The
green LED will flash during infusion.
HOLD button - Press to put the infusion on hold.
The amber LED will be lit while on hold.
MUTE - Press to silence alarm for 2 minutes. Press
and hold until 3 beeps are heard for 15 minutes
silence.
PURGE/BOLUS - Press to access PURGE or BOLUS
soft keys. Press and hold down soft key to operate.
PURGE the extension set during set up. Pump on
hold, extension set not connected to patient,
Volume Infused (VI) not added. BOLUS delivered
at an accelerated rate. Pump infusing, extension
set connected to patient, VI added.
Indicators
BATTERY - When illuminated the pump is running
on the internal battery. When flashing the battery
power is low with less than 30 minutes of use
remaining.
AC POWER - When illuminated the pump is
connected to an AC power supply and the battery
is being charged.
OPTION button - Press to access optional features
(see page 12).
PRESSURE - Use this button to display the
pumping pressure trend display and alarm level.
BLANK SOFTKEYS - Use in conjunction with the
prompts shown on the display.
Symbol Definitions
Attention (Consult accompanying
documents)
.
Functional Earth
/
RS232/Nurse Call Connector (Optional)
CHEVRONS - Double or single for
faster/slower increase or decrease of
values shown on display.
#
TIME REMAINING DISPLAY - Indicates time
before syringe will require replacing.
BATTERY ICON - Indicates battery charge
level to highlight when the battery will require
recharging.
SOFT ALERT - Indicates the pump is running at a
rate above (pointing up) or below (pointing down)
a Soft Alert. (Number of arrows vary depending
on drug name length)
LIMIT WARNING - Indicates the setting entered is
under or exceeds a Soft Alert or setting entered is
not permitted as it exceeds a Hard Limit.
DOWN MODE - Infusion status indicating
that the target concentration is below current
concentration.
0
)
*
+
,
Type CF Equipment (Degree of protection
against electrical shock)
Protected against vertically falling drops of
water
Alternating Current
Pump complies with the requirements of the
EC Directive 93/42/EEC. Registered with the
CE Mark.
Date of Manufacture
Important Information
Induction Phase Dose (Displayed on protocol
confirmation screen)
Duration of Induction Phase (Displayed on
protocol confirmation screen)
1000PB01489 Iss. 4 7/32
Maintenance Phase Dose (Displayed on
protocol confirmation screen)
Duration of Hands Free Bolus (Displayed on
bolus set-up screen)
Operating Precautions
$
%
&
This ALARIS® syringe pump has been calibrated for
use with single-use disposable syringes. To ensure
correct and accurate operation, only use 3 piece
luer-lock versions of the syringe make specified on
the pump or described in this manual. Use of nonspecified syringes or administration sets may impair
the operation of the pump and the accuracy of the
infusion.
Uncontrolled flow or syphoning may result if the
syringe is located incorrectly in the pump, or if it is
removed from the pump before the extension set
is properly isolated from the patient. Isolation may
include closing a tap in the patient line or activating
a flow stop clamp.
Secure the extension set to the pump using the
extension set hook at the rear of the pump. This
provides protection against accidental dislodging of
the syringe from the pump.
When combining several apparatus and/or
instruments with administration sets and other
tubing, for example via a 3 way tap, the performance
of the pump may be impacted and should be
monitored closely.
The pump must be mounted within 1.0m above
or below the patient’s heart. The most accurate
pressure monitoring in the extension set is achieved
when the pump is positioned close to the patients
heart level. Do not mount the pump in a vertical
position with the syringe pointing upwards as this
could lead to an infusion of air which may be in
the syringe. To protect against the introduction of
air the user should regularly monitor the progress
of the infusion, syringe, extension set and patient
connections and follow the priming procedure
specified herein.
This is a positive pressure pump designed to achieve
very accurate fluid administration by automatically
compensating for resistance encountered in the
infusion system.
The pumping pressure alarm system is not designed
to provide protection against, or detection of,
infiltration conditions which can occur at low
pressures.
Several alarm conditions detected by this pump will
stop the infusion and generate audible alarms and
lights. Users must perform regular checks to ensure
that the infusion is progressing correctly and no
alarms are operating.
This pump is protected against the effects of external
interference, including high energy radio frequency
emissions, magnetic fields and electrostatic discharge
(for example, as generated by electrosurgical and
cauterising equipment, large motors, portable
radios, cellular telephones etc.).
When using any infusion pump in conjunction with
other instruments requiring vascular access, extra
care is advised. Adverse delivery of medication or
fluids can be caused by the substantial variation in
pressures created within the local vascular system by
such instruments.
Typical examples of those instruments are used
during dialysis, bypass or cardiac assist applications.
In some circumstances the pump may be affected by
an electrostatic discharge through air at levels close to
or above 15kv; or by radio frequency radiation close
to or above 10v/m. If the pump is affected by this
external interference the pump will fail safe or reset
(after which a call back alarm will occur). Should false
alarm conditions be encountered, either remove the
source of the interference, or regulate the infusion by
another appropriate means.
This pump emits a certain level of electromagnetic
radiation which is within the levels specified by
IEC60601-2-24 and IEC6 0601-1-2:2002. If h owever
the pump interacts with other equipment, measures
should be taken to minimise the effects, for instance
by repositioning or relocation.
If this pump is dropped, subjected to excessive
moisture, fluid spillage, humidity or high temperature,
or otherwise suspected to have been damaged,
remove it from service for inspection by a qualified
service engineer. When transporting or storing the
pump, use original packaging where possible, and
adhere to temperature, humidity and pressure ranges
stated on page 16 and on the outer packaging.
An explosion hazard exists if the pump is used in the
presence of flammable anaesthetics. Exercise care
to locate the pump away from any such hazardous
sources. An electrical shock hazard exists if the units
casing is opened or removed. Refer all servicing to
qualified service personnel.
When connected to an external power source, a
three-wire (Live, Neutral, Earth) supply must be used.
If the integrity of the external protective conductor
in the installation or its arrangement is in doubt, the
equipment should be operated from the battery.
A comprehensive Technical Service Manual is available
for this pump. The part number is 1000SM00001.
All illustrations used in this manual show typical
settings and values which may be used in setting up
the functions of the pump. These settings and values
are for illustrative use only. The complete range of
settings and values are specified on page 16. Where
stated, a minimum infusion rate refers to a nominal
rate of 1.0ml/h, and an intermediate infusion rate
refers to a nominal rate of 5.0ml/h. The complete
range of infusion rates are shown on page 16.
T he em b e dd e d p um p so f t wa re i nc o rp o ra te s li mi t s a n d
pump configuration parameters. Qualified personnel
must ensure the appropriateness of the limits, the
compatibility of the drugs, and the performance of
each pump, as part of the overall infusion. Potential
hazards include drug interactions, and inappropriate
delivery rates and pressure alarms.
Do not open the RS232/Nurse Call protective covering
when not in use. Electrostatic discharge (ESD)
precautions are required when connecting RS232/
Nurse Call. Touching the pins of the connectors may
result in ESD protection failure. In order to prevent
any potential failure generated by ESD close to or
above 15kV, it is recommended that all actions must
be taken by appropriately trained personnel and the
pump should not be attached to the patient when
connecting RS232/Nurse Call.
1000PB01489 Iss. 4 8/32
Getting Started
Installation
Check that the pump is complete, undamaged and that the voltage
rating specified on the base plate is compatible with your AC power
supply. Items supplied are:
ALARIS® Asena® PK Syringe Pump
User Support CD
AC Power Cable (as requested)
Protective Packaging
Connect the pump to the AC power supply for 2½ hours to ensure
that the internal battery is fully charged prior to use.
Should the pump fail to perform correctly, replace in its original
protective packaging and contact a qualified service engineer for
investigation.
On initial start-up the pump will display the Select Language
screen.
Select the required language from the list displayed using the
keys.
Press the OK softkey to confirm your selection.
Loading a Syringe
Place the pump on a stable horizontal surface or secure as described
above.
Prepare, load and prime the single-use disposable syringe and
extension set using standard aseptic techniques.
Important: Only use a syringe of the type stated on the pump or
in this manual. Using an incorrect syringe could adversely affect
the accuracy of the infusion and the performance of the pump.
When initially loading the syringe, allow for the volume of fluid
contained in the extension set and retained in the syringe at the
end of infusion as this “dead-space” will not be infused.
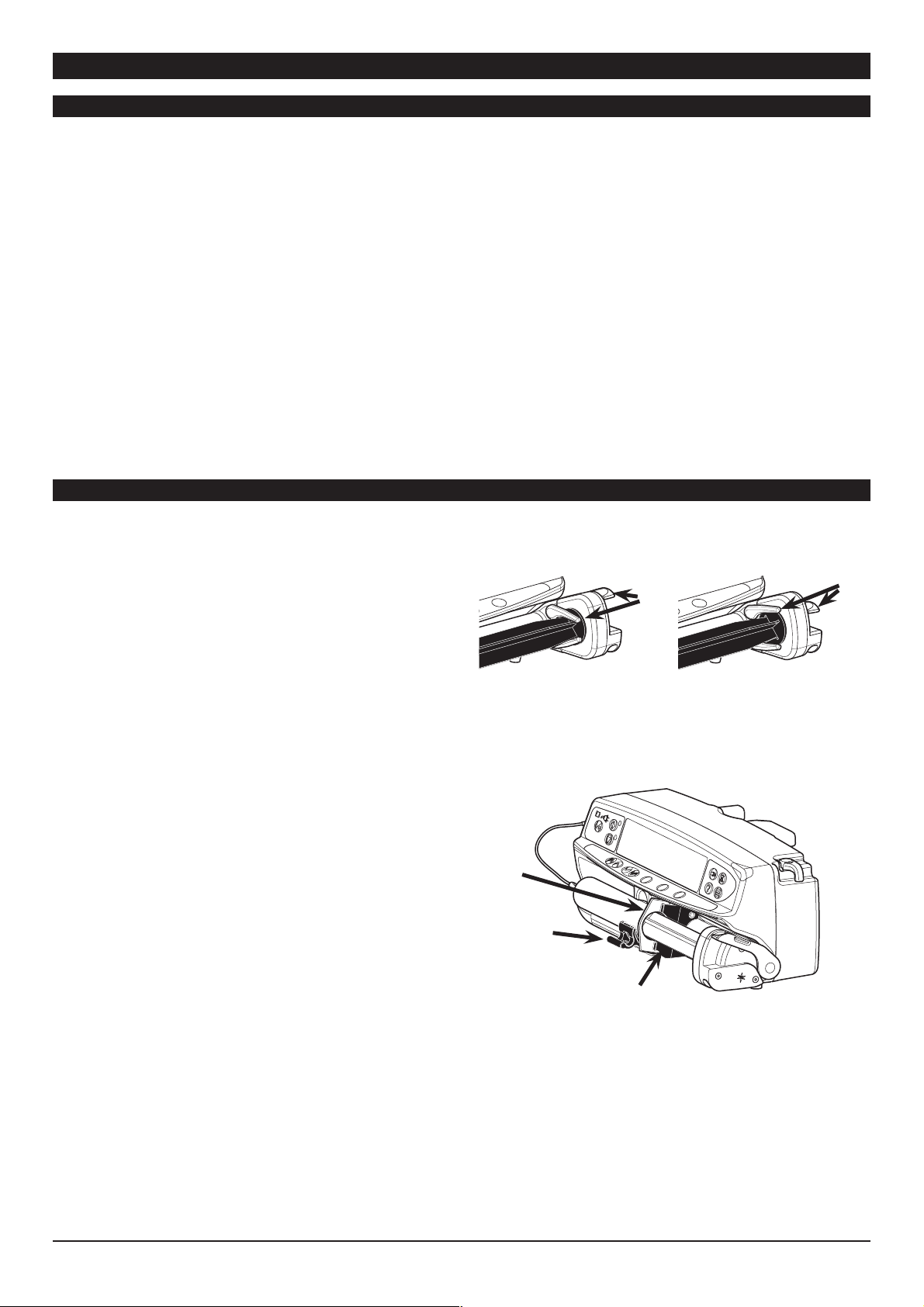
1. Squeeze the finger grips together on the plunger holder
and slide the mechanism to the right. Pull the syringe clamp
forward and down.
2. Insert the syringe ensuring that the finger flanges are located in
the slots on the syringe holder.
3. Lift the syringe clamp until it locks against the syringe barrel.
4. Squeeze the finger grips on the plunger holder and slide the
mechanism to the left until it reaches the plunger end.
5. Release the finger grips. Ensure that the plunger grippers are
securing the plunger in place and the finger grip returns to its
original position.
Important: Secure the extension set using the extension set
hook at the rear of the pump. This provides protection against
accidental dislodging of the syringe from the pump.
A pole clamp is fitted to the rear of the pump and will provide
secure fixing to standard vertical IV poles of a diameter of between
15 and 40 mm. It should be folded away when not in use.
There is a Medical Device Interface (MDI) at the rear of the pump
used for mounting the pump onto horizontal rectangular bars, for
instance the ALARIS® Asena® Docking Station. Holding the pump
horizontally push the pump firmly on to the bar. Ensure that the
pump clicks securely into position on the bar. To release, push the
release lever and pull the pump forward.
Important: Do not mount the pump with the AC power inlet or
the syringe pointing upwards. This could affect the electrical
safety in the event of a fluid spill or lead to the infusion of air
which may be in the syringe.
The pump will automatically operate from its internal battery
if the pump is switched on without being connected to the AC
power supply.
Important: Ensure that both plunger grippers are fully locked
onto the plunger flange and the upper finger grip has returned
to its original position.
Important : To ensure the syringe is loaded correctly, place the
barrel flange in the space between the syringe clamp and the
syringe flange clamp. This is correct if the syringe remains in
position before the syringe clamp is closed.
Barrel
Flange
Syringe
Clamp
1000PB01489 Iss. 4 9/32
Syringe Flange
Clamp
Getting Started (continued)
Starting the Pump
1. Connect the pump to an AC power supply using the AC power cable. Press the button.
• The pump will run a short self-test. Ensure that two beeps are activated during this test.
• Check the display test pattern and ensure that no coloured rows are missing.
• Finally check that the displayed time and date are correct.
Note: A warning - REPAIRING LOGS, may be displayed if event log information was not completely stored at the previous power
down. This is for information only, the pump will continue to power up as normal.
2. CONFIRM PROFILE? - Answering NO will display SELECT PROFILE screen, select profile and press the OK softkey. YES will display the
TCI MODE screen.
3. The TCI MODE selection is displayed - Answering YES selects the TCI Mode, NO will enter TIVA MODE.
The Asena® PK Syringe Pump allows the user to select a TCI or TIVA mode of operation. The user may, at any time, switch mode by stopping
the infusion and selecting the appropriate mode from the options menu. When in TIVA mode, if a drug with an associated model has
been selected, the current plasma and effect site concentration will be displayed. This will demonstrate to the user unfamiliar with TCI, the
Pharmacokinetics and Pharmacodynamics of the drug while still using TIVA mode.
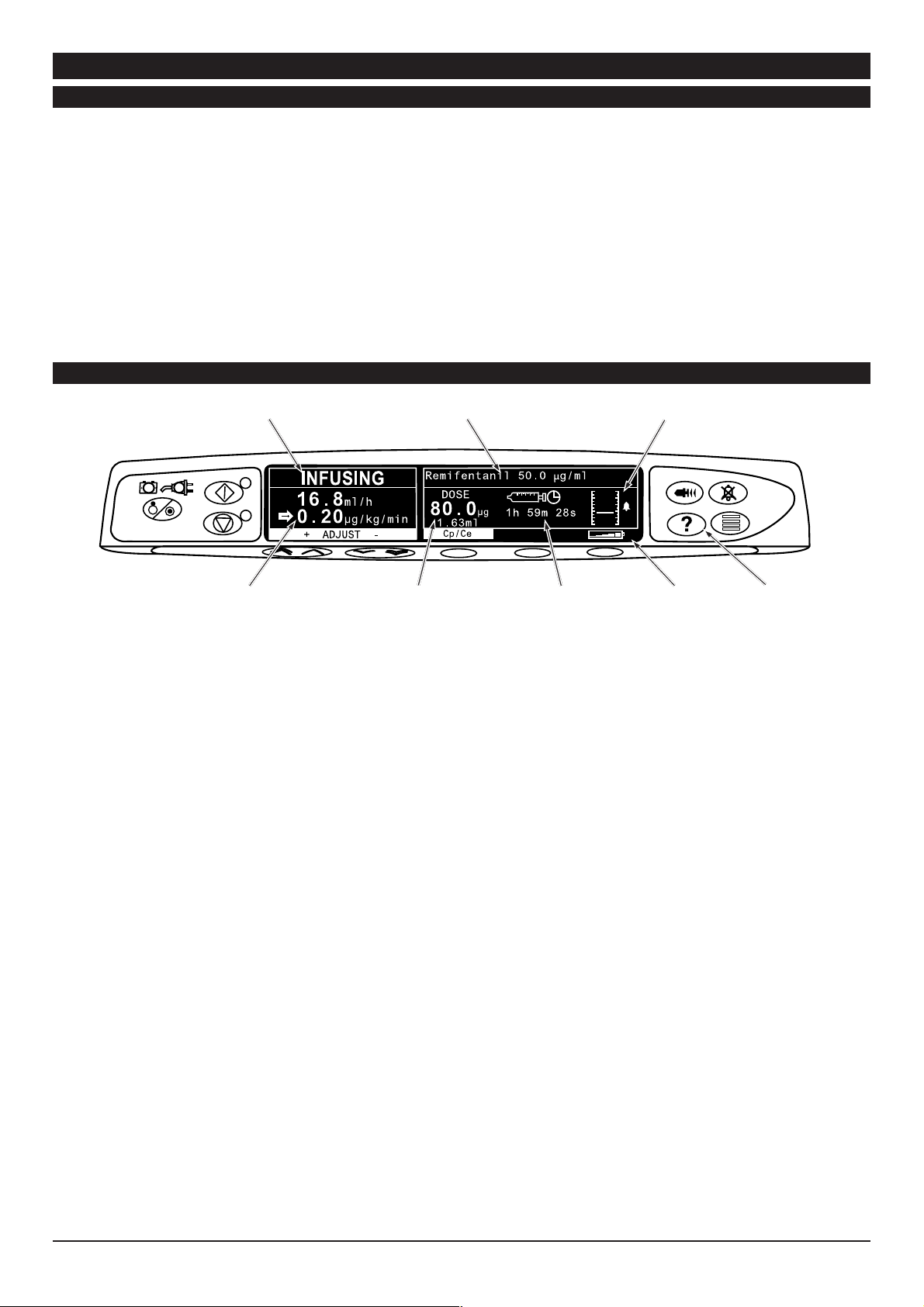
TIVA Mode (with or without prediction)
Drug Name and
Pump Status
Concentration
Pressure Information
Flow Rate and
Dose Rate
1. A list of available drugs and models will be displayed. Use the
Dose and
Volume Infused
keys to select the required drug and press the OK
softkey. If the drug has an associated model, an INFO softkey
will be displayed. Pressing the INFO softkey will show more
information on the selection. The ml/h option allows infusions
without doserate calculation.
2. CONCENTRATION -
a. Select Concentration required and OK to confirm (Only
required if more than one concentration is available).
b. Press the OK softkey to confirm Concentration or press
the MODIFY softkey to change Drug amount and diluent
volume.
3. WEIGHT - adjust the patient weight using the
press the OK softkey to confirm.
4. The remaining patient parameters for the selected drug must
be entered using the
to confirm. The required parameters may include the following
depending on the model:
• AGE
• HEIGHT
• GENDER
• LBM and BMI (Lean Body Mass and Body Mass Index. This is
for information only and is not an adjustable parameter)
5. The CONFIRM drug setup screen shows the initial infusion
parameters for the drug. Press the OK softkey to accept or
MODIFY to change the drug setup.
6. INDUCTION - Using the
dose amount per kg of patient weight (if required for dosing).
Press the OK softkey to enter. The Induction feature may be
disabled reducing the dose to zero until OFF is displayed and
press OK softkey to confirm.
7. TIME - Enter the induction time in seconds over which the
induction dose will be delivered. Press the OK softkey to enter.
keys and press the OK softkey
keys, enter the induction
keys,
Battery IconTime Remaining
8. MAINTENANCE - Set the maintenance dose rate in the drug
protocol units. Press the OK softkey to enter.
Important: Prime IV infusion set.
9. Load Syringe - Load the syringe according to the procedure in
this manual.
10. Confirm Syringe - Check that the syringe type and size being
used matches the display. If required, the make of syringe can
be changed by pressing the TYPE button. Press CONFIRM when
the correct type and size are shown.
11. Purge (if required) - Press the
hold the PURGE softkey until the fluid flows and the purging of
the IV infusion set is complete. Release the softkey. The volume
used during purging will be displayed.
12. Connect To Patient - Connect the extension set to the patient
access device.
13. Start - Press the
INFUSING will be displayed. The amber stop light will be
replaced by the flashing green start light to indicate that the
pump is in operation. If the infusion rate exceeds the Soft Alerts
then check infusion setting, to continue with infusion at set
target press the button and then confirm OVERRIDE LIMIT
by pressing the YES softkey. If OVERRIDE LIMIT is not required
press the NO softkey and adjust target concentration to be
within the Soft Alerts.
Note: If a model has been selected, the VOLUME softkey
will be replaced by a Ce/Cp softkey. This will allow
the user access to screens showing predicted target
concentrations. In this mode of operation the volume
may never be cleared.
14. Stop - Press the
will be displayed. The AMBER STOP light will replace the
GREEN START light.
button to commence operation.
button to halt the operation. ON HOLD
button and then press and
Operations
During Use
1000PB01489 Iss. 4 10/32
 Loading...
Loading...