Page 1

Agilent 2100 Bioanalyzer
Installation and Safety Guide
Page 2
Notices
CAUTION
WARNING
© Agilent Technologies, Inc.
No part of this manual may be reproduced
in any form or by any means (including electronic storage and retrieval or translation
into a foreign language) without prior agreement and written consent from Agilent
Technologies, Inc. as governed by United
States and international copyright laws.
Manual Part Number
G2938-90007 Rev B
Edition
Printed in Germany
Agilent Technologies
Hewlett-Packard-Strasse 8
76337 Waldbronn
This product may be used as a component of an in vitro diagnostic system if the system is registered with
the appropriate authorities and complies with the relevant regulations.
Otherwise, it is intended only for general laboratory use.
Warranty
The material contained in this document is provided “as is,” and is subject to being changed, without notice,
in future editions. Further, to the maximum extent permitted by applicable
law, Agilent disclaims all warranties,
either express or implied, with regard
to this manual and any information
contained herein, including but not
limited to the implied warranties of
merchantability and fitness for a particular purpose. Agilent shall not be
liable for errors or for incidental or
consequential damages in connection
with the furnishing, use, or performance of this document or of any
information contained herein. Should
Agilent and the user have a separate
written agreement with warranty
terms covering the material in this
document that conflict with these
terms, the warranty terms in the separate agreement shall control.
Technology Licenses
The hardware and/or software described in
this document are furnished under a license
and may be used or copied only in accordance with the terms of such license.
Restricted Rights Legend
If software is for use in the performance of a
U.S. Government prime contract or subcontract, Software is delivered and licensed as
“Commercial computer software” as
defined in DFAR 252.227-7014 (June 1995),
or as a “commercial item” as defined in FAR
2.101(a) or as “Restricted computer software” as defined in FAR 52.227-19 (June
1987) or any equivalent agency regulation
or contract clause. Use, duplication or disclosure of Software is subject to Agilent
Technologies’ standard commercial license
terms, and non-DOD Departments and
Agencies of the U.S. Government will
receive no greater than Restricted Rights as
defined in FAR 52.227-19(c)(1-2) (June
1987). U.S. Government users will receive
no greater than Limited Rights as defined in
FAR 52.227-14 (June 1987) or DFAR
252.227-7015 (b)(2) (November 1995), as
applicable in any technical data.
Safety Notices
A CAUTION notice denotes a
hazard. It calls attention to an
operating procedure, practice, or
the like that, if not correctly performed or adhered to, could
result in damage to the product
or loss of important data. Do not
proceed beyond a CAUTION
notice until the indicated conditions are fully understood and
met.
A WARNING notice denotes a
hazard. It calls attention to an
operating procedure, practice,
or the like that, if not correctly
performed or adhered to, could
result in personal injury or
death. Do not proceed beyond a
WARNING notice until the indicated conditions are fully understood and met.
Page 3

Contents
Contents
1 Safety Information 5
Power Cords 7
Operation 8
Safety Symbols 9
Laser Safety 10
Chemical and Biological Safety 11
Legal Notice 13
2 Site Requirements for the Agilent 2100 Bioanalyzer 15
Power Considerations 16
Bench space 17
Environment 18
Sound Emission 19
Physical Specifications of the Agilent 2100 Bioanalyzer 20
3 Unpacking the Agilent 2100 Bioanalyzer System 21
Damaged Packaging 22
4 Installing the Agilent 2100 Bioanalyzer System 25
Setting up PC and Printer 26
Setting up the Agilent 2100 Bioanalyzer 27
5 Starting the Agilent 2100 Bioanalyzer 31
Turning on the Agilent 2100 Bioanalyzer 32
Connecting the Bioanalyzer via the USB/Serial Cable 33
Starting the Agilent 2100 Bioanalyzer Software 34
Preparing the Assay 35
6 Maintenance of the Agilent 2100 Bioanalyzer 37
7 Spare Parts and Accessories 39
3
Page 4

Contents
4
Page 5

1
WARNING
Safety Information
Power Cords 7
Operation 8
Safety Symbols 9
Laser Safety 10
Chemical and Biological Safety 11
Legal Notice 13
The following general safety precautions must be observed during all phases of
operation, service, and repair of the Agilent 2100 Bioanalyzer.
All safety instructions should be read and understood before installation,
operation and maintenance of the instrument. Failure to comply with these
precautions or with specific warnings elsewhere in this manual violates safety
standards of design, manufacture and intended use of the instrument.
Agilent Technologies assumes no liability for the customer’s failure to comply
with these requirements.
A WARNING notice denotes a hazard.
It calls attention to an operating procedure, practice, or the like that, if not correctly
performed or adhered to, could result in personal injury or death.
➔ Do not proceed beyond a WARNING notice until the indicated conditions are fully
understood and met.
Agilent Technologies
5
Page 6
1 Safety Information
CAUTION
NOTE
A CAUTION notice denotes a hazard.
It calls attention to an operating procedure, practice, or the like that, if not correctly
performed or adhered to, could result in damage to the product or loss of important
data.
➔ Do not proceed beyond a CAUTION notice until the indicated conditions are fully
Instrument Safety: This is a Safety Class I instrument (provided with a terminal for
protective grounding) and has been manufactured and tested according to international
safety standards.
understood and met.
6
Page 7
Power Cords
WARNING
Safety Information
Power Cords
Different power cords are offered as options with the Agilent 2100
Bioanalyzer. The female end of all power cords is identical. It plugs into the
power-input socket at the rear of the instrument. The male end of each power
cord is different and designed to match the wall socket of a particular country
or region.
Electric Shock
The absence of ground connection and the use of an unspecified power cord can
lead to electric shock or short circuit.
➔ Never operate your instrument from a power outlet that has no ground connection.
➔ Never use a power cord other than the Agilent Technologies power cord designed
for your region.
➔ Never use cables other than those supplied by Agilent Technologies to ensure
proper functionality and compliance with safety or EMC regulations.
1
7
Page 8

1 Safety Information
WARNING
Operation
Operation
Ensure the proper usage of the equipment
The protection provided by the equipment may be impaired.
➔ The operator of this instrument is advised to use the equipment in a manner as
Before the instrument is switched on, all protective ground terminals,
extension cords, auto-transformers, and devices connected to it must be
connected to a protective ground socket. Any interruption of the protective
grounding will cause a potential shock hazard that could result in serious
personal injury. Whenever it is likely that the protection has been impaired,
the instrument must be made inoperative and be secured against any intended
operation.
To operate the instrument safely:
• Do not remove any cover of the Agilent 2100 Bioanalyzer
• Avoid any maintenance of the instrument under voltage
• Do not let liquid drip into the Agilent 2100 Bioanalyzer. It could cause a
• Do not replace components of the instruments (e.g. electrode cartridge or
• Do not operate the instrument in the presence of flammable gases or fumes.
• Do not install substitute parts or make any unauthorized modification to
specified in this manual.
shock or it could damage the Agilent 2100 Bioanalyzer.
pressure cartridge) with power turned on.
Operation of any electrical instrument in such an environment constitutes a
definite safety hazard.
the instrument.
8
Page 9
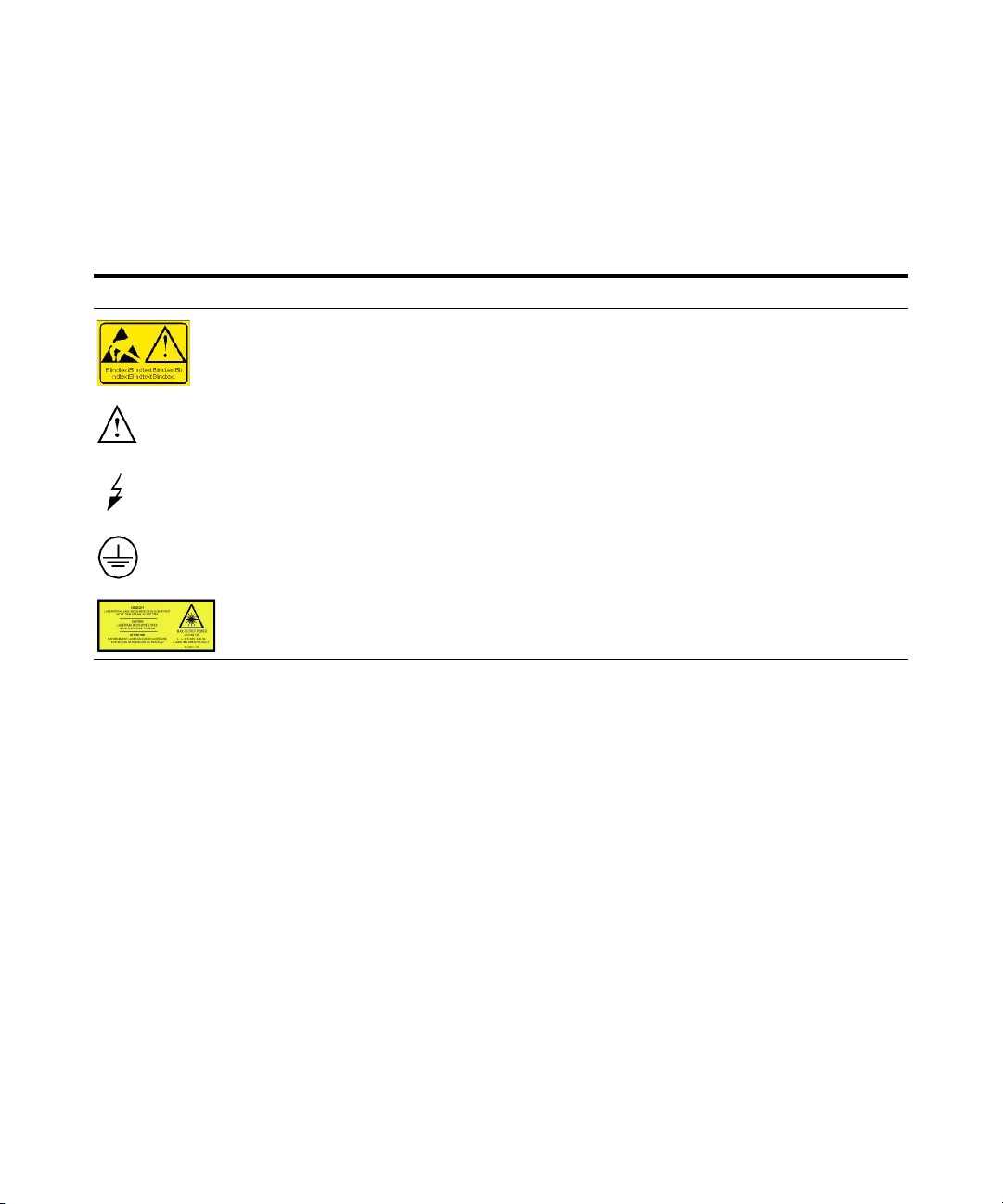
Safety Symbols
Symbol Description
Electrosensitive device.
The apparatus is marked with this symbol when the user should refer to the Installation and Safety
Manual in order to prevent risk of harm to the operator and to protect the apparatus against damage.
Indicates dangerous voltages.
Indicates a protected ground terminal.
Laser Radiation. When open avoid exposure to beam.
Safety Information
Safety Symbols
1
9
Page 10
1 Safety Information
WARNING
Laser Safety
Laser Safety
The Agilent 2100 Bioanalyzer is classified as a "Laser Class 1" product
(IEC825-1, CFR1040.10). During normal operation of the Agilent 2100
Bioanalyzer no laser light is accessible to the user.
When the laser is in use, the laser light source emits light at a power that may
be harmful to the eyes. To prevent operator exposure to potentially harmful
laser light, an interlock mechanism turns off the laser whenever the lid of the
Agilent 2100 Bioanalyzer is not closed. Furthermore, the lid can't be
completely opened and has a black light absorbing surface in order to prevent
any potential laser light reflection.
Harmful laser light
The laser light source emits light at a power that may be harmful to the eyes.
➔ Never look into the beam or direct it towards someone else.
➔ System interlocks should never be disabled.
10
Page 11

Chemical and Biological Safety
WARNING
WARNING
Toxic and hazardous solvents
The handling of solvents and reagents can hold health risks.
➔ When working with solvents observe appropriate safety procedures (for example,
goggles, safety gloves and protective clothing) as described in the material handling
and safety data sheet supplied by the solvent vendor, especially when toxic or
hazardous solvents are used.
Pathogenic, toxic, or radioactive samples
Handling and use of pathogenic, toxic, or radioactive samples and of genetically
modified organisms holds risks for health and environment.
➔ Ensure that all necessary safety regulations, guidelines, precautions and practices
are adhered to accordingly.
Safety Information
Chemical and Biological Safety
1
➔ Consult the laboratory safety officer for advise on the level of containment required
for the application, and proper decontamination or sterilization procedures to follow
if fluids escape from containers.
To operate the instrument safely:
• Observe all cautionary information printed on the original solution
containers prior to their use.
• Because leaks, spills, or loss of sample may generate aerosols, observe
proper safety precautions.
• Agilent 2100 Bioanalyzer covers have not been designed as bioseals for
aerosol or liquid containment.
• Handle body fluids with care because they can transmit disease. No known
test offers complete assurance that they are free of micro-organisms. Some
of the most virulent – Hepatitis (B and C) and HIV (I-V) viruses, atypical
mycobacteria, and certain systemic fungi – further emphasize the need for
aerosol protection.
11
Page 12

1 Safety Information
WARNING
Chemical and Biological Safety
• Always follow local state and federal biohazard handling regulation when
• Handle all infectious samples according to good laboratory procedures and
• Dispose of all waste solutions and products according to appropriate
Harmful chemical and biological substances
Residues of clinical samples may contain chemicals and biological substances that
are dangerous and harmful to persons working on the 2100 Bioanalyzer.
➔ The Agilent 2100 Bioanalyzer and any accessories must be decontaminated before
disposing of biohazardous waste material e.g. used Cell LabChips or
contaminated pressure adapters.
methods to prevent spread of disease.
environmental health and safety guidelines.
requesting service by an Agilent Technologies Field Service Representative and
before returning the 2100 Bioanalyzer to Agilent Technologies for repair or
replacement.
12
Page 13

Legal Notice
Safety Information
Legal Notice
The Agilent 2100 Bioanalyzer is sold for research use only.
By purchasing this instrument, the purchaser is granted the limited right to
use only this instrument. Purchase of this instrument does not include any
right, express or implied, to use any other patented product, method or
process, or to use any other portion or component of any patented system or
systems, software, microfluidic devices or reagents, either alone or in
conjunction with this product, unless use of such method, process or other
portion or component is separately authorized.
1
13
Page 14

1 Safety Information
Legal Notice
14
Page 15

2 Site Requirements for the Agilent 2100 Bioanalyzer
Power Considerations 16
Bench space 17
Environment 18
Sound Emission 19
Physical Specifications of the Agilent 2100 Bioanalyzer 20
Two versions of the 2100 Bioanalyzer are available (see the label at the lower
right corner at the front of your 2100 Bioanalyzer). Both have the same
specifications and requirements except for the option to do flow cytometrie.
For both versions a suitable environment is important to ensure optimal
performance.
G2938C: for electrophoretic assays and for flow cytometrie
G2939A: for electrophoretic assays only
Agilent Technologies
15
Page 16

2 Site Requirements for the Agilent 2100 Bioanalyzer
CAUTION
Power Considerations
Power Considerations
The Agilent 2100 Bioanalyzer power supply has wide ranging capabilities and
accepts any line voltage in the range 100 - 240 V ±10% with a line frequency of
50 - 60 Hz ±5% (see also Table 1 on page 20 for more details). Consequently,
there is no voltage selector in the rear of the instrument.
Damage through high line voltage
Connecting the Agilent 2100 Bioanalyzer to a line voltage higher than specified may
result in damage of the instrument.
➔ Connect the Agilent 2100 Bioanalyzer to the specified line voltage only.
16
Page 17

Bench space
Site Requirements for the Agilent 2100 Bioanalyzer
Bench space
The Agilent 2100 Bioanalyzer dimensions and weight (see Table 1 on page 20)
allow you to place it on almost any desk or laboratory bench. It needs
approximately 8 cm (3.1 inches) of clear space at the rear for air circulation
and electric connections.
2
17
Page 18

2 Site Requirements for the Agilent 2100 Bioanalyzer
CAUTION
NOTE
NOTE
Environment
Environment
Your Agilent 2100 Bioanalyzer will work within the specifications of ambient
temperatures and relative humidity described in Table 1 on page 20.
Condensation
Condensation will damage the electronics.
➔ Do not store, ship or use the instrument under conditions where temperature
fluctuations could cause condensation within the instrument.
➔ If your instrument was shipped in cold weather, leave it in its box and allow it to
warm up slowly to room temperature to avoid condensation.
The Agilent 2100 Bioanalyzer is designed to operate in a controlled electromagnetic
environment (EN61326/A1) where RF transmitters such as mobile telephones should not
be used in close proximity.
18
In order to function properly, do not place the Agilent 2100 Bioanalyzer on a vibrating
surface or near vibrating objects.
Page 19

Sound Emission
Manufacturers Declaration
This statement is provided to comply with the requirements of the German
Sound Emission Directive of 18 January 1991.
This product has a sound pressure emission (at the operator position) < 70 dB.
• Sound Pressure Lp < 70 dB (A)
• At Operator Position
• Normal Operation
• According to ISO 7779:1988/EN 27779/1991 (Type Test)
Site Requirements for the Agilent 2100 Bioanalyzer
Sound Emission
2
19
Page 20

2 Site Requirements for the Agilent 2100 Bioanalyzer
Physical Specifications of the Agilent 2100 Bioanalyzer
Physical Specifications of the Agilent 2100 Bioanalyzer
Table 1 on page 20 lists the physical specifications of the Agilent 2100
Bioanalyzer.
Ta bl e 1 Physical specifications
Type Specification Comment
Weight 10 kg (22 lbs)
Dimensions 162 — 412 — 290 mm
(6.4 — 16.2 — 11.4 in)
Line voltage 100-240 VAC ±10% Wide-ranging capability
Line frequency 50-60 Hz ±5%
Power consumption 30 W / 60 VA Maximum
Ambient operating temperature 5-40 °C (41-104 °F) The assays set-up to be analyzed on a chip
Ambient non-operating
temperature
Humidity <80%, at 5-31 °C (41-89 °F), decreasing
Operating altitude Up to 2000 m (6500 ft)
Non-operating altitude Up to 4600 m (14950 ft) For storage of the Agilent 2100 Bioanalyzer
Safety standards: IEC, CSA, UL Installation Category II, Pollution
-40-70 °C (-40-158 °F)
linearly to 50% at 40 °C (104 °F)
Degree 2
Width — depth — height
temperature of 30 °C. If the ambient
temperature is above 30 °C the assays are
analyzed using that temperature.
Non-condensing
20
Page 21

3 Unpacking the Agilent 2100 Bioanalyzer System
Damaged Packaging 22
Delivery Checklist 22
Agilent Technologies
21
Page 22

3 Unpacking the Agilent 2100 Bioanalyzer System
CAUTION
Damaged Packaging
Damaged Packaging
Upon receipt of your Agilent 2100 Bioanalyzer, computer and printer, inspect
the shipping containers for any signs of damage. If containers or cushioning
material are damaged, save them until the contents have been checked for
completeness and the 2100 Bioanalyzer, computer and printer have been
mechanically and electrically checked. If the shipping container or cushion
material is damaged, notify the carrier as well as Agilent Technologies. Save
the shipping material for the carrier's inspection.
If there are signs of damage to the Agilent 2100 Bioanalyzer:
➔ Do not attempt to install the instrument.
➔ Contact your local Agilent Technologies sales and service office.
Delivery Checklist
22
Ensure that all parts and materials have been delivered with your 2100
Bioanalyzer.
The delivery checklists valid for G2938C are shown in Table 2 on page 22 to
Table 4 on page 23. The checklist for G2939A is shown in Table 5 on page 24.
Please report any missing or damaged parts to your local Agilent Technologies
sales and service office.
Ta bl e 2 Agilent 2100 Bioanalyzer (G2938C) delivery checklist
Description Quantity
2100 Bioanalyzer instrument 1
Declaration of conformity 1
Instrument control license 1
Installation and Safety Manual 1
RS232 Connector cable 1
Page 23

Unpacking the Agilent 2100 Bioanalyzer System
Damaged Packaging
Ta bl e 2 Agilent 2100 Bioanalyzer (G2938C) delivery checklist
Description Quantity
USB/serial adapter 1
Fuses 2
Chip priming station 1
IKA vortex mixer (optional) 1
3
Agilent 2100 Bioanalyzer expert software including declaration
of system validation.
1
2 different application sets are available for the 2100 Bioanalyzer G2938C. The
2100 Bioanalyzer electrophoresis set contains an electrode cartridge required
for all electrophoretic assays.
Ta bl e 3 Agilent 2100 Bioanalyzer electrophoresis set (G2947CA) delivery checklist
Description Quantity
Electrode cartridge 1
Test chip bundle 1
Agilent 2100 Bioanalyzer software electrophoresis license 1
The flow cytometry set contains a pressure cartridge mandatory for all flow
cytometry assays.
Ta bl e 4 Agilent 2100 Bioanalyzer flow cytometry set (G2948CA) delivery checklist
Description Quantity
Pressure cartridge 1
Pressure adapter kit 1
Cell test chip kit 1
Agilent 2100 Bioanalyzer software flow cytometry license 1
Checkout kit 1
23
Page 24

3 Unpacking the Agilent 2100 Bioanalyzer System
Damaged Packaging
Ta bl e 5 Agilent 2100 Bioanalyzer (G2939A) delivery checklist
Description Quantity
Agilent 2100 Bioanalyzer instrument 1
Electrode cartridge 1
Test chip bundle 1
Declaration of conformity 1
Installation and Safety Manual 1
RS232 Connector cable 1
USB/serial adapter cable 1
Fuses 2
Chip priming station 1
IKA vortex mixer 1
Agilent 2100 Bioanalyzer expert software, including declaration
of system validation.
Instrument control license and electrophoresis license 1
1
24
Page 25

4
NOTE
Installing the Agilent 2100 Bioanalyzer System
Setting up PC and Printer 26
Setting up the Agilent 2100 Bioanalyzer 27
After unpacking and checking the completeness of the shipment, the Agilent
2100 Bioanalyzer system is ready to be installed. Depending on system
configuration, the installation requires up to three steps:
1 Setting up the PC.
2 Setting up the printer.
3 Setting up the Agilent 2100 Bioanalyzer.
If you have problems installing your Agilent 2100 Bioanalyzer, refer to the Troubleshooting
section of the Maintenance and Troubleshooting Guide.
Agilent Technologies
25
Page 26

4 Installing the Agilent 2100 Bioanalyzer System
Setting up PC and Printer
Setting up PC and Printer
For setting up the PC and printer, please refer to the instructions supplied
with the PC and printer respectively.
26
Page 27

Installing the Agilent 2100 Bioanalyzer System
BZiVaaZkZg
H]^ebZciadX`
A^Y
CAUTION
Setting up the Agilent 2100 Bioanalyzer
To set-up the Agilent 2100 Bioanalyzer instrument:
1 Locate bench space that meets the requirements described in “Bench
space” on page 17.
2 Place the instrument on the bench in an upright position.
3 Open the lid of the bioanalyzer and pull the metal lever on the inside left of
the lid to the vertical position. Remove the shipment lock and store it for
future use.
4
Setting up the Agilent 2100 Bioanalyzer
Damage by electrostatic discharge
The heater plate and electrodes are sensitive to electrostatic discharge.
➔ Make sure that you are grounded before inserting a chip to prevent the Agilent 2100
Bioanalyzer from being damaged by electrostatic discharge. The lid is grounded to
leak off electrostatic energy of the operator.
27
Page 28

4 Installing the Agilent 2100 Bioanalyzer System
A^Y
;dXjh^c\aZchZ
=ZViZgeaViZ
:aZXigdYZXVgig^Y\Z
:aZXigdYZh
8]^ehZaZXidg
EgZhhjgZXVgig^Y\Z
EgZhhjgZVYVeiZg
8]^ehZaZXidg^cedh^i^dc'
Setting up the Agilent 2100 Bioanalyzer
4 Slide the electrode or pressure cartridge into the lid and move the lever into
a flat (closed) position. Adjust the chip selector accordingly (electrode
cartridge: position1; pressure cartridge: position2).
28
Page 29

Installing the Agilent 2100 Bioanalyzer System
GH"'('edgi
EdlZg^cejihdX`Zi
EdlZghl^iX]
Setting up the Agilent 2100 Bioanalyzer
5 Connect one end of the serial cable to the RS-232 port located at the rear of
the instrument.
6 Connect the other end of the serial cable to the serial port (COM-port) of
your PC.
4
29
Page 30

4 Installing the Agilent 2100 Bioanalyzer System
NOTE
Setting up the Agilent 2100 Bioanalyzer
7 If your PC is not equipped with a serial port, use the USB/serial adapter
cable that is provided with the instrument.
8 Ensure that the power switch located at the rear of the instrument is in off
position.
The power input socket accepts a line voltage of 100-240 VAC ±10% with a line frequency
of 50-60 Hz. Maximum power consumption is 60 VA. There is no voltage selector at the rear
of the Agilent 2100 Bioanalyzer because the power supply has a wide-ranging capability.
9 Plug the female end of the power cord into the power input socket of the
Agilent 2100 Bioanalyzer instrument. Plug the male end of the power cord
into the electrical outlet.
30
Page 31

5
CAUTION
Starting the Agilent 2100 Bioanalyzer
Turning on the Agilent 2100 Bioanalyzer 32
Connecting the Bioanalyzer via the USB/Serial Cable 33
Starting the Agilent 2100 Bioanalyzer Software 34
Preparing the Assay 35
When starting the Agilent 2100 Bioanalyzer software, wait a few seconds until
the self-test procedures for the Agilent 2100 Bioanalyzer are finished.
Otherwise the software may not recognize the Agilent 2100 Bioanalyzer.
Condensation within the instrument
Temperature fluctuations could cause condensation within the instrument.
➔ Do not use the Agilent 2100 Analyzer under conditions with temperature
fluctuations.
➔ Let the instrument equilibrate to room temperature for one day to avoid damage of
the electronics.
Agilent Technologies
31
Page 32

5 Starting the Agilent 2100 Bioanalyzer
HiVijh>cY^XVidg
<gZZc"GZVYn
<gZZc![aVh]^c\"7jhn
DgVc\Z"HZa["iZhi
GZY":ggdg
Turning on the Agilent 2100 Bioanalyzer
Turning on the Agilent 2100 Bioanalyzer
1 Turn on your Agilent 2100 Bioanalyzer. The power switch is located at the
rear of the instrument, where the power cable plugs in (see ).
2 The status indicator lamp will light green if power is present and all
instrument self-tests have passed successfully.
32
If the status indicator lamp lights red, this indicates a hardware error. In
this case, please refer to the Troubleshooting section of the Agilent 2100
Bioalyzer Maintenance and Troubleshooting Guide or the Online Help.
3 Once your Agilent 2100 Bioanalyzer has passed the self- tests, it is ready to
run samples.
4 Start your PC and printer by pushing the appropriate power buttons as
outlined in the documentation.
Page 33

Starting the Agilent 2100 Bioanalyzer
Connecting the Bioanalyzer via the USB/Serial Cable
Connecting the Bioanalyzer via the USB/Serial Cable
If you connect the 2100 Bioanalyzer instrument via the USB/serial adapter
cable, the driver needs to be installed on the PC. This is preinstalled on bundle
PCs but needs to be installed manually on 3rd party PCs.
The driver can be found on the 2100 Expert Software CD. For installation
instructions please refer to the
readme file of the 2100 Expert Software.
5
33
Page 34

5 Starting the Agilent 2100 Bioanalyzer
NOTE
NOTE
Starting the Agilent 2100 Bioanalyzer Software
Starting the Agilent 2100 Bioanalyzer Software
The Agilent 2100 Bioanalyzer software is pre-installed on bundle PCs.
However, the full functionality of the software becomes available only if you
add the required license key(s). License keys are supplied with the 2100
Bioanalyzer electrophoresis set and/or with the flow cytometry set (see
Table 3 on page 23 and Table 4 on page 23).
1 Open the Agilent 2100 Bioanalyzer expert software by double-clicking the
icon on the desktop.
2 Select Help > Registration and highlight the Add License tab.
3 Add the appropriate license key.
For more details of adding license keys, refer to the Online Help .
4 To establish instrument-PC communication, assign a COM-port in the
instrument context. When the communication is established, instrument
specific information like serial number, vendor information and firmware
version is displayed.
For detailed information and in case of problems, please refer to the Troubleshooting
section of the Agilent 2100 Bioanalyzer Maintenance and Troubleshooting Guide or the
Online Help.
34
Page 35

Preparing the Assay
For information about the assay preparation, please refer to the
corresponding reagent kit guide. All kit guides are included in the online help
of the 2100 Expert software. The quick start guide is provided with every kit.
Use the reagent kit guides as a reference when preparing and running an assay
with the Agilent 2100 Bioanalyzer.
The Agilent Technologies Genomics Web site offers support and useful
information about current developments, products and technology:
http://www.agilent.com/genomics/bioanalyzer
Starting the Agilent 2100 Bioanalyzer
Preparing the Assay
5
35
Page 36

5 Starting the Agilent 2100 Bioanalyzer
Preparing the Assay
36
Page 37

6 Maintenance of the Agilent 2100 Bioanalyzer
For detailed maintenance procedures of the Agilent 2100 Bioanalyzer
instrument and its accessories please refer to:
• the Online Help of the Agilent 2100 Bioanalyzer software or
• the Maintenance part of the Troubleshooting and Maintenance Guide
Agilent Technologies
37
Page 38

6 Maintenance of the Agilent 2100 Bioanalyzer
Preparing the Assay
38
Page 39

7 Spare Parts and Accessories
The following table provides a list of spare parts and accessories that are
available for the Agilent 2100 Bioanalyzer. To buy parts, please refer to the
Agilent Online Store: http://www.agilent.com/home/buyonline.html
Ta bl e 6 Spare Parts and Accessories for the Agilent 2100 Bioanalyzer
Reorder number Part Description
RS232-61601 RS 232 Cable communication cable between PC and instrument
8121-1013 USB/Serial Adapter Cable connects RS232 cables to USB PC ports (for PCs without serial port)
2110-0007 Fuse fuse for power supply
G2938-68716 Gasket Kit contains spare parts for chip priming station: 1 plastic adapter, 1 ring and
10 gaskets
5042-1398 Adjustable Clip used in combination with a syringe to apply defined pressure for chip
priming
5065-4478 Pressure Adapter Kit contains 5 pressure adapters for use with the pressure cartridge (for
G2938C only)
5065-9966 Vortex Mixer Adapter replacement part for IKA MS2 Vortexer (3 mounting screws)
5065-4413 Electrode Cartridge no extra electrode pin set; pin-set not separately re-orderable
5065-4401 Chip Priming Station includes priming station, timer and 1 syringe clip
G2938-68300 Test Chip Kit for
Electrophoresis Assays
G2938-68200 Test Chip Kit for Flow
Cytometry Assays
5065-4428 IKA Vortexer MS3 type Bundled to all Bioanalyzer systems but NOT available from Agilent
comprises autofocus chip, electrode/diode test chip and documentation
comprises 1 Cell Autofocus Chip and documentation (for G2938C only)
standalone. Replacement vortexers must be ordered directly from IKA.
Description: "MS 3 basic S36 Agilent" IKA Part Number: "3617036"
Agilent Technologies
39
Page 40

Index
Index
A
accessories 39
assay preparation 35
B
bench space 17
bioanalyzer software 23, 24
bioanalyzer
accessories 39
delivery checklist 22
maintenance 37
spare parts 39
biological safety 11
body fluids 11
C
cable 22, 24
cartridge 23, 23
chemical safety 11
chip priming station 23, 24
communication 34
com-port 34
D
declaration of conformity 22, 24
decontamination 12
delivery checklist 22
dimensions 20
E
electrode cartridge 23
electrophoresis license 23
electrophoresis set 23
environment 18
F
flammable gases 8
flow cytometry license 23
flow cytometry set 23
frequency 16, 20
fuse 23, 24
G
Genomics Web site 35
H
humidity 20
I
installation 25
interlock mechanism 10
K
kit guide 35
L
laser safety 10
laser 10
legal notice 37
license key 34
license 23, 23, 34
M
maintenance 37
O
online store 39
operating temperature 20
P
packaging 22
physical specifications 20
power considerations 16
power consumption 20
power cord 7
power supply 16
pressure cartridge 23
R
reagent kit guide 35
requirements 15
RS232 cable 22, 24
S
safety information 5
safety standards 20
safety symbols 9
setup
bioanalyzer 27
PC 26
printer 26
site requirements 15
software license 34
software 23, 24
sound emission 19
spare parts 39
start
bioanalyzer software 34
40
Page 41

bioanalyzer 31
status indicator 32
T
turning on the Agilent 2100
Bioanalyzer 32
V
voltage 20
vortex mixer 23, 24
W
weight 20
Index
41
Page 42

www.agilent.com
© Agilent Technologies
Printed in Germany
*G2938-90007*
*G2938-90007*
G2938-90007 Rev B
Agilent Technologies
 Loading...
Loading...