Page 1

3B SCIENTIFIC® PHYSICS
... going one step further
ELECTROCHEMISTRY
U11110
ALF 05/04
Practical experiments
for secondary levels I and II
Page 2

Contents
Preface 4
Contents of the Electrochemistry case 5
Assembly and cleaning of the battery block 6
Description of the meter 7
Measuring the voltage of galvanic cells - teacher's instructions 8
Measuring the voltage of galvanic cells - student instructions 9
Measuring the voltage of a Daniell cell - teacher's instructions 10
Measuring the voltage of a Daniell cell - student instructions 11
Measuring the voltage of three Daniell cells connected in parallel - teacher's instructions 12
Measuring the voltage of three Daniell cells connected in parallel - student instructions 13
Measuring the voltage of three Daniell cells connected in series - teacher's instructions 14
Measuring the voltage of three Daniell cells connected in series - student instructions 15
Measuring the standard electrochemical potential of various metals - tea cher's instructions 16
Measuring the standard electrochemical potential of various metals - student in structions 17
Measuring the standard electrochemical potential of various non-metals - teacher's in structions 18
Measuring the standard electrochemical potential of various non-metals - student instructions 19
Measuring the voltage of a zinc-carbon (Leclanché) cell - teacher's instructions 20
Measuring the voltage of a zinc-carbon (Leclanché) cell - student instructions 21
Measuring the voltage for various electrolyte concentrations - teacher's instru ctions 22
Measuring the voltage for various electrolyte concentrations - student instructio ns 23
Measuring the voltage for various electrolyte temperatures - teacher's instructions 24
Measuring the voltage for various electrolyte temperatures - student instructions 25
Design, charging and discharging of a steel accumulator - teacher's instructions 26
Design, charging and discharging of a steel accumulator - student instructions 27
Experiment - measuring pH - teacher's instructions 28
Experiment - measuring pH - student instructions 29
Standard electrochemical potential series 30
Notes on performing experiments and disposing of materials 31
Bibliography 32
3
Page
Page 3

Preface
The electrochemistry case is a teaching aid exclusively designed for chemistry and physics
experiments in schools.
The fact that students can perform experiments themselves deepens their understanding of the
subjects being treated. The experiments should be performed in small groups of not more than two
or three students. Thus the teacher is only required to supervise and may also give suitable advice
on specific questions that students are unable to answer themselves. Careful organization of
workgroups can therefore add to the achievement, understanding and enjoyment of all students.
The accompanying literature reduces to a minimum the teacher's preparation time, which may for
school experiments in particular be quite lengthy. With each experiment there are instructions for
both teacher and students. The teacher's instructions include descriptions of all the facts required
for the conduct of the experiment. The expected results for each experiment can be found in the
teacher's instructions. (These may differ slightly from the theoretical results given in text books.)
The teacher is also given instructions on how to prepare suitable electrolyte solutions.
The student instructions can be copied by the teacher and distributed to the students. This means
that there is no need to go to the extra lengths of writing down an experiment procedure, freeing
the student to concentrate on the essentials of the experiments themselves.
To ensure safety, German R (Risk) and S (Safety) phrases and hazard symbols are included for all
chemicals used.
At the end of the instructions, notes can be found on the disposal of chemicals. In order to prevent
wastage on chemicals, we recommend that once an experiment is finished, the electrolyte
solutions that have been used should be removed from the chambers of the trough using the
supplied pipette and stored in a labelled container for use in subsequent experiments. This also
makes a contribution to the environment.
We would be grateful for comments and modifications (or notification of any errors) pertaining to
the experiments. Please contact your supplier for the Electrochemistry case.
We wish you success in carrying out the experiments.
4
Page 4

Contents of the Electrochemistry case
1 Meter for electrochemical and pH experiments,
powered by battery or mains power supply.
1 3V adapter for electrolyzing platinum gauze electrode
1 Mains power supply prim:
115..240VAC
,
1 Combined probe for measuring pH, electrode in storage vessel
2 Plastic beakers 25 ml
2 Pipettes
1 Storage box with:
2 silver electrodes, 4 zinc electrodes, 2 iron electrodes, 2 carbon electrodes,
2 aluminum electrodes, 2 nickel electrodes, 4 copper electrodes, 1 magnesium electrode
(magnesium strip to wrap around a plastic plate), 1 platinum gauze electrode
2 half cells for 4 electrodes each, 1 set of filter paper strips,
1 set of cables for electrochemistry (consisting of: 3 cables with crocodile clips, red,
each 20 cm long,
1 cable with crocodile clip and plug, red, 30 cm long, 3 cables with crocodile clips, blue,
each 20 cm long, 1 cable with crocodile clip and plug, blue, 30 cm long),
1 emery stone
1 set of instructions for experiments
50-60Hz
5
sec:
12V–500mA
Page 5

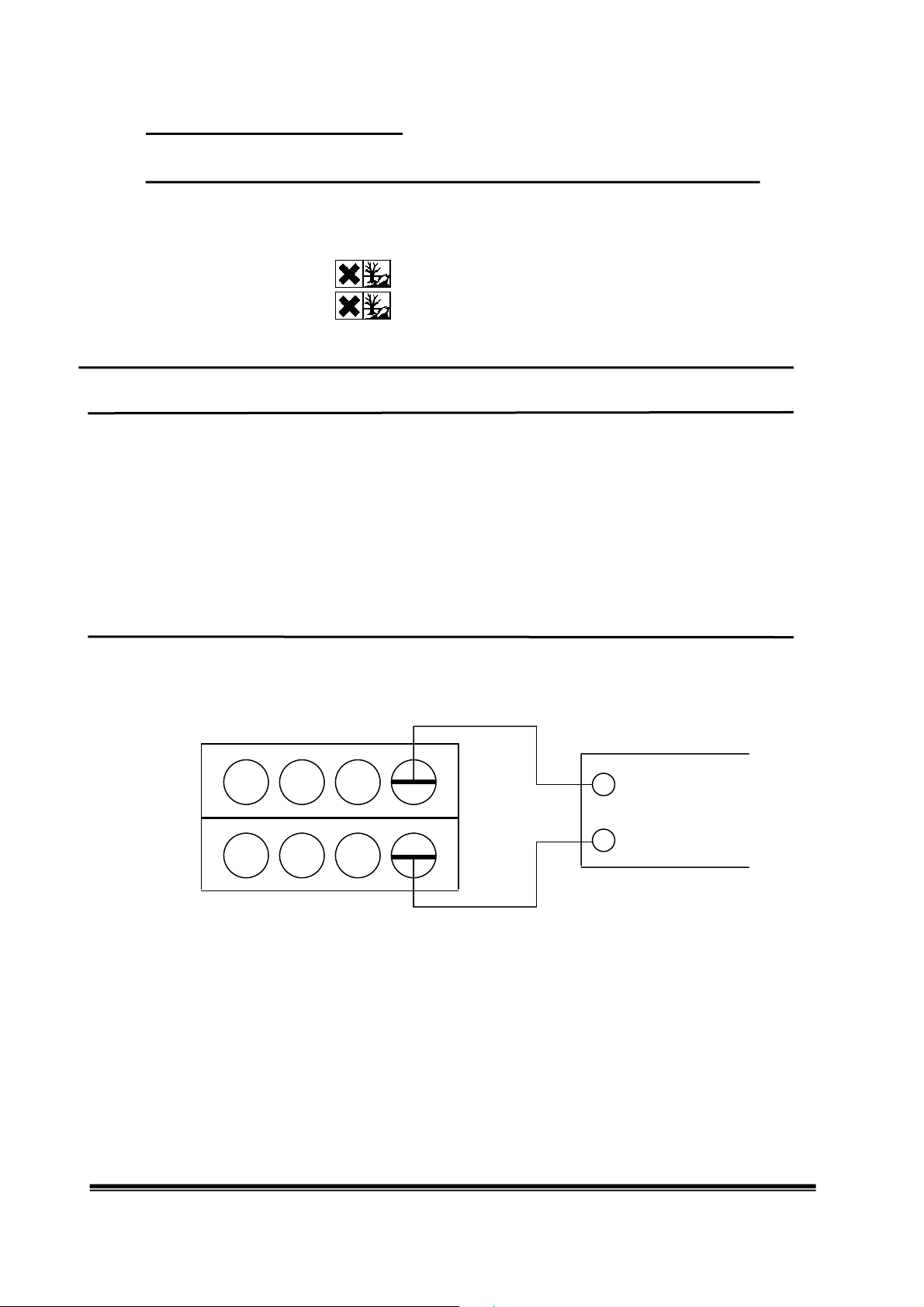
Assembly and cleaning of the battery block
The battery block is supplied fully assembled and can be used for experiments immediately. It is
stored in the storage box inside the case.
If the battery block is used, it must be disassembled after the experiment is finished by undoing the
knurled screws to completely separate the two half cells so that the electrolyte can be sucked out
and the electrolytes removed. After removing the filter paper, the two half cells should be rinsed out
with water and then thoroughly dried.
Reassemble the battery block for use in subsequent experiments. This involves placing one of the
supplied filter paper strips flush between the two half cells. When this is done, first one and then
the second knurled screw should be poked through the filter paper and the two half cells should
then be screwed firmly together. (Note: The four openings on the inside must all face the filter
paper (as in the illustration).
The 8 chambers are electrically connected via the filter paper, which becomes wet when the
chambers are filled with electrolyte.
Please take care:
After the set is used, all the components employed must be thoroughly cleaned and dried.
Remove the filter paper between the battery blocks and dry both the knurled screws.
The electrodes used should be cleaned under running water and dried in order to avoid corrosion.
It is recommended that the electrodes be rubbed with the emery stone after use in order to
thoroughly remove any chemical residues that may have been deposited.
If you follow these instructions, you should get plenty of enjoyment from your case.
6
Page 6

Meter
Front
Range display/
battery charge
――――――>
Off/range switch ―――――>
Terminal sockets ―――――>
<―――――― LCD display
<― Zero point adjuster (for pH )
Rear
Mains power supply socket ―>
<――――――― pH socket
Brief instructions for
voltage measureme
nts ―――>
Brief instructions for
<―
pH measurement
The meter is battery powered but can also be used with the power supply unit provided. The battery is
recharged completely when the device is used with the power supply and overcharging is impossible. The
power supply is plugged in to the bottom of the instrument.
In addition to voltage measurement, pH can also be measured with the meter.
1. Voltage measurement
To perform voltage measurement, the meter is turned on via the range switch and is then initially configured
for the range 0 - 2V. If higher voltages are to be measured, the instrument can be reconfigured for 20 V by
using the range switch again. Electrodes are connected to the meter via 2 mm sockets (red/blue) in order to
take measurements.
2. pH measurement
If pH is to be measured, the supplied combined pH electrode is connected to the pH socket at the bottom.
Set the range switch to “pH”. To calibrate the combined pH electrode, a buffer solution is employed (either
pH 4 or pH 9). This is a single-point calibration. After the pH electrode is immersed in the relevant buffer
solution, the zero point adjuster is altered until the displayed pH value matches that of the buffer solution. It
is now possible to conduct pH measurements. However, no further changes should be made to the
adjustment knobs.
7
Page 7
Experiment 1 - Measuring voltage
Measuring the voltage of galvanic cells - teacher's instructions
Chemicals Hazard
R phrases S phrases Equipment
Teacher's instructions
symbols
Copper (II)-sulfate-(5 H2O)
Zinc sulfate-(7H2O)
Silver nitrate
Iron (II)-sulfate-(7 H2O)
Nickel sulfate-(6H2O)
Distilled water --- ---
Warning:
Please take care:
Salts of heavy metals are poisonous!
22-36/38-50/53 22-60-61 Meter
36/38-50/53 22-25-60-61 Electrodes:
34-50/53 26-45-60-61 2 Experiment cables
22-36/38 24/25 2 Pipettes
22-40-42/43-
50/53
22-36/37-60-
61
1 Cu, 1 Zn,1 Ag, 1 Fe, 1 Ni
Experiment procedure:
1. The prepared 1.0 and 0.1 molar electrolyte solutions should be given to the students. Students require no more than
10 ml of the relevant solution each.
2. Assemble the battery block as described.
3. Fill the chambers with electrolyte using the pipette (included in the case) and insert the appropriate electrodes.
Clean the pipette thoroughly before using it add the next electrolyte.
4. After the chambers (at least 2, at most 8) have been prepared for the experiment as described, start measuring
voltages. In this experiment, each of 5 chambers is filled with a separate electrolyte and
the corresponding electrode is inserted to make up a galvanic cell:
Cu / CuSO
, Zn / ZnSO4 , Ag / AgNO3 , Fe / FeSO4 , Ni / NiSO4
4
5. To measure voltage, two experiment cables (red/blue with 2mm plugs - included in case) should be connected to the
voltmeter. The connection between the two electrodes and the meter is made by means of crocodile clips.
6. The voltage produced by the galvanic cell can be read off the meter. If a negative voltage is displayed, reverse the
polarity of the two electrodes.
Observation and evaluation:
In galvanic cells, the less electropositive metal always forms the negative pole.
Electrons always flow from negative to positive poles, i.e. for a zinc/copper combination, they flow from the zinc to the copper and in a
copper/silver combination, they flow from copper to silver.
The combinations involving zinc always have zinc as the negative pole and those involving silver always have silver as the positive pole
of the galvanic element. An electrochemical potential series for these metals thus has the following order:
Zn
Which of the electrodes forms the negative pole can be determined by reversing the polarity.
Galvanic cell Voltage (V)
Cu / Zn
Cu / Ag
Cu / Fe
Cu / Ni
Zn / Ag
Zn / Fe
Zn / Ni
Fe / Ag
Fe / Ni
Calculation of masses required to prepare a 0.1 molar solution:
The electrolyte solutions should be made up by the teacher in sufficient quantities (usually 1 liter suffices) in advance of the lesson.
1.
1 liter of 1.0 molar CuSO
2.
1 liter of 1.0 molar ZnSO
3. 1 liter of 1.0 molar AgNO
4. 1 liter of 1.0 molar FeSO
5.
1 liter of 1.0 molar NiSO4 solution: Add water to 262.70 g NiSO4 (6 H2O) up to 1 liter in a measuring flask.
To make up a 0.1 molar solution, simply use 1/10 of the quantities given above (for making a 1.0 molar solution)
and add water up to 1 liter in a measuring flask.
Ag / Ni
solution: Add water to 249.50 g CuSO4 (5 H2O) up to 1 liter in a measuring flask.
4
solution: Add water to 287.40 g ZnSO4 (7 H2O) up to 1 liter in a measuring flask.
4
solution: Add water to 169.88 g AgNO3 up to 1 liter in a measuring flask.
3
solution: Add water to 277.90 g FeSO4 (7 H2O) up to 1 liter in a measuring flask.
4
→
Fe → Ni → Cu → Ag
Electrolyte 1.0 mol/l
1.086 approx. 1.086 approx.
0.383 approx. 0.383 approx.
0.670 approx. 0.670 approx.
0.044 approx. 0.044 approx.
1.416 approx. 1.416 approx.
0.378 approx. 0.378 approx.
1.095 approx. 1.095 approx.
1.089 approx. 1.089 approx.
0.700 approx. 0.700 approx.
0.290 approx. 0.290 approx.
Electrolyte 0.1 mol/l
Voltage (V)
8
Page 8

Experiment 1 - Measuring voltage Student instructions
Measuring the voltage of galvanic cells
Chemicals Hazard
symbols
Copper (II)-sulfate-(5 H2O)
Zinc sulfate-(7H
Silver nitrate
Iron (II)-sulfate-(7 H
2
O)
2
O)
Nickel sulfate-(6H
Distilled water
2
O)
R phrases S phrases Equipment
22-36/38-50/53 22-60-61 Meter
36/38-50/53 22-25-60-61 Electrodes:
34-50/53 26-45-60-61 2 Experiment cables
22-36/38 24/25 2 Pipettes
22-40-42/43-
50/53
---
22-36/37-60-
61
---
1 Cu, 1 Zn,1 Ag, 1 Fe, 1 Ni
Warning:
Please take care:
Experiment procedure:
1. The prepared 1.0 and 0.1 molar electrolyte solutions should be given to the students. Students require no more than
10 ml of the relevant solution each.
2. Assemble the battery block as described.
3. Fill the chambers with electrolyte using the pipette (included in the case) and insert the appropriate electrodes.
Clean the pipette thoroughly before using it add the next electrolyte.
4. After the chambers (at least 2, at most 8) have been prepared for the experiment as described, start measuring
voltages.
In this experiment, each of 5 chambers is filled with a separate electrolyte and
the relevant electrodes are added to form a galvanic cell
Cu / CuSO
5. To measure voltage, two experiment cables (red/blue with 2mm plugs - included in case) should be connected to the
voltmeter. The connection between the two electrodes and the meter is made by means of crocodile clips.
6. The voltage produced by the galvanic cell can be read off the meter. If a negative voltage is displayed, reverse the
polarity of the two electrodes.
Observation and evaluation:
Enter the results of the experiment in the following table and evaluate them.
Galvanic cell Voltage (V)
Cu / Zn
Cu / Ag
Cu / Fe
Cu / Ni
Zn / Ag
Zn / Fe
Zn / Ni
Fe / Ag
Fe / Ni
Ag / Ni
Salts of heavy metals are poisonous!
, Zn / ZnSO4 , Ag / AgNO3 , Fe / FeSO4 , Ni / NiSO4
4
Electrolyte 1.0 mol/l
9
Voltage (V)
Electrolyte 0.1 mol/l
Page 9
Experiment 2 - Measuring voltage Teacher's instructions
Measuring the voltage of a Daniell cell
Chemicals Hazard
symbols
Copper (II)-sulfate-(5 H
Zinc sulfate-(7H
Distilled water
2
O)
2
O)
R phrases S phrases Equipment
22-36/38-50/53 22-60-61 Electrodes:
36/38-50/53 22-25-60-61 2 Experiment cables
--- --- 2 Pipettes
Meter
1 Cu, 1 Zn
Warning:
Please take care:
Salts of heavy metals are poisonous!
Experiment procedure:
1. The prepared 0.1 molar electrolyte solutions should be given to the studen ts. Students require no more than 10 ml of
the relevant solution each.
2. Assemble the battery block as described.
3. Fill one chamber each with electrolyte using the pipette (included in the case). Clean the pipette thoroughly before
using it add the next electrolyte.
4. Insert the appropriate electrodes into the electrolyte solutions, CuSO
5. After the chambers have been prepared for the experiment as described, connect the experiment cable s to the
meter and start measuring voltages. If a negative voltage is displayed, reverse the polarity of the two electrode
connections.
6. The experiment can also be repeated using 1.0 molar copper sulfate and zinc sulfate solutions.
Observation and evaluation:
/ Cu and ZnSO4 / Zn.
4
Sketch of experiment set-up:
+ +
Meter
Cu
-
Zn
The electrolyte solutions should be made up by the teacher in sufficient quantities (usually 1 liter suffices) in advance of the lesson.
Calculation of masses required to prepare a 0.1 molar solution:
1.
1 liter of 0.1 molar CuSO4 solution: Add water to 24.95 g CuSO4 (5 H2O) up to 1 liter in a measuring flask.
2.
1 liter of 0.1 molar ZnSO4 solution: Add water to 28.74 g ZnSO4 (7 H2O) up to 1 liter in a measuring flask.
Calculation of masses required to prepare a 1.0 molar solution:
1. 1 liter of 1.0 molar CuSO
2. 1 liter of 1.0 molar ZnSO
solution: Add water to 249.50 g CuSO4 (5 H2O) up to 1 liter in a measuring flask.
4
solution: Add water to 287.40 g ZnSO4 (7 H2O) up to 1 liter in a measuring flask.
4
When the meter is connected to the Daniell cell with Cu / CuSO4 or Zn / ZnSO
an electrolyte concentration of 0.1 mol/l. The result of the actual measurement is usually slightly less than the theoretical value at
1.06 V.
If a 1.0 molar solution is used in the Daniell cell, the measured voltage should still be 1.06 V.
the measured voltage should theoretically be 1.08 V for
4
10
Page 10

Experiment 2 - Measuring voltage Student instructions
Measuring the voltage of a Daniell cell
Warning:
Chemicals Hazard
symbols
Copper (II)-sulfate-(5 H
Zinc sulfate-(7H
Distilled water --- --- 2 Pipettes
2
Please take care:
O)
2
O)
Salts of heavy metals are poisonous!
R phrases S phrases Equipment
22-36/38-50/53 22-60-61 Electrodes:
36/38-50/53 22-25-60-61 2 Experiment cables
Meter
1 Cu, 1 Zn
Experiment procedure:
1. The prepared 0.1 molar electrolyte solutions should be given to the studen ts. Students require no more than 10 ml of
the relevant solution each.
2. Assemble the battery block as described.
3. Fill one chamber each with electrolyte using the pipette (included in the case). Clean the pipette thoroughly before
using it add the next electrolyte.
4. Insert the appropriate electrodes into the electrolyte solutions, CuSO
5. After the chambers have been prepared for the experiment as described, connect the experiment cable s to the
meter and start measuring voltages. If a negative voltage is displayed, reverse the polarity of the two electrode
connections.
6. The experiment can also be repeated using 1.0 molar copper sulfate and zinc sulfate solutions.
/ Cu and ZnSO4 / Zn.
4
Observation and evaluation:
Sketch of experiment set-up:
Results of voltage measurement:
Daniell cell
1.
1.0 molar solutions
with
CuSO
/ Cu and ZnSO4 / Zn:
4
V
Daniell cell
2.
0.1 molar solutions
with
CuSO
/ Cu and ZnSO4 / Zn:
4
V
11
Page 11

Experiment 3 - Measuring voltage Teacher's instructions
Measuring the voltage of three Daniell cells connected in parallel
Chemicals Hazard
R phrases S phrases Equipment
symbols
Copper (II)-sulfate-(5 H
Zinc sulfate-(7H2O)
2
O)
22-36/38-50/53 22-60-61 Electrodes:
36/38-50/53 22-25-60-61 6 Experiment cables
Meter
3 Cu, 3 Zn
Distilled water
--- --- 2 Pipettes
Warning:
Please take care:
Salts of heavy metals are poisonous!
Experiment procedure:
1. The prepared 0.1 molar electrolyte solutions should be given to the studen ts. Students require no more than 10 ml of
the relevant solution each.
2. Assemble the battery block as described.
3. Fill three chambers on one side of the battery block with 0.1 molar CuSO
4. Now fill three chambers on the other side of the battery block with 0.1 molar ZnSO
electrodes.
5. Connect the copper electrodes together using experiment cables with crocodile clips and then connect the last one
to the meter.
6. As described in step 5, connect the zinc electrodes together then connect them to the meter.
7. Read off the voltage from the meter.
(If a negative value is displayed on the meter, reverse the polarities of the cables to the meter.)
solution then insert the copper electrodes.
4
solution then insert the zinc
4
Observation and evaluation:
Sketch of experiment set-up:
+ +
Meter
-
Zn
Cu
In the parallel circuit, the measured voltage with three electrodes connected together should be V= 1.06 V approx. It can be seen that
connecting the electrodes in parallel does not lead to any increase in the voltage. What does increase in this case is the current as can
be measured using a multimeter.
The electrolyte solutions should be made up by the teacher in sufficient quantities (usually 1 liter suffices) in advance of the lesson.
Calculation of masses required to prepare a 0.1 molar solution:
1. 1 liter of 0.1 molar CuSO
2. 1 liter of 0.1 molar ZnSO4 solution: Add water to 28.74 g ZnSO4 (7 H2O) up to 1 liter in a measuring flask.
solution: Add water to 24.95 g CuSO4 (5 H2O) up to 1 liter in a measuring flask.
4
12
Page 12

Experiment 3 - Measuring voltage Student instructions
Measuring the voltage of three Daniell cells connected in parallel
Chemicals Hazard
symbols
Copper (II)-sulfate-(5 H
Zinc sulfate-(7H2O)
2
O)
R phrases S phrases Equipment
Meter
22-36/38-50/53 22-60-61 Electrodes:
36/38-50/53 22-25-60-61 6 Experiment cables
3 Cu, 3 Zn
Distilled water
--- --- 2 Pipettes
Warning:
Please take care:
Salts of heavy metals are poisonous!
Experiment procedure:
1. The prepared 0.1 molar electrolyte solutions should be given to the studen ts. Students require no more than 10 ml of
the relevant solution each.
2. Assemble the battery block as described.
3. Fill three chambers on one side of the battery block with 0.1 molar CuSO
4. Now fill three chambers on the other side of the battery block with 0.1 molar ZnSO
electrodes.
5. Connect the copper electrodes together using experiment cables with crocodile clips and then connect the last one
to the meter.
6. As described in step 5, connect the zinc electrodes together then connect them to the meter.
7. Read off the voltage from the meter.
(If a negative value is displayed on the meter, reverse the polarities of the cables to the meter.)
solution then insert the copper electrodes.
4
solution then insert the zinc
4
Observation and evaluation:
Sketch of experiment set-up:
Results of voltage measurement:
13
Page 13

Experiment 4 - Measuring voltage Teacher's instructions
Measuring the voltage of three Daniell cells connected in series
Chemicals Hazard
R phrases S phrases Equipment
symbols
Copper (II)-sulfate-(5 H
2
O)
22-36/38-50/53 22-60-61 Electrodes:
Zinc sulfate-(7H2O)
36/38-50/53 22-25-60-61 4 Experiment cables
Distilled water
--- --- 2 Pipettes
Meter
3 Cu, 3 Zn
Warning:
Please take care:
Salts of heavy metals are poisonous!
Experiment procedure:
1. The prepared 0.1 molar electrolyte solutions should be given to the studen ts. Students require no more than 10 ml of
the relevant solution each.
2. Assemble the battery block as described.
3. Fill three chambers on one side of the battery block with 0.1 molar CuSO
4. Now fill three chambers on the other side of the battery block with 0.1 molar ZnSO
electrodes.
5. Connect two copper electrodes to two zinc electrodes using experiment cables with crocodile clips and then connect
the last copper and the last zinc electrode to the meter.
6. Read off the voltage from the meter.
(If a negative value is displayed on the meter, reverse the polarities of the cables to the meter.)
solution then insert the copper electrodes.
4
solution then insert the zinc
4
Observation and evaluation:
Sketch of experiment set-up:
+ +
Meter
-
Zn
Cu
In the series circuit, the measured voltage with three electrodes connected together should be V= 3.18 V approx. It can be seen that
connecting the electrodes in series leads to an increase in voltage proportional to the number of cells connected. The current that flows
in this case is no higher than for the basic arrangement.
If a higher voltage is required, this can only be achieved by connecting cells in series.
The electrolyte solutions should be made up by the teacher in sufficient quantities (usually 1 liter suffices) in advance of the lesson.
Calculation of masses required to prepare a 0.1 molar solution:
1. 1 liter of 0.1 molar CuSO
2. 1 liter of 0.1 molar ZnSO4 solution: Add water to 28.74 g ZnSO4 (7 H2O) up to 1 liter in a measuring flask.
solution: Add water to 24.95 g CuSO4 (5 H2O) up to 1 liter in a measuring flask.
4
14
Page 14

Experiment 4 - Measuring voltage Student instructions
Measuring the voltage of three Daniell cells connected in series
Chemicals Hazard
R phrases S phrases Equipment
symbols
Copper (II)-sulfate-(5 H
2
O)
22-36/38-50/53 22-60-61 Electrodes:
Zinc sulfate-(7H
2
O)
36/38-50/53 22-25-60-61 4 Experiment cables
Distilled water
--- --- 2 Pipettes
Meter
3 Cu, 3 Zn
Warning:
Please take care:
Experiment procedure:
1. The prepared 0.1 molar electrolyte solutions should be given to the studen ts. Students require no more than 10 ml of
the relevant solution each.
2. Assemble the battery block as described.
3. Fill three chambers on one side of the battery block with 0.1 molar CuSO
4. Now fill three chambers on the other side of the battery block with 0.1 molar ZnSO
electrodes.
5. Connect two copper electrodes to two zinc electrodes using experiment cables with crocodile clips and then connect
the last copper and the last zinc electrode to the meter.
6. Read off the voltage from the meter.
(If a negative value is displayed on the meter, reverse the polarities of the cables to the meter.)
Salts of heavy metals are poisonous!
solution then insert the copper electrodes.
4
solution then insert the zinc
4
Observation and evaluation:
Sketch of experiment set-up:
Results of voltage measurement:
15
Page 15

Experiment 5 - Measuring voltage Teacher's instructions
Measuring the standard potential of various metals
Chemicals Hazard
R phrases S phrases Equipment
symbols
Copper (II)-sulfate-(5 H2O)
22-36/38-50/53 22-60-61 Electrodes:
Zinc sulfate-(7H
2
O)
36/38-50/53 22-25-60-61 2 Experiment cables
Meter
1 Cu, 1 Zn, 1 Ag, 1 Fe,
1 platinum gauze
Silver nitrate
Iron (II)-sulfate-(7 H2O)
Hydrochloric acid 1 mol/l
Distilled water
34-50/53 26-45-60-61 1 Mains power supply
22-36/38 24/25 2 Pipettes
36/37/38 26 1 3V adapter
--- ---
Warning:
Please take care:
Salts of heavy metals are poisonous! Hydrochloric acid is corrosive!
Experiment procedure:
1. The prepared 1.0 molar electrolyte solutions should be given to the studen ts. Students require no more than 10 ml of
the relevant solution each.
2. Assemble the battery block as described.
3. Add the 1 molar hydrochloric acid to one chamber of the battery block using the pipette and insert the pl atinum
gauze electrode into this cell.
4. Add a 1 molar CuSO
solution to a second chamber (opposite the platinum gauze electrode) and insert a copp er
4
electrode.
5. To form a normalized hydrogen electrode, a 3V adapter is connected to the power supply. Connect the negative
pole of the 3V adapter to the platinum gauze electrode and the positive pole to the copper electrod e using
experiment cables. Connect the power supply to the 230 V mains and electrolyze the platinum gauze for about 30
seconds. Hydrogen forms at the platinum gauze and completely surrounds the gauze.
6. The 3V adapter is then replaced by the meter and the voltage can then be read off.
7. Proceed as in steps 4 to 6 with each of the other metals using the corresponding electrolyte for each metal (AgNO
Ag , ZnSO
/ Zn and FeSO4 / Fe).
4
Observation and evaluation:
Pt _ connection
3V adapter
+ 230V
Fe Ag Zn Cu
(4) (3) (2) (1)
For each redox pair, the standard electrochemical potentials as measured should be:
(1) Cu / Cu
Calculation of masses required to prepare 1 liter of a 1 molar solution:
1. For a CuSO
2. For a ZnSO
3. For a AgNO
4. For an FeSO
2+
=
+ 0.34 V
4
4
3
, (2) Zn / Zn 2+ =
solution 249.50 g of CuSO
solution 287.40 g of ZnSO
solution 169.88 g of AgNO3 is needed.
solution 277.91 g of FeSO
4
4
4
, (3) Ag / Ag + =
- 0.76 V
⋅
5 H2O is needed.
⋅
7 H2O is needed.
⋅
7 H2O is needed.
4
+ 0.80 V
, (4) Fe / Fe2- =
- 0.44 V
16
/
3
Page 16

Experiment 5 - Measuring voltage Student instructions
Measuring the standard potential of various metals
Chemicals Hazard
R phrases S phrases Equipment
symbols
Copper (II)-sulfate-(5 H
2
O)
22-36/38-50/53 22-60-61 Electrodes:
Zinc sulfate-(7H
2
O)
36/38-50/53 22-25-60-61 2 Experiment cables
Meter
1 Cu, 1 Zn, 1 Ag, 1 Fe,
1 platinum gauze
Silver nitrate
Iron (II)-sulfate-(7 H2O)
Hydrochloric acid 1 mol/l
Distilled water
34-50/53 26-45-60-61 1 Mains power supply
22-36/38 24/25 2 Pipettes
36/37/38 26 1 3V adapter
--- ---
Warning:
Please take care:
Salts of heavy metals are poisonous! Hydrochloric acid is corrosive!
Experiment procedure:
1. The prepared 1.0 molar electrolyte solutions should be given to the studen ts. Students require no more than 10 ml of
the relevant solution each.
2. Assemble the battery block as described.
3. Add the 1 molar hydrochloric acid to one chamber of the battery block using the pipette and insert the pl atinum
gauze electrode into this cell.
4. Add a 1 molar CuSO
electrode.
5. To form a normalized hydrogen electrode, a 3V adapter is connected to the power supply. Connect the negative
pole of the 3V adapter to the platinum gauze electrode and the positive pole to the copper electrod e using
experiment cables. Connect the power supply to the 230 V mains and electrolyze the platinum gauze for about 30
seconds. Hydrogen forms at the platinum gauze and completely surrounds the gauze.
6. The 3V adapter is then replaced by the meter and the voltage can then be read off.
7. Proceed as in steps 4 to 6 with each of the other metals using the corresponding electrolyte for each metal (AgNO
Ag , ZnSO
/ Zn and FeSO4 / Fe).
4
solution to a second chamber (opposite the platinum gauze electrode) and insert a copp er
4
Observation and evaluation:
Sketch of experiment set-up:
Result of voltage measurement:
Cu / Cu
Zn / Zn
Ag / Ag
Fe / Fe
2+
2+
V
+
V
2+
V
V
/
3
17
Page 17

Experiment 6 - Measuring voltage Teacher's instructions
Measuring the standard electrochemical potentials of various non-metals
Chemicals Hazard
R phrases S phrases Equipment
symbols
Sodium chloride
Potassium bromide
Sodium iodide
Hydrochloric acid 1 mol/l
---
---
--- --- 2 Experiment cables
--- --- 1 Mains power supply
36/37/38 26 2 Pipettes
Distilled water
--- --- 1 3V adapter
Meter
Electrodes:
2 C, 1 Pt gauze
Warning:
Please take care:
Hydrochloric acid is corrosive!
Experiment procedure:
1. The prepared 1.0 molar electrolyte solutions should be given to the studen ts. Students require no more than 10 ml of
the relevant solution each.
2. Assemble the battery block as described.
3. Add the 1 molar hydrochloric acid to one chamber of the battery block using the pipette and insert the pl atinum
gauze electrode into this cell.
4. Add a 1 molar NaCl solution to a second chamber (opposite the platinum gauze electrode) and insert a carbon
electrode.
5. To form a normalized hydrogen electrode, a 3V adapter is connected to the power supply. Connect the negative
pole of the 3V adapter to the platinum gauze electrode and the positive pole to the carbon electrode using
experiment cables. Connect the power supply to the 230 V mains and electrolyze the platinum gauze for about 30
seconds. Hydrogen forms at the platinum gauze and completely surrounds the gauze.
6. The 3V adapter is then replaced by the meter and the Cl
7. Proceed as in steps 4 to 6 with each of the other non metals dipping the carbon electrode into potassium bromid e
and potassium iodide solutions one after the other to determine the electrochemical potentials of Br- / Br2 and I- / I2 .
Observation and evaluation:
Pt _ connection
3V adapter
+ 230V
C C C
(3) (2) (1)
For each redox pair, the standard electrochemical potentials as measured should be:
-
/ Cl
(1) Cl
Calculation of masses required to prepare 1 liter of a 1 molar solution:
1. For an NaCl solution 58.44 g of NaCl is needed.
2. For a KBr solution 119.01 g of KBr is needed.
3. For a KI solution 166.00 g of KI is needed.
=
2
+ 1.35 V
, (2) Br
-
/ Br
=
2
+ 1.06 V
, (3) I
-
/ Cl2 voltage can then be read off.
-
/ I2 =
+ 0.54 V
18
Page 18

Experiment 6 - Measuring voltage Student instructions
Measuring the standard electrochemical potentials of various non-metals
Chemicals Hazard
R phrases S phrases Equipment
symbols
Sodium chloride
Potassium bromide
Sodium iodide
Hydrochloric acid 1 mol/l
---
--- --- 2 Experiment cables
--- --- 1 Mains power supply
36/37/38 26 2 Pipettes
---
Distilled water
--- --- 1 3V adapter
Meter
Electrodes:
2 C, 1 Pt gauze
Warning:
Please take care:
Hydrochloric acid is corrosive!
Experiment procedure:
1. The prepared 1.0 molar electrolyte solutions should be given to the students. Students require no more
than 10 ml of the relevant solution each.
2. Assemble the battery block as described.
3. Add the 1 molar hydrochloric acid to one chamber of the battery block using the pipette and insert the
platinum gauze electrode into this cell.
4. Add a 1 molar NaCl solution to a second chamber (opposite the platinum gauze electrode) and insert a
carbon electrode.
5. To form a normalized hydrogen electrode, a 3V adapter is connected to the power supply. Connect the
negative pole of the 3V adapter to the platinum gauze electrode and the positive pole to the carbon
electrode using experiment cables. Connect the power supply to the 230 V mains and electrolyze the
platinum gauze for about 30 seconds. Hydrogen forms at the platinum gauze and completely surrounds
the gauze.
6. The 3V adapter is then replaced by the meter and the Cl
7. Proceed as in steps 4 to 6 with each of the other non metals dipping the carbon electrode into potassium
bromide and potassium iodide one after the other to determine the electrochemical potentials of Br
-
and I
/ I2 .
Observation and evaluation:
Sketch of experiment set-up:
Result of voltage measurement:
Cl - / Cl
2
V
Br - / Br2 V
I - / I2 V
-
/ Cl2 voltage can then be read off.
-
/ Br2
19
Page 19

Experiment 7 - Measuring voltage Teacher's instructions
Measuring the voltage of a zinc-carbon (Leclanché) cell
Chemicals Hazard
R phrases S phrases Equipment
symbols
Graphite powder
Manganese (IV) oxide
Ammonium chloride
Distilled water
Warning: Be careful when handling chemicals!
--20/22 25 2 Experiment cables
22-36 22 1 Plastic beaker
--- --- 2 Pipettes
---
Meter
Electrodes:
1 C, 1 Zn
Experiment procedure:
1. To assemble a zinc-carbon or Leclanché cell a 20% ammonium chloride solution and some depolarizing paste are
required. These should be given to the students.
2. Assemble the battery block as described.
3. Fill one chamber each with 20% ammonium chloride solution and insert the zinc electrode. Fill the cham ber opposite
with a depolarizing paste consisting of graphite powder, manganese (IV) oxide (battery manganese) and ammonium
chloride solution and insert the carbon electrode. The depolarizing paste is there to prevent hydrogen forming at the
carbon electrode.
4. Connect the two electrodes to the meter using 2 experiment cables with crocodile clips and measure the voltage
generated by the Leclanché cell.
Observation and evaluation:
Batteries are important where there is no electric current. Batteries are galvanic cells that can convert chemical energy directly into
electrical energy.
The dry cell (Leclanché cell) is an economically important type of galvanic cell. It supplies a voltage of between 1.3 and 1.4 V, as can be
read from the meter.
C
+ +
Meter
-
Zn
Calculation of the mass of NH4Cl required to prepare 1 liter of a 20% solution:
Put the beaker on a set of scales and adjust the scales to show 0.0 g. Fill the beaker with 200 g of NH
display a mass 1000 g . This makes up a 20% solution.
To make depolarizing paste for about 30 students:
45g of graphite powder should be well mixed with 225 g of manganese (IV) oxide and 225 ml of 20% ammonium chloride solution. The
resulting paste should not be stored for more than 1 day.
After use, the paste should be disposed of in the container for inorganic substances.
Cl then add water until the scales
4
20
Page 20

Experiment 7 - Measuring voltage Student instructions
Measuring the voltage of a zinc-carbon (Leclanché) cell
Chemicals Hazard
R phrases S phrases Equipment
symbols
Graphite powder
Manganese (IV) oxide
Ammonium chloride
Distilled water
Warning: Be careful when handling chemicals!
--20/22 25 2 Experiment cables
22-36 22 1 Plastic beaker
--- --- 2 Pipettes
---
Meter
Electrodes:
1 C, 1 Zn
Experiment procedure:
1. To assemble a zinc-carbon or Leclanché cell a 20% ammonium chloride solution and some depolarizing paste are
required. These should be given to the students.
2. Assemble the battery block as described.
3. Fill one chamber each with 20% ammonium chloride solution and insert the zinc electrode. Fill the cham ber opposite
with a depolarizing paste consisting of graphite powder, manganese (IV) oxide (battery manganese) and ammonium
chloride solution and insert the carbon electrode. The depolarizing paste is there to prevent hydrogen forming at the
carbon electrode.
4. Connect the two electrodes to the meter using 2 experiment cables with crocodile clips and measure the voltage
generated by the Leclanché cell.
Observation and evaluation:
Sketch of experiment set-up:
Results of voltage measurement:
The Leclanché cell supplies a voltage of V.
21
Page 21

Experiment 8 - Measuring voltage Teacher's instructions
Measuring the voltage for various electrolyte concentrations
Chemicals Hazard
symbols
Silver nitrate
Distilled water
Warning: Be careful when handling silver nitrate! Silver nitrate is corrosive!
Experiment procedure:
1. Silver nitrate solutions of various concentrations should be given to the students.
2. Assemble the battery block as described.
3. Fill two chambers located opposite one another in the battery block with 0.1 molar silver nitrate soluti on and insert a
silver electrode into both of them.
4. Fill two more chambers with 1 molar and 0.01 molar silver nitrate solution.
5. First measure the voltage for the Ag / AgNO
be connected to the meter.
6. Remove one silver electrode from the 0.1 molar silver nitrate solution, rinse thoroughly with water and insert into the
1 molar silver nitrate solution. The voltage produced by the
Ag / AgNO
0.1 mol/l
(
3
) //Ag / AgNO3 (
1.0 mol/l
7. Next remove the silver electrode from the 1 molar silver nitrate solution, rinse thoroughly with water again and insert
into the 0.01 molar silver nitrate solution. The voltage from the Ag / AgNO3 (
can now be read off from the meter.
8. For the next experiment remove the silver electrode from the 0.1 molar silver nitrate solution, rinse thoroughly with
water and insert into the 1.0 molar silver nitrate solution. The voltage from the Ag / AgNO
0.01 mol/l
(
Observation and evaluation:
Measuring a voltage from chains of electrolyte of equal concentration is impossible. To measure a voltage the electrolyte concentrations
have to be different. If the electrolyte concentrations differ by a factor of 10, the voltage measured is 0.058 V, regardless of whether
measurement is made between 1.0 mol/l and 0.1 mol/l or 0,1 mol/l and 0.01 mol/l concentrations. Each further increase by a factor of 10
increases the measured voltage by another 0.058 V. Due to the diffusion of the more concentrated electrolyte solution through the paper
diaphragm, the concentrations in the two half cells tend to become more equal so that the potential difference decreases over time.
In the more concentrated silver nitrate solution, silver ions are reduced to silver and in the more dilute solution the silver goes into
solution creating silver ions.. This means that the silver electrode in the more concentrated solution is the cathode (+ pole) and the one
in the more dilute solution is the anode (- pole).
) cell can now be read off from the meter.
Galvanic cell Measured voltage (V)
Ag / AgNO3 (0,1 mol/l) // Ag / AgNO
Ag / AgNO3 (0,1 mol/l) // Ag / AgNO
Ag / AgNO3 (0,1 mol/l) // Ag / AgNO
Ag / AgNO3 (1,0 mol/l) // Ag / AgNO3 (0,01 mol/l) 0.116 V
+ +
-
1.0 mol/l 0.01 mol/l 0.1 mol/l
To make 1 liter of the required solutions:
1. To make a 1 molar solution of AgNO
2. To make a 0.1 molar solution of AgNO
3. To make a 0.01 molar solution of AgNO
solution, 169.8 g of AgNO3 should be dissolved in a liter of water.
3
solution, 16.98 g of AgNO3 should be dissolved in a liter of water.
3
solution, 1.69 g of AgNO3 should be dissolved in a liter of water.
3
R phrases S phrases Equipment
34-50/53 26-45-60-61 Electrodes:
--- --- 2 Experiment cables
2 Pipettes
0.1 mol/l
(
3
) //Ag / AgNO3 (
) cell can now be read off from the meter.
(0,1 mol/l) --
3
(1.0 mol/l) 0.058 V
3
(0.01 mol/l) 0.058 V
3
l Meter
0,1 mol/
0.1 mol/l
0.1 mol/l
Meter
2 Ag
) cell. Both silver electrodes should
) //Ag / AgNO3 (
1.0 mol/l
(
3
0.01 mol/l
) cell
) //Ag / AgNO3
22
Page 22

Experiment 8 - Measuring voltage Student instructions
Measuring the voltage for various electrolyte concentrations
Chemicals Hazard
R phrases S phrases Equipment
symbols
Silver nitrate
34-50/53 26-45-60-61 Electrodes:
Distilled water
Warning: Be careful when handling silver nitrate! Silver nitrate is corrosive!
--- --- 2 Experiment cables
2 Pipettes
Meter
2 Ag
Experiment procedure:
1. Silver nitrate solutions of various concentrations should be given to the students.
2. Assemble the battery block as described.
3. Fill two chambers located opposite one another in the battery block with 0.1 molar silver nitrate soluti on and insert a
silver electrode into both of them.
4. Fill two more chambers with 1 molar and 0.01 molar silver nitrate solution.
5. First measure the voltage for the Ag / AgNO3 (
be connected to the meter.
6. Remove one silver electrode from the 0.1 molar silver nitrate solution, rinse thoroughly with water and insert into the
1 molar silver nitrate solution. The voltage produced by the
Ag / AgNO3 (
7. Next remove the silver electrode from the 1 molar silver nitrate solution, rinse thoroughly with water again
and insert into the 0.01 molar silver nitrate solution. The voltage produced by the
Ag / AgNO
For the next experiment remove the silver electrode from the 0.1 molar silver nitrate solution, rinse thoroughly with
8.
water and insert into the 1.0 molar silver nitrate solution. The voltage from the Ag / AgNO
0.01 mol/l
(
0.1 mol/l
0.1 mol/l
(
3
) cell can now be read off from the meter.
) //Ag / AgNO3 (
) //Ag / AgNO3 (
1.0 mol/l
0.01 mol/l
0.1 mol/l
) cell can now be read off from the meter.
) //Ag / AgNO3 (
) cell can now be read off from the meter.
0.1 mol/l
) cell. Both silver electrodes should
1.0 mol/l
(
3
) //Ag / AgNO3
Observation and evaluation:
Sketch of experiment set-up:
Result of voltage measurement:
Galvanic cell Measured
voltage (V)
Ag / AgNO3 (0.1 mol/l) // Ag / AgNO
Ag / AgNO3 (0.1 mol/l) // Ag / AgNO
Ag / AgNO3 (0.1 mol/l) // Ag / AgNO
Ag / AgNO3 (0.1 mol/l) // Ag / AgNO
(0,1 mol/l)
3
(1.0 mol/l)
3
(0.01 mol/l)
3
(1.0 mol/l)
3
23
Page 23

Experiment 9 - Measuring voltage Teacher's instructions
Measuring the voltage for various electrolyte temperatures
Chemicals Hazard
R phrases S phrases Equipment
symbols
Silver nitrate
34-50/53 26-45-60-61 Electrodes:
Distilled water
Warning: Be careful when handling silver nitrate! Silver nitrate is corrosive!
--- --- 2 Experiment cables
1 Beaker
1 Bunsen burner
1 Thermometer
2 Pipettes
Meter
2 Ag
Experiment procedure:
1. The 0.01 molar silver nitrate solution should be given to the students.
2. Assemble the battery block as described.
Put about 15 ml of 0.01 molar silver nitrate solution in a beaker and heat it to about 70-80°C .
3.
Fill one chamber of the battery block with 0.01 molar silver nitrate at room temperature and put the hot 0.01 molar
4.
silver nitrate solution into the chamber opposite.
Insert one silver electrode into both electrolyte solutions, connect the electrodes to the meter and read off the
5.
voltage.
Observation and evaluation:
By contrast with the previous observation that no voltage can be measured between electrolytes of the same concentration, it can be
observed here that a voltage is indeed measured if the equally concentrated electrolytes are at different temperatures. This means that
differences in temperature between electrolyte solutions can have an effect on the resulting potential (theoretically about 2 mV/10K). In
the experiment a voltage of about 20 mV is measured. This gradually decreases as the temperatures of the two electrolyte solutions
converge. When the temperatures are equal, the measured voltage is 0 V.
Silver nitrate solution at room temperature
+ +
0.01 mol/
-
0.01 mol/l
Silver nitrate solution at 70-80°C
To make 1 liter of the electrolyte solution:
To make a 0.01 molar solution of AgNO
, 1.69 g of AgNO3 should be dissolved in a liter of water.
3
l Meter
24
Page 24

Experiment 9 - Measuring voltage Student instructions
Measuring the voltage for various electrolyte temperatures
Chemicals Hazard
R phrases S phrases Equipment
symbols
Silver nitrate
34-50/53 26-45-60-61 Electrodes:
Distilled water
Warning: Be careful when handling silver nitrate! Silver nitrate is corrosive!
--- --- 2 Experiment cables
1 Beaker
1 Bunsen burner
1 Thermometer
2 Pipettes
Meter
2 Ag
Experiment procedure:
1. The 0.1 molar silver nitrate solution should be given to the students.
2. Assemble the battery block as described.
3. Put about 15 ml of 0.1 molar silver nitrate solution in a beaker and heat it to about 70-80° .
4. Fill one chamber of the battery block with 0.1 molar silver nitrate at room temperature and
put the hot 0.01 molar silver nitrate solution into the chamber opposite.
5. Insert one silver electrode into both electrolyte solutions, connect the electrodes to the meter and
read off the voltage.
Observation and evaluation:
Sketch of experiment set-up:
Observation during the experiment:
25
Page 25

Experiment 10 - Measuring voltage Teacher's instructions
Assembly, charging and discharging of a steel accumulator
Chemicals Hazard
R phrases S phrases Equipment
symbols
Potassium hydroxide 20%
approx.
(≈ 4 molar)
Distilled water
22-35
--- --- 2 Experiment cables
2 Pipettes
26-36/37/39-45 Electrodes:
Warning: Be careful when handling potassium hydroxide! Wear protective glasses!
Experiment procedure:
1. The suitably concentrated potassium hydroxide (20%) required for the experiment should be given to the students.
2. Assemble the battery block as described.
3. Fill two opposing half cell chambers with 20% potassium hydroxide.
4. Insert an iron electrode into one chamber and a nickel electrode into the other.
5. To charge up the cell, connect the 3V adapter so that the nickel electrode is connected to the + pole and the iron
electrode is connected to the - pole.
After connecting the 3V adapter to the mains power supply and plugging the latter into the 230V mains, allow the
6.
accumulator to charge up for about 10 minutes.
7. The 3V adapter is then replaced by the meter and the voltage produced by the accumulator can then be measured.
Observation and evaluation:
Fe
20% potassium hydroxide
_ connection
3V adapter
+ 230V
20% potassium hydroxide Ni
The voltage displayed on the meter should be read off and written down. The no-load voltage at the terminals is
1.3 V approx.
If the meter is connected for a longer period of time, it can be observed that the voltage decreases rapidly because the capacitance of
this co-called Edison accumulator is very low.
The following chemical processes take place within the Edison accumulator:
Discharging
2 NiO(OH) + Fe + 2 H
Charging
O 2 Ni(OH)2 + Fe(OH)2
2
Meter
1 Ni, 1 Fe
26
Page 26

Experiment 10 - Measuring voltage Student instructions
Assembly, charging and discharging of a steel accumulator
Chemicals Hazard
R phrases S phrases Equipment
symbols
Potassium hydroxide 20%
approx.
(≈ 4 molar)
Distilled water
22-35
--- --- 2 Experiment cables
2 Pipettes
26-36/37/39-45 Electrodes:
Warning: Be careful when handling potassium hydroxide! Wear protective glasses!
Meter
1 Ni, 1 Fe
Experiment procedure:
1. The suitably concentrated potassium hydroxide (20%) required for the experiment should be given to the students.
2. Assemble the battery block as described.
3. Fill two opposing half cell chambers with 20% potassium hydroxide.
4. Insert an iron electrode into one chamber and a nickel electrode into the other.
5. To charge up the cell, connect the 3V adapter so that the nickel electrode is connected to the + pole and the iron
electrode is connected to the - pole.
6. After connecting the 3V adapter to the mains power supply and plugging the latter into the 230V mains, allow the
accumulator to charge up for about 10 minutes.
7. The 3V adapter is then replaced by the meter and the voltage produced by the accumulator can then be measured.
Observation and evaluation:
Sketch of experiment set-up:
Observation for the experiment:
27
Page 27

Experiment 11 - measuring pH Teacher's instructions
Chemicals Hazard
symbols
Buffer solution pH 4 or 7
Hydrochloric acid
Sodium hydroxide
Sodium chloride solution
Sodium acetate solution
Warning: Be careful when handling acids and alkalis! Wear protective glasses!
The Electrochemistry case contains a combined pH probe electro de for measuring pH. The meter displays
highly accurate measurements with a single point calibration. Since the meter is bat tery powered, it can also
be used to make pH measurements outside the classroom, e.g. in lakes and rivers.
Experiment procedure:
1. Take the combined pH probe out of the case and plug it into the pH connection on the underside of the meter.
2. Change the range switch on the front of the meter from voltage measurement to pH measurement.
3. Now take the pH probe out of its storage flask and rinse it with distilled water.
4. Immerse the pH probe in the buffer solution, wait for a moment and then adjust the zero point until it shows the
correct value for the buffer solution. Make no more adjustments to the rotary knob thereafter.
5. Immerse the probe in the prepared solutions one after the other and read off the corresponding pH values. After
each measurement, thoroughly clean the probe. Take care when doing this not to damage the probe's glass head.
Observation and evaluation:
In order to determine the degree of acidity or alkalinity of fluids, one determines their pH value. The pH value can either be determined
with the help of indicators, which change their color depending on whether they are in an acid or alkaline solution, or with a combined
pH probe, which displays the pH highly accurately on the digital display of a meter. The pH value measured with the probe is much
more accurate as it can also measure fractional values. The pH value is dependent on the concentration of H
The scale runs from pH 0 to pH 14.
pH 0 – 6 = acid
pH 7 = neutral
pH 8 -14 = alkali
Experimental result:
A variety of pH values are found depending on the concentrations of the solutions under investigation Therefore no specific results for
the pH can be given. Use the table to record the pH values measured by the students for the various tested solutions.
Tested solution Measured pH value
Hydrochloric acid
Sodium hydroxide
Sodium chloride solution
Sodium acetate solution
R phrases S phrases Equipment
---
36/37/38
34
---
--- ---
28
---
26
26-36/37/39-45
---
Meter
Electrodes:
Combined pH probe
O- ions in a solution.
3
Page 28

Experiment 11 - measuring pH Student instructions
Chemicals Hazard
Buffer solution pH 4 or 7
Hydrochloric acid
Sodium hydroxide
Sodium chloride solution
Sodium acetate solution
Warning: Be careful when handling acids and alkalis! Wear protective glasses!
The Electrochemistry case contains a combined pH probe electro de for measuring pH. The meter displays
highly accurate measurements with a single point calibration. Since the meter is battery powered, it can also
be used to make pH measurements outside the classroom, e.g. in lakes and rivers.
Experiment procedure:
1. Take the pH probe out of the case and plug it into the pH connection on the underside of the meter.
2. Change the range switch on the front of the meter from voltage measurement to pH measurement.
3. Now take the pH probe out of its storage flask and rinse it with distilled water.
4. Immerse the pH probe in the buffer solution, wait for a moment and then adjust the zero point until it shows the
correct value for the buffer solution. Make no more adjustments to the rotary knob thereafter.
Immerse the probe in the prepared solutions one after the other and read off the correspondin g pH values. After
5.
each measurement, thoroughly clean the probe. Take care when doing this not to damage the probe's glass head.
Observation and evaluation:
symbols
R phrases S phrases Equipment
---
36/37/38
34
---
--- ---
---
26
26-36/37/39-45
---
Meter
Electrodes:
Single probe pH
measuring equipment
Tested solution Measured pH value
Hydrochloric acid
Sodium hydroxide
Sodium chloride
solution
Sodium acetate
solution
29
Page 29

Standard electrochemical potential series
Summary of some important theoretical standard electrochemical potentials:
Reducing agent ' Oxidizing agent + n e- Standard potential
Au
Au
Pt
Ag
Fe
Cu
Cu +
H2
Cu
Pb
Ni
Fe
Zn
Al
Mg
K
Li
'
'
'
'
'
'
'
'
'
'
'
'
'
'
'
'
'
( in V )
+
+ e- + 1.70
Au
3+
+ 3 e- + 1.42
Au
2+
+2 e- + 1.20
Pt
+
+ e- + 0.81
Ag
3+
+ e- + 0.77
Fe
2+
+2 e- + 0.34
Cu
2+
+ e- + 0.15
Cu
+
+2 e- 0.00
2 H
+
+ 3 e- - 0.036
Cu
2+
+2 e- - 0.12
Pb
2+
+2 e- - 0.23
Ni
2+
+2 e- - 0.44
Fe
2+
+2 e- - 0.76
Zn
3+
+ 3 e- - 1.66
Al
2+
+2 e- - 2.37
Mg
+
+ e- - 2.92
K
+
+ e- - 3.02
Li
Increasing concentration of Increasing concentration of
Oxidizing agent Reducing agent
Noble metals can be distinguished from less noble metals on the basis of the difference in voltage that is measured.
Noble metals have positive potentials and ignoble metals have negative p otentials.
If zinc, not a noble metal, is assigned the potential 0, then the electropositivity of metals towards the most noble metals
can be shown in an overview such as the following, based on the differences in voltage:
Zn Fe Pb Ni Cu Ag
0 0,5 1,0 1,5
Increasingly electropositive (noble)
30
Page 30

Notes on performing experiments
The teacher is responsible in all aspects for ensuring that pupils conduct the experiments in an
orderly and proper fashion. The teacher must become thoroughly familiar with the experimental
procedure and the handling of the appropriate equipment before the experiment is performed.
Students should be warned of possible dangers and be advised on the prevention of accidents.
All chemistry teachers should be informed concerning all aspects of safety regulations, accident
avoidance and prevention and are obliged to abide by these.
Safety regulations and regulations for the handling of chemicals are specified in the laws
concerning chemicals, hazardous materials and in the technical regulations for hazardous
materials. Further byelaws and guidelines for local regions are legally binding upon the school.
Instructions for disposal:
All equipment and electrodes should be cleaned and dried as thoroughly as possible after
experiments are completed in order to ensure their continued functionality.
Please dispose of residues and waste with due regard to the environment.
Those chemicals that have been used, which cannot be recycled for further use and need to be
destroyed, should be stored in special containers and disposed of in an appropriate manner.
Types of waste:
Inorganic acids
1.
Disposal:
2. Alkalis
Disposal:
3. Inorganic salts
Disposal:
4. Salts of heavy metals
Disposal:
container for acids and alkalis!
container for acids and alkalis
container for inorganic salts
to be agreed with the party responsible for their disposal.
Disposal of substances should always be agreed with the party responsible for disposal. If
so agreed, substances may be dissolved in water in order to stabilize them.
31
 Loading...
Loading...