Page 1
©2014 Welch Allyn, Inc.
MM 721462 Ver.
Fiber optic laryngoscope handles
Directions for use
1
C
Intended
About this
use
The
laryngoscope blades which are used to examine
placement of a tracheal
document
This directions for use apply to Welch Allyn reusable fiber optic laryngoscope handles:
60813, 60814, 60815. Welch Allyn
with
MacIntosh
Warnings and
WARNING: R
use.
WARNING: The reprocessing procedure and the equipment and materials described
must be followed and conducted by persons trained and familiar
reprocessing.
WARNING: Consult cleaning and disinfecting agent manufacturer
proper preparation and use.
WARNING: Repeated
inspection procedures to assure damage has not occurred to the handle.
WARNING: High level disinfection and/or sterilization are not achieved by these
methods.
WARNING:
WARNING: Discard any component that shows evidence of damage or
deterioration.
WARNING: Do not modify this equipment. Any modification of this
lead to patient injury. Any modification of this equipment voids the
WARNING: Personnel shall
appropriate personal protective equipment when handling
equipment.
WARNING:
fiber optic laryngoscope handle is an accessory used w i t h co m p a t i b l e ri g i d f i b e r op t i c
and visualize
tube.
reusable
Welch Allyn fiber optic laryngoscope blades MacIntosh
REF:
6921X, and Miller REF: 6806X.
fiber optic laryngoscope handles may be used
a patient’s airway
REF:
6906X, English
and
aid
cautions
eusable
fiber optic
laryngoscope handles
must be reprocessed
after
with
medical device
instructions
reprocessing may
Lamps, if left illuminated, could generate sufficient heat to cause burns
follow their facility policies
Laryngoscope equipment is not suitable for use in intense
degrade
elements of the handle.
product warranty.
and
procedures
potentially
and
contaminated
magnetic
for their
Follow
equipment may
wear
fields
each
CAUTION:
damage o r poor performance of the handle.
CAUTION: If the device will be unused for several months or longer, remove
batteries prior to storing the device.
Only use lamp specified. Failure
to follow these instructions may cause
the
Page 2
s
2
Directions for use
Welch Allyn fiber optic laryngoscope handle
Reprocessing
instructions
These reprocessing instructions refer to procedures for cleaning and intermediate level
disinfection. Fiber optic laryngoscope handles must be reprocessed prior to first use and
between each use using
• Cleaning
the
following method as outlined in this
and intermediate level
disinfection
Welch Allyn has validated the above instruction as being capable of preparin g these
laryngoscope handles for re-use. The user must ensure that the reprocessing as actually
performed by the user's personnel, with the user's equipment and materials, achieves the
desired result. This may require validation and routine monitoring of the user's actual proce ss
NOTE: The main handle and bottom cap components are compatible with the disinfection
solution and autoclave methods identified which are provided for facilities who wish to perform
either method after cleaning and intermediate level disinfection.
Cleaning and intermediate level disinfection instructions
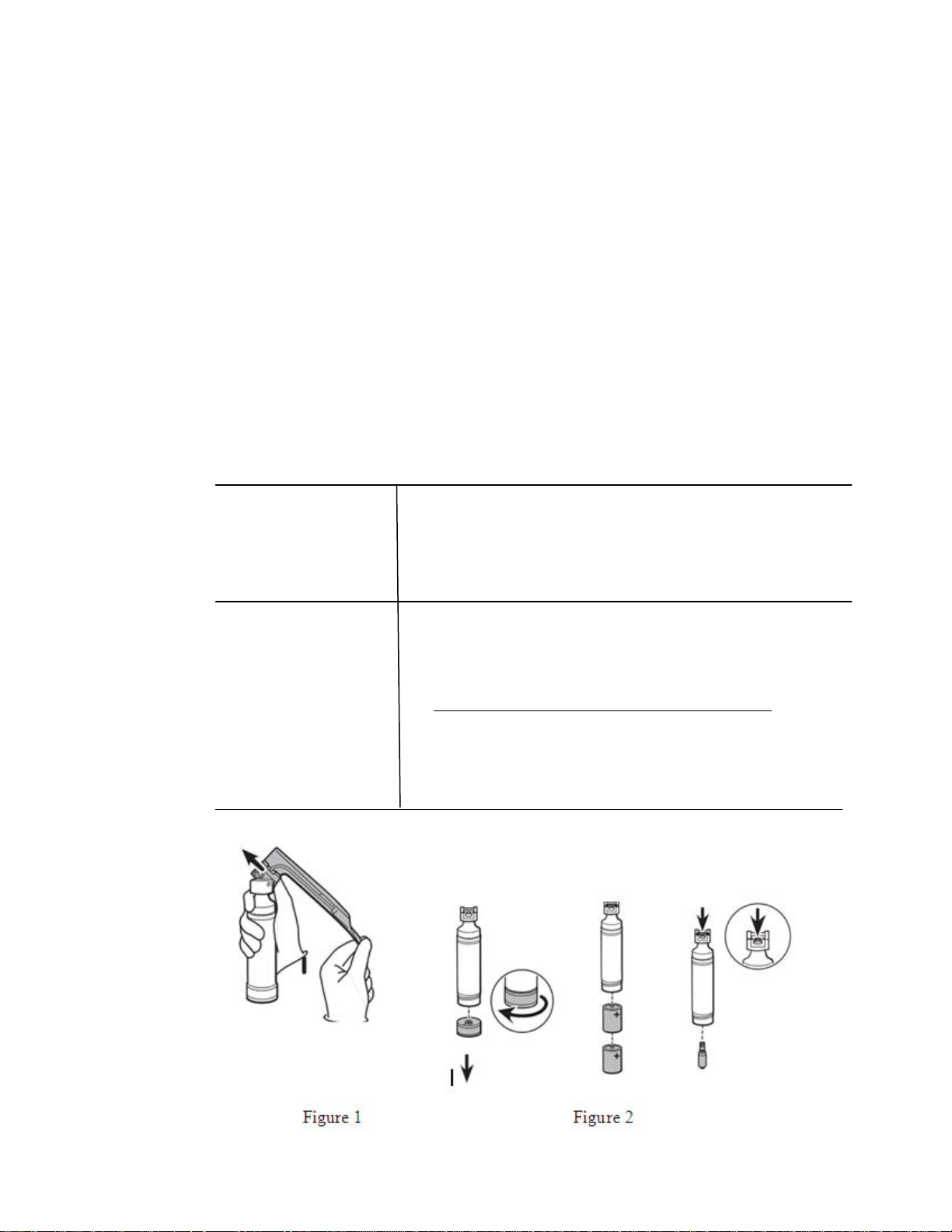
Point of use: 1.
Preparation
for
decontamination:
Separate
blade assembly from handle and place handle
into suitable containment for subsequent reprocessing
(per f igure 1). Do not place handle with sharp devices.
2. Prevent the handle from drying (i.e. wrap/cover in moist
germicidal
wipe).
1. Select an appropriate quaternary ammonium
isopropanol based germicidal cleaner labeled suitable
for use on healthcare equipment and capable
intermediate level disinfection. Reference EPAregistered disinfectants:
http://www.epa.gov/oppad001/chemregindex.htm
Outside of the U.S., please consult applicable
regulatory body for equivalent quaternary ammonium
isopropanol germicidal cleaner.
2. Remove batteries and lamp cartridge (per figure 2).
document:
Page 3
Directions for use
Cleaning
intermediate level
disinfection:
and
Welch Allyn fiber optic laryngoscope handles
3
1. Follow the ge r m i c i dal w i p e manufacturer’s instructions
to clean all exposed surfaces of the main handle, end
cap, and lamp cartridge.
2. If necessary, brush with a dry, soft-bristled brush and
re-wipe to loosen/remove excessive visible soil.
3. After all visible soil is removed, re-wipe to wet all
surfaces and allow adequate contact time for
disinfection as directed by the germicidal wipe
manufacturer.
CAUTION: Only use quaternary ammonium
isopropanol based germicidal wipes.
Drying:
Maintenance,
Inspection and Testing
Storage
End of
reprocessing
Allow components to air dry.
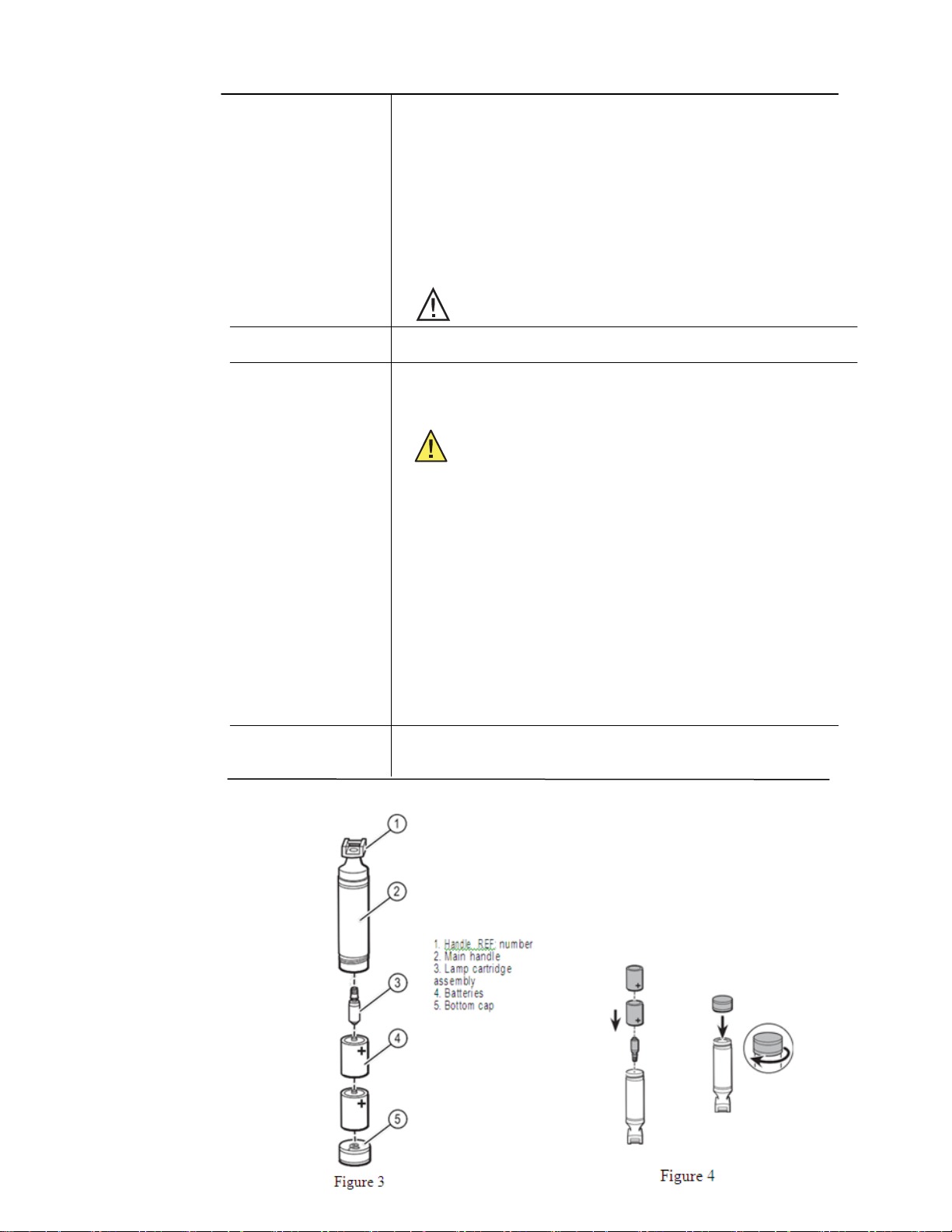
1. Inspect
each
component
area (per f
igure 3) for
damage
or
deterioration.
WARNING: Discard any component that
shows
evidence of damage or deterioration.
Contact Welch Allyn for component replacement.
2. Reassemble handle (per figure 4) with new or
batteries in known good condition.
3 . Attach handle to a clean and disinfected test blade
assembly in known working condition. Verify
•
Blade assembly engages and locks onto handle.
•
Blade assembly deploys into its locked position on
handle
•
Light output is satisfactory.
If the la mp fails to
replace the lamp and/or
AND lamp illuminates.
light or output is low
batteries.
that:
, check or
Store handle per facility practice to allow device to remain
clean, dry, and ready for service.
instructions for intermediate level disinfection.
Page 4

4
Directions for use
Cold solution disinfection instructions
NOTE: The main handle and bottom cap are compatible with this cold solution disinfection
method which is provided for facilities who wish to solution disinfect after cleaning and
intermediate level disinfection.
Disassembly: 1. Disassemble the handle and remove batteries and
Preparation
for
decontamination:
Cold solution
disinfection:
Drying: Dry all components with a clean cloth or allow to air dry.
Maintenance,
Inspection and Testing
Storage
2. Set batteries and lamp cartridge aside.
1. Select a 14 day (2.4 – 2.6%) glutaraldehyde
2.
1. Immerse main
2. Thoroughly rinse all components in potable water,
1. Inspect
2. Reassemble handle (per figure 4) with new or batteries in
3. Attach handle to a clean and disinfected test blade
Store handle per facility practice to allow device to remain
clean, dry, and ready for service.
Welch Allyn fiber optic laryngoscope handles
lamp cartridge per figures 3.
disinfectant.
Prepare
instructions.
disinfection solution per manufacturer
handle and
bottom
cap (o n l y )
in disinfection
solution, for a time duration identified by the disinfection
solu t i o n manufacturer.
softened water or per disinfection solution manufacturer
instructions to remove disinfecting
each
component
area (per f
solution.
igure
2)
for
damage
or
deterioration.
WARNING: Discard any component that
shows
evidence of damage or deterioration.
Contact Welch Allyn for component replacement.
known good condition.
assembly in known working condition. Verify
•
Blade assembly engages and locks onto handle.
•
Blade assembly deploys into its locked position on
handle AND lamp
•
Light output is satisfactory
If the la mp fails to
replace the lamp or
illuminates.
light or output is low
batteries.
that:
, check or
Page 5

Directions for use
Autoclave
Welch Allyn fiber optic laryngoscope handles
instructions
NOTE: The main handle and bottom cap are compatible with these autoclave methods which
are provided for facilities who wish to autoclave after cleaning and intermediate level
disinfection.
Disassembly: 1. Disassemble the handle and remove batteries and
lamp cartridge per figures 3.
2. Set batteries and lamp cartridge aside.
After battery and lamp cartridge removal, select ONE of the following autoclave
methods below
Gravity autoclave: Follow equipment manufacturer and
set-up and operation of autoclave equipment. Gravity autoclave settings are as
follows:
•
Temperature: 132 C (270 F)
•
Exposure time: 3 minutes (unwrapped)
•
Minimum dry time: 1
Pre-vacuum autoclave: Follow equipment manufacturer and
set-up and operation of autoclave equipment.
follows:
•
Temperature: 132 C (270 F)
•
Exposure time: 3 minutes (unwrapped)
•
Minimum dry time: 1
Maintenance,
Inspection and Testing
Storage: Store handle per facility practice to allow device to remain
for
the main handle and bottom cap:
minute
minute
1. Inspect
each
component
deterioration.
WARNING: Discard any component that
evidence of damage or deterioration.
Contact Welch Allyn for component replacement.
2. Reassemble (per figure 4) with new or batteries in known
good condition.
3. Attach handle to a clean and disinfected test blade
assembly in known working condition. Verify
•
Blade assembly engages and locks onto handle.
•
Blade assembly deploys into its locked position on
handle AND lamp
•
Light output is satisfactory.
If the la mp fails to
replace the lamp and/or
clean, dry, and ready for service.
Pre-vacuum
area (per f
illuminates.
light or output is low
facility
procedures in the
facility
procedures in the
autoclave settings are as
igure
2)
for
damage
shows
that:
, check or
batteries
or
5
Page 6

6
Directions for use
Welch Allyn standard laryngoscope handles
Maintenance
Replace
the
lamp
1. Unscrew bottom cap of handle counterclockwise and remove batteries per figure 3.
2. Remove lamp cartridge assembly from main handle by applying finger pressure in
3. Remove shroud c a p from shroud by rotating shroud cap counterclockwise per f
4. Rotate lamp counterclockwise to remove per figure 6.
5.
6. To replace lamp cartridge ass e m b l y in the main handle, invert handle, then gently slide
7. Re-insert batteries and apply slight pressure to set the cartridge in place.
8.
9. Reprocess repaired assembly as appropriate per these instructions.
instructions
WARNING: Lamps, if left illuminated, could generate sufficient heat
cause burns.
CAUTION: Use only Welch Allyn replacement lamps REF: 0 6 0 0 0 t o ensure
proper
direction shown by the arrow per figure 4.
and 6.
Replace
the cartridge down the inside of handle, tipping it side to side until the sh r o u d c a p
exits
Replace
illumination alignment.
lamp and rotate shroud cap clockwise to tighten per figure 7.
opening on
and tighten bottom cap.
top.
to
igures
the
5
Figure 3 Figure 4
Figure 5 Figure 6
1. Shroud cap
2. Lamp
3. Shroud
Figure 7
Page 7

Directions for use
Replace
the
batteries
1. Unscrew bottom cap of handle per figure 3 and remove batteries.
2. Alkaline batteries are supplied with your handle for maximum performance and
3. Insert batteries and reinstall bottom cap per figure 3.
4. Verify lamp and blade engagement/operation using a known working test blade.
5. Reprocess repaired assembly as appropriate per these instructions.
Specifications
Electrical:
For information about electromagnetic compatibility (EMC) see Welch Allyn website:
http://www.welchallyn.com
Operating:
32
(0 C)
Approvals:
Con fo rm s to AS TM F 965 and
Warranty:
One year
Service Information:
For
Welch Allyn
are recommended as replacements; however
used.
•
Medium handle, REF 60813 uses two “C” size
•
Penlight handle, REF 60814 uses two “AA” size
•
Stubby handle, REF 60815 uses two “AA” size
Storage/Transport:
104 F
(40 C)
F
-4 F
(-20 C)
120 F
(49 C)
ISO-7376-1, IEC/EN
The
CE
mark on this product indicates that it has been tested to and
with the provisions noted within the
Complies with EMC Framework of Australia
Technical
Support or to obtain information about any Welch Allyn product,
Technical
Support: w
ww.welchallyn.com/support.
93/42/EEC
Welch Allyn standard laryngoscope handles
carbon-zinc
60601-1,
Medical Device
batteries may also be
IEC/EN
60601-1-2
conforms
Directive.
contact
7
Welch Allyn, Inc.
4341 State Street Road
Skaneateles Falls, NY 13153-0220 USA
www.welchallyn.com
Authorized European Representative Address:
Regulatory Affairs Representative
Welch Allyn, Limited
Navan Business Park
Dublin Road
Navan, County Meath, Republic
of Ireland
 Loading...
Loading...