
CP 100™ 12-Lead Resting
Electrocardiograph
Directions for Use

ii Welch Allyn CP 100 Electrocardiograph
Copyright 2008, Welch Allyn. All rights are reserved. No one is permitted to reproduce or duplicate, in any
form, this manual or any part thereof without permission from Welch Allyn.
Caution: Federal US law restricts sale of the device identified in this manual to, or on the order of, a
licensed physician.
Welch Allyn assumes no responsibility for any injury, or for any illegal or improper use of the product, that
may result from failure to use this product in accordance with the instructions, cautions, warnings, or
indications for use published in this manual.
Welch Allyn is a registered trademark of Welch Allyn, Inc., and CP 100 and CardioPerfect are trademarks of
Welch Allyn, Inc.
SD is a trademark of Toshiba.
Software in this product is copyright Welch Allyn or its vendors. All rights are reserved. The software is
protected by United States of America copyright laws and international treaty provisions applicable
worldwide. Under such laws, the licensee is entitled to use the copy of the software incorporated within
this instrument as intended in the operation of the product in which it is embedded. The software may not
be copied, decompiled, reverse-engineered, disassembled or otherwise reduced to human-perceivable
form. This is not a sale of the software or any copy of the software; all right, title and ownership of the
software remains with Welch Allyn or its vendors.
For information about any Welch Allyn product, please call Welch Allyn Technical Support:
USA 1 800 535 6663
+ 1 315 685 4560
Canada 1 800 561 8797 China + 86 216 327 9631
European Call Center + 353 46 906 7790 France + 331 6009 3366
Germany + 49 747 792 7186 Japan + 81 33 219 0071
Latin America + 1 305 669 9003 Netherlands + 31 15 750 5000
Singapore + 65 6419 8100 South Africa + 27 11 777 7555
United Kingdom + 44 207 365 6780 Sweden + 46 85 853 6551
Australia + 61 29 638 3000
Reorder Number (multi-language CD): 401150
Mat. Number (manual only): 708794, Ver: F
Welch Allyn
4341 State Street Road, PO Box 220
Skaneateles Falls, NY 13153-0220
Welch Allyn LTD.
Navan Business Park
Dublin Road
Navan, County Meath, Republic of Ireland
Tel.: 353-46-90-67700
Fax: 353-46-90-67755
www.welchallyn.com
Printed in USA

Contents
1 - Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1
iii
About This Manual . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
Product Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
Intended Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
Indications for Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
Standard Features & Benefits. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
Options . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
Accessories . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
Controls, Indicators, and Connectors . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
About the Main Menu. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10
Moving Through the Menus . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
About the Patient Cable and Leads. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
Symbols . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
Using the Electrocardiograph Safely . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
General Warnings. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
General Cautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
Getting Help . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 18
2 - Setting Up the Electrocardiograph . . . . . . . . . . . . . . . . . . . . . . . . . 19
Inspecting the Electrocardiograph . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20
Connecting the Patient Cable . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20
Loading the Thermal Chart Paper . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
Powering the Electrocardiograph . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 22
Verifying Proper Operation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23
Connecting an External USB Printer (Optional). . . . . . . . . . . . . . . . . . . . . . . . . . 24
3 - Reviewing the System Settings . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25
“System Settings” Menu Tree . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 26
Reviewing the Device Configuration Settings. . . . . . . . . . . . . . . . . . . . . . . . . . . 27
Reviewing the Device Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 29
Transferring a Configuration to Another Electrocardiograph . . . . . . . . . . . . . . . . 30

iv Contents Welch Allyn CP 100 Electrocardiograph
4 - Reviewing the ECG Settings. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 31
“ECG Settings” Menu Tree . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 32
About Auto ECG Reports . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 33
Reviewing the Format Settings for Auto Reports. . . . . . . . . . . . . . . . . . . . . 35
Reviewing the Interpretation and Copy Settings for Auto Reports. . . . . . . . 36
Reviewing the Patient Data Fields Available. . . . . . . . . . . . . . . . . . . . . . . . . 37
Reviewing the Auto Report Settings. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 39
Reviewing the Miscellaneous ECG Settings. . . . . . . . . . . . . . . . . . . . . . . . . . . . 40
Turning the Augmented Pediatric Lead Set On and Off . . . . . . . . . . . . . . . . . . . 41
5 - Performing ECG Tests . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 43
Connecting the Leads to the Patient . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 44
Recording an Auto ECG . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 48
Recording a Normal Auto ECG . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 49
Recording a Stat Auto ECG . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 53
Recording a Rhythm ECG . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 54
Adjusting the ECG Waveforms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 55
6 - Maintaining the Electrocardiograph . . . . . . . . . . . . . . . . . . . . . . . . 57
Inspecting the Equipment. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 58
Cleaning the Equipment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 58
Testing the Equipment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 59
Recharging a Fully Discharged Battery . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 60
Replacing the Battery . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 61
Replacing the Battery (DC) Fuse. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 63
Replacing the AC Fuses . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 64
Storing the Equipment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 65
Discarding the Equipment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 65
7 - Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 67
Problem-Solving Suggestions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 68
Limited Warranty . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 71
Service Policy . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 72
A - Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 73
B - EMC Guidance and Manufacturer’s Declarations . . . . . . . . . . . . . 75
Glossary . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 79
Index . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 81
1
1
Introduction
About This Manual . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
Product Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
Intended Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
Indications for Use. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
Standard Features & Benefits. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
Options . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
Accessories . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
Controls, Indicators, and Connectors. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
About the Main Menu . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10
Moving Through the Menus . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
About the Patient Cable and Leads . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
Symbols . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
Using the Electrocardiograph Safely . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
Getting Help . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 18

2 Chapter 1 Introduction Welch Allyn CP 100 Electrocardiograph
About This Manual
This manual is written for clinical professionals with a working knowledge of medical
procedures and terminology as required for monitoring cardiac patients.
Before using the CP 100 electrocardiograph for clinical applications—or before setting up,
configuring, troubleshooting, or servicing the electrocardiograph—you must read and
understand this manual and all other information accompanying the electrocardiograph
and related options or accessories.
Product Overview
The Welch Allyn CP 100 electrocardiograph features a full alphanumeric keyboard, an LCD
display, full-size user-programmable reports, and the ability to operate on either battery or
AC power.
ECG tests sent to a memory card or removable USB storage device are compatible with
the Welch Allyn CardioPerfect™ workstation, which in turn can connect with other
electronic patient-information systems, such as billing and medical records.
For details, see the following sections:
• “Standard Features & Benefits” on page 3
• “Options” on page 5
• “Specifications” on page 73
Intended Use
The Welch Allyn electrocardiography and spirometry products (subject devices) are
intended for use by trained operators in health facilities. The subject devices provide the
following diagnostic functions:
• Acquiring and printing ECG waveforms using ECG front end modules (patient cables)
and associated accessories that provide signal acquisition for up to twelve (12) leads
of patient ECG waveforms through surface electrodes adhered to the body.
• Using optional algorithms to generate measurements, data presentations, graphical
presentations and interpretative statements on an advisory basis. These are
presented for review and interpretation by the clinician based upon knowledge of the
patient, the result of physical examination, the ECG tracings and other clinical
findings.

Directions for Use Chapter 1 Introduction 3
Indications for Use
The electrocardiograph is one of the tools that clinicians use to evaluate, diagnose, and
monitor patient cardiac function.
The 12-lead ECG interpretive algorithm provides a computer-generated analysis of
potential patient cardiac abnormalities which must be confirmed by a physician with other
relevant clinical information.
Standard Features & Benefits
Full alphanumeric keypad
Enter patient information quickly and easily.
LCD display
Enter data and program the system easily.
Unlimited storage on removable media
Use SD™ memory cards or removable USB storage devices to save as many ECG records
as you like. (Media are not included.) If both an SD memory card and a USB storage
device are connected, files are sent to the SD card. For media specifications, see
“Standard connectivity” on page 74.
Battery operation
Use the electrocardiograph almost anywhere. On battery power, you can print up to 100
ECGs continuously before needing to recharge.
User-definable ECG report format
Customize the report format for efficient reporting.
Removable leads for ECG patient cable
Replace leads individually if needed.
Compatibility with CardioPerfect workstation software
Store and manage data electronically by transferring records to a Welch Allyn
CardioPerfect workstation via an SD memory card or removable USB storage device
(media not included).
Compatibility with external printer
You can connect an external printer. For details, see “Connecting an External USB Printer
(Optional)” on page 24.

4 Chapter 1 Introduction Welch Allyn CP 100 Electrocardiograph
Pacemaker detection
If the software detects the possible presence of a pacemaker, it asks you whether the
patient has a pacemaker. If you say no, interpretation (if purchased) is included in the
report. If you say yes, interpretation is not included in the report, and a message
indicating that a pacemaker was detected is displayed on the report.
Support for augmented pediatric lead set
The augmented pediatric lead set, an alternate placement of the precordial leads on
pediatric patients, is easier to use on the small chests of infants and young children.
It provides optimal vectors for early development of the heart for the change of right-toleft ventricular dominance. For electrode placement locations, see Figure 28 on page 46.

Directions for Use Chapter 1 Introduction 5
Options
These options are available both for initial purchases and for upgrades.
• Automatic ECG interpretation
The optional MEANS interpretation algorithm, developed by the University of
Rotterdam in the Netherlands, provides automatic analysis of ECG tests. For more
information, see the MEANS Physicians' Manual or the PEDMEANS Physicians’
Manual on the CD that came with your electrocardiograph. The MEANS algorithm is
used for adult patients 18 years and older. The PEDMEANS algorithm is used for
pediatric patients from 1 day through 17 years old.
•Carts
Two specially designed carts are available for convenient transport and use of the
electrocardiograph, as shown here with the optional cable arm and shelf.
Figure 1. Office Cart Figure 2. Hospital Cart
Cable arm and
shelf (optional)
Free-motion
plastic wheels
Cable arm and
shelf (optional)
Durable, highquality, rubber
wheels with locks

6 Chapter 1 Introduction Welch Allyn CP 100 Electrocardiograph
Accessories
To order accessories, call Welch Allyn. For phone numbers, see page ii.
Item Customer Order Number Quantity
Resting tab electrodes
Resting tab electrode adaptors
Thermal chart paper (1 case = 5 pads, 200 sheets each)
Welch cup
Limb lead clamps, IEC
Limb lead clamps, AHA
Patient cable (Figure 11 on page 12)
•AHA
•IEC
• IEC, vacuum adapter
• Vacuum system
Lead wires (10 wires per set)
• AHA banana
• IEC banana
• AHA pinch
• IEC pinch
Battery (Figure 39 on page 61)
Dust cover
Carts
• Utility cart
•Office cart (Figure 1 on page 5)
•Hospital cart (Figure 2 on page 5)
• Cable arm & shelf option (page 5)
Interpretation upgrade option 100623 1
45008-0000
58581-0000
94018-0000
RE-ELEC-CUP
RE-ELEC-CLP
401432
400293
400294
100920
VAC-DT100-CP
401129
401122
401123
401124
100660
701586
08265-0000
401393
401394
401161
1000
1 case
1 set
1 set
1 set
1 set
10
1
4
4
1
1
1
1
1
1
1
1
1
Product information
• Electrode placement wall poster 71300-0000 1
• CP 100 12-Lead Resting Electrocardiograph Directions
for Use
• CP 100 product information multi-language CD 401150 1
708794 1
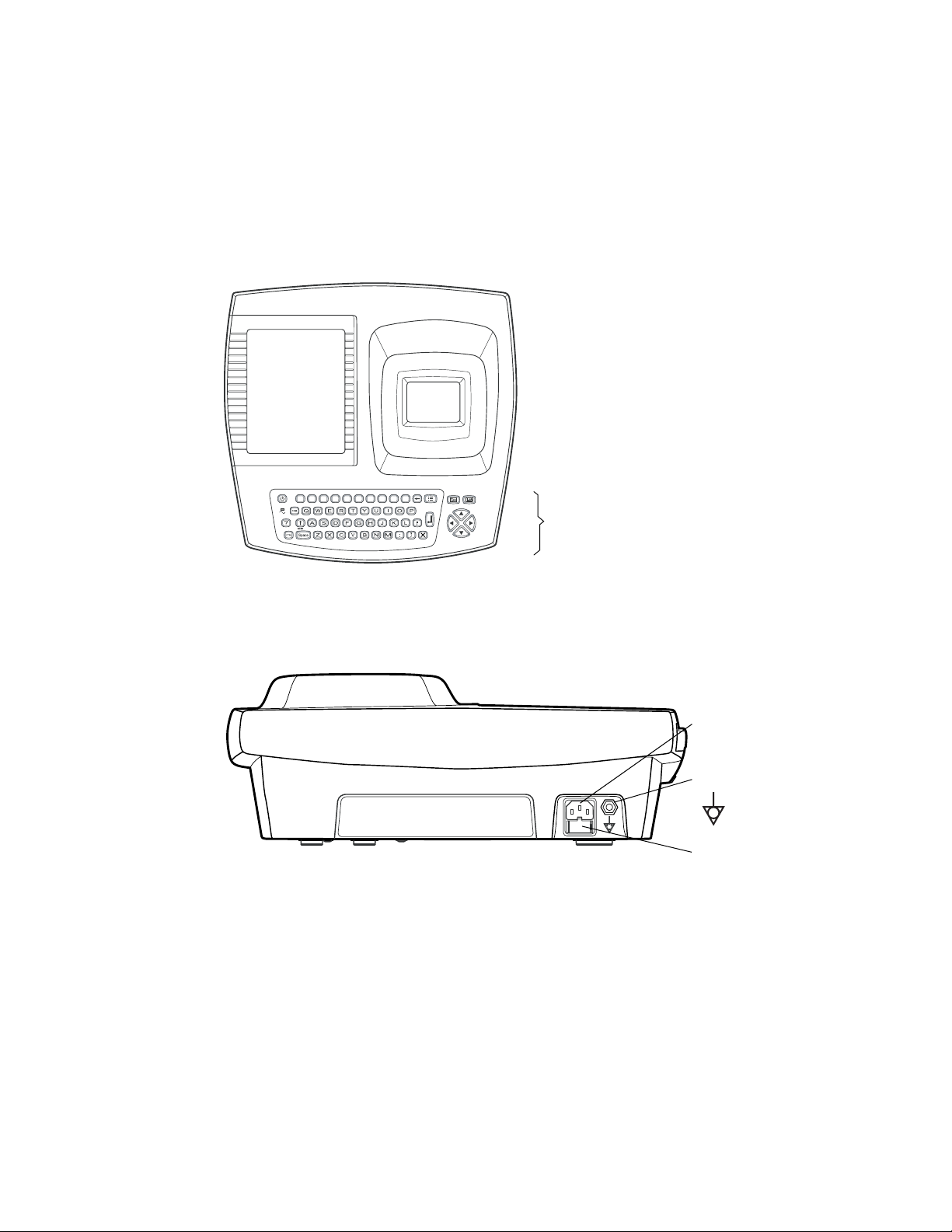
Directions for Use Chapter 1 Introduction 7
Controls, Indicators, and Connectors
This section describes the controls, indicators, and connectors that are part of the
electrocardiograph.
Figure 3. Top
)
1!2
@3#
4$5%6+7-8
(
*
9
0
Keyboard
See Figure 1 on page 9.
Figure 4. Back
AC power inlet
Equipotential stud
AC fuses
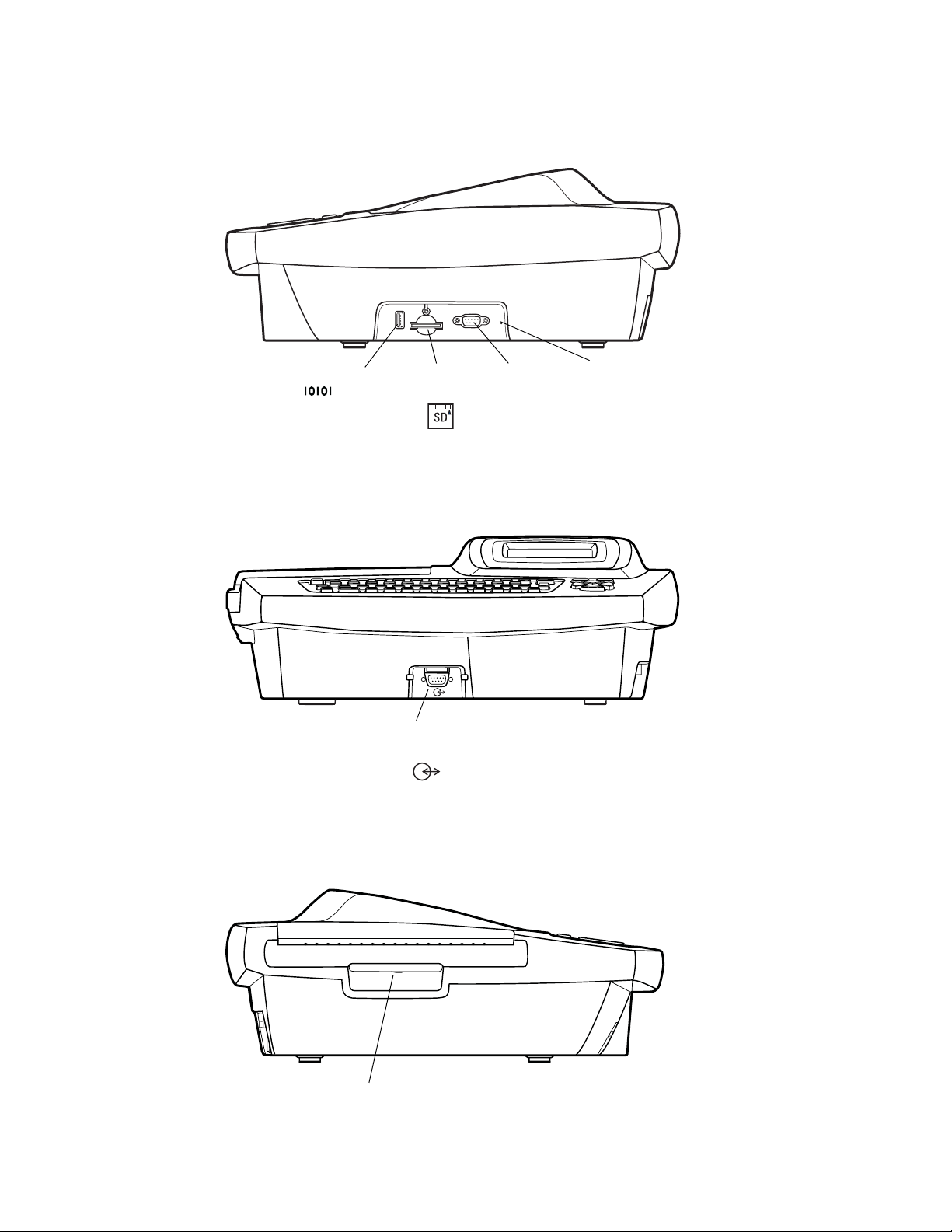
8 Chapter 1 Introduction Welch Allyn CP 100 Electrocardiograph
Figure 5. Right Side
Com port B (USB) Reset
Figure 6. Front
SD memory card
slot
Com port A
(for patient cable)
Not
functional
button
Figure 7. Left Side
Paper tray latch

Directions for Use Chapter 1 Introduction 9
Table 1. Keyboard
A
BCD
N
1!2
@3#
4$5%6+7-8
)
(
*
9
0
E
M
L
K
J
I
H
G
F
Key Function
A. On/Off See “Powering the Electrocardiograph” on page 22.
B. Backspace Deletes the character to the left of the cursor.
C. Menu See “About the Main Menu” on page 10.
D. Auto ECG Begins Auto ECGs, normal and stat. See “Recording an Auto ECG” on page 48.
E. Rhythm ECG Begins a Rhythm ECG. See “Recording a Rhythm ECG” on page 54.
F. Navigation arrows See “Moving Through the Menus” on page 11.
G. Enter See “Moving Through the Menus” on page 11.
H. Stop/Cancel Stops any current activity. See “Moving Through the Menus” on page 11.
I. Space Enters a space.
J. Control (Applicable only for keyboards with characters above certain keys.) To enter a character
above a key, hold the Ctrl key while pressing that key. To capitalize the character, hold
Shift + Ctrl while pressing the key.
K. Shift Capitalizes letters. Also enters symbols (%, #, etc.). To enter a symbol, hold the
Shift key while pressing a number key.
L. Help See “Getting Help” on page 18.
M. Tab Moves through the data-entry fields.
N. Green LED Lights up when the electrocardiograph is connected to AC power.
10 Chapter 1 Introduction Welch Allyn CP 100 Electrocardiograph
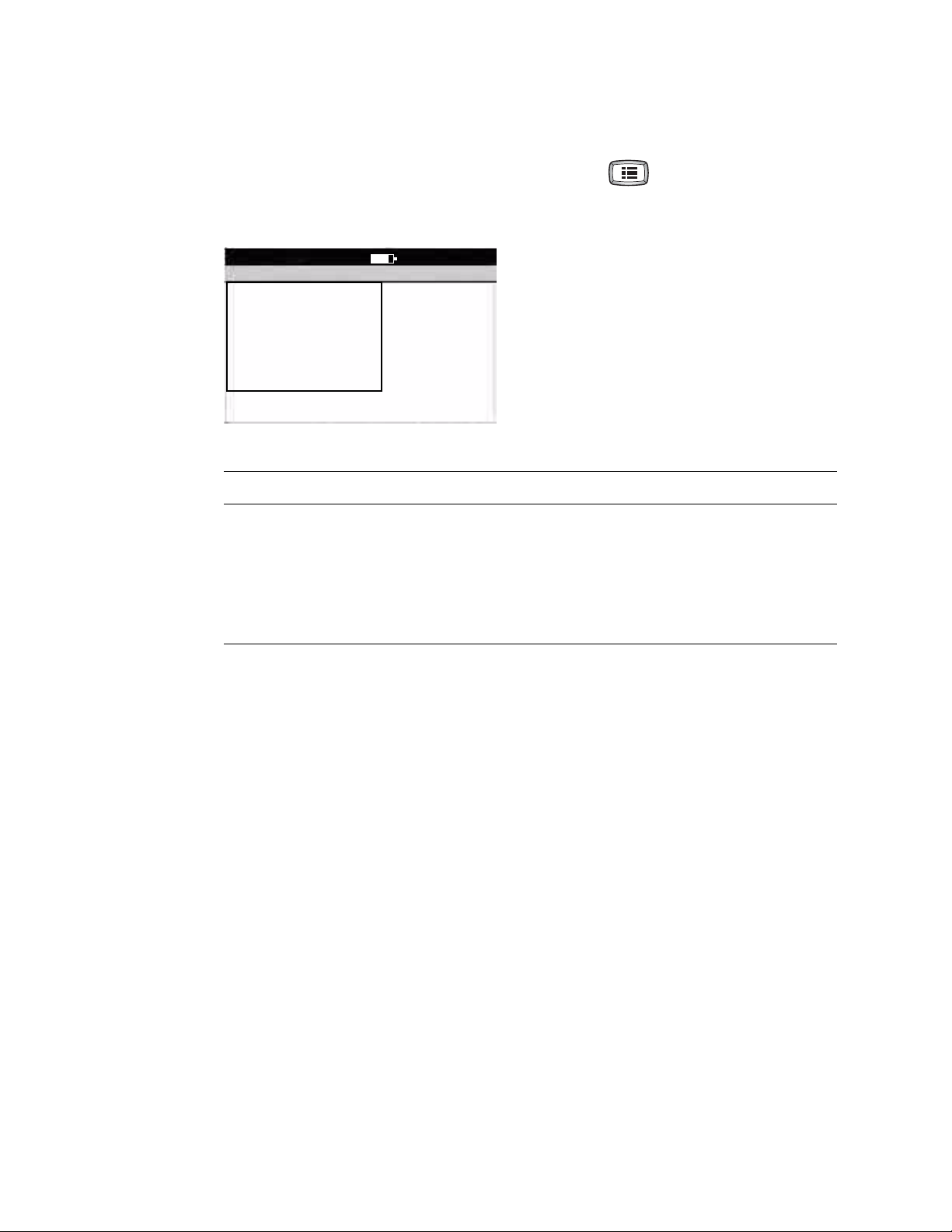
About the Main Menu
The main menu appears when you press the Menu key .
Figure 8. Main Menu
Main Menu
1 ECG Settings
2 System Settings
0 Exit
9:17AM Oct 16 08
Submenu Purpose Procedure
ECG Settings Review or change ECG settings: Auto
Report format, Rhythm Report format, and
See “Reviewing the ECG Settings” on
page 31.
so on.
System Settings Review or change system settings: device
configuration, device info, user setup, and
See “Reviewing the System Settings” on
page 25.
so on.
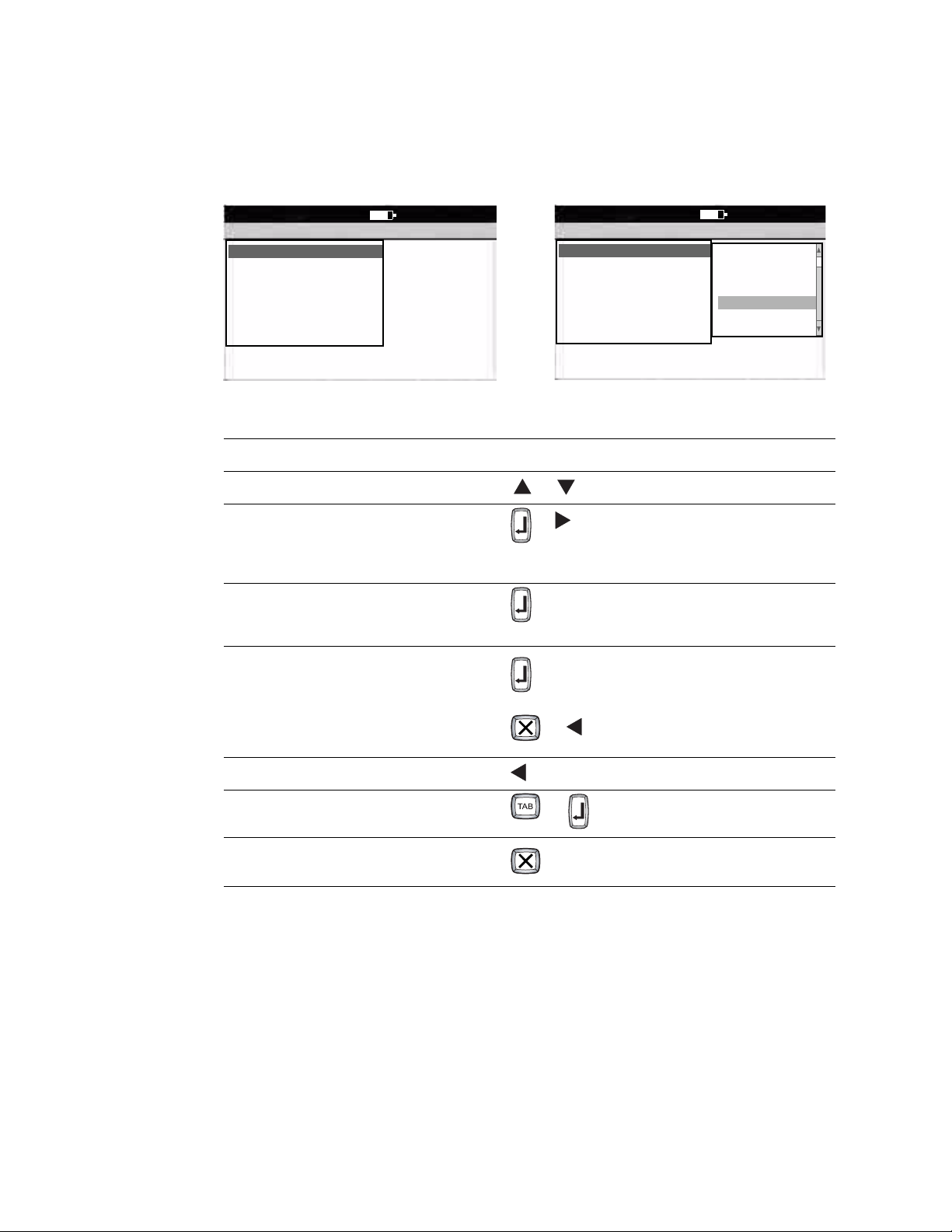
Directions for Use Chapter 1 Introduction 11
Moving Through the Menus
Figure 9. Standard Menu Figure 10. Parent Menu With Submenu
Edit Auto Report
1 Format
2 Interp Settings
3 Patient Data
0 Previous Menu
9:17AM Oct 16 08
Format
1 Lead Arrangement
2 Rhythm Lead 1
3 Rhythm Lead 2
4 Rhythm Lead 3
5 Extended Measurements
6 Average Cycles
0 Previous Menu
Desired Actions Keys to Press
To move up or down a list or
To open a standard menu (Figure 9)
or
To move from parent menu to submenu on same
screen (Figure 10)
To perform an action
To accept data
To check or uncheck a checkbox
To return to parent menu from submenu on same
screen (Figure 10)
(To select the highlighted submenu
item.)
or item’s number or letter
9:17AM Oct 16 08
3x4 +1R
3x4 +3R
6x2
12x1
6x2 50 mm/s
6x2 10 Ext.
2x6+1R
or
To move back through the menus or zero key
To move through data-entry fields or
To return to the Ready screen from a standard menu
(Figure 9)
(To make no change.)
12 Chapter 1 Introduction Welch Allyn CP 100 Electrocardiograph
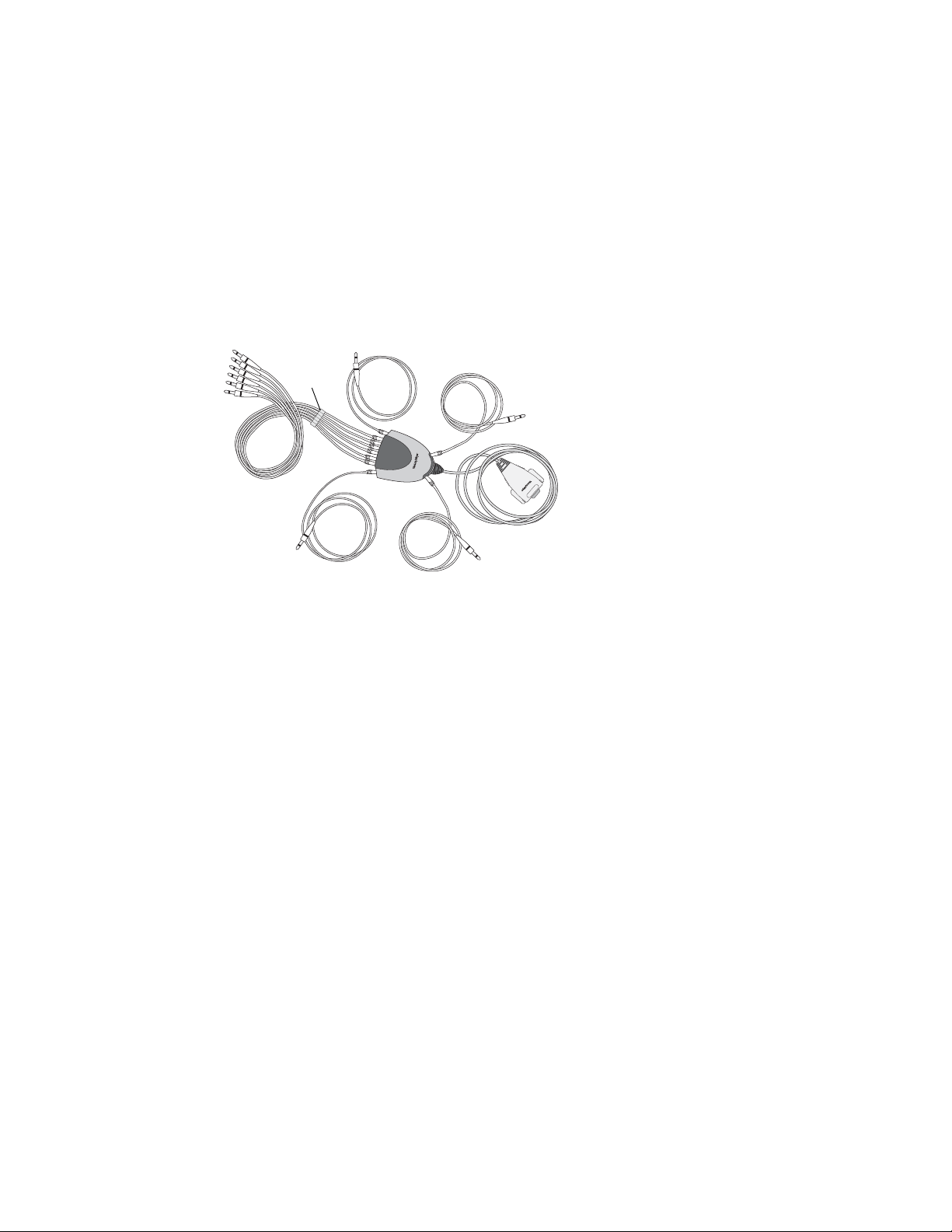
About the Patient Cable and Leads
The patient cable processes the patient’s ECG data and transmits it to the
electrocardiograph. To make handling convenient, the ten leads are arranged to point
toward the appropriate parts of the body. The cable rake, which slides easily, prevents the
chest leads from tangling.
Figure 11. Patient Cable and Leads
Chest leads
Cable
rake
Right arm lead
Left arm lead
Left leg lead
Electrocardiograph
connector
Right leg lead
Directions for Use Chapter 1 Introduction 13
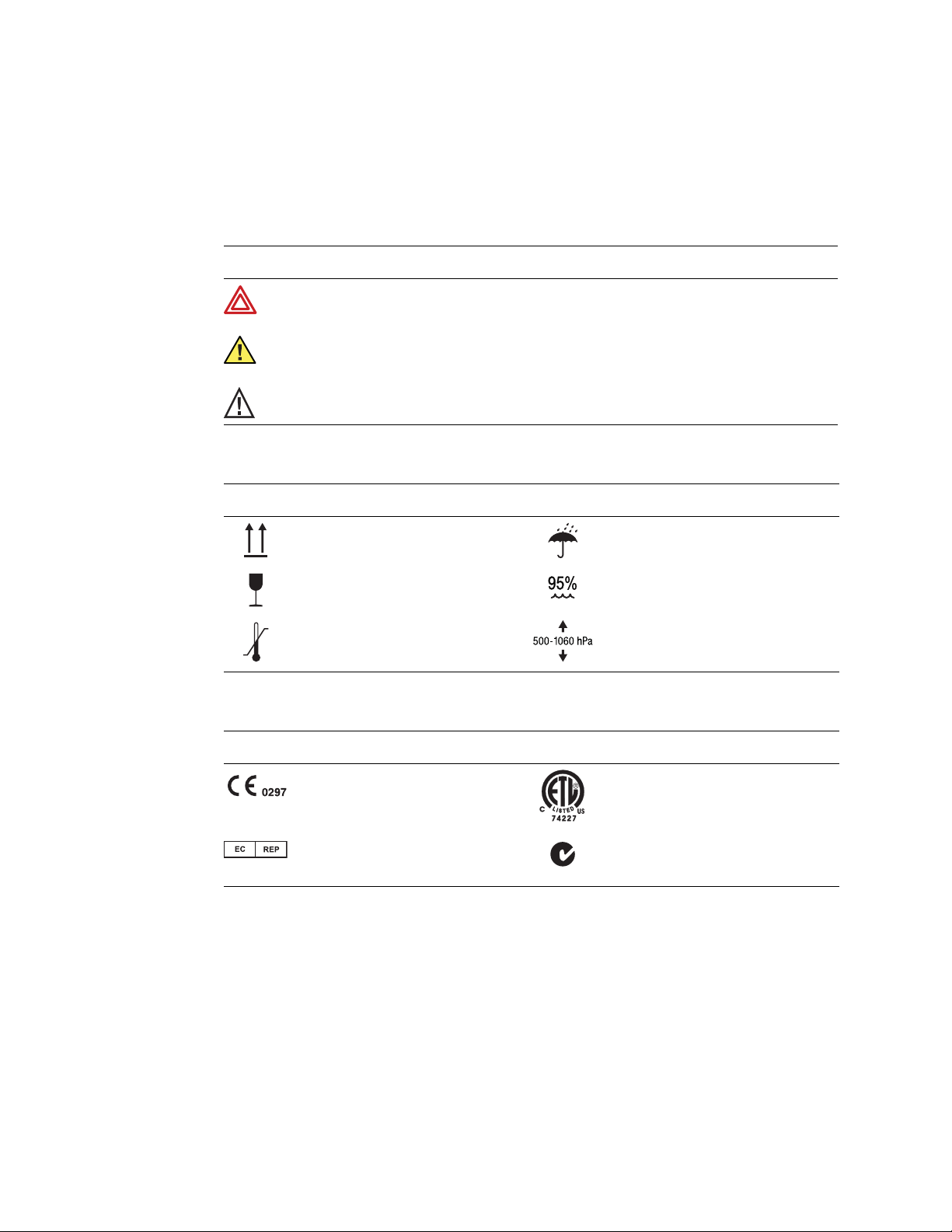
Symbols
The symbols illustrated on the following pages may appear on the electrocardiograph, on
the packaging, on the shipping container, or in this manual.
Documentation Symbols
WARNING Indicates conditions or practices that could lead to illness, injury, or
death.
Caution In this manual, indicates conditions or practices that could damage the
equipment or other property.
Caution On the product, means “Consult accompanying documentation.”
Shipping, Storing, and Environment Symbols
-20°C
This end up Keep dry
Fragile Relative humidity limit
+49°C
Temperature limits Altitude limits
Certification Symbols
Meets essential requirements of European
Medical Device Directive 93/42/EEC
European Regulatory Manager Australian registered importer
N344
Complies with applicable U.S. and Canadian
medical safety standards
14 Chapter 1 Introduction Welch Allyn CP 100 Electrocardiograph
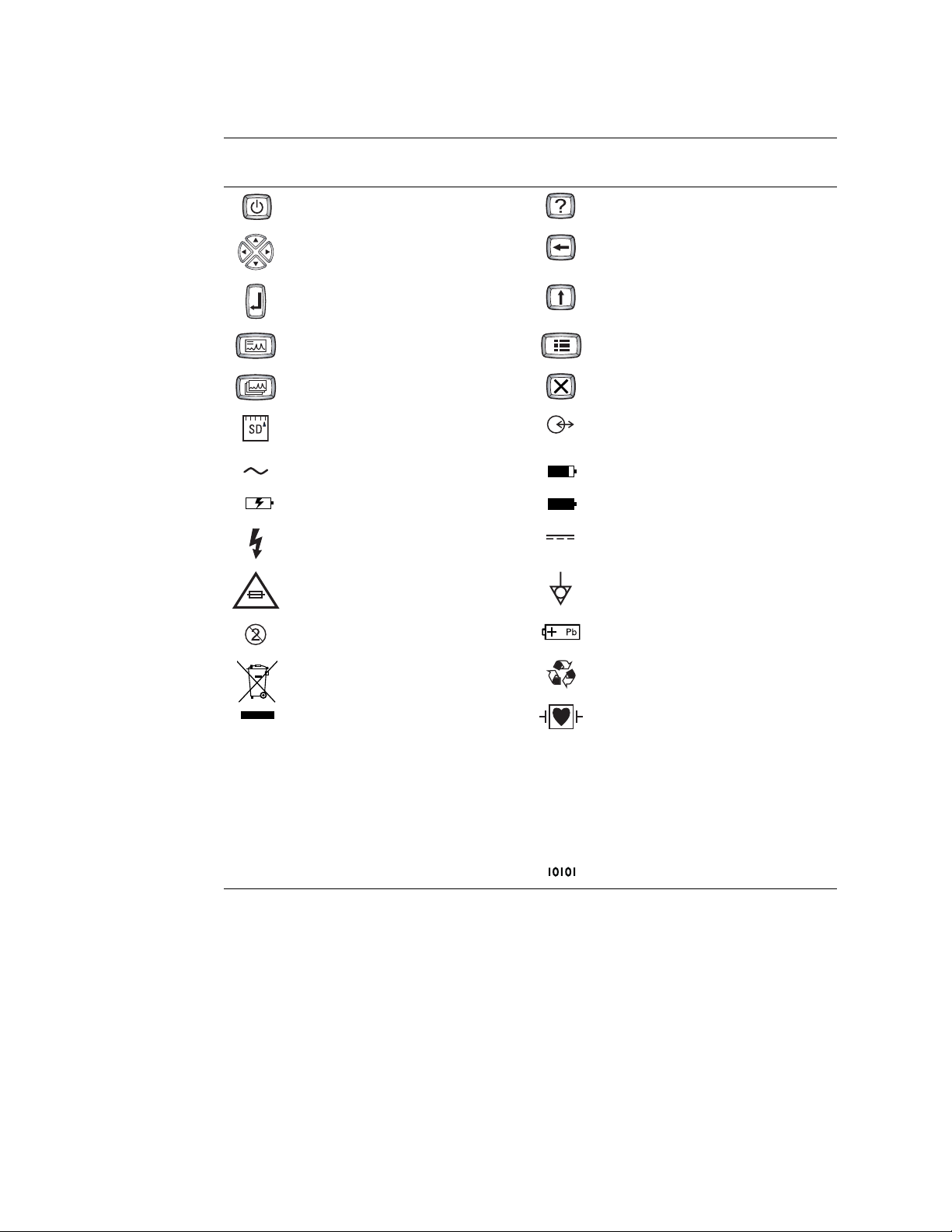
Operation Symbols
For details on the keys, see Figure 1 on page 9.
On/standby (off) Help
Navigation arrows Backspace
Enter Shift
Auto ECG Menu
Rhythm ECG Stop/Cancel
T2.0A/250V
SD memory card slot Com port A
(for patient cable)
Alternating current Battery charge level
Battery is charging. Battery is charged.
Dangerous voltage Direct current
AC fuse replacement information Ground equipotential
Do not reuse.
Do not dispose of this product as unsorted
Sealed lead-acid battery
Recycle.
municipal waste. Prepare this product for
reuse or separate collection as specified by
Directive 2002/96/EC of the European
Parliament and the Council of the European
Union on Waste Electronic and Electrical
Equipment (WEEE). If this product is
contaminated, this directive does not apply.
For more specific disposal information, see
Defibrillation-proof Type CF applied parts.
(While the electrocardiograph is safety-rated
“CF” for direct cardiac contact, it is not
intended to be connected directly to the
patient’s heart. Only surface contact with the
patient’s skin is intended.)
www.welchallyn.com/weee, or contact
Welch Allyn Customer Service at +44 207
365 6780.
Com port B (USB)

Directions for Use Chapter 1 Introduction 15
Using the Electrocardiograph Safely
Before using or servicing the electrocardiograph, you must read and understand the
following safety-related information.
General Warnings
The following warning statements apply to electrocardiograph use in general. Warning
statements that apply specifically to particular procedures, such as connecting the patient
cable or performing an ECG test, appear in the corresponding sections of the manual.
Warning statements indicate conditions or practices that could lead to illness, injury, or
death.
Warnings Related to the Environment
WARNING To ensure patient and device safety, leave 5 feet (1.5 meters) of
open area around the patient.
WARNING To avoid a possible explosion, do not use the electrocardiograph in
the presence of flammable anesthetics: mixtures containing air, oxygen, or
nitrous oxide.
WARNING When transporting the electrocardiograph on a cart, tuck the patient
cable away from the wheels so that it does not present a hazard.
Warnings Related to Accessories and Other Equipment
WARNING For operator and patient safety, peripheral equipment and
accessories that can come in direct patient contact must be in compliance with
all appropriate safety, EMC, and regulatory requirements. See “EMC Guidance
and Manufacturer’s Declarations” on page 75.
WARNING All signal input and output (I/O) connectors are intended for
connection of only devices complying with IEC 60601-1, or other IEC standards
(for example, IEC 60950), as appropriate to the device. Connecting additional
devices to the electrocardiograph might increase chassis or patient leakage
currents. To maintain operator and patient safety, consider the requirements of
IEC 60601-1-1. Measure the leakage currents to confirm that no electric shock
hazard exists. In the case of a USB printer, the printer (non-medical electrical
equipment) shall be situated outside the patient environment (reference IEC
60601-1-1). The printer used should be approved to the appropriate safety
standard for non-medical electrical equipment (IEC 60950, or its national
variants), and use of an isolation transformer is recommended. If there is a
requirement for the printer to be situated within the patient environment it is the
responsibility of the user to ensure that the system provides a level of safety in
compliance with IEC 60601-1 and 60601-1-1.
WARNING The electrocardiograph has not been designed for use with highfrequency (HF) surgical equipment and does not protect against hazards to the
patient.

16 Chapter 1 Introduction Welch Allyn CP 100 Electrocardiograph
Warnings Related to Using the Electrocardiograph
WARNING This device captures and presents data reflecting a patient’s
physiological condition. When reviewed by a trained physician or clinician, this
data can be useful in determining a diagnosis. However, the data should not be
used as a sole means for determining a patient’s diagnosis.
WARNING To avoid serious injury or death, take these precautions during
patient defibrillation:
• Avoid contact with the electrocardiograph, patient cable, and patient.
• Verify that the patient leads are properly connected. See “Connecting the
Patient Cable” on page 20.
• Place defibrillator paddles properly in relation to electrodes.
• After defibrillation, pull each patient lead out of the patient cable and inspect
the tips for charring (black carbon marks). If there is any charring, the patient
cable and individual leads must be replaced. If there is no charring, fully
reinsert the leads into the patient cable. (Charring can occur only if a lead is
not fully inserted into the patient cable before defibrillation.)
WARNING To prevent the spread of infection, take these precautions:
• Dispose of single-use components (for example, electrodes) after using
them once.
• Regularly clean and disinfect all components that come in contact with
patients. See “Cleaning the Equipment” on page 58.
• Avoid ECG testing for patients with open, infectious sores.
WARNING Avoid positioning any leads or cables so that they could easily trip
someone or become wrapped around a patient’s neck.
WARNING Satisfactory maintenance procedures must be implemented, or
equipment failure and health hazards may result.
WARNING Only qualified service personnel should attempt to repair the
electrocardiograph. In case of a malfunction, call Technical Support and precisely
describe the problem. For phone numbers, see page ii.

Directions for Use Chapter 1 Introduction 17
General Cautions
The following caution statements apply to electrocardiograph use in general. Caution
statements that apply specifically to particular procedures, such as connecting the patient
cable or performing an ECG test, appear in the corresponding sections of the manual.
Caution statements indicate conditions or practices that could damage the equipment or
other property.
Caution When removing the electrocardiograph from storage, allow it to
thermally stabilize to surrounding environmental conditions before using it.
Caution To prevent possible damage to the keypad, do not use sharp or hard
objects to press keys. Only use fingertips.
Caution Do not expose the patient cable to strong ultra-violet radiation.
Caution Do not pull or stretch the patient cable. Doing so could result in
mechanical or electrical failures. Form the patient cable into a loose loop before
storing.
Caution Avoid positioning the patient cable where it might get pinched or
stepped on. If the cable’s impedance is altered, measurements might no longer
be accurate, and repair might be necessary.
Caution Using the equipotential terminal for anything but grounding purposes
may contribute to damage of the device.
Caution Use only parts and accessories supplied with the device and available
through Welch Allyn. The use of accessories other than those specified may
result in degraded performance of this device.
Caution Portable and mobile RF communications equipment can affect the
performance of the electrocardiograph.
Caution The electrocardiograph meets the Class A requirements of IEC 606011-2:2000 regarding incidental emission of radio frequency interference. As such it
is suitable for use in commercial grade electrical environments. If the
electrocardiograph is used in residential grade electrical environments and you
experience incidental interference with other equipment that uses radio
frequency signals to operate, minimize the interference as described under
“EMC Guidance and Manufacturer’s Declarations” on page 75.
Caution Other medical equipment—including but not limited to defibrillators,
ultrasound machines, pacemakers, and other stimulators—may be used
simultaneously with the electrocardiograph. However, such devices may disturb
the electrocardiograph signal.
Caution The power cord must be disconnected from AC power before cleaning,
maintaining, or servicing.

18 Chapter 1 Introduction Welch Allyn CP 100 Electrocardiograph
Getting Help
You can get help with the electrocardiograph in a variety of ways beyond this manual.
• Press the Help key from the Ready screen or Lead Status screen for a list of
topics available to print.
• Review the other information that came with the electrocardiograph. For list, see
“Product information” on page 6.
• Contact Welch Allyn. For phone numbers, see page ii.
19
2
Setting Up the Electrocardiograph
Inspecting the Electrocardiograph . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20
Connecting the Patient Cable . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20
Loading the Thermal Chart Paper. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
Powering the Electrocardiograph. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 22
Verifying Proper Operation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23
Connecting an External USB Printer (Optional) . . . . . . . . . . . . . . . . . . . . . . . 24
20 Chapter 2 Setting Up the Electrocardiograph Welch Allyn CP 100 Electrocardiograph
Inspecting the Electrocardiograph
1. Look for obvious signs of shipping damage. If you find any damage, contact Technical
Support. For phone numbers, see page ii.
2. Verify that you have received all appropriate options and accessories. See “Options”
on page 5 and “Accessories” on page 6.
Connecting the Patient Cable
WARNING Conductive parts of the patient cable, electrodes and associated
connections of defibrillation-proof Type CF applied parts, including the neutral
conductor of the patient cable and electrode, should not come into contact with
other conductive parts, including earth ground.
WARNING To avoid injury to the patient or damage to the device, never plug
patient leads into any other device or wall outlet.
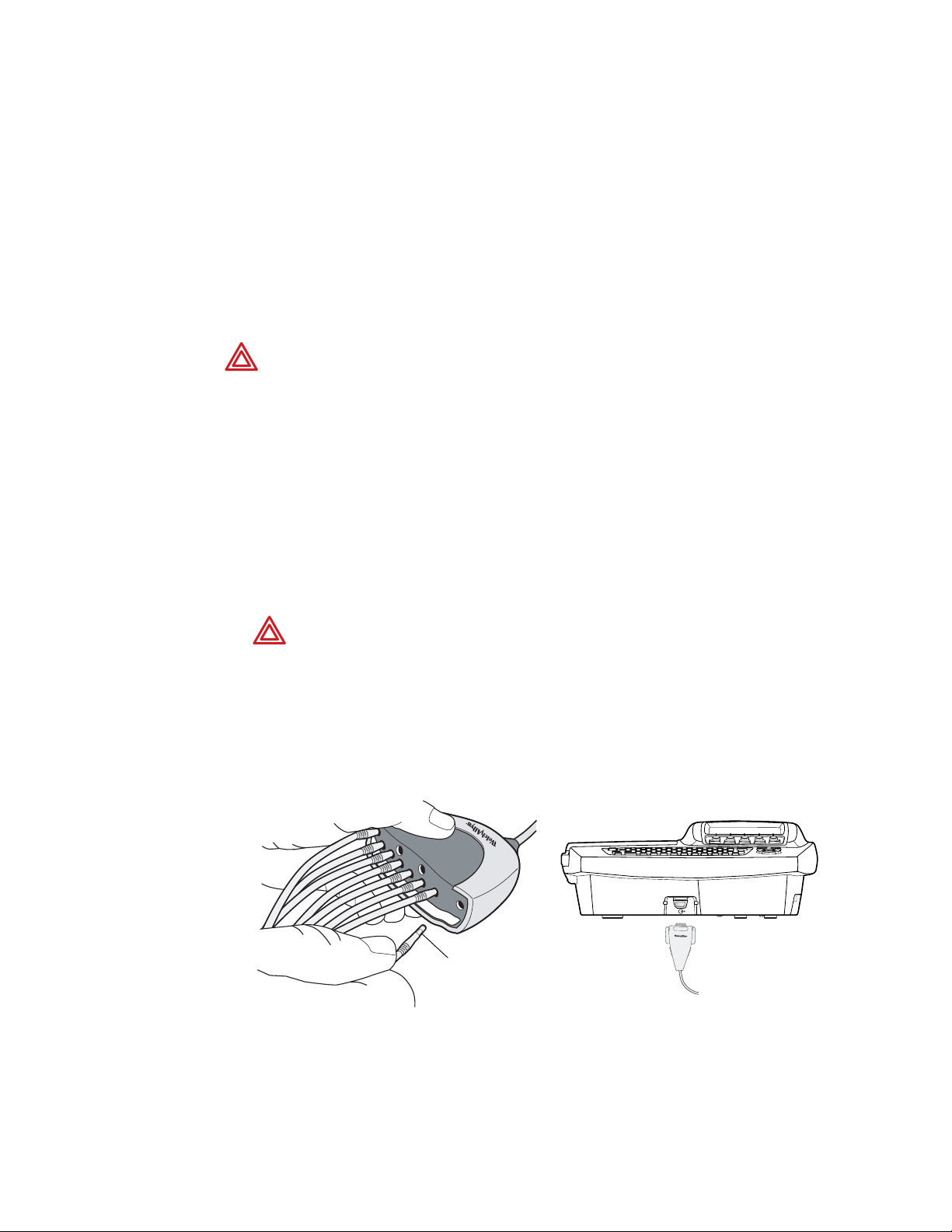
1. Insert all leads into their proper positions, as labeled on the connectors.
Insert connectors fully so that no part of the metal ring remains exposed.
For example, see Figure 12. (To see the whole patient cable with all leads inserted,
see Figure 11 on page 12.)
WARNING Failure to insert all connectors fully may result in a loss of energy
being delivered to the patient during defibrillation and damage to the patient
cable itself. For other warnings related to defibrillation, see page 16.
2. Plug the patient cable into the port on the front of the electrocardiograph.
See Figure 13.
Figure 12. Inserting the Leads Figure 13. Plugging in the Connector
Metal ring
Directions for Use Chapter 2 Setting Up the Electrocardiograph 21
Loading the Thermal Chart Paper
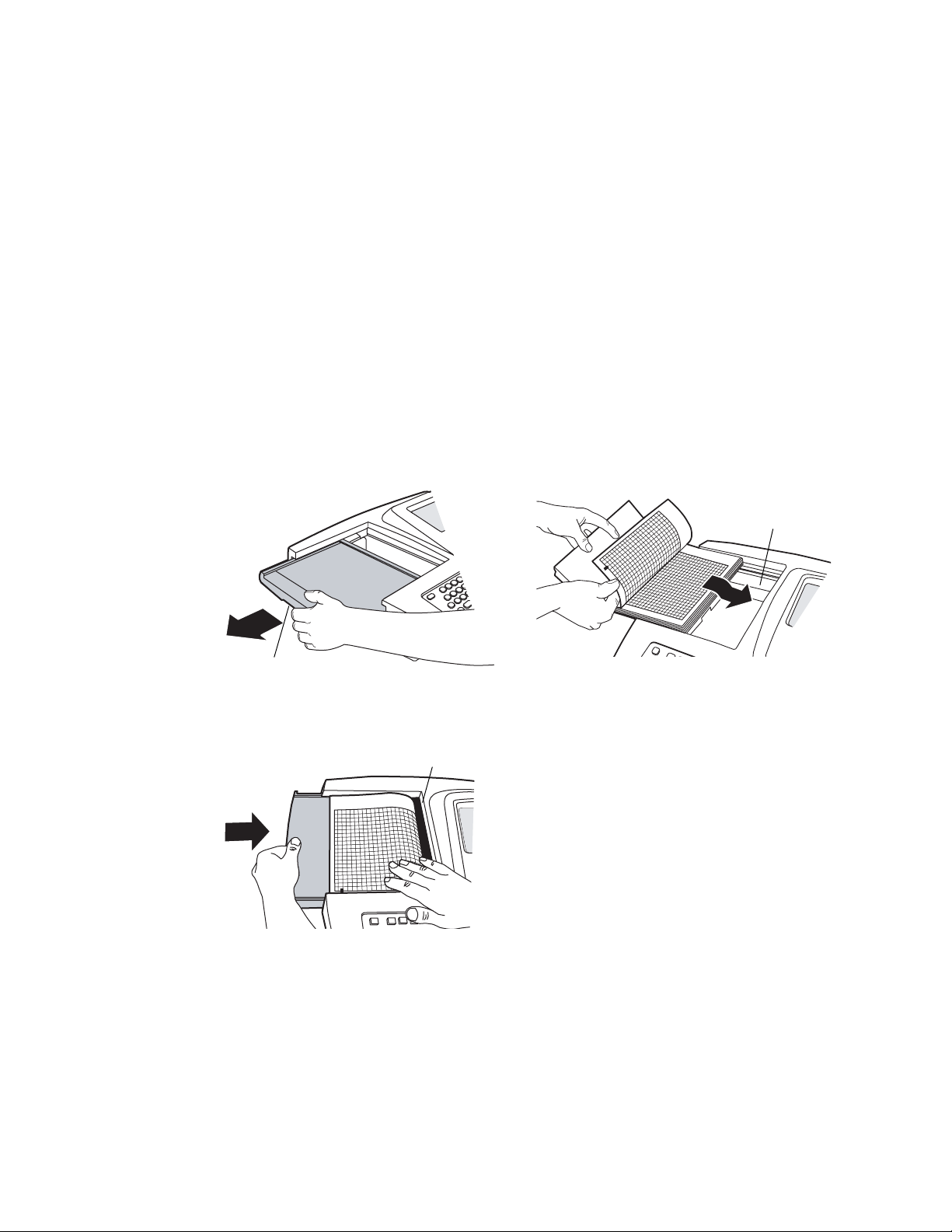
1. Squeeze the latch. Pull the paper door to the left. See Figure 14.
If any paper remains in the tray, remove it.
2. Remove the outer packaging, including the cardboard bottom, from a new pack of
paper. Pull the top sheet back so that the paper’s grid side faces up and the Welch
Allyn name is on the bottom of the paper.
3. Slide the paper into the tray. See Figure 15.
If humidity is high, remove up to 10 sheets so that the paper fits properly.
4. Lay the top sheet over the paper door. Push the door to the right until it clicks.
See Figure 16.
Figure 14. Opening the Paper Door Figure 15. Loading the Paper
Latch
Figure 16. Closing the Paper Door
Tea r bar
Paper tray
Tips for handling thermal paper:
• Store in a cool, dry, dark place.
• Avoid exposure to bright light or UV sources.
• Avoid exposure to solvents, adhesives, or cleaning
fluids.
• Do not store with vinyls, plastics, or shrink wraps.
22 Chapter 2 Setting Up the Electrocardiograph Welch Allyn CP 100 Electrocardiograph
Powering the Electrocardiograph
The electrocardiograph can run on AC or battery power.
WARNING To ensure that electrical safety is maintained when using AC power,
the device must be plugged into a hospital-grade outlet.
WARNING Where the integrity of external protective earth conductor
arrangement is in doubt, use battery power.
Caution Medical electrical equipment needs special precautions regarding EMC
and must be installed and used according to the information provided in “EMC
Guidance and Manufacturer’s Declarations” on page 75.
To Connect to AC Power
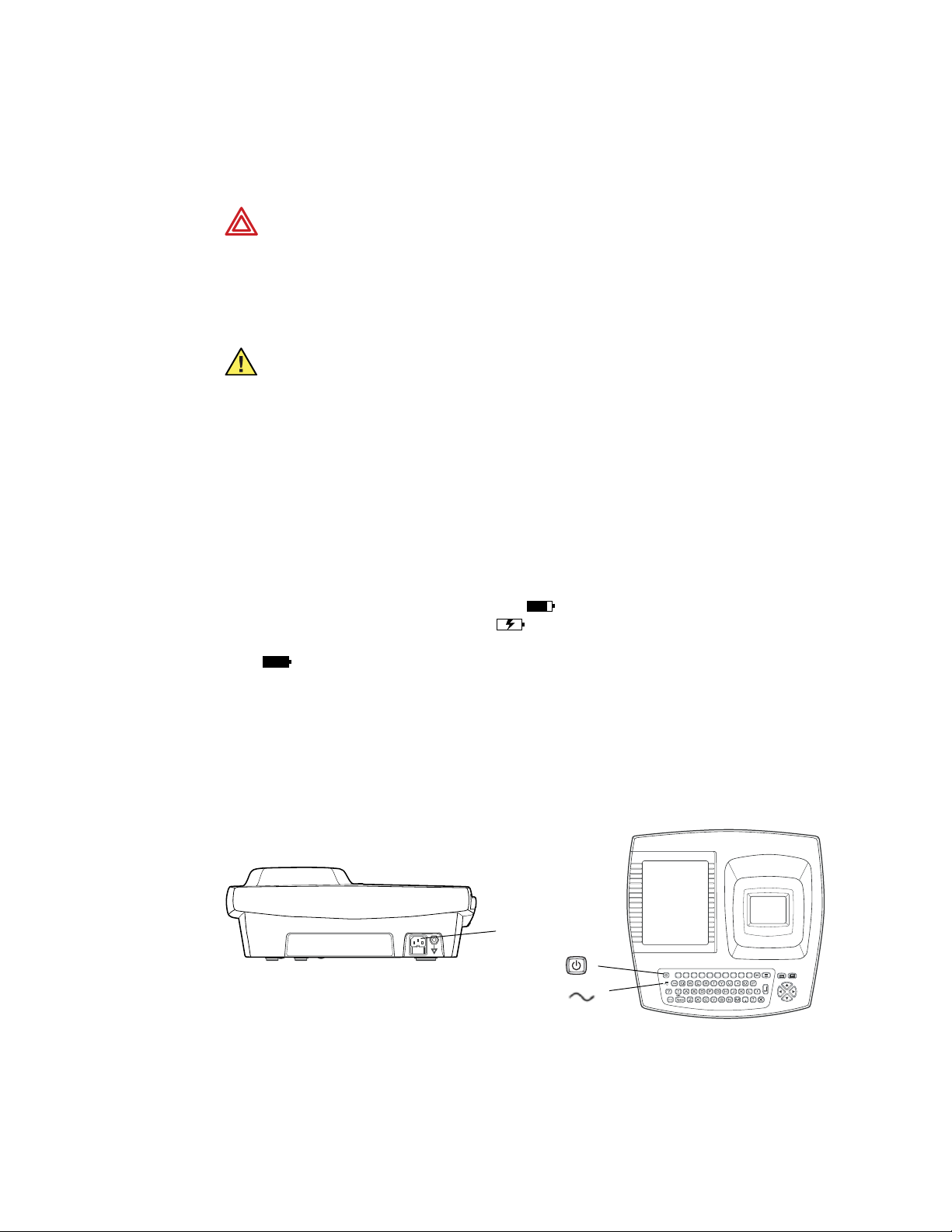
Plug one end of the power cord into the electrocardiograph’s AC power inlet. Plug the
other end into an AC outlet. The green LED on the keyboard lights up, indicating that
power is connected. See Figure 17.
To Keep the Battery Charged
Leave the electrocardiograph connected to AC power whenever possible. Battery charge
status is indicated on the screen by an icon: . Whenever the battery is charging and is
not yet fully charged, this icon appears: . For maximum battery performance, as often
as possible leave the electrocardiograph plugged in until you see the “fully charged”
icon: .
When the charge gets low, the icon flashes. When the charge gets too low to operate, a
warning message appears and the electrocardiograph beeps every 15 seconds for 1
minute, then it turns off.
For more, see “Recharging a Fully Discharged Battery” on page 60.
Figure 17. AC Power Inlet and Green LED
AC power inlet
On/Off key
Green LED
@3#
1!2
4$5%6+7-8
)
(
*
9
0
Directions for Use Chapter 2 Setting Up the Electrocardiograph 23
To Turn the Electrocardiograph On
Press .
To Turn the Electrocardiograph Off
Press and hold.
Note
If Power-Save is enabled, the electrocardiograph turns off automatically after
several idle minutes. To learn how to enable or disable Power-Save, see
“Reviewing the Device Configuration Settings” on page 27.
Verifying Proper Operation
Once your electrocardiograph is set up, verify proper operation by using an ECG simulator
to acquire and print a standard 12-lead ECG of known amplitude. See Step 2 on page 59.
Note
As part of your initial set-up, you may want to adjust the display contrast. To learn
how, see “Reviewing the Device Configuration Settings” on page 27.
You may also want to change other software settings, as described in the
following chapters:
• “Reviewing the System Settings” on page 25
• “Reviewing the ECG Settings” on page 31
 Loading...
Loading...