Page 1
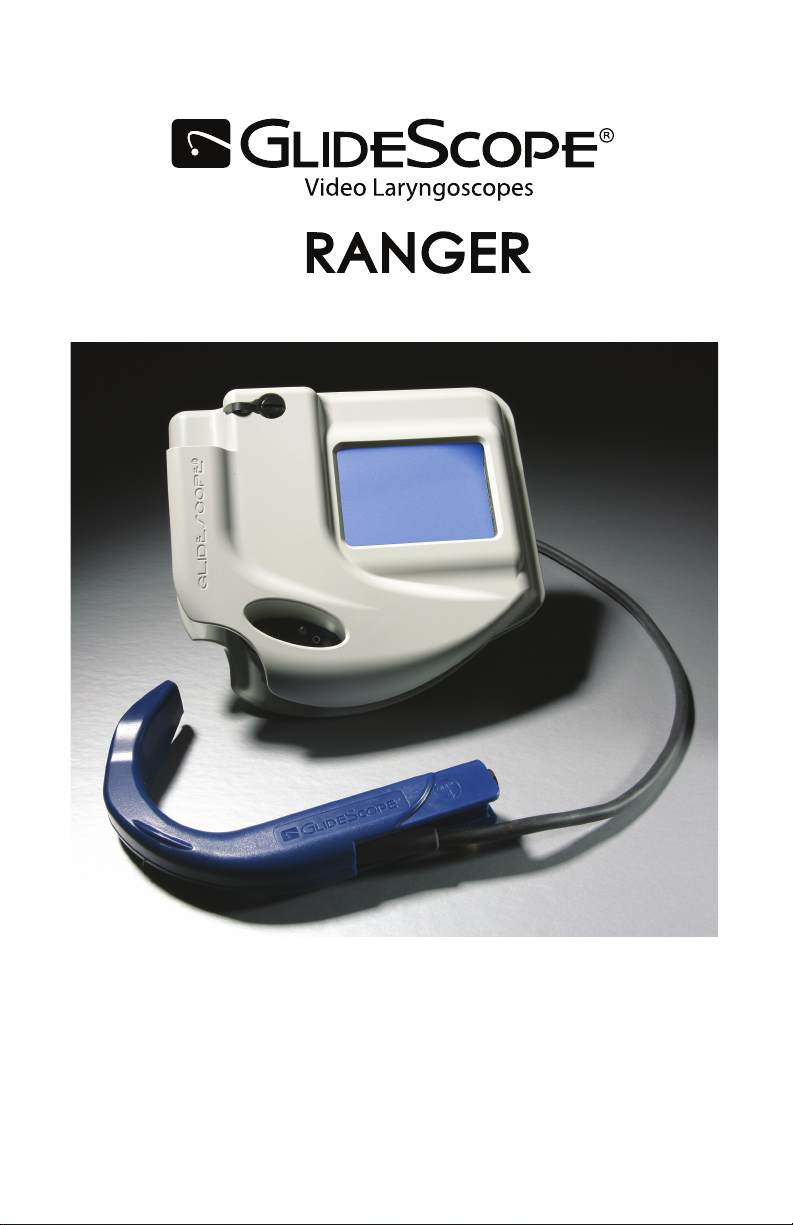
Ranger and Ranger Single Use
User’s Manual and Quick Reference Guide
0900-1307-05-20
Page 2
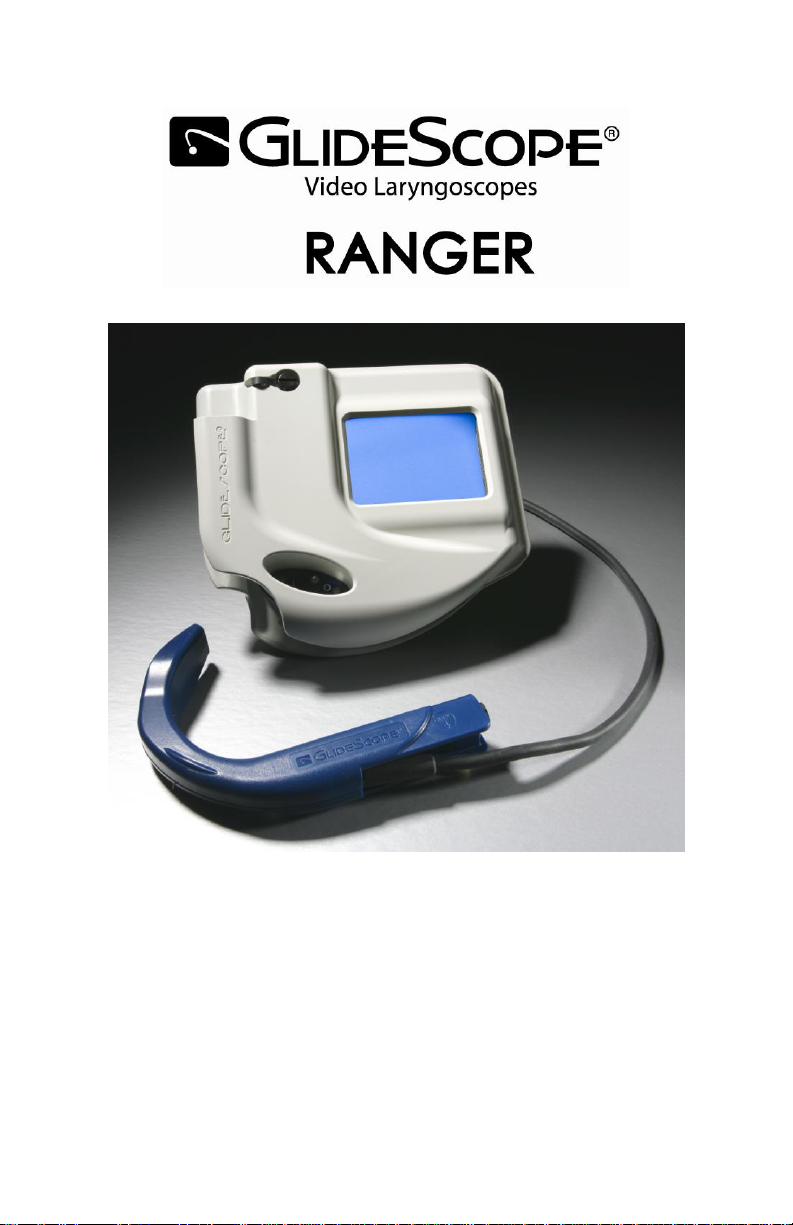
Ranger and Ranger Single Use
Quick Reference Guide
Page 3

Corporate Headquarters:
Verathon Inc.
20001 North Creek Parkway,
Bothell, WA 98011 USA
800.331.2313 (Canada and US)
425.867.1348
Fax: 425.883.2896
verathon.com
Verathon Medical (Europe) B.V.
Linnaeusweg 11
3401 MS, IJsselstein
The Netherlands
+31.30.68.70.570
Fax: +31.30.68.70.512
verathon.eu
Verathon Medical (Canada) ULC
4224 Manor Street
Burnaby, BC V5G 1B2
Canada
604.439.3009
Fax: 604.439.3039
For additional contact information please visit our corporate website:
verathon.com.
GlideScope, GVL, GlideRite, and Verathon are trademarks of Verathon
Inc. All other brand and product names are trademarks of their
respective owners.
The GlideScope® technology is covered under US Patents (6,655,377)
(6,543,447) as well as European Patent 1307131. Additional patents
pending.
Information in this User’s Manual and Quick Reference Guide may
change at any time without notice. For the most up-to-date information,
see the online manuals on verathon.com.
GlideScope® Video Laryngoscope systems are CE marked in
accordance with the Medical Device Directive, and the Verathon Inc.
quality is Quality System Certified to ISO 13485:2003 standards.
© 2009, 2010 Verathon Inc. No part of this manual may be copied or
transmitted by any method without the express written consent of
Verathon Inc.
0900-2111-02-20111-01-20 Phillips, Oct2008, www.allterrainwriter.com
Page 4
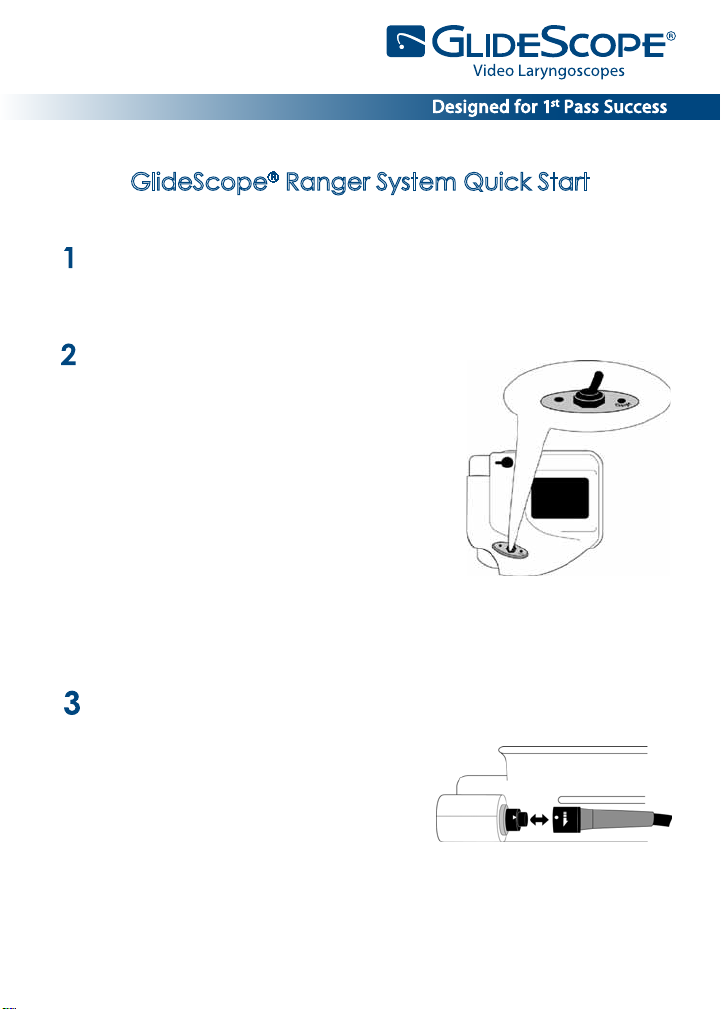
GlideScope® Ranger System Quick Start
The battery must be fully charged before rst use. The unit will not operate
while charging. If it is switched on during charging, the charging light will
ash alternately green and orange.
OFF
To ensure proper charging, follow these steps
in order:
• Make sure the power switch is in the OFF
position.
• Unscrew the DC socket protective cap
on the front of the monitor (a screwdriver
or a small coin may be used).
• Plug the power supply barrel connector
into the DC socket. Plug the AC cord
into the power supply and then into the wall.
• The charge status LED will turn orange,
indicating that the recharging cycle has begun.
• When charging is complete, the status LED will turn green.
Connect the Video Laryngoscope to the monitor.
• If using the Ranger GVL®:
Insert the Ranger GVL® cable connector
into the port located on the face of
the monitor so that the arrows on the
cable and the monitor line up.
• If using the Ranger Single Use system:
Connect the video baton cable connector to the monitor as shown for the Ranger GVL® above. Then
slide a single-use GVL® Stat over the video baton.
Position
Page 5
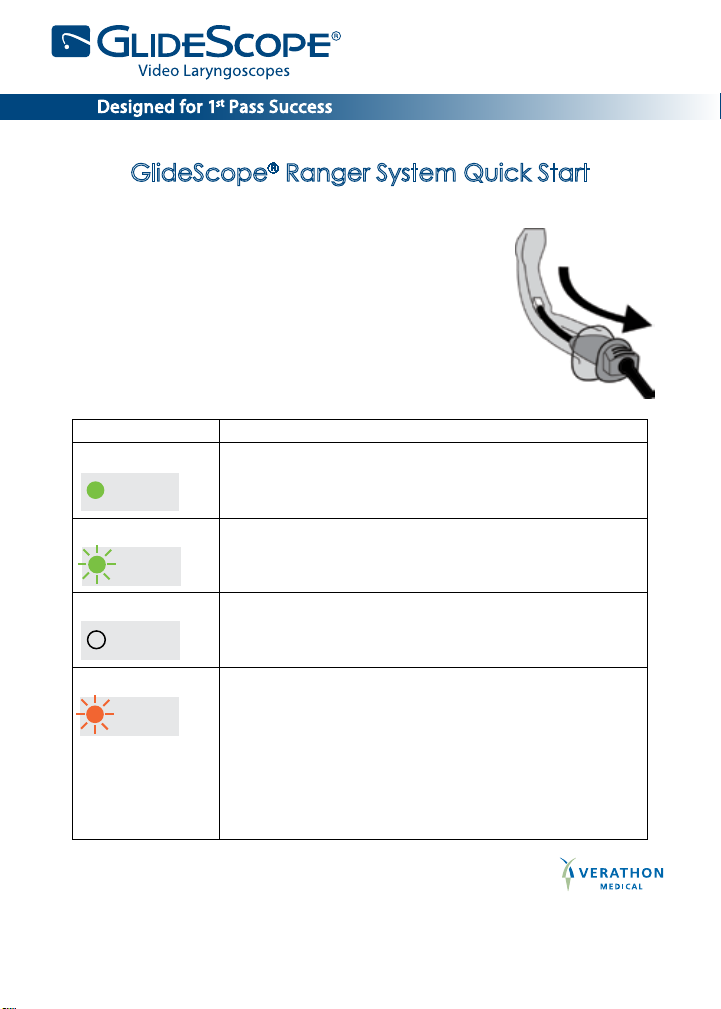
GlideScope® Ranger System Quick Start
To detach a GVL® Stat from a Ranger Video
Stat
Baton, grasp the base of the video baton
handle and pull rmly.
IMPORTANT! A used GVL® Stat is a biohazard and
should be disposed of in compliance with local
protocols.
Video Baton
LED State Meaning
Steady green
The battery is fully charged.
CHARGE
STATUS
Flashing green Battery power is low. LED ashes for approximately
CHARGE
5 minutes before shutting off.
STATUS
No LED The battery is completely depleted and needs to
CHARGE
be recharged.
STATUS
Flashing orange A ashing orange light indicates a battery problem.
CHARGE
STATUS
When this happens:
• Disconnect the DC connector.
• Make sure the switch is in the CHARGE
(or OFF) position.
• Reconnect the DC connector.
• If the orange LED is still ashing, the battery
has failed.
• Return the unit for repair.
Corporate Headquarters:
Verathon Inc.
20001 North Creek Parkway
Bothell, WA 98011, USA
Tel: 800.331.2313 (US and Canada only)
Tel: 425.867.1348 Fax: 425.883.2896
GlideScope®, GVL®, Verathon® and Verathon Medical® are registered trademarks of Verathon Inc. in the United States and/or other countries.
© 2008 Verathon Inc. GlideScope® video laryngoscope systems are CE marked in accordance with the Medical Device Directive, and the
Verathon Inc. quality system is Quality System Certied to ISO 13485:2003 standards.
Manufacturer:
Verathon Medical (Canada) ULC
4224 Manor Street
Burnaby, British Columbia
Canada, V5G 1B2
EC Representative:
Verathon Medical (Europe) B.V.
Linnaeusweg 11
3401 MS IJsselstein Netherlands
Tel: +31.30.68.70.570
Fax: +31.30.68.70.512t
www.verathon.com
0900-2116-01-60
Page 6

Cleaning and Disinfecting the GlideScope®
Ranger GVL and Ranger Single Use Video Baton
• DONOT exposetotemperaturesabove60°C(140°F).
DO NOT disinfect using devices such as autoclaves, ultrasonic cleaners or pasteurizers.
®
• The GlideScope
the Ranger GVL
Disinfectant method. High Level Disinfection is required for the Ranger GVL
visibly soiled.
• The GlideScope
intended, it is protected from direct contact with the patient by the sterile, single-use
®
Stat. Low Level Disinfection is recommended for the Ranger Video Baton after every
GVL
patient use. High Level Disinfection is required for the video baton when it is visibly soiled.
Disconnect the Ranger GVL
Place the cleaning cap over the connector as shown. Turn counter-clockwise to
lock in place.
• During cleaning, the protective cap must be inserted as shown to protect
the cable connector.
Ranger GVL® is a non sterile, reusable device. It is recommended that
®
is cleaned and disinfected after every patient use using a High Level
®
Ranger Video Baton is a non sterile, reusable device. When used as
®
or Video Baton from the monitor.
®
Ranger Single Use Video BatonRanger GVL
Video Baton
Cleaning Cap
®
when it is
®
Wash the Ranger GVL
or Video Baton manually to remove all foreign material from
the surface of the device.
• Chemical compatibility and disinfection methods are detailed in the user’s
manual.
• To clean the exterior of the monitor, wipe with IPA (70% isopropyl alcohol) bleach
(100ppm) or a mild detergent and water.
®
FormoredetailedcleaninginstructionsseetheGlideScope
RangerUser’sManual.
Page 7

Attaching/Detaching the GlideScope® Ranger
GVL or Ranger Single Use Video Baton
Insert the Ranger GVL® or Video Baton
cable into the port located on the face of
the monitor so that the arrows on the cable
and the monitor line up.
Detach the cable by turning the connector
and gently pulling away from the monitor
port.
Attaching/Detaching the
GlideScope® Ranger Video Baton and Stat
STAT Video
To ensure proper insertion, match the
GlideScope® logo on the side of the video
baton and the GVL® Stat. Insert the Ranger
Video Baton into the sterile, single-use GVL®
Stat until it clicks into place.
Detach the video baton from the GVL® Stat
by grasping the base of the video baton and
pulling rmly.
IMPORTANT!A used GVL® Stat is a biohazard and should be disposed
of in a manner consistent with local directives in the user’s jurisdiction.
CorporateHeadquarters:
VerathonInc.
20001 North Creek Parkway
Bothell, WA 98011, USA
Tel: 800.331.2313 (US and Canada only)
Tel: 425.867.1348 Fax: 425.883.2896
GlideScope, GVL, and Verathon are trademarks of Verathon Inc. © 2008, 2009 Verathon Inc. GlideScope
accordance with the Medical Device Directive, and the Verathon Inc. quality system is Quality System Certied to ISO 13485:2003 standards.
Manufacturer:
VerathonMedical(Canada)ULC
4224 Manor Street
Burnaby, British Columbia
Canada, V5G 1B2
ECRepresentative:
VerathonMedical(Europe)B.V.
Linnaeusweg 11
3401 MS IJsselstein Netherlands
Tel: +31.30.68.70.570
Fax: +31.30.68.70.512t
®
video laryngoscope systems are CE marked in
verathon.com
Baton
0900-1326-09-60
Page 8

The GlideScope
®
4-Step Technique
Looking directly into the patient’s mouth
and with the GlideScope® in the left hand,
introduce the video laryngoscope into the
midline of the oral pharynx.
With the laryngoscope inserted, look to
the monitor to identify the epiglottis, then
manipulate the scope to obtain the best
glottic view.
Looking directly into the patient’s mouth,
not at the screen, carefully guide the
distal tip of the tube into position near the
tip of the laryngoscope.
It is important to look into the mouth at this step to
avoid injuring the tonsils or soft palate.
Look to the monitor to complete the
intubation; gently rotate or angle the tube
to redirect as needed.
GlideScope® video laryngoscope systems are CE marked in accordance with the Medical Device Directive, and the Verathon Inc. quality system
is Quality System Certied to ISO 13485:2003 standards. © 2009 Verathon Inc.
Page 9

®
Tips for GlideScope
Video Laryngoscope Insertion
• Verathon® recommends inserting the GlideScope® Video
Laryngoscope down the midline of the tongue to the epiglottis.
• The GlideScope® video laryngoscope may be used to produce a
MacIntosh indirect lift of the epiglottis or a Miller lift.
• Intubations using GlideScope® Video Laryngoscopes require
approximately 0.5kg-1.5kg of lifting force.
• Use of an endotracheal tube stylet
is recommended. The GlideRite®
Rigid Stylet has been designed to
complement the angle of the
GlideScope® video laryngoscope to
facilitate intubation. A malleable
stylet may be used with a 60°- 90°
angle.
• To aid the passage of the endotracheal
tube, withdraw the stylet (approx. 5 cm)
while gently advancing the ETT. A 1 cm
adjustment (withdrawal) of the laryngoscope
also may be benecial to reduce the viewing
angle and allow the glottis to drop.
Corporate Headquarters:
Verathon Inc.
20001 North Creek Parkway
Bothell, WA 98011, USA
Tel: 800.331.2313 (US and Canada only)
Tel: 425.867.1348 Fax: 425.883.2896
GlideScope, GVL, GlideRite, and Verathon are trademarks of Verathon Inc. © 2009 Verathon Inc. All rights reserved.
Verathon Medical
(Canada) ULC
4224 Manor Street
Burnaby, British Columbia
Canada, V5G 1B2
Verathon Medical (Europe) B.V.
Linnaeusweg 11
3401 MS IJsselstein Netherlands
Tel: +31.30.68.70.570
Fax: +31.30.68.70.512
verathon.com
0900-1436-06-60
Page 10
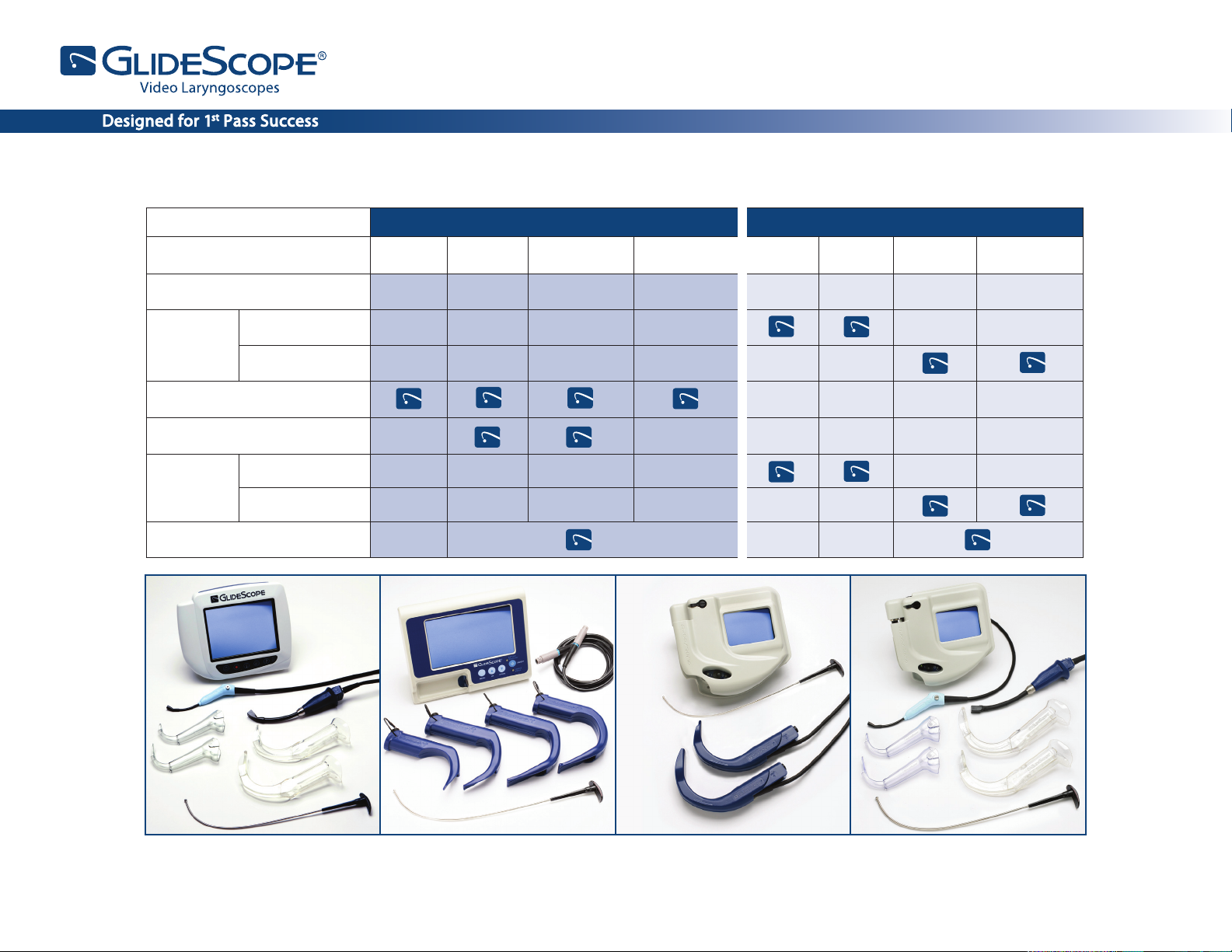
GlideScope
®
Products and Systems
REUSABLE SINGLE USE STATS
®
GVL
Size GVL® 2 GVL® 3 GVL® 4 GVL® 5 GVL® 1 GVL® 2 GVL® 3 GVL® 4
Patient Weight
Cobalt
AVL
GlideScope
Video Baton 1-2
Video Baton 3-4
®
Ranger
Ranger
Video Baton 1-2
Single
Use
GlideRite
Video Baton 3-4
®
Rigid Stylet *
GVL
1.8 - 10 kg
10 kg -
Adult
40 kg -
Morbidly Obese
40 kg -
Morbidly Obese
< 3.6 kg 1.8 - 10 kg 10 kg - Adult
40 kg -
Morbidly Obese
FPO FPO FPO
Cobalt AVL System (single use)
Note: System congurations vary. The Cobalt AVL and Ranger Single Use Video Batons are reusable.
*For use with endotracheal tubes 6.0 mm and larger.
GVL® System (reusable)
Ranger System (reusable)
Ranger Single Use System
Page 11

GlideScope
®
Product Specications
SYSTEM MONITORS
Cobalt AVL Monitor
Color, 6.4in (diagonal), VGA 640 x 480
Height: 190 mm
Width: 225 mm
Depth: 80 mm
Weight: 1.0 kg
GVL® Monitor
Color, 6.4in (diagonal), 440 x 234
Height: 167 mm
Width: 207 mm
Depth: 83 mm
Weight: 1.4 kg
SINGLE USE SYSTEM COMPONENTS
Cobalt AVL Video Baton 1-2
Length: Camera tip to SS ring to handle: 66mm
Height of camera: 6 mm
Width of camera: 7 mm
Cable length: 194.5 cm
Weight: 170 g
Ranger Video Baton 1-2
Length: Camera tip to handle: 40 mm
Height of camera: 6 mm
Width of camera: 7 mm
Cable length: 86.4 cm
Weight: 95 g
Cobalt AVL Video Baton 3-4
Length: Camera tip to SS ring: 100 mm
Height of camera: 11 mm
Width of camera: 11 mm
Cable length: 193 cm
Weight: 230 g
Ranger Video Baton 3-4
Length: Camera tip to SS ring: 104.1 mm
Height of camera: 10.7 mm
Width of camera: 10.9 mm
Cable length: 86.4 cm
Weight: 141 g
REUSABLE SYSTEM COMPONENTS
GVL® 2
Blade length (tip to handle): 47 mm
Thickness (height) at camera: 14.5 mm
Width at camera: 18 mm
GVL® 4
Blade length (tip to handle): 102 mm
Thickness (height) at camera: 14 mm
Width at camera: 27 mm
GVL® 3
Blade length (tip to handle): 82 mm
Thickness (height) at camera: 14.5 mm
Width at camera: 20mm
GVL® 5*
Blade length (tip to handle): 102 mm
Thickness (height) at camera: 14 mm
Width at camera: 27 mm
Ranger Monitor
Color, 3.4in (diagonal), 480 x 234
Height: 168 mm
Width: 173 mm
Depth: 49 mm
Weight: 0.56 kg
GVL® 1 and 2
STAT length (tip to handle): 38 mm/51 mm
Thickness (height) at camera: 8.7 mm/8.7 mm
Width at camera: 9.9 mm/10.9 mm
Note: The Cobalt AVL and Ranger Single Use Video Batons are reusable.
20001 North Creek Parkway, Bothell, WA 98011 www.verathon.com 1.800.331.2313 (U.S. & Canada Only) 425.867.1348
The GlideScope video laryngoscopy systems are CE marked in accordance with the Medical Device Directive, and the Verathon Inc. quality system is Quality System Certied to ISO 13485:2003 standards. US Patent No. 6,655,377; 6,543,447; Other patents pending.
GlideScope, GVL and Verathon are trademarks of Verathon Inc. © 2009 Verathon Inc.
GVL® 3 and 4
STAT length (tip to handle): 80 mm/95 mm
Thickness (height) at camera: 16 mm/16 mm
Width at camera: 16 mm/20 mm
Ranger GVL® 3
Blade length (tip to handle): 78mm
Thickness (height) at camera: 14.5 mm
Width at camera: 14 mm
*The GVL® 5 is designed to accommodate anatomical anomalies sometimes associated with bariatric patients.
Ranger GVL® 4
Blade length (tip to handle): 89 mm
Thickness (height) at camera: 14.5 mm
Width at camera: 19 mm
0900-2067-03-86
Page 12
GlideScope® Ranger System
Important Information
Statement of Prescription
Federal (USA) law restricts this device for sale by or on the order
of a physician.
The Ranger System should be used only by individuals who
have been trained and authorized by a physician, or by health
care providers who have been trained and authorized by the
institution providing patient care.
Intended Use
The Ranger System is intended for use by qualified medical
professionals to obtain a clear, unobstructed view of the vocal
cords for medical procedures. The Ranger System is designed
for field (military and pre-hospital) use.
Warnings and Cautions
CAUTION. Risk of permanent equipment damage.
Do not expose the Ranger GVL
temperatures above 140° F (60° C). Do not disinfect the Ranger
®
or Ranger Video Baton using devices such as autoclaves,
GVL
ultrasonic cleaners, or pasteurizers. Use of such methods will
cause permanent device damage and void the warranty
the User’s Manual for a list of approved cleaning procedures and
products.
®
or Ranger Video Baton to
. Refer to
CAUTION: Risk of equipment damage.
Failure to cover the cable connector port with the protective cap
prior to cleaning may result in water ingress and potential device
failure.
CAUTION: Risk of equipment damage.
Bleach can be used on the baton but with special attention to the
connector. Bleach may corrode the stainless steel inserts and
damage the connector pins.
CAUTION: Potential interference with other devices.
The Ranger GVL
®
or Ranger Video Baton must be used with the
supplied cables to maintain electromagnetic interference (EMI)
within certified limits.
Quick Reference Guide page 1
Page 13
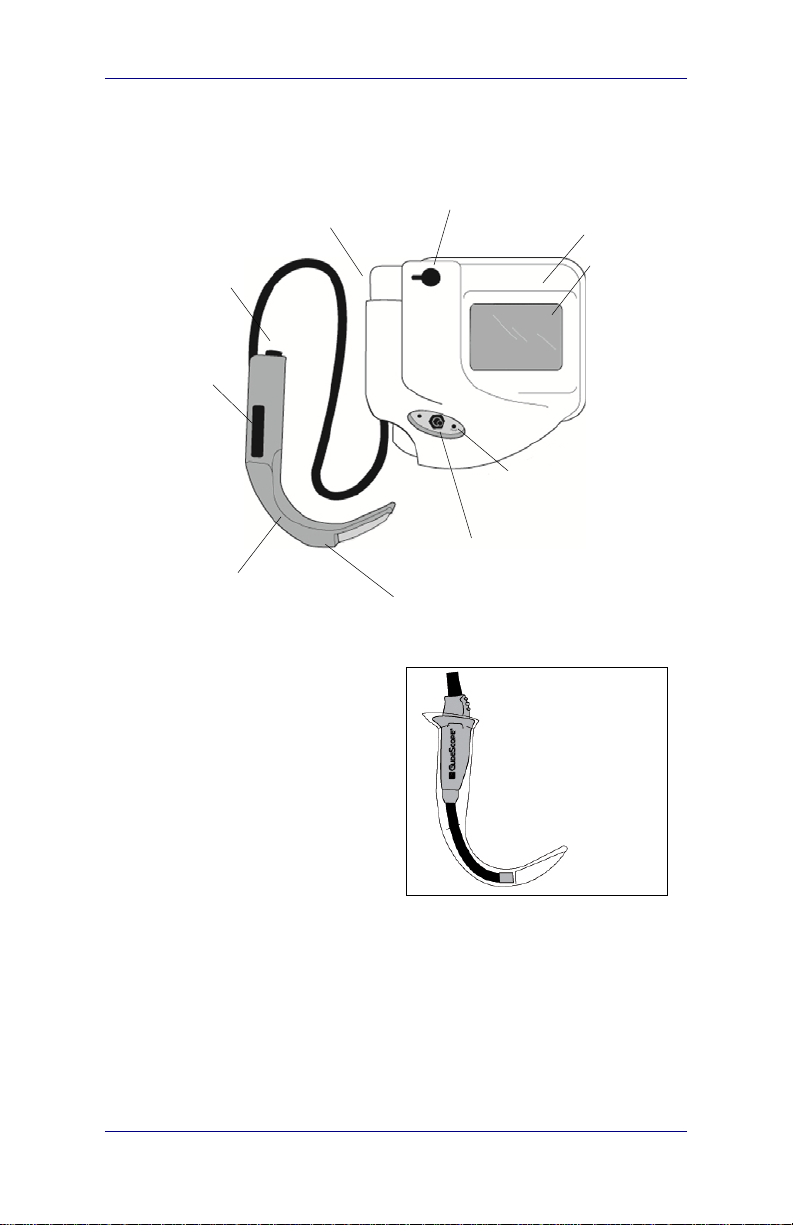
GlideScope® Ranger System
r
Components and Features
Monitor connecto
(inside cradle)
DC power socket
Monitor
Protective cap
Temperature
gauge
Ranger Video
Laryngoscope (GVL
with integrated cable
(reusable blade shown)
®
)
Battery charge
status LED
Power ON/OFF
CMOS video camera
Reusable Ranger
Video Baton
inside a sterile,
single-use
Ranger GVL
LCD
display
®
Stat
page 2 Quick Reference Guide
Page 14
GlideScope® Ranger System
Preparing for First Use
Prior to using the Ranger System for the first time:
1. Charge the monitor battery.
2. Perform a functional check:
Connect a Ranger GVL
®
or Ranger Video Baton to
the monitor
Turn the system on by pressing the on/off switch
Observe the monitor screen to verify that an image
is being received from the Ranger GVL
®
or Ranger
Video Baton
®
If the GVL
, Video Baton or Stat are stored in cold conditions,
additional warming time may be required for optimal
performance of the anti-fog feature.
Cleaning, Disinfecting, and Maintenance
To ensure patient safety, users should perform a routine
inspection of the Ranger GVL
Stat before every use to ensure that all endoscopic components
are free of unintended rough surfaces, sharp edges, protrusions
or cracks.
If inspection reveals any faults in the components, contact
Verathon Medical Customer Care or your local GlideScope
representative. All repairs must be performed by an authorized
Verathon Medical Service Center.
®
, Ranger Video Baton, or GVL®
®
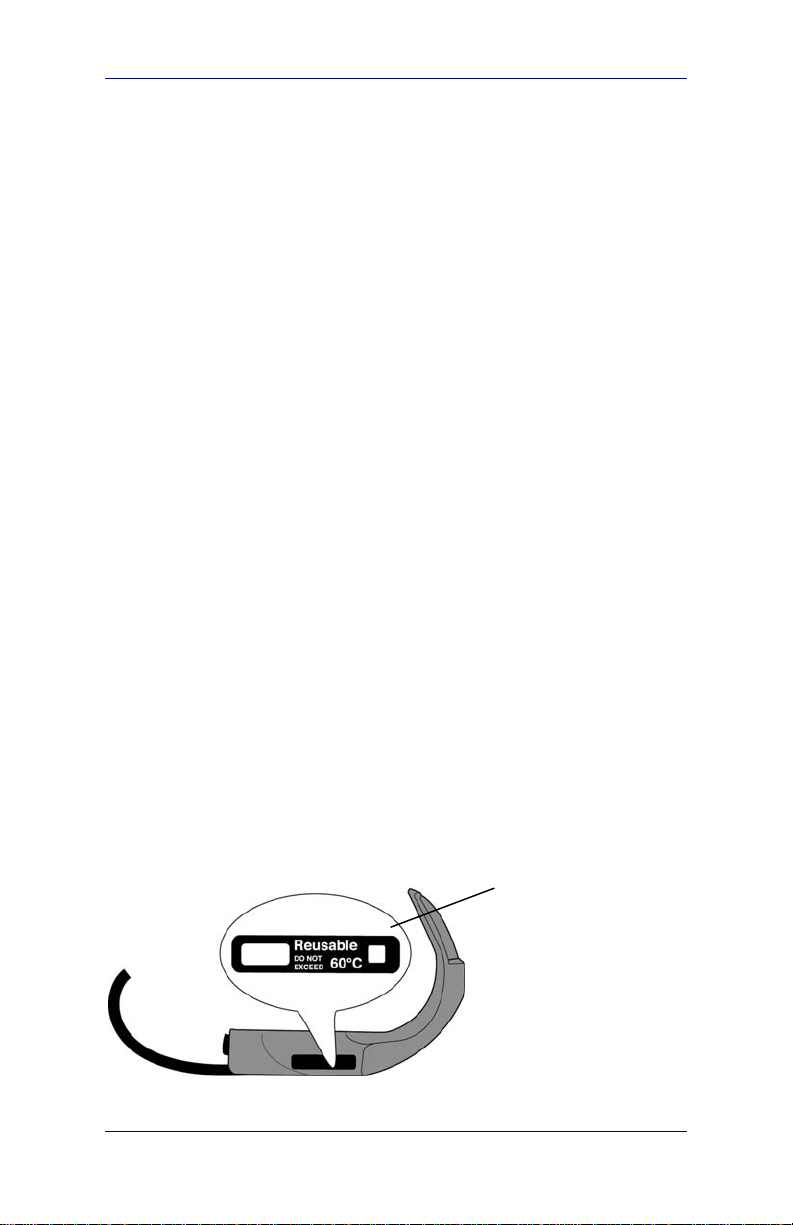
Caution: Risk of permanent device damage!
Do not expose the Ranger GVL
®
or Ranger Video Baton to
temperatures above 140° F (60°C).
The temperature
indicator turns black
when the GVL
video baton is
exposed to
temperatures above
140° F (60° C)
Quick Reference Guide page 3
®
or
Page 15
GlideScope® Ranger System
General Cleaning and Disinfection Information
Table 1: Risk Assessment
Device Sterile Use Spaulding’s
CDC
Classification
Di Sterilization sinfection
Low
Level
Int.
Level
High
Level
Ranger GVNon- Reusable Semicritical X
L® sterile
Ranger
GVL®
Stat
Ranger
Video
Baton*
Stylet Non-
Ranger
Video
Monitor
DVR for
Ranger
Cradle# Non-
Clam
Shell
Storage
Case
Sterile Single
Non-
sterile
sterile
Non-
sterile
#
Non-
#
sterile
sterile
Non-
sterile
use
Reusable Noncritical X
Reusable Semicritical X
Reusable Noncritical X
Reusable Noncritical X
Reusable Noncritical X
Reusable Noncritical X
Semicritical
It is understood that all items in this chart will be used as intended.
page 4 Quick Reference Guide
Page 16

GlideScope® Ranger System
Key:
* The Ranger Vide
protected from conta
is
intact skin by the Stat (sterile, singl
intended. Low Level Disinfection is
o aft ery High ction
Vide Baton er ev patient use.
required for the Video Bato n it is visibly soiled.
# Disinfect monitor, DVR and cradle w v soiled
and on a regular basis as per a schedule established by the
med
al ca facility or rovider.
ic re p
– Shaded a
mat
e
– C
hecked boxes (x) show minimum requirement.
– Unshaded areas show permissible levels of disinfection based
om il vi ls.
on cWapatib ity with de ce materia
rning:
inf ts in liste are r omm ded by
Dis ectan
Verat sed on compatibility with device materials. Refer to
the label in
ro c s.
app
tion: Meticulous cleaning must precede any disinfection
Cau
cess, to en removed from the surface
pro
e e. ws ing ients of the chosen
of th
cess to reach all the surfaces of the device.
pro
reas - not required/not compatible with device
rials.
ba
hon
struction si
priate
o Baton is a nonsterile, reusable device which
ct with mucous membranes and non-
e-use) when used as
recommended for the Ranger
Level Disinfe is
n whe
hen they are isibly
and clean g methods d ec en
s for gu
linical use
sure all forei
This allo
idance on di nfection efficacy and
gn matter is
the active devic red
ila of pr ies b
Ava bility
unabl st products in every market. Please use the list of
e to te
men
om de als nual com re with
rec
cts av
du a lly
pro
Quick Reference Guide page 5
cleaning oducts var y country, and we are
d chemic
ilable loca
in this ma. to pa
Page 17
GlideScope® Ranger System
Note: When using any of the chemicals listed below, read and
comply with product use instructions in all applications.
Table 2: Chemical Compatibility and Disinfectio
Methods for Ranger GVL® and Ranger Video
Batons
Active Disinfec
gredient
In
nzymatic
E
bridement General
de
agent/
etergent
d
sopropyl
I
Alcohol Solution
Glut Up to 3.4%
Orthoalaldehyde
Phth as per manufacturer’s
Per
acetic Acid 0.2%
ch (Sodium Up to
Blea
Hy
pochlorite) 8000ppm
Compatibility Conditions
hospital grade
70%
0.55%
As per instructions
70% used to wipe
down with minimum 1 L
minute exposure
2.0% – exposur
minimum 20 minutes
at 20°C or as per
manufacturer’s
instructions
0.55% – exposure for
12 minutes at 20°C or
instructions
0.2% - exposure for
minimum 12 minutes
at 50 to 56°C or as
per manufacturer’s
instructions
5000ppm – exposure
for 10 minutes at
500ppm – used to
wipe down with
minimum 1 minute
exposure
e for
20°C
Level Comm
N/A
ow
High araldehyde
High
See
comments
on right
High
Low
n
tion Caution/
ents
Surface
cleaning only
in preparation
for
disinfectant
Classifie
d as
a c
hemical
sterilant
rosive for
Cor
nnector
co
pins and SS
ring
osive
Noncorr
at ≤ 500ppm
page 6 Quick Reference Guide
Page 18
GlideScope® Ranger System
Table 3: Chemical Compatibility and Disinfection
Methods for GlideRite® Rigid Stylet
Active
gredient
Gluta Up to 3.4% 2.0% – exposure for
Ortho-
thalaldehydPh e
Peracetic Acid 0.2% 0.2% - exposure for
Isopropyl Alcohol
Solution
Bleach (Sodium
Hypochlorite)
Enzymatic
debridement
agent/ detergent
N/A
Compatibility Conditions
minimum 20 minutes
20°C or as pe
at r
anufacturer’s
m
instructions
0
12 minutes at 20°C or
as per manufacturer’s
instructions
minimum 12 minutes Scomments
per manufacturer’s
instructions
70% 70% 10
≤ 500ppm L
General
hospital grade
Tested in
autoclave
team cycle
(s )
minutes at 20°C
70% used to wipe
down with minimum 1
minute exposure
500ppm – used to
wipe down with
minimum 1 minute
exposure
As per instructions N/A Surface
Minimum 4 minute See
132°C pre-vacuum
steam sterilization
cycle
Disinfecti
on Level
High raldehyde
High 0.55% .55% – exposure for
ee
on right
Intermedia
te
Low
ow
comments
on right
CauIntion/
Comments
Classified as a
chemical
sterilant at 50 to 56°C or as
- exposure for
Noncorrosive at
≤ 500ppm
cleaning only
p
reparation for
d
isinfectant
Based on
requests from
users, an
utoclave cycle
a
has been
established for
the Stylet for
ease of use
in
Quick Reference Guide page 7
Page 19

GlideScope® Ranger System
Cleaning and Disinfecting the GlideScope®
Ranger GVL® and Ranger Video Baton
Caution:
process
of the device.
process t
Meticulous cleaning must precede a ction
, to reign move e su
This allo
o re su e.
ach all the
ws t s of the chosen
he active ingredient
rfaces of the devic
ny disinfe
d from th rface ensure all fo matter is re
The Ranger GVL® is a non- device. It is
recommended that the Rang ned and disinfected
after every patient use, using a High Level Disinfectant method.
High L
evel Disinfe
when it is
visib d.
ction is required for th
ly soile
The Ranger Video Baton i sable device which
is protected from direct contac atient by the Stat
(sterile, s se) w sed as intended,
ingle u hen u Lo
Disinfection is recommend V n aft
every patient use. High Lev req
sterile reusable
er GVL
®
is clea
e GlideScope
s a non-sterile, reu
t with the p
ed for the Ranger
el Disinfection is
®
GVL
w Level
ideo Bato
uired for the
er
Ranger Video Baton when it is visibly soiled.
CAUTION: ent damage.
Bleach can be used on the baton but wit cial attention to the
connector. Bleach may c ste d
damage the connector pin
Availability of disinfection p y country, and we are
unable to
recomme
with products available locally.
For mor
User's Ma
Risk of equipm
h spe
orrode the stainless
el inserts an
s.
roducts varies b
t products se th t of
tes in ev ase u e lis
nded disinfectants anual to compar
ery market. Ple
in the User's M
tailed cleani ystem
e de ng instructions see the Ranger S
nual on our Web site at http://www.verathon.com
e
/
gs_manuals.htm
page 8 Quick Reference Guide
Page 20

GlideScope® Ranger System
Battery Replacement and Device Repair
Under normal operating conditions, the monitor battery will last 2
- 3 years; or approximately 500 charge/discharge cycles.
The battery is not user-replaceable. In case of battery
malfunction, do not attempt to replace the monitor battery. Any
attempts to replace the battery by unauthorized service
void the technicians may cause serious harm to the user and will
warranty. Please contact your Verathon Medical Customer Care
Representative for more information on battery replacement.
Device Disposal
Disposal of this device can be coordinated through your
Verathon Service Center in accordance with WEEE
requirements.
Quick Reference Guide page 9
Page 21

GlideScope® Ranger System
Specifications
General Specifications
Classification: Electrical Class II, Applied Par
Line Voltage Range: 100–240 VAC, 50 & 60 Hz
Line Current: MAX 0.25 A
Operating and Storage Conditions:
Operating Conditions
Temperature:………………………………………...…10°C to 40°C
t BF
Relative Humidity:……………………………………..…....0
Atmospheric P
Shipping and Storage Conditions
Temperature:……………………………..……………-20°C to 45°C
Relative Humidity:………………………………………......0 to 95%
Atmospheric Pressure:…………………………….440 to 1060 hPa
Charging Conditions
Temperature:………………………………………….…0°C to 40°C
Relative Humidity:………………………………………......0 to 95%
Atmospheric Pressure:…………………………….440 to 1060 hPa
Ingress Protection
IP68 up to 3 ft (1.0 m) for 60 minutes
ressure:…………………………….440 to 1060 hPa
to 95%
page 10 Quick Reference Guide
Page 22
GlideScope® Ranger System
Standards and Approvals
ISO 13485 Certificate No. 9235, CE Ma
for Class 1 and Class 1 Sterile
(Second Edition IEC 60601-1-2:2001 Medical Directi
US UL 60601- ition), US UL 2601-1 (Second Editio
CAN/CSA C22.2 No. 601.1-M90, AU, CA, DK, IL, and US
National Differences, as per CB Bulletin 107a, FCC Report of
Measurements as per FCC CFR47 Part 18.
ymbol Directory
S
The following international regulatory symbols are found on
Ranger System components and indicate compliance with
international regulatory standards.
Symbol Meaning
, 1996), ve,
1 (First Ed n),
Devices, IEC 60601-2-18
rking requirements met
Power connected to m
Power disconnected from mains
Type BF equipment
CE marking in accordance with the Medical
Device Directive ( 0413 for Sterile Devices)
Canadian Standards Association (CSA) mark of
certification to applicable standards for electromedic
al equipment
Tested to Federal Communications Commission
Requirements
Caution – consult accompanying documents.
Read instructions before connecting or
operating.
Subject to WEEE (Waste of Electronic Electrical
Equipment) regulations.
Electrical Class II
ains
Quick Reference Guide page 11
Page 23
Ranger and Ranger Single Use
User’s Manual
Page 24

Page 25

Corporate Headquarters:
Verathon Inc.
20001 North Creek Parkway,
Bothell, WA 98011 USA
800.331.2313 (Canada and US)
425.867.1348
Fax: 425.883.2896
verathon.com
Verathon Medical (Canada) ULC
4224 Manor Street
Burnaby, BC V5G 1B2
Canada
604.439.3009
Fax: 604.439.3039
For additional contact information please visit our corporate website:
verathon.com.
GlideScope, GVL, GlideRite, and Verathon are trademarks of Verathon Inc. All
other brand and product names are trademarks of their respective owners.
The GlideScope
(6,543,447) as well as European Patent 1307131. Additional patents pending.
Information in this User’s Manual and Quick Reference Guide may change at
any time without notice. For the most up-to-date information, see the online
manuals on verathon.com.
GlideScope® Video Laryngoscope systems are CE marked in accordance with
the Medical Device Directive, and the Verathon Inc. quality is Quality System
Certified to ISO 13485:2003 standards.
© 2009 Verathon Inc. No part of this manual may be copied or transmitted by
any method without the express written consent of Verathon Inc.
®
technology is covered under US Patents (6,655,377)
Verathon Medical (Europe) B.V.
Linnaeusweg 11
3401 MS, IJsselstein
The Netherlands
+31.30.68.70.570
Fax: +31.30.68.70.512
verathon.eu
PN:0900-2112-02-60
Page 26

Page 27

GlideScope® Ranger System Contents
Contents
Important Information ............................................................... 5
Product Description .................................................................. 5
Intended Use ............................................................................ 5
Statement of Prescription ...................................................... 5
Intended Use ......................................................................... 6
Notice to All Operators ............................................................. 6
Cautions ................................................................................... 7
Introducing the Ranger System ............................................... 8
GlideScope® Ranger System Components ............................. 9
Reusable Ranger System ..................................................... 9
Ranger Single Use System ................................................. 10
Displays, Controls, and Indicators ........................................ 12
Getting Started ......................................................................... 13
Initial Inspection ..................................................................... 13
Preparing for First Use ........................................................... 13
Optional Accessory ................................................................ 23
GlideScope® DVR for Ranger ............................................. 23
Cleaning .............................................................................. 24
Clinical Application Tips ......................................................... 25
The GlideScope® 4-Step Technique ................................... 25
Tips for Ranger Video Laryngoscope Insertion ................... 26
Tips for Working with Endotracheal Tubes ......................... 27
Cleaning, Disinfecting and Maintenance............................... 28
General Maintenance Information .......................................... 28
General Cleaning and Disinfection Information ..................... 29
Cleaning and Disinfecting the Ranger Systems .................... 33
User’s Manual page 3
Page 28

Contents GlideScope® Ranger System
Cleaning the GlideScope® Ranger GVL® ............................ 34
Disinfecting the GlideScope® Ranger GVL® ....................... 35
Cleaning the GlideScope® Ranger Video Baton ................. 36
Disinfecting the Ranger Video Baton .................................. 38
Cleaning the Ranger Monitor .............................................. 38
Cleaning the Ranger DVR (Digital Video Recorder) ........... 38
Cleaning the Ranger Cradle................................................ 38
Cleaning the Ranger Clam Shell Storage Case.................. 39
Cleaning and Disinfecting the GlideRite® Rigid Stylet ........ 39
Replacing the Monitor Battery ............................................. 39
Storing the GlideScope® Ranger ........................................... 40
Storing the Reusable GVL® ................................................. 40
Storing the GlideScope® Ranger Video Baton .................... 40
Device Disposal ..................................................................... 41
GlideScope® Warranty Offerings ........................................... 42
Original First Year Total Customer CareSM Warranty ............ 42
What is Covered .................................................................. 43
Premium Customer CareSM Warranty ................................. 43
Disclaimer of Additional Warranties .................................... 43
Contact Information ................................................................ 44
Parts and Accessories ............................................................ 45
Specifications .......................................................................... 47
General Specifications ........................................................... 47
Operating and Storage Conditions: ....................................... 47
GlideScope® Ranger System Components ........................... 48
Power Supply Specifications ............................................... 49
Standards and Approvals ....................................................... 50
Symbol Directory ..................................................................... 51
page 4 User’s Manual
Page 29

GlideScope® Ranger System Important Information
Important Information
Important Information
Product Description
GlideScope® Ranger is a portable, compact video laryngoscope
that provides a clear, real-time view of a patient's airway
enabling quick intubation.
Designed for “1st Pass Success” in military and emergency
settings, the Ranger System features rugged, high-impact
construction, patented blade angulation, a patented anti-fogging
mechanism, and a non-glare monitor that is easily visible in
bright light. The device is dependable in an array of field
conditions, making it ideal for pre-hospital and critical care
situations. The GlideScope
been granted Airworthiness Certification from the United States
Army.
The Ranger is operational in seconds. It is compact for easy
carrying and storage. The integrated, rechargeable lithium
battery provides a minimum 90-minute continuous-use autonomy
and allows for approximately 20 intubations per battery cycle
(depending on usage).
®
Ranger Video Laryngoscope has
Intended Use
Statement of Prescription
Federal (USA) law restricts this device for sale by or on the order
of a physician.
The Ranger System should be used only by individuals who
have been trained and authorized by a physician, or by health
care providers who have been trained and authorized by the
institution providing patient care.
User’s Manual page 5
Page 30

Important Information GlideScope® Ranger System
Intended Use
Ranger video laryngoscopes are intended for use by qualified
medical professionals to obtain a clear, unobstructed view of the
vocal cords for medical procedures. The Ranger System is
designed for field (military and pre-hospital) use.
Notice to All Operators
All operators should read this entire User’s Manual prior to using
the Ranger System. Failure to follow these instructions may
result in patient injury, compromise the performance of the
system, and may void the system warranty.
Verathon recommends that new Ranger users:
Practice using the Ranger on a mannequin before
clinical use
Acquire clinical experience on patients without airway
abnormalities
Refer to page 25 for recommended techniques.
page 6 User’s Manual
Page 31

GlideScope® Ranger System Important Information
Cautions
Caution. Risk of permanent equipment damage.
Do not expose Ranger GVL
Video Batons to temperatures above 140° F
(60°C). Do not disinfect Ranger GVL
®
blades or Ranger
®
blades or
Ranger Video Batons using devices such as
autoclaves, ultrasonic cleaners, or pasteurizers.
Use of such methods will cause permanent
device damage and void the warranty
. Refer to
the Cleaning section for a list of approved
cleaning procedures and products.
Electrical shock hazard. Refer servicing to qualified
personnel.
This equipment has been tested and found to comply with the
standards listed in the Approvals section of this manual. These
limits are designed to provide reasonable protection against
harmful interference in typical medical installations.
This equipment generates, uses, and can radiate radio frequency
energy and if used properly is very unlikely to cause harmful
interference to any other device(s) in the vicinity.
However, there is no guarantee that interference will not occur in
a particular installation. Interference can be determined by
turning the equipment on and off. If this equipment does cause
interference with other devices, try to correct the interference by
one or more of the following measures:
Re-orient or relocate the receiving device
Increase the separation between equipment
Connect the equipment to an outlet on a circuit different from
that to which the other device(s) is (are) connected
Consult your Verathon Medical Customer Care
representative
The Ranger System must be used with the supplied
NOTE:
cables to maintain electromagnetic interference (EMI) within
certified limits.
Users should be aware that portable and mobile equipment
(cellular phones, etc.) may affect medical electrical equipment
and take appropriate precautions during operation.
User’s Manual page 7
Page 32

Introduction GlideScope® Ranger System
Introducing the GlideScope® Ranger System
Introducing the Ranger System
The Ranger System is designed to provide an unobstructed view
of the vocal cords through the use of advanced video
technology.
®
The Ranger System, based on GlideScope
an ideal tool for physicians and other healthcare providers who
need to effectively manage standard to difficult airways in remote
locations, pre-hospital, and other mobile, emergency situations.
The GlideScope
®
Ranger is available in both reusable and single
use models.
The GlideScope
®
Ranger is built to military specifications for
prehospital and EMS settings. With a non-glare color monitor
and anti-fogging mechanism, the Ranger provides a clear view of
the airway, enabling quick intubation. Compact, portable and
rugged, the Ranger is operational in seconds.
Ranger
video laryngoscopes may be useful for the following
procedures:
First use intubations, replacing Direct Laryngoscopy (DL)
Normal or restricted oropharyngeal views and
assessment of the oropharynx
GVL technology, is
Cormack-Lehane grades I - IV laryngeal views
Trauma airways - excellent when dealing with blood and
secretions in the airway
Airway management in morbidly obese patients
Patients requiring cervical spine immobilization
Preterm and neonatal intubations
Reintubation in Intensive Care Unit (ICU) settings
Teaching the anatomy of the airway
Supervision and documentation of the laryngoscopy
Nasal tracheal intubations
page 8 User’s Manual
Page 33

GlideScope® Ranger System Introduction
Insertion of transesophageal echocardiac probes
Laryngoscopic foreign body removal
Awake intubation for difficult airway management
Insertion of double lumen tubes
GlideScope® Ranger System Components
Two interchangeable video laryngoscope systems are available
for use with the Ranger Monitor:
The reusable Ranger
Ranger Single Use
Please refer to page 45 for a listing of Ranger System
components and part numbers.
Reusable Ranger System
The Ranger System includes:
The Ranger Video Monitor
One reusable GVL
®
is comprised of a reusable, medical-grade plastic
GVL
®
with an integrated video cable. The
shell that houses a high resolution, autofocusing, CMOS
camera, LED light source, an innovative anti-fogging
mechanism. The GVL
®
device is available in two sizes.
Additional units may be purchased separately.
GlideRite
®
Rigid Stylet
User’s Manual page 9
Page 34

Introduction GlideScope® Ranger System
®
®
Figure 1. Ranger GVL® System.
Ranger Video
Monitor
GlideRite
Rigid Stylet
Ranger GVL
and GVL
(Reusable)
Ranger Single Use System
The Ranger Single Use System includes:
The Ranger Video Monitor
Reusable Ranger Video Baton with integrated video
cable. The Ranger Video Baton is comprised of a
reusable, high resolution, autofocusing, CMOS camera,
LED light source, and an innovative anti-fogging
mechanism. The Ranger Video Baton is available in two
sizes and each baton supports two sizes of GVL
®
4
®
Stats.
3
page 10 User’s Manual
Page 35

GlideScope® Ranger System Introduction
®
Single use GVL® Stats (four sizes available). The GVL®
Stat is a disposable, medical-grade plastic shell that
provides mechanical support and protection for the
Ranger Video Baton during intubations.
®
GlideRite
Rigid Stylet
Figure 2. Ranger Single Use System.
Ranger Video
Monitor
Reusable
Ranger Video
Baton
(2 sizes)
Single use
®
Stats
GVL
(4 sizes)
GlideRite
Rigid Stylet
User’s Manual page 11
Page 36

Displays, Controls, and Indicators GlideScope® Ranger System
r
Displays, Controls, and Indicators
Displays, Controls, and Indicators
Ranger System components and features are illustrated in
Figure 3.
Figure 3. GlideScope® Ranger components.
Monitor connecto
(inside cradle)
Protective cap
Temperature
gauge
DC power socket
Monitor
LCD
display
Battery charge
status LED
Power ON/OFF
Ranger Video
Laryngoscope (GVL®)
with integrated cable
(reusable blade shown)
CMOS video camera
Reusable
Ranger Video
Baton inside
a sterile,
single use
®
Stat
GVL
page 12 User’s Manual
Page 37

GlideScope® Ranger System Getting Started
Getting Started
Getting Started
Initial Inspection
Upon receipt, inspect the components of the GlideScope®
Ranger System for any obvious physical damage that may have
occurred during shipment. Verathon recommends that the
inspection be performed by a biomedical engineer or other
qualified professional who is familiar with electronic medical
devices.
If any of the components are missing or damaged, notify the
carrier and Verathon Medical Customer Care immediately at:
800.331.2313 (Canada and US)
425.867.1348 (International)
+31.30.68.70.570 (Europe)
For additional contact information, please see page 44.
Preparing for First Use
Prior to using the Ranger System for the first time, perform the
following steps:
1. Charge the Battery (page 13).
2. Connect the Ranger GVL
3. Perform a functional check (page 21).
1. Charge the Battery
Important:
The battery must be fully charged before first use.
The unit will not operate while charging. If it is
switched on during charging, the charging light will
User’s Manual page 13
®
to the monitor (page 18).
Page 38

Getting Started GlideScope® Ranger System
flash alternatively green and red-orange.
WARNING! The battery can be fully charged from 0-32° C.
The battery will not fully charge if the ambient temperature is
above 90° F (32° C).
To charge the battery:
1. Ensure that the power switch is in the
OFF position.
2. Unscrew the DC socket protective cap (Figure 4) on the
front of the monitor (a screwdriver or small coin may be
used).
3. Connect the monitor to an AC power source (wall outlet)
using the provided DC power supply and AC power cord
as shown in Figure 4.
The charge status LED will turn orange, indicating that
the charging cycle has begun.
When the charge status LED turns green, the charging
cycle is complete (Figure 5).
Figure 4. Connect the Ranger Monitor to AC power to charge
the battery.
page 14 User’s Manual
Page 39

GlideScope® Ranger System Getting Started
Figure 5. The charge status LED will turn green when the
battery is fully charged.
Charge status LED
Orange: The
monitor is charging
Green: The monitor
is fully charged
User’s Manual page 15
Page 40

Getting Started GlideScope® Ranger System
Table 1: Charge status LED indicators.
Indicator Meaning
Steady green
Flashing green
No LED
Steady orange
Flashing
orange
Battery is fully charged.
Battery power is low. LED flashes for
approximately 5 minutes before shutting
off.
The battery is completely depleted and
needs to be recharged.
The monitor is connected to an AC power
source and the battery is charging.
A flashing orange charging light indicates a
battery problem. When this happens:
Disconnect the DC plug
Make sure the switch is on
CHARGE (or OFF position)
Reconnect the DC plug
If the orange LED is still flashing,
the battery has failed. Return the
unit for repair
Powering the GlideScope® Ranger System
The monitor contains a lithium battery that provides power to the
Ranger System. The system must be operated exclusively on
battery power, without a connection to an AC power source.
Under normal conditions, a fully charged battery will last
approximately 90 minutes.
page 16 User’s Manual
Page 41

GlideScope® Ranger System Getting Started
IMPORTANT! The battery needs to be fully charged on first
receipt. For instructions on charging the battery, see
“Charge the Battery” on page 13.
To supply power to the monitor:
1. Flip the power switch located on the front of the monitor
ON position.
to the
NOTE: The system will not work when it is connected to
an AC power source.
2. Observe the monitor screen to verify that an image is
being displayed.
3. Remove the GVL
®
or video baton from its cradle. The
unit is now ready for use.
Figure 6. Power switch in ON position.
User’s Manual page 17
Page 42

Getting Started GlideScope® Ranger System
2. Attaching the GlideScope® Ranger GVL® or Ranger Video
Baton to the Monitor
1. Ensure that the power switch is in the
2. Insert the Ranger GVL
®
or Ranger Video Baton cable
OFF position.
into the port located on the face of the monitor so that
the arrows on the cable and the monitor line up
(Figure 7).
Figure 7. Connecting the Ranger Video Cable to the monitor.
Inserting the Ranger Video Baton into the GVL
®
Stat
GlideScope
®
GVL® Stats are disposable, single-use, sterile
plastic shells which provide mechanical support and protection
from contamination for the reusable Ranger Video Baton during
intubation.
®
To insert the Ranger Video Baton into the GVL
1. Ensure proper insertion by matching the logos on the
side of the video baton and the GVL
®
Ranger Video Baton into the sterile, single use GVL
Stat:
Stat. Insert the
®
Stat until it clicks into place (Figure 8).
page 18 User’s Manual
Page 43

GlideScope® Ranger System Getting Started
®
Figure 8. Inserting the Ranger Video Baton into the single
use GVL
GVL
Stat
®
Stat.
Ranger Video Baton
2. Take care to avoid inserting the video baton backwards
(Figure 9)
If the video baton is inserted incorrectly and becomes
stuck, you may insert a tongue depressor into the GVL
Stat to release the video baton.
®
Figure 9. Assure that the handgrip on the video baton and
the hand grip on the GVL
®
Stat are aligned. In this image,
the handgrips are facing in opposite directions.
User’s Manual page 19
Page 44

Getting Started GlideScope® Ranger System
NOTE: Visually inspect the GVL
®
Stat to ensure that all
external surfaces are free of unintended rough surfaces,
sharp edges, protrusions, or cracks.
®
Detaching the GVL
The GVL
®
Stat is a single-use device. After each use, it should
Stat from the Ranger Video Baton
be removed from the Ranger Video Baton and disposed of
properly.
®
To detach the Ranger Video Baton from the GVL
Stat, grasp
the handle of video baton and pull firmly (Figure 10).
Figure 10. Detaching the video baton from the GVL® Stat.
IMPORTANT! A used GVL
®
Stat is a biohazard and should be
disposed of in a manner consistent with local directives in the
user’s jurisdiction.
®
GlideScope
To ensure optimal results of the anti-fog heating feature on the
GlideScope
Single Use Model:
If using the GlideScope
Anti-Fog Feature
®
Video Laryngoscope perform the following steps:
®
Ranger Single Use model, follow
steps 1, 2 and 3 recommended below.
page 20 User’s Manual
Page 45

GlideScope® Ranger System Getting Started
Reusable Models:
If using the GlideScope
®
Ranger model, attach the GVL®
blade to the system then follow steps 2 and 3.
1. Prior to use, open the GVL
®
Stat pouch, but do not
remove the Stat from the packaging (to keep the Stat
clean until ready for use). With the Stat still in the
package, insert the video baton into the Stat. The video
baton must be securely seated in the GVL
®
Stat for
efficient heating of the Stat.
2. Turn on the Ranger monitor to activate the anti-fog
heating feature.
3. After 30 seconds to 120 seconds, the anti-fog feature
should be fully effective, depending on the ambient
temperature and humidity where the equipment is being
stored and/or used.
®
If the GVL
, Video Baton or Stat are stored in cold conditions,
additional warming time may be required for optimal
performance of the anti-fog feature.
3. Perform a Functional Check
To assure that the GlideScope
®
Ranger System is functioning
properly, perform a functional check prior to first use.
To perform a functional check:
1. Fully charge the monitor battery (see page 13).
2. Connect the Ranger GVL
®
or Ranger Video Baton to the
monitor (see page 18).
3. Turn the Ranger on by moving the power switch to the
ON position.
4. Observe the monitor screen to verify that an image is
being received from the GlideScope
®
.
NOTE: The upper left corner of the LCD screen will
User’s Manual page 21
Page 46

Getting Started GlideScope® Ranger System
®
®
display a small portion of the GVL® blade
(Figure 11). The blade is captured in the view due to the
wide-angle properties of the camera lens. This view
provides a frame of reference during the intubation and
assures that the orientation of the image is correct in the
monitor.
Please contact your Verathon
Medical Customer Care
representative if your Ranger does not function as
described above.
Figure 11. When the power is on, one corner of the Ranger
®
GVL
will be visible in the display.
Edge of GVL
Reusable Ranger GVL
page 22 User’s Manual
Page 47

GlideScope® Ranger System Getting Started
Optional Accessory
GlideScope® DVR for Ranger
The GlideScope® DVR (Digital Video Recorder) is designed to
record intubations performed with the GlideScope
®
Ranger
Video Laryngoscope.
INSTRUCTIONS FOR USE
Recording
1. Ensure Monitor is OFF and SD
card no larger than 2GB is
inserted into DVR
2. Connect GVL
®
/Video Baton
cable to DVR
3. Connect DVR cable to Monitor
4. Turn Monitor ON
5. REC LED will light up in solid
RED after approximately 3 seconds
6. DVR begins recording automatically
Playback
1. Turn the Monitor OFF and wait
until REC LED turns OFF
(approximately 3 seconds)
2. Open DVR door
and remove SD
card by pushing and
releasing
3. Insert the SD card in
an SD card reader
connected to a PC
4. Browse to the DVMPG4 folder in SD card drive
5. Playback any recordings with .ASF extensions
User’s Manual page 23
Page 48

Getting Started GlideScope® Ranger System
Troubleshooting
If REC LED is blinking, there may be a problem with
recording. Possible causes:
1. Connections are not secure. Verify cable connections:
®
GVL
•
DVR to Monitor
•
/Video Baton to DVR
2. SD card is missing or not inserted properly. Turn Monitor OFF;
ensure SD card is properly inserted and try again.
3. SD card is defective. Try another SD Card.
4. Problem with GVL
function by connecting GVL
®
/Video Baton. Ensure proper
®
/Video Baton
directly to Monitor.
If the problem persists, contact Verathon Customer Care.
Cleaning
Clean the exterior of the DVR with IPA (70% Isopropyl Alcohol
Solution) wipes.
page 24 User’s Manual
Page 49

GlideScope® Ranger System Getting Started
Clinical Application Tips
The GlideScope® 4-Step Technique
Verathon recommends using the GlideScope® 4-Step Technique
as outlined below:
1. In the Mouth: looking directly into the patient’s mouth
and with the GlideScope
introduce the GlideScope
midline of the oral pharynx.
2. At the Screen: With the laryngoscope inserted, look at
the monitor to identify the epiglottis, then manipulate the
scope to obtain the best glottic view.
3. In the Mouth: looking directly into the patient’s mouth,
not at the screen, carefully guide the distal tip of the tube
into position near the tip of the laryngoscope.
4. At the Screen: Look to the monitor to complete the
intubation; gently rotate or angle the tube to redirect as
needed.
®
GVL ® in the left hand,
®
Video Laryngoscope into the
User’s Manual page 25
Page 50

Getting Started GlideScope® Ranger System
Tips for GlideScope® Ranger Video Laryngoscope Insertion
• The GlideScope® Ranger is designed to be inserted
down the midline of the tongue to the epiglottis.
®
• The Ranger GVL
indirect lift of the epiglottis or a Miller lift.
• Intubations using the Ranger GVL
approximately 1 to 3.5 lb (0.5 - 1.5 kg) of lifting force.
may be used to produce a Macintosh
®
only require
• The use of an endotracheal tube stylet is recommended.
The GlideRite
complement the angle of the GVL
®
Rigid Stylet has been designed to
®
to facilitate
intubation, and should be used with endotracheal tubes
6.0mm and larger. A malleable stylet may be used with
a 60° - 90° angle.
• To aid the passage of the endotracheal tube when at the
vocal cords, gradually withdraw the stylet approximately
2 inches (5 cm). A 1cm adjustment (withdrawal) of the
laryngoscope may be beneficial to reduce the viewing
angle and allow the glottis to drop.
Figure 12. The curvature of the GlideRite® Rigid Stylet
complements that of the Ranger GVL
®
and GVL® Stat.
page 26 User’s Manual
Page 51

GlideScope® Ranger System Getting Started
Tips for Working with Endotracheal Tubes
• Insert the ET tube behind or immediately adjacent to the
GVL
®
Stat.
• Do not insert the stylet into the larynx during intubation.
• You can bend the proximal tip of the stylet backward to
permit one-handed operation of the ET tube.
• Carefully introduce the distal end of the ET tube between
the vocal folds.
®
• When introducing the GlideScope
and/or the
endotracheal tube, look directly into the mouth to avoid
damaging the endotracheal tube cuff, the patient’s teeth,
or the soft tissues such as the soft palate or tonsils.
• Advance the ET tube while simultaneously withdrawing
the stylet with the thumb (Figure 13). The stylet should
be withdrawn approximately 5 cm (2in).
Avoid excessive lifting or pushing of the glottis by the GVL® Stat.
Maximum laryngeal exposure may not facilitate intubation;
reducing the elevation applied to the laryngoscope may make
inserting the ET tube easier.
Figure 13. The GlideRite
removal from the endotracheal tube.
®
Rigid Stylet is designed for one-handed
Removing the rigid stylet
from the endotracheal tube
User’s Manual page 27
Page 52

Cleaning and Maintenance GlideScope® Ranger System
Cleaning, Disinfecting and Maintenance
Cleaning the GlideScope® Ranger system is an important part of
maintaining the system. Make sure that the system is clean
before each use. You should also examine the system
periodically to make sure it is operating correctly.
General Maintenance Information
Periodic inspections should be performed to ensure safe and
effective operation. It is recommended that a qualified technician
perform a full visual inspection of all components at least every
three months.
The technician should check for the following items:
External damage
Damage to the power supply
Connectors and cable insulation integrity
To ensure patient safety, users should perform a routine
inspection of the GlideScope
use to ensure that all endoscopic components are free of
unintended rough surfaces, sharp edges, protrusions or cracks.
If inspection reveals any faults in the components, contact
Verathon Medical Customer Care. All repairs must be performed
by an authorized Verathon Medical Service Center.
®
Video Laryngoscope before every
Caution. Risk of permanent equipment damage.
Do not expose the Ranger GVL
Video Baton to temperatures above 140° F
®
or Ranger
(60°C). Do not disinfect the Ranger GVL
®
or
Ranger Video Baton using devices such as
autoclaves, ultrasonic cleaners, or pasteurizers.
Use of such methods will cause permanent
device damage and void the warranty
. Refer to
the Cleaning section for a list of approved
cleaning procedures and products.
page 28 User’s Manual
Page 53

GlideScope® Ranger System Cleaning and Maintenance
General Cleaning and Disinfection Information
Table 2: Risk Assessment
Device Sterile Use Spaulding’s
Ranger
GVL®
Ranger
GVL®
Stat
Ranger
Video
Baton*
Stylet Non-
Ranger
Video
Monitor
DVR for
Ranger
Cradle# Non-
Non-
sterile
Sterile Single
Non-
sterile
sterile
Non-
sterile
#
Non-
#
sterile
sterile
Reusable Semicritical X
Reusable Noncritical X
Reusable Semicritical X
Reusable Noncritical X
Reusable Noncritical X
Reusable Noncritical X
use
CDC
Classification
Semicritical
Disinfection Sterilization
Low
Level
Int.
Level
High
Level
Clam
Shell
Storage
Case
Non-
sterile
Reusable Noncritical X
It is understood that all items in this chart will be used as intended.
User’s Manual page 29
Page 54

Cleaning and Maintenance GlideScope® Ranger System
Note:
* The Ranger Video Baton is a nonsterile, reusable device which
is protected from contact with mucous membranes and nonintact skin by the Stat (sterile, single use) when used as
intended. Low Level Disinfection is recommended for the Ranger
Video Baton after every patient use. High Level Disinfection is
required for the Video Baton when it is visibly soiled.
# Disinfect monitor, DVR and cradle when they are visibly soiled
and on a regular basis as per a schedule established by the
medical care facility or provider.
– Shaded areas - not required/not compatible with device
materials.
– Checked boxes (x) show minimum requirement.
– Unshaded areas show permissible levels of disinfection based
on compatibility with device materials.
Warning:
Disinfectants and cleaning methods listed are recommended by
Verathon
based on compatibility with device materials. Refer to
the label instructions for guidance on disinfection efficacy and
appropriate clinical uses.
Caution: Meticulous cleaning must precede any disinfection
process, to ensure all foreign matter is removed from the surface
of the device. This allows the active ingredients of the chosen
process to reach all the surfaces of the device.
Availability of cleaning products varies by country, and we are
unable to test products in every market. Please use the list of
recommended chemicals in this manual to compare with
products available locally.
Note: When using any of the chemicals listed below, read and
comply with product use instructions in all applications.
page 30 User’s Manual
Page 55

GlideScope® Ranger System Cleaning and Maintenance
Table 3: Chemical Compatibility and Disinfection
Methods for Ranger GVL
Active
Ingredient
Enzymatic
debridement
agent/
detergent
Isopropyl
Alcohol Solution
Glutaraldehyde Up to 3.4%
Ortho-
Phthalaldehyde
Peracetic Acid 0.2%
Compatibility Conditions
General
hospital grade
70%
0.55%
®
and Ranger Video Batons
Disinfection
Level
As per instructions N/A
70% used to wipe
down with minimum 1
minute exposure
2.0% – exposure for
minimum 20 minutes
at 20°C or as per
manufacturer’s
instructions
0.55% – exposure for
12 minutes at 20°C or
as per manufacturer’s
instructions
0.2% - exposure for
minimum 12 minutes
at 50 to 56°C or as
per manufacturer’s
instructions
comments
on right
Caution/
Comments
Surface
cleaning only
in
preparation
for
disinfectant
Low
High
High
See
Classified as
a chemical
sterilant
5000ppm – exposure
Bleach (Sodium
Hypochlorite)
Up to
8000ppm
for 10 minutes at
20°C
500ppm – used to
wipe down with
minimum 1 minute
exposure
High
Low
Refer to page 35 for Disinfecting Steps.
User’s Manual page 31
Corrosive for
connector
pins and SS
ring
Noncorrosive
at ≤ 500ppm
Page 56

Cleaning and Maintenance GlideScope® Ranger System
Table 4: Chemical Compatibility and Disinfection Methods
for GlideRite
Active
Ingredient
Glutaraldehyde Up to 3.4% 2.0% – exposure for
Ortho-
Phthalaldehyde
Peracetic Acid 0.2% 0.2% - exposure for
Isopropyl
Alcohol
Solution
Bleach
(Sodium
Hypochlorite)
Enzymatic
debridement
agent/
detergent
N/A
®
Rigid Stylet
Compatibility Conditions
minimum 20 minutes at
20°C or as per
manufacturer’s
instructions
0.55% 0.55% – exposure for
70% 70% - exposure for 10
≤ 500ppm
General
hospital grade
Tested in
autoclave
(steam cycle)
12 minutes at 20°C or
as per manufacturer’s
instructions
minimum 12 minutes at
50 to 56°C or as per
manufacturer’s
instructions
minutes at 20°C
70% used to wipe
down with minimum 1
minute exposure
500ppm – used to wipe
down with minimum 1
minute exposure
As per instructions N/A Surface cleaning
Minimum 4 minute
132°C pre-vacuum
steam sterilization cycle
Disinfection
Level
High
High
See
comments
on right
Intermediate
Low
Low
See
comments
on right
Caution/
Comments
Classified as a
chemical
sterilant
Noncorrosive at
≤ 500ppm
only in
preparation for
disinfectant
Based on
requests from
users, an
autoclave cycle
has been
established for
the Stylet for
ease of use
Refer to page 39 for Cleaning & Disinfecting Steps.
page 32 User’s Manual
Page 57

GlideScope® Ranger System Cleaning and Maintenance
Cleaning and Disinfecting the GlideScope® Ranger Systems
Warning
Make sure that you do not overheat the Ranger GVL
®
or Ranger
Video Baton during cleaning. You can monitor the color of the
temperature gauge on the handle to avoid overheating the
Ranger GVL
Caution. Risk of permanent equipment damage.
Do not expose Ranger GVL
®
or Ranger Video Baton
®
or Ranger Video Batons to
temperatures above 140° F (60°C).
The manufacturer’s warranty is void if the product is exposed to
temperatures above 60°C (140°F).
Figure 14. Monitor the color of temperature indicator to avoid
overheating the GVL
®
or the video baton
Temperature indicator.
User’s Manual page 33
The temperature indicator turns black if the Ranger is
heated above 60°C (140°F).
A gray or white indicator does not indicate overheating.
Page 58

Cleaning and Maintenance GlideScope® Ranger System
Caution
Do not use autoclaves or pasteurizers for the Ranger systems.
Use of such methods will cause permanent device damage and
void the warranty.
Bleach can be used on the baton but with special attention to the
connector. Bleach may corrode the stainless steel inserts and
damage the connector pins.
Cleaning the GlideScope® Ranger GVL
The Ranger GVL® is a non-sterile reusable device. It is
recommended that the Ranger GVL
®
is cleaned and disinfected
®
after every patient use, using a High Level Disinfectant method
as defined in Table 3. High Level Disinfection is required for the
Ranger GVL
To clean the GlideScope
®
when it is visibly soiled.
®
Ranger GVL
®
1. Disconnect the Ranger GVL® and video cable from the
monitor.
2. Loop the video cable back and place the cable
connector over the Ranger GVL
®
protective cap as
shown in Figure 15. Turn the cable connector to lock
into place.
Caution
During cleaning, the cable connector and Ranger GVL
®
protective cap must be mated and locked into place as shown in
Figure 15.
page 34 User’s Manual
Page 59

GlideScope® Ranger System Cleaning and Maintenance
Figure 15. Correct cleaning position – protective cap covering the
electronic connector. Stow the connector as shown before cleaning the
Ranger GVL
®
.
3. Wash the Ranger GVL® manually using a hospital-grade
equipment detergent or an enzymatic debridement
agent/ detergent to remove all foreign material (e.g., soil
and organic material) from the surface of the device.
Disinfecting the GlideScope® Ranger GVL®
To disinfect use any of the chemicals listed in Table 3 and do the
following:
1. Ensure the equipment is clean (see Cleaning
Section for Ranger GVL
2. Ensure the cable connector and Ranger GVL
®
).
®
protective cap is secure (Figure 15)
3. Prepare the disinfectant solution at the concentration
and temperature recommended by the disinfectant
manufacturer. Disinfect the equipment following the
disinfectant manufacturer’s instructions or as stated
in Table 3.
4. After the disinfection process, rinse (as applicable)
and dry the Ranger GVL
®
and then store the
equipment in a clean environment.
User’s Manual page 35
Page 60

Cleaning and Maintenance GlideScope® Ranger System
Cleaning the GlideScope® Ranger Video Baton
The GlideScope® Ranger Video Baton is a non-sterile reusable
device, which is protected by the Stat and is not intended to have
direct patient contact.
Caution
Do not place the Ranger Video Baton in the cradle if it is
contaminated.
The video baton which is protected from direct contact with the
patient by the Stat, will normally only require cleaning and wiping
down between uses using either IPA or bleach. If there is
concern about its level of contamination or if it is exposed to
contamination then it can be disinfected using a High Level
Disinfection method, as defined in Table 3 on page 31.
Caution: Ensure the protective cap is properly fitted on the
Ranger Video Baton prior to immersion in water. Bleach can be
used on the video baton but with special attention to the
connector, as it can corrode the stainless steel inserts and
damage the connector pins. You can also use a soft brush to
scrub the video baton, taking care not to damage the camera
lens. However, do not use a wire brush because it may damage
the surface of the video baton.
1. Detach the GVL® Stat from the video baton as described on
page 20.
®
Note: A used GVL
disposed of in a manner consistent with local
protocols.
2. Disconnect the video baton from the monitor.
3. Place the protective cleaning cap over the connector
(Figure 16).
page 36 User’s Manual
Stat is a biohazard and must be
Page 61

GlideScope® Ranger System Cleaning and Maintenance
Figure 16. Make sure that the protective cap is in the correct
position before you clean the video baton.
4. Wash the baton manually using a hospital-grade equipment
detergent or an enzymatic debridement agent/ detergent to
remove all foreign material (e.g., soil and organic material)
from the surface of the device.
5. The Ranger Video Baton can now be disinfected.
User’s Manual page 37
Page 62

Cleaning and Maintenance GlideScope® Ranger System
Disinfecting the Ranger Video Baton
To disinfect use any of the chemicals listed in Table 3 and do the
following:
1. Ensure the equipment is clean (see Cleaning Section for
Ranger Video Baton).
2. Ensure the cable connector and protective cap lock into
place (Figure 16).
3. Prepare the disinfectant solution at the concentration and
temperature recommended by the disinfectant manufacturer.
Disinfect the equipment following the disinfectant
manufacturer’s instructions or as stated in Table 3.
4. After the disinfection process, rinse (as applicable) and dry
the Ranger Video Baton and then store the equipment in a
clean environment.
Cleaning the Ranger Monitor
Wipe the exterior of the monitor with IPA (70% isopropyl
alcohol), bleach (100ppm) or a mild detergent and water.
Cleaning the Ranger DVR (Digital Video Recorder)
Wipe the exterior of the DVR with IPA (70% isopropyl alcohol).
Cleaning the Ranger Cradle
Wipe the cradle with a standard hospital-grade surface cleaning
product.
page 38 User’s Manual
Page 63

GlideScope® Ranger System Cleaning and Maintenance
Cleaning the Ranger Clam Shell Storage Case
Wipe the Clam shell storage case with IPA (70% isopropyl
alcohol), bleach (100ppm) or a mild detergent and water.
Cleaning and Disinfecting the GlideRite® Rigid Stylet
The Stylet is a nonsterile reusable device which requires
cleaning and High Level Disinfection as defined in the risk
analysis – Table 2 on page 29.
1. Clean using a Low Level Disinfection wipe method or rinse/
brush method.
2. Using a brush, apply detergent or an enzymatic
debridement agent.
3. Rinse under clean, running water for 1 minute.
4. Disinfect the Stylet using a High Level Disinfection method
as defined in Table 4 on page 32.
Note: For end user convenience it has been determined that the
GlideRite
Table 4 on page 32.
®
Rigid Stylet is compatible with the autoclaving cycle in
Replacing the Monitor Battery
Under normal operating conditions, the battery’s projected life is
2 - 3 years; the expected number of charge-discharge cycles is
approximately 500 cycles.
In case of battery malfunction, do not attempt to replace the
monitor battery. Any attempts to replace the battery by
unauthorized service technicians will void the system
warranty. Please contact your Verathon Medical Customer Care
representative for more information on battery replacement.
User’s Manual page 39
Page 64

Cleaning and Maintenance GlideScope® Ranger System
t
Storing the GlideScope® Ranger
Storing the Reusable GVL®
When the Ranger GVL® is not in use, wind the video cable and
place it in the neoprene cover (Figure 17).
Figure 17. When not in use, stow the reusable GVL® with the
Ranger Monitor.
Storage
compartmen
Storing the GlideScope® Ranger Video Baton
When not in use, store the Ranger Video Baton and GVL® Stat
with the monitor cradle (Figure 17) or in the clam shell storage
case (Figure 18).
When using the clamshell case for storage, please insert the
video baton into the cradle as pictured. Tuck the cradle under the
straps of the clam shell to secure it snugly in place. Then wrap
the cable around the top of the cradle and zip the clamshell
closed.
page 40 User’s Manual
Page 65

GlideScope® Ranger System Cleaning and Maintenance
Figure 18. Storing the Ranger Single Use Video Baton
The Ranger GVL
®
and Ranger Video Baton can be safely used
and stored under the environmental conditions specified on page
47.
IMPORTANT: The power switch must be in the
OFF (left
side) position during storage and shipping.
Device Disposal
Disposal of this device can be coordinated through your
Verathon Service Center in accordance to the WEEE
requirements.
User’s Manual page 41
Page 66

Warranty GlideScope® Ranger System
GlideScope® Warranty Offerings
GlideScope® Warranty Offerings
SM
Original First Year Total Customer Care
Warranty
Verathon warrants the Ranger System against defects in
material and workmanship. This warranty applies for one (1) year
from the date of shipment from Verathon. This warranty is given
only to the original purchaser of the Ranger System.
If a customer’s system requires service or repair, Verathon will
either replace or provide a loaner unit within one (1) business
day from the date of customer service notification. The customer
agrees to send the defective unit to Verathon upon receipt of the
loaner or replacement unit and agrees to return the loaner unit
within two (2) business days of receipt of the repaired unit.
This warranty provides coverage for damage from
accidental drops or mishandling. It does not cover
damage due to deliberate mishandling.
This warranty does not apply if the product has been
damaged due to, or as the result of, service or
modification by anyone other than an authorized
Verathon Service Center
This warranty does not apply if there is evidence of the
equipment being exposed to temperatures in excess of
60º C
The product shall be used in accordance with the
instructions contained in this User’s Manual. Consumable
items (i.e., endotracheal tubes, Stats, stylets, etc.) shall be
used in conformance to Verathon product specifications.
Consumable items are not covered under this warranty.
page 42 User’s Manual
Page 67

GlideScope® Ranger System Warranty
What is Covered
Warranty coverage is extended to GlideScope® Ranger
Systems:
Video Monitor including the display connector cable
Reusable Ranger GVL
®
3 and GVL® 4
Ranger Single Use Video Batons
Additional GVL
®
or video baton devices purchased either
singularly or as a part of a system must be warranted separately.
Additional Video Monitors purchased either singularly or as part
of a system must be warranted separately.
Premium Customer CareSM Warranty
The Premium Customer CareSM warranty from Verathon® can be
extended for a total of up to six (6) years from date of purchase.
Disclaimer of Additional Warranties
There are no understandings, agreements, representations of
warranties expressed or implied (including warranties of
merchantability or fitness for a particular purpose) other than
those set forth in the preceding Warranty section. The contents
of this manual do not constitute a warranty.
Some states disallow certain limitations on applied warranties.
The purchaser, user, and patient should consult state law if there
is a question regarding this disclaimer. This information,
descriptions, recommendations, and safety notations in this
manual are based upon Verathon
®
experience and judgment
with the Ranger System as of October 2008. The contents of this
manual should not be considered to be all-inclusive, or to cover
all contingencies.
User’s Manual page 43
Page 68

Warranty GlideScope® Ranger System
Contact Information
To obtain additional information regarding your Ranger System,
please contact Verathon Medical Customer Care at:
Corporate HQ:
(US and Canada)
Verathon Inc.
20001 North Creek Parkway
Bothell, WA 98011-8128
USA
800.331.2313 (Canada and US)
425.867.1348
Fax: 425.883.2896
verathon.com
Verathon Medical
(Europe) B.V.
Linnaeusweg 11
3401 MS, IJsselstein
The Netherlands
+31.30.68.70.570
Fax: +31.30.68.70.512
verathon.eu/
Verathon Medical
(France) Sarl
Espace Europeen de l’Entreprise
2 allée d’Oslo
BP 10039 Schiltigheim
F-67012 Strasbourg Cedex
France
+33.(0)3.88.60.14.02
Fax: +33.(0)3.88.60.46.87
Verathon Medical
(United Kingdom) Ltd.
The Granary Manor Farm
Courtyard
Aston Sandford, Aylesbury
Buckinghamshire, HP17 8JB
United Kingdom
+44.1844.299.207
Fax: +44.1844.299.218
verathon.co.uk
Verathon Medical
(Japan) K.K.
Executive Tower Azabudai 7F
1-4-3 Azabudai
Minato-ku
Tokyo, Japan 106-0041
+81.03.3560.3501
Fax: +81.03.3560.3502
Verathon Medical
(Canada) ULC
4224 Manor Street
Burnaby, BC V5G 1B2
Canada
604.439.3009
Fax: 604.439.3039
page 44 User’s Manual
Page 69

GlideScope® Ranger System Specifications
Parts and Accessories
Parts and Accessories
Table 5. Ranger System components and accessories.
Product Description Part Number
GlideScope® Ranger System, NA 0270-0374
GlideScope® Ranger System, EU 0270-0375
GlideScope® Ranger System, UK 0270-0376
GlideScope® Ranger GVL® 4 (Reusable) 0574-0028
GlideScope® Ranger GVL® 3 (Reusable) 0574-0029
Ranger Video Baton 3-4 (Reusable) 0570-0198
Ranger Video Baton 1-2 (Reusable) 0570-0211
GVL® Stat 4 (Single Use), Qty 10 0270-0628
GVL® Stat 3 (Single Use), Qty 10 0270-0626
GVL® Stat 2 (Single Use), Qty 10 0270-0429
GVL® Stat 1 (Single Use), Qty 10 0270-0428
GVL® Stat 4 (Single Use), Qty 100 0270-0629
GVL® Stat 3 (Single Use), Qty 100 0270-0627
GVL® Stat 2 (Single Use), Qty 100 0270-0431
GVL® Stat 1 (Single Use), Qty 100 0270-0430
GlideScope® Ranger Video Monitor 0570-0186
GlideRite® Rigid Stylet (package of six) 0803-0009
GlideScope® DVR for Ranger 0570-0300
GlideRite® Guide 0803-0104
Ranger Video Baton Clam Shell Case 0800-0334
GlideScope® Ranger Large Soft Bag with
Snaps
User’s Manual page 45
0800-0349
Page 70

Specifications GlideScope® Ranger System
Product Description Part Number
Medical Power Supply 0400-0065
GlideScope® Ranger System
0900-1307
User’s Manual, (English)
AC Power Cord, 0.6 m, NA 0600-0247
AC Power Cord, 0.6 m, EU 0600-0246
AC Power Cord, 0.6 m, UK 0600-0248
Ranger GVL® Quick Reference Card 0900-1326
GlideScope® 4-Step Tips and Techniques
0900-1436
Quick Reference Card
GlideScope® GVL® and Video Baton
0900-1429
Cleaning Poster
page 46 User’s Manual
Page 71

GlideScope® Ranger System Specifications
Specifications
Specifications
Verathon reserves the right to change product specifications
without notice.
General Specifications
Classification: Electrical Class II, Applied Part BF
Line Voltage Range: 100–240 VAC, 50 & 60 Hz
Line Current: MAX 0.25 A
Operating and Storage Conditions:
Operating Conditions
Temperature:………………………………………...…10°C to 40°C
Relative Humidity:………………………………………......0 to 95%
Atmospheric Pressure:…………………………….440 to 1060 hPa
Shipping and Storage Conditions
Temperature:……………………………..……………-20°C to 45°C
Relative Humidity:…………………………………..……....0 to 95%
Atmospheric Pressure:…………………………….440 to 1060 hPa
Charging Conditions
Temperature:………………………………………….…0°C to 40°C
Relative Humidity:……………………………………..…....0 to 95%
Atmospheric Pressure:…………………………….440 to 1060 hPa
Ingress Protection
IP68 up to 3 ft (1.0 m) for 60 minutes
User’s Manual page 47
Page 72

Specifications GlideScope® Ranger System
GlideScope® Ranger System Components
Ranger Video Monitor
320 x 240 pixel, 3.5 in LCD TFT Panel
Height:
Width:
Depth:
Weight:
Ranger GVL® 4
Tip to front of handle:
Thickness (height) at camera:
Width at camera:
Blade length in front of camera:
Max blade width in front of camera:
Weight:
Ranger GVL® 3
Tip to front of handle:
Thickness (height) at camera:
Width of camera pod:
Blade length in front of camera:
Max blade width in front of camera:
Weight:
Ranger Video Baton 3-4:
Length: camera tip to SS ring:
Height of camera:
Width of camera:
Cable length:
Weight:
6.6 in
6.8 in
1.9 in
1.25 lb
3.5
in
.57
in
in
0.75
1.9
in
in
1.06
4.5 oz
3.07 in
.57 in
.55 in
1.4 in
.79 in
4.2 oz
4.1 in
.42 in
.43 in
34 in
5 oz
104.1 mm
168 mm
173 mm
49 mm
0.56 kg
89 mm
14.5
mm
mm
19
49
mm
mm
27
128 g
78 mm
14.5 mm
14 mm
36 mm
20 mm
120 g
10.7 mm
10.9 mm
864 mm
141 g
Ranger Video Baton 1-2:
Length: camera tip to handle (flexible
tube):
Height of camera:
Width of camera:
Cable length:
Weight:
page 48 User’s Manual
1.57 in
.236 in
.276 in
34 in
3.4 oz
40 mm
6 mm
7 mm
865 mm
95 g
Page 73

GlideScope® Ranger System Specifications
s
B
r
GVL® Stat 4
Tip to front of handle:
Thickness (height) at camera:
Width of camera:
Blade length in front of camera:
Max blade width in front of camera:
GVL® Stat 3:
Tip to front of handle:
Thickness (height) at camera:
Width of camera pod:
Blade length in front of camera:
Max blade width in front of camera:
GVL® Stat 2:
Tip to front of handle:
Thickness (height) at camera:
Width of camera:
Blade length in front of camera:
Max blade width in front of camera:
GVL® Stat 1:
Tip to front of handle:
Thickness (height) at camera:
Width of camera:
Blade length in front of camera:
Max blade width in front of camera:
3.7 in
.63 in
.78 in
2.1 in
1.1 in
3.15 in
.63 in
.63 in
1.46 in
.83 in
2 in
.34 in
.43 in
1.10 in
.63 in
1.5 in
.34 in
.39 in
.59 in
.51 in
95 mm
16 mm
20 mm
53 mm
27.5 mm
80 mm
16 mm
16 mm
37 mm
21 mm
51 mm
8.7 mm
10.9 mm
28 mm
16 mm
38 mm
8.7 mm
9.9 mm
15 mm
13 mm
GlideScope
Length:
Width:
Height:
Weight:
Max SD card size:
Recording on 1 GB SD card:
®
DVR for Ranger:
4.1 in
2.3 in
1.2 in
~.38 lb
2 G
~1.5 h
103.2 mm
58.2 mm
32.2 mm
~172 g
2 GB
~1.5 hr
Power Supply Specifications
Manufactured by Jerome Power Supply for Verathon Medical
(Canada) ULC. Model Name: Jerome WSC112M, Verathon
number: 0400-0065.
User’s Manual page 49
®
part
Page 74

Specifications GlideScope® Ranger System
Standards and Approvals
ISO 13485 Certificate No. 9235
CE marking requirements met for Class 1 and Class 1
Sterile Devices
IEC 60601-2-18 (Second Edition, 1996)
IEC 60601-1-2:2001 Medical Directive
US UL 60601-1 (First Edition)
US UL 2601-1 (Second Edition)
CAN/CSA C22.2 No. 601.1-M90
AU, CA, DK, IL, and US National Differences, as per
CB Bulletin 107a
FCC Report of Measurements as per FCC CFR47
Part 18
page 50 User’s Manual
Page 75

GlideScope® Ranger System Symbol Directory
Symbol Directory
Symbol Directory
The following international regulatory symbols are found on
Ranger System components and indicate compliance with
international regulatory standards.
Table 6. Ranger System labeling symbols.
Symbol Meaning
Power connected to mains
Power disconnected from mains
Type BF equipment
CE marking in accordance with the Medical
Device Directive (
0413 for Sterile Devices)
Canadian Standards Association (CSA) mark of
certification to applicable standards for electromedical equipment
Tested to Federal Communications Commission
Requirements
Caution – consult accompanying documents.
Read instructions before connecting or
operating.
Subject to WEEE (Waste of Electronic Electrical
Equipment) regulations.
Electrical Class II
User’s Manual page 51
Page 76

 Loading...
Loading...