Page 1
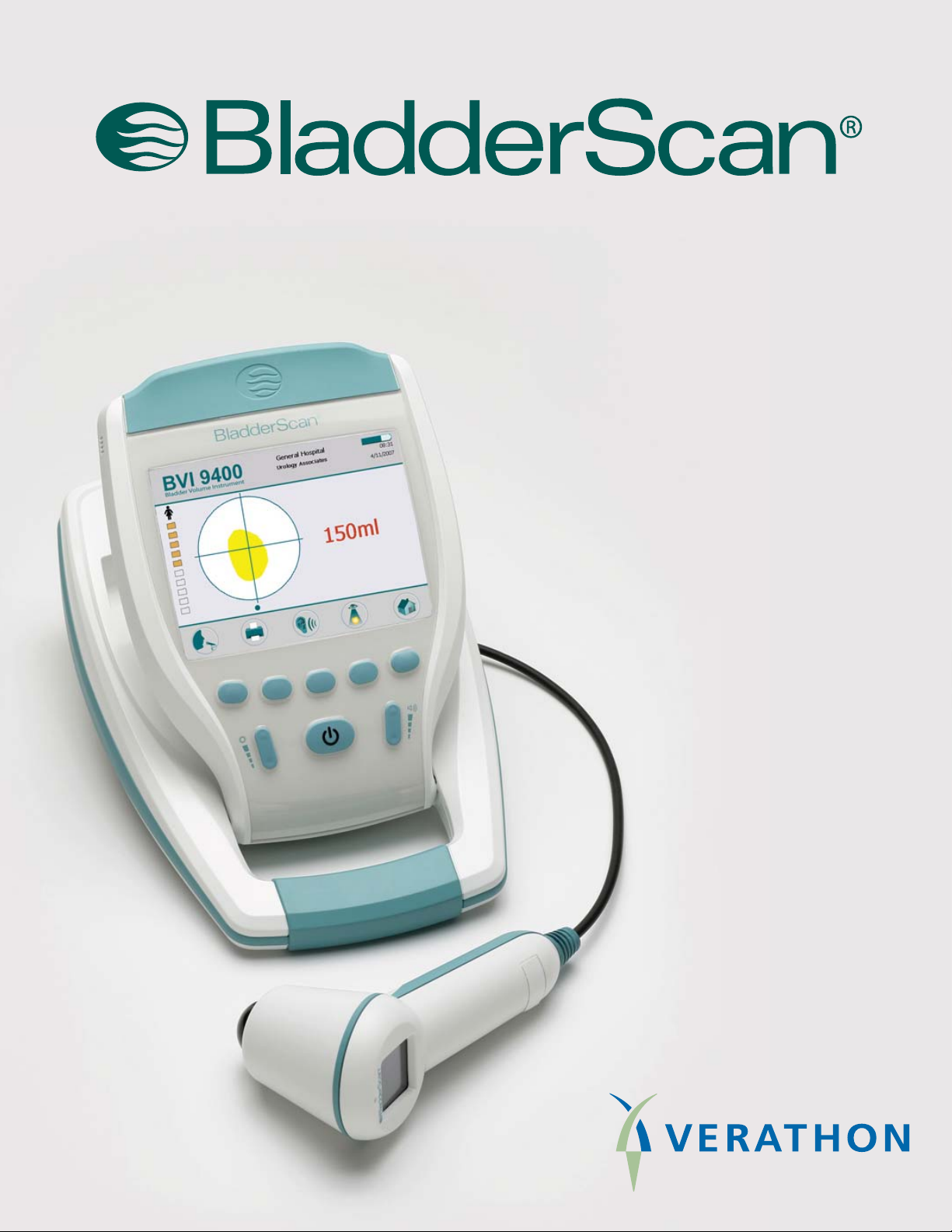
Bladder Volume Instrument
BVI 9400
User’s Manual
Page 2

Page 3

BladderScan® BVI 9400
Bladder Volume Instrument
User’s Manual
CAUTION: In the United States, federal law restricts this
device to use by or on the order of a physician.
Page 4
Copyright © 2008 by Verathon Inc.. All rights reserved. No part of this User’s Manual may be copied or transmitted by
any method without the express written consent of Verathon Inc..
®
Verathon
or registered trademarks, and Total Reliability
, Verathon Medical®, BladderScan®, ScanPoint®, Sontac®, and NeuralHarmonics™ are either trademarks
SM
Plan is a service mark of Verathon Inc..
Bluetooth® word mark and logos are owned by the Bluetooth SIG, Inc. and any use of such marks by Verathon® is
under license.
®
is a registered trademark of Advanced Sterilization Products.
Cidex
Sporocidin
®
is a registered trademark of Aporocidin International.
All other brand and product names are trademarks or registered trademarks of their respective owners.
The BladderScan
6,676,605, and 6,884,217. The ScanPoint
6,569,097. The Sontac
®
technology described in this manual is protected by U.S. Patent Numbers 4,926,871, 5,235,985,
®
ultrasound coupling pad described in this manual is protected by U.S. Patent Number
®
technology described in this manual is protected by U.S. Patent Number
5,782,767. Other international patents pending.
Information in this User’s Manual may change at any time without notice. For the most up-to-date information, see the
online manuals at http://www.verathon.com. Examples described or illustrated in this User’s Manual are fictitious and
do not in any way represent real patient or exam data.
Corporate Headquarters
20001 North Creek Parkway
Bothell, WA 98011
USA
800.331.2313 (US and Canada only)
425.867.1348
Fax: 425.883.2896
Verathon Medical (Japan) K.K.
Executive Tower Azabudai 7F
1-4-3 Azabudai
Minato-ku, Tokyo 106-0041
Japan
+81.3.3560.3501
Fax: +81.3.3560.3502
Verathon Medical (Europe) B.V.
EC REP
Boerhaaveweg 1
3401 MN IJsselstein
The Netherlands
+31.30.68.70.570
Fax: +31.30.68.70.512
Verathon Medical (France)
Postal Address
BP 10039
F-67012 Strasbourg Cedex
France
+33.3.88.60.14.12
Fax: +33.3.88.60.46.87
Office Address:
Espace Europeen de l'Entreprise
2 allée d'Oslo
67300 Schiltigheim
Verathon Medical (United Kingdom) Ltd.
The Granary Manor Farm Courtyard
Aston Sandford, Aylesbury
Buckinghamshire HP17 8JB
United Kingdom
+44.1844.299.207
Fax: +44.1844.299.218
PN: 0900-1606-00-60
Page 5

BladderScan® BVI 9400 Quick Reference - Use
User’s Manual page 5
Page 6
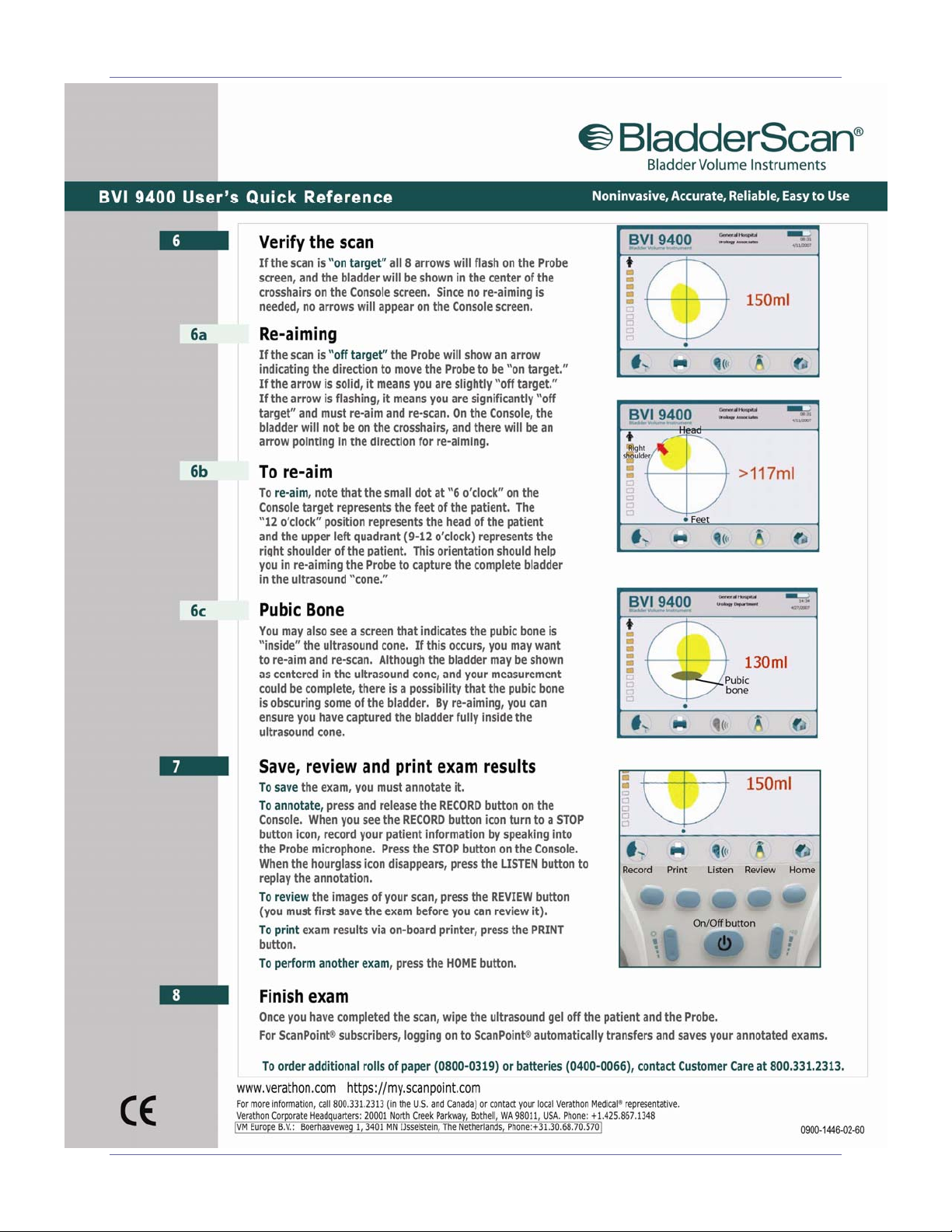
Quick Reference - Use BladderScan® BVI 9400
page 6 User’s Manual
Page 7

BladderScan® BVI 9400 Quick Reference - Calibration
NOTE: This procedure requires ScanPoint® with QuickPrint Imaging
Technology Software. If your BladderScan
calibration, and you’re not using ScanPoint
contact Verathon
®
Technical Support at 1.800.331.2313 (US &
®
BVI 9400 requires
®
with QuickPrint, please
Canada) or 425.867.1348 (International).
User’s Manual page 7
Page 8
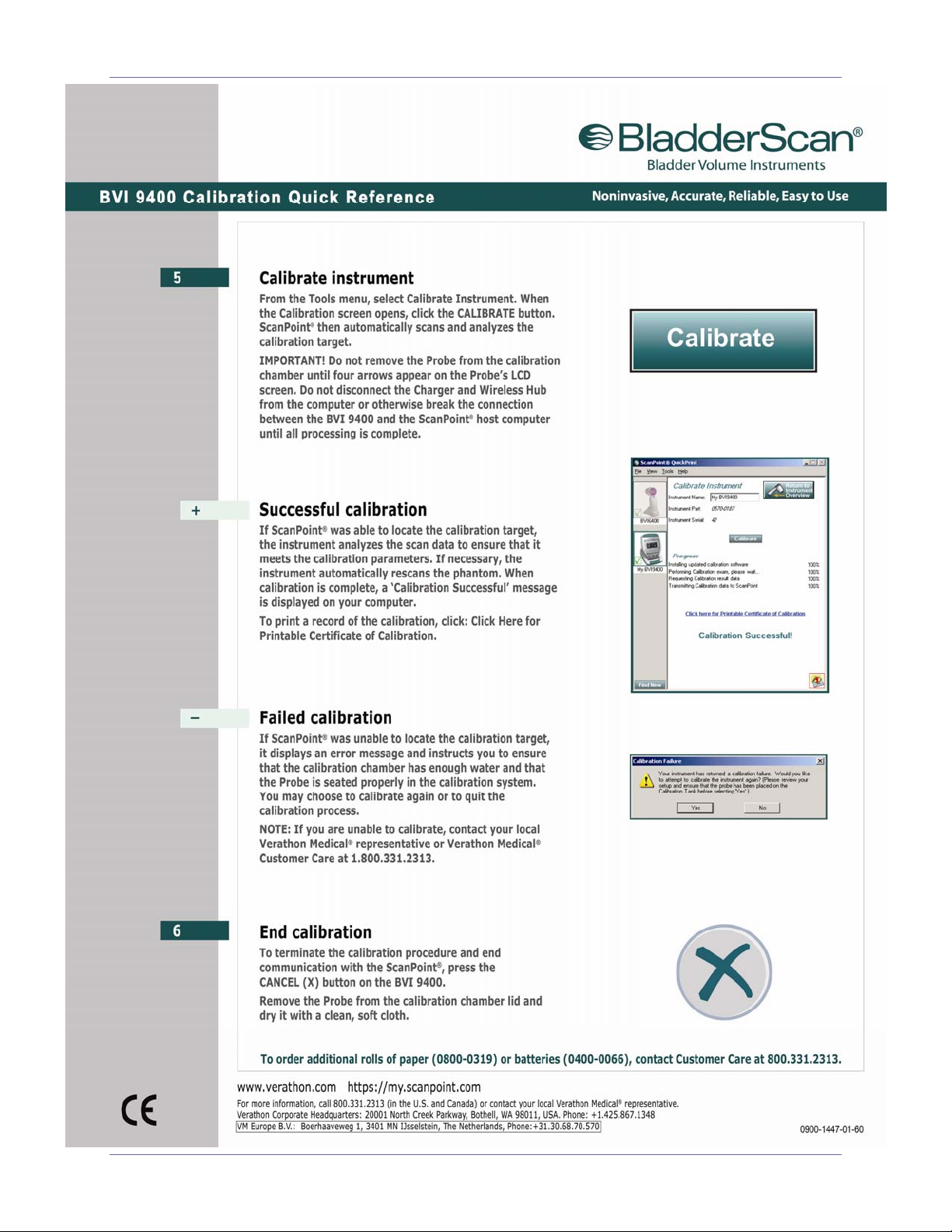
Quick Reference - Calibration BladderScan® BVI 9400
page 8 User’s Manual
Page 9

BladderScan® BVI 9400 Table of Contents
Table of Contents
Quick Reference................................................................................................................ ......................... 5
President’s Statement ..............................................................................................................................13
Important Information...............................................................................................................................14
Product Description ................................................................................................................................. 14
Intended Use Warnings and Cautions..................................................................................................... 14
Biological Safety ..................................................................................................................................14
Statement of Prescription.....................................................................................................................15
Statement of Intended Use .................................................................................................................. 15
Contraindications (U. S. Only) ............................................................................................................. 15
Bluetooth® Wireless Technology.......................................................................................................... 16
Introducing the BladderScan® BVI 9400.................................................................................................17
Product Description ................................................................................................................................. 17
BladderScan
®
Parts and Features ..........................................................................................................18
Probe Components..............................................................................................................................19
Console Components ..........................................................................................................................19
Battery Charger/Wireless Hub................................................................................................................. 20
Parts and Accessories.............................................................................................................................21
Getting Started ..........................................................................................................................................22
Unpack the Box and Check the Components .........................................................................................22
Charge the Batteries................................................................................................................................22
Install a Battery Pack in the Console....................................................................................................... 23
Battery Care.........................................................................................................................................23
Enter the Clinic Name..............................................................................................................................24
Set the Date and Time.............................................................................................................................26
Setting General Preferences and Savings Preferences ......................................................................27
Load a Roll of Thermal Paper .................................................................................................................27
Assemble the Medical Cart .....................................................................................................................28
Medical Cart Parts List.........................................................................................................................28
Install the BladderScan
Install the ScanPoint
®
on the Optional Mobile Cart............................................................................. 30
®
with QuickPrint Software (Optional)..................................................................... 30
Watch the Training Video ........................................................................................................................ 31
User’s Manual page 9
Page 10

Table of Contents BladderScan® BVI 9400
Displays, Controls, and Indicators..........................................................................................................32
Console Displays and Button Functions..................................................................................................32
Home Screen .......................................................................................................................................32
Scan Screen ........................................................................................................................................34
Results Screen..................................................................................................................................... 34
Review Screen.....................................................................................................................................36
Tutorial Screen.....................................................................................................................................37
Video Viewing Screen.......................................................................................................................... 38
Settings Screen.................................................................................................................................... 39
Alpha-Numeric Screen......................................................................................................................... 40
Date & Time Screen.............................................................................................................................41
General Preferences Screen ...............................................................................................................42
Savings Preferences Screen ...............................................................................................................43
Self-Test Screen .................................................................................................................................. 45
ScanPoint
®
Screen ..............................................................................................................................46
Sleep Mode .............................................................................................................................................46
Memory Optimization During Power Down .............................................................................................47
Measuring Bladder Volume......................................................................................................................48
Preparing for the Exam............................................................................................................................48
Measuring Bladder Volume ..................................................................................................................... 49
Additional Scanning Tips.........................................................................................................................52
Recording a Voice Annotation.................................................................................................................52
Deleting a Saved Exam........................................................................................................................... 54
Printing.....................................................................................................................................................54
Adding Addition Information................................................................................................................. 54
Histogram of Cost Savings......................................................................................................................55
Cleaning and Maintenance.......................................................................................................................56
Cleaning and Disinfecting the BladderScan
®
BVI 9400 ..........................................................................56
Regular Inspections and Maintenance .................................................................................................... 56
Weekly Inspections ..............................................................................................................................57
Using the Built-in Self-Test Utility ............................................................................................................57
Calibrating the BladderScan® BVI 9400 Using the ScanPoint
®
System .................................................57
Self-Calibration ....................................................................................................................................58
Device Repair ..........................................................................................................................................58
page 10 User’s Manual
Page 11

BladderScan® BVI 9400 Table of Contents
Unit Disposal ...........................................................................................................................................58
Troubleshooting........................................................................................................................................59
Help Resources .......................................................................................................................................59
Icons on the BladderScan
®
Console .......................................................................................................60
Diagnosing problems...............................................................................................................................61
Instrument Does Not Turn On.............................................................................................................. 61
Printer Problems .................................................................................................................................. 61
No Paper.............................................................................................................................................. 61
Too Hot ................................................................................................................................................62
Clearing a Paper Jam .......................................................................................................................... 62
Warranty.....................................................................................................................................................63
Disclaimer of Additional Warranties ........................................................................................................63
Contacting Verathon®...............................................................................................................................64
Safety and Performance Summary .........................................................................................................65
Parts and Accessories..............................................................................................................................66
BladderScan
®
BVI 9400 System Components........................................................................................ 66
Product Specifications.............................................................................................................................67
Symbol Directory ..................................................................................................................................... 67
Standards and Regulations Compliance ................................................................................................. 68
Electromagnetic Effects ....................................................................................................................... 68
BladderScan
®
BVI 9400 Instrument Specifications................................................................................. 69
Ultrasound Output Parameters ............................................................................................................69
Accuracy Specifications ....................................................................................................................... 70
BladderScan
BladderScan
BladderScan
®
BVI 9400 Operating Conditions.................................................................................... 70
®
BVI 9400 Storage Conditions....................................................................................... 70
®
BVI 9400 Displays, Controls, and Indicators ...................................................................71
Instrument Buttons...............................................................................................................................71
Instrument Display ............................................................................................................................... 73
Battery Charger/Wireless Hub Specifications ......................................................................................... 74
Operating Conditions ........................................................................................................................... 74
Storage Conditions ..............................................................................................................................75
Battery Specifications ..............................................................................................................................75
Glossary.....................................................................................................................................................76
User’s Manual page 11
Page 12

Table of Figures BladderScan® BVI 9400
Table of Figures
Figure 1. BladderScan
Figure 2. Battery Charger/Wireless Hub .....................................................................................................20
Figure 3. Home Screen............................................................................................................................... 24
Figure 4. Settings Screen............................................................................................................................24
Figure 5. Alpha-Numeric Screen................................................................................................................. 25
Figure 6. Facility Name in Display Header.................................................................................................. 25
Figure 7. Set Up Screen - Date and Time................................................................................................... 26
Figure 8. Date and Time Screen................................................................................................................. 26
Figure 9. Medical Cart, PN: 0800-0322 ......................................................................................................28
Figure 10. Medical Cart Assembly ..............................................................................................................29
Figure 11. Attaching the BVI 9400 to the Medical Cart............................................................................... 30
Figure 12. Home Screen............................................................................................................................. 33
Figure 13. Scan Screen ..............................................................................................................................34
®
BVI 9400 Controls and Indicators ........................................................................18
Figure 14. Results Screen...........................................................................................................................35
Figure 15. Review Screen........................................................................................................................... 36
Figure 16. Tutorial Screen...........................................................................................................................37
Figure 17. Video Viewing Screen................................................................................................................ 38
Figure 18. Settings Screen Start Menu.......................................................................................................39
Figure 19. Alpha-Numeric Screen............................................................................................................... 40
Figure 20. Date & Time Screen ..................................................................................................................41
Figure 21. General Preferences Screen ..................................................................................................... 43
Figure 22. Savings Preference Screen .......................................................................................................44
Figure 23. Self-Test Screen ........................................................................................................................ 45
Figure 24. ScanPoint
®
Screens................................................................................................................... 46
Figure 25. Sleep Mode Screen ...................................................................................................................47
Figure 26. Memory Optimization Alert ........................................................................................................47
Figure 27. Exam Results Printout Using the Onboard Printer .................................................................... 54
Figure 28. Calibration Warning ...................................................................................................................58
Figure 29. Printer Out of Paper Screen ......................................................................................................62
User’s Manual page 12
Page 13

BladderScan® BVI 9400 President’s Statement
President’s Statement
President’s Statement
The team at Verathon® is committed to improving health care delivery by
putting healthcare providers and their patients first.
Our products support you, the health care provider, by consistently
offering accuracy, utility, reliability and excellence.
Please contact us directly at 1.800.331.2313 (USA and Canada only) or
1.425.867.1348, if we can improve our service to you.
Sincerely,
Gerald McMorrow
Gerald McMorrow, CEO, Founder and Chairman of the Board
User’s Manual page 13
Page 14

Important Information BladderScan® BVI 9400
Important Information
Important Information
Product Description
BladderScan® instruments are the gold standard in bladder volume measurement.
The BladderScan
ultrasound instrument that provides a noninvasive measurement of urinary bladder
volume. The device consists of an ultrasound probe that scans the patient’s bladder, and
a compact, battery-operated console that provides an array of measurement-related
information.
BladderScan
and easy to use. When the user releases the scan button, within seconds, BladderScan
measures ultrasonic reflections on multiple planes inside the body and produces a threedimensional image. Based on this image, the BladderScan
displays the bladder volume. A sonographer is not required.
Patented NeuralHarmonics™
in the BVI 9400, sharpens accuracy and accelerates speed of measurement. Volume
measurements made with NeuralHarmonics™ technology are more accurate than those
from conventional two-dimensional ultrasound, as they are based on a more complex,
multifaceted image of the bladder. This technology, applying multi-spectral analysis to a
robust data set, helps reduce margin of error and minimize uncertainty in essential
measurements of bladder function.
BladderScan
transmitted, using HIPAA-compliant ScanPoint
Software (optional), to your office or facility computer for viewing, printing, or archiving.
®
is noninvasive and comfortable for the patient. It is accurate, reliable, quick
®
BVI 9400 measurements can be printed via an onboard printer or
®
BVI 9400, with patented NeuralHarmonics™ technology, is a portable
®
BVI 9400 calculates and
technology (abbreviated from “neural network harmonics”)
®
with QuickPrint Imaging Technology
®
Intended Use Warnings and Cautions
The BladderScan® BVI 9400 should be used only by individuals who have been trained
and authorized by a physician or the institution providing patient care. All users must read
this entire User’s Manual prior to using the BladderScan
operate this instrument until you thoroughly understand all instructions and procedures in
this manual. Failure to comply with these instructions may compromise the performance
of the device and the reliability of its measurements. For the most current version of the
User’s Manual, please visit the Verathon
select the BladderScan
training materials.
Biological Safety
To date, exposure to pulsed diagnostic ultrasound has not been shown to produce
adverse effects. However, ultrasound should be used only by medical professionals when
clinically indicated, using the lowest possible exposure times indicated by clinical need.
The ultrasound output power of the BladderScan
limited to the minimum level necessary for effective performance. Data on acoustic output
levels can be found in the “Technical Description” section of this manual.
®
> Customer Support links to open a link to this manual and other
®
BVI 9400. Do not attempt to
®
Web site at: http://www.verathon.com, then
®
BVI 9400 is not user adjustable and is
page 14 User’s Manual
Page 15
BladderScan® BVI 9400 Important Information
Statement of Prescription
United States federal law restricts the BladderScan
®
BVI 9400 to use by, or on the order
of, a physician. This statement is required per 21 Code of Federal Regulations (CFR)
801.109.
NOTE:
It is standard practice to have medical staff authorize the use of the BladderScan
®
BVI
9400 within its intended use throughout an institution. Individual prescriptions for use are
not required.
Statement of Intended Use
The BladderScan
®
BVI 9400 projects ultrasound energy through the lower abdomen of
the patient to obtain an image of the bladder which is used to calculate bladder volume
noninvasively.
Contraindications (U. S. Only)
The BladderScan
®
BVI 9400 is not intended for fetal use or for use on pregnant patients.
To assure safe and reliable operation for the use and the patient, please read and heed
the following warnings and cautions.
WARNING! Risk of explosion.
If you use the BladderScan® BVI 9400 in the presence of flammable anesthetics, the
hazard of potential explosion exists.
WARNING! Risk of explosion, fire, or serious injury.
The BladderScan® BVI 9400 is provided with two Lithium Ion batteries. Never short circuit
the battery pack by either accidentally or intentionally bringing the battery terminals into
contact with any other conductive object. This could cause serious injury or fire and could
also damage the battery pack and the BladderScan® device.
WARNING! Risk of explosion, fire, or serious injury.
Never expose the battery pack to abnormal shock, vibration, or pressure. The main
battery pack’s internal protective covering could fail, causing it to overheat or ignite,
resulting in caustic liquid leakage, or explosion or fire, possibly resulting in serious injury.
WARNING! Potential patient hazard.
To date, exposure to low-power, pulsed diagnostic ultrasound has not been shown to
produce adverse effects. However, medical professionals should use ultrasound only
when clinically indicated, using the lowest exposure times possible to obtain accurate
measurements. The ultrasonic output of the BladderScan® BVI 9400 is not useradjustable and is limited to the minimum level necessary for effective performance. For
more information about the acoustic output levels of this device, please refer to the
section in this manual titled BladderScan® BVI 9400 Instrument Specifications on
page 69.
CAUTION: Risk of inaccurate measurements/results.
When using the BladderScan® BVI 9400 be aware of the following conditions which can
affect ultrasound transmission and decrease the accuracy of exam results.
Use care when scanning patients who have had supra-pubic or pelvic surgery.
Scar tissue, surgical incisions, sutures, and staples can affect ultrasound
transmission and accuracy.
User’s Manual page 15
Page 16
Important Information BladderScan® BVI 9400
Do not use the BladderScan® BVI 9400 on a patient with open skin or wounds in
the suprapubic region.
Do not use the BladderScan
®
BVI 9400 on a patient with ascites.
If you scan a patient with a catheter in his/her bladder, the catheter may affect
measurement accuracy. However, the information obtained from the
measurement could still be clinically useful for detecting problems such as a
blocked catheter.
CAUTION: Observe the following precautions in the safe use and care of the
BladderScan® BVI 9400.
Hazardous materials present. Assure proper disposal.
The BladderScan
batteries, and other environmentally hazardous materials. When the BladderScan
9400 has reached the end of its useful service life, return the device, charging cradle, and
related accessories to a Verathon
®
BVI 9400 and related devices may contain lead, mineral oils,
®
Service Center for proper disposal. Alternatively,
®
BVI
follow your local protocols for hazardous waste disposal.
Assure proper computer system configuration.
When using the BladderScan
®
BVI 9400 with optional ScanPoint® software, your
computer must be minimally certified to EN / IEC / CSA / UL 60950 or 60101-1
standards. This configuration ensures that compliance to the EN/IEC 60601-1-1 system
standard is maintained. Anyone connecting additional equipment to the BladderScan
®
BVI 9400 signal input port or signal output port configures a medical system, and is
therefore responsible for ensuring that the system complies with EN/IEC 60601-1-1. If
you need assistance, contact your biomedical staff, Verathon Medical
the Verathon Medical
®
Customer Care Department at 1.800.331.2313.
Assure proper distance from patient.
When transmitting data to or from you computer, make sure that the BladderScan
®
representative, or
®
BVI
9400, accessories, and computer are outside the patient vicinity (more than six feet (2
meters) from the patient). Refer to UL 2601-1 Clause 2 deviation for the definition of
patient vicinity.
Bluetooth
The Bluetooth technology used in the BladderScan
®
Wireless Technology
®
BVI 9400 is compliant with:
Bluetooth Specification as defined and approved by The Bluetooth Special
Interests Group.
Logo certification with Bluetooth wireless technology as defined by The Bluetooth
Special Interest Group.
CAUTION: Bluetooth and Wireless LAN devices operate within the same radio frequency
range and may interfere with one another. If you are using the BladderScan® BVI 9400
Bluetooth link and Wireless LAN devices simultaneously, you may experience less than
optimal network performance or even loose your network connection. If this happens, you
may need to move the BladderScan
®
and ScanPoint® host computer to an area away
from the 2.4 GHz wireless LAN devices (40 meters/44 yards, or more).
page 16 User’s Manual
Page 17

BladderScan® BVI 9400 Introducing the BladderScan® BVI 9400
Introducing the BladderScan® BVI 9400
Introducing the BladderScan
Product Description
BladderScan® instruments are the gold standard in bladder volume measurement.
The BladderScan
ultrasound instrument that provides a noninvasive measurement of urinary bladder
volume. The device consists of an ultrasound probe that scans the patient’s bladder, and
a compact, battery-operated console that provides an array of measurement-related
information.
BladderScan
and easy to use. When the user releases the scan button, within seconds, BladderScan
measures ultrasonic reflections on multiple planes inside the body and produces a threedimensional image. Based on this image, the BladderScan
displays the bladder volume. A sonographer is not required.
Patented NeuralHarmonics™ technology (abbreviated from “neural network harmonics”)
in the BVI 9400, sharpens accuracy and accelerates speed of measurement. Volume
measurements made with NeuralHarmonics™ technology are more accurate than those
from conventional two-dimensional ultrasound, as they are based on a more complex,
multifaceted image of the bladder. This technology, applying multi-spectral analysis to a
robust data set, helps reduce margin of error and minimize uncertainty in essential
measurements of bladder function.
BladderScan
transmitted using HIPAA-compliant ScanPoint
Verathon
computer for viewing or printing. (NOTE: Use of ScanPoint
®
is noninvasive and comfortable for the patient. It is accurate, reliable, quick
®
BVI 9400 measurements can be printed via an onboard printer or
®
servers. Stored exams can be accessed at any time from your office’s
®
BVI 9400
®
BVI 9400, with patented NeuralHarmonics™ technology, is a portable
®
BVI 9400 calculates and
®
technology for storage and archiving on
®
software is optional.)
®
If needed, after a scan has been taken, a unique aiming icon guides the operator to
optimal probe placement with a comprehensive, three-dimensional display showing the
bladder in two cross-sectional images verifying that a complete scan has been achieved.
Bladder volume, patient type, directional aiming with real-time feedback, battery status,
and usage rate indicators are all displayed on the device’s LCD screen. The
BladderScan
®
BVI 9400 contains an on-board thermal printer that allows the user to print
exam results quickly with the press of a button.
A Calibration Targeting System, consisting of a spiral-shaped calibration target along with
a purpose-designed calibration container, allows the user to easily calibrate the device by
scanning a known target.
Optionally, exam results may be transmitted to a personal computer running ScanPoint
with QuickPrint software via a proprietary wireless connection. ScanPoint
®
with
®
QuickPrint allows the user to archive data, calibrate the device, update software, print,
and transfer data through a Web-based interface.
The BladderScan
®
BVI 9400 system also includes a universal battery charger for the
custom, user-replaceable Lithium Ion battery incorporated in the system.
The BladderScan
®
BVI 9400 may be mounted on a purpose-designed cart which holds
the instrument securely and provides a holder for ultrasound gel and other accessories.
User’s Manual page 17
Page 18

Introducing the BladderScan® BVI 9400 BladderScan® BVI 9400
The BladderScan® BVI 9400 is designed for simple, intuitive operation. However, to
ensure safe and effective operation, before using the device:
Familiarize yourself with the contents of this manual.
Watch the training video provided on the instrument.
BladderScan® Parts and Features
The BladderScan® BVI 9400 has two main components: The Console and the Probe. The
Console and Probe are linked by a detachable cable. The BladderScan
®
BVI 9400
controls and indicators are illustrated in Figure 1.
Figure 1. BladderScan® BVI 9400 Controls and Indicators
Console
Main display
Battery
Pack
5 Variable
Function
Buttons
Adjust
Brightness
Battery Status Indicator
Printer (behind display)
Battery Packs
Battery Charger/
Wireless Hub
Power On/Off
Adjust
Volume
Probe
(Scan button
on hand grip)
Aiming Display
Microphone
(above display)
page 18 User’s Manual
Page 19

BladderScan® BVI 9400 Introducing the BladderScan® BVI 9400
Probe Components
The Probe transmits and receives ultrasound waves, automatically moving its internal
transducer 360º to scan twelve different planes to produce a three-dimensional image of
the bladder. The Probe is attached to the Console by a cable. The Probe has three main
features:
Part Name Purpose
SCAN button
Aiming Display
Press to take a scan.
The LCD displays directional arrows to assure that the bladder
is centered within the scanning cone.
Microphone
Records voice annotations.
Console Components
The Console provides all operating controls for the scanning process by means of five
variable function buttons. The measured bladder volume and target-shaped aiming icons
are clearly displayed on the LCD screen. The Console also provides controls for
adjusting brightness and volume, turning the power on/off, interfacing with a ScanPoint
®
host computer (optional), and adjusting user settings and preferences. The Console also
houses the Battery and the Printer. The Console’s controls and features are described in
the following table:
Part Name Purpose
Main Display
Displays the bladder volume measurement, patient type,
settings, and instrument status.
Power on/off
Toggles main power on/off
Volume
Press to adjust volume up/down on voice annotation
playback, start up sound, and “scan complete” tone.
Brightness
5 Variable Function
Buttons
Press to adjust display brightness dimmer/brighter.
Depending on device mode, the buttons provide access to all
instrument functions for scanning, recording annotations,
printing, connecting to ScanPoint
®
(optional), accessing the
training video, and setting user preferences.
Printer / Printer Door
Press to release the Printer door.
User’s Manual page 19
Page 20

Introducing the BladderScan® BVI 9400 BladderScan® BVI 9400
Battery Charger/Wireless Hub
The BladderScan® BVI 9400 is powered by a Lithium Ion (Li-Ion) battery pack. The
battery charger provided with your BVI 9400 can charge two Li-Ion battery packs while
simultaneously functioning as the wireless hub linking your BVI 9400 to the ScanPoint
host computer.
NOTE: Use of ScanPoint
®
with QuickPrint software is optional.
To provide power to the battery packs, the Battery Charger/Wireless Hub must
be plugged into a wall outlet using the power cord provided.
To provide wireless communication between the BVI 9400 and the ScanPoint
host computer, plug the Battery Charger/Wireless Hub USB connector into a
USB port on the ScanPoint
®
host computer.
®
®
The Battery Charger/Wireless Hub maintains an operating distance of up to 120 feet
(36 meters) between the ScanPoint
®
computer and the BVI 9400, regardless of barriers
such as walls, ceilings, or windows.
Figure 2. Battery Charger/Wireless Hub
Charging bays for two
battery packs
Plug into wall outlet
to power the
battery charger.
Plug into a USB port on the
ScanPoint
(optional) to enable a wireless
connection with the BVI 9400.
®
host computer
page 20 User’s Manual
Page 21

BladderScan® BVI 9400 Introducing the BladderScan® BVI 9400
Parts and Accessories
Part Number Description
0570-0190
0570-0188
0570-0193
0570-0066
0900-1606
0900-1601
0900-1446
0900-1447
0800-0319
0900-1500
0800-0322
0900-1238
BVI 9400 Instrument - Console
BVI 9400 Instrument - Probe
Battery Charger/Wireless Hub
Lithium Ion Battery (2 provided)
BVI 9400 User’s Manual (on In-Service CD)
ScanPoint
®
with QuickPrint User’s Manual (on In-Service CD)
Quick Reference Card - Demo/Use
Quick Reference Card - Calibration
Thermal Paper Roll for the Printer
BladderScan
®
BVI 9400 In-Service CD (Includes User’s Manuals,
Video Tutorial, and Quick Reference Cards)
Mobile Cart (optional)
ScanPoint
ScanPoint
®
with QuickPrint Software Install CD. NOTE: Use of the
®
software is optional and not required for operation of the
BVI 9400.
0620-0340
0800-0225
0800-0005
Calibration Kit (Optional - Requires ScanPoint
®
with QuickPrint
software. Includes Calibration Container, Calibration Target, etc.)
Sontac
®
Gel Pads, QTY 50
Acoustic Coupling Gel, 0.25 liter (case of 12)
User’s Manual page 21
Page 22

Getting Started BladderScan® BVI 9400
Getting Started
Getting Started
The previous section provided basic information about the BVI 9400 parts and features.
This section provides instructions for setting up your BVI 9400 prior to first use.
To help you get up and running as quickly as possible, the next few pages explain how
to:
Unpack the BladderScan
Charge and install the batteries
Customize the display (enter your institution’s name, date, time, and other user
data)
Assemble the mobile cart (optional)
Install the ScanPoint
Unpack the Box and Check the Components
Set the shipping container right side up and carefully open the top flaps (do not insert
anything sharp through the top of the box). Remove the contents and verify that you have
received everything listed below. If anything is missing or damaged, notify your
authorized Verathon Medical
Department at 1.800.331.2313.
(1) BladderScan
®
BVI 9400 (Console and Probe)
®
BVI 9400 and related accessories
®
with QuickPrint software (optional)
®
representative or Verathon Medical® Customer Care
(1) Medical Cart (optional)
(2) Rechargeable Lithium Ion batteries
(1) Battery Charger/Wireless Hub
(1) In Service CD (includes User Manuals, Video Tutorial, and Quick Reference Cards)
(1) Training Pack
Charge the Batteries
Two Lithium Ion batteries are included with the BladderScan® BVI 9400. One battery can
be recharged in the Battery Charger/Wireless Hub while the other is installed in the
BladderScan
charger will bring the batteries to a full charge within 6 hours or less.
NOTE: Before using the BladderScan
both batteries prior to first use.
®
instrument. This ensures that there is no instrument downtime. The
®
BVI 9400 for the first time, you will need to charge
page 22 User’s Manual
Page 23
BladderScan® BVI 9400 Getting Started
To charge the batteries:
1. Plug the Battery Charger/Wireless Hub unit in to a standard wall outlet.
2. Remove the label covering the battery contacts and insert the battery into the
recess in the battery charger
NOTE: Fully charging the battery may take up to 6 hours.
Batteries may be stored in the charger. There is no danger of overcharging the
batteries.
3. Check the colored indicator lights on the battery charger to determine battery
status:
Solid green: Battery fully charged.
Amber: Battery charging.
Install a Battery Pack in the Console
WARNING! Risk of serious injury.
If the battery pack is leaking or its case is cracked, put on protective gloves to handle it
and discard it immediately. Always dispose of used battery packs in compliance with all
applicable laws and regulations. Put insulating tape, such as cellophane tape, on the
electrodes during transportation to avoid a possible short circuit, fire, or electrical shock.
Failure to do so could result in serious injury.
To insert a battery pack:
Insert the charged battery into the battery well in the Console, slide it under the ledge and
push down gently until the battery clicks into place.
NOTE: The battery pack is designed to prevent incorrect installation.
NOTE: If the battery pack does not slide into the battery well easily, move the battery and
try again. Do not force the battery into position.
Battery Care
The BladderScan
do not plan to use the BladderScan
®
BVI 9400 draws very little power when it is turned off. However, if you
®
for several weeks, you should remove the battery to
prevent it from discharging.
When batteries are not in use, they should be stored in the Battery Charger so they
remain fully charged.
User’s Manual page 23
Page 24
Getting Started BladderScan® BVI 9400
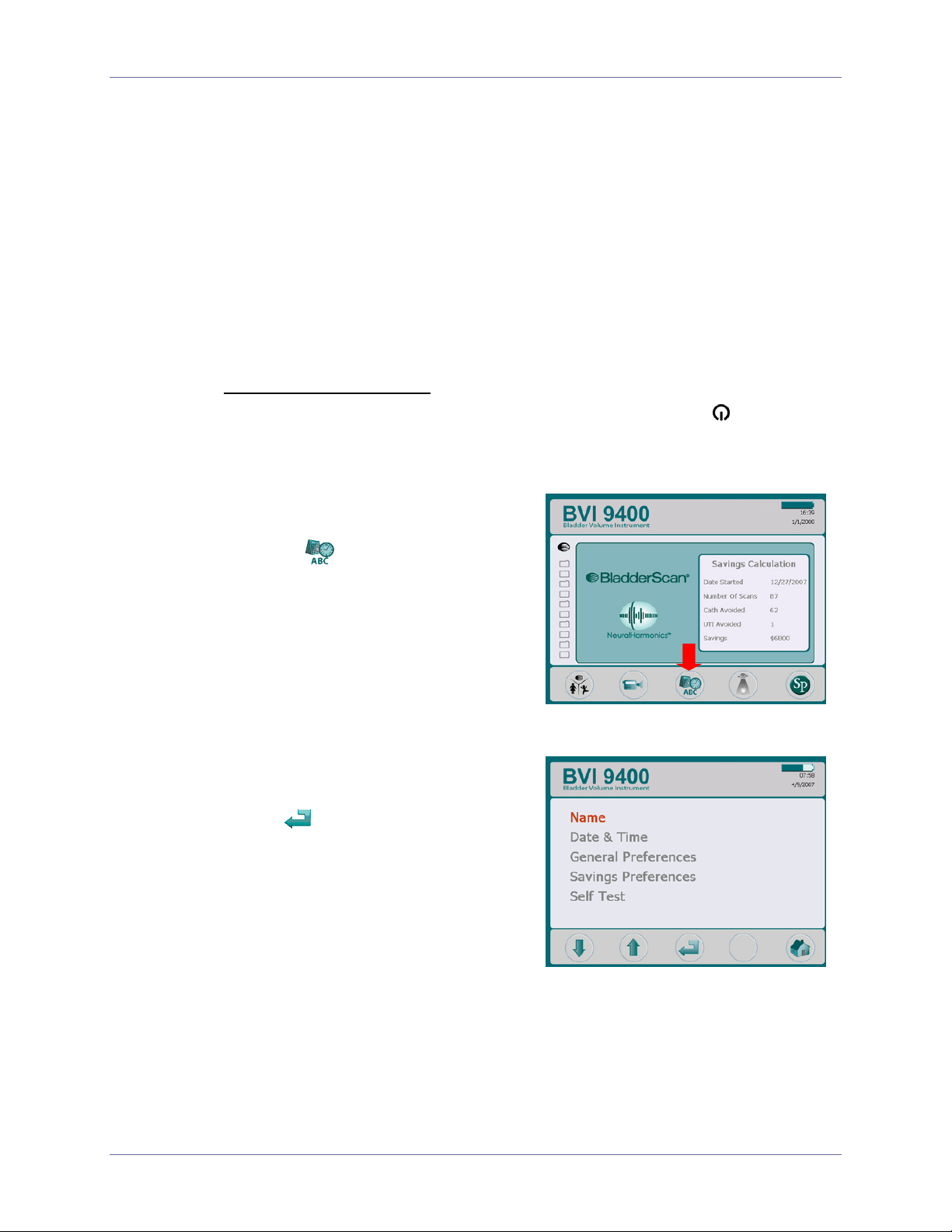
Enter the Clinic Name
You can customize your BladderScan® 9400 by entering your facility’s name and current
date and time. This information will subsequently be included on BladderScan
®
displays
and all printouts of exam results.
NOTE: Use of extended-Latin and/or non-Latin characters: The following instructions
explain how to enter a facility name that uses only standard Latin characters. Extended
Latin characters (tilde, umlaut, accents, circumflex, etc.) and/or non-Latin characters can
be entered only by using ScanPoint
extended or non-Latin characters, please refer to the instructions in the ScanPoint
®
with QuickPrint software. To enter a name that uses
®
with
QuickPrint User’s Manual.
To enter your clinic’s name:
1. Turn the BladderScan
®
BVI 9400 on by pressing the POWER button on top of the
Console.
Figure 3. Home Screen
2. When the Home screen appears
(Figure 3), press the S
button
to open the Settings
ETTINGS
screen.
Figure 4. Settings Screen
3. On the Settings screen (Figure 4),
push either the Å or Æ button until
Name is highlighted (red). Press
the
button to open the Alpha-
Numeric screen.
page 24 User’s Manual
Page 25

BladderScan® BVI 9400 Getting Started
Figure 5. Alpha-Numeric Screen
4. On the Alpha-Numeric screen
(Figure 5), use the Ä and Æ
buttons to move to the desired
character. When the desired
character is highlighted (red), press
the ® button to add it to your text.
Use the
-- button to delete
characters.
To add a space between words,
press the blank space below the
letter “x”.
To add a second line of text use
the
¶ character.
Figure 6. Facility Name in Display Header
5. Continue to select and add
characters as needed.
6. When finished, press the S
button
to return to the Settings
ETTINGS
screen. From the Settings screen,
press the H
OME button to return
to the Home screen. The facility
name will now appear in the display
header (Figure 6).
User’s Manual page 25
Page 26

Getting Started BladderScan® BVI 9400
Set the Date and Time
The clock has its own Lithium battery and maintains the correct date and time for at least
ten years.
To set the date and time:
1. If the BladderScan® instrument is turned off, turn the power on by pressing the
button on the front of the Console.
2. From the Home screen (Figure 3), press the S
Settings screen.
3. On the Settings screen, push either
the Å or Æ arrow until Date & Time
is highlighted (red) (
the
button to open the Time &
Figure 7). Press
Date screen.
4. On the Date & Time screen (Figure
8), use the Ä and Æ buttons to
move to the desired unit (hours,
minutes, month, day, year).
When the desired unit is highlighted
(red),
Press the ® button to increase
values.
Press the
-- button to decrease
values.
ETTINGS button to open the
Figure 7. Set Up Screen - Date and Time
Figure 8. Date and Time Screen
5. When the time and date are set
correctly, press the S
ETTINGS button
to return to the Settings screen.
From the Settings screen, push the
H
OME button to return to the
Home screen.
page 26 User’s Manual
Page 27

BladderScan® BVI 9400 Getting Started
Setting General Preferences and Savings Preferences
NOTE: See page 42 for information about setting General Preferences, Savings
Preferences, and Self-Test Options.
General Preferences, Savings Preferences, and Self-Test Options can be accessed from
the Settings screen.
General preferences include options for: Language, Date Format, Time Format, Time
Zone, and Calibration Warning.
Savings Preferences include: UTI Rate, UTI Cost, Catheterization Cost, Catheterization
Volume, Currency, and Savings Calculation.
Load a Roll of Thermal Paper
To load a new roll of thermal paper:
1. Open the paper compartment door (located on the base of the Console, under the
display).
2. Slide the door to the right, then lift the printer door up.
3. Insert the end of a new paper roll with the thermal side down, into the paper slot.
To verify which side is the thermal side, scratch the paper with your finger nail. If a
black mark appears, this is the thermal side.
4. Extend the end of the paper past the end of the unit.
5. Rotate the printer door down. Make sure you hear it snap into place.
6. Slide the printer door to the left.
7. Tear the excess paper off.
CAUTION! To avoid paper jams, never fold the end of the paper roll or cut it diagonally or
to a point.
NOTE: If the paper appears to be stuck in the printer, contact your authorized
BladderScan
®
Service Center, your local BladderScan® distributor, or Verathon Medical®
Customer Care at 1.800.331.2313 for service.
User’s Manual page 27
Page 28

Getting Started BladderScan® BVI 9400
Assemble the Medical Cart
NOTE: If you’re not using the medical cart, please skip to the next section, Install the
ScanPoint
®
with QuickPrint Software, on page 30.
The optional Medical Cart is illustrated in Figure 9.
Figure 9. Medical Cart, PN: 0800-0322
Medical Cart Parts List
Item No. Quantity Part Number Description
1 1 0142-0276 Tray Assembly
2 1 0810-0140 Bracket and Post
3 1 0810-0141 Medical Cart Base
4 4 0261-0107 Screw PH W Lock 25-20 x 1/2
5 1 0260-0357 Fluted Knob 3/8-16 x 1.00
6 4 0810-0142 Caster, 3 inch
7 1 0810-0143 Caster, 3 inch with Brake
page 28 User’s Manual
Page 29

BladderScan® BVI 9400 Getting Started
To assemble the cart:
While following the assemble procedure,
refer to Figure 10.
1. Align and press one 3-inch Caster
with Brake (Item 7) and four 3-inch
Casters (Item 6) into Medical Cart
Base (Item 3).
2. Align through round hole in Bracket
and Post (Item 2) bracket to through
hole in Medical Tray (Item 1).
3. Insert Bracket and Post (Item 2) into
square relief in bottom of Medical
Tray Assembly (Item 1).
4. Insert four Screws (Item 4) through
Bracket and Post (Item 2) into
molded inserts in Medical Tray (Item
1); tighten securely.
5. Place Medical Tray Assembly with
Bracket and Post into Medical Cart
Base (Item 3).
6. Place device in foot prints on
Medical Tray (Item 1).
7. Screw the Fluted Knob (Item 5)
Figure 10. Medical Cart Assembly
through Bracket and Post (Item 2)
bracket round hole and hand tighten.
To attach the BVI 9400 to the Medical Cart, refer to the instructions on page 30.
User’s Manual page 29
Page 30

Getting Started BladderScan® BVI 9400
Install the BladderScan® on the Optional Mobile Cart
Your BladderScan® BVI 9400 is completely portable and can
be easily moved and positioned for convenient use. Installing
the BVI 9400 on the optional mobile cart will allow you to
move the BladderScan
battery, Sontac
®
of paper) to the patient examining area or bedside as
necessary.
®
along with related accessories (spare
Gel Pads or ultrasound gel, and an extra roll
To install the BladderScan
®
BVI 9400 on the Mobile Cart:
NOTE: For cart assembly instructions and parts list, refer to
page 28.
To install the BVI 9400 to the Medical Cart, screw the Fluted
Knob (Item 5) through Bracket and Post (Item 2) bracket
round hole and Tray Assembly (Item 1), and into the mounting
hole on the bottom of the device. Hand tighten (Figure 11).
Figure 11. Attaching the BVI 9400 to the Medical Cart
BVI 9400
Tray Assembly
(Item 1)
Fluted Knob
(Item 5)
Bracket and Post
(Item 2)
Install the ScanPoint® with QuickPrint Software (Optional)
Optional ScanPoint® with QuickPrint software is designed to mesh seamlessly with your
BladderScan
ScanPoint
Charger/Wireless Hub - allowing further viewing, analysis, archiving, and report
generation.
To install ScanPoint
into your computer’s CD drive and follow the on-screen prompts. Please refer to the
page 30 User’s Manual
®
devices. The BVI 9400 automatically downloads exam data to the
®
host computer via a wireless connection enabled by the Battery
®
with QuickPrint, insert the ScanPoint® with QuickPrint Install CD
Page 31

BladderScan® BVI 9400 Getting Started
separate User’s Manual provided with ScanPoint® with QuickPrint for complete
installation and operating instructions.
Watch the Training Video
The training video provides an overview of how to perform an ultrasound scan of the
bladder using the BladderScan
The training video is available at the Verathon
http://www.verathon.com/Training%20Videos.htm
A training tutorial is available for review anytime on the BladderScan
®
BVI 9400. The video is approximately 5 minutes long.
®
Web site:
.
®
BVI 9400
by pushing the button from the Home screen.
User’s Manual page 31
Page 32

Displays, Controls, and Indicators BladderScan® BVI 9400
Displays, Controls, and Indicators
Displays, Controls, and Indicators
Console Displays and Button Functions
The Console LCD display presents user information and prompts that vary depending on
the current device function.
The five buttons below the display have variable functions according to device mode.
Button functions are indicated by icons in the display footer, immediately above each
button.
The following tables illustrate and describe the BladderScan
button functions:
Home Screen
The Home screen (Figure 12, page 33) appears when the BladderScan
on.
The Home screen serves as a starting point for all of the main functions of the
BladderScan
A list of saved exam results (10 maximum) saved in chronological order. Yellow
A battery status indicator.
®
. The Home screen displays:
folders hold saved exams. Grey folders represent empty spaces still available for
saving exam results.
Indicates a fully charged battery.
Indicates a battery 50% to 75% charged.
Indicates a battery 25% to 50% charged.
Indicates a battery that is nearly discharged and can power
only a few more scans.
Indicates the battery must be changed.
®
BVI 9400 displays and
®
is first powered
page 32 User’s Manual
Page 33

BladderScan® BVI 9400 Displays, Controls, and Indicators
Figure 12. Home Screen
Battery
Status
Saved
exams
(yellow)
Indicator
Available
memory
for 8 more
exams
(gray)
Home Screen Buttons
Button Function
Single button with three modes. When performing an exam, press the
button repeatedly until the desired setting appears above the saved
exams folders:
Select the “small child” icon to scan a patient with height less
Select the "female icon" to scan a female patient who has not
Select the "BladderScan
Cost
savings
calculator
than 48 inches (122 cm) tall and weight less than 60 lbs (27
kg).
had a hysterectomy.
®
icon" to scan all other patients.
Press to view the training video.
Go to the Settings screen (set the time, date, institution name, and user
preferences).
Press to review a previously saved exam.
User’s Manual page 33
Page 34

Displays, Controls, and Indicators BladderScan® BVI 9400
Button Function
®
Press to initiate communication with the ScanPoint
Saved and annotated exams will be automatically uploaded to the host
computer. (ScanPoint
®
software must be previously installed and the
computer connected to the wireless hub. Use of the ScanPoint
host computer.
®
software is optional.)
Scan Screen
The Scan screen (Figure 13) appears after you press the SCAN button on the probe and
displays a progressively updating image of the bladder outline. When the ultrasound
measurement is complete, the Results screen opens automatically.
The five buttons below the display do not function during the scan.
Figure 13. Scan Screen
Results Screen
The Results screen (Figure 14) appears automatically when an ultrasound scan is
complete.
The display presents the result of the exam: crosshairs, bladder outline, and the
calculated bladder volume. You may choose to print this result to the onboard printer
and/or to record a voice annotation to save the exam. After the annotation is recorded,
the P
LAY and REVIEW buttons become active and the newly recorded exam appears on
the main display as an yellow folder icon.
page 34 User’s Manual
Page 35

BladderScan® BVI 9400 Displays, Controls, and Indicators
Figure 14. Results Screen
Results Screen Buttons
Button Function
Press to record a voice annotation (up to 10 seconds long).
Press to print exam results to the onboard label printer.
Press to play a previously recorded voice annotation. If no voice
annotations are recorded, this button is disabled.
Press to go to the review screen. If no voice annotations are recorded,
this button is disabled.
Press to return to the Home screen.
User’s Manual page 35
Page 36

Displays, Controls, and Indicators BladderScan® BVI 9400
Review Screen
The Review screen (Figure 15) opens when you select a saved exam (yellow folder icon)
to review.
The displays shows the ultrasound images associated with the selected exam. A green
open-folder icon indicates which exam is being viewed. While reviewing saved exams,
the buttons below the display allow you to print, replay, or delete exam data.
Figure 15. Review Screen
Review Screen Buttons
Button Function
SELECT. Press to select the next exam in the list.
PRINT. Press to print the results for the currently selected exam to the
onboard label printer. While printing is in progress a flashing print icon
appears on the display. The buttons are disabled except
PLAY.
PLAY. Press to play a previously recorded voice annotation. If no voice
annotations are recorded, this button is disabled.
DELETE. Press to delete the currently selected exam.
HOME. Press to return to the Home screen.
SELECT and
page 36 User’s Manual
Page 37

BladderScan® BVI 9400 Displays, Controls, and Indicators
Tutorial Screen
To open the Tutorial Screen (Figure 16) press the
button from the Home screen
(Figure 12, page 33).
The Tutorial screen presents a menu of training modules. To select a title, press either
the Å or Æ button until the desired title is highlighted (red). Press the
button to go to
the Video Viewing screen.
NOTE: The
SCAN button (on the Probe) is disabled during module playback.
Figure 16. Tutorial Screen
Tutorial Screen Buttons
Button Function
SELECT NEXT: Press to move down one title or to skip back one
chapter in the training module
SELECT PREVIOUS: Press to move up one title or to skip forward one
module.
ENTER: Press to begin module playback. While the module is playing,
press to pause. Press again to resume play.
- No function.
HOME: Press to return to the Home screen.
User’s Manual page 37
Page 38

Displays, Controls, and Indicators BladderScan® BVI 9400
Video Viewing Screen
The Video Viewing (Figure 17) screen is activated by pushing the X button on the
Tutorial screen (Figure 16).
NOTE: The
SCAN button is disabled during video playback.
Figure 17. Video Viewing Screen
Video Viewing Screen Buttons
Button Function
- No function.
PLAY/PAUSE: Press to start video playback. Press to pause. Press
again to resume play.
BACK: Return to the training titles screen.
- No function.
HOME: Press to return to the Home screen.
page 38 User’s Manual
Page 39

BladderScan® BVI 9400 Displays, Controls, and Indicators
Settings Screen
To open the Settings screen (Figure 18), push the
SETTINGS button on the Home
screen.
The display presents a list of user-configurable settings: Name, Time & Date, General
Preferences, Savings Preferences, and Self-Test. Use the Up/Down arrows to move
through the list of setup options. To select an option, press the U-turn arrow.
NOTE: When the Settings screens are open, the
SCAN button (on the Probe) is disabled.
Figure 18. Settings Screen Start Menu
Settings Screen Buttons
Button Function
SELECT NEXT: Press to select the next setting in the list.
SELECT PREVIOUS: Press to select the previous setting in the list.
ENTER: Press to select the highlighted setting.
- No function.
HOME: Press to return to the Home screen.
User’s Manual page 39
Page 40

Displays, Controls, and Indicators BladderScan® BVI 9400
Alpha-Numeric Screen
This screen allows you to select the appropriate alpha-numeric characters for entering
your health care institution’s name.
Figure 19. Alpha-Numeric Screen
Alpha-Numeric Screen Buttons
Button Function
Press to move down in the grid.
Press to move to the right in the grid.
Press to add the highlighted character to the name.
Press to delete one character from the name.
Press to return to the Setup screen.
page 40 User’s Manual
Page 41

BladderScan® BVI 9400 Displays, Controls, and Indicators
Date & Time Screen
This screen allows you to adjust the date and time.
NOTE: If the time display is set to show a 24-hour clock, the hour units are 0 - 23. If the
clock is set to show a 12-hour clock, the hour units are 1 AM - 12 AM and 1 PM - 12 PM.
Referring to the example shown in Figure 20:
To change the time from 8:17 AM to 11:30 AM:
1. Press the Æ button until the 8 is highlighted.
2. Press the ® button 3 times until the time digits display 11:17 AM. (Alternatively,
press and hold the ® button to move more rapidly through the digits.)
3. Press the Æ button until the 17 is highlighted.
4. Press the ® button repeatedly until the minutes display 30. (Alternatively, press
and hold the ® button to move more rapidly through the digits.)
The time setting is now 11:30 AM.
5. Press the
SETTINGS button to save this entry and return to the Setup screen.
Figure 20. Date & Time Screen
User’s Manual page 41
Page 42

Displays, Controls, and Indicators BladderScan® BVI 9400
Date & Time Screen Buttons
Button Function
Move back one changeable unit.
Press to move forward to the next changeable unit (in this case, hours,
minutes, AM/PM, month, day, year).
Press to add and/or toggle digits as appropriate (hours, minutes,
AM/PM, months, day, years). Press and hold the button to move
through options more quickly.
Press to subtract or toggle digits as appropriate (same as above). Press
and hold the button to move through options more quickly.
Press to save the current date date/time setting and return to the Setup
screen.
General Preferences Screen
This screen displays a list of available settings and their current values.
Available settings:
Language: English (default), Chinese Traditional, Czech, Danish, Dutch,
Finnish, French, German, Greek, Italian, Hungarian, Japanese, Korean,
Norwegian, Portuguese (European), Spanish, Swedish, Turkish.
Date Format: mm/dd/yyyy; dd.mm.yyyy; yyyy-mm-dd.
Time Format: 12-hour or 24-hour
Calibration Warning: On (default), Off. When “On” is selected, a calibration
warning will appear in the display header when the device requires calibration.
Enable Small Child Mode (SCM): On (default), Off. Select “Off” to disable Small
Child Mode.
NOTE: If use of the small child mode is rare in your practice, you may want to
turn that option off.
page 42 User’s Manual
Page 43

BladderScan® BVI 9400 Displays, Controls, and Indicators
Figure 21. General Preferences Screen
General Preferences Screen Buttons
Button Function
SELECT PREVIOUS: Press to select the previous setting in the list.
SELECT NEXT: Press to select the next setting in the list.
For settings with a list of options, press to select the next option.
For settings with a list of options, press to select the previous option.
Press and hold to move through options more quickly.
Press to return to the Setup screen.
Savings Preferences Screen
To open the Savings Preferences screen, select Savings Preferences from the Setup
Screen (Figure 18, page 39).
Use this screen to enter base values used to calculate the savings to your organization
from using the BladderScan
User’s Manual page 43
®
BVI 9400 rather than catheterization.
Page 44

Displays, Controls, and Indicators BladderScan® BVI 9400
Preferences lists and options:
UTI Rate: 1% to 100% in increments of 1%
UTI Cost: $10 to $10,000 in increments of $10
Catheter Cost: $1 to $1000 in increments of $1
Catheter Volume: 20 ml to 1000 ml in increments of 20 ml
Currency: $ / € / £ / ¥
Saving Calculation: Since New, Since 1/1/2006, Reset Now, Print Since New,
Print Recent, Hide Savings
Figure 22. Savings Preferences Screen
Savings Preferences Screen Buttons
Button Function
SELECT PREVIOUS: Press to select the previous setting in the list.
SELECT NEXT: Press to select the next setting in the list.
For settings with a list of options, press to select the next option. Press
and hold to move through options more quickly.
For settings with a list of options, press to select the previous option.
Press and hold to move through options more quickly.
page 44 User’s Manual
Page 45

BladderScan® BVI 9400 Displays, Controls, and Indicators
Button Function
Press to save the current date date/time setting and return to the Setup
screen.
Self-Test Screen
To open the Self-Test screen, select Self-Test from the Setup menu (Figure 18, page 39).
The Self-Test screen will open and testing begins automatically.
Figure 23. Self-Test Screen
Self-Test Screen Buttons
Button Function
- No function
- No function
- No function
- No function.
Press to return to the Setup screen.
User’s Manual page 45
Page 46

Displays, Controls, and Indicators BladderScan® BVI 9400
ScanPoint® Screen
NOTE: Only available if optional ScanPoint
®
software is installed on a PC.
To open the ScanPoint
The ScanPoint
BladderScan
®
screens display information about the status of the link between the
®
instrument and the ScanPoint® host computer (Figure 24).
®
screen, press from the Home screen (Figure 12, page 18).
Figure 24. ScanPoint® Screens
Searching for ScanPoint® Connected to ScanPoint®
ScanPoint
Button Function
®
Screen Buttons
- No function.
Sleep Mode
- No function.
- No function.
CANCEL: Cancels the current action and ends communication with
ScanPoint
®
.
- No function.
The Sleep mode helps preserve battery power. After four minutes of idle time, a Sleep
mode alert message displays for 15 seconds (Figure 25). While the message is
displayed, press any button to keep the console awake and dismiss the message. After
15 seconds, the console goes to sleep. To wake the instrument from sleep, simply press
the Power button.
page 46 User’s Manual
Page 47

BladderScan® BVI 9400 Displays, Controls, and Indicators
Figure 25. Sleep Mode Screen
Memory Optimization During Power Down
When the power is turned off, the BVI 9400 begins an internal process of memory
optimization. This process takes several minutes. When complete, the device will shut
down. An alert appears on the display during the memory optimization process
(Figure 26).
Figure 26. Memory Optimization Alert
NOTE: DO NOT replace the battery until the device shuts down completely. Replacing
the battery while memory is being optimized might disable the instrument as program and
exam files might not be rewritten properly.
User’s Manual page 47
Page 48

Measuring Bladder Volume BladderScan® BVI 9400
Measuring Bladder Volume
Measuring Bladder Volume
Preparing for the Exam
Before You Begin the Exam:
Make sure you are familiar with the parts of the BladderScan® instrument (see
Introducing the BladderScan
If you are a new BladderScan
your first exam on a patient with a moderately full bladder, rather than initially
attempting to locate and scan a nearly empty bladder.
Check the instrument’s battery icon to make sure the battery has sufficient
power.
NOTE: If the battery icon is 1/4 or less full, replace the battery with a freshly
charged battery before proceeding. Place the discharged battery in the Battery
Charger to recharge.
Clean and disinfect the Probe by wiping it gently with a soft cloth soaked in
isopropyl alcohol.
Be aware of the following conditions the patient may have that could affect ultrasound
transmission and the accuracy of your exam:
®
BVI 9400 on page 17).
®
user, Verathon® recommends that you perform
A catheter in the patient’s bladder. The presence of a catheter may affect the
accuracy of the bladder volume measurement, but the measurement may still be
clinically useful (detecting a blocked catheter, for example).
Previous suprapubic or pelvic surgery. Scar tissue, surgical incisions, sutures,
and staples can affect ultrasound transmission and reflection.
Do not use the BVI 9400 on:
Patients with ascites
Patients with open skin or wounds in the suprapubic region.
Pregnant patients
page 48 User’s Manual
Page 49

BladderScan® BVI 9400 Measuring Bladder Volume
Measuring Bladder Volume
1. Turn on the BVI 9400.
Turn on the BVI 9400 by pressing the
ON/OFF button.
2. Select the exam mode.
Select to scan a patient with
height less than 48 inches
(122 cm) tall and weight less
than 60 lbs (27 kg).
Select to scan a female
patient who has not had a
hysterectomy.
Select to scan all other
patients.
3. With the patient supine apply gel.
Have the patient lie in the supine
position with the abdominal muscles
relaxed.
Palpate the patient's symphysis pubis
(pubic bone). Place an ample quantity of
gel (with as few air bubbles as possible)
midline on the patient's abdomen,
approximately one inch (3 cm) above
the symphysis pubis (pubic bone).
User’s Manual page 49
Page 50

Measuring Bladder Volume BladderScan® BVI 9400
4. Aim toward the bladder.
Standing at the patient’s right side,
place the Probe on the gel and aim it
toward the expected location of the
bladder. For most patients, this means
angling the Probe slightly toward the
patient's coccyx (tail bone) so the scan
clears the pubic bone.
5. Press the SCAN button.
Press the
SCAN button, located on the
underside of the Probe. As the scan
progress, sections of the bladder will
appear on the Console screen. When
you hear the end-scan tone, the scan is
complete.
6. Verify the scan.
If the scan is “on target” the Probe will
display eight arrows. On the Console
display, the bladder will be appear in the
center of the crosshairs.
6a. Re-aiming.
If the scan is “off-target” the Probe will
show an arrow indicating the direction to
move the Probe to be “on target.” If the
arrow is solid, it means you are slightly
“off target” and must re-aim and rescan. On the Console, the bladder will
not be in the crosshairs, and there will
be an arrow pointing in the direction for
re-aiming.
page 50 User’s Manual
Page 51

BladderScan® BVI 9400 Measuring Bladder Volume
6b. To re-aim:
Note that the crosshairs correspond to
the anatomy of the patient as follows:
The small dot at the base of the
crosshairs represents the feet of the
patient.
The top of the crosshairs represents
the patient’s head.
The upper left quadrant represents
the patient’s right shoulder.
This orientation should help you in reaiming the Probe to capture the
complete bladder fully in the ultrasound
cone.
6c. Pubic Bone.
You may also see a display indicating
that the pubic bone is inside the
ultrasound cone. If this occurs, you may
want to re-aim and re-scan. Although
the bladder may be shown as centered
in the ultrasound cone, and your
measurement may be complete, there is
a possibility that the pubic bone is
obscuring part of the bladder. By reaiming and re-scanning you can ensure
you have captured the bladder fully
inside the ultrasound cone.
7. Save, review, and print exam results.
To save the exam you must annotate
it.
To annotate, press and release the
RECORD button on the Console. When
you see the
RECORD button icon change
to a STOP button icon, record the patient
information by speaking into the Probe
microphone. When the hourglass icon
disappears, press the
LISTEN button to
replay the annotation.
To review the scan images, press the
REVIEW button. (You must first save the
exam before you can review it.)
To print exam results via the on-board
printer, press the
PRINT button.
To perform another exam, press the
HOME button.
User’s Manual page 51
Page 52

Measuring Bladder Volume BladderScan® BVI 9400
8. Finish the exam.
Once you have completed the scan, wipe the ultrasound gel off the patient and the
Probe. For ScanPoint
saves your annotated exams to your Windows
®
subscribers, logging onto ScanPoint® automatically transfers and
®
computer.
Additional Scanning Tips
Applying too much pressure when scanning will lead to a Greater Than symbol (>)
preceding the measurement. Apply less pressure and re-scan.
Hold the device steady while scanning. Movement will result in an inaccurate reading.
Volume reading will be affected by:
The presence of scar tissue
The presence of a catheter
Overly obese patients:
With obese patients, lift as much abdominal adipose tissue out of the way of the
instrument as possible. Apply more pressure to the BladderScan
instrument to reduce the amount of adipose tissue through which the ultrasound
must pass.
®
BVI 9400
To ensure accurate results, make sure that:
There are no air gaps between the Probe and the patient’s skin.
The gel pad is intact (not broken or cracked).
There are no air bubbles under the gel pad.
You are holding the instrument steady while scanning (avoid changing its
position, angle, or pressure).
You are using enough pressure to maintain good skin contact until the scan is
complete.
There is not a catheter in the patient’s bladder. The presence of a catheter may
affect the accuracy of the bladder volume measurement, but the measurement
may still be clinically useful (detecting a blocked catheter, for example).
NOTE: The BVI 9400 is able to scan rapidly and repeatedly throughout the day. If
however scans are taken quickly, in rapid succession, over a 30 minute period, some
slowing in performance may be noticed. While this may occur rarely, it is normal for the
instrument.
Recording a Voice Annotation
After performing an exam, you can record additional information about a patient to be
stored with the exam results.
The instrument can store ten scans with voice annotations, so you can perform multiple
exams on your rounds. If you are using the optional ScanPoint
page 52 User’s Manual
®
with QuickPrint software,
Page 53

BladderScan® BVI 9400 Measuring Bladder Volume
you can upload saved exams to your PC. (Refer to the ScanPoint® with QuickPrint User’s
Manual for more information.)
To record a voice annotation:
1. Press the RECORD button
(microphone). A S
displayed.
2. The microphone is located just
above the aiming display on the
TOP button is now
LISTEN
RECORD
Probe. Hold the microphone
approximately 6 inches (15 cm) from
your mouth when you are recording
the annotation, and speak clearly.
Be sure to include all relevant exam
information, including the patient’s
name and the name of the person
performing the exam.
3. When you have finished your annotation, press the R
4. When you have finished your annotation, press the S
ECORD button again.
TOP button. An hour glass
will be displayed while your annotation and exam are updated and stored. After
the annotation has been saved, to listen to your annotation, press the L
ISTEN
button.
If you are not satisfied with the recording, press
RECORD again to record a new
annotation.
IMPORTANT!
If you do not record a voice annotation for a particular exam, that exam will
be lost and the next exam you perform will overwrite the non-annotated one.
If the instrument battery runs low or the instrument goes into sleep mode,
any non-annotated exam data is lost. However, the instrument does not
erase any annotated exam results when it goes into sleep mode. To make
sure you do not lose any patient data, it is a good idea to add a voice
annotation to every patient exam.
You can make a new recording only if the instrument still displays the bladder
volume for that particular exam.
5. When you are satisfied with your recording, wait while the instrument
automatically accepts and stores the recording with the exam results. The
volume displayed on the LCD screen disappears, and the instrument is ready to
perform another measurement.
6. If you are using the BVI 9400 in conjunction with the optional ScanPoint
QuickPrint software, you can view exam results and annotations on your
Windows PC. Please refer to the ScanPoint
®
with QuickPrint User’s Manual for
®
more information.
with
User’s Manual page 53
Page 54

Measuring Bladder Volume BladderScan® BVI 9400
Deleting a Saved Exam
Saved exams are indicated by yellow folder icons along the left edge of the display. To
delete a saved exam:
Printing
1. Open the Review Screen. (From the Home Screen press
2. Press the
3. Press the
ª button until the desired exam is highlighted (blue).
button to delete the exam.
.)
You can use the BVI 9400 onboard Printer to print the ultrasound images captured during
the scan (Figure 27 is an example).
To print exam results press the
PRINT button immediately following the exam.
Adding Addition Information
The label provides fields for Patient ID, Patient Name, Operator, and Physician. This
information must be entered manually.
Figure 27. Exam Results Printout Using the Onboard Printer
NOTE: If the facility name, date, and time have not been set, those lines will be skipped
on the printout.
The BVI 9400 prints on thermal paper, which fades over time. For maximum storage life,
Verathon
®
recommends that you photocopy the printout.
page 54 User’s Manual
Page 55

BladderScan® BVI 9400 Histogram of Cost Savings
Histogram of Cost Savings
Histogram of Cost Savings
Each volume measurement from a completed scanning procedure is stored in the BVI
9400 memory in one of eleven volume ranges (each with a 100 ml increment). This data
is analyzed and can be displayed on the BVI 9400 at any time. The cost savings screen
lists: Date Started, Number of Scans, Cath Avoided, UTI Avoided, Savings.
Cost Savings Criteria
Cost savings are based on the following criteria:
Catheterizations avoided: Urinary catheterization is deemed unnecessary.
Thus, by using the BVI 9400, these catheterizations are avoided. The default
setting (for volume below which catheterization is unnecessary) is 200 ml.
UTIs avoided: Studies indicate that a certain percentage of catheterizations lead
to urinary tract infections (UTIs).
By avoiding unnecessary catheterizations, the resulting UTIs are thereby
avoided. The default setting (for percent of catheterizations leading to UTIs) is
3%.
Associated UTI costs: Literature suggests that the additional costs associated
with treating UTIs amounts to $680.00 per patient infection. The default setting is
$680.00.
Cost of catheter kits: The default setting is $3.00 per kit.
Total Cost Savings from Using the BVI 9400 =
(Catheterizations avoided x catheter costs) + (UTIs avoided x UTI costs)
NOTE: The default settings can be changed to reflect the rates and costs at your facility
by selecting from the Home screen:
page 43, “Savings Preferences Screen” for more information on customizing Savings
Preferences.
(SETTINGS) > SAVINGS PREFERENCES. See
User’s Manual page 55
Page 56

Cleaning and Maintenance BladderScan® BVI 9400
Cleaning and Maintenance
Cleaning and Maintenance
Cleaning and Disinfecting the BladderScan® BVI 9400
To Clean and disinfect the BladderScan® BVI 9400:
1. Use a soft cloth dampened with isopropyl alcohol (or an appropriate hospital cleaning
agent) to wipe the Probe until it is thoroughly cleaned.
2. If you use a detergent solution to clean the instrument, remove all residual detergent.
Dry the instrument with a clean, soft cloth.
3. If the instrument needs to be disinfected, dampen a soft cloth in any glutaraldehydebased hospital disinfectant solution such as Cidex
Sterilization Products, or Sporocidin
instrument with a dampened cloth.
4. To remove all traces of disinfectant solution and wipe the instrument with a clean soft
cloth dampened in sterile water or cleaning solution. Verathon
the device three separate times to remove all residual disinfectant.
5. Thoroughly dry the instrument with a clean, soft cloth before using.
®
from Sporocidin International. Wipe the
®
or Cidex 7® from Advanced
®
recommends wiping
IMPORTANT! Failure to heed the following warnings may cause device damage not
covered by the BladderScan® BVI 9400 warranty.
Do not immerse the instrument in disinfectant solution.
Do not use CidexPlus® to disinfect the instrument. CidexPlus will damage the plastic
enclosure.
Do not subject any part of the instrument to steam sterilization or ethylene oxide
sterilization.
Changing gel pads between patients is strongly recommended.
Regular Inspections and Maintenance
Verathon Medical® recommends that the BVI 9400 be certified by an authorized
BladderScan
inspection and testing of the instrument to ensure accurate performance in clinical use.
For more information, please contact your authorized BladderScan
local BladderScan
1.800.331.2313 (North America only. International customers, please refer to the contact
information on page 64).
NOTE: ScanPoint
accessing their ScanPoint
Online, please refer to the ScanPoint
®
Service Center once a year. Certification service includes comprehensive
®
distributor, or Verathon Medical® Customer Care Department at
®
Online customers can maintain device certification via the Internet by
®
account. For more information about using ScanPoint®
®
with QuickPrint User’s Manual.
®
Service Center, your
page 56 User’s Manual
Page 57

BladderScan® BVI 9400 Cleaning and Maintenance
Weekly Inspections
Once a week, you should inspect the Probe and cable for physical faults or cracks.
Cracks that allow the ingress of fluid may affect the performance of the instrument. Any
apparent cracks or faults in the Console, Probe, or the cable that links the Console and
the Probe, must be referred to your authorized BladderScan
BladderScan
Medical
®
distributor, your local Verathon Medical® representative, or the Verathon
®
Customer Care Department.
®
Service Center, your local
IMPORTANT! If you see any physical faults or cracks in the instrument, discontinue use
immediately and contact your local Verathon Medical® representative or the Verathon
Medical® Customer Care Department at 1.800.331.2313.
Using the Built-in Self-Test Utility
The BVI 9400 can perform a number of self-diagnostic tests. To access the Self-Test
utility:
1. From the Home screen, press the
2. When the Settings screen opens, press the
highlighted (red), then press
SETTINGS button:
Æ or Å buttons until SELF-TEST is
to open the Self-Test screen.
3. When the Self-Test screen opens, testing begins automatically with the display
providing status and results.
4. When test is complete, press the
screen, then press the
HOME button to return to the Home screen.
SETTINGS button to return to the Settings
5. If any test results were failed or abnormal, contact your authorized BladderScan
representative, or contact the Verathon Medical
®
Customer Care Department at
1.800.331.2313.
Calibrating the BladderScan® BVI 9400 Using the ScanPoint® System
You must periodically calibrate your BladderScan® BVI 9400 to make sure your
instrument is providing the most accurate results. Calibrating ensures accurate and
proper alignment of the instrument’s internal coordinate system. If you do not perform the
calibration by the prescribed date, the instrument can still be used to take scans but
measurements may be compromised.
When calibration is required, a warning appears in the display header (Figure 28).
®
User’s Manual page 57
Page 58

Cleaning and Maintenance BladderScan® BVI 9400
Figure 28. Calibration Warning
If you do not have ScanPoint
authorized Verathon
Customer Care Center for more information. For contact information, please see
Contacting Verathon
Self-Calibration
Self-calibration requires the ScanPoint
(P/N 0620-0340). For instructions on software requirements and installation, please refer
to the ScanPoint
The entire calibration process takes approximately 15 minutes. ScanPoint
allow for calibration of the instrument as often as you like. You do not need to wait until
the next scheduled calibration date. However, at a minimum, you must calibrate the
instrument once every 12 months.
Device Repair
The BladderScan® BVI 9400, Probe, and Battery Charger/Wireless Hub are completely
sealed. There are no user-serviceable components. Verathon
any type of circuit diagrams, component parts lists, descriptions, or other information that
would be required for repairing the device and related accessories.
Premium Warranty customers have access to a loaner replacement unit and free
shipping options which vary according to plan.
If you have any questions, contact your local Verathon Medical
Verathon Medical
®
®
service center for calibration. Contact your Verathon
®
on page 64.
®
with QuickPrint User’s Manual.
®
Customer Care Department at 1.800.331.2313.
with QuickPrint, you must send your instrument in to an
®
with QuickPrint System and a Calibration Kit
®
®
Medical
®
Online plans
does not make available
®
representative or the
Unit Disposal
The BladderScan® BVI 9400 and related devices may contain lead, mineral oils,
batteries, and other environmentally hazardous materials. When the BladderScan
9400 has reached the end of its useful service life, return the device, Battery
Charger/Wireless Hub, and related accessories to a Verathon
®
Service Center for proper
disposal. Alternatively, follow your local protocols for hazardous waste disposal.
page 58 User’s Manual
®
BVI
Page 59

BladderScan® BVI 9400 Troubleshooting
Troubleshooting
Troubleshooting
Help Resources
Verathon® provides an extensive array of customer service resources, described in the
table below.
You can obtain copies of this manual, Quick Reference Cards, and clinical studies by
visiting the Verathon
Verathon Medical
on page 64.
Resource Description
BladderScan® BVI 9400
In-Service CD
Onboard training modules Training modules installed on your BladderScan®
BladderScan® BVI 9400 Quick
Reference Cards
®
Web site at http://www.verathon.com or by contacting your
®
representative. A complete listing of contact information is provided
CD included with your BVI 9400 that shows how to
use the BladderScan
®
.
are available by pressing the button from the
Home Screen. Available titles include: Using the
BladderScan
®
BVI 9400 and NeuralHarmonics™
Technology.
Summary of procedures for using the BladderScan®
system.
Clinical Studies Scientific papers on BladderScan® use.
ScanPoint® Online ScanPoint® Online provides customers:
The ability to calibrate and certify devices
online anytime you wish.
Automatic data backup and archiving
(HIPAA compliant).
Automatic software upgrades.
Access to real-time troubleshooting from
Verathon
®
.
User’s Manual page 59
Page 60

Troubleshooting BladderScan® BVI 9400
Resource Description
Premium Warranty A Verathon® device warranty plan that provides all
the benefits of ScanPoint
paragraph) and also:
All repairs performed free of charge.
Instrument insurance: A perpetual warranty
against outdated technology with free
upgrade / replacement when your device is
no longer manufactured.
Free loaner program.
Free shipping.
Verathon® Web site http://www.verathon.com includes many in-service
training resources. From the Verathon
select “BladderScan
select “Customer Service.”
Phone support In North America, call: 1.800.331.2313.
International customers, please refer to the list of
Customer Care Department phone numbers on
page 64.
®
Online (preceding
®
®
.” On the BladderScan® page,
Home page,
Icons on the BladderScan® Console
The following icons may appear on the instrument’s LCD screen.
Symbol Meaning
(Solid arrow) The bladder is contained within the image cone (cone-
Ã
shaped area in which the Probe transmits ultrasound waves), but not
centered. You may be able to obtain a more accurate measurement
by re-aiming the Probe in the direction indicated by the arrow.
Battery power level:
Indicates a fully charged battery.
Indicates a battery 50% to 75% charged.
Indicates a battery 25% to 50% charged.
Indicates a battery that is nearly discharged and can power
only a few more scans.
Indicates the battery must be changed.
The patient is a small child with height less than 48 inches (122 cm)
tall and weight less than 60 lbs (27 kg).
page 60 User’s Manual
Page 61

BladderScan® BVI 9400 Troubleshooting
Symbol Meaning
The patient is a female patient who has not had a hysterectomy.
Select to scan all other patients.
>
Diagnosing problems
Instrument Does Not Turn On
If the instrument does not turn on, this is usually due to a dead or discharged battery and
can be remedied by replacing the dead battery with a charged battery. Check the battery
icon in the upper right corner of the LCD display. If the battery icon does not display any
power segments, replace the battery.
When the battery charge is too low to allow normal operation (but not too low to permit
operation of the internal circuitry) the device displays the following message:
B
ATTERY CHARGE LEVEL IS TOO LOW FOR
INSTRUMENT OPERATION. RECHARGE BEFORE NEXT USE.
In this case, the battery must be recharged or replaced with a charged one.
Printer Problems
No Paper
The BladderScan
this image when the printer is out of paper (Figure 29).
The bladder is too large to be contained within the scan cone, or the
bladder contains more than 999 ml of urine.
®
BVI 9400 senses the presence of paper and automatically displays
User’s Manual page 61
Page 62

Troubleshooting BladderScan® BVI 9400
Figure 29. Printer Out of Paper Screen
For instructions on loading paper, review the on-board training module or refer to the
section in this manual titled Load a Roll of Thermal Paper beginning on page 27.
Too Hot
The BVI 9400 displays the message T
OO HOT if the print head overheats. In this case,
turn off the BVI 9400 immediately. This condition may be the result of a paper jam. To
clear the paper jam see the following paragraph.
Clearing a Paper Jam
If the paper will not advance through the printer, gently pull the paper jam backward while
moving the thumb wheel counterclockwise.
CAUTION! Possible device damage.
If the paper jam is inaccessible, do not try to disassemble the printer. Contact you
authorized Verathon
®
Service Center or your local Verathon® distributor for service.
page 62 User’s Manual
Page 63

BladderScan® BVI 9400 Warranty
Warranty
Warranty
Verathon® Inc. warrants the BladderScan® BVI 9400 against defects in material and
workmanship as long as it is covered by the Premium Warranty.
Damage or loss insurance is available as part of the Total Reliability
this warranty, a service center authorized by Verathon
prove to be defective during the warranty period.
This warranty does not apply if the unit was misused or modified by anyone other than a
service center authorized by Verathon
The unit must be used in accordance with the instructions contained in this manual.
Consumable items are not covered in this warranty and should be used in conformance
with Verathon
For further details, consult your Premium Warranty. Warranty conditions may differ in
some countries outside the United States. Contact your local distributor for warranty
terms.
Disclaimer of Additional Warranties
There are no understandings, agreements, representations of warranties expressed or
implied (including warranties of merchantability or fitness for a particular purpose) other
than those set forth in the preceding Warranty section. The contents of this manual do
not constitute a warranty.
®
product specifications.
SM
®
will repair or replace units that
®
.
Plan. Pursuant to
Some states disallow certain limitations on applied warranties. The purchaser, user, and
patient should consult state law if there is a question regarding this disclaimer. This
information, descriptions, recommendations, and safety notations in this manual are
based upon Verathon
®
experience and judgment with BladderScan® as of December
2007. The contents of this manual should not be considered to be all-inclusive, or to
cover all contingencies.
The physician who directs the use of the BladderScan
®
BVI 9400 at the institution where
it is in use is responsible for keeping current with clinical research in bladder volume
measurements.
Please direct any questions or problems concerning bladder volume measurement, using
the instrument, or the interpretation of data to the responsible physician.
User’s Manual page 63
Page 64

Contacting Verathon® BladderScan® BVI 9400
Contacting Verathon®
Contacting Verathon®
The team at Verathon® is committed to modernizing healthcare delivery by putting
patients first. Our products support healthcare professionals by providing accuracy, utility,
and excellence. For additional product and company information, visit the Verathon
site at http://www.verathon.com. If you have any questions or comments about Verathon
products and services, please contact us at:
Corporate Headquarters (USA)
Verathon Incorporated
20001 North Creek Parkway
Bothell, WA 98011
USA
Verathon Medical B. V. (Europe)
Boerhaaveweg 1
3401 MN IJsselstein
The Netherlands
Verathon Medical Sarl (France)
Office Address:
Espace Europeen de l'Entreprise
2 allée d'Oslo
67300 Schiltigheim
France
®
Web
®
Toll free: 800.331.2313 (US & Canada Only)
Tel: 425.867.1348
Fax: 425.883.2896
Web: http://www.verathon.com
Email: customerservice@verathon.com
Tel: +31.30.68.70.570
Fax: +31.30.68.70.512
Web: http://www.verathon.eu/
Email: customerserviceeu@verathon.nl
Postal Address:
BP 10039
F-67012 Strasbourg Cedex
France
Tel: +33(0)3.88.60.14.02
Fax: +33(0)3.88.60.46.87
Email: info@verathon.fr
Verathon Medical Ltd.
(United Kingdom)
The Granary Manor Farm Courtyard
Aston Sandford, Aylesbury
Tel: +44.1844.299.207
Fax: +44.1844.299.218
Web: http://www.verathon.co.uk/
Email: customerserviceuk@verathon.co.uk
Buckinghamshire, HP17 8JB
United Kingdom
Verathon Medical K. K. (Japan)
Executive Tower Azabudai 7F
1-4-3 Azabudai
Tel: +81.03.3560.3501
Fax: +81.03.3560.3502
Email: servicejp@verathon.com
Minato-ku
Tokyo, Japan 106-0041
page 64 User’s Manual
Page 65

BladderScan® BVI 9400 Safety and Performance Summary
Safety and Performance Summary
Safety and Performance Summary
The BladderScan® BVI 9400 computes the volume of the urinary bladder based
upon twelve cross-sectional ultrasound images. For maximum accuracy, be sure
to hold the Probe motionless while scanning.
The most accurate measurements are obtained when the patient rests quietly in
a supine position.
Errors in usage tend to result in the underestimation of bladder volume, except in
cases where the Probe is moved during scanning. In this case, the measurement
may overestimate the patient’s bladder volume.
We recommend that new users use the BladderScan
patients with moderately full bladders rather than initially attempting to locate a
bladder with a low urinary volume.
To conserve battery power, the BladderScan
automatically when not in use (goes into “sleep”).
WARNING! Risk of explosion.
There is a possible risk of explosion if the BladderScan® BVI 9400 is used in the
presence of flammable anesthetics.
WARNING! Risk of patient injury.
Do not use the BladderScan® BVI 9400 on patients with open skin or wounds in the
suprapubic region.
®
BVI 9400 to measure
®
BVI 9400 shuts itself down
CAUTION: Risk of inaccurate measurements.
Accuracy is compromised if the user does not obtain an optimal, repeatable
image.
The BladderScan
®
BVI 9400 is not intended for use on pregnant patients.
The patient being scanned should not have a catheter in his/her bladder. This
could create microbubbles in the bladder, which will compromise the accuracy of
the measurement.
Use care when scanning suprapubic and pelvic surgery patients. Scar tissue,
surgical incisions, sutures, and staples can affect ultrasound transmission and
reflection.
User’s Manual page 65
Page 66

Parts and Accessories BladderScan® BVI 9400
Parts and Accessories
Parts and Accessories
BladderScan® BVI 9400 System Components
The following components are included with your BladderScan® BVI 9400 system.
Part Number Description
0570-0190
0570-0188
0570-0193
0400-0066
0800-0319
0620-0340
0800-0322
0900-1606
0900-1500
BVI 9400 system: Console and Probe. Transmits and receives
ultrasound to measure a patient’s bladder volume.
Battery Charger/Wireless Hub
Rechargeable batteries (2 included)
Roll of Thermal Paper: Properly sized paper for the on board Printer.
Calibration Kit (Optional - requires ScanPoint
software)
Medical Cart (optional)
BladderScan
BladderScan
®
BVI 9400 User’s Manual (on In-Service CD)
®
BVI 9400 In-Service CD (with User Manuals, Video
Tutorial, and Quick Reference Cards)
®
with QuickPrint
0900-1601
0900-1446
0900-1447
ScanPoint
Quick Reference Cards - Demo/Use
Quick Reference Cards - Calibration
To order any of the above parts, contact your authorized Verathon Medical® sales
representative or contact the Verathon Medical
®
with QuickPrint User’s Manual (on In-Service CD)
®
Customer Care Department at
1.800.331.2313.
page 66 User’s Manual
Page 67

BladderScan® BVI 9400 Product Specifications
Product Specifications
Product Specifications
Symbol Directory
The following table explains the industry symbols used to indicate the BladderScan®
system compliance with international and national standards and regulations.
Symbol Meaning
Marked in accordance with Directive 2002/96/EC on Waste
Electrical and Electronic Equipment (WEEE) (solid bar indicates
product was put on the market after 13 August 2005.)
Class II equipment protected throughout by double or reinforced
insulation.
Type BF applied part with EN/IEC-60601-1.
CE marked in accordance with the Medical Device Directive
(MDD).
Canadian Standards Association (CSA) mark of certification to
United States standards for electromedical equipment.
This unit is powered by a Lithium-Ion battery pack. The
Verathon
®
part number for the battery pack is 0400-0066.
User’s Manual page 67
Page 68

Product Specifications BladderScan® BVI 9400
Standards and Regulations Compliance
Verathon® certifies that all units are in compliance with all applicable international and
national standards and regulations, including but not limited to the following:
Specification Standard
International Electrotechnical
Commission
EN/IEC 60601-1 Amendments 1 and 2 and
EN/IEC 60601-1-2 (EMC)
Safety Standard EN/IEC 60601-2
Medical Device Directive MDD 93/42/EEC Annex 1
Canadian Standards Association C22.2 No. 601.1-M90 (Master Contract No.
177198)
Underwriters Laboratories, Inc. UL 60601-1
Health Insurance Portability and Accountability Act (HIPAA)*
*For details on Verathon® compliance with privacy rules, please refer to the information in
the QuickPrint Help menu (select PRIVACY AGREEMENT).
Electromagnetic Effects
There are no restrictions on the use of BladderScan
characteristics. Both the emissions from BladderScan
®
BVI 9400 due to its electromagnetic
®
and the susceptibility of this
instrument to interference from other sources are within prescribed limits of all applicable
standards at the date of manufacture. The emissions test procedure that was used is
specified in EN/ IEC55011: 1991 for Group 1, Class A equipment (per EN/IEC60601-1-2,
36.201.1.7).
BladderScan
environments, and in domestic environments under the jurisdiction of a health care
professional. An indication of adverse electromagnetic effects from BladderScan
®
BVI 9400 is suitable for use in industrial, scientific, and medical (ISM)
®
BVI
9400 on another electronic device would be a degradation of performance in the other
device when the devices are operated simultaneously. If such interference is suspected,
separate the two devices as much as possible, or discontinue simultaneous operation, if
practical, and contact Verathon
BladderScan
®
BVI 9400 will operate normally in the proximity of other potential
®
.
interference sources, and has demonstrated immunity at a field strength of 3 V/m (per
EN/IEC 60601-1-2, 36.202.2.1). You do not need to take any other precautions regarding
exposure in reasonably foreseeable environmental conditions to magnetic fields,
pressure, or variations in pressure, acceleration, or thermal ignition sources.
page 68 User’s Manual
Page 69

BladderScan® BVI 9400 Product Specifications
BladderScan® BVI 9400 Instrument Specifications
For technical performance assessment in an acoustic laboratory, the Probe cannot be
immersed in water.
Item Specification
Input Lithium Ion Battery Pack. Verathon P/N 0400-0066.
Output No load to full load at rated voltage. Refer to unit label.
Insulation The power supply is Class I with basic insulation to each
terminal.
Transient Overvoltage: Category II
Weight 5.2 lb (2.36 kg) (with battery)
Display 13.36cm W x 10.13cm H (5.26” W x 3.99” H)
(640 x 480 pixels, 120 dpi)
Integrated printer Thermal label printer
Languages English, Chinese Traditional, Czech, Danish, Dutch, Finnish,
French, German, Greek, Italian, Hungarian, Japanese,
Korean, Norwegian, Portuguese (European), Spanish,
Swedish, Turkish
Ultrasound Output Parameters
Item Specification
Maximum ultrasound I
Maximum ultrasound I
during a scan ≤ 5.0 m W/cm2
spta
during a scan ≤ 60.0 W/cm2
sppa
Maximum MI (Mechanical Index) .95 max
Transducer diameter 13 mm (0.512 inches)
Transducer resonant frequency 3.0 MHz and 1.74 MHz
Transducer bandwidth 75% at 10 dB
Time from 3D scan initiation to result
< 3 seconds
display
Penetration depth (in normal North
≥ 15 cm
American patient)
User’s Manual page 69
Page 70

Product Specifications BladderScan® BVI 9400
Accuracy Specifications
The accuracy specifications assume the instrument is being used according to the
instructions provided by Verathon
Specification Description
®
, scanning a tissue-equivalent phantom.
Bladder volume accuracy ± 15%, ± 15 ml (on a Verathon® tissue-equivalent
phantom)
Bladder volume range 0 - 999 ml (0-200ml in small child scan mode)
BladderScan
The BladderScan
®
BVI 9400 Operating Conditions
®
BVI 9400 is designed to function properly within the following
specifications:
Condition Description
Use Indoor
Ambient Temperature Range +10 - +40º Celsius (+50 - +104º Fahrenheit)
Atmospheric pressure range 700 hPa - 1060 hPa
Relative humidity 30% - 75% non-condensing
Water resistance Rated at IPX1 (indicates DRIP-PROOF, a higher than
ORDINARY level of protection from drips, leaks, and
spills)
BladderScan
The BladderScan
Condition Description
®
BVI 9400 Storage Conditions
®
BVI 9400 is designed for storage under the following conditions:
Storage Indoor
Ambient Temperature Range -20 - +60º Celsius (-4 - +140º Fahrenheit)
Atmospheric pressure range 500 hPa - 1060 hPa
Relative humidity 20% - 95% non-condensing
page 70 User’s Manual
Page 71

BladderScan® BVI 9400 Product Specifications
BladderScan® BVI 9400 Displays, Controls, and Indicators
The BladderScan® BVI 9400 provides the following controls, connectors, and features:
Instrument Buttons
Power O
N/OFF, Brightness (/), Volume (/). SCAN and RECORD buttons are on the
Probe. Five buttons below the Console LCD vary depending on current instrument mode
as follows:
Screen/Mode Active Buttons
Start up screen
Appears when the
POWER, VOLUME, BRIGHTNESS
(1) - (5) No function.
instrument is turned on.
Home Screen
Appears when the
instrument is first
turned on.
Scan Screen:
Appears when the
operator presses and
holds the S
CAN button.
Results Screen
Appears when the
operator releases the
SCAN button (after
aiming).
POWER, SCAN: opens Aim/Scan screen, (1) PATIENT
TYPE: toggle between three modes: small child, female with
uterus, all other patients, (2) TUTORIAL: opens tutorial
screen, (3) SETUP: opens Setup screen (4) REVIEW: opens
the review screen, (5) SCANPOINT
exams to ScanPoint
®
.
®
: transmits saved
POWER: No function
SCAN: Press to take a scan.
Buttons (1) - (5): No action.
POWER, SCAN: opens Aim/Scan screen, (1) RECORD:
press to record, release to replay, (2) PRINT: print to onboard label printer, (3) PLAY: press to play voice
annotations, otherwise, no function, (4) REVIEW: opens
review screen if a voice annotation has been recorded.
Otherwise, no function. (5) HOME: return to Home screen.
Review Screen
Review, replay, print, or
delete saved exams
Training Title Screen
View the menu of
training modules.
Video Viewing Screen
The video viewing
screen.
User’s Manual page 71
POWER, SCAN, (1) NEXT: select next exam in memory,
(2) PRINT: print to on-board printer, (3) PLAY: replay voice
annotation for currently selected exam, (4) DELETE: delete
currently selected exam, (5) HOME: return to Home screen.
POWER, (1) Skip back one chapter, (2) Play/Pause, (3) Skip
Forward one chapter (4) No function, (5) Home (return to
Home screen).
POWER, (1) No function, (2) Play/Pause, (3) Return to
Training Title Screen (4) No function, (5) Home (return to
Home screen).
Page 72

Product Specifications BladderScan® BVI 9400
Screen/Mode Active Buttons
Settings Screen
Start screen for editing
clinic name, date &
time, general
preferences, savings
preferences, and selftest options.
Alpha-Numeric
Screen
Displays alpha-numeric
characters for entering
information.
Date & Time Screen
Allows the user to set
the date and time
Preferences Screen
Set preferences for
language, time and
date format, time zone,
UTI rate and cost, and
currency
POWER, (1) Select next setting in list, (2) Select previous
setting in list, (3) Enter (proceed to the selected screen),
(4) No action, (5) Home (return to Home screen).
Settings: Name, Date/Time, Preferences, Self-Test.
POWER, (1) Move down, (2) Move right, (3) Add currently
selected character, (4) Delete currently selected character,
(5) Return to main setup screen.
POWER, (1) Move forward to next changeable unit,
(2) Move back to previous changeable unit, (3) Add/toggle
units as appropriate (hold down to move faster),
(4) Decrease/toggle units as appropriate (hold down to move
faster), (5) Save current date/time entries and return to main
setup screen.
POWER, (1) Select next setting in list, (2) Select previous
setting in list, (3) Select next option/Enter, (4) Select
previous option, (5) Home (return to Home screen).
Preferences list and options
Languages
: English (default), Chinese Traditional, Czech,
:
Danish, Dutch, Finnish, French, German, Greek, Italian,
Hungarian, Japanese, Korean, Norwegian, Portuguese
(European), Spanish, Swedish, Turkish.
Date format
: mm/dd/yyyy, dd.mm.yyyy, yyyy-mm-dd
Time Format: 12-hour, 24-hour
Time Zone:
Calibration Warning
scroll through available time zones
: On (default), Off. When “On” is
selected, a calibration warning will appear in the display
header when the device requires calibration.
Enable Small Child Mode (SCM)
: On (default), Off. Select
“Off” to disable Small Child Mode.
UTI Rate
UTI Cost
Catheter Cost
Catheter Volume
Currency
: 1% - 100% in increments of 1%
: $10 - $100,000 in increments of $1
: $1 to $1000 in increments of $1
: 20 ml - 100 ml in increments of 20 ml
: $ / € / £ / ¥
Savings Calculation: Since new, Since 1/1/2006, Reset
Now, Print Since New, Print Recent, Hide Savings.
page 72 User’s Manual
Page 73

BladderScan® BVI 9400 Product Specifications
Screen/Mode Active Buttons
Self-Test Screen
POWER, (1) Scroll down, (2) Scroll up, (3) No function,
(4) No function, (5) SETUP (Go back to setup screen).
ScanPoint® Screen
(NOTE: Only available when used with ScanPoint
®
with
QuickPrint software.) POWER, (1) No function, (2) No
function, (3) No function, (4) CANCEL, (5) No function.
Instrument Display
The instrument display is divided into three horizontal panes:
The center pane is the main display area. The user interface for the center pane
varies depending on device mode.
The header pane displays the institution name, date, time, and a battery status
icon.
The footer pane displays icons that indicate button functions (described in the
preceding table).
Screen/Mode Display
Start-Up Screen
Displays “BladderScan
®
” and a progress bar.
appears when the
instrument is turned
on.
Home Screen
Scan Screen
Results Screen
Review Screen
Tutorial Screen
Setup Screen
Name Screen
Memory indicator, Correct scanning technique graphic,
Savings Calculation table.
Appears when the operator presses the
SCAN button.
As bladder volume is calculated, the display refreshes and
updates until the scan is complete.
Appears when a scan is complete. Prominently displays
calculated bladder volume, patient type, available memory
and animated Print icon (if Print is pressed).
Review currently save exams. Saved exam folders on left
(currently selected folder is yellow) and ultrasound images
associated with selected exam.
The tutorial video.
Lists available setting screens.
A grid of alpha-numeric characters.
User’s Manual page 73
Page 74

Product Specifications BladderScan® BVI 9400
Screen/Mode Display
Date & Time Screen
Preferences Screen
Self-Test Screen
ScanPoint® Screen
The date and time.
List of available settings and their current values.
Displays test progress and results.
When ScanPoint
status information about the ScanPoint
example, when uploading an exams displays a progress bar.
Battery Charger/Wireless Hub Specifications
The Battery Charger/Wireless Hub is powered from a standard wall outlet (adaptable to
international power standards). The Battery Charger/Wireless Hub can charge two
batteries simultaneously.
Operating Conditions
The following are proper operating conditions for the Battery Charger/Wireless Hub:
Specification Description
Use Indoor
Ambient Temperature Range +5 - +40º Celsius (+41 - +104º Fahrenheit)
®
with QuickPrint is installed: Displays
®
communication. For
Atmospheric pressure range 70 kPa - 106 kPa
Relative humidity 30% - 75% non-condensing
Water resistance Rated at IPX 0 (ordinary equipment without protection
against ingress of water)
Computer connection USB 2.0
Charger Powered by a desktop DC power supply.
Input voltage 100 - 240 VAC RMS
Input frequency 50 - 60 Hz
Input current 1 Amp max
Input connection 2 wire IEC 60320 C7
Output 9v at 1 Amp
Insulation Class II with double insulation
page 74 User’s Manual
Page 75

BladderScan® BVI 9400 Product Specifications
Specification Description
Fuses 250 VAC, 2A, quick acting
Testing CSA 60950-1-03/UL 60950-1
Storage Conditions
Condition Description
Use Indoor
Ambient Temperature Range +5 - +40º Celsius (+41 - +104º Fahrenheit)
Atmospheric pressure range 70 kPa - 106 kPa
Relative humidity 30% - 75% non-condensing
Water resistance Rated at IPX 0 (ordinary equipment without protection
against ingress of water)
Battery Specifications
The BladderScan® BVI 9400 is provided with two Lithium Ion batteries. A battery icon on
the instrument display is always present indicating how much power remains and when
the battery needs to be changed. The user can change the battery whenever necessary.
Removing a discharged battery and replacing with a fresh battery will not erase any
saved exams or user settings. Use only the battery charger provided with the BVI 9400.
Any other battery charger may damage the battery pack.
The batteries meet the following specifications:
Condition Description
Battery type Lithium Ion
Battery life A fully charged battery can provide approximately 30
Charging time Charging time offline will take no more than six hours
exams within a 24-hour period.
from an empty battery to a full charge.
User’s Manual page 75
Page 76

Glossary BladderScan® BVI 9400
Glossary
Glossary
Term Meaning
Aiming arrows
Arrows that appear on the LCD screen to indicate the location
of the bladder relative to the Probe. A solid aiming arrow
means re-aiming is optional.
Battery
Charger/Wireless
Hub
The unit that charges the Lithium Ion batteries that power the
BladderScan
®
and simultaneously receives and sends
information from/to a BVI 9400 instrument within
communication range.
BladderScan® The BladderScan
produce an image of the patient’s bladder and determine
bladder volume.
Calibration
Checking the accuracy and function of your BladderScan
comparing it with a known standard.
Calibration Kit
Tools for calibrating your BladderScan
container has a spiral-shaped target which provides a known
reference in a spherical coordinate system. The calibration of
the instrument to this known reference increases the accuracy
of volume measurements.
Console
The main BladderScan
and communicates with the user via images and text displays
on an LCD screen.
®
instrument transmits ultrasound energy to
®
®
. The calibration
®
device that provides operating controls
by
Coupling medium
A substance, such as ultrasound gel or a gel pad, that
enhances the transmission of ultrasound waves.
Flashing Aiming
Arrows
Gel pad
Indicate the bladder is not inside the scan cone and the Probe
needs to be redirected. The arrow shows the direction to move
or tilt the BladderScan
®
to improve the measurement. Re-
aiming is required.
Sontac
®
Gel Pad: A disc-shaped, gel-filled pad that enhances
the transmission of ultrasound waves.
HIPAA
Health Insurance Portability and Accountability Act, enacted by
the US Congress in 1996.
Title II of HIPAA, the Administrative Simplification provisions
(AS), requires the establishment of national standards for
electronic health care transactions and national identifiers for
providers, health insurance plans, and employers. The AS
provisions also address the security and privacy of health data.
The standards are meant to improve the efficiency and
page 76 User’s Manual
Page 77

BladderScan® BVI 9400 Glossary
Term Meaning
effectiveness of the nation's health care system by encouraging
the widespread use of electronic data interchange in the US
health care system.
Image cone
Instrument
LCD screen
NeuralHarmonics™
Technology
Probe
SCAN button
ScanPoint® bladder
imaging
technology
During a bladder volume measurement, this is the cone-shaped
area in which the Probe transmits ultrasound waves.
In this manual, refers to the BladderScan
BladderScan
®
instrument transmits ultrasound energy to
®
BVI 9400. The
produce an image of the patient’s bladder and determine
bladder volume.
The LCD (liquid crystal display) screen on the BladderScan
®
Console that displays the bladder volume measurement,
instrument status, and other exam settings and information.
A patented BladderScan
®
technology that sharpens accuracy
and accelerates speed of three-dimensional ultrasound bladder
volume measurements.
The handheld part of the BladderScan
®
instrument that
transmits ultrasound energy to the patient.
Located on the underside of the BladderScan
®
hand grip, this
button is used to initiate the measurement.
The software and online service provided by Verathon® that
prints exam results, maintains patient records, displays
ultrasound images from exams, downloads software updates,
and calibrates the BladderScan
®
.
ScanPoint® with
QuickPrint
Sleep mode
Solid aiming arrow
Supine position
Suprapubic
Symphysis pubis
Ultrasonic
ScanPoint® with QuickPrint is a software program installed on
your computer that communicates with ScanPoint
help you quickly and easily download and print BladderScan
®
Online to
®
results.
When the BladderScan
®
shuts down to conserve energy. Press
any button to turn on the instrument.
Indicates the bladder is inside the scan cone but is not
completely centered. The arrow shows the direction to move or
tilt the BladderScan
®
to improve the measurement. Re-aiming
is optional but not required.
Refers to the patient laying on their back with their face
upward.
The area above the pubic bone.
The front of the pelvis where the pubic bones meet.
Involving ultrasound. Ultrasound is sound at frequencies above
the range that can be heard by the human ear.
User’s Manual page 77
Page 78

Page 79

For more information, call 800.331.2313 (in the U.S. and Canada) or contact your local Verathon Medical® representative.
BladderScan®, ScanPoint®, Verathon® and Verathon Medical® are registered trademarks of Verathon Inc. in the United States
and/or other countries. Copyright ©2008. All rights reserved.
Page 80

 Loading...
Loading...