Page 1

BladderScan® Bladder Volume Instruments
User’s Manual
For:
BladderScan BVI 6100 Bladder Volume Instrument
BladderScan BVI 6400 Bladder Volume Instrument
CAUTION: In the United States, federal law restricts
this device to use by or on the order of a physician.
User’s Manual
page 1
Page 2

Copyright © 2008, 2011 by Verathon Inc. No part of this User’s Manual may be copied or transmitted by any
method without the express written consent of Verathon Inc.
BladderScan, the BladderScan symbol, ScanPoint, Sontac, Vmode, Verathon, the Verathon Torch Symbol,
and Total Reliability Plan are trademarks of Verathon Inc.
Cidex® is a registered trademark of Advanced Sterilization Products. Sporocidin® is a registered trademark of
Sporocidin International.
Microsoft® and Windows® are registered trademarks of Microsoft Corporation in the United States and/or
other countries.
All other brand and product names are trademarks or registered trademarks of their respective owners.
The BladderScan® technology described in this manual is protected by U.S. Patent Numbers 5,235,985,
6,676,605 and 6,884,217. The ScanPoint® technology described in this manual is protected by U.S. Patent
Number 6,569,097. Other patents pending.
Information in this User’s Manual may change at any time without notice. For the most up-to-date information,
see the online manuals at verathon.com. Examples described or illustrated in the User’s Manual are ctitious
and do not in any way represent real patient or exam data.
Corporate Headquarters
20001 North Creek Parkway
Bothell, WA 98011
USA
800.331.2313 (US and Canada only)
425.867.1348
Fax: 425.883.2896
Verathon Medical (Europe) B.V.
REP
EC
Linnaeusweg 11
3401 MS IJsselstein
The Netherlands
+31.30.68.70.570
Fax: +31.30.68.70.512
page 2
PN: 0900-1236-02-60
User’s Manual
Page 3

BladderScan BVI 6000 Series Bladder Volume Instruments
Table of Contents
Table of Contents
Table of Figures ..........................................................................................................................................5
Statement from the CEO ............................................................................................................................6
Important Information ................................................................................................................................7
Product Description ......................................................................................................................................7
Intended Use Warnings and Cautions ..........................................................................................................7
Biological Safety ........................................................................................................................................... 7
Statement of Prescription ...................................................................................................................... 7
Statement of Intended Use....................................................................................................................8
Contraindications...................................................................................................................................8
Introducing the BladderScan® BVI 6000 Series Instruments ...............................................................10
Product Description.................................................................................................................................10
BladderScan Bladder Volume Instruments ............................................................................................. 11
Optional ScanPoint® Image Management Technology ...........................................................................12
Getting Started ..........................................................................................................................................14
Unpack the Box and Check the Components .........................................................................................14
Optional Accessories ..............................................................................................................................16
BladderScan Controls and Features .......................................................................................................17
Charging the BladderScan Instrument ....................................................................................................19
Battery Power Status Icons .................................................................................................................19
Battery Charging Notes .......................................................................................................................20
Installing ScanPoint Image Management Technology ............................................................................20
Measuring Bladder Volume .....................................................................................................................21
Preparing for the Exam ...........................................................................................................................21
Measuring Bladder Volume .....................................................................................................................22
Additional Scanning Tips.........................................................................................................................26
User’s Manual
page 3
Page 4

Table of Contents
BladderScan BVI 6000 Series Bladder Volume Instruments
Cleaning and Maintenance ......................................................................................................................28
Cleaning and Disinfecting the BladderScan® Instrument ........................................................................ 28
Regular Inspections and Maintenance....................................................................................................28
Calibrating the BladderScan Instrument .................................................................................................29
Displaying the Days Remaining Until Calibration ................................................................................ 29
Device Repair and/or Replacement ........................................................................................................30
Battery Replacement ..............................................................................................................................30
Unit Disposal ...........................................................................................................................................31
Troubleshooting .......................................................................................................................................32
Help Resources ......................................................................................................................................32
Common Problems .................................................................................................................................33
Warranty .................................................................................................................................................... 35
Disclaimer of Additional Warranties ........................................................................................................35
Contacting Verathon
®
............................................................................................................................... 36
Safety and Performance Summary ......................................................................................................... 37
Parts and Accessories ............................................................................................................................. 38
BladderScan System Components (BVI 6100 and BVI 6400) ................................................................38
Product Specifications.............................................................................................................................40
Symbol Directory.....................................................................................................................................40
Standards and Regulations Compliance.................................................................................................41
Electromagnetic Effects .......................................................................................................................41
BladderScan Instrument Specications ..................................................................................................42
Storage Conditions .................................................................................................................................43
Charging Cradle Specications...............................................................................................................43
Glossary ....................................................................................................................................................44
page 4
User’s Manual
Page 5

BladderScan BVI 6000 Series Bladder Volume Instruments
Table of Figures
Table of Figures
Figure 1. BladderScan® Report for a Physician’s Ofce ............................................................................. 12
Figure 2. BladderScan Instrument Parts ....................................................................................................17
Figure 3. Calibration Required Display .......................................................................................................29
Figure 4. BVI 6000 series instrument with replaceable battery ..................................................................30
Table of Tables
Table 1. BladderScan Controls and Features .............................................................................................17
Table 2. Display Icons.................................................................................................................................18
Table 3. Battery Status Icons ......................................................................................................................19
Table 4. Typical Scanning Scenarios and Displays ....................................................................................27
Table 5. Troubleshooting Help Resources ..................................................................................................32
Table 6. Common Problems and Solutions ................................................................................................33
Table 7. Contacting Verathon® ....................................................................................................................36
Table 8. Included Items...............................................................................................................................38
Table 9. Optional Items ...............................................................................................................................38
Table 10. Symbol Directory.........................................................................................................................40
Table 11. Standards and Regulations Compliance ..................................................................................... 41
Table 12. BladderScan Instrument Specications ......................................................................................42
Table 13. BladderScan BVI 6000 Series Storage Conditions .....................................................................43
Table 14. Charging Cradle Specications...................................................................................................43
Table 15. Charging Cradle Storage Conditions ..........................................................................................43
Table 16. Glossary ......................................................................................................................................44
User’s Manual
page 5
Page 6

Statement from the CEO
Statement from the CEO
The team at Verathon® is committed to improving health care delivery by
putting healthcare providers and their patients rst.
Our products support you, the health care provider, by consistently offering
accuracy, utility, reliability and excellence.
Please contact us directly at 1.800.331.2313 (USA and Canada only) or
1.425.867.1348, if we can improve our service to you.
Sincerely,
BladderScan BVI 6000 Series Bladder Volume Instruments
Gerald McMorrow
Gerald McMorrow, CEO and Founder
page 6
User’s Manual
Page 7
BladderScan BVI 6000 Series Bladder Volume Instruments
Important Information
Product Description
BladderScan® BVI 6000 series bladder volume instruments are wireless, battery-powered,
ultrasound instruments that provide a noninvasive measurement of urinary bladder volume.
During each scan, BladderScan instruments employ patented Vmode® technology to create
a three-dimensional image of the bladder. BladderScan instruments automatically calculate
and display measurements based upon this image. Vmode measurements tend to be more
accurate than those obtained from conventional two-dimensional ultrasound, as they are
based on a more complete, multi-faceted image of the bladder.
Optionally, exam results may be transmitted to a personal computer running ScanPoint
with QuickPrint software via a proprietary wireless connection. ScanPoint with QuickPrint
allows the user to archive data, calibrate the device, update software, print, and transfer
data through a Web-based interface.
Intended Use Warnings and Cautions
Important Information
All users must read this entire User’s Manual prior to using the BladderScan
instrument. Do not attempt to operate this instrument until you thoroughly understand
all instructions and procedures in this manual.
Failure to comply with these instructions may compromise the performance of the
device and the reliability of its measurements. For the most current version of the
User’s Manual, please visit the Verathon® Web site at verathon.com.
BladderScan instruments should be used only by individuals who have been trained
and authorized by a physician or the institution providing patient care.
Biological Safety
To date, exposure to pulsed diagnostic ultrasound has not been shown to produce adverse
effects. However, ultrasound should be used only by medical professionals when clinically
indicated, using the lowest possible exposure times indicated by clinical need.
The ultrasound output power of BladderScan BVI 6000 series instruments is not user
adjustable and is limited to the minimum level necessary for effective performance. Data
on acoustic output levels can be found in Product Specications beginning on page 40 of
this manual.
Statement of Prescription
United States federal law restricts BladderScan BVI 6000 series instruments to use by, or
on the order of, a physician. This statement is required per 21 Code of Federal Regulations
(CFR) 801.109.
User’s Manual
NOTE: It is standard practice to have medical staff authorize the use of a BladderScan
instrument within its intended use throughout an institution. Individual prescriptions for use
are not required.
page 7
Page 8
Important Information
Important Information
Statement of Intended Use
BladderScan® bladder volume instruments project ultrasound energy through the lower
abdomen of the patient to obtain an image of the bladder which is used to noninvasively
measure urinary bladder volume.
Contraindications
BladderScan instruments are not intended for fetal use or for use on pregnant patients.
Cautions and Warnings
To assure safe and reliable operation for the use and the patient, please read and heed
the following warnings and cautions.
WARNING! Risk of explosion.
If you use the BladderScan instrument in the presence of ammable anesthetics, the
hazard of potential explosion exists.
WARNING! Risk of explosion, re, or serious injury.
The BladderScan BVI 6100/6400 is provided with an internal lithium-ion battery. Never
short circuit the battery by either accidentally or intentionally bringing the battery
terminals into contact with any other conductive object. This could cause serious injury
or re and could also damage the battery and the BladderScan device.
BladderScan BVI 6000 Series Bladder Volume Instruments
Never expose the battery to abnormal shock, vibration, or pressure. The battery’s
internal protective covering could fail, causing it to overheat or ignite, resulting in
caustic liquid leakage, or explosion or re, possibly resulting in serious injury.
WARNING! Potential patient hazard.
To date, exposure to low-power, pulsed diagnostic ultrasound has not been shown to
produce adverse effects. However, medical professionals should use ultrasound only
when clinically indicated, using the lowest exposure times possible to obtain accurate
measurements. The ultrasonic output of the BladderScan instrument is not useradjustable and is limited to the minimum level necessary for effective performance.
For more information about the acoustic output levels of this device, please refer to
BladderScan Instrument Specications beginning on page 42 of this manual.
CAUTION:
Items Affecting Accuracy of Results.
When using the BladderScan BVI 6100/6400 be aware of the following conditions which
can affect ultrasound transmission and decrease the accuracy of exam results.
Use care when scanning patients who have had supra-pubic or pelvic surgery.
Scar tissue, surgical incisions, sutures, and staples can affect ultrasound
transmission and accuracy.
Do not use the BVI 6100/6400 on patients with open skin or wounds in the
suprapubic region.
Do not use the BVI 6100/6400 on patients with ascites.
If you scan a patient with a catheter in his/her bladder, the catheter may
affect measurement accuracy. However, the information obtained from the
measurement could still be clinically useful for detecting problems such as a
blocked catheter.
page 8
page 8
User’s Manual
User’s Manual
Page 9
BladderScan BVI 6000 Series Bladder Volume Instruments
Important Information
CAUTION:
Observe the following precautions in the safe use and care of the
BladderScan® instrument.
Hazardous materials present. Assure proper disposal.
The BladderScan instrument and related devices may contain lead, mineral oils,
batteries, and other environmentally hazardous materials. When the BladderScan
instrument has reached the end of its useful service life, return the device, Charging
Cradle, and related accessories to a Verathon® Service Center for proper disposal.
Alternatively, follow your local protocols for hazardous waste disposal.
Assure proper computer system conguration.
When using the BladderScan instrument with optional ScanPoint® image management
technology, your computer must be minimally certied to EN / IEC / CSA / UL 60950
or 60101-1 standards. This conguration ensures that compliance to the EN/IEC
60601-1-1 system standard is maintained. Anyone connecting additional equipment to
the BladderScan instrument signal input port or signal output port congures a medical
system, and is therefore responsible for ensuring that the system complies with EN/
IEC 60601-1-1. If you need assistance, contact your biomedical staff, Verathon Medical
representative, or the Verathon Medical Customer Care Department at 1.800.331.2313.
Assure proper distance from patient.
The BladderScan instrument, accessories, and computer used to access online
ScanPoint image archive (if needed) must be placed outside the patient vicinity (more
than six feet (2 meters) from the patient). Refer to UL 2601-1 Clause 2 deviation for the
denition of patient vicinity.
CAUTION:
Risk of Fire and Burns. Regarding the battery, do not disassemble,
heat above 60° C (140° F), or incinerate. Keep battery out of reach of children and in
original package until ready to use. Dispose of used batteries promptly according to local
recycling or waste regulations.
User’s Manual
page 9
Page 10

Introducing the BladderScan Bladder Volume Instruments
Introducing the BladderScan Bladder Volume Instruments
Product Description
The BladderScan® BVI 6100/6400 are portable ultrasound instruments that measure
bladder volume. Using patented Vmode® technology, BladderScan BVI 6000 series
instruments provide a noninvasive measurement of urinary bladder volume.
The BVI 6100/6400 consists of an ergonomic, battery-powered, handheld probe that
scans the patient’s bladder. The LCD display provides aiming assistance and displays an
array of bladder measurement information.
BladderScan instruments are quick, accurate, reliable, and easy to use. When the
user releases the scan button, within seconds, the BladderScan instrument measures
ultrasonic reections on multiple planes inside the body and produces a threedimensional image. Based on this image, the BladderScan instrument calculates and
displays the bladder volume. A sonographer is not required.
Volume measurements made with Vmode ultrasound are more accurate than those from
conventional ultrasound, as they are based on a more complex, 3D image of the bladder.
The BladderScan BVI 6400 includes an integrated microphone for recording voice
annotations.Voice annotation allows up to 10 exams to be stored on the instrument.
page 10
User’s Manual
Page 11

Introducing the BladderScan Bladder Volume Instruments
BladderScan Bladder Volume Instruments
BladderScan BVI 6100
PN: 0570-0154
The handheld, portable BladderScan® BVI 6100:
Measures bladder volume noninvasively.
Provides fast, accurate and reliable results.
Takes scans quickly providing test results in a
matter of seconds.
Is easy to operate: staff members can easily learn to
scan patients quickly and accurately.
Allows for exam results and images to be
downloaded, viewed and printed using the optional
ScanPoint® image management technology.
Is battery-operated, lightweight, and portable.
BladderScan BVI 6400
PN: 0570-0167
The handheld, portable BladderScan BVI 6400:
Measures bladder volume noninvasively.
Provides fast, accurate, and reliable results.
Takes scans quickly providing test results in a
matter of seconds.
Is easy to operate: staff members can easily learn to
scan patients quickly and accurately.
Provides the capability to voice annotate each exam
(10 seconds to record patient ID and relevant exam
information) ensuring that valuable patient and
exam data is retained.
Stores voice annotated data for up to ten exams.
Improves efciency of healthcare professionals who
examine multiple patients on their rounds.
Allows for exam results and images to be
downloaded, viewed, and printed using the optional
ScanPoint technology.
User’s Manual
Is battery-operated and easy to use.
page 11
Page 12
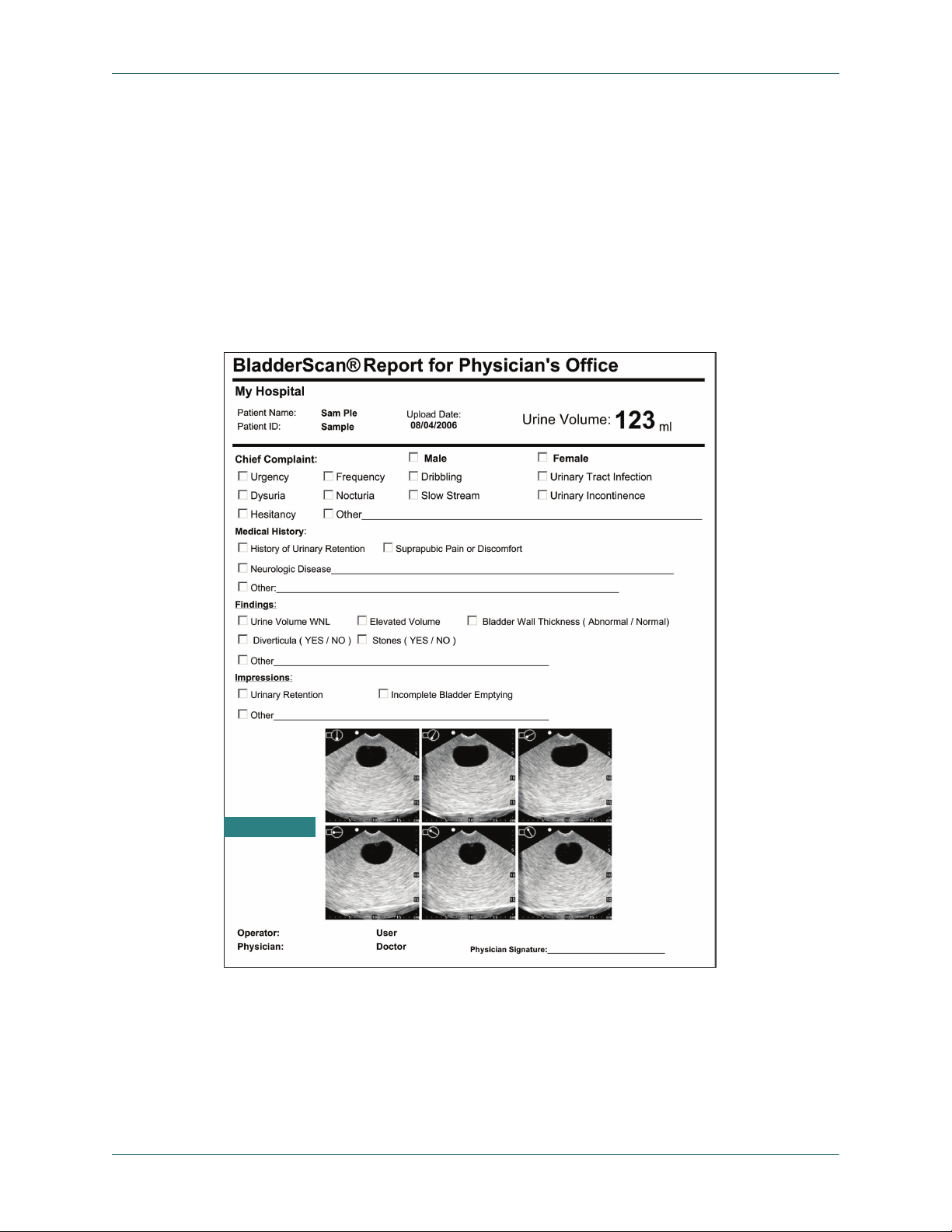
Introducing the BladderScan Bladder Volume Instruments
Optional ScanPoint Image Management Technology
Bladder volume measurements and ultrasound images may be transmitted from your
device to ScanPoint® image management software. ScanPoint software installs on a
Windows®-based computer and allows viewing, printing, and archiving of patient exam
results including ultrasound images for patient records and reimbursement (when
applicable). Exam data and ultrasound images may be printed in a variety of report
formats from adhesive labels that may be afxed to patient charts, to full letter-size
formats. Figure 1 shows an example of a ScanPoint report.
Figure 1. BladderScan Report for a Physician’s Ofce
page 12
CAUTION:
ScanPoint can also be used to calibrate your BladderScan instrument. The ScanPoint
image management technology (ScanPoint software, license, and accessories) is
available with the purchase of any BladderScan instrument. Comprehensive service and
warranty are provided under the ScanPoint Total ReliabilitySM Plan. For details, please
contact your local Verathon Medical representative at 1.800.331.2313.
User’s Manual
Page 13

Introducing the BladderScan Bladder Volume Instruments
ScanPoint® Local Client (LC) is a stand-alone, non-networked version of the
software. It is available for use with the BVI 6100, BVI 6400, BVI 9400, and
FloPoint® Elite products.
ScanPoint with QuickPrint is a network-based version of the application.
Archived patient data is stored securely on HIPAA-compliant, Verathon®maintained servers. Users can access records from any Internet-enabled
Windows®-based PC. ScanPoint with QuickPrint allows users to maintain the
most recent software for their devices, to calibrate their devices themselves
without having to send them in for service, and also enables remote diagnostics
and troubleshooting by Verathon service technicians.
ScanPoint with QuickPrint is available for use with Verathon BladderScan® BVI
6000 series, BVI 9000 series, and FloPoint Elite products.
User’s Manual
page 13
Page 14

Getting Started
BladderScan BVI 6000 Series Bladder Volume Instruments
Getting Started
To get up and running as quickly as possible, follow this sequence:
Unpack the BladderScan® instrument and related accessories.
Charge the device in the Charging Cradle. (Instructions begin on page 19)
Install optional items as desired (ScanPoint® software and ScanPoint Label
Writer)
Unpack the Box and Check the Components
Set the shipping container right side up and carefully open the top aps (do not insert
anything sharp through the top of the box). Remove the contents and verify that you
have received everything listed below. If anything is missing or damaged, notify your
authorized Verathon Medical representative or the Verathon Medical Customer Care
Department at 1.800.331.2313.
Part and Part Number Name and Description
BladderScan BVI 6100 or BVI 6400:
Handheld, wireless, battery-operated, ultrasound
bladder volume instrument.
0570-0154 (BVI 6100)
0570-0167 (BVI 6400)
Charging Cradle: Use the Charging Cradle to
charge the BladderScan instrument’s internal
battery. The Charging Cradle plugs directly
into an electrical wall outlet. Before using your
BladderScan device you must charge it for a
minimum of six hours.
0270-0234
page 14
User’s Manual
Page 15

BladderScan BVI 6000 Series Bladder Volume Instruments
Part and Part Number Name and Description
BladderScan BVI 6000 Series In-Service CD:
Includes the electronic version of the BladderScan
User’s Manual (this manual), Quick Reference
Cards, and the ScanPoint® User’s Manual.
0900-0813
Activation Tool:
Use to press the Activation button on the
instrument if needed.
Getting Started
®
0130-0181
0900-0589 (BVI 6100)
0900-0956 (BVI 6400)
BladderScan Bladder Volume Instruments
Quick Reference Cards:
Provide a summary of essential user instructions.
User’s Manual
page 15
Page 16

Getting Started
Optional Accessories
The following optional items are available to enhance the capabilities of your
BladderScan® instrument. Please contact your Verathon Medical Customer Care
Representative (1.800.331.2313) for more information on any of the following Verathon®
products.
Part and Part Number Name and Description
0900-1238
BladderScan BVI 6000 Series Bladder Volume Instruments
ScanPoint
®
with QuickPrint Install CD:
Installs ScanPoint with QuickPrint imaging software. Allows
viewing, printing, and archiving of patient exam results
with a Windows®-based computer. Archived patient data is
stored securely on HIPAA-compliant, Verathon-maintained
servers. Users can access records from any Internetenabled Windows®-based PC. ScanPoint with QuickPrint
allows users to maintain the most recent software for their
devices, to calibrate their devices themselves without
having to send them in for service, and also enables
remote diagnostics and troubleshooting by Verathon
service technicians. ScanPoint with QuickPrint works with
all BladderScan 6000 series, 9000 series, and FloPoint®
Elite devices.
ScanPoint Docking Station:
(Optional - used with ScanPoint Bladder image
management technology). Transmits data from the
BladderScan device to the ScanPoint host computer and
simultaneously recharges the device battery.
0570-0168
0620-0225
0800-0005
Calibration Kit (Requires ScanPoint with QuickPrint
software)
The calibration container holds a spiral-shaped calibration
target and 4.2 liters of water. The top has an indentation
for the instrument that places it in a known and repeatable
location with respect to the spiral target. Self-calibration
takes about 15 minutes.
Sontac
®
Ultrasound Gel
Provides the optimal coupling medium. Performance of the
BVI 6000 series instruments has been optimized for use
with Sontac Ultrasound Gel. The use of any other coupling
gel or medium may compromise the accuracy of readings.
page 16
User’s Manual
Page 17

BladderScan BVI 6000 Series Bladder Volume Instruments
BladderScan Controls and Features
The BladderScan® instrument controls and features are illustrated in Figure 2 and
explained in Table 1.
Figure 2. BladderScan Probe Instrument Parts
Getting Started
User’s Manual
Table 1. BladderScan Controls and Features
Part Name Purpose
Scan button Press to take a scan.
Probe The Probe transmits and receives ultrasound waves automatically
moving its internal transducer 360º to scan twelve different planes
to produce a three-dimensional image of the bladder.
Top button Press to select gender.
acTivaTion button Press to reactivate the BladderScan instrument if the battery
becomes completely discharged.
page 17
Page 18

Getting Started
BladderScan BVI 6000 Series Bladder Volume Instruments
Part Name Purpose
LCD Screen Displays bladder volume measurements and other scan, patient,
and instrument data.
Infrared (IR)
Window
Enables the BladderScan
ScanPoint®-equipped PC via the Docking Station.
®
instrument to communicate with a
Microphone Speak into the microphone to record voice annotations (found on
BVI 6400 only).
Table 2. Display Icons
Symbol Meaning
Battery power level. Please refer to the table on page 19 for a
completely description of battery power icons.
Female gender option is selected. Select this option only for
women who have not had a hysterectomy. Deselect for all others,
male or female.
Bladder imaging in progress. Hold instrument steady.
Flashing arrow indicates that the aim was “off target.” In order to
get an accurate bladder volume measurement, you must re-aim
the Probe in the direction of the arrow.
A solid arrow indicates that the bladder was not centered within
the scanning cone. However, the bladder volume measurement is
still accurate. Re-aiming is optional.
The patient’s actual bladder size is larger than the scanning cone.
(BVI 6400 only.) Indicates that a voice annotation is being
recorded and stored.
Indicates the number of days remaining until the next required
calibration.
page 18
User’s Manual
Page 19

BladderScan BVI 6000 Series Bladder Volume Instruments
Charging the BladderScan Instrument
Before using your BladderScan® instrument for the rst time, and subsequently if
the BladderScan instrument becomes completely discharged, you must charge your
BladderScan instrument battery for approximately six hours, or until it is fully charged.
To charge the bladder volume instrument:
1. Plug the Charging Cradle into an electrical wall outlet.
2. Place the BladderScan instrument in the Charging Cradle.
The battery icon will begin scrolling, indicating that the instrument is charging. If the
battery icon does not appear, then your bladder volume instrument was completely
discharged and may need to charge for up to six hours. The battery icon will appear
when the bladder volume instrument is charged sufciently for operation.
NOTE: Alternatively, you can charge the BladderScan instrument in the Docking
Station if you have already installed ScanPoint® on your computer, and installed the
Docking Station using the Windows® New Hardware Install Wizard.
Battery Power Status Icons
Getting Started
The battery icon is located in the lower-right corner of the bladder volume instrument
display and indicates the power level of the battery. You can recharge the bladder volume
instrument whenever you like but you must recharge the bladder volume instrument when
the power status icon shows only one segment. While charging, the battery icon displays
scrolling power segments. The following table provides more information about battery
status icons.
Table 3. Battery Status Icons
Battery Icon Description
Battery is fully charged and ready for use.
Battery is 50 - 75% charged.
Battery power is 25 - 50% charged.
Battery is nearly discharged. There is enough power for few scans.
Recharge the battery as soon as possible.
The battery is completely discharged. The bladder volume instrument
will not work until it is recharged.
User’s Manual
Scrolling segments indicate that the battery is being recharged.
page 19
Page 20

Getting Started
BladderScan BVI 6000 Series Bladder Volume Instruments
Battery Charging Notes
You may need to reactivate your BladderScan® instrument before charging the battery.
If your BladderScan instrument battery is completely discharged.
If the “scrolling segments” battery icon does not appear after the BladderScan
instrument has been in the cradle for two hours.
To reactivate your BladderScan instrument:
1. Use the tip of the activation tool to press the ActiVAtion button. The ActiVAtion
button is located just above the ScAn button (Figure 2).
2. Place the BladderScan instrument in the Charging Cradle or Docking Station until
the “full battery” icon is displayed.
NOTE: When you are not using your bladder volume instrument, Verathon
recommends that you store it in the Charging Cradle to ensure that your
instrument is always sufciently charged.
The Charging Cradle cannot overcharge the battery.
Installing ScanPoint Image Management Technology
If desired, install ScanPoint® Bladder Image Management Technology,
Please consult your ScanPoint User’s Manual for complete instructions for installation
and use of ScanPoint Image Management Technology.
The ScanPoint User’s Manual also includes instructions for setting up and installing the
ScanPoint Label Writer.
page 20
User’s Manual
Page 21

BladderScan BVI 6000 Series Bladder Volume Instruments
Measuring Bladder Volume
Preparing for the Exam
Before You Begin the Exam:
Make sure you are familiar with the parts of the BladderScan® instrument (see
Introducing the BladderScan Instrument on page 10).
If you are a new BladderScan instrument user, Verathon® recommends that you
perform your rst exam on a patient with a moderately full bladder, rather than
initially attempting to locate and scan a nearly empty bladder.
Check the instrument’s battery icon to make sure the battery has sufcient power.
NOTE: If the battery icon shows only one segment, fully charge the BladderScan
instrument before use.
Clean and disinfect the Probe by wiping it gently with a soft cloth soaked in
isopropyl alcohol.
Measuring Bladder Volume
Be aware of the following conditions the patient may have that could affect ultrasound
transmission and the accuracy of your exam:
A catheter in the patient’s bladder. The presence of a catheter may affect the
accuracy of the bladder volume measurement, but the measurement may still be
clinically useful (detecting a blocked catheter, for example).
Previous suprapubic or pelvic surgery. Scar tissue, surgical incisions, sutures,
and staples can affect ultrasound transmission and reection.
Do not use the BladderScan instrument on:
Patients with ascites.
Patients with open skin or wounds in the suprapubic region.
Pediatric patients (patients under 60 lbs and under 48” tall).
Pregnant patients.
User’s Manual
page 21
Page 22

Measuring Bladder Volume
Measuring Bladder Volume
1. Turn on the bladder volume instrument.
If the BladderScan® instrument has been stored in a Charging Cradle or Docking
Station, the power comes on automatically when you remove the bladder volume
instrument from the cradle.
If the bladder volume instrument has not been stored in the Charging Cradle or
Docking Station, it will go into sleep mode to conserve power. If the bladder volume
instrument is in sleep mode, press and release any button to turn the power on.
2. Select the patient’s gender.
If your patient is a female who has not had a
hysterectomy, press the top button located
below the display. For all other patients,
(male or female), press the top button again
to clear the gender icon from the Console
display.
BladderScan BVI 6000 Series Bladder Volume Instruments
Gender Icon
3. With the patient supine, apply gel.
Have the patient lie in the supine position
with the abdominal muscles relaxed.
Palpate the patient’s symphysis pubis (pubic
bone). Place an ample quantity of gel (with
as few air bubbles as possible) midline on
the patient’s abdomen, approximately one
inch (3 cm) above the symphysis pubis
(pubic bone). Make sure that there are as
few air bubbles as possible.
Top Button
page 22
User’s Manual
Page 23

BladderScan BVI 6000 Series Bladder Volume Instruments
4. Aim toward the bladder.
Standing at the patient’s right side, place
the Probe on the gel and aim it toward the
expected location of the bladder. For most
patients, this means angling the Probe slightly
toward the patient’s coccyx (tail bone) so the
scan clears the pubic bone.
5. Press and release the Scan button.
Press and release the ScAn button, located on
the underside of the Probe.
A scanning symbol appears in the upper right
corner of the LCD screen during the scan:
Measuring Bladder Volume
Hold the Probe steady until the scan is
nished. When you hear the end-scan tone,
the scan is complete.
After a scan, the bladder volume
measurement in milliliters (ml) is
Scan button
displayed in the top half of the screen.
NOTE: To assure the highest degree of accuracy, Verathon® recommends that you
scan the patient’s bladder at least three times per exam, to ensure the repeatability of
your measurements.
Repeatability refers to your ability to center the bladder during each measurement,
not your ability to obtain exactly the same bladder volume measurement each
time. Volume measurements should be close, but need not be identical. If you
cannot obtain an optimal, repeatable measurement, the accuracy of the result is
compromised.
6. Verify the scan.
Flashing arrow: If the scan is “off-target,”
that is, if the bladder was not enclosed within
the scanning cone, the display will show a
ashing arrow. In this case, you must re-aim
the Probe in the direction indicated by the
arrow and perform the scan again.
User’s Manual
page 23
Page 24

Measuring Bladder Volume
Solid arrow: A solid arrow indicates that the
bladder was mostly inside the scanning cone.
The results are satisfactory but re-aiming in the
direction of the arrow and re-scanning would be
advisable to assure accuracy.
No arrow: Indicates that the bladder was
completely contained within the scanning
cone. The measurement is accurate.
BladderScan BVI 6000 Series Bladder Volume Instruments
7. Record a Voice Annotation (BVI 6400 only)
After performing an exam, you can record additional information about a patient to be
stored with the exam results.
The instrument can store up to ten scans with voice annotations, so you can perform
®
multiple exams on your rounds. If you are using the optional ScanPoint
software,
you can upload saved exams to your PC or electronic health record (EHR) system.
To record a voice annotation:
a. Press and hold the top (Gender) button until you hear a tone
(approximately 3 seconds).
b. To record your annotation, continue to hold the top button and
speak into the microphone (directly above the display) Hold the
microphone approximately 6 inches (15 cm) from your mouth
and speak clearly.
Be sure to include all relevant exam information, including the
patient’s name and the name of the person performing the
exam. You have 10 seconds to record information.
c. When you have nished recording, release the top button and
listen to your annotation play back. If desired, re-record your
annotation.
page 24
User’s Manual
Page 25

BladderScan BVI 6000 Series Bladder Volume Instruments
d. If you are satised with the annotation, do not press any
buttons. After several seconds, the BVI 6400 automatically
accepts and stores the annotation with the exam. The
microphone icon on the display ashes while the exam and
voice annotation are being saved. You may now begin a new
patient exam, if desired.
NOTE: The BVI 6400 will retain at least 10 voice-annotated
exams. When the device is full, the message FULL is
displayed on the LCD screen. To clear the memory, you may
transfer all the voice-annotated exams to ScanPoint®.
IMPORTANT!
If you do not record a voice annotation for a particular exam, that exam will
be lost and the next exam you perform will overwrite the non-annotated one.
If the instrument battery runs low or the instrument goes into sleep mode, any
non-annotated exam data is lost. However, the instrument does not erase
any annotated exam results when it goes into sleep mode. To make sure you
do not lose any patient data, it is a good idea to add a voice annotation to
every patient exam.
Measuring Bladder Volume
You can make a new recording only if the instrument still displays the bladder
volume for that particular exam.
8. Record, review, and print exam results.
If you are using the BVI 6100:
If desired, manually record the bladder volume measurement, because the
measurement will be erased when you begin a new exam.
Optionally, you may also choose to view, save, and print exam results using
ScanPoint Image Management Technology. Please consult your ScanPoint User’s
Manual for more information.
IMPORTANT: If you do not return the Probe to the Charging Cradle or Docking
Station within 20 minutes of completing the exam, the Probe will go into sleep mode
to conserve battery power and bladder imaging data will be erased. To avoid loss of
data, promptly download results to ScanPoint, or record a voice annotation (BVI 6400
only).
9. Finish the exam.
Once you have completed the scan, wipe the ultrasound gel off the patient and the
Probe.
User’s Manual
page 25
Page 26

Measuring Bladder Volume
Additional Scanning Tips
IMPORTANT: Hold the BladderScan® instrument steady while scanning.
Movement will result in an inaccurate reading.
Applying too much pressure when scanning will lead to a “greater than” symbol
(>) preceding the bladder volume measurement. Apply less pressure and rescan.
Volume reading will be affected by:
o The presence of scar tissue
o The presence of a catheter
o Overly obese patients:
BladderScan BVI 6000 Series Bladder Volume Instruments
With obese patients, lift as much abdominal adipose tissue out of
the way of the instrument as possible. Apply more pressure to the
BladderScan instrument to reduce the amount of adipose tissue through
which the ultrasound must pass.
To assure accurate results, make sure that:
o There are no air gaps between the Probe and
the patient’s skin.
o There are no air bubbles in the ultrasound gel.
o You are holding the instrument steady while
scanning (avoid changing its position, angle, or
pressure).
o You are using enough pressure to maintain good
skin contact until the scan is complete.
o There is not a catheter in the patient’s bladder.
The presence of a catheter may affect the accuracy of the bladder
volume measurement, but the measurement may still be clinically useful
(detecting a blocked catheter, for example).
Table 4 illustrates typical scanning scenarios and corresponding bladder volume
information that may appear on the Probe display.
page 26
User’s Manual
Page 27

BladderScan BVI 6000 Series Bladder Volume Instruments
Table 4. Typical Scanning Scenarios and Displays
Scanning Scenario Example Display Description
Optimal Scan In an optimal scan, the bladder is
Bladder volume is
greater than 999 ml.
Measuring Bladder Volume
entirely contained within the scanning
cone. The display shows:
Bladder volume.
No > symbol.
No ashing arrow.
No solid arrow.
The bladder is entirely contained within
the scanning cone but the bladder
volume is greater than 999 ml. In this
case, the display shows:
A bladder volume of >999 ml.
No ashing arrow.
Bladder is too
large to be fully
contained within
the scan cone.
Bladder not
centered
(Optional re-scan)
Bladder not
centered
Re-scan required.
No solid arrow.
Either the bladder is too large to be
contained by the ultrasound cone, or
the user is pressing too hard with the
Probe. The display shows:
Bladder volume with a > symbol.
No ashing arrow.
No solid arrow.
Apply less pressure and rescan.
The bladder is partially contained within
scan cone. Re-scanning is optional.
The display shows:
Bladder volume.
A solid arrow indicating re-aiming
direction for optional re-scan. Move
Probe in the direction of the arrow and
re-scan.
Bladder is only partially contained within
the scan cone. A re-scan is necessary
to assure accurate bladder volume
measurement. The display shows:
User’s Manual
Bladder volume.
A ashing arrow indicating re-aiming
direction. Move Probe in the direction of
the arrow and re-scan.
page 27
Page 28

Cleaning and Maintenance
BladderScan BVI 6000 Series Bladder Volume Instruments
Cleaning and Maintenance
Cleaning and Disinfecting the BVI 6100/BVI 6400
To Clean and Disinfect the Instrument:
1. Use a germicidal wipe or soft cloth dampened with isopropyl alcohol (or an
appropriate hospital cleaning agent) to wipe the Probe until it is thoroughly cleaned.
2. If you use a detergent solution to clean the instrument, remove all residual detergent.
Dry the instrument with a clean, soft cloth.
3. If the instrument needs to be disinfected, dampen a soft cloth in any glutaraldehydebased hospital disinfectant solution such as Cidex® or Cidex 7® from Advanced
Sterilization Products, or Sporocidin® from Sporocidin International. Wipe the
instrument with a dampened cloth.
4. To remove all traces of disinfectant solution, wipe the instrument with a clean soft
cloth dampened in sterile water or cleaning solution. Verathon® recommends wiping
the device three separate times to remove all residual disinfectant.
5. Thoroughly dry the instrument with a clean, soft cloth before using.
IMPORTANT! Failure to heed the following warnings may cause device damage not
covered by the BladderScan® warranty.
Do not immerse the instrument in disinfectant solution.
Do not use CidexPlus® to disinfect the instrument. CidexPlus® will damage the
plastic enclosure.
Do not subject any part of the instrument to steam sterilization or ethylene oxide
sterilization.
Regular Inspections and Maintenance
Once a week, you should inspect the instrument for physical faults or cracks. Cracks
that allow the ingress of uid may affect the performance of the instrument. Any apparent
cracks or faults in the instrument must be referred to your authorized BladderScan
Service Center, your local BladderScan distributor, your local Verathon Medical
representative, or the Verathon Medical Customer Care Department.
IMPORTANT! If you see any physical faults or cracks in the instrument, discontinue
use immediately and contact your local Verathon Medical representative or the
Verathon Medical Customer Care Department at 1.800.331.2313.
page 28
User’s Manual
Page 29

BladderScan BVI 6000 Series Bladder Volume Instruments
Calibrating the BladderScan Instrument
You must periodically calibrate your BladderScan® instrument to make sure that it
is providing accurate results. Calibrating ensures accurate and proper alignment of
the instrument’s internal coordinate system. If you do not perform the calibration by
the prescribed date, the instrument can still be used to take scans but measurement
accuracy may be compromised.
At a minimum, Verathon® requires that you calibrate the instrument every 6 - 12 months,
depending on your Total Reliability PlanSM. Calibrating your bladder volume instrument on
a regular basis ensures the accuracy of bladder imaging.
If you are using ScanPoint® and if your BladderScan instrument is within 20 days of
a required calibration, the Calibration Warning dialog box will appear when you start
ScanPoint. Please take prompt action to ensure that your device continues to function
properly and accurately.
If you are not using ScanPoint with QuickPrint, you must send you instrument in to
an authorized Verathon service center for calibration. Contact your Verathon Medical
Customer Care Center for more information. For contact information, please see
Contacting Verathon on page 36.
Cleaning and Maintenance
If you are using ScanPoint with QuickPrint, you can easily and quickly calibrate your own
instrument. Please consult your ScanPoint User’s Manual for complete instructions.
Displaying the Days Remaining Until Calibration
Your BladderScan instrument tracks the number of days remaining until calibration is
necessary. When zero days remain, the instrument is disabled, and you will have to
calibrate the instrument before using it again. You will know your instrument is disabled
when “000” and the “setting sun” symbol appear on the instrument display (Figure 3).
Figure 3. Calibration Required Display
To display the number of days remaining until calibration:
1. Remove the instrument from the Charging Cradle or Docking Station.
2. Press and hold down the top (Gender) button, located on the back of the Probe’s
handgrip, below the display for ve seconds. The number of days remaining until
the next required calibration will be displayed.
User’s Manual
page 29
Page 30

Cleaning and Maintenance
Device Repair and/or Replacement
The BladderScan
sealed, except for some units that feature a replaceable battery, described below.
Verathon
®
does not make available any type of circuit diagrams, component parts lists,
descriptions, or other information that would be required for repairing the device and
related accessories.
ScanPoint® Total ReliabilitySM Plan customers have access to loaner units while their
instruments are under repair and free shipping options which vary according to plan.
If you have any questions, contact your local Verathon Medical representative or the
Verathon Medical Customer Care Department at 1.800.331.2313.
®
instrument, Battery Charger, and Docking Station are completely
Battery Replacement
Some BladderScan BVI 6000 series instruments were built or refurbished to
accommodate a replaceable battery. These units can be identied by the battery
replacement door on the handle (Figure 4).
Figure 4. BVI 6000 series instrument with replaceable battery
BladderScan BVI 6000 Series Bladder Volume Instruments
page 30
If your BladderScan instrument battery no longer holds a charge, or if the BladderScan
instrument requires frequent charging, a battery replacement kit may be ordered.
BladderScan BVI 6000 Li-ion battery replacement kit: P/N 0270-0435
Instructions for replacing the batteries are included in the replacement kit.
To order a battery replacement kit or if you have any questions about battery
replacement, please contact the Verathon Medical Customer Care Department at
1.800.331.2313.
User’s Manual
Page 31

BladderScan BVI 6000 Series Bladder Volume Instruments
Unit Disposal
The BladderScan® instrument and related devices may contain lead, mineral oils,
batteries, and other environmentally hazardous materials. When the BladderScan
instrument has reached the end of its useful service life, return the device, Charging
Cradle, Docking Station, and related accessories to a Verathon Service Center for proper
disposal. Alternatively, follow your local protocols for hazardous waste disposal.
Cleaning and Maintenance
User’s Manual
page 31
Page 32

Troubleshooting
Troubleshooting
Help Resources
Verathon® provides an extensive array of customer service resources, described in the
table below.
You can obtain copies of this manual, Quick Reference Cards, and clinical studies by
visiting the Verathon Web site at verathon.com or by contacting your Verathon Medical
representative. A complete listing of contact information is provided on page 36.
Table 5. Troubleshooting Help Resources
Resource Description
BladderScan® Bladder Volume
Instruments In-Service CD
BladderScan Quick Reference
Cards
Clinical Studies Scientic papers on BladderScan use available on
ScanPoint
BladderScan BVI 6000 Series Bladder Volume Instruments
CD included with your device containing User’s
Manual and Quick Reference Cards.
Summary of procedures for using the BladderScan
devices.
the Verathon Web site (verathon.com).
®
Online ScanPoint Online provides customers:
The ability to calibrate and certify devices
online anytime you wish.
Automatic data backup and archiving
(HIPAA compliant).
Automatic software upgrades.
Access to real-time troubleshooting from
Verathon.
ScanPoint Total Reliability
Plan
SM
A Verathon device warranty plan that provides:
All repairs performed free of charge.
Instrument insurance: A warranty against
outdated technology with free upgrade /
replacement when your device is no longer
manufactured.
Free loaner program.
Free shipping.
Verathon Web site For additional information and in-service training
resources, visit bladderscan.com.
Phone support In North America, call: 1.800.331.2313.
International customers, please visit verathon.com
and select “Contact Us.”
page 32
User’s Manual
Page 33

BladderScan BVI 6000 Series Bladder Volume Instruments
Common Problems
If you are having problems operating your BladderScan® device, review this list of
common issues. If you do not nd a solution here, contact your authorized BladderScan
service center, your local BladderScan distributor or Verathon Medical . In North
America, call: 1.800.331.2313. International customers, please refer to “Contact Us”
at verathon.com.
Table 6. Common Problems and Solutions
Problem Meaning
The bladder
volume
instrument does
not turn on.
The bladder
volume
instrument is
charged but will
not scan.
This problem is usually caused by an unresponsive or
discharged battery. Charge the bladder volume instrument for
a minimum of six hours. If the scrolling battery icon does not
appear after two hours, you will need to activate the bladder
volume instrument by pressing the activation button with the
Activation Tool. Once activated, place the instrument in the
Charging Cradle to continue charging. For more information
about charging the battery and activating the instrument, see
Charging the BladderScan Instrument on page 19.
If the bladder volume instrument does not measure bladder
volume when you press the
the instrument display indicates that the battery has some power
remaining, one of the following conditions may apply:
Battery power is too low to perform bladder imaging.
In this case, the battery icon will show only one segment.
Place the instrument in the Charging Cradle to recharge
the battery. (see Charging the BladderScan Instrument
on page 19.)
Troubleshooting
ScAn button, but the battery icon on
User’s Manual
The bladder volume instrument requires calibration.
If the display shows “000” as the number of days
remaining until calibration, you must calibrate the
device before you can continue to perform bladder
volume measurements. Please refer to Calibrating
the BladderScan Instrument on page 29 for more
information.
page 33
Page 34

Troubleshooting
Problem Meaning
The bladder
volume
instrument beeps
The instrument may beep in the following situations. These
beeps indicate an alert or completion of a normal instrument
function.
You turn on the bladder volume instrument.
The device goes into sleep mode to conserve battery power.
The battery power is low, and the battery requires
recharging. In this case, the battery icon will show no power
segments. Place the instrument in the Charging Cradle or
Docking Station to recharge the battery.
You need to calibrate the instrument. Please refer to
Calibrating the BladderScan® Instrument on page 29 for
more information.
The instrument performs and completes a bladder volume or
calibration measurement.
The instrument has begun or nished transmitting data to
ScanPoint®.
BladderScan BVI 6000 Series Bladder Volume Instruments
Flashing aiming
arrows appear on
the LCD display
A solid arrow
appears on the
LCD display
A (>) symbol
appears
before the
bladder volume
measurement
The calibration procedure was successfully completed.
You select or deselect the female option.
If ashing aiming arrows appear on the instrument display after a
scan, the bladder was not fully within the image cone. Re-adjust
your aim in the direction indicated by the arrow and re-scan the
patient. Repeat this process until no ashing arrows appear.
When the instrument is aimed properly, either a solid arrow or
no arrow appears with the bladder volume measurement. For
more information about using the aiming arrows, see Measuring
Bladder Volume beginning on page 22.
A solid arrow indicates an aiming suggestion. The solid aiming
arrow appears on the instrument’s LCD display when the
bladder is not completely centered in the scanning cone. In this
case, the measurement is accurate and re-aiming is optional.
If a “greater than” symbol (>) appears before the bladder
volume measurement, then the bladder is too large to be fully
contained within the image cone. In such cases, the true volume
is greater than the measurement displayed and re-aiming the
instrument will not help. This situation occurs almost exclusively
in patients with extremely high bladder volumes. At these high
volumes, measurements are clinically useful even though they
underestimate the actual bladder volume.
page 34
User’s Manual
Page 35

BladderScan BVI 6000 Series Bladder Volume Instruments
Warranty
Verathon® Inc. warrants the BladderScan® instrument against defects in material and
workmanship as long as it is covered by the ScanPoint® Total ReliabilitySM Plan.
Damage or loss insurance is available as part of the ScanPoint Total Reliability Plan.
Pursuant to this Total Reliability Plan, a service center authorized by Verathon will repair
or replace units that prove to be defective during the Total Reliability Plan period.
This Total Reliability Plan does not apply if the unit was misused or modied by anyone
other than a service center authorized by Verathon.
The unit must be used in accordance with the instructions contained in this manual.
Consumable items are not covered under this warranty and should be used in
conformance with Verathon product specications.
For further details, consult your ScanPoint Total Reliability Plan. Total Reliability Plan
conditions may differ in some countries outside the United States. Contact your local
distributor for warranty terms.
Warranty
Disclaimer of Additional Warranties
There are no understandings, agreements, representations of warranties expressed or
implied (including warranties of merchantability or tness for a particular purpose) other
than those set forth in the preceding Warranty section. The contents of this manual do not
constitute a warranty.
Some states disallow certain limitations on applied warranties. The purchaser, user,
and patient should consult state law if there is a question regarding this disclaimer.
This information, descriptions, recommendations, and safety notations in this manual
are based upon Verathon experience and judgment with BladderScan instrument as of
October 2011. The contents of this manual should not be considered to be all-inclusive,
or to cover all contingencies.
The physician who directs the use of the BladderScan instrument at the institution where
it is in use is responsible for keeping current with clinical research in bladder volume
measurements.
Please direct any questions or problems concerning bladder volume measurement, using
the instrument, or the interpretation of data to the responsible physician.
User’s Manual
page 35
Page 36

Contacting Verathon
Contacting Verathon
The team at Verathon® is committed to modernizing healthcare delivery by putting
patients rst. Our products support healthcare professionals by providing accuracy, utility,
and excellence. For additional product and company information, visit the Verathon Web
site at verathon.com. If you have any questions or comments about Verathon products
and services, please contact us at:
Table 7. Contacting Verathon
BladderScan BVI 6000 Series Bladder Volume Instruments
Corporate Headquarters (USA)
Verathon Incorporated
20001 North Creek Parkway
Bothell, WA 98011
USA
Verathon Medical B. V. (Europe)
Linnaeusweg 11
3401 MS IJsselstein
The Netherlands
Toll free: 800.331.2313 (US & Canada Only)
Tel: 425.867.1348
Fax: 425.883.2896
Web: verathon.com
Email: customerservice@verathon.com
Tel: +31.30.68.70.570
Fax: +31.30.68.70.512
Web: verathon.eu
Email: customerserviceeu@verathon.nl
page 36
User’s Manual
Page 37

BladderScan BVI 6000 Series Bladder Volume Instruments
Safety and Performance Summary
The BladderScan® instrument computes the volume of the urinary bladder based
upon twelve cross-sectional ultrasound images. For maximum accuracy, be sure
to hold the Probe motionless while scanning.
The most accurate measurements are obtained when the patient rests quietly in
a supine position.
Errors in usage tend to result in the underestimation of bladder volume, except in
cases where the Probe is moved during scanning. In this case, the measurement
may overestimate the patient’s bladder volume.
We recommend that new users use the BladderScan instrument to measure
patients with moderately full bladders rather than initially attempting to locate a
bladder with a low urinary volume.
To conserve battery power, the BladderScan instrument shuts itself down
automatically when not in use (goes into “sleep” mode).
Safety and Performance Summary
WARNING! Risk of explosion.
There is a possible risk of explosion if the BladderScan instrument is used in the
presence of ammable anesthetics.
WARNING! Risk of patient injury.
Do not use the BladderScan instrument on patients with open skin or wounds in the
suprapubic region.
CAUTION:
Accuracy is compromised if the user does not obtain an optimal, repeatable
The BladderScan instrument is not intended for use on pregnant patients.
The patient being scanned should not have a catheter in his/her bladder. This
Use care when scanning suprapubic and pelvic surgery patients. Scar tissue,
CAUTION:
or incinerate battery. Keep battery out of reach of children and in original package until
ready to use. Dispose of used batteries promptly according to local recycling or waste
regulations.
Risk of inaccurate measurements.
image.
could create microbubbles in the bladder, which will compromise the accuracy of
the measurement.
surgical incisions, sutures, and staples can affect ultrasound transmission and
reection.
Risk of Fire and Burns. Do not disassemble, heat above 60° C (140° F),
User’s Manual
page 37
Page 38

Parts and Accessories
BladderScan BVI 6000 Series Bladder Volume Instruments
Parts and Accessories
BladderScan System Components (BVI 6100 and BVI 6400)
The following components are included with your BladderScan® BVI 6000 series device.
To order any of the following items, contact your authorized Verathon Medical sales
representative or contact the Verathon Medical Customer Care Department at
1.800.331.2313.
Table 8. Included Items
Part Number Description
0570-0154
One of the following:
BladderScan BVI 6100
0570-0167
BladderScan BVI 6400
0270-0234 Charging Cradle
0130-0181 Activation Tool
0900-1236 BladderScan Bladder Volume Instruments User’s Manual
0900-0813 BladderScan Bladder Volume Instruments In-Service Training CD
(with BladderScan Bladder Volume Instruments User’s Manual,
Quick Reference Cards, and training videos.)
0900-0589
0900-0956
Table 9. Optional Items
Part Number Name and Description
One of the following:
BladderScan BVI 6100 Quick Reference Card
BladderScan BVI 6400 Quick Reference Card
0900-1009 ScanPoint® LC Software Install CD. Installs ScanPoint Image
Management System on a stand-alone (non-networked) Windows®
PC.
0900-1238 ScanPoint with QuickPrint Install CD. Installs ScanPoint with
QuickPrint software on a network-enabled Windows
®
PC
0570-0168 ScanPoint Docking Station
0570-0178 ScanPoint Label Writer: Prints exam results on adhesive label
media. Requires installation of ScanPoint software on a Windows
PC.
0600-0233 USB Cable: Connects the ScanPoint Label Writer to the ScanPoint
host computer.
0600-0232 ScanPoint Label Writer Power Cord. Connects the ScanPoint Label
Writer power adaptor to a wall outlet.
0275-0002 Power Supply: Connects the power cord to the Label Writer.
®
page 38
User’s Manual
Page 39

BladderScan BVI 6000 Series Bladder Volume Instruments
Part Number Name and Description
0125-0446 Roll of Labels: Labels in roll format properly sized for the
ScanPoint® Label Writer.
0800-0255 Acoustic Coupling Gel, 0.25 liter (case of 12)
0270-0435 Battery Replacement Kit
Parts and Accessories
User’s Manual
page 39
Page 40

Product Specications
Product Specications
Symbol Directory
The following table explains the industry symbols used to indicate BladderScan®
instrument compliance with international and national standards and regulations.
Table 10. Symbol Directory
Symbol Meaning
BladderScan BVI 6000 Series Bladder Volume Instruments
Marked in accordance with Directive 2002/96/EC on Waste
Electrical and Electronic Equipment (WEEE) (solid bar indicates
product was put on the market after 13 August 2005.)
0400-0073
Class II equipment protected throughout by double insulation or
reinforced insulation.
Type BF applied part with EN/IEC-60601-1.
CE marked in accordance with the Medical Device Directive
(MDD).
Canadian Standards Association (CSA) mark of certication to
United States standards for electromedical equipment.
Attention. Consult accompanying documents.
This unit is powered by a Lithium-Ion battery pack. The
Verathon® part number is 0400-0073. The battery pack is NOT
user-replaceable.
page 40
User’s Manual
Page 41

BladderScan BVI 6000 Series Bladder Volume Instruments
Standards and Regulations Compliance
Verathon® certies that all units are in compliance with all applicable international and
national standards and regulations, including but not limited to the following:
Table 11. Standards and Regulations Compliance
Specication Standard
International Electrotechnical
Commission
Safety Standard EN/IEC 60601-2
Medical Device Directive MDD 93/42/EEC Annex 1
Canadian Standards Association C22.2 No. 601.1-M90 (Master Contract No.
Underwriters Laboratories, Inc. UL 60601-1
Health Insurance Portability and Accountability Act (HIPAA)
*For details on Verathon compliance with privacy rules, please refer to the information in
the ScanPoint
®
Help menu (select PRIVACY AGREEMENT).
Product Specications
EN/IEC 60601-1 Amendments 1 and 2 and
EN/IEC 60601-1-2 (EMC)
177198)
Per the MDD, BladderScan® instruments are Class IIa devices.
Electromagnetic Effects
There are no restrictions on the use of the BladderScan instrument due to its
electromagnetic characteristics. Both the emissions from BladderScan instrument
and the susceptibility of this instrument to interference from other sources are within
prescribed limits of all applicable standards at the date of manufacture. The emissions
test procedure that was used is specied in EN/ IEC55011: 1991 for Group 1, Class A
equipment (per EN/IEC60601-1-2, 36.201.1.7).
BladderScan instruments are suitable for use in industrial, scientic, and medical (ISM)
environments, and in domestic environments under the jurisdiction of a health care
professional. An indication of adverse electromagnetic effects from a BladderScan
instrument on another electronic device would be a degradation of performance in the
other device when the devices are operated simultaneously. If such interference is
suspected, separate the two devices as much as possible, or discontinue simultaneous
operation, if practical, and contact Verathon.
BladderScan instruments will operate normally in the proximity of other potential
interference sources, and have demonstrated immunity at a eld strength of 3 V/m (per
EN/IEC 60601-1-2, 36.202.2.1). You do not need to take any other precautions regarding
exposure in reasonably foreseeable environmental conditions to magnetic elds,
pressure, or variations in pressure, acceleration, or thermal ignition sources.
User’s Manual
page 41
Page 42

Product Specications
BladderScan Instrument Specications
Table 12. BladderScan® Instrument Specications
Specication Description
Range Bladder volume range 0 - 999 ml (BVI 6100, BVI 6400)
BladderScan BVI 6000 Series Bladder Volume Instruments
Accuracy The following accuracy specication assumes usage per
instructions, scanning a Verathon
®
Tissue Equivalent Phantom:
Bladder Volume: ± 15%, ± 15 ml
Scan Time Less than 5 seconds
Voice
10 seconds (BVI 6400 only)
Annotation
Interval
Weight Less than 11 oz (309 grams)
Power 3.8v Li Ion rechargeable battery
Display Liquid crystal
Ultrasound
Output
Parameters
Maximum SPTA* Intensity: 1.04 mW/cm
Maximum SPPA* Intensity: 65.0 mW/cm
Mechanical Index (MI): 0.925 maximum
2
2
Ultrasound Frequency: 3.7 MHz
Scan angle: 120 degrees
Mode: Vmode® (multiple, aligned B-mode images)
Operating
Temperature: + 50º - + 104º F (+ 10° C to + 40° C)
Conditions
Atmospheric
70 kPa - 106 kPa
Pressure
Range
page 42
Relative
30% - 75% non-condensing
Humidity
Water
Resistance
Rated at IPX1 (indicates DRIP-PROOF, a higher than ORDINARY
level of protection from drips, leaks, and spills)
*SPTA = Spatial temporal average; SPPA = Spatial peak pulse average
User’s Manual
Page 43

BladderScan BVI 6000 Series Bladder Volume Instruments
Storage Conditions
BladderScan® BVI 6000 series instruments are designed for storage under the following
conditions:
Table 13. BladderScan BVI 6000 Series Storage Conditions
Condition Description
Storage Indoor
Ambient
- 4 - + 140º F (- 20 - + 60º C)
Temperature Range
Atmospheric
500 hPa - 1060 hPa
Pressure Range
Relative Humidity 20% - 95% non-condensing
Charging Cradle Specications
Product Specications
The Charging Cradle is tested to EN/EN/IEC 60601-1 requirements and is in compliance
with UL and CSA equivalent standards. The Charging Cradle is not intended for direct
patient contact. It is designed to operate within the specications and environmental
conditions identied in the following tables.
Table 14. Charging Cradle Specications
Specication Description
Input Voltage 90-264 VAC RMS
Input Frequency 47-63 Hz
Input Current 2 Amp max
Input Connection Direct plug-in AC prongs for wall plug-in units
Output 9v at 1 Amp
Insulation Class II with double insulation
Table 15. Charging Cradle Storage Conditions
Condition Description
Use Indoor
Ambient Temperature
+ 41 - + 104º F (+ 5 - + 40º C)
Range
Atmospheric Pressure
70 kPa - 106 kPa
Range
Relative Humidity 30% - 75% non-condensing
Water Resistance Rated at IPX 0 (ordinary equipment without protection
against ingress of water)
User’s Manual
page 43
Page 44

Glossary
Glossary
BladderScan BVI 6000 Series Bladder Volume Instruments
Table 16. Glossary
Term Meaning
Activate The act of turning on your instrument by placing the activation
tool in the opening just above the scan button. When your
instrument has been completely discharged, sometimes
activation will be necessary.
Activation Tool The small, metal tool used to activate your instrument.
Aiming arrows Arrows that appear on the LCD screen to indicate the location of
the bladder relative to the Probe. A ashing arrow indicates that
you must re-aim the instrument and re-scan the patient to obtain
the highest degree of accuracy. A solid aiming arrow means reaiming is optional.
BladderScan
Instrument
The BladderScan
to produce an image of the patient’s bladder and determine
®
instrument transmits ultrasound energy
bladder volume.
Calibration Checking the accuracy and function of your BladderScan
instrument by comparing it with a known standard.
Charging Cradle The cradle that is used to charge the battery of the instrument.
This cradle plugs directly into an electrical wall outlet.
Docking Station The cradle that plugs into any personal computer through a
standard USB connection and enables the bladder volume
instrument to communicate with the computer. This cradle can
also be used to recharge the battery of the instrument when the
connected computer is turned on.
Coupling Medium A substance, such as ultrasound gel, that enhances the
transmission of ultrasound waves.
Flashing Aiming
Arrows
Indicate the bladder is not inside the scan cone and the Probe
needs to be redirected. The arrow shows the direction to move
or tilt the BladderScan instrument to improve the measurement.
Re-aiming is required.
HIPAA Health Insurance Portability and Accountability Act, enacted by
the US Congress in 1996.
page 44
Title II of HIPAA, the Administrative Simplication provisions
(AS), requires the establishment of national standards for
electronic health care transactions and national identiers
for providers, health insurance plans, and employers. The
AS provisions also address the security and privacy of health
data. The standards are meant to improve the efciency and
effectiveness of the nation’s health care system by encouraging
the widespread use of electronic data interchange in the U. S.
health care system.
User’s Manual
Page 45

BladderScan BVI 6000 Series Bladder Volume Instruments
Term Meaning
Image Cone During a bladder volume measurement, this is the cone-shaped
area in which the Probe transmits ultrasound waves.
IR Window The window located at the base of the handgrip which enables
the instrument to connect with your computer via the Docking
Station.
Labeling The square label located below the
that provides important product information, including the name,
product and serial numbers, and instrument classications.
LCD Screen The LCD (liquid crystal display) screen on the BladderScan
instrument that displays the bladder volume measurement,
instrument status, and other exam settings and information.
Neurogenic Caused or affected by the nervous system.
Repeatability Repeating a scan multiple times in order to assure more
accurate exam results.
Scan Button Located on the underside of the Probe hand grip, this button is
used to initiate the measurement.
ScanPoint
Bladder Imaging
Technology
®
Verathon®-provided software that allows users to display
ultrasound images from exams, prints exam results, and
maintain patient records.
ScanPoint LC Verathon-provided software that allows users to display
ultrasound images from exams, prints exam results, and
maintain patient records on a stand-alone (non-networked) PC.
ScanPoint Online Verathon-provided software that allows users to display
ultrasound images from exams, prints exam results, and
maintain patient records on a secure HIPAA, Verathonmaintained server. Patient exam results are accessible from any
Internet-enabled PC via a password-protected “myscanpoint”
account. The network connection also enables users to calibrate
their Verathon devices by themselves, without having to send
them in for servicing, allows automatic software updates, and
remote diagnostics by Verathon service technicians.
ScanPoint with
QuickPrint
ScanPoint with QuickPrint is a software program installed on
your computer that communicates with ScanPoint Online to help
you quickly and easily download and print exam reports.
Sleep Mode When the BladderScan shuts down to conserve energy. Press
any button to turn on the instrument.
Solid Aiming
Arrow
Indicates the bladder is inside the scan cone but is not
completely centered. The arrow shows the direction to move or
tilt the BladderScan to improve the measurement. Re-aiming is
optional but not required.
Supine Position Refers to the patient laying on their back with their face upward.
Suprapubic The area above the pubic bone.
Symphysis Pubis The front of the pelvis where the pubic bones meet.
Glossary
ScAn button on the Probe
®
User’s Manual
page 45
Page 46

Glossary
BladderScan BVI 6000 Series Bladder Volume Instruments
Term Meaning
Top (Gender)
Button
The button located below the Probe’s LCD screen. This button
allows the user to select various settings and perform different
functions, depending on the device model. For example, it is
used to select or deselect the female setting when measuring
bladder volume, to record a voice annotation with the BVI
6400, and to display the days remaining until the next required
calibration.
Ultrasonic Involving ultrasound. Ultrasound is sound at frequencies above
the range that can be heard by the human ear.
page 46
User’s Manual
 Loading...
Loading...