Page 1

Warranty FloPoint® Elite Uroflow System
Warranty
Warranty
Verathon Medical® warrants the FloPoint® Elite Uroflow System against defects in
material and workmanship as long as it is covered by the Premium Warranty Total
Customer Care
Damage or loss insurance is availability as part of the Total Reliability
this warranty, a service center authorized by Verathon
prove to be defective during the warranty period.
This warranty does not apply if the unit was misused or modified by anyone other than a
service center authorized by Verathon
The unit must be used in accordance with the instructions contained in this man ual.
Consumable items are not covered in this warranty and should be used in conformance
with Verathon
For further details, consult your Premium Warranty Total Customer Care
Warranty conditions may differ in some countries outside the United States. Contact your
local distributor for warranty terms.
Disclaimer of Additional Warranties
There are no understandings, agreements, representations of warranties expressed or
implied (including warranties of merchantability or fitness for a particular purpose) other
than those set forth in the preceding Warranty section. The contents of this manual do
not constitute a warranty.
SM
Plan. This warranty does not cover equipment sold as used.
®
product specifications.
SM
®
will repair or replace units that
®
.
Plan. Pursuant to
SM
Plan.
Some states disallow certain limitations on applied warranties. The purchaser, user, and
patient should consult state law if there is a question regarding this disclaimer. This
information, descriptions, recommendations, and safety notations in this manual are
based upon Verathon
®
experience and judgment with FloPoint® Elite as of July 2007. The
contents of this manual should not be considered to be all-inclusive, or to cover all
contingencies.
The physician who directs the use of the FloPoint
®
Elite Uroflow System at the institution
where it is in use is responsible for keeping current with clinical research in uroflowmetry.
Please direct any questions or problems concerning uroflowmetry, using the instrument,
or the interpretation of data to the responsible physician.
page 94 User’s Manual
Page 2

FloPoint® Elite Uroflow System Contacting Verathon® Medical
Contacting Verathon®
Contacting Verathon®
The team at Verathon® is committed to modernizing healthcare delivery by putting
patients first. Our products support healthcare professionals by providing the reliability,
utility, and excellence. For additional product and company information, visit the
Verathon
Verathon
Corporate Headquarters (USA)
Verathon Incorporated
20001 North Creek Parkway
Bothell, WA 98011
USA
Verathon Medical B. V. (Europe)
Boerhaaveweg 1
3401 MN IJsselstein
The Netherlands
Verathon Medical Sarl (France)
Office Address:
Espace Europeen de l'Entreprise
2 allée d'Oslo
67300 Schiltigheim
France
®
Web site at www.verathon.com. If you have any questions or comments about
®
products and services, please contact us at:
Toll free: 800.331.2313 (US & Canada Only)
Tel: 425.867.1348
Fax: 425.883.2896
Web: www.verathon.com
Email: customerservice@verathon.com
Tel: +31.30.68.70.570
Fax: +31.30.68.70.512
Web: http://www.verathon.eu/
Email: customerserviceeu@verathon.nl
Postal Address:
BP 10039
F-67012 Strasbourg Cedex
France
Tel: +33(0)3.88.60.14.02
Fax: +33(0)3.88.60.46.87
Email: info@verathon.fr
Verathon Medical Ltd.
(United Kingdom)
The Granary Manor Farm Courtyard
Aston Sandford, Aylesbury
Tel: +44.1844.299.207
Fax: +44.1844.299.218
Web: www.verathon.co.uk/
Email: customerserviceuk@verathon.co.uk
Buckinghamshire, HP17 8JB
United Kingdom
Verathon Medical K. K. (Japan)
Executive Tower Azabudai 7F
1-4-3 Azabudai
Tel: +81.03.3560.3501
Fax: +81.03.3560.3502
Email: servicejp@verathon.com
Minato-ku
Tokyo, Japan 106-0041
User’s Manual page 95
Page 3

Clinical Application FloPoint® Elite Uroflow System
Clinical Application
Clinical Application
Definitions, Indications, and Output
Definitions
Uroflowmetry is a diagnostic test in which the patient urinates into a flow sensor
connected to a recording device that produces a plot of urine flow rate versus time (the
“uroflow curve”). Most Uroflow Systems measure urine flow rate by continuously weighing
the urine as it fills a catch vessel and calculating the rate of increase in urine weight. By
contrast, the FloPoint
When a flow of urine hits the disk, the motor must work harder to maintain the disk
spinning at that same speed with the added weight of the urine. The FloSensor measures
the amount of power needed to maintain the disk’s original speed and uses that
measurement to calculate the urine flow rate. The FloSensor also records the total
amount of urine discharged by the patient.
Indications
In general, uroflowmetry is used as a screening test to determine which patients with
symptoms or clinical conditions involving the lower urinary tract should be referred for
further workup (e.g., urodynamics, cystography). The most common clinical application of
uroflowmetry is to provide an objective indication of a low urine flow rate caused by
bladder outflow obstruction – especially in males with symptoms suggestive of benign
prostatic hypertrophy. Uroflowmetry is also often included in the workup of females with
urinary incontinence.
®
Elite FloSensor contains a disk that spins at a constant speed.
Clinical Condition or History Suggestive Of:
High bladder outlet resistance. Examples include prostatism (the most
common indication), lower urinary tract infection and previous lower urinary
tract surgery.
A decompensated bladder or weak detrusor contraction.
Neurologic impairment of voiding. Examples include multiple sclerosis and
spinal cord injury.
Specific Signs and Symptoms:
Prolonged or interrupted voiding.
High residual urine.
Manually assisted voiding (e.g., by applying pressure to the lower abdomen).
Excessive straining required to void.
FloPoint
The FloPoint
seconds. Several measurements are taken from the FloPoint
®
Elite Output
®
Elite Uroflow System plots urine flow rate in ml/second versus time in
®
Elite curve, either
manually by the clinician, or automatically by a digital processor.
page 96 User’s Manual
Page 4

FloPoint® Elite Uroflow System Clinical Application
FloPoint® Interpretation
Limitations and Specific Clinical Applications
A reduced urine flow rate can be caused by either a bladder outlet obstruction or by
detrusor hypocontractility. In turn, bladder outlet obstruction can be due either to an
anatomical abnormality (e.g., prostatic hypertrophy) or a neurological abnormality (e.g.,
detrusor-sphincter dyssynergia caused by multiple sclerosis). Therefore, by itself,
uroflowmetry cannot determine the cause of a reduced flow rate.
However, many clinicians with uroflowmetry experience contend that uroflowmetry can
provide useful clinical information by itself, particularly in men. For example, Boone and
2
Kim
state that if a patient complains of a reduced flow of urine, but there are no other
signs or symptoms of voiding dysfunction and uroflowmetry is normal, it is unlikely that
further urodynamic testing will reveal an abnormality. Chapple and MacDiarmid
“Simple uroflowmetry by itself is adequate investigation for uncomplicated prostatemediated bladder outflow obstruction in over 60% of patients. McLoughlin, et al
that an abnormally reduced maximum urine flow rate is a reliable indicator of obstruction
in over 90 percent of men with prostatic symptoms. Also, Abrams
Ostergard
7
state that in female patients with urinary incontinence an abnormally high flow
1
and Sand and
rate and short duration void sometimes provides a useful suggestion of detrusor
instability and/or abnormally reduced outlet resistance.
3
state:
5
found
Quantitative Measurements
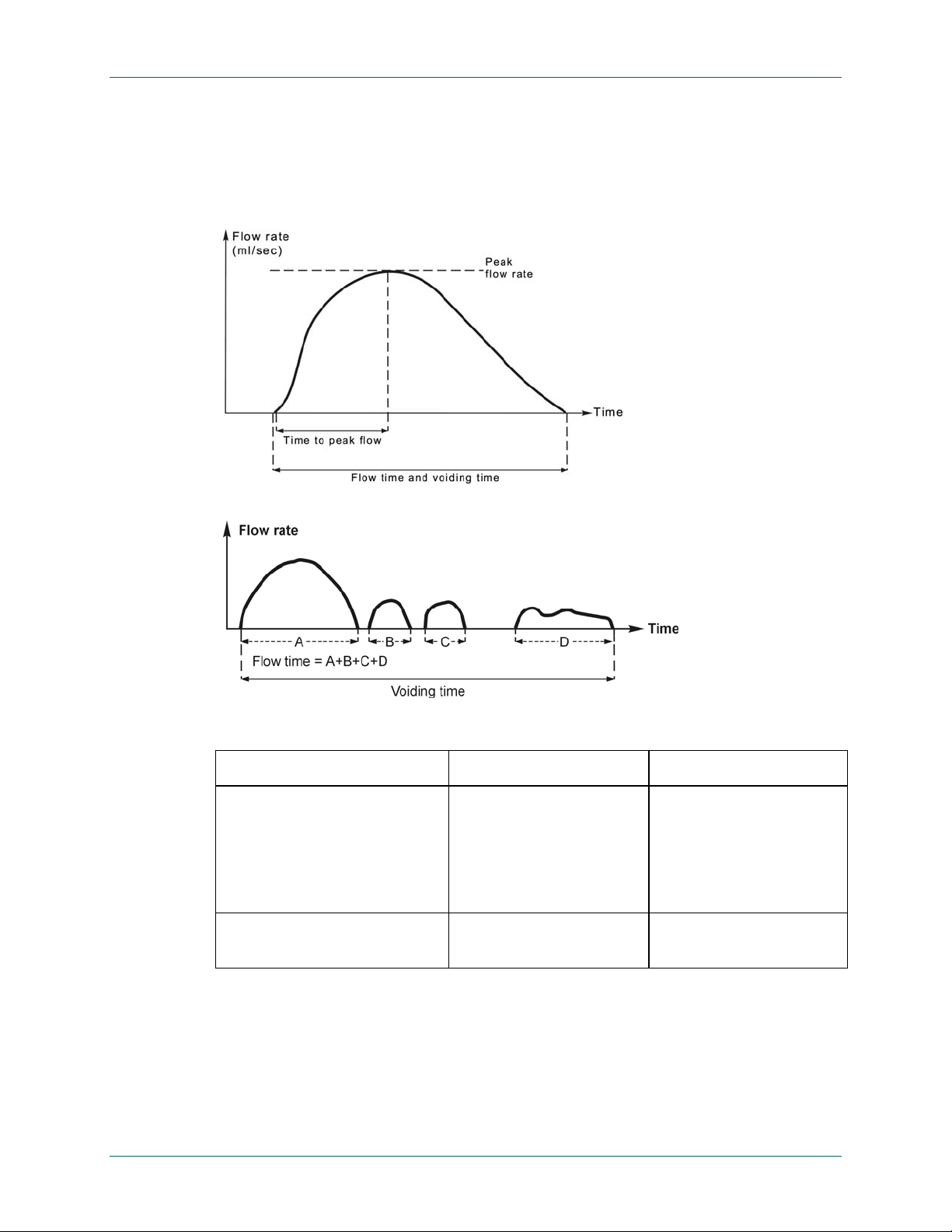
Figure 33 diagrams the measurements taken from the FloPoint
®
Elite curve. Table 1
summarizes the definitions and normal results of these measurements.
Flow Rates
Both maximum and average urine flow rates are strongly dependent on voided volume
and the patient's age and sex.
NOTE: In subsequent FloPoint Elite Report Charts (Figure 33 through Figure 42 ), flow
rate is represented by the letter Q.
User’s Manual page 97
Page 5
Clinical Application FloPoint® Elite Uroflow System
Figure 33. Measurements Taken From the FloPoint® Elite Curve
A. Measurements of the continuous uroflow curve. Average flow rate (not shown) is calculated b y
divided total volume voided by flow time. B. Measurements of flow time and voiding time from an
intermittent uroflow curve.
A.
B.
Table 1. Measurements from the FloPoint® Elite Curve (see Figure 33)
MEASUREMENTS DEFINITION NORMAL RESULTS
Peak Flow Rate Maximum measured flow
rate excluding dribbleproduced or other spikes.
Depends on age, sex and
voided volume. Rarely
exceeds 40 ml/sec.
Clinical significance is
questionable with
irregular flow patterns.
Average Flow Rate Voided volume divided by
flow time.
Typically is about half of
Peak Flow.
page 98 User’s Manual
Page 6
FloPoint® Elite Uroflow System Clinical Application
MEASUREMENTS DEFINITION NORMAL RESULTS
Voiding Time Total duration of the
micturition, including
interruptions.
Averages about 10
seconds with 100 ml
voided volume and 25
seconds with 400 ml
voided volume. No
“normal limits” defined.
Flow Time Total duration of
measurable flow.
Equal or nearly equal to
voiding time. A flow time
that is significantly shorter
than the voiding time
indicates an abnormal
intermittent FloPoint
®
Elite
pattern.
Time to Peak Flow Time from flow onset to
peak flow.
About 30 percent of
voiding time. Has no
clinical significance with
irregular flow pattern.
Measurements Related to FloPoint
®
Elite Curve Patterns
Time to peak flow rate, flow time and voiding time do not provide the same quantitative
“normal” versus “abnormal” results as do flow rates. However, as discussed in the next
section, these measurements can provide useful indications of the FloPoint
®
Elite curve’s
shape.
Continuous/Regular Patterns:
Normal: A normal FloPoint® Elite pattern is a smooth unbroken, bell-shaped
curve with peak flow occurring relatively early. (Time to peak flow averages
about 30% of total flow time.) Figure 34 shows an example of a normal
uroflow curve.
User’s Manual page 99
Page 7
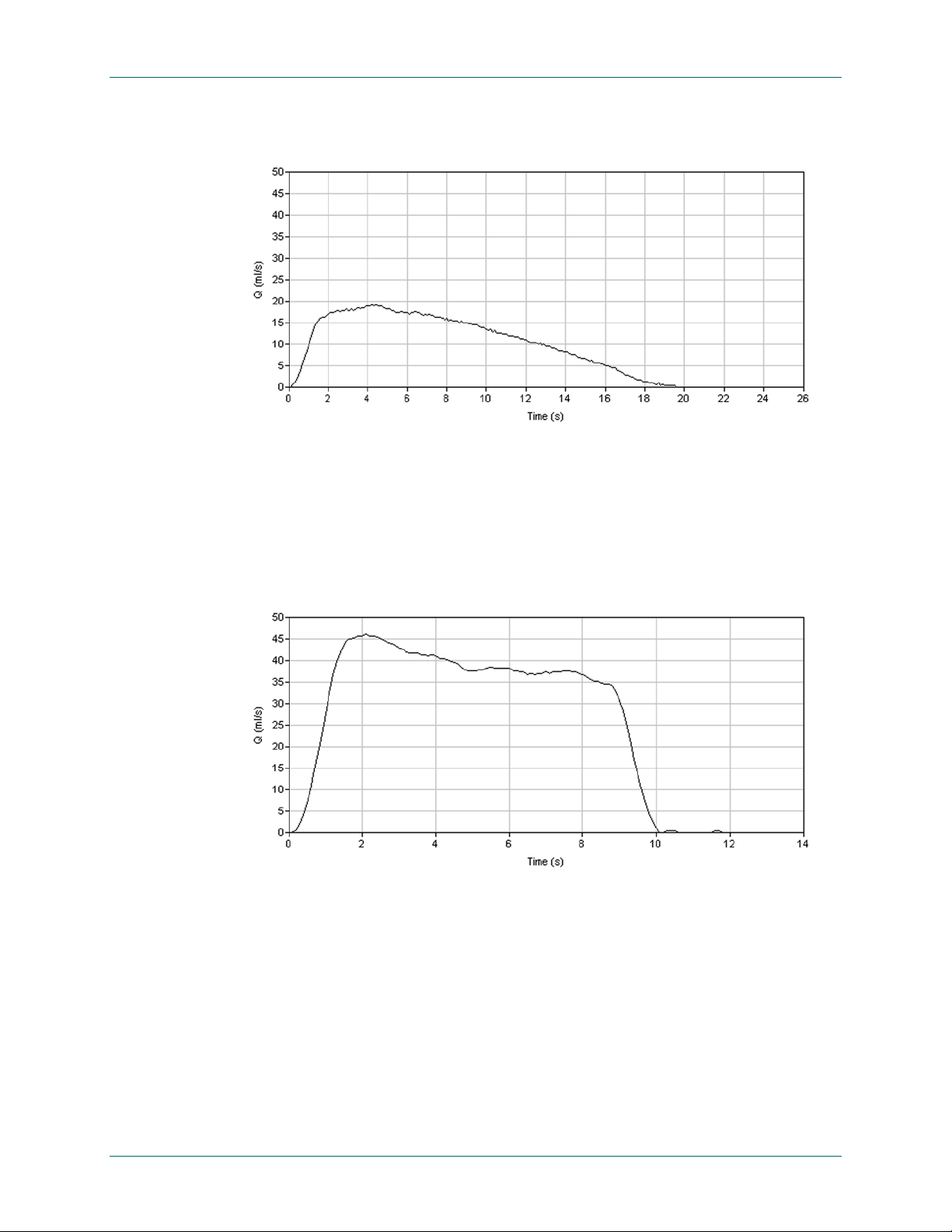
Clinical Application FloPoint® Elite Uroflow System
Figure 34. Normal Uroflow Curve
♦
Superflow: Figure 35 shows an example of a “superflow” pattern. This
pattern is characterized by a very high flow rate - usually greater 40 ml/sec. and a very short flow time. A superflow pattern is usually seen in females. It
suggests decreased outlet resistance and/or detrusor instability, hence may
be associated with urinary incontinence.
2, 6
Figure 35. Superflow Pattern
Obstructive:
Patterns suggestive of bladder outlet obstruction (BOO) are characterized by
a prolonged flow time during which a large part of the total voided volume is
voided at a constant low flow rate. Figure 36 and Figure 37 show examples
of “obstructive” flow patterns.
page 100 User’s Manual
Page 8
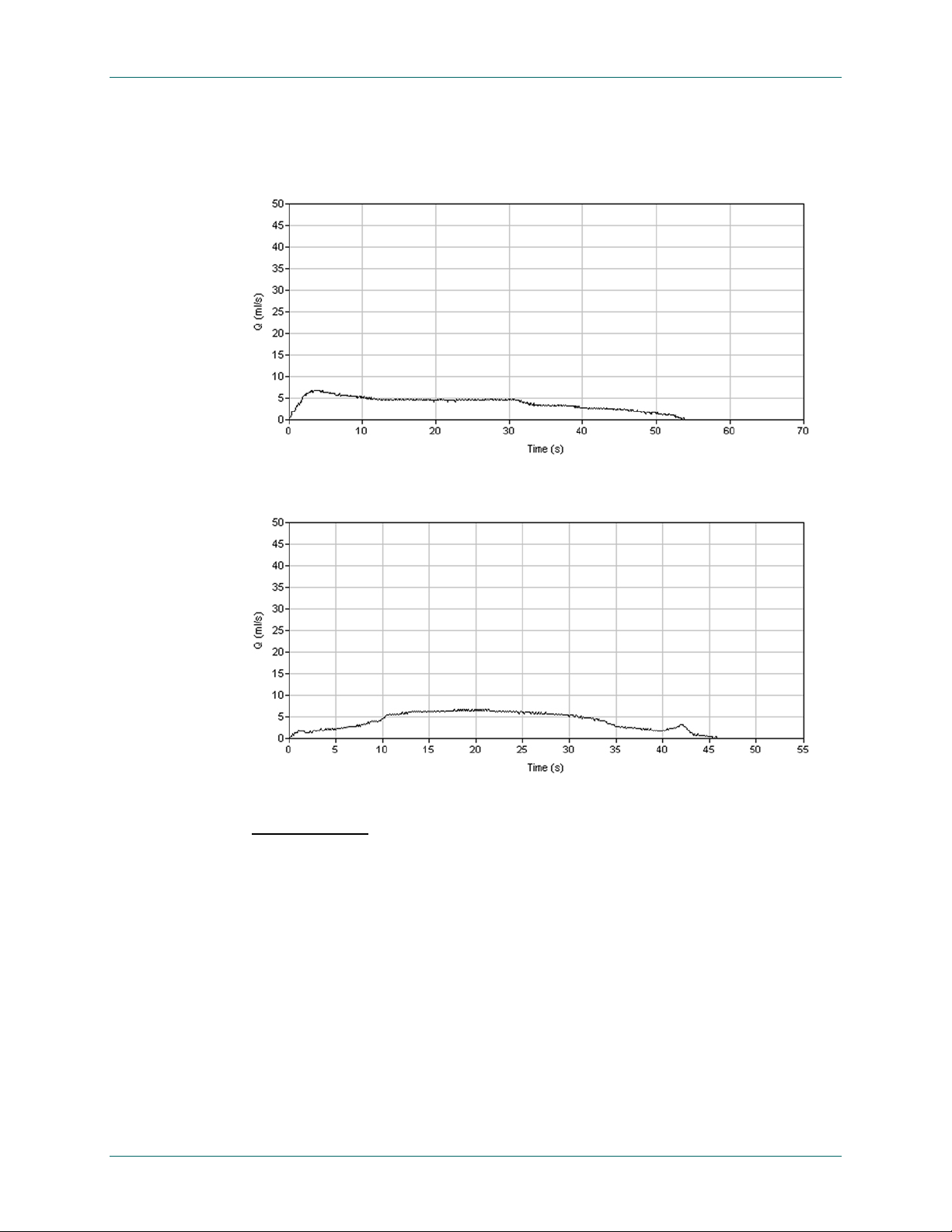
FloPoint® Elite Uroflow System Clinical Application
Figure 36. "Flat Top" Obstructive Flow Pattern
The peak on the spike at the end of the trace might have been misinterpreted by the
uroflowmetry as the peak flow.
Figure 37. Rounded Top Obstructive Flow Pattern
Irregular Patterns
With the exception of an occasional terminal spurt (e.g., Figure 36) or one or two
secondary voids in a normal man, irregular flow patterns are always either abnormal
or artifactual. There are two types of irregular flow patterns: a “fluctuating” flow
pattern, in which the repeated downward deflections do not fall below a measurable
flow rate, and an “intermittent” flow pattern characterized by the occurrence of
interruptions of varying durations between voiding episodes. Figure 38 shows an
example of a fluctuating flow pattern caused by abdominal straining: Figure 39 shows
an example of an intermittent flow pattern caused by detrusor-sphincter dyssynergia.
User’s Manual page 101
Page 9
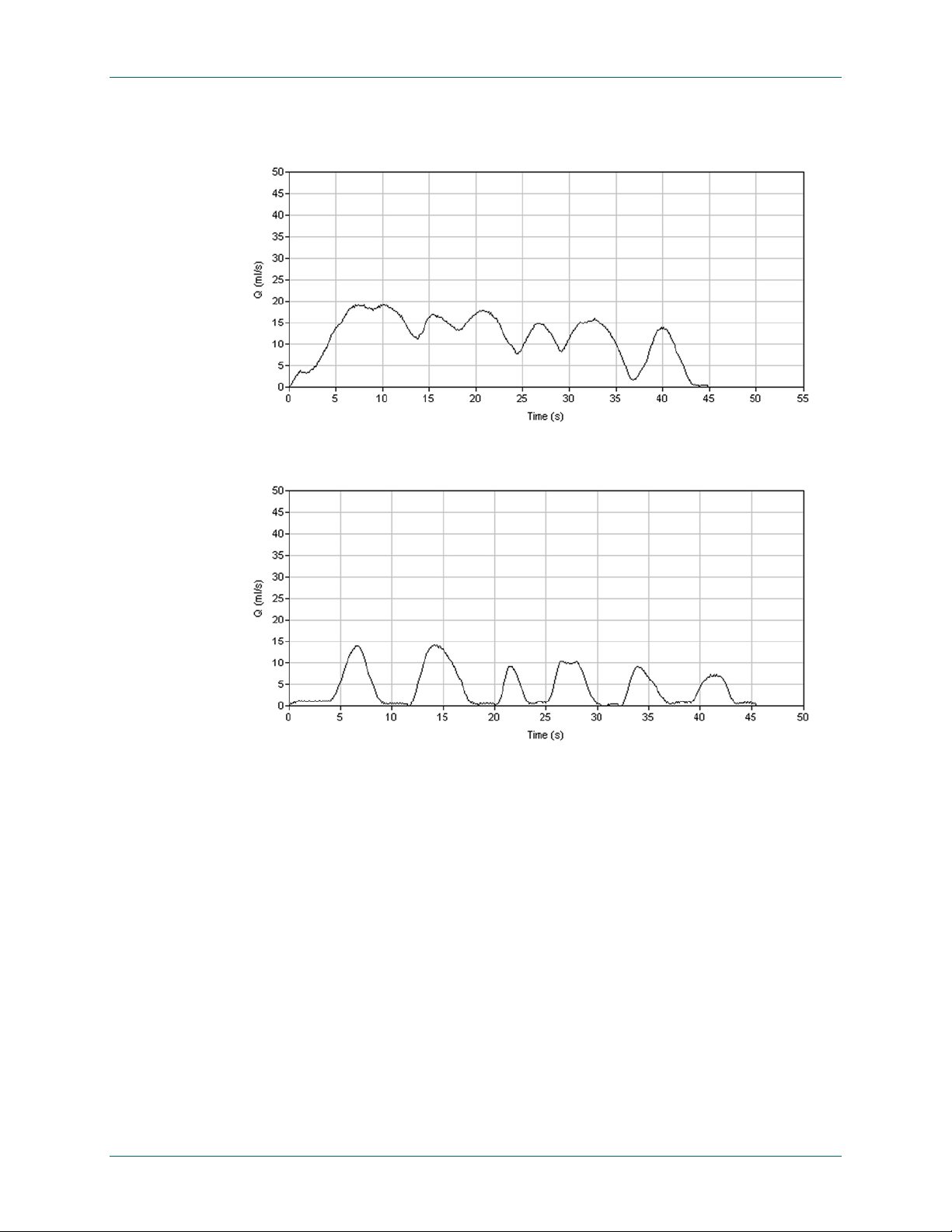
Clinical Application FloPoint® Elite Uroflow System
Figure 38. Fluctuating Pattern Caused by Abdominal Straining
Figure 39. Abnormal Intermittent Pattern, Caused By Detrusor Sphincter Dyssynergia
Clinical Significance
Whether an irregular uroflow pattern or urethral sphincter relaxation are lost cau sing the
detrusor to push intermittently against a contracted urethral sphincter, irregular traces can
also be caused by fluctuating or poorly sustained detrusor contractions, which are
generally seen in patients with a neurological abnormality – most commonly multiple
sclerosis.
1
Artifactual Causes
As discussed previously, irregular traces can be caused by a male patient moving his
stream across to the collecting funnel (“cruising”) or intermittently squeezing the tip of the
penis or foreskin during voiding.
1
Figure 40 and Figure 41 show examples of these two
types of artifactual irregular patterns. Anxiety, as might be caused by the unfamiliar
laboratory environment, can also cause an irregular trace in normals.
page 102 User’s Manual
Page 10
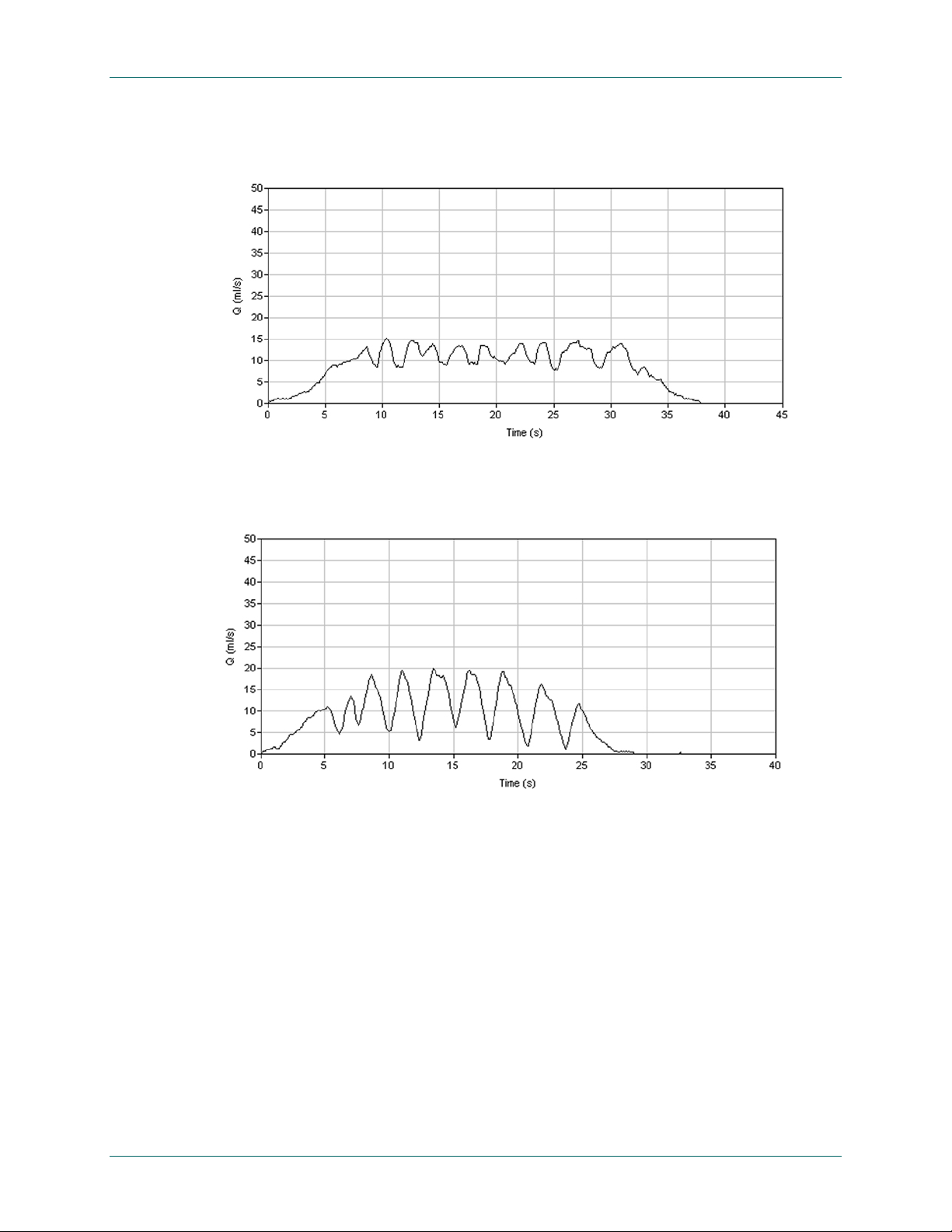
FloPoint® Elite Uroflow System Clinical Application
Figure 40. Artificial Fluctuating Pattern
The patient creates this pattern by repeatedly moving his stream across the commode outlet.
Figure 41. Artificial "Squeezing" Pattern
The patient creates this pattern by repeatedly obstructing urine flow by squeezing his foreskin or
penis.
Using Flow Time and Voiding Time to Help Identify the Curve Pattern
A long time to peak and a long flow time can help confirm a subjective impression of an
“obstructive” flow pattern.
Comparing flow time with total voiding time provides a quantitative indication of an
interrupted flow pattern’s severity – the greater the difference between voiding and flow
times, the more severe the interruption.
Summary of Diagnostic Significance
Table 2 summarizes the diagnostic significance of abnormal FloPoint
®
Elite test results.
User’s Manual page 103
Page 11

Clinical Application FloPoint® Elite Uroflow System
Table 2. Diagnostic Significance of FloPoint® Elite Results
CURVE SHAPE
Pattern Shape
Continuous Flat
(“obstructive”
pattern)
Continuous Rounded
(“obstructive”
pattern)
Continuous High Peak
short
duration and
(“superflow”
pattern)
Irregular** Fluctuating Variable No significance No
PEAK
FLOW
Low Shortened
Low Variable Normal Detrusor under-
High Shortened Shortened Detrusor
TIME TO
MAXIMUM
(Peak flow may
be difficult to
identify.)
VOID TIME CONCLUSION
Prolonged Outlet
significance
obstruction*
activity
instability and/or
normal or
reduced output
resistance
Voluntary
abdominal
straining.
Detrusorsphincter
dyssynergia.
Dysfunctional
voiding
Irregular Interrupted Variable No significance No
significance
* In female, rule out stress urinary incontinence and lower urinary tract infection.
** In male, rule out ”cruising”, (variable stream direction) and intermittent squeezing of penis or foreskin.
Same as
fluctuating
pattern.
Possibly a more
severe
condition.
Artifacts and Pitfalls
Following are some events and conditions that can lead to misinterpretation of FloPoint
Elite test results.
“Spikes” or Rapid Fluctuations in the Curve
There are several potential causes of spikes in the FloPoint® Elite curve that can lead
to misinterpretation. The most common of these are: (1) mechanical disturbance of
the flow sensor and, (2) straining either during (Figure 42) or at the end (Figure 38) of
the void.
The most significant misinterpretation that can be caused by spikes is mistaken
identification by an automatic processor of a spike peak as the void maximum.
1,2,4,6
®
page 104 User’s Manual
Page 12

FloPoint® Elite Uroflow System Clinical Application
Figure 42 shows examples of such a computer misinterpretation (solid arrow). In
situations such as this, the clinician must override the computer’s interpretation and
read the maximum manually from a smooth portion of the uroflow trace (open arrow
in Figure 42).
Figure 42. Artifactual uroflow peak produced by a spike
The (black arrow) identifies an artifactual spike that the uroflowmetry reads as the flow peak.
The correct peak flow (open arrow) must be read manually by the interpreter.
Low and High Voided Volumes
When total voided volumes are below 150 ml, “normal” flow rates become so low as
to make the test an insensitive indicator of an abnormally low flow rate. At voided
volumes of over 600 ml, the bladder may become decompensated, thereby
producing an artifactually low flow rate.
1
Therefore, it is important to avoid voided
volumes below 150ml and above 600ml. If such volumes are encountered, the test
should be repeated.
User’s Manual page 105
Page 13

Clinical Application FloPoint® Elite Uroflow System
Test Technique
Incorporating the following into the FloPoint® Elite test procedure will materially enhance
both the efficiency with which the test is done and the clinical utility of the test’s results:
1. The void should be performed with a comfortably full bladder. To accomplish this, the
patient should be instructed to drink approximately 2 pints of fluid two hours before
appearing for his or her appointment and to not void prior to arriving at the clinic.
Alternatively, the patient might be questioned about the fullness of his bladder upon
arriving for his appointment and instructed to drink water immediately if the bladder is
empty. As noted above, voids of more than 600 ml or less than 150 ml should be
repeated as the patient’s bladder has refilled.
2. Instruct the patient to void in a position that he or she is most used to (usually
standing for a male, sitting for a female).
3. A male should be instructed to direct the stream to the location marked on the funnel
and to maintain constant stream direction throughout the void.
Reimbursement Information
Physician Participation Requirement
During the performance of FloPoint
®
Elite uroflowmetry, “direct physician supervision” is
required. This means that the physician must be present in the office suite (not
necessarily in the room when the procedure is performed), and immediately available to
furnish assistance and direction throughout the performance of the procedure.
Procedure Codes
There are only two procedure codes to use for the performance of FloPoint
®
Elite
uroflowmetry:
51736: Simple uroflowmetry (uroflowmetry performed by the use of stop-watch flow rate
or a mechanical Uroflow System).
51741: Complex uroflowmetry (uroflowmetry performed using calibrated electronic
equipment).
Diagnosis Codes
Table 3 lists diagnosis codes that can be entered into a claim for FloPoint
®
Elite
uroflowmetry reimbursement. Diagnosis codes for uroflowmetry that are acceptable to
regional reimbursement bodies differ by region. Therefore, specific regions may not
authorize uroflowmetry reimbursement for all listed diagnosis.
Table 3. Diagnosis Codes Used with FloPoint® Elite
DIAGNOSIS CODE
Equina syndrome with neurogenic bladder 344.61
Atony of Bladder 596.4
page 106 User’s Manual
Page 14

FloPoint® Elite Uroflow System Clinical Application
DIAGNOSIS CODE
Bladder Neck Obstruction (acquired) 596.0
Hypertonicity of bladder 596.51
Low bladder compliance 596.52
Paralysis of bladder 596.53
Neurogenic bladder NOS 596.54
Detrusor sphincter dyssynergia 596.55
Other functional bladder disorder 596.59
Urethral stricture due to unspecified infection 598.00
Traumatic urethral stricture 598.1
Postoperative urethral stricture 598.2
Other specified causes of urethral stricture 598.8
Urethral stricture, unspecified 598.9
Hypertrophy of prostate (BPH) 600.0
Retention of urine, unspecified 788.20
Incomplete bladder emptying 788.21
Other unspecified retention of urine 788.29
Urinary incontinence, unspecified 788.30
Urge incontinence 788.31
Stress incontinence, male 788.32
Mixed incontinence 788.33
Incontinence w/o sensory awareness 788.34
Post-void dribbling 788.35
Nocturnal enuresis 788.36
Continuous leakage 788.37
Other urinary incontinence 788.39
Urinary frequency (micturition) 788.41
Polyuria 788.42
Nocturia 788.43
Splitting of urinary stream (intermittent) 788.61
Slowing of urinary stream (weak) 788.62
User’s Manual page 107
Page 15

Clinical Application FloPoint® Elite Uroflow System
REFERENCES
1. Abrams, P., Uroflowmetry, Urodynamics
, 22nd ed. London, Springer-Verlag, 1997,
pages 20-39.
2. Boone T.B. and Y. H. Kim, Chapter 4, Uroflowmetry, in Nitti, V. D. (ed), Practical
Urodynamics, Philadelphia, W. B. Saunders, 1998, pages 28-37.
3. Chapple, C.A. and S. A. MacDiarmid, Urodynamics Made Easy
, Chapter 3
Urodynamic Techniques: Flow Rate, New York, W.B. Saunders, 2000, pages 26-32.
4. Grino, P., B., et al, Maximum urinary flow rate by uroflowmetry: automatic or visual
interpretation, J. Urol
, 149, 339-341, 1993.
5. McLoughlin, J., et al, Symptoms versus flow rates versus urodynamics in the
selection of patients for prostatectomy, Brit. J. Urol.
66, 303-305, 1990.
6. Rivas, D. A. and M. B. Chancellor, Chapter, 5, Uroflowmetry, in Blaivis, J., and M.
Chancellor, (eds.), Atlas of Urodynamics
, Baltimore, Williams & Wilkins, 1996.
7. Sand, P. K. and D. R. Ostergard, Urodynamics and the Evaluation of Female
Incontinence, A Practical Guide, Chapter 3, Uroflowmetry in the Female, London,
Springer-Verlag, 1995.
page 108 User’s Manual
Page 16

FloPoint® Elite Uroflow System Parts and Accessories
Parts and Accessories
Parts and Accessories
FloPoint® Elite Uroflow System Components
The following components are included with your FloPoint® Elite Uroflow System with
ScanPoint
Part and Part Number Name and Description
0570-0175 - FloSensor
®
with QuickPrint system.
FloSensor
Measures urine volume and flow rate. The Handles
suspend the FloSensor inside the toilet.
FloCharger with Clamshell:
Stores the FloSensor, recharges the batteries, and resets
the measuring device to zero.
0570-0176 - FloCharger
0800-0337
FloPoint
®
Elite Mounting Bracket:
Attaches to the wall, securely holding the FloCharger and a
funnel box 3 feet (0.914 m) or more above the floor.
User’s Manual page 109
Page 17

Parts and Accessories FloPoint® Elite Uroflow System
Part and Part Number Name and Description
®
ScanPoint
Remote:
Acts as a remote control to start and stop the FloSensor,
displays information on flow measurements and device
status, and records voice annotation.
0570-0174
®
ScanPoint
Transmits data from the ScanPoint
ScanPoint
Docking Station:
®
host computer and recharges the Remote
®
Remote to the
batteries.
0570-0168
To order any of the above parts, contact your authorized Verathon Medical® Sales
Representative or contact the Verathon Medical
1.800.331.2313.
FloPoint® Elite Uroflow System Accessories
The following accessories are included with your FloPoint® Elite Uroflow System with
ScanPoint
Part and Part Number Name and Description
0900-1238
®
with QuickPrint system.
ScanPoint
Produces reports based on the data gathered during an
exam and submitted by the ScanPoint
FloPoint
Includes FloPoint
Reference Cards.
®
Elite In-Service CD
®
Customer Care Department at
®
with QuickPrint Installation CD:
®
Remote.
®
Elite User’s Manual and Quick
0900-1445
page 110 User’s Manual
Page 18

FloPoint® Elite Uroflow System Parts and Accessories
Part and Part Number Name and Description
Box of Paper Funnels:
Help direct the urine flow toward the FloSensor.
Box contains 40 funnels.
0800-0297
0130-0181
0264-0008
0900-1443
Activation Tool:
Use to press the Reset button on the ScanPoint
®
Remote if
the Remote needs to be reactivated.
Lanyard:
Attaches to the ScanPoint
®
Remote, if desired, to assist
with placement of the Remote in proximity to the FloSensor
®
FloPoint
Elite Setup and Use Quick Reference Card:
Provides a summary of essential operator instructions.
FloPoint
Provides instructions for the FloPoint
®
Elite Calibration Quick Reference:
®
calibration
procedure.
0900-1444
User’s Manual page 111
Page 19

Parts and Accessories FloPoint® Elite Uroflow System
Part and Part Number Name and Description
®
FloPoint
Elite Mounting Bracket Template
Aids in attaching the Mounting Bracket to the wall.
0900-1557
To order any of the above parts, contact your authorized Verathon Medical® Sales
Representative or contact the Verathon Medical
1.800.331.2313.
Other FloPoint® Elite Uroflow System Accessories
The following accessories are not included with your FloPoint® Elite Uroflow System with
ScanPoint
Part and Part Number Name and Description
0800-0331
ScanPoint® Label Writer and Related Components
®
with QuickPrint system.
Calibration Fluid Pouches:
Pouch containing unique fluid for use in calibrating the
FloPoint
with the Calibration Kit.
ScanPoint
Prints exam results.
®
Elite. The Calibration Fluid Pouches are shipped
®
Label Writer (optional):
®
Customer Care Department at
0570-0178
page 112 User’s Manual
Page 20

FloPoint® Elite Uroflow System Parts and Accessories
Part and Part Number Name and Description
0600-0233
0275-0002
0125-0446
USB Cable:
Connects the ScanPoint
®
Label Writer to the ScanPoint®
host computer.
Switching Power Adapter:
Connects the Label Writer to the wall outlet.
Roll of Labels:
Labels in roll format properly sized for the ScanPoint
®
Label
Writer.
Power Cord:
Connects the FloCharger to the wall outlet to charge the
FloSensor battery.
0600-0232
To order any of the above parts, contact your authorized Verathon Medical® Sales
Representative or contact the Verathon Medical
®
Customer Care Department at
1.800.331.2313.
User’s Manual page 113
Page 21

Specifications FloPoint® Elite Uroflow System
Specifications
Specifications
Symbol Directory
The following table illustrates and explains the symbols that may appear on the FloPoint®
Elite Uroflow System components and/or packaging. These symbols indicate the
FloPoint
regulations.
Symbol Meaning
®
Elite system compliance with international and national standards and
Underwriters Laboratories mark of certification of the FloPoint® Elite
uroflow system with respect to electrical shock, fire and mechanical
hazards only in accordance with UL 60601-1 and CAN/CSA 22.1
No. 601.1
IEC 348 symbol indicating "Attention, consult accompanying
documentation.”
Marked in accordance with Directive 2002/96/EC on Waste
Electrical and Electronic Equipment (WEEE) (solid bar indicates
product was put on the market after 13 August 2005.
Protection Class II equipment, internally powered equipment.
Type BF applied part with EN/IEC-60601-1.
CE marked in accordance with the Medical Device Directive (MDD).
Canadian Standards Association (CSA) mark of certification to
United States standards for electromedical equipment.
Standards and Regulations Compliance
Verathon® certifies that all units are in compliance with all applicable international and
national standards and regulations, including but not limited to the following:
page 114 User’s Manual
Page 22

FloPoint® Elite Uroflow System Specifications
Specification Standard
Electromagnetic compatibility standards IEC 60601-2, ICES-001
Safety Standard IEC 60601-1
Health Insurance Portability and Accountability Act (HIPAA)*
*For details on Verathon® compliance with privacy rules, please refer to the information in
the QuickPrint Help menu (select “Privacy Agreement”).
Electromagnetic Effects
There are no restrictions on the use of FloPoint
electromagnetic characteristics. Both the emissions from FloPoint
®
Elite Uroflow System due to its
®
Elite and the
susceptibility of this instrument to interference from other sources are within prescribed
limits of all applicable standards at the date of manufacture. The emissions test
procedure that was used is specified in EN/IEC55011: 1991 for Group 1, Class A
equipment (per EN/IEC60601-1-2, 36.201.1.7).
This ISM device complies with Canadian ICES-001.
Cet appareil ISM est conforme à la norme NMB-001 du Canada.
NOTE: This equipment has been tested and found to comply with the limits for a Class B
digital device, pursuant to Part 15 of the FCC Rules. These limits are designed to provide
reasonable protection against harmful interference in a residential installation. This
equipment generates, uses and can radiate radio frequency energy and, if not installed
and used in accordance with the instructions, may cause harmful interference to radio
communications. However, there is no guarantee that interference will not occur in a
particular installation. If this equipment does cause harmful interference to radio or
television reception, which can be determined by turning the equipment off and on, the
user is encouraged to try to correct the interference by one or more of the following
measures:
-- Reorient or relocate the receiving antenna.
-- Increase the separation between the equipment and receiver.
-- Connect the equipment into an outlet on a circuit different from that to which
the receiver is connected.
-- Consult the dealer or an experienced radio/TV technician for help.
Modifications to this equipment not expressly approved by Verathon
®
could void the
user's authority to operate the equipment
FloPoint
(ISM) environments, and in domestic environments under the jurisdiction of a health care
professional. An indication of adverse electromagnetic effects from FloPoint
®
Elite Uroflow System is suitable for use in industrial, scientific, and medical
®
Elite
Uroflow System on another electronic device would be a degradation of performance in
the other device when the devices are operated simultaneously. If such interference is
suspected, separate the two devices as much as possible, or discontinue simultaneous
operation, if practical, and contact Verathon
The FloPoint
®
Elite Uroflow System will operate normally in the proximity of other
®
.
potential interference sources, and has demonstrated immunity at a field strength of 3
V/m (per EN/IEC 60601-1-2, 36.202.2.1). You do not need to take any other precautions
User’s Manual page 115
Page 23

Specifications FloPoint® Elite Uroflow System
regarding exposure in reasonably foreseeable environmental conditions to magnetic
fields, pressure, or variations in pressure, acceleration, or thermal ignition sources.
Health Insurance Portability and Accountability Act
To address the growing concern about the protection and confidentiality of an individual’s
medical information, Verathon
delivery and storage of all information that is maintained, used, and presented by the
ScanPoint
®
system:
All ScanPoint
®
system communications across the Internet are transmitted using
®
has taken the following measures to ensure the safe
the Secure Socket Layer (SSL) protocol and the 128-bit Data Encryption
Standard (DES).
All Patient Health Information (PHI) specific data stored within the ScanPoint
®
database is encrypted.
The ScanPoint
®
Web site infrastructure is housed by a Tier-1 Network Service
Provider (NSP) that meets the SAS/70 certification.
Within the NSP, the ScanPoint
®
equipment is located in a “locked, steel cage”
structure, removing access from even the NSP’s personnel.
Verathon
®
has enacted all policies and procedures outlined in HIPAA to protect
our internal network from unauthorized access.
Verathon
and shall continue to monitor any changes in these laws to insure all precautions are
taken to protect PHI. For further information on how Verathon
refer to the Verathon
®
has taken these actions to meet HIPAA’s Privacy and Security requirements
®
®
Business Associate Agreement (0003-0138).
manages access to PHI,
FloCharger
Item Specification
Input Voltage 100 – 240 V AC RMS
Input Frequency 50 – 60 Hz
Input current 2 A max
Input connection Employs direct plug-in AC prongs for wall outlets.
Insulation Class II with double insulation to each terminal.
Testing To EN/EN/IEC 60601-1 requirements
Water Ingress Rated at IPX0 (not protected against ingress of water)
Compliance UL and CSA equivalent standards
page 116 User’s Manual
Page 24

FloPoint® Elite Uroflow System Specifications
FloSensor
Item Specification
Sensory Type Spinning Disk
Battery type Lithium Ion. 14.4 V, 2200 mAh
Battery life A fully charged battery can perform approximately 30 exams
within a 24-hour period.
Charging Method Charge the FloSensor using the FloCharger installed on the
Mounting Bracket.
Charging Time No more than six hours from an empty battery to a full
charge.
Water Ingress Rated at IPX7 (Water tight for 30 minutes at a depth of 1 m)
ScanPoint® Remote
Item Specification
Battery type Lithium Ion. 4.2 V, 700 mAh
Battery life A fully charged battery can perform approximately 30 exams
Charging Method Charge the FloSensor using the ScanPoint® Docking Station
Charging Time No more than eight hours from an empty battery to a full
Water Ingress Rated at IPX0 (not protected against ingress of water)
ScanPoint® Docking Station
Condition Specification
within a 24-hour period.
connected to the USB port of a computer compliant with IEC
60950 or IEC 60601-1.
charge.
Use Indoor
Water Ingress Rated at IPX0 (not protected against ingress of water)
User’s Manual page 117
Page 25

Specifications FloPoint® Elite Uroflow System
Condition Specification
Computer Regulatory
Compliance
Computer Connection USB 1.1
Design Decoupled transformer at 200 mA at 4.2 V (25 kHz)
Water Ingress IPX0
Accuracy Specifications
The accuracy specifications assume the instrument is being used according to the
instructions provided by Verathon
Specification Description
Flow rate measurement ± 3% ± 1 ml/s (as measured by Verathon
Volume measurement ± 3% ± 5 ml/s (as measured by Verathon
The computer must minimally comply with the requirements
of IEC 60950 and preferable with the requirements of IEC
60601-1.
®
.
®
accuracy
measurement test)
®
accuracy
measurement test)
Maximum Flow Rate 50 ml/s
Maximum volume measure 2 liters
Operating Conditions
All components of the FloPoint® Elite Uroflow System are designed to function properly
within the following specifications:
Condition Value
Use Indoor
Ambient Temperature Range +10 - +35º Celsius (50 - 95º Fahrenheit)
Atmospheric pressure range 70 kPa - 106 kPa
Relative humidity 30% - 75% non-condensing
page 118 User’s Manual
Page 26

FloPoint® Elite Uroflow System Specifications
Storage Conditions
All components of the FloPoint® Elite Uroflow System are designed to withstand the
following storage conditions:
Condition Description
Storage Indoor
Ambient Temperature Range -10 - +35º Celsius (14 - 95º Fahrenheit)
Atmospheric pressure range 50 kPa - 106 kPa
Relative humidity 20% - 95% non-condensing
Radio Specifications
The FloSensor and ScanPoint® Remote communicate via a radio frequency that operates
in the 2.4 GHz ISM (Industrial, Scientific, Medical) band with a range of 3 meters (10
feet). FloPoint Elite Equipment complies with the specifications contained in FCC Part 15.
Parameter Description Condition Value
RF Frequency Range 2.402 - 2.479 GHz
Range Up to 3 meters (10 feet)
Computer Hardware and Software Requirements
To use ScanPoint® with QuickPrint, your computer must meet the following hardware and
software requirements:
NOTE: ScanPoint® with QuickPrint works only with Microsoft Internet Explorer 6.0 or
later.
ScanPoint® with QuickPrint is not compatible with Apple Macintosh computers.
Requirement Minimum Recommended
Processor
Video display
PC with 800 MHz
processor
Video card and monitor
capable of 800 x 600
resolution.
PC with 2.0 GHz processor
Video card and monitor
capable of 1024 x 768
resolution.
User’s Manual page 119
Page 27

Specifications FloPoint® Elite Uroflow System
Requirement Minimum Recommended
USB Ports
Hard drive
Memory
Internet access
Two USB 1.1 ports Two USB 2.0 ports*
50 Mb of available space 5 Gb of available space
256 Mb 512 Mb
256k DSL 512k DSL, cable modem,
T1 line or other high-speed
connection
Operating System and Software Requirements
Requirement Minimum Recommended
Operating system
Browser
Microsoft .NET
Framework
Microsoft
Professional with Service
Pack 2
Microsoft Internet
Explorer
.NET Framework version
2.0 (This software is
installed with ScanPoint
®
Windows® 2000
®
version 6.0
Microsoft Windows 2000
Professional with Service
Pack 4, Windows XP.
Microsoft Internet Explorer
version 7.0
.NET Framework version
2.0 with the latest Microsoft
®
updates installed.
with QuickPrint.)
Adobe® Acrobat Reader®
*Available for free download from www.adobe.com.
Label Writer Specifications
The ScanPoint® Label Writer meets the following specifications:
Condition Description
Print method
Print resolution
Maximum print width
Maximum media width
Interface
Adobe Acrobat Reader 6.0 Adobe Acrobat Reader 7.0*
Direct thermal
300 dots per inch (118 dots per mm)
2.25” / 56 mm
2.44” / 62 mm
USB 2.0 full speed printer class device
page 120 User’s Manual
Page 28

FloPoint® Elite Uroflow System Specifications
Condition Description
Average print head life
2,000,000 linear inches (over 31 miles) / 50,800 linear
meters
Printer power requirements
Regulatory approvals
24 VDC 1.75 A
CE, FCC, cTUVus, GS and C-Tick
Mounting Bracket and Related Equipment Specifications
The ScanPoint® Label Writer meets the following specifications:
Condition Description
Mounting bracket material
Weight (mounting bracket
alone)
Weight (mounting bracket
plus funnels, charger, and
sensor
16 guage 304 stainless steel
6.5 lb (2.95 kg)
13 lb (5.9 kg)
User’s Manual page 121
Page 29

Glossary FloPoint® Elite Uroflow System
Glossary
Glossary
Term Meaning
Activate
When the ScanPoint
discharged, you may need to activate, or reset, the Remote before
you can use it again. The Remote can be reactivated by pressing
the recessed Reset button on the back of the unit using the
supplied Activation Tool.
Activation Tool
The small tool used to depress the Reset button on the back of
the Remote (see “Activate” above).
Annotation
A brief voice recording made on the ScanPoint
exam details.
Average Flow
The average flow rate during flow intervals measured in ml/s. This
calculation does not include the time between intervals.
Calibration
Checking the accuracy and function of your FloPoint
comparing it with a known standard.
®
Remote battery has been completely
®
Remote providing
®
by
ScanPoint®
Docking Station
Continuous
(Pattern)
Detailed Report
FloCharger
FloSensor
The unit that transmits exam data from the Remote to the
ScanPoint
®
host computer, and when the computer is turned on,
also charges the Remote battery.
There was one flow interval detected during the exam.
This report contains all ICS values and prints on two labels or a
single sheet of 8.5 x 11” paper. It contains: the date and time of
the exam, patient ID, patient name, operator ID, physician,
gender, position and indicator for whether or not this was a normal
flow for the patient, peak flow measurement, average flow
measurement, voided volume, flow time measurement, void time
measurement, pattern measurement, chart of flow rate vs. time,
chart of cumulative volume vs. time, part number and serial
number of the FloSensor, and the ScanPoint
®
exam ID. See also
Summary Report.
The FloPoint
®
Elite base unit that provides a storage and
transportation unit for the FloSensor and also recharges the
FloSensor batteries.
The central component of the FloPoint
®
Elite system, it contains a
rotating disc for measuring urinary flow rate and volume..
page 122 User’s Manual
Page 30

FloPoint® Elite Uroflow System Glossary
Term Meaning
Flow Interval
Ends
Flow Interval
Starts
Flow Time
HIPAA
ICS
The end of a flow interval is identified by two consecutive samples
with a flow rate less that 0.2 ml/s.
The start of a flow interval is identified by two consecutive
samples with a flow rate greater than 0.5 ml/s. The actual start of
the interval is selected to be the sample prior to the identifying
samples where the flow rate is at least 0.2 ml/s.
The total amount of time in seconds during which flow is being
measured. In the case of intermittent flow, this value will be
smaller than the Void Time as this value does not include the time
between intervals where there is no flow.
Health Insurance Portability and Accountability Act, enacted by
the US Congress in 1996.
Title II of HIPAA, the Administrative Simplification provisions (AS),
requires the establishment of national standards for electronic
health care transactions and national identifiers for providers,
health insurance plans, and employers. The AS provisions also
address the security and privacy of health data. The standards are
meant to improve the efficiency and effectiveness of the nation's
health care system by encouraging the widespread use of
electronic data interchange in the U. S. health care system.
International Continence Society. An international association of
medical professionals whose purpose is to study storage and
voiding function of the lower urinary tract, its diagnosis and the
management of lower urinary tract dysfunction, and to encourage
research into pathophysiology, diagnostic techniques and
treatment.
Intermittent
(Pattern)
LCD screen
There were two or more flow intervals detected during the exam
(see also Continuous and Undetermined).
The LCD (liquid crystal display) screen on the ScanPoint
®
Remote
that displays flow measurements, instrument status, and other
exam settings and information.
New Flow
Interval Starts
Peak Flow
A new flow interval is identified by a Flow Interval Start that is at
least 0.25 seconds after the previous Flow Interval End.
Peak flow rate measured in ml/s. The value of the peak flow
detected by the FloSensor (see also Average Flow).
QuickPrint
User’s Manual page 123
The program that process exams and prints the results.
Page 31

Glossary FloPoint® Elite Uroflow System
Term Meaning
ScanPoint
Remote
The remote control unit that receives exam data from the
FloSensor via radio contact and transmits the exam data to the
ScanPoint
®
host computer via ScanPoint® Remote Docking
®
Station.
ScanPoint®
Imaging
Technology
ScanPoint® with
QuickPrint
Sleep mode
Summary
Report
Time to Peak
Flow
The software and online service provided by Verathon® that prints
exam results, maintains patient records, displays flow diagrams
from exams, downloads software updates, and calibrates the
FloPoint
ScanPoint® with QuickPrint is a software program installed on your
computer that communicates with ScanPoint
quickly and easily download and print FloPoint
®
Elite.
®
Online to help you
®
Elite
measurements.
When the FloSensor and/or ScanPoint
conserve energy. Press the ScanPoint
®
Remote shuts down to
®
Remote button to “wake
up” the device.
Provides only peak flow measurement and prints on a single label
or a single sheet of 8.5 x 11” paper. The Summary Report
contains the date and time of the exam, patient ID, patient name,
operator ID, physician, gender, position and indicator for whether
or not this was a normal flow for the patient, peak flow
measurement, chart of flow rate vs. time, part number and serial
number of the FloSensor, and the ScanPoint
®
exam ID. See also
Detailed Report.
The amount of time, measured in seconds, between the start of
the first interval of flow to the maximum measured flow rate.
Undetermined
(Pattern)
Void Time
There were no flow intervals detected in the exam (see also
Continuous and Intermittent).
The total amount of time in seconds from the start of the first
interval to the end of the last interval, including all the time
between intervals where flow is not measured.
Voided Volume
page 124 User’s Manual
The total volume of urine measured by the FloSensor in ml.
Page 32

Inside Back Cover
Page 33

Outside Back Cover
 Loading...
Loading...