Page 1

Page 1 of 19
Reprocessed by
Instructions for Use
Reprocessed HARMONIC ACE® +7, 5mm Diameter Shears with
Advanced Hemostasis
Reprocessed Device for Single Use
Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician.
• STERILE
Explanation of Symbols
Sterilized by Ethylene Oxide Gas
Date of Reprocessing
Use by Date
Product Code
Do Not Reuse
See Instructions For Use
Stryker Sustainability Solutions, Inc. © 2016
1810 W Drake Dr.
Tempe AZ, 85283
sustainability.stryker .com
888.888.3433
Page 2
Reprocessed HARMONIC ACE®+7, 5mm Diameter Shears with
Advanced Hemostasis Page 2 of 19
Page 3

Reprocessed HARMONIC ACE®+7, 5mm Diameter Shears with
Advanced Hemostasis Page 3 of 19
Page 4
Reprocessed HARMONIC ACE®+7, 5mm Diameter Shears with
Advanced Hemostasis Page 4 of 19
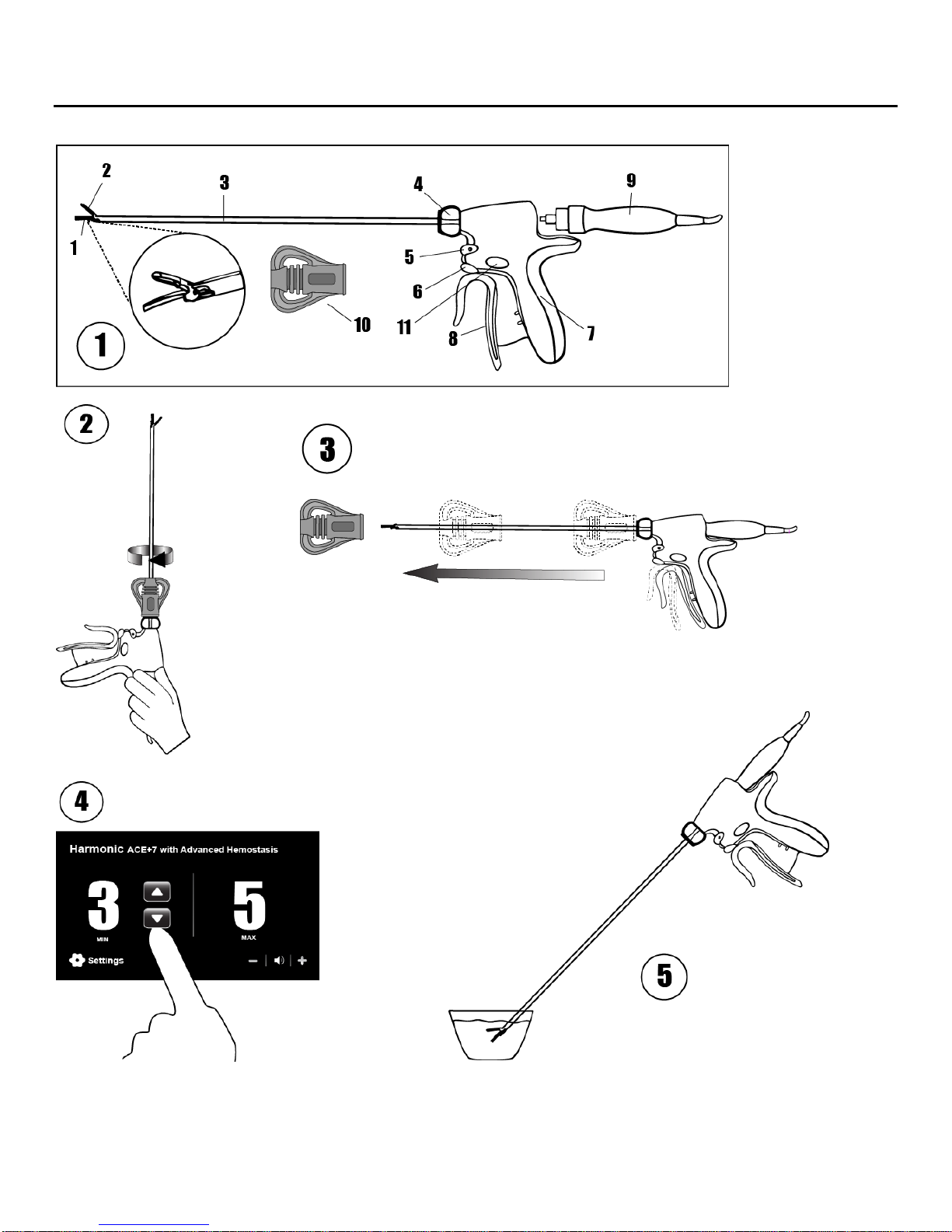
Illustration and Nomenclature (Illustration 1)
1. Coated Blade
7. Grip Housing
2. Clamp Arm and Tissue Pad
8. Trigger
3. Shaft
9. Hand Piece (not included)
4. Rotation Knob
10. Torque Wrench
5. MAX Hand Control Button (both sides of instrument)
11. Advanced Hemostasis Hand Control Button
6. MIN Hand Control Button (both sides of instrument)
Reprocessed HARMONIC ACE®+7, 5mm Diameter Shears with Advanced Hemostasis Description
Reprocessed HARMONIC ACE®+7, 5mm Diameter Shears with Advanced Hemostasis are sterile, single patient use instruments
consisting of an ergonomic grip housing assembly with hand control buttons (MIN for minimum power level, MAX for
maximum power level, and Advanced Hemostasis for large vessel sealing). An integrated audible and tactile mechanism in the
grip housing indicates full trigger closure. The instruments have a clamp arm and coated curved blade that are designed to
work through a 5 mm trocar, through a 5 mm reducer cap in a larger diameter trocar, or through an incision without the use of
a trocar. The instrument shafts can be rotated 360° to facilitate visualization and access to targeted tissue. The three dashes on
the instrument are intended to represent relative vessel size. The MAX button is typically used for smaller vessels where
cutting speed is fastest. The MIN button is typically used in slightly larger vessels and has reduced cutting speed. It is indicated
for vessels up to 5 mm in size. The Advanced Hemostasis button is designed for larger vessels and is indicated for vessels up to
7 mm in size. In this mode, cutting speed is further reduced and hemostasis is maximized. The instruments utilize Adaptive
Tissue Technology. This provides the generator with the ability to identify and monitor the instrument during use, which
enables the generator to modulate and adjust its power output as well as provide audible feedback to the user as appropriate.
Each instrument is shipped with one sterile, single-use, disposable torque wrench. Use only the gray torque wrench provided
with the instrument. The torque wrench should not be discarded until the completion of the surgical case. Do not attempt to
sterilize the disposable torque wrench.
Note: Use of HARMONIC torque wrenches other than the one provided may result in damage to the device.
The Reprocessed HARMONIC ACE®+7, 5 mm Diameter Shears with Advanced Hemostasis are designed for use exclusively
with the Generator G11 (GEN11) software version 2013_1, 2014_1, or 2016_1. Software revision can be found under "System
lnformation" in the Generator G11 (GEN11) "Settings" menu. Refer to the Generator G11 (GEN11) Operator's Manual for more
information.
Page 5

Reprocessed HARMONIC ACE®+7, 5mm Diameter Shears with
Advanced Hemostasis Page 5 of 19
Indications for Use
The Reprocessed HARMONIC ACE®+7, 5 mm Diameter Shears with Advanced Hemostasis are indicated for soft tissue incisions
when bleeding control and minimal thermal injury are desired. The instruments can be used as an adjunct to or substitute for
electrosurgery, lasers and steel scalpels in general, plastic, pediatric, gynecologic, urologic, thoracic, exposure to orthopedic
structures (such as spine and joint space), sealing and transection of lymphatic vessels, and other open and endoscopic
procedures. The instruments allow for the coagulation of vessels up to and including 7 mm in diameter, using the Advanced
Hemostasis hand control button.
Contraindications for Use
Reprocessed HARMONIC ACE®+7, 5 mm Diameter Shears with Advanced Hemostasis are contraindicated for:
• The instruments are not indicated for incising bone.
• The instruments are not intended for contraceptive tubal occlusion.
Warnings and Precautions
• Caution: Federal (USA) law restricts this device to sale by or on the order of a physician.
• Minimally invasive procedures should be performed only by persons having adequate training and familiarity with
minimally invasive techniques. Consult medical literature relative to techniques, complications, and hazards prior to
performance of any minimally invasive procedure.
• Minimally invasive instruments may vary in diameter from manufacturer to manufacturer. When minimally invasive
instruments and accessories from different manufacturers are employed together in a procedure, verify compatibility
prior to initiation of the procedure.
• A thorough understanding of the principles and techniques involved in laser, electrosurgical, and ultrasonic procedures is
essential to avoid shock and burn hazards to both patient and medical personnel and damage to the device or other
medical instruments. Ensure that electrical insulation or grounding is not compromised. Do not immerse instruments in
liquid unless the instruments are designed and labeled to be immersed.
• Verify compatibility with generators. Use device only with Ethicon Endo-Surgery Generator G11 (GEN11) software
version 2013_1, 2014_1, or 2016_1. Software revision can be found under "System Information" in the Generator G11
(GEN11) "Settings" menu. Refer to the Generator G11 (GEN11) Operator's Manual for more information.
• In case of system failure, ensure the availability of the appropriate back up equipment relevant to the specific procedure.
• Audible high-pitched ringing, resonating from the blade or Hand Piece, are an abnormal condition and an indicator that
the blade or Hand Piece is not operating properly. The ringing may be an indicator that the Hand Piece is beyond its useful
life or that the blade has not been attached properly, which may result in abnormally high shaft temperatures and user or
patient injury.
• The instruments allow for the coagulation of vessels up to and including 7 mm in diameter, using the Advanced
Hemostasis hand control button. Do not attempt to seal vessels in excess of 7 mm in diameter.
• Blood and tissue buildup between the blade and shaft may result in abnormally high temperatures at the distal end of the
shaft. To prevent burn injury, remove any visible tissue buildup at the distal end of the shaft.
• As with all energy sources (Electrosurgery, Laser, or Ultrasound), there are concerns about the carcinogenic and infectious
potential of the by-products, such as tissue smoke plume and aerosols. Appropriate measures such as protective eyewear,
filtration masks, and effective smoke evacuation equipment should be used in both open and laparoscopic procedures.
• Do not attempt to bend, sharpen, or otherwise alter the shape of the blade. Doing so may cause blade failure and user or
patient injury.
• To avoid user or patient injury in the event that accidental activation occurs, the instrument blade, clamp arm, and distal
end of the shaft should not be in contact with the patient, drapes, or flammable materials while not in use.
• During and following activation in tissue, the instrument blade, clamp arm, and distal 7 cm of the shaft may be hot. Avoid
unintended contact with tissue, drapes, and surgical gowns at all times.
• Avoid contact with any and all other instruments while the instrument is activated. Contact with staples, clips or other
instruments while the instrument is activated may result in cracked or broken blades.
• Do not introduce or withdraw the instrument with the jaws open through a trocar sleeve as this may damage the
instrument.
• Care should be taken not to apply pressure between the instrument blade and tissue pad without having tissue between
them. Clamping the tissue pad against the active blade without tissue on the full length of the blade will result in higher
blade, clamp arm and distal shaft temperatures and can result in possible damage to the instrument. If this occurs, there
may be an instrument failure, and the generator touchscreen displays a troubleshooting message.
Page 6

Reprocessed HARMONIC ACE®+7, 5mm Diameter Shears with
Advanced Hemostasis Page 6 of 19
• To avoid user or patient injury, do not activate an electrosurgical device in close proximity to the HARMONIC instruments.
The aerosols created by the activation of the HARMONIC instruments in fatty tissue are potentially flammable.
• Keep the clamp arm open when back cutting or while the blade is active without tissue between the blade and tissue pad
to avoid damage to the tissue pad and increased blade, clamp arm and distal shaft temperatures.
• The entire exposed blade tip and any exposed blade shaft are active and will cut/coagulate tissue when the instrument
blade is activated. Be careful to avoid inadvertent contact between all exposed blade surfaces and surrounding tissue
when using the instrument.
• Use only the appropriate Foot Switch, Hand Piece, instruments, and power cord to ensure that they are compatible with
the generator.
• After removing the instrument, examine the tissue for hemostasis. If hemostasis is not present, appropriate techniques
should be used to achieve hemostasis.
• Successful hemostasis may require adjunct measures when HARMONIC instruments are used on solid organs. Due to the
difficulty of visualizing internal structures, proceed slowly and do not attempt to transect large masses of tissue in one
activation. Avoid the division of large vascular/biliary bundles when using the instrument under these conditions.
• If activation is unintentionally stopped while sealing, maintain jaw closure and reactivate.
• Do not use Advanced Hemostasis mode for procedures where energy application is desired prior to full closure of the jaws
(e.g. solid organs). Energy is not delivered in Advanced Hemostasis mode until the jaws are completely closed.
• During benchtop testing of vessels >5 mm, the strongest vessel seals were achieved by allowing the Advanced Hemostasis
mode to completely transect the targeted vessel.
• Prolonged usage of Advanced Hemostasis Mode may cause tissue pad damage.
• Products manufactured or distributed by companies not authorized by Ethicon Endo-Surgery may not be compatible with
the HARMONIC system. Use of such products may lead to unanticipated results and possible injury to the user or patient.
• If during use the generator displays ''Advanced Features Are Not Available In This Device", the following functions of
Adaptive Tissue Technology are no longer available: Regulated Energy Delivery, Enhanced Audible Feedback and
Advanced Hemostasis. As a consequence, the device vessel sealing indication does not exceed 5 mm.
• Instruments or devices which come into contact with bodily fluids may require special disposal handling to prevent
biological contamination.
• Incidental and prolonged activation against solid surfaces, such as bone, may result in blade heating and subsequent blade
failure, and should be avoided.
• Dispose of all opened instruments whether used or unused.
• This device is packaged and sterilized for single use only. Multiple patient use may compromise the device integrity or
create a risk of contamination that, in turn, may result in patient injury or illness.
Directions for Use
1. Verify compatibility of all instruments and accessories prior to using this instrument (refer to Warnings and
Precautions).
2. The Hand Piece is shipped non-sterile. It must be sterilized prior to each use according to the instructions for use supplied
with the Hand Piece.
Assembly
1. Using sterile technique, remove the instrument from the package. To avoid damage, do not flip the instrument into the
sterile field.
2. While holding the Hand Piece in a vertical orientation, attach the Hand Piece to the instrument by rotating the instrument
onto the Hand Piece in a clockwise rotation as viewed from the distal end of the instrument (finger tight only).
3. Use the torque wrench (already mounted to the shaft) to tighten the blade onto the Hand Piece. Turn the torque wrench
clockwise while holding only the gray Hand Piece until it clicks twice indicating that sufficient torque has been applied to
secure the blade.
4. Note: Do not use any other means than the torque wrench to attach or detach the instrument from the Hand Piece.
5. Note: Do not torque the instrument by hand without the torque wrench or damage may occur to the Hand Piece.
6. Note: Hold only the gray Hand Piece and not the instrument handle while applying the torque wrench (Illustration 2).
7. Close the trigger. Remove the torque wrench by sliding it off of the shaft. Do not dispose of the torque wrench until the
procedure is completed. The torque wrench is used to remove the instrument from the Hand Piece following the
procedure (Illustration 3). Dispose of the torque wrench only after completing the procedure.
8. Note: Take care to avoid damage to the blade and clamp arm by closing the trigger while sliding the torque wrench onto or
off of the shaft.
Page 7

Reprocessed HARMONIC ACE®+7, 5mm Diameter Shears with
Advanced Hemostasis Page 7 of 19
9. Note: Take care to avoid injury from the blade tip while sliding the torque wrench onto or off of the shaft.
Operation
1. Connect the assembled Hand Piece and instrument to the generator and turn the generator power on.
Note: MIN is the only mode that can be adjusted.
2. Select the desired minimum power level using the INCREASE and DECREASE "buttons on the generator touchscreen.
Note: The recommended minimum starting power level is Level 3 (Illustration 4). For greater tissue cutting speed use a
higher generator power level, and for greater coagulation use a lower generator power level. The amount of energy
delivered to the tissue and resultant tissue effects are a function of many factors, including the power level selected, blade
characteristics, grip force, tissue tension, tissue type, pathology, and surgical technique.
3. For optimal performance and to avoid tissue sticking, clean the instrument blade, clamp arm, and distal end of the shaft
throughout the procedure by activating the instrument tip in saline (Illustration 5).
Note: Do not touch the instrument to metal while activated (Illustration 6).
Note: Do not clean the blade tip with abrasives. It can be wiped with a moist gauze sponge to remove tissue, if necessary.
If tissue is still visible in the clamp arm, use hemostats to remove residue, taking care not to actuate the Hand Piece. If
desired, the instrument may be unplugged (Illustration 7).
4. The blade is ultrasonically energized when either the foot switch pedal is depressed or one of the hand control buttons is
depressed. Pressing the left foot pedal of the footswitch or the lower hand control button (MIN) on the instrument
activates the selected minimum power level. Pressing the right foot pedal of the footswitch or upper hand control button
(MAX) on the instrument activates the maximum power level. Pressing the Advanced Hemostasis button on the
instrument activates the Advanced Hemostasis. Under default settings, Advanced Hemostasis is hand-activated only. Note:
The "MIN" button can be re-assigned to Advanced Hemostasis by selecting "Convert MIN to Advanced Hemostasis" on the
GEN11 "Settings” screen. Refer to the Generator G11 Operator's Manual "Settings" section (Illustration 8). Once "Convert
MIN to Advanced Hemostasis" is selected, the Advanced Hemostasis button is disabled.
Note: Energy is not delivered in Advanced Hemostasis mode until the jaws are completely closed.
Caution: If activation is unintentionally stopped while sealing, maintain jaw closure and reactivate.
Note: The generator provides an audible tone to indicate when the instrument blade is active. The generator changes to a
second activation tone as Adaptive Tissue Technology regulates the delivery of energy.
• Thermal influences such as fluids or minimal to no tissue in the jaws may affect the presence or timing of the
tone change.
• The tone change does not provide confirmation of tissue effect. When the second tone is heard, the situation
should be assessed and the intended surgical action completed, such as gradual application of tension to
facilitate transection.
• The secondary activation tone change is not a substitute for surgical experience.
Note: Scratches on the blade may lead to premature blade failure.
• Avoid accidental contact with other instruments during use.
• Do not use any means other than the torque wrench to attach or detach the instrument from the Hand Piece.
5. Close the clamp arm by closing the trigger, and insert the shaft through a trocar or incision.
6. The instrument can be used for dissection, grasping, coagulation, and cutting between the blade and clamp arm.
Note: To achieve complete sealing, the trigger should be fully closed and the vessel fully contained between clamp arm and
blade of device. An audible and tactile "click" indicates full trigger closure. To achieve full closure of the jaws of the device,
squeeze the plastic trigger until you feel it stop against the plastic handle (plastic to plastic) (Illustration 9). If full trigger
closure is released prior to or during activation on tissue, an audible and tactile "click" is evident. Increase grip force until
full trigger closure is achieved.
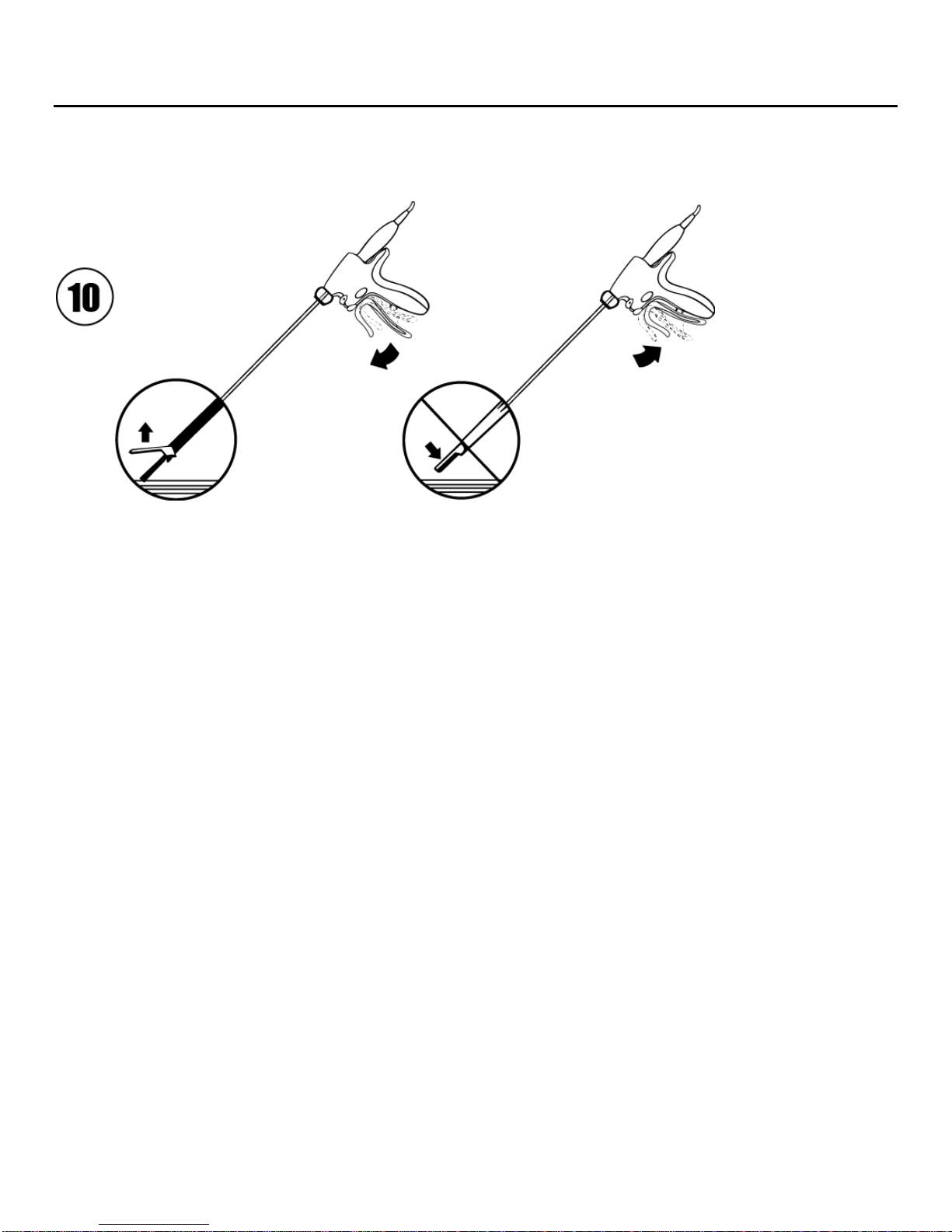
Note: Keep clamp arm open when using the inside bottom of the blade for backcutting (Illustration 10).
WARNING: Do not use Advanced Hemostasis mode for procedures where energy application is desired prior to full
closure of the jaws (e.g. solid organs). Energy is not delivered in Advanced Hemostasis mode until the jaws are completely
closed.
WARNING: During benchtop testing of vessels >5 mm, the strongest vessel seals were achieved by allowing the Advanced
Hemostasis mode to completely transect the targeted vessel.
WARNING: Prolonged usage of Advanced Hemostasis Mode may cause tissue pad damage.
7. Close the clamp arm by closing the trigger and remove the shaft from the trocar or incision.
Shaft Rotation
1. The instrument’s shaft can be rotated 360°to facilitate visualization and access to target tissue when dissecting, grasping,
coagulating, and cutting.
Page 8

Reprocessed HARMONIC ACE®+7, 5mm Diameter Shears with
Advanced Hemostasis Page 8 of 19
Diassessmbly
1. Turn the generator OFF at the power switch.
2. Close the clamp arm and slide the torque wrench over the distal end and up the shaft until the wrench aligns with the flats
on the shaft. Hold by the Hand Piece only, not the instrument handle, and loosen the instrument by turning the wrench
counterclockwise. Continue to loosen by turning the rotation knob manually to completely unscrew the instrument. Do
not untorque the instrument by hand without the torque wrench or damage may occur to the Hand Piece.
Note: Do not use any other means than the torque wrench to detach the instrument from the Hand Piece.
Note: Take care to avoid injury from the blade tip while sliding the torque wrench onto or off of the shaft.
3. Remove the torque wrench by pulling it straight back over the blade.
4. Dispose of the instrument in an appropriate container.
How Supplied
The Reprocessed HARMONIC ACE®+7, 5 mm Diameter Shears with Advanced Hemostasis are supplied sterile for single patient
use. Each instrument is shipped with one sterile, single use, gray disposable torque wrench.
Storage and Handling
• Temperature: -20°C to +50°C
• Relative Humidity: 25 to 90%
Compatibility
The Reprocessed HARMONIC ACE®+7, 5 mm Diameter Shears with Advanced Hemostasis are designed for use exclusively with
the Generator G11 (GEN11) software version 2013_1, 2014_1, or 2016_1. Software revision can be found under
"System lnformation" in the Generator G11 (GEN11) "Settings" menu. Refer to the Generator G11 (GEN11) Operator's Manual
for more information.
Warranty
Reprocessed Products
Stryker warrants all reprocessed products, subject to the exceptions provided herein, to be free from defects in reprocessing
and to substantially conform to the product specifications contained in the documentation provided by Stryker with the
products for one use in accordance with the instructions for use of such product.
STRYKER SHALL NOT BE LIABLE FOR ANY DAMAGES TO THE EXTENT CAUSED BY ANY DEFECT IN
MATERIAL, WORKMANSHIP OR DESIGN BY THE ORIGINAL MANUFACTURER OF THE PRODUCT OR ANY ACT
OR OMISSION OF THE ORIGINAL MANUFACTURER OF THE PRODUCT.
Products for which Stryker is the Original Manufacturer
Stryker warrants all products for which it is the original manufacturer, subject to the exceptions provided herein, to be free
from defects in design, materials and workmanship and to substantially conform to the product specifications contained in the
documentation provided by Stryker with the products for a period of one year from the date of purchase.
General Warranty Terms Applicable to All Products
TO THE FULLEST EXTENT PERMITTED BY LAW, THE EXPRESS WARRANTY SET FORTH HEREIN IS THE ONLY
WARRANTY APPLICABLE TO THE PRODUCTS AND IS EXPRESSLY IN LIEU OF ANY OTHER WARRANTY BY
STRYKER, EXPRESSED OR IMPLIED, INCLUDING, BUT NOT LIMITED TO, ANY IMPLIED WARRANTY OR
MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE. IN NO EVENT WILL STRYKER’S LIABILITY
ARISING IN CONNECTION WITH THE SALE OF THE PRODUCT (WHETHER UNDER THE THEORIES OF BREACH
OF CONTRACT, TORT, MISREPRESENTATION, FRAUD, WARRANTY, NEGLIGENCE, STRICT LIABILITY OR ANY
OTHER THEORY OF LAW) EXCEED THE PURCHASE PRICE, CURRENT MARKET VALUE OR RESIDUAL VALUE
OF THE PRODUCTS, WHICHEVER IS LESS. STRYKER SHALL NOT BE LIABLE FOR INDIRECT, SPECIAL,
INCIDENTAL, PUNITIVE OR CONSEQUENTIAL DAMAGES RESULTING FROM ANY BREACH OF WARRANTY OR
UNDER ANY OTHER LEGAL THEORY.
Page 9

Reprocessed HARMONIC ACE®+7, 5mm Diameter Shears with
Advanced Hemostasis Page 9 of 19
This warranty shall apply only to the original end-user purchaser of products directly from Stryker or a Stryker authorized
distributor. This warranty may not be transferred or assigned without the express written consent of Stryker.
This warranty does not apply to: (1) products that have been misused, neglected, modified, altered, adjusted, tampered with,
improperly installed or refurbished; (2) products that have been repaired by any person other than Stryker personnel
without the prior written consent of Stryker; (3) products that have been subjected to unusual stress or have not been
maintained in accordance with the instructions in the user manual or as demonstrated by a Stryker representative; (4)
products on which any original serial numbers or other identification marks have been removed or destroyed; or (5) products
that have been repaired with any unauthorized or non-Stryker components.
If a valid warranty claim is received within thirty (30) days of the expiration of the applicable warranty period, Stryker will, in
its sole discretion: (1) replace the product at no charge with a product that is at least functionally equivalent to the orig inal
product or (2) refund the purchase price of the product. If a refund is provided by Stryker, the product for which the refund is
provided must be returned to Stryker and will become Stryker’s property. In any event, Stryker’s liability for breach of
warranty shall be limited to the replacement value of the defective or non-conforming part or component.
If Stryker determines in its reasonable discretion that the claimed defect or non-conformance in the product is excluded from
warranty coverage as described hereunder, it will notify the customer of such determination and will provide an estimate of
the cost of repair of the product. In such an event, any repair would be performed at Stryker’s standard rates.
Products and product components repaired or replaced under this warranty continue to be warranted as described herein
during the initial applicable warranty period or, if the initial warranty period has expired by the time the product is repaired
or replaced, for thirty (30) days after delivery of the repaired or replaced product. When a product or component is replaced,
the item provided in replacement will be the customer’s property and the replaced item will be Stryker’s property. If a refund
is provided by Stryker, the product for which the refund is provided must be returned to Stryker and will become Stryker’s
property.
HARMONIC® and HARMONIC ACE® are trademarks of Ethicon Endo-Surgery
ULS EL10053 Rev. E 10/2017 RM702506
The OEM information listed on the label is provided as device ID prior to reprocessing and may contain the trademarks of unrelated
third parties that do not sponsor this device.
Sterilization: This product and its packaging have been sterilized with ethylene oxide gas (EtO). Even though the product then is
processed in compliance with all applicable laws and regulations relating to EtO exposure, Proposition 65, a State of California voter
initiative, requires the following notice:
Warning: This product and its packaging have been sterilized with ethylene oxide. The packaging may expose you to ethylene oxide, a
chemical known to the State of California to cause cancer or birth defects or other reproductive harm.
Page 10

Page 10 de 19
Retraité par
Mode d’emploi
Cisailles HARMONIC ACE® +7 retraitées de 5 mm de diamètre
pour hémostase avancée
Dispositif retraité à usage unique
Mise en garde : Selon la loi fédérale américaine (É.-U.), ce dispositif ne peut être vendu que
par un médecin ou sur ordonnance médicale.
• STÉRILE
Explication des symboles
Stérilisé par oxyde d’éthylène
Date du retraitement
Date de péremption
Code du produit
Ne pas réutiliser
Voir le mode d’emploi
Stryker Sustainability Solutions, Inc. © 2016
1810 W Drake Dr.
Tempe AZ, 85283 États-Unis
sustainability.stryker.com
888.888.3433
Page 11
Cisailles HARMONIC ACE®+7 retraitées de 5 mm de diamètre
pour hémostase avancée Page 11 de 19
Page 12

Cisailles HARMONIC ACE®+7 retraitées de 5 mm de diamètre
pour hémostase avancée Page 12 de 19
Page 13

Cisailles HARMONIC ACE®+7 retraitées de 5 mm de diamètre
pour hémostase avancée Page 13 de 19
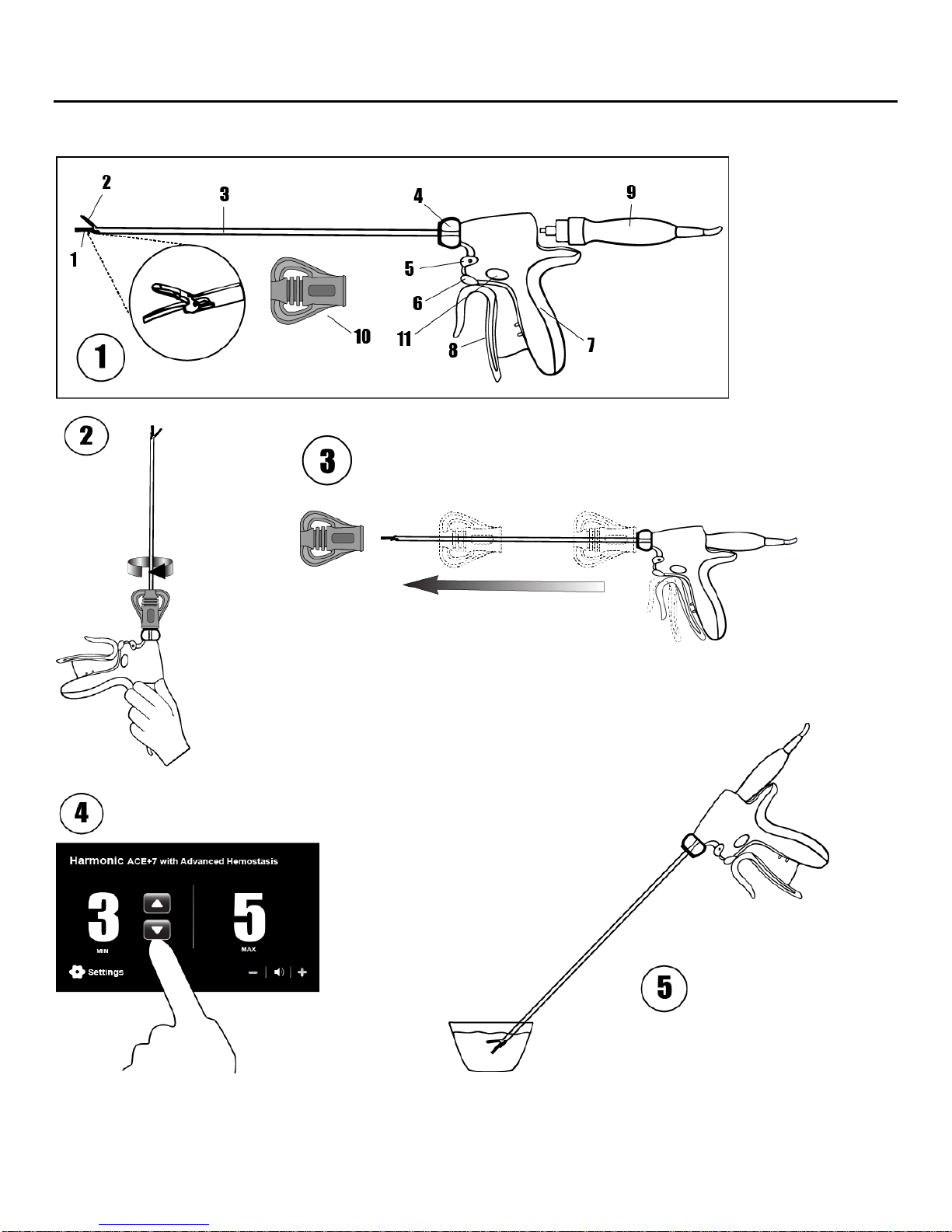
Illustration et nomenclature (Illustration 1)
1. Lame revêtue
7. Boîtier à prise facile
2. Bras de serrage et tampon
8. Déclencheur
3. Tige
9. Pièce à main (non comprise)
4. Bouton de rotation
10. Clé dynamométrique
5. Bouton de commande manuelle MAX (des deux côtés de
l’instrument)
11. Bouton de commande manuelle de l’hémostase avancée
6. Bouton de commande manuelle MIN (des deux côtés de
l’instrument)
Description des cisailles HARMONIC ACE®+7 retraitées de 5 mm de diamètre pour hémostase avancée
Les cisailles HARMONIC ACE®+7 retraitées de 5 mm de diamètre pour hémostase avancée sont des instruments stériles à
usage unique composés d’un boîtier à prise ergonomique avec boutons de commande manuelle (MIN pour puissance
minimale, MAX pour puissance maximale et hémostase avancée pour scellement des vaisseaux de grande calibre). Un
mécanisme sonore et tactile intégré dans le boîtier à prise indique la fermeture complète du déclencheur. Les instruments
possèdent un bras de serrage et une lame courbe revêtue conçus pour être utilisés avec un trocart de 5 mm, à travers un
capuchon réducteur de 5 mm dans un trocart de diamètre plus large ou grâce à une incision sans l’utilisation d’un trocart. Les
tiges de l’instrument peuvent pivoter sur 360° pour faciliter la visualisation et l’accès aux tissus ciblés. Les trois tirets affichés
sur l’instrument représentent une dimension relative du vaisseau. Le bouton MAX est généralement utilisé pour les vaisseaux
plus petits lorsque la vitesse de la coupe est plus rapide. Le bouton MIN est généralement utilisé pour les vaisseaux légèrement
plus grands et que la vitesse de coupe est plus lente. Il est indiqué pour les vaisseaux dont le calibre peut atteindre 5 mm. Le
bouton Advanced Hemostasis (hémostase avancée) est conçu pour les vaisseaux de plus grande dimension et indiqué pour les
vaisseaux dont le calibre maximal peut atteindre 7 mm. Dans ce mode, la vitesse de coupe est davantage réduite et l’hémostase
est maximisée. Les instruments utilisent la technologie adaptative des tissus. Ainsi, le générateur a la capacité d’identifier et de
surveiller l’instrument durant son utilisation, ce qui permet au générateur de moduler et d’ajuster sa puissance de sortie tout
en offrant une rétroaction sonore à l’utilisateur au besoin.
Chaque instrument est expédié avec une clé dynamométrique stérile, jetable et à usage unique. N’utilisez que la clé
dynamométrique grise fournie avec l’instrument. La clé dynamométrique ne doit pas être mise au rebut avant la fin de
l’intervention chirurgicale. Ne tentez pas de stériliser la clé dynamométrique jetable.
Remarque : L’utilisation de clés dynamométriques HARMONIC autres que celles fournies pourrait causer des dommages à
l’appareil.
Page 14

Cisailles HARMONIC ACE®+7 retraitées de 5 mm de diamètre
pour hémostase avancée Page 14 de 19
Les cisailles HARMONIC ACE®+7 retraitées de 5 mm de diamètre pour hémostase avancée sont conçues pour être
exclusivement utilisées avec le logiciel Generator G11 (GEN11) version 2013_1, 2014_1 ou 2016_1. La révision logicielle se
trouve sous « System Information » (Information système) dans le menu « Settings » (Paramètres) du générateur G11
(GEN11). Consultez le manuel de l’opérateur du générateur G11 (GEN11) pour obtenir de plus amples renseignements.
Indications
Les cisailles HARMONIC ACE®+7 retraitées de 5 mm de diamètre pour hémostase avancée sont indiquées pour les incisions
dans les tissus mous lorsqu’un contrôle du saignement et des lésions thermiques minimes sont souhaités. Les instruments
peuvent servir de complément ou de substitut aux scalpels en acier, laser ou pour électrochirurgie, utilisés dans les
procédures générales, plastiques, pédiatriques, gynécologiques, urologiques, thoraciques, l’exposition des structures
orthopédiques (espace articulaire et colonne vertébrale, par exemple), le scellement et la coupe transversale des vaisseaux
lymphatiques et autres procédures ouvertes et endoscopiques. Les instruments permettent la coagulation des vaisseaux d’un
diamètre inférieur ou égal à 7 mm, grâce au bouton de commande manuelle de l’hémostase avancée.
Contre-indications
Les cisailles HARMONIC ACE®+7 retraitées de 5 mm de diamètre pour hémostase avancée sont contre-indiquées dans les cas
suivants :
• Les instruments ne sont pas indiqués pour une incision osseuse.
• Les instruments ne sont pas conçus pour une ligature des trompes.
Avertissements et précautions
• Mise en garde : Selon la loi fédérale américaine, ce dispositif ne peut être vendu que par un médecin ou sur ordonnance
médicale.
• Les interventions à effraction minimale ne doivent être exécutées que par un personnel ayant reçu une formation
adéquate et disposant des connaissances requises concernant ce type de technique. Avant de procéder à une intervention
à effraction minimale, consultez la documentation médicale se rapportant aux techniques, aux complications et aux
risques qui y sont liés.
• Le diamètre des instruments à effraction minimale peut varier d’un fabricant à un autre. Lorsque de tels instruments et
accessoires provenant de différents fabricants sont utilisés lors d’une même intervention, leur compatibilité doit être
vérifiée avant de commencer l’intervention chirrugicale.
• Une compréhension approfondie des principes et techniques impliqués dans les procédures laser, électrochirurgicales et
ultrasoniques est essentielle pour éviter tout risque d’électrocution et de brûlure infligée à la fois au patient et au
personnel médical ainsi que pour éviter tout dommage au dispositif ou aux autres instruments médicaux. Assurez-vous
que ni l’isolation électrique ni la mise à la terre ne sont compromises. N’immergez pas ces instruments dans un liquide
sauf s’ils sont conçus à cet effet et que l’étiquette le mentionne.
• Vérifiez la compatibilité avec les générateurs. N’utilisez ce dispositif qu’avec le logiciel Ethicon Endo-Surgery Generator
G11 (GEN11) version 2013_1, 2014_1 ou 2016_1. La révision logicielle se trouve sous « System Information » (Information
système) dans le menu « Settings » (Paramètres) du générateur G11 (GEN11). Consultez le manuel de l’opérateur du
générateur G11 (GEN11) pour obtenir de plus amples renseignements.
• En cas de défaillance du système, vérifiez la disponibilité d’un équipement de secours approprié pour la procédure en
cause.
• Une sonnerie à tonalité aigüe, qui retentit de la lame ou de la pièce à main, est une condition anormale et indique un
mauvais fonctionnement de la lame ou de la pièce à main. La sonnerie pourrait indiquer que la pièce à main a dépassé sa
durée de vie utile ou que la lame est incorrectement fixée, ce qui pourrait provoquer une température anormalement
élevée de la tige et infliger des blessures à l’utilisateur ou au patient.
• Les instruments permettent la coagulation des vaisseaux d’un diamètre inférieur ou égal à 7 mm, grâce au bouton de
commande manuelle de l’hémostase avancée. Ne tentez pas de sceller des vaisseaux d’un diamètre supérieur à 7 mm.
• L’accumulation de sang et de tissus entre la lame et la tige pourrait provoquer une température anormalement élevée de
l’extrémité distale de la tige. Pour éviter les brûlures, éliminez toute accumulation visible des tissus à l’extrémité distale de
la tige.
• Comme c’est le cas avec toutes les sources d’énergie (électrochirurgie, laser ou ultrasons), les produits dérivés, comme la
fumée s’élevant des tissus et les aérosols, pourraient présenter un potentiel infectieux et cancérigène. Des mesures
adéquates, comme une protection oculaire, des masques filtrants et un équipement d’évacuation efficace de la fumée,
doivent être utilisées lors des interventions laparoscopiques ou ouvertes.
• Ne tentez pas de plier, d’aiguiser ou de modifier de quelque façon que ce soit la forme de la lame. Ce faisant, vous pourriez
provoquer une faiblesse de la lame et des blessures au patient ou à l’utilisateur.
Page 15

Cisailles HARMONIC ACE®+7 retraitées de 5 mm de diamètre
pour hémostase avancée Page 15 de 19
• Pour éviter toute blessure au patient ou à l’utilisateur en cas d’activation accidentelle, la lame de l’instrument, le bras de
serrage et l’extrémité distale de la tige ne doivent pas entrer en contact avec le patient, les champs opératoires ou les
matières inflammables lorsqu’ils ne sont pas utilisés.
• Pendant et après l’activation dans les tissus, la lame de l’instrument, le bras de serrage et l’extrémité distale de 7 cm de la tige
pourraient être chauds. En tout temps, évitez tout contact avec les tissus, les champs opératoires et les vêtements chirurgicaux.
• Évitez le contact avec tout autre instrument lorsque l’instrument est activé. Le contact avec des agrafes, des pinces ou
d’autres instruments lorsque l’instrument est activé peut provoquer des fissures ou un bris des lames.
• N’introduisez pas et ne retirez pas l’instrument dont les mâchoires sont ouvertes à travers un manchon de trocart, car cela
pourrait endommager l’instrument.
• Veillez à ne pas appliquer de pression entre la lame de l’instrument et le tampon sans avoir d’abord vérifié la présence de
tissus entre eux. Serrer le tampon de tissus contre la lame active sans que les tissus ne s’étendent sur toute la longueur de
la lame aura pour résultat une augmentation des températures de la lame, du bras de serrage et de la tige distale et
pourrait provoquer des dommages à l’instrument. Si cela se produisait, l’instrument pourrait tomber en panne et l’écran
tactile du générateur afficherait un message de dépannage.
• Pour éviter toute blessure à l’utilisateur ou au patient, n’activez jamais un dispositif électrochirurgical à proximité des
instruments HARMONIC. Les aérosols créés par l’activation des instruments HARMONIC dans les tissus adipeux sont
potentiellement inflammables.
• Pour éviter d’endommager le tampon et de provoquer une augmentation des températures de la lame, du bras de serrage
et de la tige distale, maintenez le bras de serrage ouvert lors d’une incision inversée ou lorsque la lame est active sans
aucun tissu entre la lame et le tampon de tissu.
• L’extrémité de la lame complètement exposée et la tige de lame exposée sont actives et coupent/coagulent les tissus
lorsque la lame de l’instrument est activée. Lorsque vous utilisez l’instrument, veillez à éviter tout contact accidentel entre
les surfaces exposées de la lame et les tissus environnants.
• Pour assurer que ces éléments sont compatibles avec le générateur, n’utilisez que la pédale, la pièce à main, les
instruments et le cordon d’alimentation appropriés.
• Une fois l’instrument retiré, examinez les tissus pour déceler une mauvaise hémostase. Si l’hémostase est inadéquate,
utilisez les techniques appropriées pour obtenir l’hémostase.
• Une hémostase fructueuse peut demander des mesures supplémentaires lorsque les instruments HARMONIC sont utilisés
sur des organes pleins. En raison de la difficulté de visualisation des structures internes, procédez lentement et ne tentez
pas de couper de gros amats de tissu en une seule activation. Évitez la division des faisceaux biliaires/vasculaires de
grande taille lorsque vous utilisez l’instrument dans ces conditions.
• Si l’activation est arrêtée involontairement lors du scellement, maintenez les mâchoire fermées et réactivez.
• N’utilisez pas le mode d’hémostase avancée pour les procédures où une application d’énergie est requise avant la
fermeture complète des mâchoires (p. ex., dans le cas des organes pleins). En mode d’hémostase avancée, l’énergie est
administrée uniquement lorsque les mâchoires sont entièrement fermées.
• Durant les essais sur des vaisseaux d’une dimension supérieure à 5 mm, les scellements les plus résistants ont été obtenus
lorsque le mode d’hémostase avancée a complètement sectionné le vaisseau ciblé.
• Une utilisation prolongée du mode d’hémostase avancée peut endommager les tissus.
• Les produits fabriqués ou distribués par des sociétés non autorisées par Ethicon Endo-Surgery pourraient ne pas être
compatibles avec le système HARMONIC. L’utilisation de tels produits pourrait entraîner des résultats imprévus et infliger
de possibles blessures à l’utilisateur ou au patient.
• Si, lors de l’utilisation du générateur, le message « Advanced Features Are Not Available In This Device » (les options
avancées ne sont pas disponibles sur cet appareil) s’affiche, cela signifie que les fonctions suivantes de technologie
adaptative des tissus ne sont plus disponibles : Administration d’énergie régulée, rétroaction sonore améliorée et
hémostase avancée. Par conséquent, le dispositif n'est pas indiqué pour le scellement de vaisseaux don le calibre est
supérieur à 5 mm.
• Les instruments ou dispositifs qui entrent en contact avec les liquides corporels pourraient exiger une mise au rebut
particulière afin de prévenir toute contamination biologique.
• Une activation accidentelle et prolongée sur des surfaces solides, par exemple les os, pourrait faire chauffer la lame et
entraîner une défaillance; elle doit par conséquent être évitée.
• Mettez au rebut tous les instruments ayant été ouverts, qu’ils aient été utilisés ou non.
• Cet appareil est emballé et stérilisé dans le but d’un usage unique seulement. Une utilisation sur de multiples patients
pourrait compromettre l’intégrité du dispositif ou créer un risque de contamination qui, en retour, pourrait provoquer des
blessures ou des maladies pour le patient.
Page 16

Cisailles HARMONIC ACE®+7 retraitées de 5 mm de diamètre
pour hémostase avancée Page 16 de 19
Mode d’emploi
1. Vérifiez la compatibilité de tous les instruments et accessoires avant d’utiliser l’instrument (consultez la section
Avertissements et précautions).
2. La pièce à main est expédiée non stérilisée. Elle doit être stérilisée avant chaque usage conformément au mode d’emploi
fourni avec la pièce à main.
Assemblage
1. Au moyen d’une technique stérile, retirez l’instrument de l’emballage. Afin d’éviter tout dommage, ne retournez pas
l’instrument dans le champ stérile.
2. Tout en maintenant la pièce à main verticalement, fixez-la à l’instrument en faisant pivoter l’instrument dans la pièce à
main dans le sens horaire tel que vu à partir de l’extrémité distale de l’instrument (serrer au doigt uniquement).
3. Utilisez la clé dynamométrique (déjà montée sur la tige) pour serrer la lame dans la pièce à main. Tournez la clé
dynamométrique dans le sens horaire tout en maintenant la pièce à main de couleur grise jusqu’à entendre un double dé
clic indiquant qu’un couple suffisant a été appliqué pour sécuriser la lame.
4. Remarque : N’utilisez que la clé dynamométrique pour fixer ou détacher l’instrument de la pièce à main.
5. Remarque : Ne serrez pas l’instrument manuellement sans la clé dynamométrique, au risque d’endommager la pièce à main.
6. Remarque : Maintenez seulement la pièce à main grise et non la tige de l’instrument lorsque vous utilisez la clé
dynamométrique (Illustration 2).
7. Fermez le déclencheur. Retirez la clé dynamométrique en la faisant glisser hors de la tige. Ne mettez pas au rebut la clé
dynamométrique jusqu’à ce que la procédure soit terminée. La clé dynamométrique est utilisée pour retirer l’instrument
de la pièce à main conformément à la procédure (Illustration 3). Ne mettez au rebut la clé dynamométrique qu’une fois la
procédure terminée.
8. Remarque : Prenez soin de ne pas endommager la lame et le bras de serrage lorsque vous fermez le déclencheur en
glissant la clé dynamométrique sur la tige ou hors de la tige.
9. Remarque : Veillez à ne pas infliger de lésions causées par l’extrémité de la lame lorsque vous glissez la clé
dynamométrique sur la tige ou hors de la tige.
Opération
1. Connectez la pièce à main assemblée et l’instrument au générateur et mettez le générateur en marche.
Remarque : MIN est le seul mode qui peut être ajusté.
2. Choisissez le niveau de puissance minimal voulu à l’aide des boutons « INCREASE » (augmenter) et « DECREASE »
(diminuer) qui apparaissent à l’écran du générateur.
Remarque : Le niveau de puissance minimal de démarrage recommandé est 3 (Illustration 4). Pour une vitesse accrue de
coupe des tissus, utilisez un niveau de puissance plus élevé du générateur et pour une coagulation accrue, utilisez plutôt
un niveau de puissance plus faible. La quantité d’énergie administrée aux tissus et les effets obtenus dépendent de
nombreux facteurs, incluant le niveau de puissance sélectionné, les caractéristiques de la lame, la force de prise, la tension
des tissus, le type de tissu, la pathologie et la technique chirurgicale.
3. Pour atteindre un rendement optimal et pour éviter l’adhérence des tissus, nettoyez la lame de l’instrument, le bras de
serrage et l’extrémité distale de la tige tout au long de la procédure en activant l’embout de l’instrument dans une solution
saline (Illustration 5).
Remarque : L’instrument ne doit pas entrer en contact avec du métal lorsqu’il est en fonction (Illustration 6).
Remarque : Ne nettoyez pas le bout de la lame avec des abrasifs. Au besoin, vous pouvez l’essuyer à l’aide d’une éponge de
gaze humide pour en retirer les tissus.
Si les tissus sont toujours visibles dans le bras de serrage, utilisez des pinces hémostatiques pour supprimer les résidus en
veillant à ne pas actionner la pièce à main. Si vous le désirez, vous pouvez débrancher l’instrument (Illustration 7).
4. La lame est activée par ultrasons lorsque vous enfoncez la pédale ou un des boutons de commande manuelle. Enfoncer la
pédale du côté gauche ou le bouton de commande manuelle inférieur (MIN) de l’instrument active le niveau de puissance
minimal sélectionné. Enfoncer la pédale du côté droit ou le bouton de commande manuelle supérieur (MAX) de
l’instrument active le niveau de puissance maximal. Enfoncer le bouton d’hémostase avancée situé sur l’instrument active
l’hémostase avancée. Sous les paramètres par défaut, l’hémostase avancée ne peut s’activer que manuellement.
Remarque : Le bouton « MIN » peut être réaffecté à la fonction d’hémostase avancée en sélectionnant « Convert MIN to
Advanced Hemostasis » (convertir le bouton MIN à Hémostase avancée) de l’écran « Settings » (paramètres) du GEN11.
Consultez la section « Paramètres » du manuel de l’opérateur du générateur G11 (Illustration 8). Lorsque « Convert MIN to
Advanced Hemostasis » est sélectionné, le bouton Advanced Hemostasis (hémostase avancée) est désactivé.
Remarque : En mode d’hémostase avancée, l’énergie est administrée uniquement lorsque les mâchoires sont entièrement
fermées.
Page 17

Cisailles HARMONIC ACE®+7 retraitées de 5 mm de diamètre
pour hémostase avancée Page 17 de 19
Mise en garde : Si l’activation est arrêtée involontairement lors du scellement, maintenez la fermeture de la mâchoire et
réactivez.
Remarque : Le générateur émet une tonalité pour indiquer que la lame de l’instrument est active. Le générateur passe alors à
une seconde tonalité d’activation à mesure que la technologie adaptative des tissus contrôle l’administration de l’énergie.
• Les influences thermiques, comme les liquides et la présence d’un minimum de tissus ou l’absence de tissus
dans les mâchoires, peuvent avoir une incidence sur la présence ou le moment où la tonalité change.
• Le changement de tonalité ne fournit aucune confirmation de l’effet sur les tissus. Lorsque vous entendez la
seconde tonalité, la situation doit être évaluée et l’action chirurgicale prévue est terminée, telle que
l’application graduelle de tension pour faciliter la transsection.
• Le changement de la tonalité d’activation secondaire n’est pas un substitut à l’expérience chirurgicale.
Remarque : Les éraflures sur la lame peuvent provoquer une défaillance prématurée de cette dernière.
• Évitez tout contact accidentel avec d’autres instruments durant l’utilisation.
• N’utilisez que la clé dynamométrique pour fixer ou détacher l’instrument de la pièce à main.
5. Fermez le bras de serrage en refermant le déclencheur et insérez la tige à travers un trocart ou une incision.
6. L’instrument peut être utilisé pour la dissection, la saisie, la coagulation et la coupe entre la lame et le bras de serrage.
Remarque : Pour obtenir une étanchéité complète, le déclencheur doit être complètement fermé et le vaisseau
entièrement contenu entre le bras de serrage et la lame du dispositif. Un « dé clic » audible et tactile indique une fermeture
complète du déclencheur. Pour obtenir une fermeture complète des mâchoires du dispositif, serrez le déclencheur en
plastique jusqu’à ce qu’il bute contre la tige de plastique (plastique à plastique) (Illustration 9). Si la fermeture complète
du déclencheur est dégagée avant ou durant l’activation sur les tissus, un « dé clic » audible et tactile est évident.
Augmentez la force de saisie jusqu’à obtenir la fermeture complète du déclencheur.
Remarque : Maintenez le bras de serrage ouvert lorsque vous utilisez la partie inférieure interne de la lame pour une
coupe inverse (Illustration 10).
AVERTISSEMENT : N’utilisez pas le mode d’hémostase avancée pour les procédures où une application d’énergie est
requise avant la fermeture complète des mâchoires (p. ex., dans le cas des organes pleins). En mode d’hémostase avancée,
l’énergie est administrée uniquement lorsque les mâchoires sont entièrement fermées.
AVERTISSEMENT : Durant les essais sur des vaisseaux d’un calibre supérieur à 5 mm, les scellements les plus résistants
ont été obtenus lorsque le mode d’hémostase avancée a complètement sectionné le vaisseau ciblé.
AVERTISSEMENT : Une utilisation prolongée du mode d’hémostase avancée peut endommager le tampon de tissus.
7. Fermez le bras de serrage en refermant le déclencheur et retirez la tige du trocart ou de l’incision.
Rotation de la tige
1. La tige de l’instrument peut pivoter sur 360° pour faciliter la visualisation et l’accès aux tissus cibles lors de la dissection,
de la saisie, de la coagulation et de la coupe.
Démontage
1. Éteignez le générateur (« OFF ») à la prise d’alimentation.
2. Fermez le bras de serrage et glissez la clé dynamométrique sur l’extrémité distale et tout le long de la tige jusqu’à ce que la
clé soit alignée avec les parties plates de la tige. Maintenez l’instrument par la pièce à main uniquement et non par sa tige,
puis desserrez-le en tournant la clé dans le sens antihoraire. Continuez à desserrer en tournant à la main le bouton de
rotation pour complètement dévisser l’instrument. Ne desserrez pas l’instrument manuellement sans la clé
dynamométrique, au risque d’endommager la pièce à main.
Remarque : N’utilisez que la clé dynamométrique pour détacher l’instrument de la pièce à main.
Remarque : Veillez à ne pas infliger de lésions avec l’extrémité de la lame lorsque vous glissez la clé dynamométrique sur
la tige ou hors de la tige.
3. Retirez la clé dynamométrique en la tirant en ligne droite par-dessus la lame.
4. Mettez l’instrument au rebut dans un récipient approprié.
Présentation
Les cisailles HARMONIC ACE®+7 retraitées de 5 mm de diamètre pour hémostase avancée sont livrées stériles et destinées à un
usage unique. Chaque instrument est expédié avec une clé dynamométrique stérile de couleur grise, jetable et à usage unique.
Entreposage et manipulation
• Température : -20 °C à +50 °C
• Humidité relative : 25 à 90 %
Page 18

Cisailles HARMONIC ACE®+7 retraitées de 5 mm de diamètre
pour hémostase avancée Page 18 de 19
Compatibilité
Les cisailles HARMONIC ACE®+7 retraitées de 5 mm de diamètre pour hémostase avancée ne doivent être utilisées qu’avec le
logiciel du générateur G11 (GEN11) version 2013_1, 2014_1 ou 2016_1. La révision logicielle se trouve sous « System
Information » (informations système) dans le menu « Settings » (paramètres) du générateur G11 (GEN11). Consultez le
manuel de l’opérateur du générateur G11 (GEN11) pour obtenir de plus amples renseignements.
Garantie
Produits retraités
Stryker garantit que tous les produits retraités, sous réserve des exceptions mentionnées aux présentes, sont exempts de
défectuosités dans le retraitement et qu’ils sont essentiellement conformes aux spécifications de produit contenues dans la
documentation fournie par Stryker avec les produits concernant un usage unique conforme aux modes d’emploi d’un tel produit.
STRYKER NE PEUT ÊTRE TENU RESPONSABLE D’UN QUELCONQUE DOMMAGE CAUSÉ PAR UNE
DÉFECTUOSITÉ DANS LE MATÉRIEL, LA MAIN-D’OEUVRE OU LA CONCEPTION PAR LE FABRICANT D’ORIGINE
DU PRODUIT OU PAR TOUT ACTE OU OMISSION DU FABRICANT D’ORIGINE DU PRODUIT.
Produits pour lesquels Stryker est le fabricant d’origine
Stryker garantit que tous les produits pour lesquels il est fabricant d’origine, sous réserve des exceptions mentionnées aux
présentes, sont exempts de défectuosités dans la conception, les matériaux et la main-d’oeuvre et qu’ils sont essentiellement
conformes aux spécifications de produit contenues dans la documentation fournie par Stryker avec de tels produits pour une
période d’un an à partir de la date de l’achat.
Modalités générales de garantie applicables à tous les produits
DANS TOUTE LA MESURE PERMISE PAR LA LOI, LA GARANTIE EXPRESSE MENTIONNÉE AUX PRÉSENTES EST
LA SEULE GARANTIE APPLICABLE AUX PRODUITS ET REMPLACE EXPRESSÉMENT TOUTE AUTRE GARANTIE
DE STRYKER, EXPRESSE OU IMPLICITE, INCLUANT, MAIS SANS S’Y LIMITER, TOUTE GARANTIE IMPLICITE OU
DE QUALITÉ MARCHANDE OU D’ADÉQUATION À DES FINS PARTICULIÈRES. EN AUCUN CAS LA
RESPONSABILITÉ DE STRYKER EN LIEN AVEC LA VENTE DU PRODUIT (QUE CE SOIT DANS LE CADRE DES
THÉORIES DE LA RUPTURE D’UN CONTRAT, D’UN TORT, D’UNE FAUSSE REPRÉSENTATION, D’UNE FRAUDE,
D’UNE GARANTIE, D’UNE NÉGLIGENCE, D’UNE RESPONSABILITÉ STRICTE OU DE TOUTE AUTRE THÉORIE DU
DROIT) NE PEUT ÊTRE SUPÉRIEURE AU PRIX D’ACHAT, À LA VALEUR ACTUELLE DU MARCHÉ OU À LA
VALEUR RÉSIDUELLE DES PRODUITS, SELON LE MOINDRE DE CES CAS. STRYKER NE PEUT ÊTRE TENUE
RESPONSABLE DE TOUT DOMMAGE INDIRECT, SPÉCIAL, ACCESSOIRE, PUNITIF OU CONSÉCUTIF RÉSULTANT
D’UNE QUELCONQUE VIOLATION DE GARANTIE DANS LE CADRE DE TOUTE AUTRE THÉORIE JURIDIQUE.
Cette garantie ne s’applique qu’à l’utilisateur final d’origine des produits achetés directement chez Stryker ou chez un
distributeur Stryker agréé. Cette garantie ne peut pas être transférée ou affectée sans le consentement écrit express de Stryker.
Cette garantie ne s’applique pas à : (1) des produits mal utilisés, négligés, modifiés, altérés, ajustés, trafiqués, incorrectement
installés ou remis en état; (2) des produits qui ont été réparés par toute autre personne que le personnel de Stryker sans le
consentement écrit préalable de Stryker; (3) des produits sujets à un stress inhabituel ou qui n’ont pas été entretenus
conformément aux instructions du manuel de l’utilisateur ou tel que démontré par un représentant de Stryker; (4) des
produits sur lesquels tous les numéros de série d’origine ou autres marques d’identification ont été retirés ou détruits; ou
(5) des produits réparés à l’aide de composants non autorisés ou non fabriqués par Stryker.
Si une réclamation valide au titre de la garantie est reçue dans les trente (30) jours de l’expiration de la période de garantie en
vigueur, Stryker pourra, à sa seule discrétion : (1) remplacer le produit sans frais par un produit dont la fonctionnalité est au
moins équivalente à celle du produit d’origine ou (2) rembourser le prix d’achat du produit. Si un remboursement est fourni
par Stryker, le produit pour lequel le remboursement est décidé doit être retourné à Stryker et deviendra la propriété de
Stryker. Dans tous les cas, la responsabilité de Stryker pour une violation de la garantie se limitera à la valeur de
remplacement de la pièce ou du composant défectueux ou non conforme.
Page 19

Cisailles HARMONIC ACE®+7 retraitées de 5 mm de diamètre
pour hémostase avancée Page 19 de 19
Si Stryker détermine à sa seule discrétion que la défectuosité ou la non-conformité invoquée du produit est exclue de la
garantie comme il est décrit aux présentes, elle avisera le client d’une telle décision et lui fournira une évaluation des coûts de
réparation du produit. Dans un tel cas, toute réparation sera effectuée aux tarifs standard de Stryker.
Les produits et les composants de produit réparés ou remplacés dans le cadre de cette garantie continueront à être garantis tel
que décrit aux présentes durant toute la période de garantie applicable initiale ou, si la période de la garantie initiale est
expirée au moment où le produit est réparé ou remplacé, pour une période de trente (30) jours après la livraison du produit
réparé ou remplacé. Lorsqu’un produit ou un composant est remplacé, l’article fourni en remplacement deviendra la propriété
du client et l’article remplacé la propriété de Stryker. Si un remboursement est fourni par Stryker, le produit pour lequel le
remboursement est décidé doit être retourné à Stryker et deviendra la propriété de Stryker.
HARMONIC® et HARMONIC ACE® sont des marques de commerce d’Ethicon Endo-Surgery
ULS EL10053 Rév. E 10/2017 RM702506
Les informations concernant le fabricant d’équipement d’origine fournies sur l’étiquette apparaissent sous forme d’ID de dispositif avant
le retraitement et pourraient contenir les marques de commerce de tierces parties non liées qui ne commanditent pas ce dispositif.
Stérilisation : Ce produit et son emballage ont été stérilisés à l’oxyde d’éthylène (EtO). Bien que ce produit ait été traité conformément à
toutes les lois et à tous les règlements applicables concernant l’exposition à l’EtO, la Proposition 65, une initiative des électeurs de l’État
de Californie, requiert l’avis suivant :
Avertissement : Ce produit et son emballage ont été stérilisés à l’oxyde d’éthylène (EtO). L’emballage pourrait vous exposer à l’oxyde
d’éthylène, un produit chimique reconnu par l’État de Californie comme cause de cancer ou d’anomalies congénitales ou autres
anomalies de la reproduction.
 Loading...
Loading...