Stryker Crossfire 2 User Manual

Crossre® 2
Integrated Resection and Energy System
0475-100-000


EN-1
Table of Contents
Warnings and Cautions ............................................................................................3
Product Description/Intended Use ........................................................................6
Indications ...................................................................................................................................7
Contraindications ......................................................................................................................7
Package Contents ......................................................................................................................7
Available Accessories ...............................................................................................................7
The Crossre 2 Console ...........................................................................................................8
Setup and Device Connections ............................................................................ 11
Connecting to the iSWITCH Wireless Footswitch ........................................................13
Operation ................................................................................................................. 14
Powering the Console On and O ...................................................................................14
Adjusting User and System Settings ...............................................................................15
Arthroscopy Shaver Controls .............................................................................................16
RF Ablation Controls .............................................................................................................19
Dual Controls ...........................................................................................................................23
Troubleshooting ..................................................................................................... 25
Error Codes ...............................................................................................................................26
Cleaning and Maintenance ................................................................................... 27
Cleaning .....................................................................................................................................27
Maintenance ............................................................................................................................ 27
Disposal......................................................................................................................................28
Technical Specications ........................................................................................ 29
Generator Output ...................................................................................................................30
Classications ..........................................................................................................................32
Radio Equipment Directive Compliance ........................................................................33
Electromagnetic Compatibility ......................................................................................... 34
Symbols .................................................................................................................... 37


EN-3
Warnings and Cautions
Caution: Federal law (USA) restricts this device to use by, or on order of, a physician.
Operator Prole
The Crossre2 system is intended for use only by licensed medical professionals, properly
trained in the use of arthroscopic and electrosurgical equipment and techniques. The Crossre2
system generates potentially hazardous levels of energy that can result in injury or even death if
improperly used.
General Warnings
To avoid potential serious injury to the user and the patient, observe the following warnings:
1. Read this manual thoroughly and be familiar with its contents prior to operating the
equipment.
2. Carefully unpack the device and ensure that all components are accounted for and remain
undamaged from shipment.
3. Inspect all handpieces and probes for damage to the cable insulation. If damage is found,
refer to the Stryker Standard Warranty and Return Policy (1000-401-175).
4. Before using the Crossre2 system in an actual procedure, verify that each component is
installed and functioning properly. Improper connection may cause arcing or malfunction of
the handpiece or console, which can result in injury, unintended surgical eect, or product
damage.
5. Do not use the Crossre2 system on patients with cardiac pacemakers or other electronic
device implants. Doing so could lead to electromagnetic interference and possible death.
6. Do not attempt to reuse or resterilize any product labeled “Single-Use,” as this may lead to
equipment malfunction, patient/user injury, and/or cross-contamination.
7. Shaver handpieces are provided nonsterile and must be cleaned and sterilized prior to each
use, according to the reprocessing instructions provided in the handpiece manual.
8. Do not use the Crossre2 system with non-conductive irrigants (e.g. sterile water, air, gas,
glycine, etc.). Use only conductive irrigants such as saline or Ringer’s lactate in order for the
system to function properly.
9. Do not activate the Crossre2 system for prolonged lengths of time when the attachment is
not in contact with tissue. Doing so may lead to unintentional damage to surrounding tissue.
10. Do not obstruct the fans located near the rear and side of the console. Position the console
so the fan directs the ow of air away from the patient.
11. Keep the activation indication lights and speaker in eld of view and hearing at all times
during activation. The light and sound are important safety features.

EN-4
Fire/Burn Warnings
1. Do not use this device in the presence of ammable anaesthetics, gases, or uids, such as
skin prepping agents and tinctures. Observe appropriate re precautions at all times.
2. To prevent the risk of explosion, do not use this device in oxygen-enriched atmospheres,
nitrous oxide (NO) atmospheres, or in the presence of other oxidizing agents. Ensure that
oxygen connections in the surgical environment are not leaking.
3. Electrosurgical components, such as the RF probe, may remain hot after activation. To avoid
combustion, keep all electrosurgical equipment away from ammable materials.
4. Do not use ammable agents for cleaning and disinfection of the Crossre2 console,
handpiece, or footswitch.
5. To prevent the risk of re, do not replace console fuses. If it is suspected that fuses are
damaged, return the console to Stryker for repair.
Electrical Safety Warnings
1. Install this device in an operating room that complies with all applicable IEC, CEC, and NEC
requirements for safety of electrical devices.
2. Crossre2 system components are designed to be used together as a system. Use only the
appropriate footswitch, handpiece, and disposable attachments described in this manual.
3. When the Crossre2 system is activated, the conducted and radiated electrical elds may
interfere with other electrical medical equipment. Provide as much possible distance
between the console and other electronic medical equipment.
4. Connect the power cord to a grounded receptacle. To prevent risk of electric shock, do not
use extension cords or adapter plugs.
5. Do not wrap the handpiece cable around metal objects, or the induction of hazardous
currents may result.
6. Keep the ends of the handpiece cable connectors, footswitch cable connectors, and console
receptacles away from all uids.
7. During use, the RF and shaver handpieces generate electronic noise that may interfere with
EKG readings. Before responding to any erratic EKG readings, rst power down the system to
ensure the readings are not the result of system noise.
Electrosurgery Warnings
1. Inspect electrosurgical accessories for defects prior to use. Do not use any cable or electrode
that is cut, broken, or otherwise damaged, as burns or electric shock may result.
2. Position the cables to avoid contact with the patient, electrodes, cables, and any other
electrical leads that provide paths for high frequency current.
3. To prevent the risk of shock, do not allow the patient to come into contact with grounded
metal objects or objects that have an appreciable capacitance to the earth, such as a surgical
table frame or instrument table. The use of antistatic sheeting is recommended for this
purpose.

EN-5
4. When the Crossre2 system and physiological monitoring equipment are used
simultaneously on a patient, position any monitoring electrodes as far as possible from the
surgical electrodes. Monitoring equipment using high frequency, current-limiting devices is
recommended. Needle monitoring electrodes are not recommended.
5. During use, operators should wear standard surgical gloves to help reduce the risk of electric
shock.
6. To prevent patient injury, select the lowest output power required for the intended purpose.
7. Do not exceed the rated accessory voltage of electrosurgical accessories. Only use
electrosurgical accessories that have a rated accessory voltage equal to or greater than the
maximum output voltage of the generator.
8. Do not activate the Crossre2 system until the probe is properly positioned in the patient.
9. Ensure that the probe tip, including the return electrode, is completely surrounded by
irrigant solution during use.
10. Maintain the active electrode in the eld of view at all times to avoid tissue damage.
11. Keep active electrodes isolated from the patient when not in use.
12. When not in use, remove the handpiece and disposable attachments from the surgical
site and place them away from metallic objects. Attachments should be separated from
other electrosurgical equipment to avoid inadvertent electrical coupling between devices.
Inadvertent activation may cause user/patient injury and/or product damage.
13. Failure of the system may result in an unintended increase in output power.
14. Neuromuscular stimulation may occur when RF probes are used.
15. Smoke generated during electrosurgical procedures may be harmful to surgical personnel.
Take appropriate precautions by wearing surgical masks or other means of protection.
Cautions
To avoid product damage, observe the following cautions.
1. While using the handpiece, do not touch the attachment to metal objects, such as an
endoscope or metal cannula. Damage to the attachments or other devices may result.
2. Attempt no internal repairs or adjustments, unless specied otherwise in this manual. Units
requiring repair should be returned to Stryker.
3. Pay close attention to the care and cleaning instructions in this manual. Failure to follow
these instructions may result in product damage.
4. Do not remove the cover of the console as this could cause electric shock and product
damage.
EN-6
Product Description/Intended Use
The Crossre2 Integrated Resection and Energy System is a combination powered shaver system/
electrosurgical generator that powers arthroscopic shaver handpieces and RF surgical probes for
use in a variety of arthroscopic and orthopedic surgeries.
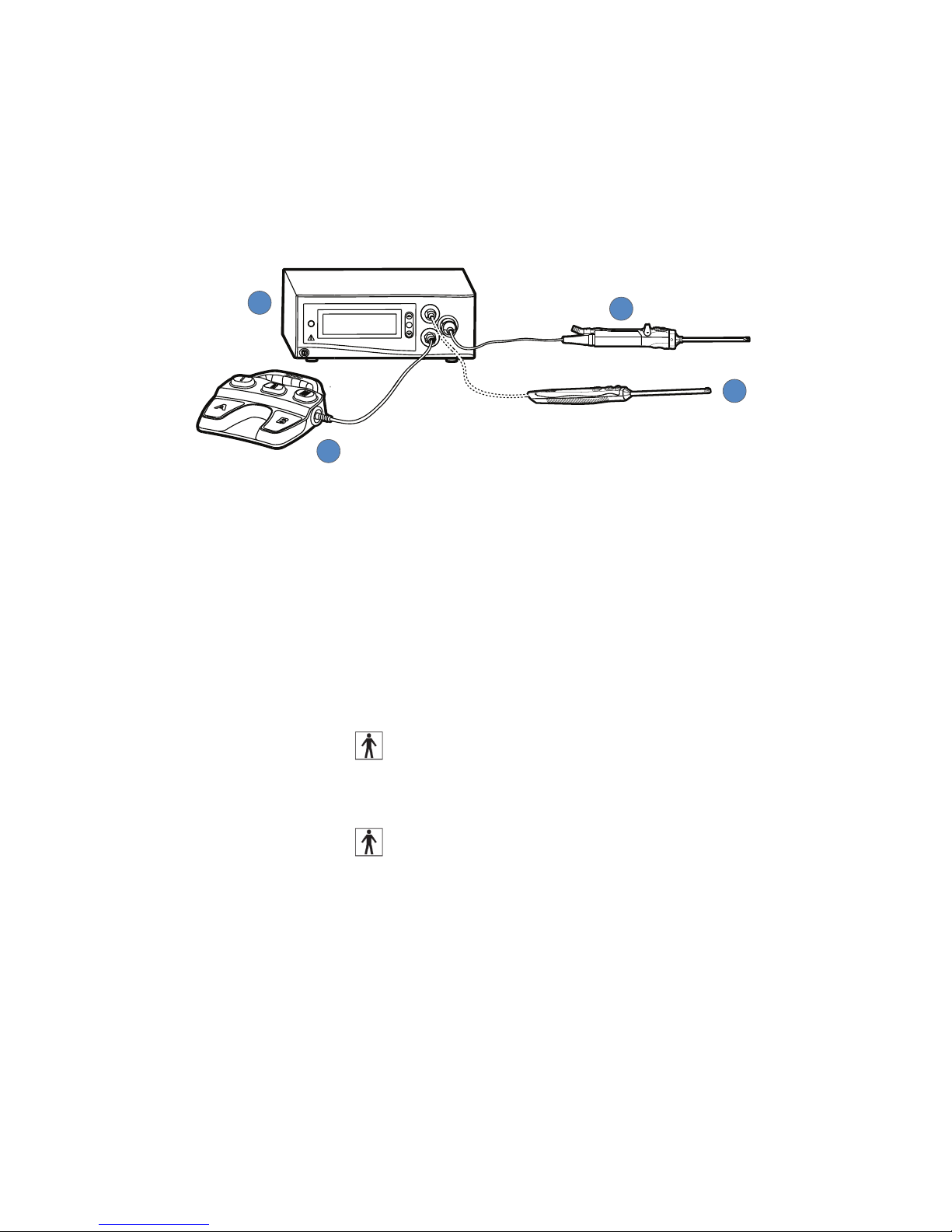
Illustrated below, the Crossre2 system consists of the following components:
1
2
3
4
1. Crossre2 Console (featured in this manual)
• Acts as a connection hub for the various components of the Crossre2 system
• Powers a motorized shaver handpiece for the mechanical cutting and debridement of
bone and soft tissue
• Generates bipolar radio frequency (RF) energy for electrosurgical cutting and
coagulation of tissue
• Provides a central user interface for operating the Crossre2 system
2. Powered Shaver Handpiece (and disposable attachments)
• Enables arthroscopic cutting and debridement
• Type BF applied part
3. Disposable RF Probe
• Enables RF cutting and coagulation
• Type BF applied part
4. Crossre Footswitch
Provides remote, foot control of the powered shaver handpiece and RF probe

EN-7
Indications
The Stryker Crossre 2 System is intended for use in orthopedic and arthroscopic procedures for
the following joints: knee, shoulder, ankle, elbow, wrist, and hip. The Crossre 2 System provides
abrasion, resection, debridement and removal of bone and soft tissue through its shaver blade;
and the ablation and coagulation of soft tissue, as well as hemostasis of blood vessels, through its
electrosurgical probe. Examples of uses of the product include resection of torn knee cartilage,
subacromial decompression, and resection of synovial tissue in other joints.
Contraindications
The electrosurgical probe should not be used in procedures where a nonconductive irrigant is
used or with patients having cardiac pacemakers or other electronic implants.
Package Contents
Carefully unpack the Crossre2 console and inspect each of the following components.
• (1) Crossre 2 Console
• (1) Hospital-grade power cord
• (1) User Guide
If damage is found, refer to the Stryker Standard Warranty and Return Policy (1000-401-175).
Available Accessories
The Crossre 2 system is compatible with the following accessories:
System Accessories
0475-000-100 Crossre Footswitch
0277-200-100 iSWITCH Universal Wireless Footswitch Receiver
0277-200-101 iSWITCH Universal Wireless Footswitch Receiver (AUS)
0277-100-100 iSWITCH Universal Wireless Footswitch
6000-001-020 Stryker rewire cable
Arthroscopy Accessories
0279-xxx-xxx SERFAS Energy family of electrosurgical probes
0375-708-500 Formula 180 Handpiece
0375-704-500 Formula Handpiece (with buttons)
0375-701-500 Formula Handpiece (without buttons)
0275-601-500 Small-Joint Shaver Handpiece
EN-8
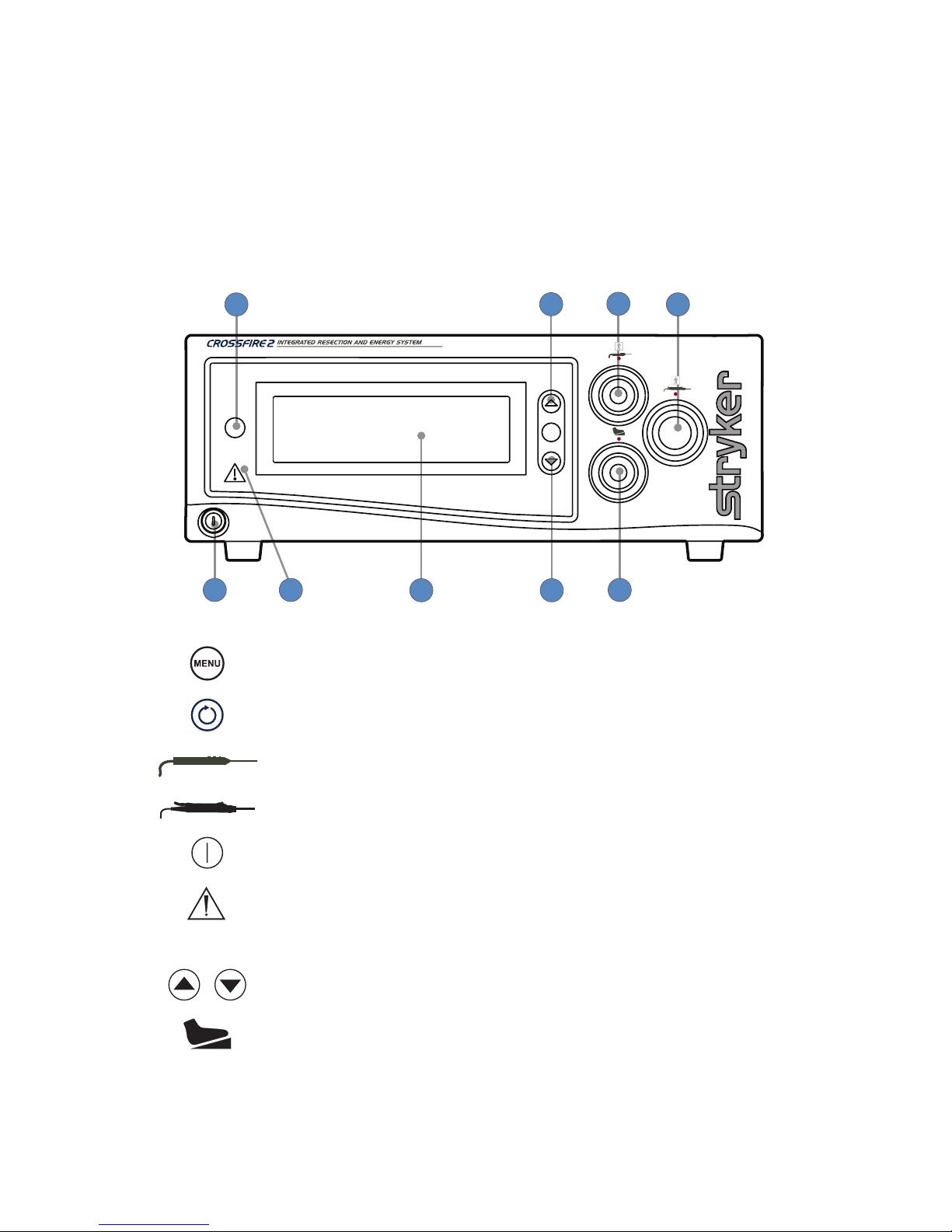
The Crossre 2 Console
The Crossre2 console is the connection hub for the components of the Crossre2 system. It
generates RF energy for ablation, powers motorized shavers, and provides user controls and
system feedback.
Front Panel
The front console panel features ports for connecting handpieces, controls for adjusting
handpiece settings, and an LCD screen to provide system feedback.
5
7 8
9
3
1 4
6
2
1. Menu Selects menu items
2. Select Selects which device displays on the LCD screen.
3. RF connector
(SERFAS Energy)
Delivers RF energy for ablation handpieces
4. Handpiece connector Powers shaver handpieces
5. Power Powers the console on and o
6. Error indicator Shines red to indicate errors (error details
appear in the LCD)
7. LCD screen Provides system feedback
8. Adjust Adjusts options for connected devices
9. Footswitch connector Connects to the Crossre Footswitch
EN-9
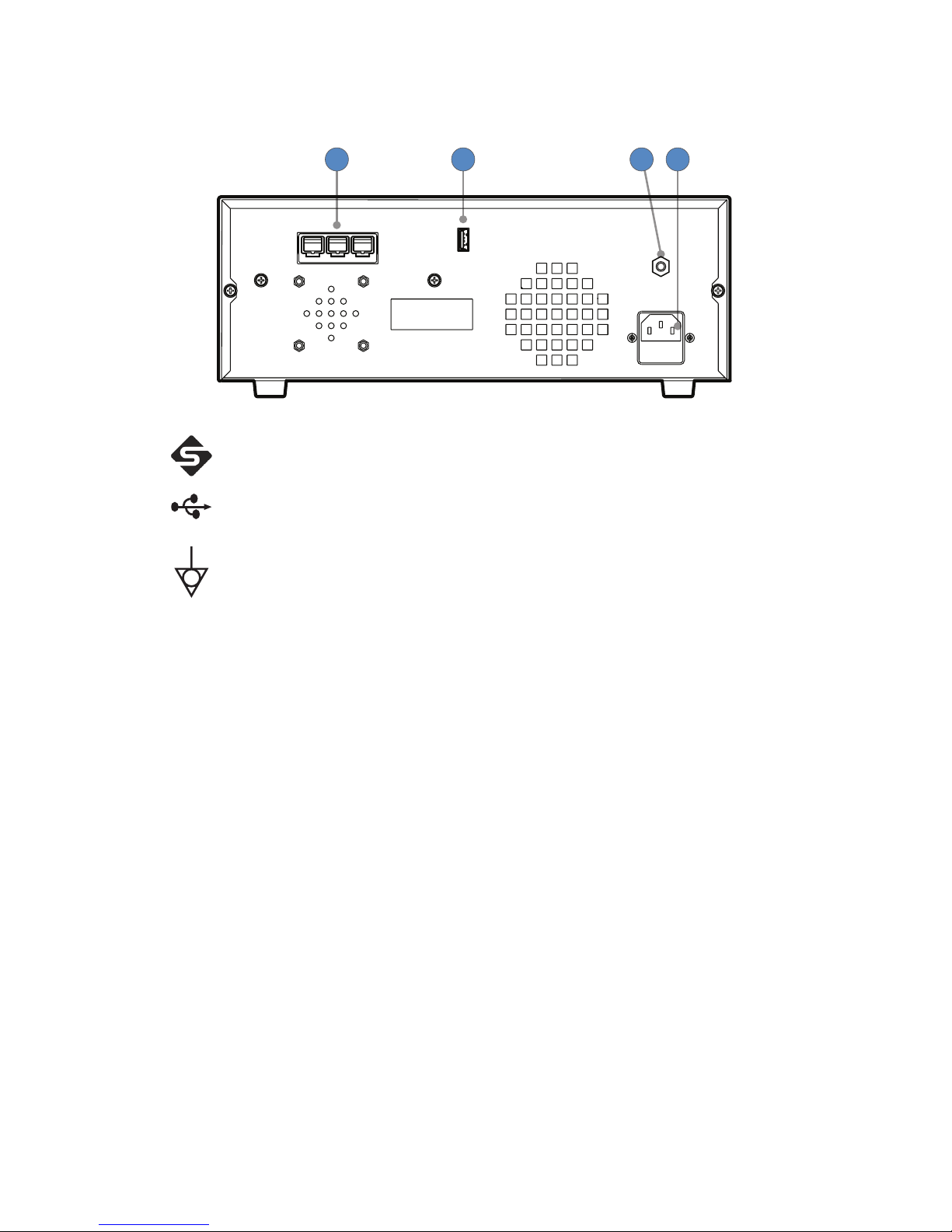
Rear Panel
The rear panel provides ports for connecting the console to other Stryker equipment.
321 4
1. Firewire
Connectors
Enables connection to other Stryker Firewire devices, such
as the iSWITCH Universal Wireless Footswitch
2. USB Drive Enables software installation from authorized service
personnel
3. Equipotential
Ground Plug
—
4. AC Power Inlet —
EN-10
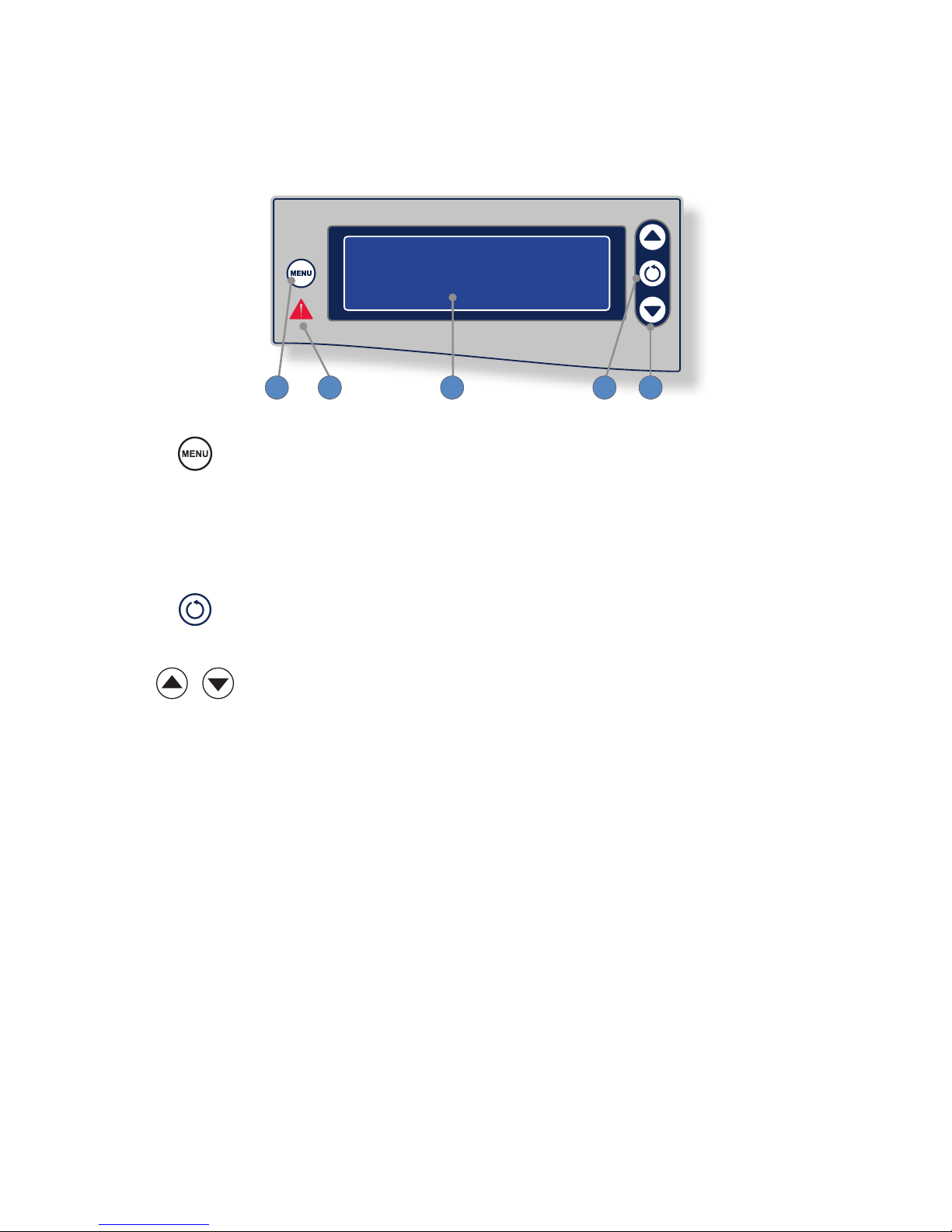
Interface
The Crossre2 interface displays system status, enables you to choose between RF ablation
and shaver modes, and enables you to adjust power and speed settings. Activating the actual
handpieces is performed through controls on the handpiece and on the Crossre Footswitch.
1 2 3 54
1. Menu The Menu button opens a menu for selecting user
and system settings.
2. Error indicator The Error indicator shines red when a system error
occurs.
3. LCD screen The LCD screen displays system status, error codes,
mode of operation, cutting speed, and power levels.
4. Select The Select button toggles between RF and Shaver
controls. The selected device can then be controlled
using the Crossre2 interface.
5. Adjust The Adjust buttons increase/decrease speed and
power settings for the selected device.
EN-11
Setup and Device Connections
Stryker Endoscopy considers instructional training an integral part of the Crossre2 system. Your
Stryker Endoscopy sales representative will perform at least one inservice at your convenience
to help you set up your equipment and instruct you and your sta on its operation and
maintenance. Please contact your local Stryker Endoscopy representative to schedule an inservice after your equipment has arrived.
Warning
• Be sure that no liquid is present between connections to the console and the
handpiece. Connection of wet accessories may lead to electric shock or electrical
short.
• To avoid the risk of electric shock, this equipment must only be connected to a
supply mains with protective earth.
• Use only hospital-grade power cables. Using other cables may result in increased RF
emissions or decreased immunity from such emissions.
• Only the handpieces and disposable attachments are suitable for use in the patient
environment. The console and footswitch are not sterile devices and should not enter
the sterile eld.
• The Crossre2 System is compatible only with the Stryker handpieces and
footswitches listed in this manual. Do not connect any equipment not specied in
this manual, as unexpected results or serious injury will occur.
• The separable AC power cord is provided as a means of emergency shutdown and
disconnection from the power source. Do not position the console in a way that is
dicult to disconnect the AC power cord.
1. Place the console on a sturdy platform, such as a Stryker cart.
• Select a location according to the recommendations in the “Electromagnetic
Compatibility” section of this user guide.
• Leave four inches of space around all sides for convection cooling.
• Do not obstruct the fans located near the rear and side of the console. Position the
console so the fan directs the ow of air away from the patient.
• Keep the activation indication lights and speaker in eld of view and hearing at all
times during activation. The light and sound are important safety features.
EN-12
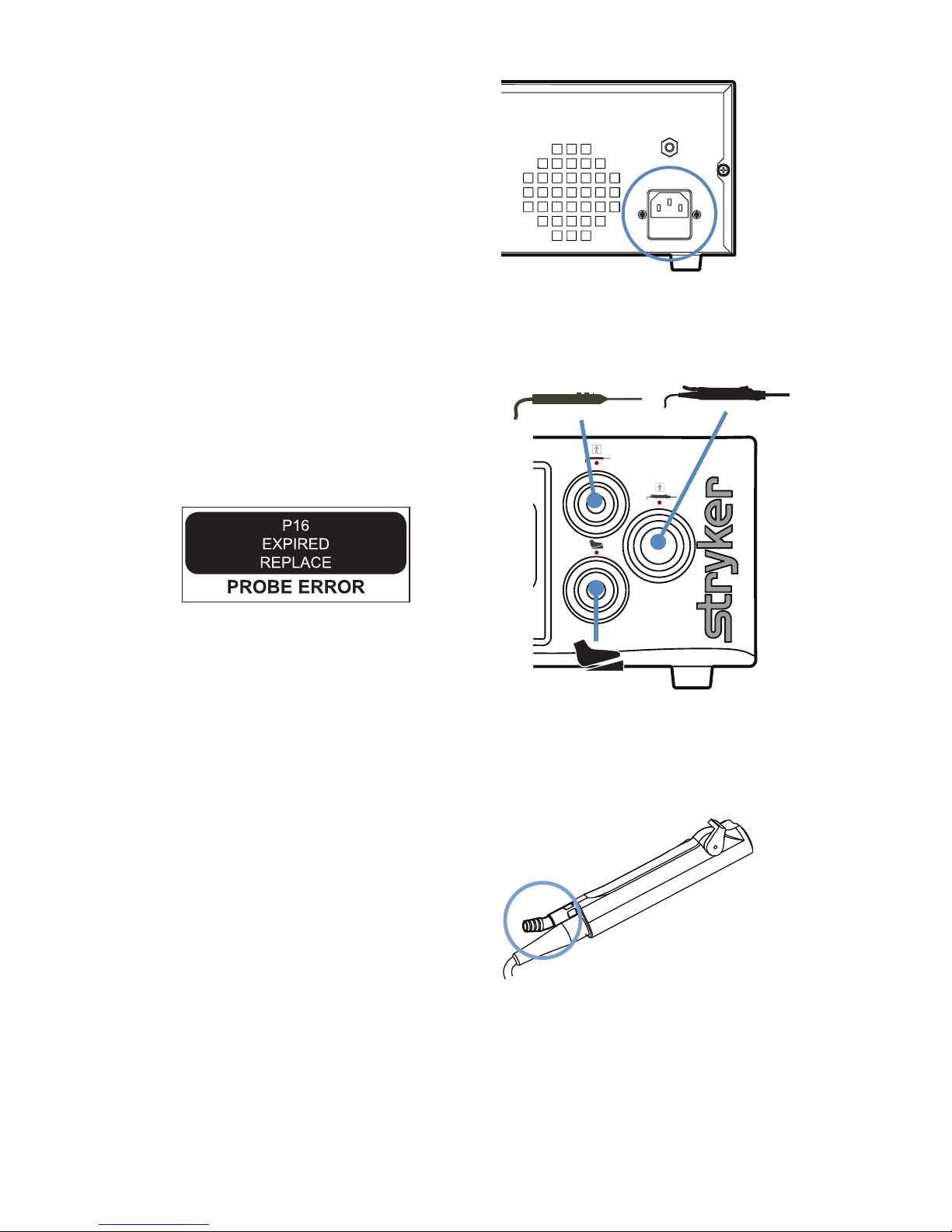
2. Connect the AC power.
3. Connect the handpieces and footswitch.
Note: The console will display an error
message if expired or used attachments are
connected:
4. Connect suction tubing (for all suctioncapable devices).
 Loading...
Loading...