Page 1

Roche Molecular Biochemicals
LightCycler Oper ator’s Manual
Version 3.0
May 1999
Page 2

Prologue
The LightCycler Instrument of Roche Molecular Biochemicals, Mannheim, Germany, is a thermocycler for the rapid analysis of PCR applications.
LightCycler technology is the most innovative and rapid possibility for carrying
out and simultaneously evaluating PCR experiments. 30 amplification steps including analysis of results can be carried out in less than 30 minutes. Fluorimetric
analysis of the PCR products formed is performed as real-time measurement either continuously or at a specifically defined time during each PCR cycle. The
analyses can be monitored online by the LightCycler’s user-friendly software, i.e.
directly during the reaction.
This Operator’s Manual contains all the necessary technical information required
to place the LightCycler instrument in operation and to carry out practical analyses. It contains background information on some of the applications and enables
the quick and safe optimization of individual applications.
Page 3
Symbols and Notes
Symbols and
Headings
Trademarks
and License
Disclaimers
The following symbols and headings are used in this manual:
Symbol Heading Description
WARNING This heading and symbol are
used to indicate that noncompliance with instructions
or procedures may lead to injury or even death.
CAUTION This heading and symbol are
used to indicate that noncompliance with instructions
or procedures may cause damage to the instrument.
NOTE This heading is used to bring
your attention to topics of importance
• The LightCycler technology is licensed from Idaho Technology Inc., Idaho
Falls, ID, USA
• LightCycler is a trademark of Idaho Technology Inc., Idaho Falls, ID, USA,
in the United States and some other countries.
• SYBR Green I is a trademark of Molecular Probes Inc., Eugene, OR, USA.
• TaqStart is a trademark of Clontech Laboratories, Inc., Palo Alto, CA, USA
• Expand is a trademark of a Member of the Roche Group.
For License statements, please refer to LightCycler reagent package inserts.
Page 4

LightCycler Software and License Agreement
Software License Agreement
Program License Agreement
Grant of License
READ THIS LICENSE AGREEMENT BEFORE REMOVING THE
PROGRAM CD (HEREINAFTER REFERRED TO AS PROGRAM MEDIA)
OR DOCUMENTATION FROM ITS PROTECTIVE COVER. REMOVING
THE PROGRAM MEDIA OR DOCUMENTATION WILL CONSTITUTE
AGREEMENT TO THE TERMS AND CONDITIONS OF THIS SINGLEUSE, END-USER LICENSE AGREEMENT. IF YOU ARE NOT WILLING TO
BE BOUND BY THE TERMS AND CONDITIONS OF THIS LICENSE
AGREEMENT, PROMPTLY RETURN THE UNOPENED PACKAGE TO
ROCHE DIAGNOSTICS GMBH WITH A COPY OF THE RECEIPT, AND
YOUR LICENSE FEE WILL BE REFUNDED.
You assume all responsibility and liability for the selection of this software
program, hereinafter referred to as Product, to achieve your intended results,
and for its installation and subsequent use.
Roche Diagnostics GmbH hereby grants to Licensee a non-exclusive, singleuse license to use the Product upon the terms and conditions contained in
this agreement. You may:
1. Use the Product on a single workstation owned, leased or otherwise con-
trolled by you, whether in a network or other configuration.
2. Make one (1) copy of the Product for backup purposes in support of your
use of the Product on the single workstation.
3. Transfer the Product and license to another party if the other party agrees
to accept the terms and conditions of this Agreement. If you transfer the
Product, you must, at the same time, either transfer thel copy of Product
to the same party, or destroy any copies not transferred.
You must reproduce and include the copyright notice on all copies of the
product documentation.
Continued on next page
Page 5
LightCycler Software and License Agreement,
Grant of Licence (continued)
YOU MAY NOT:
1. use or copy the Product, in whole or in part, except as expressly provided
in this Agreement,
2. use the Product on more than one workstation concurrently,
3. copy, rent, distribute, sell, license or sublicense, or otherwise transfer the
Product or this license, in whole or in part, to another party, except as
specifically set forth in this Agreement,
4. use the Product, or any portion of the Product, to develop, or incorporate
into, other software that can be executed without a licensed copy of the
Roche Diagnostics Image.
The use of the Product, or any portions of the Product, to develop, or incorporate into, other software capable to executing without a licensed copy of
Roche Diagnostics Image, is specifically prohibited under the terms of this
Agreement. Further, the use of the Product, or any portion of the Product on
more than one workstation at one time is specifically prohibited under the
terms of this Agreement. For further information, please contact
Terms
Roche Diagnostics GmbH
Roche Molecular Biochemicals
Sandhoferstraße 116
D-68298 Mannheim.
Germany
The license is effective until terminated. You may terminate this Agreement at
any time by destroying the Product together with the copy and documentation in any form. It will also terminate automatically and without notice from
Roche Diagnostics GmbH if you fail to comply with any term or condition of
this Agreement. You agree to destroy the Product and the copy ,if any, of the
Product upon termination of this Agreement.
Continued on next page
Page 6

LightCycler Software and License Agreement,
Limited Warranty
Limitations of
Remedies
The Product is provided as is without warranty of any kind, either expressed
or implied, including, but not limited to the implied warranties of merchantability and fitness for a particular purpose. The entire risk as to the quality and
performance of the Product is with you as Licensee, should the Product prove
to be defective. You assume the entire costs of all necessary servicing, repair,
or correction.
However, Roche Diagnostics GmbH warrants that the program media on
which the software is furnished is free from defects in materials and workmanship under normal use for a period of ninety (90) days from the date of
delivery as evidenced by a copy of your receipt. ROCHE DIAGNOSTICS
GMBH MAKES NO FURTHER WARRANTIES OR GUARANTEES NOR
EXPLICIT NOR IMPLIED.
Roche Diagnostics GmbH’s sole liability and your sole remedy shall be:
1. the replacement of the program media not meeting Roche Diagnostics
GmbH’ s limited warranty and which is returned to Roche Diagnostics
GmbH with a copy of your receipt;
2. if Roche Diagnostics GmbH is unable to deliver replacement program
media which is free of defects in workmanship, you may terminate this
Agreement by returning the Product and a copy of your receipt to Roche
Diagnostics GmbH, and your money will be refunded.
In no event will Roche Diagnostics GmbH be liable to you for any damages,
including any lost profits, lost savings, or other indirect, special, exemplary,
incidental or consequential damages, claims or actions, arising out of the use
or inability to use the Product, even if Roche Diagnostics GmbH has been advised of the possibility of such damages, claims or actions. Further, in no
event will Roche Diagnostics GmbH be liable for any claim by any other party
arising out of your use of the Product.
Continued on next page
Page 7

LightCycler Software and License Agreement,
General Information
You may not sublicence, assign or transfer the license or the Product, in
whole or in part, except as expressly provided in this Agreement. Any attempt
otherwise to sublicense, assign or transfer any of the rights, duties or obligations hereunder is void.
• This Agreement will be governed by the laws of Germany.
• Should any part of this agreement be declared void or unenforceable by a
court of competent jurisdiction, the remaining terms shall remain in full
force and effect.
• Failure of Roche Diagnostics GmbH to enforce any of its rights in this
Agreement shall not be considered a waiver of its rights, including but not
limited to its rights to respond to subsequent breaches.
By opening and using this software you acknowledge that you have read this
Agreement, understand it, and agree to be bound by its terms and conditions.
You further agree that this agreement is the complete and exclusive statement
of the Agreement between you and Roche Diagnostics GmbH and supersedes
any proposal or prior agreement, oral or written, any other communications
between you and Roche Diagnostics GmbH relating to the subject matter of
this Agreement.
Page 8

Chapter A
Technical Aspects of the LightCycler
Instrument
A 1
Page 9
1. Technical Aspects of the LightCycler Instrument
1.1 Table of Contents
1.
1.1
1.2
1.3
1.3.1
1.3.2
1.3.3
1.3.4
1.4
2.
2.1
2.2
3.
3.1
3.2
3.3
3.4
3.5
4. Operation and Maintenance of the LightCycler
Technical Aspects of the LightCycler Instrument
Table of Contents
LightCycler System
Technical Data
Specifications of the LightCycler
Specifications for Applications
Specifications for the Detectors
Temperature Control
Safety Precautions for the LightCycler
System Description
The LightCycler
PC Configuration
Installation
Installation Requirements
Installation of the LightCycler
Installation of the Computer
Installation of LightCycler Software Version 3
Dismantling the LightCycler System
Topic See Page
A 1
A 2
A 3
A 6
A 6
A 7
A 8
A 9
A 11
A 12
A 12
A 16
A 17
A 17
A 19
A 20
A 21
A 29
A 30
A 2
Page 10
1.2 LightCycler System
1
Note
Components
of the LightCycler System
Using the system improperly may compromise the integrity of the results, result in poor performance, or even permanently damage the equipment.
The components of the LightCycler System are listed in the following table.
Please note that these components may vary from country to country.
Component Description
System component 1
• LightCycler Instrument
• Sample Carousel (for Ø 1.5 mm capillary)1 pre-
mounted in LightCycler Instrument
System component 2
• LightCycler Capillaries (96 capillaries and stop-
pers/box)
• 32 LightCycler Centrifuge Adapters in an alumi-
num cooling block
1
1
• 1 LightCycler Operator’s Manual
• 1 LightCycler Software Package
1
• Cable to connect LightCycler Instrument to the
computer
• Power Cord (German)
• Power Cord (U.S.)
• Mouse Pad
Hardware
• PC with Pentium processor
2
• 64 MB SDRAM (minimum configuration)
• 24x CD-ROM drive
• Keyboard and PS/2 mouse
• Internal Iomega ZIP drive
Operating system
Monitor
Printer
• Windows NT 4.0, including Service Pack III
• 17" monitor
• Hewlett-Packard Color Inkjet Printer
2
2
Also available as single components:
LightCycler Sample Carousel
LightCycler Capillaries
LightCycler Centrifuge Adapters
LightCycler Software Package
2
Computer workstation configuration may vary from country to country and
Cat. no. 1 909 282 (1 piece)
Cat. no. 1 909 339 (1 set, 768 capillaries)
Cat. no. 1 909 312 (1 Set)
Cat. no. 1 909 304
may change due to component availability and technical advances. To confirm
exact computer configuration, please contact your local Roche Molecular Biochemicals representative.
Continued on next page
A 3
Page 11

1.2 LightCycler System, Continued
Marks of
Conformity
The LightCycler has been manufactured according to EN 61010-1 (Safety
regulations for Measuring, Control and Laboratory Instruments; Part 1: General Requirements [IEC 1010-1 + A1: 1992, modified]) and has been checked
in accordance with all relevant safety standards prior to leaving the factory.
The instrument has been approved for use by recognized testing institutions.
This is confirmed by the following test/conformity symbols:
Acronym Test Symbol Testing Institution
GS
CE
UL
Certified by TÜV Product Service
The instrument conforms to
current directives as issued by
the European Union
Certified by Underwriters Laboratories Inc.
CUL
Equipment to be connected must fulfill the standards set by IEC 950 (Information security in technical equipment, including electronic business machines).
Certified by Underwriters Laboratories for Canada – a testing
facility recognized by the Standards Council of Canada (SSC)
Continued on next page
A 4
Page 12

1.2 LightCycler System, Continued
Classification
Note on Use
with Infectious
Material
The LightCycler Instrument is classified as:
• ISM instrument (Industrial Scientific Medical Device), medium-sized, for
industrial, laboratory and domestic use.
• Designed for stationary operation.
• Intended for worldwide use.
• Intended for evaluating preprocessed biological material.
• The instrument may not be used to analyze infectious materials unless ad-
ditional safety measures to ensure safe sample handling (e.g., placing the
instrument in a laminar flow biological safety cabinet) are taken beforehand.
A 5
Page 13

1.3 Technical Data
1.3.1 Specifications of the LightCycler
General Data
Environmental
Parameters
Dimensions Length:
Width:
Height:
Weight 19.2 kg
Power supply 110–240 V, +/–10%, 47–63 Hz
Wattage max. 800 W
Maximum current Europe:
US:
Noise in accordance with DIN 43635 <60 dBA
Heat emission, including PC, monitor
and printer
Safety symbols CE, GS, UL, CSA or CUL
Temperatures allowed during operation 15° to 35°C
Temperatures required to maintain
specifications during operation
Temperatures allowed during transportation/storage/packaging
Relative humidity 20 to 80%, no condensation
Altitude/pressure 0 to 2000 m above sea level,
max. 850 W
18° to 30°C
–20° to +60°C
1030 to 850 hp
45 cm
30 cm
40 cm
4 A (220 V)
8 A (110 V)
Samples
Number of samples per run 32
Sample volume 10 to 20 µl
A 6
Page 14
1.3.2 Specifications for Applications
Temperature
for PCR
Temperature range 40° to 98°C
Accuracy of “capillary temperature” at thermal equilibrium
Accuracy of “displayed temperature” with respect to
capillary temperature at thermal equilibrium
Capillary Heating Rates Volume : Heating
Heating rate 40° to 95°C (non-linear)
Heating rate 50° to 72°C (non-linear)
Heating rate 72° to 95°C (non-linear)
Capillary Cooling Times Volume : Cooling
Cooling rate 95° to 40°C (non-linear)
Cooling rate 95° to 60°C (non-linear)
Temperature Tolerances, Short Term
Precision of capillary temperature over all capillary positions when measured for 30 s at 95°C
Precision of capillary temperature over all capillary positions when measured for 30 s at 70°C
Precision of capillary temperature over all capillary positions when measured for 30 s at 45°C
Temperature Difference, Melting Curves
Systematic difference between sensor temperature and
capillary temperatures when chamber is heated from
50°C to 95°C at a rate of 0.2°C/s
±0.4°C
±0.4°C
Time Required
10 µl: ≤13 s
20 µl: ≤15 s
10 µl: ≤6 s
20 µl: ≤8 s
10 µl: ≤6 s
20 µl: ≤8 s
Time Required
10 µl: ≤23 s
20 µl: ≤24 s
10 µl: ≤7 s
20 µl: ≤7 s
±1.5°C
±1.0°C
±0.5°C
<0.1°C
A 7
Page 15

1.3.3 Specifications for the Detectors
Excitation
Type LED
Median wavelength 470 nm
Wattage at 470 to 490 nm 0.1 mW
Filter
Detector 1 Bandpass 530 nm, HBW 20 nm, dichroic
Detector 2 Bandpass 640 nm, HBW 20 nm, dichroic
Detector 3 Bandpass 710 nm, HBW 40 nm, dichroic
Detector
Type Photohybrid
Sensitivity at 530 nm, 20 µl sample volume 10 fM fluorescein
Resolution 12 bit
Range of detection sensitivity Adjustable by a factor of 1 to
256
Typical Time
Signal acquisition time for 32 capillaries
≈ 5.0 s
A 8
Page 16

1.3.4 Temperature Control
Visual Display
of Temperature Profiles
The LightCycler software graphically displays the current temperature in the
thermal chamber. These graphs appear under Temperature History on the
Running Screen (see below).
The graphs show “overshoots” and “undershoots” for each temperature transition (see arrows below).
A 9
Continued on next page
Page 17
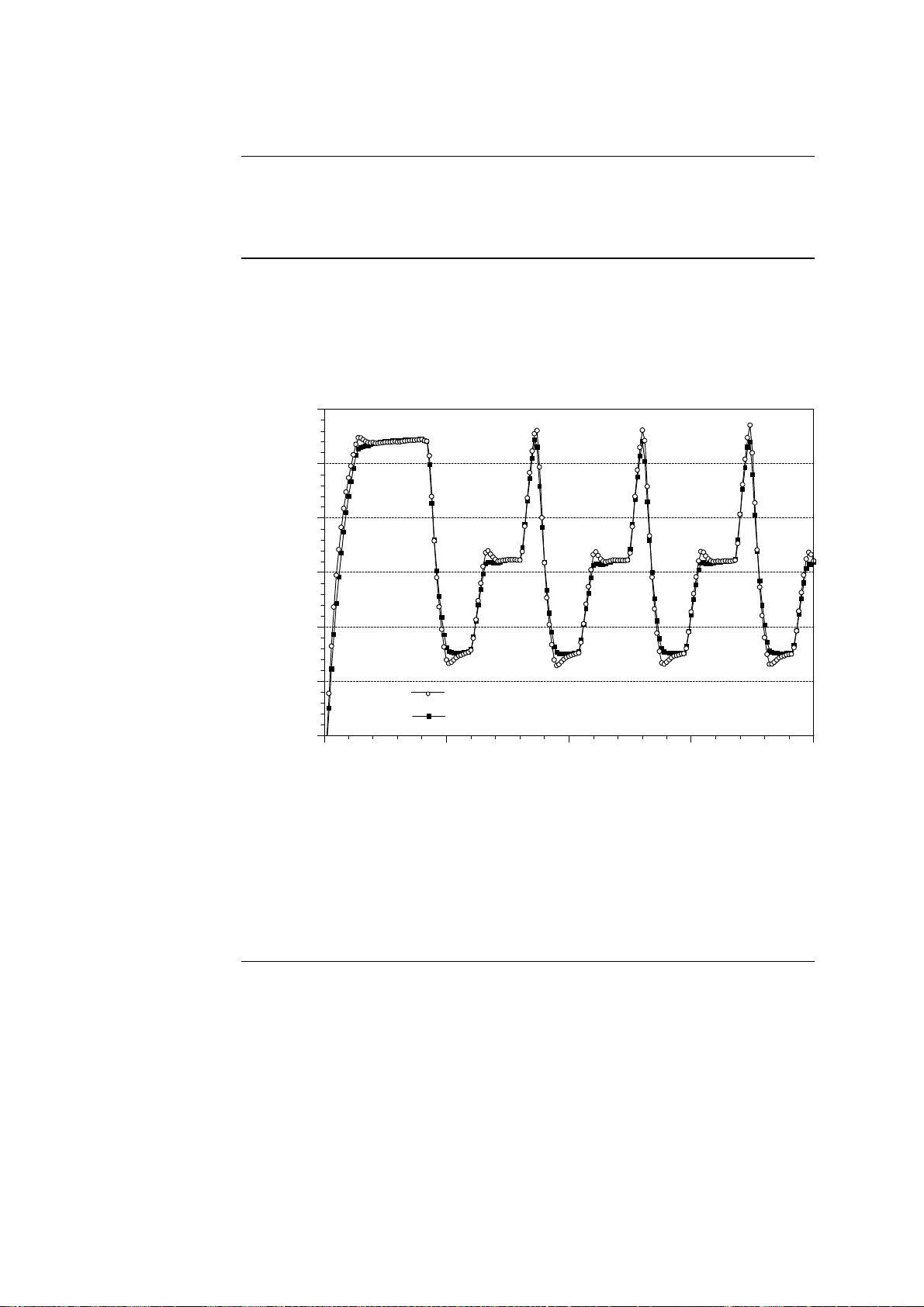
1.3.4 Temperature Control, Continued
100
Temperature [ C]
200
Fluid temperature in capillary
Ambient temperature in thermal chamber
Autocorrection
Effectiveness of
Autocorrection
Autocorrection compensates for physical differences in heat capacity in air
and water. This specific autocorrection ensures that every programmed temperature is adjusted within the sample capillaries during thermal cycling.
The graph below shows the changes in temperature in both the thermal
chamber and a sample capillary during thermal cycling. Differences at any
given time between the temperature in the two locations are shown as spaces
between the two temperature curves.
90
80
o
70
60
50
40
0 50 100 150
Time [sec]
Conclusion: This graph demonstrates that:
• The temperature profile shown under Temperature History on the Running Screen exactly corresponds to the capillary temperature, and
• The fluid temperature in the capillaries coincides with the programmed
profile.
A 10
Page 18
1.4 Safety Precautions for the LightCycler
General Safety
Precautions
Always observe these precautions when using the LightCycler Instrument:
• Follow all safety instructions printed on, or attached to, the analytical in-
struments.
• Observe all general safety precautions which apply to electrical instruments.
• Never touch switches or power cord with wet hands.
• Do not open the housing of the LightCycler.
Note: Only authorized service personnel should perform service or repairs
required for this unit.
• Do not open the LightCycler thermal chamber during operation.
• The chamber lid and the sample rotor are hot while the instrument is op-
erating.
Note: The corresponding symbol is attached to the front side of the
LightCycler.
• Always wear safety goggles and gloves when dealing with toxic, caustic, or
infectious materials .
• When analyzing infectious materials, use the LightCycler only in specially
equipped rooms with controlled exhaust equipment, e.g., biological safety
cabinets.
Safety Precautions for Centrifuge Adapters
Loading capillaries to perform fluorescence measurement with the LightCycler requires a centrifugation step in a tabletop centrifuge with specially designed adapters. A centrifuge adapter with capillary weighs approximately 6.2
grams. Always observe the following precautions during the centrifugation
step:
• Always distribute the adapters in the rotor so the weight in the rotor is
balanced, and
• Adjust the speed of the centrifuge so the centrifugal force does not exceed
700 x g.
Note: For details on the maximum weight that can be processed in your centrifuge, refer to the product specification sheet for that centrifuge.
A 11
Page 19

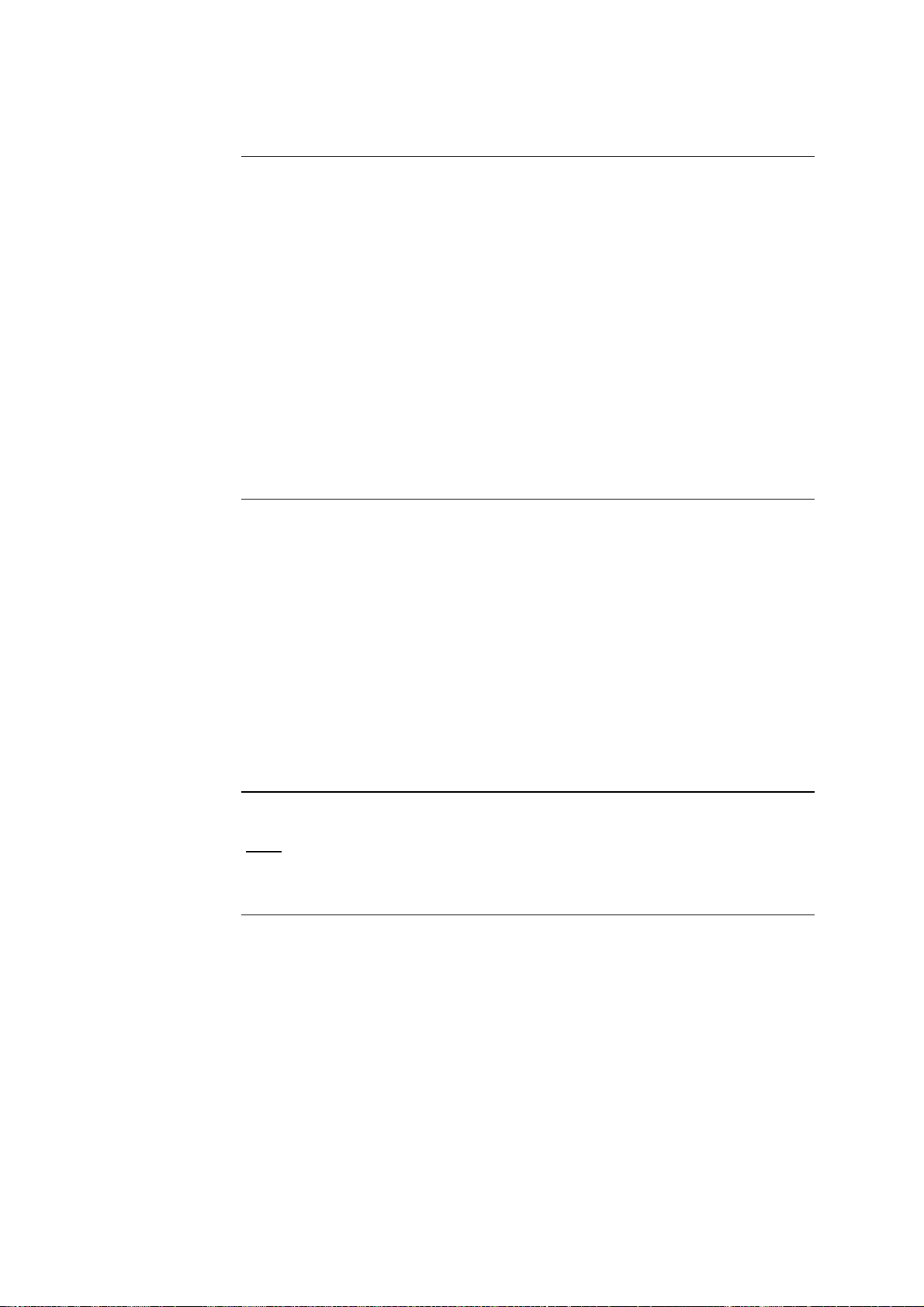
2.1. The LightCycler
Schematic of
the LightCycler
2. System Description
A 12
Continued on next page
Page 20
2.1 The LightCycler, Continued
Description of
the Instrument
Communication between
LightCycler
and PC
The LightCycler basically consists of an upper unit and a lower unit. The upper unit contains the heating coil. The lower unit contains the thermal chamber, fluorimeter, drive units, electronic boards and power supply. The various
elements are mounted on a 10-mm cast aluminum base plate. This guarantees
stability, especially for the thermal chamber and fluorimeter.
Hot or ambient temperature air, introduced into the thermal chamber, regulates the temperature of the sample capillaries. A heating coil heats the air,
which is then fed into the chamber by a fan. The fan ensures efficient air circulation and temperature homogeneity during the heating cycle.
During the cooling cycle, the fan operates at a higher speed to ensure adequate cooling.
During measurements, a stepper motor rotates the sample carousel to position the capillary tip precisely at the focal point of the fluorimeter optics. The
fluorimeter itself is positioned radially to the maximum signal to compensate
for any radial deviation of the capillary tip.
For online display, data are transmitted to and from the PC via a serial interface.
Control is made possible by three microprocessors that are integrated into the
instrument:
• Processor 1 is for communication,
• Processor 2 regulates the temperature, and
• Processor 3 controls the measurement procedures as well as the move-
ment of the rotor and fluorimeter.
Users enter data on samples (number of samples, name, concentration, etc.)
and on the experimental protocol into the PC; the PC transmits the data to
the LightCycler. The PC also monitors the temperatures and fluorescence signals during the PCR run and operates all the analytical programs.
Power Supply
Unit
All high-voltage components are located in the power-supply unit.
Note: Electrical safety information and test symbols apply to this unit.
To minimize interference to the detector electronics, the electronics that control the drive units are located on a separate circuit board.
Continued on next page
A 13
Page 21
2.1 The LightCycler, Continued
Temperature
Setting of the
Thermal
Chamber
Fluorimeter
Temperature is controlled with hot air and air at ambient temperature. Varying the voltage supplied to the heating coil regulates the temperature. A sensor
provides reference values for control purposes.
During the heating phase, the fan in the thermal chamber operates at low
speeds to ensure homogenous distribution of temperature.
During the cooling phase, the fan operates at higher speeds so that the capillaries and the heating coil can be cooled efficiently.
Two sensors are integrated into the LightCycler to prevent unduly high temperatures:
• Sensor I is located in the thermal chamber and switches off the heat when a
temperature of 125°C has been reached.
• Sensor II monitors the temperature on the aluminum base plate, and
switches off the entire LightCycler to protect the electronics when the temperature exceeds 55°C.
A three-channel fluorimeter is used for detection purposes. A blue diode
(LED) with maximum emission of 470 nm serves as the energy source for
sample excitation. Screens are used to diffuse the light emitted by the LED to
ensure uniformity. The exhaust and ventilation channels have been designed
to prevent ambient light from entering directly into the thermal chamber.
Fluorescence is detected at 530 nm, 640 nm, and 710 nm with the aid of
photohybrids.
Note: Acquisition time for fluorescence acquisition time is 20 msec (16.6 msec
in the U.S.).
Measurement
of Samples
The thermal chamber and the fluorimeter are placed optimally for sample
measurement. Only the sample carousel, which holds the capillary, rotates
during measurement of the various samples.
Continued on next page
A 14
Page 22

2.1 The LightCycler, Continued
LightCycler
Diodes
Position of
Diode
Top Power on Green On Instrument is switched on
Middle Cycling Red On Instrument is running
Bottom Run Yellow On PCR run is completed
Three diodes are located at the front of the LightCycler. All of the diodes
come on when the instrument is switched on. In this way, the instrument tests
for proper functioning of the diodes.
During instrument operation, the diodes function as described in the table
below:
Label Color of Diode Function Indication
Off
Flashing Instrument is defective
completed Off Instrument is running
• No power
• Instrument is defective
A 15
Page 23

2.2 PC Configuration
General
The computer workstation contains the following components:
Hardware
• PC with a Pentium processor
1
• 64 MB SDRAM (minimum configuration)
• 24x CD-ROM drive
• Keyboard and PS/2 mouse
• Internal Iomega ZIP drive
Operating system
Monitor
Printer
1
Computer workstation configuration may vary from country to country and
• Windows NT 4.0, including Service Pack III
• 17" monitor
• Hewlett-Packard Color Inkjet Printer
1
1
may change due to component availability and technical advances. To confirm exact computer configuration, please contact your local Roche Molecular
Biochemicals representative.
A 16
Page 24
3. Installation
3.1 Installation Requirements
Note
Space and
Power Requirements
• Do not place the LightCycler next to instruments that cause electromag-
netic interference or have high inductance (e.g., centrifuges or mixers).
• Peripheral instruments connected to the LightCycler System must meet
the IEC 950 (UL 1950) standard.
• All plugs used in the LightCycler System (PC, printer, monitor) should
have the same phasing in order to prevent switch-on peaks and electronic
noise generated by other instruments or by the power supply itself. We
recommend using a dedicated multiple-outlet surge protector for the
LightCycler System.
• Use only the supplied power lines and RS 232 connector.
Place the instrument in a site that can support the following instrument requirements:
Dimensions The LightCycler is 30 cm wide, 45 cm long, and 40 cm
high. .
Weight Approximately 20 kg
Voltage Requirements
The LightCycler operates between 120 and 240 V (50 to 60
Hz).
Note: The LightCycler adjusts automatically to the avail-
able voltage when the instrument is plugged in. The user
does not have to set the instrument to the correct voltage
manually.
Power Consumption
Do not open the LightCycler
housing.
The LightCycler uses 800 W max.
PC and printer consume an additional 500 W (approx.).
Continued on next page
A 17
Page 25

3.1 Installation Requirements, Continued
Environmental
Parameters
Storage Conditions
The instrument requires the following environment for accurate operation:
Ambient temperature 15° to 35°C
Ambient temperature required
to main specifications during
operation
Humidity 20 to 80%, no condensation
Altitude Sea level to 2000 m
Excess voltage Category II
Degree of contamination 2
The LightCycler can be stored under the following conditions:
Ambient temperature –20° to +60°C
Humidity 20 to 80%, no condensation
18° to 30°C
A 18
Page 26
3.2 Installation of the LightCycler
Installation of
the LightCycler
Instrument
Your Roche Molecular Biochemicals representative will normally install the
LightCycler Instrument at your site. Should this not be possible, follow these
steps to install the instrument successfully:
Step Action
1 Unpack the instrument.
2 Position the instrument on the workbench. Allow 10-cm spaces to
the left, right and behind the instrument to ensure sufficient cooling of the electronic components.
Note: The workbench should not be covered with paper.
3 Make the following electrical connections:
• Connect the LightCycler to the PC using the RS 232 cable (serial interface) provided with the system.
• Connect the LightCycler, PC, monitor, and printer to the same
multiple-outlet distributor plug.
Ensure that PC, monitor, and printer have
been set to the correct voltage.
A 19
Page 27
3.3 Installation of the Computer
Installation of
the PC
To install the PC, do the following:
Step Action
1 Connect mouse, keyboard, and monitor to the computer.
2 Connect the LightCycler to the computer with the RS 232 cable
(serial interface) provided with the system.
3 Connect the computer, monitor, and LightCycler to the same
multiple-outlet distributor plug.
The computer is now ready for operation.
A 20
Page 28

3.4 Installation of LightCycler Software Version 3
Logging In
Installation of
LightCycler
Software
A prerequisite for the software installation is that the operating system WINNT 4.0 (Service Pack 3) has been installed. For the installation of the software
and the setting up of users it is recommended that you log in as administrator.
All instructions are based on the English WIN NT 4.0 version. The operating
system programs and folders in the German versions have different names.
Insert the CD with the LightCycler Software version 3 into the CD-ROM drive
of your computer. The autorun function will automatically start the Setup
program. If the autorun function is not available, the CD must be opened
from the Explorer, and the 'setup' program started manually by doubleclicking on it. The Setup program displays the following start screen:
Click the “Next” button to continue with the installation. Click “Cancel” to
abort the program.
Continued on next page
A 21
Page 29
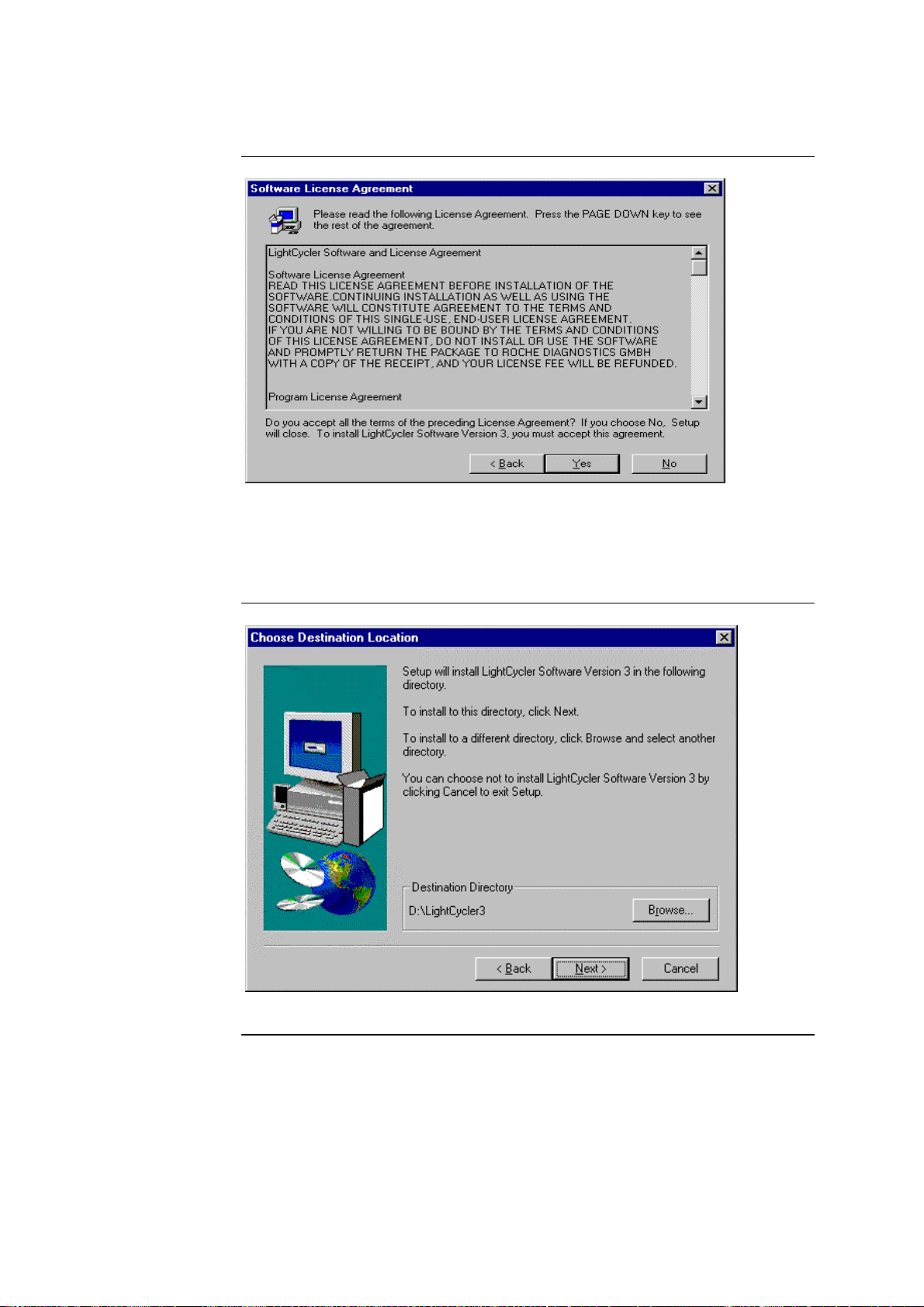
3.4 Installation of LightCycler Software Version 3, Continued
License
Agreement
Information of
LightCycler
Software
Here, the customer is provided with a display of the license agreement. Scroll
bars can be used to view the entire text.
Choose “Yes” to accept the license agreement. If you choose “No”, the program will be exited. Clicking on “Back” will redisplay the start screen.
Installation
Directory of
LightCycler
Software
Continued on next page
A 22
Page 30

3.4 Installation of LightCycler Software Version 3, Continued
Installation
Directory of
LightCycler
Software,
Continued
The software will be installed to the recommended directory “LightCycler3”.
The disk drive corresponds to the drive to which WIN NT was installed, here:
drive “D”.
If “Next” is clicked on, the installation will be continued. If “Cancel” is chosen, the installation will be exited, and “Back” will redisplay the previous
screen.
If you wish to change the disk drive or the directory, click the “Browse” button. A popup window will display permitting the desired path and directory to
be entered.
The installation to the “LightCycler3” directory will have no consequence for
any previous installations of version V1.22 since that version was installed in
the directory named “LightCycler”. It is therefore recommended that directory “LightCycler3” be used so as to avoid any conflict with respect to future
versions and to be able to quickly locate the relevant versions.
Continued on next page
A 23
Page 31

3.4 Installation of LightCycler Software Version 3, Continued
LightCycler
Software Program Folder
The program folder contains icons to start the various LightCycler Programs.
A folder named “LightCycler3” is set up in the Start menu under 'Programs'.
This name should be retained, if possible, to be able to locate the folder
quickly.
Click “Next” to continue with the installation. If “Cancel” is chosen, the installation will be exited, and ”Back” will redisplay the previous screen.
Starting the
LightCycler
Software Installation
Prior to the start of the installation, a summary of the current settings is displayed. You are provided with one last chance to make changes, or to exit the
installation.
Click “Next” to start the installation. If “Cancel” is chosen, the installation
will be exited, and 'Back' will redisplay the screen below.
Continued on next page
A 24
Page 32

3.4 Installation of LightCycler Software Version 3, Continued
Starting the
LightCycler
Software Installation, Con-
tinued
LightCycler
Software Installation Progress
After the start of the installation, a progress bar is displayed indicating the
progress of the installation. Please wait until the installation is complete and
the “Setup Complete” screen appears.
It is possible to exit the installation by clicking “Cancel”. This should, however, only be done in emergencies. Any program parts that have been installed
up to this point will not be removed automatically.
At the end of the installation, the program registration takes place.
A start icon for the “Front” program will be installed on the desktop and
added to the Start menu. The folder “LightCycler3” containing the programs
“Front”, “BMRun” and “LCDA” will be set up in the Start menu under “Programs”.
Continued on next page
A 25
Page 33

3.4 Installation of LightCycler Software Version 3, Continued
LightCycler
Software Installation Progress, Continued
Terminating
Setup
The installation has been carried out successfully. Click “Finish” to complete
the Setup program. If the check box “Launch the program file” is selected, the
LightCycler program “LightCycler3 Front Screen” (Front) will start when the
“Finish” button is clicked.
A 26
Continued on next page
Page 34

3.4 Installation of LightCycler Software Version 3, Continued
System File For the LightCycler3 program to be able to run, the system file “mfc42.dll” is
required. If an earlier version of this file is installed on your computer, it will
be overwritten with the more recent version in the directory
Winnt\system32\.
Acrobat
Reader
Num2Dot
The Acrobat Reader is required to be able to read the Help files. This program
is also included on the CD. It needs only to be installed if the program has not
previously been installed on the computer.
To install Acrobat-Reader, open the CD from the Explorer and start the
“ar40eng” program by double-clicking on it in the “Acrobat Reader” subdirectory. The installation procedure is similar to that of the LightCycler Software.
Num2Dot is a tool that allows use of the “comma” of the numerical pad of
your keyboard to enter a decimal point. As the LightCycler Software is designed to be run under english country settings, the Software expects a “point”
as a decimal point. The ability to convert the decimal “comma” on a variety of
non-english keyboards to write a “point” instead significantly improves the
convenience of the system.
Note: It is not required to install Num2Dot if your keyboard´s numerical pad
is equipped with a “point” as decimal point.
To install Num2Dot, open the CD from the Explorer and start the install
Num2Dot by double-clicking “Setup.exe” in the “Num2Dot” subdirectory.
The installation procedure is similar to that of the LightCycler Software.
Num2Dot will automatically be started whenever you launch Windows-NT
on your PC.
Verify activation of the numerical pad on your keyboard. The green light built
into the “Num” key must be on.
After installation of Num2Dot, the “comma” key will be converted to a
“point” key.
The Short cut command “AltGr + DecimalComma” can be used to deactivate
Num2Dot, as well as to reactivate the conversion.
Continued on next page
A 27
Page 35

3.4 Installation of LightCycler Software Version 3, Continued
Uninstall To uninstall programs under WIN NT, use the “Add/Remove Programs”
tool. This auxiliary program appears in the Start menu under Settings-Control
Panel. A list of the programs installed is displayed. Select the program you
wish to uninstall from the list and remove it by clicking the “Add/Remove”
button.
A 28
Page 36

3.5 Dismantling the LightCycler System
Dismantling
the System
To dismantle the LightCycler system, do the following:
Step Action
1 Switch off the instrument.
2
3 Ship instrument in its original packaging.
• Disconnect the RS 232 cable and power cables.
• Clean the instrument (according to the guidelines in Topic 4 of
this chapter).
• Decontaminate the instrument if necessary.
A 29
Page 37

4. Operation and Maintenance of the LightCycler
Guidelines for
Operation
Transport and
Storage
• The LightCycler instrument should only be operated in locations which
are protected from the weather. It may not be operated in buildings that
lack facilities for regulating temperature. If necessary, additional drying
agents may be used to eliminate humidity. Neither ice nor moisture
should be allowed to form on the instrument.
• The instrument should be placed on an even work surface and protected
from direct sunlight and outside light.
• The instrument should not be operated near dripping, spraying, splashing
or running water.
• The instrument is suitable for use according to classification 3K3 in accor-
dance with Standard EN 60721-3-3.
• The instrument should be protected from effects generated by fauna and
flora.
• The instrument may be used at locations subject to noticeable or signifi-
cant vibration. However, it should not be exposed to higher levels of
shock waves.
• The instrument is suitable for use according to classification 3M4 in ac-
cordance with EN 60721-3-3. The instrument is able to tolerate vibrations
up to classification 3M6.
When storing or transporting the instrument, do not allow it to be exposed to
extreme cold (e.g., placing it in an airfreight cargo bin). Avoid temperatures
lower than –25°C (since such temperatures may damage optical systems).
The optical systems have open ventilation systems and should therefore be
protected from dirt and humidity.
Guarantee
The terms of the guarantee are incorporated in the purchase agreement. For
further details, please contact your Roche Molecular Biochemicals representative.
Continued on next page
A 30
Page 38

4. Operation and Maintenance of the LightCycler, Continued
General Maintenance
Cleaning the
Instrument
The instrument is maintenance-free.
• Use 70% ethanol for disinfecting the thermal chamber.
• Clean the optical window with alcohol and an optical polishing cloth.
• Clean the housing with a mild commercial household detergent.
• Use a mild commercial household detergent for cleaning the housing.
• Do not pour fluids into the thermal chamber.
A 31
Page 39

B 1
Chapter B
LightCycler Software
Version 3
Page 40
B 2
1.1. Table of Contents
1. Software
Topic See Page
1. Software
1.1 Table of Contents B 2
1.2 Introduction B 4
1.3 The Front Screen B 7
1.4 Functions of Buttons on the Front Screen B 8
1.5 Edit Graphics Defaults Window B 9
2. Programming a Run
2.1 Programming Screen B 11
2.1.1 Menu Options Available on the Programming Screen B 14
2.1.2 Programming Screen: User / Experiment Window B 17
2.1.3 Programming Screen: Experiment / RUN / EXIT Window B 19
2.1.4 Programming Screen: Sample Data Entry B 21
2.1.4.1 Loading Screen Activated by the Edit Samples Button B 22
2.1.4.2 Naming Samples at the Beginning of a Run B 25
2.1.5 Real Time Fluorimeter (RTF) B 29
2.1.6 Programming Screen: Color Compensation Window B 32
2.1.7
Programming Screen: Display Mode / Fluorimeter Gains
Window
B 1
B 11
B 34
2.1.8 Programming Screen: Cycle Program Data and
Temperature Targets Window
2.1.9 Programming Screen: Graphic Simulation Window B 40
2.1.10 Programming Screen: Cycle Program Overview Window B 41
3. Running the LightCycler
3.1 LightCycler Run Screen B 42
Continued on next page
B 36
B 42
Page 41

B 3
1.1 Table of Contents (continued)
Topic See Page
4. Data Analysis
4.1 Introduction B 46
4.2 Select Data File / Data Analysis Window B 47
4.3 LCDA Front Screen B 49
4.3.1 LCDA Front Screen: Temperature vs. Time Graph B 50
4.3.2
4.3.3 LCDA Front Screen: Using Color Compensation Files B 54
4.4 LCDA Front Screen: Data Retrieval B 56
4.5 Further Analysis B 56
Fluorescence vs. Cycle Number / Time / Temperature
Graph
B 46
B 52
5. Display and Analysis of Quantification Data
5.1 Introduction B 57
5.2 Quantification: Selection of Analysis Method B 58
5.3 Quantification Step 1: Baseline B 61
5.4 Quantification Step 2: Noise Band B 64
5.5 Quantification Step 3: Analysis B 66
5.6 A Procedure for Analyzing Quantification Data B 72
6. Display and Analysis of Melting Curve Data
6.1 Introduction B 85
6.2 Melting Curve Step 1: Melting Peaks B 87
6.3 Melting Curve Step 2: Peak Areas B 91
6.4 Melting Curve Step 3: Manual T
6.5 A Procedure for Analyzing Melting Curve Data B 95
m
B 57
B 85
B 93
Page 42

B 4
1.2. Introduction
Before
Beginning
Program Start
Your LightCycler should have been installed and set up according to the
instructions described in Section 3 of Chapter A.
During installation, the LightCycler software will automatically be loaded
into the part of the hard disc that contains the Windows NT operating
system.
Prior to the start of the program, an account must be set up for each user
granting him access privileges that enable him to use the computer. Such
accounts must be used by the users to log on to Win NT.
If the check box 'Yes, launch the program file' was selected at the end of the
installation, the 'LightCycler3 Front Screen' (Front) program will now start.
Log On
Procedure
The current user may log on using the 'Log On As Different User' button.
The information message to the effect that all programs will be closed must
be acknowledged by clicking 'OK'. Subsequently, all programs are closed and
WIN NT displays its log-on screen. The user must now log on by entering his
user name and password.
Note: The LightCycler does not allow to log on as a different user as long as a
LightCycler Run is performed to prevent loss of data by interrupting the run.
Continued on next page
Page 43

B 5
1.2. Introduction, Continued
Program Start, In the event that the check box 'Yes, launch.....' was not selected at the end
of the installation, the user needs to log on first. This may be accomplished
in the Start menu using the 'Shut Down' command.
Select the option button 'Close all Programs and log on as different user'
and click the 'Yes' button. Windows now closes all programs and displays
its log-on screen. The user must now log on by entering his user name and
password.
Following the log-in procedure, the LightCycler3 program may be started.
The start should always be carried out from the desktop or the Start menu
by clicking the 'LightCycler3 Front Screen' icon. Only this procedure will
ensure that the user is logged on properly to the program and that the
required work directories are created upon the initial startup.
Continued on next page
Page 44

B 6
1.2. Introduction, Continued
How to Start Follow the steps in the table to start.
Step Action
1 Turn on the computer by pressing the power button on the front
of the computer.
Result: Windows NT will automatically load.
2 Type in your User Name and Password to log into Windows NT
and the LightCycler software.
Note: User management requirements for the LightCycler software
are set up through the Windows NT operating system.
3
4
Flip the switch on the back panel of the LightCycler to the On
position.
Click on the LightCycler 3 Front Screen icon; this will bring up the
LightCycler Front Screen.
Note: Alternatively, you may use Windows NT Explorer to start
each software function independently, without first entering the
LightCycler Front Screen. For instance, from Windows NT
Explorer, do the following:
Important
Note about
the PC
To start from... Click on...
LightCycler Front Screen
Programming / Run Screen
LightCycler Data Analysis Front Screen
Tip: Accessing the screens through Windows NT Explorer allows
you to graphically display and evaluate data generated in previous
experiments while other experimental protocols are in progress.
The PC supplied with the LightCycler should be used exclusively with the
LightCycler software. We strongly advise against installing and running
additional software on this PC.
Front.exe
(BM) Run32.exe
LCDA.exe
Continued on next page
Page 45

B 7
1.3. The Front Screen
LightCycler
Front Screen
In Version 3 of the LightCycler software, the front screen looks like this:
Introduction When you click on the LightCycler Front Screen icon on the Windows NT
desktop, the LightCycler software is activated and the LightCycler Front
Screen will appear on the monitor. This screen provides access to the
functions of the LightCycler software via a set of menus.
Alternatively, you may also directly access the most important instrument
functions by clicking on these screen buttons:
• Run
• Data Analysis
• Edit Graphics Defaults
• Log On As Different User
• Exit
Note: The functions of these buttons are explained in more detail on the
pages of the next section.
Page 46

B 8
1.4. Functions of Buttons on the Front Screen
Run Button The Run button starts the LightCycler Run Software which allows you to
program and execute cycle experimental protocols.
Note: The Front Screen Run menu may be used for the same purpose.
Data Analysis
Button
Edit Graphics
Defaults
Button
Log On As
Different User
Button
The Data Analysis button provides access to the LightCycler Data Analysis
(LCDA) software.
Note: The Front Screen Analysis menu may be used for the same purpose.
The Edit Graphics Defaults button allows a user to change the colors and line
styles used in the data display graphs (see Section 1.5 of this chapter).
Note: The Front Screen Options menu may be used for the same purpose.
Each user only has access to a unique set of files. To access files belonging to a
different user, click on the Log On As Different User button. This allows you
to exit all LightCycler programs and reactivate the software with a different
user name and password (as prompted by the system).
Note: The LightCycler does not allow to log on as a different user as long as a
LightCycler Run is performed to prevent loss of data by interrupting the run.
The Front Screen Options menu may be used for the same purpose.
Exit Button The Exit button allows you to quit the LightCycler program.
Note:
• You may also quit the program by closing the Front Screen window. The
Exit function is also part of the Front Screen File menu.
• After exiting the software, you still must turn off the LightCycler instrument
separately.
Help Menu The Operator Manual can be called by Acrobat Reader.
Note: Acrobat reader 4.0 is provided as part of the Software CD.
Page 47

B 9
1.5. Edit Graphics Defaults Window
Edit Graphics
Defaults
Window
The Edit Graphics Defaults button allows a user to change the color and style
of the line used to represent each sample in LightCycler data displays. When
you click on the button, the Edit Graphics Defaults window will appear (as
shown below):
Continued on next page
Page 48

B 10
1.5. Edit Graphics Defaults Window, Continued
Changing the
Line Display
After entering the Edit Graphics Defaults window, change the appearance of
the graph lines as follows:
Caution: Changes made to graphics defaults will not be accepted if the
LightCycler Data Analysis (LCDA) software is active. Before changing the
graphics defaults, first close LCDA. Then, edit and save your graphic defaults
and reload LCDA. The changes are then accepted by LCDA.
User specific default settings are stored in a file „defaults.ini“ in the user
directory. You may store your settings with another user by copying your
user default.ini file into the other user directory.
Step Action
1
2
3
4
5 If you do not like the appearance of the sample lines in the data
Right-Click on the sample number you wish to change.
Result: A pop-up menu will appear. The menu contains the
following graphics options: Color, Point Style and Line Style.
From the menu, click on the display option you wish to change.
Result: One of the Graphics Default Options windows (pictured
below) will appear.
Click on the option you prefer and then click the OK button.
Result: The Graphics Default Options window will close.
Note: Changes made to the graphic defaults of one user do not
affect the graphics defaults of any other user.
From the Edit Graphics Defaults window, click on the Save button
to save your new graphics defaults.
Note: After saving them, you may always return to your display
options (defined in Steps 1–4) at any time by clicking on the
Restore button (in the Edit Graphics Defaults window).
display, you may open the Edit Graphics Defaults window and do
one of the following:
• Click on the Restore Defaults button to return all options to the
system default settings.
• Repeat Steps 1–4 above and choose different options.
Graphics
Default
Options
Windows:
Color, Point
Styles and Line
Styles
Page 49

B 11
2. Programming a Run
2.1. Programming Screen
Introduction The descriptions given in the following pages explain how to use the
Programming Screen for:
• Defining the parameters of a PCR protocol,
• Starting a PCR run, or
• Viewing online a PCR experiment that is currently in progress.
Activation of
Programming
Screen
To set up a new experimental protocol or to order a new run with a protocol
that has already been defined and stored, you must first activate the Run
function from the LightCycler Front Screen. Follow the steps below to
activate the Run function and open the Programming Screen:
Step Action
1
2
Click the Run button on the LightCycler Front Screen.
Result: A data-entry screen opens. This screen will henceforth be
referred to as Programming Screen.
If you have not already switched on the LightCycler instrument,
the software will prompt you to do so.
Note: It is possible to ignore this message, by simply clicking OK.
This will allow you to become familiar with the LightCycler
software without running an experiment.
Continued on next page
Page 50

B 12
2.1. Programming Screen, Continued
Programming
Screen
Functions
Available on the
Programming
Screen
Defining Cycle
Programs
The rest of the topics in Section 2.1 of this chapter describe the software
functions that may be activated by either using the menu bars or clicking on
various buttons available on the Programming Screen. Menu options are
listed in the top-to-bottom order in which they appear on each menu.
Function buttons are described according to the screen areas in which they
appear.
Note: Use the Programming Screen diagram above to locate the screen areas,
menus, and buttons as they are described below.
Use the Programming Screen to define the cycle programs for an
Experimental Protocol (*.exp-file). Once you have defined your
Experimental Protocol, you will be ready to run a LightCycler experiment.
Tip: You can use the descriptions in the following sections to familiarize
yourself with the actions necessary to define an Experimental Protocol. For
instance, as you read the descriptions below, you may activate each function
by clicking on the menu option or button, and then enter typical
experimental parameters in the tables or fields that appear.
Continued on next page
Page 51

B 13
2.1. Programming Screen, Continued
Program 3:
Melt
Program 1:
Program 2:
Amplification
Program 4:
Cooling
Initial
Denaturation
Definitions:
Protocol /
Program /
Segment
Experimental Protocol
An Experimental Protocol in general contains one or more Cycle Programs.
In the PCR profile shown above, a typical Experimental Protocol contains
four programs: 1) an Initial Denaturation Program, 2) an Amplification
Program, 3) a Melting Program and 4) a Cooling Program.
A Program (e.g. Cycle Program) contains several Temperature Segments,
each of which defines the time and temperature parameters that will be used
for denaturation, annealing, extension and/or melting, cooling as well as the
fluorescence acquisition mode used to monitor the amplification signal.
Page 52

B 14
2.1.1. Menu Options Available on the Programming Screen
Options
Available on
the
Programming
Screen Menus
The Menu bar on the Programming Screen offers a variety of options for
programming, managing, and displaying Experimental Protocols (*.exp-
files). The following list gives you a brief description of the menu functions.
Note: You may also activate a number of the options listed on these menus
key by clicking the buttons found in various areas on the screen. The use of
these buttons is discussed in detail in later sections of this chapter.
Menu Offers these options
File Allows you to:
• Create or Open existing *.exp-files,
• Save the current *.exp-file,
• Print the current window contents, and
• Exit the Run program.
Note: Experimental files (*.lin-files) made with older
versions of LightCycler software will be automatically
converted to *.exp-files when they are first opened.
If you had used the Lightcycler Software 1.2x, we
recommend converting all *.lin-files into *.exp-files
and then deleting the *.lin- and *pro-files. As a *.profile may have been used by several *.lin-files, *.profiles should only be deleted after converting all *.linfiles.
Edit
Allows you to:
• Cut, Copy, and Paste,
• Edit Sample Defaults ,
• Display Cycle Program Simulation,
• Edit Experimental Notes to your *.exp-files, and
• Show Experimental Notes.
Note: The Cycle Program Simulation and
Experimental Notes will appear in the window in the
right portion of the Programming Screen under the
User / Experiment window.
Continued on next page
Page 53

B 15
2.1.1. Menu Options Available on the Programming Screen,
Continued
Options Available on the Programming Screen Menus (continued)
Menu Offers these options
Tools
Options
Allows you to:
• Display the Real Time Fluorimeter, and
• Activate a Service Utility function.
Note:
1) The Real Time Fluorimeter option permits
continuous fluorescence measurement of samples to
allow adjustment of fluorimeter gain by the user.
2) The Service Utility function facilitates adjusting
and testing to permit the instrument to be serviced
by an authorized specialist from Roche Molecular
Biochemicals Technical Support.
Caution: Do not activate the Service Utility function
unless Roche Molecular Biochemicals service
personnel ask you to do so. Settings of the Service
Utility function will not be stored and used by the
Run program.
Allows you to:
• Choose (color compensation,-*.ccc-)-files
containing color compensation data, or
• Disable (color compensation,-*.ccc-)-files,
• Set LED Power (to turn the LED light source on
and off),
• Edit Sample Defaults (to specify and alter sample
information), or
• Use Seek Threshold.
Continued on next page
Page 54

B 16
2.1.1. Menu Options Available on the Programming Screen,
Continued
Options (continued)
Menu Offers these options
Options
Note:
1) Choose (color compensation, -*.ccc-)-files
activates the Color Compensation function for use
with multicolor reaction systems (see Section 4.3.3 of
this chapter and Section 5 of Chapter D for more
information).
2) The numerical value below the instrument switch
indicates the current LED level on a scale of 0-100%.
If the Calibrated box is checked, the LED power is set
to 75% and cannot be adjusted. If the Calibrated box
has been cleared, choosing Set LED Power causes a
yellow box to be displayed on the Programming
Screen, indicating the LED light source level chosen
and allowing the value to be changed.
Help
Note: We strongly recommend to use Calibrated
LED.
3) The Use Seek Threshold function may be
inactivated when a capillary, containing no
fluorescent dye is not found by the “Seek” function at
the beginning of a calibration run.
Allows you to
• Access the Online LightCycler Manual.
Note: The Operator Manual can be called by Acrobat
Reader. Acrobat reader 4.0 is provided as part of the
Software CD.
Page 55

B 17
2.1.2. Programming Screen: User / Experiment Window
Location of
Window
Contents and
Functions of
User /
Experiment
Window
The User / Experiment Window is in the right upper corner of the
Programming Screen.
• The following information is available in the User / Experiment Window
(left photo above):
• The User name that you used to log onto the LightCycler software appears
in the User Name Field.
• The Experiment Field displays the name of the Experimental Protocol
(*.exp)-file that is currently loaded into the programming software.
• The Cycle Program list (below the Experiment Field) displays the names
of the cycle programs contained in the active *.exp-file.
• Use the Cycle Program list in the User / Experiment Window, along with
the buttons below the list to:
• Define a new *.exp-file, or
• Modify an already existing *.exp-file.
Note: See the next page for a description of the functions activated by
these buttons.
Caution: Although you can edit an *.exp-file in this window, you must use
another window to open or create that file. Go to the left side of the
Programming Screen, find the Experiment / RUN / EXIT window (right
photo above) and:
• Click on the Open Experiment File button to open an existing file that you
want to edit, or
• Click on the New Experiment button to create a new *.exp-file.
Continued on next page
Page 56

B 18
2.1.2. Programming Screen: User / Experiment Window, Continued
Cycle
Programs
Order of Cycle
Programs
Cycle Program
Buttons
As mentioned in Section 2.1 of this chapter, each Experimental Protocol
(*.exp-file) contains one or more cycle programs that are defined by the user,
e.g.:
Cycle Program No. Example of operation described in program
1 Initial denaturation
2 PCR amplification, with repeated cycles of
denaturation, annealing, and elongation
3 Melting curve for analysis of the PCR data
4 Cooling
In the Cycle Program window, all cycle programs in the current *.exp-file
are displayed in the order they will be executed during a run.
Use the buttons below the Cycle Program window to perform the following
modifications of the programs in a new or existing *.exp-file.
Use this button To modify an *.exp-file in this way
Add To create a new program in the *.exp-file
Remove To delete a program from the *.exp-file
Import To import cycle programs from other *.exp-files
Move Up To change the order in which programs will be
executed
Continued on next page
Page 57

B 19
2.1.3. Programming Screen: Experiment / RUN / EXIT Window
Location of
Window
Experiment /
RUN / EXIT
Window
Functions of
Window
Buttons
The Experiment / RUN / EXIT Window is on the left side of the
Programming Screen.
The Experiment / RUN/ EXIT Window contains seven buttons. Use the
buttons to perform the following software functions:
Use this button To perform this function
New
Create a new *.exp-file (see Section 2.1.2 above).
Experiment
Open
Select and open an existing *.exp-file.
Experiment File
Note: Any *.lin-files, used in the previous version of the
LightCycler software (Versions 1.2 and 1.22) to contain
protocol information, are automatically converted when
opened with this button. The *.lin-files are copied,
converted, and saved with an exp suffix.
Save
Save a newly defined or modified *.exp-file.
Experiment File
Continued on next page
Page 58

B 20
2.1.3. Programming Screen: Experiment / RUN / EXIT Window,
Continued
Functions of Window Buttons (continued)
Use this button To perform this function
Edit Samples
Real Time
Fluorimeter
RUN
Name samples in a given run and include additional
sample information, e.g. carousel position, type of sample,
whether the sample is a replicate of another sample,
concentration (if known), and descriptive notes.
Note: See the next section (Section 2.1.4) in this chapter
for details on sample information.
Monitor fluorescence over time without running a cycle
program. This function allows you to set optimum
fluorimeter gain values for your specific protocol.
Note: See Section 2.1.5 in this chapter for details on the
Real Time Fluorimeter.
Start a LightCycler run.
EXIT
Note: When you click on this button, the Loading Screen
window will appear and you will be prompted to enter
your sample data.
Leave the Programming Screen window.
Page 59

B 21
2.1.4. Programming Screen: Sample Data Entry
Introduction The LightCycler offers you several opportunities to name and describe your
samples. You may define sample data in either of two similar windows:
• Click on the Edit Samples button in the Experiment / RUN / EXIT window
(left side of screen).
Result: The Loading Screen will appear. This screen contains a Sample Data
table (see Section 2.1.4.1 below for details).
Note: This option allows you to include sample information in the *.exp-file
so it will be available for every run performed with that file. If you are going
to repeatedly run the *.exp-file with the same number of samples, use this
option.
• Click on the RUN button (in the Experiment / RUN / EXIT window) to
start the LightCycler run. Whether or not you have previously stored sample
data in the active *.exp-file, the software will prompt you to enter sample
data for this particular run.
Result: The Loading Screen with the Sample Data table will appear. Note,
however, that the options available at this stage differ slightly from those
available when the Edit Samples button is used (see Section 2.1.4.2 below
for details).
Note: Use this option if the number or the type of samples varies from run
to run.
Page 60

B 22
2.1.4.1. Loading Screen Activated by Edit Samples Button
Appearance of
Loading Screen
Activated by
Edit Samples
Button
When you click on the Edit Samples Button in the Experiment / RUN / EXIT
window, the Loading Screen below appears. The software will prompt you to
enter your sample default into the Sample Data table.
Note: Start entering data for carousel position #1 and continue through the
last carousel position that contains samples, including any gaps in the sample
order.
Fields in
Sample Data
Table
To define sample data that will become a part (i.e., the sample default data)
of the *.exp-file, describe a sample set in these fields of the Sample Data table:
This field Contains this sample data
Rotor Position Indicates the position of the sample in the 32-sample
carousel.
Sample Name Lists the name of the sample as it will appear in the
sample loading dialog and the analysis results.
Type Indicates whether a sample is a positive control, negative
control, quantification standard, or an unknown.
Replicate of Indicates whether a sample is a replicate of another
sample located at another position in the carousel.
Note: Enter the carousel position of the replicated sample.
Concentration Lists the concentration of a sample if it is known, e.g. if
the sample is a quantification standard.
Notes Allows additional sample information to be included.
Continued on next page
Page 61

B 23
2.1.4.1. Loading Screen Activated by Edit Samples Button,
Continued
Additional
Fields on This
Loading Screen
After entering the sample description into the fields of the Sample Data table,
enter additional sample information into three fields along the bottom of the
Loading Screen:
Field Location Contains this information
Temperature
Number of
Samples
Left lower corner
of Loading Screen
Left lower corner
of Loading Screen
Lists the temperature that the sample
chamber holds during the “Seek
Process” at the beginning of a run. As
a default during editing the sample list
the LightCycler holds a temperature of
30°C.
Examples: For instance when
preparing for an RT-PCR, the
temperature during editing the sample
list and the subsequent seek may be set
to a higher temperature (e.g. 55°C).
Higher temperature during the seek
may also be set, when using low
concentrations of probes, that are
fluorescent only at higher
temperatures (e.g. beacon probes).
Lists the total number of samples in
the carousel.
Concentration
Units
Right lower corner
of Loading Screen
Note: If you want to leave space in
between the capillaries, indicate the
number of samples and the number of
free positions in between the
capillaries.
Lists the units of concentration to be
used during data analysis (i.e., the
units appropriate for the values in the
Concentration fields).
Note: This information is only used to
correctly label the axis of the analysis
program. It has no relevance for
calculation of data as such.
Page 62

B 24
2.1.4.1. Loading Screen Activated by Edit Samples Button,
Continued
Additional
Functions on
This Loading
Screen
This Loading Screen contains four buttons that perform the following useful
functions:
Use this button To do the following
Done To confirm the values entered in the sample data
fields and return to the Programming Screen.
Cancel To interrupt the entry of sample data.
Clear Sample List To make all fields in the Sample Data table blank.
Default Sample List To reset the values in the Sample Data table to
values previously defined and stored in the *.exp-
file by the user.
Page 63
B 25
2.1.4.2. Naming Samples at the Beginning of a Run
Introduction When you start a run (by clicking on the RUN button in the Experiment /
RUN / EXIT window, the software will offer you an opportunity to enter
descriptions of the samples in this particular run.
Note: This option is useful if you have not previously defined a default set of
samples in the active *.exp-file. Even if you have previously defined the
samples in the active *.exp-file, you may wish to define a different sample set
that is unique to this run.
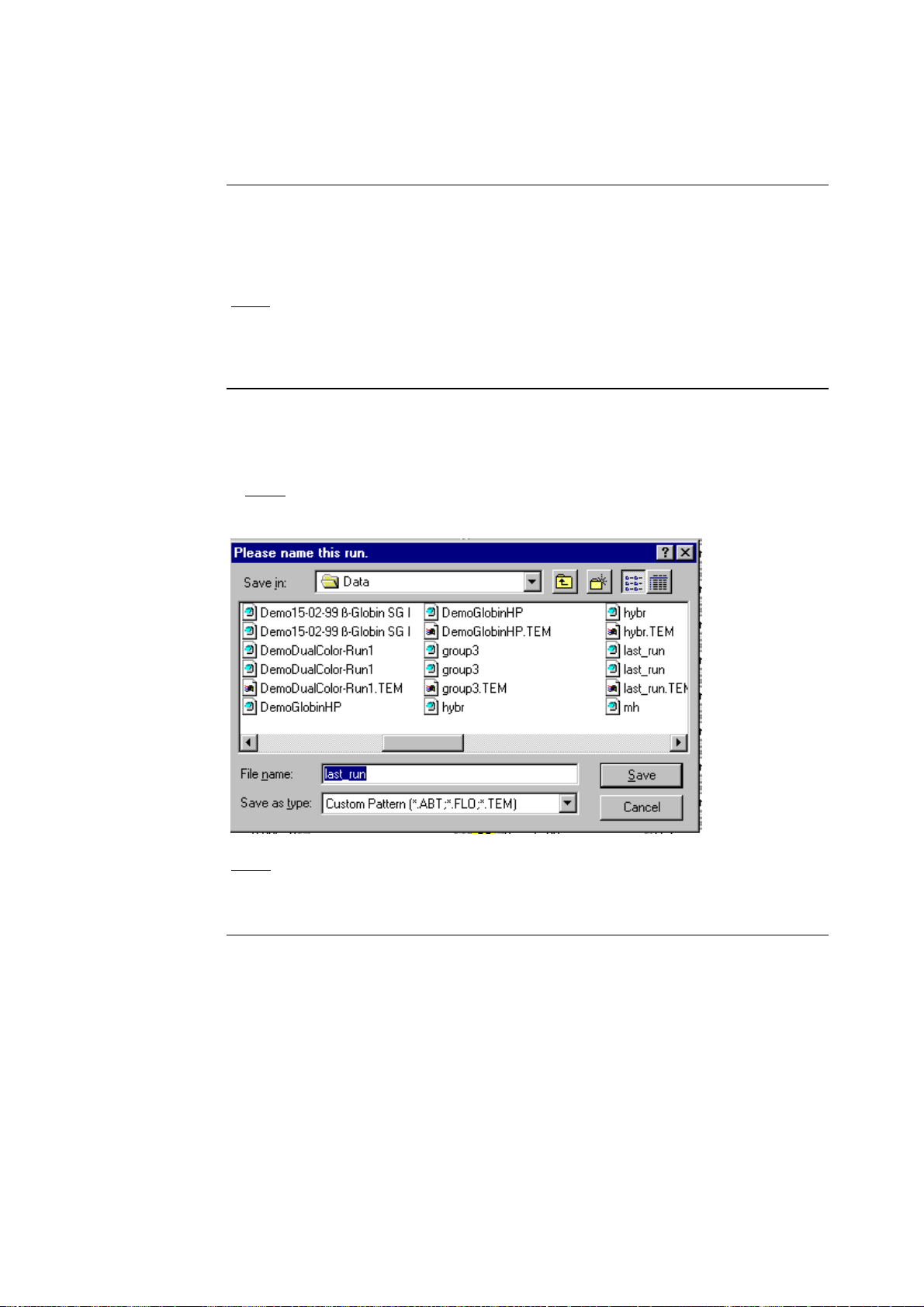
Create a Run
Data File
• After you click on the Run button, a dialog box (shown below) will open
and prompt you to name the file where the run data will be stored.
• Enter a name for the run data file and click the Save button.
Note: To close the dialog box without specifying a file name, click on the
Cancel button.
Note: After you save the data run file and the dialog box closes, the Loading
Screen will appear and the software will prompt you to enter sample data for
the current run (see next page).
Continued on next page
Page 64

B 26
2.1.4.2. Naming Samples at the Beginning of a Run, Continued
Loading Screen
that Appears at
the Beginning
of a Run
After you click on the RUN button in the Experiment / RUN / EXIT Window,
the Loading Screen (shown below) will appear. The software will prompt you
to enter sample data for this run in the Sample Data table.
Note: Start entering data for rotor position #1 and continue through the last
rotor position that contains samples, including any gaps in the sample order.
Fields in
Sample Data
Table
To define sample data for the current run, describe your samples in these
fields of the Sample Data table:
This field Contains this sample data
Rotor Position Indicates the position of the sample in the 32-sample
carousel.
Sample Name Lists the name of the sample as it will appear in the
sample loading dialog and the analysis results.
Type Indicates whether a sample is a positive control, negative
control, quantification standard, or an unknown.
Replicate of Indicates whether a sample is a replicate of another
sample located at another position in the carousel.
Note: Enter the carousel position of the replicated sample.
Concentration Lists the concentration of a sample if it is known, e.g. if
the sample is a quantification standard.
Notes Allows additional sample information to be included.
Continued on next page
Page 65

B 27
2.1.4.2. Naming Samples at the Beginning of a Run, Continued
Additional
Fields on This
Loading Screen
After entering the sample description into the fields of the Sample Data table,
enter additional sample information into three fields along the bottom of the
Loading Screen:
Field Location Contains this information
Temperature
Number of
Samples
Left lower corner
of Loading Screen
Left lower corner
of Loading Screen
Lists the temperature that the sample
chamber holds during the “Seek
Process” at the beginning of a run. As
a default during editing the sample list
the LightCycler holds a temperature of
30°C.
Examples: For instance when
preparing for an RT-PCR, the
temperature during editing the sample
list and the subsequent seek may be set
to a higher temperature (e.g. 55°C).
Higher temperature during the seek
may also be set, when using low
concentrations of probes, that are
fluorescent only at higher
temperatures (e.g. beacon probes).
Lists the total number of samples in
the carousel.
Concentration
Units
Right lower corner
of Loading Screen
Note: If you want to leave space in
between the capillaries, indicate the
number of samples and the number of
free positions in between the
capillaries.
Lists the units of concentration to be
used during data analysis (i.e., the
units appropriate for the values in the
Concentration fields).
Note: This information is only used to
correctly label the axis of the analysis
program. It has no relevance for
calculation of data as such.
Page 66

B 28
2.1.4.2. Naming Samples at the Beginning of a Run, Continued
Additional
Functions on
This Loading
Screen
This Loading Screen contains four buttons that perform the following useful
functions:
Use this button To do the following
Done Closes the Loading Screen and opens the Run
Screen.
Note: The instrument will begin to search for the
indicated number of samples.
Enter Samples Later Allows user to define sample data after the
beginning of the run.
Note: The Edit Samples button on the Run Screen
will flash red as a reminder that sample data still
must be entered.
Clear Sample List Makes all fields in the Sample Data table blank.
Default Sample List To reset the values in the Sample Data table to
values previously defined and stored in the *.exp-
file by the user.
Page 67

B 29
2.1.5. Real Time Fluorimeter (RTF)
Introduction The Real Time Fluorimeter (RTF) permits continuous monitoring of
fluorescence, even when a cycle program is not running.
Whenever you set up a new experiment, e.g. with new primers, nucleic acid
templates, or with a new detection format, use the RTF to optimize the
measuring sensitivity of the LightCycler fluorimeter. For example, you may
adjust the fluorimeter gains to optimize the fluorescence response to
variations in temperature or illumination.
Real Time
Fluorimeter
(RTF) Screen
From the Programming Screen, click on the Real Time Fluorimeter button.
The RTF screen will appear (as in the photo below)
RTF Data
Display
The graph that will appear on the gridlines of the RTF screen shows
temperature and fluorescence output of a sample over time. The Real Time
Fluorimeter reads the raw output from the fluorimeters, so fluorescence
outputs are plotted in tenths of a volt (i.e. a reading of 67 units equals 6.7
volts). Time is measured in seconds.
Note: You may adjust the scale on the temperature axis by selecting the upper
limit on the graph and typing in a new upper limit value. You may use the
scroll bar at the bottom of the screen to move the display back and forth along
the time axis.
Continued on next page
Page 68

B 30
2.1.5. Real Time Fluorimeter (RTF), Continued
RTF Settings Use the fields at the bottom of the RTF screen to enter all RTF settings
described below. Fields are listed as they appear on screen from left to right.
Use this field... To alter this value
Target Temp.
Gains
Changes the temperature in the thermal chamber. The current
temperature in the thermal chamber is displayed in the Value field to
the left of Target Temp.
Note: To adjust the temperature, you may either type in a value, or
adjust the value in the window incrementally by clicking on the small
arrow tabs.
Caution: You must press Enter on the keyboard after typing in a
temperature.
Changes the enhancement factors in the fluorescence measuring
channels. The range is 1-256. The default setting is 1, 15 and 30 for the
respective channels F1, F2 and F3. Each channel can be individually
switched on and off by clicking on the colored On/Off radio button to
the left of the Gains field.
LED
Calibrated
Seek Sample
Note: As in other data screens, Channel 1 (F1) is optimized to detect
emissions from SYBR Green I. Channel 2 (F2) is optimized to detect
emissions from LC-Red 640. Channel 3 (F3) is optimized to detect
emissions from LC-Red 705. The colored lines in the left lower corner
of the screen show the color in which each parameter is displayed on
the graph.
Turns the LED light source on and off. The numerical value below the
switch indicates the current LED level on a scale of 0-100%
Note: . We recommend using Calibrated LED only. If the Calibrated
box is checked, the LED power is at 75% and cannot be adjusted
Determines the power of the LED.
Note: We recommend leaving the LED power at its normal setting by
leaving the Calibrated box checked x. If you would like to change the
power setting, click on the x to clear the box; a scroll box will appear
which allows you to make the necessary changes.
When accessing the RTF, the LightCycler will seek sample #1. Enter the
number of sample you wish to be graphed in the data display. Click on
the Seek Sample button and the LightCycler will move the indicated
sample into the optimal measuring position and acquire any other
fluorescence signal of that sample.
Continued on next page
Page 69

B 31
2.1.5. Real Time Fluorimeter (RTF), Continued
Finding the
Optimum Gain
Setting
To find the optimum gain setting for your experiment, proceed as described
below:
Step Action
1 Prepare a capillary containing a complete PCR reaction mixture.
Note: As template, use the highest starting nucleic acid
concentration that you plan to use in your experiment.
2
3 Depending on the detection format you plan to use, enter the
4 Verify that the correct measuring channel is turned on (i.e., the
5
6
• Place the capillary into the carousel.
• Load the rotor into the LightCycler.
temperature at which the fluorescence will be acquired during your
experiment.
Example: For SYBR Green I format, set the temperature at 72°C
(temperature at end of extension phase). For Hybridization Probes
format, set the temperature at the annealing temperature of the
probes (usually in the range of 50°-60°C, at the end of annealing
phase).
correct LED is lit).
Note: Click on the F1 button for SYBR Green I; F2 button for
Hybridization Probes.
Click on the Seek Sample button to bring the capillary into the
focus of the fluorimeter.
Adjust the fluorimeter Gain (by clicking on the small arrow tabs)
until the displayed fluorescence signal is between 10 and 20.
Caution: Gain values determined in the RTF will not be saved
automatically when you exit the RTF and return to the
Programming or Front Screen. However when returning to the
Programming Screen you will be asked to confirm whether you
wish to use the settings, chosen in the RTF in your experiment.
Exit RTF To quit the RTF and return to the Programming Screen or Front Screen,
either click on the Exit RTF button or choose “Exit RTF” from the File menu.
Page 70

B 32
2.1.6. Programming Screen: Color Compensation Window
Location of
Window
Color
Compensation
Window
Function of
Color
Compensation
Window
The Color Compensation Window is in the upper left corner of the
Programming Screen.
The Color Compensation Window allows you to select a software file (*.ccc file) containing information which can improve the display of data obtained
with multicolor analysis systems (e.g., Dual Color Detection experiments).
The use of color compensation is restricted to Hybridization probes. The
DNA binding dye SYBR Green I is analyzed in channel 1 with no color
compensation.
Color compensation is also not required when a single color detection with
LC Red 640 or LC Red 705 is performed.
Note: See Section 5 of Chapter D for an example of a system that uses color
compensation files.
How to Load a
ccc File
If you select a *.ccc-file in the Color Compensation Window, color
compensated run data will be displayed during a PCR run. To view the effect
of color compensation during a run, do the following:
Step Action
1
2
3
Note:
• You may also choose and/or disable and enable Color compensation during
a Run.
• When Color Compensation is chosen, the Color Compensation file content
will be stored as part of the data-file, generated during the a Run.
• The Display of Fluorescence History however will not switch the displayed
fluorescence values back to the uncorrected values if you turn color
compensation off. Data will be stored without color compensation after
the run.
In the Programming Screen, open the *.exp-file (i.e., file
name.exp).
In the Color Compensation Window, click on “Choose CCC File”
and select the appropriate color compensation file from the
Calibration directory window.
Color Compensation is automatically enabled.
Click on Use Color Compensation to disable Color Compensation.
Page 71

B 33
2.1.6. Programming Screen: Color Compensation Window,
Continued
Use of *.ccc Files for Data
Analysis
In addition to using a *.ccc-file to generate a color compensated run display,
you can use the file to analyze multicolor experiments more accurately. This
important use of *.ccc-files is described more fully in Section 4.3.3 of this
chapter.
Page 72

B 34
2.1.7. Programming Screen: Display Mode / Fluorimeter Gains Window
Location of
Window
Display
Mode /
Fluorimeter
Gains Window
Display Mode
The Display Mode / Fluorimeter Gains Window is in the upper center
portion of the Programming Screen.
To select the fluorescence display used during LightCycler runs, use the pulldown menus in the Display Mode window. One menu sets the numerator of
the selection; the second sets the denominator of the selection. Choices for
numerator and denominator include F1, F2, F3, and 1.
Note: The F1 channel is optimized to detect emissions from SYBR Green I or
fluorescein, at wavelengths around 530 nm. The F2 channel detects emissions
from LC-Red 640. The F3 channel is optimized to detect emissions from LCRed 705 (see Section 1.3.2 of Chapter C).
Examples: Use the following settings for typical experiments:
• For quantification runs with SYBR Green I alone, select F1/1.
• If fluorescein is used alone, select F1/1.
• If fluorescein is used with another fluorophore as a FRET pair (see Section
1.3.2 of Chapter C), select either F2/1 or F3/F1, depending on whether the
second fluorophore is LC-Red 640 (F2/1) or LC-Red 705 (F3/F1).
Note: Channel Settings may also be selected and altered during a Run.
For more information on the use of these fluorescence display settings in
various applications, see Chapter D.
Continued on next page
Page 73

B 35
2.1.7. Programming Screen: Display Mode / Fluorimeter Gains
Window, Continued
Fluorimeter
Gains
Different applications require different detection sensitivity. Use the
Fluorimeter Gains window to set the correct detection sensitivity for your
application in each of the fluorimeter channels. The gains may be adjusted on
a scale from 1 to 256. You may either type in a gain number or click on the
small tabs to adjust the number gradually. The default setting is 1, 15 and 30
for the respective channels F1, F2 and F3.
Note: Grain Settings cannot be changed during a Run, but must be set to their
optimum values as described earlier. To determine the correct gain
experimentally, use the Real Time Fluorimeter (RTF) (see Section 2.1.5 of this
Chapter). If the signal for a channel in the RTF is too high, select a lower gain
number. If the signal level is too low, select a higher gain number.
Page 74

B 36
2.1.8. Programming Screen: Cycle Program Data and Temperature Targets Window
Location of
Window
Cycle Program
Data and
Temperature
Targets
Window
The Cycle Program Data and Temperature Targets Window is in the center
portion of the Programming Screen.
Cycle Program
Data
The Cycle Program Data section automatically appears when a new cycle
program is added to a protocol (e.g. by clicking the Add button under the
Cycle Programs list as described in Section 2.1.2 above).The section accepts
the following information:
Part Option
Cycles Field
Analysis Mode
Menu
Note: Information entered in this window is automatically saved without
onscreen confirmation.
Type in the number of cycles to be run with the cycle
program parameters to be defined in the Temperature
Targets Window.
Depending on the application and the cycle program, use
the pull down menu to select the correct analysis mode:
• None,
• Quantification, or
• Melting Curves.
Note: The default setting is None. Change the setting if
the data acquired during the cycle program is to be used
for quantification or melting curve analysis.
Continued on next page
Page 75

B 37
2.1.8. Programming Screen: Cycle Program Data and
Temperature Targets Window, Continued
Temperature
Targets
Field
Description
Field Purpose
Target
Temperature
(°C)
Incubation Time
(h:min:s)
Temperature
Transition Rate
(°C/s)
Use the Temperature Targets section to define the temperature profiles for
each individual program. Type the appropriate settings in the horizontal fields
between the Ins and the Del buttons.
Each temperature segment is inserted or deleted as follows:
Button Click on the button...
Ins
(Green)
Del
To enter a new temperature segment or to add a
temperature segment in a new position.
To delete a temperature segment.
(Red)
Enter the following information into the fields in the Temperature Targets
section:
Defines the temperature of the segment in °C, e.g., 94°C for
denaturation or 72°C for elongation phase.
Defines the holding time of a temperature segment. Use the following
guidelines for setting typical incubation times:
• For denaturation: 0 s
• For annealing with SYBR Green I: 0–10 s
• For annealing with hybridization probes: 0–15 s
• For elongation: As a general rule, divide the length of fragment in bp
by 25 to determine the incubation time (in seconds).
Note: If you enter a single number, it indicates the time is in seconds. To
enter a time in minutes, you have to use a colon to separate minutes and
seconds, e.g. ”10:30” means 10 minutes and 30 seconds. When entering
60 seconds or more "seconds" when leaving this field an automatic
conversion from seconds to minutes occurs (e.g. 100 seconds will be
converted to "1:40").
Defines rates at which the instrument changes temperature between
temperature targets. The slowest rate is 0.1°C/s and the fastest rate is
20°C/s.
Note: A fast transition rate (20°C/s) works well for amplification
programs. Slower rates (0.1–0.2 °C/s) are recommended for melting
curves.
Continued on next page
Page 76
B 38
2.1.8. Programming Screen: Cycle Program Data and
Temperature Targets Window, Continued
Field Description (continued)
Field Purpose
Secondary Target
Temperature
(°C)
Step Size
(°C)
Step Delay
(cycles)
Acquisition Mode
Defines a second target temperature within a segment beginning at a
defined cycle number (see Step Delay below).
Note: This is suitable for a so-called ”touch down” PCR. Here, annealing
occurs at the upper temperature limit to ensure specificity during the
first 15-20 cycles. However, this reduces the efficiency of amplification.
After 15-20 cycles, the annealing temperature is decreased by as much as
10°C to the Secondary Target Temperature where annealing occurs
with lower specificity to facilitate greater amplification efficiency.
Defines the degree change per cycle used to step up/step down from the
Target Temperature to the Secondary Target Temperature.
Note: A decrease of 1°C/cycle is commonly used in ”touch down” PCR.
Defines the cycle number at which the step up/step down from the
Target Temperature to the Secondary Target Temperature begins.
Defines the time in each PCR cycle at which fluorescence measurement
of samples is made. The available choices are:
None
Single Measures fluorescence once at the end of the
Cont.
(continuous)
No fluorescence measurement.
temperature segment selected. For the
Hybridization Probe format, measurement
occurs at the end of annealing phase; for the
SYBR Green I format, it occurs at the end of
the elongation phase.
Monitors fluorescence data continuously from
the first sample to the last one. The
temperature continues to increase during
measurement.
Note: This acquisition mode is suitable for
melting curve analysis.
Continued on next page
Page 77

B 39
2.1.8. Programming Screen: Cycle Program Data and
Temperature Targets Window, Continued
Field Description (continued)
Field Purpose
Acquisition mode
(continued)
Step
(stepwise)
Measures fluorescence at each temperature
transition.
Example: If you use Step to acquire
fluorescence during a melting curve analysis,
enter 0.2oC/s as your Temperature Transition
Rate. The system will heat up the samples by
0.2°C, hold the temperature and measure all
samples. It will then stepwise increase the
temperature by 0.2°C and measure at the
respective temperatures.
Note: This acquisition mode is suitable for
melting curve analysis.
Page 78

B 40
2.1.9. Programming Screen: Graphic Simulation Window
Location of
Window
Graphic
Simulation
Window
Cycle Program
Simulation
The Graphic Simulation window is just below the Cycle Program list on the
right side of the Programming Screen.
Click on the Simulation button in the Graphic Simulation window to display
the Cycle Program Simulation. This simulation shows a graph of a single cycle
of the program named in the Cycle Program list (directly above the window).
Use this visual aid to confirm your cycling parameters
Experimental
Notes
Note: In the simulation, the temperature profile is displayed as a red line,
while the fluorescence acquisition profile is graphed in green.
In addition to the Cycle Program Simulation display, the Graphic Simulation
Window can display notes about the current program. Use the buttons
beneath the window to do the following:
Use this button To do this
Experimental Notes
Edit Exp. Notes
Display notes on the program highlighted in the
Cycle Program list.
Alter the notes displayed in the window.
Page 79
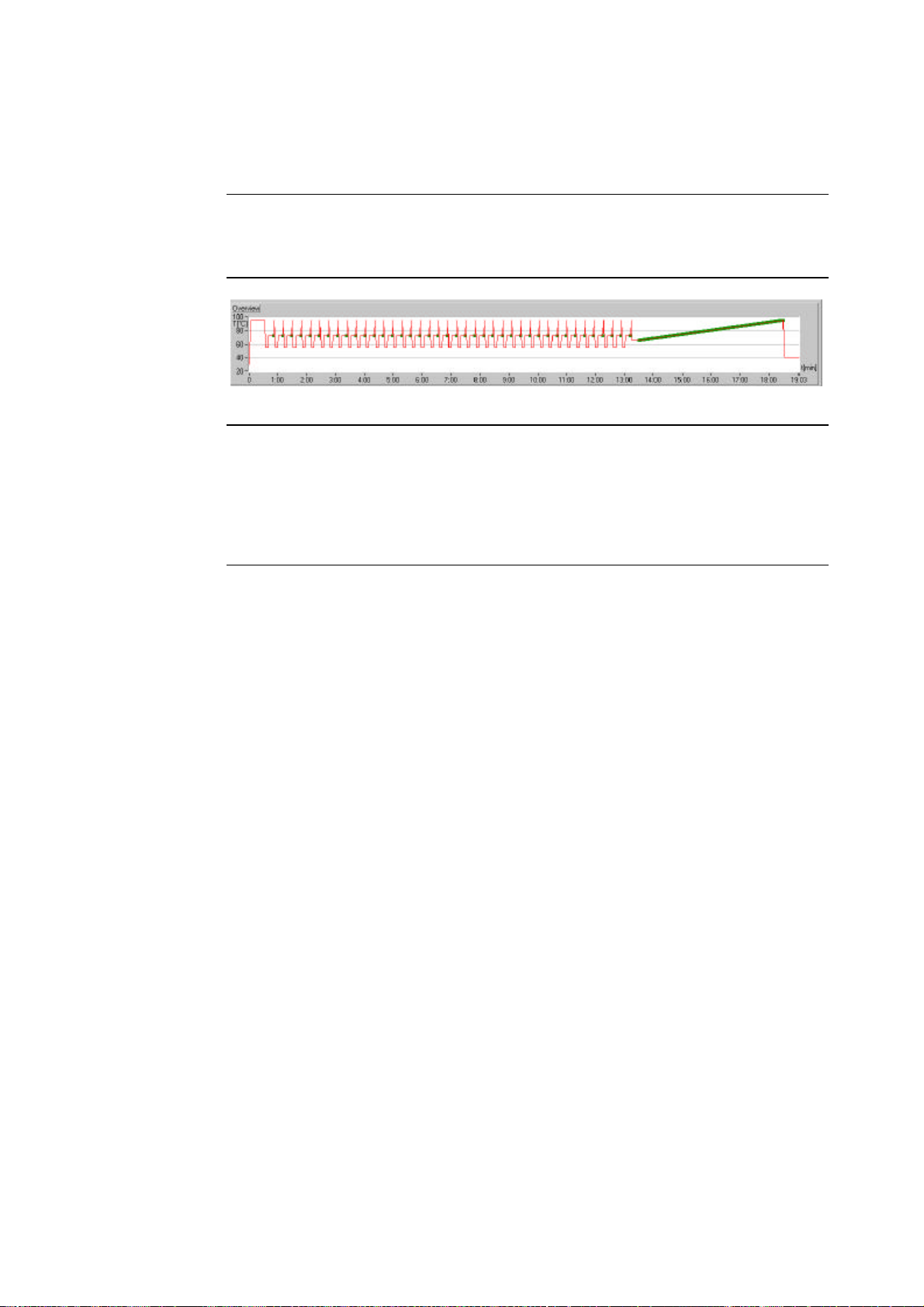
B 41
2.1.10. Programming Screen: Cycle Program Overview Window
Location of
Window
Cycle Program
Overview
Window
Temperature
Profile vs.
Time
The Cycle Program Overview window stretches across the bottom of the
Programming Screen.
The Cycle Program Overview window displays a graph of the current
experimental protocol. The temperature profile is graphed in red, while the
fluorescence acquisition profile is graphed in green. The time for the
programmed Experimental Protocol is indicated as rough estimate.
Page 80

B 42
3. Running the LightCycler
3.1. LightCycler Run Screen
Introduction When a PCR run is started, the monitor switches from the Programming
Screen to the Run Screen. This screen displays actual fluorescence values, the
development of fluorescence during the run, and the actual temperature
online.
Note: A picture of the Run Screen is shown on the next page.
Run Screen
Display
After the instrument detects the samples, temperature cycling begins and the
Run Screen will display three graphs:
• Fluorescence vs. Time (Fluorescence History)
• Temperature vs. Time (Temperature History)
• Relative fluorescence of each sample (Current Fluorescence bar graph).
The screen also displays the following:
• Cycle Program Name
• Cycle Program Segment
• Cycle Number (currently in progress)
• Current Fluorescence Display Mode.
• Samples Names (and the corresponding line color used to display the
progress of that sample).
Note: If the LightCycler is unable to detect one or more samples, a dialog box
will tell the user how many samples were found at which position and offer a
chance to abort the run or continue it.
This behavior also applies, when intentionally one position of the sample
carousel was left free.
In case the system was not able to find a capillary, repeat the seek with "Use
Seek Threshold" disabled in the Options pull-down of the Programming
Screen.
Position #1 is always measured. Here, if no capillary is found, a default
position, determined during instrument calibration is used.
Continued on next page
Page 81

B 43
3.1. LightCycler Run Screen, Continued
LightCycler
Run Screen
Fluorimetry
Controls
A panel at the top of the Run Screen controls several fluorimetry display and
acquisition (measurement) parameters.
Area Function
CCC File (fields)
Display Mode
(pull down menus)
Fluorimeter Gains
(fields)
If you are using a color compensation file, this
area will list the name of that file.
May be used to select the fluorescence channel(s)
displayed during LightCycler runs.
Note: Display mode is usually specified during
programming (see Section 2.1.7 above), but may
be changed here.
Displays the detection sensitivity in each of the
fluorimeter channels.
Note: Gains cannot be changed during a run.
Gains are set during the programming phase of
the experiment (see Section 2.1.7 above).
Continued on next page
Page 82
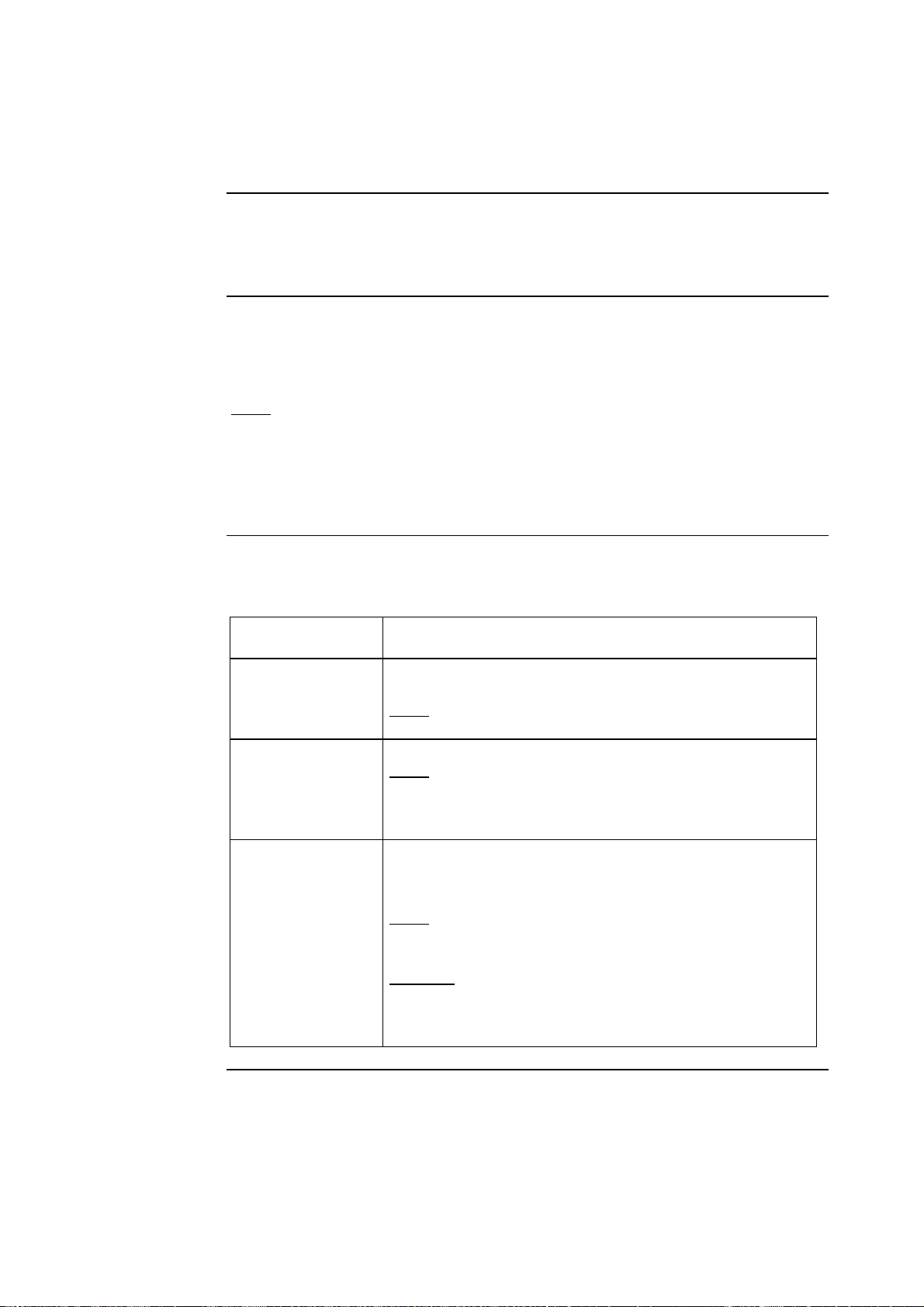
B 44
3.1. LightCycler Run Screen, Continued
Program Name
/ Segment /
Cycle Number
Sample
Names/
Default Colors
Other Buttons
The right section of the top panel shows 1) the name of the program (e.g.
PCR, Annealing), 2) the number of the temperature segment now in progress,
and 3) the number of the cycle now in progress.
A window in the far right corner of the screen lists 1) the names of the samples
in the current run, 2) the rotor position of each sample, and 3) the color of the
line (or bar) that represents each sample in the graphs.
Note: You can change the color coding by just clicking on the color squares to
the left of the samples. Changes are effective in the current run and shall not
be forwarded to the Graphics Defaults settings.To reset the color defaults that
you defined earlier (see Section 1.5 above), click on the Default Colors button
(lower right corner of the screen). This does not affect data display in the
LCDA module.
In the lower right corner of the screen, there is a set of buttons. Use these
buttons to perform the following functions:
Click on this
button...
End Program
Add 10 Cycles
Exit Run
Stop the cycle program that is currently running and
move onto the next cycle program.
Note: If the current program is the last in the protocol,
clicking this button will return you to the Front Screen.
Add ten more cycles to the program in progress.
Note: Use this button if your amplification reaction is
not complete or your reaction is proceeding slowly. This
change is only effective for the current run and shall not
be stored in the *.exp-file.
End the run immediately and offer you two options:
• Save, or
• Cancel.
Note: If there was an error in the program or you do not
want to save the data click on Cancel. Otherwise, click
on Save to save the run data for later analysis.
Caution: If a protocol is run twice, both sets of data
cannot be saved. You can replace the first set of data
with the second set, rename the second data set, or hit
Cancel to save the original data.
To do this...
Continued on next page
Page 83

B 45
3.1. LightCycler Run Screen, Continued
End of Run
• If the LightCycler completes a run, it will automatically save the program.
• After the LightCycler finishes a run, it will automatically open a data
analysis window that displays the data acquired in the current run.
Note: When at the end of a Run another experiment is being analyzed, the
current data will not be displayed atomatically.
Page 84

B 46
4.1. Introduction
4. Data Analysis
Starting Data
Analysis
Analysis
Formats
Format Select by clicking Description
Quantification
Melting Curve
To begin data analysis, do either of the following:
• Simply allow the LightCycler to complete a run. When the protocol has
finished, the software will automatically display the collected data on the
LightCycler Data Analysis (LCDA) Front Screen.
• If no run has been performed, open the LCDA Front Screen by clicking on
the Data Analysis button on the LightCycler Front Screen.
The LightCycler Data Analysis (LCDA) software allows a LightCycler user to
analyze quantification data or melting curve data acquired during a
LightCycler reaction. The LCDA software can display and analyze data in two
formats:
Quantification button
(at top of screen)
Melting Curve button
(at top of screen)
Estimates the original number of target DNA
copies in a sample by comparing it to at least two
known concentrations of a standard. It displays
the amplification profile of a PCR.
Displays a fluorescence curve profile obtained
during a slow denaturation of PCR products, and
includes options for differentiating melting
curves to give melting peaks, integrating the area
under the melting peaks, and defining the
melting temperature of amplified products.
In this Section
Note: You must select the segment of the run to be analyzed and define other
analysis parameters before the data can be analyzed. For more information,
see the following sections of this chapter for details:
• See Section 5 for details on defining parameters for the Quantification
format, including setting the baseline and crossing line, determining
crossing points, and selecting data from a set of standards to generate a
standard curve.
• See Section 6 for details on defining parameters for the Melting Points
format, which are similar to those used for the Quantification format.
The rest of Section 4 gives a brief overview of the various parts of the LCDA
Front Screen. See Sections 5 and 6 for detailed information on using these
parts to set up a Quantification or Melting Curve analysis.
Page 85

B 47
4.2. Select Data File / Data Analysis Window
Select Data File
to Analyze
Searching for a
Data File
You must choose the data to be analyzed by doing the following:
• Select “Open” from the File menu on the LCDA Front Screen. A Data
Analysis Window (shown below) will open and display a list of LightCycler
data files. For each file, the list gives the File Name, User Name, date / time
Created, and date / time Analyzed
Note: You will see only those files that belong to the current user (e.g., the
user whose log on name was used to enter the LightCycler software. Every
user has an individual data folder.
• Use the pointer to highlight the file containing the data.
• Click on the Open button.
Result: The LCDA Front Screen will open and display the selected data file in
a new window.
Note: A new window is created for each open file. Thus, you may open and
analyze several data sets by moving between windows.
If you are not certain which data file you want, you may use the fields at the
bottom of the Data Analysis Window to search for a select subset of files.
These fields allow you to search for files by File name, User name, Date
Created or Date Analyzed.
Data Analysis
Window
Continued on next page
Page 86

B 48
4.2. Select Data File / Data Analysis Window, Continued
Additional
Information
on Data Files
File Open
Default
Settings
After a file is highlighted (but before it is opened), additional information
about the file will be displayed in the box at the right of the Data Analysis
Window. This information includes:
• File name
• Number of linked programs that constitute the run, with a brief description
of each program
• Number of samples
• List of all sample names.
Note: If the samples were designated as standards of known concentration,
the concentrations will also be listed next to the sample name.
The Preferences option under the pull down "Help" menu of the LCDA Front
screen allows customizing the file open default settings.
Select by Clicking Description
Initial Directory Allows the user to define the directory from
which he wants to start in when the open file
dialog appears for the first time.
Export Data Allows the user to define the format of numbers
when data will be exported.
Page 87

B 49
4.3. LCDA Front Screen
Introduction When you open a data file from the Data Analysis Window, the data will
automatically be displayed in the LCDA Front Screen shown below. The data
will eventually be displayed in two sections of the window:
• Initially, the top section of the window will display the run data as a
Temperature versus Time graph. On this graph, you must define the section
of the run that will be analyzed by bracketing the selected section with the
green cursors (see Section 4.3.1).
• After you set the cursors, the lower section of the window will display the
fluorescence data acquired during the selected section of the run (as shown
below).
Note: In this and all subsequent data analysis screens, you can enlarge or
reduce the size of each graph by clicking on the horizontal divider between the
graphs and moving it up or down.
LCDA Front
Screen
Continued on next page
Page 88

B 50
4.3.1. LCDA Front Screen: Temperature vs. Time Graph
Set Cursors for
Quantification
Analysis
To use Quantification analysis to analyze the data (i.e., data acquired once
per cycle), set the green cursors to bracket the section of the graph that
represents the amplification program (as shown in the photo below).
Note: To automatically bracket an amplification program, click the Select a
Program button and then select the program segment in which amplification
took place. (For example, see the upper left corner in the picture of the LCDA
Front Screen on the previous page. The name of the selected segment appears
in the window below the Select a Program button.)
To further refine the data to be analyzed grab and move the green cursors
manually.
Set Cursors for
Melting Curve
Analysis
To analyze melting curve data (i.e., data acquired continuously), set the green
cursors to bracket the section of the graph that represents the melt program
(as shown in the photo below).
Note: To automatically bracket a melt program, click the Select a Program
button and then select the program segment of the protocol in which melting
took place. To further refine the data to be analyzed grab and move the green
cursors manually.
Continued on next page
Page 89

B 51
4.3.1. LCDA Front Screen: Temperature vs. Time Graph, Continued
Upper Graph:
Temperature vs.
Time
Change Graph
Settings
The graph in the top section of the LCDA Front Screen displays the
temperature cycles (black line) during the run, as well as the temperatures at
which fluorescent data was acquired (pink line).
To define the area of this graph that will be analyzed, do one of the following:
• Use the mouse to move the green cursors until they bracket the area to be
analyzed.
• Click on the button at the left corner of the graph to open a window (as
shown below). In this window, you may enter a number in the Value field
to specify the position of each cursor (expressed as a particular time, in
h:min:s format). After you have entered the times for each cursor, click the
OK button to set the cursors.
To change any axis values of the graphs displayed click on the button on the
top left corner of the graph.
This will bring up a Customize Graph screen. In this screen the user can
define values for the axis which will then be carried into the LCDA display.
Deselect the Automatic Fit option and enter values for the axis in the
appropriate fields.
Page 90

B 52
4.3.2. Fluorescence vs. Cycle Number / Time / Temperature Graph
Fluorescence
vs. Cycle
Number
Select x-Axis
After you set the cursors in the top graph (see Section 4.3.1 above), the
bottom graph (as shown in the photo below) will display the fluorescence data
acquired during the bracketed section of the protocol.
In the pull down menu below the bottom graph, you may select any of three
options for the parameter plotted on the x-axis: Time, Temperature, or
Cycles. Use the following guidelines to select the appropriate parameter:
If you will analyze... Then select...
Fluorescence data acquired once per cycle (i.e.,
quantification data)
Fluorescence data from a melt
Any experiment (default setting, for data viewing only)
Cycles
Temperature
Time
Continued on next page
Page 91

B 53
4.3.2. Fluorescence vs. Cycle Number / Time / Temperature
Graph, Continued
Select
Fluorescence
Display
Guidelines for
Fluorescence
Display
To set the fluorescence channel to be displayed in the lower graph, highlight
the appropriate item in the pull-down menu to the right of the y-axis. You
may choose to display fluorescence data from a single channel (F1, F2, or F3)
or a ratio of data from two channels (F1/F2, F1/F3, F2/F1, F2/F3, F3/F1, or
F3/F2).
Use the following guidelines to determine which fluorescence display to use
for various applications:
Use this fluorescence display... For assays containing...
F1
F2
F2/F1
F3,
F3/F1
• SYBR Green 1
• Fluorescein
• LC-Red 640
• LC-Red 640 / Fluorescein
Hybridization probe pair
• LC-Red 705
• LC-Red 705 / Fluorescein
Hybridization probe pair
Note: When opening a file the first program with data acquisition is bracketed
and the channel setting is chosen in the *.exp-file selected.
Example: For examples of experiments which use different fluorescence
display settings, see the applications described in Chapter D.
Page 92

B 54
4.3.3. LCDA Front Screen: Using Color Compensation Files
Color
Compensation
Preparing a
Color
Compensation
File
Saving a Color
Compensation
File
The following dyes can be used for multicolor analysis with the LightCycler:
Analysis Format Channel 1 Channel 2 Channel 3
Hybridization
Probes
Each of the fluorophores listed above has a different emission maximum, and
the LightCycler detection filters have been optimized to these maxima.
However, when more than one of these dyes is used in a single run, there is
some crosstalk between fluorimeter channels. LCDA color compensation
software compensates for this between-channel crosstalk.
To use color compensation, you must first create a *.ccc (ccc)-file in a
calibration experiment with the dyes listed above (see Section 5 of Chapter D
for details).
Note: We offer a LightCycler Color Compensation Set (Cat No. 2 158 580),
with ready-to-use solutions and optimized conditions for the generation of
ccc files.
After completing a calibration run, do the following:
Fluorescein LC Red 640 LC Red 705
Step Action
1 Open the acquired data in the Programming Screen.
2
3
4 Save the color compensation data by naming the calibration file.
Note: Once you have created and saved a color compensation calibration file,
you can use that data to correct many future data sets (as long as you use the
same combination of fluorophors).
In the Select a Program pull-down menu in LCDA, highlight the
program segment that includes the melting program.
From the Color Compensation menu, select “Calibration”.
Note: The file name must end in “.ccc”, e.g. data1.ccc.
Continued on next page
Page 93

B 55
4.3.3. LCDA Front Screen: Using Color Compensation Files,
Continued
Using a Color
Compensation
File During
Data Analysis
To use a calibration file to color compensate run data, follow the steps
described below:
Step Action
1
2
3 Under the Color Compensation menu, select „Load Calibration
4 To see what your data looks like after color compensation, do
5
From the Data Analysis Window, open the file containing the run
data (see Section 4.2 of this chapter).
Result: The LCDA Front Screen will appear and display the run
data.
Bracket the program segment to be analyzed in the Temperature
vs. Time screen (see Section 4.3.1 of this chapter).
Data“, and click on the name of the stored ccc (calibration) file.
Note: You may also click on the Select CC Data button (top center
portion of screen) to find the stored ccc file.
either of the following:
• Click on the Color Compensation button (top center portion of
screen), or
• Select „Enable“ under the Color Compensation pull-down
menu.
Click on the Color Compensation button again to return to the
raw data.
Page 94

B 56
4.4. LCDA Front Screen: Data Retrieval
Introduction
Print Data
You can retrieve permanent copies of the data displayed on the LCDA Front
Screen. This section describes options for data retrieval.
You can print a hard copy of the data displayed on the screen.
To print either the upper or lower graph:
• From the File pull-down menu, select “Print Window”, and
• On the Report pull-down menu, highlight the graph to be printed. The
Report function will launch the LightCycler Software report tool
"Graphworks".
4.5. Further Analysis
Further
Analysis of
Amplification
or Melting
Curve Data
If you have collected data in specific formats, you can use the buttons at the
top of the LCDA Front Screen to prepare them for further analysis, as
follows:
If the data is displayed
as...
Fluorescence vs. Cycle
(e.g., fluorescence data
that was acquired once
per cycle)
Fluorsence vs.
Temperature
(e.g., fluorescence data
acquired continuously
from a melting
experiment)
For further analysis,
click...
Quantification button
Melting Curve button
And...
Use Section 5 of this
chapter to set up a
Quantification analysis
Use Section 6 of this
chapter to set up a
Melting Curve analysis
Page 95

B 57
5. Display and Analysis of Quantification Data
5.1. Introduction
Quantification
on the
LightCycler
Fluorescence
Acquisition for
Quantification
Analysis
Use of
Crossing Line
to Quantify
Unknowns
The LightCycler performs a quantification analysis by comparing the
fluorescence of a PCR product of unknown concentration with the
fluorescence of several dilutions of an external standard. For this analysis, the
instrument considers only fluorescence values measured in the log-linear
phase of amplification.
Note: To perform a quantification analysis the user must have defined at least
two standards of known concentration in the Loading Screen. Ideally,
standards are amplified under the same conditions as the unknowns and have
the same amplification efficiency. We recommend using the same primer set
to amplify both standards and unknowns.
In order to quantify PCR product with the LightCycler, the instrument must
be set up to acquire fluorescence once per cycle. This will generate a
fluorescence curve that increases in value with each cycle as the product
accumulates.
The most useful data occurs during the few cycles in which the fluorescence
readings are above background and increase in a log-linear fashion.
The LCDA quantification software is designed to generate a best-fit line from
this log-linear region of each curve.
This best-fit line will be parallel to the x-axis and is called a Crossing Line. The
point at which the Crossing Line intercepts the log-linear region of each
fluorescence curve may be used to calculate the concentration of the sample
that generated the fluorescence curve.
The instrument plots a standard curve of the Crossing Line intercepts of the
standards vs. the known concentrations of these standards. By comparing the
Crossing Line intercept of an unknown sample with the standard curve, the
instrument generates a quantitative estimate of the starting copy number of
the target DNA in the unknown.
Page 96

B 58
5.2. Quantification: Selection of Analysis Method
Quantification
Analysis
Methods
Fit Points
Method
(Default
Method)
Clicking on the Quantification button on the LCDA Front Screen will open
the Quantification Analysis window. Once that window is open, the first step
of the data analysis is to select the appropriate analysis method. There are two
choices. The descriptions given below should help you determine the most
suitable analysis.
Note: To choose either of the two methods, click on one of these buttons:
• Fit Points, or
• Second Derivative Maximum
Alternatively, you can choose either of the methods under “Analysis Method”
in the Quantification menu. Note that the Fit Points option is called Fit N
Points Above Threshold in this menu, while Second Derivative Maximum
has the same name on both the menu and the button.
This method is possible with all applications. When quantitating samples
containing low copy numbers of target DNA (typically less than 1000 copies),
the Fit Points method often gives better results than Second Derivative
Maximum. Fit Points also works well at high copy numbers, so if the template
concentration in your samples ranges from low to high, you should use Fit
Points. Additionally this method shows backward compatibility to the old
software.
Second
Derivative
Maximum
Method
Note: Set up and analysis by the Fit Points method requires three steps: Step 1:
Baseline, Step 2: Noise Band and Step 3: Analysis. These steps are described in
detail in Sections 5.3–5.5 of this chapter.
The Second Derivative Maximum method is better for analyzing high copy
numbers (above 1000 copies per tube) since it requires no user input or bias.
However, this method may have problems analyzing low copy numbers that
lead to less well-behaved reactions.
The Second Derivative Maximum Method is a semiautomated method, that
uses the shape of the curve to determine the target copy number. It is
independent of user-borne influences and will produce identical data e.g.
irrespective of baseline subtraction method.
Note: Set up and analysis by the Second Degree Maximum method requires
only two steps: Step 1: Baseline, Step 2: Analysis. This method does not
require setting a noise band (Step 2 under Fit Points above). See the next page
for more information.
Continued on next page
Page 97

B 59
5.2. Quantification: Selection of Analysis Method, Continued
Tabs on the
Quantification
Analysis
Screen
The Quantification Analysis screen will display fluorescence curves for all the
samples in the selected run.
If you chose the Fit Points method, you will see three tabs displayed at the top
of the screen: Step 1: Baseline, Step 2: Noise Band, and Step 3: Analysis. Note:
For more details on each stage, see the section of this chapter listed in the table
below).
If you chose the Second Derivative Maximum method, you will see only two
tabs: Step 1: Baseline and Step 2: Analysis.
To set up and perform the analysis, you must select each tab in turn, and
define the appropriate data analysis parameters in the screen that appears. The
following parameters must be defined:
Stage Description See
Section
Step 1:
Baseline
Step 2:
Noise Band
(Fit Points
method only)
Step 3:
Analysis
For valid comparison, all samples should have
baseline fluorescence values close to zero. In
this screen, you will define any baseline
corrections that are necessary prior to analysis.
In this screen, you will set the noise band that
defines non-informative fluorescence
data.Typically, this is data that cannot be
distinguished from the fluorescence noise in the
earlier cycles of amplification.
Note: Not required for the Second Derivative
Maximum method. This method is a semiautomated approach to quantitative PCR. It
solely interprets the shape of the curve to
determine copy number. Consequently, it does
not require setting a Noise Band, nor a
Crossing Line. Also Baseline correction is
solely performed to free the graph from effects
of background variability. It will not have any
effect on quantification as such.
In this screen, you will determine the point of
intersection between the fluorescence curves
and a defined crossing line. You will select the
type of threshold setting from several options.
Note: The values for the crossing points are
used to generate a standard curve which plots
Cycle Number vs. Log Concentration and this
standard curve will be used to calculate
concentrations for unknown samples.
5.3
5.4
5.5
Continued on next page
Page 98

B 60
5.2. Quantification: Selection of Analysis Method, Continued
How to Use
this Part of the
Manual
Each of the steps in the table above are described more fully in Sections 5.2–
5.5 of this chapter. You may use these sections to familiarize yourself with the
steps of a Quantification Analysis.
After you are familiar with the required steps, use the convenient procedure
(Section 5.6 of this chapter) to set up and perform Quantification Analysis.
Page 99

B 61
5.3. Quantification Step 1: Baseline
Step 1: Baseline
Screen
Continued on next page
Page 100

B 62
5.3. Quantification Step 1: Baseline, Continued
Step 1: Baseline
Quantification
Regardless of which analysis method you choose, you must use the Baseline
Screen to define any baseline corrections necessary for data analysis.
Note: Baseline fluorescence may vary from sample to sample for several
reasons including differences in sample preparation, differences in DNA
content from tube to tube or pipetting error. However, in order to make
sample to sample comparisons easier, you must set the baseline fluorescence
for all samples to zero.
The Fluorescence vs. Cycle Number graph (shown on the previous page) has
been carried over from the LCDA front screen and is used for baseline
adjustment. As you select a method for baseline adjustment, this graph will
change. The corrected graph will then be carried over to the next screen.
There are four options available for baseline adjustment:
Baseline
adjustment
None No baseline adjustment.
Arithmetic
(Default option)
Proportional
Recommended for any application with high sample-tosample background variation.
In this method, the mean value of the five lowest
measured data points for each sample will be calculated,
then subtracted from each reading point in the sample.
Note: This method is best for SYBR Green I reactions,
since the template DNA will sometimes give a signal that
is removed most efficiently by this method.
When target DNA presents no baseline problem, the
Proportional method can remove some of the tube-totube variations due to pipetting.
Description
Normalization
Note: This method is best for hybridization probes,
TaqMan probes, Beacons, and especially suited for Dual
Color experiments.
All fluorescence curves are displayed as a percent of
maximum fluorescence. One hundred percent
fluorescence is represented by the value 100. This brings
all fluorescence curves to the same maximum value.
When used with the Fit Points Method, it will alter the
curves such, that incorrect quantification data may be
produced.
Note: This method is not suited for analysis.
Continued on next page
 Loading...
Loading...