Page 1

®
cobas
6000 analyzer series
Operator´s Manual – Version 8.2
Software Version 06-03
Page 2

Publication information
cobas®6000 analyzer series
Revision history
Publication
version
1.0 01-01 June 2006 First version
1.1 02-01 September 2007 General update
2.0 02-01 – 02-07 March 2008 General update
3.0 03-01 June 2008 General update
4.0 04-01 February 2009 General update
5.0 05-01 March 2010 General update
5.1 05-01 April 2011 Two safety alerts added
6.0 05-02 July 2013 General update
6.0.1 05-02 March 2014 Minor changes concerning
7.0 06-01
7.1 06-01
8.0 06-01
8.1 06-02
8.2 06-03
(a) Also valid for software version 05-02
(b) Also valid for software version 06-01
(c) Also valid for software version 06-02
Software version Revision date Change description
expired reagents and controls
(a)
(a)
(a)
(a) (b)
(a) (b) (c)
December 2014 General update
January 2015 Minor changes
September 2016 General update
October 2017 Online Help also applies to
software version 06-02.
December 2018 General update including the
addendum 1.0, 2.0, and 3.0.
Edition notice This publication is intended for operators of the cobas 6000 analyzer series.
Every effort has been made to ensure that all the information contained in this
publication is correct at the time of publishing. However, the manufacturer of this
product may need to update the publication information as output of product
surveillance activities, leading to a new version of this publication.
Where to find information The Online Help contains all information about the product, including the
following:
o Routine operation
o Maintenance
o Safety
o Troubleshooting information
o A software reference
o Configuration information
o Background information
e
See The help system of the instrument on page B-15
The Operator´s Manual contains information about safety, hardware modules and
operating the system as well as maintenance and troubleshooting.
The manual Interlock function cobas c 501 with ISE describes maintenance actions
of the cobas 6000 analyzer series that can only be performed by special trained
person.
Roche Diagnostics
2 Operator´s Manual · Version 8.2
Page 3

cobas®6000 analyzer series
Training Do not carry out operation tasks or maintenance actions unless you have received
Images The screenshots and hardware images in this publication have been added exclusively
Warranty Any customer modification to the system renders the warranty or service agreement
General attention
To avoid serious or fatal injury, ensure that you are familiar with the system and safety
information before you use the system.
o Pay particular attention to all safety precautions.
o Always follow the instructions in this publication.
o Do not use the instrument in a way that is not described in this publication.
o Store all publications in a safe and easily retrievable place.
training from Roche Diagnostics. Leave tasks that are not described in the user
documentation to trained Roche Service representatives.
for illustration purposes. Configurable and variable data in screenshots, such as tests,
results, or path names visible therein must not be used for laboratory purposes.
null and void. For conditions of warranty, contact your local sales representative or
refer to your warranty contract partner. Always leave software updates to a Roche
Service representative, or perform such updates with their assistance.
Copyright 2001-2018, Roche Diagnostics GmbH. All rights reserved.
License information cobas 6000 analyzer series software is protected by contract law, copyright law, and
License agreement for
UltraVNC software
Open Source and Commercial
Software
international treaties. cobas 6000 analyzer series contains a user license between F.
Hoffmann-La Roche Ltd. and a license holder, and only authorized users may access
the software and use it. Unauthorized use and distribution may result in civil and
criminal penalties.
UltraVNC is a piece of free software for all commercial uses. It is installed on the
control unit PC of the cobas 6000 analyzer series.
You can redistribute the software and/or modify it under the terms of the GNU
General Public License (version 2 or later), as published by the Free Software
Foundation. A copy of the GNU General Public License (version 2) is stored on the
control unit PC. The path for the license is C:\Program Files\uvnc bvba\UltraVNC.
The software is distributed without warranty. There is no implied warranty of
merchantability or fitness for a particular purpose. For more information, see the
GNU General Public License at http://www.gnu.org/licenses.
The source code for the software is stored on the control unit PC. The path for the
source code is C:\DriversAndTools\UltraVNC.
cobas 6000 analyzer series may include components or modules of commercial or
open-source software. For further information on the intellectual property and other
warnings, as well as licenses pertaining to the software programs included in
cobas 6000 analyzer series, refer to the electronic distribution included with this
product.
This open source and commercial software and cobas 6000 analyzer series as a whole
can constitute a device regulated in accordance with applicable law. For more
detailed information, refer to the user manual and labeling.
Please note that the respective authorization is no longer valid according to the
corresponding legislation should any unauthorized changes be made to cobas 6000
analyzer series.
Roche Diagnostics
Operator´s Manual · Version 8.2 3
Page 4
cobas®6000 analyzer series
Trademarks The following trademarks are acknowledged:
COBAS, COBAS C, COBAS INTEGRA, ELECSYS and LIFE NEEDS ANSWERS are
trademarks of Roche.
All other trademarks are the property of their respective owners.
Feedback Every effort has been made to ensure that this publication fulfills the intended use. All
feedback on any aspect of this publication is welcome and is considered during
updates. Contact your Roche representative, should you have any such feedback.
Approvals The cobas 6000 analyzer series meets the requirements laid down in:
Directive 98/79/EC of the European Parliament and of the Council of 27 October
1998 on in vitro diagnostic medical devices.
Directive 2011/65/EU of the European Parliament and of the Council of 8 June 2011
on the restriction of the use of certain hazardous substances in electrical and
electronic equipment.
Compliance with the applicable directives is provided by means of the Declaration of
Conformity.
The following marks demonstrate compliance:
For in vitro diagnostic use.
Complies with the IVD directive 98/79/EC on in vitro diagnostic
medical devices
Issued by Underwriters Laboratories, Inc. (UL) for Canada and the
US.
Instrument approvals Furthermore, the instrument is manufactured and tested according to the following
international safety standards:
o IEC 61010-1
o IEC 61010-2-101
The instrument complies with the emission and immunity requirements described in
standard IEC 61326-2-6/EN 61326-2-6.
The Operator’s manual meets the European Standard DIN EN ISO 18113-3.
Fluorinated greenhouse gas The product contains a fluorinated greenhouse gas in the hermetically sealed
refrigeration.
Type Charge weight (kg) CO2 equivalent
(tonne)
R-134a 0.102 0.14 1430
Roche Diagnostics
4 Operator´s Manual · Version 8.2
Global warming
potential
Page 5
cobas®6000 analyzer series
Contact addresses
Inside the European Union and
EFTA member states
Outside the European Union
and EFTA member states
Manufacturer of the
cobas 6000
analyzer series
instrument
Authorized
representative
Manufactured by: Hitachi High-Technologies Corporation
Manufactured for: Roche Diagnostics GmbH
Hitachi High-Technologies Corporation
1-24-14 Nishi-Shimbashi
Minato-ku, Tokyo 105-8717
Japan
Roche Diagnostics GmbH
Sandhofer Strasse 116
68305 Mannheim
Germany
Sandhofer Strasse 116
68305 Mannheim
Germany
Roche Diagnostics
Operator´s Manual · Version 8.2 5
Page 6

cobas®6000 analyzer series
Roche Diagnostics
6 Operator´s Manual · Version 8.2
Page 7
cobas®6000 analyzer series
Table of contents
Publication information 2
Contact addresses 5
Table of contents 7
Intended use 9
Symbols and abbreviations 9
What is new in publication Version 8.2? 13
System description Part A
1 General safety information
Safety classifications A–5
Safety precautions A–6
Safety labels of the system A–22
Safety information for barcode readers A–30
Interlock function A–32
Disposal of the instrument A–33
2 Overview modules
Overview A–37
Modules of the cobas 6000 analyzer series A–38
3 Control unit, cobas link and core unit
Control unit A–47
cobas link A–49
Core unit cu 150 A–54
Trays, racks, tubes, and cups A–61
4 c 501 module
Overview A–73
Sampling area components A–76
Reagent area components A–78
Reaction disk area components A–83
Behind the front doors A–92
Rear view A–94
ISE area components A–95
5 e 601 module
Overview A–101
Reagent area components A–103
Reaction area components A–105
Pre-wash area components A–107
Consumables area components A–109
Auxiliary reagents and cleaning solutions A–112
6 Specifications
General system specifications A–117
Control unit A–124
Core unit cu 150 A–124
c 501 module A–125
e 601 module A–128
Operation Part B
7 Software basics
General description of the user interface B–5
The help system of the instrument B–15
Main menus B–24
8 Daily operation
Overview B–33
Starting the analyzer B–34
Preroutine operation B–47
Routine operation B–65
Shutting down the analyzer B–78
9 Orders and results
Overview B–83
Barcode and non-barcode mode B–84
Test Selection screen B–85
Data Review screen B–93
Calib. Review screen B–101
Tracking samples on the analyzer B–103
10 Reagents
Reagent concept – c 501 B–109
Reagent concept – e 601 B–119
Operator-related reagent management B–129
Reagent Setting screen B–130
Reagent Status screen B–135
Reagent Overview button B–146
11 Calibration
Calibration concept B–159
Calibration concept – c 501 B–162
Calibration concept – e 601 B–166
Calibration concept – masking B–169
Overview B–170
Calibration Status screen B–170
Calibration Calibrator screen B–182
Calibration Install screen B–185
System Overview screen B–198
12 QC
QC concept B–201
Overview B–206
QC Status screen B–206
QC Run Status screen B–211
QC Individual screen B–213
QC Cumulative screen B–221
QC Control screen B–222
QC Install screen B–224
Automatic QC measurement B–232
Roche Diagnostics
Operator´s Manual · Version 8.2 7
Page 8
cobas®6000 analyzer series
13 Extended operation
Instrument status B–237
Interlock function: Reset after stop B–239
Masking functions B–240
Backup operation B–244
Data storage B–248
14 Configuration
Application B–255
Description of application parameters B–264
System configuration B–281
Module Set B–291
Calculated Tests (c 501) B–295
Special Wash B–301
Report Format B–310
Saving system parameters B–314
Restoring system parameters B–316
Maintenance Part C
15 General maintenance
Overview C–5
Maintenance pipes C–10
Maintenance types C–18
Maintenance report C–22
Maintenance schedules C–23
Automating instrument care
with maintenance pipes C–32
Background and parallel maintenance C–35
List of maintenance items C–39
List of maintenance checks C–43
18 Maintenance e 601
Maintenance schedule C–119
Daily maintenance C–120
Weekly maintenance C–123
Every two weeks maintenance C–139
Every three months maintenance C–142
As needed C–145
Troubleshooting Part D
19 Data alarms
Introduction D–7
Data alarm list D–10
Data alarms c 501 (ISE) D–12
Data alarms c 501 (P) D–19
Data alarms e 601 module D–30
Data alarms for calibrations D–40
Data alarms for controls D–52
Data problems without alarm D–57
Rerun list D–60
20 Troubleshooting
General troubleshooting D–65
Instrument troubleshooting D–68
c 501 (ISE) troubleshooting D–72
c 501 (P) troubleshooting D–77
e 601 troubleshooting D–84
Glossary Part E
16 Maintenance cu 150
Maintenance schedule C–47
Daily maintenance C–48
Monthly maintenance C–51
Every six months maintenance C–56
As needed C–59
17 Maintenance c 501 with ISE
Maintenance schedule C–67
Periodic replacement of parts C–69
Daily maintenance C–70
Weekly maintenance C–79
Monthly maintenance C–86
Every three months maintenance C–100
Every six months maintenance C–103
As needed maintenance C–108
Index Part F
Notes Part G
Roche Diagnostics
8 Operator´s Manual · Version 8.2
Page 9
cobas®6000 analyzer series
Intended use
The cobas 6000 analyzer series is a fully automated, random-access,
software-controlled system for immunoassay and photometric analysis intended for
qualitative and quantitative in vitro determinations using a wide variety of tests. The
cobas 6000 analyzer series is a powerful tool for complete diagnostic laboratory
automation. It is optimized for high throughput workloads using a combination of an
ion selective electrode (ISE) and photometric analysis (c 501 module), and an
immunoassay analysis module (e 601 module).
This publication has detailed descriptions of features of the cobas 6000
analyzer series and general operational concepts, specification functions and use of
controls, operating techniques, emergency procedures, product labeling and
maintenance procedures.
Observe the instructions of the Operator’s Manual for safe operation of the system
o If the system is used in a manner not specified in this Operator’s Manual, the
protection provided by the system may be impaired.
o Keep this manual in a safe place to ensure that it is not damaged and remains available
for use.
o This Operator´s Manual should be easily accessible at all times.
Symbols and abbreviations
Product names Except where the context clearly indicated otherwise, the following product names
and descriptors are used.
Product name Descriptor
cobas 6000 analyzer series System
cobas c 501 module c 501 module
cobas e 601 module e 601 module
cobas cu 150 module cu 150 module
cobas c pack reagent cassette
cobas e pack reagent cassette
cobas e-library Software
cobas link Software
Sample Cup standard cup
Micro-Sample Cup micro cup
AssayTip/AssayCup tray magazine
WasteLiner solid waste box
Roche Diagnostics
Operator´s Manual · Version 8.2 9
Page 10
cobas®6000 analyzer series
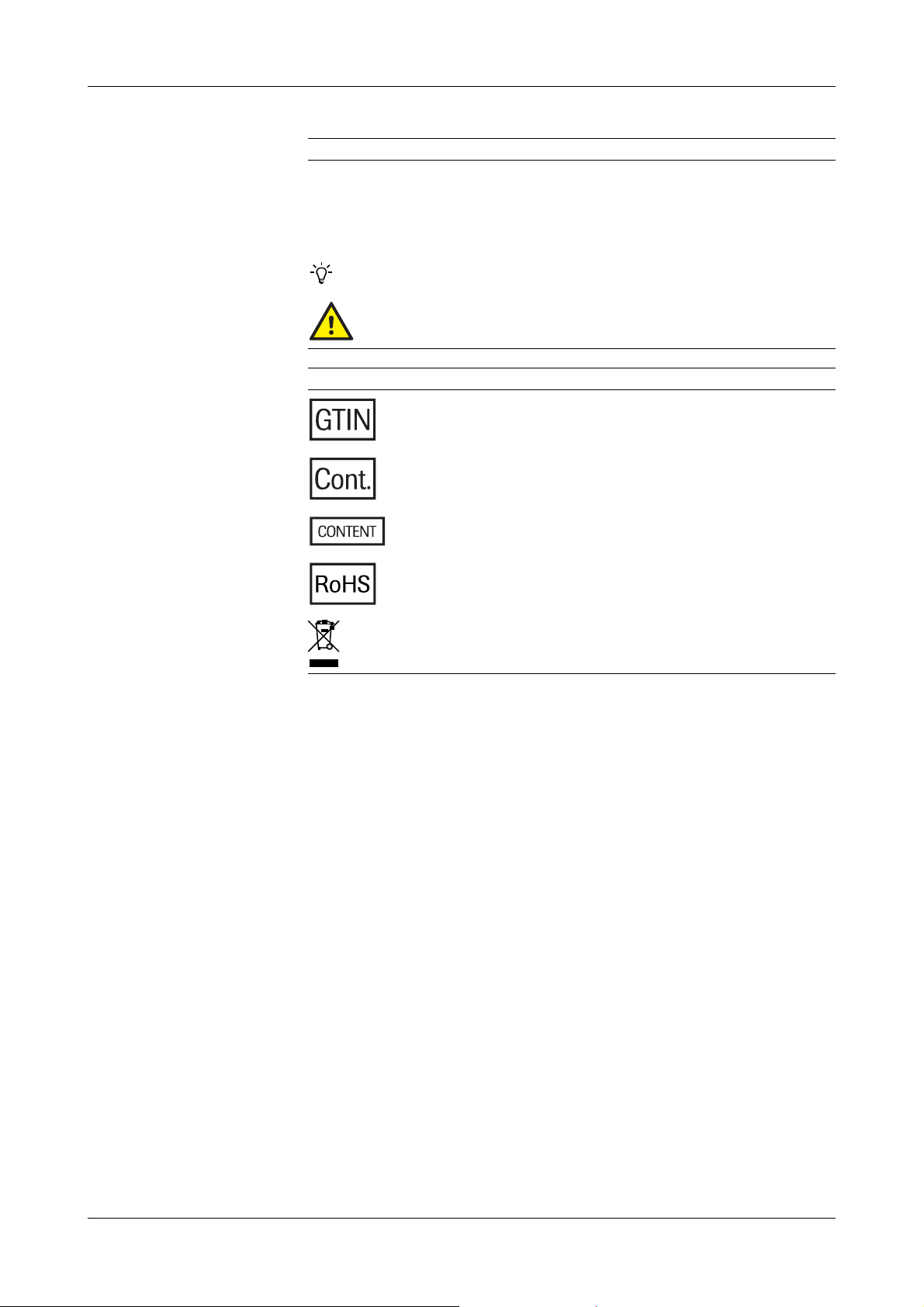
Symbols used in the publication
Symbols used on products
Symbol Explanation
a Start of procedure
End of procedure
o List item
e Cross-reference
Tip
Safety alert
Symbol Explanation
Global Trade Item Number.
Quantity contained in the package.
Quantity contained in the package.
Complies with the directive 2011/65/EU on RoHS.
Disposal of electronic equipment.
Roche Diagnostics
10 Operator´s Manual · Version 8.2
Page 11

cobas®6000 analyzer series
Abbreviations The following abbreviations are used:
Abbreviation Definition
A
Abs Absorbance
ACN Application code number
ANSI American national standards institute
A.U. Analytical unit (module short name and measuring cell, if applicable)
C
c 501 (ISE) ISE unit of the c 501 module
c 501 (P) Photometric unit of the c 501 module
CLAS 2 Clinical laboratory automation system 2
CSA Canadian standards association
CSF Cerebrospinal fluid
D
DIL Diluent
E
e 601 cobas e 601 module for immunoassay analysis
EC European community
ECL Electrochemiluminescence
EN European standard
F
FBT False bottom tube
H
HbA1c Hemoglobin A1c, glycosylated hemoglobin
HPE High priority elecsys
I
IEC International electrical commission
IS Internal standard (ISE module)
ISE Ion selective electrode
IVD In vitro diagnostic
K
KCL Potassium chloride, reference solution for the ISE unit of the c 501
module
kVA Kilovolt-ampere. unit for expressing rating of AC electrical
machinery.
L
LDL Lower detection limit see analytical sensitivity
LIS Laboratory information system
LLD Liquid level detection
Roche Diagnostics
Operator´s Manual · Version 8.2 11
Page 12

cobas®6000 analyzer series
Abbreviation Definition
M
MPA MODULAR PRE-ANALYTICS
N
n/a Not applicable
NaCl Sodium chloride
NG Not good (calibration)
P
PC/CC ProCell M (ProCell) /CleanCell M (CleanCell)
PRE Pretreatment reagent for immunoassays
PSM Process system manager (software)
Q
QC Quality control
R
RCM Reaction calculation mode
REF Reference solution for ISE module
RF Radio frequency
R.M. Result message
RoHS Restriction of Hazardous Substances Directive
R.P. or R. pack cobas e pack (reagent pack)
RRM Rack reception mode
S
SCCS Special cell cleaner solution
SD Standard deviation
SMS Selective mode solution (Detergent for special washes of reagent
probes)
STAT Short turn-around time
Std Standard, commonly used for calibrator
SVGA Super video graphics adapter
T
TPA Tripropylamine
U
UL Underwriters laboratories Inc.
UPS Uninterruptible power supply
W
WEEE Waste Electrical and Electronic Equipment
Roche Diagnostics
12 Operator´s Manual · Version 8.2
Page 13

cobas®6000 analyzer series
What is new in publication Version 8.2?
This section provides an overview of all major changes in this publication.
Software version This documentation is valid for the software version 05–02, 06-01, 06–02, and 06–03.
Instrument approvals The international standard IEC 61010-2-081 and IEC 61010-2-010 have been deleted
in section Instrument approvals.
CAN/CSA 61010-1 has been deleted.
UL 61010-1 has been deleted.
e
See Instrument approvals on page 4.
Safety information for barcode
readers
Correct alignment of sample
tubes on a rack
ISE calibrations For ISE calibrations, the software now uses separate compensated limit checks for
Maintenance
General maintenance In section Recommended maintenance pipes the descriptions for the following pipes
The section has been revised.
A new optional laser barcode reader has been added to the description.
The maximum LED radiation output power of the e 601 reagent barcode reader has
increased to 166.9 μW.
e
See Safety information for barcode readers on page A-30.
The specification of the safety precaution about the tube alignment has been changed
to WARNING.
e
See Correct alignment of sample tubes on a rack on page A-66.
slope and calibrator S3 concentration.
have been revised:
o Extended power ON (2-17 days – only e 601)
o Extended power OFF (2-17 days – only e 601)
e
For associated topics see:
Extended power ON 2 (2 to17 days – only e 601) on page C-34
Extended power OFF (2 to17 days – only e 601) on page C-34
Maintenance cu 150 As needed maintenance Cleaning rack tray surfaces:
Safety message has been revised.
e
See Cleaning rack tray surfaces on page C-62.
Roche Diagnostics
Operator´s Manual · Version 8.2 13
Page 14

cobas®6000 analyzer series
Maintenance c 501 with ISE During product surveillance, a risk of personal injury at the edges of the cell cover
above the ultrasonic mixers was found.
A safety message was added to all maintenance actions that involve handling of the
cell cover above the ultrasonic mixers.
e
For associated topics see:
Cleaning the cell covers on page C-81
Cleaning the incubator bath on page C-90
Cleaning the ultrasonic mixers on page C-100
Replacing the photometer lamp on page C-103
Cleaning instrument surfaces Materials required were revised concerning the use of alcohol or bleach.
e
For associated topics see:
Cleaning instrument surfaces on page C-59
Cleaning instrument surfaces on page C-112
Cleaning instrument surfaces on page C-152
Maintenance e 601 The procedures for extended power on and power off have been reworked.
e
See Extended Power OFF procedures on page C-154.
Roche Diagnostics
14 Operator´s Manual · Version 8.2
Page 15
System description
1 General safety information . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-3
2 Overview modules . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-35
3 Control unit, cobas link and core unit . . . . . . . . . . . . . . . . . . . A-45
4 c 501 module . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-71
5 e 601 module . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-99
A
6 Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-115
Page 16

Page 17
cobas®6000 analyzer series 1 General safety information
Table of contents
General safety information
Before operating with the cobas 6000 analyzer series it is essential that the warnings,
cautions, and safety requirements contained in this manual, as well as the explanation
of safety labels on the system are read and understood by the user.
In this chapter
Safety classifications ........................................................................................................ A-5
Safety precautions ........................................................................................................... A-6
Operator qualification .............................................................................................. A-6
Commissioning and decommissioning ................................................................. A-6
Relocation and transportation .......................................................................... A-7
Disposal ................................................................................................................ A-7
Operating conditions ................................................................................................ A-7
Power interruption ............................................................................................. A-7
Electromagnetic compatibility .......................................................................... A-8
Safe and proper use of the instrument ................................................................... A-8
Personal protection measures ........................................................................... A-9
Biohazardous materials ...................................................................................... A-9
Electrical safety .................................................................................................. A-11
Mechanical safety .............................................................................................. A-12
Reagents and working solutions ........................................................................... A-13
Skin contact ....................................................................................................... A-13
Fire and burns ................................................................................................... A-13
Incorrect results ...................................................................................................... A-13
Samples ............................................................................................................... A-14
Reagents ............................................................................................................. A-15
Instrument covers ............................................................................................. A-16
Others ................................................................................................................. A-16
Sample loss ............................................................................................................... A-17
Software and data security ..................................................................................... A-18
Unauthorized access ......................................................................................... A-18
Incorrect or corrupt data ................................................................................. A-19
USB storage devices .......................................................................................... A-19
Computer viruses .............................................................................................. A-19
Third-party software ........................................................................................ A-19
Backup ................................................................................................................ A-20
Roche Diagnostics
Operator´s Manual · Version 8.2 A-3
Chapter
1
Page 18

1 General safety information cobas®6000 analyzer series
Table of contents
Instrument damage ................................................................................................. A-20
Moving parts ..................................................................................................... A-20
Spillage ............................................................................................................... A-20
Cleaning agents ................................................................................................. A-21
Excessive weight ................................................................................................ A-21
Safety labels of the system ............................................................................................ A-22
Front view ................................................................................................................ A-24
Side view ................................................................................................................... A-26
Top view ................................................................................................................... A-27
Rear view .................................................................................................................. A-29
Safety information for barcode readers ..................................................................... A-30
Interlock function ......................................................................................................... A-32
Disposal of the instrument ........................................................................................... A-33
Roche Diagnostics
A-4 Operator´s Manual · Version 8.2
Page 19
cobas®6000 analyzer series 1 General safety information
WARNING
CAUTION
NOTICE
Safety classifications
Safety classifications
This section explains how precautionary information is presented in the manual.
The safety precautions and important user notes are classified according to
ANSI Z535.6 Standards. Familiarize yourself with the following meanings and icons:
Safety alert
The safety alert symbol is used to alert you to potential physical injury hazards. Obey all
safety messages that follow this symbol to avoid possible damage to the system, injury, or
death.
These symbols and signal words are used for specific hazards:
Warning
Indicates a hazardous situation which, if not avoided, could result in death or serious
injury.
Caution
Indicates a hazardous situation which, if not avoided, could result in minor or moderate
injury.
Notice
Indicates a hazardous situation which, if not avoided, may result in damage to the
instrument.
Important information which is not safety relevant, is indicated with the following
icon:
Tip
Indicates additional information on correct use or useful tips.
Roche Diagnostics
Operator´s Manual · Version 8.2 A-5
Page 20

1 General safety information cobas®6000 analyzer series
Safety precautions
Safety precautions
Pay attention to the following safety precautions!
If you ignore them you may be killed or suffer serious injuries.
Each precaution is important.
Keep in mind that the hazard warnings in this manual and on the instrument cannot
cover every possible case, as it is impossible to predict and evaluate all circumstances
beforehand.
Just following the given directions may, therefore, be inadequate for operation.
Always be alert and use your common sense.
Operator qualification
As a user, you are required to be a licensed person according to your country’s
specific laws. You must have sound knowledge of relevant safety precaution
guidelines and standards and of the information and procedures contained in the
Operator´s Manual.
o Do not carry out operation and maintenance unless you have been trained by
Roche Diagnostics to do so.
o Leave maintenance, installation, or service that is not described in the Operator´s
Manual to the Roche service representative.
o Carefully follow the procedures specified in the Operator´s Manual for the
operation and maintenance.
o Follow standard laboratory practices, especially when working with biohazardous
material.
Commissioning and decommissioning
Only the Roche service representatives can perform commissioning.
For installation described in the Operator´s Manual (such as Installing an
application), follow the installation instructions carefully.
Correct grounding of the power supply cord is essential for the correct function of
the analyzer. The system can only be connected to a power supply source with the
specified power supply cord and only by authorized personnel.
o Contact your Roche service representative for any changes to the power supply
cord.
Roche Diagnostics
A-6 Operator´s Manual · Version 8.2
Page 21

cobas®6000 analyzer series 1 General safety information
Safety precautions
Relocation and transportation
Do not attempt to relocate or transport the instrument.
o Leave relocation and transportation to trained and authorized Roche Diagnostics
personnel.
Disposal
e
For information about disposal of the instrument, see Disposal of the instrument on
page A-33.
Operating conditions
Operation outside the specified ranges may lead to incorrect results or malfunction of
the instrument.
o Never modify the instrument.
o Ensure that the instrument is operated in compliance with the system
specifications.
o Use the instrument indoors only and avoid heat and humidity.
o Make sure that the instrument's ventilation openings remain unobstructed at all
times.
o Perform maintenance according to the specified intervals to maintain the
operating conditions of the instrument.
o Keep the Operator’s Manual in a safe place to ensure that it is not damaged and
remains available for use. This Operator’s Manual must be easily accessible at all
times.
Power interruption
A power failure or momentary drop in voltage may damage the instrument or lead to
data loss.
o Operate only with an uninterruptible power supply (UPS).
o Ensure regular maintenance of the UPS.
o Perform regular backups of measurement results.
o Do not switch off the power while the PC accesses the hard disk or another
storage medium.
Roche Diagnostics
Operator´s Manual · Version 8.2 A-7
Page 22

1 General safety information cobas®6000 analyzer series
Safety precautions
Electromagnetic compatibility
This instrument complies with the standard IEC 61326-2-6/EN 61326-2-6. It has
been designed and tested to CISPR 11 Class A. In a domestic environment it may
cause radio interference, in which case, you may need to take measures to mitigate
the interference.
o The electromagnetic environment should be evaluated prior to operation of the
instrument.
o Do not operate this instrument in close proximity to sources of strong
electromagnetic fields (for example, unshielded intentional radio frequency
sources), as they may interfere with proper operations.
o Do not operate the following devices in close proximity to the instrument:
O Mobile phone
O Transceiver
O Cordless phone
O Other electrical devices that generate strong electromagnetic fields
Safe and proper use of the instrument
Fatigue due to long hours of
operation
Instrument unused for an
extended period of time
Approved parts Use of non-approved parts or devices may result in malfunction of the instrument
For safe and proper use of the instrument, observe the following information.
Looking at the monitor screen over an extended period of time may lead to eye strain
or body fatigue.
o Take a break for 10 to 15 minutes every hour to relax.
o Avoid spending more than 6 hours per day looking at the monitor screen.
If the instrument is not used for an extended period of time, the main circuit breaker
must be switched to OFF.
o Remove, cap, and refrigerate any remaining reagents.
o For information on power off for a period up to 7 days and power on after this
time, see the corresponding maintenance chapter.
e
See Extended Power OFF procedures on page C-154.
o For information on power off for a period more than 7 days and power on after
this time, call the Roche service representative.
The necessary procedure is performed by your Roche service representative.
and incorrect results. It also may render the warranty null and void.
o Only use parts and devices approved by Roche Diagnostics.
Roche Diagnostics
A-8 Operator´s Manual · Version 8.2
Page 23

cobas®6000 analyzer series 1 General safety information
Safety precautions
Personal protection measures
This section describes how you can minimize your own risk during operation or
maintenance.
Personal protective equipment Wear appropriate protective equipment, including, but not limited to: safety glasses
with side shields, fluid-resistant lab coat, and approved disposable gloves.
Wear a face shield if there is a chance of splash or splatter.
Handling of reagents and other
working solutions
Biohazardous materials
Reagents, calibrators, and controls must be handled, stored, and disposed of
according to the instructions for use.
You are responsible for handling, storing, and disposing of samples and chemicals in
accordance with the appropriate standards.
Contact with samples containing material of human origin (for example, sample,
waste) may result in infection. All materials and mechanical components (for
example, reaction system, waste systems) associated with samples of human origin
are potentially biohazardous.
o Follow standard laboratory practices, especially when working with biohazardous
material.
o Keep the top and the front cover closed while the system is operating.
o Always switch the system to maintenance or shutdown mode before working with
an opened cover (for example, cleaning or maintenance).
o Wear appropriate protective equipment.
e
See Personal protective equipment on page A-9.
o If any biohazardous material is spilled, wipe it up immediately and apply
disinfectant.
o If sample or liquid waste comes into contact with your skin, wash it off
immediately with soap and water and apply a disinfectant. Consult a physician.
Spilled sample The sample containers may vibrate during rack transport. Sample may be spilled on
the transport mechanisms.
o Avoid overfilling sample tubes and cups.
o Only use specified sample tubes and cups.
o Leave approximately 10 mm clearance between the liquid and the top of the
sample tube or cup.
o If sample is spilled on the instrument, wipe it up immediately and apply
disinfectant. Be sure to wear protective equipment.
Sharp objects Contact with probes may result in infection.
o When wiping probes, use several layers of gauze and wipe from the top down.
o Be careful to not puncture yourself.
o Wear appropriate protective equipment. Take extra care when working with
protective gloves; these can easily be pierced or cut, which can lead to infection.
e
See Personal protective equipment on page A-9.
Roche Diagnostics
Operator´s Manual · Version 8.2 A-9
Page 24

1 General safety information cobas®6000 analyzer series
Safety precautions
Instrument mechanism Aerosols could be formed inside the instrument, which are potentially hazardous.
Contact with the sampling mechanism or other mechanisms may result in
biohazardous contamination, causing personal injury and/or infection.
o Whenever possible, keep all of the instrument’s covers closed and in place.
O Do not open any cover while the instrument is operating or is performing
maintenance.
O Before starting any operation or maintenance, be sure to close and lock the top
cover.
O When working with an open top cover while the instrument is powered on
(for example, for cleaning probes), always switch the system to Maintenance
or Shut Down mode first. Observe all instructions given in the Operator´s
Manual very carefully.
O Allow only trained personnel access to the keys of the instrument covers.
O Do not use the key for any purpose other than locking and unlocking the
cover.
o During cleaning and maintenance, wear appropriate protective equipment. Take
extra care when working with protective gloves; these can easily be pierced or cut,
which can lead to infection.
e
See Personal protective equipment on page A-9.
o Do not touch any parts of the instrument other than those specified. Keep away
from moving parts during system operation.
o During operation and maintenance of the instrument, proceed according to the
instructions.
Roche Diagnostics
A-10 Operator´s Manual · Version 8.2
Page 25

cobas®6000 analyzer series 1 General safety information
Safety precautions
Waste Contact with liquid waste may result in infection. All materials and mechanical
components associated with the waste systems are potentially biohazardous.
o Wear appropriate protective equipment.
e
See Personal protective equipment on page A-9.
o If any biohazardous material is spilled, wipe it up immediately and apply
disinfectant.
o If liquid waste comes into contact with your skin, wash it off immediately with
water and apply a disinfectant. Consult a physician.
Waste must be treated in accordance with the relevant laws and regulations. Any
substances contained in reagents, calibrators, and quality controls, which are legally
regulated for environmental protection, must be disposed of according to the
relevant water discharge facility regulations. For the legal regulations on water
discharge, please contact the reagent supplier.
Two kinds of liquid waste are discharged by the instrument:
o Concentrated liquid waste that contains highly concentrated reaction solution.
Treat this waste as infectious waste. Dispose of this waste according to the
appropriate local regulations.
o Dilute waste: A non-concentrated liquid waste diluted with rinsing water from
cell wash or water from the incubator bath. When using NaOH-D for washing the
reaction cells, alkaline concentration is 0.1 to 1.0 mmol/L in terms of sodium
hydroxide equivalence. Dilute waste is discarded through tubes at the rear of the
instrument.
Electrical safety
When disposing of any waste generated by the instrument, do so according to the
relevant laws and local regulations.
Liquid waste and replacement parts such as reaction cells and ISE electrodes have to
be treated as infectious medical waste.
e
For information on disposal of the instrument, see Disposal of the instrument on
page A-33.
Removing the covers of electronic equipment can cause electric shock, as there are
high voltage parts inside. In addition, opening the top cover of a photometric module
(c 501) during operation can also cause electric shock.
o Do not attempt to work in any electronic compartment.
o Do not remove any covers from the instrument other than those specified in the
Operator's Manual.
o Only authorized and qualified Roche Diagnostics personnel are authorized to
perform an installation, service, and repair of the system.
Roche Diagnostics
Operator´s Manual · Version 8.2 A-11
Page 26

1 General safety information cobas®6000 analyzer series
Safety precautions
Mechanical safety
Contact with the instrument mechanism could result in personal injury.
o Keep all covers closed and in place while the system is operating.
o Always switch the system to Maintenance or Shut Down mode first, before
working with an open cover (for example, cleaning or maintenance).
o Only trained and authorized Roche Diagnostics personnel should have access to
the keys for the protective covers of the system.
o Do not touch any parts of the system other than those specified. Keep away from
moving parts during operation.
o Do not put your hands into the pathway of any moving parts during instrument
operation.
o During operation and maintenance, proceed according to the instructions.
Fan Contact with the cooling fan during operation of the instrument may result in
personal injury. There is a grille to protect the user from coming into contact with the
moving fan.
o Do not insert your fingers into the opening of the ventilation grille.
o Shut down the instrument before cleaning. If you clean the fan while the
instrument is not in shutdown mode, you risk personal injury.
Pierce pin A pierce pin for cobas c packs is located adjacent to the reagent probe rinse station.
Contact with the pierce pin may result in personal injury.
o Do not touch the pierce pin and gripper during operation, cleaning, or
maintenance.
Rack transfer mechanism The rack transfer mechanism can cause personal injury.
o Only load or unload racks when the green light on the rack loader is on.
o Do not insert your fingers or any objects into the rack loading/unloading area
while the instrument is operating.
o Keep all covers closed and in place while the instrument is operating.
Cover Be careful when opening or closing a top cover. If you let go of the handle, the top
cover may drop onto your fingers.
o Always keep a firm grip on the handle and do not let go when opening or closing
a top cover.
o If a top cover does not stay open properly, please contact your local Roche service
representative.
Open cover Injury to your head or body may be caused by an open cover.
o Be careful not to hit yourself when working with open covers (for example,
during maintenance).
Roche Diagnostics
A-12 Operator´s Manual · Version 8.2
Page 27

cobas®6000 analyzer series 1 General safety information
Safety precautions
Reagents and working solutions
The cobas 6000 analyzer series uses reagent and working solutions, such as cleaners
or diluents. Observe the following information on reagents and working solutions.
Skin contact
Direct contact with reagents, detergents, cleaning solutions, or other working
solutions may cause skin irritation, inflammation, or burns.
o When handling reagents, exercise the precautions required for handling
laboratory reagents.
o Wear appropriate protective equipment.
e
See Personal protective equipment on page A-9.
o Observe the cautions given in the instructions for use.
o Observe the information given in the Material Safety Data Sheets (available for
Roche Diagnostics reagents and cleaning solutions).
o Be careful when removing open reagent packs from the instrument. They may
contain residues of reagents.
o If a reagent, detergent, or other working solution comes into contact with your
skin, wash it off immediately with water and apply disinfectant. Consult a
physician.
Fire and burns
Incorrect results
Alcohol is a flammable substance.
o Keep all sources of ignition (for example, sparks, flames, or heat) away from the
system when conducting maintenance or checks using alcohol.
o When using alcohol on or around the system, use no more than 20 mL at a time.
An incorrect result may lead to an error in diagnosis, therefore posing danger to the
patient.
o For proper use of the instrument, run QC tests and monitor the instrument
during operation.
o Do not use reagents or consumables that have exceeded their expiration date,
otherwise inaccurate data may be obtained.
o For diagnostic purposes: always assess the results in conjunction with the patient's
medical history, clinical examination, and other findings.
Varying factors can influence the accuracy or precision of measured results. Pay
particular attention to the following information on risks for incorrect results.
Roche Diagnostics
Operator´s Manual · Version 8.2 A-13
Page 28

1 General safety information cobas®6000 analyzer series
Safety precautions
Samples
Any problem regarding the samples can cause incorrect results. Observe the
following information on sample handling.
Sample mismatch Be aware that in non-barcode mode there is a risk of sample mismatch. If samples are
not placed in the proper positions, incorrect measurement results may occur.
o When operating in non-barcode mode, make sure to load the samples according
to the Requisition List as provided by the instrument.
o Do not exchange or remove samples.
o Avoid empty positions within the racks. Do not place non-registered samples in
any empty rack position.
o Do not insert any gray rack into the STAT port when operating in non-barcode
mode because the predefined sequence of samples will be disrupted by the routine
rack inserted through the STAT port.
o Do not reload an unloaded rack when working in non-barcode mode. In
non-barcode mode, pink racks have to be used to reload samples.
Contamination Insoluble contaminants in samples, bubbles, or film inside a sample container may
cause blockage or pipetting volume shortage leading to compromised measurement
accuracy.
o Make sure that samples contain no insoluble contaminants such as fibrin, dust, or
bubbles.
o Make sure to prepare enough sample volume for measurement.
If foreign material falls inside the rack loading area, samples may get contaminated.
o Keep the cover of the rack loading area closed while the instrument is operating.
o Do not place anything on the cover of the rack loading area.
Evaporation of samples Evaporation of samples may lead to incorrect results. For critical analytes in terms of
on-board time, you must ensure a short turn-around time.
o After pipetting, do not leave sample, calibrators, or QC materials in a sample
container for any length of time.
Foam or films Foam, fibrin clots, or bubbles in sample types may cause pipetting volume shortage
and lead to incorrect results.
o When loading samples, calibrators, and controls, make sure that they do not
contain any foam, fibrin clots, or bubbles.
o Avoid the formation of foam in all sample types (specimens, calibrators,
controls).
Carryover Traces of analytes or reagents may be carried over from one test to the next.
o Take adequate measures to safeguard additional testing and to avoid potentially
false results.
Sample volume When using standard cups or micro cups, the correct sample cup type must be
selected. Otherwise, the sample pipetting may be inaccurate and thus lead to
inaccurate measurement results.
o Be sure to specify the sample cup type for standard cups and micro cups.
Roche Diagnostics
A-14 Operator´s Manual · Version 8.2
Page 29

cobas®6000 analyzer series 1 General safety information
Safety precautions
Reagents
Wrong reagent handling could cause incorrect results. Observe the following
information on reagent handling.
Reagent volume In case of an undetected loss of reagent, the instrument pipettes no reagent or the
wrong volume of reagent. The system will calculate incorrect results.
o Do not reuse a reagent pack from which reagent was spilled.
o Reload a reagent pack only if you are sure the reagent volumes remained
unchanged while it was not on the instrument.
o Do not use a single reagent pack for different instruments.
Reagents, auxiliary reagents, calibrators, and controls are sensitive regarding
temperature and/or light, especially if the container is already open.
o Store reagents, calibrators, and controls always according to specified storage
conditions.
When using cobas c pack MULTI, it must be filled with the exact filling volume
specified in the instructions for use. Failure to do so may lead to incorrect results.
Additionally, an alarm (reagent short) may occur if the instrument detects low
reagent volume.
Expired reagents or mixing
reagents
Foam or films Foam on top or bubbles in reagents can cause pipetting volume shortage and lead to
o Ensure that the cobas c pack MULTI contains the correct filling volume. Refer to
the instructions for use.
Results obtained using expired reagents are not reliable. Mixing new reagent and
residues of old reagent may cause concentration changes or carryover, leading to
incorrect results.
o Do not use expired reagents.
o Always store reagents according to the specified storage conditions.
o Do not top off old reagents with new reagents. When a bottle is empty, replace it
with a new one.
o Do not leave reagents on the instrument for any length of time.
incorrect results.
o Avoid the formation of foam in all reagent types.
o Do not shake reagents.
Roche Diagnostics
Operator´s Manual · Version 8.2 A-15
Page 30

1 General safety information cobas®6000 analyzer series
Safety precautions
Instrument covers
The instrument comprises various covers. Some covers are important for correct
results. Observe the following information for instrument covers.
ISE measuring system If the cover of the ISE measuring system is not reinstalled after maintenance, the
temperature level or the noise level may be affected, leading to incorrect results.
o Only perform measurements if the cover of the ISE measuring system is closed.
o Before opening or closing the cover of the ISE measuring system, make sure the
c 501 module is in standby or powered off.
e 601 module If the covers of the reagent compartment or the incubator are not reinstalled after
maintenance, the temperature may become inaccurate, leading to incorrect results.
o Only perform measurements, when the covers are closed.
Others
Other causes can lead to incorrect results. Observe the following information on
other causes.
Foam on ProCell/CleanCell Foam on the surface of the e 601 ProCell/CleanCell reservoirs may also lead to
incorrect results.
o Before start of analysis, check that no foam has accumulated on the surface of the
e 601 ProCell/CleanCell reservoirs.
o Clean the ProCell/CleanCell reservoirs if foam has accumulated.
Incubator temperature Measuring before the incubator has reached the correct temperature may lead to
incorrect results and to incorrect results for the photometer check (maintenance
(3) Photometer Check).
Measuring with open top cover or reagent disk cover may lead to abnormal
temperature control, which may also cause incorrect results. Depending on the
ambient temperature, it can take up to 30 minutes to reach the correct temperature
after switching on the analyzer or after exchange of incubation water (maintenance
item (5) Incubation Water Exchange).
o Before starting any measurement, check that the temperature of the incubator is
within 37 ±0.1 °C (98.6 ±0.2 °F). Select each module on the System Overview
screen to display the temperature of the incubator bath of the selected module.
o Be sure to close the covers before starting analysis.
Roche Diagnostics
A-16 Operator´s Manual · Version 8.2
Page 31

cobas®6000 analyzer series 1 General safety information
Safety precautions
Standard cups Although non-Roche Diagnostics sample cups look similar, they may have a different
inner geometry and may have been produced using non-specified materials. The
liquid level detection and the level tracking are influenced by the inner geometry of
the cup. Thus, air may be pipetted when non-Roche Diagnostics cups are used. This
might not be detected under certain circumstances.
Sample cups from other vendors may cause inaccurate sample pipetting, which may
lead to incorrect results.
o Use only cups certified by Roche Diagnostics.
Only the following original standard cups are certified by Roche Diagnostics:
o Hitachi standard cup
o cobas sample cup (catalog no. 10394246001)
Barcodes Undetected barcode read errors may lead to sample mismatch and, therefore, to
incorrect results.
o Use only barcodes with check digits.
Sample loss
In case of sample loss, there may not be enough sample left for measuring and,
therefore, it is impossible to report results for diagnosis. This may lead to false
diagnosis.
Stop (global button) Choosing Stop (global button) stops all sampling and sample processing, and results
in process are lost.
o Before choosing Stop (global button), be sure sample processing has finished.
Interlock system The interlock system of c 501 senses top cover opening and immediately stops the
operation by cutting off the power. All pipetted samples are lost and must be
reloaded.
o Before starting operation or maintenance, be sure to close and lock the top cover.
o Do not open the top cover during operation.
Roche Diagnostics
Operator´s Manual · Version 8.2 A-17
Page 32

1 General safety information cobas®6000 analyzer series
Safety precautions
Software and data security
The control unit comprises a personal computer which controls the instrument,
monitors the instrument functions, and provides and stores data (for example, test
results, patient data, calibration, and control results, and so on). Therefore, there is a
risk of unauthorized access and data loss due to malicious software and hacker
attacks.
Unauthorized access
Portable storage media can be infected with and transmit computer malware, which
may be used to gain unauthorized access to data or cause unwanted changes to
software.
The instrument is not protected against malicious software and hacker attacks.
The users are responsible for IT security of their IT infrastructure and for protecting
it against malicious software and hacker attacks. Failure to do so may result in data
loss or render the instrument unusable.
Roche Diagnostics recommends the following precautions:
o Access to your data and the configuration should only be granted to authorized
experts. All user interventions are logged in the system.
o Allow connection to authorized external devices only.
o Ensure that all external devices are protected by appropriate security software.
o Ensure that access to all external devices is protected by appropriate security
equipment. Roche Diagnostics strongly recommends the use of a cobas IT
firewall.
o Do not copy or install any software on the instrument unless it is part of the
system software or you are instructed to do so by a Roche service representative.
o If additional software is required, contact your Roche service representative to
ensure validation of the software in question.
o Do not use the USB ports to connect other storage devices unless you are
instructed to do so by official user documentation or a Roche service
representative.
o Exercise utmost care when using external storage devices such as USB flash
drives, CDs, or DVDs. Do not use them on public or home computers.
o Before using a removable storage medium, it should be scanned by an antivirus
program.
o Keep all external storage devices in a secure place and ensure that they can be
accessed by authorized persons only.
Roche Diagnostics
A-18 Operator´s Manual · Version 8.2
Page 33

cobas®6000 analyzer series 1 General safety information
Safety precautions
Incorrect or corrupt data
The aim of passwords is to ensure that only authorized personnel have access to a
system or information source. The combination of your unique user ID and
password tells the system who you are and whether you are permitted access or not.
SECURE HANDLING OF YOUR PASSWORD IS CRUCIAL
o Always enter your password unobserved.
o Do not write down your password.
o Never note down the password in a contact form, in the address book, or in a file
on the computer.
o Do not disclose your password to anyone.
o Roche Diagnostics will never ask you for your password.
o If you ever disclose your password to anyone, change it immediately afterwards.
o If you think your account has been compromised, then contact your Roche
service representative.
USB storage devices
USB storage devices can be used for several kinds of data backups and restores.
Wrong handling of a USB storage device may result in data loss or malfunction of the
instrument.
Computer viruses
Third-party software
o Insert or remove a USB storage device only when the instrument is in standby
mode.
o At any one time only one USB storage device can be in use. Before inserting a USB
storage device, check that no other USB storage device is inserted.
o Only remove USB storage devices after choosing USB (global button).
o Use only USB storage devices that are tested and installed by your local Roche
service representative.
If you detect an unexpected operation or program/data damage, the PC may be
infected with a computer virus.
o Never use a program or storage medium that is suspected of containing a virus.
o If you think your PC is infected with a computer virus, call your local Roche
service representative. Your local Roche service representative will disinfect your
PC and will check the restoration.
Installation of any third-party software that is not approved by Roche Diagnostics
may result in incorrect behavior of the instrument.
o Do not install any non-approved third-party software.
o Do not install a commercially available program on the control unit computer or
change any PC settings.
o If additional software is required, contact your Roche service representative to
ensure validation of the software in question.
Roche Diagnostics
Operator´s Manual · Version 8.2 A-19
Page 34

1 General safety information cobas®6000 analyzer series
Safety precautions
Backup
Data may get lost due to aging of the hard disk or due to a hard disk failure because of
electric power failure.
o Back up your data (measurement results and system parameters) at regular
intervals.
o Use the backup function in a daily maintenance pipe to store relevant data on the
hard disk.
o Make a backup copy if you have changed any system parameters.
Instrument damage
During operation, always check for any abnormal sound, water leakages, or other
abnormal conditions. If a problem occurs, take suitable safety measures according to
the condition and contact your Roche service representative.
Should one of the instrument circuit breakers or fuses blow, do not attempt to
operate the instrument before contacting your Roche service representative.
Moving parts
Spillage
Contact with moving parts may bend the probes or damage some other component.
If the system detects a collision, an alarm will sound, stopping the operation
immediately.
o Keep all covers closed and in place during operation.
o Do not touch any parts of the instrument other than those specified. Keep away
from moving parts during operation.
o Only load or unload racks when the green status LEDs at the rack loading area are
on.
Maintenance If the instrument power is turned ON when performing manual maintenance, parts
or tools may contact the instrument mechanisms and damage the instrument.
o Do not power on the instrument while performing manual maintenance.
Any liquid spilled on the instrument may result in malfunction or damage of the
instrument.
o Do not place samples, reagents, or any other liquid on the surface of the
instrument.
o When removing and replacing the reaction cells, be careful not to spill water from
the incubator bath.
o If liquid does spill on the instrument, wipe it up immediately and apply
disinfectant. Wear protective equipment.
e
See Personal protective equipment on page A-9.
Roche Diagnostics
A-20 Operator´s Manual · Version 8.2
Page 35

cobas®6000 analyzer series 1 General safety information
Safety precautions
Cleaning agents
Some cleaning agents may damage the instrument. Observe the following
instructions to avoid instrument damage during cleaning.
Alcohol or bleach Alcohol or bleach may damage the surfaces of the instrument.
o Do not use alcohol or bleach to clean the instrument surfaces.
Acid or alkaline solutions The pre-wash mixer, the incubator, and the AssayCup vortex mixer are made of
aluminum. Acid solution or an alkaline solution degrades the metal.
o Do not use an acid solution or an alkaline solution to clean these parts of the
instrument.
o Use deionized water to clean these parts of the instrument.
Excessive weight
Excessive weight on the instrument may damage the instrument.
c 501 module Excessive weight on the cassette table of the c 501 module may lead to damage.
o Do not place anything other than cobas c packs on the table.
o Maximum load of the table is 2 kg.
e 601 module Excessive weight on the magazine drawer of the e 601 module may lead to damage.
o Do not open the front door of the magazine drawer and do not pull out the
magazine drawer when the indicator lamp is off or blinking.
o Pull out the magazine drawer gently and do not lean on the magazine drawer.
Roche Diagnostics
Operator´s Manual · Version 8.2 A-21
Page 36

1 General safety information cobas®6000 analyzer series
Safety labels of the system
Safety labels of the system
Warning labels have been placed on the analyzer to draw your attention to areas of
potential hazards. The labels and their definitions are listed below according to their
location on the instrument.
The safety labels on the system comply with the following standards: ANSI Z535,
IEC 61010, IEC 60417, or ISO 7000.
If the labels are damaged, they must be replaced by your local Roche service
representative. For replacement labels, contact your local Roche service representative.
Spillage warning
This label indicates the instrument may be damaged if a spillage
occurs within the vicinity of this label. Do not place liquids in this
area.
Protective equipment warning
This label indicates protective goggles and gloves should be worn
when working within the vicinity of this label as there is a danger of
coming into contact with corrosive material.
Warning
This label indicates there is a danger of hazardous situations within
the vicinity of this label, which may result in death or serious injury.
Refer to the Operator’s Manual for safety operation.
Biohazard warning
This label indicates there are potential biohazards within the vicinity
of this label. Follow standard laboratory practices for working with
biohazardous materials.
Electrical warning
This label indicates there is a danger of coming into contact with
electrical components when gaining access to parts of the system
marked with this label.
Mechanical parts warning
This label indicates there is a danger of coming into contact with
moving mechanical parts within the vicinity of this label.
Sharp object warning
This label indicates there is a danger of coming into contact with
sharp objects which may result in injuries.
Hot surface warning
This label indicates the area within the vicinity of this label may be
hot. Do not touch this area as you may be burned.
Roche Diagnostics
A-22 Operator´s Manual · Version 8.2
Page 37

cobas®6000 analyzer series 1 General safety information
Safety labels of the system
Maximum weight
This label indicates the maximum weight. Do not place anything
heavier than the specified weight on the label.
2 kg Max.
Green button light states
This label indicates the meaning of the status of the green button
lights. DO NOT perform an action unless the correct state is
indicated.
The following sections describe the meaning of the safety labels on the instrument in
a short form.
e
For more information about the safety labels on the instrument, see:
Front view on page A-24
Side view on page A-26
Top view on page A-27
Rear view on page A-29
Safety information for barcode readers on page A-30
In addition to safety labels on the instrument there are safety notes in the
corresponding parts of the Operator’s Manual and Online Help.
e
For more information, see:
Part Operation on page B-1
Part Maintenance on page C-1
These safety notes give more detailed information about potentially hazardous
situations in the context of all kinds of working procedures.
When working with the analyzer be sure to observe both safety labels on the
instrument and safety notes in the Operator’s Manual and Online Help.
Roche Diagnostics
Operator´s Manual · Version 8.2 A-23
Page 38

1 General safety information cobas®6000 analyzer series
F-1 F-2 F-3 F-9
F-10 F-12
F-11 F-13
F-4
F-5
F-6
F-7 F-17F-15
F-8 F-14 F-16 F-19F-18
Safety labels of the system
Front view
Figure A-1 Front view of the analyzer
F-1 Attention to interlock!
F-2 Caution against contact with pierce pin!
F-3 Caution against contact with mechanism!
F-4 Caution against pinch by syringe!
F-5 Caution against false result due to loose tube
F-6 Caution against infection due to contact with sipper
F-7 Caution against infection due to contact with waste
connector!
syringe!
from the vacuum tank!
F-8 Caution against irritation by detergent and/or
reagent!
F-9 Caution against pinch by syringe
Roche Diagnostics
A-24 Operator´s Manual · Version 8.2
Page 39

cobas®6000 analyzer series 1 General safety information
Safety labels of the system
F-10 Caution against injury and infection due to contact
with mechanical parts!
F-11 Attention to handling the magazine drawer!
F-12 Attention to auxiliary reagent setting!
F-13 Attention to auxiliary reagent misplacement!
F-14 Caution against irritation by detergent and/or
reagent!
F-15 Caution against irritation by detergent and/or
reagent!
F-16 Caution against irritation by detergent and/or
reagent!
F-17 Caution against injury by contact with needle!
F-18 Attention to the status of the green lights!
F-19 Caution against infection due to contact with tips
and AssayCups!
Roche Diagnostics
Operator´s Manual · Version 8.2 A-25
Page 40

1 General safety information cobas®6000 analyzer series
Safety labels of the system
Side view
Figure A-2 Right side of the analyzer
S-1 Caution against infection due to contact with waste
solution in waste solution tank!
Roche Diagnostics
A-26 Operator´s Manual · Version 8.2
Page 41

cobas®6000 analyzer series 1 General safety information
Safety labels of the system
Top view
Figure A-3 Top view of the analyzer
T-1 Attention to rack direction when loading racks at
T-2 Attention to sampler cover!
T-3 Caution against pinch by rotor mechanism!
T-4 Attention to rack direction when loading racks at
T-5 Caution against burn at photometer lamp
T-6 Caution against entanglement by reagent disk!
T-7 Attention during incubator maintenance!
the STAT port!
the backup operation port!
replacement!
T-8 Instrument damage by water!
T-9 Instrument damage by water!
Roche Diagnostics
Operator´s Manual · Version 8.2 A-27
Page 42

1 General safety information cobas®6000 analyzer series
2 kg Max.
2 kg Max.
Safety labels of the system
T-10 Reminder to use cover for KCL bottle replacement
T-11 Attention when opening or closing ISE cover!
T-12 Attention to cassette direction when loading
cassettes!
T-13 Caution against infection due to contact with parts
of the ISE compartment!
T-14 Attention to cassette table maximum weight!
T-15 Instrument damage by water!
T-16 Attention to cassette table maximum weight!
T-17 Instrument damage by water!
T-18 Attention when opening or closing reagent disk
cover!
T-19 Caution against infection due to contact with
mechanism!
T-20 Instrument damage by water!
Roche Diagnostics
A-28 Operator´s Manual · Version 8.2
Page 43

cobas®6000 analyzer series 1 General safety information
Safety labels of the system
Rear view
Figure A-4 Rear view of the analyzer
R-1 Caution against contact with mechanism!
R-2 Attention to cover with a key!
R-3 Caution against entanglement by rack rotor!
R-4 Attention during water filter maintenance!
R-5 Attention to electric shock at the back side of the
R-6 Caution against infection due to contact with ISE
R-7 Attention to electric shock at the lower part of the
instrument!
waste solution!
rack sampler unit!
Roche Diagnostics
Operator´s Manual · Version 8.2 A-29
Page 44

1 General safety information cobas®6000 analyzer series
WARNING
Safety information for barcode readers
Safety information for barcode readers
The cobas 6000 instrument uses barcode readers to scan the barcodes on samples and
racks.
o Most of the barcode readers use LED technology with very low output power.
o One optional laser barcode reader is used in the core unit. The laser beam is fully
covered during normal operation.
The cobas 6000 instrument complies with the lowest laser class (Class 1) as long as
the laser barcode reader is covered. However, the following safety message must be
observed.
Loss of sight caused by intense light of barcode readers
The intense light of a laser or LED barcode reader may severely damage your eyes or result
in hazardous radiation exposure.
o Do not stare into the beam of a LED barcode reader.
o Do not remove covers from barcode readers.
o Do not perform any maintenance actions on barcode readers. If problems concerning
the barcode readers occur, contact your local Roche service representative.
The cobas 6000 core unit with optional laser barcode reader is a class 1 laser product
(which is the lowest class).
The mentioned classes refer to the standard IEC 60825-1:2014
o Class 1: Eye-safe under normal operating conditions.
o Class 2: Visible lasers. Eye-safe for accidental viewing. However, it may not be
safe for a person who deliberately stares into the laser beam for longer than 0.25 s,
by overcoming their natural aversion response to the very bright light.
The following figure shows the position of the barcode readers and the directions of
the LED apertures used by the cobas 6000 analyzer series:
Figure A-5 Top view of the analyzer - localization of barcode readers
Roche Diagnostics
A-30 Operator´s Manual · Version 8.2
Page 45

cobas®6000 analyzer series 1 General safety information
Safety information for barcode readers
The following table gives technical information about the intensity of the barcode
readers:
Barcode no. Module Barcode reader used for Maximum radiation output
power
LED class / Laser class
Classified standard
BC-1 cu 150 Rack ID and sample ID 10.0 μW Class 1 LED product
IEC 60825-1, +A2:2001BC-2 c 501 Rack ID and sample ID 10.0 μW
BC-3 Reagent 10.0 μW
BC-4 e 601 Reagent 166.9 μW
BC-5 Rack ID 10.0 μW
BC-1
(laser option)
Table A-1 Barcode readers of the cobas 6000 analyzer series
cu 150 Rack ID and sample ID 304.8 μW Class 1 Laser product
IEC 60825-1:2014
Roche Diagnostics
Operator´s Manual · Version 8.2 A-31
Page 46

1 General safety information cobas®6000 analyzer series
A
Interlock function
Interlock function
The top cover of the c 501 module is equipped with an interlock function to protect
the operator from being injured by moving parts. If the top cover of the c 501 module
is opened, the interlock function cuts the power to and stops all moving parts. An
alarm is triggered.
If you want to continue operation or perform a maintenance item, you have to put
the instrument back to standby.
e
To put the instrument back to standby, see Interlock function: Reset after stop on
page B-239.
A Top cover
Figure A-6 c 501 module
Roche Diagnostics
A-32 Operator´s Manual · Version 8.2
Page 47

cobas®6000 analyzer series 1 General safety information
Disposal of the instrument
Disposal of the instrument
The instrument must be treated as potentially biohazardous waste. Decontamination
(that is, a combination of processes, including cleaning, disinfection and/or
sterilization) is required before reuse, recycling, or disposal of the instrument.
Dispose of the instrument according to the appropriate local regulations. Before
disposing of the instrument, contact your Roche service representative.
Disposal of control unit components
Components of your control unit (such as the computer, monitor, keyboard) that are
marked with this symbol are covered by the European Directive on Waste Electrical and
Electronic Equipment (WEEE, 2002/96/EC).
These items must be disposed of through designated collection facilities appointed by
government or local authorities.
For more information about disposal of your old product, please contact your city office,
waste disposal service, or your Roche service representative.
Constraint:
It is left to the responsible laboratory organization to determine whether control unit
components are contaminated or not. If contaminated, treat in the same way as the
instrument.
Roche Diagnostics
Operator´s Manual · Version 8.2 A-33
Page 48

1 General safety information cobas®6000 analyzer series
Disposal of the instrument
Roche Diagnostics
A-34 Operator´s Manual · Version 8.2
Page 49

cobas®6000 analyzer series 2 Overview modules
Table of contents
Overview modules
This chapter provides an overview of all modules of the cobas 6000 analyzer series. A
description of possible configuration is provided, as well as system specifications and
environmental conditions.
In this chapter
Overview ......................................................................................................................... A-37
Modules of the cobas 6000 analyzer series ................................................................ A-38
Control unit and cobas link ................................................................................... A-39
Core unit .................................................................................................................. A-40
c 501 module ............................................................................................................ A-41
Photometric unit ............................................................................................... A-41
ISE unit ............................................................................................................... A-42
e 601 module ............................................................................................................ A-42
Second rotor module .............................................................................................. A-43
Chapter
2
Roche Diagnostics
Operator´s Manual · Version 8.2 A-35
Page 50

2 Overview modules cobas®6000 analyzer series
Table of contents
Roche Diagnostics
A-36 Operator´s Manual · Version 8.2
Page 51

cobas®6000 analyzer series 2 Overview modules
Overview
Overview
The cobas 6000 analyzer series is a fully automated, software-controlled system for
clinical chemistry and immunoassay analysis. It is designed for both quantitative and
qualitative in vitro determinations using a large variety of tests for analysis. It is ready
to use 24 hours per day. The cobas 6000 analyzer series:
o is fully automated
o is modular
o is computerized
o uses serum/plasma, urine, CSF, supernatant and whole blood sample types
o performs in vitro quantitative and qualitative tests on a wide range of analytes
o performs photometric assays and ion-selective electrode measurements on
c 501 modules as well as electrochemiluminescence assays on e 601 modules
Roche Diagnostics
Operator´s Manual · Version 8.2 A-37
Page 52

2 Overview modules cobas®6000 analyzer series
Modules of the cobas 6000 analyzer series
Modules of the cobas 6000 analyzer series
The cobas 6000 analyzer series comprises of a control unit, core unit cu 150 and the
following hardware units, which can be combined in various combinations:
o c 501 module
o e 601 module
Figure A-7 The cobas 6000 analyzer series
The modularity of the system allows for up to seven combinations of the above
hardware units and thus provides tailored solutions for all medium workload
requirements:
Clinical chemistry
ECL technology
Hybrid combinations
< c > < c c > Up to 2000 tests/h (photometric and ISE)
for a <cc> system
Up to 1000 test/h for a single <c> system
Up to 60 cobas c packs per c 501 module
< e > < e e > Up to 340 tests/h for a <ee> system
Up to 170 test/h for a single <e> system
Up to 25 cobas e packs per e 601 module
< c e > < c c e > < c e e > Up to 2170 tests/h
Up to 145 reagent packs
Roche Diagnostics
A-38 Operator´s Manual · Version 8.2
Page 53

cobas®6000 analyzer series 2 Overview modules
A
B
cobas link Control unit
Modules of the cobas 6000 analyzer series
Control unit and cobas link
The control unit uses a graphical user interface to control all instrument functions.
The cobas link PC is used as the gateway for retrieving and distributing
information—such as important notes and test- and lot-specific analyzer
settings—from Roche TeleService-Net to cobas analyzers.
The following figure shows the control unit together with the cobas link PC:
Acobaslink PC B Control unit computer
Figure A-8 Control unit and cobas link PC
Any component of the control unit is subject to change without prior notice.
Roche Diagnostics
Operator´s Manual · Version 8.2 A-39
Page 54

2 Overview modules cobas®6000 analyzer series
A
B
ED
C
F
G
Modules of the cobas 6000 analyzer series
Core unit
The core unit comprises several components that manage the transport of samples to
each assigned analytical module.
A Rack sampler unit
B STAT port
C Barcode reader
D Rack rotor
Figure A-9 Core unit cu 150
E Conveyor line (depending on system
configuration)
F Rack loader
G Rack unloader
Other core module components (installed into the rack sampler unit) are:
o Water supply
o System interface port
o Power switches
Roche Diagnostics
A-40 Operator´s Manual · Version 8.2
o Main circuit breaker
Page 55

cobas®6000 analyzer series 2 Overview modules
Modules of the cobas 6000 analyzer series
c 501 module
The c 501 module comprises a photometric unit and an ISE unit (for ion-selective
electrode [ISE] determinations).
Photometric unit
Sampling system The sampling system is composed of a sample pipetter (consisting of a pipetter arm
Reagent system The reagent system is composed of a refrigerated reagent compartment consisting of
Reaction disk system The reaction disk system is composed of a reaction disk, immersed in an incubator
Figure A-10 c 501 module
The photometric unit provides the analyzer with a flexible photometric method of
assaying up to 600 in vitro tests per hour on a wide range of analytes. The following
are the main components of the c 501 module:
o Sampling system
o Reagent system
o Reaction disk system
and the sample probe), a sample syringe, and a rinse station for internal and external
rinsing of the sample probe.
two storage rings for reagent cassettes, and a reagent pipetting system with two rinse
stations for internal and external rinsing of the reagent probes.
Another integral part of the reagent system is the cassette management system, which
provides a fully automated management of reagent cassettes—from the point of
loading of new cassettes all the way to the disposal of empty cassettes.
bath, three ultrasonic mixing units, a photometric measuring system, and a cell rinse
unit for cleaning the reaction cells once test measurement is complete.
Roche Diagnostics
Operator´s Manual · Version 8.2 A-41
Page 56

2 Overview modules cobas®6000 analyzer series
Modules of the cobas 6000 analyzer series
ISE unit
Moreover, the c 501 module has an integrated ISE unit, which provides the analyzer
with a potentiometric method for assaying sodium, potassium and chloride samples.
The ISE unit can process up to 200 samples per hour. The following are the main
components of the ISE unit:
o ISE measuring compartment with measuring cartridges for Cl
-
, K+, Na+ and
Reference cartridge
o ISE pipetter
o ISE sipper
o IS bath
o ISE reagent compartment
e 601 module
The e 601 module is an immunoassay analyzer with a throughput of up to 170 tests
per hour. The cobas 6000 analyzer series can be configured with up to two e 601
modules.
Figure A-11 e 601 module
The following are the main components of the e 601 module:
o Reagent area
o Measuring area
o Consumables area
o Pre-wash area
Reagent area The reagent area comprises the left side of the analyzer and consists of a reagent disk,
a barcode reader, a cap open/close mechanism, a microbead mixer, a reagent probe
and two rinse stations.
Roche Diagnostics
A-42 Operator´s Manual · Version 8.2
Page 57

cobas®6000 analyzer series 2 Overview modules
Modules of the cobas 6000 analyzer series
Measuring area The measuring area is in the middle of the analyzer and consists of an incubator, a
sample probe, two sipper probes, two sipper rinse stations, and the two measuring
cells.
Pre-wash area The Pre-wash station, located in the middle at the back of the analytical module,
carries out a pre-wash step to remove special contents of serum from the reaction
solution before measuring if required by the assay protocol. The Pre-wash station
consists of a Pre-wash gripper, Pre-wash sipper, Pre-wash dispenser, rinse station,
separation stations, and the vortex mixing station.
Consumables area The consumables area is on the right side of the e 601 and consists of the gripper, the
mixing station, the AssayTip station, the magazine lifter trays, two solid waste
containers, the magazine waste compartment, and the auxiliary reagents and cleaning
solutions.
Second rotor module
Only cobas 6000 analyzer series with a specific configuration have a second rotor
module.
Figure A-12 Second rotor module
The second rotor module routes racks from the core unit to the second and third
analyzer modules and back to the first rack rotor. The second rack rotor also acts as
rack buffer for the automatic rerun function.
Systems with the following configurations have a second rotor module:
o <c 501> <c 501>
o <c 501> <c 501> <e 601>
o <c 501> <e 601> <e 601>
The second rotor module is always placed between the first and the second analyzer
module.
Roche Diagnostics
Operator´s Manual · Version 8.2 A-43
Page 58

2 Overview modules cobas®6000 analyzer series
Modules of the cobas 6000 analyzer series
Roche Diagnostics
A-44 Operator´s Manual · Version 8.2
Page 59

cobas®6000 analyzer series 3 Control unit, cobas link and core unit
Table of contents
Control unit, cobas link and core unit
This chapter provides a detailed description of the control unit, the cobas link
platform, and the core unit. The description of the core unit includes the rack
sampler unit, the rotor, and the conveyor line, as well as line movements within these
components. The sample containers, sample racks, and the rack identification are
also described in this chapter.
In this chapter
Control unit ................................................................................................................... A-47
Control unit computer ........................................................................................... A-47
Touchscreen Monitor ............................................................................................. A-48
Keyboard .................................................................................................................. A-48
Mouse ....................................................................................................................... A-48
Printer ....................................................................................................................... A-48
cobas link ........................................................................................................................ A-49
Overview .................................................................................................................. A-49
Main functions ........................................................................................................ A-51
Working with cobas link functions ...................................................................... A-52
Using e-barcodes via download ...................................................................... A-52
Using the backup function .............................................................................. A-53
Using e-package inserts ................................................................................... A-53
Core unit cu 150 ............................................................................................................ A-54
Rack sampler unit ................................................................................................... A-55
Rack loader/unloader ....................................................................................... A-56
STAT port .......................................................................................................... A-57
Barcode reader / cup sensor ............................................................................ A-57
Power switches .................................................................................................. A-58
System interface port ........................................................................................ A-58
Water supply ..................................................................................................... A-59
Rack rotor ................................................................................................................ A-60
Chapter
3
Roche Diagnostics
Operator´s Manual · Version 8.2 A-45
Page 60

3 Control unit, cobas link and core unit cobas®6000 analyzer series
Table of contents
Trays, racks, tubes, and cups ....................................................................................... A-61
Rack trays ................................................................................................................. A-61
Racks ......................................................................................................................... A-62
Rack types .......................................................................................................... A-62
Rack number ranges ......................................................................................... A-63
Rack ID ............................................................................................................... A-63
Rack classes and colors of standard racks ..................................................... A-64
Rack classes and colors of MPA racks ........................................................... A-65
Correct alignment of sample tubes on a rack ............................................... A-66
Sample containers ................................................................................................... A-68
Sample tubes and cups ..................................................................................... A-68
Sample identification ....................................................................................... A-69
Maximum fill height ......................................................................................... A-69
Roche Diagnostics
A-46 Operator´s Manual · Version 8.2
Page 61

cobas®6000 analyzer series 3 Control unit, cobas link and core unit
A
B
C
D
E
F
G
Control unit
Control unit
The control unit uses a graphical user interface to control all instrument functions.
The following figure shows the control unit together with the cobas link PC:
A Monitor cobas link
B Keyboard/Mouse cobas link
Ccobaslink PC
Figure A-13 Control unit and cobas link PC
Do not place the computer, touchscreen or any data storage media in the vicinity of
electric or magnetic fields, since this may led to damage and/or data loss.
D Touchscreen Monitor
E Keyboard/Mouse
F Control unit computer
G Ergonomic PC stand
Control unit computer
The control unit computer monitors the system functions, and operation modes for
all modules.
o A hard disk drive is used to store the operating system, the analyzer software, the
Online Help system and data, for example patient data, calibration data, QC data
and system parameters.
o A 31/2 inch floppy disk drive is available for reading and writing parameters and
other information for backup purposes.
o A CD or a DVD drive is available for loading software updates.
Roche Diagnostics
Operator´s Manual · Version 8.2 A-47
Page 62

3 Control unit, cobas link and core unit cobas®6000 analyzer series
Control unit
Touchscreen Monitor
The system uses a 17" SVGA with color monitor touchscreen adapter to:
o Display information
o Navigate through the software
o Initiate instrument functions
To use a touchscreen, touch what you want to request or change directly on the
screen. Most of the items within the software can be accessed using the touchscreen.
Touch the item desired (for example, menu bar, list box, text box, button, etc.) to
complete your task. For example, to display the Data Review screen in the
Workplace menu, touch Workplace, then the Data Review tab.
When touching the screen, be sure to “tap” not “press”. The tap must be of short duration.
When touching the screen, you can use your finger or a pointing device.
Selecting Items To select a consecutive range of items in a list, press <Shift> and touch the first item
in the range. While continuing to press <Shift>, touch the last item in the range. All
items, including the first and last items touched in the range, are highlighted. You
may also touch the first item in the list and drag your finger to the last item in the list.
Keyboard
Mouse
Printer
To select multiple, nonconsecutive items, press <Ctrl>, then touch the desired items.
A 101-key enhanced keyboard is used to navigate through the software and to enter
information.
Most items that can be accessed via the touchscreen can also be accessed via the
keyboard.
e
For more information, see Shortcut Keys on page B-13.
A mouse is available to navigate through the software.
The mouse can be used to select items on the screen and to place the cursor at an
insertion point in a text box. To select an item using the mouse, move the mouse over
the item and then click.
The system uses a graphics-capable printer. Patient results can be printed in report
format (long) or in monitor format (short). The printer can be ordered as an optional
accessory.
e
See Report Format on page B-310.
Roche Diagnostics
A-48 Operator´s Manual · Version 8.2
Page 63

cobas®6000 analyzer series 3 Control unit, cobas link and core unit
A
C
B
cobas link
cobas link
This section explains the principles of the cobas link platform and gives an
introduction to working with cobas link.
Overview
cobas link platform The cobas link platform is the gateway for retrieving and distributing information
from the Roche remote service infrastructure to cobas analyzers in the laboratory
(Figure A-14). Information such as instructions for use, value sheets, important
notes, as well as test-specific and lot-specific analyzer settings are made available
through cobas link
cobas link is an integral and mandatory part of the cobas modular platform
analyzers.
A Remote service infrastructure
Bcobas analyzers
Figure A-14 cobas link platform
Ccobaslink PC
A number of applications and other information, for example about controls and
calibrators, are available on the cobas link, which automates manual tasks, improving
efficiency and reducing errors.
Roche Diagnostics
Operator´s Manual · Version 8.2 A-49
No patient names or patient IDs are transferred to or stored within the remote service
infrastructure.
Page 64

3 Control unit, cobas link and core unit cobas®6000 analyzer series
cobas link
Remote service infrastructure Remote service infrastructure is the technical infrastructure which provides the cobas
analyzers and operators with important product information from Roche
Diagnostics. Remote service infrastructure offers several applications to manage and
display data and information gathered from remotely connected instruments which
are uploaded by cobas link located in the laboratory systems.
cobas link PC A dedicated desktop computer consisting of monitor, keyboard, mouse, and printer
located in the customer laboratory, which has been configured to act as a gateway
between Roche Diagnostics systems and the internet. As well as providing a
communication link, the cobas link PC also stores data and documentation for assay
processing.
cobas e-library cobas e-library runs on the cobas link platform. cobas e-library receives data, from
the remote service infrastructure, about assay parameters as well as labeling
information for the applications, controls, and calibrators used on cobas analyzers.
This data is then made available to the connected analyzers, ensuring that they always
have the current data when it is required. Laboratory staff can also view and print this
data, when needed, through cobas e-library.
cobas e-library is updated daily by an automatic download if it is connected to a
network. New catalogue numbers, calibrator lots, and control lots are displayed by
default.
Regular updates
It is important to update cobas e-library regularly to ensure you receive all important
information as it becomes available.
There is a separate Operator’s Manual for the cobas e-library available.
e
For information on working with the cobas e-library, refer to the cobas e-library
Operator’s Manual.
If your laboratory does not have an online connection to remote service
infrastructure, the cobas e-library data is provided on a CD, distributed by your local
Roche service representative.
e
For information on working with CDs, refer to the cobas e-library Operator’s Manual.
Roche Diagnostics
A-50 Operator´s Manual · Version 8.2
Page 65

cobas®6000 analyzer series 3 Control unit, cobas link and core unit
Analyzer control unit
Download
e-barcodes
Upload backups
Provide e-package inserts
cobas link PC
cobas link
Main functions
From the operator’s point of view the main functions of cobas link are:
Figure A-15 cobas link - main functions
e-package inserts (e-PI) The cobas e-library stores many documents (e-package inserts) which can be viewed,
printed, and saved:
o Instructions for use for the applications
o Value sheets of controls and calibrators
o Important notes (for example, reassigned control values)
o Announcements from Roche service representatives (for example, customer
letter)
e
See Using e-package inserts on page A-53.
e-barcodes (e-BC) Providing e-barcodes like application parameters to be downloaded from cobas link
to the analyzer by the operator. e-BCs are readable by the instrument only.
e
See Using e-barcodes via download on page A-52.
e-reference documents e-reference documents provide current product information.
Screen sharing Screen sharing is a function to support the instrument and the user.
Backup function Providing the possibility of data backup for the cobas 6000 analyzer series.
e
See Using the backup function on page A-53.
Roche Diagnostics
Operator´s Manual · Version 8.2 A-51
Page 66

3 Control unit, cobas link and core unit cobas®6000 analyzer series
cobas link
Working with cobas link functions
This section explains the working principles of the main cobas link functions.
Using e-barcodes via download
The operator downloads the required e-barcodes from the cobas link PC to the
analyzer.
The following types of e-barcodes are available:
o Application data
o Calibrator data
o Control data
The following figure gives an overview of the installation process.
Figure A-16 Installation process of a new cobas application
If the data of a particular application (application parameters, calibrator data, and
control data) is not available on the analyzer, it has to be downloaded from
cobas link.
e
For more information, see Installing an application on page B-256.
A detailed description for installing new applications, calibrators, and controls is
given in the corresponding chapters.
e
For a more detailed description see:
To download application parameters from cobas link on page B-259
To download calibrator data from cobas link on page B-190
To download control data from cobas link on page B-226
Roche Diagnostics
A-52 Operator´s Manual · Version 8.2
Page 67

cobas®6000 analyzer series 3 Control unit, cobas link and core unit
cobas link
Using the backup function
Instrument and process related data can be stored on the hard disk of the cobas link
PC for recovery. In order to initiate this backup function it is necessary to include the
cobas link upload function (Smart. Com Essential information upload) in a
maintenance pipe that is carried out daily (for example, as part of the Power ON
pipe).
This item can only be performed in a maintenance pipe.
e
For more information, see:
Defining and editing maintenance pipes on page C-11
Recommended maintenance pipes on page C-33
Smart. Com Essential information upload on page C-42
Perform daily backups to preserve the operational availability of the analyzer!
Include the cobas link upload function (Smart. Com Essential information upload) into a
maintenance pipe that is executed daily. If a hard disk error on the control unit occurs, the
last backup can be restored from the cobas link PC by a Roche service representative.
Using e-package inserts
You can view, print, and save e-package inserts from the cobas e-library.
The e-package inserts (e-PI) consists of the following electronic documents:
Instructions for use
Value sheets of calibrators and controls
Important notes (for example, reassigned control values)
Announcements from Roche service representatives (for example, customer
letter)
Check your cobas e-library daily to obtain important information
It is important to check your cobas e-library daily (New Entries) because important
information that is necessary for analysis—like reassigned control values—is announced
through the cobas e-library.
e
For information on working with the cobas e-library, refer to the cobas e-library
Operator’s Manual.
Roche Diagnostics
Operator´s Manual · Version 8.2 A-53
Page 68

3 Control unit, cobas link and core unit cobas®6000 analyzer series
A
B
ED
C
F
G
Core unit cu 150
Core unit cu 150
The core unit comprises several components that manage the conveyance of samples
to each assigned analytical module. Therefore, the actual composition depends on the
current module configuration of the analyzer. The core unit comprises at least the
rack sampler unit and one rack rotor as main components. Conveyor line(s) and
second rack rotor are possible extensions.
Roche Diagnostics
A-54 Operator´s Manual · Version 8.2
A Rack sampler unit
B STAT port
C Barcode reader
D Rack rotor
Figure A-17 Core unit cu 150
E Conveyor line (depending on system
configuration)
F Rack loader
G Rack unloader
Page 69

cobas®6000 analyzer series 3 Control unit, cobas link and core unit
C
D
A
B
Core unit cu 150
The following are main components of the core unit:
o Rack sampler unit
o Rack rotor(s)
o (Conveyor line[s])
Other core unit components (installed into the rack sampler unit) are:
o Rack loader/unloader
o STAT port
o Barcode reader (for racks and samples)
o Water supply
o System interface port
o Power switches
o Main circuit breaker
Rack sampler unit
A Rack loader/unloader
B STAT port
Figure A-18 Rack sampler unit
C Operation power switch
D Main circuit breaker
All central control and supply functions of the core unit are installed into the rack
sampler unit.
The following subsections describe the rack loader/unloader, STAT port, barcode
reader, and power switches. Then, we turn to the rear of the rack sampler unit and
discuss the system interface and the water supply.
Roche Diagnostics
Operator´s Manual · Version 8.2 A-55
Page 70

3 Control unit, cobas link and core unit cobas®6000 analyzer series
A
D
C
B
Core unit cu 150
Rack loader/unloader
A Rack loader
B Rack unloader
Figure A-19 Rack loader/unloader
C Rack tray (with racks)
D Green LED
Viewed from above, the rack sampler unit features two lanes where sample racks (or
rack trays with sample racks) are to be inserted; the left lane is the rack loader, the
right lane is the rack unloader. Both the loader and the unloader have a capacity of
150 samples, corresponding to 30 racks each. The racks are supplied on rack trays.
Make sure the rack barcode label is on the right side when placing a rack on a tray. The
barcode label can be read only when racks are supplied in correct orientation.
The rack loader is designed for continuous loading. That is, the operator can load
new samples while others are still being processed. When the analyzer is in operation,
the green LEDs indicate when a rack tray can be exchanged. That is, when new
sample racks can be inserted or when a rack tray can be removed from the unloader.
When the analyzer is in operation, rack loading proceeds automatically. When the
analyzer is in standby, rack loading initiates after the operator starts the run.
Rack loading The rack loader automatically pushes the sample racks forward. One after another,
the racks are pushed onto a feeder, which transports each rack into the rack rotor.
When transporting the racks into the rack rotor, a barcode reader scans the barcode
and a sensor checks the height of each sample container. From the rack rotor, the
racks move to the analytical module where their samples are analyzed.
After analysis, the racks move from the analytical module via the rack rotor to the
unloader. The unloader collects the processed sample racks up to a maximum of 30
racks.
Roche Diagnostics
A-56 Operator´s Manual · Version 8.2
Page 71

cobas®6000 analyzer series 3 Control unit, cobas link and core unit
Core unit cu 150
STAT port
Barcode reader / cup sensor
Figure A-20 STAT port
Use the STAT port to send any racks directly onto the rack rotor, bypassing all racks
on the trays of the rack loader. A rack loaded through the STAT port will be
processed with higher priority than other racks in rack rotor.
e
To locate the STAT port on the rack sampler unit, see Figure A-18 on page A-55.
Make sure the rack barcode label is on the right side when inserting a rack into the STAT
port. The barcode label can be read only when racks are supplied in correct orientation.
Any rack can be put into the processing track at any time by using the STAT port.
However, when operating in non-barcode mode, routine (gray) racks must not be put
into the processing track by using the STAT position! Doing so would disrupt the
predefined sample sequence and lead to sample mismatch for all subsequent samples.
e
For information on the different types of racks, see Racks on page A-62.
When racks are loaded onto the analyzer, either via rack loader or via STAT port,
they pass by a barcode reader and a height sensor before they enter the rack rotor.
This barcode reader performs the following tasks:
o Scans rack barcode of each rack
o Detects if there is a sample in each of the five rack positions
o Detects the type of sample tube or cup present
o Scans barcodes on each sample within a rack
The height sensor cannot distinguish between sample cups and microcups.
Roche Diagnostics
Operator´s Manual · Version 8.2 A-57
Page 72

3 Control unit, cobas link and core unit cobas®6000 analyzer series
Core unit cu 150
Power switches
There are two types of power switches: Superordinate power switches and power
switches for each module.
Superordinate power switches (on the left of the rack sampler unit):
o Operation power switch
o Main circuit breaker
e
To locate the superordinate power switches, see Figure A-18 on page A-55.
Power switches for each module (at the rear of each module):
o Module power switch for the rack sampler unit
o Module power switch for the c 501 module and the e 601 module
Power switch Dependent components
Operation power switch Whole analyzer except for cooling unit
Main circuit breaker Whole analyzer including the cooling unit
Module power switch for the rack sampler
unit
Module power switch for the c 501 module
and the e 601 module
Table A-2 Power switches and dependent components
Rack sampler unit (including rack rotor)
c 501 module, e 601 module
System interface port
The following components take part in the data communication within the analyzer
and its network environment:
o Rack sampler unit
o Connected analytical modules
o cobas link
o Host computer
There are two devices to accomplish the data communication: A serial port for
bidirectional communication with the Host computer and a hub for network
connections. The hub is located on the PC stand.
e
For technical information about the serial interface, see System interface on page A-119.
Roche Diagnostics
A-58 Operator´s Manual · Version 8.2
Page 73

cobas®6000 analyzer series 3 Control unit, cobas link and core unit
A
C
E
D
B
Core unit cu 150
Water supply
A Fitting with inlet filter
B System interface port
C Water tank
Figure A-21 Water supply
D Tap for water tank
E High concentration waste container
The deionized water supply system consists of the water tank, located behind the rack
sampler unit, connecting tubing, and a series of electronic valves. Water is
automatically added to the water tank when necessary. Water from this source is
supplied directly to the cell rinse unit, to the rinse stations, and to the incubator bath.
e
For more information about water consumption and specifications, see Water
requirements on page A-118.
Roche Diagnostics
Operator´s Manual · Version 8.2 A-59
Page 74

3 Control unit, cobas link and core unit cobas®6000 analyzer series
Core unit cu 150
Rack rotor
Figure A-22 Rack rotor
The rack rotor is located at the rear between the rack sampler unit and the analytical
module (c 501 or e 601, depending on system configuration). The rotor has 20
positions for sample racks. It receives sample racks from the rack loader, passes them
on to the c 501 module or the conveyor line, receives sample racks from the c 501
module after pipetting or from the conveyor line, and passes them on to the unloader.
In addition, the rack rotor can hold special racks (auto QC) readily available and
reserve special positions for STAT racks. Any of the 20 positions is freely definable
for STAT or auto QC.
If your cobas 6000 analyzer is equipped with two c 501 modules or with one c 501
and two e 601 modules, then a module with a second rack rotor is placed between the
first and the second analyzer module.
Roche Diagnostics
A-60 Operator´s Manual · Version 8.2
Page 75

cobas®6000 analyzer series 3 Control unit, cobas link and core unit
A
B
C
Trays, racks, tubes, and cups
Trays, racks, tubes, and cups
This section describes the different containers and components used to transport
samples.
Rack trays
A Rack Trays B Sample racks with different tubes, cups and
cup on tube
C Standard cup, micro cup
Figure A-23 Trays, racks, tubes, and cups
Samples are supplied to the analyzer in sample tubes or cups. These sample
containers are inserted in sample racks, which are placed on rack trays.
Rack trays are used to transport sample racks to and from the rack loader/unloader.
Each tray has a capacity of 15 racks. This corresponds to a number of 75 samples that
can be loaded onto the analyzer with one tray.
Roche Diagnostics
Operator´s Manual · Version 8.2 A-61
Page 76

3 Control unit, cobas link and core unit cobas®6000 analyzer series
BA
C
D
Trays, racks, tubes, and cups
Racks
For the cobas 6000, different rack types and classes are available. This chapter
explains all rack details.
Rack types
For the cobas 6000, two rack types are available: Standard racks and MPA racks.
o Standard racks are used as transport carriers for all types of sample containers
throughout the cobas 6000. You can mix 13 and 16 mm sample tubes on one
standard rack.
o MPA racks are used as transport carriers either for 13 mm or 16 mm sample
tubes.
A Standard rack
B Spring clips, allow the use of different tube
diameters
C Rubber bottom, ensures the proper
orientation for reading of sample barcode
Figure A-24 Comparison of racks
Characteristics Standard rack MPA rack
Tube diameter Flexible, ∅ 13 mm – 16 mm
D MPA rack (without spring clips and rubber
bottom)
Fixed, ∅ 13 mm or 16 mm
Cup adapters available for
tubes with ∅ 13 mm or less
Cup on rack
Can be used Not supported
(standard or micro cup)
Sample classes All For routine, STAT, and rerun
samples only
Color of routine rack Gray Different colors and rack
ranges available for different
Table A-3 Rack types and characteristics
e
For more information, see:
sample types
Rack classes and colors of standard racks on page A-64
Rack classes and colors of MPA racks on page A-65
Roche Diagnostics
A-62 Operator´s Manual · Version 8.2
Page 77

cobas®6000 analyzer series 3 Control unit, cobas link and core unit
A
B
Trays, racks, tubes, and cups
Rack number ranges
Different sample types, such as Ser/Pl or urine, can be processed on the analyzer. To
process these different sample types on the different rack types (routine, rerun,
STAT), rack number ranges must be defined for each sample type and rack type.
As an example, some number ranges are presented below:
o Serum: Rack number range 001–100
o Urine: Rack number range 401–500
Rack ID
Each rack is equipped with an identification number. This unique number is called
rack ID.
Every rack is identified and registered when its barcode ID is scanned by the barcode
reader of the core unit.
A Rack ID – operator readable B Barcoded rack ID – system readable
(displayed in user interface)
Figure A-25 Rack ID positions
The rack labels displayed in the software differ from those printed on the racks.
Roche Diagnostics
Operator´s Manual · Version 8.2 A-63
Page 78

3 Control unit, cobas link and core unit cobas®6000 analyzer series
Trays, racks, tubes, and cups
Rack classes and colors of standard racks
Racks are available in different colors which are allocated to individual sample
classes. This simplifies the handling of sample classes.
The table below provides an overview of rack classes.
Rack class Rack color Display in software Rack ID on label
Routine
Gray N00001-N03999 0001-3999
STAT
Rerun
Calibrator
QC
Wash rack
Red E00001-E00999 S001–S999
Pink R00001-R00999 R001–R999
Note: Pink racks are only used for manual reruns in non-barcode mode!
Black S00001-S00999 C001–C999
White C00001-C00999 Q001–Q999
Green W00999 W999
Table A-4 Rack classes and colors of standard racks
Roche Diagnostics
A-64 Operator´s Manual · Version 8.2
Page 79

cobas®6000 analyzer series 3 Control unit, cobas link and core unit
Trays, racks, tubes, and cups
Rack classes and colors of MPA racks
MPA racks for routine samples are available in different colors. This allows the use of
racks in different colors for each sample type, such as Ser/Pl or urine, according to the
rack ranges defined under Utility > System > Rack Assignment.
Overlapping of the rack ranges
The MPA racks share part of the same rack range with the standard racks. Therefore, only
one type can be used within the same rack range, either MPA or standard racks.
If you load a rack into the instrument and another rack with the same rack ID is already
being processed, the samples on the second rack will not be processed.
The table below provides an overview of MPA racks:
Rack class Rack color Display in software Rack ID on label
Routine
13 mm
Navy blue
Light green
Yellow
Brown
60001–90000
(a)
1001–4000
(a)
STAT
13 mm
Routine
16 mm
Navy blue S4501–S4600 S501–S600
Light blue
60001–90000
(a)
1001–4000
Light green
(a)
Yellow
STAT
16 mm
Table A-5 MPA racks for 13 mm and 16 mm sample tubes
(a) Each color has its specific ID range.
Navy blue S4801–S4900 S801–S900
The racks for 13 and 16 mm tubes use different colors and rack ranges, so that you can
also distinguish between them.
Please contact your local Roche Diagnostics sales representative for a detailed ordering
list for MPA racks.
Roche Diagnostics
Operator´s Manual · Version 8.2 A-65
Page 80

3 Control unit, cobas link and core unit cobas®6000 analyzer series
WARNING
BA
Trays, racks, tubes, and cups
Correct alignment of sample tubes on a rack
Take special care to place sample tubes correctly on the rack. This is especially
important for 13 mm tubes which are narrower and more likely to tilt if placed
incorrectly. If the tubes are not correctly seated in an upright position on the rack, the
sample probe may attempt to sample outside the tube. Or the sample probe may hit
the side of the tube, causing errors and incorrect results.
Incorrect results when sample tubes are not aligned vertically
Incorrect placement of sample tubes in racks may cause incorrect sample pipetting, which
may lead to false low results, especially when testing immunoassays.
o Ensure that sample tubes are always placed vertically in the racks.
o For sample tubes with an outer diameter of 13 mm or less, use the Roche Diagnostics
cup adapter or use 13 mm MPA racks alternatively.
e
See Sample tube alignment on page A-66.
o Only use sample tubes specified for use with the cobas 6000 analyzer series.
e
See Sample cups and tubes on page A-122.
Sample tube alignment The following figure illustrates correct and incorrect alignment of a sample tube on
the rack.
The tube position must be straight vertically for correct sampling. The vertical
position also reduces possible barcode reading errors and incorrect pipettings.
Roche Diagnostics
A-66 Operator´s Manual · Version 8.2
A Incorrect - position misaligned B Correct - position perfectly aligned
Figure A-26 Sample tube alignment
Page 81

cobas®6000 analyzer series 3 Control unit, cobas link and core unit
CA B
A
B
Trays, racks, tubes, and cups
Cup adapters for tubes ≤13 mm To improve the alignment of tubes with an outer diameter ≤ 13 mm, Roche
Diagnostics strongly recommends that Roche Diagnostics cup adapters are used.
Cup adapters are inserted into standard racks as shown in the following figure.
Conditions for 13 mm tubes
without cup adapters
A Roche Diagnostics cup adapter
B Insert adapter into rack
Figure A-27 Placing a cup adapter into a rack
C 13 mm tube placed in a rack position with
cup adapter
If immunoassays are requested, cup adapters must be used for sample tubes with an
outer diameter ≤ 13 mm.
Do not use cup adapters for tubes with an outer diameter > 13 mm, because the
barcode label might be damaged.
For MPA racks, the cup adapters cannot be used.
You can omit the cup adapters for 13 mm sample tubes if the following preconditions
are fulfilled:
o The cobas 6000 is connected with a cobas
8100 automated workflow series. In
this clinical laboratory automation system, a gripper arm places the sample tubes
into the racks.
o The sample probes are precisely adjusted in horizontal and vertical direction on
all modules. The adjustment of the sample probes must be performed by your
Roche Service representative.
o All spring clips are symmetrically bent in the used racks so that the sample tubes
are held vertically.
A Incorrect - spring clips are not symmetric B Correct - spring clips are symmetric
Table A-6 Spring clips in racks
Roche Diagnostics
Operator´s Manual · Version 8.2 A-67
Page 82

3 Control unit, cobas link and core unit cobas®6000 analyzer series
A
B
C
D
E
F
Trays, racks, tubes, and cups
Sample containers
There are three general kinds of sample containers: Sample tubes, sample cups, and
calibrator or control vials.
Sample tubes and cups
Sample tubes are 13 mm or 16 mm in diameter and 75 mm or 100 mm in length.
Sample cups (both standard and micro cups) can be inserted into 16-millimeter
sample tubes (cup on tube) or they can be used without tubes.
e
For sample container specifications, see Sample cups and tubes on page A-122.
A Sample cup on rack
B 16 mm x 75 mm tube
C Sample cup on 16 mm x 75 mm tube
Figure A-28 Sample container heights
D 16 mm x 100 mm tube
E Sample cup on 16 mm x 100 mm tube
F Micro cup
Restrictions for the use of micro cups
When the end of sample probe touches the inner wall of a micro cup this may result in
improper sample aspiration.
Roche Diagnostics
A-68 Operator´s Manual · Version 8.2
o Do not use micro cups on e 601 modules.
o Do not use micro cups for calibrators and controls.
Page 83

cobas®6000 analyzer series 3 Control unit, cobas link and core unit
WARNING
-10 mm
Trays, racks, tubes, and cups
Non-standard tubes
Sample tubes with the following dimensions can also be used:
o Length between 73 mm and 102 mm
o Outer diameter between 12 mm and 16 mm
Non-standard tubes and false bottom tubes can be used for patient samples and controls.
Please contact your Roche service representative for more information about the use of
non-standard tubes and other sample containers.
Sample identification
When operating in barcode mode, each sample’s barcode label is scanned by the
barcode reader of the rack sampler unit. The sample barcode label provides the
sample ID, which is used for sample identification and test selection purposes.
e
For label dimensions and placement specifications, see Barcode types on page A-120.
The combination of the sample ID and the rack identification number provides
tracking for the system. To track samples by means of the software, select Sample
Tracking from the System Overview screen.
When operating in non-barcode mode, samples are identified by a sequence number,
the rack number, and their position in the sample rack. This assignment has to be
done on the Workplace > Test Selection screen.
Maximum fill height
To prevent any splashing during processing of sample containers, the maximum fill
height should not exceed 10 mm below the top edge of each container.
Figure A-29 Maximum fill height for containers
Infection and danger by spilled sample during operation
The sample containers may vibrate during rack transport. Sample may be spilled on the
transport mechanisms.
o Avoid overfilling sample tubes and cups.
o Only use specified sample tubes and cups.
o Leave approximately 10 mm clearance between the liquid and the top of the sample
tube or cup.
o If sample is spilled on the instrument, wipe it up immediately and apply disinfectant. Be
sure to wear protective equipment.
Roche Diagnostics
Operator´s Manual · Version 8.2 A-69
Page 84

3 Control unit, cobas link and core unit cobas®6000 analyzer series
Trays, racks, tubes, and cups
Roche Diagnostics
A-70 Operator´s Manual · Version 8.2
Page 85

cobas®6000 analyzer series 4 c 501 module
Table of contents
c 501 module
This chapter provides a detailed description of the c 501 module, its hardware
components and technical specifications.
In this chapter
Overview ......................................................................................................................... A-73
System characteristics ............................................................................................. A-73
Components of the c 501 module ......................................................................... A-74
Sampling area components .......................................................................................... A-76
Sample pipetter ........................................................................................................ A-76
Sample probe rinse station .................................................................................... A-77
Sample syringe ......................................................................................................... A-77
Reagent area components ............................................................................................ A-78
Reagent storage compartment .............................................................................. A-78
Reagent pipetting system ....................................................................................... A-79
Reagent pipetters .............................................................................................. A-79
Reagent syringes ............................................................................................... A-80
Reagent probe rinse stations ........................................................................... A-80
Cassette management system ................................................................................ A-81
Cassette loading port ........................................................................................ A-81
Cassette preparation station ............................................................................ A-82
Piercer and Gripper .......................................................................................... A-82
Cassette disposal ............................................................................................... A-82
Reaction disk area components .................................................................................. A-83
Reaction disk ........................................................................................................... A-84
Ultrasonic mixers .................................................................................................... A-84
Incubator bath ......................................................................................................... A-84
Photometer .............................................................................................................. A-85
Cell rinse unit .......................................................................................................... A-86
Flow of photometric analysis ................................................................................ A-88
Main functions on the reaction disk area ...................................................... A-88
Main steps of photometric analysis ................................................................ A-89
Reaction monitoring .............................................................................................. A-91
Chapter
4
Roche Diagnostics
Operator´s Manual · Version 8.2 A-71
Page 86

4 c 501 module cobas®6000 analyzer series
Table of contents
Behind the front doors ................................................................................................. A-92
Auxiliary reagents and cleaning solutions ........................................................... A-93
Vacuum system ....................................................................................................... A-93
Rear view ........................................................................................................................ A-94
ISE area components .................................................................................................... A-95
ISE pipetting system ............................................................................................... A-96
Internal standard bath ............................................................................................ A-96
ISE sipper mechanism ............................................................................................ A-96
ISE measuring system ............................................................................................. A-97
ISE rinse station ...................................................................................................... A-97
ISE reagent compartment ...................................................................................... A-97
Flow of an ISE analysis ........................................................................................... A-98
Roche Diagnostics
A-72 Operator´s Manual · Version 8.2
Page 87

cobas®6000 analyzer series 4 c 501 module
Overview
Overview
The c 501 module is a fully automated, discreet, computerized analyzer for in vitro
tests on a wide range of analytes. It is designed to use serum/plasma, urine, CSF,
supernatant and whole blood sample types. The c 501 module performs photometric
assays as well as ion-selective electrode (ISE) determinations. The throughput is up to
1000 tests per hour for a combination of photometric and ISE tests.
System characteristics
Figure A-30 c 501 module
This chapter describes the c 501 module. The rack rotor and rack sampler unit belong
to the core unit.
e
For a detailed description of the core unit, see Chapter 3 Control unit, cobas link and core
unit.
o 131 on board pre-programmable applications
o STAT sample processing within less than 2 min.
o Automatic reagent cassette loading and unloading
o Automated maintenance functions
o Automatic rerun capability
o Automatic calibration notification
o Automatic sample dilution capabilities
o Non-contact ultrasonic mixing
o Reduced water consumption
o HbA1c whole blood support
o Backup solution for core unit
Roche Diagnostics
Operator´s Manual · Version 8.2 A-73
Page 88

4 c 501 module cobas®6000 analyzer series
F
C
D
G
B
E
Overview
Components of the c 501 module
The c 501 module contains an ISE unit and a photometric unit. Figure A-31 sketches
the different areas from a top-view perspective:
A Rack sampling position
B Sampling area
C Reaction disk area
D ISE area
Figure A-31 Areas of the c 501 module
E Reagent cassette loading port
F Reagent pipetters (pipetting area)
G Reagent area
Roche Diagnostics
A-74 Operator´s Manual · Version 8.2
Page 89

cobas®6000 analyzer series 4 c 501 module
Overview
The c 501 module can be subdivided into the following areas:
o Sampling area
e
See Sampling area components on page A-76.
o Reagent area
e
See Reagent area components on page A-78.
o Reaction disk area
e
See Reaction disk area components on page A-83.
o Behind the front doors
e
See Behind the front doors on page A-92.
o Rear side
e
See Rear view on page A-94.
o ISE area
e
See ISE area components on page A-95.
Roche Diagnostics
Operator´s Manual · Version 8.2 A-75
Page 90

4 c 501 module cobas®6000 analyzer series
A
B
C
D
E
Sampling area components
Sampling area components
The sampling area of the c 501 module consists of the following components:
o A rack sampling position.
o A sample pipetter (consisting of pipetter arm and probe) for pipetting samples
from the sample tubes to the reaction cells on the reaction disk.
o A rinse station for internal and external rinsing of the sample probe.
A Pipetter arm
B Sample probe
C Shield pipe (against electrostatic noise)
Figure A-32 Sample pipetting system
D Drying cylinder (whole blood applications)
E Sample probe rinse station
Sample pipetter
The sample pipetter consists of the pipetter arm and the sample probe. When a rack
is in the sampling position, the pipetter transports sample liquid from the sample
tube to a reaction cell. When aspirating, liquid level detection is accomplished by a
highly sensitive capacitance measurement, as well as clot detection by means of
pressure measurements.
To protect the probe against electrostatic noise, which would interfere with the
capacitance measurement, a metal shield pipe is mounted over the sampling position.
The sample probe is equipped with an extra sensitive level detection and clot detection.
That is, it is not identical in construction and therefore not exchangeable with the ISE
pipetter probe.
After sample has been aspirated, the probe is raised from the sample and is moved to
the reaction disk. The sample probe arm lowers the probe into the reaction cell at the
sample dispense position. Sample is dispensed while the beveled sample probe tip is
in contact with the bottom of the reaction cell. This ensures that a precise volume of
sample is deposited into the bottom of the cell even when using a low dispense
volume. The sample probe is spring-mounted on the arm to avoid damage to the
probe or reaction cell.
Roche Diagnostics
A-76 Operator´s Manual · Version 8.2
Page 91

cobas®6000 analyzer series 4 c 501 module
A
B
Sampling area components
Sample probe rinse station
The sample probe rinse station is located between the sample aspiration position at
the sample tubes in the sample racks and the sample dispense position at the reaction
disk. To prevent carryover, the sample probe is rinsed here with deionized water both
externally and internally before aspirating from a new sample.
The sample probe stops at the drying cylinder only when whole blood is pipetted for
HbA1c tests.
The rinse station is the home position for the sample probe when the pipetter is in
standby.
Sample syringe
The sample pipetter is connected by tubing to the sample syringe, which controls the
pipetting action.
A Sample syringe B Reagent syringes
Figure A-33 Sample syringe
The sampling syringe, which is located behind the left front door of the module, is
filled with degassed and deionized water. The syringe uses positive displacement to
aspirate and dispense samples via the sample probes.
The syringe motor retracts the plunger within the chamber of the syringe, and sample
is aspirated into the tip of the sample probe. The pipetter arm moves the sample
probe to the reaction disk. The sample probe lowers into the reaction cell and the
syringe motor reverses to dispense the sample. The pipetter arm lifts the sample
probe from the reaction cell and moves it to the sample probe rinse station.
Roche Diagnostics
Operator´s Manual · Version 8.2 A-77
Page 92

4 c 501 module cobas®6000 analyzer series
B
C
DE
A
F
Reagent area components
Reagent area components
The reagent area of the c 501 module consists of the following components:
o A refrigerated reagent compartment for storing up to 60 reagent cassettes.
o Two reagent pipetters for aspirating and dispensing reagents from the reagent
compartment to the reaction cells on the reaction disk.
o Two rinse stations for internal and external rinsing of the reagent probes.
o A fully automated cassette management system
Reagent storage compartment
A Reagent pipetters
B Shutter
C Cutouts for R1 reagent pipetter probe
D Cutouts for R2 reagent pipetter probe
Figure A-34 Reagent storage compartment
E Reagent compartment (cover closed)
F Reagent compartment (cover opened)
Reagent cassettes are stored in a closed, temperature-controlled (5-12°C)
compartment containing two concentric rings with a total of 60 positions for reagent
cassettes. There are 24 positions on the inner and 36 positions on the outer ring.
To prevent reagents from evaporating, the reagent compartment is equipped with a
cover. By design, this cover is not meant to be opened or removed. Cassettes are
placed into and removed from the compartment by way of the shutter and cutouts in
the cover allow reagent probes to access the reagents.
Roche Diagnostics
A-78 Operator´s Manual · Version 8.2
Page 93

cobas®6000 analyzer series 4 c 501 module
A
B
C
Reagent area components
Reagent pipetting system
The reagent pipetting system is composed of two reagent pipetters—R1 and R2—and
two reagent syringes. The pipetters are mounted on two independent x-y-motion
mechanisms.
Reagent pipetters
A R2 pipetter B R1 pipetter
C Rinse stations
Figure A-35 Reagent pipetters
Two reagent pipetters, which are mounted above the reagent disk, transport the
reagents from the reagent compartment to the reaction disk.
Before each pipetting, the reagent probes are externally and internally rinsed with
deionized water and dried. After reagent has been aspirated, the probe moves from
the reagent compartment to the reaction disk. There, the reagent volume is dispensed
into a reaction cell containing the sample. Unlike the sample probe, the reagent
probes are not lowered into the reaction cell. Reagent is dispensed from the top of the
reaction cell.
The mechanical cycle of the c 501 module allows for three different reagent timings:
R1, R2, and R3. The R1 pipetter pipettes reagents at R1 timing. The R2 pipetter
pipettes reagents at R2 and R3 timing.
In contrast to R2, the R1 reagent probe is equipped with a pressure sensor to detect
the reagent fill level of manually filled cobas c packs at the preparation station. Ready
to use prefilled cobas c packs are not checked. The gripper and the piercer are
mounted on the same x-y-motion mechanism as the R1 reagent probe.
e
See Cassette preparation station on page A-82.
Roche Diagnostics
Operator´s Manual · Version 8.2 A-79
Page 94

4 c 501 module cobas®6000 analyzer series
A
B
Reagent area components
Reagent syringes
A R1 syringe B R2 syringe
Reagent probe rinse stations
Figure A-36 Reagent syringes
The reagent syringes are located behind the left front door of the module. They are
filled with degassed and deionized water, using positive displacement to aspirate and
dispense reagents.
The reagent probe rinse stations are located between the reagent compartment and
the reaction disk. After each reagent dispense, water is flushed through the probes
and onto their outside surfaces. The probes are then dried at a drying cylinder.
The rinse stations are the home position for the reagent probes when the module is in
standby.
Roche Diagnostics
A-80 Operator´s Manual · Version 8.2
Page 95

cobas®6000 analyzer series 4 c 501 module
A
B
D
C
Reagent area components
Cassette management system
Reagents for all Roche Diagnostics applications are provided in reagent cassettes.
These cassettes contain one to three specially designed reagent bottles and carry
barcode labels with detailed reagent and test related information.
From the time of check-in until an empty cassette is discharged the c 501 manages the
registration, internal transportation, and placement fully automatically. This rules
out any possibility of misplacement or usage of inappropriate reagents. The cassette
management system consists of the following components:
o Cassette loading port
o Cassette preparation station
o Piercer and Gripper
o Cassette disposal
A Cassette preparation station
B Cassette loading port
Figure A-37 Components of the cassette management system
C Cassette table
D Cassette disposal
Cassette loading port
The cassette loading port is located behind the reagent cassette table at the front of
the c 501 module. It is used for loading reagent cassettes onto the module.
When loading a reagent cassette, it is important that its barcode label is facing to the
right.
After loading, the system handles the cassette without any further intervention of the
operator: The cassette is pulled in to the preparation station where a barcode reader
scans the cassette’s barcode label and checks its integrity.
Roche Diagnostics
Operator´s Manual · Version 8.2 A-81
A cassette is rejected and not loaded but pushed out of the loading port again in the
following cases:
o The cassette’s barcode is unreadable
o The cassette has been on the analyzer before and it was previously dumped
(discarded) by the system
Page 96

4 c 501 module cobas®6000 analyzer series
Reagent area components
Cassette preparation station
The cassette preparation station is located directly behind the cassette loading port.
When a new cassette is pulled in to the preparation station, a barcode reader scans
the following data from the cassette’s barcode label:
o System-ID (for example, 07-3755-0)
o Lot number
o Production date
o Cassette number (serial number, for example, 01983)
o Expiration date
o Bottle configuration information
In case, the current cassette has been registered before, the shutter of the reagent
compartment opens and the gripper loads the cassette.
If the current cassette is new, the system proceeds with the following actions while the
cassette is at the preparation station:
o By reading the cassette barcode, the system checks the availability of the
corresponding test application.
o The piercer pierces the reagent bottle caps.
Piercer and Gripper
Cassette disposal
Now, the cassette is ready to be transported to the reagent compartment by the
gripper.
The piercer and gripper are mounted—together with the R1 pipetter—on an x-y-z
motion mechanism. When a cassette is ready to be transported to the reagent
compartment, the gripper lowers down on the cassette, grips it, lifts it, and moves it
to the shutter. The shutter automatically opens and the cassette is placed in a
registered position in the reagent compartment.
Empty reagent cassettes are automatically transported to the cassette disposal. With
the aid of gravity, the cassettes drop down the cassette disposal shaft at the end of
which they can be removed by the operator. The cassette disposal has a capacity of 10
cassettes.
e
To locate the cassette disposal, see Figure A-37 on page A-81.
Roche Diagnostics
A-82 Operator´s Manual · Version 8.2
Page 97

cobas®6000 analyzer series 4 c 501 module
A
B
C
D
E
F
G
C
H
I
Reaction disk area components
Reaction disk area components
The reaction disk area of the c 501 module consists of the following components:
o A reaction disk with the reaction cells, which are immersed in the incubator bath.
o Three ultrasonic mixing units for non-contact mixing of reaction mixtures
(15 levels).
o A photometric measuring system which continually measures the absorbance of
the reaction mixture in each of the reaction cells.
o A cell rinse unit for cleaning the reaction cells once test measurement is complete.
o The auxiliary reagents Sample Cleaner 2 (acid wash), Sample Cleaner 1 (basic
wash), and EcoTergent.
A Reaction disk
B Water level sensor
C Ultrasonic mixing units
Figure A-38 Reaction disk area
Roche Diagnostics
Operator´s Manual · Version 8.2 A-83
D Photometric unit
E Incubator bath
F Reaction cell segment
G (1) Sample Cleaner 1 (basic wash)
(2) Sample Cleaner 2 (acid wash)
H Reaction cell rinse unit
I EcoTergent
Page 98

4 c 501 module cobas®6000 analyzer series
Reaction disk area components
Reaction disk
The reaction disk of the c 501 module carries 160 reusable plastic reaction cells
(cuvettes). These reaction cells are grouped together in eight segments with 20 cells
each. All reaction cells are placed in a temperature-controlled bath. This incubator
bath maintains the cells at the required temperature of 37°C.
The reaction cells should be replaced once a month since they gradually deteriorate over
time. Carry out cell blank measurement once a week after rinsing the reaction system to
check the integrity of all cells.
e
For more information, see:
To perform a cell blank measurement on page C-80
Replacing reaction cells on page C-88
Ultrasonic mixers
The ultrasonic mixing units mix the reagents within each of the reaction cells to
ensure a homogeneous distribution of reactants. Corresponding to the three reagent
timings R1, R2, and R3, there are three independent mixing units.
Incubator bath
To avoid spillage, the water level of the incubator bath is checked before mixing by
calculating the volume. If the liquid level is too low or too high, an alarm (Mix.E) is
issued and mixing is not performed.
Contamination on the surface of the ultrasonic mixing units causes inadequate
mixing. It should be cleaned at least once every three months. The ultrasonic output
intensity is continually monitored. If the intensity falls below a certain limit, an alarm
(<Mix) is issued, and replacement of the ultrasonic mixing unit is required. Contact
your Roche service representative for the replacement.
e
See Cleaning the ultrasonic mixers on page C-100.
The circular incubator bath, positioned beneath the reaction disk, maintains the
reaction mixtures in the reaction cells at a temperature of 37°C. Water in the
incubator bath is circulated by a pump through a refrigeration unit where it is cooled
and then onto the heater where it is heated, as necessary, to maintain the temperature
(±0.1 °C).
Two glass windows (inner and outer) are positioned in opposite walls of the
incubator bath. These windows permit light from the photometer lamp to pass
through the incubator bath water and through the reaction cells in the bath. The light
beam emerges from the outer window of the incubator bath and enters the
instrument photometer.
A liquid level sensor detects the water level of the bath. Deionized water is
automatically added to the incubator bath, as determined by the liquid level sensor, to
compensate for evaporation. This occurs even in standby.
Roche Diagnostics
A-84 Operator´s Manual · Version 8.2
Page 99

cobas®6000 analyzer series 4 c 501 module
Reaction disk area components
Bath detergent EcoTergent is a non-ionic, bacteriostatic detergent automatically added to the
incubator bath by the ISE pipetter whenever the water is exchanged. It acts as a
surfactant to minimize the formation of bubbles that could potentially interfere with
the photometer readings. EcoTergent is located between the ISE reagent
compartment and the reaction disk. EcoTergent is the substitute product to the
previously used Hitergent.
e
To locate the reagent bottle for EcoTergent, see Figure A-38 on page A-83.
Photometer
The c 501 module is equipped with a photometer to measure the absorbencies of the
reaction mixtures in the reaction cells.
The photometer lamp is located against the inner ring of the incubator bath beneath
the reaction disk. The detector is outside the incubator bath ring, behind the ISE
reagent compartment.
e
To locate the photometer unit, see Figure A-38 on page A-83.
Measurements are taken of all the 160 reaction cells, as the reaction disk is turning.
C EADFGHIJBK
N
A Photometer lamp
B Water jacket
C Infrared cut filter
D Mask
E Condenser lens
Figure A-39 Illustration of light path through photometer components
F Slit (in)
G Incubator bath
H Reaction cell and contents
I Slit (out)
J Imaging lens
K Slit
L Photometer
M Grating
N Detector
L
M
Photometer lamp The photometer lamp beneath the reaction disk, is encased in a constant-temperature
water jacket, which helps to maintain a constant energy output from the lamp, and
also extends the lamp life.
e
For more information about replacing the photometer lamp, see Replacing the photometer
lamp on page C-110.
Roche Diagnostics
Operator´s Manual · Version 8.2 A-85
Page 100

4 c 501 module cobas®6000 analyzer series
A
C
B
Reaction disk area components
Light path The light from the photometer lamp passes through the following main components:
o Inner incubator bath window
o Incubator bath water
o Reaction cell and its contents
o Incubator bath water
o Outer incubator bath window
o ...and into the photometer
When the light beam enters the photometer, it strikes a diffraction grating. This
separates the light into its constituent wavelengths and reflects them onto a fixed
array of 12 photodiodes. Each photodiode is permanently positioned to detect light at
a different wavelength.
The instrument computer uses the available assay parameter information to select the
wavelengths and the times at which a reaction mixture’s absorbance is read and
results are calculated.
The instrument computer keeps tracks of which test is being performed in each
reaction cell. The instrument computer also knows when each reaction cell passes
through the photometer light path. The instrument computer uses this tracking
ability and the programmed read instructions to obtain test results.
Cell rinse unit
A Cell rinse unit B Cell rinse nozzles
C Reaction disk
Figure A-40 Reaction cell rinsing system
The cell rinse unit is located to the left of the reaction disk. It cleans, rinses, and dries
the reaction cells once the chemical reaction of the reaction measurement is
completed. To ensure cell integrity (optical characteristics), a photometric reading of
the cell containing water is performed during the cleaning process (cell blank) and
compared with the stored value from the weekly cell blank measurement.
Roche Diagnostics
A-86 Operator´s Manual · Version 8.2
 Loading...
Loading...