Physiomed Bodyflow-P2CH, Bodyflow-P1CH User manual

S E R V I C E M A N U A L
BodyFlowTM-P2CH/-P1CH
Revision: 11 / 2007

Bodyflow International PTY LTD
Legal Notice
Distribution, reproduction and translation of the software and its documentation (or excerpts thereof) are prohibited without the prior written consent of Bodyflow™ International PTY LTD. Bodyflow™ International PTY LTD reserves the right to change the software and associated data as well as the documentation without notice. All other rights reserved.
Bodyflow™ International PTY LTD
Suite 12 Level 1
134 Cambridge Street
Collingwood
Victoria Australia 3066 1300 BODYFLOW 1300 26393569
www.bodyflow.com.au DISCLAIMER
The Bodyflow™ portable stimulation unit ("the Product") is manufactured in Germany by PHYSIOMED ELEKTROMEDIZIN AG and is distributed in Australia by its distributor, Bodyflow™ International PTY LTD.
Bodyflow™ International PTY LTD has endeavoured to ensure that the data analysis and assessment of such data and other information as provided by the manufacturer is accurate.
Any projected information contained in the operating instructions is based on Bodyflow's or PHYSIOMED ELEKTROMEDIZIN AG's analysis and subjective estimates and assumptions and may be about circumstances, scenarios and events which may take place. As such, no representations are made by Bodyflow™ International PTY LTD or PHYSIOMED ELEKTROMEDIZIN AG as to the accuracy of such information.
The product must be used strictly in accordance with the operating instructions. Neither Bodyflow™ International PTY LTD or PHYSIOMED ELEKTROMEDIZIN AG will not be liable for any liability arising from any loss, damage or injury caused through any use of the product outside the scope of the operating instructions.
To the extent permitted by law, neither PHYSIOMED ELEKTROMEDIZIN AG or
Bodyflow™ International PTY LTD assume any liability for any loss or damages incurred directly or indirectly from any use of the Product or as a result of any person acting or refraining to act in reliance of any information contained in any assessment or operating instructions relating to the Product or in respect of any negligent act.
Where any Act implies any condition or warranty in respect to the use or supply of the Product, Bodyflow™ International PTY LTD's liability will be limited to the re-supply of the relevant Product by Bodyflow™ International PTY LTD.
Bodyflow™ is made in Germany in compliance with the quality requirements of ISO 9001 and the applicable safety standards and regulations of the Council Directive 93/42/EEC of 14 June 1993 concerning medical devices.
A conformity check acc. to Annex II, approved by the notified body 1275, was carried out.
2 |
BodyFlowTM-P2CH/-P1CH |
|
|
|
Bodyflow International PTY LTD |
|
|
||
Table of Contents |
|
||
1 |
Technical Data ........................................................................................................... |
4 |
|
2 |
Instrument Overview .................................................................................................. |
6 |
|
|
2.1 |
Setmenue.......................................................................................................... |
7 |
|
2.2 |
Introduction....................................................................................................... |
7 |
|
2.3 |
General Notes .................................................................................................... |
7 |
|
2.4 |
Instrument Description ........................................................................................ |
7 |
3 |
Calibration................................................................................................................. |
9 |
|
|
3.1 |
Additional Equipment Required ............................................................................. |
9 |
4 |
Test Menu ............................................................................................................... |
10 |
|
5 |
Selftest ................................................................................................................... |
12 |
|
6 |
Controls and Indicators ............................................................................................. |
13 |
|
|
6.1 |
Display <1>..................................................................................................... |
13 |
|
Symbols in the Upper Status Bar ................................................................................ |
13 |
|
|
6.2 |
Function Keys <2> ........................................................................................... |
13 |
|
6.3 |
Intensity Control Circuit I <3> and Circuit II <4> ................................................. |
14 |
|
6.4 |
Output Indicator <5>........................................................................................ |
14 |
|
6.5 |
Power Connector <6> ....................................................................................... |
14 |
|
6.6 |
Power Switch <7> ............................................................................................ |
14 |
|
6.7 |
Patient Lead Connector <9>............................................................................... |
15 |
7 |
Operation of the Device............................................................................................. |
16 |
|
|
7.1 |
Mains and Battery Operation .............................................................................. |
16 |
|
7.2 |
Notes on Handling the Batteries .......................................................................... |
16 |
|
7.3 |
Battery Charger................................................................................................ |
17 |
|
7.4 |
Economy Mode ................................................................................................. |
17 |
8 |
Monitoring Notes ...................................................................................................... |
18 |
|
|
8.1 |
Selftest ........................................................................................................... |
18 |
|
8.2 |
Therapy........................................................................................................... |
18 |
|
8.3 |
Battery Charger................................................................................................ |
18 |
9 |
Appendix................................................................................................................. |
19 |
|
3 |
BodyFlowTM-P2CH/-P1CH |
Bodyflow International PTY LTD
1 Technical Data
Table 1: Treatment
Protection class acc. to VDE 0750 / IEC
Battery Mode Only, Type BF
601
Table 2: Charging
Protection class acc. to VDE 0750 / IEC 601
Input voltage
Input current
Power Supply
Table 3: General Technical Data
II
17 VDC
0.8 |
ADC |
|
|
|
|
7,2 |
V 1350mAh Ni-MH Accumulator |
|
|
CE characterization
Class acc. to Council Directive concerning medical devices
Ambient temperature (operation) Storage temperature Dimensions (W x H x D)
Weight
acc. to Council Directive concerning medical devices (93/42 EEC)
IIa
+10 °C ... + 40 °C
+10 °C ... + 40 °C
17.5 cm x 4.5 cm x 10 cm
0.485 kg
Table 4: Battery Charger
Type (to be used exclusively) |
|
|
|
Switchmode Charger FW 7219 / NI 4-10 NTC |
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Mains supply |
|
|
|
100 ... 240 VAC |
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Input current |
|
|
|
0.1 ... 0.3 A |
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Mains frequency |
|
|
|
50 ... 60 Hz |
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Output voltage |
|
|
|
17 VDC |
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Output current |
|
|
|
0.8 ADC |
|
||
|
|
|
|
|
|
|
|
Table 5: Stimulation Current Output Parameters |
|
||||||
|
|
|
|
|
|
|
|
|
|
Standard |
|
|
|
Light |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current |
|
40 mA |
|
|
|
40 mA |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Resistance |
|
500 Ohm |
|
|
|
500 Ohm |
|
|
|
|
|
|
|
|
|
4 |
BodyFlowTM-P2CH/-P1CH |
Bodyflow International PTY LTD
Manufacturer Address
PHYSIOMED ELEKTROMEDIZIN AG
Hutweide 10
91220 Schnaittach/Laipersdorf
Germany
5
BodyFlowTM-P2CH/-P1CH
Bodyflow International PTY LTD
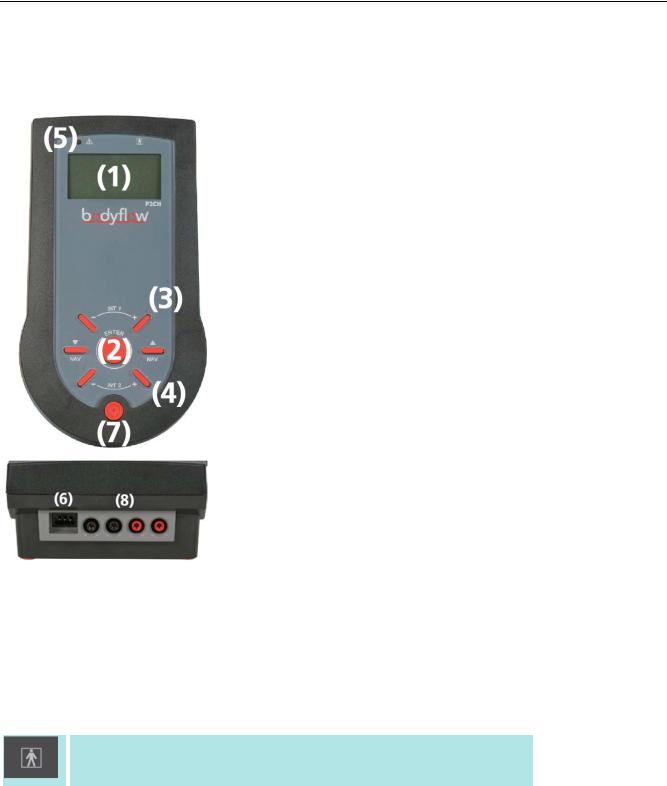
2 Instrument Overview
Table 6: Legend
1 |
|
Display |
|
2 |
|
Function Keys |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3 |
|
Intensity Control Circuit I |
|
4 |
|
Intensity Control Circuit II (only BodyFlowTM-P2CH) |
|
|
|
|
|
|
|
5 |
|
Output Indicator |
|
6 |
|
Power Connector |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
7 |
|
Power Switch |
|
8 |
|
Patient Lead Connector |
|
|
|
|
|
|
|
Table 7: Symbols
Type BF component, not connected to protective ground wire!
6 |
BodyFlowTM-P2CH/-P1CH |

Bodyflow International PTY LTD
2.1 Setmenue
In the Setmenue, you can adjust the following device parameters:
Symbol |
|
Meaning |
|
|
|
Contrast of the Display <1>
 Brightness of the Display <1>
Brightness of the Display <1>
 Back to start screen
Back to start screen
2.2 Introduction
With your Bodyflow™ you have acquired a high-quality and extremely versatile unit for stimulation current therapy. The instrument will only show its true potential, however, if you are well informed about its functions. For this reason, carefully read the Operating Instructions and familiarize yourself with the use of the instrument.
2.3 General Notes
The instrument complies with the technical specifications of IEC 601, VDE 0750 and is assigned to class IIa according to the Council Directive concerning Medical Devices.
The instrument may only be operated by qualified personnel who have undergone special training. You must operate the instrument properly, i.e. in accordance with the Operating Instructions.
It is not intended for operation in explosion hazard zones or hydrotherapy rooms. Drastic temperature changes should be avoided, since condensation could be caused within the instrument. Do not start up the instrument until it is in temperature equilibrium with its environment!
Operating the instrument in the proximity (e.g. 1 m) of a short-wave or micro-wave therapy unit may cause output irregularities and should be avoided for this reason. Simultaneous connection of the patient to high-frequency surgical instrument should also be avoided.
Using the electrodes near the chest can increase the risk of heart beat irregularities.
2.4 Instrument Description
Bodyflow™ is a portable stimulation current therapy unit. The device is equipped with a rechargeable battery and is intended to be used as a mobile unit, e.g. in situations where no connection to the mains is available. This unit can only be used on battery power and not whilst plugged into mains power.
The function of Bodyflow™ is controlled by a microprocessor. Essential components are permanently controlled by the processor and thus malfunctions are prevented. After switching on, all instrument functions are checked during an automatic self-test routine.
7 |
BodyFlowTM-P2CH/-P1CH |

Bodyflow International PTY LTD
The instrument complies with all current safety standards. It meets the requirements of the EC directive concerning medical devices (93/42/EEC) and is therefore CE-labelled.
Bodyflow™ has two modes of operation:
•Treatment: In this mode, the instrument is disconnected from the mains. When the battery charger is plugged in, the instrument cannot be switched on and treatment is not possible. Plugging in the battery charger into the instrument during treatment has the consequence that treatment is being interrupted and the intensity will be automatically turned down to zero and the instrument switches off.
•Charging: Charging is only possible when the device is switched off (refer to Mains and Battery Operation on page 16).
8 |
BodyFlowTM-P2CH/-P1CH |
 Loading...
Loading...