Philips M1351, M1353 Service manual

Obstetrical Care
SERVICE GUIDE
Series 50 A
M1351A
Series 50 IP-2
M1353A
Fetal/Maternal Monitors
FETAL MONITORING

Printed in Germany 10/04
*M1353-9000K*
Part Number M1353-9000K
4512 610 04691
S

Series 50 Fetal Monitors
Series 50 A (M1351A)
Series 50 IP-2 (M1353A)
SERVICE AND INSTALLATION GUIDE
M1353-9000K
Printed in Germany October 2004

Notice
Philips makes no warranty of any kind with regard to this material, including, but
not limited to, the implied warranties of merchantability and fitness for a particular
purpose. Philips Medical Systems shall not be liable for errors contained herein or for
incidental or consequential damages in connection with the furnishing, performance
or use of this material.
This document contains proprietary information that is protected by copyright. All
rights are reserved. No part of this document may be photocopied, reproduced or
translated to another language without prior written consent of Philips Medical
Systems.
The information contained in this document is subject to change without notice.
Philips assumes no responsibility for the use or reliability of its software on
equipment that is not furnished by Philips.
Purchase of this instrument confers no express or implied license under any Nellcor
patent or copyright to use this instrument with any fetal oximetry sensor that is not
manufactured or licensed by Nellcor.
Dinamap is a trademark of General Electric.
Press-Mate is a trademark of the COLIN Corporation.
Federal Law (US) restricts this device to sale by or on the order of a physician.
Caution
Failure on the part of the responsible individual hospital or institution
employing the use of this equipment to implement a satisfactory
maintenance schedule may cause undue equipment failure and possible
health hazards.
ii

Printing History
New editions are complete revisions of the manual. Update packages, which are issued
between editions, contain additional and replacement pages to be added to the manual. The
dates on the title page change only when a new edition or a new update is published.
Edition 1: June 1992
Edition 2: August 1993
Edition 3: February 1995
Edition 4: March 1997
Edition 5: February 1998
Edition 6: May 2000
Edition 7: April 2002
Edition 8: October 2004
1990-2004 Koninklijke Philips Electronics N.V.
All Rights Reserved.
iii

iv

Contents
1. General Information. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1
Who Should Read This Guide . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1
What to do Next . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1
Repair Strategy . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1
Conventions and Symbols Used in this Guide. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
Initial Inspection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
Claims for Damage. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
Repacking . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
Major Keys and Parts at a Glance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
Monitor Control and Display Panel. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
M1351A Single Ultrasound Model . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
M1351A Dual Ultrasound Twins Model . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
M1353A Model . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
Accessories. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
Series 50 A (M1351A) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
Series 50 IP-2 (M1353A) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
Documentation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10
Options . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
2. Technical Specifications. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
Monitor. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
Power Requirements. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
Environment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
Weight and Dimensions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
Displays . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
Inputs . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
Recorder . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
Self-Test Facilities . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16
Combined Interface Module. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16
Modem Interface Module . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16
Remote Event Marker (15249A) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16
Transducers and Cables . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
Brown Toco Transducer (M1355A). . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
Blue Toco Transducer (M1355A). . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
Brown Ultrasound Transducer (M1356A) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
1
Blue
Ultrasound Transducer (M1356A) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
DECG Transducer (M1357A) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 18
MECG Transducer (M1359A) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 18
DECG/MECG Patient Module (M1364A). . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 18
IUP Quartz Transducer (1290C option J05). . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 19
IUP Pressure Transducer (CPJ840J5). . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20
3. Installing the Monitor. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
Fitting the Monitor to a Surface. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
Fitting the Monitor to the Angle Mount. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
Fitting the Monitor to a Wall . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 22
Wall Mount Dimensions. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 22
Fitting the Paper Take-Up Tray. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23
Contents v

Cart-mounted Paper Tray . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23
Carts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .24
Fitting the Barcode Reader Holder . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .24
4. Configuring the Monitor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25
Introduction. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .25
Configuring the Monitor Using Pushbuttons . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .26
Examples: How to Change the Time Format and IUP Format using Pushbuttons . . . . . . . . . . . . . . . . . . . . 28
Configuring the Monitor Using Barcodes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .29
Configuring the Monitor Using a PC . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .29
Installing the Service Program. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 30
Connecting the PC to the Monitor. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 31
Loading the Service Program . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 31
Using the Service Program . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 31
Error Log Messages . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .44
5. Setting Time, Date, and Paper Speed . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 47
Time and Date. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .47
Paper Speed. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .48
6. Theory of Operation. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 49
System Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .49
Booting and Self Tests . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .50
Front End Board . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .52
M1351A (M1353-66501 and M1353-66511) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 52
M1353A (M1353-66512) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 52
Frontend Board for M1353A (M1350-66517) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 54
Power Supply Board (M1353-66502) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .55
CPU Board (M1353-66503 and M1353-66513) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .56
CPU Board M1353-66503. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 56
CPU Board M1353-66513. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 56
Display Board (M1350-66520) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .58
Recorder Interface Board (M1353-66510) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .59
Interface Boards . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .60
Combined Interface Board. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 60
Modem Interface Board. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 63
7. Tests and Error Messages . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 65
Service Philosophy . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .65
Overview of the Service Tests. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 65
Performance Assurance Tests. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .65
Self Test. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 65
Quick Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 66
Parameter Test. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 68
Operator Error Messages . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .69
Permanent Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .70
FSpO2. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .70
8. Troubleshooting Flowcharts. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 71
Introduction. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .71
Error 500: General Failure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .72
Error 501, 511, 512, 516, 517: Front End Board . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .74
Error 502: Power Supply . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .76
Contents vi

Error 503 and 513: CPU Board. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .78
Error 510: Recorder Board . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .79
Error 531: Combined Interface Board . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .80
Error 532: Modem Interface Board . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .81
Error 70: Modem Not Responding. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .82
Error 77: Modem Transmission Failure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .83
Error 601: Paper Feed. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .84
Error 610: No Loudspeaker . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .85
Error 611: Loudspeaker . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .86
Ultrasound Parameter Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .87
DECG Parameter Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .88
MECG Parameter Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .89
Toco Parameter Test. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .90
Maternal NIBP with the Dinamap 1846/8100 Monitor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .91
Maternal NIBP with the COLIN Model BP-8800 Monitor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .92
Fetal Pulse Oximetry with Nellcor N-400 or Compatible M onitor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .93
Paper Sensing Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .94
9. Preventive Maintenance,
Care and Cleaning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 95
Introduction. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .95
Cleaning the Monitor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .95
Regular Maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .96
Mechanical Inspection. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 96
Recorder Maintenance. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 96
Accessory Testing. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .97
Testing Toco Transducers . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 97
Testing Ultrasound Transducers . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 98
Testing Patient Modules (M1364A) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 99
IUP. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 99
Safety Testing. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .100
Safety Test Procedures . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 100
When to Perform Safety Tests. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 102
Test and Inspection Matrix . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 103
Safety Tests . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 104
10. Peripherals. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 107
Fitting the Combined Interface Module . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .107
Connecting Peripheral Devices. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .108
RS232 Serial Interface . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .109
Barcode Reader . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 110
NIBP Monitor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 111
Maternal Measurements on the FHR Trace. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 112
FSpO2 Monitor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 113
Telemetry System. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .115
80235A (OBMS), M1370A (ODIS), and OB TraceVue . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .117
11. Replacing Parts. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 119
Introduction. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .119
Ordering Parts. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .119
Safety Test Requirements. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .119
Service Tools . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .120
Lists of Parts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .120
Contents vii

Boards . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 120
Monitor Parts. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 122
Recorder Parts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 126
Monitor Housing Color . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 127
Toco Transducer (Blue, M1355A) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 128
Ultrasound Transducer (Blue, M1356A) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 129
DECG Transducer (M1357A) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 130
MECG Transducer (M1359A). . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 131
Toco Transducer (Brown). . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .132
Ultrasound Transducer (Brown) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .134
Patient Module (M1364A) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .136
Parts List . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 136
Troubleshooting. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 136
Fuses . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .137
Batteries . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .138
Top Cover. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .139
Front End Board . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .140
Power Supply Board . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .141
CPU Board . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .142
Combined Interface Module . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .143
Modem Interface Module . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .143
Chassis . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .144
On/Off Switch . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .145
Recorder Board . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .146
Display Board . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .147
Switch Board . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .148
Loudspeaker . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .149
Transformer . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1 50
Drawer Assembly . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .151
Thermal Printhead. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .152
Recorder Sensing Assembly . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .154
Stepper Motor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .155
A. Modem Interface Module . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 157
Introduction. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .157
Fitting the Modem Interface Module . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .157
Connecting Peripheral Devices. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .158
Barcode Reader Interface . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 159
RS232 Serial Interface . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 160
Entering, Storing, and Transmitting Data. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .161
Setting the Receiver Phone Number . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 162
Setting the Patient Phone Number. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 162
Setting the Patient ID . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 162
Clearing Memory. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 163
Starting Storage . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 163
Displaying Memory . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 164
Stopping the Storage or Transmission of Data . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 164
Transmitting Data . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 164
PCMCIA Card Modem. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .165
Modem Compatibility . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 166
Modem Initialization . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 166
Using the Modem Setup Barcodes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 166
List of Parts. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .167
Troubleshooting and Error Messages . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1 67
Contents viii

Telephone Connection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 168
Modem Setup Barcodes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .170
Service Barcodes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 170
B. Safety and Environment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 173
Safety Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .173
Protective Earth . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 173
Patient Safety. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 173
Series 50 A. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 174
Series 50 IP-2. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 174
Environment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .174
Spillage . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .175
Electromagnetic Compatibility (EMC) Specifications. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .175
Emissions and Immunity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .175
Electromagnetic Emissions. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .176
Electromagnetic Immunity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .176
Finding Recommended Separation Distances . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .177
Recommended Separation Distances from Portable and Mobile RF Communication Equipment . . . . . . . . . .179
C. Upgrade Key. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 181
Introduction. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .181
Upgrade Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .181
Contents ix

x Contents

Introduction
1
General Information
This guide tells you how to install, service, and repair an Series 50 A (M1351A) and an
Series 50 IP/IP-2 (M1353A) fetal monitor. Throughout this book, the M1353A is referred
to as the IP-2. This also covers the IP, unless stated otherwise. It describes the system
hardware and software, and tells you how to test the system and diagnose operating and
service problems.
It gives instructions for both the Series 50 A and the Series 50 IP/IP-2 monitors. The
features available on the monitor you are installing or servicing depend on which options
have been purchased. If your monitor does not have a described parameter, you can skip
that part of the instruction and move on to the next point.
Who Should Read This Guide
What to do Next
Repair Strategy
The manual is for anyone who services and repairs Series 50 A (M1351A) and Series
50 IP/IP-2 (M1353A) fetal monitors. You must understand English and be familiar with
current conventional technical terms.
Familiarize yourself with the contents of this guide before attempting to install or service the
monitor.
Reading operating error messages and the error log helps you to determine whether a fault is
a hardware or software problem. Faults may be repaired by replacing a board when possible,
or exchanging the monitor. Repair or replacement of components on the boards should not
be attempted.
After any repairs you must carry out the following tests:
! Performance test, by running the Self Test (see page 65).
! Quick Test (see page 66).
! Parameter Test (see page 68).
You must also perform the appropriate safety tests (see the section “Safety Testing” on
page 100).
A series of preventive maintenance tasks and performance assurance tests must be carried
out regularly to ensure the proper functioning of the monitor: these are described in
“Regular Maintenance” on page 96.
Chapter 1 General Information 1

Conventions and Symbols Used in this Guide
Conventions and Symbols Used in this Guide
This guide uses the following conventions for notes, cautions, and warnings:
Warning
A warning alerts you to a potential serious outcome, adverse event or safety
hazard. Failure to observe a warning may result in death or serious injury to
the user or patient.
Caution
A caution alerts you to situations where special care is necessary for the safe
and effective use of the product. Failure to observe a caution may result in
minor or moderate personal injury or damage to the product or other
property, and possibly in a remote risk of more serious injury.
Note— A note calls your attention to an important point in the text.
On your monitor, this sign indicates that there is detailed
information in this book and the Instructions For Use which
you must read before proceeding with your task
Equipotential Terminal
This symbol is used to identify terminals which are connected together, bringing various
parts of an equipment or system to the same potential, not necessarily being earth potential
(the value of potentials of earth may be indicated adjacent to the symbol).
Protective Earth Terminal
This symbol identifies the terminal for connection to an external protective earth.
2 Chapter 1 General Information

Initial Inspection
Initial Inspection
The monitor and any supporting options ordered are supplied packed in protective
shipping cartons. Before unpacking, visually check the packaging and ensure that there are
no signs of mishandling or damage.
Claims for Damage
If the shipping cartons show signs of having been mishandled, contact the carrier and
arrange for his agent to make an inspection.
If any of the equipment supplied is damaged, you should contact both the carrier and your
local Philips Medical Service Organization. Arrangements will then be made for repair or
replacement, as appropriate.
Repacking You are advised to retain the original packing carton and material. You will find it useful if it
becomes necessary to return a piece of equipment to Philips for service. If you need to
repack the equipment but cannot locate the original packing materials, Philips can advise
you on alternatives.
Chapter 1 General Information 3

Overview
Overview
This guide describes three different Series 50 Fetal Monitor models:
! M1351A Single Ultrasound model (with US and Toco channels).
! M1351A Dual Ultrasound Twins model (with US1, Toco and US2 channels).
! M1353A model (with US1, Toco and US2/ECG channels).
The M1351A single and double ultrasound model is for external monitoring of FHR and
uterine activity in the antenatal period from early gestation (approximately 20 to 25 weeks)
to term. The M1353A is for monitoring FHR, including twins, maternal heart rate and
uterine activity. FHR and uterine activity can be monitored externally in the antenatal
period from early gestation to term, and internally throughout labor and delivery. The basic
capabilities of the three models are summarized in the table below.
Table 1-1 Fetal Monitor Parameters
Parameter
M1351A
Single
M1351A
Twin
M1353A
Monitor FHR using ultrasound Yes Yes Yes
Monitor twin FHRs using ultrasound No Yes Yes
Monitor twins using DECG and ultrasound No No Yes
Monitor FHR using DECG No No Yes
Monitor uterine activity using Toco ext Yes Yes Yes
Monitor IUP No No Yes
Monitor maternal heart rate No No Yes
Detect fetal movements
1
Yes Yes Yes
Mark events Yes Yes Yes
Record nursing notes
1
Transmission of fetal trace information
1
Fetal trace memory
1
Yes Yes Yes
Yes Ye s Yes
Yes Yes Yes
Interfacing to fetal pulse oximetry Yes Yes Yes
1. May be ordered as an option for all models
4 Chapter 1 General Information
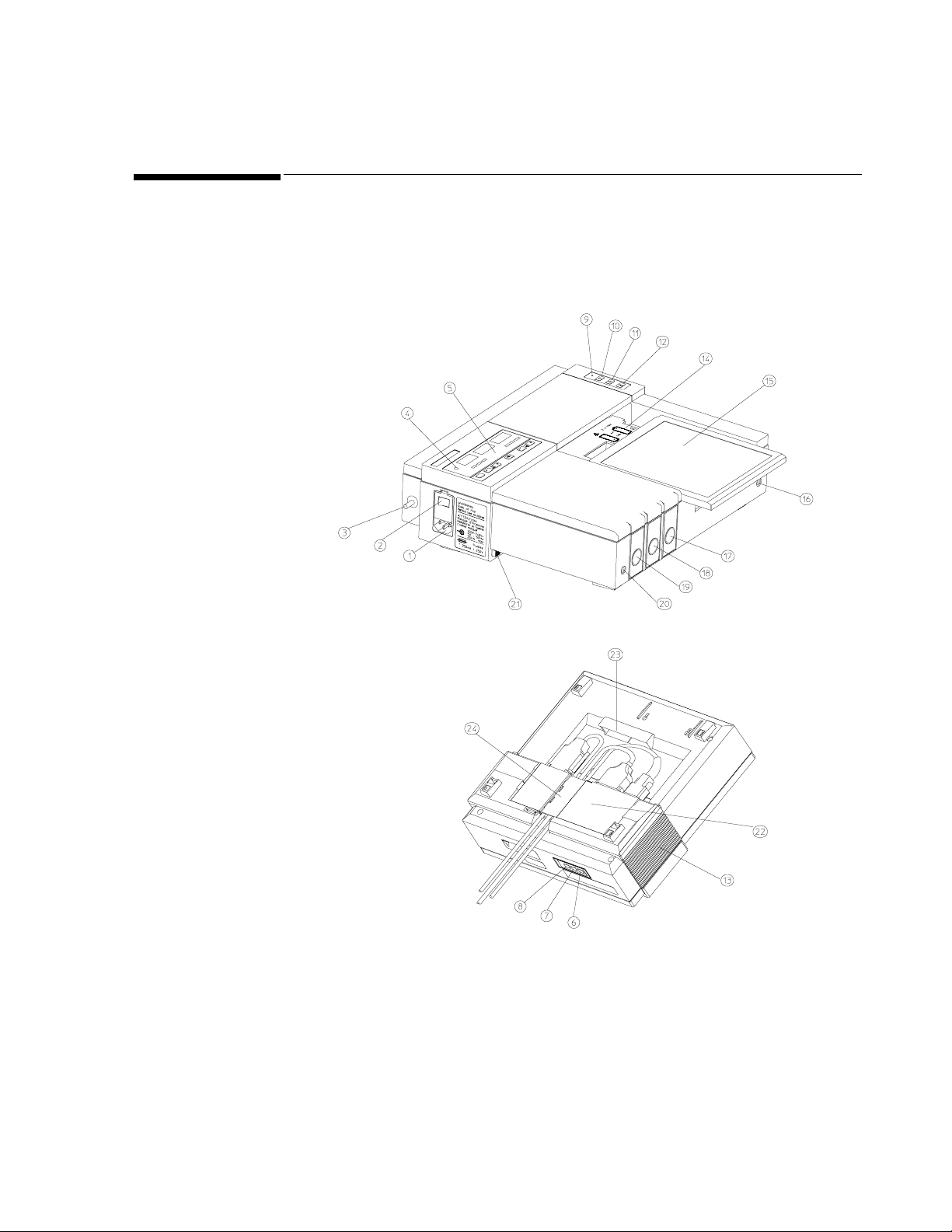
Major Keys and Parts at a Glance
Major Keys an d Parts at a Glance
Figure 1-1 General Layout of the Series 50 A and Series 50 IP-2 Fetal
Monitors
Chapter 1 General Information 5

Major Keys and Parts at a Glance
10. Recorder on/off key
11. Event marker key (Alert acknowledge key)
12. Paper advance key
13. Loudspeaker
14. Battery compartment
15. Paper table
16. Service socket
17. Series 50 A: US2 Socket (not present on Single Ultrasound model)
18. Toco socket
19. Series 50 A: Single Ultrasound Model: US Socket
20. Socket for remote event marker
21. Lock-release button
22. Combined interface module
23. Integrated carrying handle
24. Cable clamp
1. Mains socket
2. Monitor on/off switch
3. Equipotential grounding point
4. Monitor on/off light
5. Display panel
6. Time and date key
7. Paper speed key
8. Test key
9. Recorder on/off light
Series 50 IP-2: US2/ECG Socket
Double Ultrasound Model: US1 socket
Series 50 IP-2:US1 socket
6 Chapter 1 General Information

Monitor Control and Display Panel
Monitor Control and Display Panel
M1351A Single Ultrasound Model
M1351A Dual Ultrasound Twins Model
Figure 1-2 Layout of the Monitor Control and Display Panel
1. Monitor On/Off Light.
2. Telemetry Indicator. On when the Fetal Telemetry Receiver is connected and
switched on.
3. Function Key. Used to switch FMP and Fetal Alerting on and off.
4. US Display. Shows the FHR detected by the US transducer.
5. US Signal Quality Indicator. Indicates the quality of the signal detected by the US
transducer:
" Green (optimum).
" Yellow (fair to potentially poor).
" Red (unacceptable).
6. US Speaker Light. On when you are hearing the US heartbeat.
7. US Volume Keys. Sets the volume and selects the US heartbeat.
8. Toco Display. Shows uterine activity.
9. Toco Baseline Key. Zeroes the Toco display and trace to 20 units.
1. Monitor On/Off Light.
2. Telemetry Indicator. On when the Fetal Telemetry Receiver is connected and
switched on.
3. Function Key. Used to switch Twins Offset, FMP, and Fetal Alerting on and off.
4. US1 Display. Shows the FHR detected by the US1 transducer.
Chapter 1 General Information 7

Monitor Control and Display Panel
5. US1 Signal Quality Indicator. Indicates the quality of the signal detected by the US1
transducer.
6. US1 Speaker Light. On when you are hearing the US1 heartbeat.
7. US1 Volume Keys. Sets the volume and selects the US1 heartbeat.
8. Toco Display. Shows uterine activity.
9. Toco Baseline Key. Zeroes the Toco display and trace to 20 units.
10. US2 Display. Shows the FHR detected by the US2 transducer.
11. US2 Signal Quality Indicator. Indicates the quality of the signal detected by the US2
transducer.
12. US2 Speaker Light. On when you are hearing the US2 heartbeat.
13. US2 Volume Keys. Sets the volume and selects the US2 heartbeat.
M1353A Model 1. Monitor On/Off Light.
2. Telemetry Indicator. On when the Fetal Telemetry Receiver is connected and
switched on.
3. Function Key. Used to switch Twins Offset, Logic, FMP, and Fetal Alerting on and
off.
4. US Display. Shows the FHR detected by the US transducer.
5. US1 Signal Quality Indicator. Indicates the quality of the signal detected by the US1
transducer:
6. US1 Speaker Light. On when you are hearing the US1 heartbeat.
7. US1 Volume Keys. Sets the volume and selects the US1 heartbeat.
8. Toco Display. Shows uterine activity.
9. Toco Baseline Key. Zeroes the Toco display and trace to 20 units (when monitoring
externally) or 0 units (when monitoring internally).
10. US2/ECG Display. Shows the FHR detected by the ECG transducer.
11. US2/ECG Signal Quality Indicator. Indicates the quality of the signal detected by the
ECG transducer.
12. US2/ECG Speaker Light. On when you are hearing the ECG heartbeat.
13. US2/ECG Volume Keys. Sets the volume and selects the ECG heartbeat.
8 Chapter 1 General Information

Accessories
Accessories
Series 50 A (M1351A)
The following accessories are supplied as standard with the Monitor:
! One external Toco transducer (M1355-60011).
! One ultrasound transducer (M1356-60011) (or two with the Dual Ultrasound
Model).
! Two reusable transducer belts (M1562A) (or three with Dual Ultrasound Model).
! Three transducer knob adapters (M1356-43201).
! One power cord.
! One pack of paper:
" M1910A (USA/Canada)
" M1911A (Europe)
" M1913A (Japan)
! One bottle of gel: 40483A (Acquasonic gel)
! One User’s Guide.
! One Installation and Service Guide
! One remote event marker (15249A).
Series 50 IP-2
The following accessories are supplied as standard with the Monitor:
(M1353A)
! One external Toco transducer (M1355-60011).
! One ultrasound transducer (M1356-60011).
! One patient module M1364A with cables:
" One DECG legplate adapter cable (M1362B)
" One MECG adapter cable (M1363A)
! Five spiral electrodes:
" 15133D Single spiral (USA).
" 15133E Double spiral (Europe).
! Three reusable transducer belts.
! Three transducer knob adapters (M1356-43201).
! One power cord.
! Equipotential grounding cable:
Chapter 1 General Information 9

Accessories
" 8120-2961 (USA).
" 8120-4808 (Europe).
! One pack of paper:
" M1910A (USA/Canada)
" M1911A (Europe)
" M1913A (Japan)
! One bottle of gel: 40483A (Acquasonic gel)
! One Instructions for Use.
! One Installation and Service Guide.
Documentation The following documentation is available for the Series 50 A and Series 50 IP-2 fetal
monitors. Unless otherwise specified localized versions are available.
! Technical Data Sheets: Contain features and benefits, technical specifications,
accessories, ordering, upgrading and re-ordering information.
! Service Documentation: All service documentation is in English.
! Instructions for Use: Detailed operating information, care and cleaning, and safety
requirements.
! Video Tapes: 30-minute VHS video tapes demonstrating the Monitor.
! Barcode Booklets: Labels and cards, and instructions on how to customize sheets of
nursing notes.
! Digital Interface Protocol Specifications: Written as a programmer's guide, describing
the data exchange between the Series 50 Fetal Monitors and an Information
Management System such as OB
TraceVue. English only.
10 Chapter 1 General Information

Accessories
Options The following accessories can also be supplied when the appropriate option is ordered.
Accessories Option Model
Barcode Reader, including a reader and barcode booklet. This
H15 Series 50 A and Series 50 IP
requires Option J10 or J15.
Combined Interface Module for telemetry and obstetrical
J10
1
Series 50 A and Series 50 IP
surveillance systems (e.g. Philips OB Tr a c e Vu e ) and barcode
reader
Combined Interface Module for telemetry and obstetrical
J13
1
Series 50 A and Series 50 IP
surveillance systems (e.g. Philips OB Tr a c e Vu e ) , includes an
interface cable M1350-61609.
• for Dinamap 1846 or
• COLIN Press-Mate/Nippon Colin Listmini Model BP-
8800 NIBP Monitor
Combined Interface Module for telemetry and obstetrical
J14
1
Series 50 A and Series 50 IP
surveillance systems (e.g. Philips OB Tr a c e Vu e ) , includes an
interface cable M1353-61614
• for Nellcor OxiFirst™ Fetal Oxygen Saturation Monitor
(N-400)
Modem Interface Module allows the transmission of fetal
J15
1
Series 50 A
trace data from a Series 50 A to a receiver (e.g., an OB
TraceVue system)
Fetal Movement Profile C02 Series 50 A and Series 50 IP
IUP Pressure Transducer (CPJ840J5) C07 Series 50 IP
Disposable IUP Catheter. This includes 1 x box M1333A
C08
2
Series 50 IP
(containing 10 catheters) disposable intrauterine sensor-tip
pressure catheters and M1334A reusable connector cable
1. Options J10, J13, J14 and J15 cannot be fitted at the same time
2. Not available in all countries.
Chapter 1 General Information 11

Accessories
Accessories Option Model
Service and Installation Guide 0B3 Series 50 A and
Series 50 IP
Installation and Operating Guide Video
•VHS ⁄ NTSC
0B5 Series 50 A and
Series 50 IP
•VHS ⁄ PAL
Wall mounting kit 1AB Series 50 A and
Series 50 IP
Paper take-up tray
1
1AC Series 50 A and
Series 50 IP
Angled mounting kit 1AD Series 50 A and
Series 50 IP
Mobile cart 2AE Series 50 A and
Series 50 IP
1. Not compatible with the wall mounting kit.
12 Chapter 1 General Information

Monitor
2
Technical Specifications
Power Requirements
The monitor is set for the correct voltage at the factory. Before you connect power, however,
ensure that the voltage label shows the correct setting for your country.
Operating Voltage: 100V - 120V∼ or 220V - 240V (±10%).
Line Frequency: 50 to 60Hz ±5%.
Power Consumption: 25VA max.
Battery Type: 2 x 1.5V (AA size). Lifetime > 1 year.
Environment The monitor should be used in an environment which is reasonably free from vibration,
dust, corrosive or explosive gases, extremes of temperature, humidity, etc. It operates within
specifications at ambient temperatures between 0 and 55°C. Ambient temperatures which
exceed these limits can affect the accuracy of the monitor and cause damage to the
components and circuits. Allow at least 5cm (2in) clearance around the monitor for proper
air circulation.
Operating Temp: 0 to +55°C (32°F to 131°F).
Storage Temp: -40 to +75°C (-40°F to 167°F), excludes transducers: -40 to
Relative Humidity: 5 to 95%.
+60°C (-40°F to +140°F)
Weight and
Height: 115mm (4.5in).
Dimensions
Width: 340mm (13.4in).
Depth: 308mm (12.1in).
We ig ht : 5.74kg (12.6lb) (without transducers).
Chapter 2 Technical Specifications 13

Monitor
Displays
Numerical Display M1351A Single
Ultrasound Model: One heart rate display (orange) and one uterine activity display
(green).
M1351A Dual
Ultrasound Twins Model: Two heart rate displays and one uterine activity display.
M1353A Model: Two heart rate displays and one uterine activity display.
Ty pe : 7-segment LEDs (10mm).
FHR Range: 50 to 240 bpm.
Uterine Activity Range: -99 to +127 relative units.
Instrument Display Telemetry Mode is displayed if Option J10 is fitted and an M2720A Avalon CTS Cordless
Fetal Transducer System or M1310A or 80240A Fetal Telemetry System is connected and
switched on.
M1351A Single
Ultrasound Model: One signal quality indicator.
M1351A Dual
Ultrasound Twins Model: Two signal quality indicators.
M1353A Model: Two signal quality indicators.
Inputs M1351A Single
Ultrasound Model US socket accepts the M1356A ultrasound transducer. Toco
M1351A Dual
Ultrasound Twins Model US1 and US2 sockets accept M1356A ultrasound transducers.
M1353A Model US1 socket accepts the M1356A ultrasound transducer. Toco
socket accepts the M1355A Toco transducer. Socket for the
Remote Event Marker (15249A), and another for servicing. The
monitor automatically selects the correct operating mode.
Toco socket accepts the M1355A Toco transducer. Socket for the
Remote Event Marker (15249A), and another for servicing. The
monitor automatically selects the correct operating mode.
socket accepts the M1355A external Toco or the M1350A/
8040A compatible internal Toco transducer. US2/ECG socket
accepts either the M1356A ultrasound transducer, or the
M1364A DECG/MECG patient module or the M1357A
DECG or the M1359A MECG transducer. There is a socket for
the remote event marker (15249A), and another for servicing.
The monitor automatically selects the correct operating mode.
14 Chapter 2 Technical Specificati ons

Ultrasound Mode System: Pulsed Doppler oscillation.
Frequency: 998.4 kHz.
Repetition Rate: 3.2 kHz.
Ultrasound Intensity: 1.5mW/cm² average for each of the seven active surfaces.
Monitor
DECG and MECG
Mode
External Labor Signal Range: 0 to 127 units.
See Specifications for Transducers and Cables on page 17.
Offset Compensation: ±200 units.
Internal Labor Signal Range: -99 to +127 mmHg.
Patient Leakage Current:
≤10 µA
rms
.
Sensitivity: 40 µV/V/mmHg (M1348A).
5 µV/V/mmHg (M1334A and CPJ840J5).
Recorder Mechanism: 3-channel, high-resolution (8 dots/mm) thermal array recorder
with paper-end detection.
Paper Speeds: 1, 2 or 3 cm/min.
Recording Time Per Pack
of Paper: 1 cm/min (25 h).
2 cm/min (12 h 30 min).
3 cm/min (8 h 20 min).
Paper Advance Speed: 24 cm/min (with automatic stop at the paper-end mark).
Annotation: Time of day, date, and paper speed are printed automatically
every ten minutes. Monitoring mode is printed with every
alteration of parameter.
Paper: Fanfold paper with numbered pages.
FHR Scale: USA: 30 to 240 bpm @ 30 bpm/cm.
Other countries: 50 to 210 bpm @ 20 bpm/cm.
Labor Scale: 0 to 100 units @ 25 units/cm.
Chapter 2 Technical Specifications 15

Monitor
Self-Test Facilities
Combined Interface Module
Modem Interface Module
Self-test facilities include:
System test: With no transducers connected (includes a display and recorder
test).
Parameter test: With the appropriate transducer connected, the monitoring
mode (ultrasound or uterine activity) is tested.
Te l e me t r y : M1310A Fetal Telemetry System.
System: M1383A/B/C OB TraceVue.
Either
Barcode Reader: SmartWand.
or
Maternal NIBP Monitor: Dinamap1846/8100.
COLIN Press-Mate/Nippon Colin Listmini Model BP-8800.
or
Nellcor OxiFirst Fetal Oxygen Saturation Monitor (N-400) or
compatible.
Modem: Interface socket for an Philips-approved PCMCIA card modem.
Fetal Trace Memory: Local fetal trace storage.
Barcode Reader: Smart Wand.
Remote Event Marker (15249A)
RS232 Serial Interface: For internal use only.
Length: 2.8m/9ft 2in.
We ig ht : 75g/2.65oz.
16 Chapter 2 Technical Specificati ons

Transducers and Cables
Transducers can be stored at temperatures between -40 and +60°C.
Transducers and Cables
Brown Toco Transducer (M1355A)
Blue1 Toco
Transducer
(M1355A)
Brown Ultrasound Transducer (M1356A)
Blue1 Ultrasound Transducer (M1356A)
System: Passive Straingauge.
Dynamic Range: 0 to 12N (overload protected).
We ig ht : 180g/6.3oz.
Cable Length: 2.5m/8ft 2in.
System: Passive Straingauge.
Dynamic Range: 0 to 12N (overload protected).
We ig ht : 180g/6.3oz.
Cable Length: 2.5m/8ft 2in or 0.7m/2ft 3in.
System: Pulsed Doppler.
Oscillator Frequency: 998.4kHz.
We ig ht : 185g/6.5oz.
Cable Length: + 2.5m/8ft 2in.
Size: 75mm/2.95in diameter.
System: Pulsed Doppler.
Oscillator Frequency: 998.4kHz.
We ig ht : 185g/6.5oz.
Cable Length: 2.5m/8ft 2in or 0.7m/2ft 3in.
Size: 75mm/2.95in diameter.
1. Indicates transducer is waterproof.
Chapter 2 Technical Specifications 17

Transducers and Cables
DECG Transducer (M1357A)
MECG Transducer (M1359A)
Input Impedance: >10MΩ (differential, dc to 50/60Hz).
CMRR: >110dB (with patient cable, 51.5kΩ/0.047µF imbalance at line
frequency).
Noise: <4µV
(referred to input with 25kΩ).
p
Contact Potential
To l e ra n c e: ±400mV.
Input Voltage Range: 20µV
Patient Leakage Current: <10µA
to 3mVp.
p
@ 120V/60Hz.
rms
Patient Auxiliary Current:<0.1µA (dc).
Dielectric Strength: 1500V
(spark-gap protected).
rms
We ig ht : 185g/6.5oz.
Cable Length: 2.5m/8ft 2in or 0.7m/2ft 3in.
Input Impedance: >10MΩ (differential, dc to 50/60Hz).
CMRR: >90dB (with patient cable, 51.5kΩ/0.047µF imbalance at line
frequency).
Noise: <4µV
(referred to input with 25kΩ).
p
Contact Potential
To l e ra n c e: ±400mV.
DECG/MECG Patient Module (M1364A)
Input Voltage Range: 80µV
Patient Leakage Current: <10µA
to 4mVp.
p
@ 120V/60Hz.
rms
Patient Auxiliary Current:<0.1µA (dc).
Dielectric Strength: 1500V
(spark-gap protected).
rms
We ig ht : 175g/6.2oz.
Cable Length: 2.5m/8ft 2in.
The patient module has a 7-pin ECG connector into which you can plug either DECG
cable (M1362A or B) or MECG cable (M1363A).
Overall length: 2706mm (+30, -100mm)
Length of free cable: 2618mm (+30, -100mm)
Weight: 120 grams
Size: 88x42x30mm
Socket: DECG or MECG connection
18 Chapter 2 Technical Specificati ons
 Loading...
Loading...