Panasonic MHE-UN4025CW User Manual

MHE-PF4025CW
MHE-UN4025CW
Integrated Cell
Processing Work
Station
CPWS Features:
• Cost effective, space-saving solution
for GMP and GTP compliant regenerative
medicine and cell therapy research.
• Minimizes the expense of a cleanroom laboratory
Panasonic… the new name for SANYO
www.panasonic.com/biomedical
MHE-PF4025CW
MHE-UN4025CW
Panasonic CPWS Integrated Cell Processing Work Station
Minimizes Cleanroom Expense
Self-Contained
Space-Saving
Quick to Acquire and Install
GMP Compliant for Aseptic Process
User Friendly
Energy Efcient, Green Design
The Essential Work Station for Cell Therapeutics
Continued worldwide development of new methods and processes in cell therapy and
regenerative medicine research requires renewed emphasis on tools and technologies
required to establish and maintain aseptic conditions demanded of the clinical environment.
Panasonic has met that challenge.
In designing the industry’s rst viable alternative to a conventional Class 10,000 cleanroom for
both Class 100 air quality and barrier isolation, Panasonic has introduced the Cell Processing
Work Station (CPWS) to bring the potential of cell therapeutics to more facilities while
mitigating acquisition and operation costs and protracted time lines.
Ophthalmalogy and
Organ Replacement/
Preservation
Dental/Oral
Skin
Urology
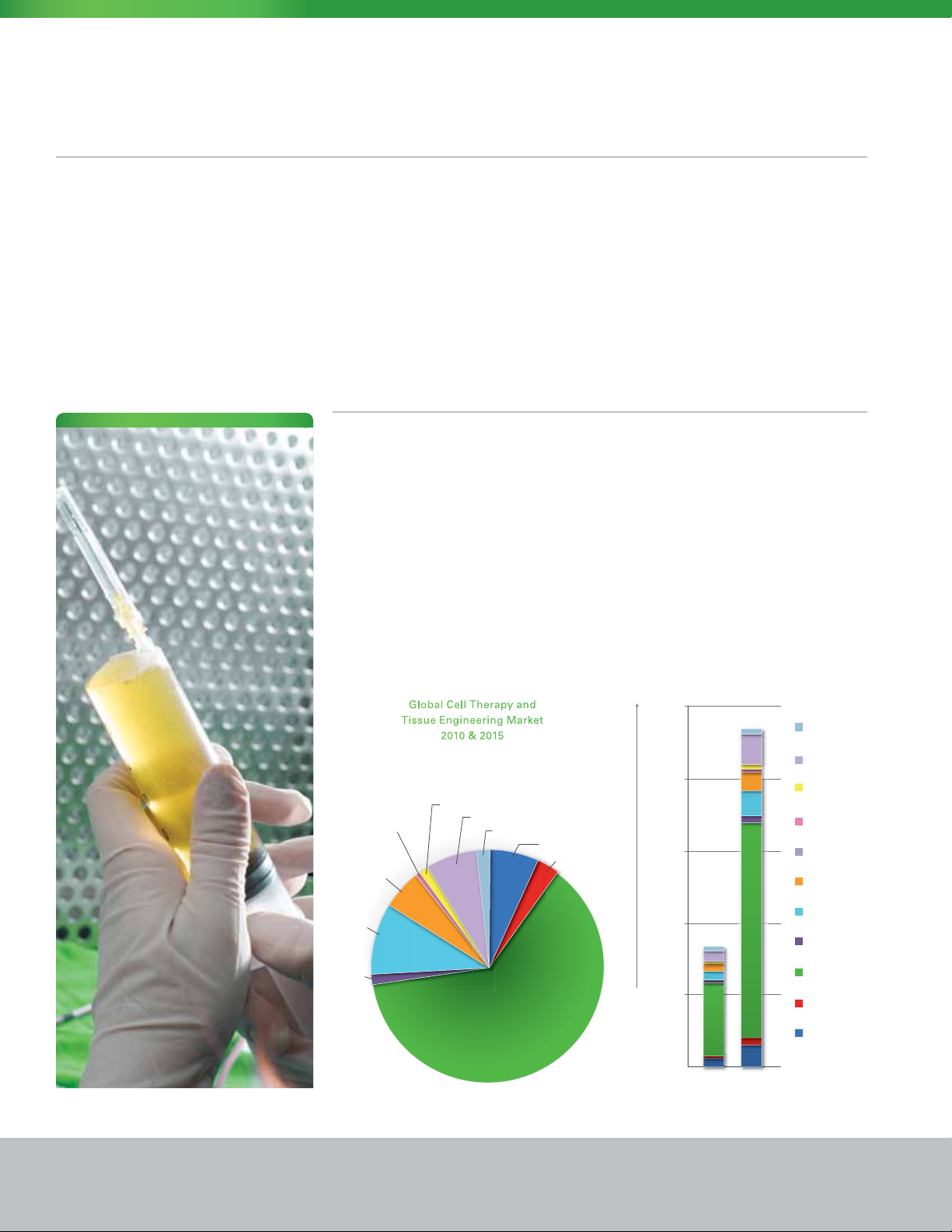
Global Cell Therapy and
Tissue Engineering Market
2010 & 2015
General
Cancer
Cord Blood and Cell Banking
Cardiology
Neurology
Orthopedics/Spine
$25,000
$20,000
$15,000
$10,000
$5,000
Cell/Tissue
Banking
Cancer
General Surgery,
GI, Gyn, Others
Ophthalmalogy
Organ
Dental
Skin
Urology
Ortho
Neuro
Cardio
2 CWPS Integrated Cell Processing Work Station www.panasonic.com/biomedical
Millions of Dollars
$0
2010 2015

The Market
Immediate beneciaries of the Panasonic
CPWS include both private and public
institutions serving the life science,
clinical, biotechnology and pharmaceutical
markets. These include mainstream
and scale-up pharmaceutical companies
focused on drug and medical device
development related to human cells,
medical researchers applying cellular
therapies in clinical trials, and hospitals
now deploying approved cellular therapeutics to treat a broad range
of diseases.
As the science progresses and results
are propagated throughout the medical
and scientic community, the need for
bench-level research and production is
expanding exponentially. As a result, the
availability of the Panasonic free-standing,
self-contained work station apart from
the conventional layered cleanroom
approach to containment and protection,
is bringing the capability for on-site
cellular therapeutics to more facilities
more quickly and at a lower capital and
operational cost, all within the GMP
performance envelope required of the
cell technology itself.
Self-Contained System,
Small Footprint
The Panasonic concept of a self-contained work station is enabled by the
company’s demonstrated prociency
for in situ decontamination required to
separate processes from one patient
to the next, or one protocol to another.
By using a highly effective H
tamination process, the CPWS can be
completely decontaminated without heat
and prepared for the next protocol within
two hours as compared to a cleanroom
decontamination function can take days
or weeks.
By increasing throughput within the
compliant parameters of GMP criteria,
the CPWS is deployed for both research,
cellular manipulation and growth, cell
product extraction and emerging processes that fall within similar guidelines.
Current and future applications of the
CPWS include organ and tissue regeneration such as skin, cartilage, alveolar bone,
2O2
decon-
cornea, cardiac muscle, nerve, liver and
pancreas regeneration. Immunotherapy
applications may extend to dendritic cells,
T-cells and more.
Making it Work
Improving efciency in human interaction
is a primary design attribute of the CPWS.
All work must be performed without
human error, in an aseptic environment
with detailed documentation to assure
quality and compliance. Gown-up time,
expense and inconvenience is minimized
or eliminated altogether. Integrated
systems within the CPWS permit cellular
extraction, preparation, culturing and
administration with aseptic assurance as
well as economic practicality.
Because conventional autoclaving is
not possible, the Panasonic H
2O2
decontamination process diminishes both time
and labor associated with this critical step
between patients, positioning the CPWS
squarely in the equation for cost/benet
justication associated with investment
decision-making. Allowances for integrated centrifuge, microscopy, data acquisition and incubation functions are important considerations in the CPWS design;
a unique docking station permits interface
and exchange with unlimited number
of cell culture incubators dedicated to
individual patients or cell lines.
On the Work Surface
Since humans remain the single most
common source of contamination, the
CPWS provides both physical and
process benets to minimize contamination and cross-contamination in the
work area. Within the four-port glove
box the CPWS delivers more than a
conventional Class II, Type A2 biological
safety cabinet typically installed within a
cleanroom to achieve the same objective.
Here, the manually initiated, automatically
deployed H
supplements continuous HEPA ltration.
As 0.3 micron particles are removed
from the fresh air exchange, H
contamination neutralizes contaminants
brought forth by instrumentation or
equipment. A number of decontamination
sequences are available to protect the
aseptic environment.
decontamination process
2O2
2O2
de-
The glove box design offers barrier isolation protection for the operator and the
work inside. User comfort and ergonomics are inherent to the CPWS design, including a sloped front for ease of access
and glare reduction.
What You Don’t Need
The relatively small footprint permits
installation into existing or new Class
100,000 lab space with conventional
utilities and minimal site preparation.
Multi-layered airlocks in multiple treated
rooms are avoided. Capital intensive
expenses are lowered and lead times
from decision to operation are shortened.
Once in place, operational costs are
highly contained and predictable, with decontamination available more frequently
and at a fraction of conventional cost.
The Panasonic Difference
For over forty years, Panasonic has
established a reputation as a premier
manufacturer of precision biomedical and
laboratory equipment. Known throughout
the world as a leading brand in consumer
electronics and appliances, Panasonic
addresses global needs such as energy,
food, housing, healthcare and information
technology.
As a part of the Panasonic product line
worldwide, the Cell Processing Work
Station exemplies our unique Vertical Component Integration approach to
product development, combining ideas
and innovations from our global industrial
and consumer products network into an
integrated product featuring advanced
technology, controls, construction and
performance attributes.
The CPWS and subcomponent systems
have been extensively tested to meet the
toughest quality standards for performance, ergonomics and cost of ownership. The CPWS is designed to minimize
its carbon footprint through energy savings and environmental stewardship.
3

Panasonic CPWS Series Technical Attributes
The Panasonic CPWS work station is
designed to deliver efcient, costeffective and GMP compliant cell therapy
and manufacturing capability without the
expense and inconvenience of a class
10,000 cleanroom. The CPWS offers
signicant advantages over conventional
hard wall cleanroom construction.
• The CPWS is less expensive than a
cleanroom.
• It is quicker to acquire and place
into operation.
• The small footprint increases options
for location and orientation.
• The user-friendly glove box design
eliminates gowning and improves
operator comfort and convenience.
• Operating costs are lower than
cleanroom costs
• Work is easily suspended and resumed
without the need to de-gown and regown, improving user comfort.
• Fast decontamination and changeover improve productivity, increase
throughput and deliver quicker return
on investment.
• Recordkeeping and process
documentation are easier to manage.
Components and operating systems are
congured around a central work station
with a HEPA ltration and air management
system designed to deliver Class 100 air
to the work surface within the glove box.
• Central barrier isolator
• Pass box interchange
• Integrated H
decontamination
2O2
system
• Optional cell observation system with
microscope and monitor
• Optional centrifuge integrated into the
work surface
• Optional CO
incubator with
2
docking collar
• The optional incubator and optional
centrifuge operate within a Class 100
environment.
CPWS Work Station
The Panasonic CPWS work station is a
component-based design that permits
long-term or quick turnover self-contained
protocols with efciency and safety for
the product as well as personnel. As an
integrated system, all functions associated with good laboratory technique,
environmental control and ergonomic
comfort are selected for compatibility
and complementary functional performance.
1. Modular CO
cart, docked to barrier isolator.
2. Incubator cart with locking casters;
offset casters nest with
frame assembly when docked.
3. Lid cam latch
4. Centrifuge controller
5. Centrifuge module accessible
for the aseptic work area.
6. Glove port
incubator, shown on
2
1
2
14
18
15
3
7. Hinged front access assembly;
front lifts up when total interior
access is required.
8. Interchange pass box with manually
initiated, automatic sequence H
2O2
decontamination system.
9. System controller
10. H
liquid supply cartridge
2O2
11. HEPA supply and exhaust ltration
blower motor assembly
12. Electrical compartment
13. Interior uorescent lamps
14. Electropolished interior surfaces
15. Angled 6° front for user comfort,
reduced glare
16. Locking screws to secure
hinged front.
17. Adjustable leveling feet
18. Optional cell monitor LCD
11
7
13
6
5
16
4
17
9
8
10
12
4 CWPS Integrated Cell Processing Work Station www.panasonic.com/biomedical

Panasonic CPWS Series Applications
Clinical Applications of Cell Therapies
Disease States Cell Therapies
Cancer
Hematopoietic Stem Cell
(HSC) Transplantation
Immunotherapy
Orthopedic
Neurodegenerative Disorders/Trauma
Cardiovascular Disease
Organ Replacement
Pancreas (diabetes)
Liver (failure, metabolic disorders) Bioarticial liver; Isolated hepatocy tes; Hepatocyte stem cells
Kidney (failure) Bioarticial kidney
Wound Healing
Infectious Diseases
Genetic Deciencies
Hemophilia Gene Therapy
SCID Gene Therapy
Cystic Fibrosis Gene Therapy
Autologous and allogeneic HSC; Ex vivo expansion of HSC;
‘Suicide’ T-cells – gene transfer; Stem cell transportation
Dendritic cells; NK /T cells; Macrophage -activated killer cells;
T-cell expansion; NK cells; Co- stimulatory molecules (gene transfer)
Expanded chondrocytes; Mesenchymal stem cells
Adult stem cell-derived cells; Embryonic stem cell- derived neural cells
Infusion of marrow/blood- derived angio blasts;
CD34 stem cells; cardiac cells*
Pancreatic islet cells; Embryonic stem cell-derived islet cells;
Adult stem cell-derived islet cells
Keratinocytes; Skin stem cells
Antigen -loaded dendritic cells; Lymphocyte expansion; Macrophages
Applications
The Panasonic CPWS enables a broader
access to cellular therapeutic research
related to both minimally manipulated
and non-minimally manipulated cell
products by lowering the cost of entry,
extending the process to the widest
range of applications, and minimizing
operating expenses when compared to a
conventional cleanroom environment.
• Minimally manipulated products
are associated with cell washing,
enrichment, selection, HSC (PB,
BM, CB), cancer therapies and
other under GTP requirements.
• Non-minimally manipulated
products are associated with
expanded, differentiated or
transformed cells (DC, MSC, ESC,
TC) in cancer centers, biotech labs,
stem cell institutes and contract
manufacturing facilities operating
under GMP requirements.
• GMPs (Good Manufacturing Practices)
are mandated by the United States
Food and Drug Administration to
ensure that drug development
and manufacturing is safe, quality
controlled for repeatability and
thoroughly documented.
• GMPs typically require expensive
hard wall laboratories and laboratory
suites using biological safety cabinets
in Class 10,000 cleanrooms surrounded
by a Class 100,000 room.
Autoimmune Diseases
Immunotherapy
HSC, hematopoietic stem cells; NK, Natural Killer; SCID, severe combined immunodeciency
* Currently in clinical studies.
Dendritic cells; T-cells, Mesenchymal stem cells*;
Lymphocy te expansion; Natural Killer cells
5
 Loading...
Loading...