Page 1
Introduction
Intended Use
This device is a digital monitor intended for use in measuring blood pressure and
pulse rate in adult patient population. The device detects the presence of irregular
heartbeats during measurement and gives a warning signal with the measurement
result.
Please follow this instruction manual thoroughly for your safety.
Please keep for future reference. For specific information about your own blood
pressure, CONSULT YOUR PHYSICIAN.
Important Safety Information
Warning: Indicates a potentially hazardous situation which, if not avoided,
could result in death or serious injury.
General Usage
DO NOT adjust medication based on measurement results from this blood
pressure monitor. Take medication as prescribed by your physician. Only a
physician is qualified to diagnose and treat high blood pressure.
Consult your physician before using the device for any of the following
conditions: common arrhythmias such as atrial or ventricular premature beats or
atrial fibrillation, arterial sclerosis, poor perfusion, diabetes, age, pregnancy,
pre-eclampsia, renal diseases.
Note that PATIENT motion, trembling, shivering may affect the measurement
reading.
Do not use the device on an injured arm or an arm under medical treatment.
Stop using the device and consult your physician if you experience skin irritation
or other troubles.
Do not apply the arm cuff on the arm while being on an intravenous drip or blood
transfusion.
Consult your physician before using the device on the arm with an arterio-venous
(A-V) shunt.
Do not use the device with other medical electrical (ME) equipment
simultaneously.
Do not use the device in the area of high frequency (HF) surgical equipment,
magnetic resonance imaging (MRI), or computerized tomography (CT) scanner
exists, or in the oxygen rich environment.
The air tube or the AC adapter cable may cause accidental strangulation in
infants.
Contains small parts that may cause a choking hazard if swallowed by infants.
Data Transmission
Do not use this product on an aircraft or in hospitals. Please remove the battery
and AC adapter from the device. This product emits radio frequencies (RF) in the
2.4 GHz band, use of this product in locations where RF is restricted is not
recommended.
The use of RF in this product is licensed for use by the FCC, for further
information on any potential restrictions refer to documentation on Bluetooth
®
usage by the FCC.
AC Adapter (optional accessory)
Do not use the AC adapter if the device or the power cord is damaged. Turn off
the power and unplug the power cord immediately.
Plug the AC adapter into the appropriate voltage outlet. Do not use in a
multi-outlet plug.
Never plug in or unplug the power cord from the electric outlet with wet hands.
Do not disassemble or attempt to repair the AC adapter.
Battery Usage
Keep the battery out of reach of children.
Caution: Indicates a potentially hazardous situation which, if not avoided,
may result in minor or moderate injury to the user or patient or
damage to the equipment or other property.
General Usage
Always consult your physician. Self-diagnosis of measurement results and
self-treatment are dangerous.
Consult your physician before using the device for any of the following
conditions:
•If you have had a mastectomy.
•People with severe blood flow problems or blood disorders as cuff inflation can
cause bruising.
Do not take measurements more often than necessary. It may cause bruising
due to blood flow interference.
Remove the arm cuff if it does not start deflating during the measurement.
Do not use this device on infants or persons who cannot express their intentions.
Do not use the device for any purpose other than measuring blood pressure.
Use only the approved arm cuff for this device. Use of other arm cuffs may result
in incorrect measurement results.
Do not use a mobile phone or other devices that emit electromagnetic fields near
the device except when in use for wireless communications. This may result in
incorrect operation of the device.
Do not disassemble or attempt to repair the device or components. This may
cause an inaccurate reading.
Do not use in a location with moisture, or a location where water may splash on
the device. This may damage the device.
Do not use the device in a moving vehicle. For example, the car or airplane.
Read “What to do if your systolic pressure is more than 210 mmHg” of this
instruction manual, if your systolic pressure is known to be more than 210
mmHg. Inflating to a higher pressure than necessary may result in bruising of the
arm where the cuff is applied.
Data Transmission
Do not replace the battery or unplug the AC adapter when in use for wireless
communications. This may result in incorrect operation of the device or damage
to the data.
Do not place integrated circuit cards, magnets, metal objects, or other devices
that emit electromagnetic fields near the device when in use for wireless
communications. This may result in incorrect operation of the device or damage
to the data.
AC Adapter (optional accessory)
Fully insert the power plug into the outlet.
When disconnecting the power plug from the outlet, be sure to safely pull from
the power plug. Do not pull from the power cord.
When handling the power cord, take care not to do the following:
Wipe any dust off of the power plug.
Unplug the monitor when not in use.
Disconnect the power plug before cleaning.
Battery Usage
Do not insert the batteries with their polarities incorrectly aligned.
Use only 4 “AA” alkaline or manganese batteries with this device. Do not use
other types of batteries. Do not use new and used batteries together.
Remove the batteries if the device will not be used for three months or more.
If battery fluid should get in your eyes, immediately rinse with plenty of clean
water. Consult a physician immediately.
Use the battery within recommended period mentioned to it.
General Precautions
• Do not forcibly crease the arm cuff or the air tube excessively.
• Do not fold or kink the air tube while taking a measurement. This may cause
harmful injury by interrupting blood flow.
• To unplug the air plug, pull on the air plug at the connection with the monitor, not
the tube itself.
• Do not drop the monitor or subject the device to strong shocks or vibrations.
• Do not inflate the arm cuff when it is not wrapped around your arm.
• Do not use the device outside the specified environment. It may cause an
inaccurate reading.
• Use only an AC adapter, arm cuff, batteries and etc, specified for this device. Use
of unsupported adapters, arm cuff and batteries may damage and/or may be
hazardous to the device.
• Dispose of the device, components and optional accessories according to
applicable local regulations. Unlawful disposal may cause environmental pollution.
• Please check (for example, by observation of the limb concerned) if the device is
not causing a prolonged impairment of PATIENT blood circulation.
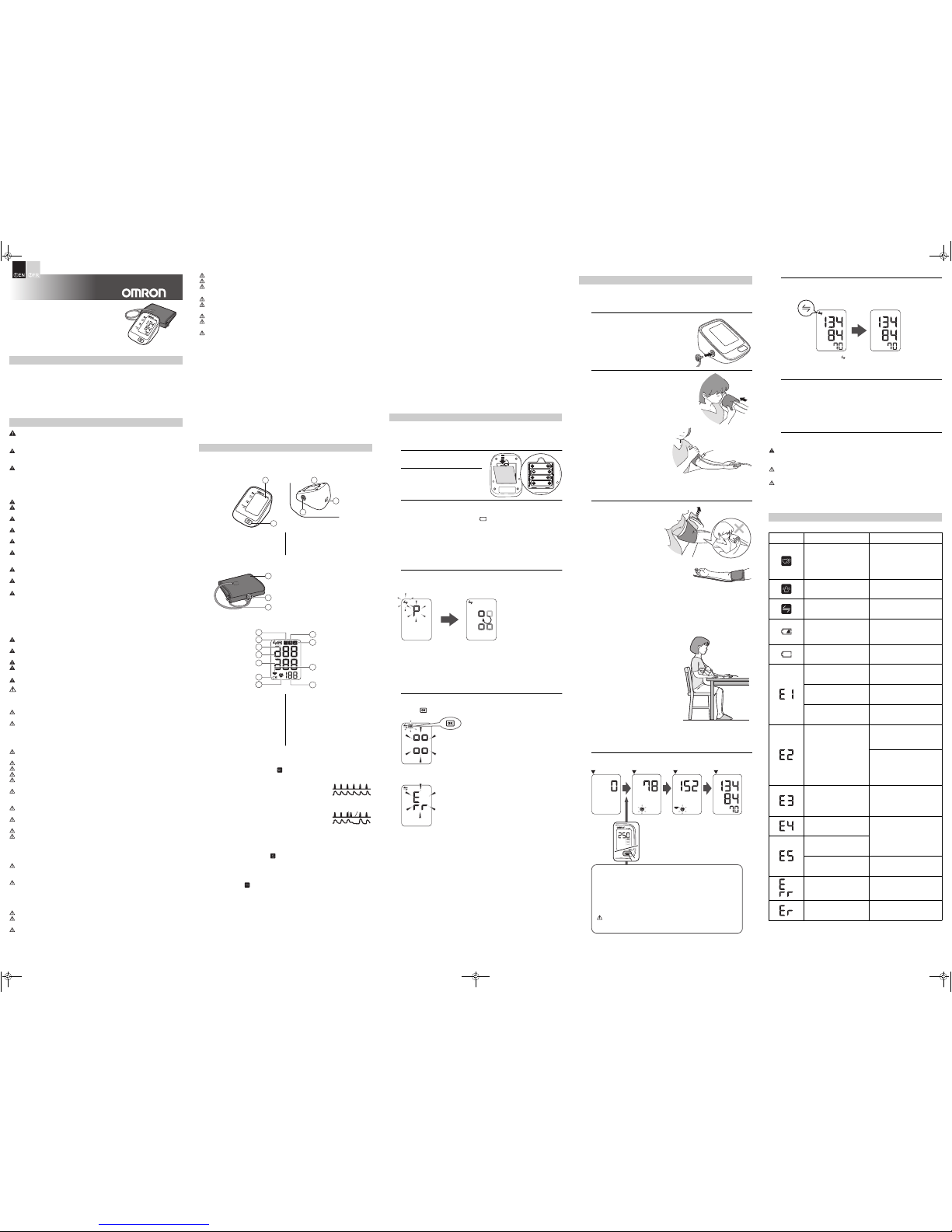
1. Know Your Device
Contents:
Monitor, arm cuff, battery set, instruction manual, quick start guide
Monitor:
Arm cuff:
Arm circumference 22 - 42 cm (9" - 17")
Display:
1.1 Display symbols
Irregular Heartbeat Symbol ( )
When the monitor detects an irregular
rhythm two or more times during the
measurement, the irregular heartbeat
symbol will appear on the display with the
measurement values.
An irregular heartbeat rhythm is defined as
a rhythm that is 25% less or 25% more
than the average rhythm detected while
the monitor is measuring the systolic and
diastolic blood pressure.
If the irregular heartbeat symbol displays with your measurement results, we
recommend you consult your physician. Follow the directions of your
physician.
Movement Error Symbol ( )
The movement error symbol is displayed if you move your body during the
measurement. Please remove the arm cuff, and wait 2 - 3 minutes.
Take another measurement, remain still during measurement.
SYNC Symbol ( )
The SYNC symbol is displayed if the device is not connected to Telehealth
service receiver or if measured data is not transmitted successfully. Please
refer to “Connection failure. / Data is not being transmitted.” in section 4.2.
1.2 Wireless Function
Before use, thoroughly read the instruction manual included with the
Telehealth service receiver (in some cases, receiver may be your
smartphone) being used with this blood pressure monitor for instruction about
your monitor to Telehealth service receiver, and receiver transmission range.
It will be necessary for the blood pressure monitor to be within the receiver’s
transmission range to successfully transfer data. This Omron blood pressure
monitor is designed to connect to specific Bluetooth Smart receivers, and is
not guaranteed to connect to all Bluetooth Smart compatible devices and
Telehealth service receivers. “OMRON HEALTHCARE Co., Ltd. cannot
accept liability for any damages incurred due to impaired operation or
data loss, etc. that occur through the use of this product.”
1.3 Before Taking a Measurement
To help ensure an accurate reading, follow these directions:
1. Avoid bathing, drinking alcohol or caffeine, smoking, exercising and eating
for 30 minutes before taking a measurement.
2. Rest for at least 5 minutes before taking the measurement.
3. Stress raises blood pressure. Avoid taking measurements during stressful
times.
4. Measurements should be taken in a quiet place.
5. Remove tight-fitting clothing from your arm.
2. Preparation
Battery Installation
Below connection process (step 4 and 5) can only be completed when
inserting batteries.
1. Remove the battery cover.
2. Insert 4 “AA” batteries as
indicated, into the battery
compartment.
3. Replace the battery cover.
Notes:
• When the depleted battery symbol “ ” appears on the display, turn the
monitor off and remove all the batteries. Replace with 4 new batteries at the
same time. Long life alkaline batteries are recommended.
• The measurement values continue to be stored in memory even after the
batteries are replaced.
• The supplied batteries may have a shorter life than new batteries.
• Disposal of used batteries should be carried out in accordance with the
national/local regulations for the disposal of batteries.
4. The device will start the connection process.
As soon as inserting the batteries, it will automatically start to connect to the
Telehealth service receiver, as below.
If the display shown above does not appear, refer to “Connection failure.
/ Data is not being transmitted.” in section 4.2.
To retry connecting the Telehealth service receiver, remove batteries
and press [START/STOP] button for 2-3 times. Then start with step 2
again.
Note: If your Telehealth service receiver asks for a PIN code, enter the digits
of the PIN code located on the rating label at the bottom of the device.
5. Confirm the device is successfully connected.
If the device is connected successfully to the Telehealth service receiver, OK
symbol “ ” will appear on the display, as shown below.
If “Err” appears, refer to “Connection failure. / Data is not being
transmitted.” in section 4.2 for more detail.
Notes:
• We recommend keeping batteries in the device at all times, even if you
choose to use the AC adapter.
• If only the AC adapter is used without keeping the batteries in the device, the
device connection process (steps 4 and 5) is necessary each time you
unplug and plug back the AC adapter.
3. Using the Device
3.1 Applying the Arm Cuff
Remove tight-fitting clothing from your left upper arm. Do not place the arm
cuff over thick clothing.
1. Connect the air plug to the unit.
2. Wrap the arm cuff firmly in
place around your left upper
arm.
The bottom edge of the arm cuff
should be 1/2 inch (1 to 2 cm) above
the elbow. The air tube is on the inside
of your arm and aligned with your
middle finger.
Note: Please refer to the operating instruction of the HEM-RXL31 for applying
the extra large cuff.
3. Securely close with the
fabric fastener.
Notes:
• When you take a measurement on the
right arm, the air tube will be at the side of
your elbow. Be careful not to rest your arm
on the air tube.
• The blood pressure can differ between the right arm and the left arm, and
the measured blood pressure values can be different. Omron recommends
to always use the same arm for measurement. If the values between both
arms differ substantially, please check with your physician as to which arm
to use for your measurements.
3.2 How to Sit Correctly
To take a measurement, you need to
be relaxed and comfortably seated, at
a comfortable room temperature.
• Sit in a chair with your legs uncrossed
and your feet flat on the floor.
• Sit upright with your back straight.
• Sit with your back and arm being
supported.
• The arm cuff should be placed on your
arm at the same level as your heart.
3.3 Taking a Measurement
Notes:
• To stop the measurement, press the
[START/STOP] button once to deflate the arm cuff.
• Remain still and do not talk while taking a measurement.
• The reading is stored in the memory and cannot be viewed on the display.
1. Press the [START/STOP] button.
The arm cuff will automatically start to inflate.
2. Transfer your readings.
As soon as the measurement is completed, your readings will be
automatically transferred, as shown below.
If the connection symbol “ ” does not appear, refer to
“Connection failure. / Data is not being transmitted.” in section 4.2 for
more detail.
3. After data transfer is completed, press the [START/STOP]
button to turn the monitor off.
It will automatically turn off after 2 minutes.
Note: Wait 2-3 minutes before taking another measurement. Waiting between
measurements allows the arteries to return to their prior condition to
taking a measurement.
4. Remove the arm cuff.
DO NOT adjust medication based on measurement results from this blood
pressure monitor. Take medication as prescribed by your physician. Only a
physician is qualified to diagnose and treat high blood pressure.
Always consult your physician. Self-diagnosis of measurement results and
self-treatment are dangerous.
Read “What to do if your systolic pressure is more than 210 mmHg” of this
instruction manual, if your systolic pressure is known to be more than 210
mmHg. Inflating to a higher pressure than necessary may result in bruising of the
arm where the cuff is applied.
4. Error Messages and Troubleshooting
4.1 Error Messages
Do not damage. Do not break it.
Do not tamper with it. Do not forcibly bend or pull.
Do not twist. Do not bundle during use.
Do not pinch. Do not place under heavy objects.
2800028-0A
Automatic Upper Arm
Blood Pressure Monitor
Model HEM-9210T
Instruction Manual
A. Display
B. START/STOP button
C. Battery compartment
D. Air jack
E. AC adapter jack
(for optional AC adapter)
I. SYNC symbol
J. Connection symbol
K. OK symbol
L. Systolic blood pressure
M. Diastolic blood pressure
N. Battery symbol
(low/depleted)
O. Heartbeat symbol
(Flashes during measurement.)
P. Movement error symbol
Q. Irregular heartbeat symbol
R. Deflation symbol
S. Pulse display
C
D
E
A
B
F
H
G
F. Arm cuff
G. Air plug
H. Air tube
L
M
N
LO
K
Q
P
R
S
I
J
Irregular Heartbeat
Normal Heartbeat
Pulse
Blood pressure
Blood pressure
Short
Long
Pulse
®
®
1 - 2 cm
(1/2 inch)
START INFLATING DEFLATING COMPLETED
What to do if your systolic pressure is more than
210 mmHg
After the arm cuff starts to inflate, press and hold the
[START/STOP] button until the monitor inflates 30 to 40 mmHg
higher than your expected systolic pressure.
Notes:
• The monitor will not inflate above 299 mmHg.
Inflating to a higher pressure than necessary may result in
bruising of the arm where the cuff is applied.
Error Display Cause Solution
Irregular heartbeat is
detected.
Remove the arm cuff. Wait 2 - 3
minutes and then take another
measurement. Repeat the steps
in section 3.3. If this error
continues to appear, contact
your physician.
Movement during
measurement.
Carefully read and repeat the
steps in section 3.3.
Connection failure. /
Data is not being
transmitted.
Refer to “Connection failure. /
Data is not being transmitted.” in
section 4.2.
The batteries are low.
Recommend to replace 4
batteries with new ones at this
time.
Refer to chapter 2.
The batteries are depleted.
Immediately replace the 4
batteries with new ones.
Refer to chapter 2.
Air plug is disconnected.
Insert the plug securely.
Refer to section 3.1.
Arm cuff is applied too
loosely.
Apply the arm cuff tighter.
Refer to section 3.1.
Air is leaking from the arm
cuff.
Replace the arm cuff with a new
one.
Refer to section 5.3.
Movement during
measurement and the arm
cuff has not been inflated
sufficiently.
Repeat measurement. Remain
still and do not talk during
measurement.
Refer to section 3.3.
If “E2” appears repeatedly,
inflate the arm cuff manually
until it is 30 to 40 mmHg above
your previous measurement
result.
Refer to section 3.3.
The arm cuff was inflated
exceeding the maximum
allowable pressure, and
then deflated automatically.
Do not touch the arm cuff and/or
bend the air tube while taking a
measurement. Do not inflate the
arm cuff more than necessary.
Refer to section 3.3.
Movement during
measurement.
Repeat measurement. Remain
still and do not talk during
measurement.
Refer to section 3.3.
Movement during
measurement.
Clothing is interfering with
the arm cuff.
Remove any clothing interfering
with the arm cuff.
Refer to section 3.1.
Communication failed.
Refer to “Connection failure. /
Data is not being transmitted.” in
section 4.2.
Device error.
Contact your Telehealth service
provider.
HEM-9210T-Z_A_M08_160209.pdf
Page 2
4.2 Troubleshooting
5. Maintenance and Storage
5.1 Maintenance
To protect your device from damage, please follow the directions
below:
•Store the device and the components in a clean, safe
location.
•Do not use any abrasive or volatile cleaners.
•Do not wash the device and any components or immerse
them in water.
•Do not use gasoline, thinners or similar solvents to clean the
device.
•Use a soft dry cloth, or a soft cloth moistened with neutral
soap to clean on the monitor and the arm cuff.
•Changes or modification not approved by the manufacturer
will void the user warranty. Do not disassemble or attempt to
repair the device or components.
5.2 Storage
1. Unplug the air plug from the air jack.
2. Gently fold the air tube into the arm cuff.
Note: Do not bend or crease the air tube
excessively.
Do not store the device in the following situations:
• If the device is wet.
• Locations exposed to extreme temperatures,
humidity, direct sunlight, dust or corrosive vapors such as bleach.
• Locations exposed to vibrations, shocks or where it will be at an angle.
5.3 Optional Accessories
Note: Please refer to the operating instruction of the HEM-RXL31 for applying
the extra large cuff.
Optional Accessories List
Note: Please check with your Telehealth service provider or local OMRON
representatives for the appropriate optional parts models
Using the Optional AC Adapter
Note: Make sure to use an easily accessible power socket in which to connect
and disconnect the AC adapter.
1. Insert the AC adapter plug into the
AC adapter jack on the rear side of
the monitor.
2. Plug the AC adapter into an
electrical outlet.
To disconnect the AC adapter, unplug the AC adapter from the electrical
outlet first, and then remove the AC adapter plug from the monitor.
6. Specifications
Notes:
• These specifications are subject to change without notice.
• In the clinical validation study, K5 was used on 85 subjects for determination
of diastolic blood pressure.
• This device is clinically investigated according to the requirements of
ISO81060-2:2013.
• This device has not been validated for use on pregnant patients.
• IP classification is degrees of protection provided by enclosures in
accordance with IEC 60529. This device is protected against solid foreign
objects of 12 mm diameter and greater such as a finger.
• This device fulfils the provisions of EC directive 93/42/EEC (Medical Device
Directive).
• This blood pressure monitor is designed according to the European Standard
EN1060, Non-invasive sphygmomanometers Part 1: General Requirements and
Part 3: Supplementary requirements for electromechanical blood pressure
measuring systems.
• This OMRON product is produced under the strict quality system of OMRON
HEALTHCARE Co., Ltd., Japan. The Core component for OMRON blood pressure
monitors, which is the Pressure Sensor, is produced in Japan.
7. FCC/IC/RE Statement and Trademarks
FCC CAUTION
Changes or modifications not expressly approved by the party responsible for
compliance could void the user’s authority to operate the equipment.
Note:
This equipment has been tested and found to comply with the limits for a Class B
digital device, pursuant to part 15 of the FCC Rules. These limits are designed to
provide reasonable protection against harmful interference in a residential
installation. This equipment generates, uses and can radiate radio frequency
energy and, if not installed and used in accordance with the instructions, may cause
harmful interference to radio communications. However, there is no guarantee that
interference will not occur in a particular installation. If this equipment does cause
harmful interference to radio or television reception, which can be determined by
turning the equipment off and on, the user is encouraged to try to correct the
interference by one or more of the following measures:
• Reorient or relocate the receiving antenna.
• Increase the separation between the equipment and receiver.
• Connect the equipment into an outlet on a circuit different from that to which the
receiver is connected.
• Consult the dealer or an experienced radio/TV technician for help.
This transmitter must not be co-located or operated in conjunction with any other
antenna or transmitter.
This equipment complies with FCC/IC radiation exposure limits set forth for an
uncontrolled environment and meets the FCC radio frequency (RF) Exposure
Guidelines and RSS-102 of the IC radio frequency (RF) Exposure rules. This
equipment has very low levels of RF energy that are deemed to comply without
testing of specific absorption ratio (SAR).
The Bluetooth® Smart word mark and logos are registered
trademarks owned by Bluetooth SIG, Inc. and any use of such
marks by OMRON HEALTHCARE Co., Ltd. is under license.
Other trademarks and trade names are those of their respective
owners.
Hereby, OMRON HEALTHCARE Co., Ltd., declares that the radio equipment type
HEM-9210T is in compliance with Directive 2014/53/EU.
The full text of the EU declaration of conformity is available at the following internet
address: www.omron-healthcare.com
8. Limited Warranty
Your HEM-9210T Automatic Upper Arm Blood Pressure Monitor, excluding
batteries, is warranted to be free from defects in materials and workmanship
appearing within 2 years from the date of purchase, when used in accordance with
the instructions provided with the monitor. The above warranty extends only to the
original retail purchaser.
We will, at our option, replace without charge any monitor or arm cuff covered by the
above warranty. Replacement is our only responsibility and your only remedy under
the above warranty.
THE FOREGOING IS THE SOLE WARRANTY PROVIDED BY OMRON IN
CONNECTION WITH THIS PRODUCT, AND OMRON HEREBY DISCLAIMS ANY
OTHER WARRANTIES, EXPRESS OR IMPLIED, INCLUDING IMPLIED
WARRANTIES OF MERCHANTABILITY AND FITNESS FOR A PARTICULAR
PURPOSE. IMPLIED WARRANTIES AND OTHER TERMS THAT MAY BE
IMPOSED BY LAW, IF ANY, ARE LIMITED IN DURATION TO THE PERIOD OF
THE ABOVE EXPRESS WARRANTY.
OMRON SHALL NOT BE LIABLE FOR LOSS OF USE OR ANY OTHER SPECIAL,
INCIDENTAL, CONSEQUENTIAL OR INDIRECT COSTS, EXPENSES OR
DAMAGES.
This warranty provides you with specific legal rights, and you may have other rights
which vary from state to state. Some states do not allow limitations on how long an
implied warranty lasts, so the above limitation may not apply to you. Some states do
not allow the exclusion or limitation of incidental or consequential damages, so the
above limitation or exclusion may not apply to you.
Should guarantee service be required, please contact your Telehealth service
provider.
9.
Guidance and Manufacturer’s Declaration
OMRON Blood Pressure Monitor (BPM) including AC-adapter
Information for accompanying documents in the scope of IEC60601-1-2:2007
Problem Cause and Solution
No power.
No display appears on the monitor.
Replace all batteries with new ones.
Check the battery installation for
proper placement of the battery
polarities.
Refer to chapter 2.
Measurement values appear too high
or too low.
Blood pressure varies constantly.
Many factors including stress, time of
day, and how you wrap the cuff, may
affect your blood pressure.
Review the section 1.3, 3.2 and 3.3.
Connection failure. /
Data is not being transmitted.
The blood pressure monitor might not
be properly placed within the receiver’s
transmission range and is too far from
the receiver. If there are no causes of
data transmission interference found
near the blood pressure monitor, move
the blood pressure monitor within 5 m
(16 ft.) of the receiver and try again.
The Bluetooth feature on the receiver
is turned off. Turn on the Bluetooth
feature on the receiver. To retry
connecting the Telehealth service
receiver, remove batteries and press
[START/STOP] button for 2-3 times.
The blood pressure monitor did not
pair successfully to the receiver. Try to
pair the devices again.
Refer to chapter 2.
The application on the receiver or
destination device is not ready. Check
the application then try to transmit the
readings again.
Refer to chapter 2. If the “Err” symbol
is on the screen after checking the
application, contact your Telehealth
service provider.
Arm cuff
Arm circumference
42 - 50 cm (17" - 20")
Arm circumference
22 - 42 cm (9" - 17")
Arm circumference
17 - 22 cm (7" - 9")
Extra Large Cuff Wide Range Cuff
• Same as the arm cuff
provided with the
product.
Small Cuff
AC Adapter
®
®
Name
Arm
Circumference
Model Sales area
Extra Large
Cuff
42-50 cm / 17"-20" HEM-RXL31
North America,
Asia
Wide Range
Cuff
22-42 cm / 9"-17"
CD-WR17 North America
HEM-RML31 Asia
Small Cuff 17-22 cm / 7"-9"
CD-CS9 North America
HEM-CS24 Asia
AC Adapter -
HEM-ADPTW5 North America
AC ADAPTER-S Asia
Omron representative in North
America
Omron representative in Asia
Call: 1-800-634-4350
Visit: OmronHealthcare.com
Visit: www.omronhealthcare-ap.com
Model
HEM-9210T
Display LCD digital display
Cuff pressure range Pressure: 0 to 299 mmHg
Measurement range Pressure: 20 to 280 mmHg
Pulse: 40 to 180 beats / min.
Accuracy Pressure: ±3 mmHg
Pulse: ±5% of display reading
Inflation Fuzzy-logic controlled by electric pump
Deflation Automatic pressure release valve
Measurement method Oscillometric method
Transmission method
Bluetooth
®
Version 4.0 (Low Energy support)
Wireless
communication
Frequency range : 2.4 GHz (2400 - 2483.5 MHz)
Modulation : GFSK
Effective radiated power : <20 dBm
IP classification IP 20
Rating DC6 V 4 W
Power source
4 “AA” batteries 1.5V or optional AC adapter (INPUT AC100-240V
50/60Hz 0.12A)
Battery life Approximately 1000 measurements (using new alkaline batteries)
Durable period
(Service life)
Monitor : 30000 times
Cuff : 10000 times
Operating conditions 10°C to 40°C (50°F to 104°F) / 15 to 90% RH / 700 to 1060 hPa
Storage / transport
conditions
-20°C to 60°C (-4°F to 140°F) / 10 to 95% RH / 700 to 1060 hPa
Weight Monitor : Approximately 290 g (10 oz.)
not including batteries
Arm cuff : Approximately 170 g (6 oz.)
Dimensions Monitor : Approximately 107 (w) mm × 79 (h) mm × 141 (l) mm
(4 1/4" × 3 1/8" × 5 1/2")
Arm cuff : Approximately 145 mm × 594 mm (air tube: 750 mm)
(5 3/4" × 23 1/2" (air tube: 29 1/2"))
Cuff circumference 22 to 42 cm (9" to 17")
Contents Monitor, arm cuff, battery set, instruction manual, quick start guide
Applied part Type BF
Protection against
electric shock
Internally powered ME equipment (When using only the batteries)
Class II ME equipment (Optional AC adapter)
Symbols description
Need for the user to consult the instruction
manual
Need for the user to follow the instruction manual
thoroughly for your safety
Applied part - Type BF
Degree of protection against electric shock
(leakage current)
Applied part - Type B
Degree of protection against electric shock
(leakage current)
(Optional AC adapter)
Class II equipment. Protection against electric
shock
(Optional AC adapter)
Indication of connector polarity
(Optional AC adapter)
For indoor use only
(Optional AC adapter)
To indicate generally elevated, potentially
hazardous, levels of non-ionizing radiation, or to
indicate equipment or systems e.g. in the medical
electrical area that include RF transmitters or that
intentionally apply RF electromagnetic energy for
diagnosis or treatment.
Serial number
Temperature limitation
Humidity limitation
Atmospheric pressure limitation
LOT number
Identifier of cuffs compatible for the device
Cuff positioning indicator for the left arm
Marker on the cuff to be positioned above the
artery
Range pointer and brachial artery alignment
position
Range indicator of arm circumferences to help
selection of the correct cuff size.
Product production date is integrated in a Serial or LOT number, which placed on
the Rating Label and sales package: the first 4 digits mean year of production, the
next 2 digits - month of production.
About a wireless communication interference
This Product operates in the unlicensed ISM band at 2.4GHz. In case this
Product is used around the other wireless devices including microwave and
wireless LAN, which operate same frequency band of this Product, there is a
possibility that interference occurs between this Product and such other devices.
If such interference occurs, please stop the operation of other devices or relocate
this Product before using this Product or do not use this Product around the other
wireless devices.
Correct Disposal of This Product
(Waste Electrical & Electronic Equipment)
This marking shown on the product or its literature, indicates that
it should not be disposed of, with other household wastes at the
end of its working life. To prevent possible harm to the
environment or human health from uncontrolled waste disposal,
please separate this product from other types of wastes and
recycle it responsibly to promote the sustainable reuse of material
resources.
Household users should contact either the retailer where they purchased this
product, or their local government office, for details of where and how they can
return this item for environmentally safe recycling.
Business users should contact their supplier and check the terms and conditions
of the purchase contract. This product should not be mixed with other
commercial waste for disposal.
or
or
Important information regarding Electro Magnetic Compatibility (EMC)
With the increased number of electronic devices such as PC’s and mobile (cellular)
telephones, medical devices in use may be susceptible to electromagnetic interference from
other devices. Electromagnetic interference may result in incorrect operation of the medical
device and create a potentially unsafe situation. Medical devices should also not interfere with
other devices.
In order to regulate the requirements for EMC (Electro Magnetic Compatibility) with the aim to
prevent unsafe product situations, the IEC60601-1-2 standard has been implemented. This
standard defines the levels of immunity to electromagnetic interferences as well as maximum
levels of electromagnetic emissions for medical devices.
Medical devices manufactured by OMRON Healthcare conform to this IEC60601-1-2:2007
standard for both immunity and emissions.
Nevertheless, special precautions need to be observed:
• The use of accessories and cables other than those specified by OMRON, with the exception
of cables sold by OMRON as replacement parts for internal components, may result in
increased emission or decreased immunity of the device.
• The medical devices should not be used adjacent to or stacked with other equipment.
In case adjacent or stacked use is necessary, the medical device should be observed to
verify normal operation in the configuration in which it will be used.
• Refer to further guidance below regarding the EMC environment in which the device should
be used.
• The MEDICAL ELECTRICAL EQUIPMENT BPM including AC-adapter needs special
precautions regarding EMC and needs to be installed and put into service according to the
EMC information provided in this documentations.
• The Essential Performance of the BPM including AC-adapter is to measure a blood pressure
and a pulse rate and using the memory function.
The BPM including AC-adapter may be interfered with by other equipment, even if that other
equipment complies with CISPR EMISSION requirements.
Guidance and manufacturer’s declaration - electromagnetic emissions
OMRON BPM including AC-adapter is intended for use in the electromagnetic environment specified
below. The customer or the user of this OMRON BPM including AC-adapter should assure that it is
used in such environment.
Emissions test Compliance Electromagnetic environment - guidance
RF emissions
CISPR 11
Group 1
The OMRON BPM including AC-adapter uses RF energy
only for its internal function. Therefore, its RF emissions
are very low and are not likely to cause any interference in
nearby electronic equipment.
RF emissions
CISPR 11
Class B
The OMRON BPM including AC-adapter is suitable for use
in all establishments, including domestic establishments
and those directly connected to the public low-voltage
power supply network that supplies buildings used for
domestic purposes.
Harmonic
emissions
IEC 61000-3-2
Class A
Voltage
fluctuations/
flicker emissions
IEC61000-3-3
Complies
Guidance and manufacturer’s declaration - electromagnetic immunity
OMRON BPM including AC-adapter is intended for use in the electromagnetic environment specified
below. The customer or the user of this OMRON BPM including AC-adapter should assure that it is
used in such environment.
Immunity test
IEC 60601 test
level
Compliance level
Electromagnetic environment -
guidance
Electrostatic discharge
(ESD)
IEC 61000-4-2
±6 kV contact
±8 kV air
±6 kV contact
±8 kV air
Floor should be wood, concrete,
or ceramic tile. If floors are
covered with synthetic material,
the relative humidity should be at
least 30 %.
Electrical fast
transient/burst
IEC 61000-4-4
±2 kV for power
supply lines
±1 kV for
input/output lines
±2 kV for power
supply lines
±1 kV for
input/output lines
Mains power quality should be
that of a typical commercial
and/or hospital environment.
Surge
IEC 61000-4-5
±1 kV line(s) to
line(s)
±2 kV line(s) to
earth
±1 kV line(s) to
line(s)
±2 kV line(s) to
earth
Mains power quality should be
that of a typical commercial
and/or hospital environment.
Voltage dips, short
interruptions and
voltage variations on
power supply inputlines
IEC 61000-4-11
<5 % U
T (>95 % dip
in U
T)
for 0.5 cycle
<5 % U
T (>95 % dip
in U
T)
for 0.5 cycle
Mains power quality should be
that of a typical commercial
and/or hospital environment. If the
user of the OMRON BPM
including AC-adapter requires
continued operation during power
mains interruption, it is
recommended that the OMRON
BPM including AC-adapter be
powered from an uninterruptible
power supply.
40 % U
T (60 % dip
in U
T)
for 5 cycles
40 % U
T (60 % dip
in U
T)
for 5 cycles
70 % U
T (30 % dip
in U
T)
for 25 cycles
70 % U
T (30 % dip
in U
T)
for 25 cycles
<5 % U
T (>95 % dip
in U
T)
for 5 sec.
<5 % U
T (>95 % dip
in U
T)
for 5 sec.
Power frequency
(50/60 Hz) magnetic
field
IEC 61000-4-8
3 A/m 3 A/m
Power frequency magnetic fields
should be at levels characteristic
of a typical location in a typical
commercial or hospital
environment.
Note: UT is the A.C. mains voltage prior to application of the test level.
Guidance and manufacturer’s declaration - electromagnetic immunity
OMRON BPM including AC-adapter is intended for use in the electromagnetic environment specified
below. The customer or the user of this OMRON BPM including AC-adapter should assure that it is
used in such environment.
Immunity test
IEC 60601 test
level
Compli
ance
level
Electromagnetic environment - guidance
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
3 V rms
150 kHz to 80
MHz
3 V/m
80 MHz to 2.5
GHz
3 V rms
3 V/m
Portable and mobile RF communications equipment
should be used no closer to any part of the OMRON BPM
including AC-adapter and cables, than the recommended
separation distance calculated from the equation
appropriate to the frequency of the transmitter.
Recommend separation distance
d = 1.2
d = 1.2
80 MHz to 800 MHz
d = 2.3
800 MHz to 2.5 GHz
where P is the maximum output power rating of the
transmitter in watts (W) according to the transmitter
manufacturer and d is the recommended separation
distance in meters (m).
Field strengths from fixed RF transmitters as determined
by an electromagnetic site survey,
a
should be less than
the compliance level in each frequency range.
b
Interference may occur in the vicinity of equipment
marked with the following symbol:
Note1: At 80 MHz and 800 MHz, the higher frequency range applies.
Note2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by
absorption and reflection from structures, objects, and people.
a
Field strengths from fixed transmitters, such as base stations for radio (cellular/ cordless) telephones
and land mobile radio, AM and FM radio broadcast, and TV broadcast cannot be predicted
theoretically with accuracy. To assess the electromagnetic environment due to fixed RF transmitters,
an electromagnetic site survey should be considered. If the measured field strength in the location in
which the OMRON BPM including AC-adapter is used exceeds the applicable RF compliance level
above, the OMRON BPM including AC-adapter should be observed to verify normal operation. If
abnormal performance is observed, additional measures may be necessary, such as reorienting or
relocating the OMRON BPM including AC-adapter.
b
Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3 V/m.
Recommended separation distance between portable and mobile RF communications
equipment
and the OMRON BPM including AC-adapter
OMRON BPM including AC-adapter is intended for use in an electromagnetic environment in which
radiated RF disturbances are controlled. The customer or the user of this OMRON BPM including
AC-adapter can help prevent electromagnetic interference by maintaining a minimum distance
between portable and mobile RF communications equipment (transmitters) and the OMRON BPM
including AC-adapter as recommended below, according to the maximum output power of the
communications equipment.
Output Power of Transmitter in
Watt
Separation distance according to frequency of transmitter
in meter
150 kHz to 80 MHz
d = 1.2
80 MHz to 800 MHz
d = 1.2
800 MHz to 2.5GHz
d = 2.3
0.01 0.12 0.12 0.23
0.1 0.38 0.38 0.73
1 1.2 1.2 2.3
10 3.8 3.8 7.3
100 12 12 23
For transmitters rated at a maximum output power not listed above, the recommended separation
distance d in meters (m) can be estimated using the equation applicable to the frequency of the
transmitter, where P is the maximum output power rating of the transmitter in watts (W) according to
the transmitter manufacturer.
Note: At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
Note: These guidelines may not apply in all situations. Electromagnetic propagation is affected by
absorption and reflection from structures, objects, and people.
Manufacturer
OMRON HEALTHCARE Co., Ltd.
53, Kunotsubo, Terado-cho, Muko, KYOTO,
617-0002 JAPAN
Distributor
OMRON HEALTHCARE, INC.
1925 West Field Court, Lake Forest, IL 60045 U.S.A.
OmronHealthcare.com
Asia Pacific HQ
OMRON HEALTHCARE SINGAPORE PTE LTD.
438A Alexandra Road, #05-05/08
Alexandra Technopark, Singapore 119967
www.omronhealthcare-ap.com
EU-representative
OMRON HEALTHCARE EUROPE B.V.
Scorpius 33, 2132 LR Hoofddorp, THE NETHERLANDS
Made in Japan
P
P
P
PPP
 Loading...
Loading...