Page 1
Main modules described in this manual
MVX10
MVX-2RE
MVX-CA2X
SZX-STAD1
SZH-STAD1
SZX-STAD2
SZ2-FO
SZH-SG
SZH-SC
INSTRUCTIONS
MVX10
RESEARCH MACRO ZOOM
SYSTEM MICROSCOPE
This instruction manual is for the Olympus MVX Research Macro Zoom System Microscope. To
ensure the safety, obtain optimum performance and familiarize yourself fully with the use of this
attachment, we recommend you study this manual thoroughly before operating the microscope.
Retain this instruction manual in an easily accessible place near the work desk for future reference.
A X 7 4 4 1
Page 2

This device complies with the requirements of directive 98/79/EC concerning in vitro diagnostic medical
devices. CE marking means the conformity to the directive.
Page 3
MVX10
CONTENTS
Correct assembly and adjustments are indispensable for the microscope to manifest its full performance. If you
want to assemble the microscope by yourself, refer to Chapter 11, “ASSEMBLY” before other chapters. (P. 36 to P. 44)
IMPORTANT — Be sure to read this section for safe use of the equipment. —
1 NOMENCLATURE
2 CONTROLS
3
SUMMARY OF REFLECTED FLUORESCENT LIGHT OBSERVATION PROCEDURE
4 OPERATION
4-1 Base ....................................................................................................................................................................................................................... 10
1 Using the Stage Plate 2 Placing the Specimen
4-2 Microscope Body and Focusing Assembly........................................................................................ 10, 11
1 Adjusting the Focus
1-4
5
6, 7
8, 9
10-17
2 Adjusting the Rotation Tension of the Coarse Focus Adjustment Knob
3 Engaging and Disengaging the Zooming Knob Click Stop Position
4 Adjusting the Aperture Iris Diaphragm 5 Using the Objective Correction Collar
4-3 Coaxial Fluorescence Illuminator....................................................................................................................... 12-15
1 Selecting the Fluorescence Mirror Unit 2 Turning the Burner ON
3 Opening/Closing the Shutter 4 Using the Field Iris Diaphragm
5 Switching the Filter Slider Knob
4-4 Observation Tube ...................................................................................................................................................................... 15-17
1 Adjusting the Tilt 2 Adjusting the Interpupillar Distance
3 Adjusting the Diopter (Zoom Parfocality Adjustment)
4 Using the Eye Shades 5 Using the Eyepiece Micrometer Disk
6 Selecting the Light Path 7 Switching the Monaural/Stereo View
5 TV OBSERVATION AND PHOTOMICROGRAPHY
1 Selecting the C-Mount Adapter Magnification
18, 19
2 Attaching the C-Mount Adapter 3 Selecting the TV Camera Light Path
4 Adjusting the Parfocality Between Observation Image and Monitor Image
Page 4

6 TRANSMITTED LIGHT OBSERVATION
20
7 TROUBLESHOOTING GUIDE
8 SPECIFICATIONS
9 OPTICAL CHARACTERISTICS
1011OPERATION OF OTHER MODULES
10-1 Revolving Nosepiece MVX-2RE..................................................................................................................... 24, 25
10-2 Magnification Changer MVX-CA2X ....................................................................................................................... 26
10-3 BX Stage Adapter Type 1 SZX-STAD1 .................................................................................................... 27-29
10-4 Stage Adapter Type 1 SZH-STAD1 ......................................................................................................................... 29
10-5 BX Stage Adapter Type 2 SZX-STAD2 .................................................................................................. 30, 31
10-6 Vertical-Movement Stage SZ2-FO ................................................................................................................ 31-33
10-7 Gliding Stage SZH-SG ................................................................................................................................................. 33, 34
21
22
23
24-35
10-8 Cup Stage SZH-SC........................................................................................................................................................... 34, 35
ASSEMBLY
11-1 Assembly Diagram ........................................................................................................................................................................ 36
11-2 Detailed Assembly Procedures ........................................................................................................................ 37-43
11-3 Centration of the Mercury (Xenon) Burner and Field Iris Diaphragm ...... 44, 45
PROPER SELECTION OF THE POWER SUPPLY CORD .................................................................... 46, 47
36-45
Page 5
MVX10
IMPORTANT
SAFETY PRECAUTIONS
1. After observation of a specimen that involves the risk of infection, be sure to clean the positions that contacted the
specimen to prevent infection.
· To avoid the risk that the specimen drops and splatters, be sure to remove the specimen before moving the microscope.
· If a specimen is destroyed due to an erroneous operation, immediately take the infection prevention measures.
· The microscope becomes unstable when its height is increased by a mounted attachment. To prevent the specimen
from dropping if the microscope topples down, be sure to take the toppling prevention measures when mounting an
attachment.
2. The applicable ultrahigh-pressure mercury burners are the USH-103OL (OLYMPUS) and HBO103W/2 (OSRAM), both of
which are DC mercury burners available from Olympus.
3. Ensure that the burners are mounted and that the cords are connected securely.
4. The inside of the lam housing is very hot while the burner is on and immediately after it is turned off. Do not open the lamp
housing in these periods (see page 42).
5. The power supply unit contains high-voltage parts inside. Do not attempt to disassemble it.
6. Always use the power cord provided by Olympus. If no power cord is provided, please select the power cord by referring
to the section “PROPER SELECTION OF THE POWER SUPPLY CORD” at the end of this instruction manual. If the proper
power cord is not used, Olympus can no longer warrant the electrical safety performance of the equipment.
Before plugging the power cord into the wall outlet, ensure that the main switch of the power supply unit is set to “ ”
(OFF).
7. Always ensure that the grounding terminal of the power supply unit is properly grounded. If the equipment is not grounded,
Olympus can no longer warrant the electrical safety performance of the equipment.
8. Before opening the lamp housing for replacing the burner, be sure to set the main switch to “ ” (OFF), unplug the lamp
housing output connector of the power supply unit and wait at least 10 minutes or until the burner and lamp housing
have cooled down.
9. The top of the lamp housing becomes very hot. To avoid the risk of a fire, never block the ventilation of this part.
Make sure you leave a space of 10 cm or more around the lamp housing and power supply unit to dissipate heat.
10. The power cord is also used to shut off the power supply unit in case of emergency by unplugging. To facilitate this,
locate the power supply unit or wall outlet so that the power cord connector (on the rear of the power supply unit) or the
wall outlet is easily accessible in case of emergency.
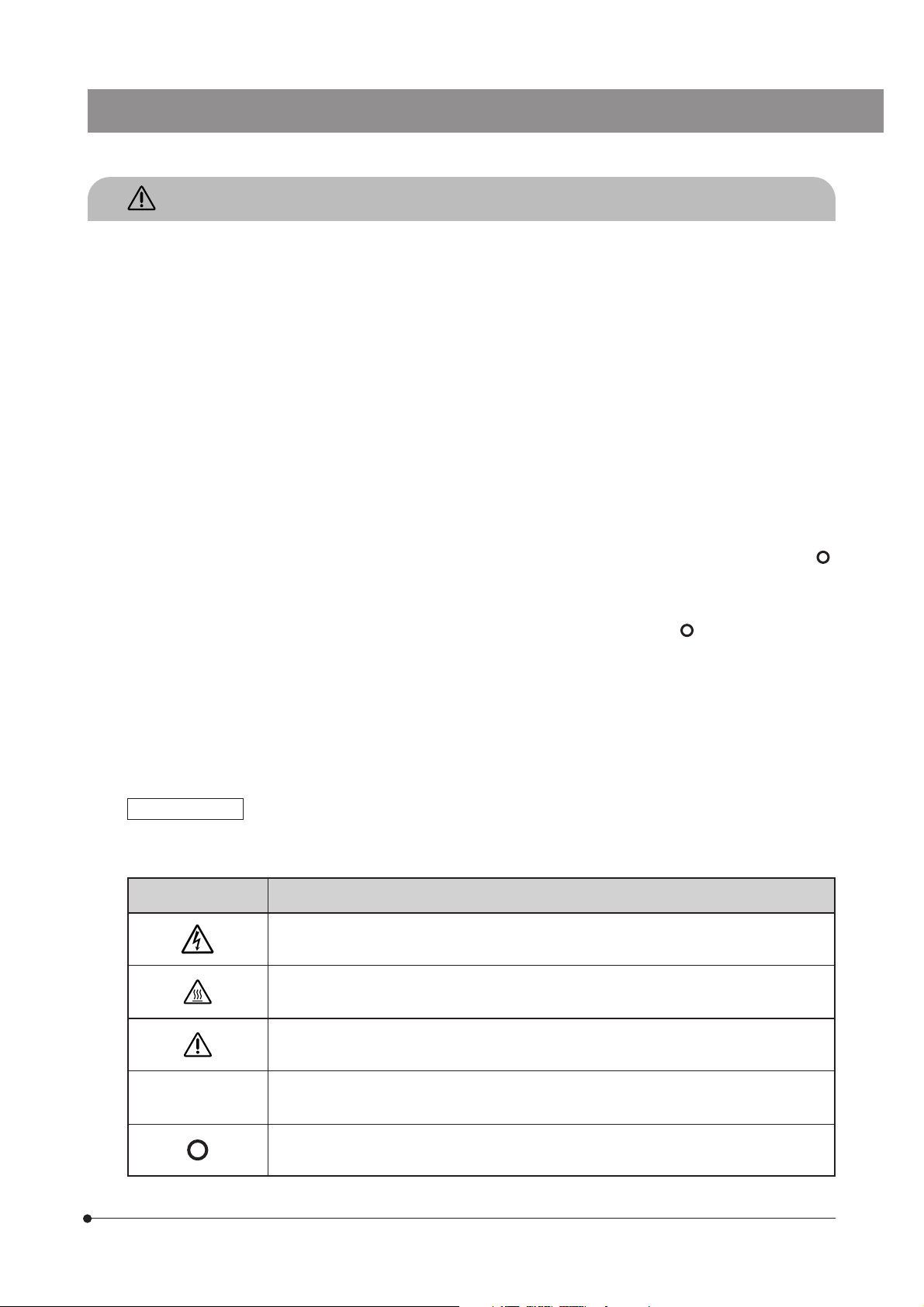
Safety Symbols
The following symbols are found on the microscope. Study the meaning of the symbols and always use the equipment
in the safest possible manner.
Symbol
l
Indicates the presence of a high voltage (1 kV or more), which should not be touched to
prevent an electric shock.
Indicates that the surface becomes hot, and should not be touched with bare hands.
Before use, carefully read the instruction manual. Improper handling could result in injury to
the user and/or damage to the equipment.
Indicates that the main switch is ON.
Indicates that the main switch is OFF.
Explanation
1
Page 6
Caution indications
Caution indications are affixed at parts where special precaution is required when handling and using the microscope.
Always heed the cautions.
· Lamp housing
Caution indication
positions
Getting Ready
1
}This instruction manual pertains only to the operating procedures of the Research Macro Zoom System. Also read the
instruction manuals for the modules used in combination with the microscope system so that you can understand the
comprehensive operating procedures of the system.
(U-LH100HG, U-LH10HGAPO)
· Power supply unit [High voltage caution]
1. A microscope is a precision instrument. Handle it with care and avoid
subjecting it to sudden or severe impact. Also be careful in handling
the limiting or stopper mechanisms because an excessive force may
destroy them.
2. Do not use the microscope where it is subjected to direct sunlight, high
temperature and humidity, or vibration.(For the operating environment,
see Section 8, “SPECIFICATIONS” on page 22.)
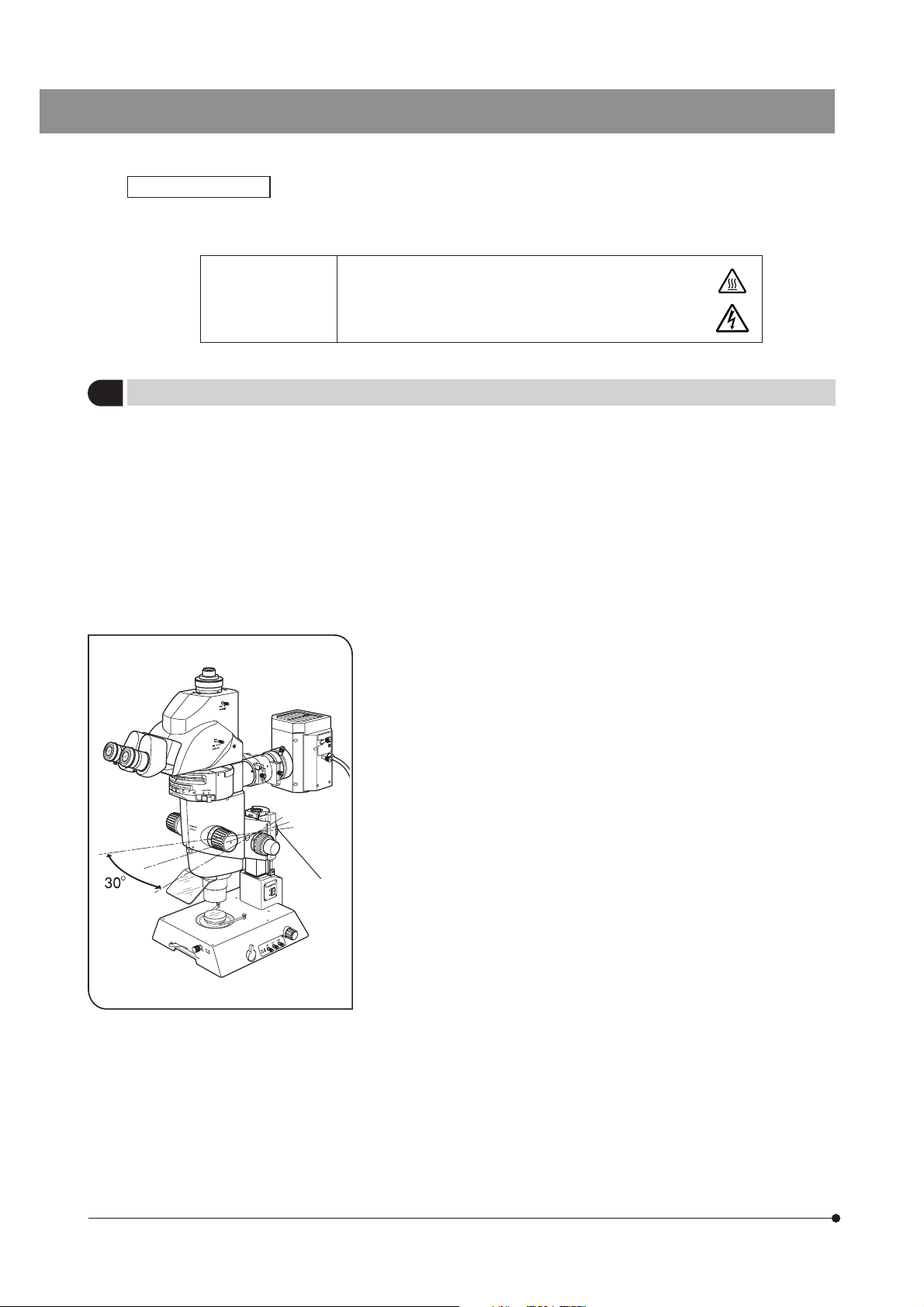
3. To prevent the microscope body from turning over, its pivot angle
must be limited to 30° and the tilt angle of the desk surface must
be limited to 5° as shown in Fig.1.
4. Care is required when using an auxiliary pillar (SZH-P400/P600) because
it increases the height of the microscope body and makes it unstable.
5. To adjust the microscope body height, be sure to hold the focusing
assembly with one hand and then loosen the focusing assembly
clamping knob @. (Fig. 1)
(Use the drop prevention collar (SZX-R) to prevent a hazard from
occurring.)
Be careful not to pinch your finger during adjustment.
[High temperature caution]
2
@
Fig. 1
Page 7
MVX10
6. Observe the following cautions when operating the coarse focus
adjustment knobs or the zooming knobs.
²
³
Fig. 2
|
@
Operation
Focusing Coarse focus
Zooming
7. Before moving the microscope, detach the modules including the tilting
trinocular head and lamp housing to reduce the total weight. Then hold
it by the base, not by the zoom microscope body.
Manipulated
Controls
adjustment
knobs @ (Fig. 2)
Zooming knobs
² (Fig. 2)
Caution
1. If the knob hits the upper or lower limiting mechanism violently or it is rotated
after it hits a limiting mechanism, the
internal mechanism may be damaged.
2. If the knobs on the left and right are
rotated in opposite directions, the internal mechanism will be damaged.
(The rotation tension of the knob
should be adjusted using the rotation
tension adjustment ring ³ on the
knob. See page 10.)
1. If the knob hits the upper or lower limiting mechanism violently or it is rotated
after it has hit a limiting mechanism,
the internal mechanism may be damaged.
2. If the knobs on the left and right are
rotated in opposite directions, the internal mechanism will be damaged.
Maintenance and Storage
2
1. To clean the lenses and other glass components, simply blow dirty away using a commercially available blower and
wipe gently using a piece of cleaning paper (or clean Gauze).
If a lens is strained with fingerprints or oil smudges, wipe it gauze slightly moistened with commercially available
absolute alcohol.
Since the absolute alcohol is highly flammable, it must be handled carefully.
Be sure to keep it away from open flames or potential sources of electrical sparks - for example, eletrical equipment that is being switched on or off.
Also remember to always use it only in a well-ventilated room.
2. The equipment uses plastic resins extensively in its external finish. Do not attempt to use organic solvents to clean the
non-optical components of the microscope. To clean these components, use a lint-free, soft cloth lightly moistened with
a diluted neutral detergent.
3. Never disassemble any part of the microscope as this could result in malfunctions or reduced performance.
4. When not using the microscope, keep it covered with the dust cover provided. Ensure that the lamp housing is cool
before covering the microscope.
5. When the hour counter on the power supply unit indicates 300 hours (USH-103OL, HBO103W/2), set the main switch
to “ ” (OFF) for safety, wait for more than 10 minutes and then replace the burner (see page 42). Unlike the fluorescent
lamps, the mercury burner seals high-pressure gas inside. If it used after the specified service life has been exceeded,
the glass tube may be distorted accumulatively and may eventually burst, though this happens very rarely. The used
mercury burner should be disposed of as an industrial waste. If you cannot dispose of it properly, contact Olympus.
6. When disposing of the microscope. Check the regulations and rules of your local government and be sure to observe
them. To dispose of only the gas spring (|, Fig. 2) used in the counterbalance of the focusing assembly, follow the
precautions provided with the gas spring.
3
Page 8
Caution
3
If the microscope is used in a manner not specified by this manual, the safety of the user may be imperiled. In addition,
the microscope may also be damaged. Always use the microscope as outlined in this instruction manual.
The following symbols are used to set off text in this instruction manual.
: Indicates that failure to follow the instructions in the warning could result in bodily harm to the
user and/or damage to equipment (including objects in the vicinity of the equipment).
# : Indicates that failure to follow the instructions could result in damage to equipment.
} : Indicates commentary (for ease of operation and maintenance).
4
Page 9
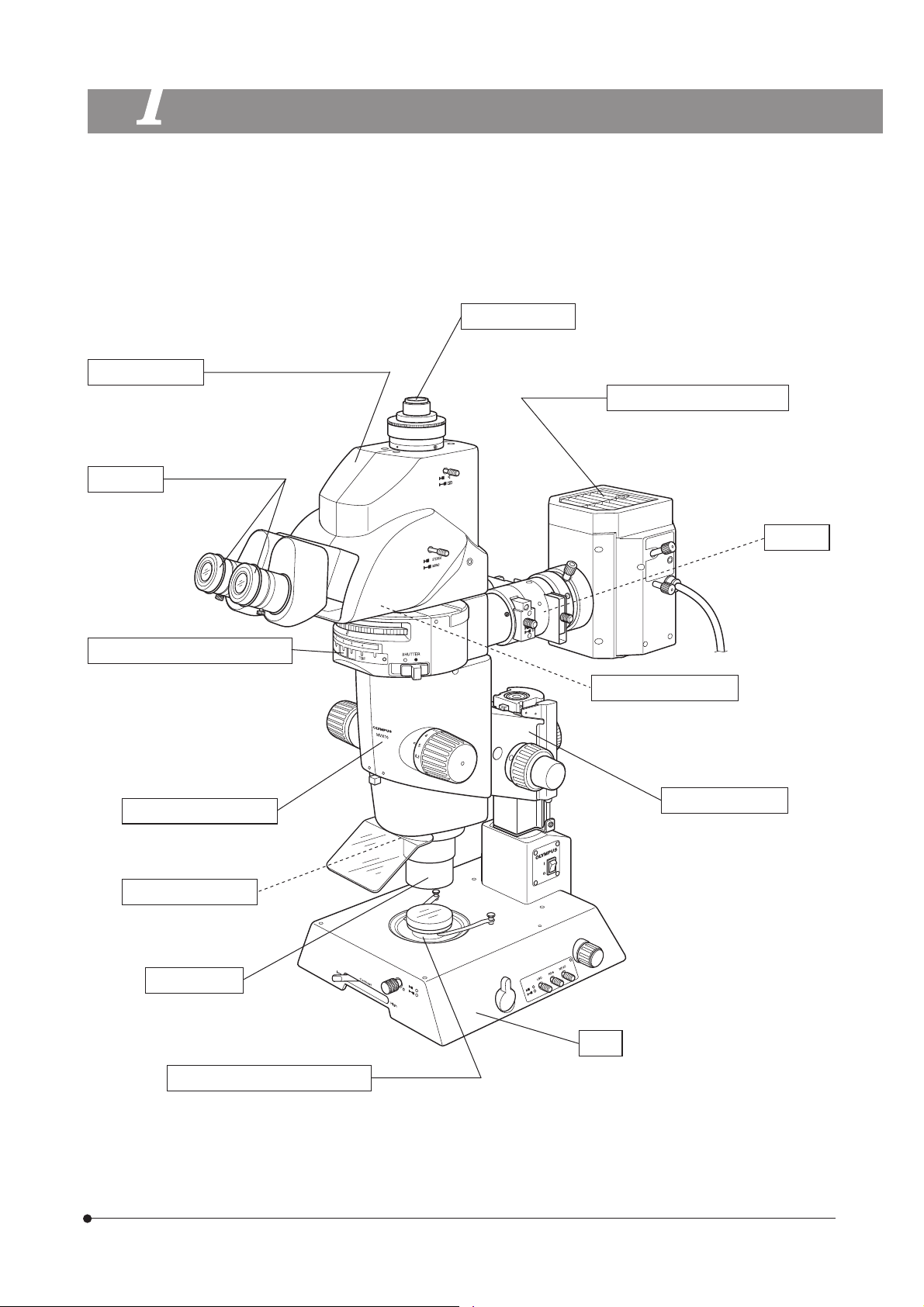
MVX10
1
}The module names listed below are typical ones that can be used with the system, and the illustration shows the system
composed of modules marked {. Certain other modules are also usable even when they are not mentioned below. For
these modules, refer to the latest catalogues or contact Olympus.
For the modules marked *, refer to their instruction manuals.
Observation Tube
{ Tilting Trinocular Head: MVX-TTRS
· Tube Lens Unit: MVX-TLU
Eyepieces
{ WHN10X-H
NOMENCLATURE
C-Mount Adapter
{ MVX-TV 1XC
· MVX-TV 0.63XC
High-Intensity Lamp Housing
{ U-LH100HG (for mercury burner)
· U-LH100HGAPO (for mercury burner)
· U-LH75XEAPO* (for xenon burner)
ND Filter
· 32ND6
· 32ND12
· 32ND25
· 32ND50
Coaxial Fluorescence Illuminator
{ MVX-RFA
<< Fluorescence mirror units >>
· U-MCFPHQ/XL
· U-MGFPHQ/XL
· U-MYFPHQ/XL
· U-MRFPHQ/XL
· U-MGFP/XL
· U-MGFPA/XL
Zoom Microscope Body
{ MVX-ZB10
Revolving Nosepiece
· MVX-2RE
Objective Lens
{ MVPLAPO 1X
· MVPLAPO 0.63X
· MVPLAPO 2XC
Stage Glass & base accessories
{ Stage Glass: SP-C
· Fluorescence Center Plate: SP-FL
· Stage Plate (B&W): SP-BW-2
· Base accessories
Magnification Changer
· MVX-CA2X
Focusing Assembly
{MVX-FOF
Base
{ Transmitted Illumination Base : SZX-ILLB2*
· Transmitted Illumination Base : SZX-ILLD2*
· Transmitted Illumination Base : SZX-ILLK*
· Large Base : SZX-STL
5
Page 10
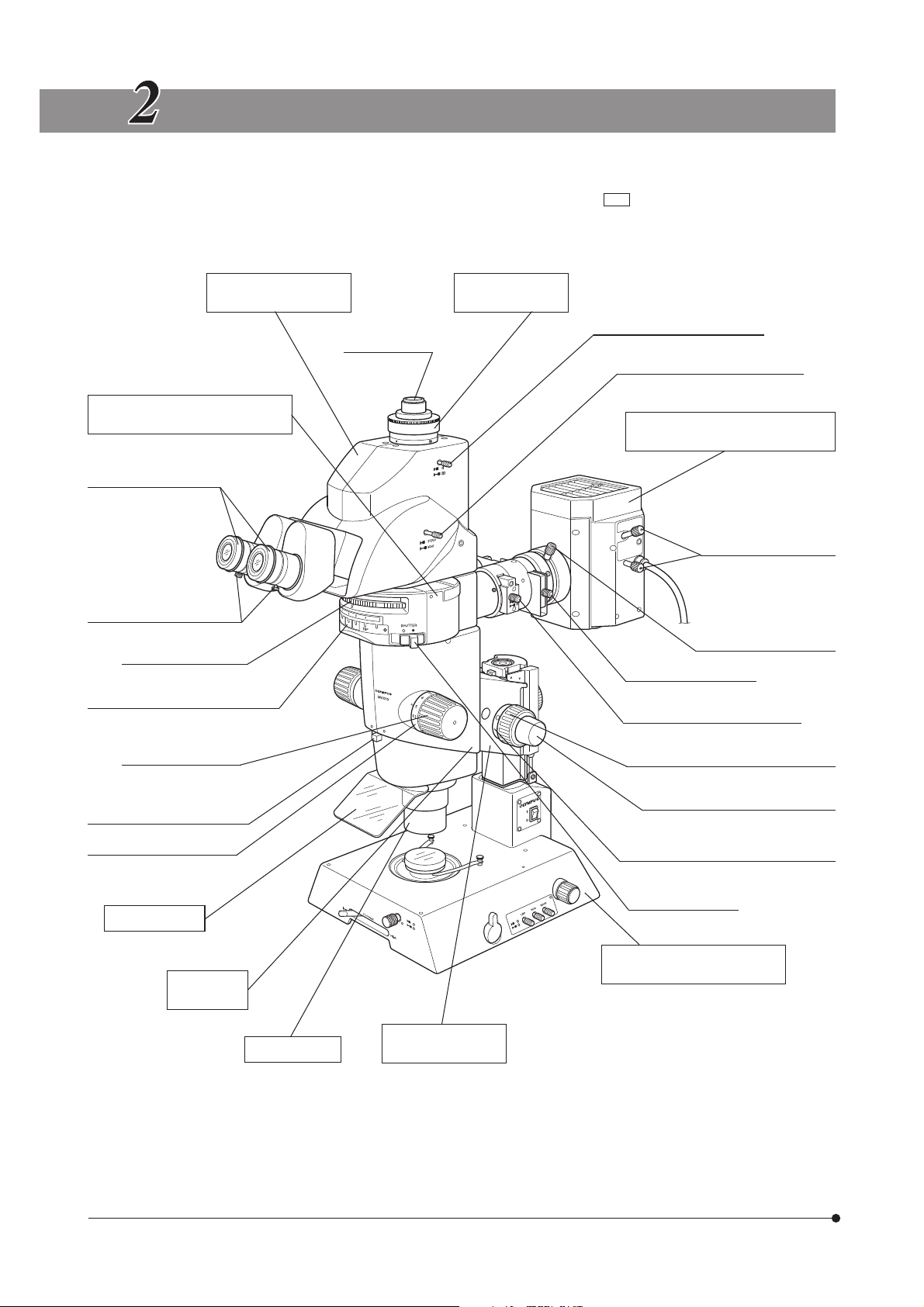
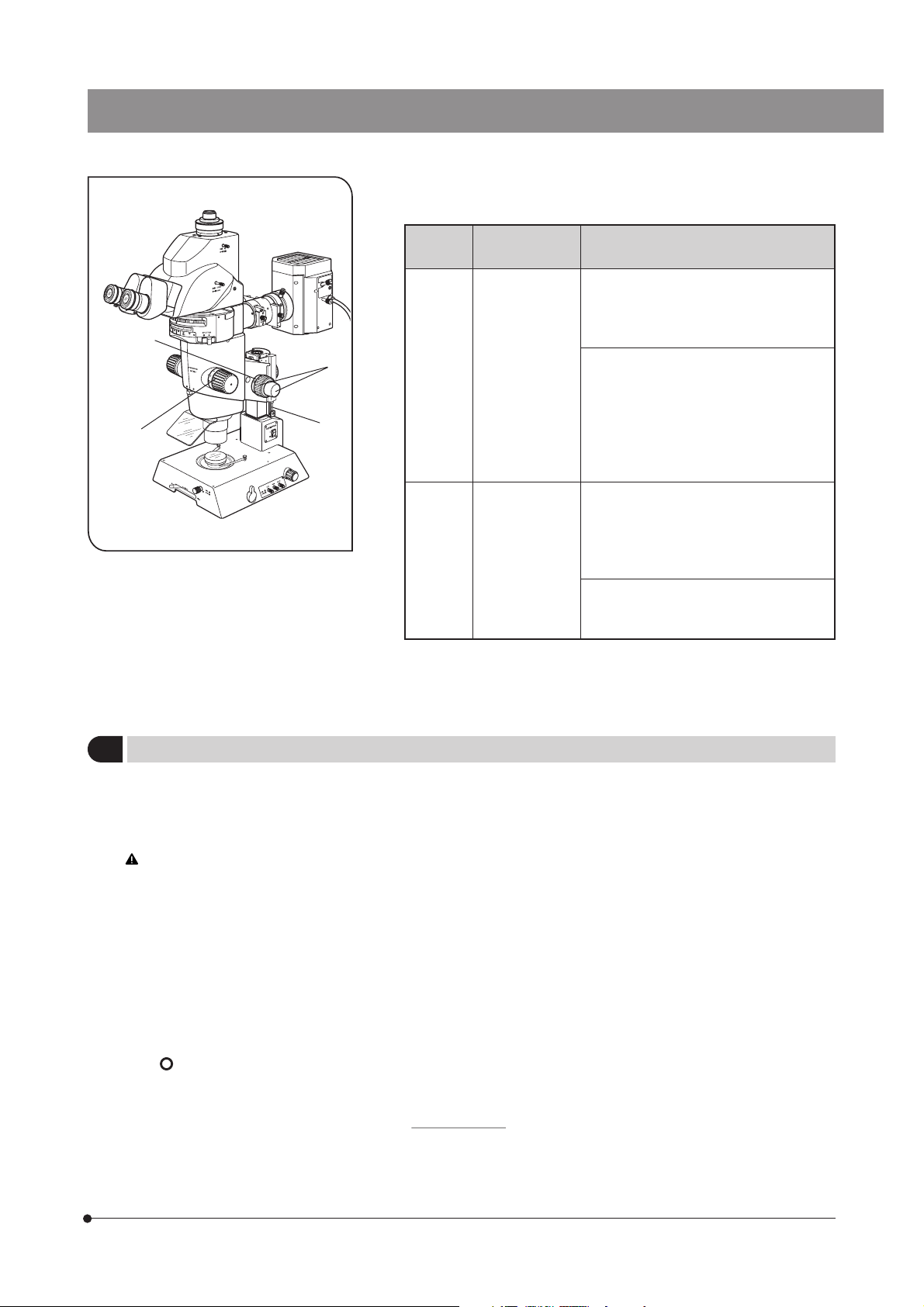
CONTROLS
}If the microscope is not yet assembled, go to Chapter 11, “ASSEMBLY” (pages 36 to 45).
The following illustration shows the systems with the modules enclosed in . For other ancillary modules, see
subsequent pages.
Tilting Trinocular Head
MVX-TTRS
Coaxial Fluorescence Illuminator
MVX-RFA
Diopter adjustment ring
(P. 16)
Eyepiece clamping knobs
Mirror unit turret (P. 12)
Mirror unit indicator pocket (P. 38)
C-Mount Adapter
MVX-TV 1XC
Light path selector lever (P. 17)
C-mount (P. 18)
Mono/stereo selector lever (P. 17)
Lamp Housing for Mercury Burner
U-LH100HG/U-LH100HGAPO
Burner centering knobs
(P. 44)
Collector lens focusing
knob (P. 44)
Filter slider knob (P. 15)
Field iris diaphragm lever (P. 14)
Zooming knob (P. 16)
Aperture iris diaphragm knob
(P. 11)
Zoom ratio indicator (P. 11)
UV shield plate
Zoom Body
MVX-ZB10
MVX objective
Coarse focus adjustment knob (P. 10)
Fine focus adjustment knob (P. 10)
Coarse focus adjustment knob rotation
tension adjustment ring (P. 10)
Shutter knob (P. 14)
Transmitted Illumination Base
SZX-ILLB2
(Refer to the separate instruction manual.)
Focusing Assembly
MVX-FOF
6
Page 11

MVX10
Fluorescence mirror units
U-MCFPHQ/XL
U-MGFPHQ/XL
U-MYFPHQ/XL
U-MRFPHQ/XL
U-MGFP/XL
U-MGFPA/XL
Power Supply Unit
U-RFL-T
}For details, see the instruction manual provided with the U-RFL-T.
}Up to three fluorescence mirror units can be mounted by placing them
in every other dovetail of the six dovetails of the MVX-RFA.
#Each fluorescence mirror unit is composed of the dichroic mirror,
barrier filter and excitation filter that match a specific fluorescent
protein.
}When fabricating an original fluorescence unit by your own, it is
recommended to use the U-MF/XL mirror unit frame (which does
not incorporate a filter). (P. 39)
Use the blank indicator sheets provided with the illuminator to write the
original mirror unit name.
Lamp housing connector
Hour counter
Lamp ON LED
Main switch
I : ON
: OFF
Xenon Lamp Housing
U-LH75XEAPO
Power Supply Unit
U-RX-T
}For details, see the instruction manual provided
with the U-LH75XEAPO and U-RX-T.
Power cord connector
Tube Lens Unit
MVX-TLU
C-mount adapter mount dovetail
Mount dovetail
7
Page 12
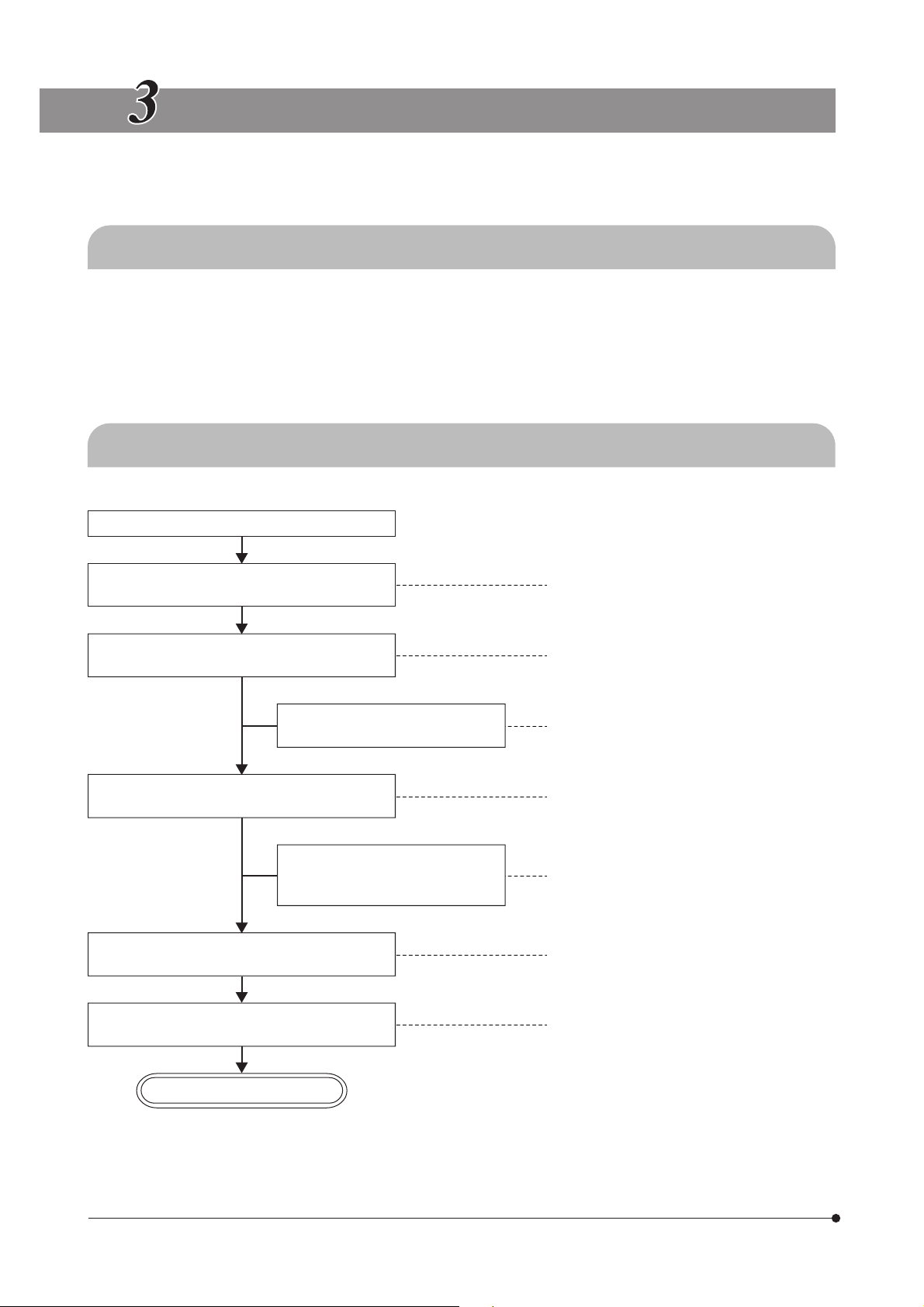
SUMMARY OF REFLECTED FLUORESCENT LIGHT OBSERVATION PROCEDURE
}For the observation procedures of other methods, see page 20.
3-1 Preparation
1. Set the shutter knob to “{” to close the shutter. (Page 14)
2. Mount the fluorescence mirror unit(s) that match the desired observation. (Page 38)
3. Turn the mirror unit turret to engage the desired fluorescence mirror units in the light path. (Page 12)
4. Turn on the mercury burner and wait until the arc stabilizes (for about 5 to 10 minutes).
(If the burner is not centered, center it.) (Page 14)
3-2 Observation Procedure
(Controls) (Page)
Place the specimen on the base.
(P. 10)
Engage the optimum mirror unit for the specimen in the light path.
Set the shutter knob to “¦” to open the shutter, and bring the specimen into focus.
Engage the ND filter in the light
path as required.
Adjust the field of view so that it is uniform
and brightest.
Adjust the tilt.
Adjust the interpupillar distance.
Adjust the diopter.
Open the field iris diaphragm and adjust the
aperture iris diaphragm.
Set the zooming knob for the desired zoom ratio
and bring the specimen into focus.
@ Mirror unit turret (P. 12)
² Shutter knob (P. 14)
³ Coarse/fine focus adjustment knobs (P. 10)
| ND filter (P. 15)
ƒ Collector lens focusing knob (P. 44)
… Binocular assembly (P. 15)
… Binocular assembly (P. 16)
† Diopter adjustment ring (P. 16)
‡ Field iris diaphragm lever (P. 14)
Š Aperture iris diaphragm knob (P. 11)
‰ Zooming knob (P. 16)
³ Coarse/fine focus adjustment knobs (P. 10)
START OF OBSERVATION
}To interrupt observation for a short period, close the shutter. ² Shutter knob (P. 14)
8
Page 13
MVX10
†
@
…
²
ƒ
‡
|
³
Š
}Make a photocopy of this spread and post it near the microscope for quick reference.
‰
9
Page 14
OPERATION
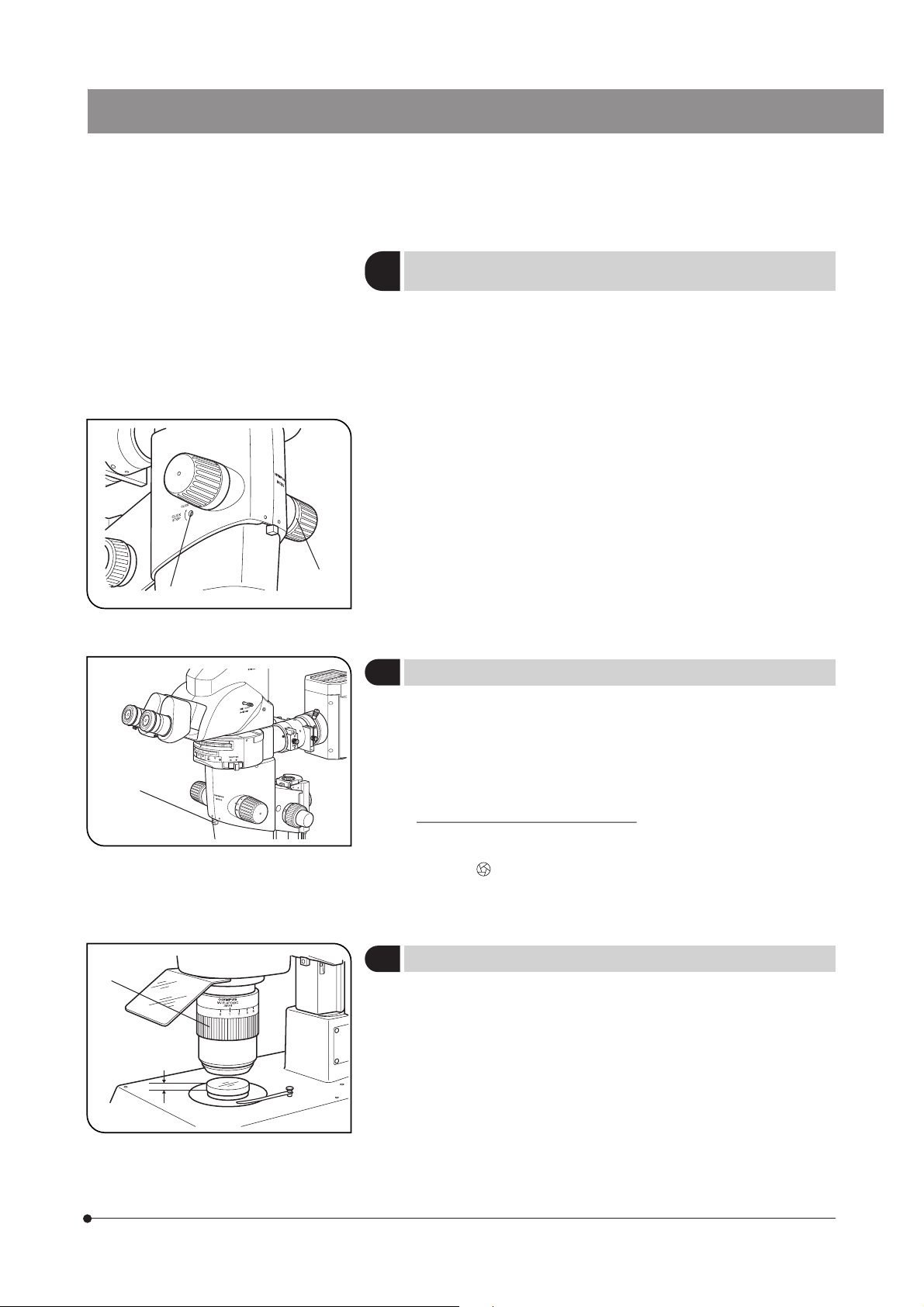
4-1 Base
Using the Stage Plate
1
1. When performing reflected light observation, place the stage plate with the white or black side up according to the
specimen.
2. When performing transmitted light observation, use the transparent stage glass.
Placing the Specimen
2
Place the specimen on the approximate center of the stage plate (or stage glass). Hold the specimen with the specimen
holder as required.
4-2 Microscope Body and Focusing Assembly
|
³
Fig. 3
@
²
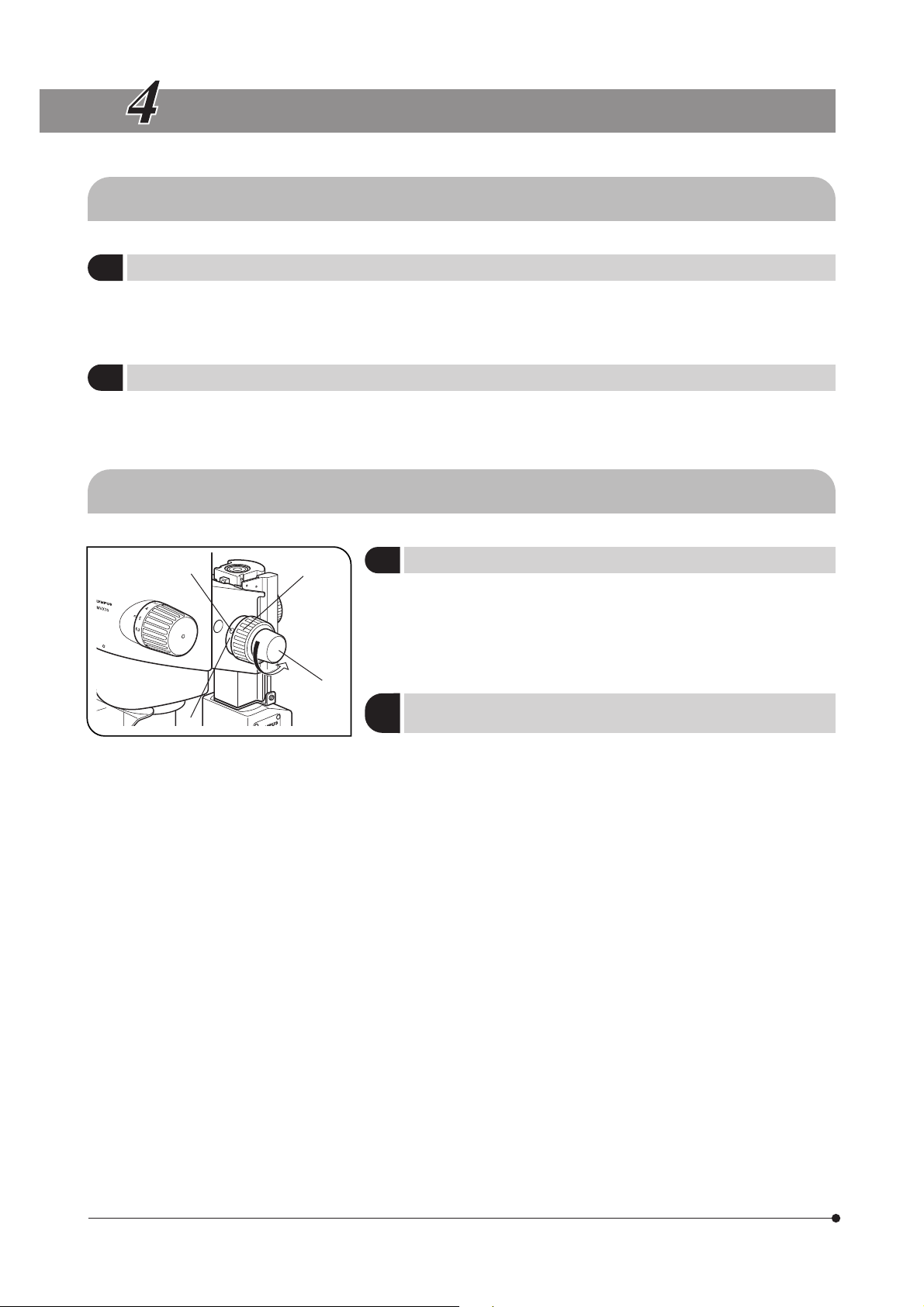
Adjusting the Focus
1
With both the coarse focus adjustment knob @ and fine focus adjustment knob ², rotating the knob in the direction of the arrow lowers the
microscope body.
· Stroke per turn of the coarse focus adjustment knob: 36. 8mm.
· Stroke per turn of the fine focus adjustment knob : 1.5 mm.
Adjusting the Rotation Tension of the
2
Coarse Focus Adjustment Knob
#The rotation tension of the coarse focus adjustment knob can be
adjusted with the rotation tension adjustment ring ³. Do not rotate
the knobs on the left and right in opposite directions, for this will
damage the internal mechanism.
}This adjustment is intended to facilitate the rotation of the knob while
preventing the microscope body from lowering spontaneously. For best
ease of use, it is recommended to adjust the rotation tension slightly
tighter than the level at which spontaneous lowering of the microscope
body occurs.
1. Rotate the rotation tension adjustment ring ³ by inserting the Allen
screwdriver into the hole | on the ring periphery.
Rotating the ring clockwise increases the rotation tension of the coarse
focus adjustment knob, and rotating counterclockwise decreases it.
#If the microscope body falls down by its own weight or the focus
obtained by fine focusing is lost immediately, the rotation tension
adjustment may be too light. In this case, rotate the ring clockwise
to increase the rotation tension.
#If the rotation tension adjustment is too tight, delicate focusing
will be impossible and the knob may be damaged. Particularly,
never rotate the fine focus adjustment knob quickly while its
rotation tension is extremely tight.
(Fig. 3)
(Fig. 3)
10
Page 15
MVX10
@
@
Fig. 4
Fig. 5
²
Engaging and Disengaging the
3
Zooming Knob Click Stop Position
}When the click stop knob is engaged to ON, the click stop function is
engaged for each zoom ratio indicated with the zooming knob. When
the knob is disengaged to OFF, the zoom magnification can be varied
continuously and finely near the click groove.
}Click stop can be provided for the nine zoom ratios from 0.8 to 5. The
click stop is engaged when the microscope leaves the factory.
1. To disengage the click stop function, rotate the click stop ON-OFF screw
@ counterclockwise (by about two turns from the ON position, in the
direction opposite to the arrow) using the Allen screwdriver.
#Do not rotate too much, or the cover may be damaged.
2. To engage the click stop function, rotate the click stop ON-OFF screw @
fully clockwise (in the direction of the arrow) using the Allen screwdriver.
The zooming knob then stops at every position corresponding to the
zoom ratio indicated on the zoom ratio indicator ².
Adjusting the Aperture Iris Diaphragm
4
}Adjusting the aperture iris diaphragm improves the insufficiency of
brightness in the peripheral section light at intermediate zoom ratios
as well as the depth of focus.
However, setting the aperture iris diaphragm too narrowly degrades
resolution.
When performing fluorescent light observation, it is recommended to
open the aperture iris diaphragm fully for bright observation.
1. Adjust the aperture iris diaphragm knob @ to the left or right.
Rotating the ring toward the left “¦” opens the aperture; rotating it toward
the right “ ” closes it.. Adjust while monitoring the observed image to
confirm the contrast and focal depth improvement effects.
# Do not close the aperture too much, for this may cause degradation in
resolution and/or insufficiency of brightness in the peripheral section.
(Fig. 4)
(Fig. 5)
@
Water depth 5 mm
or less
Fig. 6
Using the Objective Correction Collar
5
}The MVPLAPO 2XC objective is equipped with a correction collar @,
which corrects the aberration produced by a medium such as water or
plastic container.
When observing the specimen through a liquid or petri dish cover, rotate
the correction collar to find the position that provides the best contrast,
· Correction is possible for aberrations equivalent to a water depth of about
5 mm.
· The effect of the correction collar may be less sensible when the zoom
ratio is low or the aperture iris diaphragm is stopped down.
}When the MVX-2RE revolving nosepiece is used, hold the objective so
that the revolving nosepiece does not displace from the position when
turning the correction collar @.
(Fig. 6)
11
Page 16
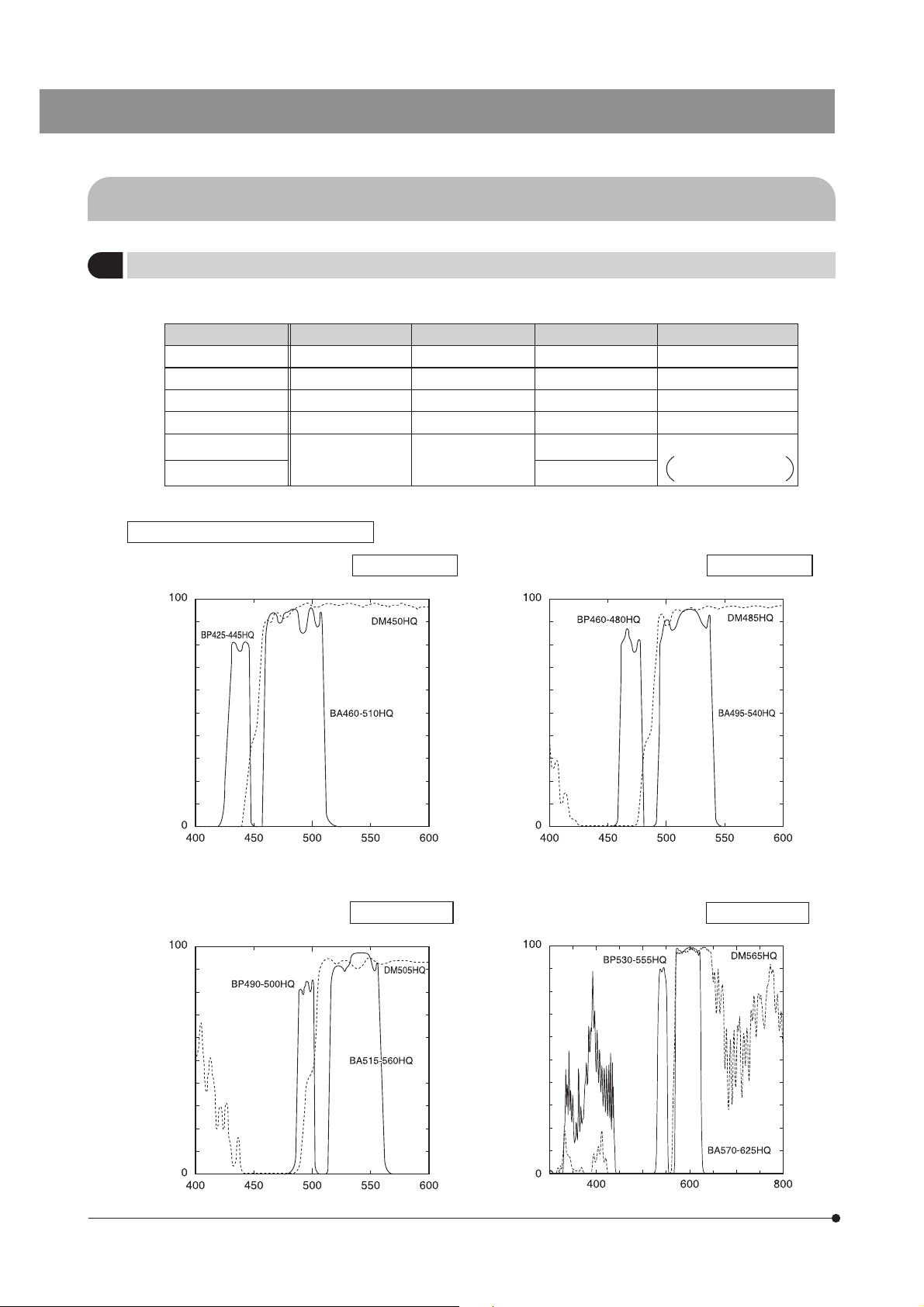
4-3 Coaxial Fluorescence Illuminator
Selecting the Fluorescence Mirror Unit
1
}Use the fluorescence mirror unit that is optimum for each purpose of observation.
Mirror unit name Dichroic mirror Excitation filter Barrier filter Application
U-MCFPHQ/XL DM450HQ BP425-445HQ BA460-510HQ ECFP
U-MGFPHQ/XL DM485HQ BP460-480HQ BA495-540HQ EGFP
U-MYFPHQ/XL DM505HQ BP490-500HQ BA515-560HQ EYFP
U-MRFPHQ/XL DM565HQ BP530-555HQ BA570-625HQ RFP
U-MGFP/XL
U-MGFPA/XL BA510-550
Spectral Characteristics of the Filters
1. Filter for fluorescent protein CFP U-MCFPHQ/XL 2. Filter for fluorescent protein GFP U-MGFPHQ/XL
DM505 BP460-490
BA510IF
EGFP
U-MGFPA/XL:
For dye separation
Transmittance %
Wavelength nm
3. Filter for fluorescent protein YFP U-MYFPHQ/XL 4. Filter for fluorescent protein RFP U-MRFPHQ/XL
Transmittance %
Wavelength nm
Transmittance %
Wavelength nm
Transmittance %
Wavelength nm
12
Page 17

MVX10
5. IB excitation filter (wide band) U-MGFP/XL
Transmittance %
Wavelength nm
6. IB excitation filter (wide band) U-MGFPA/XL
Transmittance %
Wavelength nm
13
Page 18

Turning the Burner ON
2
#The burner should be centered before the first use after it has been
mounted or replaced. (see page 45) When resetting the burner hour
counter, press and hold the reset button until the reading becomes
“0.0”.
Set the main switch of the power supply unit to “ ” (ON). The arc image
of the burner will stabilize in 5 to 10 minutes after the burner is ignited.
}A discharge-type mercury burner may not turn on by the first try due to
the characteristics of the burner. If a burner does not turn on, set the main
switch to “ ” (OFF), wait for 5 to 10 seconds and set the main switch to
“ ” (ON) again.
#To avoid shortening the service life of the burner, do not turn a burner
off for 2 hours after turning it on but use the shutter in this period.
#When turning on a mercury burner that has been turned off, wait for
about 10 minutes after it is turned off, because it cannot be turned
on unless the mercury vapor in the burner has cooled down and
liquefied.
}If the lamp housing is opened while the burner is on, the power supply
stops to ensure safety. In this case, set the main switch to “ ” (OFF) and
wait for more than 10 minutes before retrying to turn it on again. Do not
open the lamp housing unless it has cooled down sufficiently.
@
Fig. 7
Fig. 8
@
Opening/Closing the Shutter
3
}The shutter can be used to close the shutter temporarily when changing
the mirror unit turret or interrupting observation for a short period.
1. When the shutter knob @ is set to “{”, the shutter is engaged to shut off
the excitation light.
2. When the shutter knob @ is set to “¦”, the shutter is disengaged and
opened.
Using the Field Iris Diaphragm
4
}The centering of the field iris diaphragm can be adjusted (see page 45).
}Push in or pull out the field iris diaphragm lever @ as required to prevent
color fading due to fluorescence in parts other than the observed region.
(Fig. 7)
(Fig. 8)
14
Page 19

MVX10
@
Fig. 9
4-4 Observation Tube
Switching the Filter Slider Knob
5
}Hold the filter slider knob @ of coaxial fluorescence illuminator and move
it toward the left or right to select one of the two filter pockets or idle
position.
The idle position is selected when the knob is pulled to the rightmost
stop position. Pushing the knob at the click position one step after the
idle position selects filter pocket 1, and pushing it further to the leftmost
stop position selects filter pocket 2.
To adjust the brightness using filters, mount the required ND filter
(32ND6, 32ND12, 32ND25, 32ND50) or frost filter (32FR80) in the filter
pockets and select the desired filter pocket with the filter slider knob.
When the mercury burner has been turned on for an extended
period, the metallic part of the filter slider knob and the ND filter
get very hot. Be careful when switching the filter slider knob or
changing the filter.
Adjusting the Tilt
1
}Adjust the height and tilt of the eyepieces of the binocular assembly to
the most comfortable viewing position.
Holding the binocular assembly with both hands, raise or lower it to the
desired position.
#Do not attempt to force the binocular assembly past the upper or
lower stop positions. Applying excessive force could destroy the
mechanism.
(Fig. 9)
(Fig. 10)
Fig. 10
15
Page 20

@
Fig. 11
Adjusting the Interpupillar Distance
2
#Be sure to hold the binocular assembly @ with both hands to
make this adjustment.
While looking through the eyepieces, hold the left and right of the binocular assembly @ and adjust by opening or closing it for binocular
vision until the left and right fields of view coincide completely.
(Fig. 11)
|
³
@
²
Fig. 12
Adjusting the Diopter
3
(Zoom Parfocality Adjustment)
}Make sure that the eyepiece clamping knobs @ are tightened. Adjust-
ment of the diopter according to the user’s eyesight ensures parfocality
even when the zoom magnification is changed.
1. Looking into the right eyepiece, rotate the diopter adjustment ring ² so
that the outer periphery of the field of view is as sharp as possible.
2. Place an easy-to-observe specimen on the stage.
3. Rotate the zooming knob ³ to a low zoom ratio and focus the specimen
using the coarse and fine focus adjustment knobs.
4. Rotate the zooming knob ³ to the highest zoom ratio and focus the
specimen using the coarse and fine focus adjustment knobs.
5. Rotate the zooming knob ³ to the lowest zoom ratio, then focus the
specimen by rotating the left and right diopter adjustment rings |
instead of the coarse and fine focus adjustment knobs.
When Using the Micrometer Eyepiece
1. Look into the micrometer eyepiece and focus on the micrometer scale
by rotating the diopter adjustment rings ².
2. Place an easy-to-observe specimen on the stage.
3. Rotate the zooming knob ³ to a low zoom ratio and focus on the
specimen looking into the micrometer eyepiece and using the coarse
and fine focus adjustment knobs.
4. Rotate the zooming knob ³ to the highest zoom ratio and focus on the
specimen using the coarse and fine focus adjustment knobs.
5. Rotate the zooming knob ³ to the lowest zoom ratio, then focus the
specimen by rotating only the diopter adjustment ring | of the nonmicrometer eyepiece instead of the coarse and fine focus adjustment
knobs.
(Fig. 12)
16
}Note (or memorize) the diopter readings of the left and right eyepiece
scales so that they can be duplicated quickly in the next observation.
Page 21

MVX10
Fig. 13
Fig. 14
²
@
Using the Eye Shades
4
When Wearing Eyeglasses
Use with eye shades in their normal folded-down position. This will prevent eyeglasses from being scratched by the eyepieces.
When Not Wearing Eyeglasses
Extend the folded eyeshades in the direction of the arrow. This makes
observation easier by preventing the inverse incidence of light from between the eyepieces and your eyes.
Using the Eyepiece Micrometer Disk
5
WHN10X-H
1. Unscrew and remove the ring @ from the bottom of an eyepiece.
2. Clean an eyepiece micrometer disk ² ( 24 mm dia., 1.5 mm thick) to
remove dust and dirt, then place the disk into the ring @ so that the
side with reticule ³ faces downward.
3. Attach the ring @ with the eyepiece micrometer disk by gently screwing
it clockwise onto the eyepiece
(Fig. 13)
(Fig. 14)
³
Fig. 15
@
²
Selecting the Light Path
6
Slide the light path selector lever @ to select the desired light path.
Light path selector lever
Pushed in 100% for binocular assembly.
Pulled out
Switching the Monaural/Stereo View
7
}Slide the mono/stereo selector lever ² for stereo observation based on
pupil division.
Mono/stereo selector lever Indication Observation
Pushed in* Stereo
Pulled out Standard
* The brightness of the peripheral section of the field becomes insufficient
when the aperture iris diaphragm knob ³ is stopped down.
Indication Intensity Ratio
100% for TV observation and
photomicrography.
STEREO
MONO
(Fig. 15)
(Fig. 15)
17
Page 22

TV OBSERVATION AND PHOTOMICROGRAPHY
}When TV observation or photomicrography is required, use the MVX-TTRS tilting trinocular head or MVX-TLU tilting lens
unit.
The MVX-TV1XC/TV0.63XC exclusive C-mount adapter can be installed on this microscope.
}When the MVX-TV0.63XC C-mount adapter is used, insufficiency of brightness in the peripheral section may sometimes
be noticeable at low to intermediate zoom ratios. In this case, stop down the aperture iris diaphragm knob on the front
of the microscope body.
Selecting the C-mount Adapter Magnification
1
Set the magnification of the C-mount adapter according to the size of the CCD in the TV camera or digital camera.
The following figures show the TV observation areas when the WHN10X-H eyepieces with a field diameter of 22 are used.
1-in. CCD
2/3-in. CCD
1/2-in. CCD
Field number 22
When the 0.63X C-mount adapter is used
(The field may be cut off when the 1-inch CCD is used)
Attaching the C-Mount
2
@
1. Using the Allen screwdriver, fully loosen the straight tube clamping screw
@ of the straight tube mount on the top of the tilting trinocular head.
2. Fit the round dovetail ² of the C-mount adapter into the straight tube
mount of the trinocular head, and tighten the clamping screw @.
3. Mount a TV camera with C-mount on the C-mount adapter.
Selecting the TV Camera Light Path
3
Pull out the light path selector knob ³ to select the TV & Photo 100%
light path setting.
²
³
Fig. 16
When the 1X C-mount adapter is used
(Fig. 16)
(Fig. 16)
18
Page 23

MVX10
…
²
Fig. 17
Fig. 18
ƒ
@
|
³
|
Adjusting the Parfocality Between
4
Observation Image and Monitor Image
}The parfocality adjustment makes correction of focusing unnecessary
when the observation image is switched to the image monitored with the
TV camera.
1. Looking into the eyepieces, select a high zoom ratio and focus on the
specimen.
2. Select the TV/Photo light path to view the TV monitored image and select
a low zoom ratio.
When Using the MVX-TV0.63XC (Fig. 17)
3. Using the Allen screwdriver, loosen the parfocality adjustment clamping
screw (LOCK) @ on the C-mount adapter.
4. While observing the monitored image, turn the parfocality adjustment
screw (FOCUS) ² slowly to focus on the specimen.
5. When the specimen is focused, tighten the clamping screw @ using the
Allen screwdriver.
When Using the MVX-TV1XC (Fig. 18)
3. Using the Allen screwdriver, loosen the parfocality adjustment clamping
screw ³ and C-mount adapter clamping screw |.
4. While observing the monitored image, hold the upper part ƒ of the Cmount adapter and rotate the lower part … to focus on the specimen.
5. When the specimen is focused, tighten the clamping screws ³| using
the Allen screwdriver.
(Figs. 17 & 18)
Adjusting the TV Camera Angle
1. Loosen only the C-mount adapter clamping screw | and rotate the TV
camera to adjust its angle.
2. After adjusting the angle, tighten the clamping screw |.
19
Page 24

TRANSMITTED LIGHT OBSERVATION
}When an SZX series transmitted illumination base is used, the microscope can be used in transmitted light brightfield/
darkfield, focal light and simplified transmitted polarized light observations.
To perform these observations, rotate the mirror unit turret on the coaxial fluorescence illuminator to select the light path
without fluorescence mirror unit (No. 4/TBF).
The following table shows the main specifications of the transmitted illumination base.
Base
Transmitted Illumination Base
Item
Max. illumination
field diameter
Brightfield observation Available
Darkfield observation Not Available Available
Focal light observation Available Not Available
Simplified transmitted polarized light
observation
Note 1. When the MVPLAPO2XC objective is used and the zoom ratio is 2.5X or less, insufficiency of brightness in the
peripheral section becomes noticeable. To prevent this, place the diffusion plate ( 45 mm, made of plastic)
provided with the microscope body below the stage glass of the SZX transmitted illumination base.
When the SZX-ILLB2 is used and the contrast selector lever is set to the “High” position, irregularities may be
noticeable in the image.
Note 2. To reduce background noise during fluorescence observation, it is recommended to use the SP-BW-2 stage
plate (B&W) or the SP-FL fluorescence center plate (with metallic plate).
However, if transmitted light observation is also necessary, use the SP-C stage glass or the SP-FL fluorescence
center plate (with glass plate) instead, and place the ND6 filter ( 45 mm), provided with the microscope, in the
position for the diffusion plate described in Note 1) above. If both the diffusion plate and ND6 filter are used, they
can be stacked in the position.
SZX-ILLK
40 mm
(Note) The SZX-AN analyzer/SZX-PO polarizer are required.
#The MVPLAPO 2XC objectives cannot be used.
High-Class Transmitted
Illumination Base
SZX-ILLB2
Available
Transmitted Brightfield/
Darkfield Illumination Base
SZX-ILLD2
63mm: Brightfield
33 mm: Darkfield
20
Page 25

MVX10
TROUBLESHOOTING GUIDE
Under certain conditions, performance of this unit may be adversely affected by factors other than defects. If a problem
occurs, please review the following list and take remedial action as needed. If you cannot solve the problem after
checking the entire list, please contact your local Olympus representative for assistance.
Problem Cause Remedy
1. Incomplete binocular vision. Interpupillary distance is not correctly adjusted.
Diopter adjustment is incomplete.
2. Field of view is cut off or illuminated
unevenly.
3. Dust is visible in the field of view. Dust on the specimen. Remove dust.
4. Excessive image contrast.
5. Resolution problems:
· Image is not sharp.
· Insufficient contrast.
6. Specimen image blurs when
zoom ratio is changed.
7. Coarse focus adjustment knobs
rotate with too much resistance.
8. Zoom microscope body drops or
specimen goes out of focus during observation.
9. Image looks doubled. The MVX-CA2X is set to an intermedi-
10. Background noise is noticeable in
the fluorescence.
Stereo observation is performed and the
aperture iris diaphragm is stopped down
excessively.
The trinocular head and intermediate
attachment are not correctly mounted.
Light path selector knob is stopped
midway.
Mirror unit turret or filter slider knob is in
an intermediate position.
Dust on eyepiece. Remove dust.
Aperture is stopped down excessively. Open the aperture to proper diameter.
Objective is not correctly mounted. Mount it correctly until it is stopped.
Dust on objective front lens. Clean lens surfaces.
Dust on top or lower lens of zoom microscope body.
Diopter of the eyepieces is not correctly
adjusted.
Not in complete focus on specimen.
Rotation tension adjustment ring is too
tight or a module is attached on the
microscope body.
Rotation tension adjustment ring is too
loose.
ate position.
The excitation light is too strong. Insert the ND filter and set the excita-
Adjust it correctly.
Complete diopter adjustment.
Open the aperture.
Mount them correctly.
Set correctly to the desired position.
Set them in the correct positions.
Adjust it correctly.
Focus specimen correctly at a high
zoom ratio.
Loosen the ring properly.
Tighten the ring properly.
Push or pull the lever into a stop
position.
tion light to the minimum required
intensity level.
Page
16
16
11
40,41
17
15
3
3
11
37
3
16
16
10
10
26
40
21
Page 26

SPECIFICA TIONS
Item
1. Zoom microscope body
MVX-ZB10
2. Objective
3. Eyepiece
* 24 mm dia., 1.5 mm thick eyepiece
micrometer disk can be inserted.
4. Focusing assembly
MVX-FOF
5. Tilting trinocular head
MVX-TTRS
6. Coaxial fluorescence illuminator
MVX-RFA
7. Lamp housing for mercury burner U-LH100HG: Lamp housing for 100 W mercury burner.
Zoom magnification system.
Zoom drive system: Horizontal knob.
Click stop ON-OFF switchable per zoom ratio.
Zoom ratios: 10 (0.63X to 6.3X).
Zoom ratio indications: 0.63, 0.8, 1, 1.25, 1.6, 2, 2.5, 3.2, 4, 5, 6.3.
Objective mount: Threaded mount
Built-in aperture iris diaphragm.
MVPLAPO 0.63X: NA 0.15, WD 87 mm, FN 22.
MVPLAPO 1X: NA 0.25, WD 65 mm, FN 22.
MVPLAPO 2XC: NA 0.5, WD 20 mm, FN 22., with correction collar.
WHN10X-H*: FN 22, with diopter adjustment ring.
Focusing system: Rack &pinion roller guide (with coarse focus adjustment knob
rotation tension adjustment ring).
With built-in high-load counterbalance.
Coaxial coarse/fine focus adjustment knobs.
Coarse focus adjustment knob stroke: 80 mm
Coarse knob stroke per turn: 36.8 mm.
Fine focus adjustment knob stroke: 80 mm.
Fine knob stroke per turn: 1.5 mm.
Tilting angle: 0° to 23°
Light path selection: 2 steps (Bi 100% or TV/Photo 100%).
Interpupillary distance adjustment: 51 to 76 mm.
Eyepiece clamping knob provided.
Eyepiece: WHN10X-H
Illumination magnification: 1X (FN 22).
Observation switching: Mirror unit turret.
Number of mountable fluorescence mirror units: 3 (with idle positions).
Field iris diaphragm provided (centering possible).
Shutter provided.
Filter slider knob provided (2 filter pockets + 1 idle position).
Available observation methods: Reflected light fluorescence and transmitted light.
U-LH100HGAPO: Apo lamp housing for 100 W mercury burner.
Mercury burner: USH-103OL (OLYMPUS) or HBO103W/2 (OSRAM)
Power Supply Unit: U-RFL-T
Specification
22
8. Operating environment · Indoor use.
· Altitude: Max. 2000 meters (6440 ft/).
· Ambient temperature:5° to 40°C (41° to 104°F).
· Relative humidity: 80% for temperatures up to 31°C (88°F), decreasing linearly
through 70% at 34°C (93°F), 60% at 37°C (99°F) to 50%
relative humidity at 40°C (104°F).
· Supply voltage fluctuations: ±10%.
· Pollution degree: 2 (in accordance with IEC60664).
· Installation (overvoltage) category: II (in accordance with IEC60664)
Page 27

OPTICAL CHARACTERISTICS
MVX10
Total Magnification/Actual Field
Objective WHN10X-H (Field number: 22)
MVPLAPO 0.63X 4X to 40X 55.4 to 5.54
MVPLAPO 1X 6.3X to 63X 34.9 to 3.49
MVPLAPO 2XC 12.5X to 125X 17.4 to 1.74
· Total magnification = Objective power x Zoom ratio x Eyepiece magnification
· Actual field area =
Total magnification Actual field (mm)
Objective power x Zoom ratio
Eyepieces
Eyepiece FN
23
Page 28

OPERATION OF OTHER MODULES
10-1 Revolving Nosepiece MVX-2RE
}The revolving nosepiece accepts two MVPLAPO series objectives to enable observation using a wider variety of magni-
fications. The objectives can be switched quickly by simply rotating the revolving nosepiece horizontally.
External View
1
Hand guard
Allen wrench
(for M3 screws)
Clamping screws
M3, 6 mm long (x 4)
Nosepiece mount
Objective mounts
2
Assembly
²
@
(Fig. 19)
1. Remove the trinocular head and coaxial fluorescence illuminator from
the zoom microscope body.
2. Remove the zoom microscope body and focusing assembly from the
pillar, and place them upside down on a flat desk surface.
#Place a soft sheet made of rubber or similar material on the desk
surface.
3. Remove the objective from the zoom microscope body and then remove
the objective mount ² by loosening the four clamping screws @ using
the provided Allen wrench (for M3 screws). (Fig. 19)
Fig. 19
24
Page 29

ƒ
|
Fig. 20
³
4. Place the nosepiece mount ³ (with its objective mounts | facing
…
upward) where the objective has been by aligning the screw holes.
Using the Allen wrench (for M3 screws), clamp the nosepiece mount with
the provided four clamping screws (M3, 6 mm long) ƒ. (Fig. 20)
#As the screw holes may be hidden behind the objective mounts |,
clamp the nosepiece mount while rotating it. (Fig. 20)
# Before installing the focusing assembly on the pillar, adjust the coarse
and fine focus adjustment knobs so that the hand guard … does not
protrude from the lower end of the focusing assembly †. (Fig. 21)
5. Install the focusing assembly on the pillar, and install the observation
tube and coaxial fluorescence illuminator which have been removed
above in the original positions.
6. Attach two objectives onto their respective objective mounts | by
screwing. (Fig. 20)
MVX10
†
…
Fig. 21
Operation
3
Hold the objectives and gently rotate the nosepiece until a click position
where the objective to be used is engaged in the light path.
Fig. 22
Caution
4
#When transporting the microscope, do not hold it by the revolving nosepiece.
#The parfocal property when the objective is switched is not completely guaranteed.
#The revolving nosepiece cannot be used in combination with the SZ2-FO stage (because the objectives interfere
with the stage).
(Fig. 22)
25
Page 30

10-2 Magnification Changer MVX-CA2X
}When the magnification changer has been installed on the MVX-TTRS tilting trinocular head by the dealer, the
observation magnification can be switched between 1X and 2X with a selector lever.
Pushed-in position: 1X
Pulled-out position: 2X
Be sure to push in or pull out the selector lever in a position where it is stopped securely.
#Not to spoil the optical performance, always have your dealer perform the assembly work.
Assembly (Operation performed by the dealer)
#The MVX-CA2X is shipped with grease applied on it. Be careful not to stain the lenses with the grease during
assembly.
@ Using the Allen wrench, remove the four AB3
MVX-TTRS
³
|
Cover
ƒ
Magnification indication
sticker
AB4 x 6 screws
MVX-CA2X
@
|
Selector lever
²
x 8 screws from the mount dovetail on the
MVX-TTRS.
² Using the two AB4 x 6 screws and the Allen
screwdriver, attach the MVX-CA2X onto the
mount dovetail by aligning the positioning
pin.
³ Place the mount dovetail with the MVX-CA2X
on the original position by aligning the positioning pin, and then clamp using the four
AB3 x 8 screws.
| Remove the cover of the lever insertion hole
of the MVX-TTRS and screw the selector lever
into it.
ƒ Attach the magnification indication sticker
below the lever.
26
Mount dovetail
AB3 x 8 screws
Page 31

10-3 BX Stage Adapter Type 1 SZX-STAD1
}This adapter is for installation of a U-SRG or U-SRP rotary stage on the SZX-STL large base or a SZX series illumination
base. When the U-SRP rotary stage is used together with the U-FMP mechanical stage, X-Y directional movement
becomes possible, which is convenient for framing during photomicrography and TV observation.
Applicable Bases & Restrictions
1
Base Applicable Objectives Restrictions
Large base
SZX-STL
None.
MVX10
Transmitted illumination base
SZX-ILLK
SZX-ILLB2
SZX-ILLD2
MVPLAPO 0.63X
MVPLAPO 1X
MVPLAPO 2XC
The same restrictions apply regardless of whether the stage
adapter is used or not.
(Refer to the SZX illumination base’s instruction manual.)
Note that the field illuminated by the transmitted light is
limited depending on the diameter of the opening of the
stage plate in use.
#Darkfield observation is not possible using the
SZX-ILLD2.
#The illumination intensity may be reduced when a
frosted filter is used.
27
Page 32

2
Assembly
Mechanical stage
U-FMP
Rotary stage
U-SRP
BX stage adapter type 1
SZX-STAD1
Base
Clamping screws
2
1
Rotary stage
U-SRG
Allen wrench
Clamping screw
Attaching hole
Mounting threaded hole
28
Mounting the Polarizer (SZX-PO)
When simplified transmitted polarized light observation is required, install the polarizer on the SZX-STAD1 BX stage
adapter type 1.
To install the polarizer, place the polarizer frame in the polarizer mount on the upper part of the SZX-STAD1.
(The polarizer oscillation directions <––> are basically horizontal.)
Polarizer frame
Polarizer mount
SZX-STAD1
Page 33

Simplified Transmitted Polarized Light Observation
MVX10
²
³
Fig. 23
}The SZX-AN analyzer is required.
1. Loosen the analyzer clamping knob @, fit the analyzer into the front of the
objective ² and tighten the clamping knob (by placing it on the right
@
side of the microscope body).
2. With no specimen placed on the rotary stage, rotate the analyzer rotation
ring ³ so that the field of view is darkest (cross-Nikol adjustment).
3. Place a specimen on the rotary stage and rotate it to perform the
polarized light observation.
10-4 Stage Adapter Type 1 SZH-STAD1
This adapter has the same function as the SZX-STAD1 BX stage adapter type 1, but the usable stages with this adapter
is only the BH2-SH horizontal knob stage.
The assembly and polarizer (SZX-PO) installation procedures for this adapter are identical to those for the SZX-STAD1.
Refer to the previous section (page 28).
29
Page 34

10-5 BX Stage Adapter Type 2 SZX-STAD2
This adapter is for installing the U-SIC4 large stage* on the SZX-STL large base**. When this stage adapter is used, the
provided SZH-P400 auxiliary pillar should be used to cover the height of the stage adapter.
* The U-SVL or U-SVR BX stage can also be used but the operability is degraded, and the U-SVLB and U-SVRB cannot
be used due to the long stage knobs.
** The SZX-STAD2 can also be used with an SZX series illumination base, but its built-in transmitted illumination cannot
be used.
Assembly
1
Large stage U-SIC4
Frosted filter
BX stage adapter type 2
SZX-STAD2
Mirror knob
Large base
SZX-STL
#For simplified transmitted light observation, place the mirror knob on the front and use a frosted filter.
Simplified Transmitted Light Observation
2
Zoom microscope
body
Frosted
filter
Mirror
Base
Fig. 24
Objective
Stage
External light
source
2
1
1. Illuminate the specimen with an external light source (illuminator, light
guide illuminator, etc.). Light the external light source as shown in Fig. 24
and irradiate the mirror assembly.
2. Eliminate irregularities in illumination.
1) Align the microscope body center with the center of the SZX-STAD2.
2) Set the zooming knob on the microscope body to the minimum
3) While observing through the eyepieces, rotate the mirror knob to
}When observing using a focal light illuminator, remove the frosted filter
and, while observing through the eyepieces, tilt the mirror gradually until
optimum contrast can be obtained.
3
Allen wrench (provided with the base)
Clamping screw
Mounting threaded hole
zoom ratio and focus the stage top surface.
adjust the mirror angle so that the entire field of view is illuminated
uniformly.
(Fig. 24)
30
Page 35

Caution
3
#Do not project the image of the external light source filament on the frosted surface of the frosted filter. Otherwise
the frosted filter may deteriorate.
#Use neutral detergent to clean the frosted filter.
#In transmitted light observation at a total magnification of no more than 10X, the field of view may be obscured in
the peripheral sections depending on the stage in use.
10-6 Vertical-Movement Stage SZ2-FO
#The MVX-2RE revolving nosepiece cannot be used in combination with this stage (because the objectives
interfere with the stage).
MVX10
Module System Diagram
1
Cup Stage
Gliding Stage
Stage glass
Stage plate
Vertical-Movement Stage
SZ2-FO
Clamping knob
Stage
· When using a filter or polarized simplified light module in combination with a transmitted illumination base, install the filter
holder or polarizer on the base before installing the vertical-movement stage.
· The vertical-movement stage can also be installed so that the focus adjustment knob comes on the side of the observer
(i.e. in a 180° opposite orientation to the above figure).
SZH-SG
SZH-SC
SZX-STL
SZX illumination base
Focus adjustment
knob
Modules Combinable with the SZ2-FO
· Stage plate : SP-C, SP-FL, SP-BW-2
· Base : SZX-STL, SZX illumination base
· Stage : SZX-SC, SZH-SG*
* The movement in the front-rear direction is possible only in the section in front of the center due to
interference with the focus adjustment knob of the SZ2-FO.
31
Page 36

External View & Specifications
2
Focus adjustment knobs
Stroke: Approx. 21 mm
Rotation tension adjustable.
Clamping screw holes
Stage
Load capacity: 1 kg
Clamping knob
Assembly
3
1. Attach the SZ2-FO vertical-movement stage inside the stage plate mounting hole of an applicable base by using the
provided clamping screws and the Allen wrench.
The stage can also be mounted so that the focus adjustment knob comes on the front side.
2. Loosen the clamping knob of the stage, attach the stage plate SZH-SG or SZH-SC and tighten the clamping knob again.
Operation
4
@
Push up.
Clamp.
Fig. 25
Fig. 26
To increase.
To decrease.
Removing the Stage Plate (Fig. 25)
Loosen the clamping knob @ and push up the stage plate from its
bottom side.
Adjusting the Rotation Tension of the Focus Adjustment Knob (Fig. 26)
}This adjustment is intended to facilitate the rotation of the knob while
preventing the vertical-movement stage from lowering spontaneously.
For best ease of use, it is recommended to adjust the rotation tension
slightly tighter than the level at which spontaneous lowering of the stage
@
occurs.
a. Holding the left and right focus adjustment knobs @ with both hands,
fix the left knob and rotate only the right knob to increase or decrease
the rotation tension.
b. If the rotation tension is too large, fine focusing will be unavailable
and the mechanism may eventually be damaged.
32
Page 37

Set to the middle of stroke.
Fig. 27
Applicable Bases & Restrictions
5
²
Adjusting the Focus (Fig. 27)
1. Rotate one of the focus adjustment knobs @ of the SZ2-FO verticalmovement stage to set the stage in the position corresponding to the
middle of the focusing stroke.
2. Place a specimen on the stage and rotate the focus adjustment knob
@
² of the base to bring the specimen into approximate focus. Then
proceed to fine focus adjustment using the focus adjustment knob @
of the vertical-movement stage.
MVX10
Base
Large base
SZX-STL
Transmitted illumination base
SZX-ILLK
SZX-ILLB2
SZX-ILLD2
Applicable Objectives
MVPLAPO 0.63X
MVPLAPO 1X
MVPLAPO 2XC
10-7 Gliding Stage SZH-SG
External View & Nomenclature
1
Stage plate
Restrictions
None.
The same restrictions apply regardless of whether the stage
adapter is used or not.
(Refer to the SZX illumination base’s instruction manual.)
Note that the field illuminated by the transmitted light is
limited depending on the diameter of the opening of the
stage plate in use.
#Darkfield observation is not possible using the
SZX-ILLD2.
#The illumination intensity may be reduced when a
frosted filter is used.
Finger hook
Mount
Gliding stage
Illumination field : 40 mm
Movement range : 40 mm
33
Page 38

2
Assembly
Stage plate
Stage plate mounting
hole
Gliding surfaces
³
²
@
Operation
3
Hold the gliding stage by the surrounding edge and move it horizontally.
Use the one provided
with the base.
Gliding stage
Mount
Stage plate mounting
threaded hole
Applicable base
10-8 Cup Stage SZH-SC
Note 1) If dirt or metallic powder is attached on the
gliding surfaces, be sure to clean them.
Note 2) When placing the gliding stage parts, be sure
to avoid bringing the gliding surfaces in contact with the desk surface.
Note 3) Clean the gliding surfaces periodically.
34
External View & Nomenclature
1
#This stage is applicable only to reflected light illumination.
Finger hook
Stage plate
Specimen holder mount holes
(x 4)
Tube
Cup stage
Tilting limit angle: 30°
Specimen holder
Mount
Same dimensions as the stage plate.
Page 39

Assembly
2
#Before assembly, remove dirt and dust from the parts and perform the operation cautiously.
MVX10
Stage plate
Gliding surfaces
Stage plate
mounting hole
Operation
3
³
²
@
Specimen holder
Specimen holder mounting hole
|
Cup stage
Mount
Applicable base
Place a specimen on the stage plate, hold the edge of the cut stage and
tilt it slowly. (Fig. 28)
}If the specimen slips off, fix it using the provided specimen holder.
@ Fit the cut stage mount into the stage plate
mounting hole of an applicable base.
² Place the cup stage on the mount.
Before placing, clean the gliding surfaces on
the cup stage and mount by wiping with a clean
cloth.
³ Attach the stage plate.
| Attach the specimen holder.
}Clean the gliding surfaces periodically.
(Figs. 28 & 29)
Fig. 28
}To fix a container such as a petri dish, fit the provided tube around the
specimen holder and hold the container with it. (Fig. 29)
Fig. 29
Notes
#Take care not to touch the gliding surfaces of the cup stage and mount with a hand. If they are contaminated,
clean with neutral detergent before use.
#The cup stage may move spontaneously if a load of 20 g or more is applied to its outer periphery.
#When the cup stage is tilted while a specimen with large height is placed on it, the image may be defocused. If
this happens, adjust focusing again.
35
Page 40

ASSEMBLY
11-1 Assembly Diagram
The diagram below shows how to assemble the various modules. The numbers indicate the order of assembly.
#When assembling the microscope, make sure that all parts are free of dust and dirt, and avoid scratching any
parts or touching glass surfaces.
Tilting Trinocular Head
MVX-TTRS
Eyepieces
WHN10X-H
To be assembled by
the dealer
Magnification Changer*
MVX-CA2X
Fluorescence mirror units
U-MCFPHQ/XL
U-MGFPHQ/XL
U-MYFPHQ/XL
U-MRFPHQ/XL
U-MGFP/XL
U-MGFPA/XL
C-Mount Adapter
MVX-TV1XC
MVX-TV0.63XC
Tube Lens Unit
MVX-TLU
ND Filter
32ND6
32ND12
32ND25
32ND50
Coaxial Fluorescence
Illuminator
MVX-RFA
Zoom Microscope
Body
MVX-ZB10
Required tools
Allen screwdriver
(provided with the zoom microscope body)
High-Intensity Lamp Housing
U-LH100HG
U-LH100HGAPO
U-LH75XEAPO
Power Supply Unit
(for mercury/xenon burner)
36
UV shield plate
Revolving Nosepiece*
MVX-2RE
Objective Lens
MVPLAPO 0.63X
MVPLAPO 1X
MVPLAPO 2XC
Stage Glass & base
accessories*
Focusing Assembly
MVX-FOF
SZX illumination base
SZX-ILLK/ILLB2/ILLD2
Large Base
SZX-STL
The assembly methods of the modules marked * are described in Chapter 10, “OPERATION OF OTHER MODULES”.
Page 41

11-2 Detailed Assembly Procedures
MVX10
30° or less
Fig. 30
Fig. 31
²
@
³
Mounting the Focusing Assembly
1
1. First loosen the focusing assembly clamping knob @ completely and,
while holding the focusing assembly with both hands, insert the pillar ³
into the mounting hole ² from below. (Fig. 30)
#Insert slowly. Do not apply excessive force.
2. Lower the focusing assembly until it stops, then tighten the focusing
assembly clamping knob @. (Fig. 30)
To prevent the microscope from turning over, the focusing assembly
must be installed as shown in the illustration marked “¦” in Fig. 31,
and its pivot angle must be limited to 30°. If the focusing assembly
is placed on the wrong side, the microscope will turn over.
#If the clamping knob @ is tightened while the pillar ³ is not com-
pletely inserted into the mounting hole ², the plate spring supporting the pillar from the inside will deform and the pillar will not be
able to penetrate into the hole. (Fig. 30)
(Fig. 30)
|
³
³
²
Fig. 32
Fig. 33
²
@
@
2 Mounting the Zoom Microscope Body
1. Remove the cap @ on the focusing assembly by inserting a thin object
into the notch.
2. Using the provided Allen screwdriver, loosen the dovetail mount
clamping screw inside the cap on the focusing assembly by rotating
it by 2 or 3 turns (counterclockwise).
3. Gently insert the dovetail mount ³ on the rear of the microscope
body into the dovetail mount ² on the focusing assembly.
#Do not insert the mount at an angle or with excessive force, for this
may cause malfunctions.
4. When the microscope body has been inserted until it stops, tighten the
clamping screw using the Allen screwdriver.
5. Place the cap @ in the original position.
Mounting the Objective
3
1. Since the objective is heavy, there is a risk of dropping it during mounting
or dismounting. To prevent this, take the following countermeasures.
· Attach the provided cap @ on the objective.
· Protect the base against the dropped objective, place the other cap ²,
a notebook or a protecting material on the base.
2. Mount the objective | on the objective mount thread ³ by rotating the
objective in the direction of the arrow.
(Fig. 32)
(Fig. 33)
37
Page 42

²
Fig. 34
@
Attaching the UV Shield Plate
4
Align the mounting groove ² of the UV shield plate with the two
mounting pins @ on the lower part of the zoom microscope body
and fit the groove with a strong force.
(Fig. 34)
ƒ
²
Fig. 35
³
Fig. 36
@
|
Mounting the Fluorescence Mirror Units
5
}Fluorescence mirror units can be mounted by in every other dovetail,
or in positions with turret numbers 1, 2 and 3, of the six dovetails of
the mirror unit.
1. Using the Allen screwdriver, loosen the clamping screw hole @ on
the right end of the illuminator.
2. Slide out the turret and place it upside down.
}After completion of assembly of the microscope, it is difficult to slide
out the turret because the trinocular head comes in the way. In this
case, hold the two sides of the turret with two hands and pull.
3. Rotate the mirror unit so that the mirror unit position number (one of
1, 2 or 3) comes at the turret number indicator ².
4. Loosen the clamping screw ³ using the Allen wrench.
5. Insert the desired mirror unit | all the way into the dovetail position so
that the abbreviation of the mirror unit name is upside down, and then
tighten the clamping screw ³ firmly,
#If the clamping screw ³ is loose, it may interfere with the inner surface
of the turret cover, making impossible to rotate the turret.
6. Select the optimum indicator plate from the provided indicator plate sheet,
and insert the plate upside down in the mirror unit indicator pocket ƒ.
}If the optimum indicator plate is not present, write the original name in a
blank indicator plate using an oil-ink pen.
7. Mount the other required mirror units by repeating the above steps.
8. Place the turret in the original position and tighten the clamping screw @
while pushing the turret.
(Figs. 35 & 36)
38
Page 43

MVX10
How to Fabricate an Optional Mirror Unit
}A custom ordered or commercially available mirror unit can be fabricated by assembling a barrier filter, excitation filter
and dichroic mirror, all of which are available commercially, in the U-MF/XL mirror unit frame.
However, the barrier filter and dichroic mirror should be the Olympus products. If a commercially available product is
used, Olympus cannot guarantee the safety of operation.
}The arrow inscribed on the side of an Olympus barrier filter or excitation filter indicates the filter mounting orientation.
Dimensional Conditions of the Optical Parts
· Barrier filter : 32 mm, max. thickness 4 mm.
· Excitation filter : 25 mm, max. thickness 6 mm.
· Dichroic mirror : See figure on the right.
– 0.1
– 0.2
– 0.1
– 0.2
Barrier filter (made to custom order)
Push ring spring
#Be careful not to dam-
age the projection.
Dichroic mirror
(made to custom order)
Interference film
surface
Filter push ring
Excitation filter
(made to custom order or
commercially available)
Thickness: 1.5 ± 0.05 mm
#When replacing the dichroic mirror, be particularly careful not to contaminate it with fingerprints, etc.
}A mirror unit of the UIS series can also be used. However, in this case, the NA is limited in the low to medium zoom ratios
due to the diameter of the filter.
39
Page 44

²
³
Fig. 37
@
Mounting the Coaxial Fluorescence
6
Illuminator
1. Using the Allen screwdriver, loosen the illuminator clamping screw @.
2. Fit the round dovetail ² at the bottom of the illuminator into the mount
dovetail ³ on the microscope body, and tighten the clamping screw @.
}To prevent the microscope from turning over after the illuminator is
installed, the rotation angle should be limited to within ±60° from the
rear direction.
(Fig. 37)
|
Fig. 38
²
ƒ
³
@
Mounting the ND Filter
7
}Color fading of specimen can be delayed by attenuating the excitation
light intensity using an ND filter. Use the ND filters as far as they do not
hinder observation.
}When it is difficult to mount the ND filter, detach the knob on the slider
and remove the slider. Then try to mount the ND filter again.
1. Slide out the filter slider @ of the coaxial fluorescence illuminator so that
the filter pocket ² is visible.
2. Insert the ND filter ³ in the filter pocket ², and insert it so that the notch
of the filter push ring | comes on the bottom side.
}When the filter is thick (i.e. 5 mm or more), the filter will not lay down so it
is not necessary to use the push ring |.
3. Apply the filter push ring | against the filter so that it will not lay down.
4. Drop the light leak prevention cap ƒ into the filter slider.
#Even when no filter is used, the filter push ring | should be
mounted and the light leak prevention cap ƒ should be dropped
in to prevent light leak.
#Insert the filter so that the side with the indication faces the ob-
serving side. Otherwise, the filter may crack due to heat.
#Before replacing the filter, ensure that it is cooled down.
When the mercury burner has been turned on for an extended period, the filter and its metallic parts get very hot. Be careful not to
burn yourself. Also do not leave the filter slider knob in a position
other than the click stop positions.
}A filter other than the ND filter can also be mounted provided that its
diameter is no more than 32 mm and the thickness is no more than 6
mm.
#Always use reflection type ND filters (32ND6, 12, 25, 50). If an
absorption-type 32LND filter series or a commercially available
ND filter is used, the filter crack or burn may result.
(Fig. 38)
40
Page 45

MVX10
@
³
²
Fig. 39
Fig. 40
²
³
@
Mounting the Tilting Trinocular Head
8
1. Using the Allen screwdriver, loosen the clamping screw @ of the coaxial
fluorescence illuminator.
2. Fit the round dovetail ² on the bottom of the trinocular head into the
mount dovetail ³ of the illuminator and tighten the clamping screw.
Mounting the Eyepieces
9
1. Remove the eyepiece dust caps @ and loosen the eyepiece clamping
knobs ² completely.
2. Gently insert the eyepiece (WHN10X-H) ³ into the left and right eyepiece sleeves all the way until they stop.
3. Tighten both eyepiece clamping knobs ².
(Fig. 39)
(Fig. 40)
41
Page 46

³
Fig. 41
Fig. 42
@
²
|
Mounting the Lamp Housing for
10
Mercury Burner
}For the lamp housing for xenon burner, refer to the instruction manual
provided with the lamp housing. The centering method is identical to
that for the mercury burner.
Attaching the Mercury Burner
1. Using the Allen screwdriver, loosen the socket clamping screw @.
2. Hold the upper section of the lamp housing and pull it upward to remove
the socket section.
#To prevent malfunction, do not hold the lamp housing by the
centering knobs ².
3. Place the socket section upside down as shown in Fig. 42.
}The lamp housing is equipped with the holder for transportation in the
factory shipment condition or with an old burner after the burner is replaced. Remove the holder or old burner by loosening the two burner
holding screws ³.
4. Attach the + (positive) pole of a specified mercury burner | to the fixed
mount on the upper side, then the - (negative) pole to the mount on the
lower side.
#Be sure to use the USH-103OL (OLYMPUS) or the HBO103W/2
(OSRAM) mercury burner.
Be careful and avoid leaving fingerprints or contaminants on the
mercury burner. Otherwise, there is a danger of explosion due to
distortion of glass caused by the strains. If the burner is contaminated, clean it by wiping gently with gauze slightly moistened with
alsolute alcohol.
5. Attach the socket section with burner to the original position and tighten
the socket clamping screw @.
# Align the external edges of the lamp housing with those on the socket
section, and push the lamp housing straight downward.
(Figs. 41 to 43)
42
Burner Service Life
USH-103OL: 300 hours
}This value assumes light cycles composed of 2 hours of lighting
and 30 minutes of extinction. Do not turn it on and off at a shorter
cycle than the above, for this will shorten the service life of the burner.
After replacing the burner, reset the hour counter to “0.0” as
outlined above.
Page 47

@
MVX10
Attaching the Lamp Housing
1. Using the Allen screwdriver, loosen the two clamping screws @ on
the lamp housing mount hole.
2. Fit the lamp housing all the way as shown in Fig. 43.
3. Tighten the clamping screws @ using the Allen screwdriver.
#The surroundings of the lamp housing become very hot during
and after operation. When installing the microscope system,
ensure sufficient space around the lamp housing, particularly
above it. Also do not mount the lamp housing obliquely.
Fig. 43
@
Fig. 44
Fig. 45
²
Mounting (Removing) the Stage Plate
11
Place the stage plate (white, black on back side) or a stage glass into the
mounting hole on the base.
To remove, press the stage plate at the edge nearest to the pillar with
your fingertip. The opposite end will rise from the base so the stage plate
can be picked up easily.
Attaching the Specimen Holder
}Use the specimen holder when a specimen is hard to be fixed.
Insert the specimen holder @ into the two holes ² on the top surface of
the base.
(Fig. 44)
43
Page 48

11-3 Centration of the Mercury (Xenon) Burner and Field Iris Diaphragm
}For centering of the xenon burner, use the same procedure as the mercury burner.
@
³
A
B
Fig. 46
|
²
ƒ
1 Centering the Mercury Burner
}Set the main switch to “ ” (ON) and wait until the arc image stabilizes
(for 5 to 10 minutes after ignition) before proceeding to the centering.
1. Set the shutter knob @ to “{” to close the shutter.
2. Set the mirror unit turret to “TBF”, place a sheet of white paper on the
specimen surface, focus on the white sheet and set a low zoom ratio.
3. Engage the fluorescence mirror unit in the light path.
The fluorescence illumination may contain wavelengths harmful to
your eyes. The specimen must always be observed through the UV
shield plate.
4. Remove the objective.
5. Pull out the field iris diaphragm lever ² (to stop down the diaphragm).
6. Rotate the aperture iris diaphragm knob ³ toward the left (to open the
diaphragm).
7. Set the shutter knob @ to “¦” to open the shutter.
8. Rotate the collector lens focusing knob | to project the arc image on
the white sheet. (A)
9. Rotate the burner centering knobs ƒ to bring the arc image on the center of the left (or right) half of the field. (B)
10. Insert the Allen screwdriver into the mirror focusing screw hole (… in
Fig. 51) on the rear of the lamp housing, and turn the screw to focus
on the mirror-reflected image. (C)
11. Rotate the burner centering knob ƒ until the arc image and mirrorreflected image overlap.
(Figs. 46 & 47)
44
}After starting observation, rotate the collector lens focusing knob | as
required to render the observation field uniform.
}The mercury burner needs not be centered again until the time it is
replaced.
C
D
Page 49

Precise Centering of the Mirror
MVX10
²
Fig. 47
@
Fig. 48
‡
…
†
}The position of the mirror has been adjusted and locked before ship-
ment. Only if you want more precise adjustment of the mirror position,
proceed to the following steps immediately after the procedure in the
previous paragraphs.
Note that, once the following steps are completed, it is no longer possible to restore the mirror position in the factory shipped condition.
1. Using a pair of tweezers, etc., peel off the two blind stickers † on the rear
of the lamp housing.
2. Fit the Allen screwdriver into each of the screws hidden below the stickers and loosen them. Loosening the two screws releases the locking of
the mirror.
3. Peel off other two blind stickers ‡ to expose the mirror centering holes.
4. Insert the Allen screwdriver into the screw in each mirror centering hole
and adjust the centering of the mirror-reflected image.
Centering the Field Iris Diaphragm
2
1. Set the shutter knob to “{” to close the light path with the shutter.
2. Rotate the mirror unit turret to engage a mirror unit in the light path.
3. Set the shutter knob to “¦” to open the shutter.
4. Place a specimen on the center of the base and bring the specimen into
approximate focus.
5. Pull out the field iris diaphragm lever @ on the illuminator to stop down
the field iris diameter near the minimum size.
6. Insert the Allen screwdriver, provided with the microscope body, into the
two field iris diaphragm centering screw ² and adjust so that the field iris
image comes on the center of the field.
7. Push in the field iris diaphragm lever @ until the field iris image inscribes
the field of view. If the field iris image is found to be eccentric, adjust
centering again.
8. Open the iris until the field iris image size becomes equivalent to the
field size (i.e. until the field iris image circumscribes the field of view).
(Fig. 48)
45
Page 50

PROPER SELECTION OF THE POWER SUPPLY CORD
If no power supply cord is provided, please select the proper power supply cord for the equipment by referring to “Specifications”
and “Certified Cord” below:
CAUTION: In case you use a non-approved power supply cord for Olympus products, Olympus can no longer warrant
the electrical safety of the equipment.
Specifications
Voltage Rating
Current Rating
Temperature Rating
Length
Fittings Configuration
125V AC (for 100-120V AC area) or, 250V AC (for 220-240V AC area)
6A minimum
60°C minimum
3.05 m maximum
Grounding type attachment plug cap. Opposite terminates in molded-on IEC configuration appliance coupling.
Table 1 Certified Cord
A power supply cord should be certified by one of the agencies listed in Table 1, or comprised of cordage marked with
an agency marking per Table 1 or marked per Table 2. The fittings are to be marked with at least one of agencies listed
in Table 1. In case you are unable to buy locally in your country the power supply cord which is approved by one of the
agencies mentioned in Table 1, please use replacements approved by any other equivalent and authorized agencies
in your country.
Country Agency
Argentina IRAM
Australia SAA Japan
Certification
Mark
Country Agency
Italy IMQ
JET, JQA, TÜV,
UL-APEX / MITI
Certification
Mark
46
Austria ÖVE Netherlands KEMA
Belgium CEBEC Norway NEMKO
Canada CSA Spain AEE
Denmark DEMKO Sweden SEMKO
Finland FEI Switzerland SEV
France UTE
Germany VDE U.S.A. UL
Ireland NSAI
United
Kingdom
ASTA
BSI
Page 51

Table 2 HAR Flexible Cord
APPROVAL ORGANIZATIONS AND CORDAGE HARMONIZATION MARKING METHODS
MVX10
Approval Organization
Comité Électrotechnique Belge
(CEBEC)
VDE Verband der Elektrotechnik
Elektronik Informationstechnik e.V.
Union Technique de l’Électricité
(UTE)
Istituto Italiano del Marchio di Qualità
(IMQ)
British Approvals Service for Cables
(BASEC)
N.V. KEMA KEMA-KEUR <HAR> 10 30 30
SEMKO AB Svenska Elektriska
Materielkontrollanstalten
Österreichischer Verband für
Elektrotechnik (ÖVE)
Danmarks Elektriske Materielkontrol
(DEMKO)
Printed or Embossed Harmonization
Marking (May be located on jacket
or insulation of internal wiring)
CEBEC <HAR> 10 30 10
<VDE> <HAR> 30 10 10
USE <HAR> 30 10 30
IEMMEQU <HAR> 10 30 50
BASEC <HAR> 10 10 30
SEMKO <HAR> 10 10 50
<ÖVE> <HAR> 30 10 50
<DEMKO> <HAR> 30 10 30
Alternative Marking Utilizing
Black-Red-Yellow Thread (Length
of color section in mm)
Black Red Yellow
National Standards Authority of Ireland
(NSAI)
Norges Elektriske Materiellkontroll
(NEMKO)
Asociación Electrotécnica Española
(AEE)
Hellenic Organization for
Standardization (ELOT)
Instituto Português da Qualidade (IPQ) np <HAR> 10 10 90
Schweizerischer Elektrotechnischer
Verein (SEV)
Elektriska Inspektoratet SETI <HAR> 10 30 90
Underwriters Laboratories Inc. (UL) SV, SVT, SJ or SJT, 3 X 18AWG
Canadian Standards Association (CSA) SV, SVT, SJ or SJT, 3 X 18AWG
<NSAI> <HAR> 30 30 50
NEMKO <HAR> 10 10 70
<UNED> <HAR> 30 10 70
ELOT <HAR> 30 30 70
SEV <HAR> 10 30 90
47
Page 52

EC REP
Shinjuku Monolith, 3-1, Nishi Shinjuku 2-chome, Shinjuku-ku, Tokyo, Japan
Wendenstraße 14-18, 20097 Hamburg, Germany
3500 Corporate Parkway, P.O. Box 610, Center Valley, PA 18034-0610, U.S.A.
One Corporate Drive, Orangeburg, NY 10962, U.S.A.
491B River Valley Road, #12-01/04 Valley Point Office Tower, Singapore 248373
31 Gilby Road, Mount Waverley, VIC., 3149, Australia
Blue Lagoon Drive, Suite 290 Miami, FL 33126, U.S.A.
5301
01/10
 Loading...
Loading...