Page 1

INSTRUCTIONS
Rotatable Clip Fixing Device
HX-110LR HX-110QR HX-110UR
Clip
HX-610-090 HX-610-135
Long Clip
HX-610-090L
Short Clip
HX-610-090S HX-610-135S
Colored Short Clip
HX-610-090SC
AUTOCLAVABLE
Page 2

Page 3

Contents
Contents
Symbols.................................................................. 1
Important Information – Please Read Before Use 2
Intended Use ..................................................................... 2
Instruction Manual ............................................................. 3
User Qualifications............................................................. 3
Instrument Compatibility .................................................... 3
Reprocessing and Storage ................................................ 4
Repair and Modification ..................................................... 4
Signal Words ..................................................................... 5
Warnings, Cautions and Notes .......................................... 5
Chapter 1 Checking the Package Contents..... 7
1.1 Checking the Package Contents .............................. 7
Chapter 2 Instrument Nomenclature and
Specifications................................... 9
2.1 Nomenclature and Functions.................................... 9
2.2 Specifications............................................................ 10
Chapter 3 Preparation,
Inspection and Operation ................ 16
3.1 Preparation ............................................................... 18
3.2 Inspection ................................................................. 19
3.3 Operation .................................................................. 24
Chapter 4 Emergency Treatment...................... 41
4.1 Emergency Treatment .............................................. 41
Chapter 5 Reprocessing.................................... 45
5.1 General Policy .......................................................... 45
5.2 Required Reprocessing Equipment .......................... 49
5.3 Cleaning.................................................................... 51
5.4 Lubrication ................................................................ 53
5.5 Sterilization ............................................................... 54
Rotatable Clip Fixing Device
i
Page 4

Contents
Chapter 6 Storage .............................................. 57
6.1 Inspection Before Storage ........................................ 57
6.2 Storage ..................................................................... 58
ii
Rotatable Clip Fixing Device
Page 5
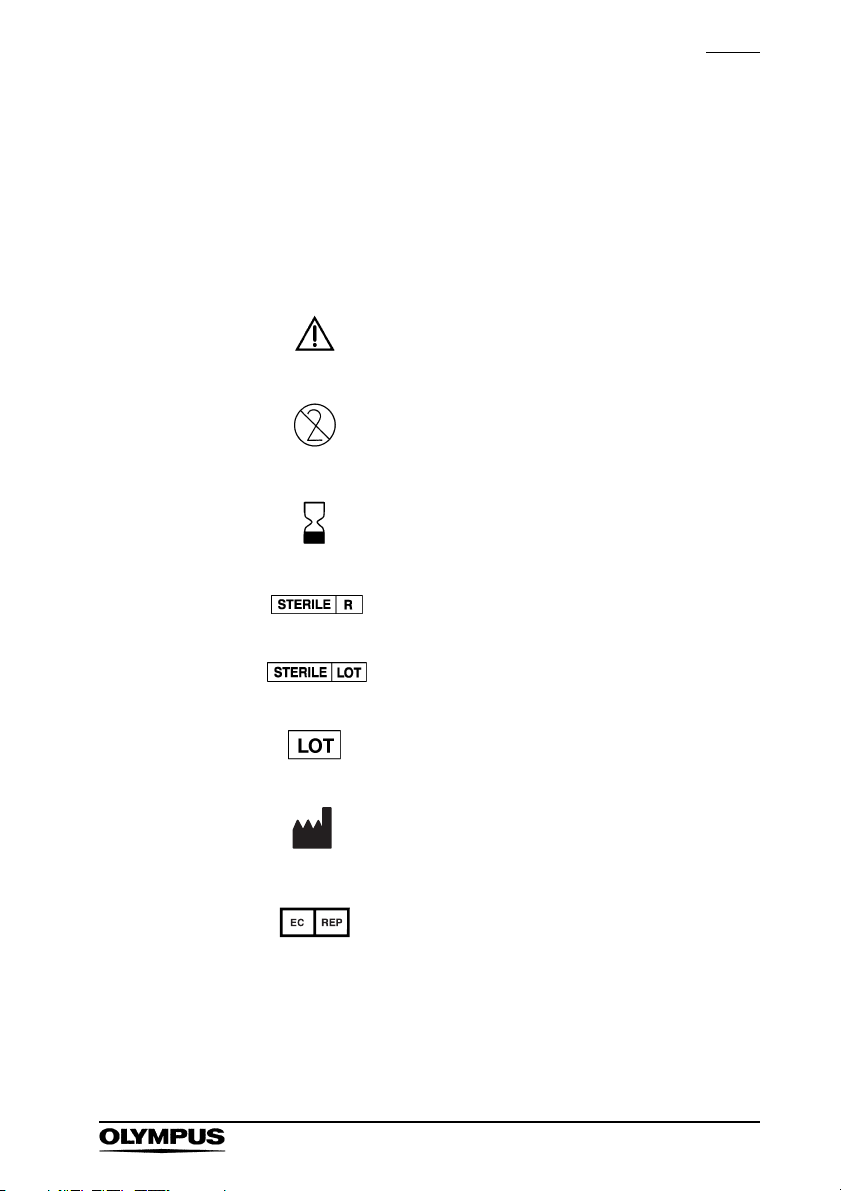
Symbols
Symbols
The meaning(s) of the symbol(s) shown on the package,
the back cover of this instruction manual and/or this
instrument are as follows:
Refer to instructions.
Single use only
Use by (expiration date)
Sterilized using irradiation
Sterilization lot number
Lot number
Manufacturer
Authorised representative in
the European Community
Rotatable Clip Fixing Device
1
Page 6
Important Information – Please Read Before Use
Important Information – Please
Read Before Use
Intended Use
This instrument has been designed to be used with an
Olympus endoscope for endoscopic clip placement
within the gastrointestinal (GI) tract for the purpose of
(1) endoscopic marking,
(2) hemostasis for
(a) mucosal/sub-mucosal defects < 3 cm,
(b) bleeding ulcers,
(c) arteries < 2 mm,
(d) polyps < 1.5 cm in diameter,
(e) diverticula in the colon,
(3) as a supplementary method, closure of GI tract
luminal perforations < 20 mm that can be treated
conservatively.
Do not use this instrument for any purpose other than its
intended uses.
Due to the range of procedures, the
indications for use of this instrument
should be evaluated by the physician,
taking into account factors such as the
anatomical site, histology, lesion type
and the patient’s condition. In addition,
the cautions and notes contained in
“Warnings, Cautions and Notes” on
page 5 should be thoroughly reviewed
before starting the procedure.
2
Rotatable Clip Fixing Device
Page 7

Instruction Manual
This instruction manual contains essential information on
using this instrument safely and effectively. Before use,
thoroughly review this manual and the manuals of all
equipment which will be used during the procedure and
use the instruments as instructed.
Keep this and all related instruction manuals in a safe,
accessible location.
If you have any questions or comments about any
information in this manual, please contact Olympus.
User Qualifications
The operator of this instrument must be a physician or
medical personnel under the supervision of a physician
and must have received sufficient training in clinical
endoscopic technique. This manual, therefore, does not
explain or discuss clinical endoscopic procedures.
Important Information – Please Read Before Use
Instrument Compatibility
Refer to the tables in Section 2.2, “Specifications” to
confirm that this instrument is compatible with the
ancillary equipment being used. Using incompatible
equipment can result in patient injury or equipment
damage.
Rotatable Clip Fixing Device
3
Page 8

Important Information – Please Read Before Use
Reprocessing and Storage
This instrument was not sterilized before shipment.
Before using this instrument for the first time, reprocess it
according to the instructions given in Chapter 5,
“Reprocessing”.
After using this instrument, reprocess and store it
according to the instructions given in Chapter 5,
“Reprocessing” and Chapter 6, “Storage”. Improper and/
or incomplete reprocessing or storage can present an
infection control risk, cause equipment damage or
reduce performance.
Clips are shipped in a sterile condition. Store them
following the instructions given in Chapter 6, “Storage”.
Improper storage can present an infection control risk,
cause equipment damage or reduce performance.
All clips are single-use, disposable items that are not to
be reprocessed after use. Do not reuse or attempt to
sterilize them after use.
Repair and Modification
This instrument and clip do not contain any
user-serviceable parts. Do not disassemble, modify or
attempt to repair them; patient or user injury and/or
equipment damage can result.
4
Rotatable Clip Fixing Device
Page 9
Signal Words
The following signal words are used throughout this
manual:
Important Information – Please Read Before Use
Indicates a potentially hazardous
situation which, if not avoided, could
result in death or serious injury.
Indicates a potentially hazardous
situation which, if not avoided, may result
in minor or moderate injury. It may also
be used to alert against unsafe practices
or potential equipment damage.
Indicates additional helpful information.
Warnings, Cautions and Notes
Follow the warning, cautions and notes described below
when handling this instrument and clip. This information
is to be supplemented by the warnings, cautions and
notes described in each chapter.
• Operation of this instrument is based on
the assumption that open surgery is
possible as an emergency measure if the
clip cannot be detached from the
instrument or if any other unexpected
circumstance takes place. In this case,
refer to Chapter 4, “Emergency
Treatment”.
Rotatable Clip Fixing Device
5
Page 10
Important Information – Please Read Before Use
• It might be impossible to stop bleeding
• Re-bleeding may occur on the clipping
• Do not use this instrument when
• Do not perform MRI procedures on
depending on the hemorrhage situation
because the clip performance for
hemostasis is limited. Prepare more than
one hemostatic device and select
appropriate hemostatic device or use it
together to respond to different
hemorrhage situations appropriately.
Choose a surgical hemostasis if
necessary.
site, depending on the local condition.
Check the patient for any re-bleeding
after the operation as appropriate.
hemostasis cannot be verified visually
within the endoscopic field of view after
application.
patients who have clips placed within
their gastrointestinal tracts. This could be
harmful to the patient.
• Limited studies indicate that lesions
located in the esophagus and the lesser
curvature of the stomach may be difficult
to treat with a forward-viewing
endoscope.
• Limited studies indicate that the
treatment of esophageal varices may
require clipping in combination with a
sclerosing agent.
• Limited studies indicate that clipping hard
or severely fibrotic lesions to achieve
hemostasis may be more difficult.
6
Rotatable Clip Fixing Device
Page 11

Important Information – Please Read Before Use
• Limited studies have shown that the
number of clips required for hemostasis
may vary depending upon the anatomical
site, histology, lesion type and patient
condition and history. A sufficient
quantity of clips should be prepared in
consideration of all of these factors prior
to the procedure.
• Limited studies indicate that the
hemostasis clips remain in place for an
average of 9.4 days; re-bleeding may
occur if the clips detach within 24 hours.
• Limited studies indicate that the use of
clips in the presence of bacterial
contamination may potentiate or prolong
infection.
Rotatable Clip Fixing Device
7
Page 12
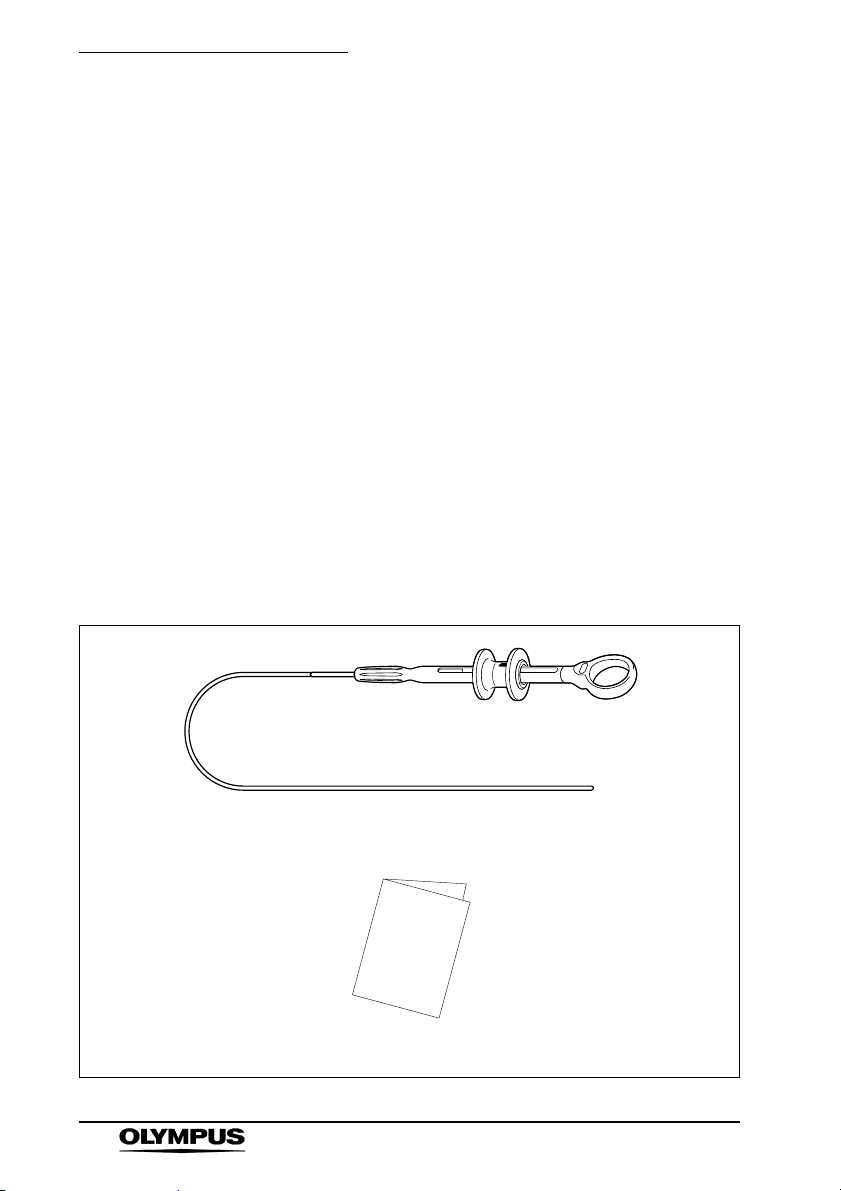
Chapter 1 Checking the Package Contents
Chapter 1 Checking the Package
Contents
1.1 Checking the Package Contents
Match all items in the package with the components
shown below. Inspect each item for damage. If the
instrument or clip is damaged, a component is missing or
you have any questions, do not use the instrument or
clip; immediately contact Olympus.
This instrument was not sterilized before shipment.
Before using this instrument for the first time, reprocess it
according to the instructions in Chapter 5,
“Reprocessing”.
Rotatable Clip Fixing Device
Rotatable clip fixing device (Reusable)
Instruction manual
8
Rotatable Clip Fixing Device
Page 13
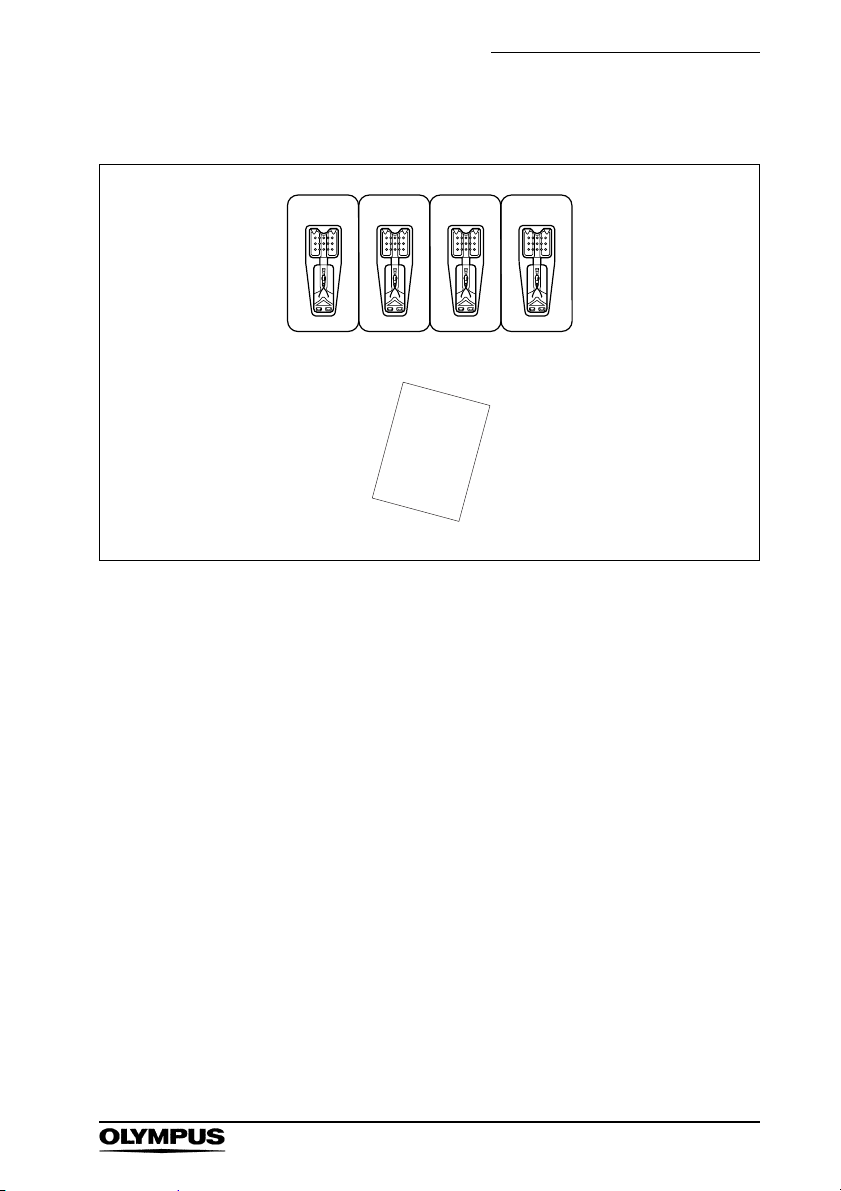
Chapter 1 Checking the Package Contents
Clip (HX-610-090 or HX-610-135)
Clip (Sterile, single use only, 40 pieces)
Access information sheet
Long Clip (HX-610-090L)
Long clip (Sterile, single use only, 40 pieces)
Access information sheet
Short Clip (HX-610-090S or
HX-610-135S)
Short clip (Sterile, single use only, 40 pieces)
Access information sheet
Colored Short Clip (HX-610-090SC)
Colored short clip (Sterile, single use only, 24 pieces)
Access information sheet
Rotatable Clip Fixing Device
9
Page 14
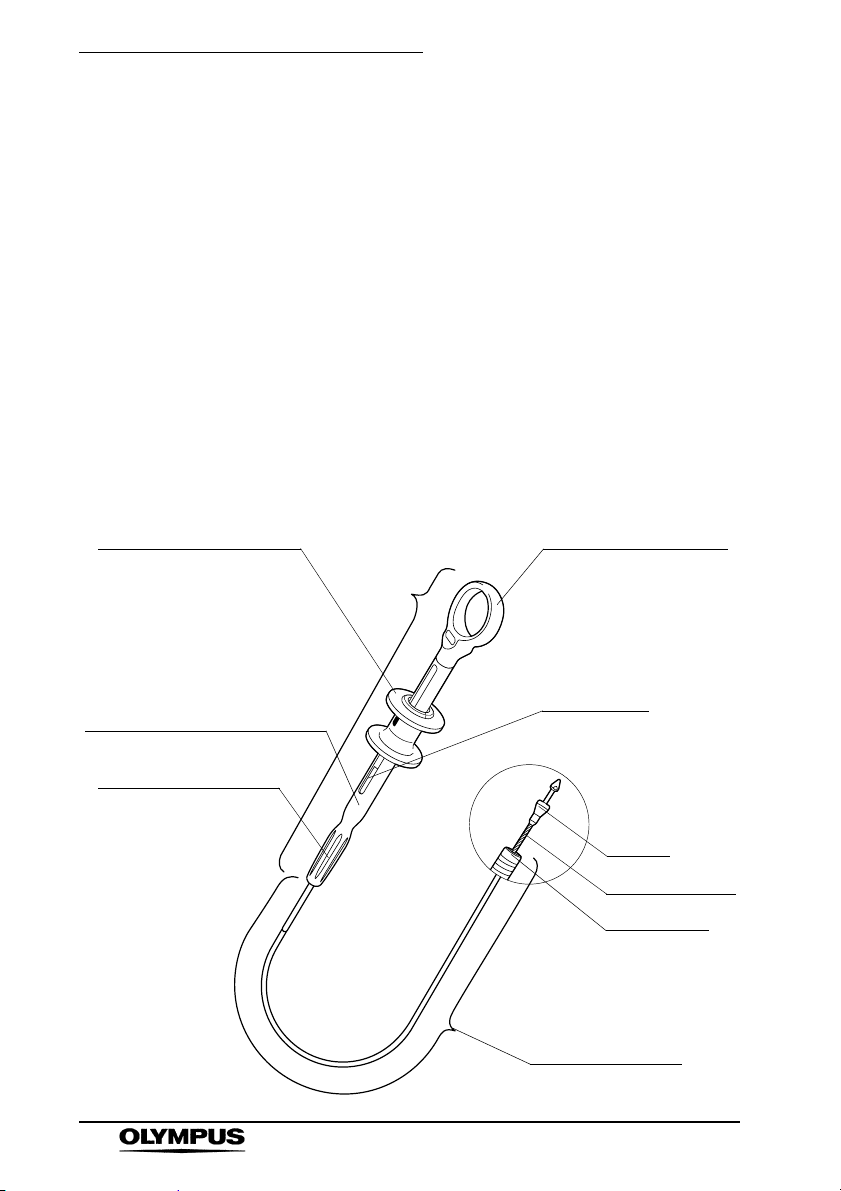
Chapter 2 Instrument Nomenclature and Specifications
Chapter 2 Instrument
Nomenclature and
Specifications
2.1 Nomenclature and Functions
This instrument must be used in combination with the
clip.
Rotatable Clip Fixing Device (Reusable)
Slider
Extends the hook from the
distal end of the coil sheath
when pushed. Retracts the
hook into the coil sheath when
pulled. The green color of this
slider indicates that the
instrument is autoclavable.
Model reference label
Indicates the product number.
Rotation grip
Rotating the rotation grip
will rotate the clip.
Handle
Ring (Yellow)
The color of the ring
indicates the minimum
instrument channel
diameter required for the
endoscope to be
compatible.
Lot number
Distal portion
Hook
Operation wire
Coil sheath
Insertion portion /
Working length
10
Rotatable Clip Fixing Device
Page 15
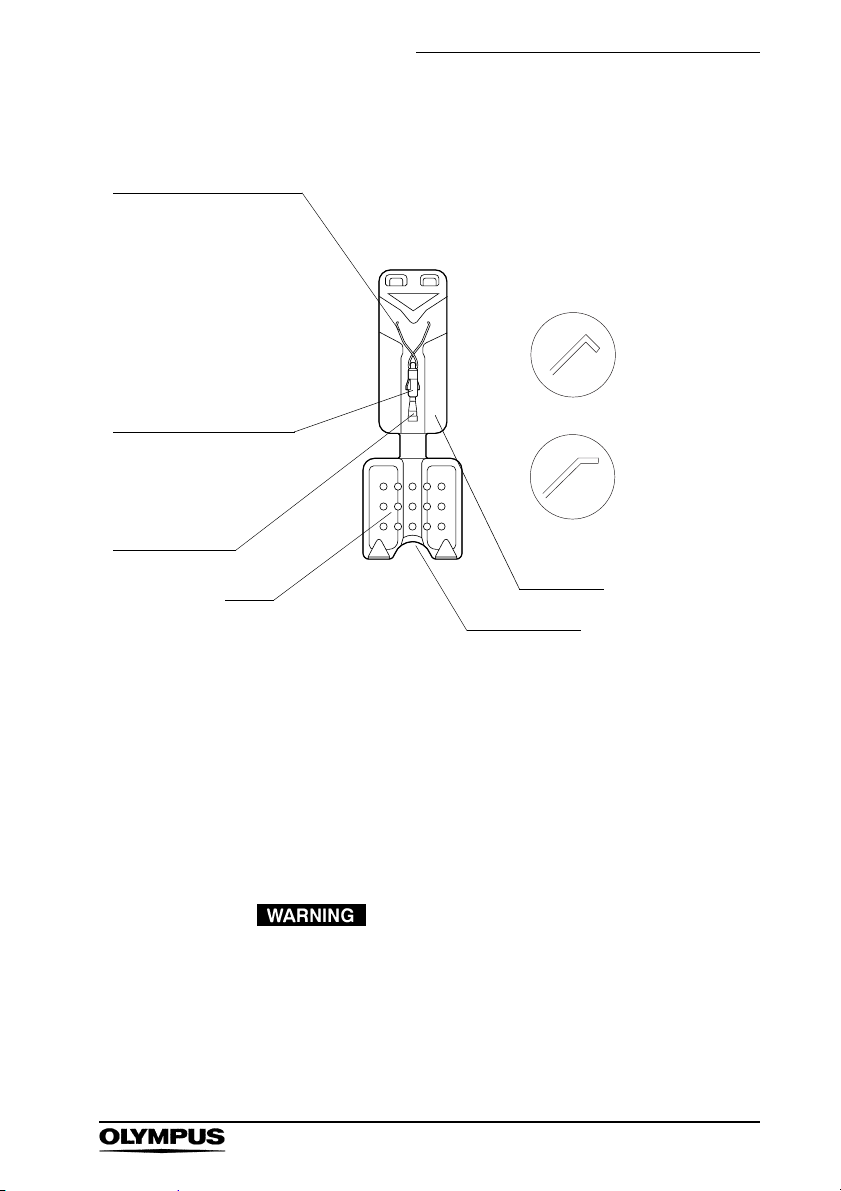
Clip (Single use only)
Clip
The clip of the
HX-610-090SC is coated
to minimize thermal injury
when electrosurgical
accessories are used
after clipping. The
HX-610-090SC is not
guaranteed to be
insulated.
Clip pipe
This part of the
HX-610-090SC will have
a red, yellow or white
coating.
Clip connector
Chapter 2 Instrument Nomenclature and Specifications
Right angle
HX-610-090
HX-610-090L
HX-610-090S
HX-610-090SC
Obtuse angle
HX-610-135
HX-610-135S
Grip
2.2 Specifications
The compatible Olympus endoscopes are listed in the
tables on the following pages. New endoscopes released
after the introduction of this instrument and clip may also
be compatible for use in combination with this instrument
and clip. For further details, contact Olympus.
Rotatable Clip Fixing Device
Cartridge
Insertion port
Use this instrument and clip only in
combination with products recommended
by Olympus. If combined with products
not recommended by Olympus, patient
or operator injury, malfunction or
equipment damage may result.
11
Page 16

Chapter 2 Instrument Nomenclature and Specifications
Operating Environment
Ambient Temperature 10 to 40°C (50 to 104°F)
Relative Humidity 30 to 85%
Air Pressure 700 to 1060 hPa
Specifications
Model HX-110LR HX-110QR
Shape of the
distal end of
the insertion
portion
Maximum
insertion
portion
diameter
(mm)
Working
length (mm)
Compatible
Olympus
endoscopes
(All of these
parameters
should be met.)
Length and
model
Channel inner
diameter
(mm)
(Color code)
(0.71 to 1.08 kgf/cm
(10.1 to 15.4 psia)
ø 2.75
1650 1950
Working
length
less than 1200
mm;
EF, GIF, OGF,
CF (Exclude I-,
L-length), OSF
ø 2.8, ø 3.2
(Yellow);
ø 3.7, ø 6
(Orange)
2
)
Working
length
less than 1500
mm;
EF, GIF, OGF,
CF (Exclude
L-length),
PCF (I-length
only), OSF
ø 2.8, ø 3.2
(Yellow);
ø 3.7, ø 4.2,
ø 6 (Orange)
12
Rotatable Clip Fixing Device
Page 17

Chapter 2 Instrument Nomenclature and Specifications
Model HX-110UR
Shape of the
distal end of
the insertion
portion
Maximum
insertion
portion
diameter
(mm)
Working
length (mm)
Compatible
Olympus
endoscopes
(All of these
parameters
should be met.)
Length and
model
Channel inner
diameter
(mm)
(Color code)
EF, GIF, OGF, CF, PCF,
ø 3.7, ø 4.2, ø 6 (Orange)
ø 2.75
2300
Working length
less than 1850 mm;
SIF (SIF-10 only)
ø 2.8, ø 3.2 (Yellow);
Model HX-610-090 HX-610-135
Shape of the clip
Color of the packages Yellow Pink
Clip arm length Standard
General application Hemostasis
Rotatable Clip Fixing Device
13
Page 18

Chapter 2 Instrument Nomenclature and Specifications
Model HX-610-090L
Shape of the clip
Color of the packages Blue
Clip arm length Long
General application Hemostasis (for large tissue
Model HX-610-135S
Shape of the clip
Color of the packages Green
Clip arm length Short
General application Hemostasis (for smaller tissue
retention)
retention)
14
Rotatable Clip Fixing Device
Page 19

Chapter 2 Instrument Nomenclature and Specifications
Model HX-610-090SC HX-610-090S
Shape of the clip
Color of the packages Red
White
Yell ow
Clip arm length Short
General application Marking (for smaller tissue
retention)
White
Medical Device
Directive
This device complies with
the requirements of Directive
93/42/EEC concerning
medical devices.
Classification: Class II a
Rotatable Clip Fixing Device
15
Page 20

Chapter 3 Preparation, Inspection and Operation
Chapter 3 Preparation,
Inspection and
Operation
The clips were shipped in a sterile condition.
• Do not use the clips after the expiration
date displayed on the sterile package.
Doing so may pose an infection control
risk or cause tissue irritation.
• Before each case, prepare and inspect
the instrument and clip as instructed
below. Inspect other equipment to be
used with the instrument and clip as
instructed in their respective instruction
manuals. Should the slightest irregularity
be suspected, do not use the instrument
or clip; contact Olympus.
Damage or irregularity may compromise
patient or user safety by, for example:
posing an infection control risk, causing
tissue irritation, perforation, bleeding or
mucous membrane damage. It may also
result in more severe equipment
damage.
• The instrument was not sterilized before
shipment. Before using the instrument for
the first time, reprocess it according to
the instructions in Chapter 5,
“Reprocessing”.
Do not use an instrument that has not
been cleaned and sterilized. This poses
an infection control risk or can cause
tissue irritation.
16
Rotatable Clip Fixing Device
Page 21

Chapter 3 Preparation, Inspection and Operation
• Before use, inspect several coil sheaths
as instructed and make sure they are not
crushed, bent or deformed. Do not use
the clip fixing device if the coil sheath is
damaged.
• Do not strike or crush the coil sheath
during operation or reprocessing. Doing
so can damage the distal end of the
sheath, which could cause make it
impossible to detach the clips after
hemostasis. If a clip cannot be detached
during use, follow the instructions given
in Chapter 4, “Emergency Treatment”.
• Before use, confirm that the hook is not
corroded, dented or discolored; do not
use the instrument if any of these
conditions are observed. A damaged
hook may fall off the instrument’s distal
end.
• Always monitor the endoscopic image
during the procedure. Make sure that the
instrument appears and operates
normally. If the hook comes off of the
distal end, retrieve it with a grasping
forceps.
• Do not coil the insertion portion with a
diameter of less than 20 cm. This could
damage the insertion portion.
• Never use excessive force to operate the
instrument and clip. This could damage
the instrument and/or clip.
Rotatable Clip Fixing Device
17
Page 22

Chapter 3 Preparation, Inspection and Operation
3.1 Preparation
Equipment and Personal Protective Equipment
Prepare all equipment and personal protective
equipment which will be used with the instrument and
clip in accordance with their respective instruction
manuals. Appropriate personal protective equipment
may include: eye wear, a face mask, moisture-resistant
clothing and chemical-resistant gloves.
Spare Instrument and Clips
Always have a spare instrument and clips available.
Reprocessing Equipment
Prepare the reprocessing equipment as described in
Section 5.2, “Required Reprocessing Equipment” for
immediate reprocessing after use.
18
Equipment to be Used in an Emergency
Always have pliers and/or wire cutters ready to cut the
coil sheath, tube sheath and operation wire in case the
clip cannot be detached from the instrument.
Rotatable Clip Fixing Device
Page 23

3.2 Inspection
Wear the personal protective equipment as specified in
the table on page 50.
Before each case, always inspect the instrument and clip
according to the following procedures.
If an abnormality in the instrument or clip is detected, use
a spare instrument or clip, inspecting it thoroughly before
use.
Inspection of the Sterile Package
Chapter 3 Preparation, Inspection and Operation
The clip is a single-use, disposable item.
Do not use or attempt to sterilize it.
Reusing the clip could pose an infection
control risk, cause tissue irritation or
malfunction.
Inspect the sterile package for tears, inadequate sealing
or water damage. If the sterile package shows any
irregularities, the sterile condition of the instrument or clip
may have been compromised. Use a spare instead.
Rotatable Clip Fixing Device
19
Page 24

Chapter 3 Preparation, Inspection and Operation
Appearance Inspection
If any of the following steps reveals irregularities, do not
use the instrument or clip; use a spare instead.
• Do not use the instrument if the distal
• Do not use the instrument if the coil
end of the coil sheath is deformed. Using
an instrument in this condition may result
in the clip catching on the rim of the coil
sheath after clipping and make it
impossible to remove the clip from the
coil sheath.
sheath has any slips, the buckling and
collapse. Using an instrument in this
condition may become impossible to
move through the endoscope
channel.When you forcibly insert the
instrument, it will protrude abruptly from
the distal end of the endoscope. This
could cause patient injury, such as
perforation, bleeding or mucous
membrane damage.
20
1. Confirm that the distal end of the coil sheath is
perfectly round (not deformed or crushed), and that
there are no sharp protrusions, burrs or edges when
viewing the distal end of the coil sheath from the
direction shown in Figure 3.1.
Rotatable Clip Fixing Device
Page 25

Chapter 3 Preparation, Inspection and Operation
Coil sheath
Viewing direction.
Perfectly round Crushed, deformed
Figure 3.1
2. Gently run your fingertips over the entire length of
the insertion portion to check for any slips, the
bucking and collapse broken areas or other damage
(see Figure 3.2, 3.3).
slips
Figure 3.2
Rotatable Clip Fixing Device
Coil sheath
21
Page 26

Chapter 3 Preparation, Inspection and Operation
Figure 3.3
3. Move the slider back and forth. Confirm that the coil
sheath is free from disconnection or looseness.
4. Push the slider to extend the hook from the distal
end of the coil sheath. Confirm that the hook
appears exactly as shown in the tables in Section
2.2, “Specifications” on page 11 and is not
damaged.
Coil sheath
buckling
22
5. Make sure that there are not cracks on the handle.
Rotatable Clip Fixing Device
Page 27

Inspection of Operation
If the instrument does not operate smoothly and as
intended, do not use the instrument; use a spare instead.
1. Holding the instrument as shown in Figure 3.4, form
a loop in the insertion portion approximately 20 cm
in diameter.
Approximately 20 cm.
Chapter 3 Preparation, Inspection and Operation
Extend Retract
Figure 3.4
2. Operate the slider and confirm that the hook retracts
into and extends from the coil sheath smoothly and
as intended.
Rotatable Clip Fixing Device
23
Page 28

Chapter 3 Preparation, Inspection and Operation
3.3 Operation
The operator of the instrument and clip must be a
physician or medical personnel under the supervision of
a physician and must have received sufficient training in
clinical endoscopic technique. This manual, therefore,
does not explain or discuss clinical endoscopic
procedures. It only describes basic operation and
precautions related to the operation of this instrument
and clip.
• Operation of this instrument is based on
• When using the instrument and clip,
the assumption that open surgery is
possible as an emergency measure if the
clip cannot be detached from the
instrument or if any other unexpected
circumstances take place. In this case,
refer to Chapter 4, “Emergency
Treatment”.
always wear appropriate personal
protective equipment. Otherwise, blood,
mucus and other potentially infectious
material from the patient could pose an
infection control risk. Appropriate
personal protective equipment may
include: eye wear, a face mask,
moisture-resistant clothing and
chemical-resistant gloves that fit properly
and are long enough so that your skin is
not exposed.
24
Rotatable Clip Fixing Device
Page 29

Chapter 3 Preparation, Inspection and Operation
• Do not insert the instrument into the
endoscope unless you have a clear
endoscopic field of view. If you cannot
see the distal end of the insertion portion
in the endoscopic field of view, do not
use it. This could cause patient injury,
such as perforation, bleeding or mucous
membrane damage. It may also damage
the endoscope, instrument and/or clip.
• Do not angulate the bending section of
the endoscope abruptly while the distal
end of the insertion portion is extended
from the distal end of the endoscope.
This could cause patient injury, such as
perforation, bleeding or mucous
membrane damage.
• Do not force the distal end of the
insertion portion against body cavity
tissue. This could cause patient injury,
such as perforation, bleeding or mucous
membrane damage.
• When electrosurgical accessories are
used after clipping, they could cause
patient injury, such as thermal injury of
body cavity tissue contacting the clip.
Activate output only after checking the
tissue around the clip.
When using the instrument with a two
channel endoscope, never use
electrosurgical accessories at the same
time. This could cause patient, operator
or assistant injury, such as thermal
injury.
Rotatable Clip Fixing Device
25
Page 30

Chapter 3 Preparation, Inspection and Operation
Attaching the Clip
• Do not use a clip that has not been
• After attaching the clip, dispose of the
• After retracting the clip into the coil
• Do not disassemble a used cartridge and
properly attached. Otherwise, the clip
may not operate correctly or it could be
damaged.
cartridge properly. Otherwise, infection
may result.
sheath, do not extend the clip out from
the tube sheath until the clip is actually
going to be used. If the clip is extended
unnecessarily, it may be impossible to
insert it into the endoscope.
attempt to reuse the clip in it. Otherwise,
the clip cannot be attached properly or
may be damaged.
26
To attach additional clips after the first
one, detach the clip connector from the
hook by following the instructions in
“Detaching the Clip Connector” on
page 38.
If the clip cannot be attached securely, do not use the
clip and/or instrument; use a spare instead.
1. Open the package containing the cartridge.
2. Carefully pull the slider up to the ring (yellow).
Rotatable Clip Fixing Device
Page 31

Chapter 3 Preparation, Inspection and Operation
3. Place the cartridge on the coil sheath (see Figure
3.5).
Figure 3.5
4. Hold the coil sheath in position by pinching the grip
of the cartridge. At this time, confirm that the coil
sheath can be suspended (see Figure 3.6).
Figure 3.6
Rotatable Clip Fixing Device
27
Page 32

Chapter 3 Preparation, Inspection and Operation
5. Push the slider forward (distally) until it clicks, then
pull it toward you (proximally) until it stops (see
Figure 3.7 ). The clip is now attached inside the coil
sheath.
Keep pinching the grip of the cartridge
until the clip has been attached
completely. Otherwise, the positioning
between the coil sheath and cartridge
may be deviated and the clip may be
unable to be mounted.
28
Figure 3.7
Do not push the slider too much forward.
Otherwise, the clip may be damaged.
6. Confirm that the clip has been successfully removed
from the cartridge and that it is not extending from
the coil sheath.
7. After attaching the clip, dispose of the cartridge
properly.
Rotatable Clip Fixing Device
Page 33

Chapter 3 Preparation, Inspection and Operation
Insertion Into the Endoscope
• Do not force the instrument if resistance
to insertion is encountered. Reduce the
angulation of the endoscope until the
instrument passes smoothly. Attempting
to force the instrument could cause
patient injury, such as perforation,
bleeding or mucous membrane damage.
It may also damage the endoscope and/
or instrument.
• Hold the slider still when inserting the
instrument into the endoscope.
Otherwise, the clip will open and may
extend from the distal end of the
endoscope abruptly. This could cause
patient injury, such as perforation,
bleeding or mucous membrane damage.
It could also damage the endoscope,
instrument and/or clip.
• Do not advance or extend the instrument
abruptly. This could cause patient injury,
such as perforation, bleeding or mucous
membrane damage. It could also
damage the endoscope, instrument and/
or clip.
• When inserting the instrument into the
endoscope, make sure that the clip is
completely retracted into the coil sheath.
Otherwise, patient injury, such as
perforation, bleeding or mucous
membrane damage may result. It could
also damage the endoscope, instrument
and/or clip.
Rotatable Clip Fixing Device
29
Page 34

Chapter 3 Preparation, Inspection and Operation
• When inserting the instrument into the
• Insert the instrument slowly. Abrupt
• As the distal end of the coil sheath is
1. Confirm that the entire clip is retracted into the coil
sheath.
endoscope, hold it close to the biopsy
valve and keep it as straight as possible
relative to the biopsy valve. Otherwise,
the insertion portion could be damaged.
insertion could damage the endoscope
and/or instrument. If the insertion portion
of the instrument is damaged, the
rotation function will be impaired.
thicker than other parts, resistance may
be felt when it passes through the
section near the biopsy valve. In this
case, do not advance it forcibly but gently
advance it upright with respect to the
biopsy valve. Otherwise, deformation of
the coil sheath may result.
30
2. Carefully insert the instrument into the biopsy valve
(see Figure 3.8).
Keep as straight as
possible.
Hold the
insertion portion
close to the
biopsy valve.
Biopsy valve
Figure 3.8
Rotatable Clip Fixing Device
Page 35

3. Advance the instrument until the distal end of the
insertion portion appears within the endoscopic field
of view.
Clipping Tissue
Chapter 3 Preparation, Inspection and Operation
• Do not extend the clip abruptly from the
distal end of the coil sheath. Also, when
pushing the clip out of the coil sheath,
keep a sufficient distance between the
distal end of the coil sheath and the
mucous membrane. If the clip is
extended without keeping this distance,
the clip may hit against the tissue
unintentionally, and perforation,
hemorrhage or mucous membrane
damage or dropping off of the clip, or
break up of the clip may result.
• Do not force the clip against body cavity
tissue. The clip may be deformed and
does not close properly. This could result
in reduced performance.
• Do not try to forcibly remove the clip if it
becomes caught on the distal end of the
coil sheath. Forcible removal of the clip
could cause patient injury such as
perforation, bleeding or mucous
membrane damage.
• Do not withdraw the instrument if clipping
is not completely finished (when the
slider is not pulled out all the way in the
proximal direction). Doing so may tear
tissue inside the body cavity, resulting in
patient injury, such as perforation,
bleeding or mucous membrane damage.
Rotatable Clip Fixing Device
31
Page 36

Chapter 3 Preparation, Inspection and Operation
• When clipping tissue, do not change the
• When aspirating body fluid via the
• If the clip does not extend when the
angulation of the endoscope before the
clip is detached from the instrument.
Doing so may result in patient injury,
such as perforation, bleeding or mucous
membrane damage.
endoscope, do not aspirate a clip or clip
connector which has been dropped
inside the body cavity, as this could
disable the endoscope's suction function.
If a clip or clip connector is accidentally
aspirated into the endoscope, follow the
procedure given in “Removal of an
Aspirated Clip or Clip Connector” on
page 39.
slider is pushed, straighten the angulated
distal end of the endoscope until the clip
can be extended smoothly. Otherwise,
the endoscope and/or instrument may be
damaged.
32
After the clip is deployed, do not move
the slider in the distal direction prior to
withdrawing the instrument from the
patient. Doing so could extend the hook
from the coil sheath, which could cause
the clip connector to fall off into the
patient.
Rotatable Clip Fixing Device
Page 37

Chapter 3 Preparation, Inspection and Operation
1. Gently push the slider so that the clip projects from
the coil sheath until the white part of the clip is
visible. At this time, the clip should appear in the
endoscopic image as shown in Figure 3.9(b).
Once the clip of the rotatable clip fixing
device is extended from the coil sheath,
the clip cannot be accommodated in the
coil sheath again. If it is required to
discontinue clipping after extending the
clip from the distal end of the sheath,
either close the clip and withdraw the
entire endoscope or let the clip inside the
body and then collect the clip.
(a)
(b)
Figure 3.9
Rotatable Clip Fixing Device
White part
33
Page 38

Chapter 3 Preparation, Inspection and Operation
2. Pull the slider slowly towards you to open the clip
(see Figure 3.10).
Figure 3.10
3. Hold only the ring (yellow) and rotate the rotation
grip to orient the clip so that it may be applied to the
tissue (see Figure 3.11).
Do not pull the slider quickly. This will
open and close the clip.
34
Figure 3.11
Rotatable Clip Fixing Device
Page 39

Chapter 3 Preparation, Inspection and Operation
• Rotating the rotation grip also causes the
slider to rotate together. Therefore, be
sure to remove your finger from the slider
when rotating the rotation grip.
• Rotate the rotation grip slowly. If it is
rotated quickly, the clip rotation may
jump.
• If the endoscope is bent sharply, there
may be a delay between the rotation of
the rotation grip and that of the clip.
• If the clip will not rotate smoothly,
straighten out the part of the insertion
tube extending from the forceps port as
straight as possible and rotate the
rotation grip (see Figure 3.12).
Figure 3.12
4. Press the clip against the targeted lesion.
Rotatable Clip Fixing Device
35
Page 40

Chapter 3 Preparation, Inspection and Operation
5. Pull the slider firmly to close the clip on the target
site (see Figure 3.13).
Figure 3.13
6. Gently pull the slider up to the thumb ring (yellow)
gently to detach the closed clip from the coil sheath
(see Figure 3.14).
36
Figure 3.14
Rotatable Clip Fixing Device
Page 41

Chapter 3 Preparation, Inspection and Operation
Withdrawing the Instrument From the Endoscope
Do not withdraw the instrument from the
endoscope quickly. This could scatter
blood, mucus or other patient debris and
pose an infection control risk.
• Do not withdraw the instrument from the
endoscope if the forceps elevator is up.
This could damage the endoscope and/
or instrument.
• Do not withdraw the instrument from the
endoscope if the hook is not completely
retracted into the coil sheath. This could
damage the endoscope and/or
instrument.
• Do not withdraw the instrument abruptly
from the endoscope. Otherwise, damage
to the endoscope or instrument may
result.
• As the distal end of the coil sheath is
thicker than other parts, resistance may
be felt when it passes through the
section near the biopsy valve. In this
case, do not withdraw it forcibly but
gently pull it upright with respect to the
biopsy valve. Otherwise, deformation of
the coil sheath may result.
1. Lower the forceps elevator when using an
endoscope equipped with a forceps elevator.
2. Withdraw the instrument from the endoscope.
Rotatable Clip Fixing Device
37
Page 42

Chapter 3 Preparation, Inspection and Operation
Detaching the Clip Connector
1. Push the slider so that the hook is extended from the
coil sheath. Now bend the clip connector with
respect to the hook and remove (see Figure 3.15).
After removing the clip connector,
dispose of it properly. Otherwise,
infection may result.
38
Figure 3.15
2. After removing the clip connector, dispose of it
properly.
Rotatable Clip Fixing Device
Page 43

Chapter 3 Preparation, Inspection and Operation
Removal of an Aspirated Clip or Clip Connector
If a clip or clip connector is accidentally aspirated into the
endoscope, follow the procedure below to remove it.
1. Withdraw the endoscope from the body cavity,
keeping the insertion portion and bending section
straight. Leave the biopsy valve mounted on the
endoscope.
2. Remove the suction tube and connect a syringe
filled with tap water to the endoscope’s suction
connector (see Figure 3.16).
Figure 3.16
3. While gently pressing the suction valve, inject the
tap water into the endoscope’s suction connector
(see Figure 3.17).
Rotatable Clip Fixing Device
39
Page 44

Chapter 3 Preparation, Inspection and Operation
Figure 3.17
4. Irrigation should discharge the clip or clip connector
from the endoscope. If one injection is not enough to
discharge the clip or clip connector, repeat steps 2.
and 3. until the clip or clip connector is discharged.
Push the suction valve.
40
Rotatable Clip Fixing Device
Page 45

Chapter 4 Emergency Treatment
Chapter 4 Emergency Treatment
4.1 Emergency Treatment
Do not try to forcibly withdraw the
instrument from the endoscope if the clip
cannot be detached from the instrument.
Forcibly withdrawing the instrument
could cause patient injury such as
perforation, bleeding or mucous
membrane damage.
If the clip cannot be detached from the instrument, follow
the procedures described in this section.
If the distal end of the coil sheath or clip pipe is crushed
or deformed, it may not be possible to detach the clip
from the instrument.
Rotatable Clip Fixing Device
41
Page 46

Chapter 4 Emergency Treatment
Keep as straight as
possible.
Straighten out the portion of
the endoscope that extends
from the patient.
Keep as straight
as possible.
Straighten out the portion of
the instrument that extends
from the biopsy valve.
Confirm that the slider has been
pulled out as far as possible.
When the
clip cannot
be detached
from the
instrument.
Keep as straight as possible.
Straighten out the coil
sheath that extends from
the distal end of the
endoscope.
42
Rotatable Clip Fixing Device
Page 47

Chapter 4 Emergency Treatment
1. Push the slider to extend
the hook from coil
sheath.
3. Pull the slider to withdraw
the hook inside the coil
sheath, and remove the
clip from the rotatable clip
fixing device.
2. While adjusting the
endoscope angle, push
the coil sheath to bend
the clip with respect to
the hook.
When the clip cannot be detached from
the instrument.
Rotatable Clip Fixing Device
43
Page 48

Chapter 4 Emergency Treatment
Cut the coil sheath and
operation wire using pliers
and/or wire cutters.
Withdraw the endoscope.
A certain amount of force is sometimes
required to withdraw the endoscope.
44
Rotatable Clip Fixing Device
Leave the clip in place
until tissue necrosis
occurs and the clip
comes free. This may
take some time.
Carry out open surgery or
other possible treatment.
Page 49

Chapter 5 Reprocessing
This instrument was not sterilized before
shipment. Before using this instrument
for the first time, reprocess it according to
the instructions in this Chapter. Do not
use an instrument that has not been
cleaned and sterilized. This poses an
infection control risk or can cause tissue
irritation.
5.1 General Policy
• The medical literature reports incidents of patient
cross contamination resulting from improper
cleaning or sterilization. It is strongly
recommended that reprocessing personnel have
a thorough understanding of and follow all
national and local hospital guidelines and
policies.
A specific individual or individuals in the
endoscopy unit should be responsible for
reprocessing endoscopic equipment. It is highly
desirable that a trained backup be available
should the primary reprocessing individual(s) be
absent.
• All individuals responsible for reprocessing
should thoroughly understand:
Chapter 5 Reprocessing
• your institution’s reprocessing
procedures
• occupational health and safety
regulations
• national and local hospital guidelines and
policies
Rotatable Clip Fixing Device
45
Page 50

Chapter 5 Reprocessing
• the instructions in this manual
• the mechanical aspects of endoscopic
equipment
• pertinent germicide labeling
Olympus endo-therapy accessories are compatible with
2.0% to 3.2% glutaraldehyde solution. However, routine
biological monitoring is not feasible with glutaraldehyde
and, therefore, it should not be used to sterilize reusable
medical devices that are compatible with other methods
of sterilization that can be biologically monitored, such as
steam sterilization.
• Failure to properly clean and sterilize the
instrument after each examination can
compromise patient safety. During use,
the instrument normally comes in contact
with intact mucous membranes. To
minimize the risk of transmitting diseases
from one patient to another, after each
examination the instrument must
undergo thorough cleaning followed by
sterilization.
• If the instrument is not cleaned
meticulously, effective sterilization
cannot be obtained. Clean the instrument
thoroughly before sterilization to remove
microorganisms or organic material
which can limit the effectiveness of the
sterilization process.
46
Rotatable Clip Fixing Device
Page 51

Chapter 5 Reprocessing
• Patient debris and reprocessing
chemicals are hazardous. Wear personal
protective equipment to guard against
dangerous chemicals and infectious
material. During cleaning and
sterilization, always wear appropriate
personal protective equipment, such as
eye wear, a face mask,
moisture-resistant clothing and
chemical-resistant gloves that fit properly
and are long enough so that your skin is
not exposed. Always remove
contaminated protective clothing before
leaving the reprocessing area.
• The reprocessing procedures described
in this manual should be completed the
same day the instrument has been used.
If reprocessing is delayed, residual
organic debris will solidify and it may be
difficult to effectively reprocess the
instrument.
• Reprocess the instrument immediately
after use, first by immersing it in a
neutral, low-foaming, medical-grade
detergent solution, then following the
remaining steps as instructed in this
chapter. Failure to reprocess the
instrument immediately after use, or
using other than a medical-grade
detergent may cause corrosion at the
instrument’s hook. This could cause the
hook to break and/or come off inside the
patient.
Rotatable Clip Fixing Device
47
Page 52

Chapter 5 Reprocessing
• With the cleaning and sterilization
methods stated in this instruction
manual, prions, which are considered to
be the pathogenic substance of the
Creutzfeldt-Jakob disease (CJD) cannot
be destroyed or inactivated. When using
this instrument on a patient with CJD or
variant Creutzfeldt-Jakob disease
(vCJD), be sure to use this product for
such patient only and/or immediately
dispose of this product after use in an
appropriate manner. For methods to
handle CJD, please follow the respective
guidelines in your country.
• This instrument is not durable, or does
not have sufficient durability against the
respective methods stated in the
guidelines of each country for destroying
or inactivating prions. For information on
the durability against each method,
please contact Olympus. If cleaning and
sterilization methods not stated in this
instruction manual are performed,
Olympus cannot guarantee the
effectiveness, safety and durability of this
instrument. Make sure to confirm that
there is no abnormality before use, and
use under responsibility of a physician.
Do not use if any abnormality is found.
48
Rotatable Clip Fixing Device
Page 53

Chapter 5 Reprocessing
5.2 Required Reprocessing Equipment
Wear the personal protective equipment as specified in
the Table on page 50.
1. Prepare the following equipment. The required
amount of detergent solution, lubricant and other
equipment depends on the number of instruments to
be reprocessed.
2. Fill an immersion basin with detergent solution and
fill a second immersion basin with lubricant at the
temperatures and concentrations recommended by
the manufacturers. Also fill the ultrasonic cleaner
with a detergent solution appropriate for ultrasonic
cleaning.
Equipment Needed for Reprocessing
To perform proper reprocessing, the equipment in the
following table is required. For details on preparation and
directions for use of the following equipment, refer to the
respective instruction manuals or contact the equipment
manufacturer.
Contact Olympus for the names of specific brands of
detergent solutions and lubricants.
Rotatable Clip Fixing Device
49
Page 54

Chapter 5 Reprocessing
Equipment Needed
Protective
equipment
Immersion basin
for detergent
solution
Detergent solution
for immersion
Ultrasonic cleaner Use a medical grade ultrasonic cleaner with
Detergent solution
for ultrasonic
cleaning
Lubricant Use a medical grade water soluble or
Immersion basin
for lubricant
Lint-free cloths
Packages for steam
sterilization
Appropriate personal protective equipment
may include: Eye wear, face mask,
moisture-resistant clothing and
chemical-resistant gloves.
Use a basin with a depth and diameter large
enough to allow complete immersion of the
instrument when the insertion portion is
coiled with a diameter of not less than 20 cm.
Use a neutral pH, low-foaming, medical
grade detergent solution.
a frequency range of 38 to 47 kHz, and with a
depth and a diameter large enough to allow
complete immersion of the instrument when
the insertion portion is coiled with a diameter
of not less than 20 cm.
Compatible ultrasonic cleaners include
OLYMPUS ULTRASONIC CLEANER KS-2.
Use a neutral pH, low-foaming, medical
grade detergent solution with no abrasive.
low-viscosity emulsion type lubricant.
Use a basin with a depth and diameter large
enough to allow complete immersion of the
instrument when the insertion portion is
coiled with a diameter of not less than 20 cm.
Use packages compatible with steam
sterilization (autoclaving). The packages
should be large enough to accommodate the
instrument when the insertion portion is
coiled with a diameter of not less than 20 cm.
50
Rotatable Clip Fixing Device
Page 55

Chapter 5 Reprocessing
Sealing device for
packages
Autoclave Use an autoclave that will operate at the
5.3 Cleaning
Sealing the packages may require a device
such as a heat sealer. Prepare an
appropriate sealing device according to the
packages to be used.
conditions specified in Section 5.5,
“Sterilization”.
When cleaning, avoid exposure to the
reprocessing chemicals. It may pose an
infection control risk or cause skin
irritation.
• When reprocessing, do not coil the
insertion portion with a diameter of less
than 20 cm. This could damage the
insertion portion.
• Never use excessive force to operate the
instrument. This could damage the
instrument.
Immersion
Rotatable Clip Fixing Device
Immerse the instrument in detergent
solution immediately after use. If the
instrument is not cleaned immediately, it
may be difficult to effectively reprocess,
and this could result in reduced
performance.
51
Page 56

Chapter 5 Reprocessing
Ultrasonic Cleaning
Rinsing
1. Immerse the entire instrument in the detergent
solution for the time specified in manufacturer’s
instructions. If no time is specified, immerse for
between 5 minutes and 3 hours.
2. Remove the instrument from the detergent solution.
1. Immerse the entire instrument in the ultrasonic
cleaner containing detergent solution.
2. Clean ultrasonically for 30 minutes. For details on
operation of the ultrasonic cleaner, refer to the
instruction manual of the ultrasonic cleaner.
3. Remove the instrument from the detergent solution.
52
• After ultrasonic cleaning, rinse the
instrument thoroughly to remove residual
detergent. Residual detergent solution
could cause tissue irritation in the next
patient.
• Do not forcefully squeeze, wipe or scrub
the instrument. This could cause damage
to the instrument or result in reduced
performance.
1. Rinse the instrument under clean running tap water.
2. Confirm that no debris is left on the surfaces of the
instrument.
3. Wipe the exterior of the instrument with a clean, dry
lint-free cloth.
Rotatable Clip Fixing Device
Page 57

5.4 Lubrication
1. Immerse the insertion portion in the lubricant for 2 to
3 seconds.
2. Remove the instrument from the lubricant.
3. Move the slider back and forth two or three times to
retract the hook into and extend it from coil sheath.
Chapter 5 Reprocessing
When lubricating, avoid exposure to the
lubricant. It may pose an infection control
risk or cause skin irritation.
• Do not coil the insertion portion with a
diameter of less than 20 cm. This could
damage the insertion portion.
• Never use excessive force to operate the
instrument. This could damage the
instrument.
4. Wipe the exterior of the instrument with a clean, dry
lint-free cloth and allow the instrument to air dry.
Rotatable Clip Fixing Device
53
Page 58

Chapter 5 Reprocessing
5.5 Sterilization
Sealing the Package
Before placing the instrument in the
package, always retract the hook into the
coil sheath. Otherwise, they could tear
the package during sterilization or
storage and compromise its sterility,
which could pose an infection control risk
or cause tissue irritation.
• Do not coil the insertion portion with a
diameter of less than 20 cm. This could
damage the insertion portion.
• Never use excessive force to operate the
instrument. This could damage the
instrument.
54
1. Before sterilization, the instrument must be
thoroughly cleaned and dried. Residual moisture
inhibits sterilization.
2. Coil the insertion portion and place the instrument in
the package.
3. Seal the package. For details on sealing, refer to the
instruction manual of the package and the sealing
device.
Rotatable Clip Fixing Device
Page 59

Steam Sterilization (Autoclaving)
• Use biological indicators as
recommended by your hospital’s policy
and follow the manufacturer’s
instructions, all national and local
hospital guidelines and policies.
• Always leave space between the
packages in the autoclave. If the
packages are placed too close together,
effective sterilization will not be possible.
• Allow the packages to dry within the
autoclave using the autoclave’s drying
cycle (if applicable) or by opening the
door of the autoclave and allowing the
packages to air dry. Handling a wet
package can compromise its sterility.
• The results of sterilization depend on
various factors such as how the sterilized
instrument was packed or the
positioning, method of placing and
loading of the instrument in the
sterilization device. Please verify the
sterilization effects by using biological or
chemical indicators. Also follow the
guidelines for sterilization issued by
medical administrative authorities, public
organizations or the infection
management sections at each medical
facility, as well as the instruction manual
of the sterilization device.
Chapter 5 Reprocessing
1. Place the sealed package containing the instrument
in the autoclave and sterilize in accordance with the
conditions listed below. For details on operation of
the autoclave, refer to the instruction manual for the
autoclave or other manufacturer instructions.
Rotatable Clip Fixing Device
55
Page 60

Chapter 5 Reprocessing
2. After steam sterilization, let the instrument gradually
cool down to room temperature. Sudden changes in
temperature may damage the instrument.
Autoclavable products have a green
reference label. Products that do not
have green reference labels are not
autoclavable.
Temperature Exposure Time
Prevacuum
Table 5.1 Recommended steam sterilization
(autoclaving) conditions
132 to 134
(270 to 274
°C
°F)
5 minutes
56
Rotatable Clip Fixing Device
Page 61

Chapter 6 Storage
• Do not store the instrument in a sterile
package that is damaged, wet or
improperly sealed. Otherwise, the
sterility of the instrument may be
compromised and could pose an
infection control risk or cause tissue
irritation.
• Do not store the sterile packages
containing the instrument in places
where they will become damaged, wet or
improperly sealed. Otherwise, the
sterility of the instrument may be
compromised and pose an infection
control risk or cause tissue irritation.
Chapter 6 Storage
Do not coil the insertion portion with a
diameter of less than 20 cm. This could
damage the insertion portion.
6.1 Inspection Before Storage
Prior to storage, inspect the sterile package as follows:
Confirm that the sterile package containing the
instrument is free from tears, inadequate sealing or water
damage. If tears, inadequate sealing or water damage is
detected, repackage and sterilize again as described in
Section 5.5, “Sterilization”.
Rotatable Clip Fixing Device
57
Page 62

Chapter 6 Storage
6.2 Storage
Store the instrument in the sterile package at room
temperature in a clean and dry environment. Do not store
it in direct sunlight. Ensure that the packaged instrument
is not crushed by surrounding objects during storage.
Follow any additional storage instructions provided by
the manufacturer of the sterile package.
58
Rotatable Clip Fixing Device
Page 63

©2004 OLYMPUS MEDICAL SYSTEMS CORP. All rights reserved.
No part of this publication may be reproduced or distributed without the
express written permission of OLYMPUS MEDICAL SYSTEMS CORP.
OLYMPUS is a registered trademark of OLYMPUS CORPORATION.
Page 64

Manufactured by
2951 Ishikawa-cho, Hachioji-shi, Tokyo 192-8507, Japan
Fax: (042)646-2429 Telephone: (042)642-2111
Distributed by
3500 Corporate Parkway, P.O. Box 610 Center Valley, PA
Fax: (484)896-7128 Telephone: (484)896-5000
One Corporate Drive, Orangeburg, N.Y. 10962, U.S.A.
Fax: (845)398-9444 Telephone: (845)398-9400
5301 Blue Lagoon Drive, Suite 290 Miami, FL 33126-2097, U.S.A.
Fax: (305)261-4421 Telephone: (305)266-2332
(Premises/Goods delivery) Wendenstrasse 14-18, 20097 Hamburg, Germany
(Letters) Postfach 10 49 08, 20034 Hamburg, Germany Telephone: (040)237730
KeyMed House, Stock Road, Southend-on-Sea, Essex SS2 5QH, United Kingdom
8F, Hyundai Marines Bldg., 646-1, Yeoksam-Dong, Kangnam-Gu, Seoul 135-080 Korea
491B, River Valley Road #12-01/04, Valley Point Office Tower, Singapore 248373
Fax: (01702)465677 Telephone: (01702)616333
117071, Moscow, Malaya Kaluzhskaya 19, bld. 1, fl.2, Russia
Fax: (095)958-2277 Telephone: (095)958-2245
Room 1202, NCI Tower, A21 Jianguomenwai Avenue Chaoyang
Fax: (10)6569-3545 Telephone: (10)6569-3535
Fax: (02)6255-3499 Telephone: (02)1544-3200
31 Gilby Road, Mount Waverley, VIC., 3149, Australia
Fax: (03)9543-1350 Telephone: (03)9265-5400
18034-0610, U.S.A.
District Beijing 100022 PRC
Fax: 6834-2438 Telephone: 6834-0010
GK4803 08
Printed in Japan 20050411 *0000
 Loading...
Loading...