Nikon C1si User Manual

Page 1 of 7
Nikon C1si
Spectral Laser Scanning Confocal Microscope
User Guide
Contents:
C1Si Turn-On/ShutDown Procedures............................................................................................. 2!
Overview ......................................................................................................................................... 4!
Setup for epi-illumination to view through the eyepieces: ............................................................... 5!
Setup for confocal imaging:............................................................................................................. 5!
Notes:.............................................................................................................................................. 6!
Troubleshooting ..............................................................................................................................7!
Owners Consortium:
Department of Pathology
SABRE
Proctor Foundation
Page 2 of 7
C1Si Turn-On/ShutDown Procedures
Turn On:
1. Turn on arc lamp (Nikon Intensilight on shelf above table). This should be left on for
at least 30 minutes at a run and turned off for at least 30 minutes prior to turning on
again, this prevents the arc from flickering and wearing out prematurely
2. Turn on the lasers that you will use: (you don’t need to turn them all on)
a. 2x Diodes on the laser bench: turn key from 12 o’clock to 3 o’clock.
b. 1x Diode on top of the control box: turn key and then push ‘laser on’ (green
button).
c. Argon-Ion multi-line: Turn the key clockwise (do not turn off switch).
3. Turn on the remote focus accessory: switch is on left hand side of box.
4. Turn on the control box (Nikon D-Eclipse C1). Don’t start software until ‘ready’ light
is on.
5. Make sure the Z-stepper motor slider (located back-right of microscope) is pushed
down for manual operation (or up if you are going to use the remote focus
accessory).
6. Choose and carefully load 1 or 2 objective lenses. Center the lever so that both
lens sockets are in the up position. Use both hands and gently thread it until it is
finger tight. Do not over tighten it or it will get stuck.
Available Objectives
Dipping objectives are designed to form an image without a cover slip, whereas
immersion lenses require a cover slip. For ideal imaging, always use #1.5 cover slips.
You can then switch between the two different objectives that you have loaded by
turning the lever on the front so that it is in the middle (this is very important because
if it is not in the middle the objectives can come crashing down and get damaged),
pressing the black button on the top right, and carefully moving the carriage forward
or backwards.
Number
Objective
Theoretical
Resolution
(nm)
Depth of
field (um)
Suggested
step size
(um)
Transmitted
light
Working
Distance
Comments
1
10x/0.30 NA
W Plan Fluor
1017
14.4
5.76
DIC N1/10x
0.3 mm
Water
Dipping
2
20x/0.75 NA
air CFI Plan
Apo
407
2.31
0.924
DIC N2/20x
0.75 mm
Air
3
40x NIR/ 0.8
NA W
381
2.03
0.812
DIC N2/40x
III
3.5 mm
Water
Dipping
4
40x/1.30 NA
oil Plan
Fluor
235
0.77
0.308
DIC N2/40x
II
0.20 mm
Oil
Immersion
5
60x/1.2 NA
W NIR Apo
250
~0.7
~0.4
DIC N2/60x I
0.27 mm
Water
Immersion
6
60x/1.4 NA
oil Plan Apo
VC
218
0.66
0.264
DIC N2/60x I
0.21 mm
Oil
Immersion
Page 3 of 7
7. Turn on computer:
8. Launch software (EZ-C1 3.80).
a. Choose the colors you are going to use: 408/488/561, 488/561/638, or
457/514 (If you plan on doing spectral imaging it doesn’t matter what you
choose in the software).
9. Choose standard or spectral detector.
a. Standard detector: Flip lever on side of the scanhead diagonal.
b. Spectral detector: Flip lever on side of scanhead vertical.
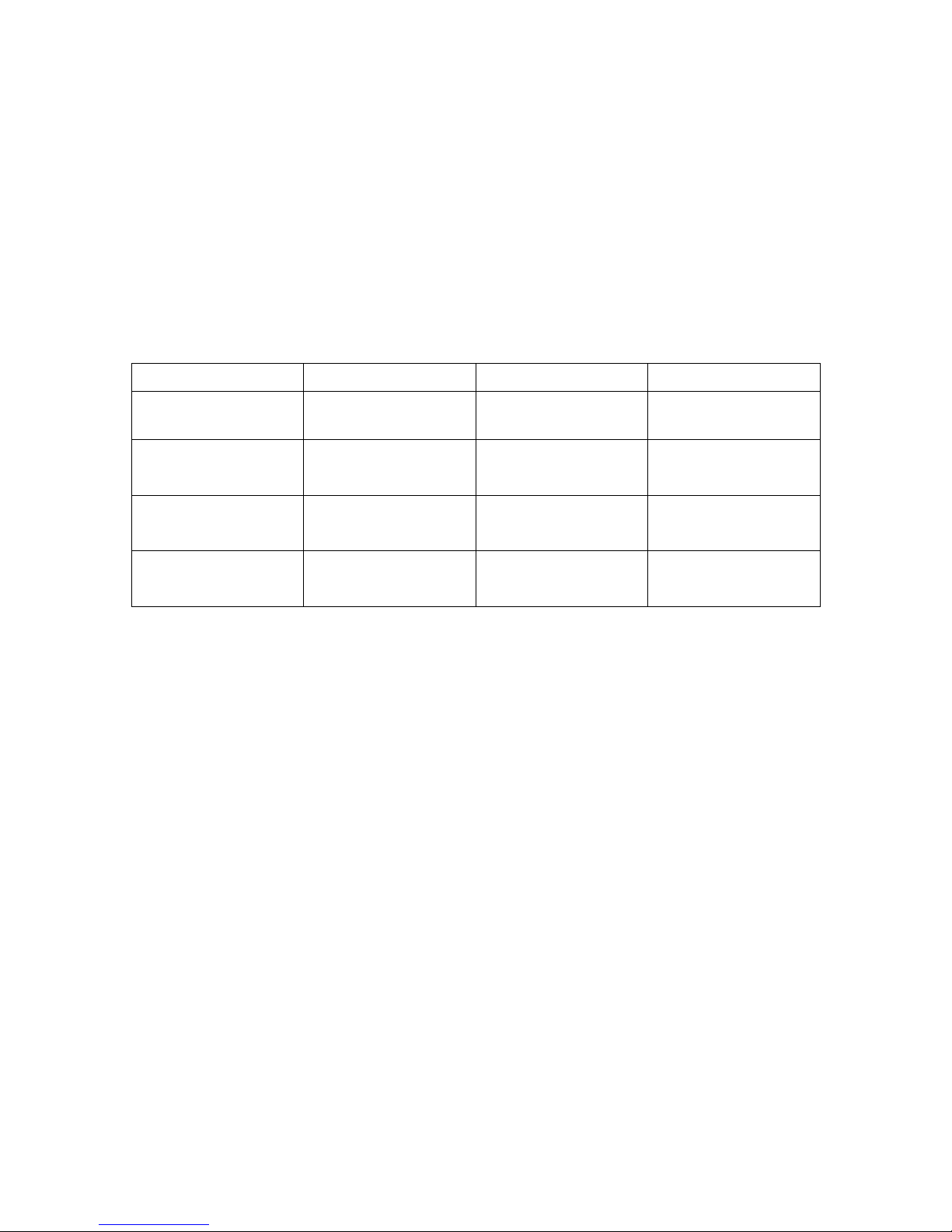
10. Choose excitation/emission wavelengths and put in scanhead dichroics and filter
cubes:
Scanhead Dichroic
Left Filter Cube
Right Filter Cube
Beam splitter
(spectral detector)
BS 20/80
n/a
n/a
Blue/Green/Red
408/488/561
595/50
525/50
450/40
Green/Red/Far Red
488/561/638
685/70
595/50
525/50
CFP/YFP
457/514
550/50
485/30
11. Align pinhole for your particular scanhead dichroic that you have selected:
a. Place a green fluorescent slide under your objective and focus on it using the
arc lamp and the eyepieces (you need to push the eyepiece slider in, open
the shutter, and choose the GFP filter cube) – it gets very bright so you can
put in the neutral density filters on the back right of the scope so you don’t
blind yourself.
b. Engage the confocal (pull eyepiece slider out, close shutter, and move filter
cube to open position – #6).
c. Turn the software onto live and set it up so the color changes with intensity,
256x256 pixels, green laser and green detector are on, and then go live.
d. Turn up the gain on the green detector until you get signal.
e. Use a 2.5mm hex key to align the pinhole using the two screws on the bottom
right of the scan head.
i. This is done by ‘walking’ the pinhole: Move one screw until it gets as
bright as possible, then move the other screw to the brightest point, return
to the first one, and repeat until you get the brightest image possible. If
the image gets too bright so that all the pixels are saturated just turn
down the detector gain.
12. Take off the fluorescent slide and start imaging!
13. If using transmitted light, turn on halogen lamp (Lamp with dial on shelf) or turn off
for fluorescence.
 Loading...
Loading...