Hill Rom 041115 User Manual
WatchCare™ Incontinence Management
DRAFT 26-FEB-2019
System
User and Service Manual
Product No. P006979
196414 REV 2

DRAFT 26-FEB-2019

REVISION
DRAFT 26-FEB-2019
© 2019 by Hill-Rom Services, Inc. ALL RIGHTS RESERVED.
Distributed by:
HILL-ROM, INC.
1069 STATE ROUTE 46 E
BATESVILLE, IN 47006-9167 USA
800-445-3720
Manufactured by:
HILL-ROM, INC.
1069 STATE ROUTE 46 E
BATESVILLE, IN 47006-9167 USA
No part of this text shall be reproduced or transmitted in any form or by
any means, electronic or mechanical, including photocopying, recording,
or by any information or retrieval system without written permission from
Hill-Rom Services, Inc. (Hill-Rom).
The information in this manual is confidential and may not be disclosed to
third parties without the prior written consent of Hill-Rom.
The information in this manual is subject to change without notice. HillRom makes no commitment to update or keep current, the information in
this manual.
Hill-Rom reserves the right to make changes without notice in design,
specifications, and models. The only warranty Hill-Rom makes is the
express written warranty extended on the sale or rental of its products.
The WatchCare™ Incontinence Management System product may be
covered by one or more patents. For a list of applicable patents, go to
www.hill-rom.com/patents. The Hill-Rom companies are the proprietors
of European, US, and other patents and pending patent applications.
This product may contain software known as “free” or “open source”
software (FOSS). Hill-Rom uses and supports the use of FOSS. We believe
that FOSS makes our products more robust and secure, and gives us and
our customers greater flexibility. To learn more about FOSS that may be
used in this product, please visit our FOSS website at www.hill-
rom.com/opensource. Where required, a copy of FOSS source code is
available on our FOSS website.
Product images and labels are for illustrative purposes only. Actual
product and label may vary.
Second Edition, DRAFT 2019-03
WatchCare™ Incontinence Management System User and Service Manual (196414 REV 2)
i

First Printing, 2018
DRAFT 26-FEB-2019
Bluetooth® is a registered trademark of Bluetooth Sig, Inc.
Clorox HealthCare® is a registered trademark of Clorox Professional
Products Company, Inc.
OxyCide® is a registered trademark of Ecolab USA Inc.
Torx® is a registered trademark of Acument Intellectual Properties, LLC.
Virex® is a registered trademark of Diversey, Inc.
Wex-Cide™ is a trademark of Wexford Labs, Inc.
Quick View™, StayInPlace™, and WatchCare™ are trademarks of Hill-Rom
Services, Inc.
Centrella®, Hill-Rom®, NaviCare®, Progressa®, Safe Skin®, SafeView®,
SideCom®, SmartSync®, and VersaCare® are registered trademarks of HillRom Services, Inc.
Replace this manual (196414) if it is damaged and/or can not be read.
For product support or to order additional copies of this manual (196414),
contact your local Hill-Rom representative or go to www.hill-rom.com.
Reference Documents
Centrella® Smart+ Bed User Manual (193587)
Centrella® Smart+ Bed Service Manual (193588)
Progressa® Bed User Manual (171528)
Progressa® Bed Service Manual (171748)
VersaCare® Bed User Manual (USR119 (A through J models); 161956 (K
model and newer))
VersaCare® Bed Service Manual (MAN333 (A through J models); 161955 (K
model and newer))
WatchCare™ Incontinence Management System User and Service Manual (196414 REV 2)
ii

Table of Contents
DRAFT 26-FEB-2019
Intended Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1
Symbols . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
Document Symbols . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
Product Symbols . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
Safety Instructions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
FCC Guidance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
Quick View™ List of Features . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
Standard Features . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
Indicator Light—Visual Identification . . . . . . . . . . . . . . . . . . . . . 8
Prepare the System for Use. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
Replace the WatchCare™ smart pad. . . . . . . . . . . . . . . . . . . . . . 12
Transport The Bed . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
Cleaning and Disinfecting. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
Recommendations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
Cleaning and Disinfection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
Prepare for Cleaning and Disinfecting. . . . . . . . . . . . . . . . . . . . 15
STEP 1: Cleaning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16
STEP 2: Disinfection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
Prepare for Use. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
Preventive Maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
Storage and Handling. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
Expected Life . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
Troubleshooting. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 18
Replacement Parts. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
Expendable Parts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
Service Parts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
Service Part Replacement Procedures . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25
Setup . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25
Reader Replacement . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 27
Power Cable Replacement . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 30
Indicator Light Assembly Replacement. . . . . . . . . . . . . . . . . . . . . . . 34
VersaCare® Bed . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 35
Progressa® Bed. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 35
WatchCare™ Incontinence Management System User and Service Manual (196414 REV 2)
iii

Table of Contents
DRAFT 26-FEB-2019
Foot-End Cover With Holes . . . . . . . . . . . . . . . . . . . . . . . . . 36
Foot-End Cover without Holes . . . . . . . . . . . . . . . . . . . . . . 37
Final Steps. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 39
Antenna Assembly Replacement . . . . . . . . . . . . . . . . . . . . . . . . . . . . 42
Graphic Replacement for the Head, Seat, and Thigh Sections,
and Deck Filler—Progressa® Bed. . . . . . . . . . . . . . . . . . . . . . . . . . . . . 45
Equipment Call Cable Replacement. . . . . . . . . . . . . . . . . . . . . . . . . . 48
Label Replacement. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 52
WatchCare™ System Function Check. . . . . . . . . . . . . . . . . . . . . . . . . 53
Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 54
Electromagnetic Emissions Guidance . . . . . . . . . . . . . . . . . . . . . . . . 56
Electromagnetic Immunity Guidance . . . . . . . . . . . . . . . . . . . . . . . . 57
Wireless Connectivity Specifications . . . . . . . . . . . . . . . . . . . . . . . . . 60
Standards . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 60
Encryption. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 60
Extensible Authentication Protocol Types (EAP Types) . . . 61
Regulatory Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 61
USA—Federal Communications Commission (FCC) Radiation
Exposure Statement. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 62
Interference Statement for FCC. . . . . . . . . . . . . . . . . . . . . . . . . . 62
Wireless System Characteristics. . . . . . . . . . . . . . . . . . . . . . . . . . 63
RFID Characteristics. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 65
FCC ID . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 65
FCC ID WiFi and Bluetooth® . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 65
Limited Warranty . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 66
WatchCare™ Incontinence Management System User and Service Manual (196414 REV 2)
iv

Intended Use
NOTE:
DRAFT 26-FEB-2019
INTENDED USE
The Incontinence Monitor is intended to detect and provide a timely alert
when the patient’s skin is exposed to incontinence (both urine and liquid
fecal events).
INTRODUCTION
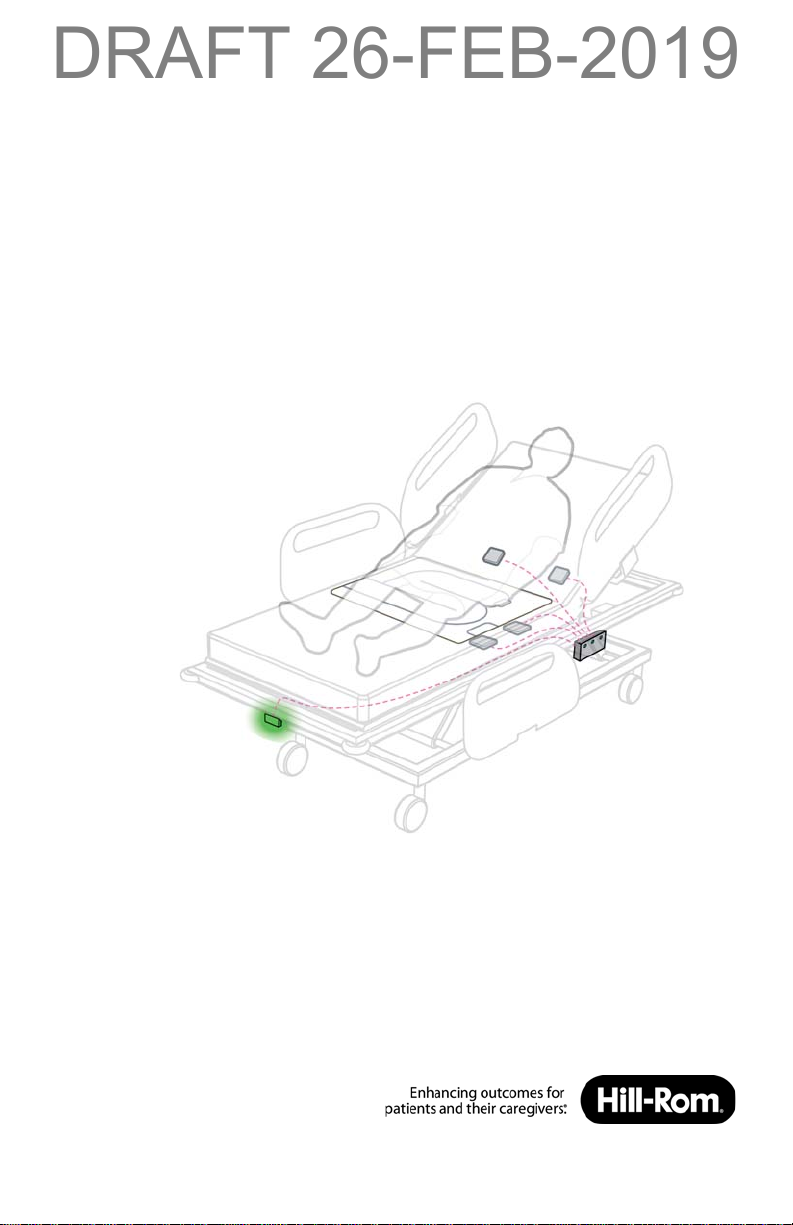
The WatchCare™ Incontinence Management System (system) provides a
discreet visual alert and optional equipment call alerts after moisture is
detected on the WatchCare™ smart pad.
This system is designed to discreetly alert the caregiver of an incontinence
event and in doing so, may prevent prolonged exposure to moisture
against the skin.
This system is most applicable in the critical care and medical/surgical
settings, but it may be used in other clinical areas as well.
Before you operate the system, make sure that you read and understand
in detail the contents of this manual. It is important that you read and
obey the aspects of the safety content in this manual.
Any reference to a side of the bed is from the patient’s view lying in the
bed on his or her back.
The system has been designed and tested for compatibility with the
hospital beds shown below and their compatible Hill-Rom® mattresses. To
determine if the system can be used on a hospital bed not listed, contact
Hill-Rom.
• The Centrella® Smart+ Hospital Bed
• The Progressa® Hospital Bed
• The VersaCare® Hospital Bed
The system is installed by Hill-Rom representatives to receive its power
from the bed.
WatchCare™ Incontinence Management System User and Service Manual (196414 REV 2)
1
Symbols
DRAFT 26-FEB-2019
SYMBOLS
D
OCUMENT SYMBOLS
This manual contains different typefaces and symbols to make the
content easier to read and understand:
• Standard text—used for regular data.
• Boldface text—emphasizes a word or phrase.
• NOTE:—sets apart special data or important instruction clarification.
•WARNING or CAUTION
– A WARNING identifies situations or actions that may have an
effect on patient or user safety. To ignore a warning could
cause patient or user injury.
– A CAUTION identifies special procedures or precautions that
persons must obey to help prevent equipment damage.
PRODUCT SYMBOLS
Symbol Description
WatchCare™ indicator light
Wireless indicator—identifies the connection status of
the system to the facility wireless network
Connected indicator—identifies the connection status
of the system to the NaviCare® SmartSync® System
Location indicator—identifies the connection status of
the Location feature
WatchCare™ Incontinence Management System User and Service Manual (196414 REV 2)
2
Symbol Description
DRAFT 26-FEB-2019
Identifies these WatchCare™ System components (Progressa® and VersaCare® Beds):
• WatchCare™ 1/4" communication cable
• Facility equipment call jack designated for the
WatchCare™ 1/4"communication cable
• WatchCare™ connector on the bed
Manufacturer
Model or type reference
Serial number
Manufacture date
Symbols
ATTENTION: Consult accompanying documents
Federal Communications Commission
Degree of protection against Ingress of Water
Type B applied part according to IEC 60601-1
Medical - General Medical Equipment as to Electrical
Shock, Fire and Mechanical Hazards only in accordance
with ANSI/AAMI ES60601-1 (2005) + AMD 1 (2012),
CAN/CSA-22.2 No. 60601-1 (2014)
WatchCare™ Incontinence Management System User and Service Manual (196414 REV 2)
3
Safety Instructions
WARNING:
DRAFT 26-FEB-2019
Symbol Description
Do not dispose as Unsorted Municipal Waste
SAFETY INSTRUCTIONS
Obey these safety instructions to help prevent injury and/or equipment
damage:
• Warning—Read and understand all warnings in this manual and
on the unit itself prior to use with a patient.
• Warning—The potential for electrical shock exists with electrical
equipment. Failure to follow facility protocols may cause death or
serious injury.
• Warning—The system is to be installed only by Hill-Rom
representatives.
• Warning—To avoid risk of electrical shock, this equipment must
only be connected to supply mains with protective earth.
• Warning—The system is not suitable for use in an oxygenenriched environment.
• Warning—The system has no user serviceable parts. Only
facility-authorized service persons should service the system.
• Warning—With the exception of the WatchCare® smart pads, do
not discard components of the system as unsorted municipal
waste. See your local distributor for collection and/or recycling
systems available in your country.
• Warning—Do not modify the system.
• Warning—Do not use the smart pad(s) to transfer the patient
from one surface to another.
• Warning—This product can expose you to chemicals including
Titanium Dioxide, which is known to the State of California to
cause cancer. For more information go to
www.P65Warnings.ca.gov.
• Warning—Make sure the position of the bed is such that you can
quickly, without obstruction, unplug the power cord from the
main power supply if necessary.
WatchCare™ Incontinence Management System User and Service Manual (196414 REV 2)
4

Safety Instructions
NOTE:
DRAFT 26-FEB-2019
• Warning—To safely stop the operation of the system, unplug the
bed and/or the external power supply from the power outlet.
FCC GUIDANCE
Pursuant to FCC 15.21 of the FCC rules, changes not expressly approved by
Hill-Rom might cause harmful interference and void the FCC authorization
to operate this product.
This equipment has been tested and found to comply with the limits for a
Class B digital device, pursuant to Part 15 of the FCC Rules. These limits are
designed to provide reasonable protection against harmful interference in
a residential installation. This equipment generates, uses, and can radiate
radio frequency energy and, if not installed and used in accordance with
the instructions, may cause harmful interference to radio
communications. However, there is no guarantee that interference will not
occur in a particular installation. If this equipment does cause harmful
interference to radio or television reception, which can be determined by
turning the equipment off and on, the user is encouraged to try to correct
the interference by one or more of the following measures:
• Reorient or relocate the receiving antenna.
• Increase the separation between the equipment and receiver.
• Connect the equipment into an output on a circuit different from
that to which the receiver is connected.
• Consult the dealer or an experienced radio/TV technician for
help.
WatchCare™ Incontinence Management System User and Service Manual (196414 REV 2)
5
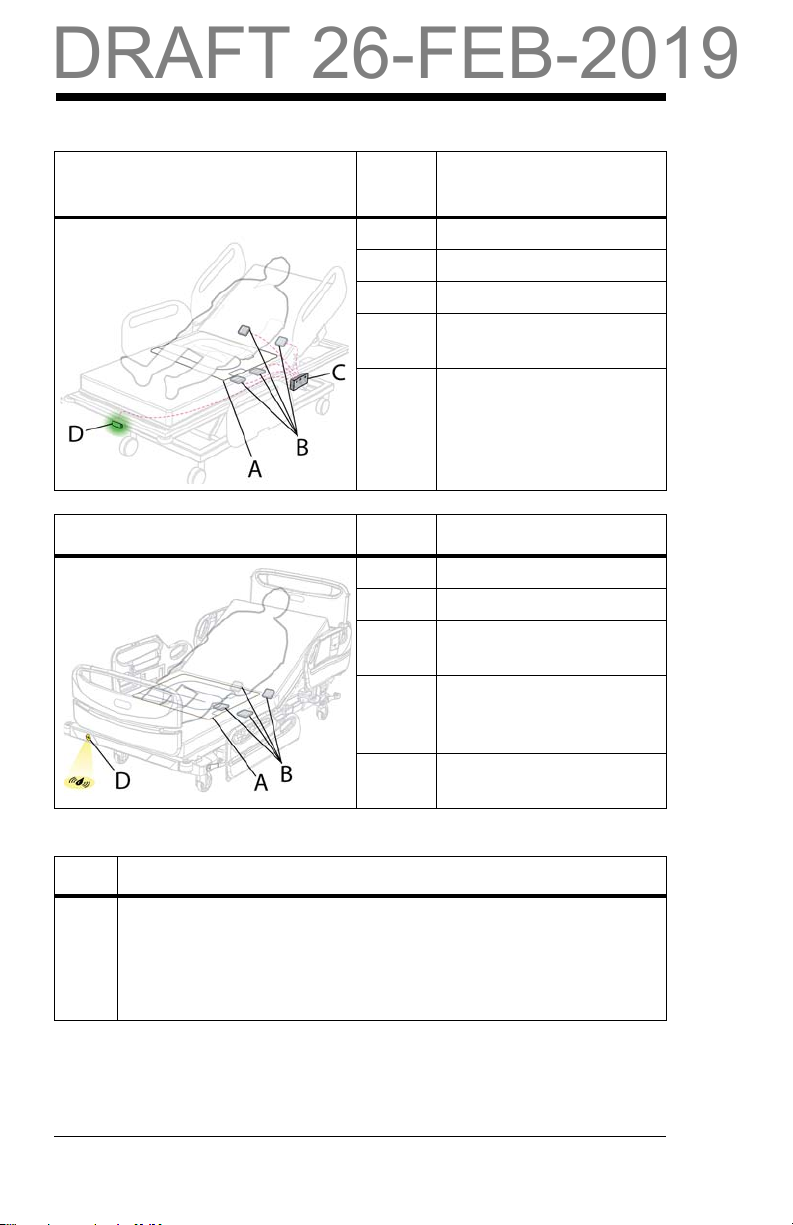
Quick View™ List of Features
DRAFT 26-FEB-2019
QUICK VIEW™ LIST OF FEATURES
PROGRESSA® AND VERSACARE®
EDS
B
CENTRELLA® SMART+ BED
Item Feature
AWatchCare™ smart pad
B WatchCare™ antennas
C WatchCare™ reader
D WatchCare™ indicator
light
E (not
shown)
Item Feature
AWatchCare™ smart pad
B WatchCare™ antennas
C (not
shown)
D SafeView®+ Alerts—
E (not
shown)
WatchCare™ connector
(on the bed) and WatchCare™ 1/4" communication cable
WatchCare™ reader
WatchCare™ indicator
light
Nurse call system connection
S
TA ND AR D FEATURES
Item Description
A WatchCare™ smart pad
The WatchCare™ smart pad (smart pad) is an incontinence pad,
with a radio-frequency identification (RFID) tag, that detects
moisture to notify the WatchCare™ RFID reader of an incontinence event.
WatchCare™ Incontinence Management System User and Service Manual (196414 REV 2)
6
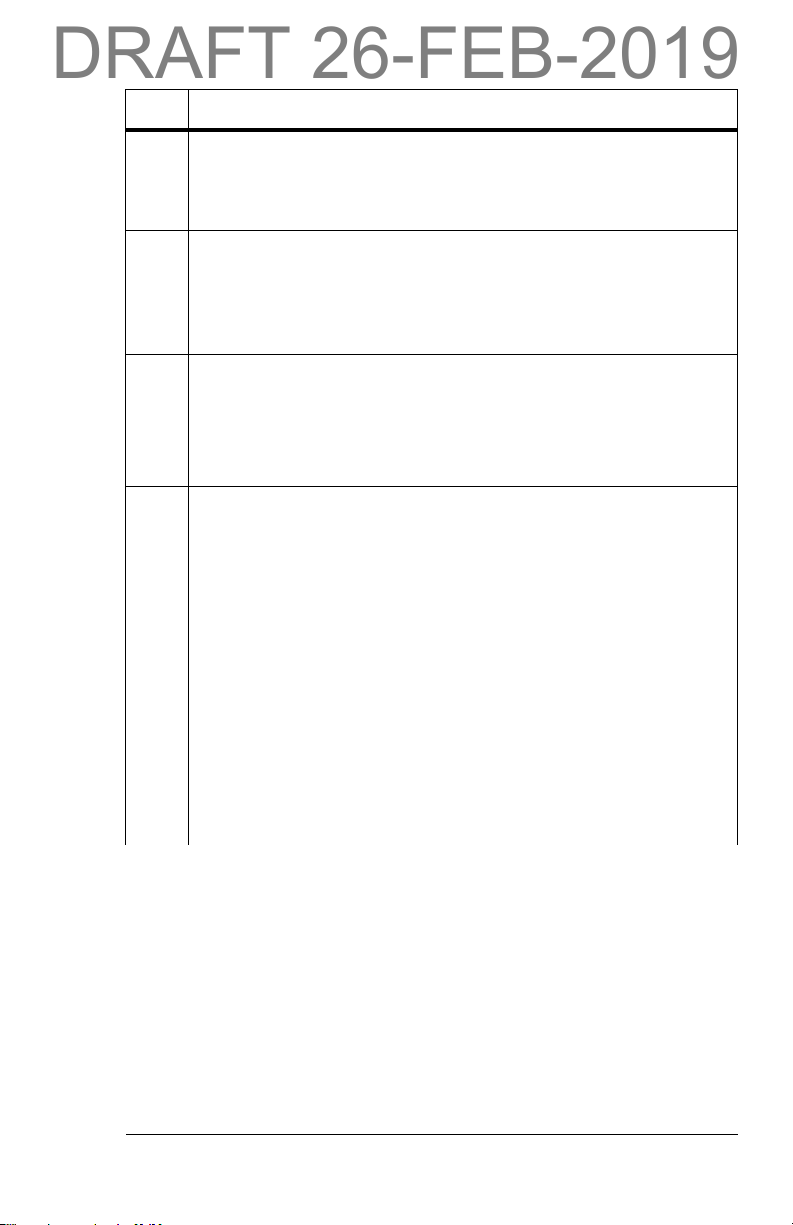
Item Description
DRAFT 26-FEB-2019
B WatchCare™ Antennas
There are four WatchCare™ antennas (antenna(s)) located under
the mattress. They identify that a smart pad is present and send
a signal to the WatchCare™ reader.
C WatchCare™ Reader
The WatchCare™ RFID reader (reader) is located on the left side
of the bed near the seat section. The RFID reader sends a signal
prompting the WatchCare™ indicator light as to the smart pad’s
status.
D
WatchCare™ Indicator Light
The WatchCare™ indicator light (indicator light) is located on the
foot end of the bed. The indicator has multi-color (green, amber,
and white) lights that indicate the system status as identified in
the table that follows.
Quick View™ List of Features
E
WatchCare™ Communication
• Progressa® and VersaCare® Beds—for the WatchCare™
System to send customizable incontinence alerts to
caregivers through the facility’s nurse call system, connect
the WatchCare™ 1/4" communication cable to the
WatchCare™ connector (under the head end of the bed, on
the patient’s left) and to the facility’s equipment call jack
designated for the WatchCare™ System.
• Centrella® Bed—for the WatchCare™ System to send
customizable incontinence alerts to caregivers through the
facility’s nurse call system, do one of these as applicable:
Centrella® Bed with the NaviCare® System—connect the
SideCom® cable to the bed and to the facility’s equipment
call jack.
WatchCare™ Incontinence Management System User and Service Manual (196414 REV 2)
7
Quick View™ List of Features
DRAFT 26-FEB-2019
Item Description
E Centrella® Bed with a different
nurse call system—
a. Connect the two-cable
end of the WatchCare™
adapter to the bed.
b. Connect the 37-pin end of the adapter cable to the
facility’s communication cable.
c. Connect the remaining end (the 1/4" cable
connector) of the adapter cable to the facility’s
equipment call jack designated for the WatchCare™
System.
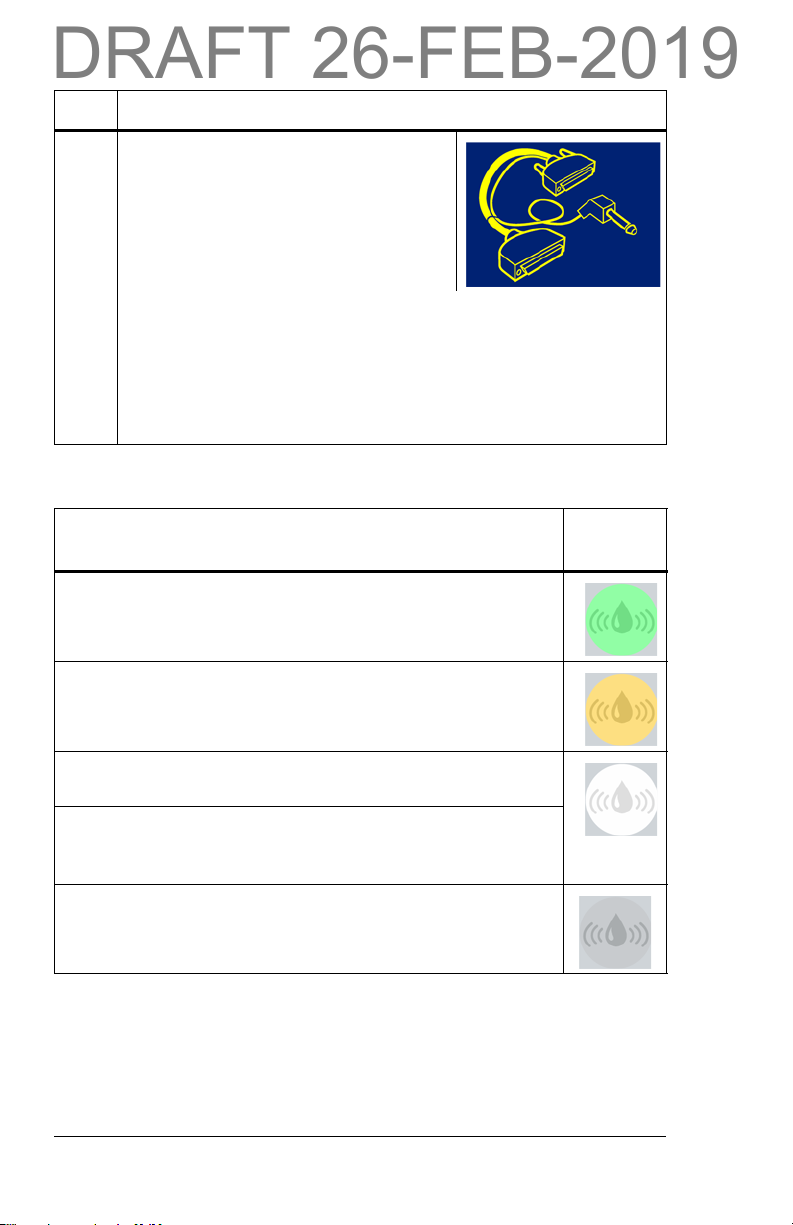
Indicator Light—Visual Identification
Status
Solid green light—identifies that a smart pad is present and
is being monitored. No moisture is detected at this time.
Flashing amber light—identifies that the smart pad is wet.
This visual alert will project on the floor.
Solid white light—identifies that the monitor system is on,
but the reader does not detect any smart pads on the bed.
Alternating white and green light—identifies that the
monitor system can not operate effectively because more
than four smart pads are detected.
No light—the monitor system is not active. Make sure the
bed is plugged into a power outlet.
Indicator
Light
WatchCare™ Incontinence Management System User and Service Manual (196414 REV 2)
8

Prepare the System for Use
WARNING:
NOTE:
NOTE:
DRAFT 26-FEB-2019
PREPARE THE SYSTEM FOR USE
To help prevent injury and/or equipment damage, obey these warnings:
• Warning—Keep cables out of the patient foot fall area.
• Warning—Connect the WatchCare™ 1/4" communication cable
or WatchCare™ adapter cable (as applicable) to an equipment call
jack designated for the WatchCare™ System only. Patient injury
could occur if the nurse call system is not operational.
1. Make sure the bed’s power cord is plugged into a power outlet.
Once the bed is plugged in, it may take up to 2 minutes for the indicator
light to turn white.
2. If the WatchCare™ System interfaces with a nurse call system, make
sure of these:
• Centrella® Bed—
– Bed with the NaviCare® System—the SideCom® cable is
connected to the bed and to the facility’s equipment call
jack.
– Bed with a different nurse call system—the adapter cable
is connected to the bed, and the cable’s 1/4" cable
connector is connected to the facility’s equipment call jack
designated for the WatchCare™ System’s incontinence
alerts.
The location of the adapter cable differs depending on your bed
version. An earlier version bed is shown on the left in the photos
below.
WatchCare™ Incontinence Management System User and Service Manual (196414 REV 2)
9

Prepare the System for Use
DRAFT 26-FEB-2019
Centrella® Bed Centrella® Bed
P7900A0, P7900B0 P7900B1 and Newer Facility Connection
• Progressa® and VersaCare® Beds—the WatchCare™
1/4"communication cable is connected to the WatchCare™
connector on the bed and to the facility’s equipment call jack
designated for the WatchCare™ System’s incontinence alerts.
Progressa® Bed VersaCare® Bed Facility Connection
WatchCare™ Incontinence Management System User and Service Manual (196414 REV 2)
10
Prepare the System for Use
DRAFT 26-FEB-2019
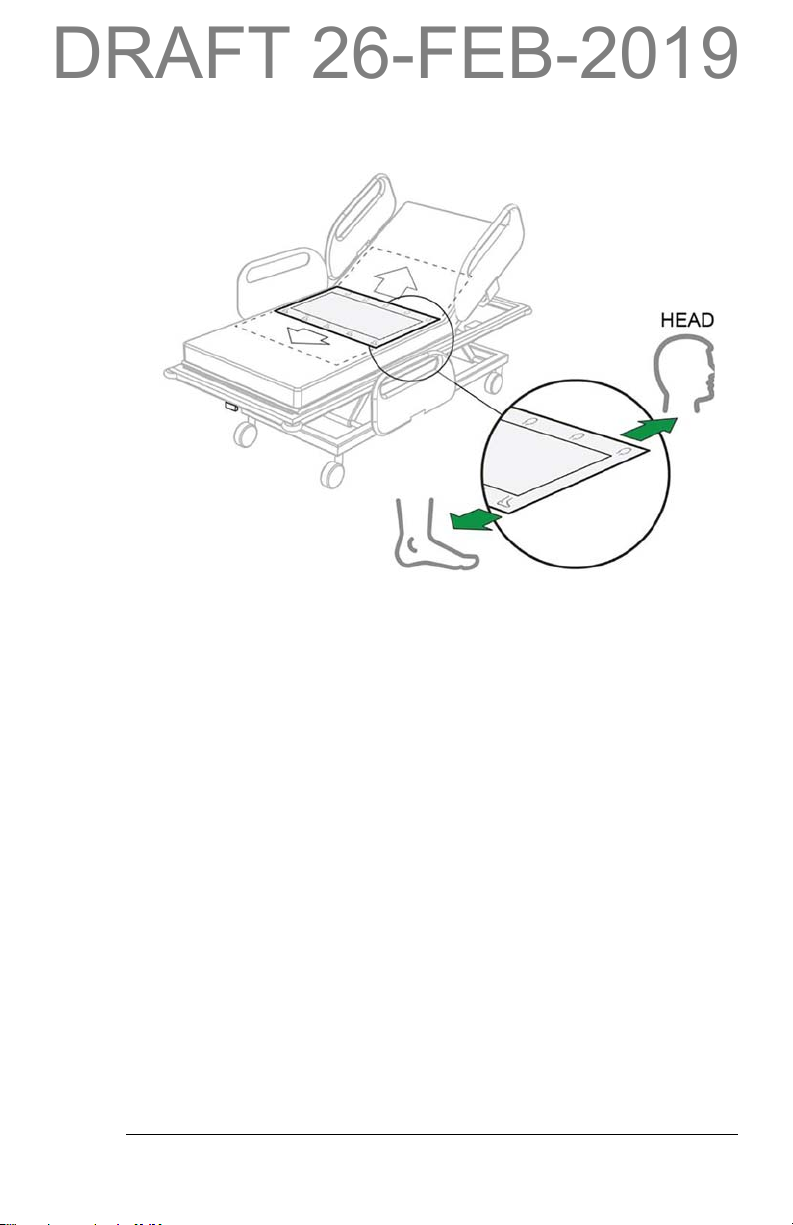
3. Put a smart pad(s) in the middle of the bed with the head icon toward
the head end of the bed and foot icon toward the foot end.
smart pad Placement
4. Listen for a single beep, and make sure the indicator light has turned
green. These let you know that the pad has been detected and the
incontinence monitoring has begun.
NOTES:
• Store unused, clean smart pads at least 2 feet (61 cm) from the
bed.
• If the indicator light does not operate as specified, see “Indicator
Light—Visual Identification” on page 8 and/or “Troubleshooting”
on page 18.
• The WatchCare™ Incontinence Management System can monitor
up to four WatchCare™ smart pads on a bed at one time; however,
Hill-Rom recommends to use the minimum number of smart
pads to minimize the risk of skin breakdown (refer to www.hillrom.com, Hill-Rom® Safe Skin® Program).
• Placing devices that contain metallic components or metallic
materials (such as a chair exit pad) under or on top of the smart
pad could interfere with incontinence monitoring.
WatchCare™ Incontinence Management System User and Service Manual (196414 REV 2)
11
Transport The Bed
NOTE:
DRAFT 26-FEB-2019
Replace the WatchCare™ smart pad
The indicator light will flash amber to indicate an incontinence event. If
you are using a nurse call system, customizable incontinence alerts can be
sent to—
• The nurse call console
• The nurse call dome light over the door, outside the patient’s
room
• The applicable mobile devices available at your facility
• The nurse call status board
1. Remove the soiled smart pad(s), and discard the pad(s) at least 2 feet
(61 cm) from the bed so that the reader no longer detects the soiled
pad(s).
2. Put a new smart pad on the bed, and listen for the single beep to
make sure the new pad is being monitored.
• If there is no beep, look at the indicator light. If the light is white,
the smart pad is not detected. Make sure the pad is in the correct
head/foot orientation and intended location (see Step 3 on page
11).
• If you are using more than one smart pad, make sure the
indicator light is green after the soiled pad is removed.
When the new pad is detected, the alert will clear in the WatchCare™
System.
TRANSPORT THE BED
1. Prepare the bed for transport per the bed’s user manual.
2. Disconnect the bed cable (WatchCare™ 1/4"communication cable,
SideCom® cable, or WatchCare™ adapter cable) from the equipment
call jack on the wall.
3. Transport the bed per facility protocol.
4. After the transport, do these:
a. Plug in the bed’s power cord.
b. Connect the bed cable (WatchCare™ 1/4" communication cable,
SideCom® cable, or WatchCare™ adapter cable) to the equipment
call jack on the wall.
WatchCare™ Incontinence Management System User and Service Manual (196414 REV 2)
12

Cleaning and Disinfecting
NOTE:
WARNING:
DRAFT 26-FEB-2019
CLEANING AND DISINFECTING
This section does not apply to the smart pads. The smart pads are
intended for single use only. Discard a soiled smart pad at least 2 feet (61
cm) from the bed so that the reader no longer detects the soiled pad.
To help prevent injury and/or equipment damage, obey these warnings:
• Warning—The potential for electrical shock exists with electrical
equipment. Failure to follow facility protocol could cause death
or serious injury.
• Warning—Do not reuse wiping material for multiple steps or on
multiple products.
• Warning—Harmful cleaning solutions may cause skin rash
and/or irritation upon contact. Follow the manufacturer’s
instructions found on the product label and Safety Data Sheet
(SDS).
• Warning—Lift and move items correctly. Do not twist, and seek
assistance when necessary. Make sure the bed is at a correct
height to lift items off the bed.
• Warning—Fluid spills on to the system’s electronics could cause
a hazard. If such a spill occurs, unplug the bed and remove it from
service. When fluid spills occur outside of what is seen in normal
use, immediately do as follows:
a. Unplug the bed from its power source.
b. Remove the patient from the bed.
c. Clean the fluid spill from the system.
d. Have maintenance examine the system completely.
e. Do not use the system until it is completely dry, tested, and
found to be safe to operate.
WatchCare™ Incontinence Management System User and Service Manual (196414 REV 2)
13

Cleaning and Disinfecting
CAUTION:
DRAFT 26-FEB-2019
To help prevent equipment damage, obey these cautions:
• Caution—Do not steam clean or power wash the system.
Pressure and excessive moisture can damage the protective
surfaces of the system and its electrical components.
• Caution—Do not use harsh cleansers/detergents, heavy duty
grease removers, solvents such as toluene, xylene, or acetone,
and do not use scouring pads (you may use a soft bristle brush).
• Caution—Do not use bleach as your primary everyday
cleaner/disinfectant.
RECOMMENDATIONS
For proper cleaning and disinfection, staff members should be trained.
The trainer should carefully read the instructions and follow them when
the trainee is being trained. The trainee should:
• Be given time to read the instructions and to ask any questions.
• Clean and disinfect the product while the trainer supervises.
During, and/or after this process, the trainer should correct the
trainee of any differences from the instructions for use.
The trainer should supervise the trainee until the trainee can clean and
disinfect the system as instructed.
Hill-Rom recommends to clean and disinfect the system’s components
between patient use and regularly during extended patient stays.
Some fluids used in the hospital environment, such as iodophor and zinc
oxide creams can cause permanent stains. Remove temporary stains by
wiping vigorously with a lightly-dampened wiping cloth.
CLEANING AND DISINFECTION
Cleaning and disinfection are distinctly different processes. Cleaning is the
physical removal of visible and non-visible soil and contaminants.
Disinfection is intended to kill microorganisms.
WatchCare™ Incontinence Management System User and Service Manual (196414 REV 2)
14
Cleaning and Disinfecting
DRAFT 26-FEB-2019
Table 1 below summarizes the approved cleaners/disinfectants for use
with the associated contact time for disinfection.
Table 1: Approved Cleaners/Disinfectants
Recommended
Cleaner/
Disinfectant
Wex-Cide™ Germicidal Detergent ready-touse
Virex® II 256 Yes No
OxyCide® Daily
Disinfectant
Cleaner
Clorox HealthCare® Bleach
Germicidal
Cleaner readyto-use
Clorox HealthCare® Bleach
Germicidal
Wipes
*Bleach is not recommended as the primary cleaner/disinfectant.
for Routine
Cleaning and
Disinfection
Yes N o
Yes Yes 3 minute s
No*Yes 5 minutes
No*Yes 3 minutes
Recommended
for Disinfection
against
Clostridium
Difficile (C.Diff)
Maintain
Wetness
(Disinfection
Contact Time)
10 minutes
10 minutes
Remove any disinfectant residue prior to and after the use of bleach
with a new or clean cloth/wipe soaked in tap water.
When you perform the detailed cleaning steps, please note the following:
• A microfiber cloth or the Clorox HealthCare® Bleach Germicidal
Wipe is recommended as the wiping cloth
• Always replace the wiping cloth when visibly soiled.
• Always replace the wiping cloth between steps (spot clean, clean,
and disinfect).
• Always use Personal Protective Equipment (PPE).
.
Prepare for Cleaning and Disinfecting
a. Unplug the bed.
WatchCare™ Incontinence Management System User and Service Manual (196414 REV 2)
15

Cleaning and Disinfecting
DRAFT 26-FEB-2019
b. Adjust the mattress position as necessary to get access to the
antennas. Refer to the bed’s service manual.
STEP 1: Cleaning
a. As necessary, first remove visible soil from the reader, indicator
light, and antennas using a wiping cloth soaked with an
approved cleaner/disinfectant (see “Table 1: Approved
Cleaners/Disinfectants” on page 15).
• A soft bristle brush may be used to loosen hardened soil.
• Use as many wiping cloths as needed to remove the soil.
It is important to remove all visible soil from all areas before
continuing to remove non-visible soil.
b. With a new wiping cloth soaked in an approved
cleaner/disinfectant, use firm pressure to wipe all surfaces of the
reader, indicator light, and antennas. Use a new or clean wiping
cloth as often as necessary. Make sure the following areas are
cleaned:
• Seams around the antennas
Centrella® Bed
• Progressa® or VersaCare® Bed—seam around the reader
WatchCare™ Incontinence Management System User and Service Manual (196414 REV 2)
16
Progressa® Bed VersaCare® Bed
Preventive Maintenance
NOTE:
DRAFT 26-FEB-2019
STEP 2: Disinfection
a. With a new or clean wiping cloth soaked in an approved
cleaner/disinfectant, use light pressure to wipe all exterior
surfaces of the reader, indicator light, and antennas.
b. Make sure all surfaces remain wet with the
cleaner/disinfectant for the specified contact time. Re-wet
surfaces with a new wiping cloth as necessary. See “Table 1:
Approved Cleaners/Disinfectants” on page 15 for the contact
time.
If bleach is used with another cleaner/disinfectant, use a new or clean
cloth/wipe soaked in tap water to remove any disinfectant residue
prior to and after the bleach application.
Prepare for Use
a. Make sure the mattress is in position for use and is connected
to the bed. Refer to the bed’s service manual.
b. Plug the bed into an applicable power outlet.
PREVENTIVE MAINTENANCE
The WatchCare™ System does not require preventive maintenance.
STORAGE AND HANDLING
Store the WatchCare™ smart pads in a dry location that is at least 2 feet (61
cm) from a bed with the WatchCare™ System.
EXPECTED LIFE
The expected life of the WatchCare™ System hardware is 10 years. The
smart pads are intended for single use only.
WatchCare™ Incontinence Management System User and Service Manual (196414 REV 2)
17
 Loading...
Loading...