GE Healthcare Lung VCAR Brochure
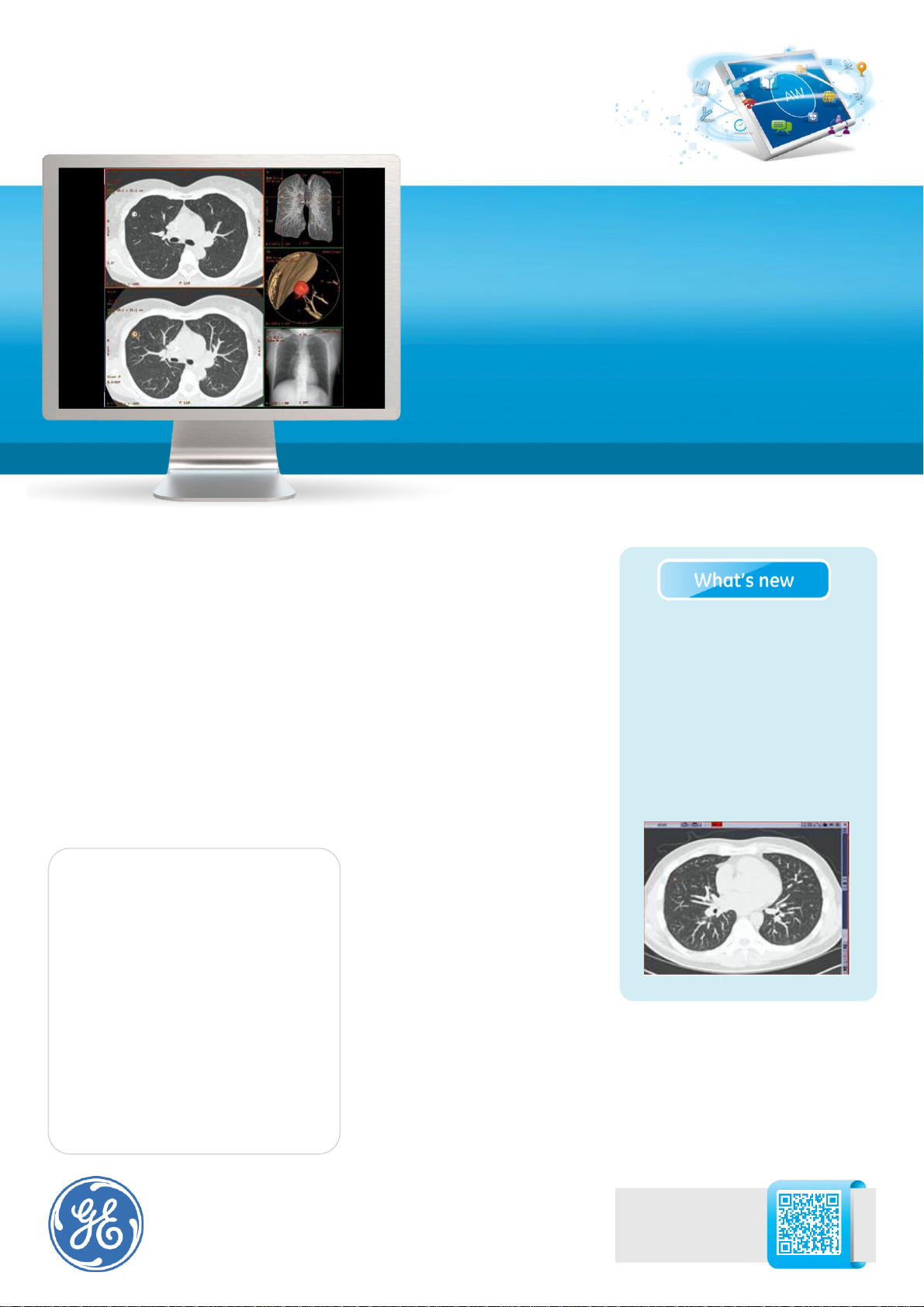
For lung nodule analysis - automatic
visualization, measurement, reporting
and follow-up
GE Healthcare
CT Scanning often is utilized as the exam of choice for intricate
CT exams to visualize and analyze complex lung pathology. The
detection of pulmonary nodules and assessment of their evolution
with CT are of major importance in chest imaging.
Lung VCAR
Visit us:
www.gehealthcare.com/aw/
applications/lung-vcar/
Overview
Lung VCAR brings efficient CT
pulmonary nodule assessment and
diagnosis. The innovative Digital
Contrast Agent (DCA) feature
automatically visualizes lung nodules to
help you confirm the presence or
absence of suspicious lesions from 2 to
12 mm in size. Lung VCAR allows
automated follow-up for lesion
matching by the registration of two or
more datasets, automatic lesion
classification, and a customizable
reporting tool.
Synchronized 2D, Digital
Contrast Agent (DCA) and
segmentation analysis.
Automatic nodule visualization.
Automatic nodule analysis
(volume, doubling time, %
growth).
Automatic follow-up
Customizable and interactive
report

Features
Review
Ability to synchronize multiple
images for nodule comparison.
Ability to review single or multiple
exams and compare axial, sagittal,
oblique, coronal, and volumerendered images.
Automatically propagates previous
exam bookmarks to current exam.
Automatically segments both right
and left lung to reduce visual
distraction.
Digital Contrast Agent (DCA)
automatically highlights spherical
shapes to enhance visualization of
suspicious nodules.
Analysis
Performs automatic segmentation
of all nodule types.
Provides automatic nodule
analysis, including % growth,
doubling time
Automatic Nodule Contour to verify
pixels within the volume
Reporting
Features a customizable,
interactive patient reporting tool
System requirements
AW Workstation
AW Server
Indications for Use
Lung VCAR/ AdvantageALA is an
image analysis software package for
AW systems, which allows the user to
study suspicious lesions within the
lungs using CT helical- and axialacquired images.
Standards/Regulations
This product complies with the
European CE marking regulation
following Medical Devices Directive:
Directive 93/42/EEC.
© 2012 General Electric Company.
All rights reserved. Data subject to change.
GE and GE Monogram are trademarks of General Electric Company.
* Trademark of General Electric Company.
 Loading...
Loading...