Page 1

Solar® SpO2 Module
with Masimo SET
Service Manual
2004407-001 Revision A
®
Page 2

127(Due to continuing product innovation, specifications in this manual are subject to change without
notice.
Listed below are GE Medical Systems Information Technologies trademarks. All other trademarks contained
herein are the property of their respective owners.
900 SC, ACCUSKETCH, AccuVision, APEX, AQUA-KNOT, ARCHIVIST, Autoseq, BABY MAC, C Qwik
Connect, CardioServ, CardioSmart, CardioSys, CardioWindow, CASE, CD TELEMETRY, CENTRA, CHART
GUARD, CINE 35, CORO, COROLAN, COROMETRICS, Corometrics Sensor Tip, CRG PLUS, DASH,
Digistore, Digital DATAQ, E for M, EAGLE, Event-Link, FMS 101B, FMS 111, HELLIGE, IMAGE STORE,
INTELLIMOTION, IQA, LASER SXP, MAC, MAC-LAB, MACTRODE, MANAGED USE, MARQUETTE,
MARQUETTE MAC, MARQUETTE MEDICAL SYSTEMS, MARQUETTE UNITY NETWORK, MARS,
MAX, MEDITEL, MEI, MEI in the circle logo, MEMOPORT, MEMOPORT C, MINISTORE, MINNOWS,
Monarch 8000, MULTI-LINK, MULTISCRIPTOR, MUSE, MUSE CV, Neo-Trak, NEUROSCRIPT,
OnlineABG, OXYMONITOR, Pres-R-Cuff, PRESSURE-SCRIBE, QMI, QS, Quantitative Medicine,
Quantitative Sentinel, RAC RAMS, RSVP, SAM, SEER, SILVERTRACE, SOLAR, SOLARVIEW, Spectra
400, Spectra-Overview, Spectra-Tel, ST GUARD, TRAM, TRAM-NET, TRAM-RAC, TRAMSCOPE, TRIM
KNOB, Trimline, UNION STATION, UNITY logo, UNITY NETWORK, Vari-X, Vari-X Cardiomatic,
VariCath, VARIDEX, VAS, and Vision Care Filter are trademarks of GE Medical Systems Information
Technologies registered in the United States Patent and Trademark Office.
12SL, 15SL, Access, AccuSpeak, ADVANTAGE, BAM, BODYTRODE, Cardiomatic, CardioSpeak, CD
TELEMETRY
Cumulus, Event-Link Nimbus, HI-RES, ICMMS, IMAGE VAULT, IMPACT.wf, INTER-LEAD, IQA,
LIFEWATCH, Managed Use, MARQUETTE PRISM, MARQUETTE
MicroSmart, MMS, MRT, MUSE CardioWindow, NST PRO, NAUTILUS, O
®
-LAN, CENTRALSCOPE, Corolation, EDIC, EK-Pro, Event-Link Cirrus, Event-Link
®
RESPONDER, MENTOR,
SENSOR, Octanet, OMRS, PHi-
2
Res, Premium, Prism, QUIK CONNECT V, QUICK CONNECT, QT Guard, SMART-PAC, SMARTLOOK,
Spiral Lok, Sweetheart, UNITY, Universal, Waterfall, and Walkmom are trademarks of GE Medical Systems
Information Technologies.
© GE Medical Systems Information Technologies, 2000. All rights reserved.
T-2 Masimo SET SpO2 Module Revision A
2004407-001 15 December 2000
Page 3

Contents
1 Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-1
Manual Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-3
Revision History . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-3
Purpose . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-3
Intended Audience . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-3
Safety Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-4
Responsibility of the Manufacturer . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-4
Intended Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1 -4
Definitions of Warnings, Cautions, and Notes . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-5
Equipment Symbols . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-5
Service Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-6
Service Requirements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-6
Equipment Identification . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-6
2 Equipment Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-1
System Components . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-3
Solar SpO
Solar 7000 Patient Monitor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-4
Solar 8000 Patient Monitor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-4
Solar 8000M Patient Monitor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-5
Tram-rac Housing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-5
Technical Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-6
Insert Module . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-8
Module with Masimo SET . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-3
2
Revision A Solar SpO
2004407-001
with Masimo SET i
2
Page 4

3 Maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-1
Maintenance Schedule . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-3
Recommended Maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-3
Recommended Frequency . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-3
Safety Tests . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-3
Inspection and Cleaning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-4
Visual Inspection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-4
Cleaning Precautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-4
Exterior Cleaning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-4
Checkout Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-5
General . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-5
Required Tools/ Equipment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-5
Preparation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-6
SpO
Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-6
2
Electrical Safety Tests . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-8
Recommendations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-8
Required Tests . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-8
Test Conditions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-8
AC Hi-Pot Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-9
Test Frequency . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-9
Required Tools/Equipment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-9
Procedures . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-9
Current Leakage Tests . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-10
Preparation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-10
Patient (Source) Leakage Current Test . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-11
Patient (Sink) Leakage Current Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-12
Completion . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-13
PM Form . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-13
Repair Log . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-14
ii Solar SpO
2004407-001
with Masimo SET Revision A
2
Page 5

4 Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-1
General Fault Isolation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-3
First Things to Ask . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-3
Visual Inspection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-3
Troubleshooting Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-5
System OK LED . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-6
MS-3 Communications OK LED . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-6
Isolated Power Supply OK LED . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-6
Theory of Operation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-7
Hardware Functions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-7
Software Functions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-7
System Processor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-7
Masimo MS-3 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-8
Host Patient Monitor Communications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-9
Power Condition and Soft Start . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-9
Isolated Power Supply . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-9
Patient Connector Flex Circuit . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-9
External Connectors . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-10
SpO
Signals . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-10
2
Probe Schematic Diagram . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-11
Host Patient Monitor Connector . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-11
Software Updates . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-13
5 Parts Lists and Drawings . . . . . . . . . . . . . . . . . . . . . . . . . 5-1
Ordering Parts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-3
General . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-3
Field Replaceable Units . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-3
Disassembly Procedures . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-4
Assembly Housing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-4
Masimo SET SpO
Masimo SET SpO
Masimo SET SpO
Reassembly . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-4
Testing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-4
Solar SpO
Revision A Solar SpO
Module with Masimo SET 2001891-001A . . . . . . . . . . . . . . . . . . . . . . 5-5
2
2004407-001
MS-3 PCB . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-4
2
Module Flex PCB . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5 -4
2
Module PCB . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-4
2
with Masimo SET iii
2
Page 6

iv Solar SpO
2004407-001
with Masimo SET Revision A
2
Page 7

1 Introduction
Revision A Solar SpO2 Module with Masimo SET 1-1
2004407-001
Page 8

For your notes
1-2 Solar SpO2 Module with Masimo SET Revision A
2004407-001
Page 9

Manual Information
Revision History
Each page of the document has the document part number and revision
letter at the bottom of the page. The revision letter changes whenever
the document is updated.
Purpose
This manual provides technical information for maintaining the
equipment. Use it as a guide for maintenance and electrical repair of
parts considered field repairable.
Introduction: Manual Information
Revision Date Comment
A 15 December 2000 Initial release.
Intended Audience
Users of this manual are expected to have a background in electronics,
including analog and digital circuitry with RF and microprocessor
architectures. It is intended for service representatives and technical
personnel who maintain, troubleshoot, or repair this equipment.
Revision A Solar SpO2 Module with Masimo SET 1-3
2004407-001
Page 10

Introduction: Safety Information
Safety Information
Responsibility of the Manufacturer
GE Medical Systems Information Technologies is responsible for the
effects of safety, reliability, and performance only if:
n
assembly operations, extensions, readjustments, modifications, or
repairs are carried out by persons authorized by GE Medical Systems
Information Technologies, Inc;
n
the electrical installation of the relevant room complies with the
requirements of the appropriate regulations; and
n
the device is used in accordance with the instructions for use.
Intended Use
This device is intended for use under the direct supervision of a licensed
health care practitioner.
This device is not intended for home use.
Federal law restricts this device to be sold by or on the order of a
physician.
Contact GE Medical Systems Information Technologies for information
before connecting any devices to the equipment that are not
recommended in this manual.
Parts and accessories used must meet the requirements of the applicable
IEC 60601 series safety standards, and/or the system configuration must
meet the requirements of the IEC 60601 medical electrical systems
standard.
Periodically, and whenever the integrity of the device is in doubt, test all
functions.
The use of ACCESSORY equipment not complying with the equivalent
safety requirements of this equipment may lead to a reduced level of
safety of the resulting system. Consideration relating to the choice shall
include:
u
use of the accessory in the PATIENT VICINITY; and
u
evidence that the safety certification of the ACCESSORY has
been performed in accordance to the appropriate IEC 60601-1
and/or IEC 60601 harmonized national standard.
If the installation of the equipment, in the USA, uses 240V rather than
120V, the source must be a center-tapped, 240V, single-phase circuit.
1-4 Solar SpO2 Module with Masimo SET Revision A
2004407-001
Page 11

Introduction: Safety Information
Definitions of Warnings, Cautions, and Notes
Warnings, cautions, and notes are used throughout this manual to
designate a degree or level of hazardous situations. Hazard is defined as
a source of potential injury to a person.
'$1*(5
indicates an imminent hazard which, if not avoided, will
result in death or serious i njury.
:$51,1*
indicates a potential hazard or unsafe practice which, if
not avoided, could result in death or serious injury.
&$87,21
indicates a potential hazard or unsafe practice which, if
not avoided, could result in minor personal injury or
product/property damage.
Equipment Symbols
127(provides application tips or other useful information to assure
that you get the most from your equipment.
The following symbols appear on the equipment.
ATTENTION: Consult accompanying documents before using the
equipment.
Revision A Solar SpO2 Module with Masimo SET 1-5
2004407-001
Page 12
Service Information
Service Requirements
n
n
n
n
n
Introduction: Service Information
Refer equipment servicing to GE Medical Systems Information
Technologies authoriz ed service personnel only.
Any unauthorized attempt to repair equipment under warranty voids
that warranty.
It is the user’s responsibility to report the need for service to GE
Medical Systems Information Technologies or to one of their
authorized agents.
Failure on the part of the responsible individual, hospital, or
institution using this equipment to implement a satisfactory
maintenance schedule may cause undue equipment failure and
possible health hazards.
Regular maintenance, irrespective of usage, is essential to ensure
that the equipment will always be functional when required.
Equipment Identification
Every GE Medical Systems Information Technologies device has a
unique serial number for identification. The serial number appears on
the product label on the base of each unit.
D 0 XX 0005 G XX
Month
Manufactured
A = January
B = February
C = March
D = April
E = May
F = June
G = July
H = August
J = September
K = October
L = November
M = December
Year
Manufactured
0= 2000
1= 2001
2= 2002
(and so on)
Product Code
Two-character
product descriptor
Product Sequence
Number
Manufacturing
number
(of total units
manufactured.)
Division
F = Cardiology
G = Monitoring
J = G.W. Labs
Device Characteristics
One or 2 letters that further
describe the unit, for example:
P = prototype not conforming to
marketing specification
R = refurbished equipment
S = special product documented
under Specials part numbers
U = upgraded unit
1-6 Solar SpO2 Module with Masimo SET Revision A
2004407-001
Page 13

2 Equipment Overview
Revision A Solar S pO2 Module with Masimo S ET 2-1
2004407-001
Page 14

For your notes
2-2 Solar SpO2 Module with Masimo SET Revision A
2004407-001
Page 15
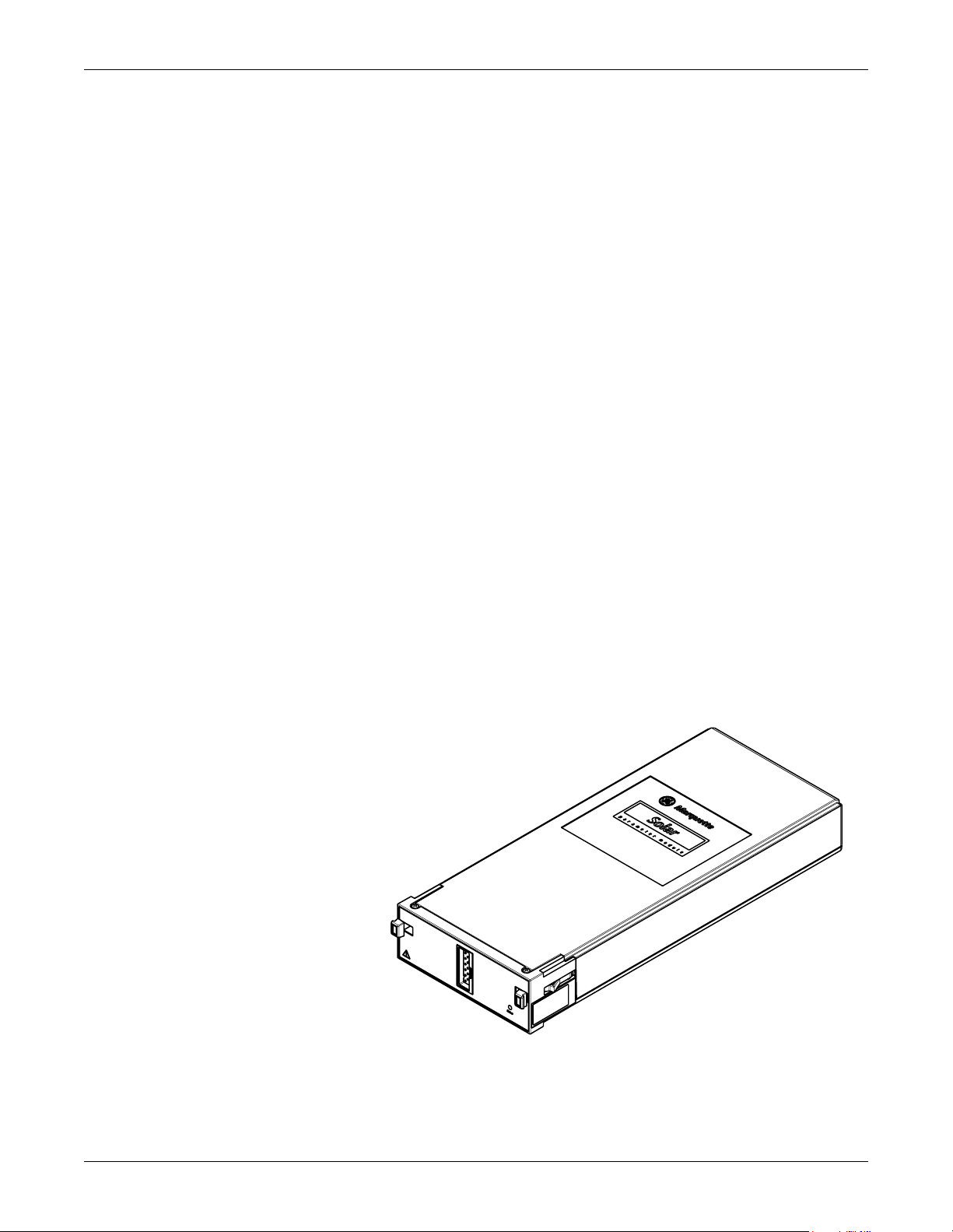
Equipment Overview: System Components
System Components
Solar SpO2 Module with Masimo SET
The Solar SpO2 module with Masimo SET, hereafter called the Masimo
module, and accessories is intended for continuous noninvasive
monitoring of functional oxygen saturation of arterial hemoglobin (SpO
and pulse rate (measured by an SpO
neonatal patients in hospitals and hospital-type environments. The
Masimo module provides all the necessary patient isolation. The Masimo
module is compatible with GE Medical Systems Information
Technologies Solar 7000, 8000, and 8000M modular products including
Tram-rac 4A. The device is housed in a standard single-high 7000 series
module enclosure.
The Masimo module uses spectrophotometric analysis to determine the
percent of oxygen saturation of hemoglobin in arterial bl ood also known
as pulse oximetry. When inserted into a Tram-rac 4A housing connected
to any Solar-based patient monitor, the module provides monitoring of
peripheral oxygen saturation and pulse rate for an adult, pediatric, or
neonatal patient.
sensor) for adult, pediatric, and
2
)
2
It uses Masimo LNOP cables. Other hardware functions include power
conditioning (soft-start live-insertion capability, short-circuit protection)
and isolation of the patient connected circuitry from earth ground.
Software may be updated using a laptop computer connected to any
compatible Solar based patient monitor.
The Masimo module shown below monitors pulse oximetry.
Revision A Solar S pO2 Module with Masimo S ET 2-3
2004407-001
Page 16
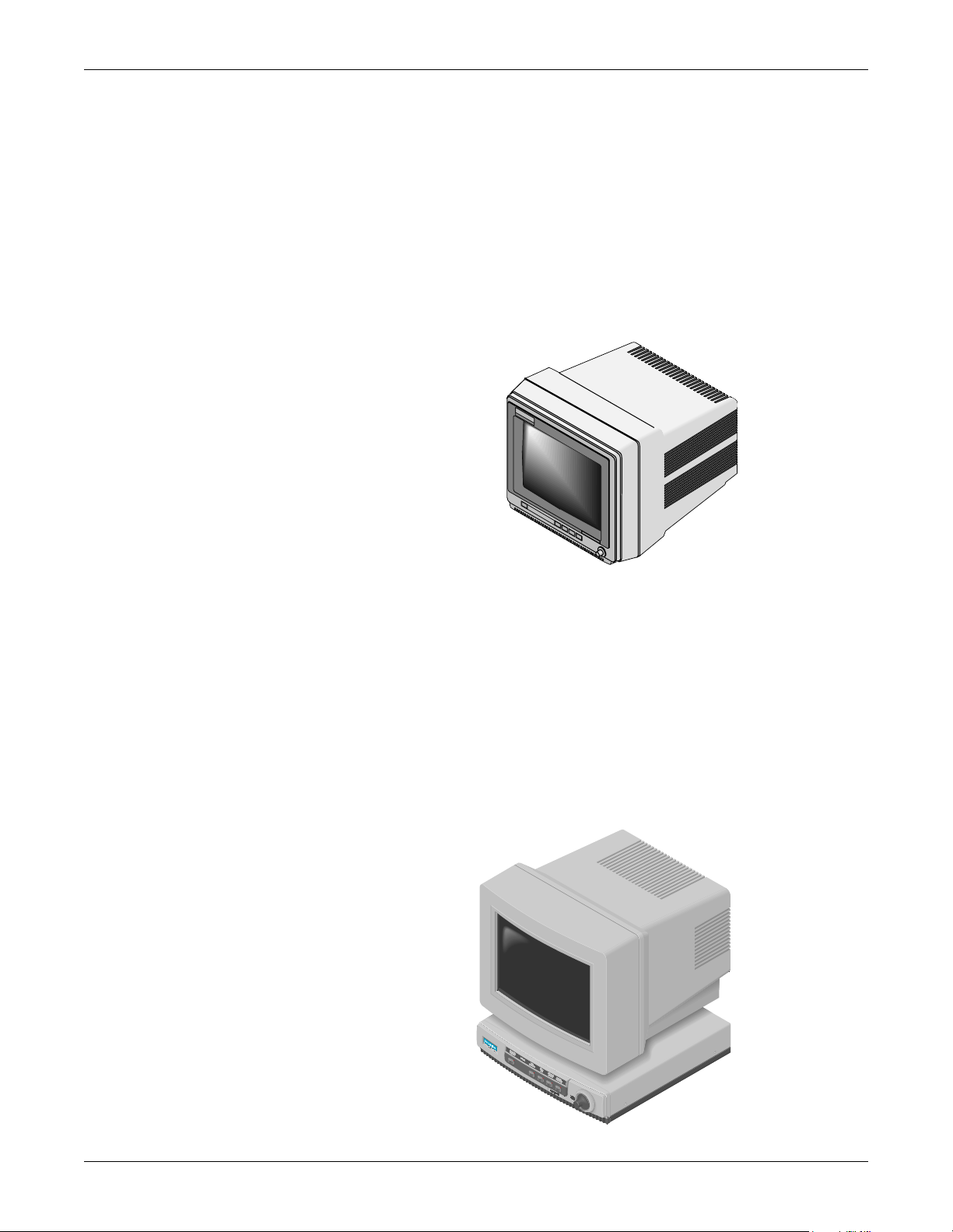
Equipment Overview: System Components
Solar 7000 Patient Monitor
One Solar based patient monitor used with the Masimo module is the
Solar 7000 patient monitor (software version 3C, 4B, or later). It is an
intelligent terminal, containing the display, all of the user controls, and
processors to communicate with patient monitor peripherals and analyze
patient data. It is capable of displaying up to six or eight different
waveforms at one time. System software may be updated by a laptop
computer at the monitor or through the Unity Network using a central
station.
Solar 7000
Patient Monitor
Solar 8000 Patient Monitor
The Solar 8000 patient monitor system (software version 3C, 4B, or
later) consists of a Solar 8000 processing unit with a compatible display.
The processing unit provides the user controls, processors to
communicate with patient monitor peripherals, and analyzes patient
data. It is capable of displaying up to six or eight different waveforms at
one time on the compatible 12, 15, 17, or 19-inch display. System
software may be updated using a laptop computer conne cted to t he Solar
8000 processing unit or through the Unity Network using a central
station.
2-4 Solar SpO2 Module with Masimo SET Revision A
2004407-001
Page 17

Equipment Overview: System Components
Solar 8000M Patient Monitor
The Solar 8000M patient monitor (software version 1A or later) consists
of a Solar 8000M processing unit with compatible display.
The processing unit is the center of the Solar 8000M Patient Monitoring
system. It provides the user con trols, t he processors t o communicate with
various patient monitoring modules, and it analyzes patient data. It can
display up to eight di fferent waveforms at one time. System software
may be updated using a laptop computer connected to the Solar 8000M
processing unit or from a central station on the Unity Network.
Tram-rac Housing
The Tram-rac housing (remote acquisition case) acquires patient data for
the patient monitor. The Tram-rac Housing Service Manual has more
information.
Only compatible Solar based monitors and the Tram-rac 4A (software
version 6C or later) housing support the Masimo module.
Shown below is a Tram-rac 4A housing.
Revision A Solar S pO2 Module with Masimo S ET 2-5
2004407-001
Page 18

Equipment Overview: Technical Specifications
Technical Specifi ca ti ons
127(
Nellcor, GE Medical Systems Information Technologies, and Masimo
pulse oximetry is calibrated to display functional saturation.
Ohmeda pulse oximetry is calibrated to display fractional saturation.
Item Description
Display Messages ARTIFACT DETECTED, LOW QUALITY, PROBE IS OFF
Measurement Range
Saturation
Pulse Rate
Perfusion
Performance Specifications
THE PATIENT, PROBE OR MODULE MALFUNCTION,
POOR SIGNAL QUALITY DETECTED, PULSE SEARCH
1 to 100% SpO
25 to 240 bpm
0.02 to 20%
2
Accuracy
Saturation, no motion
Saturation, motion
Pulse Rate, no motion
Pulse Rate, motion
Item Description
Maximum Power Consumption
(non-isolated)
Item Description
Operating Conditions
Temperature
Relative Humidity
SpO
over the range 70 to 100%, below 69% is unspecified
2
±2 digits for adults and pediatrics, ±3 digits for neonates
SpO
over the range 70 to 100%, below 69% is unspecified
2
±3 digits for adults, pediatrics, and neonates
25 to 240 bpm, ±3 bpm
25 to 240 bpm, ±5 bpm
Power Requirements
+16.5V, 150mA
+5V, 150mA
Environmental Specifications
0°C to 40°C (32°F to 104°F)
15 to 90% (non-condensing)
Storage Conditions
Temperature
Relative Humidity
Altitude -305 to 1830m (-1000 to 6,000ft.)
Atmospheric Pressure 645 to 795mmHg (860 to 1060hPa)
2-6 Solar SpO2 Module with Masimo SET Revision A
2004407-001
–40°C to +70°C (–40°F to +140°F)
0 to 95% (non-condensing)
Page 19

Equipment Overview: Technical Specifications
Alarm Specifications
Item Description
Alarm Limit Range SpO2: 1% to 105%
Pules: 40 BPM to 235 BPM
Type Audible
Visual
Physical Specifications
Item Description
Height 4.0 cm (1.6 in)
Width 11.4 cm (4.5 in)
Depth 28.6 cm (11.25 in)
Weight 0.50 Kg (1.11 lb)
Cooling Method Natural convection
Heat Dissipation 8.75Btu/Hr (2.5W), maximum
Certification UL 2601-1 Classified.
UL Classified for CAN/CSA C22.2 No. 601.1.
CE Marking for the 93/42/EEC medical Device Directive.
IEC 60601-1 Certified.
Warranty One year (accessories may differ)
Revision A Solar S pO2 Module with Masimo S ET 2-7
2004407-001
Page 20

Insert Module
Equipment Overview: Insert Module
1. Insert the module in one of the bottom two slots of a Tram-rac 4A
housing.
TRAMSCOPE 12
MARQUETTE
2. Connect the patient cable assembly to the SpO
connector on the
2
module.
Make sure the following eve nts tak e pl ace .
u
The front panel LED of the module illuminates steady green
after all self-tests of the processor are complete.
u
The module identifies itself on the monitor screen with a patient
parameter box.
127(
The module will not identify itself on the monitor if the patient cable
assembly is not connected.
127(
When used with a powered Tram-rac, the patient isolated circuitry
voltage is enabled by the host monitor as indicated by the PWR LED.
3. If the module does not identify itself on the screen and the patient
cable assembly is connected, refer to chapter 4, Troubleshooting.
Otherwise, go to chapter 3, Maintenance and perform the Checkout
Procedures and Safet y Tests.
2-8 Solar SpO2 Module with Masimo SET Revision A
2004407-001
Page 21

3 Maintenance
Revision A Solar SpO2 Module with Masimo SET 3-1
2004407-001
Page 22

For your notes
3-2 Solar SpO2 Module with Masimo SET Revision A
2004407-001
Page 23

Maintenance: Maintenance Schedule
Maintenance Schedule
Recommended Maintenance
A regular equipment maintenance program helps prevent unnecessary
equipment failures and also reduces possible health hazards. This
chapter contains instructions for the following recommended
maintenance:
n
Inspecting and cleaning the module
n
Checkout procedure to verify the unit is working properly
n
Leakage tests to verify the equipment does not propose a safety
hazard
Recommended Frequency
To help you establish a systematic maintenance routine, GE Medical
Systems
maintenance procedures presented in this chapter
Information Technologies
recommends that you perform all
Safety Tests
n
upon receipt of the module,
n
every twelve months thereafter,
n
each time a circuit board is removed or replaced, and
n
record the results on the Repair Log included at the end of this
chapter.
It is recommended that all safety tests be performed if a unit has been
opened for any reason or repaired. Listed below are the safety tests
recommended.
n
All leakage tests described in this chapter,
n
All hi-pot tests described in this chapter,
:$51,1*
Failure to implement a satisfactory maintenance
schedule may cause undue equipment failure and
possible health hazards. Unless you have an Equipment
Maintenance Contract, GE Medical Systems Information
Technologies does not in any manner assume the
responsibility for performing the recommended
maintenance procedures. The sole responsibility rests
with the individual or institution using the equipment.
GE Medical Systems Information Technologies service
personnel may, at their discretion, follow the procedures
provided in this manual as a guide during visits to the
equipment site.
Revision A Solar SpO2 Module with Masimo SET 3-3
2004407-001
Page 24

Maintenance: Inspection and Cleaning
Inspection and Cleaning
Visual Inspection
Remove module before making an inspection or cleaning the module.
n
Check the case for cracks or other damage.
n
Regularly inspect cables for fraying or other damage.
n
Inspect all plugs, cables, and connectors for bent prongs or pins.
n
Verify that all cables and connectors are securely seated. Note that
replacement of components should be performed only by qualified
service personnel.
Cleaning Precautions
Recommended cleaning supplies:
n
ammonia (diluted), or
n
Cidex solution, or
n
sodium hypochlorite bleach (diluted), or
n
mild soap (diluted), and
n
lint-free cloth, and
n
dust remover (compressed air)
Exterior Cleaning
To avoid damage to the equipment surfaces, do not use the following
cleaning agents:
n
organic solvents,
n
ammonia based solutions,
n
acetone solution,
n
alcohol based cleaning agents,
n
Bentadine solution,
n
a wax containing a cleaning substance, or
n
abrasive cleaning agents.
Clean the exterior surfaces with a clean, lint-free cloth and one of the
cleaning solutions listed above.
n
Wring the excess water from the cloth. Do not drip any liquid into
open vents, plugs, or connectors.
n
Dry the surfaces with a clean cloth or paper towel.
3-4 Solar SpO2 Module with Masimo SET Revision A
2004407-001
Page 25

Maintenance: Checkout Procedure
Checkout Procedure
General
This procedure tests the functions of the module. The checkout
procedures consist of the SpO
These procedures are based on the assumption that the modu le under
test is used with known good cables and known good test equipment. It
also assumes that you are at least somewhat familiar with the operation
of all devices required for the procedures. For more information
concerning the operation of these components, consult the appropriate
operator’s manuals.
Required Tools/Equipment
The following lists the test equipment, adapters, and cables necessary to
complete the checkout procedures.
n
Compatible Solar monitor and a Tram-rac 4A housing to power the
module.
n
Simulators and cables listed below provide waveforms and patient
vital signs.
tests.
2
Required Simulators and Cables
Item
SpO
Simulator 408610-001 or equivalent
2
Simulator Cable 2006011-001 or equivalent
SpO
2
n
Any patient cable or leadwire set that you would usually use on
patients.
Manufacturer and
Part Number/Model
Revision A Solar SpO2 Module with Masimo SET 3-5
2004407-001
Page 26

Preparation
Maintenance: Checkout Procedure
1. Install the module in a Tram-rac 4A housing.
TRAMSCOPE 12
MARQUETTE
2. Apply power to the monitor by turning the rear panel power switch to
the ON position.
3. Turn the display ON by pressing the DISPLAY ON/OFF or
POWER key on the front panel of the monitor.
SpO2 Test
4. Ensure that the power indicator of the monitor is on.
5. Ensure that the power indicator of the module is illuminated green.
127(
Do not connect the simulator to an AC power supply for these tests.
Operate the simulator on b attery power.
1. Turn the SpO
simulator power switch OFF.
2
2. Connect the simulator cable to the module.
3. Set the simulator as follows:
127(
Use the white-colored values on the simulator.
u
Set the MODE to NELLCOR.
u
Set the SpO2% to 99.
u
Set the PRR to 70 beats/minute.
u
Turn the power ON.
4. Verify the following are displayed at the monitor: (It might be
necessary to turn the SpO
parameter on.)
2
u
A waveform with an SpO2 label.
u
An SpO2% reading between 97 - 100%.
u
A PRR reading between 67 and 72 beats per minute.
3-6 Solar SpO2 Module with Masimo SET Revision A
2004407-001
Page 27

Maintenance: Checkout Procedure
5. Test the accuracy of these SpO
% settings.
2
SpO
Settings
2
Simulator Setting Displayed Value
99% 96 - 100%
90.6% 87 - 94%
80.3% 77 - 83%
6. Test the accuracy of these PPR settings.
PPR Settings
Simulator Setting Displayed Value
70 67 – 73
100 97 – 103
7. Return the simulator to these conditions:
u
Set the SpO2% to 99.
u
Set the PPR to 70 beats/minute.
8. Set these alarms on the monitor:
u
Set SpO2% LO to 90.
u
Set PPR HI to 90.
9. Set PPR on the simulator to 100.
10. Make sure the PPR value on the monitor flashes, and it sounds an
alarm.
11. Return PPR on the simulator to 70.
12. Set SpO
% on the simulator to 80.3.
2
13. Make sure the SpO2% value on the monitor flashes, and it sounds an
alarm.
14. Disconnect the simulator cable from the module.
Revision A Solar SpO2 Module with Masimo SET 3-7
2004407-001
Page 28
Maintenance: Electrical Safety Tests
Electrical Safety Tests
Electrical safety tests provide a method of determining if potential
electrical safety hazards to the patient or operator of the device exist.
Recommendations
GE Medical Systems Information Technologies recommends electrical
safety tests be performed:
n
upon receipt of the module,
n
every twelve months thereafter, and
n
each time the module is opened or repaired.
Record the date and results on the Repair Log included at the end of this
chapter.
Required Tests
Test Conditions
To help you establish a systematic maintenance routine, GE Medical
Systems Information Technologies recommends that you perform all
safety tests presented in this chapter.
These instructions are intended for every module in the system. The
Tram-rac housing should remain connected to the host during the safety
tests. Listed below are the safety tests.
n
Hi-Pot Tests
These tests are mandatory when a module is opened or repaired.
n
Leakage Current Tests
These tests are performed after the hi-pot tests.
If a module under test fails the leakage tests, call Tech Support for
assistance. (Refer to “How to Reach Us” in front of this manual.)
All electrical safety tests may be performed under normal ambient
temperature, humidity, and pressure conditions with the module
inserted in a Tram-rac 4A.
3-8 Solar SpO2 Module with Masimo SET Revision A
2004407-001
Page 29

AC Hi-Pot Test
Test Frequency
Maintenance: Electrical Safety Tests
Hi-pot (high-potential) tests protect the patient from possible electrical
safety hazards. They are recommended for any repaired patientconnected devices to ensure patient isolation after the repair.
This test is requ ired each time the module is op ened or repaired.
:$51,1*
Failure to perform hi-pot tests may cause undue
equipment failure and possible health hazards. GE
Medical Systems Information Technologies does not in
any manner, unless an Equipment Maintenance
Contract exists, assume the responsibility for performing
this recommended health test. The sole responsibility
rests with the individual or institution using the
equipment.
Required Tools/Equipment
Procedures
Equipment required to perform the test is listed below. Equivalent
equipment may be substituted.
Required Tools/Equipment
Item
AC Hi-Pot Generator 0 - 5000 Vac
Masimo Test Body Cable Assembly 2006036-001
:$51,1*
Shock hazard. DO NOT perform this test on any of the
other connectors.
1. Install the Masimo test body in the SpO
Specifications or
Part Number
connector of the module.
2
2. Connect the hi-pot generator output lead to the exposed lead of the
test body and connect the hi-pot return to any connector shell on the
back of the Tram-rac.
3. Set the HIGH VOLTAGE switch to ON.
4. Slowly increase output voltage to 4000 volts.
Revision A Solar SpO2 Module with Masimo SET 3-9
2004407-001
Page 30

Current Leakage Tests
Preparation
Maintenance: Electrical Safety Tests
5. Wait for 60 seconds. There should be no indication of breakdown
(warning lamp or buzzer).
6. Turn off the hi-pot tester and disconnect the leads.
7. If your module fails this test, contact GE Medical Systems
Information Technologies Tech Support.
The leakage current tests are safety tests to ensure that the equipment
poses no electrical safety hazards. It is recommended after performing
the hi-pot tests.
127(
These procedures test the integrity of this modu le only, not the entire
system.
:$51,1*
Failure to perform leakage tests may cause undue
equipment failure and possible health hazards. GE
Medical Systems Information Technologies does not in
any manner, unless an Equipment Maintenance
Contract exists, assume the responsibility for performing
this recommended health test. The sole responsibility
rests with the individual or institution using the
equipment.
The module must be installed in a Tram-rac 4A housing. Equipment and
tools are listed be low .
Required Tools/Equipment
Item
Leakage Current Tester Equivalent to the circuits shown below
Digital Multimeter (DMM) 0 - 200 AC millivolts
Masimo Test Body Cable Assembly 2006036-001
Specifications or
Part Number
3-10 Solar SpO2 Module with Masimo SET Revision A
2004407-001
Page 31
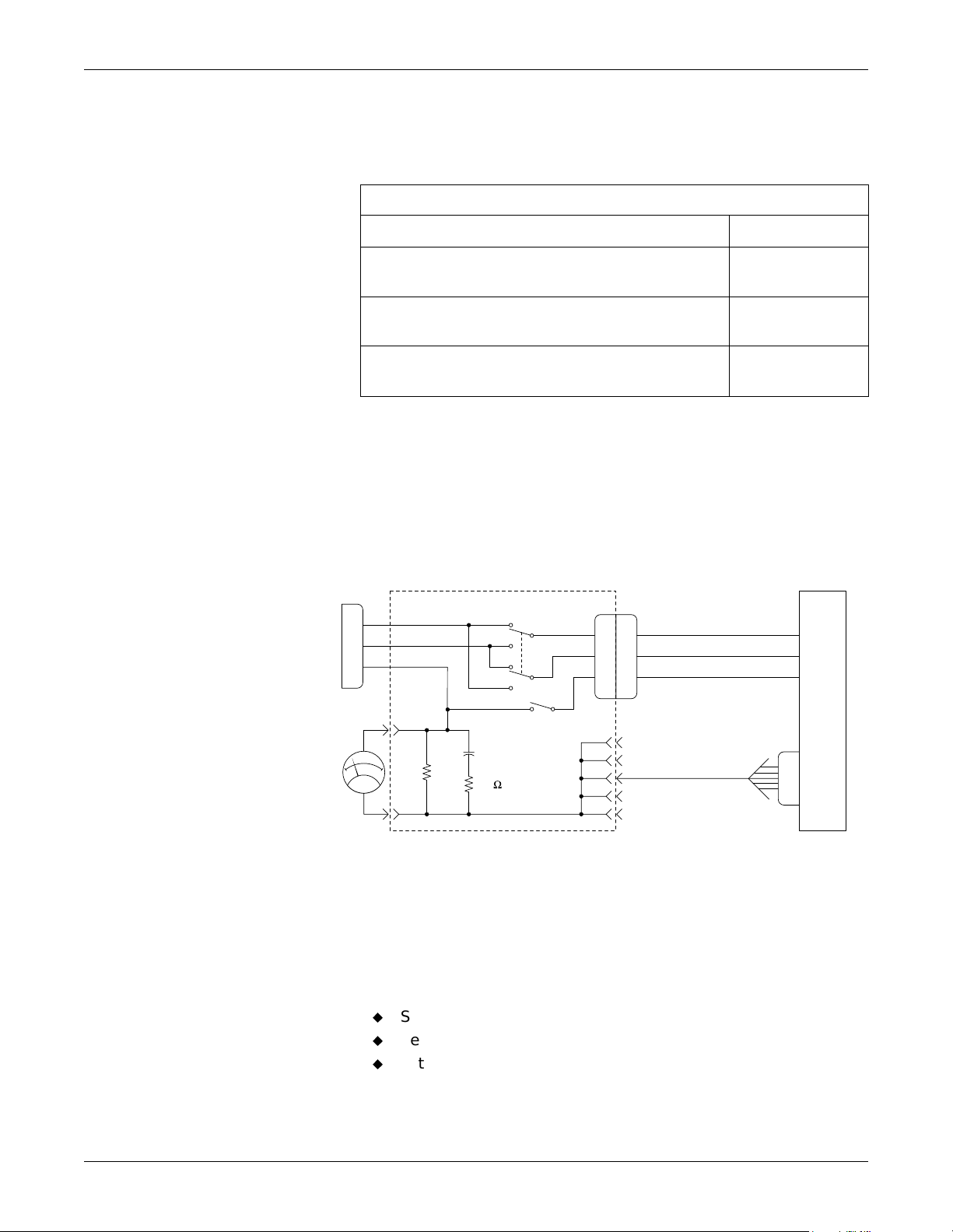
Maintenance: Electrical Safety Tests
Use the table below to determine the maximum allowable leakage
currents. For international leakage limits, refer to the internal standards
agencies of the particular country.
Maximum Allowable Leakage Currents
Test Maximum Current
Patient (Source) leakage current
Ground closed, normal & reverse polarity
Patient (Source) leakage current
Ground open, normal & reverse polarity
Patient (Sink) leakage current
Ground closed, normal & reverse polarity
Patient (Source) Leakage Current Test
This test checks leakage current from the patient cable connector of the
module to ground.
1. Prepare the sy stem according to instructio ns on page 3-6.
2. Configure the leakage tester like the circuit shown below.
LEAKAGE TESTER
HIGH
LOW
GND
POWER CORD
Measuring
Device
V*
1K
0.15µF
10
NORM
RVS
GND
10 µA
50 µA
50 µA
POWER CORD
UNIT
UNDER
TEST
TEST BODY
1-mV meter reading = 1-µA leakage current
3. Connect the host power cord to the power outlet on the leakage
tester.
4. With the power switch of the leakage tester off, connect the power
cord of the leakage tester to a correctly wired and properly grounded
ac power outlet.
5. Set leakage tester switches as follows:
u
Set the GND switch to GND OPEN.
u
Set the polarity switch to NORM/FORWARD.
u
Set the power switch to OFF.
6. Set the leakage tester power switch to ON.
Revision A Solar SpO2 Module with Masimo SET 3-11
2004407-001
Page 32

Maintenance: Electrical Safety Tests
7. Set the host rear panel power switch to ON.
8. Read the leakage current indicated on the measuring device.
9. Change the leakage tester polarity switch to the REVERSE position.
10. Read the leakage current indicated on the measuring device.
127(
If either reading is greater than 50 µA, the module fails this test.
Contact GE Medical Systems Information Technologies Tech
Support.
11. Change the GND switch to the CLOSED position.
12. Read the leakage current indicated on the measuring device.
13. Change the leakage tester polarity switch to the REVERSE position.
14. Read the leakage current indicated on the measuring device.
15. Set the power switch of the leakage tester to OFF.
If either reading is greater than 10 µA, the module fails this test.
Contact GE Medical Systems Information Technologies Tech
Support.
127(
The AAMI and IEC single fault condition (ground open) limit is
50 µA, whereas the normal condition (ground closed) limit is 10 µA.
Patient (Sink) Leakage Current Test
This tests the patient cable leakage current from a 115 or 220V ac source
into the SpO
1. Prepare the sy stem according to instructio ns on page 3-6.
2. Configure the leakage tester like the circuit shown below.
LEAKAGE TESTER
HIGH
LOW
GND
POWER CORD
Measuring
Device
V*
connector of the module.
2
NORM
RVS
120K
0.15µF
1K
10
GND
POWER CORD
(Keep cable length
short as possible.)
TEST BODY
UNIT
UNDER
TEST
1-mV meter reading = 1-µA leakage current
3. On the leakage tester, leave the GND switch set to CLOSED and set
the polarity switch to NORM/FORWARD.
3-12 Solar SpO2 Module with Masimo SET Revision A
2004407-001
Page 33

Completion
Maintenance: PM Form
:$51,1*
Shock hazard. The following step causes high voltage at
the test body. Do not touch the test body.
4. Set power switch on the leakag e tester to ON.
5. Read leakage current indicated on measuring device.
6. Change the leakage tester polarity switch to the REVERSE position.
7. Read the leakage current indicated on the measuring device.
8. Set the power switch on the leakage tester to OFF.
If either reading is greater than 50 µA, the module fails this test.
Contact GE Medical Systems Information Technologies Tech
Support.
1. Disconnect all test equipment from the module.
2. Disconnect the host power cord from leakage tester.
PM Form
3. Disconnect the tester from the power outlet.
Due to continuing product innovation and because specifications in this
manual are subject to change without notice, a PM form is not included
with this manual. For the latest PM form regarding this product, contact
a GE Medical Systems Information Technologies service representative.
On the following pages is a repair log to record the repair history of this
product.
Revision A Solar SpO2 Module with Masimo SET 3-13
2004407-001
Page 34

Repair Log
Unit Serial N umber:
Institution Name:
Date Maintenance/Repair Technician
Maintenance: Repair Log
3-14 Solar SpO2 Module with Masimo SET Revision A
2004407-001
Page 35

Maintenance: Repair Log
Revision A Solar SpO2 Module with Masimo SET 3-15
2004407-001
Page 36

Maintenance: Repair Log
3-16 Solar SpO2 Module with Masimo SET Revision A
2004407-001
Page 37

4 Troubleshooting
Revision A Solar SpO2 Module with Masimo SET 4-1
2004407-001
Page 38

For your notes
4-2 Solar SpO2 Module with Masimo SET Revision A
2004407-001
Page 39

Troubleshooting: General Fault Isolation
General Fault Isolation
First Things to Ask
If the unit is not work ing properly ask these basic questions.
u
Is the module seated correctly?
u
Is the monitor and Tram-rac housing power cord connected?
u
Is the monitor turned ON at the rear of the monitor?
u
Is the display LED illuminated? Are all the communication
cables firmly connected?
u
Were there any changes in the use, location, or environment of
the equipment that could cause the failure?
u
Has the unit been modified in any way, either in software or
hardware?
Is operator error the cause of the problem? Try to repeat the use r’s
scenario exactly and compare that to the pr oper operation of the
equipment. Check the operator’s manual as necessary.
Visual Inspection
A thorough visual inspection of the equipment can save time. Small
things—disconnected cables, foreign debris on circuit boards, missing
hardware, loose components—can frequently cause symptoms and
equipment failures that may appear to be unrelated and difficult to
track.
Take the time to make all the recommended visual checks (refer to the
visual inspection chart on the next page) before starting any detailed
troubleshooting procedures.
Revision A Solar SpO2 Module with Masimo SET 4-3
2004407-001
Page 40

Area Look for the following problems:
I/O Connectors and Cables
Troubleshooting: General Fault Isolation
Visual Inspection List
n
Fraying or other damage
n
Bent prongs or pins
n
Cracked housing
n
Loose screws in plugs
Interface Cables
Circuit Boards
Note: If module is opened,
perform Electrical Safety Tests
in chapter 3, Maintenance.
Ground Wires/Wiring
Mounting Hardware
Power Source
n
Excessive tension or wear
n
Loose connection
n
Strain reliefs out of place
n
Moisture, dust, or debris (top and bottom)
n
Loose or missing components
n
Burn damage or smell of over-heated components
n
Socketed components not firmly seated
n
Solder problems: cracks, splashes on board, incomplete feedthrough, prior
modifications or repairs
n
Loose wires
n
Faulty wiring
n
Wires pinched or in vulnerable position
n
Loose or missing screws or other hardware
n
Faulty connection between PCBs
n
Power source problems may cause static discharge, resetting problems, and
noise.)
:$51,1*
Solder multilayer and surface mount PCB assemblies at
your own risk! Improper repair methods can damage the
PCB assemblies even further. Only qualified service
personnel with the proper laboratory equipment should
attempt to repair PCB assemblies.
4-4 Solar SpO2 Module with Masimo SET Revision A
2004407-001
Page 41

Troubleshooting: Troubleshooting Procedure
Yes No
Yes No
Yes No
Troubleshooting Procedure
To use this troubleshooting procedure, read through the steps in the
table until you find a similar problem to the one you are having. Do not
perform any invasive procedures listed in this procedures unless you are
a trained field or bio-medical engineer.
:$51,1*
This procedure is intended for use by service personnel
with advanced troubleshooting skills. The consequences
of the following steps may cause loss of patient data and
disruption of the entire Unity Network.
Troubleshooting Chart
Is LED on front panel
green?
Is patient data displayed at
the monitor?
This problem has many sources, so try one or more of the following solutions.
1. Verify Tram-rac 4A housing is functional.
n
Verify Tram-rac power LED is ON.
n
Check for a loose or faulty cable from the patient monitor to the Tram-rac housing.
n
Power cycle Tram-rac 4A housing if it has an external power supply.
n
Swap Tram-rac 4A housing with a known good one.
2. If module LED is off or PROBE OR MODULE MALFUNCTION displays on the screen, there is a
hardware problem with the module.
n
Reseat Solar SpO2 module in the Tram-rac 4A housing.
n
Remove the sensor from the module, then reseat the Masimo module in the Tram-rac housing.
n
Verify Tram-rac housing is a Tram-rac 4A housing and turned ON.
n
Verify the host is a Solar-based monitor and turned ON.
n
Verify Solar-based monitor is running V2A or later software.
n
Verify Solar-based monitor is not resetting itself.
n
Swap module with a known good one.
Try one of the following solutions.
1. Verify correct patient or simulator leadwire connections to module.
2. Contact GE Medical Systems Information Technologies Tech Support.
Is the parameter box
displayed at the monitor?
End This is the extent of troubleshooting steps.
Revision A Solar SpO2 Module with Masimo SET 4-5
Verify that the patient cable is connected.
2004407-001
Page 42

Troubleshooting: Troubleshooting Procedure
System OK LED
The system LED, DS1, is red and is located inside the module. This LED
indicates whether the software is operating normally. The status of the
LED toggles each time the module completes transmitting a packet to
the host patient monitor.
MS-3 Communications OK LED
The MS-3 communications LED, DS2, is yellow and is located inside the
module. This LED indicates whether communications between the
Masimo MS-3 analyzer and the system processor are operating normally.
The LED toggles if a complete data packet is received from the Masimo
MS-3 analyzer pcb.
Isolated Power Supply OK LED
The isolated power supp ly LED, D S3, is gre en an d is lo cated on t he front
panel of the module. This LED indicates whether the isolated power
supply is operating normally. The LED is illuminated if power is applied
to the Masimo MS-3 analyzer pcb.
4-6 Solar SpO2 Module with Masimo SET Revision A
2004407-001
Page 43

Troubleshooting: Theory of Operation
Theory of Operation
Hardware Functions
The Masimo SET SpO2 Module, hereafter referred to as the Masimo
module, provides all the hardware necessary to communicate with a host
patient monitor and continuously monitor the functional oxygen
saturation of arterial hemoglobin (SpO
features include the following items:
n
n
n
n
n
Software Functions
) and pulse rate. The hardware
2
Bedside communications using the Synchronous Serial Shift Register
Interface protocol.
Masimo MS-3 analyzer communications and control
Input power conditioning and soft-start current limiting
Code download - from host or BDM
4000 Volt patient isolation
System Processor
The Masimo module PCB performs the communications with the Masimo
MS-3 Analyzer PCB and host bedside communications. The device
performs the following functions under software control:
n
Bedside communications and error detection
n
Masimo MS-3 Analyzer communications and control
n
SpO2 value calculation
n
Respiration rate calculation
n
Error detection and alarm indication
n
Initialization and self test
The system processor uses external memory for operation. There is a
512K x 8 static RAM for temporary data storage.
127(The static RAM is not battery bac ked-up, therefore this device is
not a transportable patient monitor such as the TRAM module.
The static RAM is also used as temporary storage of Masimo MS-3
program code during program download of the MS-3 software. The
program code is stored in a 128K x 8 sectored FLASH EPROM. This
device can be erased on an individual sector basis. The first sector of
FLASH is used for storing the BOOT code. This insures that even if a
download of code fails the module still attempts another download of
code. The other seven sectors of FL ASH store the MAIN code. The MAIN
code operates during normal operation of the module. This code can be
updated using the host patient monitor.
Revision A Solar SpO2 Module with Masimo SET 4-7
2004407-001
Page 44

Troubleshooting: Theory of Operation
Below is the overall block di agram for the Masimo module.
Power Status LED
Masimo SET SpO2 Module PCB
FLASH
Program
Memory
SRAM
Data
Memory
Serial Shift Register Interface Signals
Module Communications Processor
Bendix Connector
+5V
+16.75V
Supports serial shift register interface to Tram-rac 4A
Power Condition
and Soft Start
Isolated Switching Power Supply
Communication
Processor
MC68332
+5V
Soft Starte d
+16.75V
Soft Starte d
Power Supply Enable
Power
Supply
Primary
4000 VAC Isolation Barrier
Tx
Opto
Rx
Opto
Reset
Opto
Feedback
Opto
Power
Supply
Isolated
Secondary
Masimo MS-3 Board
FLASH
Program
Memory
DSP
SRAM
Data
Memory
J3 J1
+5V
ISO
+15V ISO
-15V ISO
LED Drive / Sensor ID
Detector Signal
Instrument
(Flex) Cable
Adapter Signal
Masimo
Sensor
Connector
Receptacle
Masimo MS-3
The principles of operation of the Masimo MS-3 pulse oximeter is that
oxyhemoglobin and deoxyhemoglobin differ in their absorption of red and
infrared light (spectrophotometry), the volume of arterial blood in tissue
(and hence, light absorption by that blood) changes during the pulse
(plethysmography), and that arterio-venous shunting is highly variable
and that fluctuating absorbance by venous blood is a major component of
noise during the pulse. Because oxyhemoglobin and deoxyhemoglobin
differ in light absorption, the amount of red and infrared light absorbed
by the blood is related t o hemoglobin oxygen saturati on. The Masimo
MS-3 pulse oximeter decomposes the red and infrared pulsatile
absorbance signal into an arterial signal plus a noise component and
calculates the ratio of the arterial signals without noise. The ratio of the
two arterial pulse-added absorbance signals and its value is used to find
the SpO
MS-3’s software.
4-8 Solar SpO2 Module with Masimo SET Revision A
saturation in an empirically derived equation into the Masimo
2
2004407-001
Page 45

Troubleshooting: Theory of Operation
Host Patient Monitor Communications
The Masimo module communicates with the host patient monitor using
the synchronous Serial Shi f t Register Interface Signals. The
communication processor performs this function, operating in slave mode
it receives clock and enable signals from the host patient monitor.
All integrated circuits with signals connected to the backplane are
powered directly from the backplane +5 volts not the soft-started power
supplies. This is so the signals won’t load down the synchronous Serial
Shift Register Interface S ignals upon insertion of the module.
Power Condition and Soft Start
The Masimo module receives power from the host Tram- rac. The module
is designed for insertion into a live Tram-rac. All the power supply pins
incorporate soft-start circuitry to limit inrush currents. This module uses
the +5V and +16.5V power supplies from the host patient monitor.
Isolated Power Supply
The Masimo module uses an isolated power supply to power the Masimo
MS-3 board. The power supply is designed to provide 4000 volts of
patient isolation from the host patient monitor. The isolated power
supply generates +5V, +15V, and -15V and is controlled by the system
processor.
The system processor moni tors signal PS_FAULT whic h indicates a fault
condition with the power supply. If this signal is active (logic high) for
more than 200ms, the system processor shuts down the isolated power
supply. The system processor does not monitor this line for the first
100ms after the power supply is enabled allowing the isolated power
supply time to stabilize.
Patient Connector Flex Circuit
The flex circuit a ssemb ly p r ovide s t he conn ectio n fro m t he Masi mo MS-3
analyzer to the patient receptacle on the front panel of the module. The
Masimo Flex PCB includes a ferrite core to reduce electromagnetic
emissions/susceptibility and a shield around the front panel connector to
reduce susceptibility to external interference.
Revision A Solar SpO2 Module with Masimo SET 4-9
2004407-001
Page 46

Troubleshooting: External Connectors
External Connectors
Pin-by-pin descriptions and the signal names for each connector on the
front panel of the module are described in this section.
SpO2 Signals
The SpO2 patient cable attaches to the fron t panel 11-pin Blue Nicolay
SpO
Signal Name Pin Type Description
Detector Anode 1 I Detector Anode
Detector Cathode 2 I Detector Cathode
connector.
2
SpO
Connector
2
NC 3 X No connection
NC 4 X No connection
IR LED Cathode 5 O IR LED Cathode/Red LED Anode
Outer Shield 6 O Outer shield of patient cable
Inner Shield 7 O Inner shield (shield around detector wires of
patient cable)
NC 8 X No connection
NC 9 X No connection
RED LED
Cathode
Outer Shield 11 O Outer shield of patient cable
10 O Red LED Cathode/IR LED Anode
1
7
2
8
3
9
4
10
5
11
6
Front View
of Module
4-10 Solar SpO2 Module with Masimo SET Revision A
2004407-001
Page 47

Troubleshooting: External Connectors
Probe Schematic Diagram
The schematic diagram of the Nellcor probe sensor is presented below.
RED_LED CATHODE
10
IR_LED CATHODE
5
NC
3
NC
4
DETECTOR ANODE
1
DETECTOR CATHODE
2
INNER SHIELD
7
NC
8
NC
9
ISOGND
11
FSHIELD
6
Host Patient Monitor Connector
The Masimo module uses a 2 x 15 pin female Bendix connector with
bristle brush pins to mate with the Tram-rac housing. The following
table lists signal names, input pins, type, and descriptions. Signals with
an asterisk (*) indicate a low signal.
151413121110987654321
B
151413121110987654321
A
Revision A Solar SpO2 Module with Masimo SET 4-11
2004407-001
Page 48

Troubleshooting: External Connectors
Signal Name Pin Type Description
GND A1 I +16.5 volt power supply return
WF_A B1 O Not Used
MOD_EN_B A2 I Not Used
MOD_EN_A B2 I MODULE CHANNEL A ENABLE: This signal is the
enable for this module. This signal is asserted high to
enable the module to communicate via the synchronous
serial shift register interface.
AGND A3 I Not Used
WF_B B3 O Not Used
SNYC_ECG A4 I Not Used
+16.5V B4 I +16.5 volt power supply
MOD_DATA_CLK* A5 I MODULE DATA CLOCK*: This signal is the 7000-series
module data clock used in the synchronous serial shift
register interface. The falling edge is used to shift data
both into and out of the module.
PWRBUS B5 I/O Not Used
MOD_DATA_IN* A6 I MODULE LOAD, MODULE DATA IN: The module load
function is used in the synchronous serial shift register
interface to pre-load the module’s output shift register
prior to transferring data out of the module to the Tram-
rac. When this interface is used, active-low binary input
data is transferred to the module’s input serial shift
register on this signal with the falling edge of the module
data clock signal.
WF_OUT1 B6 I/O Not Used
MOD_DATA_OUT* A7 O MODULE DATA OUT*: When the 7000-series
synchronous serial shift register interface is used,
active-low binary output data is transferred from the
module’s output shift register on this signal with the
falling edge of the module data clock signal.
WF_OUT2 B7 I/O Not Used
MOD_DATA_LAT* A8 I MODULE DATA LATCH*: This signal is the 7000-series
module data latch strobe used in the synchronous serial
shift register interface. After 8 data bits of input data
have been shifted into the module using the module
data clock signal, this signal strobes the input data into
the modules latch.
WF_OUT3 B8 I/O Not Used
AGND A9 I Not Used
4-12 Solar SpO2 Module with Masimo SET Revision A
2004407-001
Page 49

Troubleshooting: External Connectors
Signal Name Pin Type Description
123KHZ B9 I Not Used
CALIBRATE* A10 I Not Used
WF_OUT5 B10 O Not Used
Software Updates
GND A11,
B11
TN_ENA* A12 O TRAMNET ENABLE*: When high this signal identifies
+5 B12 I +5V DIGITAL POWER: +5V power supply to the module
+15V A13,
B13
-15V A14,
B14
AGND A15,
B15
Two types of downloading procedures are available for the Masimo
module. Software may be downloaded from a programmed software
diskette using a patient monitor by either of the following two ways:
I GROUND: These pins are the logic reference and the
+5V power supply return lines.
the device’s communication protocol as the
synchronous serial shift register interface. When low this
signal identifies the device’s communication protocol as
Tramnet.
for the device digital circuitry.
I Not Used
I Not Used
I Not Used
n
from a laptop personal computer or terminal, or
n
across the Unity Network from a central station.
These procedures are explained in detail in the software upgrade kit.
Contact your GE Medical Systems Information Technologies service
representative for information.
Revision A Solar SpO2 Module with Masimo SET 4-13
2004407-001
Page 50

For your notes
Troubleshooting: External Connectors
4-14 Solar SpO2 Module with Masimo SET Revision A
2004407-001
Page 51

5 Parts Lists and Drawings
Revision A Solar SpO2 Module with Masimo SET 5-1
2004407-001
Page 52

For your notes
5-2 Solar SpO2 Module with Masimo SET Revision A
2004407-001
Page 53

Ordering Parts
General
The parts lists and assembly drawings in this chapter supply enough
detail for you to order parts for the assemblies considered field
serviceable. If you require additional information or troubleshooting
assistance, contact Tech Support.
To order parts, contact Service Parts at the address or telephone number
listed on the “How to Reach Us...,” page found at th e front of th is manual.
Field Replaceable Units
The tables below list the most commonly replaced assemblies ordered in
the service spare circuit board kits.
Parts Lists and Drawings: Ordering Parts
Field Replaceable Units
Item Part Number
Masimo SET SpO
Masimo SET SpO2 Module Flex PCB 2001861-001
Masimo SET SpO
The following is a list of all accessories available for the Masimo SET
SpO2 Module.
Cable Assy Patient Adapter Masimo SpO2 12 FT 2002592-001
Cable Assy Patient Adapter Masimo SpO2 8 FT 2002592-002
Kit Samples Masimo Adult/Pediatric Sensors 2002797-001
Masimo LNOP Adt Adult Sensor N/A
Masimo LNOP Pdt Pediatric Sensor N/A
Kit Samples Masimo Neonatal Sensors 2002798-001
Module PCB 2001857-001
2
MS-3 PCB 2002271-001
2
Accessories
Description Part Number
Masimo LNOP Neo Neonatal Sensor N/A
Masimo LNOP NeoPt Neonatal Sensor N/A
Masimo LNOP DC1P Reusable Finger Sensor Pediatric 2002799-001
Masimo LNOP DC1 Reusable Finger Sensor Adult 2002800-001
Revision A Solar SpO2 Module with Masimo SET 5-3
2004407-001
Page 54

Parts Lists and Drawings: Disassembly Procedures
Disassembly Procedures
Refer to the exploded vi ew that f ollows the se procedu res f or part lo cation.
Assembly Housing
1. Remove 2 screws from the module top and 2 screws from t he module
bottom.
2. Slide the housing away from the bezel.
Masimo SET SpO2 MS-3 PCB
1. Remove the assembly housing according to the steps above.
2. Remove 3 screws from the MS-3 PCB and pull the PCB up and away
from the spacers.
3. Disconnect the Masimo module flex PCB from the MS-3 PCB and
remove the MS-3 PCB.
Masimo SET SpO2 Module Flex PCB
1. Remove the assembly housing and the Masimo module MS-3 PCB
according to the steps above.
2. Disconnect ends of the flex assembly an d remove.
Masimo SET SpO2 Module PCB
When the assembly housing, the MS-3 PCB, and the flex PCB are
removed according to the steps above, the Masimo module PCB is loose
and can be removed.
Reassembly
Reverse the above st eps to reassemble the Masimo module.
Testing
Perform the Electrical Safety Tests described in chapter 3, Maintenance.
5-4 Solar SpO2 Module with Masimo SET Revision A
2004407-001
Page 55

Parts Lists and Drawings: Solar SpO2 Module with Masimo SET 2001891-001A
Solar SpO2 Module with Masimo SET 2001891-001A
Find
Number
1 2001857-001 PCB MASIMO SET SPO2 MODULE 1
2 2001861-001 PCB FLEX MASMO SET SPO2 MODULE 1
4 2002271-001 PCB MS-3 MASIMO SET SPO2 1
5 2002326-001 SPACER 5.5MM HEX M3 F/F 20.1LG NYLON 3
6 45209-411 SCREW PH M3 X 8MM 6
7 404525-008 LABEL BLANK 1.2IN X .6IN 1
8 406019-001 LATCH SPRING TRAM 2
9 406037-001 LATCH PIN TRAM 2
10 410124-020 MDL FR BEZEL NICOLAY SGL 11P 1
11 410337-001 LATCH MODULE 2
12 412669-001 ASSY MODULE HOUSING 1
13 414536-001 ADAPTER CONN NICOLAY 11P 1
14 2004668-001 LABEL MODULE COVER SOLAR 1
Item Number Item Description Qty
15 4521-304 NUT,ESNA,4-40, 3
16 4551-312 SPRING COMPRESSION .50 LG 2
17 4760-014 SCREW PH 2-56X3/16 COATED 4
18 404525-001 LABEL BLANK 2 X 3/4 1
19 4550-014 WASH NYL .28 OD .12 ID .03THK 3
24 2004407-001 MNL SVCE MASIMO SET SPO2 MODULE ENG .25
Revision A Solar SpO2 Module with Masimo SET 5-5
2004407-001
Page 56

Parts Lists and Drawings: Solar SpO2 Module with Masimo SET 2001891-001A
Schematic Diagram
5-6 Solar SpO2 Module with Masimo SET Revision A
2004407-001
Page 57

Page 58

 Loading...
Loading...