Page 1

TM
INCU
Incubator Analyzer
Operators Manual
PN 2206965
April 2005
© 2005 Fluke Corporation
All product names are trademarks of their respective companies.
All rights reserved. Printed in USA
Page 2
INCU
Operators Manual
Notices
Fluke Biomedical
6920 Seaway Blvd.
Everett, WA 98203
USA
Customer Service and Sales
USA and Canada: 800.648.7952
Outside the USA: 775.883.3400
Sales Fax: 775.883.9541
Sales E-Mail: sales@flukebiomedical.com
Service: 888.993.5853
Service Fax: 775.886.6320
Service E-mail: techservices@flukebiomedical.com
Internet: www.flukebiomedical.com
For additional sales or service information, contact your local Fluke Biomedical Distributor or Fluke
Electronics office.
Restrictions and Liabilities
Information in this document is subject to change and does not represent a commitment by Fluke
Biomedical. Changes made to the information in this document will be incorporated in new editions of
the publication. No responsibility is assumed by Fluke Biomedical for the use or reliability of software
or equipment that is not supplied by Fluke Biomedical or its affiliated dealers.
All Rights Reserved
Copyright 2005, Fluke Biomedical. No part of this publication may be reproduced, transmitted,
transcribed, stored in a retrieval system, or translated into any language without the written
permission of Fluke Biomedical.
ii
Page 3
Incubator Analyzer
Notices, Warranty and Contents
Safety Considerations
Warnings and Cautions
Use of this instrument is restricted to qualified personnel who recognize shock hazards and are
familiar with safety precautions used when operating electrical equipment. Read the manual carefully
before operating the INCU.
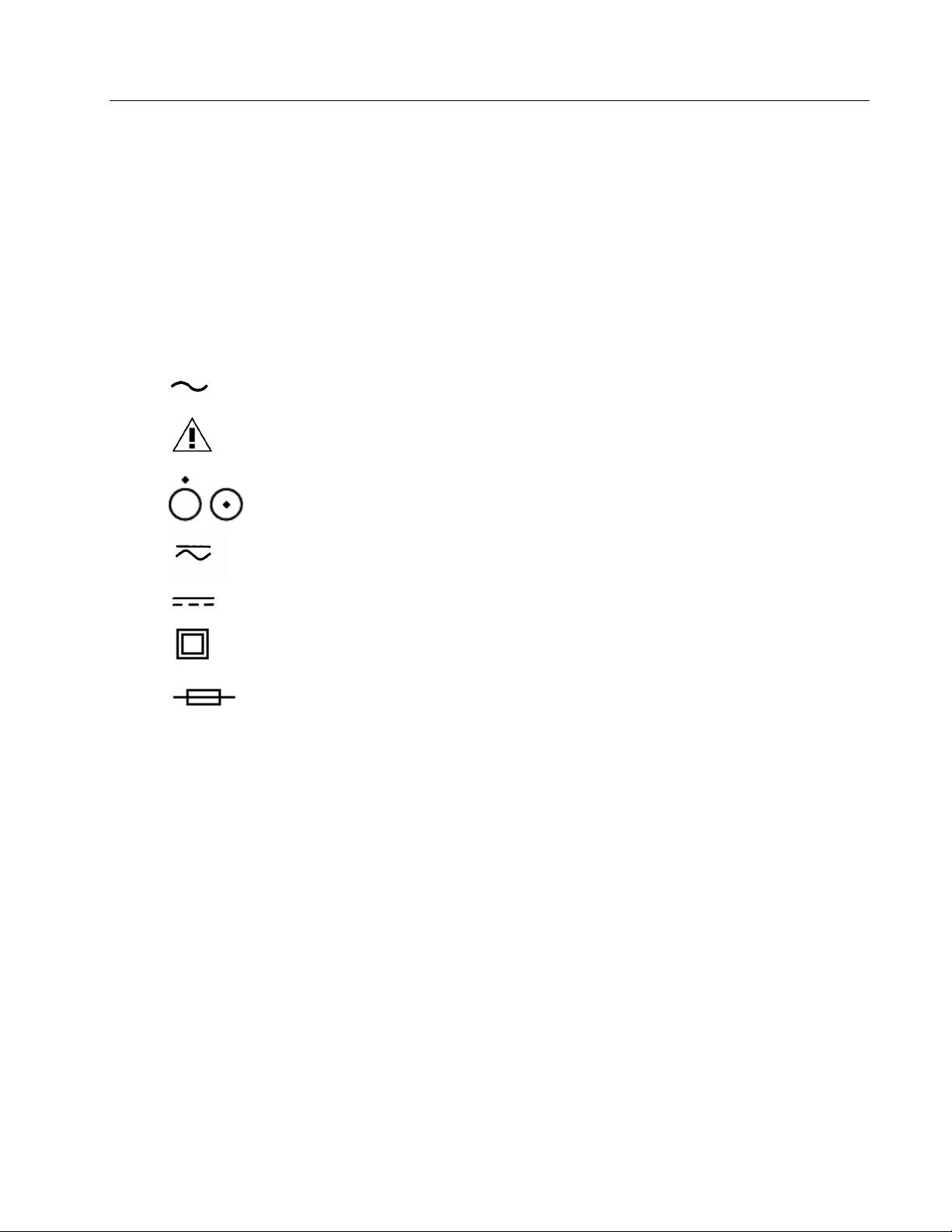
☛ The following warning and informational symbols may be found on the INCU.
Symbol Description
I P 30
Alternating Current
Caution: Refer to accompanying
documentation
Master ON/OFF,switch. Push for OFF or ON
Direct / Alternating Current
Direct Current
Enclosure with double insulation or reinforced
insolution
Fuse
Class of protection see Appendix A
iii
Page 4

INCU
Operators Manual
Hazard Warnings
☛ Warning! Power Rating. The INCU mains power input must be connected using an
☛ Warning! Internal Voltage. Always turn off the power switch and unplug the power
☛ Warning! Liquids. Avoid spilling liquids on the analyzer; fluid seepage into internal
☛ Warning! “Do not use in the presence of oxygen”. The Air Flow sensor is hot wire
☛ Warning! “Use only approved battery charger." The INCU contains a lead-acid
Precautions
The following precautions are provided to help you avoid damaging the system:
☛ Caution: Service. Authorized service personnel should service the INCU. Only
☛ Caution: Environmental Conditions. Do not expose the system to temperature
☛ Caution: Do NOT Immerse. Clean only with a mild detergent, and gently wipe down
☛ Caution: Electromagnetic radiation may affect the noise measurement.
external power supply that provides voltage and current within the specified rating for
the system.
cord before cleaning the outer surface of the INCU.
components creates a potential shock hazard. Do not operate the instrument if
internal components are exposed to fluid.
technology and is a source of combustion if used in the presence of oxygen within
the incubator. Use the INCU only in ambient oxygen conditions.
rechargeable battery. Use only the approved charger with proper voltage and current
ratings; otherwise, damage to the unit may result.
qualified technical personnel should perform troubleshooting and service procedures
on internal components.
extremes. Ambient temperatures should remain between 18°C to 40°C. System
performance may be adversely affected if temperatures fluctuate above or below this
range.
with a clean, lint-free cloth.
iv
Page 5

Incubator Analyzer
Notices, Warranty and Contents
Applicable Testing Standards
The INCU has been tested by an independent laboratory and meets the requirements listed here.
Safety Requirements
EC Directive
73/23/EEC
IEC 1010-1, Safety requirement for electrical equipment for
measurement, control and laboratory use, Part 1: General
Requirements.
North America
Battery Charger is UL marked.
Electromagnetic Interference and Susceptibility
The system meets the requirements of EC Directive 89/336/EEC Electromagnetic Compatibility (see
page viii).
☛ USA FCC Class A
Warning: Changes or modifications to this unit not expressly approved by the manufacturer could
void the user's authority to operate the equipment.
This equipment has been tested and found to comply with the limits for a Class A digital device,
pursuant to Part 15 of the FCC Rules.
These limits are designed to provide reasonable protection against harmful interference when the
equipment is operated in a commercial environment. Like all similar equipment, this equipment
generates, uses, and can radiate radio frequency energy and, if not installed and used in accordance
with the instruction manual, may cause harmful interference to radio communications. Operation of
this equipment in a residential area is likely to cause interference, in which case the user will be
required to correct the interference at his/her own expense.
☛ Canadian Department of Communications Class A
This digital apparatus does not exceed Class A limits for radio emissions from digital apparatus set
out in the Radio Interference Regulations of the Canadian Department of Communications.
Le present appareil numerique n'met pas du bruits radioelectriques depassant les limites applicables
aux appareils numerique de la Class A prescrites dans le Reglement sur le brouillage radioelectrique
edicte par le ministere des Communications du Canada.
v
Page 6
INCU
Operators Manual
EC Directive 89/336/EEC Electromagnetic Compatibility
☛ Emissions - Class B
NFEN 55022 Class B (12/94) Radio disturbance
Immunity
☛
The system has been type tested by an independent, accredited testing laboratory and found to meet
the requirements for Immunity. Verification of compliance was conducted to the limits and methods of
the following:
EN 61000-4-2 (1995) Electrostatic Discharge
EN 61000-4-3 (1984) Radiated EM Fields (electromagnetic radiation may affect the noise
measurement)
EN 61000-4-4 (1995) Electrical Fast Transient/Burst
Based on the testing standards below,
this device bears the
mark.
EN 61000-4-5 (1995) Surge Immunity
EN 61000-4-6 (1996) Conducted Disturbances
EN 61000-4-11 (1994) Voltage Dips, Short Interruptions and Variations (N/A; Battery buffers
transients)
EC Directive 73/23/EEC Low Voltage (User Safety)
The system has been type tested by an independent testing laboratory and found to meet the
requirements of EC Directive 73/23/EEC for Low Voltage. Verification of compliance was conducted
to the limits and methods of the following:
IEC 1010-1 1990 and Amendments 1 1992 and Amendments 2 1995
“Safety Requirements for Electrical Equipment for Measurement, Control and Laboratory Use,
Part 1: General requirements” (including amendments 1 and 2).
vi
Page 7

Incubator Analyzer
Notices, Warranty and Contents
Warranty
This Warranty is limited and applies only to new products, except for computer-based software, which
is covered under a separate Warranty Policy, manufactured by Fluke Biomedical. Fluke Biomedical
makes no warranty whatsoever regarding the condition of used products.
Fluke Biomedical warrants the instrument (hereinafter collectively referred to as “Products” or
“Product”) for a period of one (1) year from the original purchase date against defective materials or
workmanship. This Warranty is limited to the original purchaser (the “Purchaser”) and cannot be
assigned or transferred. All claims under this Limited Warranty must be made in writing to Fluke
Biomedical, Attention: Service Department. Purchaser must ship the Product to Fluke Biomedical,
postage pre-paid. Fluke Biomedical shall either repair or replace with new or like new, at its option
and without cost to the Purchaser, any Product which in Fluke Biomedical’s sole judgment is
defective by reason of defects in the materials or workmanship.
This Warranty is VOID if the Product has been damaged by accident or misuse, or has been
damaged by abuse or negligence in the operation or maintenance of the Product, including without
limitation unsafe operation, operation by untrained personnel, and failure to perform routine
maintenance. This Warranty is VOID if the Product has been repaired or altered by persons not
authorized by Fluke Biomedical, or if the Product has had the serial number altered, effaced, or
removed. This Warranty is VOID if any of the Products has not been connected, installed or adjusted
strictly in accordance with written directions furnished by Fluke Biomedical. Batteries, fuses, light
bulbs, and other “consumable” items used in any of the Products are not covered by this Warranty.
Software utilized in conjunction with any of the Products is not covered by the terms of this Warranty
but may be covered under a separate Fluke Biomedical software warranty.
We will continue to stock parts for a maximum period of five (5) years after the manufacture of any
equipment has been discontinued. Parts shall include all materials, charts, instructions, diagrams,
and accessories that were furnished with the standard models.
THIS WARRANTY CONTAINS THE ENTIRE OBLIGATION OF FLUKE BIOMEDICAL, AND NO
OTHER WARRANTIES, EXPRESSED, IMPLIED, OR STATUTORY ARE GIVEN. PURCHASER
AGREES TO ASSUME ALL LIABILITY FOR ANY DAMAGES AND/OR BODILY INJURY OR DEATH
THAT MAY RESULT FROM THE USE OR MISUSE OF ANY EQUIPMENT OR INSTRUMENT BY
THE PURCHASER, HIS EMPLOYEES, AGENTS, OR CUSTOMERS, OTHER THAN THE EXPRESS
WARRANTY CONTAINED HEREIN. WE SHALL NOT BE RESPONSIBLE FOR ANY DIRECT OR
CONSEQUENTIAL DAMAGES OF ANY KIND. THIS WARRANTY SHALL NOT BE CHANGED OR
MODIFIED IN ANY WAY WITHOUT THE EXPRESS WRITTEN PERMISSION OF AN OFFICER OF
FLUKE BIOMEDICAL.
THIS WARRANTY IS VOID UNLESS THE PURCHASE REGISTRATION CARD HAS BEEN
COMPLETED AND MAILED TO US WITHIN TEN (10) DAYS OF PURCHASE.
vii
Page 8

INCU
Operators Manual
About This Manual
This manual provides a complete description of the INCU Incubator Analyzer and its applications.
The manual is organized as follows:
Chapter 1, Overview: An introduction to the INCU and optional accessories
•
•
Chapter 2, Installation: How to connect the INCU and install the PC software
•
Chapter 3, General Operation: INCU keypad functions, setup, and stand-alone operation
•
Chapter 4, PC Software Operation: PC software operation
• Chapter 5, Safety, Displayed Messages, Troubleshooting, and Support
• Appendix A, INCU Specifications
• Appendix B, INCU Report Examples
• Appendix C, DAT File Format
viii
Page 9

Incubator Analyzer
Notices, Warranty and Contents
Contents
Notices ................................................................................................................... ii
Restrictions and Liabilities ........................................................................ ii
All Rights Reserved................................................................................... ii
Safety Considerations............................................................................................iii
Warnings and Cautions............................................................................ iv
Hazard Warnings............................................................................... iv
Precautions........................................................................................ iv
Applicable Testing Standards ................................................................................ v
Safety Requirements ................................................................................ v
Electromagnetic Interference and Susceptibility....................................... v
EC Directive 89/336/EEC Electromagnetic Compatibility................... vi
Emissions............................................................................................ vi
Immunity ............................................................................................. vi
EC Directive 73/23/EEC Low Voltage (User Safety) ............................... vi
Warranty............................................................................................................. vivii
About This Manual ...............................................................................................viii
Chapter 1: Overview ................................................................................ 1-1
Introducing the INCU™.......................................................................................1-2
INCU Measurements...........................................................................................1-3
Getting to Know the INCU................................................................................... 1-4
AAMI/IEC Standards...........................................................................................1-6
INCU Applications ..................................................................................1-6
Accessories.........................................................................................................1-8
Chapter 2: Installation ............................................................................. 2-1
Unpacking and Inspection................................................................................... 2-2
Connecting INCU ................................................................................................2-3
AC-DC Battery Charger Connection ......................................................2-3
Internal Battery.......................................................................................2-3
RS-232 Port ...........................................................................................2-3
Air Flow Sensor......................................................................................2-4
Radiant Baby Assembly.........................................................................2-4
PC Software System Requirements ...................................................................2-5
Installing the PC Software................................................................................... 2-6
Installing the Software............................................................................ 2-6
ix
Page 10

INCU
Operators Manual
Chapter 3: General Operation................................................................. 3-1
INCU Keypad Functions ..................................................................................... 3-2
Key Details............................................................................................. 3-3
INCU Setup......................................................................................................... 3-5
Placement of the INCU in the Incubator................................................ 3-5
Infant Radiant Warmers......................................................................... 3-9
INCU Operation ................................................................................... 3-10
Chapter 4: INCU PC Software Operation ............................................... 4-1
Introduction ......................................................................................................... 4-2
Configuring the INCU for Data Acquisition ......................................................... 4-2
Data Acquisition.................................................................................................. 4-4
Transferring Data to the PC Software ................................................................ 4-5
Graphs ................................................................................................................ 4-7
Software Options ................................................................................................ 4-8
Open Files ............................................................................................. 4-8
Save Files .............................................................................................. 4-8
Printer Configuration.............................................................................. 4-8
Print Folder ............................................................................................ 4-8
Cascade................................................................................................. 4-9
Heading ............................................................................................... 4-10
Graph Colors ....................................................................................... 4-11
Com Port.............................................................................................. 4-12
Language............................................................................................. 4-12
Zoom.................................................................................................... 4-13
Chapter 5: Safety, Displayed Messages, Troubleshooting,
and Support........................................................................... 5-1
Electrical Safety .................................................................................................. 5-2
Cleaning................................................................................................. 5-2
Air Flow Sensor ..................................................................................... 5-2
Displayed Messages .......................................................................................... 5-3
Troubleshooting.................................................................................................. 5-4
Support ............................................................................................................... 5-6
Appendix A: Specifications ....................................................................A-1
Appendix B: Report Examples ...............................................................B-1
Appendix C: DAT File Format.................................................................C-1
x
Page 11

Incubator Analyzer
Notices, Warranty and Contents
List of Illustrations
Figure 1-1. Closed Incubator With Forced Convection.......................................1-3
Figure 1-2. Infant Warmer...................................................................................1-3
Figure 1-3. Descriptions and Locations of INCU Sensors .................................. 1-4
Figure 1-4. View of Right Side of INCU ..............................................................1-5
Figure 1-5.
Figure 2-1. Radiant Baby Assembly ...................................................................2-4
Figure 2-2. INCU Setup Screen .......................................................................... 2-6
Figure 3-1. The INCU Front Panel ...................................................................... 3-2
Figure 3-2. INCU Keypad Display.......................................................................3-3
Figure 3-3. INCU Inside a Closed Incubator.......................................................3-5
Figure 3-4. Placement of Temperature Sensors T1-T3 ...................................... 3-6
Figure 3-5. Placement of Air Flow Sensor ..........................................................3-7
Figure 3-6. Placement of Temperature Sensor T4 .............................................3-8
Figure 3-7. Placement of an INCU Inside an Infant Warmer ..............................3-9
Figure 3-8. Placement of a Radiant Baby Adapter on Top of the INCU .............3-9
Figure 3-9. Data Acquisition Mode Screen .......................................................3-11
Figure 4-1. PC Interconnected With the INCU....................................................4-2
Figure 4-2. Using the Config Menu ..................................................................... 4-3
Figure 4-3. INCU Keypad Display.......................................................................4-4
Figure 4-4. INCU File Transfer Menu..................................................................4-5
Figure 4-5. INCU Comments Entry Window ....................................................... 4-6
Figure 4-6. INCU Parameter Options Menu........................................................4-7
Figure 4-7. INCU Parameter Screen...................................................................4-7
Figure 4-8. INCU File Menu Screen....................................................................4-8
Figure 4-9. INCU Window Menu Screen.............................................................4-9
Figure 4-10. INCU Parameters Menu Screen..................................................... 4-9
Figure 4-11. INCU Heading Screen ..................................................................4-10
Figure 4-12. INCU Graph Colors Screen..........................................................4-11
Figure 4-13. INCU Communication Menu Screen ............................................4-12
Figure 4-14. INCU Language Menu Screen .....................................................4-12
Figure 4-15. INCU Zoom Menu Screen ............................................................4-13
Figure 4-16. INCU Zoom on Window Screen ...................................................4-13
Figure B-1. Sample Monitoring Sheet................................................................ B-3
Figure B-2. Sample History Graph Sheet .......................................................... B-4
Figure B-3. Sample Parameter Numerical List .................................................. B-5
View of Left Side of INCU.................................................................1-5
xi
Page 12

INCU
Operators Manual
xii
Page 13

Chapter 1: Overview
1
Inside This Chapter
• Introducing INCUTM
TM
• INCU
• Getting to Know INCU
• AAMI/IEC Standards
• Accessories
Measurements
TM
1-1
Page 14

INCU
Operators Manual
Introducing INCUTM
The INCU Incubator Analyzer is a portable device designed to verify the
proper operation and environment of infant incubators. This unit records
parameters important to the care of infants over time, such as airflow, sound
level, temperature (four individual measurement probes), and relative
humidity. Event markers can be placed on the recording to identify certain
activities or periods. The rechargeable battery allows the unit to be placed
within the incubator chamber for up to 24 hours without compromising the
integrity of the environment.
The INCU can operate stand-alone or with the use of a personal computer.
With a PC, the user selects the desired record time/interval via the software,
and then initiates the start of the test from the INCU. After completion of the
test, the user uploads the data collected by INCU into the PC software for
display and analysis. The user may store the recorded data in a file or print
the data to a report. In stand-alone mode, the unit displays all measured
parameters repeatedly in cycle fashion, and no data is recorded.
Features on the INCU include:
Portability: Sits in place of the infant within the incubator.
•
Multiple Sensors: Measures and documents multiple key infant
•
incubator parameters simultaneously.
Compliance: Measures most parameters according to IEC and
•
AAMI standards.
Efficient: Saves time in testing critical infant incubators.
•
•
Data Collection: Uses Microsoft
®
Windows® software for
evaluation and documentation.
•
Reporting: Allows printing of numerical and graphical reports.
Recording Flexibility: Provides adjustable measurement intervals.
•
Two Modes of Operation: Operates stand-alone or fully
•
functioning, requiring a computer.
1-2
Page 15

Incubator Analyzer
Getting to Know the INCU
INCU is to be used by service personnel or Biomedical institutions to verify
and test infant incubators. INCU is designed to support testing of two types
of incubators, as shown in Figures 1-1 and 1-2.
Figure 1-1. Closed incubator with forced convection
INCU Measurements
INCU is an autonomous acquisition system that is used to measure and save
the operational parameters of an empty incubator, and then transmit data via
a serial communications port. INCU
measurement systems that allow you to record the multiple parameters
simultaneously.
The following measurement parameters are recorded:
Temperature – 4 Sensors T1-T4
Relative Humidity – 1 Sensor
Air Flow – 1 Sensor
Sound – 1 Sensor
See Appendix A, Specifications for performance specifications.
Figure 1-2. Infant warmer
provides, in a single unit, the
1-3
Page 16
INCU
Fig
Operators Manual
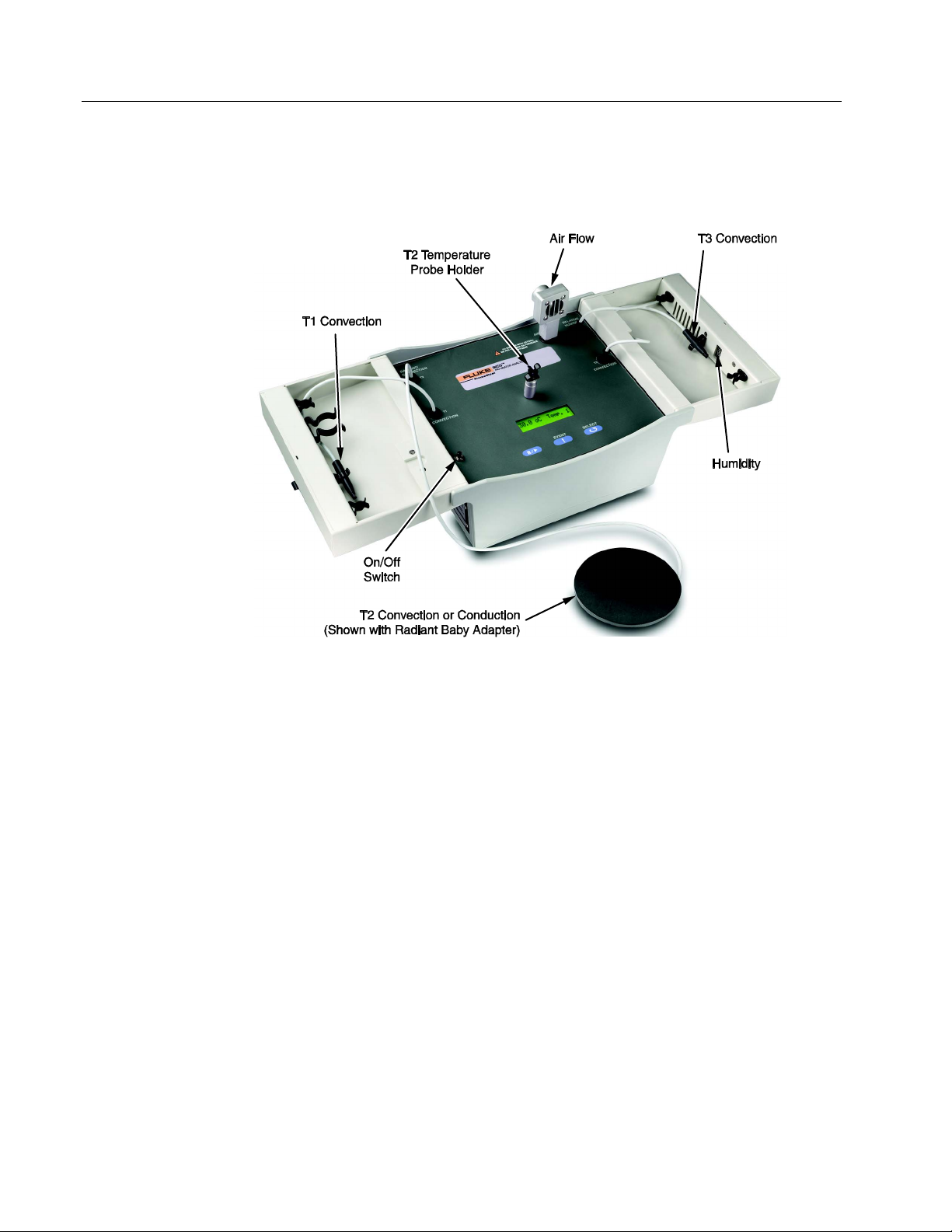
Getting to Know the INCU
Sensors are integrated and stored within INCU. Open INCU by releasing the
latch on the top cover. Fold the covers open to expose the sensors when
taking measurements. Figure 1-3 shows the location of each sensor.
ure 1-3. INCU sensors
• Temperature Sensor T1: Used for Convection measurements.
Temperature Sensor T2: Used for Convection or Radiant
•
measurements; used with radiant baby adapter supplied with INCU.
Temperature Sensor T3: Used for Convection measurements.
•
•
Relative Humidity: Located in right top cover (cover must be open
for proper measurements).
Air Flow: Detachable for storage.
•
ON/OFF Switch: Top cover engages this switch to turn power off to
•
INCU automatically, if the master On/Off switch is left in the ON
position and the top covers are closed (master switch is on the left
side of the exterior).
Temperature Probe Holder: Used to hold temperature probe T2
•
when taking convection measurements. This is a mechanical
connection only; there is no electrical connection.
1-4
Page 17
Incubator Analyzer
Fig
Fig
AAMI/IEC Standards
ure 1-4. View of right side of INCU
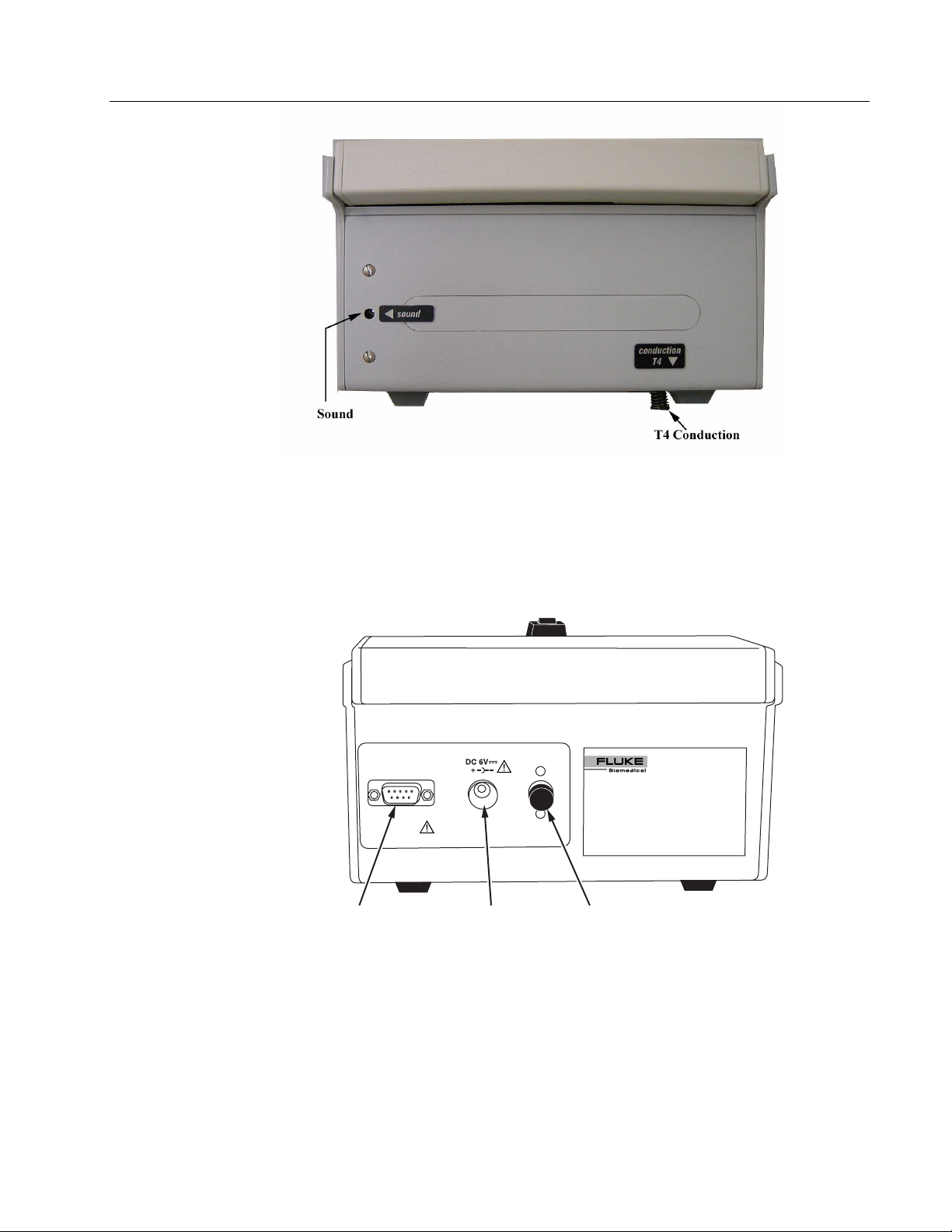
• Sound Sensor: Internal Microphone used for sound measurements.
Temperature Sensor T4: Used for Mattress Temperature
•
Measurements, made by conduction.
INCU
INCUBATOR ANALYZER
RS 232
Port
RS 232
DO NOT USE WITH OXYGEN
NE PAS UTILISER EN PRESENCE
D'OXYGENE
Power
DC in Jack
On/Off Switch
ure 1-5. View of left side of INCU
• RS-232 Port: 9 Pin D Sub type Male Connector.
•
Power DC IN Jack: Use only the specified charger. AC-DC 6V
600 mA.
Master On/Off Switch: Push for OFF or ON.
•
1-5
Page 18
INCU
Operators Manual
AAMI/IEC Standards
The AAMI and IEC standards specify sound levels, CO2 concentration, and
thermal characteristics for incubators. The standards are used by
manufacturers when designing and manufacturing incubators. INCU was
designed with consideration of the standards, and can perform testing to
satisfy many testing requirements.
Incubator Standards
Standard# Description
IEC 601-2-19 Infant Incubator Tester Safety Requirements
IEC 601-2-20 Safety Requirements for Transport Incubators
IEC 601-2-21 Infant Radiant Warmer Standard
ANSI/AAMI 1136-1997 Infant Incubators
Note:
Note:
For up-to-date standards, please visit AAMI.org or IEC.org.
The following are examples of standards for description
proposed. Please reference the current application to determine
your testing protocol.
INCU Applications
The following section provides information describing how INCU
can be
used to perform testing in consideration of the standards.
Temperature – Standard: During steady state condition, the
1.
INCUBATOR TEMPERATURE shall not differ from the AVERAGE
INCUBATOR TEMPERATURE by more than 0.5°C (1°C transportable)
during at least 1 hour at the control temperatures of 32°C and 36°C.
INCU: The user shall check the oscillation from min to max in steady
state.
Temperature - Standard: The AVERAGE TEMPERATURE in each
2.
point A,B,C,D,E, shall not differ from the AVERAGE INCUBATOR
TEMPERATURE (test at a set T of 32°C - 36°C) by more than ±0.8°C
(±1.5°C transportable).
1-6
INCU: In any position of the tilted mattress, it shall not differ by more
than
±
1.0°C (±2.0°C transportable). Calculate manually the difference
between the mean value at the center and the other sensors readings.
3.
Temperature - Standard: The TEMPERATURE as measured by the
skin temperature sensor shall not differ from a reference sensor
temperature by more than 0.3°C in steady temperature condition.
INCU: Check the difference between the value given by the T2 sensor
and the value displayed by the skin sensor when placed in close
proximity to the T2 sensor.
Page 19

Incubator Analyzer
AAMI/IEC Standards
4. Temperature - Standard: The INCUBATOR shall be provided with an
indicator of the internal temperature. The mean value of the reading of
this device shall not differ from the average incubator temperature
measured by a standard thermometer by more than ±0.8°C (±1°C
transportable), less the standard thermometer
error. The standard
thermometer shall be accurate within ±0.05°C.
INCU: Check the difference between the value of the mean value at the
center and the one displayed by the indicator.
Temperature - Standard: Working as an air-controlled INCUBATOR,
5.
the average INCUBATOR TEMPERATURE shall not differ from the
control temperature by more than ±1.5°C (±2°C transportable).
INCU: Check the difference between the value of the mean value at the
center and the set value.
Temperature - Standard: The warm-up time of the equipment shall not
6.
differ by more than 20% from the warm-up time specified in the
instructions for use.
INCU: Check the difference between the time stated by the manufacturer
and the time to raise the temperature by 11 C, starting at the
environmental conditions with a setting temperature 12°C above the
ambient.
Temperature - Standard: After adjusting the temperature from 30°C to
7.
34°C or Transit 32°C to 36°C, the overshoot in the incubator temperature
shall not exceed 2°C.
INCU: Calculate the overshoot manually.
Standard: Any indicated value of relative humidity shall have an
8.
accuracy of ±10% (±15% transportable) of the actual measured value.
INCU: Read the value and compare it with the set value.
Sound Pressure - Standard: In normal use, the sound level within the
9.
baby compartment shall not exceed 60 dB except as specified in 102.2.
INCU: Read the value and compare it with the threshold.
Sound Pressure - Standard: When any incubator alarm is sounding,
10.
the sound level within the baby compartment shall not exceed 80 dB.
INCU: Activate an alarm, read the value, and compare it with the
threshold.
Sound Pressure - Standard: Audible alarms shall have a sound level of
11.
at least 80 dB at a distance of 3 m perpendicular to the front of the
control unit.
INCU: Activate an alarm, move the INCU
outside, read the value, and
compare it with the threshold.
1-7
Page 20
INCU
Operators Manual
Accessories
12. Air Flow – Standard: In normal use, air velocity over the mattress shall
not exceed 0.35 m/s.
INCU: Read the value and compare it with the threshold.
The following accessories are available for your INCU Incubator Analyzer.
Description Part No.
DB9 Serial Cable 2238834
Transport Bag 2248900
Radiant Baby Assembly 2239002
Outside Temperature Probe Holder 2213928
Service Manual 2206983
1-8
Page 21

Chapter 2: Installation
2
Inside This Chapter
• Unpacking and Inspection
TM
• Connecting INCU
• PC Software System Requirements
• Installing the PC Software
2-1
Page 22

INCU
Operators Manual
Unpacking and Inspection
Before unpacking the INCU, visually inspect the shipping box for damage.
If no damage is evident, unpack the INCU and use the checklist below to
ensure that you have received the instrument accessories. Save the foam
inserts and shipping box. You must use the original packing materials when
shipping the INCU for service or re-calibration. If the original shipping
carton and packing materials are not available, call a Fluke service
representative for assistance.
If the shipping box is damaged, unpack the analyzer and inspect it for visible
defects.
If the instrument is damaged, notify the carrier and your local dealer or
service center. Keep the shipping cartons and packing materials for the
carrier's inspection. Call a Fluke service representative to arrange for repair
or replacement of your instrument without waiting for the claim against the
carrier to be settled.
After unpacking the INCU, use the following checklist to ensure that you
have received everything. In addition to the analyzer and this manual
(P/N 3901000), you should have the following:
• A Transport Bag, P/N 2248900
• An Air Flow Sensor, P/N 2239025
• PC Software CD ROM, P/N 2213919
• AC Battery Charger with Country Adapters for the USA, Europe,
Australia, or the UK, P/N 2213937
• 9-Pin to 9-Pin Serial Cable, P/N 2238834
• Temperature Probe Holder, P/N 2213928
• Radiant Baby Assembly, P/N 2239002
• Certificate of Calibration
• Declaration of Conformity
• Fluke Biomedical Warranty Card Information
2-2
Page 23

Incubator Analyzer
Connecting INCU
Connecting INCU
AC-DC Battery Charger Connection
The INCU can operate either on its internal battery or with AC power.
Connect the specified adapter to the INCU
Plug the opposite end into the AC power source. The universal charger can
be plugged into AC power sources ranging from 90 VAC to 240 VAC,
50/60 Hz. Use the charger to recharge the internal batteries when a “low
battery” warning is displayed. Regular charging of the battery will increase
its life.
DC jack on the side of the unit.
Internal Battery
RS-232 Port
Warning:
To reduce the risk of damage to the battery and to the INCU,
only use the specified Fluke Biomedical Charger, P/N 2213937,
AC-DC 8V 800 mA. Failure to do so will void the warranty.
The system is equipped with a lead-acid battery, giving a maximum of 24
hours of operation. Use the AC Charger if recording parameters for longer
than 24-hour periods.
Connect the INCU RS 232 port to an available COM port on the PC using
the supplied 9-pin to 9-pin serial cable.
2-3
Page 24

INCU
Operators Manual
Air Flow Sensor
Plug the Air Flow Sensor into the jack on top of the INCU where it is labeled
"Air Flow." Ensure that the Air Flow Sensor is installed before attempting to
use the INCU. Otherwise, a “Sensor Fault” condition will result, indicating
the absence of this sensor. To bypass the “ Fault sensor : 6” message, press
any key. INCU operation will continue with normal operation with the
exception of recording Air Flow parameters. Results will be displayed as
9.99 m/s.
The Air Flow Sensor is calibrated for each INCU unit. If the Air Flow Sensor
is lost or damaged, the unit must be returned for service. See "Support" in
Chapter 5, Safety, Displayed Messages, Troubleshooting, and Support.
Warning! Do not use in the presence of oxygen!
uses a hot-wire technology that can be a source of ignition if
used in oxygen-enriched environments. INCU should not be
The Air Flow Sensor
used in conditions where oxygen levels are greater than
ambient.
Radiant Baby Assembly
Figure 2-1 shows the radiant baby assembly.
2-4
Figure 2-1. Radiant baby assembly
Page 25

Incubator Analyzer
Installing the PC Software
PC Software System Requirements
To utilize the full capabilities of your INCU program, the following
minimum environment is required:
A PC or portable computer equipped with:
• Pentium 133 MHz microprocessor
• 16 MB free hard disk space
• SVGA color monitor
• Serial port COM 1-4 (one com port available)
• Mouse compatible with Microsoft® operating system
• 1 CD ROM disk drive
• Microsoft® Windows® 95B, Windows® 98, Windows NT® 4.0, or
Windows® 2000 operating system
• A laser or inkjet printer
Note: Microsoft® Windows® 3.0 and Windows® 3.1 for Workgroup
3.11 are not supported by this application.
Note: Certain portable computers are equipped with an infrared serial
communications port. The presence of such a port, typically
COM 2, may create operating difficulties on the COM 1 serial
port. In this case, delete the infrared COM 2 port from the
peripheral driver.
2-5
Page 26

INCU
Operators Manual
Installing the PC Software
Installing the Software
The INCU PC Software is provided with both French and English
installation instructions. If desired, the language for the PC Software may be
selected after installation.
1. Insert the INCU PC Software for Windows CD ROM into the CD
ROM drive on your PC. Under Microsoft Windows 95/98/NT, select
Run from the Start menu.
For English, when the
d:\incugb\setup.exe. If your disk drive is not "D", then substitute the
correct drive letter. Click
For French, when the
d:\incufr\setup.exe. If your disk drive is not “D”, then substitute the
correct drive letter. Click
Run dialog box is displayed, type in
OK.
Run dialog box is displayed, type in
OK.
2-6
Figure 2-2. INCU setup screen
2. To exit the installation program, click on
3. As shown in Figure 2-2, click on
Change Directory to select a location
Exit Setup.
on your computer in which the program will be installed.
4. Simply follow the instructions given by the installation program. If no
changes are made to the default installation options, INCU PC Software
will be installed to
C:\Program Files\INCU, and the shortcuts to the
program will be in the INCU group.
5. To start the program, double-click on the INCU icon.
Page 27

Chapter 3: General Operation
3
Inside This Chapter
• INCU Keypad Functions
• INCU Setup
• INCU Operation
3-1
Page 28

INCU
Operators Manual
INCU Keypad Functions
Figure 3-1 illustrates the INCU keypad. The keys and their functions are
described on the following pages.
RADIANT/
CONVECTION
T2
T1
CONVECTION
DO NOT USE WITH OXYGEN
NE PAS UTILISER EN PRESENCE
D'OXYGENE
INCU
INCUBATOR ANALYZER
EVENT SELECT
AIR FLOW
RELATIVE
HUMIDITY
T3
CONVECTION
!
Figure 3-1. The INCU front panel
3-2
Page 29

Incubator Analyzer
INCU Keypad Functions
Key Details
Start/Pause Key
1. Press this key for approximately 1 second to
begin recording. This function is only active
once the INCU has been connected to a PC and
a measurement cycle has been initialized.
2. A second display line appears (see Figure 3-2)
with the following information:
• Measurements that have already elapsed
• Total measurements
• The number of events recorded
(maximum 5).
20.4 C Temp. 1
º
1:210 Evt. 0
event select
Figure 3-2. INCU keypad display
3. The sensor number that is displayed flashes
to indicate that the measured values have
been recorded.
4. Press this key a second time to pause (to
allow time for adjustment, for example).
During this pause phase, the channel
number stops flashing.
5. Press this key a third time to resume
recording. There is no limit to the number
of interruptions possible.
3-3
Page 30

INCU
Operators Manual
event
!
Event Key
Press this key for approximately 1 second to mark a
particular value or a pause in the recording sequence.
As a result, the action is recorded and then displayed
on the History Graph Sheet and Parameter Numerical
List (see Appendix B, Report Examples).
For an explanation of the significance of each marker,
see "Transfer Data to the PC Software" in Chapter 4,
INCU PC Software Operation.
Note: It is possible to store 5 events. The stored
events cannot be deleted.
select
Sensor Key
1. First mode:
• Each time this key is pressed for
approximately 1 second, the next
sensor is displayed (one of the 7
currently active sensors, in order).
2. Second mode:
• Pressing this key two times quickly
allows you to scroll through the
channels. Press the key a third time to
freeze the display.
3-4
Page 31

Incubator Analyzer
INCU Setup
INCU Setup
Placement of the INCU in the Incubator
With both covers open and fully extended, the INCU has dimensions (length
and width) similar to those of a typical infant (non-preemie). The INCU
should therefore be placed in an isolette (incubator), or infant warmer in the
same manner as an infant would be positioned for normal operation; i.e.,
centered on the mattress ensuring that air circulation vents, temperature
probes, etc. are not blocked or impinged. Figure 3-3 shows the INCU inside
a closed incubator.
Closed Incubators
Figure 3-3. INCU inside a closed incubator
3-5
Page 32

INCU
Operators Manual
T1, T2, and T3 Convection Sensors: Figure 3-4 shows the recommended
placement for temperature sensors T1-T3 for closed incubators utilizing
forced convection. With the sensors mounted to the provided clips,
temperature accuracy, response, and uniformity can be monitored throughout
the area occupied by the infant at the height of approximately 10 cm above
the mattress as specified by standards. In this case, T2 is being utilized as a
convection temperature sensor.
Humidity Sensor (Fixed): The humidity sensor is fixed and is located on the
inside of the right-hand cover as shown in Figure 3-4.
T1 Convection
CONVECTION
T2 Convection
or Conduction
R
A
D
IA
N
T
/
C
O
N
V
E
C
T
IO
N
T
2
T1
EVENT
Air Flow
D
O
N
O
T
U
N
S
E
E
P
W
A
S
I
T
U
A
H
T
O
IR
IL
X
I
F
Y
S
G
E
L
R
E
O
E
N
W
N
P
D
R
'O
E
X
S
Y
INCU
IN
CU
B
AT
O
R
!
SELECT
E
G
N
E
C
N
E
E
A
N
A
LYZ
ER
R
E
L
A
T
IV
E
H
U
M
ID
IT
Y
T3
CO
NVECTIO
N
On/Off Switch
Temperature
Probe Holder
T3 Convection
Humidity
Figure 3-4. Placement of temperature sensors T1-T3
Note: To achieve a better temperature slew rate, for T1, T2, it
is preferable to clip on the sensor cable instead of the
body sensor.
3-6
Page 33

Incubator Analyzer
INCU Setup
Air Flow Sensor: The Air Flow Sensor is detachable; however, it must be
inserted to perform measurements. An error "fault sensor" will be displaced
if not inserted or if the Hot Wire is broken in the sensor. The INCU will not
perform airflow measurements in this condition.
The Air Flow Sensor (Figure 3-5) rotates 360 degrees. For best results,
position the Air Flow Sensor perpendicular to the airflow within the
incubator. Incubators utilize different airflow patterns; thus, the user needs to
be familiar with the particular airflow pattern utilized by the incubator under
test to properly position the Air Flow Sensor.
Figure 3-5. Placement of Air Flow Sensor
Warning! Do not use in the presence of oxygen! The Air
Flow Sensor should
environment. The Air Flow Sensor utilizes a hot-wire technique,
which can be a source of ignition.
not be used in an oxygen-enriched
3-7
Page 34

INCU
Operators Manual
T4 Conduction Sensor: As shown in Figure 3-6, the T4 conduction sensor
is fixed and is located on the bottom of the INCU. T4 provides the
temperature of the mattress.
Sound Sensor (Fixed): The sound sensor is fixed and is located on the right
side of the INCU.
Figure 3-6. Placement of temperature sensor T4
3-8
Page 35

Incubator Analyzer
INCU Operation
Infant Radiant Warmers
INCU sensor placement for testing infant radiant warmers is treated in the
same manner as closed incubators utilizing forced convection, with the
exception of T2. As discussed above, T2 is used as a third convection
temperature sensor for testing closed incubators with forced convection. In
contrast, when testing infant radiant warmers, T2 is utilized as a radiant
temperature sensor.
For testing infant radiant warmers, T2 is clipped to the underside of the
radiant baby adapter that is provided. The radiant baby adapter is a stand-
alone circular-shaped piece that simulates the absorption properties of an
infant. This adapter must be placed on top of the INCU when testing. Align
the adapter with the heater in the warmer.
Other temperature sensors (T1, T3) may be used to monitor ambient
temperature conditions.
Figure 3-7 shows the placement of the INCU in an infant warmer.
Figure 3-8 shows the placement of a radiant baby adapter on top of the
INCU.
Figure 3-7. Placement of an INCU inside
an infant warmer
3-9
Figure 3-8. Placement of a radiant baby adapter
on top of the INCU
Page 36

INCU
Operators Manual
INCU Operation
1. Place the INCU into position within the incubator or infant warmer.
Refer to Figures 3-3 and 3-7 for placement of the INCU.
2. Place the sensors into position.
Place temperature sensors T1, T2, and T3 into position, and connect the
Air Flow Sensor.
To conduct a test on an infant warmer, clip the end of probe T2 under the
radiant baby adapter as shown in Figure 2-1.
3. Turn on the power.
If acquiring data for periods longer than 24 hours, use the AC Charger.
Open both covers to expose the INCU panel and display. Push the
On/Off switch located on the left-hand-side panel of INCU to turn on the
INCU.
Note: The INCU is equipped with a secondary On/Off switch on the
top panel. This is a safety switch used to prevent INCU power
from remaining on when the covers are closed.
Caution: Pressing the On/Off switch located on the top panel during
data acquisition will initialize the INCU and stop the data
acquisition session.
The unit will display the following:
Line 1: INCU. ANALYZER
Line 2:
Ver : 3.00
followed by:
Line 1:
Self Testing
If error conditions are present, the unit will display an error.
For detailed error condition information, refer to Chapter 5, Safety,
Displayed Messages, Troubleshooting, and Support.
3-10
Page 37

Incubator Analyzer
INCU Operation
4. Obtain Readings
The unit is now ready either to be used as a measuring device in manual
mode, or in an automated data acquisition mode as configured by the PC
software. For further information on configuring INCU for data
acquisition, refer to Chapter 4, INCU PC Software Operation.
5. Observe Readings – Manual Mode
Sensor data is displayed on the first line. To manually cycle through the
available sensors, press the
quickly and then released, the INCU will cycle through and display each
of the available sensor channels automatically. To revert to manual
mode, press the
Select key again. No data is stored within the INCU in
this mode. The INCU must be configured for using the PC software prior
to starting any automated data acquisition.
6. Data Acquisition Mode Using the PC Software
After being configured for data acquisition, the INCU will display
Ready. Press the Pause/Play key to begin data acquisition.
The second line of the display shows the following (Figure 3-9):
Select key. If the Select key is pressed too
• Measurement samples already recorded
• Total samples
• The number of events memorized (maximum 5)
20.4 C Temp. 1
º
1:210 Evt. 0
event select
Figure 3-9. Data acquisition mode screen
The sensor channel number on the display flashes, indicating that the
measured values have been stored.
Press the
Pause/Play key a second time to pause data acquisition (to allow
time for adjustment, for example). During this pause phase, the channel
number stops flashing.
To resume data acquisition, press the
Pause/Play key again. There is no
limit to the number of interruptions possible.
3-11
Page 38

INCU
Operators Manual
3-12
Page 39

Chapter 4: INCU PC Software Operation
4
Inside This Chapter
• Introduction
• Configuring the INCU for Data Acquisition
• INCU Data Acquisition
• Transferring Data to the PC Software
• Graphs
• Software Options
4-1
Page 40

INCU
Operators Manual
Introduction
The full capability of the INCU is realized when used in conjunction with the
provided PC application.
The provided PC software for INCU is used to:
• Select the measurement interval (1-10) minutes
• Select the total recording period (35 hours max)
• Display recorded data in graphical form
• Print a provisional monitoring sheet
• Create backup files that can be used by a spreadsheet program such
as Microsoft® Excel.
Configuring the INCU for Data Acquisition
1. Connect all sensors
2. Press the On/Off button for approximately one second.
3. Wait until the INCU has completed the initialization sequence.
4. Using the supplied RS-232 cable, connect the INCU to a PC that is
loaded with the INCU PC software as shown below.
5. Double-click on the installed icon to start the INCU PC software.
Figure 4-1 shows an example of a PC interconnected with an INCU.
COM PORT
4-2
Figure 4-1. PC interconnected with the INCU
Page 41

Incubator Analyzer
Data Acquisition
6. Once the PC program is up and running, select the
command and then
Config.
The
Config command is used to select the measurement interval and
total recording period, and to document the serial number of the
incubator under test as shown in Figure 4-2.
Figure 4-2. Using the Config menu
Connect
7. To select the measurement interval, click on the scrolling arrows
located next to
Sampling interval. You can choose from 1 minute
up to 10 minutes in one-minute intervals.
The measurement interval specifies how often the sensors are
sampled for data. That is, if the measurement interval is one minute,
all seven INCU sensors are then polled every minute and the results
are recorded.
8. To select the recording period, click on the scrolling arrows located
next to
Total measurement time. You can choose up to 35 hours for
the recording period (with a 10-minute sampling period).
The recording period specifies the duration of the test. That is, if the
measurement interval is one minute and the recording period is set
for one hour, the test will last for one hour, and during that time, 60
measurements for each sensor will be recorded. The maximum
measurement capacity of the INCU is 1470 (210 records x 7
parameters).
9. Enter the serial number for the incubator under test in the field next
to
Incubator serial no.
Note: You must enter a serial number in this field to proceed.
10. From the
File menu, choose Print to print out a monitoring sheet
(see Figure B-1 in Appendix B, Report Examples). This sheet
contains the INCU configuration information along with fields for
manufacturer, type, department, etc. Use the
monitoring sheet to
record events that occur during the incubator-testing phase.
4-3
Page 42

INCU
Operators Manual
Data Acquisition
After the INCU is configured for data acquisition, Ready will be displayed.
Disconnect the INCU from the PC and place it into an incubator or infant
radiant warmer under test as described in Chapter 3. When properly
configured and positioned, the INCU is ready for data acquisition.
Start/Pause Key
1. Press this key for approximately 1 second to begin
recording. This function is only active once the
INCU has been connected to a PC and a
measurement cycle has been initialized.
2. A second display line appears (see Figure 4-3)
with the following information:
• Measurements that have already elapsed
• Total measurements
• The number of events recorded
(maximum 5).
20.4 C Temp. 1
º
1:210 Evt. 0
event select
Figure 4-3. INCU keypad display
3. The sensor number that is displayed flashes to
indicate that the measured values have been
recorded.
4. Press this key a second time to pause (to allow
time for adjustment, for example). During this
pause phase, the channel number stops
flashing.
5. Press this key a third time to resume
recording. There is no limit to the number of
interruptions possible.
4-4
Page 43

Incubator Analyzer
Transferring Data to the PC Software
Transferring Data to the PC Software
After data acquisition is complete, data transfer can be executed. Data
transfer is the act of transferring the data in the INCU from the previous
acquisition to a PC running INCU PC software. When data is transferred to a
PC, it can be displayed in graphical or numerical form, and/or saved as a file
for future reference (see Appendix B for examples of graphs and numerical
data). Numerical data can be entered into Microsoft® Excel or some other
spreadsheet program to customize your own graphs.
6. Press the
1 second to mark a particular value or a pause
in the recording sequence. As a result, the
action is recorded and then displayed on the
History Graph Sheet and Parameter Numerical
List (see Figures B-2 and B-3 in Appendix B,
Report Examples).
7. Describe these events on the
sheet
acquisition. It is possible to record up to five
events.
When the acquisition phase is complete,
measurements
Events key for approximately
monitoring
if one was printed before data
will be displayed.
end of
To initiate a data transfer from the INCU, connect the INCU to a PC running
the INCU PC software via the RS-232 port utilizing the supplied RS-232
cable. Click on connect and transfer as shown in Figure 4-4 to initiate data
transfer. During the transfer phase, the mouse pointer indicates the progress
of data transfer.
Figure 4-4. INCU file transfer menu
4-5
Page 44

INCU
Operators Manual
At the end of transmission, the comments entry window appears as shown
below. This window allows you to enter comments for or update
monitoring sheet.
the
Figure 4-5. INCU comments entry window
At download the user can enter either the start or end of time of the
acquisition by clicking on the checkbox. Use the two scrollbars to specify the
hour and the minutes. If
PC software defaults to
The PC software will calculate the
selected and the
end of acquisition time entry.
Start time of the acquisition: is not checked, the
end of acquisition time.
start time based on the sample interval
4-6
Page 45

Incubator Analyzer
Graphs
Graphs
This menu is accessible after the recorded data has been loaded. When the
graph menu option is selected, four parameters are displayed.
Figure 4-6. INCU parameter options menu
When a parameter is selected, a window is displayed presenting data in
graphical form (see Appendix B for examples of graphs). The upper section
of the window is shown in Figure 4-7.
Figure 4-7. INCU parameter screen
First Scrolling Bar: Selects the specific time during the acquisition period in
1
hours and minutes. The associated dialogue box shows the value of the data
item for the time selected.
Second Scrolling Bar: Selects the time interval for calculating an average
2
for the data item. The average is based on the time specified by the first
scrolling bar and is updated when the first scroll bar is adjusted (rolling
average).
Print Key: Prints the graph displayed on a single page.
3
INFO/Marker Key: Loads the Comments Window associated with an event
when an event marker is encountered by moving the
key is only active when an event is encountered.
first scrolling bar. The
4-7
This box shows the value of the data item selected by the mouse pointer.
5
Page 46

INCU
Operators Manual
Software Options
Open Files
INCU PC File menu features shown in Figure 4-8 are described below.
Figure 4-8. INCU File menu screen
To open a document:
Save Files
Printer Configuration
1. In the
2. In the area listing the folders and files, double-click on the name of
3. Double-click on each sub-folder in turn until you reach the sub-
To save a file:
1. In the
2. If you have not yet saved your file, type the name you wish to give it
This window enables you to access the different parameters of your printer.
These functions are variable and depend on the type of printer used.
File name field, click on the drive containing the document.
the folder in which the document is stored
folder containing the document.
File menu of the program you are working in, click on Save.
File name field.
in the
4-8
Print Folder
Click on
sheet
Appendix B.
Print Folder to print the recorded data including the monitoring
, the history graph sheet, and the parameter numerical list shown in
Page 47

Incubator Analyzer
Software Options
Cascade
INCU PC
Vertical tile, are shown in Figure 4-9 and are described below.
Window menu features, including Cascade, Horizontal tile, and
Figure 4-9. INCU Window menu screen
To organize windows so that they overlap each other, click on the menu
Window, then on Cascade. To position windows side by side without any
overlap, click on the
position windows from top to bottom without any overlap, click on the
Window menu, and then click on Horizontal Tile.
Window menu, and then click on Vertical tile. To
Figures 4-10 through 4-14 show the INCU PC Parameters menu features,
described below.
Figure 4-10. INCU Parameters menu screen
4-9
Page 48

INCU
Operators Manual
Heading
Figure 4-11. INCU Heading screen
This menu is used to customize the monitoring sheet. All fields shown in
red must be completed when you enter this menu.
• Site name
• Address
• Department name
• Operator name
The only exception is the
the
Comments heading in the Comments entry window.
Information field, intended for customization of
4-10
Page 49

Incubator Analyzer
Software Options
Graph Colors
Figure 4-12. INCU Graph colors screen
Use this menu to apply the color of each graph from 15 available colors. The
Desk key allows you to set the background color of the INCU window.
When you exit the INCU program, your choices are automatically saved in
the file
setup.cfg.
4-11
Page 50

INCU
Operators Manual
Com Port
Figure 4-13. INCU Communication menu screen
This menu allows you to select the serial communications port
(Com). Com2 port is the default. Select the Com1, 3,or 4 port as
shown in Figure 4-13. When you exit the INCU program, your
choices are saved in the file
setup.cfg.
Language
Figure 4-14. INCU Language menu screen
This menu allows you to select the language by clicking on French or
English text.
4-12
Page 51

Incubator Analyzer
Software Options
Zoom
The
Zoom menu screen is shown in Figures 4-15 and 4-16. The Zoom
command is only applicable when a graph is displayed.
Figure 4-15. INCU Zoom menu screen
The Zoom menu applies only to the last graph activated. It consists of two
elements:
1. Zoom X for the horizontal axis (x-coordinate: real-time scale on
graphs)
2. Zoom Y for the vertical axis (y-coordinate).
Zoom X
Figure 4-16. INCU Zoom on window screen
This command is active when a graph is displayed. It allows for inspection of
a small interval of the total acquisition time. Maximum zoom is equal to 30
times the sample interval. When you close a graph, the zoom x menu
becomes inactive.
Zoom Y
This is an optional command. It is used to define the Y-axis of the graph
between the minimum value and the maximum value of the parameter at the
time under observation.
If you wish to use the
again through the Graph menu.
4-13
Zoom feature on an inactive graph, open the graph
Page 52

INCU
Operators Manual
4-14
Page 53

Chapter 5: Safety, Displayed Messages,
Troubleshooting, and Support
5
Inside This Chapter
• Electrical Safety and Maintenance
• Displayed Messages
• Troubleshooting
• Support
5-1
Page 54

INCU
Operators Manual
Electrical Safety
Cleaning
Air Flow Sensor
Warning:
For safe operation, use only the specified AC Charger with the
INCU. Use of any charger other than that specified may result
in a hazardous condition.
The system is to be cleaned with a damp cloth on the exterior only. A
solution of 70% isopropyl alcohol may be used to remove stains and clean
the system. No other solvents are recommended.
The Air Flow Sensor has been calibrated for use with each INCU. If an Air
Flow Sensor is lost or misplaced, the unit must be returned to the factory or
to a certified service center for recalibration. Failure to do so will result in
invalid Air Flow readings.
Preventive Maintenance
Only authorized, qualified technicians should perform annual verification
and calibration of the INCU unit according to the manufacturer's
recommended procedures.
5-2
Page 55

Incubator Analyzer
Troubleshooting
Displayed Messages
Possible INCU displayed messages are described below.
Displayed Message Description
Self Testing
Low Battery
INCU is performing internal self-checks to
determine proper operating condition before taking
measurements. Any errors encountered during this
test will be displayed on Line 1. Refer to Chapter 5
for an explanation of possible error conditions from
the INCU and possible cause and resolution.
The battery should be charged without delay.
When the battery voltage is less than 5.35 volts
DC, the message will be displayed. The battery
voltage is displayed on power-up during the selftest routine.
When the message appears, use the AC Charger
for normal operation and/or to recharge the internal
battery. When the internal battery voltage is greater
than 5.85 volts DC, the message will not be
present.
Note: Regular charging of the battery increases its
life considerably. The battery must never be
completely discharged.
Note: When this message appears, no data
transfer between the INCU and the PC is possible.
Use the AC charger. When the message
disappears, data transfer is possible.
End of Measurements
Data in Memory
Ready
Fault Sensor
Indicates that the data acquisition is complete.
Connect the INCU to the PC to transfer data
between the INCU and the PC.
Note: The recorded measurements can be
retrieved using the PC even if the cycle has been
interrupted before this message is displayed.
The data transfer has not been completed. Using
the PC Software, transfer the data to the PC. Data
must be transferred to the PC in order to take
further readings in memory.
Displayed after a new configuration has been sent
from the PC software and stored in the INCU unit.
There is an error with the subject sensor. Refer to
Chapter 5 to determine corrective action for each
respective sensor error.
5-3
Page 56

INCU
Operators Manual
Troubleshooting
Problem Possible Cause Corrective Action
INCU does not initialize
when the top covers are
opened.
INCU does not
communicate with the
computer
Incorrect cable Verify the cable used to
Correct COM port not
Operating system not
Internal Battery Low Recharge Battery or
Connect AC Charger for
operation.
Master ON/OFF switch
on side panel not turned
on.
RS-232 cable not
plugged in.
selected
supported.
Push switch to turn the
INCU on.
Verify that the INCU is
connected properly.
connect the RS-232 is a
straight-through cable. Null
modem cables will not
work properly.
Select the COM port used
via the PC Software –
Tools menu.
The INCU PC Software
requires 16550 UART.
This is not present in
earlier windows operating
systems Windows 3.0, 3.1,
and Workgroup for
Windows 3.11. These
operating systems are not
supported. More
information is available on
this issue from the
Microsoft website.
INCU not initialized Turn the analyzer off then
on to reinitialize the unit.
Fault sensor: 1 to 4
during starting sequence
The sensor temperature
Fault sensor: 2 Sensor is connected to
5-4
Problem with
Temperature sensor
is out of the range
(4°C to 70°C).
the radiant baby
assembly prior to
conducting the self-test.
Return the INCU for
service.
Allow the sensor to reach
ambient room
temperature, then
reinitialize the unit by
turning the power off then
back on.
Disconnect the T2 sensor
from the radiant baby
assembly. Allow the
sensor to reach ambient
room temperature, then,
reinitialize the unit by
turning the power off then
back on.
Page 57

Incubator Analyzer
Support
Troubleshooting (Cont.)
Problem Possible Cause Corrective Action
Fault sensor: 6 Air Flow Sensor not
plugged in.
Hot Wire broken in Air Flow
Sensor
INCU did not record all
the measurements that it
was configured for.
The stop key was
If the low battery
message appeared while
in the middle of data
collection, then the INCU
will stop the recording at
that time.
pressed prior to
completion of the desired
recording cycle.
Plug in Air Flow Sensor, or
press any key to bypass
the error condition. The
INCU will perform all other
measurements with the
exception of Air Flow. All
Air Flow readings will be
displayed and stored as
9.99 m/s.
Replace Air Flow Sensor; unit
must be sent back to Factory
for recalibration.
Recharge the battery, or
use the AC Charger. All
data is stored in the INCU
up to the point of the low
battery message. Transfer
the results to the PC using
the PC Software program.
All data is stored in the
INCU up to the point of the
low battery message.
Transfer the results to the
PC using the PC Software
program.
5-5
Page 58

INCU
Operators Manual
Support
Troubleshooting
If your new INCU
Department for assistance.
Service
Before returning the INCU
Department for further information.
Phone: 888.993. 5853
Fax: 425.446.5560
Internet:
Shipping Requirements
1. Pack the unit carefully. Enclose a completed Service Return Form.
2. Insure the unit for full retail value and ship to:
Fluke Biomedical
1420 – 75
Everett, WA 98203
USA
fails to operate successfully, call or e-mail Fluke's Service
for factory service, contact Fluke's Service
www.flukebiomedical.com
th
Sreet SW
5-6
Page 59

Appendix A: Performance Specifications
A
Inside This Appendix
• Performance Specifications
A-1
Page 60

INCU
Operators Manual
Performance Specifications
This appendix provides the device specifications for the INCU Incubator
Analyzer. For more information, please contact your Fluke Biomedical
Service representative.
Temperature Measurements
Conduction: 1 sensor in contact with mattress
Convection
(Inside the incubator): 3 sensors (T1, T3, T4)
Radiation: 1 sensor (T2) connected to the so-called “radiant
Convection
(Inside or outside the
incubator): 1 sensor T2
Measuring Range: 5°C to 70°C
(bottom of unit)
baby”
Resolution: 0.1°C
Accuracy: Reading Value ±0.5°C ± 1 LSB for the range
from 25°C to 40°C
Storage Temperature -20°C to 50°C
Operating Temperature: 10°C to 40°C
Dimensions
Relative Humidity
: 27 x 14 x 20 cm
Measuring Range: 0% RH to100% RH
Resolution: 0.1% RH
Accuracy: ±5% RH (for range 0 to 90% RH
@ 25°C to 40°C) or,
± 5.3 % RH (for range 0 to 100% RH
@ 25°C to 40°C)
Humidity must be without condensation.
Air Flow
Measuring Range: 0.1-0.7 m/s
Resolution: 0.01 m/s
Accuracy: From 0.1 m/s to 0.5 m/s reading
±0.1 m/s @ temperature 25°C to 40°C
and humidity 50% RH ± 15% RH
A-2
Page 61

Incubator Analyzer
Performance Specifications
Noise
Measuring Range: 30- 80 dbA
Resolution: 0.1 dbA
Accuracy: ±5 dbA @ 30-80 dbA
Display
2 x 16 Super Twist LCD
Measuring Interval
Via PC: Adjustable from 1 to 10 minutes
Internal Memory
Capacity: 1470 measurements
(210 records of 7 parameters)
Supply
Power Requirements: Maximum over-voltage: 264 VAC
Input voltage range: 100 to 240 VAC
Input frequency range: 47 to 63 Hz
Power consumption: < 60 Volt Amperes
Fuse rating: 2A Slow Blow
Battery: Rechargeable sealed lead-acid type
NP7-6 YUASA, 6V, 7 Ah
dimensions 151 x 34.x 101.0 mm
Operates for 24 hours continuously without the
low-battery warning
Charger 600 mA minimum, 8 VAC-DC
(Charger must be agency-approved, depending
on the country)
A-3
Page 62

INCU
Operators Manual
Protection Factor: IP 30
The first index digit -3 means that the unit is
protected from object access of solid foreign
matter larger than 2.5 mm in diameter.
The second index digit - 0 means no protection.
Weight: 3 kg
A-4
Page 63

Appendix B: Report Examples
B
Inside This Chapter
• Monitoring Sheet
• History Graph Sheet
• Parameter Numerical List
B-1
Page 64

INCU
Operators Manual
Report Examples
This appendix provides examples of reports (Figures B-1 through B-3) that
can be generated and/or printed from the INCU Incubator Analyzer. Refer to
Chapter 4, INCU PC Software Operation for details on how to print these
reports.
B-2
Page 65

Incubator Analyzer
Report Examples
Fluke Biomedical
Incubator Analyzer
Ver 2.05
Manufacturer :
Type :
Incubator serial no. : 1234
Marker 1: M1
Marker 2:
Marker 3:
Marker 4:
Marker 5:
Temperature order :
Sampling interval : 08 min
12/10/2001
Monitoring sheet
Hygrometry order :
Number of samples : 145
Start time of the acquisition : 08:24:00
Department name :
Procedure no. :
Information :
Check :
Operator name : Operator name
Signature :
Site name
Address
Ver 2.05
C:\Program Files\INCU\DEMO205.DAT
Successful [ ] Failed [ }
Page 1/9
Figure B-1. Sample monitoring sheet
B-3
Page 66

INCU
Operators Manual
Figure B-2. Sample history graph sheet
B-4
Page 67

Incubator Analyzer
Report Examples
Figure B-3. Sample parameter numerical list
B-5
Page 68

INCU
Operators Manual
B-6
Page 69

Appendix C: DAT File Format
C
Inside This Chapter
• DAT File Format
C-1
Page 70

INCU
Operators Manual
The DAT File is a text file that may be imported into popular spreadsheet programs for further processing
and/or analysis. The following is the format of the DAT file stored by the INCU PC Software.
File *.DAT
ASCII Hex Hex
Key word for INCU file identification LFCL 0D 0A
Incubator serial number 0123 0D 0A
Total measurement time 0145 0D 0A
Sampling interval 0010 0D 0A
Name of channel 1 Convection 1 (T1) 0D 0A
0187 0D 0A
Data sent by the sensor. Written in tenths. 0222 0D 0A
0258 0D 0A
Event markers indicated by an M preceding the value M295 0D 0A
Name of channel 2 Radiation/Ext. (T2) 0D 0A
0197 0D 0A
Data sent by the sensor, in tenths 0205 0D 0A
0224 0D 0A
0247 0D 0A
Name of channel 3 Convection 2 (T3) 0D 0A
0186 0D 0A
Data sent by the sensor, in tenths 0232 0D 0A
0271 0D 0A
0302 0D 0A
Name of channel 4 Conduction (T4) 0D 0A
0162 0D 0A
Data sent by the sensor, in tenths 0175 0D 0A
0190 0D 0A
0209 0D 0A
Name of channel 5 Relative humidity 0D 0A
0291 0D 0A
Data sent by the sensor, in tenths 0288 0D 0A
0280 0D 0A
0275 0D 0A
Name of channel 6 Air flow 0D 0A
0040 0D 0A
Data sent by the sensor, in hundredths 0030 0D 0A
0030 0D 0A
0030 0D 0A
Name of channel 7 Noise 0D 0A
0200 0D 0A
Data sent by the sensor, in tenths 0498 0D 0A
0498 0D 0A
0485 0D 0A
Name of channel 8 **** 0D 0A
Future Option 0000 0D 0A
0000 0D 0A
0000 0D 0A
0000 0D 0A
C-2
Page 71

Incubator Analyzer
DAT File Format
File *.DAT
ASCII Hex 0A
Incubator serial number fields (14 characters maximum) 1234 0D 0A
Marker 1 comment field (28 characters maximum) M1 0D 0A
Marker 2 comment field (28 characters maximum) 0D 0A
Marker 3 comment field (28 characters maximum) 0D 0A
Marker 4 comment field (28 characters maximum) 0D 0A
Marker 5 comment field (28 characters maximum) 0D 0A
Marker 5 comment field (28 characters maximum) 0D 0A
Incubator Type fields (8 characters maximum) 0D 0A
Procedure n fields (28 characters maximum) 0D 0A
Temperature Settings fields (28 characters maximum) 0D 0A
Humidity Settings fields (28 characters maximum) 0D 0A
Start time of the acquisition 08:00:00 0D 0A
End Of File (7E hex) ~ 0D 0A
C-3
Page 72

INCU
Operators Manual
C-4
 Loading...
Loading...