Page 1
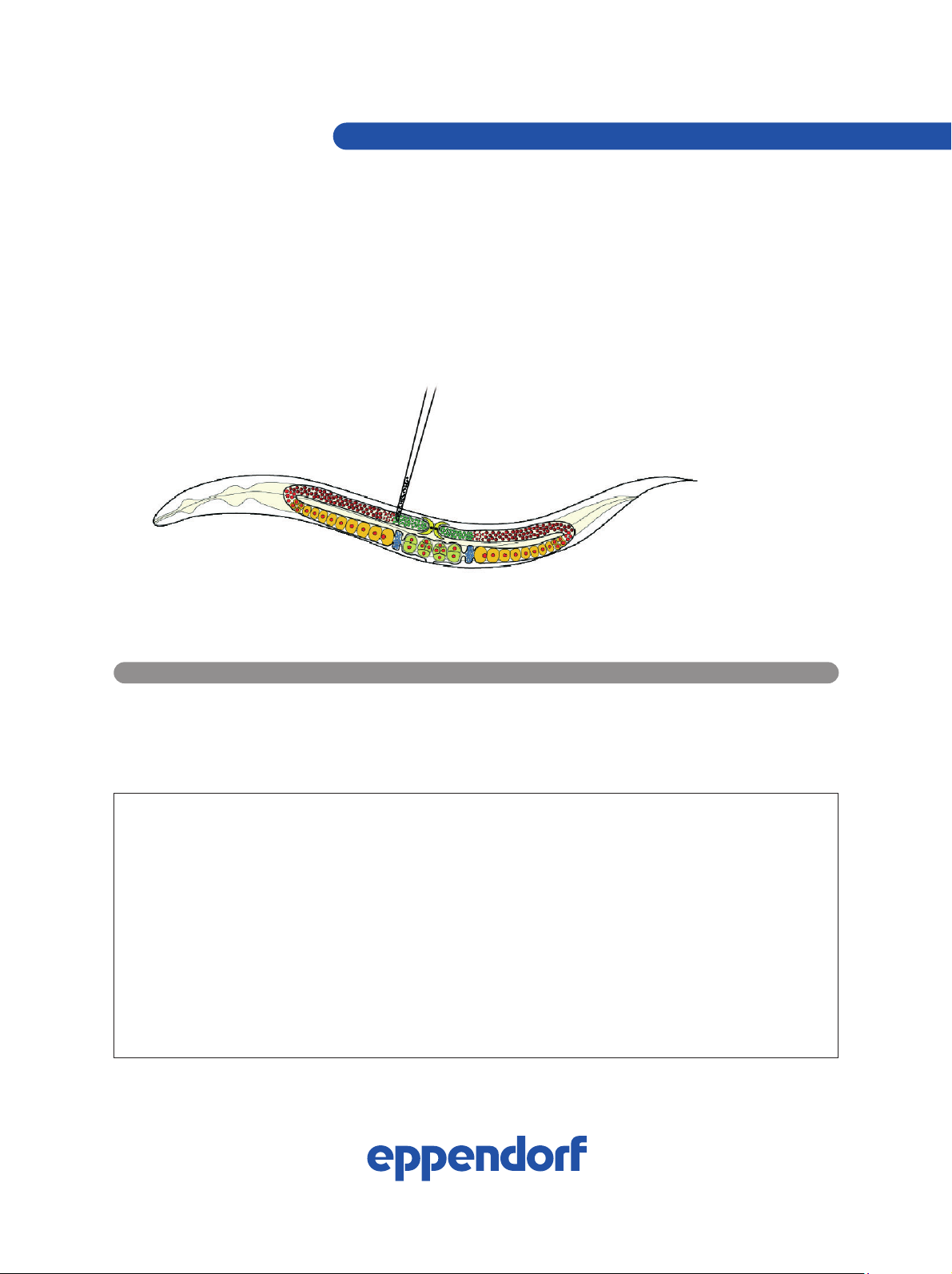
Microinjection of plasmid DNA or double
stranded RNA into the gonads of
C. elegans
FemtoJet express | No 012/06
Userguide
Author: Ryuji Minasaki, Max Planck Institute for Developmental Biology,
Abteilung IV (Evolutionsbiologie), Tübingen, Germany
FIG. 1: Intragonadal injection in C. elegans
Introduction
C. elegans was established as a model organism three decades ago by Sydney Brenner (Brenner 1974).
Two major applications for microinjection in C. elegans are the production of transgenic worms (Mello et al.
1991) and the knockdown of genes by RNA interference (Fire et al. 1998).
Here, we describe a microinjection technique that has been established in our laboratory for these purposes.
Applications for microinjection in C. elegans
•
Injection of DNA to generate transgenic lines
•
Injection of dsRNA to induce RNAi
•
Injection of proteins
Research interests
Transgenic animals can be generated for a wide range of purposes. Some of the more popular uses are outlined below:
•
Direct transformation of mutant strains using cloned DNA by germline microinjection
•
Expression pattern analysis (e.g., GFP fusion constructs)
•
Structure/function analysis
•
Promoter Bashing
•
Identification of gain-of-function phenotype through overexpression / ectopic expression of an endogenous
or foreign gene
Table 1: Applications for microinjection in worm
Page 2

Equipment
•
Inverted microscope (Axiovert®135, Carl Zeiss AG, Germany)
•
Micromanipulator TransferMan NK2 (Eppendorf AG, Germany)
•
Microinjector FemtoJet express, (Eppendorf AG, Germany)
•
Pipette puller (Sutter Instruments, Model P-87, USA)
•
Vacuum drying chambers (Binder, Germany)
•
Research Pipette (0,5 – 10 µl, Eppendorf AG, Germany)
Methods
Preparation of agarose pad
Place a 2% agarose drop (60°C) on the center of a cover slip and gently sandwich by dropping another cover slip on
top. This flattens the agarose into a thin pad. Air bubbles should be avoided. It is more useful to have a large agarose
pad because more worms can be mounted per pad. Remove one of the cover slips and air dry the pad overnight. On the
next day, bake the pad in the vacuum drying chambers for 2 - 4 hours before use. It is important to use dry agar pads
otherwise the worms will not stick to the pad. Store the agarose pads in an air-tight container, preferably in a desiccator.
Preparation of injection pipettes and loading the injection mixture
Injection pipettes are produced using a mechanical pipette puller. The needle-pulling parameters (heat, pull, velocity and
time) are adjusted in a “trial-and-error” manner. Good needles taper quickly to a sharp-open point, although each user
may develop unique preferences. The shape of the tip may change depending on the position of the heating filament. If
the needle is produced with a closed tip, the tip has to be broken prior to microinjection.
The injection mixture is loaded into the injection pipette by a very long and thin Microloader pipette tip inserted through
the wide back-end.
Mounting and breaking the tip
Assemble the injection capillary to the handle of the micromanipulator. The capillary holder is connected to a FemtoJet
express microinjector, which is attached to a pressurized nitrogen supply. Place a glass tube on a cover slip and cover
the glass tube completely with Voltalef oil. Transfer this cover slip to the Zeiss Axiovert 135 inverted microscope. Align
the glass tube perpendicular to the angle of the capillary. Center the glass tube and the needle tip in the field at low
magnification (e.g., 40x). Be careful to let the tip of the capillary neither touch the glass tube nor the surface of the cover
slip. Change to a higher magnification (e.g., 100x). Break the tip by gently pressing the tip against the glass tube. Please
keep in mind that if the breakage is clearly visible, the capillary will not be sharp enough for successful injections.
Mounting the worms on an agarose pad
Place a drop of Voltalef oil on the agarose pad. Pick a worm and let it swim in the oil to get rid of residual OP50 culture
(OP50 is an E. coli strain fed to C. elegans). Transfer the worm with plenty of oil to the pad. Attach the worm carefully to
the surface of the pad with a pick or tweezers. Straighten the worm and align it perpendicular to the injection needle.
Getting the animal to stick to the pad can be a challenge: If the pad is not freshly vacuum dried, it is difficult to attach
the worm.
Materials
•
Agarose (VWR International)
•
Voltalef oil (VWR International)
•
Micropipette glass tubes; 100 mm long, 1.2 mm diameter (World Precision Instruments, Inc., USA)
•
Cover slips; 48 mm x 60 mm, 1 mm thick (Menzel Glaeser, Germany)
•
Microloader 20 µl (Eppendorf AG, Germany)
Page 3

Injection
The parameters (injection pressure (Pi), compensation pressure (Pc), injection time (Ti)) of the FemtoJet express microinjector should be adjusted according to the quality of the needle and how the needle is broken. There aren´t any fixed
parameters for injection pressure nor for the duration of injection.
The FemtoJet express offers an "automatic" and a "manual" injection mode. The injection is triggered by hand control
or an optional foot control. "Automatic" means that the duration of injection is preset on the device by defining the injection time (Ti). In the "Manual" mode the injection time is determined individually, which means that injection proceeds as
long as the foot or hand control is triggered. In our laboratory we use this mode as injection is performed until some
liquid can be seen in the gonads.
Inject into the mitotic region of the worm gonad by gently pushing the microscope stage (the worm is moving accordingly) towards the needle and carefully move the micromanipulator towards the worm. Inject the mixture by pressing the
left mouse button. The success of injection can be checked by observing whether the germ line cells are disturbed or
not. Pull out the capillary by moving the microscope stage away from the needle. It is essential to check whether or not
the needle tip is blocked. If the injection mixture does not flow out easily, press the right mouse button (Clean function)
until the blockage is removed.
Changing the capillary
If the capillary keeps being blocked, the needle has to be changed. Be very careful: the pressure can cause the needle
to shoot out like an arrow. To avoid this, always push the Menu button – "Option 0" – which will remove any pressure in
the needle.
Recovery
By gently touching the side of the worm with a picking rod, it will start to float in the oil. Remove the worm from the oil
and place it to a OP50 culture. After one hour, transfer the worm to a new OP50 culture plate to remove residual oil.
References
Brenner, S., 1974 The genetics of Caenorhabditis elegans. Genetics 77: 71-94.
Fire, A., S. Xu, M. K. Montgomery, S. A. Kostas, S. E. Driver et al., 1998 Potent and specific genetic interference by
double-stranded RNA in Caenorhabditis elegans. Nature 391: 806-811.
Mello, C. C., J. M. Kramer, D. Stinchcomb and V. AMBROS, 1991 Efficient gene transfer in C.elegans:
extrachromosomal maintenance and integration of transforming sequences. Embo J 10: 3959-3970.
Additional literature
Evans, Thomas C., Transformation and microinjection, WormBook, The C. elegans Research Community, WormBook,
http://www.wormbook.org.
Ordering information:
Article
FemtoJet express
Description
Programmable microinjector with external
Order no. international
5248 000.017
North America
920010521
pressure supply
TransferMan NK2
InjectMan NI2
Proportional micromanipulator for microinjection
Dynamic micromanipulator for microinjection,
5188 000.012
5181 000.017
920000011
920000029
can be combined with FemtoJet express
PatchMan NP2
Microloader
Dynamic micromanipulator for microinjection
Special pipette tips for filling Femtotips from
5183 000.014
5242 956.003
920000037
930001007
the rear
is a registered trademark
®
eppendorf
●
Research Pipette
Your local distributor: www.eppendorf.com/worldwide Application · Support: E-Mail: support@eppendorf.com
Eppendorf North America, Inc. · USA . Tel. +1 516 334 7500 · Toll free phone 800 645 3050 · E-Mail: info@eppendorf.com
0.5 – 10 µl
Eppendorf AG · Germany · Tel. +49 40 538 01-0 · E-Mail: eppendorf@eppendorf.com
3111 000.122
022471902
AU 012 WW 020/PDF/0206/KW
 Loading...
Loading...