Page 1

Model 385 and 395
Gradient Former
Instruction Manual
Catalog Numbers
165-2000 and 165-2001
For Technical Service Call Your Local Bio-Rad Office or in the U.S. Call 1-800-4BIORAD (1-800-424-6723)
Page 2

Table of Contents
Page
Section 1 Introduction..........................................................................................1
1.1 Specifications ....................................................................................................2
Section 2 Pouring a Linear Gradient Gel ...........................................................2
2.1 Calculating the Chamber Volumes ....................................................................2
2.2 Pouring the Gel from the Top ............................................................................2
2.3 Pouring the Gel from the Bottom.......................................................................3
Section 3 Pouring an Exponential Gradient Gel ................................................4
3.1 Concave Exponential Gradient Gel....................................................................4
3.2 Convex Exponential Gradient Gel .....................................................................5
Section 4 Pouring Two Gradient Gels.................................................................5
Section 5 Pouring Multiple Gradient Gels in a Gel Casting Chamber .............6
Section 6 Gradient Former Care and Maintenance...........................................7
Section 7 Theory of Linear and Exponential Gradient Gels .............................7
7.1 Linear Gradients................................................................................................7
7.2 Exponential Gradients .......................................................................................7
Section 8 Protocols .............................................................................................10
8.1 Preparation of Stock Solutions for Laemmli SDS-PAGE Slab Gels ................10
8.2 Calculating the Volume of the Gel...................................................................11
8.3 Gel Preparation................................................................................................12
8.4 Linear Gradient Gels .......................................................................................13
8.5 Exponential Gradient Gels...............................................................................14
Section 9 Equipment and Accessories...............................................................15
9.1 Model 385 and Model 395 Gradient Former and Accessories .........................15
9.2 Additional Required Equipment ......................................................................15
9.3 PROTEAN®II xi and Mini-PROTEAN®II
Vertical Electrophoresis Cells and Accessories ...............................................15
9.4 Electrophoresis Chemicals...............................................................................17
Page 3
Section 1
Introduction
The Model 385 and Model 395 Gradient Formers are primarily used to construct reproducible linear and exponential polyacrylamide gradient gels. Both gradient formers can also
be used to construct sucrose density gradients for ultracentrifugation or elution gradients for
column chromatography. To obtain optimal chromatography elution gradients, tubing with an
inside diameter • 1/16" must be used.
The Model 385 Gradient Former has a 30-100 ml capacity, making it ideal for the construction of one or two identical standard (16 x 20 x 0.15 cm) polyacrylamide gradient gels,
or up to 10 identical miniature (8 x 7 x 0.15 cm) gradient gels. The Model 395 Gradient
Former has a 100-700 ml capacity, making it ideal for the construction of up to 12 identical
standard (16 x 20 x 0.15 cm) gradient gels using a gel casting chamber.
The mixing and reservoir chambers of both gradient formers have identical dimensions.
The outlet from the mixing chamber leads through a peristaltic pump (not required for linear
gradients) to a single gel, to a Y for two gels, into a gel casting chamber for multiple gels. The
gradient may be cast from the top or bottom of the gel sandwich. When the gradient is formed
in a gel casting chamber, the gradient can be cast only from the bottom. Exponential pistons,
available as accessories for both gradient formers, are used to fix the volume in mixing chamber to produce concave or convex exponential gradients.
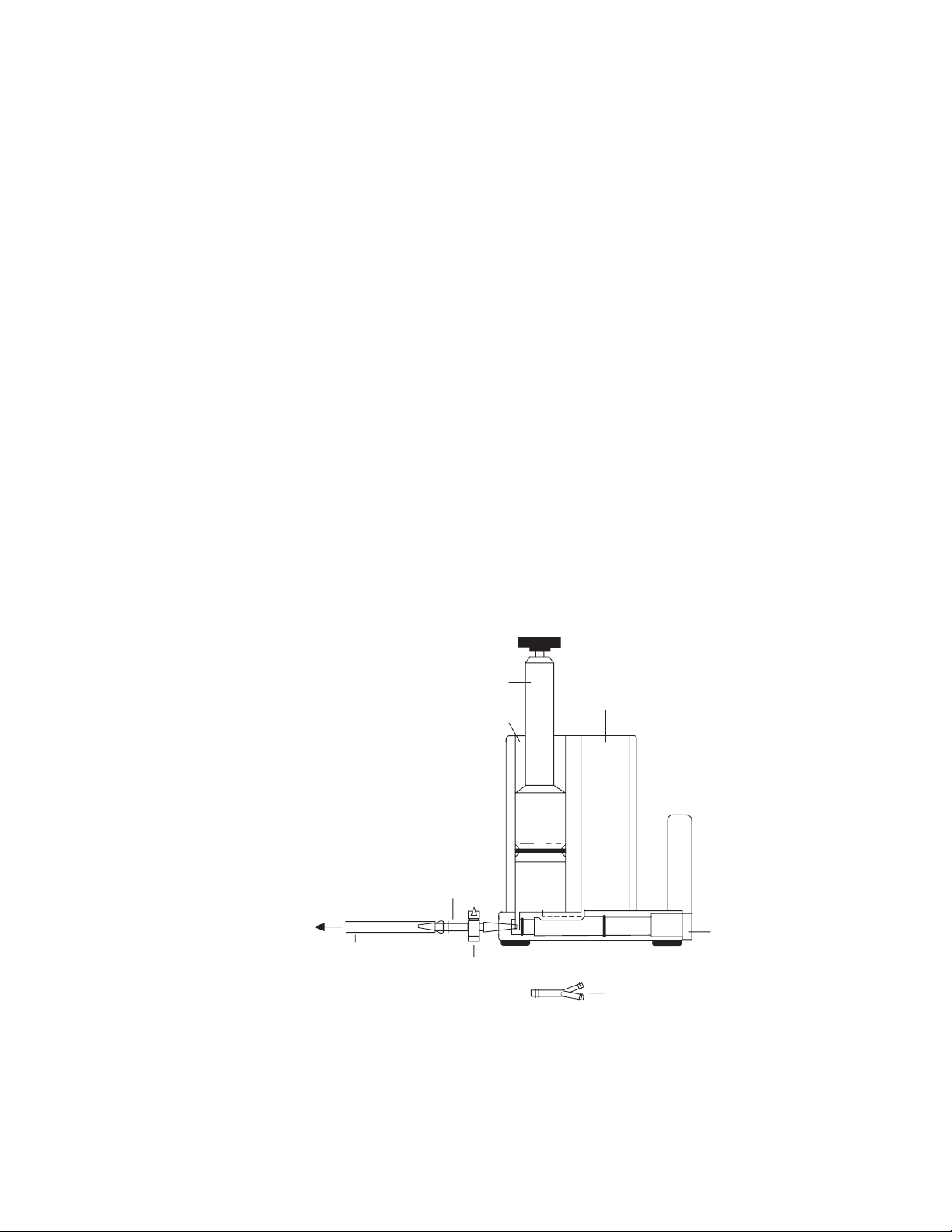
Fig. 1.1. Model 385 and 395 Gradient Formers, major parts.
1
Exponential piston
Reservoir chamber
Luer taper coupling
Mixing chamber
Peristaltic
pump
Valve stem
Stopcock
Tubing
Y-connector
Page 4

1.1 Specifications
Model 385 Gradient Former
Overall size 10 cm x 15 cm x 15 cm (with piston in chamber)
Weight 405 g without piston; piston 65 g
Capacity 2 x 56 ml
Model 395 Gradient Former
Overall size 16.5 cm x 11.2 cm x 18 cm (without piston)
Weight 730 g without piston; piston 310 g
Chamber volume 2 x 400 ml
Practical capacity 2 x 350 ml
Construction of Model 385 and 395 Gradient Formers
Body machined from an acrylic block
Valve stem Delrin
®
Stopcock one-way plastic stopcock, Luer taper
Piston Delrin
Tubing Tygon
®
1/8" ID
Section 2
Pouring a Linear Gradient Gel
2.1 Calculating the Chamber Volumes
Each chamber's volume is one-half the total volume of the gel. The volume of the tubing
and the stir bar, however, can affect the measurement of the total volume. Section 2.2, step 10,
discusses tubing volume.
Adding the stir bar to the mixing chamber increases the volume in the mixing chamber.
If not compensated for, this increased volume would divide itself between the two chambers
when the valve stem was opened. However, when the stirring motor is turned on, a vortex is
created, which compensates for the increase in mixing chamber volume, since the bottom of
the vortex becomes the level at which the other chamber equalizes.
To calculate the volume of solution required for each chamber, multiply the spacer thickness in cm by the length of the gel in cm, by the width of the gel in cm, and divide by 2.
2.2 Pouring the Gel from the Top
The heavy solution is poured into the mixing chamber since it enters the sandwich first,
flowing to the bottom. The light solution, which flows into the gel last, is poured into the
reservoir chamber.
Note: We advise you to familiarize yourself thoroughly with the sequence of operations
starting with step 6. The best guarantee of reproducibility from gradient to gradient is
careful technique on the part of the operator.
1. Set up the gel sandwich in the usual manner.
2. Arrange the tubing in the peristaltic pump so there is as short a distance as possible
between the stopcock opening and the pump, and between the pump and the gel sandwich.
Cut the tubing as necessary.
3. Attach the needle to the end of the tubing and tape it to the top of the gel sandwich near
the center.
2
Page 5

If a peristaltic pump is not used, the level of the gradient former stopcock must be above
the top of the gel sandwich by a distance sufficient to create a hydrostatic head large
enough to pour the entire volume of the gel within 10 minutes from the time the initiators
are added to the light solution. All the acrylamide should be in the gel sandwich before
polymerization begins, so that polymerization will be uniform throughout the gel.
Rather then using a needle on the end of the tubing, you may cut the end of the tubing at
an angle before taping it to the sandwich. Face the tapered opening toward the glass plate.
4. Place a 1" stir bar (Model 385) or 2" stir bar (Model 395) in the mixing chamber (with
valve stem closed).
5. Degas the heavy and light solutions.
6. Add the initiators to the light solution, swirl it 8 to 10 times, and pour it into the reservoir
chamber. (This is the start of the 10 minutes.) Leave the valve stem closed.
7. Add the initiators to the heavy solution, swirl it 8 to 10 times, and pour it into the mixing
chamber.
8. Start the stirring motor and adjust the speed so that you get good mixing and still the bot-
tom level of the vortex matches the acrylamide level in the reservoir chamber.
9. Quickly open the valve stem and stopcock, and turn on the peristaltic pump.
Note: The peristaltic pump must be able to pump the whole gradient within 10 minutes
from the time you add the initiators to the first solution. This is generally in the range of
5 to 10 ml/min for the Model 385 and 70 to 100 ml/min for the Model 395.
10. Run the pump until all the solution is pushed into the sandwich.
If a peristaltic pump is not used, the acrylamide will flow down the tubing to the gel sand-
wich by gravitational force when you open the stopcock.
Note: If tubing is left dangling below the top of the sandwich, you will have to lift it up
and drain it into the top of the gel sandwich to get the entire volume of acrylamide into
the sandwich. The tubing volume should not be a problem because the tubing will retain
a negligible amount of acrylamide following gradient formation.
11. Overlay the acrylamide with water or water saturated isobutanol. For a continuous buffer
system, insert a comb.
Note: Immediately after the gradient is cast, the system must be flushed with water to pre-
vent polymerization of residual acrylamide within the gradient former or pumping system.
12. Immediately remove the needle (or tubing) from the top of the sandwich and transfer to
a waste receptacle. Pour rinse water into both the mixing and reservoir chambers and turn
the peristaltic pump on to its maximum speed. The water should immediately flow from
the gradient former, through all connectors and tubing of the pumping system, and out the
needle (or tubing) to a waste receptacle.
2.3 Pouring the Gel from the Bottom
In this case, the light solution goes in the mixing chamber, and the heavy solution goes
in the reservoir. The light solution enters the gel from the bottom and travels to the top, and
the heavy solution follows, filling the bottom of the gel.
1. Set up the gel sandwich, gradient former, tubing, and peristaltic pump as described in
Section 2.2, steps 1, 2, 4, and 5. For a continuous gradient, insert the comb at an angle.
3
Page 6

2. Insert the needle into the sandwich through the gasket on the bottom of the casting stand.
3. To start the linear gradient, use the procedure described in Section 2.2, steps 6-9, except
pour the light solution into the mixing chamber and the heavy solution into the reservoir.
4. After the stirring motor is turned on, the vortex level adjusted, the valve stem and stop-
cock opened, run the peristaltic pump until the last of the acrylamide enters the needle fit-
ting. Do not allow air bubbles to enter the gel, as this could cause the mixing of the
gradient. Remove the needle from the bottom of the casting stand.
5. Overlay the gel. For a continuous gel, the comb, which should be positioned at an angel
between the gel sandwich, is straightened and inserted fully to form the wells.
6. Rinse the residual acrylamide from the gradient former, tubing, and needle as described
in Section 2.2, step. 12.
Section 3
Pouring an Exponential Gradient Gel
In some cases, the number of proteins (polypeptides) in a certain area of the gel will justify pouring an exponential gradient. Whether or not a linear gradient gives adequate resolution is determined empirically for each sample. If there are many bands near the top of the gel,
then a concave gradient is indicated, whereas if there are many bands toward the bottom of
the gel, a convex gradient should be poured. (See Theory of Linear and Exponential Gradient
Gels, Section 7, especially Figures 7.3 and 7.5). The Model 385 gradient former piston (catalog number 165-2006) or the Model 395 gradient former piston (catalog number 165-2005)
limits the volume of the mixing chamber so that either a concave or a convex gradient can be
formed.
3.1 Concave Exponential Gradient Gels
Concave gradient gels are formed by delivering the acrylamide from the top, with the
small volume of heavy solution in the mixing chamber and the large volume of the light solution in the reservoir chamber. See Figure 7.2.
1. Calculate the chamber volumes.
Calculate the volume of the gel (spacer thickness in cm x gel width in cm x gel length in
cm). The volume of the heavy solution, for the mixing chamber, is one-fourth the total vol-
ume of the gel. The volume of the light solution, for the reservoir chamber, is equal to the
total volume of the gel. Because the volume of the mixing chamber is fixed, not all of
the gel solution will be delivered to the gel sandwich. One-fourth the volume will remain
in the mixing chamber at the end of the delivery and must be discarded.
2. Set up the equipment as in Section 2.2, steps 1-4.
3. Mix and degas the small volume of heavy acrylamide solution and the large volume of
light acrylamide solution, add the initiators to the light acrylamide solution, swirl 8 to 10
times, and pour into the reservoir chamber, keeping the valve stem closed.
4. Add the initiators to the heavy solution, swirl 8 to 10 times, and pour it into the mixing
chamber.
5. immediately fix the volume of the mixing chamber by inserting the piston into the cham-
ber to 1 cm above the level of the acrylamide and tightening the screw top handle to hold
the piston in place.
4
Page 7

6. Quickly turn on the stirring motor (to a speed which gives good mixing without foaming),
open the valve stem and the stopcock, and start the peristaltic pump (see Note on the
speed of the peristaltic pump).
7. As the pump removes acrylamide from the mixing chamber, the reservoir solution will be
drawn into the mixing chamber and the gradient will be formed.
8. When the acrylamide solution reaches the desired level in the gel sandwich, stop the
pump, remove the tubing from the gel sandwich, and transfer it to a waste receptacle.
Overlay the gel with water or water-saturated isobutanol. For a continuous system, add the
comb to form the wells.
9. Remove the exponential piston from the mixing chamber and restart the pump to remove
any remaining acrylamide. When the chambers are empty, add rinse water to both the mix-
ing and reservoir chambers and flush out the system as described in Section 2.2, step 12.
3.2 Convex Exponential Gradient Gels
Convex gradient gels are formed by delivering the acrylamide from the bottom, with the
small volume of light solution in the mixing chamber and the large volume of heavy solution
in the reservoir chamber (see Figure 7.4).
1. Calculate the volumes of light and heavy solutions as in Section 3.1, except that the light
solution will be 1/4 the total gel volume and the heavy solution will be equal to the total
gel volume.
2. Set up the equipment as described in Section 2.3, with the needle entering the gel sand-
wich from the bottom through the casting stand gasket. For a continuous gradient, insert
the comb into the sandwich at an angle.
3. Mix and degas the small volume of light acrylamide solution and large volume of heavy
acrylamide solution.
4. Add the initiators to the light solution, swirl 8 to 10 times, and pour it into the mixing
chamber, leaving the valve stem closed.
5. Immediately fix the volume with the piston as described in Section 3.1, step 5.
6. Add the initiators to the heavy solution, swirl 8 to 10 times, and pour into the reservoir.
7. Cast the gradient as described in Section 3,1, steps 6-8.
8. Rinse out the system as described in Section 3.1, step 9.
Section 4
Pouring Two Gradient Gels
Double the volumes of heavy and light solution, and use the Y-connector provided. The
procedures are the same as above. It is essential that the tubing from the Y to the two gels be
the same length, and that the rate of flow be the same from each needle. We advise that this
be checked with water and two measuring cylinders ahead of time.
Note: It is difficult to attain reproducible gradients when pouring two gradient gels.
5
Page 8

Section 5
Pouring Multiple Gradient Gels
in a Gel Casting Chamber
Note: Gel casting chambers are designed for bottom filling, therefore concave exponen-
tial gradient gels can not be found using a gel casting chamber.
1. The total gel volume required depends on several factors, including gel thickness, num-
ber of gels, gel length, and amount of space-filling. To make a precise determination of
the volume required for a particular application, set up the casting chamber with the glass
plates, spacers, and combs (for continuous gradients) to form the required number of gel
sandwiches and measure the volume of deionized distilled water required to fill it to the
desired level. Then disassemble the chamber and rinse and dry the parts.
2. Calculate the chamber volumes,. For a linear gradient, each chamber's volume is one-
half the total gel volume required. For a convex exponential gradient, the light solution
in the mixing chamber will be one-fourth the total gel volume and heavy solution in the
reservoir chamber will be equal to the total gel volume.
3. Reassemble the multi-gel casting chamber as described in step 2. Be sure the glass plates
and spacers are clean and dry.
4. Arrange the tubing in the peristaltic pump so there is as short a distance as possible
between the stopcock opening and the pump, and between the pump and the inlet to the
gel casting chamber. Attach a 3 cm piece of tubing to the gel casting chamber inlet with
a pinch clamp to close it off. Attach one end of a Luer taper coupling to this 3 cm tubing
and attach the tubing from the pump to the other end of the Luer taper coupling. Cut the
tubing as necessary.
5. For a linear gradient, set up the gradient former as described in Section 2.2, steps 4-9,
except pour the light solution into the mixing chamber and the heavy solution into the
reservoir chamber. For a convex exponential gradient, set up the gradient former as
described in Section 3.2, steps 3-7.
6. Run the pump so that the acrylamide flows through the tubing into the gel casting chamber,
until the acrylamide reaches the desired level in the gel sandwiches. Stop before any air
enters the gel casting chamber. For bottom filling of linear gradient, the tubing should con-
tain a negligible amount of acrylamide following gradient formation. For bottom filling of
a convex exponential gradient, one-fourth the volume will remain in the mixing chamber.
7. Overlay each gel sandwich with an equal volume of water. For a continuous gel, straight-
en the pre-positioned combs and insert them fully to form the wells.
8. Following gel casting, pinch off the tubing with the pinch clamp, remove the tubing from
the Luer taper coupling, and transfer it to a waste receptacle. Pump out any remaining
acrylamide from the chambers and pour rinse water into both the mixing and reservoir
chamber. Turn the peristaltic pump on to its maximum velocity to flush out the system.
Water should flow from the gradient former through the peristaltic pump and down all the
tubing to keep it from being plugged with any polymerized residual acylamide.
6
Page 9

Section 6
Gradient Former Care and Maintenance
After use, disassemble the gradient former and rinse all parts with distilled water. If the
gel polymerizes in the gradient former during casting or before the system can be flushed
with water, the unit can be easily cleaned. The valve stem and stopcock are easily removed.
The needle will probably have to be replaced (catalog number 165-2007). The polymerized
gel can usually be removed from the tubing, the Luer coupling, and Y-connector with a little
effort, or you can replace them with the Tubing Connection Kit (catalog number 165-2008).
Use no organic solvents, strong acid solutions, or ethanol in the gradient former.
Section 7
Theory of Linear and Exponential Gradient Gels
7.1 Linear Gradients
Linear gradients are described by the equation:
Ct=C
M
+ (CR- CM)
Vt/2V
o
where Ct= concentration of gradient being delivered at any time,
CM= initial concentration in mixing chamber,
CR= concentration in reservoir,
Vt= volume delivered at time t,
Vo= original volume in each chamber.
A typical linear gradient is shown in Figure 7.1. The slope of the gradient is changed by
changing the acrylamide concentration in the mixing and/or reservoir chamber.
7.2 Exponential Gradients
Both convex and concave exponential gradients are described by the equation
Ct= CR- (CR- CM) e - Vt/V
M
where e = natural base (2.718), and
VM= volume of acrylamide in the mixing chamber; the other symbols are as above.
Figure 7.2 and 7.3 show how a concave gradient is set up in the gradient former, how the
gel pore size varies with the length of the gel, and how the % acrylamide varies with the
amount of acrylamide delivered.
Figure 7.4 and 7.5 show how a convex gradient is set up in the gradient former, how the
gel pore size varies with the length of the gel, and how the % acrylamide varies with the
amount of acrylamide delivered.
7
Page 10

Fig. 7.1. Linear gradient gel. a. 5-30% gradient. b. 10-20% gradient.
Fig. 7.2. Concave gradient—relation of heavy and light solutions in the mixing and reservoir
chambers. Intensity of shading in the slab gel sandwich indicates change in pore size.
8
% Acrylamide
% Acrylamide
25 50 75 100
Amount delivered (%)
a
b
5
10
15
20
25
30
Peristaltic
pump
30
25
20
15
10
5
25 50 75 100
Amount delivered (%)
a
b
Page 11
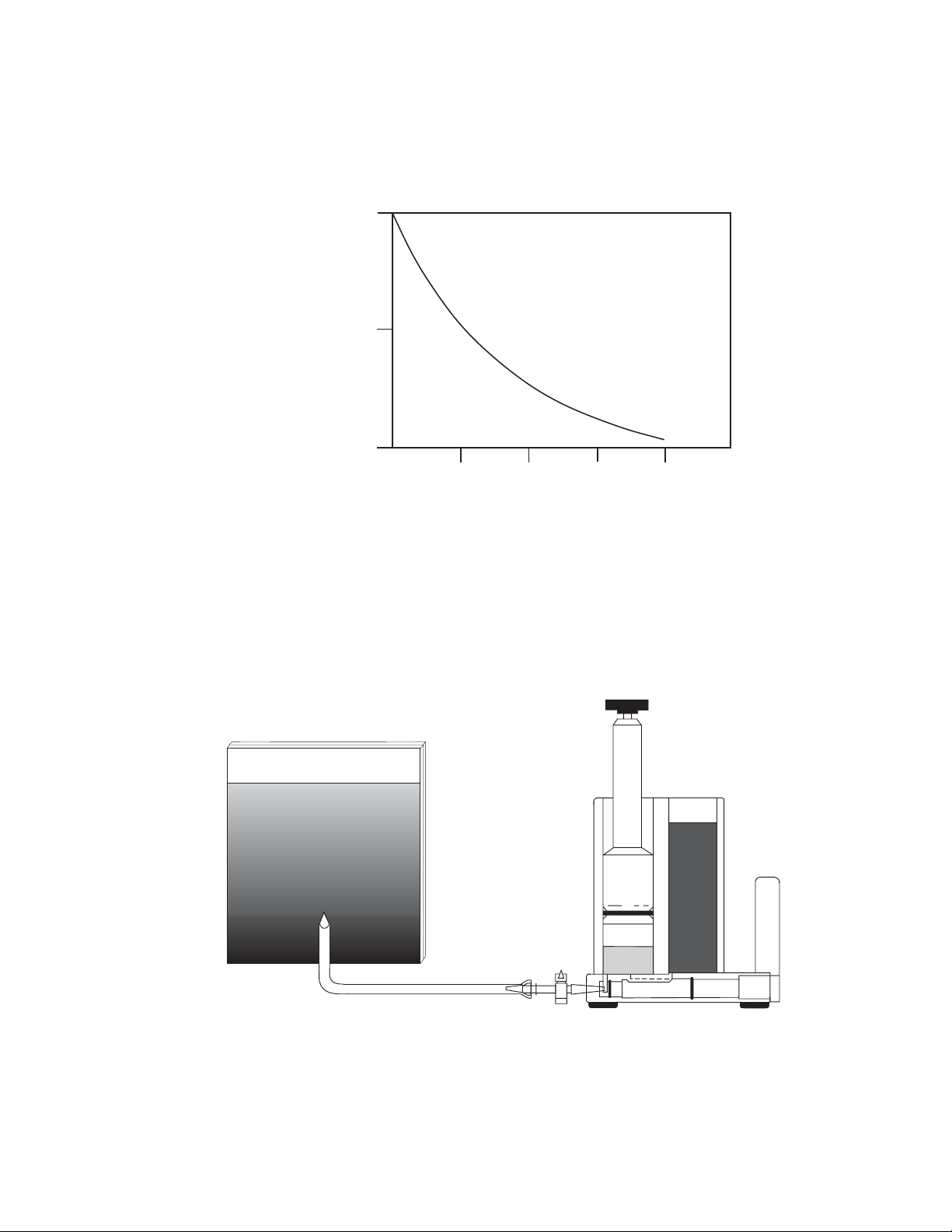
Fig. 7.3. Concave gradient gel.
Fig. 7.4. Convex gradient—relation of light and heavy solutions in the mixing and reservoir chambers. Intensity of shading in the slab gel sandwich indicates change in pore size.
9
% Acrylamide
20
15
10
25
50 75 100
Amount delivered (%)
20
15
10
% Acrylamide
25 50 75 100
Amount delivered (%)
Page 12

Fig. 7.5. Convex gradient gel.
Section 8
Protocols
8.1 Preparation of Stock Solutions for SDS-PAGE Slab Gels
(Laemmli* buffer system)
A. Acrylamide/Bis (30%T, 2. 67%C)
Acrylamide 146.0 g
N'N'-BIS-methylene-acrylamide 4.0 g
(Preweighed Acrylamide/Bis 37.5:1 mixture may be substituted)
Distilled water to 500 ml. Filter and store at 4 °C in the dark. Maximum shelf life under
these conditions is 30 days.
B. 1.5 M Tris-HCl, pH 8.8
54.45 g Tris base
150 ml distilled water
Adjust to pH 8.8 with 1 N HCl. Distilled water to 300 ml. Store at 4 °C.
C. 0.5 M Tris-HCl, pH 6.8
6 g Tris base
60 ml distilled water
Adjust to pH 6.8 with HCl. Distilled water to 100 ml. Store at 4 °C.
D. 10% (w/v) SDS
Dissolve 10 g SDS in water with gentle stirring. Distilled water to 100 ml.
E. 10% Ammonium Persulfate (w/v)
100 mg ammonium persulfate. Distilled water to 1 ml. Make fresh daily.
10
% Acrylamide
20
15
10
25 50 75 100
Amount delivered (%)
Page 13

F. Sample Buffer (SDS reducing buffer: 62.5 mM Tris-HCl, pH 6.8, 10% glycerol,
2% SDS, 5% b-mercaptoethanol)
Distilled water 4.0 ml
0.5 M Tris-HCl pH 6.8 1.0 ml
Glycerol 0.8 ml
10% SDS 1.6 ml
β-mercaptoethanol 0.4 ml
0.05% (w/v) bromophenol blue (in water) 0.2 ml
8.0 ml
Dilute the sample at least 1:4 with sample buffer. Heat at 95 °C for 4 minutes.
* Laemmli, U. K., Nature, 227, 680 (1970).
G. 5x Electrode (Running) Buffer (25 mM Tris, 192 mM glycine, 0.1% SDS, pH 8.3)
Tris base 45.0 g
Glycine 216.0 g
SDS 15.0 G
Distilled water in 3 L. Do not adjust the pH with acid or base. Store at 4 °C. Warm to
37 °C before use if precipitation occurs. Dilute 300 ml of 5x stock with 1.2 L distilled water
to make 1.5 L 1x buffer.
8.2. Calculating Gel Volumes
The total gel volume required is described by the equation:
VT= (W) (L) (T) (N)
VT= Total gel volume; W = Gel width (cm); L = Gel length (cm); T = Gel
thickness (cm); N = Number of gel slabs required
Table 8.2 Calculated volumesarequired per gel for the
PROTEAN II xi and Mini-PROTEAN II cells
PROTEAN II Mini-PROTEAN II
Gel Volume
b
GelVolume
b
Spacer 16 cm 20 cm 7 cm
Thickness length length length
0.50 mm 12.8 ml 16.0 ml 2.9 ml
0.75 mm 19.2 ml 24.0 ml 4.4 ml
1.00 mm 25.6 ml 32.0 ml 5.8 ml
1.50 mm 38.4 ml 48.0 ml 8.8 ml
3.00 mm 76.8 ml 96.0 ml
Note: With a multi-gel casting chamber, the total gel volume required depends on several
factors, including gel thickness, number of gels, gel length, and amount of space-filling.
To make a precise determination of the volume required for a particular application, set
up the casting chamber with the glass plates, spacers, and combs to form the required
number of gel sandwiches and measure the volume of deionized distilled water required
to fill it to the desired level. Then disassemble the chamber and rinse and dry the parts.
a. The volumes reported completely fill the gel sandwich to the top of the inner plate. The
amount of separating gel may be adjusted depending on application (with or without
comb, with or without stacking gel, etc.).
11
Page 14
b. The total volume required for multiple gels can be determined by multiplying the vol-
ume of a single gel by the number of gels required.
8.3 Gel Preparation
Monomer concentrations as high as 22% T can be prepared from a 30% T stock solution
as presented in Table 8.3. When monomer concentrations greater than 22% T are required, the
Acrylamide/Bis stock solutions must be increased to 40% T 0r 50% T.
Table 8.3. Formulations for SDS-PAGE Separating
and Stacking Gels
Separating Gel Stacking Gel
(0.375 M Tris, pH 8.8) (125 M Tris, pH 6.8)
Monomer Concentration (%T, 2.67%C)
C
10% 20% X%(<15%) X%>(15%) 4.0%
Acrylamide/Bis (30%T, 2.67%C) Stock 33.3 ml 66.6 ml d ml d ml 1.3 ml
Distilled water 40.2 ml 6.9 ml e ml e ml 6.1 ml
1.5 M Tris-HCl, pH 8.8 25.0 ml 25.0 ml 25.0 ml 25.0 ml
.5 M Tris-HCl, pH 6.8 2.5 ml
10% (w/v) SDS 1.0 ml 1.0 ml 1.0 ml 1.0 ml 100 ml
10% ammonium persulfate (fresh)
f
500 µl 500 µl 500 µl 500 µl 50 µl
TEMEDf(monomer concentration <15%) 50 µl 50 µl 10 µl
f
TEMED
f,g
(monomer concentration >15%) 25 µl 25 µl
TOTAL MONOMER
h
100 ml 100 ml 100 ml 100 ml 10 ml
c. The pore size of the polyacrylamide gel can be changed by adjusting either the total
monomer concentration (%T) or by adjusting the crosslinking monomer concentration
(%C). The most common method of changing the pore size is to adjust the total monomer
concentration (%T). In diluting a stock solution, the crosslinking monomer concentra-
tion (%C) is independent of the final total monomer concentration (%T).
%T=[g Acrylamide + g Bis-Acrylamide/Total Volume] x 100
%C=[g Bis-Acrylamide/(g Acrylamide + g Bis-Acrylamide)] x 100
d. Calculate the volume of Acrylamide/Bis stock required for the desired total monomer
concentration with the following formula: volume 30% T, 2.67% C Stock = (X % T) x
(3.33). For example, the volume of Acylamide/Bis stock required to prepare 100 ml final
volume of 10% T monomer solution would be 33.0 ml; 10 x 3.33 = 33.3 ml.
e. Calculate the volume of water required for the desired total monomer concentration with
the following formula: volume water = 73.5 - (volume 30% T, 2.67% C stock used). For
example, the volume of water required to prepare 100 ml final volume of a 10% T
monomer solution would be 40.4 ml: 73.5 - 33.3 ml = 40.2 ml.
f. Prepare the monomer solution by combining all reagents except ammonium persulfate and
TEMED. Dearate the solution under vacuum for at least 15 minutes. Add the two cata-
lysts just prior to casting the gels.
g. Higher concentrations of monomer polymerize more evenly with lower concentrations of
TEMED.
h. One can prepare any desired volume of monomer solution by multiplying to 100 ml recipe
by the desired multiplying factor.
i. Higher TEMED concentrations and faster polymerization are required for the stacking
gel because of the inhibitory effect of atmospheric oxygen associated with the comb.
12
Page 15

8.4 Linear Gradient Gels
For linear gradients, the volume in the mixing chamber (Vm) equals the volume in the
reservoir chamber (Vr). Try volume in each chamber is one-half of the total gel volume (VT):
VT= Vm+ V
r
or Vm= Vr= 0.5 V
T
First calculate the total gel volume required. Then determine the amount needed for the
mixing and reservoir chambers. It is easier to prepare a volume slightly more than the required
gel volume and then quantitatively transfer the required volume into mixing or reservoir
chamber.
Example 1: Describe how to prepare a linear 10-20% gradient for casting six 16 x 20
x 0.15 cm gels in a PROTEAN II multi-casting chamber. The slab gels
will be used for 2-D work and a stacking gel is not required.
1. When a gel casting chamber is used, the gradient must be poured by bottom filling.
Therefore, the mixing chamber of the Model 395 Gradient Former must contain the lighter
(10%) monomer solution and the reservoir chamber must contain the heavier (20%)
monomer solution.
2. When using a multi-gel casting chamber, the total gel volume required depends on sev-
eral factors, including gel thickness, number of gels, gel length, and amount of space-
filling. To precisely determine the volume required for six 16 x 20 x 0.15 cm gels, set up
the casting chamber with he glass plates, spacers and the necessary space fillers and mea-
sure the volume of deionized distilled water required for a 20 cm length. In this example,
let us assume that the total gel volume needed is 310 ml (your actual required gel volume
may differ slightly from this hypothetical value). Disassemble the chamber and rinse and
dry the parts.
3. Reassemble the multi-gel chamber as described in step 2.
4. The amount of monomer required for each chamber will be 155 ml (310/2). It is best to
prepare 170 ml each of a 10% and 20% monomer solution and to quantitatively transfer
155 ml of each solution into their respective chambers. The formulation for each monomer
solution can be calculated using Table 8.2 and a multiplying factor of 1.7 (170 ml required
volume/100 ml volume in Table 8.2).
Monomer Concentration 10% 20%
30% stock 56.6 ml 113.2 ml
Distilled water 68.3 ml 11.7 ml
1.5 M Tris-HCl, pH 8.8 42.5 ml 42.5 ml
10% (w/v) SDS 1.7 ml 1.7 ml
Degas
10% ammonium persulfate 850 µl 850 µl
TEMED 85 µl 42.5 µl
Total monomer volume 170 ml 170 ml
155 ml of the 10% solution will be transferred to the mixing chamber and 155 ml of the
20% solution will be transferred to the reservoir chamber.
5. Set up and cast the gradient as outlined in Section 5, steps 4-8. Overlay the gels with an
equal volume of water.
13
Page 16

8.5 Exponential Gradient Gels
For an exponential gradient gel, the volume in the mixing chamber is one fourth the total
required gel volume (0.25 VT) and the volume in the reservoir chamber equals the total
required gel volume (1.0 VT).
First calculate the total gel volume required. Then determine the amount needed for the
mixing and reservoir chambers. It is easier to prepare a volume slightly more than the required
gel volume and then quantitatively transfer the required volume into mixing or reservoir
chambers.
Example 2: How to prepare a concave 5-15% exponential gradient for one 16 x 16 x
0.15 cm gel with a 2 cm stacking gel to be cast in the PROTEAN II xi casting stand.
1. A concave exponential gradient must be cast from the top. The heavier 15% monomer will
be placed in the mixing chamber and the lighter 5% monomer will be placed in the reser-
voir chamber of the Model 385 Gradient Former.
2. From Table 8.1, 38.4 ml total gel volume is required. 9.6 ml (0.25 x 38.4 or 0.25 VT) of
the 15% monomer solution is required for the mixing chamber and 38.4 ml (VT) of the
5% monomer solution is required for the reservoir chamber. It is convenient to prepare 15
ml of the 15% solution and 50 ml of the 5% solution and quantitatively transfer the
required volumes into the respective chamber.
3. The multiplying factor for the 15% monomer solution is 0.15 (15/100) and the multiply-
ing factor for the 5% monomer solution is 0.5 (50/100). Prepare the gel solution using
Table 8.2:
Monomer Concentration 5% 15%
30% stock 8.3 ml 7.5 ml
Distilled water 28.4 ml 3.5 ml
1.5 M Tris-HCl, pH 8.8 12.5 ml 3.8 ml
10% (w/v) SDS .5 ml 50 µl
Degas
10% ammonium persulfate 250 µl 75 µl
TEMED 25 µl 7.5 µl
Total monomer volume 50 ml 15 ml
9.6 ml of the 15% solution will be transferred to the mixing chamber and 38.4 ml of the
5% solution will be transferred to the reservoir chamber.
4. Set up and pour the gradient as outlined in Section 3.1, steps 2-8. Overlay the gels with
water saturated isobutanol. After the separating gel has polymerized, remove the water sat-
urated isobutanol and wash the top of the gel with distilled deionized water. Prepare the
stacking gel as described in Table 8.2. Cast the stacking gel and insert the comb to form
the wells.
14
Page 17

Section 9
Equipment and Accessories
9.1 Model 385 and Model 395 Gradient Formers and
Accessories
Catalog
Product Description Number
Model 385 Gradient Former, includes main body assembly with 165-2000
valve stem, and tubing connection kit (stopcock, Luer taper
coupling, Y-connector, and tubing)
Model 395 Gradient Former, includes main body assembly with 165-2001
valve stem, and tubing connection kit (stopcock, Luer taper
coupling, Y-connector, and tubing)
Exponential Piston, Model 395 165-2005
Exponential Piston, Model 385 165-2006
Gradient Pouring Needles for Model 385 and 395, 2 165-2007
Tubing Connection Kit (stopcock, Luer taper coupling, tubing 165-2008
(1/8" ID, 3 ft), and Y-connector)
9.2 Additional Required Equipment (not included)
Peristaltic pump capable of delivering 10 ml/min for Model 385 Gradient Former
Peristaltic pump capable of delivering 100 ml/min for Model 395 Gradient Former
1" stir bar for Model 385 Gradient Former
2" stir bar for Model 395 Gradient Former
Magnetic stirring motor
9.3 PROTEAN II xi and Mini-PROTEAN II Vertical
Electrophoresis Cells and Accessories
Catalog
Product Description Number
PROTEAN II xi 16 cm Slab Cell* 165-1801
1.5 mm, 15 well 165-1802
1.0 mm, 15 well 165-1803
0.75 mm, 15 well 165-1804
PROTEAN II xi 20 cm Slab Cell* 165-1811
1.5 mm, 15 well 165-1812
1.0 mm, 15 well 165-1813
0.75 mm, 15 well 165-1814
All PROTEAN II xi slab cells include the central cooling core with gaskets and core caps,
lower buffer chamber, lid with power cables, 2 sets of glass plates, 4 sandwich clamps, 2
combs, an upper buffer dam, a casting stand with gaskets, a leveling bubble, PAGE starter kit,
and instructions.
* Cells marked with (*) contain all of the above except spacers and combs (order separately).
15
Page 18

Catalog
Product Description Number
PROTEAN II xi 2-D Cell, 1.0 mm, 16 cm 165-1931
PROTEAN II xi 2-D Cell, 1.5 mm, 16 cm 165-1932
PROTEAN II xi 2-D Cell, 1.0 mm, 20 cm 165-1933
PROTEAN II xi 2-D Cell, 1.5 mm, 20 cm 165-1934
PROTEAN II xi 2-D cells include central cooling core, lower buffer chamber, lid with
power cables, 2 sets of glass plates (with bevel), 4 sandwich clamps, 24 glass tubes, 2 tube cell
adapters, 16 stoppers, 48 grommets (4-8 mm O.D. tubes), 2 2-D combs, 4 spacers, 1 upper
buffer dam, slab casting stand, leveling bubble, PAGE starter kit, and instructions.
Catalog
Product Description Number
PROTEAN II xi Multi-Cell, includes 3 central cooling cores with 165-1951
gaskets, tank, lid with power cables, 1 upper buffer dam,
PROTEAN II xi Multi-Gel casting chamber with accessories,
leveling bubble, and instructions (order spacers, plates, clamps
and combs separately)
PROTEAN II xi Multi-Gel Casting Chamber, includes casting 165-2025
chamber, sealing plate Luer taper connector, silicone gasket,
package of 15 separation sheets, set of 4 acrylic blocks, and instructions
PROTEAN II xi and PROTEAN II xi Multi-Cell Glass Plates
16 cm cell 20 cm cell
Inner Plate (shorter), 2 165-1821 165-1823
Outer Plate (longer), 2 165-1822 165-1824
Frosted Inner Plate, 2 (agarose gels)* 165-1825 165-1826
Beveled Inner Plates, 2 (2-D procedures)* 165-1827 165-1828
* Used in conjunction with regular outer plate.
Protean II xi Cell Spacers (set of 4)
0.5 mm 0.75 mm 1.0 mm 1.5 mm 3.0 mm
25 well — 165-1861 165-1862 165-1863 —
20 well 165-1865 165-1866 165-1867 165-1868 165-1869
15 well 165-1870 165-1871 165-1872 165-1873 165-1874
10 well 165-1875 165-1876 165-1877 165-1878 165-1879
5 well — 165-1881 165-1882 165-1883 165-1884
3 well — — 165-1887 165-1888 165-1889
Blank 165-1890 165-1891 165-1892 165-1893 165-1894
2-D (1 ref well)- — 165-1897 165-1898 165-1899
16
0.5 mm 0.75 mm 1.0 mm 1.5 mm 3.0 mm
16 cm 165-1841 165-1842 165-1843 165-1844 165-1845
20 cm 165-1846 165-1847 165-1848 165-1849 165-1850
PROTEAN II xi Cell PTFE Combs
Page 19

Catalog
5 well 10 well 15 well (1 Reference Well)
0.5 mm 165-1915 165-2919 165-2923 —
0.75 mm 165-2916 165-2920 165-2924 165-2927
1.0 mm 165-2917 165-2921 165-2925 165-2928
1.5 mm 165-2918 165-2922 165-2926 165-2929
Mini-PROTEAN II Spacers (set of 4)
0.5 mm 0.75 mm 1.0 mm 1.5 mm
165-2930 165-2931 165-2932 165-2933
9.4 Electrophoresis Chemicals
Acrylamide, 99.9% 100 g 161-0100
500 g 161-0101
1 kg 161-0107
2 kg 161-0103
Preweighed Acrylamide/Bis, 37.5:1 mixture 30 g 161-0122
150 g 161-0125
Preweighed Acrylamide/Bis. 29.5:1 mixture 30 g 161-0121
150 g 161-1024
Preweighed Acrylamide/Bis, 19:1 mixture 30 g 161-0120
150 g 161-0123
Bis (N, N'-methylene-bis-acrylamide) 5 g 161-0200
50 g 161-0201
Tris 500 g 161-0716
1 kg 161-0719
Glycine 250 g 161-0717
1 kg 161-0718
17
Product Description Number
Mini-PROTEAN II Slab Cell, 10 well combs, 0.75 mm spacers, 165-2940
includes inner cooling core with gaskets, lower buffer chamber, lid
with power cables, 3 sets of glass plates, 2 clamp assemblies, 10
well, 0.75 mm thick combs (2), 0.75 mm thick spacers (4), casting
stand with gaskets, leveling bubble, PAGE starter kit, and instructions
Mini-PROTEAN II Slab Cell, same as 165-1940 without comb and spacers 165-2941
Mini-PROTEAN II Multi-Casting Chamber, includes casting 165-2950
chamber body with front clamp screws, cover with gasket, 10 sets
of glass plates, 15 separation sheets, set of 4 acrylic blocks, stopcock,
Luer fitting, and instructions
Mini-PROTEAN II Glass Plates, 10 sets, a set consists of 1 shorter 165-2912
(inner) plate and 1 longer (outer) plate
Mini-PROTEAN II PTFE Combs
2-D/Preparative
Page 20

Catalog
Product Description Number
SDS (sodium dodecylsulfate) 25 g 161-0300
100 g 161-0301
1 kg 161-0302
Boric Acid 500 g 161-0750
1 kg 161-0751
Ammonium Persulfate 10 g 161-0700
TEMED 5 ml 161-0800
50 ml 161-0801
Dithiothreitol 1 g 161-0610
5 g 161-0611
2-mercaptoethanol 25 ml 161-0710
SDS-PAGE Standards, 10,000-100,000 MW 161-0304
SDS-PAGE Standards, 40,000-250,000 MW 161-0303
Pre-stained SDS-PAGE Standards, 15,000-100,000 MW 161-0305
Biotinylated SDS-PAGE Standards, Low Range 161-0306
Biotinylated SDS-PAGE Standards, High Range 161-0311
Biotinylated SDS-PAGE HMW Standards Kit, Avidin HRP 161-0312
Biotinylated SDS-PAGE LMW Standards Kit, Avidin HRP 161-0307
Biotinylated SDS-PAGE HWM Standards Kit, Avidin AP 161-0313
Biotinylated SDS-PAGE LMW Standards Kit, Avidin AP 161-0308
Silver Stain Kit, includes 1 bottle oxidizer concentrate, 1 bottle 161-0443
silver reagent concentrate and 4 bottle developer. Enough to
stain approximately 24 gels.
Coomassie Blue R-250 10 g 161-0400
Bromophenol Blue 10 g 161-0404
Triton X-100 500 ml 161-0407
CHAPS 1 g 161-0460
CHAPSO 1 g 161-0465
Urea 250 g 161-0730
1 kg 161-0731
Delrin is the trademark of E.I. DuPont de Nemours Co., Inc.
Tygon is the trademark of the Norton Company.
18
Page 21

Bio-Rad
Laboratories
ISO 9001
registered www.bio-rad.com
Life Science
Group
Bio-Rad Laboratories Main Office 2000 Alfred Nobel Drive, Hercules, California 94547, Ph. (510) 741-1000, Fx. (510)741-5800
Also in: Australia Ph. 02-9914-2800, Fx. 02-9914-2889 Austria Ph. (1)-877 89 01, Fx. (1) 876 56 29 Belgium Ph. 09-385 55 11, Fx. 09-385 65 54
Canada Ph. (905) 712-2771, Fx. (905) 712-2990 China Ph. (86-10) 2046622, Fx. (86-10) 2051876 Denmark Ph. 39 17 9947, Fx. 39 27 1698
Finland Ph. 90 804 2200, Fx. 90 804 1100 France Ph. (1) 43 90 46 90, Fx. (1) 46 71 24 67 Germany Ph. 089 31884-0, Fx. 089 31884-100
Hong Kong Ph. 7893300, Fx. 7891257 India Ph. 91-11-461-0103, Fx. 91-11-461-0765 Israel Ph. 03 951 4127, Fx. 03 951 4129
Italy Ph. 02-21609.1, Fx. 02-21609.399 Japan Ph. 03-5811-6270, Fx. 03-5811-6272 The Netherlands Ph. 0313 18-540666, Fx. 0313 18-542216
New Zealand Ph. 09-443 3099, Fx. 09-443 3097 Singapore Ph. (65) 272-9877, Fx. (65) 273-4838 Spain Ph. (91) 661 70 85, Fx. (91) 661 96 98
Sweden Ph. 46 (0) 8 627 50 00, Fx. 46 (0) 8 627 54 00 Switzerland Ph. 01-809 55 55, Fx. 01-809 55 00
United Kingdom Ph. 0800 181134, Fx. 01442 259118
M1652000 Rev C
 Loading...
Loading...