Page 1

Service Manual
105953 Rev. C, 2003-02
Page 2

© 1998 Bayer Corporation. All rights reserved.
No part of this manual or the products it describes may be reproduced by
any means or in any form without prior consent in writing from
Bayer Corporation.
The Rapidpoint 400 system is for In Vitro Diagnostic Use.
Rapidpoint, Rapidlink, RapidQC, Quick, and CompleNet are trademarks
of Bayer Corporation.
Intel and Pentium are trademarks of Intel Corporation.
Northbridge is a trademark of Hyundai.
Panellink is a trademark of Silicon Image, Inc.
Sharp is a trademark of Sharp Corporation.
Sound Blaser is a trademark of Creative Technology Ltd.
Velcro is a trademark of Velcro Industries B.V.
Origin: UK
The information in this manual was correct at the time of printing.
However, Bayer Corporation continues to improve products and
reserves the right to change specifications, equipment, and
maintenance procedures at any time without notice.
If the system is used in a manner differently than specified by
Bayer Corporation, the protection provided by the equipment may be
impaired. See warning and hazard statements.
Page 3
Table of Contents
Preface 19
Conventions Used in this Manual 19
Understanding the Symbols 20
Understanding Result Symbols 22
Mechanical Descriptions 23
Overview 25
The Base Model 26
Measurement Cartridge 31
Measurement Cartridge Fluidics 33
Sample Entry 33
Sliding Valve 33
Tubing 35
Reagent System 35
Sensor Module 36
KCl Reservoir 40
Wash/Waste Cartridge 41
Cartridge Interface Module 43
AutomaticQC Cartridge 46
AutomaticQC Cartridge Fluidic Components 47
Rev. C 3
Page 4
AutomaticQC Cartridge Interface 49
Mechanical Components 51
Electronic Components 51
Pumps 52
Display 53
Display Module 53
Liquid Crystal Display 53
Touch Screen 53
Printer 54
Display Board 54
Display Support 54
CO-oximeter Components 55
CO-ox Measurement Components 56
Illumination Housing Assembly 57
Neon Board Assembly 57
Fiber Bundle Assembly 58
Sample Chamber Interface Assembly 58
Co-ox Sample Chamber 60
Polychromator Module 60
Electronic System Overview 63
Overview 67
Electronic System 68
4 Rev. C
Page 5

Contact PC Board 69
Digital Signals 69
Digital Input Signals 69
Digital Output Signals 69
Analog and Other Input Signals 70
Analog and Other Output Signals 72
Circuit Description 74
Amperometric Circuits 74
Potentiometric Circuits 75
Hematocrit/Fluid Detector Circuits 75
Display PC Board 77
Digital Signals 77
Digital Input Signals 77
Digital Output Signals 78
Analog Signals 79
Analog and Other Input Signals 79
Analog and Other Output Signals 80
Circuit Description 80
Microcontroller and Peripheral Circuitry 80
Touch Screen Switches 81
DC-DC Converter Circuit 81
LCD Backlight Inverter Circuit 81
Dualopto PC Board 82
Sensor Circuit 82
Main PC Board 83
Real-Time Processor (RTP) 83
RTP 68332 Processor Circuitry 83
RTP 68332 Address and Data Bus Circuitry 83
RTP EPROM and PSRAM Circuitry 84
Rev. C 5
Page 6

RTP Address Bus Decoder and Memory Controller
Complex Programmable Logic Device Circuitry
RTP Data Bus I/O Latch CPLD Circuitry 85
84
Data Acquisition System (DAS) Front End Circuitry 85
DAS Analog-to-Digital Converter Circuitry 86
DAS Fluid Detector Circuitry 86
RTP/UIP First In First Out (FIFO) Memory
Communications Circuitry
UIP Main Interconnect Board ISA Interface Circuitry 87
UIP Display Module, COM1, COM2/LPT1, and
Utility Circuitry
UIP COM3 Circuitry 87
UIP ISA Address Bus Decoder, Data Bus I/O
Latch CPLD, and Communications Circuitry
Control Micro-stepper Processor, and Sample
and Wash Pump Driver Circuitry
Control Heater DAC, Driver Circuitry, and Valve
Driver Circuitry
86
87
88
88
89
Power System Status Circuitry, Diagnostic LEDs,
and Switches
Control Automatic Quality Control (AQC) Valve
Stepper Circuitry
Power System Protection and Input Circuitry 90
89
90
UIP Main Interconnect Board ISA Interface 92
Digital Input Signals 92
Digital Output Signals 92
Digital Bi-directional Signals 93
UIP COM1 Interface 94
Digital Input Signals 94
Digital Output Signals 94
UIP COM2/LPT1 Interface 95
Digital Input Signals 95
Digital Output Signals 95
Flat Panel Interface 96
Digital Output Signals 96
6 Rev. C
Page 7

UIP Utility Interface 96
Digital Output Signals 96
Display Board Interface 97
Digital Input Signals 97
Digital Output Signals 97
Multiplexer Board Interface 98
Digital Input Signals 98
Digital Output Signals 98
Analog and Other Output Signals 99
Main Interconnect PC Board 100
ISA Connectors 100
Multiplexer PC Board 101
Digital Signals 102
Digital Input Signals 102
Digital Output Signals 102
Analog Signals 103
Analog and Other Input Signals 103
Analog and Other Output Signals 104
Circuit Descriptions 105
Amperometric Isolation Circuits 105
Digital Control Circuit 106
Temperature Circuits 106
Programmable Gain Amplifier Circuitry 106
User Interface Processor PC Board 107
Miscellaneous Functions 108
Keyboard Controller 108
Battery 108
Miscellaneous Function Connector 108
Peripheral Interfaces 109
Serial Ports 109
Rev. C 7
Page 8
Parallel Port 109
Floppy Disk Controller 109
Co-oximeter OMZ Heater PC Board 110
Analog Signals 111
Analog and Other Input Signals 111
Analog and Other Output Signals 111
System Diagrams and Cable Drawings 113
System Diagrams 115
Cable Drawings 130
Calibrations 141
Calibrating the System 143
Calibrating the Touch Screen 145
Emergency Calibration of the Touch Screen 147
Troubleshooting 149
Troubleshooting Problems 155
Button and Parameter Status 155
Symbols on Screens and Reports 162
No Results on Screens and Reports 164
8 Rev. C
Page 9
Unexpected Sodium and Potassium Results 166
Replacing the Measurement Cartridge 167
Replacing the AutomaticQC Cartridge 168
Bar Codes 169
Scanning Technique 172
Bar Code Quality 172
Resetting the Bar Code Scanner 172
Printer 173
Touch Screen 174
Communication 175
Viewing the Events Log 176
System Messages 177
AQC Cartridge Expired 177
AQC Cartridge Not Valid 177
AQC Connector is Open 177
AQC Pending 177
Additional Cal Required 177
Additional Wash Required 178
Analysis is turned off by a remote computer. 178
Bubbles in the Sample. The system cannot
complete analysis. Touch Continue to begin
the sequence to clear the system. Replace
the sample port when prompted. 178
Cal Overdue 179
Cal Not Done 179
Cal Pending 179
COox Chamber Temp Error 179
COox Sample Temp Out of Range 180
D2 Excessive Drift Qualifiers: pH, pO2,
pCO2, Na+, K+, Ca++, Cl-, Glu 181
D2 Excessive Drift: Hct 181
Rev. C 9
Page 10

D2 Excessive Drift: tHb 182
D3 Slope Error: pH, pO2, pCO2, Na+,
K+, Ca++, Cl-, Glu 184
D3 Slope Error: Hct 184
D3 Slope Error: tHb 185
D4 Offset Error: pH, pCO2, Na+, K+,
Ca++, Cl-, Hct 186
D4 Offset Error: Glu, pO2, 186
D21 Processing Error 188
D23 Reagent Error: 1-8 or 10-13 189
D23 Reagent Error: 9 190
D24 AQC Material Error 192
D33 Valve Error: 1 193
D33 Valve Error: 2 194
D35 Electronics Error: 1-13 196
D35 Electronics Error: 14 196
D38 Temp Error: 1 198
D38 Temp Error: 2–13 198
D39 Obstruction 200
D40 Wash Not Detected 201
D41 No AQC Material Detected 202
D60 Communications Error 204
D70 Optics Error: 2 205
D70 Optics Error: 3, 4, 7, 11 205
D70 Optics Error: 9, 12 206
D73 COox Chamber Position Error 209
D75 Lamp Failure 210
D76 COox Electronics Error: 211
D77 Coox Temperature Error 212
Door Error 214
Excessive Bubbles in COox Sample. 214
Incorrect M Cartridge 215
Installation error. Unable to complete
the installation. Try again. 215
10 Rev. C
Page 11

Insufficient Sample Volume. The system
cannot complete analysis. Touch
Continue
to begin the sequence to clear the system.
Replace the sample port when prompted. 216
M Cartridge Expired 216
M Cartridge Not Valid 216
No AQC Cartridge 217
No M Cartridge 217
No Paper in Printer 218
No W Cartridge 219
Out of Reporting Range 219
QC Lot Not Defined 219
QC Material Expired 219
Question Result: 219
Required QC Due 220
Sensors Unavailable For QC 220
SulfHb > 1.5% 220
System Error. Please wait. The system is
trying to recover from the error. 220
System Error. Turn the power switch off.
Wait 10 seconds then turn the switch on. 221
System requires operator attention. 221
Temp Not Ready 222
Temp Out of Range 223
Temp Warning 225
The language cannot be selected because
the current version is not installed. Install
the latest language version to select the language. 226
The system detected an obstruction and
cannot complete analysis. Touch
Continue
to begin the sequence to clear the obstruction.
Replace the sample port when prompted. 227
The system did not detect a sample. Remove
the sample device if present and touch
Continue.
Replace the sample port when prompted 227
This password is about to expire. Renew the
password before access to the system is denied. 228
Rev. C 11
Page 12

This password is expired. Renew the
password to access the system. 228
Uncorrected: 228
Unrecoverable System Error.
Call your service representative for assistance. 228
Unsuccessful Connection. Review the setup
values. Ensure that the cables are connected
and that the network is operating. 229
W Cartridge Expired 229
Diagnostics 230
Printing Diagnostic Reports 231
Testing the Printer 233
Definitions for a Printed Temperature Report 235
Testing the Measurement Valve in Diagnostics 235
Testing the AutomaticQC Cartridge Valve in
Diagnostics
237
Testing the Pumps 238
Using the Measurement Cartridge Simulator 240
Testing the Measurement Channels 240
Testing the Pump Flow 242
Testing the Measurement Cartridge
Valve Alignment
244
Using the AutomaticQC Cartridge Simulator 246
Testing the Valve Alignment 247
Testing the IDEE ROM and Cartridge
Connector Switch.
251
Lamp Calibration Test 252
Wavelength Calibration Test 253
Lamp On/Off Test 254
Sample Chamber Test 255
Shutting Down the System 256
12 Rev. C
Page 13

Recovering from a Power Loss 256
Illustrated Parts Lists 259
Main Assembly Illustrations 261
Main Assembly Parts List 278
Cartridge Interface Assembly Illustrations 281
Cartridge Interface Assembly Parts List 286
Power Module Assembly Illustrations 287
Power Module Assembly Parts List 293
CO-ox Module Components Illustrations 294
Replacing Components 299
Replacing the Sample Port 303
Replacing the Fuses 306
Replacing the CO-ox Lamp 308
Replacing the Cartridge Interface Assembly 309
Rev. C 13
Page 14

Replacing the Connector Block Assembly 313
Removing the AutomaticQC Cartridge 318
Replacing the AutomaticQC
Frame Assembly 320
Replacing the Diskette Drive Assembly 323
Replacing the Display/Printer Assembly 326
Replacing the Paper Cover 330
Replacing the Door Lock Module 333
Replacing the Hard Drive 334
Replacing the Power Supply Module 337
Replacing the Fan 339
Replacing the Power Entry Module
With Switch 340
Replacing the Rear Interconnect Board 343
Replacing the Pump Roller Cages 345
14 Rev. C
Page 15

Replacing the Pump Motors 349
Replacing the Wash/Waste Switch 354
Replacing the Fiber Bundle Assembly 356
Replacing the Neon Board Assembly 358
Replacing the Illumination
Housing Assembly 360
Replacing the Polychromator Module 362
Replacing the Sample Chamber
Interface Assembly 365
Replacing the Main Board 367
Replacing the UIP Board 370
Replacing the Main Interconnect Board 372
Removing the Rear Cover and Power
Module Assembly 375
Installing the Rear Cover, Cartridge Interface
Assembly, and Power Module Assembly 378
Rev. C 15
Page 16
Spare Parts 381
Spare Parts Listing 383
Biohazards and Warnings 387
Protecting Yourself from Biohazards 387
Protecting Yourself from Electrical Shock
Hazards 389
References 390
Installing and Relocating the
Rapidpoint 400 Series
Installing the System 391
Installing the AutomaticQC Cartridge 394
Relocating the System 396
Shipping or Storing the System 397
System 391
Connecting to a Computer System 401
Connecting to a Rapidlink System 401
Using a CompleNet Network Connection 401
Entering IP Addresses 402
Using DHCP 403
Using a Serial (RS-232) Connection 405
Connecting to a Laboratory Information System 407
16 Rev. C
Page 17
Connecting the Bar Code Scanner 409
Swapping Out a Rapidpoint 400 Series
System 411
Removing the Current Rapidpoint 400 Series
System
Installing the New Rapidpoint 400 Series System 414
Restore Setup and Correct the Date and Time 415
Rapidlink Setup 415
Setting Up RapidQC Complete in the
Rapidlink Database
Setting Up RapidQC Hct in the Rapidlink System 417
Finishing the Rapidpoint Setup 417
411
416
Rapidpoint 400 Series Cartridge Usage 419
Overview 419
Verifying Modes of Operation 420
Setting the Rapidpoint 400 Series System to
the FOR DEMONSTRATION ONLY Mode
What Can Go Wrong? 423
Setting the Rapidpoint 400 Series System to
the USER Mode
What Can Go Wrong? 425
Using Simulator Cartridges in the USER Mode 425
421
423
About Rapidpoint 400 Series Calibrations 427
Rev. C 17
Page 18
Overview 427
Calibration Types 427
Retrospective Calibration (Retrocal) 428
Calibration Schedule 429
Interruptible and Uninterruptible Calibrations 429
Cartridge Initialization (Init) 430
Initialization Calibration Timeline 431
Remote Access Setup to the
Rapidlink System 433
Installing and Configuring Remote Access
Setup (RAS) 434
Configuring RAS To Start Automatically 435
Adding Rapidpoint to Users and Groups 435
Adding Upload and Download Directories 436
Index 439
18 Rev. C
Page 19
Preface
This manual is to be used with the Bayer Diagnostics Rapidpoint® 400
series system. It provides the information and procedures necessary to
service the Rapidpoint 400 series system. Related documents include the
following:
• Rapidpoint 400 Series Operator’s Manual
• Rapidpoint 400 Series Reference Guide
• Rapidpoint 400 Series Interface Specification Manual
This manual is designed to meet the needs of Bayer Diagnostics Service
Representatives and biomedical engineers who perform installation,
replace parts, and troubleshoot the Rapidpoint 400 series system.
Conventions Used in this Manual
This manual uses the following text and symbol conventions.
Convention Description
Bold Bold type indicates a button on the screen. For example, if the word
“setup” appears as Setup, it refers to the button labeled Setup.
Buttons that you use frequently are represented on the screen with
a symbol instead of text. In this manual, these button names
appear in bold with the word button after them. For example, “the
Continue button” refers to the button in the lower right corner of the
screen that you touch to advance to the next screen.
You can refer to the Rapidpoint 400 Series Reference Guide on the
system or to Buttons in Section 5 of the Rapidpoint 400 Series
Operator’s Manual to identify the buttons that are represented by
symbols.
italic
Italic type refers to the title of a document or a section title in this
manual. For example, Mechanical Descriptions refers to the first
section of this manual.
Information appropriate for Rapidpoint 405 systems.
Biohazard statements alert you to potentially biohazardous
conditions.
(Continued)
Rev. C Preface 19
Page 20
Convention Description
Warning statements provide information about a condition that
may cause personal injury.
Caution statements provide information about conditions that may
cause product damage or loss of data.
NOTE
Note statements alert you to important information that requires
your attention.
Understanding the Symbols
This section describes the symbols that may appear on the exterior of the
system. The symbols provide you with the location of certain components
and with warnings for proper operation.
Symbol Description
This symbol reminds you to push the measurement cartridge
firmly to lock it in place.
This symbol identifies the area on the wash/waste cartridge
where you push to install the cartridge correctly.
This symbol identifies the ampule breaker where you insert
ampules to break off the top.
This symbol indicates where you insert the sample device
(syringe, capillary, or ampule) to perform analysis.
This symbol cautions you about the risk of exposure to
biohazards.
This symbol indicates a hazard or danger is associated with the
product.
This symbol cautions you about the risk of exposure to
potential electrical hazards.
This symbol indicates that the input electricity is alternating
current.
This symbol alerts you to important information about the
fuses.
(Continued)
20 Rev. C
Page 21
Symbol Description
This symbol identifies that the system is type B equipment,
which provides a particular degree of protection against
electric shock.
I
Class 1 This symbol indicates that the system is class 1 type
This symbol indicates that the main power supply is on.
This symbol indicates that the main power supply is off.
equipment, which has basic insulation and additional safety
grounding precautions. Reference Underwriters Laboratories
Document for Medical Electrical Equipment, Part 1: General
Requirements for Safety, UL 2601-1.
This symbol indicates that the system is approved by UL as
meeting U.S. requirements for safety.
This symbol indicates that the system meets the requirements
of the European Union.
This symbol indicates that the system is approved by CSA as
meeting the U.S. and Canadian requirements for safety.
This symbol indicates the type of measurement cartridge that
can be installed on the system.
This symbol indicates the area to write the date the cartridge is
installed on the system, if required.
This symbol cautions you not to spray this area with cleaning
solutions or other fluids that may damage sensitive parts of the
system.
In vitro diagnostic device
Consult instructions for use
Temperature limitation (2°C - 8°C)
Contains sufficient for (n) tests (250 tests)
Catalog number
(Continued)
Rev. C Preface 21
Page 22

Symbol Description
Serial number
Batch code
Manufactured by
Authorized Representative
Understanding Result Symbols
The following symbols identify results that are out of range or that need
your attention. These symbols and results appear in red on the screen.
They also appear on the report. The ranges can appear on the printed
report, if this option is selected in Setup.
This symbol . . . Indicates that . . .
the result is above range.
the result is below range.
– – – – –
– – – – – the result is below the reporting range.
? the system has an atypical response when measuring this
u the hematocrit (Hct) result was not corrected for sodium (Na+)
the result is above the reporting range.
parameter. The system does not report results for other
parameters that use the affected parameter. For example, if ?
appears with the chloride (Cl-) result, the system does not
report a result for anion gap (AnGap).
When this symbol appears with the hematocrit (Hct) result, it
may indicate that the result was not corrected for sodium
(Na+) because sodium failed Required QC analysis.
Analyze the sample again, if possible.
because the sodium sensor is out of calibration or because
the sodium measurement is beyond the reporting range.
22 Rev. C
Page 23
Mechanical Descriptions
Overview 25
The Base Model 26
Measurement Cartridge 31
Measurement Cartridge Fluidics 33
Sample Entry 33
Sliding Valve 33
Tubing 35
Reagent System 35
Sensor Module 36
KCl Reservoir 40
Wash/Waste Cartridge 41
Cartridge Interface Module 43
AutomaticQC Cartridge 46
AutomaticQC Cartridge Fluidic Components 47
AutomaticQC Cartridge Interface 49
Mechanical Components 51
Electronic Components 51
Pumps 52
Display 53
Display Module 53
Rev. C Mechanical Descriptions 23
Page 24

Liquid Crystal Display 53
Touch Screen 53
Printer 54
Display Board 54
Display Support 54
CO-oximeter Components 55
CO-ox Measurement Components 56
Illumination Housing Assembly 57
Neon Board Assembly 57
Fiber Bundle Assembly 58
Sample Chamber Interface Assembly 58
Co-ox Sample Chamber 60
Polychromator Module 60
24 Rev. C
Page 25

Overview
This section describes the mechanical components of the Rapidpoint 400
series systems. Each component contains individual modules that perform
a series of related functions. The Rapidpoint 400 series systems consists
of the following components:
• Measurement cartridge
• Wash/waste cartridge
• AutomaticQC cartridge
•Pumps
• Display
• CO-oximeter
Rev. C Mechanical Descriptions 25
Page 26
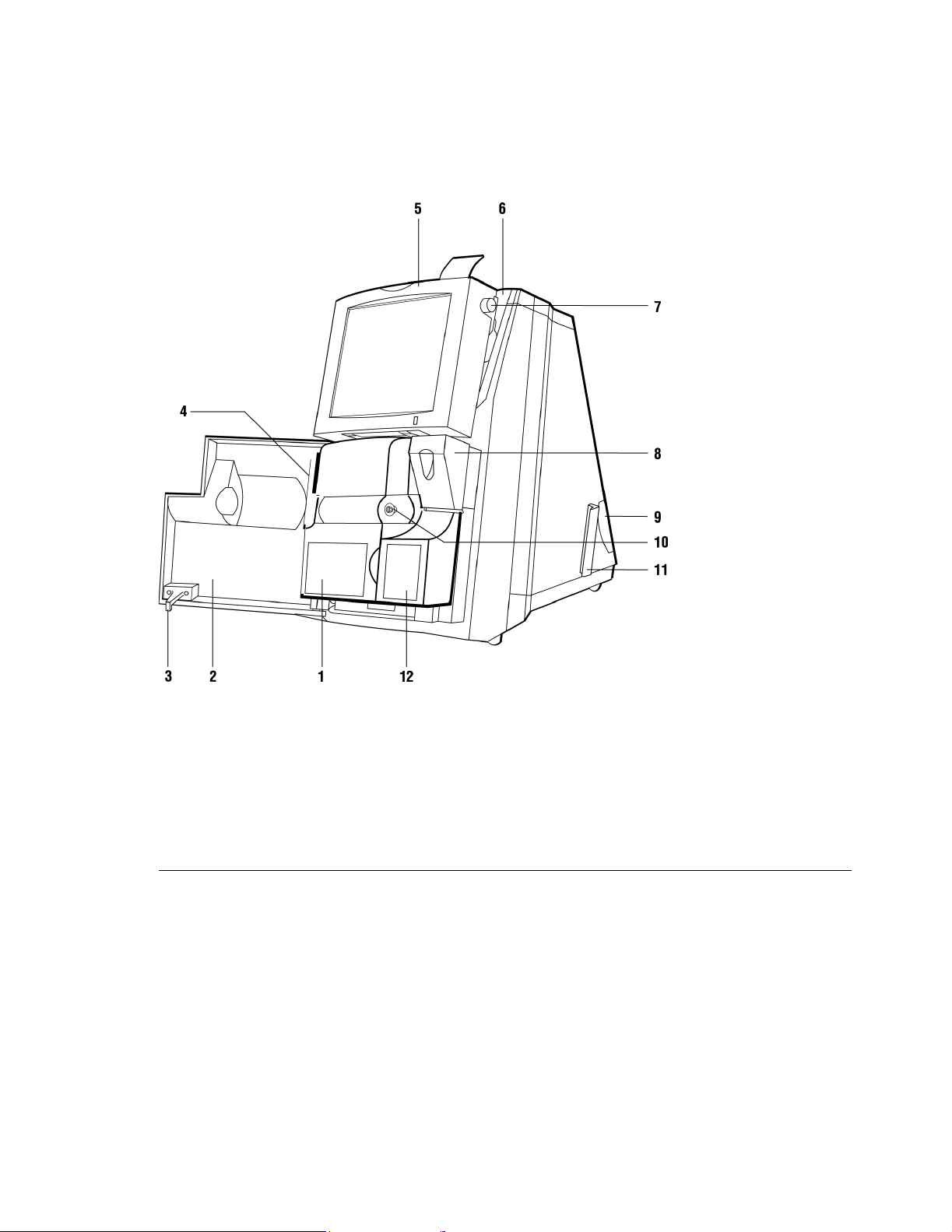
The Base Model
The base model refers to the 400 system model.
Rapidpoint 400 Series System (door open)
1 Measurement cartridge 7 Paper advance knob
2 Door 8 Ampule breaker
3 Door latch 9 Air filter carrier
4 Cartridge handle 10 Sample port
5 Display 11 Support bracket for AutomaticQC cartridge
6 Printer 12 Wash/waste cartridge
26 Rev. C
Page 27
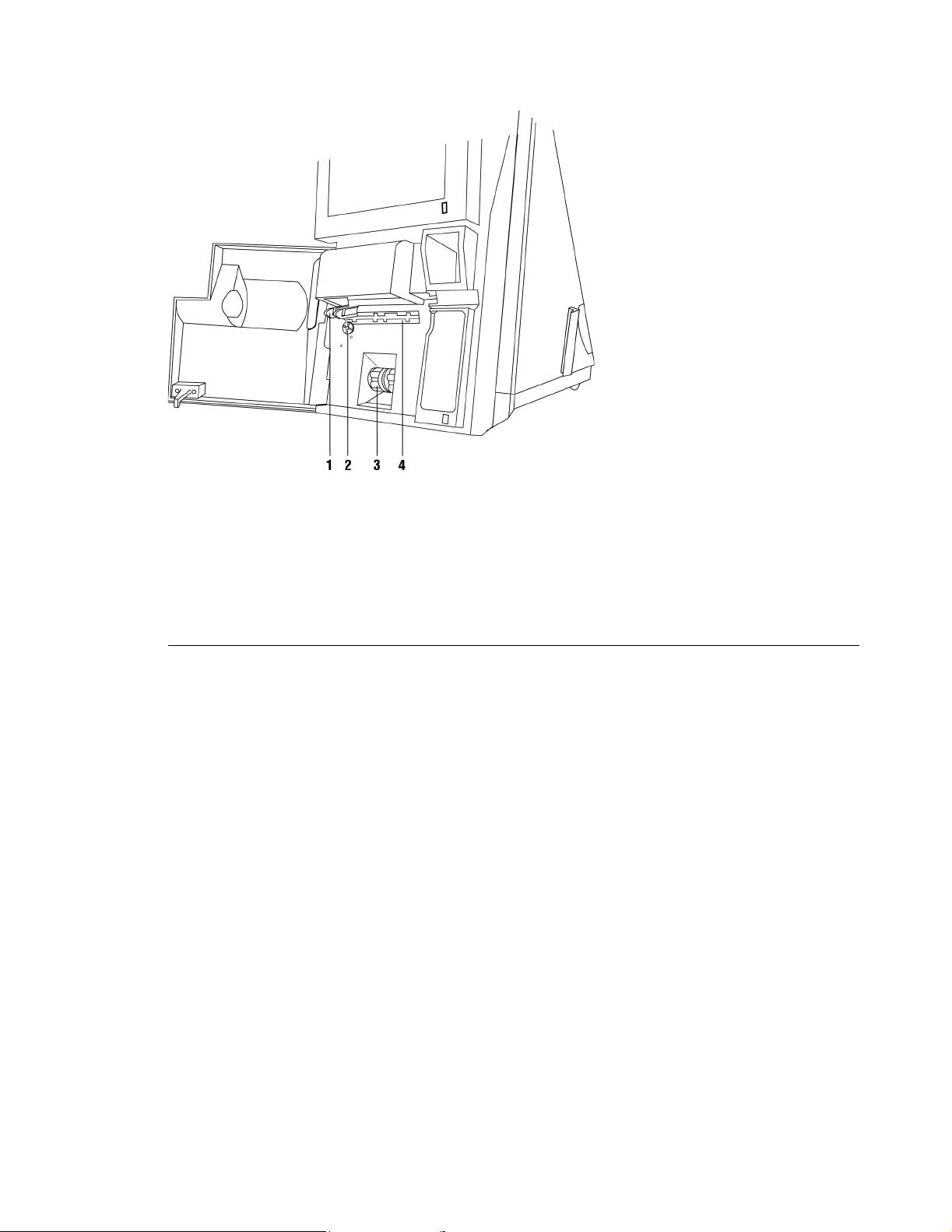
Rapidpoint 405 System (door open)
1 Optic Head Assembly: Delivers and collects light from the CO-ox sample
chamber.
2 Drive Wheel: Opens and closes the CO-ox sample chamber.
3Pumps: Move samples and reagents through the measurement and wash/waste
cartridges.
4 Valve Actuator: Moves the valve that controls flow of the sample and reagents.
Rev. C Mechanical Descriptions 27
Page 28
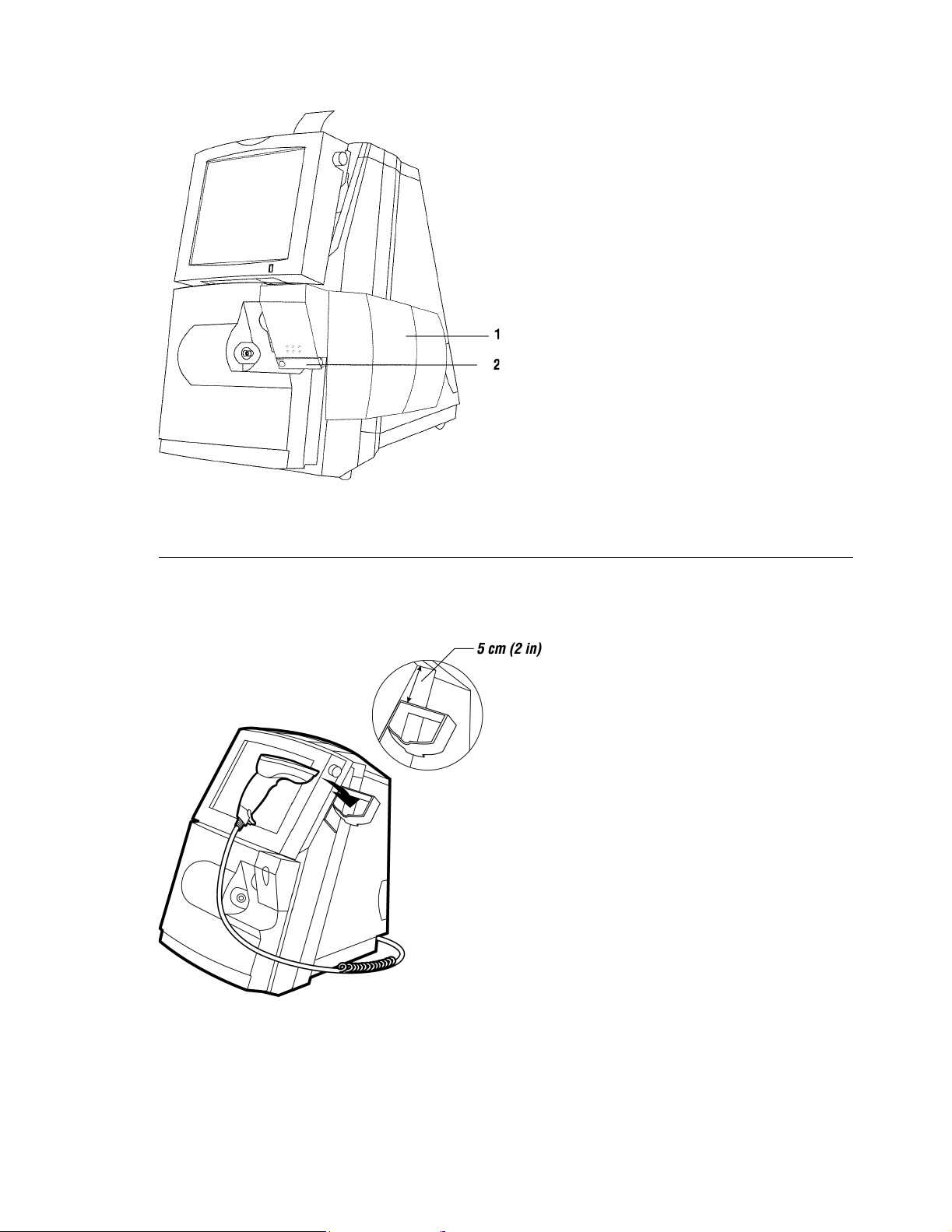
Rapidpoint 400 Series System with AutomaticQC Cartridge Installed
1 AutomaticQC cartridge
2 Cartridge connector
Rapidpoint 400 Series System with Bar Code Scanner Holder
28 Rev. C
Page 29
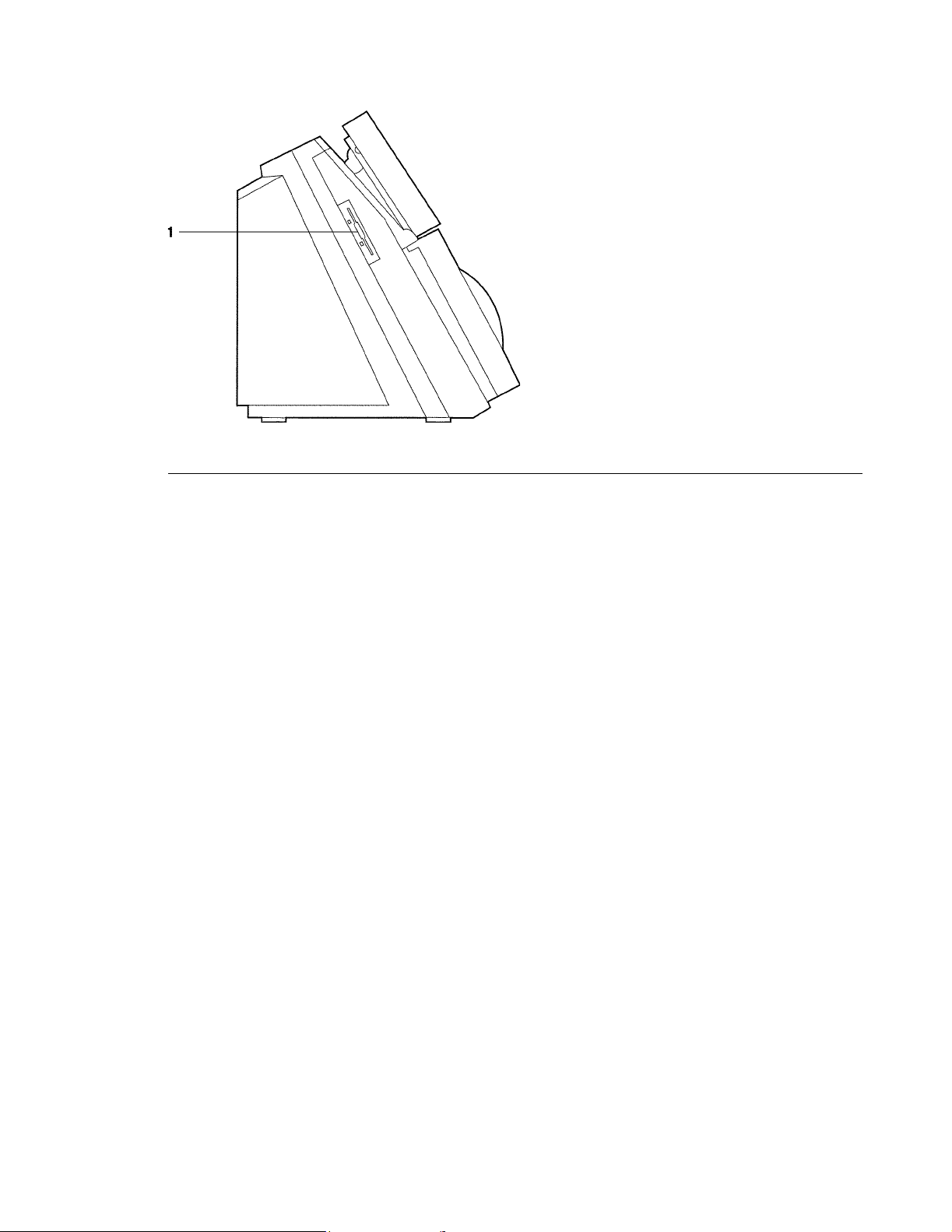
Rapidpoint 400 Series System (left side)
1 Diskette drive
Rev. C Mechanical Descriptions 29
Page 30
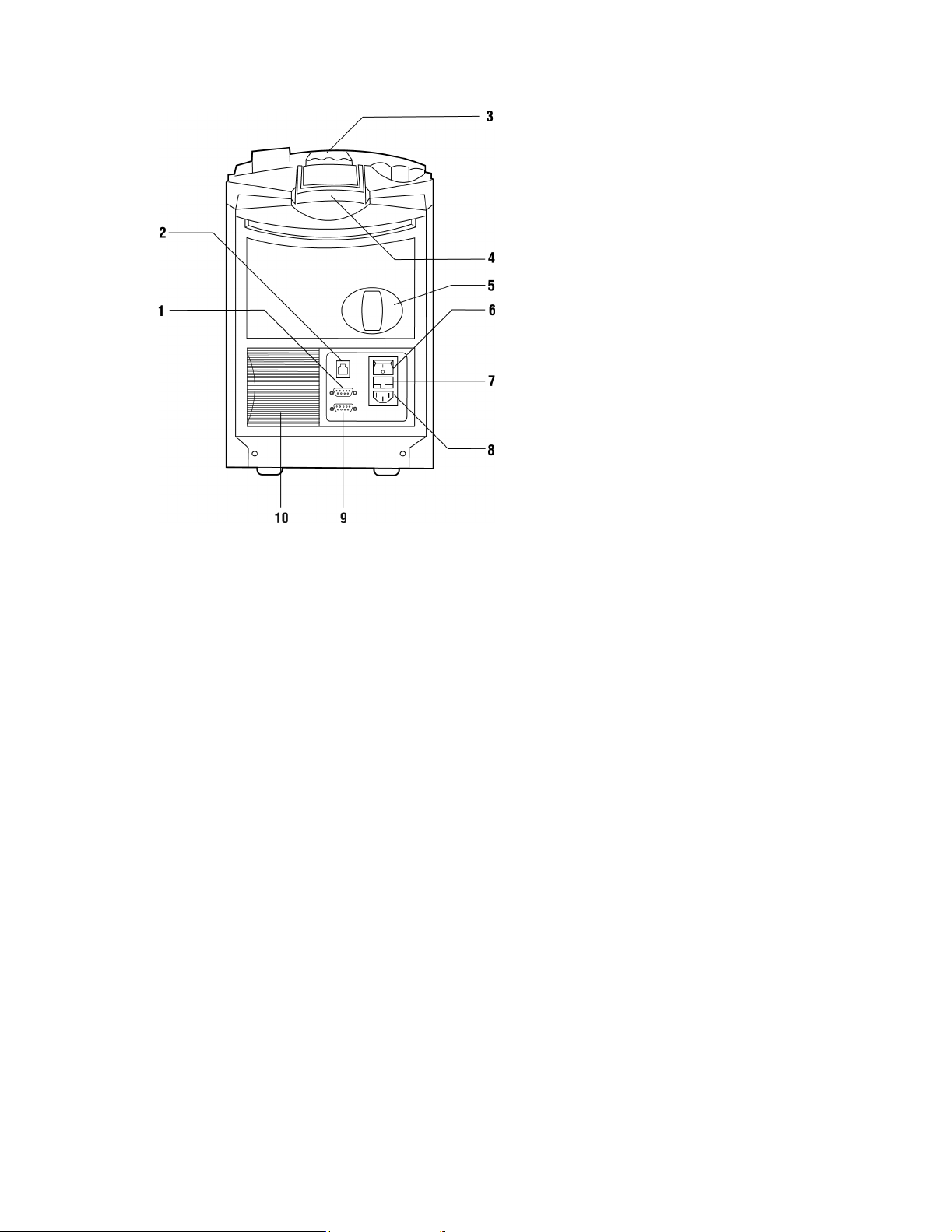
Rapidpoint 400 Series System (rear view)
1 Serial Port: Allows serial connection to a Rapidlink data management system or
an LIS.
2 Network Port: Allows network connection to a Rapidlink data management system.
3 Screen Latch: Allows you to adjust the viewing angle of the screen.
4 Handle: Helps you to move the system.
5 Co-ox Lamp: Appears only in Rapidpoint 405 systems.
6 Power Switch
7 Fuse Compartment
8 Power Input
9 Bar Code Port: Allows you to connect the optional bar code scanner to the system.
The scanner allows you to identify patient and QC samples by scanning their bar
codes.
10 Air Filter
30 Rev. C
Page 31

Measurement Cartridge
The self-contained measurement cartridge is non-serviceable. It consists
of the fluidic components, reagents, and sensors that measure patient and
QC samples.
Measurement Cartridge
1 Internal fluidic components and reagents
2 Sensor module
3 Sample port
4 Input to waste bag
5 Primary alignment pin
6 Output from wash bag
7 Secondary alignment pin
In addition, the measurement cartridge contains the mechanical
components that interface with the following components:
• the reagent system with the peristaltic pumps
• the sensor module with the electronic components
• the wash/waste cartridge
• the thermal control
The external area of the cartridge consists of the platen area which
contains the pump tubing. When the measurement cartridge is installed
and locked into place, the roller cage of the base unit is positioned against
the pump tubing to move the reagents, the samples, and the wash fluids
through the system.
Rev. C Mechanical Descriptions 31
Page 32

The measurement cartridge is protected by a door that locks in place. The
door provides an electrostatic shield for the measurement system. A
mechanical microswitch senses when the door is open or closed.
5 Pump tubing
6 Sliding valve
7 CO-ox Sample chamber
8 Drive wheel interface
Measurement cartridges can analyze a total of 750 patient and/or
QC samples, and measure blood gases, electrolytes, glucose, total
hemoglobin, and hemoglobin derivatives in arterial, venous and capillary
whole blood samples. Each cartridge is stable for 28 days after installation
on the system when the cartridge is installed by the date on the label. The
Install-by date indicates the last date on which the cartridge can be
installed and still have 28 days of use before expiration.
System Parameters Determined and Reported
400 pH, pCO2, pO2, Na+, K+, Ca++, Cl-, glucose, Hct
405 pH, pCO2, pO2, Na+, K+, Ca++, Cl-, glucose, tHb, FO2Hb, FHHb,
FMetHb, FCOHb
If a cartridge is installed after the Install-by date, it is stable for the number
of days remaining before expiration, that is, 28 days minus the number of
days past the Install-by date. View the Status screen to see the number of
days remaining and the expiration date. You cannot install a cartridge if
only 1 day of use is remaining. The system prompts you when you need to
replace the cartridge.
Store cartridges refrigerated at 2 to 8°C. Cartridges can also be stored at
room temperature, not to exceed 25°C, for up to 14 days.
32 Rev. C
Page 33

Measurement Cartridge Fluidics
The fluidic components of the measurement cartridge are part of the
fluidics system, which involves the measurement cartridge, the wash/
waste cartridge, and the pumps. The measurement cartridge fluidics
consist of the following components:
• sample entry
• sliding valve
• tubing
• reagent system
• sensor module
• KCl reservoir
Sample Entry
The sample entry performs the following functions:
• provides an insertion point for syringes and capillaries
• holds syringes, capillaries, or aspiration adapters during sample
aspiration
• connects to the wash and waste ports for automatic cleaning
The sample entry components consist of a luer and capillary seal. The
capillary seal has two passages that provide pathways for the wash and
waste fluids. The sample port attaches to the sliding valve assembly with
two snaps. The capillary seal provides a seal to the sample probe during
aspiration from a capillary.
Sliding Valve
The sliding valve performs the following functions:
• aspirates the wash fluid
• aspirates QC samples
• selects and aspirates reagents
• automatically aspirates samples from capillaries and syringes
• provides a fluid path from the reagent bag to the sensor module
Rev. C Mechanical Descriptions 33
Page 34

The sliding valve consists of a luer, a luer mount, a probe, a probe clamp,
a luer mount cover, a luer mount extension, and a valve seal. The luer and
capillary seal are replaceable. Valve movement is controlled by a valve
actuator motor and a position detector system. The position detector
system accurately positions the valve using two position sensors, which
are part of the cartridge interface.
The sliding valve has nine positions, each of which allows fluid, air, or
sample to be pumped through a thin stainless-steel probe in the center of
the valve. The probe remains stationary while the valve moves back and
forth around the probe to change the hydraulic path of the system.
The following table shows the valve positions and their functions:
Position Name Function
SYRINGE_POS moves the valve to place the probe tip into the syringe
creating the fluid path for sample aspiration
Vent AQC controls the aspiration of an external QC sample, using the
front hole of the probe
Zero Reagent Load aspirates the Zero Calibration Reagent into the sensor
module, using the rear hole in the probe
Hematocrit Reagent
Load
200 Reagent Load aspirates the 200 Calibration Reagent into the sensor
Wash Reagent Load delivers steady wash to the sensor module, using the rear
Luer Wash empties the luer area from a port in the bottom of the sample
Capillary Aspiration aspirates samples from capillary tubes, using the front hole
Vent provides an opening to advance syringe samples while the
Syringe Aspiration aspirates syringe or QC samples
aspirates the Hematocrit Calibration Reagent into the sensor
module, using the rear hole in the probe
module, using the rear hole in the probe
hole in the probe
entry, using the front hole of the probe and aspirates capillary
samples while the device is in place
in the probe
device is in place
34 Rev. C
Page 35

Tubing
All tubing is enclosed within the cartridge, except for the pump tubing in the
platen area at the rear of the cartridge. The platen area has four tubes. The
two tubes on the right serve as the flow paths for foam wash and connect
the measurement cartridge to the wash/waste cartridge. The two tubes on
the left serve as the flow paths for KCl, the sample, and reagents.
The sample tube pulls the sample or reagent through the sensors and
pushes it out into the wash/waste cartridge.
Reagent System
The reagent system delivers reagents and gases to the measurement
cartridge. The measurement cartridge contains enough reagents to
operate the system for 28 days.
The reagents are contained in three foil bags. Each bag contains a fitting.
When the measurement cartridge is installed and locked into place, pierce
pins, which are part of the interface assembly, puncture each bag creating
a fluid path. The pierce pins create a seal between the pierce probes and
fitting on each bag.
The following table describes the measurement cartridge reagents:
Reagent Volume Ingredients Cartridge
Zero 75 mL gases (oxygen, carbon dioxide, nitrogen),
salts (alkali halides), organic buffers,
catalyst, and surfactant
RCx 60 mL gases (oxygen, carbon dioxide, nitrogen),
salts (alkali halides), organic buffers,
surfactant, and preservative
200 230 mL gases (oxygen, carbon dioxide, nitrogen),
salts (alkali halides), organic buffers,
glucose, surfactant, and preservative
Reference 16 mL potassium chloride, silver chloride, and
surfactant
Wash 250 mL gases (oxygen, carbon dioxide, nitrogen),
salts (alkali halides), surfactant, and
preservative
measurement
measurement
measurement
measurement
wash/waste
Rev. C Mechanical Descriptions 35
Page 36

The following table lists the calibration points for each analyte in the
reagents:
Analyte High Calibration Point Low Calibration Point
pH 7.4 6.8
pCO
2
pO
2
+
Na
+
K
++
Ca
-
Cl
Glu 180 mg/dL 0 mg/dL
Hct 45% 0%
tHb 15 g/dL 0
70 mmHg 35 mmHg
154 mmHg 0 mmHg
159 mmol/L 116 mmol/L
8.0 mmol/L 4.0 mmol/L
1.25 mmol/L 0.62 mmol/L
98 mmol/L 69 mmol/L
Sensor Module
The sensor module consists of the following sensors:
• reference sensor
• analyte sensors
• hematocrit sensor
• CO-ox sample chamber
The sample arrives at the sensor module after traveling from a stainless
steel probe, through the sample tubing, and to the preheater. The sample
then passes over the sensors for analysis.
The sensor module provides measurements for pH, pO
potassium, ionized calcium, chloride, glucose, Hct,
total hemoglobin, and
, pCO2, sodium,
2
hemoglobin derivatives in arterial, venous and capillary whole blood
samples.
36 Rev. C
Page 37

The parameters that the Rapidpoint 400 series systems report depend
on the parameters selected in Setup. Refer to Appendix C in the
Rapidpoint 400 Series Operator’s Manual for a detailed description of the
parameters that are available.:
Category Parameter Description
blood gases partial pressure of carbon
dioxide; pCO
2
partial pressure of oxygen;
pO
2
+
H
partial pressure of carbon dioxide
partial pressure of oxygen
hydrogen ion concentration
pH negative log of the hydrogen ion
concentration
electrolytes sodium; Na
potassium; K
ionized calcium; Ca
+
+
++
sodium ion concentration
potassium ion concentration
concentration of ionized calcium, the
physiologically active form of calcium
in the blood
chloride; Cl
-
chloride ion concentration
metabolites glucose; Glu glucose concentration
oxygenation hematocrit; Hct
*
the volume occupied by red blood cells
in a given volume of blood; value is
determined by conductimetric method
metabolic
parameters
temperaturecorrected
parameters
bicarbonate ion;
HCO3act, HCO3-std
the bicarbonate ion concentration and
the bicarbonate ion concentration
normalized to a pCO2 of 40 mmHg
base excess; BE(B),
BE(ecf)
an approximation of the amount of
acid or base needed to titrate a liter of
blood to a pH of 7.4
total carbon dioxide; ctCO2the sum of the dissolved carbon
dioxide and the plasma bicarbonate
temperature-corrected
pH, pCO2, and pO2; pH(T),
blood gas values corrected for entered
patient temperature
pCO2(T), and pO2(T)
respiratory index; RI(T) the ratio of the alveolar-arterial blood
oxygen-pressure difference to arterial
pO2 when both values are corrected
for patient temperature
Rev. C Mechanical Descriptions 37
Page 38

Category Parameter Description
oxygenation
parameters
alveolar-arterial oxygen
tension difference;
pO2(A-a)(T)
arterial-alveolar oxygen
tension ratio; pO2(a/A)(T)
physiologic shunt; Qsp/
†
Qt(T)
estimated physiologic
shunt; Qsp/Qt(T)est
†
estimated oxygen
saturation; O2SAT(est)
hematocrit, Hct
†
estimated total
hemoglobin; ctHb(est)
pO2/FIO
2
an index of gas exchange within the
lungs
an index of oxygenation
percent of blood that does not
participate in external respiration
estimated value for the percent of
blood that does not participate in
external respiration
the ratio of the volume of oxygen
carried to the maximum volume of
oxygen that the hemoglobin can carry
an calculated value determined from
the total hemoglobin value
an estimation of the hemoglobin
contained in the sample
the ratio of arterial pO2 to the fraction of
inspired oxygen
a-v study
parameters
calcium adjusted for pH
7.4; Ca++ (7.4)
the ionized calcium concentration of
blood normalized to pH 7.4
anion gap; AnGap an approximation of the difference
between unmeasured cations and
unmeasured anions in the sample
arterial-venous oxygen
content difference;
†
(a-v)
ctO
2
arterial oxygen content;
ctO2(a)
†
mixed venous oxygen
content; ctO2(v)
†
oxygen consumption rate;
†
VO
2
oxygen delivery; DO
†
2
the difference between arterial and
venous blood oxygen content
the oxygen content of arterial blood
the oxygen content of mixed venous
(pulmonary artery) blood
the volume of oxygen consumed by
the body per minute
the volume of oxygen that is
transported to the tissues per minute
a-v extraction index;
ctO2([a-v]/a)
†
the arterial-venous oxygen content
difference as a percent of arterial
oxygen content
38 Rev. C
Page 39

Category Parameter Description
CO-oximetry
Parameters
total hemoglobin; tHb
oxyhemoglobin; FO2Hb
†
the total of all measured hemoglobin
fractions
†
hemoglobin that is reversibly bound to
oxygen
carboxyhemoglobin;
FCOHb
†
hemoglobin covalently bound to
carbon monoxide
methemoglobin; FMetHb†hemoglobin whose iron is oxidized to
its ferric state and is unable to bind
oxygen
hemoglobin oxygen
saturation; sO
†
2
the ratio of the amount of hemoglobin
bound to oxygen to the total amount of
hemoglobin able to bind oxygen
oxygen binding capacity;
†
BO
2
the maximum amount of oxygen that
can be carried by the hemoglobin in a
given quantity of blood
oxygen tension at 50%
saturation; p50
†
the partial pressure of oxygenated
which the hemoglobin oxygen
saturation is 50%
* These parameters are available only on Rapidpoint 400 systems.
†
These parameters are available only on Rapidpoint 405 systems.
In addition to sensor chips, the sensor module contains:
• fluid detector chips
• a preheater chip
• an IDEE ROM chip
The Rapidpoint 400 series systems use two Hct sensors as fluid detectors.
Fluid detector 1 (FD1) is located in front of the first sensor. Fluid detector 2
(FD2) is located after the last sensor.
The fluid detector chips detect the presence and the continuity of fluids in
the system, and measure sample fluid using alternating current (AC)
conductivity. The system uses information from the detectors to monitor
and control the movement and the positioning of fluids during the system
sequences.
Rev. C Mechanical Descriptions 39
Page 40

The preheater chip is located in front of the measurement block. The
sample passes through the preheater during aspiration. The preheater
temperature range is 37.0 to 37.6°C, with a target preheater temperature
of 37.3°C.
The IDEE ROM chip informs the system that the correct measurement
cartridge is installed and helps in diagnosing cartridge problems. The
IDEE Rom Identifies the following:
• analyzer software version
• date installed on the analyzer
• date removed fro analyzer (if removed normally)
• analyzer serial number
KCl Reservoir
The KCl reservoir continuously refreshes the KCl concentration on the
reference sensor. Each measurement cartridge includes a KCl vial
containing 16 mL of liquid KCl.
The KCl reservoir is clamped closed to prevent it from leaking during
shipment. The tubing on the KCl vial is automatically unclamped allowing
the KCl to flow when the measurement cartridge is installed.
40 Rev. C
Page 41

Wash/Waste Cartridge
The wash/waste cartridge is self contained and non-serviceable. It is
protected by a door that locks in place. The door provides an electrostatic
shield for the measurement system. A mechanical microswitch senses
when the door is open or closed.
The cartridge contains the wash fluid, which cleans the sample path after
analysis and calibration. The cartridge also contains the waste bag, which
stores liquid waste. The wash/waste cartridge consists of the following
components:
• seal septums
• pierce probes
• wash bag
• waste bag
Wash/Waste Cartridge
1 Connectors to measurement cartridge
2 Septum seal for waste bag
3 Primary alignment pin locator hole
4 Septum seal for wash bag
5 Secondary alignment pin locator hole
Rev. C Mechanical Descriptions 41
Page 42

Two seal septums serve as the coupling between the hydraulics of the
wash/waste and the measurement cartridges. When the wash/waste
cartridge is installed, it connects to two wash/waste cartridge fittings that
are located on the outside edge of the measurement cartridge cover.
These fittings provide the interface between the measurement cartridge
and the wash/waste cartridge. The top fitting is the path into the waste bag.
The bottom fitting is the path out of the wash bag. In addition to the wash/
waste fittings on the measurement cartridge, there is a primary and a
secondary alignment pin. These pins align the wash/waste cartridge
fittings with the septums.
When the wash/waste cartridge is placed in the system, the fittings on the
measurement cartridge are inserted into the wash/waste cartridge, and the
pierce probes penetrate the wash and waste bags. In the case of the wash
bag, the pierce probe penetrates the bag and creates a seal inside the
fitting. However, the waste bag is already open and the pierce probe seals
inside the fitting in the bag. Two bag clamps, one at the top of each bag,
hold the bags in position. Two hangers, one at the top of the cartridge and
one at the bottom of the cartridge, hold the waste bag in place as it fills.
Only the replaceable cartridges of the Rapidpoint 400 series systems
come in contact with biohazardous waste fluid. Biohazardous waste fluid
never contacts other components of the system. The waste fluid is
completely enclosed in the waste bag, which accommodates 500 mL of
waste. The waste collection system has sufficient reserve capacity to
prevent overfilling, provided the cartridges are replaced when the operator
is prompted to do so by the system.
The wash/waste cartridge contains 250 mL of wash reagent. The wash
reagent washes the valve and the sample flow path. It consists of gases
(oxygen, carbon dioxide, nitrogen), salts (alkali halides), a surfactant, and
a preservative.
Each wash/waste cartridge is stable for 10 days after installation on the
system and contains enough reagent for 300 patient and QC sample
analyses. The system prompts the user when the cartridge needs to be
replaced.
The wash/waste cartridges may be stored refrigerated or at room
temperature (2 to 25°C).
42 Rev. C
Page 43

Cartridge Interface Module
The cartridge interface module attaches the measurement cartridge to the
system. It consists of the following components:
• interface frame
• location pins
• bag pierce pins
• cable clamps
• cartridge handle
• valve actuator
• optical detector board with bidirectional stepper motor and position
detectors
• pump and motor assembly (refer to Pumps on page 52 for more
detailed information)
• thermal cover
• camshaft
• connector block
The interface frame is secured to the front of the system and holds all
devices and parts necessary to interface the system with the measurement
cartridge.
The location pins position the measurement cartridge on the cartridge
interface frame. Three bag pierce pins are below the location pins. The bag
pierce pins force the pierce probes to puncture the reagent bags creating
a fluid path. The pierce probes engage with the fittings on the reagent bags
to make a seal.
Four cable clamps secure the cables that extend from the connector block,
the stepper motor, and the optical detector board to the main board.
The cartridge handle lifts to unlock and release the measurement cartridge
from the interface module, or pulls down to lock the measurement cartridge
in place on the cartridge interface module.
Rev. C Mechanical Descriptions 43
Page 44

The valve actuator moves the sliding valve. The luer mount extension on
the sliding valve connects with the valve actuator when the measurement
cartridge locks on the cartridge interface module. During cartridge
initialization, the actuator moves back and forth until it automatically
engages the luer mount extension. A stepper motor, which has nine
positions and a left and a right limit stop, controls the actuator.
The bidirectional valve actuator motor and the position detectors, which
are part of the optical detector board, move and position the valve. Two
sensors in conjunction with the position detectors determine valve
position.
The thermal cover attaches to the cartridge interface module and protects
and guides the connector block. The cam shaft, used in conjunction with
the cartridge handle, raises and lowers the connector block.
The connector block provides the electrical and the thermal connections to
the sensors, and connections to the IDEE ROM. The connector block
consists of the following components:
• preheater
• main heater
• contact board
• multiplexer board
• contact system protector
• alignment and locking pins
The preheater contacts the preheater chip in the sensor module to heat
samples to 37°C. The preheater is thermally protected, so that the surface
temperature never exceeds 55°C.
The main heater contacts the heater plate of the sensor module to maintain
the temperature of the sensors and fluids at 37°C. The main heater also
controls the temperature of the preamplifiers. The main heater is thermally
protected, so that the surface temperature never exceeds 55°C.
44 Rev. C
Page 45

The contact board provides the junction between the connector block and
the sensor module. It processes signals from the sensors and provides
biased, filtered, and amplified results to the system. The sample thermistor
is located on the contact board. The sample thermistor senses the
temperature on the back of the sensor chips. If the thermistor detects that
the temperature of the chips is less than 37°C, it signals the main heater to
increase the temperature of the chips to 37°C.
The multiplexer board takes the signals from the contact board and
simultaneously transmits them into one signal that travels back to the
system. A programmable gain amplifier on the multiplexer board provides
the gain and the offset to the signals. The circuitry on the board also
amplifies the signal from the thermistor. Additionally, the multiplexer board
provides various excitation signals to the contact board, such as power and
digital timing signals.
The contact system protector shields the contacts from becoming
damaged when no measurement cartridge is present.
There are four alignment pins on the connector block assembly: two small
pins align the sensor module with the connector block, and two larger pins
guide the connector block into position.
Rev. C Mechanical Descriptions 45
Page 46

AutomaticQC Cartridge
The AutomaticQC cartridge is self-contained and non-serviceable. It
consists of the fluidic, electronic, and mechanical components needed to
deliver QC material to the system’s measurement components. The QC
material is uniquely formulated to provide performance verification at
several points in the clinical range of analytes measured by the
Rapidpoint 400 series systems.
The AutomaticQC cartridge is mounted on the right side of the system
where a support bracket secures it in place. The ampule breaker and an
access panel must be removed before the cartridge is installed. Four
standoffs attach the cartridge to the system where the ampule breaker was
previously mounted.
AutomaticQC cartridges supply sufficient QC material to run at least three
samples of each level three times per day for the life of the cartridge. Each
cartridge is stable for 14 days after installation on the system. The system
prompts the user when the cartridge needs to be replaced.
The cartridges must be stored refrigerated at 2 to 8°C.
AutomaticQC Cartridge Connector in the Open Position
1 Cartridge connector in the open position
46 Rev. C
Page 47

AutomaticQC Cartridge Fluidic Components
The AutomaticQC cartridge contains the following fluidic components:
• valve assembly
• QC material
• tubing
The valve assembly consists of a sliding valve, valve seal, valve lubricant,
and AutomaticQC probe.
The sliding valve is controlled by a stepper motor and a position detector
system that moves the valve and accurately positions it using two position
sensors. The valve has five positions, each of which enables one of the five
QC reagents to be pumped through the stainless steel probe in the center
of the valve. The probe remains stationary while the valve travels vertically
about the probe to change the hydraulic path between the AutomaticQC
cartridge and the measurement cartridge.
The QC material is supplied in five foil laminated bags, each of which holds
one of five levels of QC material. Each bag is firmly secured in place with
an inner and outer polystyrene clamp. Five nylon tubes, each of which
connects one of the five bags to the valve assembly, serve as flow paths for
the QC reagents.
Rev. C Mechanical Descriptions 47
Page 48

AutomaticQC Cartridge
1 Bracket to secure the cartridge to the side of the system
2 Standoffs (4)
3 EEPROM
4 Cartridge connector
5 Cartridge Lever
AutomaticQC Cartridge Indicating Lever Closed Position
1 Cartridge connector
2 Standoffs (4)
48 Rev. C
Page 49

3 Press Cartridge Lever to pierce reagent bags
The following table lists the contents of the AutomaticQC reagent bags:
Level Volume Ingredients
1 75 mL buffered bicarbonate solution with Na+, K+, Ca++, Cl-, carbon
dioxide, oxygen, nitrogen, dye, glucose, surfactant, and
preservative
2 115 mL buffered bicarbonate solution with Na+, K+, Ca++, Cl-, carbon
dioxide, oxygen, nitrogen, dye, glucose, surfactant, and
preservative
3 155 mL buffered bicarbonate solution with Na+, K+, Ca++, Cl-, carbon
dioxide, oxygen, nitrogen, dye, glucose, surfactant, and
preservative
A 60 mL buffered bicarbonate solution with Na+, K+, Ca++, Cl-, carbon
dioxide, oxygen, nitrogen, surfactant, and preservative
B 60 mL buffered bicarbonate solution with Na+, K+, Ca++, Cl-, carbon
dioxide, oxygen, nitrogen, surfactant, and preservative
AutomaticQC Cartridge Interface
Removal of the ampule breaker and an access panel provides access to
the latch assembly where the AutomaticQC cartridge connects to the
system. A sliding metal latch plate in the latch assembly engages four
standoffs on the AutomaticQC cartridge to secure the cartridge to the
system.
A valve actuator that extends through the latch assembly moves the sliding
valve on the AutomaticQC cartridge. Driven by a lead screw follower on the
motor, the actuator moves until it engages a vertical slot in the sliding valve.
The bidirectional stepper motor and position detectors move the valve
along the probe to select the required fluid path for flow of QC material from
the AutomaticQC cartridge to the measurement cartridge. The stepper
motor has five stop positions.
With the AutomaticQC cartridge engaged to the system, the cartridge
connector located on the front of AutomaticQC cartridge connects the
AutomaticQC cartridge to the measurement cartridge. Fluidic connectivity
between the two cartridges is provided by a single 0.5mm I.D. tube that
passes from the base of the sliding valve in the AutomaticQC cartridge
through the cartridge connector and into the measurement cartridge. The
cartridge connector must be closed for the system to operate.
Rev. C Mechanical Descriptions 49
Page 50

The AutomaticQC cartridge is disengaged from the system through a
software procedure that causes the stepper motor to move the sliding
metal latch plate and release the standoffs. If power is lost, the
AutomaticQC cartridge can also be disengaged from the system by
moving a release tab located beneath the stepper motor.
AutomaticQC Interface
1 Latch assembly
2 Locating holes (4)
3 Actuator
4 IDEE Pins
50 Rev. C
Page 51

Mechanical Components
The AutomaticQC cartridge contains the following mechanical
components:
• cartridge lever
• bag pierce pins
• cartridge connector
The cartridge lever is a spring-loaded panel that, when closed (flush with
the AutomaticQC cartridge base plate), causes pins on its underside to
actuate the pierce probes. The pierce probes engage fittings on the
reagent bags to provide a seal and to puncture the bags to create fluid
paths for the QC material to flow from the AutomaticQC cartridge to the
measurement cartridge. A total of three pierce probes service the five
bags. The AutomaticQC cartridge is shipped with the lever in its open
position.
The cartridge connector is mounted on the front of the AutomaticQC
cartridge. During installation of the cartridge, the connector is moved
horizontally and connects with the measurement cartridge. It provides the
sample path from the AutomaticQC cartridge to the measurement
cartridge. The cartridge connector slide must be fully engaged for the
AutomaticQC cartridge to function.
Electronic Components
The AutomaticQC cartridge contains the following electronic components:
• IDEE assembly
• microswitch
The IDEE assembly consists of a PC board and cable. An EEPROM chip
on the PC board signals the system when the AutomaticQC cartridge is
installed. The EEPROM chip also contains target ranges for each level of
QC material in the cartridge. These ranges are automatically downloaded
to the system when the AutomaticQC cartridge is installed. The cable
connects the IDEE PC board to the cartridge connector microswitch.
The microswitch is located where the cable attaches to the bottom of the
valve enclosure. It determines if the cartridge connector is open or closed.
Rev. C Mechanical Descriptions 51
Page 52

Pumps
The pumps move the reagents, samples, and wash reagent through the
Rapidpoint 400 series systems. The sample pump moves the sample and
the reagents through the system. The sample pump also moves KCl
through the reference electrode. The wash pump delivers a mixture of
wash fluid and air to the sample entry port.
Each pump consists of a roller cage, a motor, a pump housing, a motor
mount, a spring plate, and springs. The roller cage presses onto the motor
shaft.
The pump that services the measurement cartridge generates flow by
compressing the tubing against the fixed platen of the cartridge. As the
individual rollers rotate through the platen area, a segment of the tubing is
pinched in two places by adjacent rollers. The fluid is pushed through the
tubing by the peristaltic action of the moving rollers.
A stepper motor drives each pump. Each motor moves 1.8 degrees per
step and 200 steps per revolution. The electrical drive and control for the
pump stepper motors are provided by a microstepping circuit on the main
board. The software controls the speed and direction of each pump.
Stepper Motor Components
52 Rev. C
Page 53

Display
The display is the user interface component of the Rapidpoint 400 series
systems. The display tilts on a horizontal axis and locks at the chosen tilt.
The display consists of the following components:
• display module
• liquid crystal display (LCD)
• touch screen
•printer
• display board
• display support
Display Module
The display module contains the LCD, the touch screen, the printer, the
display board and the display support. In addition, the display module
contains the roll of printer paper and the paper cover.
Liquid Crystal Display
The LCD is a 265 mm (10.4 inch) diagonal display with active matrix color
LCD that provides 256 colors. The display supports an active matrix
color LCD.
Touch Screen
The touch screen is overlaid onto the LCD. The touch screen consists of
two electrically resistive sheets that are superimposed over the viewing
area of the display. The sheets are separated from each other by a small air
gap. When the screen is touched, the two sheets are electrically shorted.
The resolution of the touch screen is determined by the control circuitry.
Rev. C Mechanical Descriptions 53
Page 54

Printer
The built-in thermal-roll printer provides reports for calibrations, samples,
and diagnostics.
The printer consists of a platen, a motor and gear mechanism, a thermal
printhead, and two sensors. One sensor detects when the printhead is up
and the other sensor detects when the printer is out of paper.
The printer is controlled by the display board. The printer cable carries the
signals for the data, the motor control, the paper-empty sensor, and the
printhead-up sensor. The display board communicates with the main
board.
Display Board
The display board contains all the circuitry necessary to drive and control
the LCD, the printer, and the touch screen. There are no service settings or
jumpers on the display board.
Display Support
The display support holds the display to the system and the pawl provides
stability through the stepped mechanism when the display is tilted into the
varying tilt positions.
54 Rev. C
Page 55

CO-oximeter Components
The Rapidpoint 405 system contains the CO-oximeter (CO-ox)
measurement module that provides the capability for measuring the
concentration of total hemoglobin and hemoglobin fractions (tHb, FO
FHHb, FMetHb, FCOH) in whole blood samples.
Measurement occurs as the sample flows through the sample chamber.
Light is transmitted, through a fiber bundle, to the optics head. As the
sample flows through the sample chamber, the light is focused from one
side of the optics head through the sample. The light is collected at the
other side of the optics head and then is carried through a fiber bundle to
the polychromator to be measured.
CO-ox Measurement System
Hb,
2
Rev. C Mechanical Descriptions 55
Page 56

CO-ox Measurement Components
The Rapidpoint 405 system CO-ox components consist of the following:
• illumination housing assembly
• neon board assembly
• fiber bundle assembly
• sample chamber interface
• sample chamber
• polychromator module
• photo-feedback sensor
The CO-ox components are shown in the following figure.
Co-ox Measurement Components
56 Rev. C
Page 57

Illumination Housing Assembly
The CO-ox illumination housing assembly holds the replaceable halogen
lamp. The housing contains optical filters that limit the lamp’s output light
(spectrum) to the visible range, and lenses that collect and focus light into
the fiber bundle.
The lamp’s output intensity is controlled by a closed-loop optical feedback
system. This is accomplished by routing some of the output fibers from the
fiber bundle, which is connected to the illumination housing assembly, to a
photo-feedback sensor located on the main PB board. This sensor
continuously monitors lamp output and generates a signal that regulates
power to the lamp.
Illumination Housing Assembly
Neon Board Assembly
The neon board assembly contains a neon gas discharge lamp, lenses, a
narrow bandpass interference filter, and a driver board. The output light
(monochromatic) from this assembly is used as a reference to check the
polychromator’s wavelength measurement accuracy. This check occurs at
the same time and frequency as the automatic tHb zero. Any optical or
mechanical misalignment in the polychromator, which may cause
wavelength measurement inaccuracies, are corrected during the zero
calibration.
Rev. C Mechanical Descriptions 57
Page 58

Fiber Bundle Assembly
The fiber bundle assembly contains many small diameter optical fibers that
are combined to form a fiber optic cable with four terminals. The bundle
connects to the neon board assembly, illumination housing assembly,
sample chamber interface, and to the photo-feedback sensor on the main
PC board.
The bundle transmits light to the CO-ox components during sample
analysis.
• the fiber bundle transmits light from the illumination housing module to
the optics head of the sample chamber interface, where it is focused
onto the sample chamber
• transmits a small portion light from the illumination module to the
photo-feedback sensor, which controls the halogen lamp output
• transmits light from the neon reference lamp to the sample chamber,
which is used to check the polychromator’s accuracy
NOTE: A separate fiber bundle is attached to the polychromator. This
bundle, which is connected to one side of the optical head, transmits light
from the sample chamber (originating from the neon lamp and from the
halogen lamp) to the polychromator.
Sample Chamber Interface Assembly
The sample chamber interface assembly consists of the following
components:
• optical head with spring link
• detector housing module
• motor
• drive wheel
• PC board
58 Rev. C
Page 59

The optical head contains the optics that deliver light to and collect light
from the sample chamber. The optical head houses four lenses, two
mirrors, and the detector module.Two lenses and mirror direct the light
from the fiber bundle onto the sample chamber. On the other side of the
optical head another mirror and set of lenses collect the light that is not
absorbed by the sample. A fiber bundle, connected from the optical head
to the polychromator, relays the light for measurement. The spring link
holds and locates the optical head.
The detector module uses a thermistor to ensure that the CO-ox sample
chamber temperature is kept constant, and a flag detector system to detect
the position of the sample chamber during measurement. The flag detector
system consists of two emitters and two photo detectors that detect the
position of the sample chamber.
The drive wheel is attached to the drive shaft of the motor, and engages a
mechanism in the measurement cartridge that moves the sample chamber
to the open and closed positions.
CO-ox Sample Chamber Interface
Rev. C Mechanical Descriptions 59
Page 60

Co-ox Sample Chamber
The CO-ox sample chamber is part of the measurement cartridge.The
sample chamber consists of the following components:
•base
• gasket
• seal
• shunt/flag
• sample chamber body
•spring
• cover
•heater chip
The sample chamber is in its measurement position when the motor moves
the shunt into the closed position.
When the shunt returns to the open position, the measurement
cartridge reagent system cleans the sample chamber. A heater chip
ensures that the sample temperature is kept at 37 ± 0.3°C.
Polychromator Module
The polychromator module measures the intensities of light passed
through the sample at a number of different wavelengths.
The polychromator module has the following components:
60 Rev. C
Page 61

• fiber bundle
• diffraction grating
• grating imaging mirror
• collimating mirror
• diode array PC board
• polychromator PC board
The fiber bundle carries light collected by the optics head after passing
through the sample and relays it to the polychromator. The fibers at the end
of the fiber bundle form a line that defines the input slit to the polychromator.
The first mirror collimates light from the slit so that nearly parallel light falls
on the diffraction grating. The diffraction grating disperses the light coming
from the collimating mirror into its component wavelengths or colors. The
second mirror images the light from the grating onto the photodiode array
detector where the various wavelength intensities are measured.
The diode array board converts light into an electrical signal. The
polychromator board converts the signal from the diode array board from
an analog to a digital value for further processing.
Rev. C Mechanical Descriptions 61
Page 62

62 Rev. C
Page 63

Electronic System Overview
Overview 67
Electronic System 68
Contact PC Board 69
Digital Signals 69
Digital Input Signals 69
Digital Output Signals 69
Analog and Other Input Signals 70
Analog and Other Output Signals 72
Circuit Description 74
Amperometric Circuits 74
Potentiometric Circuits 75
Hematocrit/Fluid Detector Circuits 75
Display PC Board 77
Digital Signals 77
Digital Input Signals 77
Digital Output Signals 78
Analog Signals 79
Analog and Other Input Signals 79
Analog and Other Output Signals 80
Circuit Description 80
Microcontroller and Peripheral Circuitry 80
Touch Screen Switches 81
DC-DC Converter Circuit 81
LCD Backlight Inverter Circuit 81
Rev. C Electronic System Overview 63
Page 64

Dualopto PC Board 82
Sensor Circuit 82
Main PC Board 83
Real-Time Processor (RTP) 83
RTP 68332 Processor Circuitry 83
RTP 68332 Address and Data Bus Circuitry 83
RTP EPROM and PSRAM Circuitry 84
RTP Address Bus Decoder and Memory Controller
Complex Programmable Logic Device Circuitry 84
RTP Data Bus I/O Latch CPLD Circuitry 85
Data Acquisition System (DAS)
Front End Circuitry 85
DAS Analog-to-Digital Converter Circuitry 86
DAS Fluid Detector Circuitry 86
RTP/UIP First In First Out (FIFO) Memory
Communications Circuitry 86
UIP Main Interconnect Board ISA Interface Circuitry 87
UIP Display Module, COM1, COM2/LPT1, and Utility
Circuitry 87
UIP COM3 Circuitry 87
UIP ISA Address Bus Decoder, Data Bus I/O Latch
CPLD, and Communications Circuitry 88
Control Micro-stepper Processor, and Sample and
Wash Pump Driver Circuitry 88
Control Heater DAC, Driver Circuitry, and Valve
Driver Circuitry 89
Power System Status Circuitry, Diagnostic LEDs,
and Switches 89
Control Automatic Quality Control (AQC) Valve
Stepper Circuitry 90
Power System Protection and Input Circuitry 90
UIP Main Interconnect Board ISA Interface 92
Digital Input Signals 92
Digital Output Signals 92
Digital Bi-directional Signals 93
64 Rev. C
Page 65

UIP COM1 Interface 94
Digital Input Signals 94
Digital Output Signals 94
UIP COM2/LPT1 Interface 95
Digital Input Signals 95
Digital Output Signals 95
Flat Panel Interface 96
Digital Output Signals 96
UIP Utility Interface 96
Digital Output Signals 96
Display Board Interface 97
Digital Input Signals 97
Digital Output Signals 97
Multiplexer Board Interface 98
Digital Input Signals 98
Digital Output Signals 98
Analog and Other Output Signals 99
Main Interconnect PC Board 100
ISA Connectors 100
Multiplexer PC Board 101
Digital Signals 102
Digital Input Signals 102
Digital Output Signals 102
Analog Signals 103
Analog and Other Input Signals 103
Analog and Other Output Signals 104
Circuit Descriptions 105
Amperometric Isolation Circuits 105
Digital Control Circuit 106
Temperature Circuits 106
Programmable Gain Amplifier Circuitry 106
Rev. C Electronic System Overview 65
Page 66

User Interface Processor PC Board 107
Miscellaneous Functions 108
Keyboard Controller 108
Battery 108
Miscellaneous Function Connector 108
Peripheral Interfaces 109
Serial Ports 109
Parallel Port 109
Floppy Disk Controller 109
Co-oximeter OMZ Heater PC Board 110
Analog Signals 111
Analog and Other Input Signals 111
Analog and Other Output Signals 111
66 Rev. C
Page 67

Overview
This section describes the electronic systems for the Rapidpoint 400 series
systems. It provides circuit descriptions for each printed circuit board in the
Rapidpoint 400 series systems. Each subsection is organized by board
name and circuit description. Assembly drawings for each printed circuit
board are included in Replacing Parts.
The electronic system directs the operation of all Rapidpoint 400 series
systems components, including the fluidic system, the measurement
system, and the sensors. It also receives information from these
components regarding their various functions.
The electronic system performs all calculations required to determine
reported parameters and controls the data communication with the
devices connected to the Rapidpoint 400 series systems.
Rev. C Electronic System Overview 67
Page 68

Electronic System
The Rapidpoint 400 series systems electronic system consists of the
following PC boards:
• Contact
• Display
• Dualopto
•Main
• Main Interconnect
• Multiplexer
• UIP (User Interface Processor)
68 Rev. C
Page 69

Contact PC Board
The Contact PC board amplifies signals from the electrodes before they
are processed by the rest of the system. The Contact board provides the
following:
• low bias, current-transimpedence output amplifiers and bias amplifiers
for four amperometric sensors
• high-impedance differential amplifier for seven potentiometric sensors
• AC current, source and synchronous, differential amplifier for two
hematocrit/fluid detector sensors
• connections for three thermistors organized in a thermal-control loop
that regulate the sample temperature to +37°C
• connections to an EEPROM device located within the sensor module
for storing calibration and configuration information specific to the
measurement cartridge
Digital Signals
Digital Input Signals
Input Signal Description
ID_SDA Serial data line to the EEPROM located in the sensor module.
This signal is bi-directional.
ID_SCK Serial clock line to the EEPROM.
ASAMP Control signal for the charge transfer process in the
hematocrit circuits.
BSAMP Control signal for the charge transfer process in the
hematocrit circuits. The BSAMP signal is out of phase with
the ASAMP signal.
DRIVE This signal provides the AC excitation voltage for the
hematocrit sensors.
Digital Output Signals
Output Signal Description
ID_SDA Serial data line to the EEPROM located in the sensor module.
This signal is bi-directional.
Rev. C Electronic System Overview 69
Page 70

Analog and Other Input Signals
Input Signal Description
+5_VDC_HCT Positive-power supply for the hematocrit circuits. Voltage is
+5.00 V ±5% at 5 mA.
-5_VDC_HCT Negative power supply for the hematocrit circuits. Voltage is
-5.00 V ±5% at 5 mA.
+5_VDC_PO2 Isolated positive power supply for the oxygen circuit. Voltage
is -3.5 ±0.5 V at 250 µA.
-5_VDC_PO2 Isolated negative power supply for the oxygen circuit. Voltage
is +3.5 ±0.5 V at 250 µA.
5_RTN_PO2 Isolated power supply return for the oxygen circuit.
+5_VDC_GLU Isolated positive power supply for the glucose circuit. Voltage
is -3.5 ±0.5 V at 250 µA.
-5_VDC_GLU Isolated negative power supply for the glucose circuit.
Voltage is -3.5 ±0.5 V at 250 µA.
5_RTN_GLU Isolated power supply return for the glucose circuit.
+5_VDC_LAC Isolated positive power supply for the lactate circuit. Voltage
is -3.5 ±0.5 V at 250 µA. For future use.
-5_VDC_LAC Isolated negative power supply for the lactate circuit. Voltage
is -3.5 ±0.5 V at 250 µA. For future use.
5_RTN_LAC Isolated power supply return for the lactate circuit. For future
use.
+5_VDC_CRE Isolated positive power supply for the creatinine circuit.
Voltage is +3.5 ±0.5 V at 250 µA. For future use.
-5_VDC_CRE Isolated negative power supply for the creatinine circuit.
Voltage is -3.5 ±0.5 V at 250 µA. For future use.
5_RTN_CRE Isolated power supply return for the creatinine circuit. For
future use.
REFV_PO2 Isolated +800 mV reference voltage for the oxygen electrode.
REFV_GLU Isolated -400 mV reference voltage for the glucose electrode.
REFV_LAC For future use.
REFV_CRE For future use.
+5_VDC Positive power supply for the potentiometric amplifiers.
Supplies +5.00 V ±5% at 5 mA.
(Continued)
70 Rev. C
Page 71

Input Signal Description
-5_VDC Negative power supply for the potentiometric amplifiers.
Supplies -5.00 V ±5% at 5 mA.
ID_PWR Power supply for the EEPROM in the sensor module.
Supplies +5.00 V ±5% at 5 mA.
ID_RET Power supply return signal for the EEPROM.
PO2_SIG Current output signal of the pO2 electrode.
GLU_WB Current output signal of the glucose (active) electrode.
GLU_WA Current output signal for an inactivated twin of the glucose
electrode.
LAC_WB For future use.
LAC_WA For future use.
CRE_WB For future use.
CRE_WA For future use.
PH_VO Voltage output signal of the pH sensor.
K_VO Voltage output signal of the K+ sensor.
CA_VO Voltage output signal for the Ca++ sensor.
MG_CL_VO Voltage output of the Cl- sensor.
NA_VO Voltage output signal of the Na+ sensor.
BUN_VO For future use.
HCO3_VO Voltage output signal of the HCO3 sensor.
REF_VO Voltage output signal of the reference sensor. The range is
-60 to 140 mV. Impedance is about 10K ohms.
HCT1_HS Signal output of the hematocrit 1 sensor. This sensor
connects to the positive input of the synchronous sampling
differential amplifier on the Contact board. The amplifier
circuitry measures the impedance (conductance) between
the HCT_HF and the HCT_LF contacts of the hematocrit 1
sensor.
HCT1_LS Signal reference of the hematocrit 1 sensor. This sensor
connects to the negative input of the synchronous sampling
differential amplifier on the Contact board.
(Continued)
Rev. C Electronic System Overview 71
Page 72

Input Signal Description
HCT2_HS Positive signal output of the hematocrit 2 sensor. This sensor
connects to the positive input of the synchronous sampling
differential amplifier on the Contact board. The amplifier
circuitry measures the impedance (conductance) between
the HCT_HF and HCT_LF contacts of the hematocrit 2
sensor.
HCT2_LS Signal reference of the hematocrit 2 sensor. This sensor
connects to the negative input of the synchronous sampling
differential amplifier on the Contact board.
THM_BLK_HI One terminal of the block thermistor.
THM_BLK_LO The other terminal of the block thermistor.
THM_PRE_HI One terminal of the preheater thermistor.
THM_PRE_LO The other terminal of the preheater thermistor.
THM_SMP_HI One terminal of the sample thermistor.
THM_SMP_LO The other terminal of the sample thermistor.
Analog and Other Output Signals
Output Signal Description
PO2_SIG Amplified and buffered pO2 sensor signal.
LAC_A For future use.
LAC_B For future use.
GLU_A Amplified and buffered glucose (inactive) sensor signal.
GLU_B Amplified and buffered glucose (active) sensor signal.
CRE_A For future use.
CRE_B For future use.
PH_SIG Amplified and buffered pH sensor signal.
HCO3_SIG Amplified and buffered HCO3 sensor signal.
BUN_SIG For future use.
K_SIG Amplified and buffered K+ sensor signal.
MG_CL_SIG Amplified and buffered Cl- sensor signal.
NA_SIG Amplified and buffered Na+ sensor signal.
CA_SIG+ Amplified and buffered Ca++ sensor signal.
(Continued)
72 Rev. C
Page 73

Output Signal Description
REF_SIG Amplified and buffered reference sensor signal.
HCT1_SIG Amplified and buffered hematocrit 1 sensor signal.
HCT2_SIG Amplified and buffered hematocrit 2 sensor signal.
THM_BLK_HI The pass-through signal connected to one end of the block
thermistor.
THM_BLK_LO The pass-through signal connected to the other end of the
block thermistor.
THM_PRE_HI The pass-through signal connected to one end of the
preheater thermistor.
THM_PRE_LO The pass-through signal connected the other end of the
preheater thermistor.
THM_SMP_HI The pass-through signal connected to one end of the sample
thermistor.
THM_SMP_LO The pass-through signal connected to the other end of the
sample thermistor.
PO2_RF The voltage sensed internal to the pO2 sensor.
PO2_CN The voltage control electrode for the pO2 sensor.
GLU_RF The voltage sensed internal to the glucose sensor. The
voltage is maintained at -400 mV ±4% and constant ±1 mV
between calibration cycles with respect to 5_RTN_GLU.
GLU_CN The voltage control electrode for the glucose sensor. The
voltage adjusts such that the signal at GLU_RF is -400 mV
with respect to 5_RTN_GLU.
LAC_RF For future use.
LAC_CN For future use.
CRE_RF For future use.
CRE_CN For future use.
HCT1_HS The excitation signal for the hematocrit 1 sensor.
HCT1_LS Signal return for the hematocrit 1 sensor.
HCT2_HS The excitation signal for the hematocrit 2 sensor.
HCT2_LS Signal return for the hematocrit 2 sensor.
(Continued)
Rev. C Electronic System Overview 73
Page 74

Output Signal Description
ID_PWR Power supply for the EEPROM in the sensor module. It
supplies +5.000 V ±5% at a current of 5 mA. This signal is
generated on the Multiplexer board and is a pass-through to
the sensor module.
ID_RET Power supply return signal for the EEPROM. This is a pass-
through signal from the Multiplexer board to the sensor
module.
Circuit Description
Amperometric Circuits
Because the amperometric circuits (oxygen, glucose, lactate, and
creatinine) are nearly identical, a description of only the glucose circuit is
provided.
The glucose sensor connects to P1-5, P1-6, P2-5, and P2-6 on the Contact
board. This sensor requires that a bias potential be forced to exist on P1-6,
with respect to P1-5 and P2-6 of -400 mV.
Pin 1 of U9 forces a voltage on GLU_CN until the voltage sensed on
GLU_RF equals REFV_GLU. The voltage on REFV_GLU is -400 mV, with
respect to 5_RTN_GLU.
Limited by a diffusion rate, the electrode reaction dominates the current
output. The concentration of glucose in the sample limits the diffusion rate,
so that the output current is proportional to the glucose concentration in the
sample.
Another current output, GLU_WA, is from the inactive electrode of the
sensor. This current corrects the glucose signal current for interfering
substances and for diagnostics. To prevent leakage currents from
corrupting the electrode current output, the entire electrode current output
net is guarded by 5_RTN_GLU.
NOTE: The oxygen sensor does not have an inactive output, so that
circuitry is deleted.
74 Rev. C
Page 75

Potentiometric Circuits
All the ion selective electrodes (ISEs) are potentiometric. Each ISE
contains a membrane that isolates the electrode from the sample and that
selectively passes the desired ion from the sample to the electrode. The
change in potential difference between the ISE output voltage and the
reference electrode output voltage is proportional to the logarithm of the
concentration of the selected ion in the sample.
The eight amplifiers in U1 through U4 are configured as a differential
amplifier, with the signal from the reference electrode subtracted from all
the other signals. REF_VO connects to pin 5 of U4 through R23.
R23 protects U4 from electrostatic discharge.
The feedback voltage at the inverting input of the respective amplifier
completely guards each ISE output. This prevents leakage currents from
corrupting the high impedance signal at the ISE output.
Hematocrit/Fluid Detector Circuits
The Contact board has two hematocrit/fluid detector circuits. These
circuits are conductometric; that is, they measure the electrical
conductance (resistance) of the fluid appearing between the HCT_HS and
HCT_LS terminals. Because both circuits are nearly identical, a
description of only the hematocrit 1 circuit is provided.
The signal labeled DRIVE is a +5V peak-to-peak square wave. R61 and
C43 transforms this voltage into a current source with an amplitude of
approximately 16.67 µA peak-to-peak at the same duty cycle and
frequency as the DRIVE signal. This current is then applied to the sensor,
and a differential voltage results between the HCT_HS and HCT_LS
terminals from the excitation current flowing through the fluid.
The conductance (resistance) of the fluid in the sensor is determined using
a known current and a measured voltage.
When the excitation current goes to its positive state, all of the switches are
toggled. This action effectively disconnects C44 from ground and connects
it to C23 instead. This connection allows the charge stored in C44, which
represents the peak-to-peak voltage excursion at the HCT_HS terminal,
to C23.
Rev. C Electronic System Overview 75
Page 76

Simultaneously, a similar process is taking place with C16 and C21. U5 and
feedback resistors R35 to 38 form a differential amplifier with a gain of 51.
The HCT_SIG signal is the peak-to-peak voltage at HCT_HS minus the
peak-to-peak voltage at HCT_LS multiplied by a gain of 51.
Hematocrit circuit 2 is identical to the circuit 1, except that C50, a capacitor
AC, couples this hematocrit circuit to prevent a ground loop in the sample
path.
76 Rev. C
Page 77

Display PC Board
The Display PC board provides the electronics necessary to operate the
following components:
• thermal printer
• touch screen
• liquid crystal display (LCD)
• speaker
In addition, the display board provides the following electrical functions:
• circuitry to provide diagnostic capability
• conversion of ASCII data from the host to actual printed reports
• detection, location, measurement, and reporting of touches on the
touch screen
• generation of the voltage necessary to turn the LCD backlight on
and off
Digital Signals
Digital Input Signals
Input Signal Description
LCD_ENAB Enables operation of the LCD.
LCD_DIM Turns inverter (providing power to the LCD backlight) on and
off.
LCD_DU0..7 Upper byte of LCD data signals.
LCD_DL0..7 Lower byte of LCD data signals.
LCD_YD Control signal for the LCD. This signal indicates the start of
a scan (analogous to a vertical sync signal).
LCD_LP Control signal for the LCD. This signal indicates the start of
a new line (analogous to a horizontal sync signal).
LCD_XCK Clock signal for the LCD.
ENAVEE Control signal. This signal enables the negative supply for
the LCD.
(Continued)
Rev. C Electronic System Overview 77
Page 78

Input Signal Description
ENAVDD Control signal. This signal enables the positive supply for the
LCD.
LPT1DB<0..7> Centronics type parallel port data bus.
LPT1STB- The signal that indicates that valid data is present on
LPT1DB<0..7>.
LPT1INIT- The signal that initializes (resets) the printer control circuitry.
SPKR Speaker input signal.
COM1TX Serial port transmit signal. This signal receives data on the
serial port.
Digital Output Signals
Output Signal Description
LPT1ERROR- Indicates that there is an error associated with the printer.
LPT1PE Indicates that the printer is out of paper.
LPT1BUSY Handshake signal. This signal indicates that the printer is
currently busy and cannot accept any new data at this time.
LPT1ACK- Handshake signal. This signal asserts when LPT1BUSY is
becoming de-asserted.
LPT1SLCT The signal that indicates that the printer is online.
PRN_DATA_IN Data signal to the printer. Data is loaded into the printer in a
serial fashion (much like loading a shift register).
PRN_CLOCK The signal that indicates that valid data is available on the
PRN_DATA_IN line.
PRN_LATCH- The signal asserted to transfer the data from the shift register
to the buffers that drive the thermal elements internal to the
printer.
DST1..6 Thermal head strobe lines. These signals assert when data
have been loaded into the print head to burn the data.
78 Rev. C
Page 79

Analog Signals
Analog and Other Input Signals
Input Signal Description
GND_PLANE Ground reference signal for the electronics.
PWR_RET Ground reference signal for the power devices (such as,
printer, backlight, and inverter).
P24V The +24 V supply voltage. This signal conforms to the
requirements outlined in the Digital Inputs Signals section
above.
VCC Electronics 5 V power supply. This signal conforms to the
requirements outlined in the Digital Inputs Signals section
above.
POWER5V Power device 5 V power supply. This signal conforms to the
requirements outlined in the Digital Inputs Signals section
above.
PAPER_OUT The signal that indicates that the printer is out of paper. It is
the open collector output of an optocoupler and requires a
100K ohms resistor pullup to the VCC line to operate. A
paper-out condition is indicated by a voltage of greater than
2.5 V on this line.
HEAD_UP The signal from the mechanical switch that is internal to the
printer and that indicates the position of the print head. This
signal is pulled up to VCC through a 10K ohms resistor. A
voltage greater than 4.5 V indicates that the head is in the up
position, and a voltage less than 0.2 V indicates that the head
is in the down position.
PRN_TH_HI The signal from the thermistor within the print head that
determines the temperature of the print head.
TS_X+ and TS_X- The two drive signals to the X axis on the touch screen. The
TS_X+ signal connects through a switch to the VCC line. The
TS_X- signal connects through a switch to ground.
TS_Y+ and TS_Y- The two drive signals to the Y axis on the touch screen.
Rev. C Electronic System Overview 79
Page 80

Analog and Other Output Signals
Output Signal Description
POWER5V Power device for the 5 V power supply that powers the printer
motor. This signal conforms to the requirements outlined in
the Digital Inputs Signals section above.
VCC Electronics 5 V power supply. This signal conforms to the
requirements outlined in the Digital Inputs Signals section
above.
PRN_7V Power supply signal for the print head. The voltage on this
line is between 4.2 and 7.0 V, with the ability to supply from
0 to 10 amps of current.
GND_PLANE Ground reference signal for the electronics.
PWR_RET Ground reference signal for the power devices, such as
printer, backlight, and inverter.
PHASE 1..4 Signals that drive the printer motor.
LED_DRIVE Anode of the diode in the paper sensing optocoupler. This
signal is pulled up to VCC through a 200 ohms resistor.
VCC Electronics 5 V power supply. This signal conforms to the
requirements outlined in the Digital Inputs Signals section
above.
GND_PLANE Ground reference signal for the electronics.
BL1..2 LCD backlight power supply voltage.
Circuit Description
Microcontroller and Peripheral Circuitry
U21, a 80C552 microcontroller, controls the thermal printer and the touch
screen. This controller has a built-in, 10-bit ADC that measures diagnostic
signals as well as determines touch locations on the touch screen.
U12, a 64-macrocell PLD, expands the input/output (I/O) capability of the
microcontroller. This expanded I/O latches the data arriving at the parallel
port and provides output latching to control the printer motor and the touch
screen switches. It also includes latches for the lower address byte for
accesses to RAM and ROM.
80 Rev. C
Page 81

C45, C46, and U22, and U21 on-board oscillator circuit, make up the
system clock. The nominal frequency (11.0592 MHz) facilitates the
generation of the 9600-baud serial signal. U27 translates the serial signal
from TTL to RS-232 signal levels and vice versa.
A µC monitor IC, U11, provides a reset pulse of at least 140 mS on
power-up, power-down, and low-voltage brown-out conditions. In this
configuration, it holds µC in reset when the +5 V power supply goes below
approximately 4.65 V. U12 also uses this signal to hold its output latches in
a known state until the µC has established control of the circuit.
U13, a 32K x 8 static RAM device, provides memory beyond what is
available on the µC. This additional memory is intended for the
downloadable font feature. U14, a 128K x 8 flash EEPROM device, is for
program memory.
U10 is a power logic, octal, D-type latch.
Touch Screen Switches
Transistors Q2 to 9, along with their associated diodes, resistors, and
capacitors, are the switches that interface with the touch screen.
DC-DC Converter Circuit
U19 is a synchronous-switching regulator controller that converts the
+5 V power supply input to the display board to the +7 V power supply that
powers the thermal printer. U20, R48 to 51, C29, and C39 form a
+12 V regulator to power U19.
LCD Backlight Inverter Circuit
U15 is the module that provides the power to run the LCD backlight. The
backlight typically requires a starting voltage of ~1100 V
state voltage of ~500 V
at a supply current of ~6-7 mA
RMS
and a steady
RMS
. U24 and its
RMS
associated drive circuitry provide the ability to turn the backlight on and off.
Rev. C Electronic System Overview 81
Page 82

Dualopto PC Board
The two Dualopto PC boards provide the luer position mechanism a way to
determine where the valve actuator is located for the measurement and
AutomaticQC valves. Two optical interrupter switches accomplish this
task.
Sensor Circuit
All signals arrive from the main board through a 4-pin, male, IDC
connector, J1. The two LEDs inside the interrupters, U1 and U2, connect
in series and can be checked at test points TP3(+) and TP4(-) for a voltage
of approximately 2.5V.
The output signals from each interrupter route to J1 and to test points
TP1 (LUER_POS1) and TP2 (LUER_POS2), respectively. Their voltage
levels are approximately 0.5V for the unblocked condition and
approximately 4V for the blocked condition. All voltages are measured
with respect to ground TP4.
82 Rev. C
Page 83

Main PC Board
The Main PC board provides the following:
• system requirements for user interface processing
• real-time processing
• user/real-time first-in-first-out (FIFO) communications
• data acquisition
• control and utility functions
Real-Time Processor (RTP)
RTP 68332 Processor Circuitry
U47 is a 68332 32-bit microprocessor based on the 68000 core CPU32. It
has additional functionality such as memory chips selects and extensive
timer-counter functions.
The data bus, DR0 to DR15, has 32 bits internally and 16 bits externally.
This bus is separated into a fast local bus for memory and a slower buffered
bus for all other devices on the Main board.
The microprocessor uses a frequency synthesizer, Y1, for generating both
its internal and external system clock.
U55 is a power supervisor and watchdog. The output pin 11 of the
integrated circuit (IC) provides the microprocessor with a correctly
generated, active low reset pulse. Pin 11 monitors the 5 V power on pin 15
and generates a reset if the voltage does not remain within the specified
+
5% tolerance range. Pin 2 of IC U55 is the asynchronous active low reset
input.
RTP 68332 Address and Data Bus Circuitry
The 68332 data bus, DR0 to DR15, is pulled up to VCC through resistor
pack RN23. The exception is DR3, which is actively pulled to ground during
RTP system reset by probe CR17.
Data bus, DM0 to DM15, is conditioned and passed to the memory section
and to data bus drivers U49 and U58.
Rev. C Electronic System Overview 83
Page 84

The address bus, named AM1 to AM19, is passed to the memory section
and then to address latches inside U11. These latch a valid address on the
falling edge of signal AS-. The address signals to these latches are pulled
up by RN28, R235, R236, R237, and R238 to form the address bus A1 to
A15. This address bus is then sent to address decoder CPLD U37.
DEBUG/XRAY connector J30 is an expansion connector that connects the
main board to a diagnostic board.
RTP EPROM and PSRAM Circuitry
This section of the Main board contains the code memory EPROM and
data memory PSRAM devices U57, U48, U56, and U41.
The data in the PSRAMs is volatile and is refreshed periodically by the
68332 TPU section.
RTP Address Bus Decoder and Memory Controller Complex Programmable Logic Device Circuitry
This section of the Main board contains a group of circuits located in the
single complex programmable logic device (CPLD), U37. The majority of
this IC takes the address bus signals A1 to A15, chip select signals CS9 to
CS10 and other 68332 control signals, such as R/W, and forms various
strobe signals that latch data in and out of U38.
Another function of U37 is to provide clock signal ADS_2 MHz for use by
the main A/D converter. This clock is enabled only when the A/D converter
busy signal is active.
U37 also arbitrates when a memory refresh cycle is needed by the
PSRAMs U56 and U41 by responding to a refresh request from the 68332
TPU unit on pin 147 of U37. It responds by delaying the start of a normal
memory request by inhibiting the pin 150 (DSACK1) signal. This delay
causes the 68332 to insert wait states until the refresh cycle is completed.
The IC also enables and buffers all of the interrupt signals coming from the
FIFO, A/D, debug and power-fail circuits. All outputs and the internal timing
of U37 are synchronized to the 68332 master clock (RCLK1) on pin 139. All
outputs on U37 become tri-state by pulling pin 140 to a high signal level.
84 Rev. C
Page 85

RTP Data Bus I/O Latch CPLD Circuitry
This section of the Main board contains a group of circuits located in a
CPLD, U38. The majority of the IC takes the data bus signals, D0 to D15
and strobe signals from U37 and other 68332 control signals, such as
DS-, to latch data in and out of U38.
Latch outputs in the logic array block LAB-A direct data to the
micro-stepper microcontroller U39.
Latch inputs in LAB-B direct data to the luer and AQC motor drivers.
Latch outputs in LAB-C and LAB-D direct data to the Multiplexer board
assembly located in the measurement block module.
Latch outputs in LAB-E and LAB-F connect the system data bus (D0 to
D15) to U37, and use the control strobes generated by U37.
Latch outputs in LAB-G and LAB-H receive data from the various system
status sensors and flag signals from the FIFO circuitry.
Data Acquisition System (DAS) Front End Circuitry
This section of the Main board contains the interface to the Multiplexer
board located in the measurement block module. This module receives
parallel data bits from the U38 latches. U38 returns the properly
conditioned sensor analog signal to the 40-pin connector J8. This
connector returns the temperature sensor bridge signals for the preheater
and block heater located in the measurement block module. J8 supplies
+5 V, +12 V, and -12 V to the measurement block module.
The disposable cartridge connected to the measurement block module
contains a small EE memory to retain information about the cartridge
calibration and its history. This EE memory communicates to the Main
board through J8.
There is a 12-bit digital-to-analog converter (DAC) IC U22 contained in this
section of the DAS, and it sends a programmable analog voltage to the
measurement block module to offset each sensor into proper calibration
range. All the analog signals arriving at J8 are in a differential form and
connected to two 16-channel multiplexers, U8 and U9. The MUX0-3
signals generated from latches on U38 control U8 and U9. The selected
differential pair of signals is then sent to the next section.
Rev. C Electronic System Overview 85
Page 86

DAS Analog-to-Digital Converter Circuitry
This section of the Main board contains the balanced low-pass filter that
eliminates and averages noise received from the multiplexers, U8 and U9.
The filter output is sent to IC U14. U14 is an instrumentation amplifier that
converts differential signals to a single-ended signal.
The ADC IC U23 is a 16-bit converter. From the converter, the output is sent
to U38 in a serial digital form. U38 converts the data from serial to parallel
and sends it to the 68332 microprocessor.
The ADC IC U23 also needs a stable reference voltage to compare the
unknown input voltage. Reference source U15 and buffer amplifier U16,
along with significant filter capacitors, create an extremely stable 5 V
source for the ADC.
DAS Fluid Detector Circuitry
This section of the Main board contains the circuitry from which the two
analog fluid detector signals are monitored. The fluid detector signals are
sent to a pair of comparators located in U17.
The non-inverting input pins of these comparators connect to a dual, 12-bit
DAC, U24. The DAC allows the comparators to have programmable trip
points, so each fluid detector has its own voltage level for comparison. The
output of each comparator is sent to U12 as digital zero- or one-bit.
This section of the Main board also contains an eight-channel, singleended multiplexer, U13, that monitors various system voltages, such as
DAC heater temperatures and motor reference voltages.
RTP/UIP First In First Out (FIFO) Memory Communications Circuitry
This section of the Main board contains the circuitry that allows the user
interface processor (UIP) and the RTP 68332 processor to communicate
with each other. Communication occurs using two FIFO memories, U21
and U29. U21 and U29 are cross-coupled to each data path, which allows
full communication in both directions. Status flags on both memories
indicate to the microprocessors when the FIFOs are full or empty.
86 Rev. C
Page 87

UIP Main Interconnect Board ISA Interface Circuitry
This section of the Main board contains the circuitry to connect the UIP
processor to the Main board through the Main Interconnect board. The
connection is through a 98-pin industry standard architecture (ISA)
connector, J1.
UIP Display Module, COM1, COM2/LPT1, and Utility Circuitry
This section of the Main board contains the signals to connect the UIP
processor to the Main board through various cables. Connector J16
contains the COM1 serial port signals. These signals originate on the UIP
Processor board and exit the Main board through J6. This Main board
section controls the touch panel on the display module assembly.
Connector J19 contains the COM2 serial port signals. These signals
originate on the UIP Processor board and exit the Main board through
20-pin connector, J2. This provides the LIS communications port on the
Rear Interconnect board assembly.
Connector J19 also contains the LPT1 parallel port signals. These signals
originate on the UIP Processor board and exit the Main board through J6.
This circuit transmits data to the thermal printer on the display module
assembly.
The utility connector, J17, on the UIP Processor board utility allows the
power supply to warn the board of an impending power failure through
signal PWRFAILX-. This connector also allows the onboard speaker signal
to exit the Main board through J6 on its way to the speaker located behind
the LCD on the display module assembly.
Connector J18 is the UIP Processor board flat panel interface. J18 sends
an upper and lower data byte and control signals to the LCD through series
resistor terminators RN9 and RN25.
UIP COM3 Circuitry
This section Main board contains an IC U36, which is a USART. The U36
allows the UIP Processor board to have a third RS-232C serial port. This
IC 16C550 is a standard PC compatible component and has full modem
signals with programmable baud rates to 19,200 kilobaud.
Rev. C Electronic System Overview 87
Page 88

IC U33 takes the 0 to 5 V levels from the USART and converts them to ±7 V
signals following RS-232C standards. This serial port is used to connect a
bar code reader to the instrument. The connector, J2, is the interface
between the Main board and the Rear Interconnect board located at the
back of the instrument.
UIP ISA Address Bus Decoder, Data Bus I/O Latch CPLD, and Communications Circuitry
This section Main board contains an IC U20, which is a CPLD. The IC U20
allows expansion of the UIP Processor board using the ISA bus connector.
The LAB-A of the IC inputs the UIP address bus signals, A0 to A9, along
with other ISA control signals such as IOW- and IOR-, to form various chip
select for the other peripherals on the ISA bus.
The LAB-B of IC U20 takes the data bus signals, USD0 to USD3, in and out,
as well as inputs for the FIFO status flags and door and waste switch
signals.
The LAB-C decodes chip selects for the Controller IC and serial port
COM3.
The LAB-D decodes chip selects for FIFO memory interface and buffers
interrupt signals from the FIFO and door/waste switches.
IC U12 takes parallel UIP data and converts it to serial bus protocol.
Control Micro-stepper Processor, and Sample and Wash Pump Driver Circuitry
This section Main board contains an 87C52 microcontroller, IC U27. The
IC U27 formats data for micro-stepping DAC ICs, U10 and U18. These two
ICs take digital sine and cosine wave data and convert them to analog
voltage reference signals for the power ICs, U4 and U11. These
components work together to give the Main board the ability to drive two
23-frame size stepping motors. The wash pump motor and the sample
pump motor interfaces through connector J3.
88 Rev. C
Page 89

Control Heater DAC, Driver Circuitry, and Valve Driver Circuitry
This section Main board contains IC U25, which is an AD7247 dual DAC.
This IC takes digital data from the 68332 and creates two analog voltages
with a resolution of 12 bits. These heater voltages are sent to operational
amplifier U54, which multiplies the voltage by four and drives power
transistors Q4 and Q3.
Section A of DAC IC is for the block heater and section B is for the
preheater. These signals leave the Main board on connector J7 and are
sent to the heater resistance pads on the measurement block assembly.
Thermistor R229 is mounted near the power transistor heatsinks and
monitors for failures such as air flow restrictions). When R229 detects a
failure, it notifies the DAS section.
This section Main board also contains IC U28, which takes digital latched
signals from RTP CPLD U38 and forms the proper sequencing signals to
drive the luer position motor located in the equipment wall module. These
driver signals exit the Main board through J3.
Power System Status Circuitry, Diagnostic LEDs, and Switches
This section Main board contains the MC34161 power supply monitors:
ICs U39, U40, and U43. These ICs are voltage window comparators, which
verify if the ±12 and +24 V power supplies are within the rated tolerance.
The 68332 reads signals ±12 V_STS and +24 V_STS from the RTP data
bus through transparent latch U12.
This section Main board also contains IC U44, which is a dual comparator
that checks the motor power supply. U44 ensures that motors are
operational by measuring the voltage drop across resistors R188. The
68332 reads signals MOTOR_STS from the RTP data bus through
transparent latch U38.
This section Main board has eight diagnostic light-emitting diodes (LEDs),
CR2 to CR9, that are latched by U59 onto the RTP data bus. There is also
an eight position dip switch, SW1, which is read by the RTP data bus
through transparent latch U50.
Rev. C Electronic System Overview 89
Page 90

Control Automatic Quality Control (AQC) Valve Stepper Circuitry
This section Main board contains IC U32, which takes digital latched
signals from RTP CPLD U38 and forms the proper sequencing signals to
drive the automatic quality control (AQC) position motor that will be located
in the AQC module. The driver signals exit the Main board through
connector J4. This connector also provides +5 V power and dualopto
signals for the AQC module.
Power System Protection and Input Circuitry
This section Main board contains the 24-pin connector, J25. This
connector delivers all power supply voltages from the system power
supply.
Power Supply
Voltage Description
+12_VDC supply Overvoltage protected by transient protector U42 and is
current limited from shorts by R174.
-12_VDC supply Overvoltage protected by transient protector U51 and is
current limited from shorts by R173.
+5_VDC supply Overvoltage protected by transient protector U52 and is
current limited from shorts by R243, R218, R244.
VCC supply Overvoltage protected by transient protector U52 and is
current limited from shorts by R243, R218, R244.
AUX5V supply Overvoltage protected by transient protector U52 and is
current limited from shorts by R245.
DIS5V supply Drives the thermal printer print head and is current limited
from shorts by R196, R195, and R230.
DISVCC supply Drives the logic ICs in the display module and is current
limited from shorts by R2.
BKLTVCC supply Drives the backlight inverter in the display module and is
current limited from shorts by R41.
J24 floppy power
connector
Drives the diskette drive on the main assembly and is current
limited from shorts by R1.
Transient protector U59 protects the +24 V supply from overvoltage. The
+24 V supply is current limited from shorts by fuse F2. This supply drives
motors through diode U53, which clamps the motor current monitoring
circuit to 0.2 V. This supply also splits to drive heaters through power
transistor.
90 Rev. C
Page 91

Signal HEAT24V_EN controls power to the heaters and is enabled by a
latched bit on IC U38 on the RTP data bus. LEDs CR10 to CR13 indicate
which of the four power supply voltages (VCC, +12 V, -12 V, and +24 V) is
present.
Rev. C Electronic System Overview 91
Page 92

UIP Main Interconnect Board ISA Interface
Digital Input Signals
Input Signal Description
+12_VDC +12 VDC from the power supply.
-12_VDC -12 VDC from the power supply.
+5_VDC +5 VDC from the power supply.
GND Ground return for the +5 VDC to the power supply.
DRQ0-DRQ3
DRQ5-DRQ7
I/OCHCK I/O channel check. This signal provides the system board
I/OCHRDY I/O channel ready. A memory or I/O device pulls this signal
I/OCS16- I/O 16-bit chip select.
IRQ3-IRQ7
IRQ9-IRQ12
IRQ 14, IRQ15
Master- Signal used with DRQ to gain system control.
MEMCS16- Memory 16-bit chip select.
0WS Zero wait-state. This signal directs the microprocessor to
DMA requests. These signals are asynchronous channel
requests used by peripheral devices and a microprocessor to
gain DMA service or control of the system.
with parity (error) information about memory or devices on
the I/O channel.
low to lengthen I/O or memory cycles.
Interrupt request lines. These lines signal the
microprocessor when an I/O device requires attention. An
interrupt request is generated when an IRQ line is raised
from low to high. The line is high until the microprocessor
acknowledges the interrupt request.
complete the present bus cycle without inserting any
additional wait cycles.
Digital Output Signals
Output Signal Description
AEN Address enable. This signal disconnects the microprocessor
and other devices from the I/O channel and enables DMA
transfers.
BALE Buffered address latch enable. This signal originates from
the bus controller and is used on the system board to latch
valid addresses and memory decodes from the
microprocessor.
(Continued)
92 Rev. C
Page 93

Output Signal Description
CLK 8-MHz system clock.
DACK0- to DACK3DACK5- to DACK7-
RESETDRV Resets or initializes system logic at power up or during a low
OSC Oscillator. This signal is a high speed clock with a 70-nS
SMEMR- Instructs the memory devices to drive data onto the data bus.
SMEMW- Instructs the memory devices to store the data present on the
T/C Terminal count. This signal provides a high pulse when the
Used to acknowledge DMA requests. These signals are
active low.
voltage condition. This signal is active high.
period (14.31818 MHz) and is not synchronous with the
system clock. It has a 50% duty cycle.
SMEMR- is active only when the memory decode is within
the low 1M of memory space. SMEMR- is derived from
MEMR- and the decode of the low 1 MB of memory. This
signal is active low.
data bus. SMEMW- is active only when the memory decode
is within the low 1 MB of the memory space. SMEMWoriginates from MEMW- and the decode of the low 1 MB
memory. This signal is active low.
terminal count for any DMA channel is reached.
Digital Bi-directional Signals
Bi-directional
Signals Description
IOR- I/O Read signal. This signal instructs an I/O device to drive
its data onto the data bus. The system microprocessor or
DMA controller, or a microprocessor or DMA controller
resident on the I/O channel, drives the signal. This channel
is active low.
IOW- I/O Write signal. This signal instructs an I/O device to read
the data from the data bus.
LA17-LA23 Unlatched signals address memory and I/O devices.
MEMR- Instructs the memory devices to drive data onto the data bus.
MEMW- Instructs the memory devices to store the present data onto
the data bus.
REFRESH- Indicates a refresh cycle. This signal is driven by a
microprocessor on the I/O channel and is active low.
(Continued)
Rev. C Electronic System Overview 93
Page 94

Bi-directional
Signals Description
SA0-SA19 Address signals 0 to 19 are used to address memory and I/
O devices.
SBHE System bus high enable.
SD0-SD15 Provides data bus bits 0-15 for the microprocessor, memory,
and I/O devices.
UIP COM1 Interface
Digital Input Signals
Input Signals Description
COM1RX Transmit signal for the touch screen serial port to the UIP
Processor board from the Main board.
COM1CTS Clear to send signal for the touch screen serial port from the
UIP Processor board to the Main board.
Digital Output Signals
Output Signal Description
COM1TX Transmit signal for the touch screen serial port from the UIP
Processor board to the Main board.
COM1RTS Ready to send signal for the touch screen serial port to the
UIP Processor board from the Main board.
94 Rev. C
Page 95

UIP COM2/LPT1 Interface
Digital Input Signals
Input Signal Description
COM2RX Transmit signal for the serial port to the UIP Processor board
from the Main board.
COM2CTS Clear to send signal for the LIS serial port to the UIP
Processor board from the Main board.
COM2DSR Data set ready signal for the LIS serial port to the UIP
Processor board from the Main board.
COM2DCD Data carrier detect signal for the LIS serial port to the UIP
Processor board from the Main board.
COM2RI Ring indicator signal LIS serial port to the UIP processor from
the Main board.
LPT1ERROR Printer error. Possible error sources are print head
temperature out of range, print head voltage out of range,
print head in the up position, or printer out of paper.
LPT1PE Printer is out of paper.
LPT1BUSY Printer is currently busy and cannot accept any new data at
this time.
LPT1ACK Signal that is asserted when LPT1BUSY is becoming de-
asserted.
LPT1SLCT Printer is online.
Digital Output Signals
Output Signal Description
COM2TX Transmit signal LIS serial port from the UIP Processor board
to the Main board.
COM2RTS Ready to send signal for the LIS serial port from the UIP
Processor board to the Main board.
COM2DTR- Data terminal ready signal for the LIS serial port from the UIP
Processor board to the Main board.
LPT1DB<0..7> Centronics-type parallel port.
LPT1STB Valid data on LPT1DB<0..7>.
LPT1INIT Initializes printer control circuitry.
Rev. C Electronic System Overview 95
Page 96

Flat Panel Interface
Digital Output Signals
Output Signal Description
LCD_ENAB Enables operation of the LCD.
LCD_DIM Turns the LCD backlight inverter on and off.
LCD_DU0..7 Upper byte of LCD data signals.
LCD_DL0..7 Lower byte of LCD data signals.
LCD_YD/VSYNC LCD frame start signal.
LCD_/HSYNC LCD line start signal.
LCD_XCK/CK LCD clock signal.
ENAVEE LCD negative supply control signal.
ENAVDD LCD positive supply control signal.
UIP Utility Interface
Digital Input Signals
Input Signal Description
PWRFAILX- Active low signal from the Main board to the UIP processor
that indicates a system power failure.
RESET- Active low signal from the Main board to the UIP processor
that indicates a system restart.
Digital Output Signals
Output Signal Description
SPKR Audio output signal from the UIP Processor board to the
Main board.
96 Rev. C
Page 97

Display Board Interface
Digital Input Signals
Input Signal Description
LPT1ERROR Printer error. Possible error sources are print head
temperature out of range, print head voltage out of range,
print head in the up position, or printer out of paper.
LPT1PE Printer is out of paper.
LPT1BUSY Printer is currently busy and cannot accept any new data at
this time.
LPT1ACK- Signal asserted when LPT1BUSY is becoming de-asserted.
LPT1SLCT Printer is online.
Digital Output Signals
Output Signal Description
LCD_ENAB Enables operation of the LCD.
LCD_DIM Turns the LCD backlight inverter on and off.
LCD_DU0..7 Upper byte of LCD data signals.
LCD_DL0..7 Lower byte of LCD data signals.
LCD_YD/VSYNC LCD frame start signal.
LCD_/HSYNC LCD line start signal.
LCD_XCK/CK LCD clock signal.
ENAVEE LCD negative supply control signal.
ENAVDD LCD positive supply control signal.
LPT1DB<0..7> Centronics-type parallel port data bus.
LPT1STB Indicates valid data on LPT1DB<0..7>.
LPT1INIT- Initializes the printer control circuitry.
SPKR Speaker input signal.
COM1TX Transmit signal for the touch screen serial port to the Display
board from the Main board.
COM1CTS- Clear to send signal for the touch screen serial port.
Rev. C Electronic System Overview 97
Page 98

Multiplexer Board Interface
Digital Input Signals
Input Signal Description
PREMUX_SIG_HI Multiplexed sensor signal, offset adjusted and properly
amplified output of the Multiplexer board.
PREMUX_SIG_LO Signal low sense return for PREMUX_SIG.
THM_BLK_HI Connected to the reference side of the bridge. Typical
voltage is +595.9 mV.
THM_BLK_LO High terminal of the block thermistor.
THM_PRE_HI Connected to the reference side of the bridge. Typical
voltage is +595.9 mV.
THM_PRE_LO High terminal of the preheater thermistor.
HCT1_SIG Output of the first hematocrit circuit. This signal originates on
the Contact board and passes through the Multiplexer board
on its way to the DAS circuitry.
HCT2_SIG Output of the second hematocrit circuit. This signal
originates on the Contact board and passes through the
Multiplexer board on its way to the DAS circuitry.
Digital Output Signals
Output Signal Description
PREMUX0..7 8-bit latched bus.
GAIN0..5 6-bit latched bus.
ID_SDA Serial data line to the EEPROM located in the sensor
module. It is a bi-directional signal.
ID_SCK Serial clock line to the EEPROM.
98 Rev. C
Page 99

Analog and Other Output Signals
Signal Description
DAC_HI 12-bit binary D/A signal from the DAS circuitry.
DAC_LO Signal return line for the DAC signal.
VREF +5 V measurement system voltage reference from the DAS
circuitry.
+12_VDC +12 VDC from the filtered supply.
-12_VDC -12 VDC from the filtered supply.
12_RET 12 VDC ground return from the filtered supply.
+5_VDC +5 VDC from the filtered supply.
5_RTN 5_VDC ground return from the filtered supply.
Rev. C Electronic System Overview 99
Page 100

Main Interconnect PC Board
The Main Interconnect PC board provides the ISA bus interface from the
UIP Processor board to the Main board.
ISA Connectors
All the ISA bus signals are sent from the UIP Processor board through a
98-pin female edge connector (J1). This connector routes all signals to the
Main board through 98-pin male edge connector (P1). This board is of
multilayer construction and includes power and ground on these layers.
100 Rev. C
 Loading...
Loading...