Page 1

Analyzer
Operating
Manual
®
Page 2

50241739 ii Revised 6/03
© 2003 Bayer HealthCare LLC
All Rights Reserved
Printed in U.S.A.
Unless otherwise noted, all
®
Trademarks are the property
of Bayer HealthCare LLC
Page 3

SECTION 1. Introduction Page
System and Intended Use ......................................................................... 1.1
Features .......................................................................................... 1.1
Specifications..................................................................................... 1.1
Hazards........................................................................................... 1.1
Safety Standards...................................................................................1.1
Symbols Used .....................................................................................1.2
SECTION 2. Unpacking, Getting Acquainted and Set Up
Unpacking........................................................................................ 2.1
Carton Contents................................................................................... 2.1
Getting Acquainted
DCA 2000
®
Analyzer (Front Panel) ........................................................... 2.2
DCA 2000
®
Analyzer (Back Panel)............................................................2.3
Screen Saver....................................................................................2.3
Audible Tones.................................................................................. 2.3
Blinking Colon in Displayed Time.............................................................. 2.3
When To Turn the Power Off ................................................................... 2.3
Set Up
Placing the Instrument/Connecting the Power Cord/Inserting Program Card..................... 2.4
Checking to Verify Instrument Functions Properly .............................................. 2.5
Viewing Factory Settings ....................................................................... 2.6
Options ........................................................................................ 2.8
Accepting Factory Settings.................................................................... 2.10
Changing Factory Settings..................................................................... 2.10
Setting Date and Time......................................................................... 2.14
Setting Creatinine Concentration Units (Microalbumin/Creatinine Assay ONLY)............... 2.16
Running the Optical Test Cartridge (Standard 1) —
Prior To Analyzing Samples for the First Time .............................................. 2.17
SECTION 3. Menu
Menu ............................................................................................. 3.1
RECALL PREVIOUS TESTS?................................................................. 3.2
SET SEQUENCE NUMBER?.................................................................. 3.4
RECALL CONTROL RESULTS? .............................................................. 3.5
VIEW CALIBRATION STATUS?.............................................................. 3.8
SET DATE/TIME?............................................................................ 3.10
INSTRUMENT SETUP?...................................................................... 3.13
SET CREATININE UNITS? .................................................................. 3.15
INSTRUMENT TEST? ....................................................................... 3.16
RUN CONTROL?............................................................................. 3.17
TABLE OF CONTENTS
Revised 6/03 iii
Page 4

SECTION 4. Operating Instructions — Hemoglobin A
1c
Page
Step 1: Turning the Power On ..................................................................... 4.1
Step 2: Calibration ................................................................................ 4.2
Step 3: Preparing Patient Samples and Controls.................................................... 4.3
Step 4: Analyzing the Patient Sample.............................................................. 4.8
Analyzing DCA 2000
®
Hemoglobin A1cControls, ONLY......................................... 4.12
Cancelling a Test ................................................................................ 4.15
SECTION 5. Operating Instructions — Microalbumin/Creatinine Page
Step 1: Turning the Power On ..................................................................... 5.1
Step 2: Calibration ................................................................................ 5.2
Step 3: Preparing Patient Samples and Controls.................................................... 5.4
Step 4: Analyzing the Patient Sample.............................................................. 5.8
Analyzing DCA 2000
®
Microalbumin/Creatinine Controls, ONLY................................ 5.12
Cancelling a Test ................................................................................ 5.15
SECTION 6. Error and Warning Messages, Error Codes and Troubleshooting
Chart of Contents................................................................................. 6.1
Error and Warning Messages ...................................................................... 6.2
Error Codes....................................................................................... 6.4
Troubleshooting ................................................................................. 6.10
SECTION 7. Instrument Care and Routine Maintenance
Instrument Care................................................................................... 7.1
Routine Maintenance Chart ....................................................................... 7.1
Exterior of Instrument and Bar Code Window (includes disinfection) .............................. 7.2
Changing Air Filter ............................................................................... 7.3
Cartridge Compartment ........................................................................... 7.4
Running Optical Test Cartridge.................................................................... 7.6
SECTION 8. Minor Repair
How to Replace the Fuse.......................................................................... 8.1
SECTION 9. Service Information
How to Report the Problem ....................................................................... 9.1
Accessory Items and Replacement Parts........................................................... 9.3
APPENDIX.................................................................................... App.1.1
iv Revised 6/03
Page 5

INTR ODUCTION
Revised 6/03 1.1
The DCA 2000®System
consists of:
•
DCA 2000® Analyzer
•
DCA 2000®Reagent Cartridges, Capillary
Holders and Calibration Card
•
DCA 2000®Controls
•
Optical T est Cartridge
INTENDED USE
The DCA 2000® Analyzer quantitatively
measures:
•
the percent concentration of Hemoglobin A
1c
in blood
•
the concentrations of Microalbumin and
Creatinine in urine
FEATURES
•
easily calibrated using the bar code card
provided with each reagent kit
•
stores calibrations for up to two lots of reagent
•
stores up to 16 test results
•
stores up to 16 control results
•
can be connected to a computer and/or printer
SPECIFICATIONS
Power Required:
Instrument Model No. 5031C: 100–240 VAC,
@ 0.4 amps
50/60 Hz
Dimensions/Weight:
Depth 27.2 cm (10.7 in) without power line cord
Width 24.1 cm (9.5 in)
Height 23.9 cm (9.4 in)
Weight 5 kg (11 lbs)
Ambient Operating Temperature:
(15–32°C)—Hemoglobin A
1c
(18–30°C)—Microalbumin/Creatinine
Relative Humidity Range:
10% – 90% RH (non-condensing)
NOTE: If an instrument I/O port is utilized, the
cable used should be 100% shielded to guard
against EMI and RFI. There should be continuity
between the cable shield and connector shell that
mates with the instrument.
Instrument Safety Design
The instument is for professional, in vitro
diagnostic use and must be used in the manner
specified in the Operating Manual in order to provide
the safety and standard performance standards
specified. The instrument will operate safely in the
conditions
listed below; however, results will only
be correct for the system specifications listed above:
•
indoors
•
5°– 40°C (41°– 104°F)
•
installation category II (IEC 1010.1)
•
safety tested to comply to IEC 1010.1
HAZARDS
To alert you to potential electrical or operational
hazards, warning and caution statements are
provided where applicable. To ensure your safety,
comply with all warning and caution statements.
SAFETY STANDARDS
Underwriters’Laboratories, Inc. (UL) and the
Canadian StandardsAssociation (CSA) as certified
and complies with the safety standards specified in
UL 3101 and CSA-C22.2, No. 1010.1.
The instrument meets the provisions of the IVD
Directive 98/79/EC (Oct./1998), which includes
the EMC Directive 89/336 Amendment
92/31/EEC, and the Low Voltage Safety Directive
73/23/EEC.
WARRANTY INFORMATION
Contact your Authorized Bayer Representative for
complete warranty information.
1
INTRODUCTION
32
15
18
30
Page 6
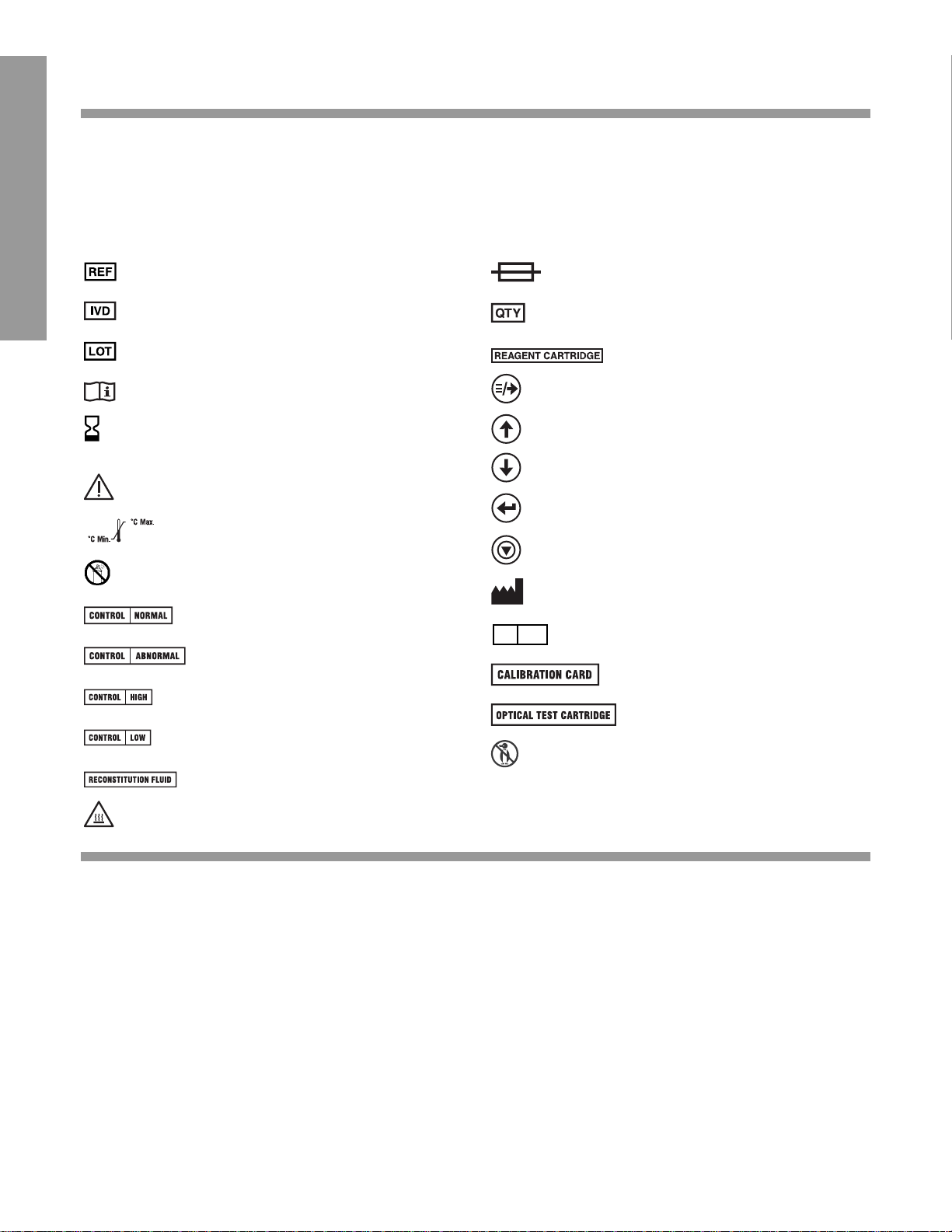
1
INTRODUCTION
1.2 Revised 6/03
Catalog number
In vitro diagnostic device
(LOT) Batch code
Consult operating instructions
(EXP) Use by
Warning/Attention: consult
instructions for use
Temperature limitations
Do not use spray
Normal control
Abnormal control
High control
Low control
Reconstitution fluid
High temperature part
Fuse
Quantity
Reagent cartridge
Menu/Next
Increase
Decrease
Enter
Escape
Manufactured by
Authorized representative
Calibration card
Optical test cartridge
Do not freeze
SYMBOLS USED
The following symbols are used throughout the product labeling for the DCA 2000®System (Instrument,
Instrument Manual, Quick Reference Guide, DCA Reagent Labeling and Instructional inserts and the
DCA Control packaging and instructional inserts).
U.S. PATENTS
6,043,043, 5,822,071, 5,610,073, 5,385,847, 5,372,948, 5,305,093, 5,272,093, 5,258,311, 5,220,161,
5,162,237, 5,151,369, 5,084,397, 4,990,075, 4,970,171, 4,968,472, 4,898,824, 4,847,209, 4,727,036,
4,658,022, 4,647,654, 4,629,692, D400,673.
2
8
EC REP
Page 7
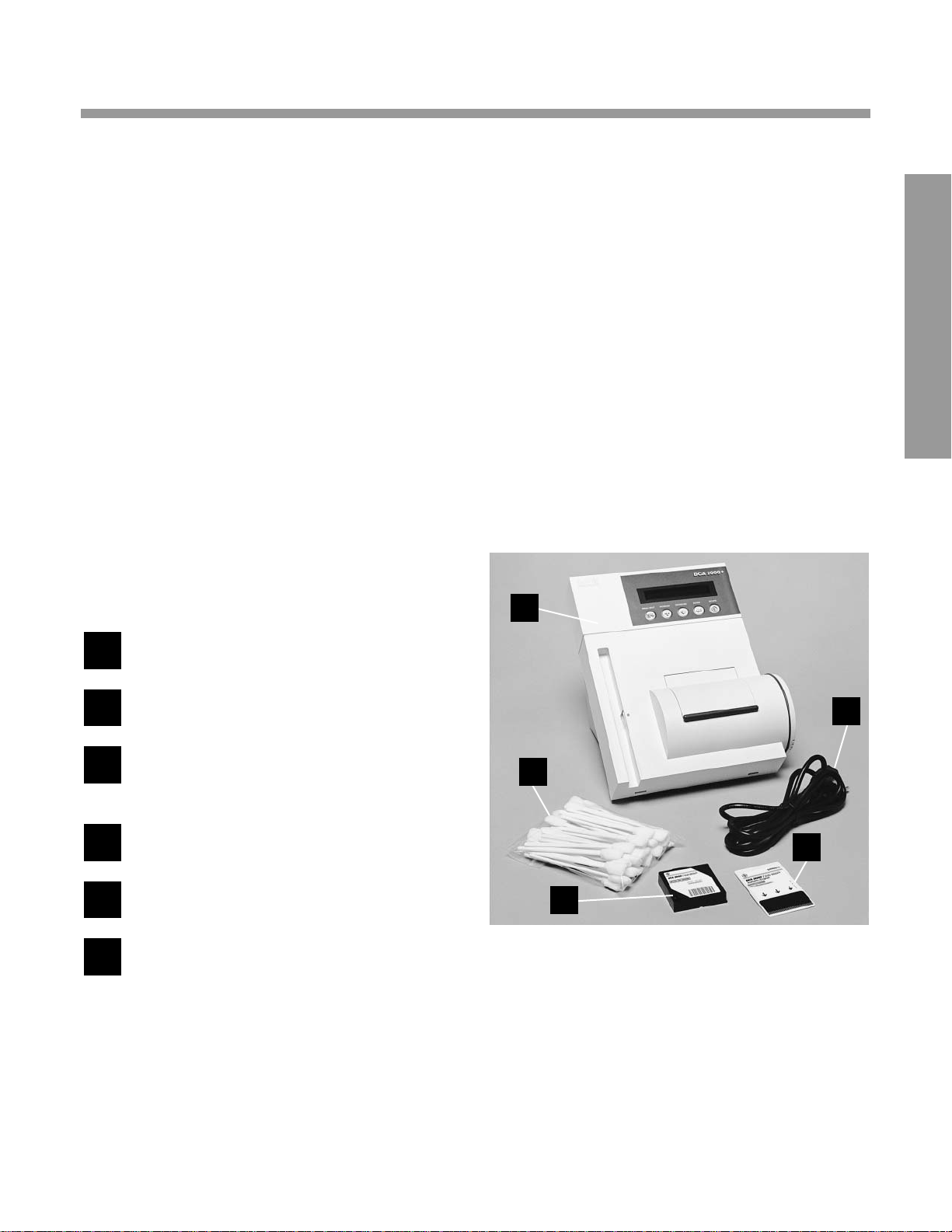
2
UNPACKING, GETTING
ACQUAINTED AND SET UP
Revised 6/03 2.1
UNP ACKING
The DCA 2000®System arrives in one shipping
carton.
Inspect the carton for shipping damage.
Unpack the carton.
•
Use extreme care when unpacking and handling
the instrument. The instrument contains
sensitive electronic and optical parts.
Check each item for shipping damage.
•Report shipping damage to the representative of
the carrier or to your Bayer HealthCare Sales
Representative.
CAR T ON CONTENTS
*Some instruments will require a separately packed Installation Kit
containing language specific instructional materials (Program
Card, Operating Manual and Quick Reference Guide), Cleaning
Kit, Air Filter Replacement Kit and the appropriate Power Cord.
DCA 2000® Analyzer
Cleaning Kit*
Optical T est Cartridge
(in instrument cartridge compartment)
Program Card*
Power Line Cord*
Other (not shown)
• Operating Manual*
•Replacement Fuse (stored in fuse holder
located inside the instrument)
• Air Filter Replacement Kit*
• HbA
1c
Quick Reference Guide*
6
5
4
3
2
1
UNPACKING, GETTING
ACQUAINTED AND SET UP
1
2
5
4
3
Missing Items?...Contact the nearest Bayer
HealthCare office or authorized distributor.
Page 8

2
UNPACKING, GETTING
ACQUAINTED AND SET UP
2.2 Revised 6/03
UNPACKING, GETTING ACQUAINTED AND SET UP
Unlock and then remove the optical test cartridge
according to the following instructions.
1. Open the cartridge compartment door.
2. Locate the button on the right side of the
cartridge compartment. Push and hold it down
with your right hand.
3. With your left hand, gently push the plastic tab
on the cartridge to the right; this action releases
(unlocks) cartridge.
4. Pull test cartridge out of compartment.
5. Put test cartridge aside for later use (page 2.17).
6. Make sure the cartridge return spring inside
the cartridge compartment is intact (refer
to Section 7, page 7.4, for information
regarding this spring).
GETTING ACQUAINTED
DCA 2000® Analyzer
FRONT PANEL
Display—indicates date, time, error
messages, test results, procedural
prompts, etc.
Keys—allow you to provide input for
system set-up, menu items, etc.
Reagent Cartridge Compartment
Access Door—covers and protects the
reagent cartridge; closing door starts test
timing (after a 5 second delay)
Reagent Cartridge Compartment—
holds one reagent cartridge during
sample analysis
Bar Code Reader Window—covers and
protects the bar code reader
Bar Code Track—area where reagent
cartridge, calibration card or control card
is placed prior to scanning bar code
6
5
4
3
2
1
1
6
5
2
3
4
➠
Page 9
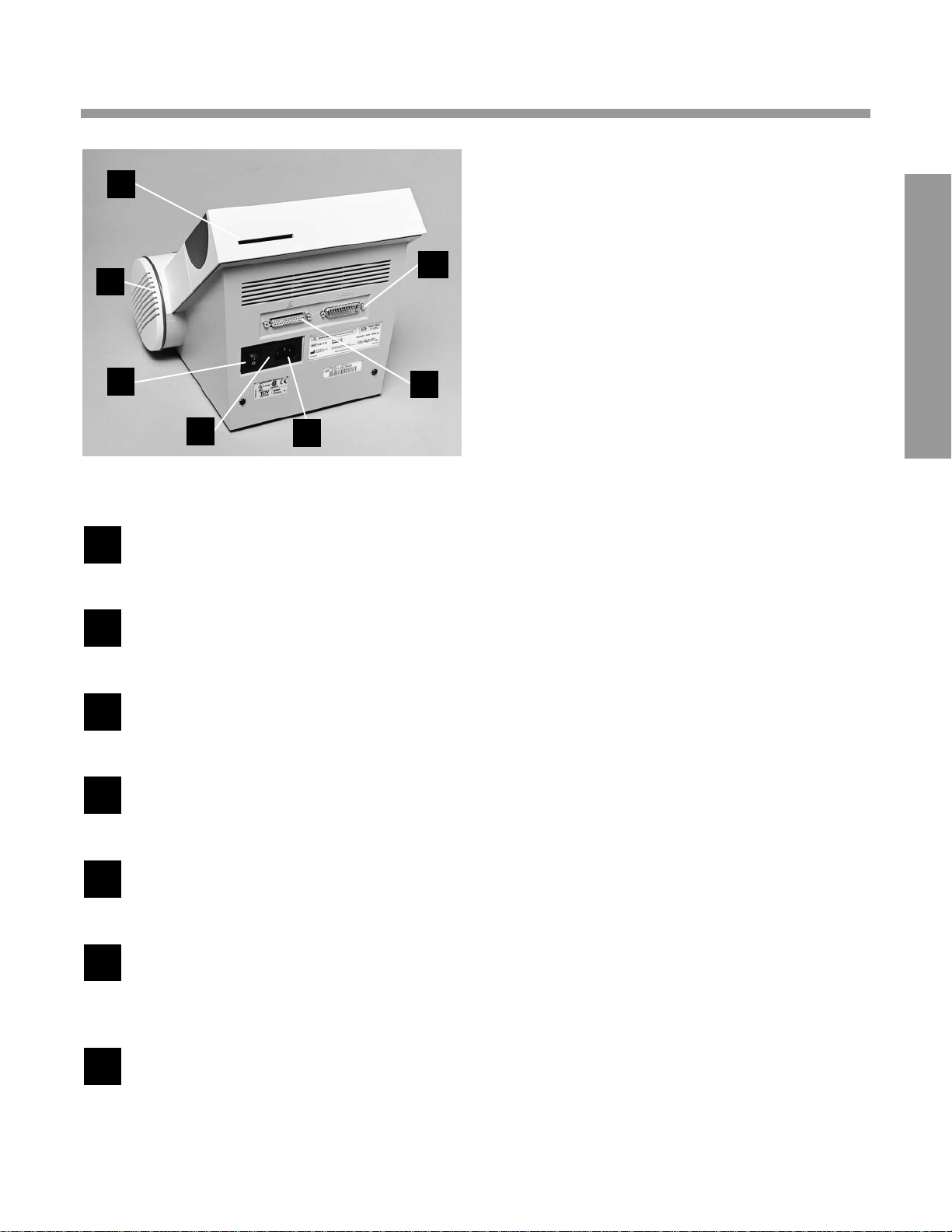
2
UNPACKING, GETTING
ACQUAINTED AND SET UP
Revised 6/03 2.3
BA CK PANEL
Power Switch—turns the power to the
instrument ON and OFF
Filter Holder—contains replaceable air
filter that prevents dust contamination
Program Card Connector—accepts the
program card
EIA-232-D Output—accepts the plug for
the computer interface cable
Printer Output—accepts the plug for the
printer cable
Power Cord Connector—accepts the
plug for connecting the power cord to
the instrument
Fuse Holder Compartment—holds two
fuses (one fuse is the replacement fuse)
AUDIBLE TONES
Beep—a short audible tone; indicates successful
completion of an activity (such as
scanning the bar code)
Buzz—a long audible tone; indicates an error
condition or reminds you to perform an
activity such as removing a reagent
cartridge
SCREEN SAVER
When the instrument is not in use for more than
5 minutes, the display will change to a block
shaped moving cursor. If the screen saver is on,
press any key to return to a normal display before
performing any other steps.
BLINKING COLON IN
DISPLAYED TIME
The current time is displayed using a “blinking”
colon. When the colon does not blink, the time
displayed is the time the assay began.
WHEN TO TURN THE POWER OFF
When the instrument is not in use, the power may
be turned OFF without loss of stored results.
However, when the po wer is subsequently restored,
a warm up period of one to eight minutes is
required.
IMPORTANT: If power is turned OFF or
interrupted while a test is in progress, the test must
be discarded.
Turn the power off when inserting or removing
the program card and whenever instructed
to do so by the particular procedure (maintenance, etc.) in use.
7
6
5
4
3
2
1
1
2
3
5
4
6
7
Page 10

2
UNPACKING, GETTING
ACQUAINTED AND SET UP
2.4 Revised 6/03
UNPACKING, GETTING ACQUAINTED AND SET UP
SET UP
PLA CING THE INSTRUMENT/
CONNECTING THE POWER CORD/
INSERTING PROGRAM CARD
IMPORTANT: Do not place the instrument
where it would be subjected to extreme
temperature variations, direct sunlight,
excessive humidity or air current, or excessive
particulate matter.
1. Place the instrument on a firm, level* surface
near a properly grounded electrical outlet.
0. *If the surface is not level, the instrument will
not function properly.
2. Set power switch to OFF (O).
3. Plug in program card (contacts facing
instrument; label side up).
0. IMPORTANT: The program card can be
damaged if inserted when the power is ON (I).
4. Connect the power cord to the power cord
connector on the instrument.
5. Plug in power cord to a properly grounded
outlet.
6. Move the instrument into place on the
designated work space.
0.
•
Allow at least 2 inches of air space between
the wall (or other surface) and the back and
right sides of the instrument (ventilation
panels).
Page 11

2
UNPACKING, GETTING
ACQUAINTED AND SET UP
Revised 6/03 2.5
CHECKING TO VERIFY INSTRUMENT FUNCTIONS PROPERL Y
Set the power switch to ON (I).
•
After about 8 seconds, the software version
is displayed.
(displayed for about 8 seconds)
•
Copyright information is displayed for 3
seconds.
•
Then:
*
The instrument is checking internal optics and
proper operation of mechanical features.
•
Then:
Instrument does not function? Above display(s) fail(s) to
appear?...Contact the nearest Bayer HealthCare office or
authorized distributor. Otherwise, continue with “Viewing
Factory Settings” (next).
INSTRUMENT SETUP
PRESS [<--] TO CONTINUE
INITIALIZING
KEEP DOOR CLOSED
COPYRIGHT 1991–2003
BY BAYER CORPORATION
SOFTWARE VERSION
E3.11/01.04
Page 12
2
UNPACKING, GETTING
ACQUAINTED AND SET UP
2.6 Revised 6/03
VIEWING FACTORY SETTINGS—
Upon Receipt of a New or Factory-Serviced Instrument
Only upon receipt of a new or factory-serviced
instrument, the following display appears (just after
“INITIALIZING / KEEP DOOR CLOSED”).
Before the instrument can analyze samples for the
first time, it is necessary for you to either accept
or change factory settings.
• To view factory settings, press .
Line 1 shows the factory setting numbers.
Line 2 shows the factory setting options.
The options in Line 2 correspond directly to
the number above them in Line 1.
The following chart defines the factory setting now
active for each available option.
Before deciding to accept factory settings, review
the chart under OPTIONS, on the following pages.
FACTORY SETTING
OPTION NUMBER DEFINITION
T (Time) 1 AM/PM
D (Date) 1 Month/Day/Year
L (Labels 4 Time Assay Began;
displayed — (0) Sequence Number (reset
with results) —(0) daily, automatically
—(0) at midnight)
C (Controls) 0 Use of DCA 2000
—(0) Controls, only
P (Port) 0 Computer Port is turned
—(0) Off (O)
1 1 4 0 00000000000000
TDLCP0000000000000
INSTRUMENT SETUP
PRESS [<--] TO CONTINUE
UNPACKING, GETTING ACQUAINTED AND SET UP
Page 13

2
UNPACKING, GETTING
ACQUAINTED AND SET UP
Revised 6/03 2.7
(This page left blank on purpose.
Intended for future use)
Page 14

2
UNPACKING, GETTING
ACQUAINTED AND SET UP
2.8 Revised 6/03
UNPACKING, GETTING ACQUAINTED AND SET UP
OPTIONS
All available settings are shown in the following chart.
•
Review this chart before you decide to accept or change
the factory setting for each option. An asterisk (*)
marks factory settings.
OPTION SETTING NUMBER DEFINITION OF SETTING NUMBER
T (Time 1* AM/PM*
Format)
2 24 hour format
D (Date 1* Month/Day/Year*
Format)
2Day
•
Month•Year
3Year–Month–Day
L (Labels displayed 0 No labels
with results)
1 Sequence number
(reset daily, automatically at midnight)
2 Sequence number
(continuous, 001 – 999)
3Time assay began
4* Time assay began; sequence number
(reset daily, automatically at midnight)*
5Time assay began; sequence number
(continuous, 001 – 999)
C (Controls) 0* DCA 2000 Controls, only*
•
The control bar code card enables the
instrument to label the control result. The
control result is then stored in the control
memory (separate from patient results).
1 ANY CONTROL
•
If using controls other than DCA 2000
Controls, use the MENU (just prior to each
control assay) to label the control result.
The control result, once labeled, is stored in
the control memory.
• IMPORTANT: When the MENU is used
to label control results, only the next
sample analyzed is labeled (and stored in
the control memory).
Page 15

2
UNPACKING, GETTING
ACQUAINTED AND SET UP
Revised 6/03 2.9
OPTION SETTING NUMBER DEFINITION OF SETTING NUMBER
P (Computer Port 0* OFF*—Computer port is OFF.
Configuration)
2ON— Computer port is ON.
All subsequent results will be
transferred to the computer.
NOTE: The options below (baud rate through modem control)
are selectable only if the computer port is turned ON.
B (Baud Rate) 9* 9600 bps*
4 4800 bps
2 2400 bps
1 1200 bps
3 300 bps
U (Data Bits) 8* 8 bits*
77 bits
V (Parity) 0* None*
1 Odd
2Even
W (Stop Bits) 1* 1*
22
X (Xon—Xoff 0* Off*
Protocol)
1On
Y (Block 0 Off
Transfer)
1* On*
Z (Modem 0* Off*
Control) 1 On
Page 16
2
UNPACKING, GETTING
ACQUAINTED AND SET UP
2.10 Revised 6/03
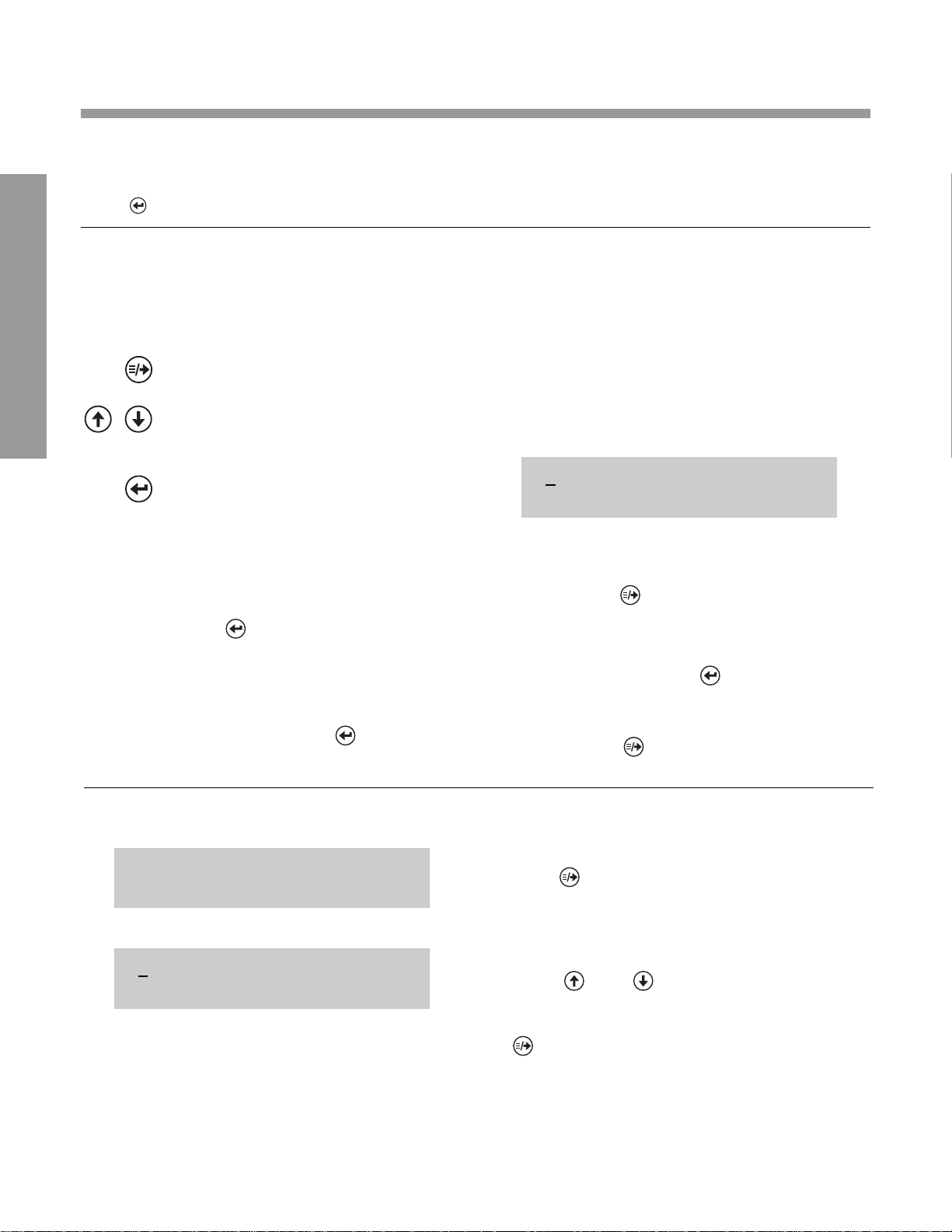
ACCEPTING FACT ORY SETTINGS
If the factory setting for each option is acceptable,
press . If not, see below. If acceptable, you are
now ready to set the current date and time. Refer
to instructions on page 2.14.
CHANGING FACTORY SETTINGS
Before attempting to change factory settings, read
the following information on “Keys” and “Cursor.”
Keys:
—moves the cursor* (underline) under
setting you desire to change
—cycles through each setting choice,
such as AM/PM or 24 HR for time
format
—accepts all displayed settings (for
time, date, labels, controls and
computer port) and immediately
advances to “SET DATE/TIME”
display.
Important: If you (prematurely)
press before you are finished
changing factory settings, refer to
Section 3, MENU for instructions on
how to access “INSTRUMENT
SETUP?”
HINT: Don’t press until all
settings on Line 1 reflect your
choices.
*Cursor
The cursor is the underline in the display. The
cursor shows you which setting is ready for
change.
Reminder: If the underlined number is
acceptable, press . The cursor then moves
horizontally (right) to the next number on line 1
while line 2 defines the corresponding option and
setting choice. Only when all numbers shown in
Line 1 are acceptable, press .
*The cursor does not move in reverse (left).
To change a number to the left of the cursor,
repeatedly press until the cursor returns to the
desired location.
1 1 4 0 0 nnnnnnnnnnnn
TIME FORMAT AM/PM
DISPLA Y WHA T Y OU DO
1. Press (places a cursor under factory
setting for first option, TIME FORMAT).
2. Press and to cycle through choices
for TIME FORMAT.
0. When the desired choice is displayed, press
0. (moves cursor under setting for next
0. option).
1 1 4 0 0 mmmmmmmmmmmm
TIME FORMAT AM/PM
1 1 4 0 0 mmmmmmmmmmmm
T D L C P mmmmmmmmmmmm
UNPACKING, GETTING ACQUAINTED AND SET UP
Page 17
2
UNPACKING, GETTING
ACQUAINTED AND SET UP
Revised 6/03 2.11
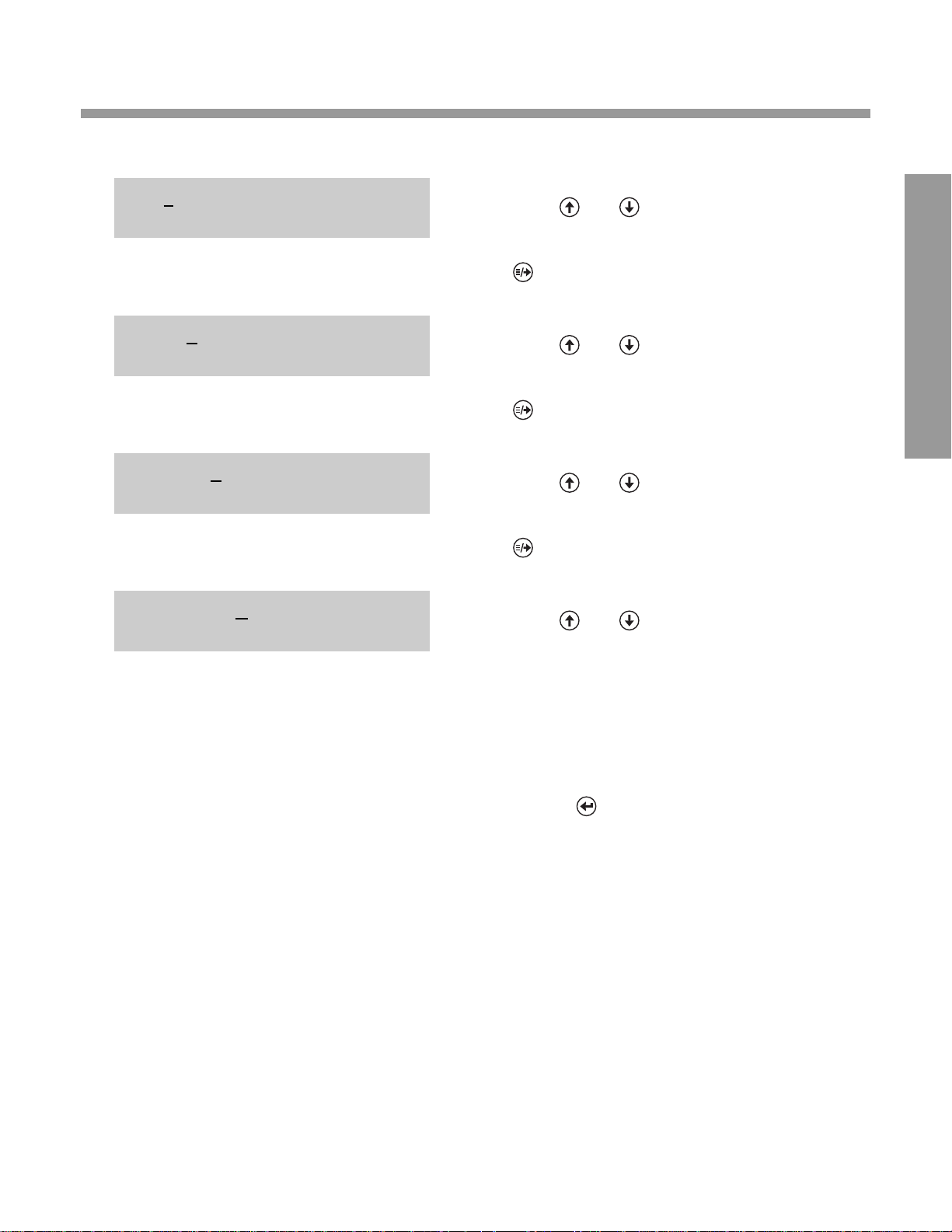
DISPLA Y WHA T Y OU DO
3. Press and to cycle through choices for
DA TE FORMAT.
0. When the desired choice is displayed, press
0. (moves cursor under setting for next
0. option).
4. Press and to cycle through choices for
LABELS.
0. When the desired choice is displayed, press
0. (moves cursor under setting for next
0. option).
5. Press and to cycle through choices for
CONTROLS.
0. When the desired choice is displayed, press
0. (moves cursor under setting for next
0. option).
6. Press and to cycle through choices for
COMPUTER PORT.
0. If you have selected “0” for COMPUTER
PORT OFF:
0.
•
Check to make sure all numbers on Line 1
reflect your choices.
0.
• Reminder: Options Chart is found on pages
2.8–2.9.
0.
•
Press (accepts all settings and
0.
• advances display to SET DATE/TIME?)
0.
•
Continue with instructions on page 2.14,
“Setting the Date and Time.”
0. If you have selected “2” for
COMPUTER PORT ON:
0.
•
Continue with instructions on page 2.12,
“Changing Factory Settings For: Computer
Port ‘ON’Option.”
1 1 4 0 0mm mmmmmmmmmm
COMPUTER PORT OFFmmmmm
1 1 4 0 0 mmmmmmmmmmmm
CONTROLS DCA 2000
1 1 4 0 0 mmmmmmmmmmmm
LABELS USED TIME/DAILY
1 1 4 0 0 mmmmmmmmmmmm
DATE FORMAT MM/DD/YY
Page 18
2
UNPACKING, GETTING
ACQUAINTED AND SET UP
2.12 Revised 6/03
UNPACKING, GETTING ACQUAINTED AND SET UP
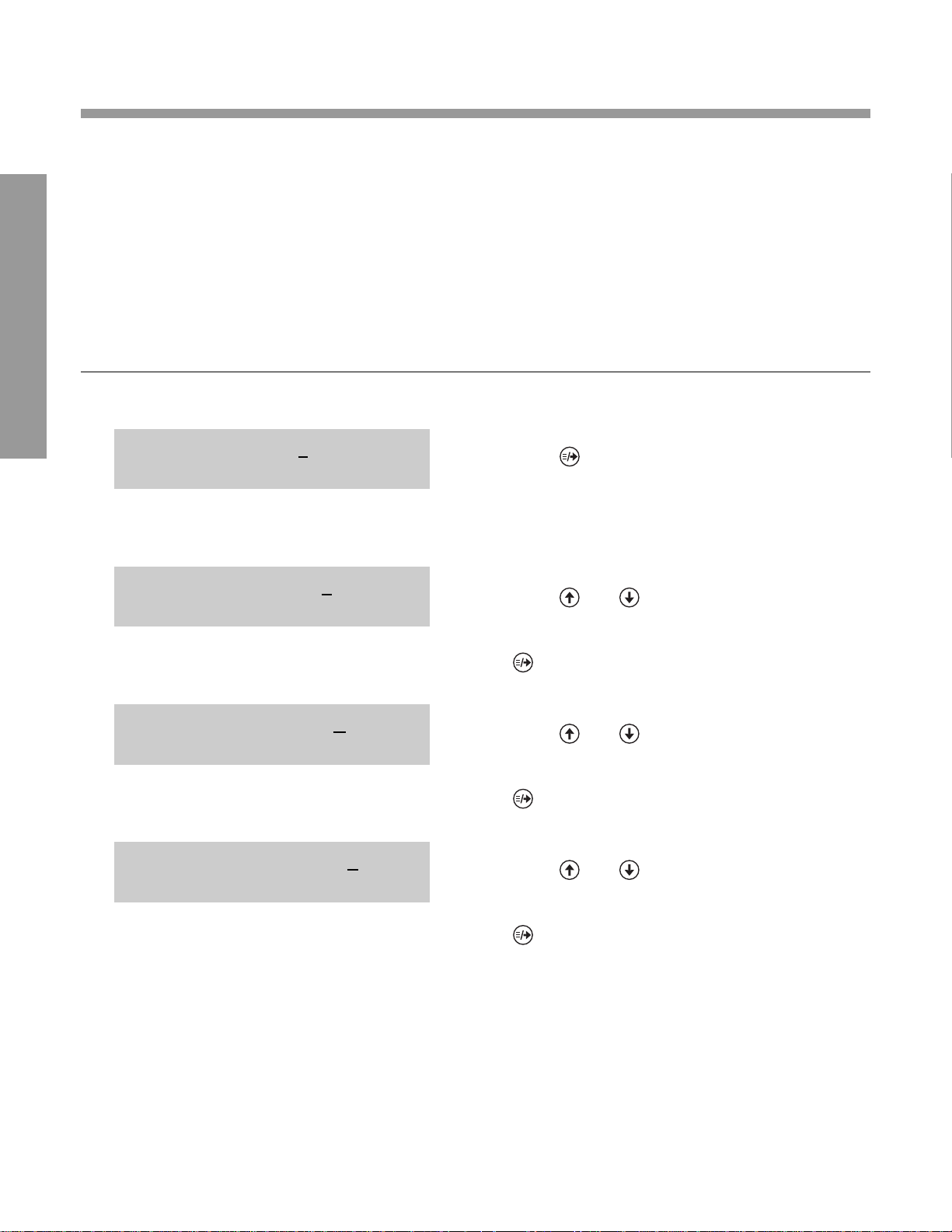
CHANGING FA CTOR Y SETTINGS FOR:
COMPUTER PORT “ON” OPTION
Refer to the chart on page 2.9 for Computer Port
Configurations.
NOTE: The first four numbers in each display
(below) are factory settings. The display on your
instrument may be different for the first four
numbers (depending on whether the first four
factory settings were accepted or changed).
DISPLA Y WHA T YOU DO
1. Press (places a cursor under factory
0. setting for first computer port option).
2. Press and to cycle through choices for
BAUD RATE.
0. When the desired choice is displayed, press
0. (moves cursor under setting for next
0. option).
3. Press and to cycle through choices
for DATA BITS.
0. When the desired choice is displayed, press
0. (moves cursor under setting for next
0. option).
4. Press and to cycle through choices for
PARITY.
0. When the desired choice is displayed, press
0. (moves cursor under setting for next
0. option).
1 1 4 0 2 9801010
PARITY NONE
1 1 4 0 2 9801110
DATA BITS EIGHT
1 1 4 0 2 9801010
BAUD RATE 9600
1 1 4 0 2 9801110
COMPUTER PORT ON
Page 19
2
UNPACKING, GETTING
ACQUAINTED AND SET UP
Revised 6/03 2.13
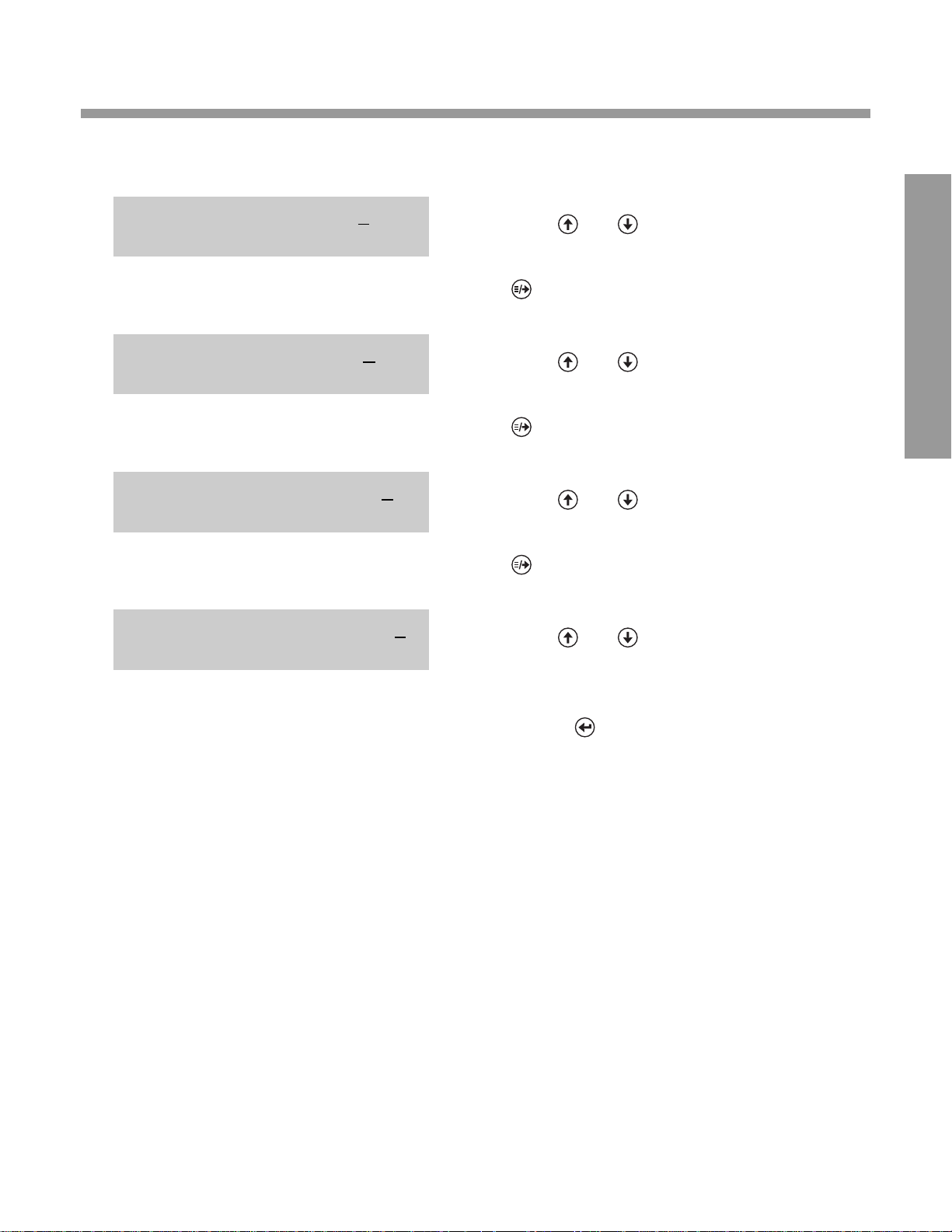
DISPLA Y WHA T Y OU DO
5. Press and to cycle through choices for
STOP BITS.
0. When the desired choice is displayed, press
0. (moves cursor under setting for next
0. option).
6. Press and to cycle through choices for
XON/XOFF.
0. When the desired choice is displayed, press
0. (moves cursor under setting for next
0. option).
7. Press and to cycle through choices for
BLOCK TRANSFER.
0. When the desired choice is displayed, press
0. (moves cursor under setting for next
0. option).
8. Press and to cycle through choices for
MODEM.
0.
•
Check to make sure all numbers on Line 1
represent desired settings.
0.
•
Press (accepts all settings and
0.
• advances display to SET DATE/TIME).
1 1 4 0 2 9801110
MODEM OFF
1 1 4 0 2 9821000
BLOCK XFER OFF
1 1 4 0 2 9821010
XON/XOFF OFF
1 1 4 0 2 9821010
STOP BITS ONE
Page 20
2
UNPACKING, GETTING
ACQUAINTED AND SET UP
2.14 Revised 6/03
UNPACKING, GETTING ACQUAINTED AND SET UP
DISPLA Y WHA T Y OU DO
The display on your instrument is displaying the
date and time in your chosen format.
1. Press or until the correct two digits are
displayed.
0. Press .
2. Press or until the correct two digits are
displayed.
0. Press .
3. Press or until the correct two digits are
displayed.
0. Press .
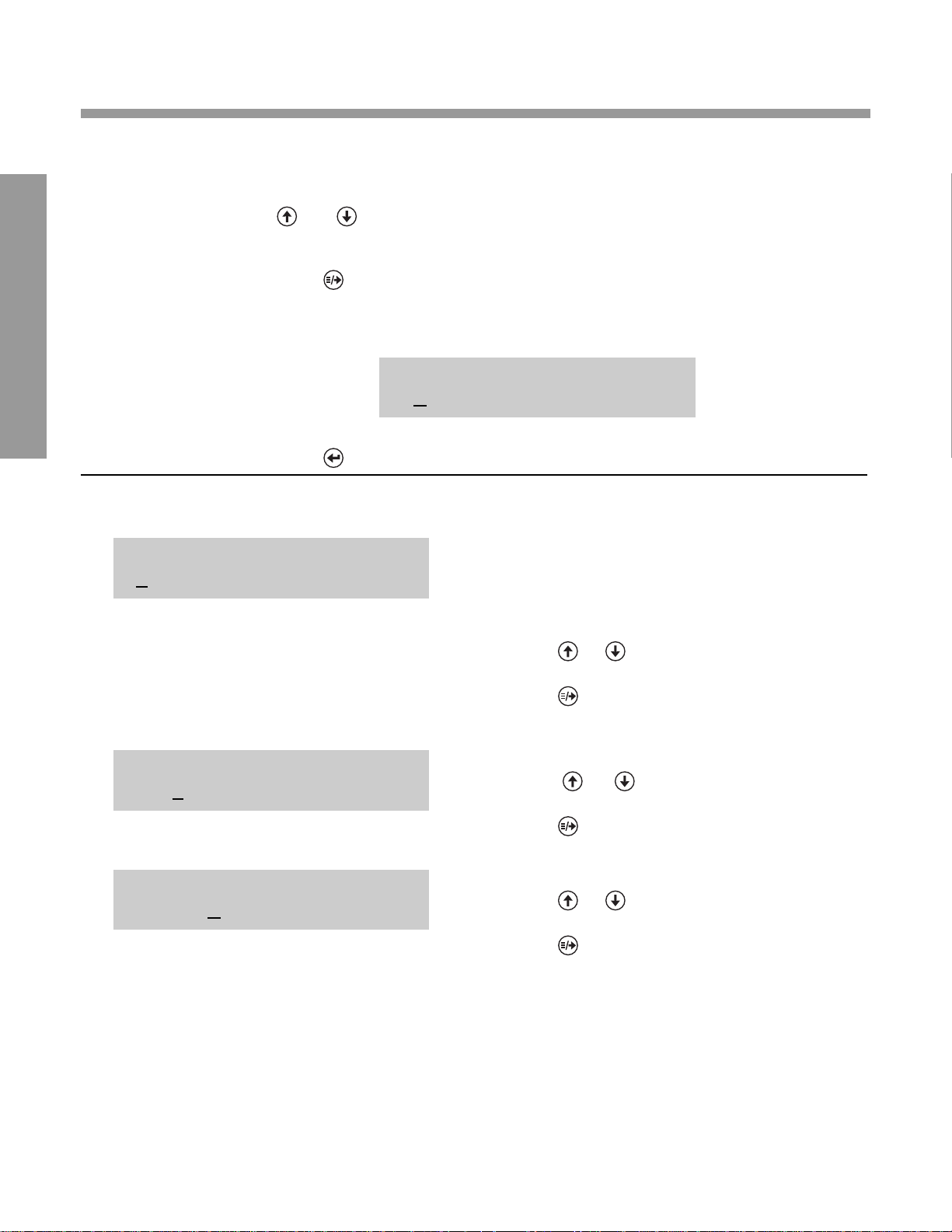
SET DATE/TIME
02/24/03 6:20AM
SET DATE/TIME
02/24/03 6:20AM
SET DATE/TIME
02/24/03 6:20AM
SETTING THE D ATE AND TIME
Use the following keys:
and To cycle through each available setting (e.g.,
digits for day/month/year and time, or AM/PM)
To move cursor under setting you desire to
change (e.g., “2” in display below is marked by
cursor and indicates “26” is ready for change)
To set the displayed date and time in the instrument
SET DATE/TIME
02/24/03 6:20AM
Page 21
2
UNPACKING, GETTING
ACQUAINTED AND SET UP
Revised 6/03 2.15
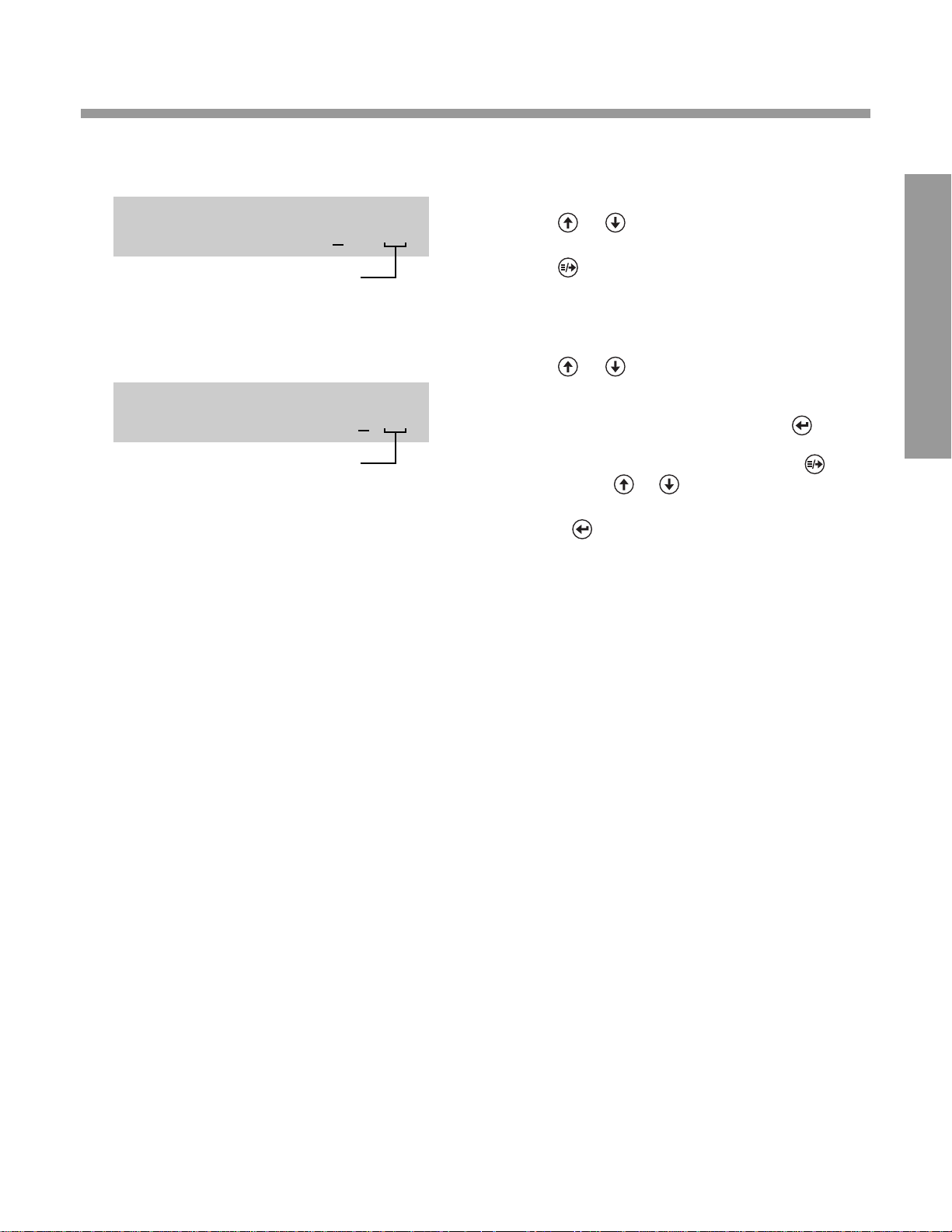
DISPLAY
AM or PM if AM/PM
format is selected
AM or PM if AM/PM
format is selected
WHA T Y OU DO
4. Press or until the correct two digits are
displayed.
0. Press .
5. Press or until the correct two digits are
displayed.
0.
•
If 24 HR format is selected, press .
0.
•
If AM/PM format is selected, press .
0.
• Then press or until the correct
choice is displayed.
0.
• Press .
SET DATE/TIME
02/24/03 6:20AM
SET DATE/TIME
02/24/03 6:20AM
Page 22
2
UNPACKING, GETTING
ACQUAINTED AND SET UP
2.16 Revised 6/03
UNPACKING, GETTING ACQUAINTED AND SET UP
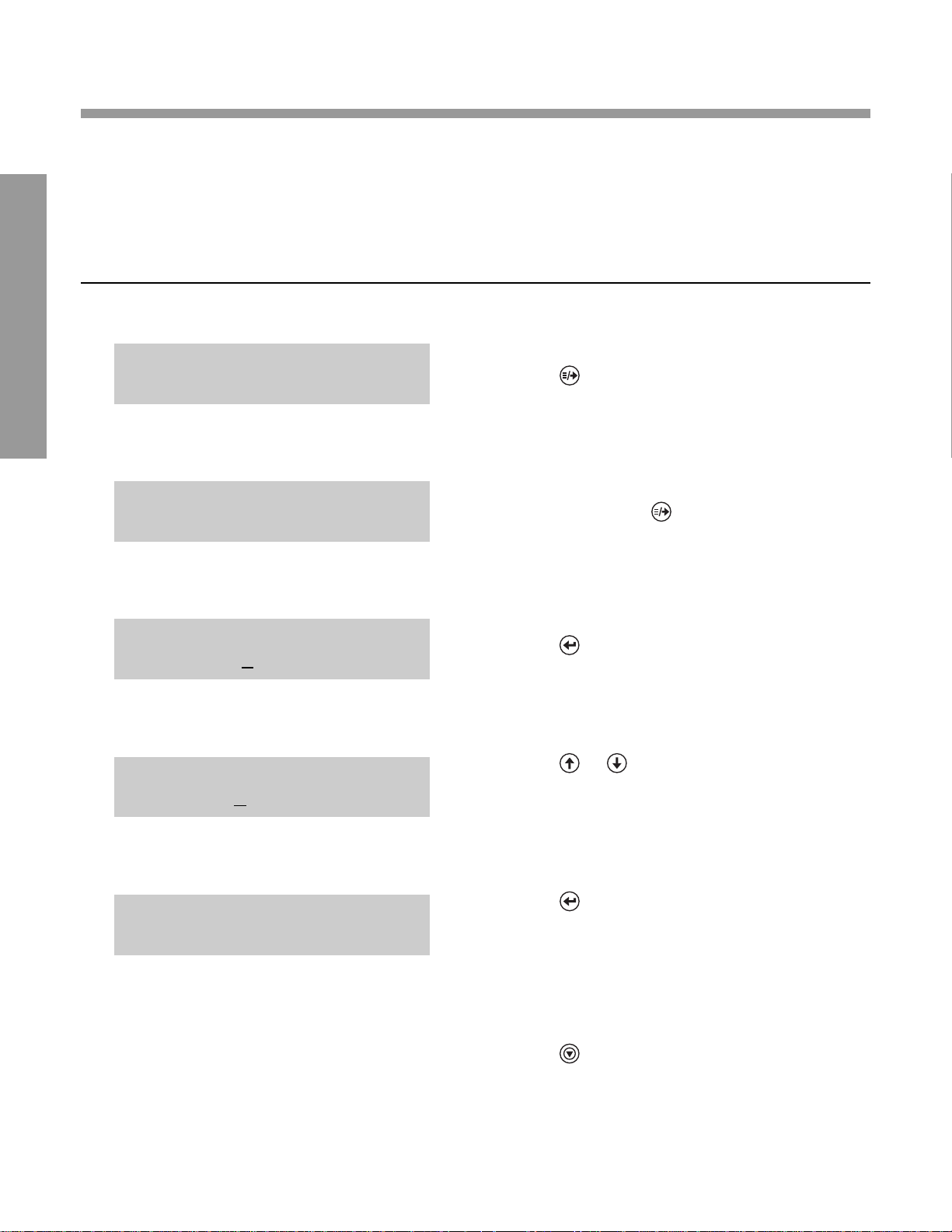
SETTING CREATININE CONCENTRA TIONUNITS
(Microalbumin/Creatinine Assay ONL Y)
The concentration units reported for creatinine are selectable between “mg/dL” and “mmol/L”.
The factory setting is “mg/dL”.
DISPLA Y WHA T YOU DO
1. Press .
0.
2. Repeatedly press , until
“SET CREATININE UNITS?” is displayed.
3. Press . This places a cursor below the “m” in
“mg/dL”, and the question mark disappears.
4. Press or to display “mmol/L” or
“mg/dL”.
5. Press to accept desired units.
6. Press to exit the MENU.
SET CREATININE UNITS?
mmol/L
SET CREATININE UNITS
mmol/L
SET CREATININE UNITS
mg/dL
SET CREATININE UNITS?
mg/dL
READY: SCAN BAR CODE
02/24/03 2:09PM
Page 23
2
UNPACKING, GETTING
ACQUAINTED AND SET UP
Revised 6/03 2.17
The provided optical test cartridge allows you to
monitor the performance of the optical system over
time. (The optical test cartridge holds the cartridge
return spring in place and also simulates the mass
of a reagent test cartridge.)
Before samples are analyzed for the first time,
run the optical test cartridge.
IMPORTANT!
Keep a permanent record of the results
obtained (i.e., Mean Transmittance, Standard
Deviation and Drift).* These initial values will
be used for comparison, as in control charting
(and also to isolate the cause of an instrument
malfunction in conjunction with instructions
provided by our Bayer HealthCare Customer
Service Department Representative).
*It is recommended that you record the results on
the page provided in the appendix of this manual.
After the optical test cartridge is run initially,
it is recommended that the optical test cartridge
be run:
•
quarterly
•
after cleaning the cartridge compartment
•
after changing the air filter
•
when instructed to do so by our Customer
Service Representative
NOTE: Refer to Section 7, page 7.6, Instrument
Care and Routine Maintenance, for information
regarding the comparison of initial values obtained
for your instrument with values obtained
thereafter.
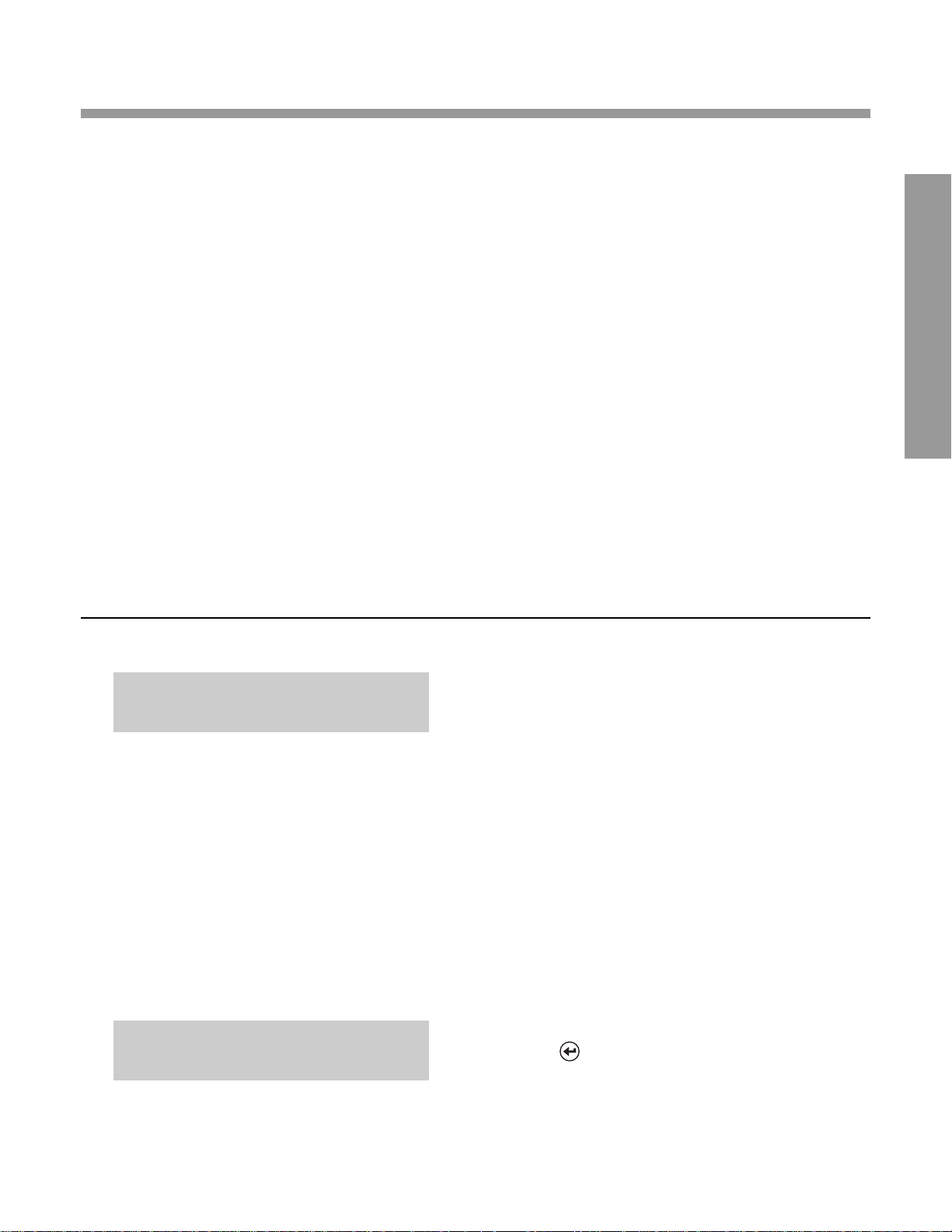
RUNNING THE OPTICAL TEST CARTRIDGE (Standard 1)
—Prior to Analyzing Samples f or the First Time
DISPLA Y WHA T Y OU DO
1. Locate the bar code on the optical test
cartridge.
2. Hold the cartridge so that the bar code faces
right.
3. Insert the cartridge (above dot on instrument)
into the bar code track.
4. Quickly (within 1 second) and smoothly, slide
the cartridge down past the dot.
0. A beep sounds to signal a successful scan.
0.
•
If no beep sounds, repeat the procedure. If a
beep repeatedly fails to sound, refer to
Troubleshooting, Section 6.
5. Press .
RUN STANDARD 1?
READY: SCAN BAR CODE
02/24/03 2:09PM
Page 24

2
UNPACKING, GETTING
ACQUAINTED AND SET UP
2.18 Revised 6/03
DISPLAY
6 MIN = total test time
After 1 minute:
Upon completion of test:
WHA T Y OU DO
6. Open the cartridge compartment door.
7. Hold the optical test cartridge so that the bar
code faces right.
0. Insert the cartridge into the compartment
until a subtle snap is heard/felt.
0. HINT: The cartridge is designed to fit only
one way into the instrument.
8. Close door.
09. Record the displayed results in the blanks
provided on the last page of this manual
(appendix).
10. Remove cartridge.
00. a) Open cartridge compartment door.*
00. b) Locate the button on the right side of the
cartridge compartment. Push and hold it
down with your right hand.
00. c) With your left hand, gently push the plastic
tab on the cartridge to the right; this action
releases (unlocks) cartridge.
00. d) Pull cartridge out of compartment.
*If the door is opened (within 15 minutes after assay
completion), the test result is displayed for only 30 seconds.
*If the door is not opened, the test result will remain
displayed for 15 minutes.
*At 15 minutes, an audible tone (error buzz) sounds and the
display changes to “READY: REMOVE TEST.”
1.0001 T 0.00012 SDnnn
S1 0.00387 DRIFT
1.0001 T 0.00012 SD
S1 5 MIN 10:03AM
PROCESSING STANDARD
S1 6 MIN 10:02AM
STANDARD 1
LOAD, CLOSE DOOR
UNPACKING, GETTING ACQUAINTED AND SET UP
Page 25
Revised 6/03 3.1
3
MENU
MENU
The MENU consists of up to nine (9) items
listed below. Note that item number two and item
number nine are optional (may or may not be
active—depends upon choices made during
“Instrument Setup”).
RECALL PREVIOUS TESTS?
SET SEQUENCE NUMBER? (optional)
RECALL CONTROL RESULTS?
VIEW CALIBRATION STATUS?
SET DATE/TIME?
INSTRUMENT SETUP?
SET CREATININE UNITS?
INSTRUMENT TEST?
RUN CONTROL? (optional)
To access the MENU (and display the first item):
•
Press
To display each additional item:
•
Repeatedly press
To select the item displayed:
•
Press
To exit the MENU:
•
Press
Information and instructions for each MENU item
are provided in this section.
9
8
7
6
5
4
3
2
1
Page 26
3.2 Revised 6/03
3
MENU
MENU
RECALL PREVIOUS TESTS?
•
Up to 16 test results can be recalled (and
printed if a printer is in use).
Recalled Test Result Format
Assay Result Reagent Lot No.
Date of Assay Sequence No. Time Assay Began
Date of Assay (optional) Time A. (optional)
6.0 % HbA1c LOT:0325
02/24/03 #011 11:24PM
1
DISPLAY
OR
OR
WHA T Y OU DO
1. Press .
(Test in progress is not aborted when MENU is
pressed.)
TEST IN PROGRESS
#001 6 MIN 10:15AM
READY: SCAN BAR CODE
02/24/03 2:09PM
WAIT: WARMING UP
02/24/03 2:09PM
Date of Assay
Test
Type
Sequence No.
(optional)
Time Assay Began
Page 27
Revised 6/03 3.3
3
MENU
DISPLAY
(example)
OR
(example)
OR
(example)
OR
(example)
(after all results are displayed)
WHA T Y OU DO
2. Press .
0.
•
The test result for the sample most recently
assayed is displayed first.
3. Press to recall up to 16 test results.
NOTES:
•
To print the displayed test result, press (if a printer
is in use).
–If is pressed for more than 3 seconds, all stored
test results are printed.
•
To return display to “RECALL PREVIOUS TESTS?,”
press once anytime during recall of test results.
•
To exit the MENU, press twice.
•
Albumin (A), creatinine (C), and the albumin/
creatinine ratio (A/C) results for the same specimen
are shown sequentially on separate displays, and are
labeled with the same sequence number (optional) and
date/time.
This display is retained until:
•
a calibration card bar code is read
•
or is pressed
– is pressed, the display returns to the first test result
previously recalled (this is the result for the most recent
sample assayed).
–If is pressed once, the display returns to “RECALL
PREVIOUS TESTS?”
–If is pressed twice, you exit the MENU.
NO MORE RESULTS STORED
IN INSTRUMENT
A/C=76 LOT:0325
02/24/03 #010 10:24PM
C=72.1mg/dL LOT:0325
09/13/02 #010 10:24PM
A=55.0mg/L LOT:0325
09/13/02 #010 10:24PM
6.0 % HbA1c LOT:0325
02/24/03 #011 11:24PM
RECALL PREVIOUS TESTS?
02/17/90 #001 11:47AM
Page 28
3.4 Revised 6/03
3
MENU
MENU
SET SEQUENCE NUMBER? (optional)
2
DISPLAY
OR
OR
WHA T Y OU DO
1. Press .
2. Press .
NOTE: If a sequence number is selected while a test is
in progress, the sequence number for the test in progress
is changed to the newly selected sequence number.
3. Press .
4. Press or to select the digit above the
cursor.
0. Press to move cursor (right) to next
0. digit.
0.
•
Repeat procedure to select second and
third digits.
0. Press .
5. To exit the MENU, press .
0. To display the next MENU item, press .
SET SEQUENCE NUMBER?
SET SEQUENCE NUMBER
#009
SET SEQUENCE NUMBER?
TEST IN PROGRESS
#001 6 MIN 10:15AM
READY: SCAN BAR CODE
02/24/03 2:09PM
WAIT: WARMING UP
02/24/03 2:09PM
Page 29
Revised 6/03 3.5
3
MENU
RECALL CONTROL RESULTS?
Format for Recalled Results (examples)
DCA 2000 CONTROLS, ONLY
Control Result Reagent Lot Number
mmmmmmmmmmmmmmmmmmmmmmmmm
**Cl—is replaced with “C2” for the DCA 2000 Abnormal or High Control and
“C1out” or “C2out” for an out-of-range control
ALL CONTROLS EXCEPT DCA 2000 CONTROLS*
Control Result Reagent Lot Number
*unless a DCA 2000 Control was run without scanning the DCA 2000
Control bar code card provided with the control kitmmmmmmmmn
9.8% HbA1c LOT:0325
02/24/03 c1 LOTC:0223
9.8% HbA1c LOT:0325
02/24/03 C1 LOTC:0223
3
• Up to 16 control results can be stored and
recalled (and also printed if a printer is in use).
• Upper case “C” in display indicates a result for a
DCA 2000
®
Control.
•Lower case “c” in display indicates a result for a
control other than a DCA 2000 Control*.
•A control lot number is displayed only for DCA
2000 Controls.
Date of Assay C1— indicates
a result for the
DCA 2000
Normal or Low
Control**
DCA 2000
Control Lot Number
Date of Assay c1—indicates control
level “1” of 9 possible
levels
Test
Type
Test
Type
—continued on next page
Page 30
3.6 Revised 6/03
3
MENU
MENU
Recall Control Results
DISPLAY
OR
OR
WHA T Y OU DO
1. Press .
2. Repeatedly press , until “RECALL
CONTROL RESULTS?” is displayed.
(Test in progress is not aborted.)
3. Press .
0.
•
The control result for the control most
recently assayed is displayed first.
RECALL CONTROL RESULTS?
TEST IN PROGRESS
#001 6 MIN 10:15AM
READY: SCAN BAR CODE
02/24/03 2:09PM
WAIT: WARMING UP
02/24/03 2:09PM
Page 31

Revised 6/03 3.7
3
MENU
DISPLAY
(example)
OR
(example)
OR
(example)
Reminder: Upper case “C” indicates
result for a DCA 2000
®
Control
(after all control
results are displayed)
WHA T Y OU DO
4. Press to recall up to 16 control
0. results.
NOTES:
•
To print the control result, press (if a printer is
in use).
– If is pressed for more than 3 seconds, all stored
control results are printed.
•
To return display to “RECALL CONTROL
RESULTS,” press once anytime during recall of
results.
•
To exit the MENU, press twice.
•
Albumin (A) and creatinine (C) for the same control
are shown sequentially on separate displays, and are
labeled with the same date.
This display is retained until:
• a bar code is scanned
•
or is pressed
– If is pressed, the display returns to the first control
result previously recalled (this is the result for the most
recent control assayed).
– If is pressed once, the display returns to “RECALL
CONTROL RESULTS?”
– If is pressed twice, you exit the MENU.
NO MORE CONTROLS STORED
IN INSTRUMENT
C=54.1mg/dL LOT:0105
02/24/03 C2 LOTC:0345
A=24.6mg/L LOT:0105
02/24/03 C2 LOTC:0345
9.8% HbA1c LOT:0325
02/24/03 C1 LOTC:0223
Page 32

3.8 Revised 6/03
3
MENU
MENU
DISPLAY
OR
OR
WHA T Y OU DO
1. Press .
0. (A test in progress is not aborted.)
2. Repeatedly press , until
0. “VIEW CALIBRATION STATUS?” is
displayed.
3. Press .
0.
•
The calibration status for the most recent
calibration is displayed first.
VIEW CALIBRATION STATUS?
TEST IN PROGRESS
#001 6 MIN 10:15AM
READY: SCAN BAR CODE
02/24/03 2:09PM
WAIT: WARMING UP
02/24/03 2:09PM
VIEW CALIBRATION STATUS?
The instrument stores up to two calibrations for
each DCA 2000 Reagent Test. The two calibrations
must be for two different lot numbers.
4
Page 33

Revised 6/03 3.9
3
MENU
DISPLAY
(example)
OR
(example)
(after all stored
calibrations are displayed)
WHA T Y OU DO
4. Press to recall status of each
0. calibration stored.
NOTES:
•
To return display to “VIEW CALIBRATION
STATUS?,” press once anytime during calibration
status recall.
•
To exit the MENU, press twice.
This display is retained until:
•
a bar code is scanned
•
or is pressed
– If is pressed, the display returns to the first
calibration status previously recalled (this is the status
for the most recent calibration).
– If is pressed once, the display returns to “VIEW
CALIBRATION STATUS?”
– If is pressed twice, you exit the MENU.
NO MORE CALIBRATIONS
STORED IN INSTRUMENT
LOT:0222 Malb/C
02/24/03 11:30PM
LOT:0183 HbA1c
02/24/03 11:23PM
Page 34

3.10 Revised 6/03
3
MENU
MENU
SET DATE/TIME?
Use the following keys:
and To cycle through each available setting (e.g.,
digits for day/month/year and time, or AM/PM)
To move cursor under setting you desire to
change (e.g., “2” in display below is marked by
cursor and indicates “26” is ready for change)
To set the displayed date and time in the instrument
SET DATE/TIME...
02/24/03 6:20AM
5
DISPLAY
OR
WHA T Y OU DO
1. Press .
2. Repeatedly press until
0. “SET DATE/TIME?” is displayed.
3. Press .
SET DATE/TIME?
02/24/03 6:19AM
READY: SCAN BAR CODE
02/24/03 2:09PM
WAIT:WARMING UP
02/24/03 2:09PM
Page 35

Revised 6/03 3.11
3
MENU
DISPLAY
AM or PM if AM/PM
format is selected
AM or PM if AM/PM
format is selected
WHA T Y OU DO
The display on your instrument is displaying the
date and time in your chosen format.
4. Press or until the correct two digits are
displayed.
0. Press .
5. Press or until the correct two digits are
displayed.
0. Press .
6. Press or until the correct two digits are
displayed.
0. Press . The cursor will move back to the first
digit of the date
7. Press to advance the cursor to the time
setting.
8. Press or until the correct two digits are
displayed.
0. Press .
9. Press or until the correct two digits are
displayed.
0. Press .
10.
•
If 24 HR format is selected, press .
10.
•
If AM/PM format is selected, press to
advance cursor to the AM/PM setting.
0. Press or until the correct choice is
displayed.
0. Press .
0. Press .
SET DATE/TIME
02/24/03 6:20AM
SET DATE/TIME
02/24/03 6:20AM
SET DATE/TIME
02/24/03 6:20AM
SET DATE/TIME
02/24/03 6:20AM
SET DATE/TIME
02/24/03 6:20AM
Page 36

3.12 Revised 6/03
3
MENU
(This page left blank on purpose.
Intended for future use.)
Page 37

Revised 6/03 3.13
3
MENU
DISPLAY
OR
OR
If computer port is Off.
OR
If computer port is On.
WHA T Y OU DO
1. Press .
2. Repeatedly press , until
0. “INSTRUMENT SETUP?” is displayed.
(Test in progress is not aborted.)
3. Press .
4. Refer to the chart (pages 2.8 and 2.9) to
determine the definition of each number and
abbreviated option appearing on your
instrument’s display.
0. Then continue with step 5 on page 3.14.
1 1 4 0 2 9801010mmmmm
T D L C P BUVWXYZmmmmm
1 1 4 0 0 MMMMMMMMMMM
T D L C P MMMMMMMMMMM
INSTRUMENT SETUP?
TEST IN PROGRESS
#001 6 MIN 10:15AM
READY: SCAN BAR CODE
02/24/03 2:09PM
WAIT: WARMING UP
02/24/03 2:09PM
INSTRUMENT SETUP?
6
Page 38

3.14 Revised 6/03
3
MENU
MENU
DISPLAY
If computer port is Off.
OR
If computer port is On.
If computer port is Off.
OR
If computer port is On.
If computer port is Off.
OR
If computer port is On.
WHA T Y OU DO
5. Press (places a cursor under the
0. setting for the first option, TIME FORMAT).
6. Press and to cycle through choices for
TIME FORMAT.
0. When the desired choice is displayed, press
0. (moves cursor under setting for
0. next option).
7.
Repeat step 6 to change settings for each option
as desired.
1 1 4 0 2 9801010mmmmm
T D L C P BUVWXYZmmmmm
1 1 4 0 0 mmmmmmmmmmmm
T D L C P Mmmmmmmmmmmm
1 1 4 0 2 9801010mmmmm
T D L C P BUVWXYZmmmmm
1 1 4 0 0 mmmmmmmmmmmm
TIME FORMAT AM/PM
1 1 4 0 2 9801010mmmmm
T D L C P BUVWXYZmmmmm
1 1 4 0 0 9801010mmmmm
T D L C Pmmmmmmmmmmmmm
INSTRUMENT SETUP DISPLAY OPTIONS/SETTINGS continued
Page 39

Revised 6/03 3.15
3
MENU
SET CREATININE UNITS
(Microalbumin/Creatinine Assay ONL Y)
The concentration units reported for creatinine are selectable between “mg/dL” and “mmol/L”.
The factory setting is “mg/dL”.
7
DISPLA Y WHA T YOU DO
1. Press .
0.
2. Repeatedly press , until
“SET CREATININE UNITS?” is displayed.
3. Press . This places a cursor below the “m”
in “mg/dL”, and the question mark disappears.
4. Press or to display “mmol/L” or
“mg/dL”.
5. Press to accept desired units.
6. Press to exit the MENU.
SET CREATININE UNITS?
mmol/L
SET CREATININE UNITS
mmol/L
SET CREATININE UNITS
mg/dL
SET CREATININE UNITS?
mg/dL
READY: SCAN BAR CODE
02/24/03 2:09PM
Page 40

3.16 Revised 6/03
3
MENU
MENU
INSTRUMENT TEST?
This menu item is to be used only under the
guidance of our Bayer HealthCare Customer
Service Department Representative.
•
Using this menu item allows you and our
Customer Service Representative to determine
existing problems with the keyboard, display,
bar code reader, printer port, memory,
and computer port functions.
8
Page 41

Revised 6/03 3.17
3
MENU
DISPLA Y WHA T Y OU DO
1. Press .
2. Repeatedly press until
0. “RUN CONTROL?” is displayed.
3. Press .
4. Press or until the desired
control number (choices 1–9) is displayed.
0. Press .
5. Follow instructions in Section 4, page 4.3, or
Section 5, page 5.4, under “Preparing Patient
Samples and Controls.”
SCAN BAR CODE
02/24/03 c1 11:10AM
SET CONTROL NUMBER
c1
RUN CONTROL?
02/17/90 #001 11:47AM
READY: SCAN BAR CODE
02/24/03 2:09PM
RUN CONTROL? (optional)
•
Available only if selected via “INSTRUMENT
SETUP” procedure
•
Marks the control result with a lower case “c”
• lower case “c” indicates controls other than
DCA 2000 Controls (unless a DCA 2000
Control was analyzed without scanning
the control bar code card provided with the
control kit)
9
Page 42

Revised 6/03 4.1
4
OPERATING INSTRUCTIONS
HEMOGLOBIN A
1C
OPERATING INSTRUCTIONS — HEMOGLOBIN A
1c
NOTE: The instructions in this section are for use
after “INSTRUMENT SETUP” has been performed
(a one time requirement upon receipt of a new or
factory-serviced instrument).
STEP 1: Turning the P ower ON
DISPLAY
After about 8 seconds:
(displayed for 8 seconds)
(displayed for 3 seconds)
(a beep is heard)
WHA T Y OU DO
1. Set the power switch to ON.
0. IMPORTANT:The program card must be
inserted or removed only when the power
switch is set to OFF. If the card is inserted
when the power is ON, the card can be
permanently damaged.
2. OPTIONAL: While the instrument is warming
up (usually 1 – 2 minutes but can take up to 8
minutes), you may access certain MENU items.
Refer to Section 3 for instructions.
0. Reminder: Current time is denoted by
“blinking colon.” Time assay began is denoted
by stationary colon.
READY: SCAN BAR CODE
02/24/03 2:09PM
WAIT: WARMING UP
02/24/03 2:09PM
INITIALIZING
KEEP DOOR CLOSED
COPYRIGHT 1991–2003
BY BAYER CORPORATION
SOFTWARE VERSION
E3.11/01.04
9.8% HbA1c LOT:0183
02/17/90 #001 11:47AM
Page 43

4.2 Revised 6/03
4
OPERATING INSTRUCTIONS
HEMOGLOBIN A
1C
OPERATING INSTRUCTIONS — HEMOGLOBIN A
1c
STEP 2: Calibration
When to Calibrate:
Calibrate the System for each new lot number of
reagent cartridges.
Materials Required:
•
Calibration Card (provided in DCA 2000
®
Hemoglobin A1cReagent Kit)
DISPLAY
OR
Any result or menu display
as long as testing is not in progress.
(After 5 seconds, the display
returns to the display in effect
prior to calibration.)
WHA T Y OU DO
1. Locate the dot (on the instrument) next to the
bar code track.
2. Locate the bar code on the calibration card.
3. Hold the card so that the bar code faces right.
4. Insert the card into the bar code track (above
dot). Hold card gently against the right side
of track.
0.
5. Quickly (within 1 sec.) and smoothly, slide
the card down past the dot.
0. A beep sounds to signal a successful scan.
0.
•
If no beep sounds, repeat procedure. If a
beep repeatedly fails to sound, refer to
Troubleshooting, Section 6.
CALIBRATION DATA SAVED
LOT:0132 HbA1c
READY: SCAN BAR CODE
02/24/03 2:09PM
NOTES:
•
The instrument stores up to two calibrations
for the DCA 2000 Hemoglobin A
1c
Reagent
Test. The two calibrations must be for two
different lot numbers.
•
The calibration stored in the instrument first
is deleted when a calibration card for a third lot
number is scanned.
Page 44

Revised 6/03 4.3
4
OPERATING INSTRUCTIONS
HEMOGLOBIN A
1C
STEP 3: Preparing Patient
Samples and Controls
Materials Required:
•
DCA 2000 Hemoglobin A1cReagent Kit
•
Patient Sample, DCA 2000®Hemoglobin A
1c
Control Kit or other control
•
Lint-free tissue
•
Clock or timer
OPENING THE FOIL PACKAGE
(Containing Reagent Cartridge)
IMPORTANT: Do not use scissors to cut open
foil package. Scissors can damage the reagent
cartridge, the flexible plastic pull-tab on the
cartridge or the sack containing desiccant.
1. Remove one foil package (containing a reagent
cartridge) from storage.
2. Refer to “Recommended Procedures
for
Handling Reagent Cartridges” in the
DCA 2000
Hemoglobin A1cReagent Kit
package insert for instructions on how and
when to open foil package.
INSPECTING THE CONTENTS
OF THE FOIL PACKAGE
The foil package contains:
1. reagent cartridge with flexible pull-tab and bar
code label
2. small sack (filled with desiccant)
0.
When handling the reagent cartridge, do not touch
or otherwise contaminate the optical window or
erroneous test results may occur.
0.
Discard the reagent cartridge if:
•
the cartridge is damaged
•
the flexible pull-tab is loose or missing
•
the small sack (desiccant) is missing or open
•
loose desiccant particles are found inside the
foil package
•
(refer to Service Information, Section 9)
OPTICAL
WINDOW
Page 45

4.4 Revised 6/03
4
OPERATING INSTRUCTIONS
HEMOGLOBIN A
1C
OPERATING INSTRUCTIONS — HEMOGLOBIN A
1c
CAPILLARY HOLDER
Unused capillary holders may be saved and used
with any lot of Hemoglobin A
1c
reagent cartridges.
1. Open plastic wrap by tearing wrap at serrated
1. edge.
2. Inspect the capillary holder for the presence of:
0. absorbent pad
0. glass capillary
0. latching mechanism
0.
If the capillary holder is missing any of the above
parts, discard the capillary holder (refer to Service
Information, Section 9).
3
2
1
1
2
3
Page 46

Revised 6/03 4.5
4
OPERATING INSTRUCTIONS
HEMOGLOBIN A
1C
FILLING THE CAPILLARY
WITH WHOLE BLOOD
(Instructions for filling capillary with
control sample are found in the
DCA 2000 Hemoglobin A
1c
Control package
insert.)
IMPORTANT PLEASE READ CAREFULLY:
Once the capillary is filled with sample, analysis must begin within 5 minutes.
There is no need to rush. Five minutes allows enough time for proper
completion of procedures.
Within 5 minutes after filling glass capillary (step 1, next page), complete steps
2-5 (page 4.7) and one of the following, whichever applies.
EITHER
•
For a Patient Sample or any
control other than
DCA 2000 Control — Steps 1 through 9 under “WHAT
YOU DO” on pages 4.8-4.9
OR
•
For DCA 2000 Control — Steps 1 through 14 under “WHAT
YOU DO” on pages 4.12-4.13
IMPORTANT: If sample analysis does not begin within 5 minutes after filling
glass capillary, discard capillary. If capillary is in the reagent cartridge, discard
both capillary and reagent cartridge.
WARNING! POTENTIAL BIOHAZARD
All products or objects which come into contact with human blood, even after cleaning, should be handled as if
capable of transmitting viral diseases.
The user should follow the recommendations for prevention of blood-borne transmissible diseases in healthcare
settings, as recommended for potentially infectious human blood specimens in National Committee for Clinical
Laboratory Standards, Protection of Laboratory Workers from Instrument Biohazards and Infectious Disease
Transmitted by Blood, Body Fluids and Tissues: Approved Guideline. NCCLS Document M29-A [ISBN 1-56238339-6] NCCLS, 940 West Valley Road, Suite 1400, Wayne, Pennsylvania 19087-1898, USA, 1997. This document
has complete information on the topic of user protection and can be used as background material for instruction.
VAROITUS! MAHDOLLINEN TARTUNTAVAARA
Terveydenhoitohenkilökunnan, joka käyttää tätä laitetta useiden ihmisten tutkimiseen, tulee ottaa huomioon, että
kaikki tuotteet, jotka joutuvat kosketukseen ihmisveren kanssa, ovat myös puhdistuksen jälkeen mahdollisia
viirustartuntalähteitä.
Tartuntojen välttämiseksi suosittelemme laboratorion omien turvallisuusohjeiden ehdotonta noudattamista.
Page 47

4.6 Revised 6/03
4
OPERATING INSTRUCTIONS
HEMOGLOBIN A
1C
OPERATING INSTRUCTIONS — HEMOGLOBIN A
1c
FILLING THE CAPILLARY WITH WHOLE
BLOOD—continued
1. Complete step A) OR step B) depending upon
the blood sample source. Continue with step 2
on the next page.
0. IMPORTANT: Do not allow blood to contact
the plastic part of the capillary holder. Any
blood touching the plastic will be transferred
into the reaction buffer, along with the blood in
the glass capillary. This can cause an invalid
HbA
1c
result or possibly an error message.
If blood contacts the plastic part of the
capillary holder, discard capillary holder.
0. A) If filling glass capillary with blood from
finger prick:
0. A) 1. Hold the capillary holder at an angle.
0. A) 2. Touch only the tip of the capillary to a
small drop of blood on the finger until
the capillary is filled.
A) 0.
0. B) If filling glass capillary with blood
obtained by venipuncture:
0. B) 1. Mix sample well (by inversion or use of
an aliquot mixer) to prevent separation
of red blood cells and plasma.
0. B) 2. Remove stopper from blood collection
tube in such a way that a small sample
of blood remains on stopper.
0. B) 3. Hold the capillary holder at an angle.
0. B) 4. Touch only the tip of the capillary to
blood sample on stopper.
0. B) 0.
•
Do not attempt to fill capillary by
touching glass capillary to blood in
a blood collection tube. Attempting
to fill capillary in this manner most
often results in blood touching the
capillary holder. If blood touches
the capillary holder, discard
capillary holder.
B) 0.
Page 48

Revised 6/03 4.7
4
OPERATING INSTRUCTIONS
HEMOGLOBIN A
1C
2. Using a lint-free tissue, carefully wipe the
outside of the glass capillary.
0. Do not allow the tissue to touch the open end
of the glass capillary. Contact with the open
end of the capillary could result in loss of
sample (by wicking into tissue). If sample loss
is obvious, discard capillary holder; then repeat
procedure using a new capillary holder.
3. Inspect the glass capillary for the presence of
bubble(s). If bubble(s) are obvious, discard
capillary holder; then repeat procedure using a
new capillary holder.
4. Position the capillary holder in the correct
orientation for insertion into the reagent
cartridge.
0.
5. Carefully insert the capillary holder into the
reagent cartridge until the holder gently snaps
into place.
0. IMPORTANT: Avoid harsh insertion of
capillary holder. It is important not to dislodge
sample from glass capillary or erroneous results
may occur.
Page 49

4.8 Revised 6/03
4
OPERATING INSTRUCTIONS
HEMOGLOBIN A
1C
OPERATING INSTRUCTIONS — HEMOGLOBIN A
1c
DISPLA Y WHA T Y OU DO
1. Locate the dot (on the instrument) next to the
bar code track.
2. Locate the bar code on the reagent cartridge.
3. Hold the reagent cartridge so that the bar code
faces right.
4. Insert the reagent cartridge (above dot) into bar
code track.
0.
5. Quickly (within 1 sec.) and smoothly, slide
the reagent cartridge down past the dot.
0. A beep sounds to signal a successful scan.
0.
•
If no beep sounds, repeat procedure. If a
beep repeatedly fails to sound, refer to
Troubleshooting, Section 5.
READY: SCAN BAR CODE
02/24/03 2:09PM
STEP 4:Analyzing the Patient Sample
Page 50

Revised 6/03 4.9
4
OPERATING INSTRUCTIONS
HEMOGLOBIN A
1C
DISPLA Y WHA T YOU DO
6. Open the cartridge compartment door.
7. Hold the reagent cartridge so that the bar code
faces right.
0. Insert the reagent cartridge into the cartridge
compartment until a subtle snap is heard/felt.
High temperature part
Huom. Kuumenee käytettäessä
0.
0.
HINT: The cartridge is designed to fit only one
way into the instrument. Do not force cartridge
into instrument.
8. Using a smooth, slow, continuous motion,
pull flexible pull-tab completely out of reagent
cartridge.
9. Close door.
Dispose of flexible pull-tab.
0.
•
Five (5) seconds after the door is closed, a
beep sounds and the assay begins.
0.
• NOTE: If you accidentally close the door before you
pull the flexible plastic tab, you have 5 seconds to
re-open the door; the display returns to “LOAD
CARTRIDGE.” You may now pull the tab or correct
existing problem(s).
LOAD CARTRIDGE
PULL TAB, CLOSE DOOR
Page 51

4.10 Revised 6/03
4
OPERATING INSTRUCTIONS
HEMOGLOBIN A
1C
OPERATING INSTRUCTIONS — HEMOGLOBIN A
1c
DISPLAY
(colon does not blink)
After test is completed:
(colon does not blink)
WHA T Y OU DO
10. Record the displayed result before removing
the reagent cartridge.
11. Remove the reagent cartridge.
00. a) Open cartridge compartment door.*
00. b) Locate the button on the right side of the
cartridge compartment. Push and hold it
down with your right hand.
00. c) With your left hand, gently push the tab on
the cartridge to the right; this action
releases (unlocks) cartridge.
00. d) Pull the reagent cartridge out of the
compartment.
00. e) Discard cartridge in proper container,
according to your standard laboratory
procedures.
0.
*If the door is opened (within 15 minutes after assay
completion), the test result is displayed for only 30
seconds.
00. If the door is not opened, the test result will remain
displayed for 15 minutes.
00. At 15 minutes, an audible tone (error buzz) sounds
and the display changes to “READY: REMOVE
TEST.”
00. HINT: If the displayed test result was not recorded,
use the MENU to recall up to 16 test results (refer to
SECTION 3).
%HbA1c mmm
#001 10:21
TEST IN PROGRESS
#001 6 MIN 10:15AM
Page 52

Revised 6/03 4.11
4
OPERATING INSTRUCTIONS
HEMOGLOBIN A
1C
RESULTS:
The displayed test result requires no further
calculation. Hemoglobin A
1c
concentrations in the
following range are reported:
2.5% to 14.0%mm
The test is linear throughout this range.
Result preceded by a less than sign (<):
A less than sign in the display indicates a
concentration below the lower limit of the test
(under range). Report the result as being less than
2.5% Hemoglobin A
1c
. This method does not
provide for re-assay using a larger sample aliquot.
Results less than 2.5% Hemoglobin A
1c
are rare
and may indicate that the sample contains
substantial amounts of fetal hemoglobin (does not
react in the immunoassay); or that the patient may
be suffering from hemolytic anemia or
polycythemia (conditions which often result in a
significant decrease in the life span of red blood
cells).
Result preceded by a greater than sign (>):
A greater than sign in the display indicates a
concentration above the upper limit of the test
(over range). Report the result as being more than
14.0% Hemoglobin A
1c
. This method does not
provide for re-assay using a diluted sample. To
obtain a more quantitative test value at levels
greater than 14%, use another test method.
All laboratory tests are subject to random error. If
the test result is questionable, or if clinical signs
and symptoms appear inconsistent with test
results, re-assay the sample or confirm the result
using another method.
Page 53

4.12 Revised 6/03
4
OPERATING INSTRUCTIONS
HEMOGLOBIN A
1C
OPERATING INSTRUCTIONS — HEMOGLOBIN A
1c
Analyzing DCA 2000
®
Hemoglobin A1cControls, ONLY
NOTE: Follow instructions on page 4.8, if
analyzing a control other than a DCA 2000
Hemoglobin A
1c
Control.
Controls are analyzed in the same manner as the
patient sample.
•
A specially designed control bar code (that
enters the control lot number, etc.) is provided
with DCA 2000 HbA
1c
Controls.
DISPLAY
•
Therefore, when analyzing DCA 2000
Hemoglobin A
1c
Controls, use instructions that
contain steps for scanning the control bar code
card found on pages 4.12–4.14.
•
If using controls other than DCA 2000
Hemoglobin A
1c
Controls, refer to Section 3,
page 3.17, for information on labeling and
storing the control result.
WHA T Y OU DO
1. Locate the dot (on the instrument) next to the
bar code track.
2. Locate the bar code on the control card.
0.
NOTE: The control card is double-sided; one side for
normal, the other for abnormal. Make sure you are using
the correct side of the control card for the particular
DCA 2000 Control level in use.
C1 = = Normal
C2 = = Abnormal
3. Hold the control card so that the bar code
faces right.
4. Insert the control card into the bar code track
(above dot). Hold card gently against the right
side of track.
5. Quickly (within 1 sec.) and smoothly, slide
the card down past the dot.
0. A beep sounds to signal a successful scan.
0.
•
If no beep sounds, repeat procedure. If a
beep repeatedly fails to sound, refer to
Troubleshooting, Section 6.
6. Press .
RUN CONTROL C1?
LOTC:1112 HbA1c
READY: SCAN BAR CODE
02/24/03 2:09PM
Page 54

Revised 6/03 4.13
4
OPERATING INSTRUCTIONS
HEMOGLOBIN A
1C
DISPLA Y WHA T Y OU DO
07. Locate the bar code on the reagent cartridge.
08. Hold the reagent cartridge so that the bar code
faces right.
09. Insert the reagent cartridge (above dot) into
bar code track.
10. Quickly (within 1 sec.) and smoothly, slide
the reagent cartridge down past the dot.
00. A beep sounds to signal a successful scan.
00.
•
If no beep sounds, repeat procedure. If a
beep repeatedly fails to sound, refer to
Troubleshooting, Section 6.
11. Open the cartridge compartment door.
12. Hold the reagent cartridge so that the bar code
faces right.
00. Insert the reagent cartridge into the cartridge
compartment until a subtle snap is heard/felt.
00. HINT: The reagent cartridge is designed to fit
only one way into the instrument. Do not
force cartridge into instrument.
13. Using a smooth, slow continuous motion,
pull flexible pull-tab completely out of
reagent cartridge.
14.
Close door. Dispose of flexible pull-tab.
00. NOTE: If you accidentally close the door before you
pull the tab, you have 5 seconds to re-open the door;
the display returns to “LOAD CARTRIDGE.” You
may now pull the tab or correct existing problem(s).
LOAD CONTROL
PULL TAB, CLOSE DOOR
SCAN BAR CODE: HbA1c
02/24/03 C1 11:10AM
Page 55

4.14 Revised 6/03
4
OPERATING INSTRUCTIONS
HEMOGLOBIN A
1C
OPERATING INSTRUCTIONS — HEMOGLOBIN A
1c
DISPLAY
(colon does not blink)
Then:mmmmmmmmnmmmmmm.
After test is completed:
For a result within the acceptable control
range printed in the control package insert
OR
For a result outside the acceptable control
range printed in the control package insert
WHA T Y OU DO
15. NOTE: If “CONTROL OUT OF RANGE” is
displayed, press to display value of out-ofrange control.
15. Record the displayed result before removing
the reagent cartridge.
16. Remove the reagent cartridge.
00. a) Open cartridge compartment door.*
00. b) Locate the button on the right side of the
cartridge compartment. Push and hold it
down with your right hand.
00. c) With your left hand, gently push the tab on
the cartridge to the right; this action
releases (unlocks) cartridge.
00. d) Pull the reagent cartridge out of the
compartment.
00. e) Discard cartridge in proper container,
according to your standard laboratory
procedures.
00.
*If the door is opened (within 15 minutes after assay
completion), the test result is displayed for only 30
seconds.
If the door is not opened, the test result will remain
displayed for 15 minutes.
At 15 minutes, an audible tone (error buzz) sounds and
the display changes to “READY: REMOVE TEST.”
15.
CONTROL OUT OF RANGE
PRESS [ESC] TO PROCEED
%HbA1cmmmmm
0 C1 10:21AM
PROCESSING CONTROL
C1-HbA1c 6 MIN 11:11AM
Page 56

Revised 6/03 4.15
4
OPERATING INSTRUCTIONS
HEMOGLOBIN A
1C
CANCELLING A TEST
You may cancel a test anytime.
Important: If a test in progress is cancelled, the
test must be discarded.
DISPLAY
OR
(colon does not blink)
(an error buzz sounds)
WHA T Y OU DO
1. Press .
2. Press within 15 seconds.
0.
NOTE: After pressing , “PLEASE WAIT”
is displayed until the cartridge returns to original
loading position.
0. If is not pressed within 15 seconds:
0.
•
the display returns to the original display
(i.e., either display shown in Step 1).
0.
•
the test in progress continues without
interruption
CANCEL TEST?
PRESS [<--] TO CONFIRM
TEST IN PROGRESS
#001 6 MIN 10:15AM
LOAD CARTRIDGE
PULL TAB, CLOSE DOOR
Page 57

4.16 Revised 6/03
4
OPERATING INSTRUCTIONS
HEMOGLOBIN A
1C
OPERATING INSTRUCTIONS — HEMOGLOBIN A
1c
DISPLAY
–
displayed if the test is cancelled after the bar
code is scanned but before the cartridge
compartment door is opened
–a beep is heard
OR
–displayed if the test is cancelled during
sample analysis (cartridge is in instrument)
–an error buzz is heard
WHA T Y OU DO
3. The test is cancelled.
0.
•
Scan bar code
ORmm
0.
•
Open cartridge compartment door and
remove cartridge.
CANCELLED: DISCARD TEST
02/24/03 10:18AM
READY: SCAN BAR CODE
02/24/03 2:09PM
Page 58

Revised 6/03 5.1
5
OPERATING INSTRUCTIONS
MICROALBUMIN/CREATININE
OPERATING INSTRUCTIONS —
MICROALBUMIN/CREATININE
NOTE: The instructions in this section are for use
after “INSTRUMENT SETUP” has been performed
(a one time requirement upon receipt of a new or
factory-serviced instrument).
STEP 1: Turning the P ower ON
DISPLAY
After about 8 seconds:
(displayed for 8 seconds)
(displayed for 3 seconds)
(a beep is heard)
WHA T Y OU DO
1. Turn the power switch to ON.
0. IMPORTANT:The program card must be
inserted or removed only when the power
switch is set to OFF. If the card is inserted
when the power is ON, the card can be
permanently damaged.
2. OPTIONAL: While the instrument is warming
up (usually 1 – 2 minutes but can take up to 8
minutes), you may access certain MENU items.
Refer to Section 3 for instructions.
0. Reminder: Current time is denoted by
“blinking colon.” Time assay began is denoted
by stationary colon.
READY: SCAN BAR CODE
02/24/03 2:09PM
WAIT: WARMING UP
02/24/03 2:09PM
INITIALIZING
KEEP DOOR CLOSED
COPYRIGHT 1991-2003
BY BAYER CORPORATION
SOFTWARE VERSION
E3.11/01.04
9.8% HbA1c LOT:0183
02/17/90 #001 11:47AM
Page 59

5.2 Revised 6/03
5
OPERATING INSTRUCTIONS
MICROALBUMIN/CREATININE
OPERATING INSTRUCTIONS — MICRO ALB UMIN / CREATININE
STEP 2: Calibration
When To Calibrate:
Calibrate the System for each new lot number of
reagent cartridges.
Materials Required:
•
Calibration Card (provided in DCA 2000
®
Microalbumin/Creatinine Reagent Kit)
NOTES:
•
The calibration card has two bar codes, one on
each side of the card. Side 1 and Side 2 are
identified on the card. Either side may be
scanned first. After the first scan, the display
will indicate the next side to be scanned. Side 1
is Malb/C; Side 2 is Malb-2.
•
It is necessary to scan both bar codes to
calibrate the system for each new lot of reagent
cartridges.
•
The instrument stores up to two calibrations for
the DCA 2000 Microalbumin/Creatinine
Reagent Test. The two calibrations must be for
two different lot numbers.
•
The calibration stored in the instrument first is
deleted when a calibration card for a third lot
number is scanned.
DISPLAY
OR
Any result or menu display
as long as testing is not in progress.
WHA T Y OU DO
1. Locate the dot (on the instrument) next to the
bar code track.
2. Locate the bar code on the calibration card.
3. Hold the card so that the bar code faces right.
4. Insert the card into the bar code track (above
dot). Hold card gently against the right side
of track.
0.
READY: SCAN BAR CODE
02/24/03 2:09PM
Page 60

Revised 6/03 5.3
5
OPERATING INSTRUCTIONS
MICROALBUMIN/CREATININE
DISPLAY
After 5 seconds, the instrument then sounds a
2 second beep and displays:
WHA T Y OU DO
5. Quickly (within 1 sec.) and smoothly, slide
the card down past the dot.
0. A beep sounds to signal a successful scan.
0.
•
If no beep sounds, repeat procedure. If a
beep repeatedly fails to sound, refer to
Troubleshooting, Section 6.
6. Repeat Steps 1–5 above using the other side of
the card.
0. If the second bar code is not scanned within 5
minutes:
0.
•
the display returns to the original display
and the calibration process must start over.
(See previous page.)0.
CALIBRATION DATA SAVED
LOT:0432 Malb-2
SCAN CALIBRATION CARD
LOT:0432 Malb-2
CALIBRATION DATA SAVED
LOT:0432 Malb/C
Page 61

5.4 Revised 6/03
5
OPERATING INSTRUCTIONS
MICROALBUMIN/CREATININE
OPERATING INSTRUCTIONS — MICRO ALB UMIN / CREATININE
STEP 3: Preparing Patient
Samples and Controls
Materials Required:
•
DCA 2000 Microalbumin/Creatinine Reagent Kit
•
Patient Sample, DCA 2000®Microalbumin/
Creatinine Control Kit or other control
•
Lint-free tissue
OPENING THE FOIL PACKAGE
(Containing Reagent Cartridge)
IMPORTANT: Do not use scissors to cut open
foil package. Scissors can damage the reagent
cartridge, the flexible pull-tab on the cartridge or
the sack containing desiccant.
1. Remove one foil package (containing a reagent
cartridge) from storage.
2. Refer to “Recommended Procedures
for
Handling Reagent Cartridges” in the
DCA 2000
Microalbumin/Creatinine Reagent
Kit package insert for instructions on how and
when to open foil package.
INSPECTING THE CONTENTS
OF THE FOIL PACKAGE
The foil package contains:
1. reagent cartridge with flexible pull-tab and bar
code label
2. small sack (filled with desiccant)
0.
When handling the reagent cartridge, do not touch
or otherwise contaminate the optical window or
erroneous test results may occur.
0.
Discard the reagent cartridge if:
•
the cartridge is damaged
•
the flexible pull-tab is loose or missing
•
the small sack (desiccant) is missing or open
•
loose desiccant particles are found inside the
foil package
•
(refer to Service Information, Section 9)
OPTICAL
WINDOW
Page 62

Revised 6/03 5.5
5
OPERATING INSTRUCTIONS
MICROALBUMIN/CREATININE
CAPILLARY HOLDER AND PLUNGER
Unused capillary holders may be saved and used
with any lot of microalbumin/creatinine reagent
cartridges.
1. Remove a capillary holder and plunger from
plastic bag.
2. Inspect the capillary holder for the presence of:
0. absorbent pad
0. glass capillary
0. latching mechanism
0. starch plug
0.
3. If the capillary holder is missing any of the
above parts, discard the capillary holder; also
discard if the starch plug is at the bottom of the
capillary tube (refer to Service Information,
Section 9).
4
3
2
1
plunger
1
2
2
3
4
Page 63

5.6 Revised 6/03
5
OPERATING INSTRUCTIONS
MICROALBUMIN/CREATININE
OPERATING INSTRUCTIONS — MICRO ALB UMIN / CREATININE
FILLING THE CAPILLARY WITH
URINE
1. Complete step A) OR step B) depending upon
the volume of urine specimen available.
Continue with Step 2 on the next page.
1. IMPORTANT: Do not allow urine to contact
either the plastic part of the capillary holder or
the adsorbent material in the capillary holder.
Any urine touching the plastic will be
transferred into the reaction buffer, along with
the urine in the glass capillary. This can cause
an invalid microalbumin/creatinine result or
possibly an error message. If urine contacts
the plastic part of the capillary holder,
discard capillary holder.
A) If filling capillary tube with urine from a
container with a large sample volume:
A) 1. Immerse the tip of the capillary tube in
the urine specimen to a level just above
the plug in the capillary.
A) 2. Allow enough time for the urine
specimen to flow into the capillary tube
and come in contact with the starch
plug, approximately 5 seconds. Wetting
the starch plug seals the capillary tube
and keeps the urine within the tube.
A) 3. Remove the capillary tube from the
urine specimen. If the urine flows back
down the tube, re-immerse the capillary
tube in the urine specimen again,
allowing enough time to ensure that the
starch plug becomes saturated.
(Instructions for filling capillary with
control sample are found in the DCA 2000
Microalbumin/Creatinine Control package insert.)
B) If filling capillary tube with urine from a
container with a small sample volume:
B) 1. Immerse the tip of the capillary tube
in the urine specimen. Tilt sample
container and capillary holder to a more
horizontal position to increase the rate of
flow into the capillary. Take care not to
spill the urine specimen.
B) 2. Allow enough time for the urine
specimen to flow into the capillary tube
and come in contact with the starch
plug. Wetting the starch plug seals the
capillary tube and keeps the urine within
the tube.
B) 3. Remove the capillary tube from the
urine specimen. If the urine flows back
down the tube, re-immerse the capillary
tube in the urine specimen again,
allowing enough time to ensure that the
starch plug becomes saturated.
➠
➠
Page 64

Revised 6/03 5.7
5
OPERATING INSTRUCTIONS
MICROALBUMIN/CREATININE
2. Using a lint-free tissue, carefully wipe the
outside of the glass capillary tube. Do not
allow the tissue to touch the open end of the
glass capillary. Contact with the open end of
the capillary tube could result in loss of sample
(by wicking into tissue). If sample loss is
obvious, discard the capillary holder; then
repeat procedure using a new capillary holder.
3. Inspect the glass capillary tube for the
presence of bubble(s). If bubbles are obvious,
discard the capillary holder; then repeat
procedure using a new capillary holder.
4. Position the capillary holder in the correct
orientation for insertion into the reagent
cartridge.
5. Carefully insert the capillary holder into the
reagent cartridge until the holder gently snaps
into place.
IMPORTANT: Avoid harsh insertion of the
capillary holder. It is important not to dislodge
sample from the capillary tube, or erroneous
results may occur.
Page 65

5.8 Revised 6/03
5
OPERATING INSTRUCTIONS
MICROALBUMIN/CREATININE
OPERATING INSTRUCTIONS — MICRO ALB UMIN / CREATININE
DISPLA Y WHA T Y OU DO
1. Locate the dot (on the instrument) next to the
bar code track.
2. Locate the bar code on the reagent cartridge.
3. Hold the reagent cartridge so that the bar code
faces right.
4. Insert the reagent cartridge (above dot) into bar
code track.
0.
5. Quickly (within 1 sec.) and smoothly, slide
the reagent cartridge down past the dot.
0. A beep sounds to signal a successful scan.
0.
•
If no beep sounds, repeat procedure. If a
beep repeatedly fails to sound, refer to
Troubleshooting, Section 6.
READY: SCAN BAR CODE
02/24/03 2:09PM
STEP 4:Analyzing the Patient Sample
Page 66

Revised 6/03 5.9
5
OPERATING INSTRUCTIONS
MICROALBUMIN/CREATININE
DISPLA Y WHA T YOU DO
6. Open the reagent cartridge compartment door.
7. Hold the reagent cartridge so that the bar code
faces right.
0. Insert the reagent cartridge into the cartridge
compartment until a subtle snap is heard/felt.
0. HINT: The cartridge is designed to fit only
one way into the instrument. Do not force
cartridge into instrument.
8. Insert plunger into hole in top of capillary
holder.
0. Depress plunger into capillary holder fully.
Plunger will lock into the capillary holder.
0. Press .
High temperature part
Huom. Kuumenee käytettäessä
00.
9. Using a smooth, slow, continuous motion,
pull flexible pull-tab completely out of
reagent cartridge.
10. Close door.
Dispose of flexible pull-tab.
0.
•
Five (5) seconds after the door is closed, a
beep sounds and the assay begins.
0.
• NOTE: If you accidentally close the door before
you pull the flexible tab, you have 5 seconds to
re-open the door; the display returns to “LOAD
CARTRIDGE.” You may now pull the tab or correct
existing problem(s).
—continued on next page
PULL TAB, CLOSE DOOR
LOAD CARTRIDGE, INSERT
PLUNGER, PRESS [<--]
Page 67

5.10 Revised 6/03
5
OPERATING INSTRUCTIONS
MICROALBUMIN/CREATININE
OPERATING INSTRUCTIONS — MICRO ALB UMIN / CREATININE
DISPLAY
(colon does not blink)
After test is completed:
(colon does not blink)
WHA T Y OU DO
11. Record the displayed result before removing
cartridge.
11. NOTE: If creatinine units are mg/dL, then the
Albumin/Creatinine (A/C) ratio is reported as
mg/g. If the creatinine units are mmol/L, then
the Albumin/Creatinine (A/C) ratio is reported
as mg/mmol.
12. Remove cartridge.
00. a) Open cartridge compartment door.*
00. b) Locate the button on the right side of the
cartridge compartment. Push and hold it
down with your right hand.
00. c) With your left hand, gently push the tab on
the cartridge to the right; this action
releases (unlocks) cartridge.
00. d) Pull cartridge out of compartment.
00. e) Discard cartridge in proper container,
according to your standard laboratory
procedures.
00. *If the door is opened (within 15 minutes
after assay completion), the test result is
displayed for only 30 seconds.
00. If the door is not opened, the test result will
remain displayed for 15 minutes.
00. At 15 minutes, an audible tone (error buzz)
sounds and the display changes to “READY:
REMOVE TEST.”
00. HINT: If the displayed test result was not
recorded, use the MENU to recall up to 16
test results (refer to SECTION 3).
A=102mg/L C=141mg/dL
A/C=72 #001 10:17AM
TEST IN PROGRESS
#001 6 MIN 10:15AM
Page 68

Revised 6/03 5.11
5
OPERATING INSTRUCTIONS
MICROALBUMIN/CREATININE
RESULTS:
Albumin: The displayed test result requires no
further calculation. Albumin concentrations in the
following range are reported: 5 to 300 mg/L. The
test is linear throughout this range.
Creatinine: The displayed test result requires no
further calculation. Creatinine concentrations in
the following range are reported: 15 to 500 mg/dL
or 1.3 to 44.2 mmol/L. The test is linear
throughout this range.
Albumin/Creatinine Ratio: The displayed test
result requires no further calculation. Albumin/
Creatinine ratios can be reported in the following
range: 1 to 2,000 mg/g or 0.11 to 226 mg/mmol.
Albumin or creatinine result preceded by a less
than sign (<): A less than sign in the display
indicates a concentration below the lower limit of
the test (under range). This method does not
provide for re-assay using a larger sample.
Albumin or creatinine result preceded by a
greater than sign (>): A greater than sign in the
display indicates a concentration above the upper
limit of the test (over range). This method does not
provide for re-assay using a diluted sample. To
obtain a more quantitative test value, use another
test method.
Ratio result preceded by a less than (<) sign or
greater than (>) sign or (---): If the albumin
and/or creatinine result is under or over range, the
ratio will also be reported as under or over range.
In certain cases, no ratio will be reported (---).
Example 1: If the albumin result is >300 mg/L
and the creatinine result is 100 mg/dL
(8.84 mmol/L), then the ratio will be reported
as >300 mg/g (>26.5 mg/mmol).
Example 2: If the albumin result is 75 mg/L
and the creatinine result is <15 mg/dL
(<1.33 mmol/L), then the ratio will be reported
as >500 mg/g (>56.4 mg/mmol).
Example 3: If the albumin result is >300 mg/L
and the creatinine result is >500 mg/dL (>44.2
mmol/L), then no ratio will be reported (---).
Example 4: If the albumin result is <5 mg/L and
the creatinine result is <15 mg/dL (<1.33 mmol/L),
then no ratio will be reported (---).
All laboratory tests are subject to random error. If
the test result is questionable, or if clinical signs
and symptoms appear inconsistent with the test
results, re-assay the sample or confirm the result
using another method.
Page 69

5.12 Revised 6/03
5
OPERATING INSTRUCTIONS
MICROALBUMIN/CREATININE
OPERATING INSTRUCTIONS — MICRO ALB UMIN / CREATININE
Analyzing DCA 2000
®
Controls, ONLY
NOTE: Follow instructions on page 5.8, if
analyzing a recommended control other than a
DCA 2000 Microalbumin/Creatinine Control.
Controls are analyzed in the same manner as the
patient sample.
•
A specially designed control bar code (that
enters the control lot number, etc.) is provided
with DCA 2000 Microalbumin/Creatinine
Controls.
DISPLAY
•
Therefore, when analyzing DCA 2000
Microalbumin/Creatinine Controls, use
instructions that contain steps for scanning the
control bar code card found on pages 5.12–5.14.
•
If using controls other than DCA 2000
Microalbumin/Creatinine Controls, refer to
Section 3, page 3.17, for information on labeling
and storing the control result.
WHA T Y OU DO
1. Locate the dot (on the instrument) next to the
bar code track.
2. Locate the bar code on the control card.
0.
NOTE: The control card is double-sided; one side for
Low, the other for High. Make sure you are using the
correct side of the control card for the particular DCA
2000 Control level in use.
C1 = = Low
C2 = = High
3. Hold the control card so that the bar code
faces right.
4. Insert the control card into the bar code track
(above dot). Hold card gently against the right
side of track.
5. Quickly (within 1 sec.) and smoothly, slide
the card down past the dot.
0. A beep sounds to signal a successful scan.
0.
•
If no beep sounds, repeat procedure. If a
beep repeatedly fails to sound, refer to
Troubleshooting, Section 6.
6. Press .
RUN CONTROL C1?
LOTC:1112 Malb/C
READY: SCAN BAR CODE
02/24/03 2:09PM
Microalb umin / Creatinine
Page 70

Revised 6/03 5.13
5
OPERATING INSTRUCTIONS
MICROALBUMIN/CREATININE
DISPLA Y WHA T Y OU DO
07. Locate the bar code on the reagent cartridge.
08. Hold the cartridge so that the bar code faces
right.
09. Insert the cartridge (above dot) into bar code
track.
10. Quickly (within 1 sec.) and smoothly, slide
the cartridge down past the dot.
00. A beep sounds to signal a successful scan.
00.
•
If no beep sounds, repeat procedure. If a
beep repeatedly fails to sound, refer to
Troubleshooting, Section 6.
11. Open the cartridge compartment door.
12. Hold the reagent cartridge so that the bar code
faces right.
00. Insert the cartridge into the cartridge
compartment until a subtle snap is heard/felt.
00. HINT: The cartridge is designed to fit only
one way into the instrument. Do not force
cartridge into instrument.
13. Insert plunger into hole in top of capillary
holder.
00. Depress plunger into capillary holder fully.
Plunger will lock into the capillary holder.
00. Press .
14. Using smooth, slow continuous motion, pull
flexible pull-tab completely out of reagent
cartridge.
15.
Close door. Dispose of flexible pull-tab.
00. NOTE: If you accidentally close the door before you
pull the tab, you have 5 seconds to re-open the door;
the display returns to “LOAD CARTRIDGE.” You
may now pull the tab or correct existing problem(s).
PULL TAB, CLOSE DOOR
LOAD CARTRIDGE, INSERT
PLUNGER, PRESS [<--]
SCAN BAR CODE Malb/C
02/24/03 C1 11:10AM
— continued on next page
Page 71

5.14 Revised 6/03
5
OPERATING INSTRUCTIONS
MICROALBUMIN/CREATININE
OPERATING INSTRUCTIONS — MICRO ALB UMIN / CREATININE
DISPLAY
(colon does not blink)
After test is completed:
For a result within the acceptable control
range printed in the control package insert
NOTE: The albumin/creatinine ratio is not
calculated for controls.
OR
For a result outside the acceptable control
range printed in the control package insert
WHA T Y OU DO
16. NOTE: If “CONTROL OUT OF RANGE” is
displayed, press to display value of out-ofrange control.
15. Record the displayed result before removing
cartridge.
17. Remove cartridge.
00. a) Open cartridge compartment door.*
00. b) Locate the button on the right side of the
cartridge compartment. Push and hold it
down with your right hand.
00. c) With your left hand, gently push the
flexible tab on the cartridge to the right;
this action releases (unlocks) cartridge.
00. d) Pull cartridge out of compartment.
00. e) Discard cartridge in proper container,
according to your standard laboratory
procedures.
00.
*If the door is opened (within 15 minutes after assay
completion), the test result is displayed for only 30
seconds.
If the door is not opened, the test result will remain
displayed for 15 minutes.
At 15 minutes, an audible tone (error buzz) sounds and
the display changes to “READY: REMOVE TEST.”
CONTROL OUT OF RANGE
PRESS [ESC] TO PROCEED
A=102mg/L C=141 mg/dL
0 C1 11:11AM
PROCESSING CONTROL
C1-Malb/C 7 MIN 11:11AM
Page 72

Revised 6/03 5.15
5
OPERATING INSTRUCTIONS
MICROALBUMIN/CREATININE
CANCELLING A TEST
You may cancel a test anytime.
Important: If a test in progress is cancelled, the
test must be discarded.
DISPLAY
OR
OR
(colon does not blink)
(an error buzz sounds)
WHA T Y OU DO
1. Press .
2. Press within 15 seconds.
0.
NOTE: After pressing , “PLEASE WAIT” is
displayed until the cartridge returns to original
loading position.
0. If is not pressed within 15 seconds:
0.
•
the display returns to original display (i.e.,
either display shown in Step 1).
0.
•
the test in progress continues without
interruption
CANCEL TEST?
PRESS [<--] TO CONFIRM
TEST IN PROGRESS
#001 6 MIN 10:15AM
PULL TAB, CLOSE DOOR
LOAD CARTRIDGE, INSERT
PLUNGER, PRESS [<--]
— continued on next page
Page 73

5.16 Revised 6/03
5
OPERATING INSTRUCTIONS
MICROALBUMIN/CREATININE
OPERATING INSTRUCTIONS — MICRO ALB UMIN / CREATININE
DISPLAY
–
displayed if the test is cancelled after the bar
code is scanned but before the cartridge
compartment door is opened
–a beep is heard
OR
–displayed if the test is cancelled during
sample analysis (cartridge is in instrument)
–an error buzz is heard
WHA T Y OU DO
3. The test is cancelled.
0.
•
Scan bar code
ORmm
0.
•
Open cartridge compartment door and
remove cartridge.
CANCELLED: DISCARD TEST
02/24/03 10:18AM
READY: SCAN BAR CODE
02/24/03 2:09PM
Page 74

Revised 6/03 6.1
6
ERROR AND WARNING MESSAGES,
ERROR CODES & TROUBLESHOOTING
ERROR AND WARNING MESSAGES,
ERR OR CODES AND TROUBLESHOOTING
Use the chart below to quickly find the correct page for
the desired error and warning message, error code, or
troubleshooting information.
ERROR AND WARNING MESSAGES
Error and warning messages are provided in alphabetical
order.
page
CANCELLED: DISCARD TEST/(date)(time) ............................. 6.2
DOOR OPEN ERROR .................................................................. 6.2
OUT OF DATE CONTROL.......................................................... 6.2
OUT OF DATE REAGENT.......................................................... 6.2
PLEASE WAIT ............................................................................. 6.2
SCAN CALIBRATION CARD..................................................... 6.2
SEE OPERATING MANUAL (COMPUTER
PORT ERROR-51)........................................................................ 6.2
SEE OPERATING MANUAL ERROR 90—RAM MEMORY .. 6.3
SEE OPERATING MANUAL/TEST ERROR XXX................... 6.3
SEE OPERATING MANUAL (ERROR Xx)............................... 6.3
SEE OPERATING MANUAL
(TEST UNKNOWN)
................... 6.3
WRONG CARD............................................................................ 6.3
ERROR CODES
E-1 ................................................................................................. 6.4
E-2.................................................................................................. 6.4
E-3.................................................................................................. 6.4
E-4.................................................................................................. 6.5
E-5................................................................................................. 6.5
E-6.................................................................................................. 6.5
TROUBLESHOOTING
..................................................................................................... 6.10
Hemoglobin A
1c
ERROR 101 ........................ 6.6
ERROR 102 ........................ 6.6
ERROR 103 ........................ 6.6
ERROR 104 ........................ 6.6
ERROR 105 ........................ 6.6
ERROR 106 ........................ 6.6
ERROR 107 ........................ 6.6
ERROR 108 ........................ 6.7
ERROR 109 ........................ 6.7
ERROR 110 ........................ 6.7
ERROR 111 ........................ 6.7
ERROR 112 ........................ 6.7
ERROR 113 ........................ 6.7
ERROR 114 ........................ 6.7
ERROR 115 ........................ 6.7
ERROR 116 ........................ 6.7
Microalbumin / Creatinine
ERROR 301 ........................ 6.8
ERROR 302 ........................ 6.8
ERROR 303 ........................ 6.8
ERROR 304 ........................ 6.8
ERROR 305 ........................ 6.8
ERROR 306 ........................ 6.8
ERROR 307 ........................ 6.8
ERROR 308 ........................ 6.8
ERROR 309 ........................ 6.9
ERROR 310 ........................ 6.9
ERROR 311 ........................ 6.9
ERROR 313 ........................ 6.9
ERROR 315 ........................ 6.9
ERROR 316 ........................ 6.9
Page 75

6.2 Revised 6/03
6
ERROR AND WARNING MESSAGES,
ERROR CODES & TROUBLESHOOTING
ERROR AND WARNING MESSAGES, ERR OR CODES AND TROUBLESHOO TING
ERROR AND WARNING MESSAGES
ERROR OR WARNING CAUSE SOLUTION
The cartridge compartment door was Open the cartridge compartment door.
opened while testing was in progress. Remove and discard the reagent
The door was then closed and “PLEASE cartridge.
WAIT” was displayed prior to
Repeat the test.
“CANCELLED: DISCARD TEST.”
• If the error message “SEE OPERATING
OR
•
MANUAL/(any system error)” is
was pressed in response to
• displayed again, refer to Section 9, for
“SEE OPERATING MANUAL/
• instructions on “How to Report the
(any system error) ” • Problem.”
The cartridge compartment door was Close door.
opened while testing was in progress.
Wait for next display “PLEASE WAIT.”
The bar code for an out-of-date control Discard out-of-date control(s).
(past expiration date) has been read.
(displayed for 15 seconds)
Control lot number and test
name above are examples, only.
The bar code for an out-of-date (past Discard out-of-date reagent cartridge(s).
expiration date) reagent cartridge has
been read.
(displayed for 15 seconds)
Reagent lot number and test
name above are examples, only.
The cartridge compartment door was Wait until cartridge is in correct position for
opened while testing was in progress. removal (until “CANCELLED: DISCARD
Then the door was closed. TEST” is displayed).
OR
A System Error was detected and
was pressed.
The calibration card for the reagent Scan the correct calibration card.
cartridge in use has not been scanned.
•
Check name and lot number of cartridge
• in use.
Lot number and test name
above are examples, only
The computer port is ON and/or: 1. Check to make sure cable is securely
•
the instrument buffer is full of
0. connected to both instrument and
•
untransmitted results
0. computer.
•
the computer will not accept results
2. If the problem persists, refer to Section 9,
0. for instructions on “How to Report the
0. Problem.”
0. HINT: To facilitate testing (without
0. transmitting results to a computer) turn
0. off the computer port (refer to Section 3,
0. MENU).
SEE OPERATING MANUAL
COMPUTER PORT ERROR-E51
SCAN CALIBRATION CARD
LOT:1154 HbA1c
PLEASE WAIT
02/17/90 #001 11:47AM
OUT OF DATE REAGENT
LOT:1154 HbA1c
OUT OF DATE CONTROL
LOTC:1154 HbA1c
DOOR OPEN ERROR
CLOSE DOOR
CANCELLED: DISCARD TEST
02/24/03 10:18AM
Page 76

Revised 6/03 6.3
6
ERROR AND WARNING MESSAGES,
ERROR CODES & TROUBLESHOOTING
ERROR OR WARNING CAUSE SOLUTION
When the power was turned ON (I), a Press key.
failure was detected in the non-volatile
“INSTRUMENT SETUP/PRESS
memory.
[<--]
TO CONTINUE” is displayed.
All factory settings (defaults) are now active.
•
Turn to Section 2 and follow the instruc-
•
tions beginning with “Viewing Factory
•
Settings.” You must now, once again,
•
either accept or change factory settings.
An error relating to a test measurement 1.Discard the test.
parameter has been detected (e.g.,
2.See pages 6.6, 6.7, 6.8, and 6.9.
abnormal hemoglobin levels, high C.V.’s,
3.Repeat the test using a new reagent
out of range hemoglobin transmission, etc.).
0.cartridge and sample.
4. If the problem persists, record the test
error identification number; then refer to
•
Section 8, for instructions on “How to
•
Report the Problem.”
* The instrument has detected an instrument
•
If testing is in progress, press .
error (motor or optical failure, temperature,
• Follow instructions under error message
etc.).
• “CANCELLED: DISCARD TEST.”
*OR:
•
If testing is not in progress, turn the
ERROR 2x—OPTICAL
• power OFF, then ON.
ERROR 3x—TEMPERATURE
If the problem persists, record the error
ERROR 4x—BAR CODE
message. Then refer to Section 9, for
ERROR 9x—INTERNAL
instructions on “How to Report the Problem.”
The instrument does not recognize the Contact Bayer HealthCare Customer Service
reagent test in use. Department. See Section 9, Service
Information.
In response to “SCAN CALIBRATION Scan correct calibration card.
CARD,” the wrong calibration card is
• Check name and lot number of cartridge
scanned.
•
in use.
(displayed for 5 seconds—then “SCAN Both sides of the calibration card must
• Repeat calibration process making certain
CALIBRATION CARD” appears) be scanned to enter the calibration for
• to scan both sides of the calibration card.
Microalbumin / Creatinine. •
WRONG CARD
SEE OPERATING MANUAL
LOT:2234 TEST: UNKNOWN
SEE OPERATING MANUAL
ERROR 11-MOTOR
SEE OPERATING MANUAL
TEST ERROR 115
SEE OPERATING MANUAL
ERROR 90-RAM MEMORY
ERROR—101 TO 116 for HbA
1c
ERROR—301 to 316 for
Microalbumin/Creatining
Page 77

6.4 Revised 6/03
6
ERROR AND WARNING MESSAGES,
ERROR CODES & TROUBLESHOOTING
ERROR AND WARNING MESSAGES, ERR OR CODES AND TROUBLESHOO TING
ERROR CODES
ERROR CODE CAUSE SOLUTION
An internal system error has been Contact Bayer HealthCare Customer
detected. Service Department. See Section 9, Service
Information.
When the power switch was set to ON (I), Set the power switch to OFF (O).
the instrument detected an error in the
Contact Bayer HealthCare Customer
system’s non-volatile memory.
Service Department. See Section 9, Service
Information.
1. The wrong program card is 1. Set the power switch to OFF (O).
0. plugged into the instrument.
0. Remove the wrong program card.
0. Plug the correct program card completely
0. into the instrument.
0. Set the power switch to ON (I).
2. No program card is plugged into 2. Set the power switch to OFF (O).
0. the instrument.
0. Plug the correct program card completely
0. into the instrument.
0. Set the power switch to ON (I).
3. The correct program card is correctly 3. Set the power switch to OFF (O).
0. plugged into the instrument but the
0. Use a pencil eraser to gently clean the
0. contacts on the program card need
0. contacts on the program card.
0. cleaning or are defective.
0. Use a lint-free tissue to wipe particles
0. from the contacts.
0. Correctly insert the program card.
0. Set the power switch to ON (I).
0.
•
If the problem persists, contact
0.
• Bayer HealthCare Customer Service
0.
•
Department. See Section 9, Service
0.•Information.
E3
E2
E1
Page 78

Revised 6/03 6.5
6
ERROR AND WARNING MESSAGES,
ERROR CODES & TROUBLESHOOTING
ERROR CODE CAUSE SOLUTION
1. The wrong program card is plugged 1. Set the power switch to OFF (O). Plug
0. into the instrument. 0. the correct program card completely into
0. the instrument. Set the power switch to
0. ON (I).
2. The correct program card is not 2. Refer to step 1.
0. completely plugged into the
0. instrument.
3. The contacts on the correct program 3. Set the power switch to OFF (O).
0. card need to be cleaned or are 0. Remove the program card.
0. defective.
0. Use a pencil eraser to gently clean the
0. contacts on the program card.
0. Use a lint-free tissue to wipe particles
0. from contact.
0. Correctly insert the program card.
0. Set the power switch to ON (I).
0. If the problem persists, contact Bayer
0. HealthCare Customer Service
0. Department. See Section 9, Service
0. Information.
When the power switch was set to ON (I), 1. Set the power switch to OFF (O).
the instrument detected use of a defective
2. Remove the program card.
program card.
3. Plug a replacement program card into the
0.
instrument. (Refer to Section 9, SERVICE
0. INFORMATION, for instructions on
0. where to order replacement program card.)
4. Set the power switch to ON (I).
0. If the problem persists, contact Bayer
0. HealthCare Customer Service
0. Department. See Section 9, Service
0. Information.
When the power switch was set to ON (I), Set the power switch to OFF (O).
the instrument detected an error in
Contact Bayer HealthCare Customer
system timing.
Service Department. See Section 9, Service
Information.
E6
E5
E4
Page 79

6.6 Revised 6/03
6
ERROR AND WARNING MESSAGES,
ERROR CODES & TROUBLESHOOTING
ERROR AND WARNING MESSAGES, ERR OR CODES AND TROUBLESHOO TING
ERROR CODE CAUSE SOLUTION
101
Buffer Reading Out of Limits — High
102
Buffer Reading Out of Limits — Low
103
High Variation in Readings for Buffer
104
106
Reading for Hemoglobin Value — Low
105
107
Reading for Hemoglobin Value — High
No cartridge present.
Optical alignment problem.
Condensation on cartridge
— cartridge not allowed to warm up
Cartridge not located properly in instrument.
Buffer tab not pulled.
Buffer tab removed before cartridge inserted.
Cartridge defect in optical window.
Cartridge optical window blocked or dirty.
Condensation on cartridge
— cartridge not allowed to warm up
Rare.
Particulate contamination.
No or low blood in reaction
— capillary underfill or air bubble
— blood dried in capillary
— no capillary holder inserted
— improper reconstitution of controls
or use of non-DCA 2000 controls
Hemoglobin < 7 g/dL
— anemic patient, abnormally low
hemoglobin
Buffer tab not pulled or buffer not
released from tray.
Excess blood on capillary holder.
Blood not lysing
— cartridge not allowed to warm up
— irregularity in patient red blood cells (rare)
Hemoglobin > 24 g/dL
— patient has abnormally high hemoglobin
— very high triglycerides
Repeat test with cartridge in place.
If problem persists, contact Bayer
HealthCare Customer Service Department.
See Section 9, Service Information.
Allow at least 10 minutes after cartridge is
removed from refrigerator before starting test.
Ensure that cartridge is inserted completely
into instrument.
Pull buffer tab after inserting cartridge
into holder.
If problem persists, contact Bayer
HealthCare Customer Service Department.
See Section 9, Service Information.
Allow at least 10 minutes after cartridge
is removed from refrigerator before starting
test.
If problem persists, contact Bayer
HealthCare Customer Service Department.
See Section 9, Service Information.
Ensure that capillary is completely filled
with no air bubbles.
Wait no more than 5 minutes after filling
capillary before starting test.
Repeat test with capillary holder in place.
Review procedure for reconstitution of
DCA 2000 controls.
Perform test by another method.
Pull buffer tab before closing door.
Take care in sampling the blood to avoid
excess blood on capillary holder.
Ensure that capillary is wiped before
inserting into cartridge.
Allow at least 10 minutes after cartridge is
removed from refrigerator before starting test.
Freeze/thaw specimen before use, or perform
test by another method.
Perform test by another method.
See reagent kit insert under Limitations.
Page 80

Revised 6/03 6.7
6
ERROR AND WARNING MESSAGES,
ERROR CODES & TROUBLESHOOTING
ERROR CODE CAUSE SOLUTION
108
High Variation in Readings for Hemoglobin
109
110
111
112
Readings for Glycated Hemoglobin
Out of Limits
113
114
116
Irregular Reaction Kinetics
for Glycated Hemoglobin
115
Final Hemoglobin Reading Greater Than
Reading at Earlier Checkpoint
Rare.
Particulate contamination.
Cartridge exposed to excessive
temperature and/or humidity.
Cartridge exposed to excessive temperature
and/or humidity.
Particulate contamination.
Blood left too long in capillary.
If problem persists, contact Bayer
HealthCare Customer Service Department.
See Section 9, Service Information.
Ensure proper storage of reagent kits.
Open foil package just prior to use
of cartridge.
Ensure proper storage of reagent kits.
Open foil package just prior to use
of cartridge.
If problem persists, contact Bayer
HealthCare Customer Service Department.
See Section 9, Service Information.
Wait no more than 5 minutes after
filling capillary before starting test.
Page 81

6.8 Revised 6/03
6
ERROR AND WARNING MESSAGES,
ERROR CODES & TROUBLESHOOTING
ERROR AND WARNING MESSAGES, ERR OR CODES AND TROUBLESHOO TING
ERROR CODE CAUSE SOLUTION
301
Buffer Mean Out of Limits — Low
Absorbance
302
Buffer Mean Out of Limits — High
Absorbance
303
High Variation in Readings for Buffer
304
Sample Blank Mean Out of Limits — Low
305
Sample Blank Mean Out of Limits — High
306
High Variation in Readings for
Sample Blank
307
308
Readings for Albumin
Out of Limits
No cartridge present.
Optical alignment problem.
Condensation on cartridge
— cartridge not allowed to warm up
Cartridge not located properly in instrument.
Buffer tab not pulled.
Buffer tab removed before cartridge inserted.
Cartridge defect in optical window.
Cartridge optical window blocked or dirty.
Rare.
Particulate contamination.
Particulate contamination.
Urine sample is turbid or highly pigmented.
Rare.
Particulate contamination.
Rare.
Cartridge exposed to excessive
temperature and/or humidity.
Repeat test with cartridge in place.
If problem persists, contact Bayer
HealthCare Customer Service Department.
See Section 9, Service Information.
Allow at least 15 minutes after cartridge is
removed from refrigerator before starting test.
Ensure that cartridge is inserted completely
into instrument.
Pull buffer tab after inserting cartridge
into holder.
If problem persists, contact Bayer
HealthCare Customer Service Department.
See Section 9, Service Information.
If problem persists, contact Bayer HealthCare
Customer Service Department. See Section 9,
Service Information.
Repeat test.
If problem persists, contact Bayer HealthCare
Customer Service Department. See Section 9,
Service Information.
Centrifuge sample before assaying if sample
is very turbid or cloudy.
If sample contains visible amounts of blood,
or is highly pigmented, obtain a fresh sample.
If problem persists, contact Bayer HealthCare
Customer Service Department. See Section 9,
Service Information.
Repeat test.
If problem persists, contact Bayer HealthCare
Customer Service Department. See Section 9,
Service Information.
Ensure proper storage of reagent kits.
Open foil package just prior to use
of cartridge.
Page 82

Revised 6/03 6.9
6
ERROR AND WARNING MESSAGES,
ERROR CODES & TROUBLESHOOTING
ERROR CODE CAUSE SOLUTION
309
High Variation in Albumin Readings
310
311
Readings for Creatinine
Out of Limits
313
Irregular Reaction Kinetics
for Creatinine
315
Normalization Factor has not been set
316
Normalization Factor outside allowable range
Rare.
Particulate contamination.
Rare.
Cartridge exposed to excessive
temperature and/or humidity.
Cartridge exposed to excessive temperature
and/or humidity.
Particulate contamination.
Instrument has not been adjusted to
run the Microalbumin/Creatinine assay.
Normalization Cartridge is not located
properly in instrument.
Normalization Cartridge is dirty or defective.
Repeat test.
If problem persists, contact Bayer
HealthCare Customer Service Department.
See Section 9, Service Information.
Ensure proper storage of reagent kits.
Open foil package just prior to use
of cartridge.
Ensure proper storage of reagent kits.
Open foil package just prior to use
of cartridge.
If problem persists, contact Bayer
HealthCare Customer Service Department.
See Section 9, Service Information.
Contact Bayer HealthCare Customer
Service Department. See Section 9,
Service Information.
Ensure that cartridge is inserted completely
into instrument.
If problem persists, contact Bayer
HealthCare Customer Service Department.
See Section 9, Service Information.
Page 83

6.10 Revised 6/03
6
ERROR AND WARNING MESSAGES,
ERROR CODES & TROUBLESHOOTING
ERROR AND WARNING MESSAGES, ERR OR CODES AND TROUBLESHOO TING
TROUBLESHOOTING
PROBLEM SOLUTION
Beep repeatedly fails to sound after scanning a bar code. 1. Insert the cartridge or expanded bottom of card (whichever
0. applies) into the bar code track below the dot.
2. Quickly (within 1 second) and smoothly, slide the card or
0. cartridge up past the dot.
0. If a beep continually fails to sound, refer to Section 8 for
0. instructions on “How to Report the Problem.”
Capillary will not fill completely with sample. Discard capillary holder.
Flexible tab tears off before it is pulled completely out of Discard the reagent cartridge.
reagent cartridge.
•
Refer to Section 9 for instructions on “How to Report the
•
Problem.”
Page 84

Revised 6/03 7.1
7
INSTRUMENT CARE
AND ROUTINE MAINTENANCE
INSTR UMENT CARE
AND R OUTINE MAINTEN ANCE
INSTRUMENT CARE
The DCA 2000® Analyzer contains sensitive
electronics and optics.
•
IMPORTANT! Do not use sprays. Sprays will
permanently damage the optical system.
•
Handle the instrument with extreme care. Severe
mechanical shocks can damage and/or dislodge
internal parts and connections.
•
Do not block the ventilation panels found on the
back and right sides of the instrument. Allow at
least two inches of air space between the wall
(or other surface) and the back and right sides of
the instrument.
• Do not operate the instrument beyond the
recommended maximum ambient operating
temperature or relative humidity ranges
listed below.
• Maximum Ambient Operating T emperature:
•
(15–32°C)—Hemoglobin A
1c
(18–30°C)—Microalbumin/Creatinine
Relative Humidity: 10% – 90% RH
•
Do not place the instrument where it would be
exposed to direct sunlight, extreme temperature
variations, particulate matter, excessive humidity
or air currents.
•
Do not smoke in the room where you have
placed the instrument. Smoke may cause a film
to form on the internal optical surfaces
affecting the optical transmission qualities of
the instrument.
ROUTINE MAINTENANCE CHART
NOTE: Maintenance requirements for each laboratory must
be assessed individually. Use the above chart as a guide. It is
good laboratory practice to properly maintain your instrument.
As Required (by spillage,
troubleshooting or
Weekly Quarterly contamination)
Exterior Cartridge Compartment Exterior
Bar Code Windo w Change Air Filter Change Air Filter
Run Optical Test Cartridge Cartridge Compartment
Bar Code Window
Run Optical Test Cartridge
32
15
18
30
Page 85

7.2 Revised 6/03
7
INSTRUMENT CARE
AND ROUTINE MAINTENANCE
INSTRUMENT CARE AND ROUTINE MAINTENANCE
EXTERIOR OF THE
INSTRUMENT AND
BAR CODE WINDOW
WARNING: Turn off the power and
unplug the power cord before cleaning
exterior of instrument.
VAROITUS: Kytke virta pois ja irrota
virtajohto ennen kuin alat puhdistaa
laitteen ulkopintoja.
Do not allow water or other cleaning fluid to drip
inside instrument (bar code window area, program
card area and key pad are especially vulnerable).
1. Clean the exterior of the instrument, including
the display panel and bar code window with a
lint-free cloth dampened with water. A cloth
dampened with ethanol may also be used.
0. If you desire to disinfect the exterior of the
instrument, expose the surface to either of the
following for 10 minutes.
1
Remove liquid blood
(as much as possible) prior to disinfection.
0. a. 0.5% sodium hypochlorite (see household
bleach, below)
0. b. 2% glutaraldehyde
0.a.
1
This is as referenced in the National
Committee for Clinical Laboratory
Standards, Protection of Laboratory Workers
from Instrument Biohazards and Infectious
Disease Transmitted by Blood, Body Fluids
and Tissues:Approved Guideline. NCCLS
Document M29-A [ISBN 1-56238-339-6].
2. After the exterior is clean and dry, attach and
plug-in power cord.
Most household bleach is (approx.) a 5%
solution of sodium hypochlorite (read the label).
Dilute household bleach containing
(approx.) 5% sodium hypochlorite as
follows to obtain (approx.) a 0.5% solution
of sodium hypochlorite.
•
10 mL of household bleach + 90 mL of
water or alternatively:
•
1 part household bleach + 9 parts water
Page 86

Revised 6/03 7.3
7
INSTRUMENT CARE
AND ROUTINE MAINTENANCE
CHANGING THE AIR FIL TER
1. Remove the filter holder from the right side of
the instrument by rotating the holder counterclockwise (until it stops) and pulling it off.
2. Dispose of the old air filter.
3. Place a new air filter into the filter holder.
4. Place the filter holder back on the instrument
and rotate the holder clockwise (until it stops).
Filter
Holder
Filter
Holder
Air
Filter
Page 87

7.4 Revised 6/03
7
INSTRUMENT CARE
AND ROUTINE MAINTENANCE
INSTRUMENT CARE AND ROUTINE MAINTENANCE
CAR TRIDGE
COMPARTMENT
WARNING: Turn off the power and
unplug the power cord before cleaning
the cartridge compartment.
VAROITUS: Kytke virta pois ja irrota
virtajohto, ennen kuin puhdistat
kasettipesän.
Do not allow liquid to drip into instrument.
If liquid drips into instrument, optics can be
destroyed.
1. Open the cartridge compartment door as far as
possible.
2. Using a lint-free cloth dampened with water
or ethanol, wipe the inside surface of the
compartment door and surfaces on both sides
of the cartridge holder.
0.
•
Dry surfaces using a clean, dry, lint-free
cloth.
3. Locate the cartridge return spring inside the
cartridge holder. Note top and bottom holes.
0.
4. Insert the tip of a straightened paper clip
(or other like device) into the top hole on
the spring.
0. •
Gently, pull metal end toward center of
cartridge compartment to release one side of
the spring from the cartridge holder.
0. •
Repeat procedure to release other side of
spring from cartridge holder.
5. Pull cartridge return spring completely out of
instrument.
6. Clean cartridge return spring using any of the
following:
0.
•
warm solution of mild detergent and water
(you may immerse cartridge return spring)
0.
•
lint-free cloth dampened in water or ethanol
0. IMPORTANT: Make sure the leaf springs are
not bent or damaged while cleaning. Damaged
leaf springs will not function properly.
0.
7. Dry cartridge return spring with a clean,
lint-free cloth. Set spring aside.
TOP
HOLE
BOTTOM
HOLE
CARTRIDGE
RETURN
SPRING
LEAF SPRINGS
Page 88

Revised 6/03 7.5
7
INSTRUMENT CARE
AND ROUTINE MAINTENANCE
08. Using a clean, dry, sponge swab (provided in
“Cleaning Kit,” Part No. 95001901) remove
spilled liquid from the cartridge holder.
00. IMPORTANT: Do not use a cotton swab.
Cotton fibers may be left on surface and
interfere with instrument optical system.
0.0 With compartment door partially closed,
rotate the cartridge holder as shown to locate
and remove any additional liquid.
00.
00.
00.
00.
NOTE: Cartridge holder cannot be rotated if
compartment door is completely open.
09. Dampen (do not soak) a sponge swab with
water or ethanol. Clean cartridge holder
(rotating cartridge holder as necessary).
00. IMPORTANT: Do not allow liquid to drip
off
sponge swab into instrument. If liquid
drips
into instrument, optics can be
destroyed.
10. Look inside the cartridge compartment and
locate the vertical grooves (tracks). Next,
locate the front and back slots (found near the
top lip of the compartment).
11. Locate the leaf spring on one side of the
cartridge return spring.
12. With the leaf spring orientated toward the
back of the instrument, lower the leaf spring
into the instrument as follows:
00. a. Hold onto both sides of the cartridge
return spring (near the holes).
00. b. Pinch the sides together (push toward each
other) and lower the spring into instrument
by sliding the sides of the spring between
the vertical grooves (tracks) in the
compartment. Release (let go of) spring.
00. b. NOTE: When “step b,” above, has been
properly completed, the return spring
should be free to slide up and down within
the cartridge compartment. If not, repeat
“step b” above.
00. b. IMPORTANT: Never force the spring into
the cartridge compartment! Forcing the
spring into the compartment will damage
the spring.
00. c. Gently and carefully, push down on edge
of cartridge return spring (with hole) and
insert edge into slot.
00. c. Repeat “step c” to attach opposite side
of cartridge return spring to cartridge
compartment.
00. c. IMPORTANT:When the cartridge return
spring is placed back into the instrument,
the leaf spring must be orientated toward
the back of the instrument and cartridge
return spring must be securely attached via
the grooves (tracks) and slots.
00. c. IMPORTANT: Failure to properly
replace return spring can result in
erroneous test results.
13. Run optical test cartridge according to
instructions that follow.
00. (Allows you to verify that the optical system
was not damaged or contaminated by dust,
etc., during this maintenance procedure.)
➠
➠
➠
Page 89

7.6 Revised 6/03
7
INSTRUMENT CARE
AND ROUTINE MAINTENANCE
INSTRUMENT CARE AND ROUTINE MAINTENANCE
RUNNING THE OPTICAL
TEST CAR TRIDGE (Standard 1)
The provided optical test cartridge allows you to
monitor the performance of the optical system
over time.
IMPORTANT! Keep a permanent record of the
results obtained (i.e., Mean Transmittance,
Standard Deviation and Drift).*
*It is recommended that you record the results on
the page provided in the appendix of this manual.
Comparing New Values With Initial Values
Compare the new values obtained with the results
recorded initially for your instrument (see
appendix). The mean Transmittance should be
within the range of 0.9500 to 1.0500, and should
not have varied by more than ±0.0100. The
Standard Deviation should be less than 0.00150
and the Drift should be less than 0.01400.
DISPLA Y WHA T Y OU DO
1. Locate the bar code on the optical test
cartridge.
2. Hold the cartridge so that the bar code faces
right.
3. Insert the cartridge (above dot on instrument)
into the bar code track.
4. Quickly (within 1 second) and smoothly, slide
the cartridge down past the dot.
0. A beep sounds to signal a successful scan.
0.
•
If no beep sounds, repeat the procedure. If a
beep repeatedly fails to sound, refer to
Troubleshooting, Section 6.
5. Press .
RUN STANDARD 1?
READY: SCAN BAR CODE
02/24/03 2:09PM
Page 90

Revised 6/03 7.7
7
INSTRUMENT CARE
AND ROUTINE MAINTENANCE
DISPLAY
6 MIN = total test time
After 1 minute:
WHA T Y OU DO
6. Open the cartridge compartment door.
7. Hold the optical test cartridge so that the bar
code faces right.
0. Insert the cartridge into the compartment until a
subtle snap is heard/felt.
0. HINT: The cartridge is designed to fit only one
way into the instrument.
8. Close door.
1.0001 T 0.00012 SD0
S1 5 MIN 10:03AM
PROCESSING STANDARD
S1 6 MIN 10:02AM
STANDARD 1
LOAD, CLOSE DOOR
Page 91

7.8 Revised 6/03
7
INSTRUMENT CARE
AND ROUTINE MAINTENANCE
INSTRUMENT CARE AND ROUTINE MAINTENANCE
DISPLAY
Upon completion of test:
WHA T Y OU DO
09. Record the displayed results in the blanks
provided on the last page of this manual
(appendix).
10. Remove cartridge.
00. a) Open cartridge compartment door.*
00. b) Locate the button on the right side of the
cartridge compartment. Push and hold it
down with your right hand.
00. c) With your left hand, gently push the plastic
tab on the cartridge to the right; this action
releases (unlocks) cartridge.
00. d) Pull cartridge out of compartment.
11. Compare the results obtained with results
obtained initially.
00. If the results are not within the limits found
on page 7.6 and in the appendix, refer to
Section 9 for information on “How to Report
the Problem.”
00.
*If the door is opened (within 15 minutes after assay
completion), the test result is displayed for only 30
seconds.
00.
If the door is not opened, the test result will remain
displayed for 15 minutes.
00. At 15 minutes, an audible tone (error buzz) sounds and
the display changes to “READY: REMOVE TEST.”
1.0001 T 0.00012 SDmm
S1 0.00387 DRIFT
Page 92

Revised 6/03 8.1
8
MINOR REPAIR
MINOR REPAIR
You can:
•
replace the fuse
If your instrument requires any other type of
repair, refer to Section 9, Service Information.
How to Replace the Fuse
Tool Required: Screwdriver
Fuse Required:
250 V, T-1.25A (Instrument Model No. 5031C)
One replacement fuse is stored in the fuse holder.
The fuse holder is located on the back panel of the
instrument between ON/OFF switch and power
cord connector.
1.
WARNING: Set the power switch to
OFF. Unplug power cord from wall
outlet. Unplug power cord from instrument.
VAROITUS: Kytke virta pois (off
-asento). Irrota virtajohto
seinäpistokkesta. Irrota virtajohto laitteesta.
2. Locate the groove on the side of the fuse holder
that faces power cord connector.
3. Insert the tip of a screwdriver into the groove.
Then, exert pressure to unsnap fuse holder
from instrument.
4. Remove the fuse holder from instrument.
replacement fuse
blown fuse
fuse block
5. Remove and dispose of blown fuse.
6. Push the replacement fuse out of the black box
using the tip of a screwdriver or similar device.
7. Insert replacement fuse onto fuse block.
0.
WARNING: For continued protection
against fire hazard, replace only with
the indicated type and rating of fuse.
0. VAROITUS: Paloturvallisuussyistä,
käytä vain suositeltuja sulakkeita.
8. Re-insert fuse holder into instrument (snap
gently into place.)
3
2
1
1
2
3
Groove
Page 93

Revised 6/03 9.1
9
SERVICE INFORMATION
SERVICE INFORMATION
When you ha v e a problem with
the System
•
Refer to Troubleshooting, Section 6, of this
Manual
If Section 6 cannot assist you in solving the
problem, answer all of the following questions
before contacting us for help. The requested
information will help identify the cause of the
particular problem.
How to Report the Problem
First
Record the following information.
Instrument Serial Number ___________________
Installation Date___________________________
Test Type ________________________________
Reagent Cartridge Lot Number _______________
Control Lot Number _______________________
Control Results ___________________________
Optical Test Cartridge Results:
Initial Current
Mean Transmittance ________ _______
Standard Deviation ________ _______
Drift ________ _______
Second
Complete the following “Preservice Questionnaire.”
1. Has Section 6, Trouble- YES NO
0. shooting been reviewed?
2. Have you performed the
YES NO
0. required maintenance
0. procedures (Section 7)?
3. Set the power switch to OFF.
YES NO
0. Make sure the program card
0. is securely plugged in. Set
0. the power switch to ON. Are
0. the following displayed?
(if instrument is not within
operating temperature range)
4. If displays do not appear:
0.
•
is the instrument plugged YES NO
0.
• into a live,AC electrical
0.
• outlet?
0.
•
Is the line fuse defective? YES NO
0.
• Refer to Section 7, Minor
0.
• Repair.
5. Do any characters on the dis-
YES NO
0. play appear partially defective?
READY: SCAN BAR CODE
02/24/03 2:09PM
xWAIT: WARMING UP
02/24/03 2:09PM
INITIALIZING
KEEP DOOR CLOSED
COPYRIGHT 1991-2003
BY BAYER CORPORATION
SOFTWARE VERSION
E3.11/01.04
Page 94

9.2 Revised 6/03
9
SERVICE INFORMATION
SERVICE INFORMATION
6.
If your instrument is connected
YES NO
0. to a printer or computer,
are all cables securely
attached?
7. List error messages that have appeared. ______
0. ______________________________________
0. ______________________________________
0. ______________________________________
0. ______________________________________
0. ______________________________________
8. Record the exact sequence of events that took
place when the failure occurred and any results
obtained.
9. Be prepared to perform a complete test
procedure when you call for assistance.
Third
Contact the nearest Bayer Diagnostics office or
authorized distributor.
Page 95

Revised 6/03 9.3
9
SERVICE INFORMATION
ACCESSORY ITEMS
Part Number Description
Hemoglobin A
1c
5035C DCA 2000®Reagent Kit
5068A DCA 2000
®
Normal &
Abnormal Control Kit
Microalbumin/Creatinine
6011A DCA 2000
®
Reagent Kit
6012A DCA 2000
®
Low & High
Control Kit
NOTE: Part numbers are subject to change
without notice.
TO ORDER:
Contact the nearest Bayer HealthCare office or
authorized distributor.
Distributed by:
Bayer HealthCare LLC
Elkhart, IN 46515 USA
REPLA CEMENT PAR TS
Part Number Description
SR002810 Program Card for Instrument
English
40330028 N.A. Power Cord for Instrument
40330046 Euro Power Cord for Instrument
40330050 U.K. Power Cord for Instrument
50214149 Filter Holder
50546216 Cartridge Return Spring
625-0127-01 Fuse: T-1.25A, Slow Blow; 250V
95001901 Cleaning Kit
95002911 Optical T est Cartridge
95002117 Air Filter (8) Replacement Kit
95002836 Operating Manual
NOTE: Part numbers are subject to change
without notice.
Bayer House
Strawberry Hill
Newbury, Berkshire
RG14 1JA UK
Ascensia™ Diabetes Support
0845 600 6030–UK
1 890 920 111–Republic of Ireland
diabetes@bayer.co.uk
Bayer plc
MERA
(Middle East, Eastern Europe,
Russia, Africa)
Bayer House
Strawberry Hill
Newbury
Berkshire RG14 1JA
UK
+44 1635 563000
Bayer Inc.
Diagnostics Division
Toronto, Ontario M9W 1G6
Bayer (South East Asia), Pte Ltd
No.9 Benoi Sector
Singapore 629844
Tel: (65) 6261 3389
Fax: (65) 6266 3376
Bayer Australia Ltd.
ABN 22 000 138 714
Diagnostics Division
875 Pacific Hwy
Pymble NSW 2073
AUSTRALIA
1 800 028 251 (toll free)
Page 96

Revised 6/03 App. 1.1
APPENDIX
APPENDIX
OPTICAL TEST CARTRIDGE RESULTS
Initial V alues
Date
Mean Transmittance
Standard Deviation
Drift
DA TE MEAN TRANSMITTANCE STANDARD DEVIATION DRIFT
Comparing New Values With Initial Values
Compare the new values obtained with the initial
values. The Mean Transmittance should be within
the range of 0.9500 to 1.0500, and should not have
varied by more than 0.0100. The Standard
Deviation should be less than 0.00150 and Drift
should be less than 0.01400.
Page 97

Revised 6/03 Index 1.1
APPENDIX
A
aborted: 3.8
air: 2.4
analysis: 2.2
analyze: 2.6
assay: 2.3
authorized: 1.1
B
bar code: 2.2
beep: 4.8
biohazard: 4.5
blood: 1.1
bubbles: 4.7
buzz: 4.10
C
calibration: 5.2
capillary: 4.5, 5.5
card: 3.3
caution: 1.1, 4.1
cleaning: 2.1
compartment: 2.2
computer: 1.1, 3.16
computer port: 2.9
concentration: 2.16
copyright: 2.5
cursor: 2.10
D
definition: 3.13
displayed: 2.3
E
electrical: 1.1, 2.4
electronic: 2.1
error: 6.1
F
finger: 4.6
foil: 4.3
format: 3.2
G
glutaraldehyde: 7.2
grounded: 2.4
H
humidity: 1.1, 2.4
I
important: 2.3
insert: 5.4
installation: 1.1
IVD: 1.1
L
laboratories: 1.1
M
mechanical: 2.5
memory: 2.8
menu: 2.8
mg/dL: 2.16
mmol/L: 2.16
O
operational: 1.1
optical: 2.1
P
power: 2.1
printer: 1.1, 3.16
procedures: 4.3
progress: 3.8
prompts: 2.2
R
ratio: 3.3
reagent: 1.1
recall: 3.7
reconstitution: 1.2
INDEX
Page 98

Index 1.2 Revised 6/03
APPENDIX
S
safety: 1.1
sequence: 2.8, 3.4
sodium hypochlorite: 7.2
software version: 2.5
specifications: 1.1
spring: 2.2
standards: 1.1
sunlight: 2.4
T
tab: 2.2
temperature: 1.1, 2.4
U
unpacking: 2.8
urine: 1.1, 5.6
use by: 1.2
V
venipuncture: 4.6
W
warning: 1.1, 4.5
warranty: 1.1
INDEX
 Loading...
Loading...