Page 1

EN
* Thank you very much for selecting ARCHOS Blood Pressure Monitor.
* Please do read the user manual carefully and thoroughly so as to ensure the safe
usage of this product, and keep the manual well for your further reference in case
you have problems.
Manuel_BPM_book.indd 1 06/06/2014 10:21:20
Version:1.0
User Manual
ARCHOS Blood Pressure Monitor
Page 2
CATALOGUE
INTRODUCTION ......................................................................................................................................................................... 2
Safety Information
LCD Display Signal
Monitor Components
BEFORE YOU START ..................................................................................................................................................................6
Installing and Replacing the Batteries
Setting Date and Time
MEASUREMENT........................................................................................................................................................................ 11
Tie the Cuff
Start Measurement
DATA MANAGEMENT............................................................................................................................................................ 13
Recalling the Records
Deleting the Records
INFORMATION FOR USER..................................................................................................................................................... 15
Tips for Measurement
Maintenance
ABOUT BLOOD PRESSURE.................................................................................................................................................... 17
What are systolic pressure and diastolic pressure?
What is the standard blood pressure classification?
Why does my blood pressure fluctuate throughout the day?
Why the blood pressure I get from the hospital is different from home?
If the result is the same if measuring on the right wrist?
TROUBLESHOOTING.............................................................................................................................................................. 19
SPECIFICATIONS ..................................................................................................................................................................... 20
CONTACT INFORMATION..................................................................................................................................................... 21
COMPLIED EUROPEAN STANDARD LIST .........................................................................................................................21
EMC GUIDANCE .......................................................................................................................................................................22
2
Manuel_BPM_book.indd 2 06/06/2014 10:21:20
Table of Contents
Page 3
EN
INTRODUCTION
General Description
Thank you for selecting ARCHOS Blood Pressure Monitor. The monitor features blood
pressure measurement, pulse rate measurement and the result storage. The design
provides you with two years of reliable service.
Reading taken by the Blood Pressure Monitor are equivalent to those obtained by a
trained observer using the cuff and stethoscope auscultation method.
This manual contains important safety and care information, and provides step by
step instructions for using the product.
Read the manual thoroughly before using the product.
FEATURES:
* Systolic Blood Pressure
* Diastolic Blood Pressure
* Pulse Rate
* Memory: Up to 60 pieces of records
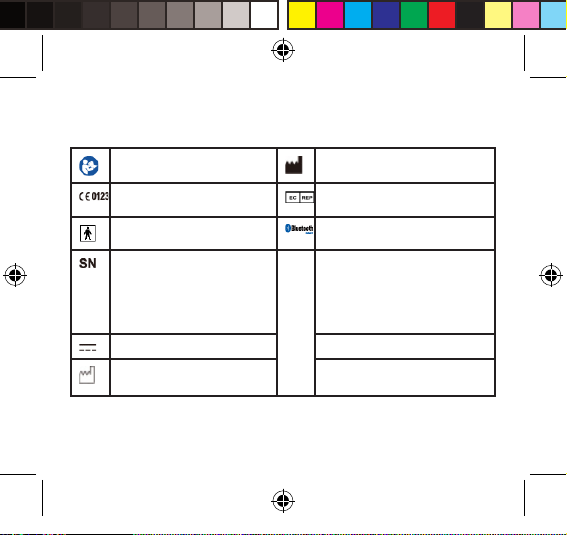
Safety Information
The below signs might be in the user manual, labeling or other components. They
are the requirement of standard and using.
Manuel_BPM_book.indd 3 06/06/2014 10:21:20
3
Page 4
4
Manuel_BPM_book.indd 4 06/06/2014 10:21:20
Symbol for “THE OPERATION GUIDE
MUST BE READ”
Symbol for “COMPLIES WITH
MDD93/42/ECC REQUIREMENTS”
Symbol for “TYPE BF APPLIED
PARTS”
Symbol for “SERIAL NUMBER” Symbol for “ENVIRONMENT
Symbol for “DIRECT CURRENT”
Symbol for “MANUFACTURE DATE”
Symbol for “MANUFACTURER”
Symbol for “Authorized Representative
in the European Community”
The Bluetooth Combination Mark
PROTECTION – Waste electrical
products should not be disposed of
with household waste. Please recycle
where facilities exist. Check with your
local authority or retailer for recycling
advice”
Page 5
EN
Please do read this user manual carefully and thoroughly before use.
This device is intended for adult use only.
This device is intended for non-invasive measuring and monitoring of arterial blood
pressure. It is not intended for use on extremities other than the wrist or for functions
other than obtaining a blood pressure measurement.
Do not confuse self-monitoring with self-diagnosis. This unit allows you to monitor
your blood pressure. Please start or end medical treatment basing solely on
physician’s treatment advice.
If you are taking medication, consult your physician to determine the most
appropriate time for your measurement. Never change a prescribed medication
without your physician’s consent.
This unit is not suitable for continuous monitoring during medical emergencies or
operations.
If the pressure of the cu exceeds 40 kPa (300 mmHg), the unit will automatically
deate. Should the cu not deate when its pressure exceeds 40 kPa (300 mmHg),
detach the cu from the wrist and press the START/STOP button to stop ination.
Do not use the monitor under the conditions of strong electromagnetic eld (e.g.
medical RF equipment) that radiates interference signal or electrical fast transient /
burst signal.
The maximum temperature that the applied part can be achieved is 42.5℃ while the
environmental temperature is 40℃.
The device is not AP/APG equipment. It is not suitable for use in the presence of a
ammable anesthetic mixture with air (or oxygen, nitrous oxide).
Please keep the unit out of reach of infants or children, since inhalation or swallowing
of small parts is dangerous or even fata.
Please use ACCESSORIES and detachable parts specied / authorized by
Manuel_BPM_book.indd 5 06/06/2014 10:21:20
CAUTION
5
Page 6
MANUFACTURER. Otherwise, it may cause damage to the unit or danger to the user
/ patient.
Manufacturer will make available on request circuit diagrams, component parts
listed.
Sensitive people, including pregnant women and those who implanted medical
electronic
Instrument, should avoid using the unit whenever possible.
This unit is not suitable for continuous monitoring during medical emergencies or
operations.
After the cu inated long time, the patient’s wrist and ngers will is insucient,
anesthesia, distending pain and ecchymosis.
Please use the device under the environment which was provided in the user
manual.
Otherwise, the performance and lifetime of the device will been impacted and
reduced.
During using, the patient will contact with the cu. The materials of the cu have
been tested and found to comply with requirements of ISO 10993-5:2009 and ISO
10993-10:2010. It will not cause any potential allergic reaction or contact injury.
The device has been evaluated clinically used manual cu/ stethoscope auscultation
as the reference.
The device doesn’t need to be calibrated in two years of reliable service.
When the device was used to measure patients who have common arrhythmias such
as atrial or ventricular premature beats or atrial brillation, the test result may occur
deviation. Please consult your physician about the result.
This device is contraindicated for any female subject who may be suspected of, or is
pregnant.
Besides provided inaccurate readings, the eects of this device on the fetus are
unknown.
6
Manuel_BPM_book.indd 6 06/06/2014 10:21:20
Page 7
EN
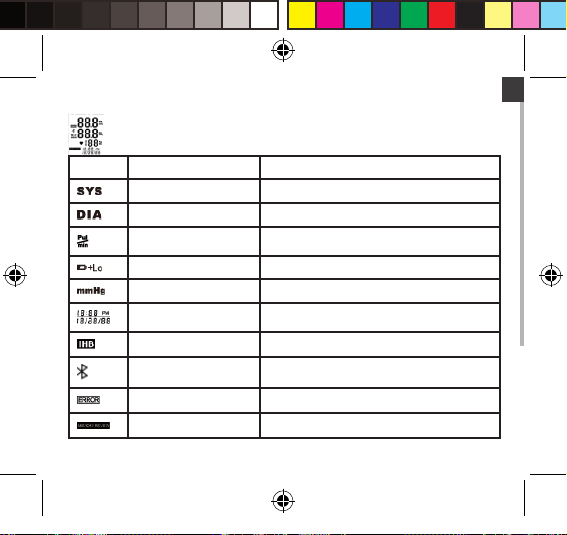
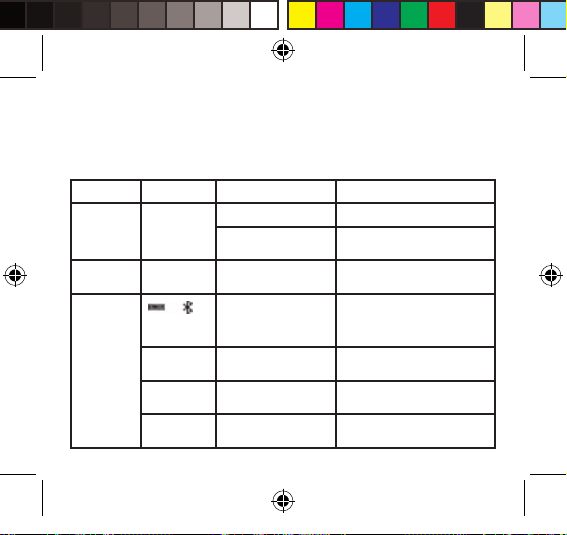
LCD Display Signal
SYMBOL DESCRIPTION EXPLANATION
Manuel_BPM_book.indd 7 06/06/2014 10:21:20
Systolic Blood Pressure High blood pressure
Diastolic Blood Pressure Low blood pressure
Pulse beat/minute
Low Battery Low battery and please replace the batteries.
Unit Measurement unit of blood pressure
Time Hour: Minute (Month/Day/Year)
IHB Detector Irregular Heartbeat Detector
Bluetooth Successful Bluetooth Connection
Error Error
Memory Recalling the history records
7
Page 8
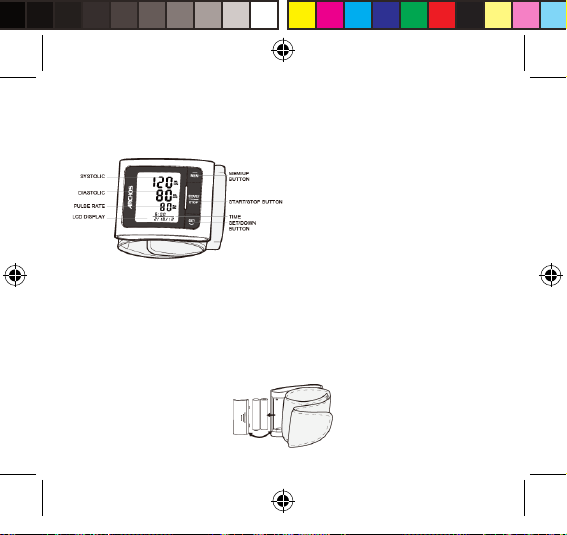
Monitor Components
Component List:
1. PCBA; 2. Air Pipe; 3. Pump; 4. Valve; 5. Cu.
List
1. Wrist Blood Pressure Monitor 2. Two AAA-size batteries 3. User Manual
BEFORE YOU START
Installing and Replacing the Batteries
•.Open the battery door.
•.Insert the batteries according to the polarity indications. (Always select the
authorized / specied battery: Two LR03 AAA-size batteries).
•.Close the battery door.
8
Manuel_BPM_book.indd 8 06/06/2014 10:21:21
Page 9

EN
Battery Life: Approx. 57 days
(Battery capacity: 600 mAH. If measured 3 times per day, each measurement takes
30s, and memory checked once per day, each checking takes 60s. The current for
measurement is 350 mA, and that for records display is 50 mA while the current
when shutdown is 25 uA.)
Setting Date and Time
Please proceed to time setting before your initial use so as to ensure each piece of
record is labeled with a time stamp. (Year Range: 2012-2052; Time Format: 12 Hours)
1. When the monitor is OFF, press and hold “SET” button for 3 seconds to enter Time
Setting Mode.
Manuel_BPM_book.indd 9 06/06/2014 10:21:21
9
Page 10
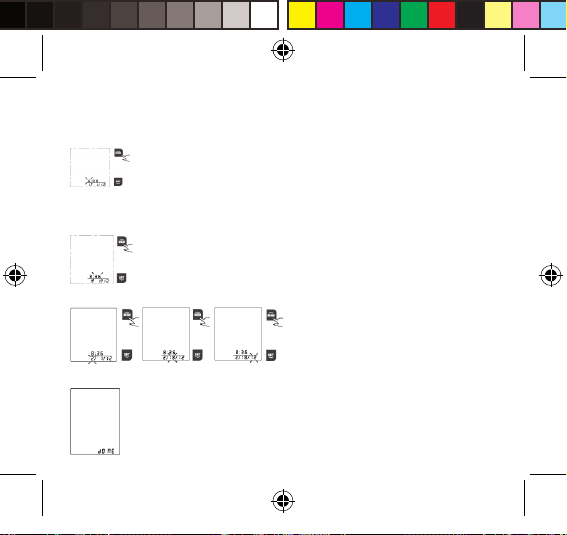
2. As pictured in the right, the blinking numeral “6” representing [HOUR]. Press “MEM”
button to change the numeral. Each press will increase the numeral by one in a
cycling manner.
3. Press “SET” button again to conrm [HOUR]. Then the numeral representing
[MINUTE] blinks.
4. Repeat step 2 and 3 to conrm [MINUTE].
5. Repeat step 2 and 3 to conrm [MONTH], [DAY] and [YEAR].
6. After conrming [YEAR], the LCD will display “DONE” and the monitor will shut o.
10
Manuel_BPM_book.indd 10 06/06/2014 10:21:21
Page 11
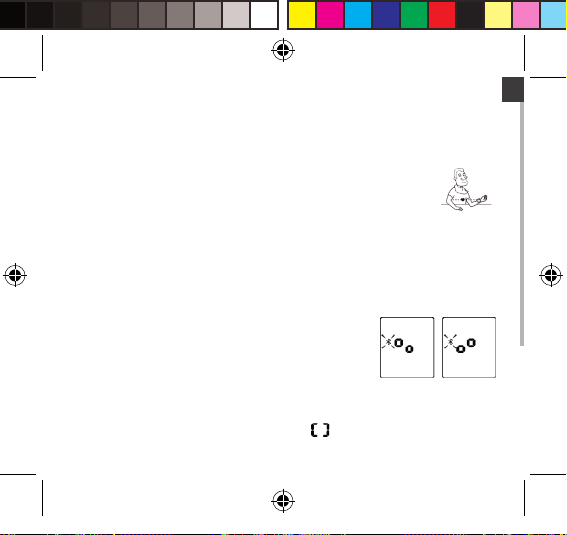
EN
Tie the Cu
1. Remove all accessories (watch, bracelet, etc.) from your wrist. If your physician has
diagnosed you with poor circulation in your wrist, use the other wrist.
2. Roll or push up your sleeve to expose the skin.
3. Apply the cu to your wrist with your palm facing up.
4. Position the edge of the cu about 1-1.5 cm.
5. Fasten the wrist cu around your wrist, leaving no extra room
between the cu and your skin. If the cu is too loose, the measurement
will not be accurate.
Resting for 5 minutes before measuring.
Wait at least 3 minutes between measurements. This allows your blood circulation
to recover.
For a meaningful comparison, try to measure under similar conditions. For example,
take daily measurements at approximately the same time, on the same wrist, or as
directed by a physician.
Pair-up the Blood Pressure Monitor with Your Device
1. Turn on Bluetooth and the app. M ake sure both are ON
when pair-up is proceeding.
2. When the monitor is OFF, press and hold the START button
for 2 seconds to start pair-up. The symbol and the symbol will be shown on the LCD
alternatively, indicating pair-up is proceeding.
If SUCCEED, symbol will be shown on the LCD.
Manuel_BPM_book.indd 11 06/06/2014 10:21:21
11
Page 12
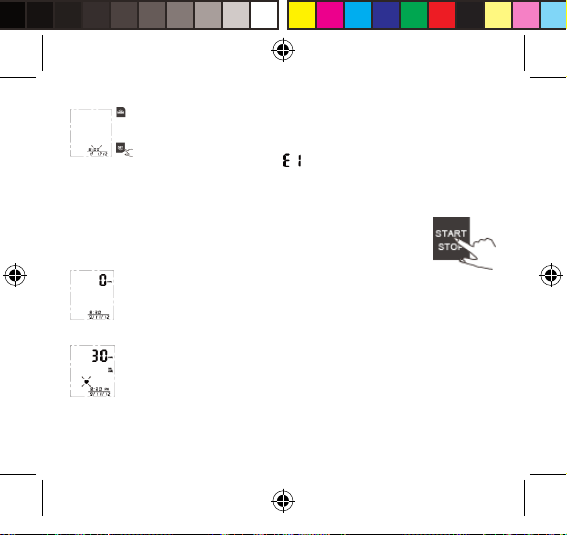
If FAIL, symbol will be shown on the LCD.
3. The monitor will shut o automatically after Pair-up process is complete.
MEASUREMENT
Start Measurement
1. After correctly positioning the cu, press START button to turn on the
monitor, and it will complete the measurement process automatically.
Adjust to zero.
Inating and measuring.
12
Manuel_BPM_book.indd 12 06/06/2014 10:21:21
Page 13
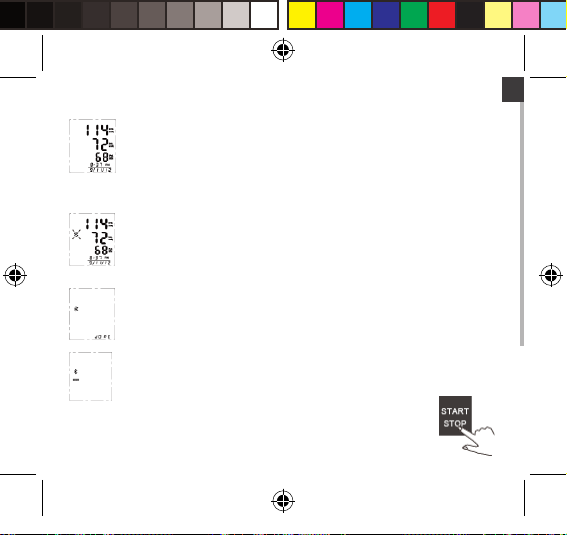
EN
Display and save the measuring result.
2. This device will proceed to data transmission automatically after measurement.
The Bluetooth symbol blinks.
3. If the data is successfully transmitted, the LCD will display as pictured to the right.
If the data transmission fails, the LCD will display “ERROR” instead.
4. Press STOP button to turn o the monitor. O therwise it will power o
automatically.
Manuel_BPM_book.indd 13 06/06/2014 10:21:21
13
Page 14
DATA MANAGEMENT
Recalling the Records
1. Press “MEM” button to access the memory.
2. Press “MEM/UP ” button or “SET/DOWN” button to rotate the history records. “MEM/
UP” to go forward; “SET/DOWN” to go backward.
Deleting the Records
When you did not obtain the accurate measurement, you can clear all the measuring
results by following below steps.
1. Under Memory Recalling Mode, press and hold both the “MEM”
button and the “SET” button for 3 seconds.
14
Manuel_BPM_book.indd 14 06/06/2014 10:21:21
Page 15
EN
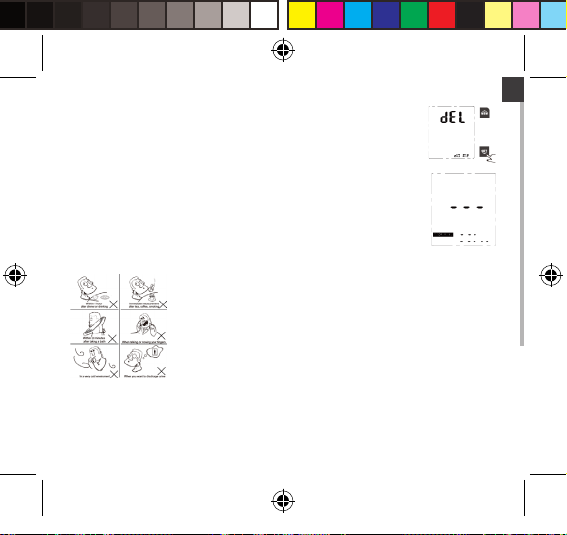
2. The LCD will display “dEL dONE”, indicating that memory clearing is
complete.
3. If you wish to give up clearing, press “START/STOP” to turn o the
monitor.
4. When there is no memory in the monitor, if you press the “MEM”
button to look up
History, the LCD will display as pictured to the right.
INFORMATION FOR USER
Tips for Measurement
It can cause inaccuracy if the measurement is taken in the following circumstances.
Manuel_BPM_book.indd 15 06/06/2014 10:21:21
15
Page 16
Maintenance
To obtain the best performance, please follow below instructions.
Cleaning: Dust environment may aect the performance of the unit. Please use the
soft cloth to remove the dirt before use.
Please make sure the unit functions safely and it is in proper working conditions
before use.
Please follow the instructions for correct replacement of interchangeable or
detachable parts specied by SERVICE PERSONNEL of MANUFACTURER as
“Replaceable”.
Disposal: Degraded sensors or loosened electrodes may degrade the unit’s
performance or even cause other problems. Please dispose of ACCESSORIES,
detachable parts, and the ME EQUIPMENT according to the local guidelines.
ABOUT BLOOD PRESSURE
What are systolic pressure and diastolic pressure?
16
Manuel_BPM_book.indd 16 06/06/2014 10:21:22
Page 17

EN
When ventricles contract and pump blood out of the heart, the blood pressure
reaches its maximum value in the cycle, which is called systolic pressure. When the
ventricles relax, the blood pressure reaches its minimum value in the cycle, which is
called diastolic pressure.
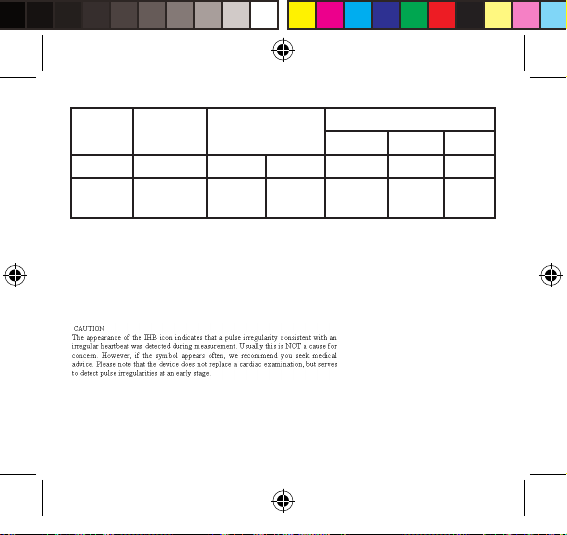
What is the standard blood pressure classication?
The blood pressure classication published by World
Health Organization (WHO) and International Society of
Hypertension (ISH) in 1999 is as follows:
Manuel_BPM_book.indd 17 06/06/2014 10:21:22
17
Page 18
Level
BP (mm
Hg)
SYS <120 121-130 131-140 141-160 161-180 ≥180
DIA <80 81-85 86-90 91-100 101-110 ≥110
Irregular Heartbeat Detector
This Wrist Blood Pressure Monitor is equipped with an intelligent function of Irregular
Heartbeat (IHB) Detector. During each measurement, this equipment records the
heartbeat intervals and works out the standard deviation. If the heartbeat intervals
compare with the average intervals, the deviation more than 3 is over 25% or more
than 5 is over 15%, this equipment will light up the IHB symbol on the screen when
displaying the measuring result.
Why does my blood pressure uctuate throughout the day?
1. Individual blood pressure varies every in one day, it also aected by the way you t
i.e. your cu and your measurement position, so please take the measurement at the
same condition.
18
Manuel_BPM_book.indd 18 06/06/2014 10:21:22
Optimal Normal Hypertension
G1 G2 G3
Page 19
EN
2. The varies of the pressure is greater if the person take medicine.
3. Waiting at least 3 minutes for another measurement.
Why the blood pressure I get from the hospital is dierent from home?
The blood pressure is dierent even during 24 hour because of the weather, emotion,
exercise etc., especially the “white coat” in hospital which makes the results are higher
than the ones at home.
If the result is the same if measuring on the right wrist?
It is ok for both wrists, but there will be some dierent results for dierent person, so
suggest you measure the same wrist every time.
The attention need to pay when you measure you blood pressure at home:
If the cu is tied properly.
If the cu is too tight or too loose.
If the cu is tied on the wrist.
If you feel anxious pressured.
You had better take deep breath 2-3 times before beginning.
Manuel_BPM_book.indd 19 06/06/2014 10:21:22
19
Page 20
Advice: adjust yourself for 4-5 minutes until you calm down.
TROUBLESHOOTING
This section includes a list of error messages and frequently asked questions for
problems you may encounter with your wrist blood pressure monitor. If the products
not operating as you think it should, check here before arranging for servicing.
PROBLEM SYMPTOM CHECK THIS REMEDY
No power Display will
Low
batteries
Error
massage
not light up.
Display is dim
or Display
shows
Batteries are exhausted. Replace with new batteries
Batteries are inserted
incorrectly.
Batteries are low. Replace with new batteries
Data communication
has failed
Insert the batteries correctly
Check if the APP is on or not, try
data transmission again.
20
Manuel_BPM_book.indd 20 06/06/2014 10:21:22
Error 1 shows Ination is slow or the
Error 2 shows The cu is very tight Readjust the cu, not too loose or
Error 3 shows The pressure of the cu
cu is not secure.
is excess.
Refasten the cu and then
measure again.
too tight and then measure again.
Refasten the cu and then
measure again.
Page 21
EN
Error
massage
Error
massage
SPECIFICATIONS
Power supply 2*AAA batteries
Display mode Digital LCD V.A.36x41mm
Manuel_BPM_book.indd 21 06/06/2014 10:21:22
Error 5 or Error 6 shows System error
Error 10 or Error 11
shows
Error 20 shows The measurement
Error 21shows on the
display.
occurred.
The monitor detected
motion, talking or
the pulse is too poor
while measuring.
process does not
detect the pulse
signal.
The treatment of the
measurement failed.
Retake the measurement.
If the problem persists,
contact the retailer or our
customer service department
for further assistance. Refer
to the warranty for contact
information and return
instructions.
Relax for a moment and then
measure again.
Loosen the clothing on the
wrist and then measure again.
Relax for a moment and then
measure again.
21
Page 22
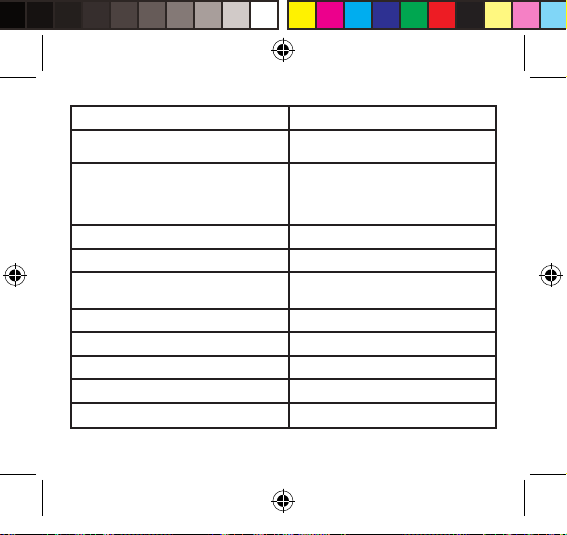
Measurement mode Oscillographic testing mode
Measurement range Pressure:0kpa-40kpa℃0mmHg-300mmHg℃
Accuracy Pressure:
Normal working condition Temperature:5℃-40℃
Relative humidity ≤80% Atmospheric pressure: 86kPa to 106kPa
Storage & transportation condition Temperature:-20℃ to 60℃
Measurement perimeter of the wrist About 13.5cm-21.5cm
Net Weight Approx.120g(Excluding the dry cells)
External dimensions Approx.80×65×22mm
Attachment 2*AAA batteries, user manual
Mode of operation Continuous operation
22
Manuel_BPM_book.indd 22 06/06/2014 10:21:22
pulse value:(40-199)beat/minute
5℃-40℃within±0.4kpa(3mmHg)
0℃-45℃(out of 5℃-40℃)
within±0.7kpa(5mmHg) pulse value:±5%
Relative Humidity: 10% to 93% RH
Page 23
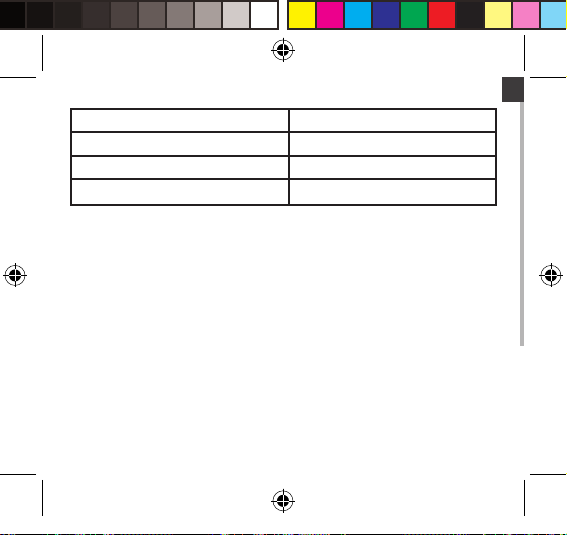
EN
Degree of protection Type BF applied part
Protection against ingress of water IP22
Software version V01
Device classication Internally Powered ME Equipment
WARNING: No modication of this equipment is allowed.
CONTACT INFORMATION
Contact Information
For more information about our products, please visit www.archos.com you can get
customer service, usual problems and customer download, ARCHOS will serve you
anytime.
Manufactured by: GUANGDONG TRANSTEK MEDICAL ELECTRONICS CO., LTD
Company: GUANGDONG TRANSTEK MEDICAL ELECTRONICS CO., LTD
Address: Zone A, 5/F., Investment Building, No. 12, Huizhan East Rd., Torch
Development District, Zhongshan, Guangdong, 528437, China
Authorized European Representative:
Company: MDSS - Medical Device Safety Service GmbH
Address: Schigraben 41, 30175 Hannover, Germany
Manuel_BPM_book.indd 23 06/06/2014 10:21:22
23
Page 24
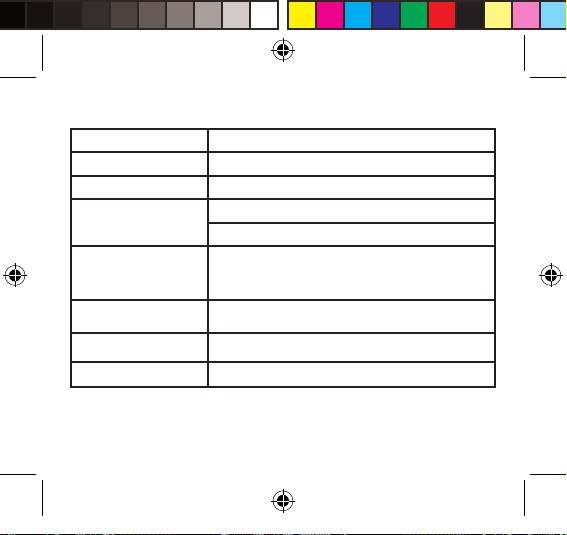
Complied European Standards List
Risk Management EN/ISO 14971:2007
Labeling EN 15223:2012
User Manual EN 1041:2008
General Requirements
for Safety
Non-invasive
Sphygmomanometers
General
Requirements
Electromagnetic
Compatibility
Software Lifetime EN 62304:2006/AC:2008
Usability EN 60601-1-6:2010
EMC Guidance
Table 1 – Guidance and MANUFACTURER’S declaration – ELECTROMAGNETIC
EMISSIONS – for all ME EQUIPMENT and ME SYSTEM
24
Manuel_BPM_book.indd 24 06/06/2014 10:21:22
EN 60601-1:2006/AC2010
EN 60601-1-11:2010
EN 1060-1:1995+A2:2009
EN 1060-3:1997+A2:2009
EN 1060-4:2004
EN 60601-1-2:2007/AC:2010
Page 25
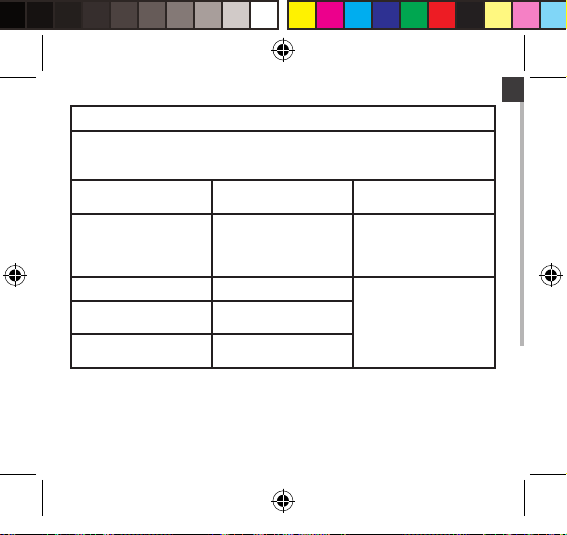
EN
Guidance and manufacturer’s declaration – electromagnetic emissions
The device is intended for use in the electromagnetic environment specified
below. The customer or the user of the device should assure that it is used in such
an environment.
Emissions test Compliance Electromagnetic environment
RF emissions CISPR 11 Group 2 The device must emit
RF emissions CISPR 11 Class B
Harmonic emissions IEC
61000-3-2
Voltage fluctuations / flicker
emissions IEC 61000-3-3
Manuel_BPM_book.indd 25 06/06/2014 10:21:22
Not applicable
Not applicable
– guidance
electromagnetic energy in
order to perform its intended
function. Nearby electronic
equipment may be affected.
25
Page 26
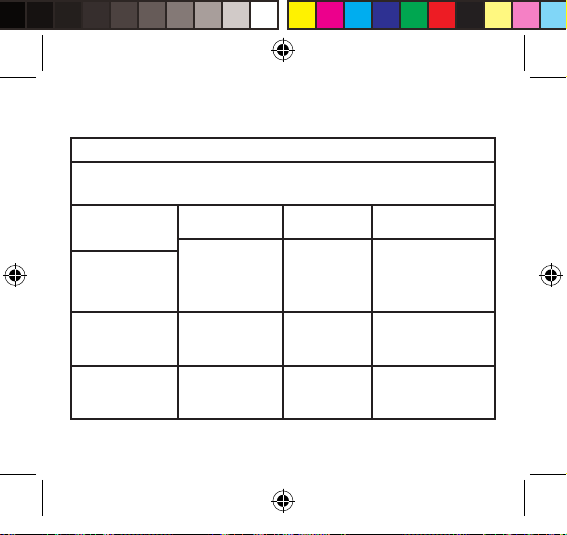
Table 2 – Guidance and MANUFACTURER’S declaration – electromagnetic IMMUNITY
– for all ME EQUIPMENT and ME SYSTEMS
Guidance and manufacturer’s declaration – electromagnetic immunity
The device
is intended for use in the electromagnetic environment specified below. The customer or the user
of the device should assure that it is used in such an environment.
IMMUNITY test IEC 60601 test level Compliance level Electromagnetic
Electrostatic
discharge (ESD)
IEC 61000-4-2
Electrical fast
transient / burst
IEC 61000-4-4
Surge
IEC 61000-4-5
26
Manuel_BPM_book.indd 26 06/06/2014 10:21:22
±6 kV contact
±8 kV air
±2 kV for power
supply lines
±1 kV for input /
output lines
±1 kV line(s) to line(s)
±2 kV line(s) to earth
environment – guidance
±6 kV contact
±8 kV air
Not applicable Mains power quality
Not applicable Mains power quality
Floors should be wood,
concrete or ceramic tile.
If floors are covered with
synthetic material, the
relative humidity should
be at least 30%.
should be that of a
typical commercial or
hospital environment.
should be that of a
typical commercial or
hospital environment.
Page 27
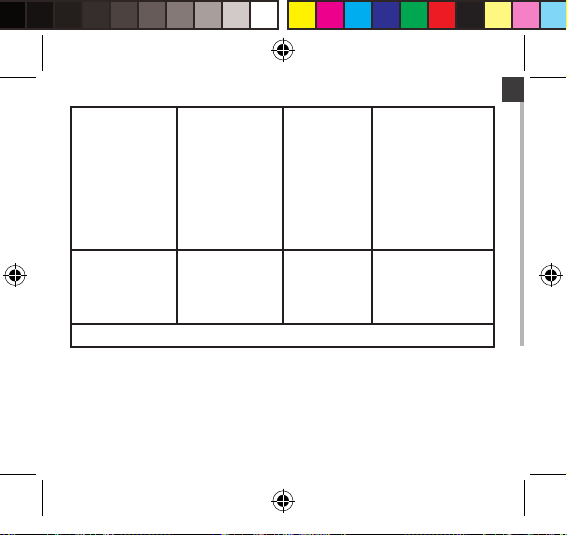
EN
Voltage dips, short
interruptions and
voltage variations on
power supply input
lines IEC 61000-4-11
Power frequency
(50/60 Hz)
magnetic field
IEC 61000-4-8
NOTE UT is the a.c. mains voltage prior to application of the test level.
Manuel_BPM_book.indd 27 06/06/2014 10:21:22
℃5% UT (℃95% dip in
UT) for 0.5 cycle
40% UT (60% dip in
UT) for 5 cycles
70% UT (30% dip in
UT) for 25 cycles
℃5% UT (℃95% dip in
UT) for 5 s
3 A/m 3 A/m Power frequency
Not applicable Mains power quality
should be that of a
typical commercial or
hospital environment.
If the user of device
requires continued
operation during power
mains interruptions, it
is recommended that
device be powered from
an interruptible power
supply or a battery.
magnetic fields should
be at levels characteristic
of a typical location in
a typical commercial or
hospital environment
27
Page 28
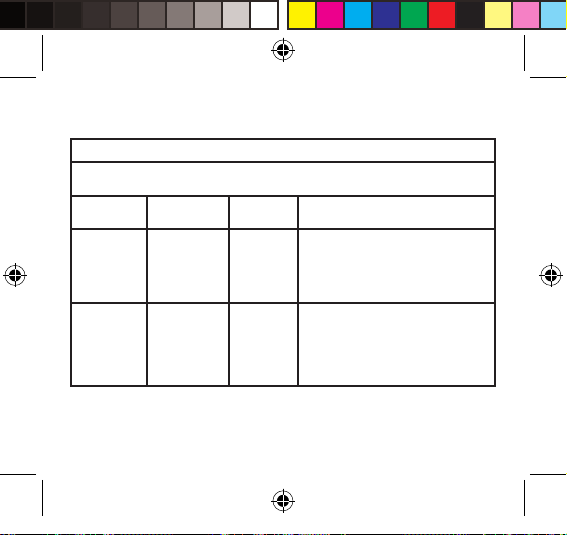
Table 3 – Guidance and MANUFACTURER’S declaration – electromagnetic IMMUNITY
– for ME EQUIPMENT and ME SYSTEMS that are not LIFE-SUPPORTING
Guidance and manufacturer’s declaration – electromagnetic immunity
The device is intended for use in the electromagnetic environment specified below. The customer
or the user of the device should assure that it is used in such an environment.
IMMUNITY
test
28
Manuel_BPM_book.indd 28 06/06/2014 10:21:22
IEC 60601 test
level
Compliance
level
Electromagnetic environment – guidance
Portable and mobile RF communications
equipment should be used no closer to any
part of the device, including cables, than
the recommended separation distance
calculated from the equation applicable to
the frequency of the transmitter.
Portable and mobile RF communications
equipment should be used no closer to any
part of the device, including cables, than
the recommended separation distance
calculated from the equation applicable to
the frequency of the transmitter.
Recommended separation distance
Page 29
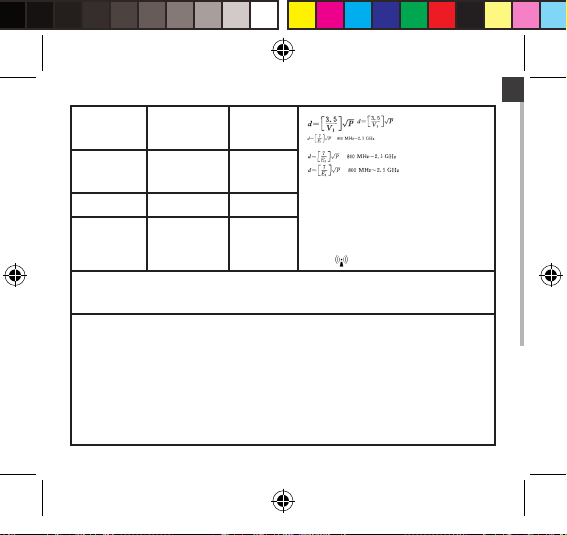
EN
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected
by absorption and reflection from structures, objects and people.
Field strengths from fixed transmitters, such as base stations for radio (cellular / cordless)
telephones and land mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast
cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due
to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured
field strength in the location in which the device is used exceeds the applicable RF compliance
level above, the device should be observed to verify normal operation. If abnormal performance is
observed, additional measures may be necessary, such as re-orienting or relocating the device.
b Over the frequency range 150 kHz to 80 MHz, field strengths should be less than[V1] V/m.
Manuel_BPM_book.indd 29 06/06/2014 10:21:23
2.333
3 Vrms
150 kHz to 80
MHz
3 V/m
80 MHz to 2.5
GHz
Not
applicable
3V/m
where P is the maximum output power rating of the
transmitter in watts (W) according to the transmitter
manufacturer and d is the recommended separation
distance in meters (m). Field strengths from fixed RF
transmitters, as determined by an electromagnetic
site survey, a should be less than the compliance level
in each frequency range. b Interference may occur in
the vicinity of equipment marked with the following
symbol:
29
Page 30
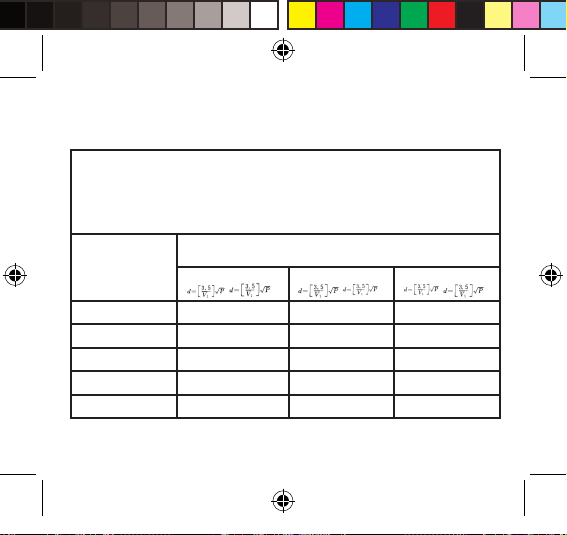
Table 4 – Recommended separation distances between portable and mobile RF
communications equipment and the ME EQUIPMENT or ME SYSTEM – for ME
EQUIPMENT and ME SYSTEMS that are not LIFE-SUPPORTING
Recommended separation distances between
portable and mobile RF communications equipment at the device
The device is intended for use in an electromagnetic environment in which radiated RF
disturbances are controlled. The customer or the user of the device can help prevent
electromagnetic interference by maintaining a minimum distance between portable and mobile RF
communications equipment (transmitters) and the device as recommended below, according to
the maximum output power of the communications equipment.
Rated maximum
output power of
transmitter
(W)
0.01 Not applicable 0.117 0.233
0.1 Not applicable 0.369 0.738
1 Not applicable 1.167 2.333
10 Not applicable 3.690 7.378
100 Not applicable 11.67 23.33
30
Manuel_BPM_book.indd 30 06/06/2014 10:21:23
Separation distance according to frequency of transmitter (m)
150 kHz to 80 MHz 80 MHz to 800 MHz 800 MHz to 2.5 GHz
Page 31

EN
For transmitters rated at a maximum output power not listed above, the recommended separation
distance d in meters (m) can be determined using the equation applicable to the frequency of the
transmitter, where P is the maximum output power rating of the transmitter in watts (W) according
to the transmitter manufacturer. NOTE 1 At 80 MHz and 800 MHz, the separation distance for
the higher frequency range applies. NOTE 2 These guidelines may not apply in all situations.
Electromagnetic propagation is affected by absorption and reflection from structures, objects and
people.
Manuel_BPM_book.indd 31 06/06/2014 10:21:23
31
Page 32

* Merci d’avoir choisi le tensiomètre ARCHOS.
* Veuillez lire attentivement l’intégralité de ce manuel de l’utilisateur an de garantir
l’utilisation en toute sécurité de ce produit, et conserver ce document an de le
consulter ultérieurement en cas de problèmes.
32
Manuel_BPM_book.indd 32 06/06/2014 10:21:23
Version:1.0
Manuel de l’utilisateur
Tensiomètre ARCHOS
Page 33

FR
CATALOGUE
INTRODUCTION
Informations relatives à la sécurité
Symboles de l’écran LCD
Composants du moniteur
AVANT DE COMMENCER
Installation et remplacement des piles
Réglage de la date et de l’heure
MESURES
Positionnement du brassard
Début des mesures
GESTION DES DONNÉES
Mémorisation des enregistrements
Suppression des enregistrements
INFORMATIONS DESTINÉES À L’UTILISATEUR
Conseils relatifs aux mesures
Maintenance
À PROPOS DE LA PRESSION ARTÉRIELLE
Que sont la pression systolique et la pression diastolique?
Quelle est la classication standard de la pression artérielle?
Pourquoi ma pression artérielle varie-t-elle pendant la journée?
Pourquoi la pression artérielle mesurée à l’hôpital est-elle diérente à la maison?
Le résultat est-il identique sur le poignet droit?
DÉPANNAGE
SPÉCIFICATIONS
INFORMATIONS DE CONTACT
LISTE DES NORMES EUROPÉENNES À RESPECTER
DIRECTIVES CEM
Manuel_BPM_book.indd 33 06/06/2014 10:21:23
Sommaire
33
Page 34

INTRODUCTION
Description générale
Merci d’avoir choisi le tensiomètre ARCHOS. Ce moniteur propose une mesure
de la pression artérielle, de la fréquence du pouls ainsi qu’une sauvegarde des
résultats. Sa conception est le gage d’un service able d’une durée de deux ans.
Les mesures prises grâce au tensiomètre sont équivalentes à celles obtenues
par un observateur formé à l’aide de la méthode d’auscultation utilisant un
brassard et un stéthoscope.
Le présent manuel contient des informations importantes relatives à la
sécurité et à l’assistance, et fournit des instructions étape par étape concernant
l’utilisation du produit.
Veuillez lire attentivement ce manuel avant toute utilisation du produit.
CARACTÉRISTIQUES:
* Pression artérielle systolique
* Pression artérielle diastolique
* Fréquence du pouls
* Mémoire: Jusqu’à 60 enregistrements
Informations relatives à la sécurité
Les symboles ci-dessous peuvent apparaître dans le manuel de l’utilisateur,
l’étiquetage ou d’autres composants. I ls constituent l’exigence d’une utilisation
standard.
34
Manuel_BPM_book.indd 34 06/06/2014 10:21:23
Page 35

FR
Symbole «LE MANUEL DOIT
ÊTRE LU»
Symbole «CONFORME AUX
EXIGENCES MDD93/42/ECC»
Symbole du «FABRICANT»
Symbole «Représentant
autorisé au sein de la
Communauté européenne»
Manuel_BPM_book.indd 35 06/06/2014 10:21:23
Symbole «PIÈCES
APPLIQUÉES DE TYPE BF»
Symbole du «NUMÉRO DE
SÉRIE»
Symbole «COURANT
CONTINU»
Symbole de la «DATE DE
FABRICATION»
Marque combinée Bluetooth
Symbole «PROTECTION
DE L’ENVIRONNEMENT» –
Les déchets des produits
électroniques ne doivent en
aucun cas être mis au rebut
avec les déchets ménagers.
Veuillez recycler ces produits
en utilisant les sites prévus
à cet eet. Renseignez-vous
auprès de votre autorité ou
de votre détaillant local pour
obtenir des informations
relatives au recyclage»
35
Page 36

AVERTISSEMENT
Veuillez lire attentivement l’intégralité du présent manuel de l’utilisateur avant toute utilisation.
Cet appareil est conçu uniquement pour une utilisation par un adulte.
Cet appareil est destiné à une mesure non invasive et une surveillance de la pression sanguine
artérielle. Il n’est pas prévu pour être utilisé sur des parties du corps autres que le poignet ou pour des
fonctions autres que l’obtention d’une mesure de la pression artérielle.
Ne pas confondre auto-surveillance et auto-diagnostic. Cette unité vous permet de surveiller votre
pression artérielle. Veuillez commencer ou interrompre un traitement médical en vous appuyant
uniquement sur les conseils de traitement d’un médecin.
Si vous prenez des médicaments, consultez votre médecin an de déterminer l’heure de mesure
la plus adaptée. Ne changez jamais un médicament prescrit sans l’autorisation préalable de votre
médecin.
Cette unité n’est pas adaptée pour une sur veillance continue pendant des urgences ou opérations
médicales.
Si la pression du brassard est supérieure à 40kPa (300mmHg), l’unité dégonera automatiquement.
Si le brassard ne se dégone pas lorsque la pression est supérieure à 40kPa (300mmHg), retirez le
brassard du poignet puis appuyez sur la touche START/STOP (MARCHE/ARRÊ T) pour interrompre le
gonage.
Ne pas utiliser le moniteur dans des conditions de fort champ électromagnétique (par exemple un
équipement médical RF) qui émet des signaux d’interférence ou des signaux transitoires électriques
rapides / des signaux en rafale.
La température maximale pouvant être atteinte par la partie appliquée est de 42.5℃ alors que la
température environnementale est de 40℃.
L’appareil n’est pas un équipement AP/APG. Il n’est pas adapté pour une utilisation en présence d’un
mélange anesthésique inammable à l’air (ou à l’oxygène, à l’oxyde d’azote).
Veuillez maintenir l’unité hors de portée des nourrissons ou des enfants, puisque l’inhalation ou
l’ingestion de petites pièces est dangereuse, voire mortelle.
Veuillez utiliser les ACCESSOIRES et les pièces détachables spéciées / autorisées par le FABRICANT.
36
Manuel_BPM_book.indd 36 06/06/2014 10:21:23
Page 37

FR
Dans le cas contraire, ceci peut entraîner un endommagement de l’unité ou un danger pour
l’utilisateur / le patient.
Le fabricant fournira sur demande des schémas de circuit, les éléments des composants mentionnés.
Les personnes sensibles, notamment les femmes enceintes et les personnes possédant un dispositif
implanté électronique médical doivent éviter dans la mesure du possible d’utiliser l’unité.
Cette unité n’est pas adaptée pour une sur veillance continue pendant des urgences ou opérations
médicales.
Un gonement du brassard pendant une longue période peut entraîner une insusance du poignet
et des doigts du patient, une anesthésie, des douleurs lors d’un mouvement et des ecchymoses.
Veuillez utiliser l’appareil dans l’environnement proposé dans le manuel de l’utilisateur.
Dans le cas contraire, la performance et la durée de vie de l’appareil seront aectées et réduites.
Pendant l’utilisation, le patient sera en contact avec le brassard. Les matériaux du brassard ont été
soumis à essai et jugés conformes aux exigences de l’ISO 10993-5:2009 et de l’ISO 10993-10:2010. Ils
ne provoqueront aucune réaction allergique potentielle ni aucune blessure due au contact.
L’appareil a fait l’objet d’une évaluation clinique à l’aide d’une auscultation utilisant un brassard
manuel / un stéthoscope servant de référence.
L’appareil n’exige aucun étalonnage pendant deux années d’utilisation en toute abilité.
Si l’appareil a été utilisé pour eectuer des mesures sur des patients atteints d’arythmie fréquente,
telle que des extrasystoles atriales ou ventriculaires ou une brillation auriculaire, les résultats d’essai
peuvent engendrer des écarts. Veuillez consulter votre médecin concernant les résultats.
Cet appareil est contre-indiqué pour toute femme enceinte ou soupçonnant une grossesse.
Outre les résultats imprécis mentionnés, les eets de cet appareil sur le fœtus ne sont pas connus.
Manuel_BPM_book.indd 37 06/06/2014 10:21:23
37
Page 38

Symboles de l’écran LCD
SYMBOLE
38
Manuel_BPM_book.indd 38 06/06/2014 10:21:23
DESCRIPTION EXPLICATION
Pression artérielle systolique Pression artérielle élevée
Pression artérielle diastolique Pression artérielle faible
Pouls battements/minute
Batterie faible
Unité Unité de mesure de la pression artérielle
Temps Heure: Minute (Mois/jour/année)
Détecteur IHB Détecteur de pouls irrégulier
Bluetooth Connexion Bluetooth réussie
Erreur Erreur
Mémoire
Batterie faible, veuillez remplacer
les piles.
Mémorisation des enregistrements de
l’historique
Page 39

FR
Composants du moniteur
Liste de composants:
1. PCBA; 2. Tuyau d’air; 3. Pompe; 4. Valve; 5. Brassard.
Liste
1. Moniteur de pression artérielle pour poignet 2. Deux piles AAA 3. Manuel de l’utilisateur
AVANT DE COMMENCER
Installation et remplacement des piles
•.Ouvrez le compartiment à piles.
•.Insérez les piles conformément aux instructions de polarité. (Choisissez toujours la pile
autorisée / spéciée: deux piles AAA LR03).
•.Fermez le compartiment à piles.
Manuel_BPM_book.indd 39 06/06/2014 10:21:23
39
Page 40

Durée de vie des piles: Environ 57 jours
(Capacité des piles: 600mAH. En cas de mesure eectuée 3 fois par jour, chaque
mesure exige 30s, et si la mémoire est vériée une fois par jour, chaque vérication
prend 60s. Le courant de mesure est de 350mA, le courant utilisé pour l’achage
des enregistrements est de 50mA, tandis que le courant à l’arrêt est de 25uA.)
Changez les piles dans les cas suivants:
achages sur l’écran LCD
La luminosité de l’écran LCD est faible.
Lors de la mise sous tension du moniteur, l’écran LCD ne s’allume pas.
* Retirez les piles si l’appareil n’est pas utilisé pendant une période prolongée.
* Les piles usagées sont nocives pour l’environnement. Ne les jetez avec les déchets
quotidiens. * Retirez les anciennes piles de l’appareil en respectant vos directives de
recyclage locales. * Ne jetez pas les piles au feu. Les piles peuvent en eet exploser
ou fuir.
Réglage de la date et de l’heure
Veuillez procéder au réglage de l’heure avant votre première utilisation an
de garantir que chaque enregistrement soit marqué d’un horodatage. (Plage
d’années: 2012-2052; Format de l’heure: 12 heures)
1. Lorsque le moniteur est sur la position OFF, appuyez puis maintenez enfoncée
la touche «SET» (RÉGLER) pendant 3 secondes an de lancer le mode Réglage de
40
Manuel_BPM_book.indd 40 06/06/2014 10:21:23
AVERTISSEMENT
Page 41

FR
l’heure.
2.Comme le montre l’image de droite, le chire «6» représentant l’[HEURE] clignote.
Appuyez sur la touche «MEM» pour modier le chire. Chaque pression augmentera
la valeur d’un chire de façon cyclique.
3.Appuyez à nouveau sur la touche «SET» pour conrmer l’[HEURE]. Le chire
représentant les [MINUTES] clignote alors.
4.Répétez les étapes 2 et 3 pour conrmer les [MINUTES].
5.Répétez les étapes 2 et 3 pour conrmer le [MOIS], le [JOUR] et l’[ANNÉE].
Manuel_BPM_book.indd 41 06/06/2014 10:21:24
41
Page 42

6.Après avoir conrmé l’[ANNÉE], l’écran LCD achera «DONE» (TERMINÉ) puis
s’éteindra.
Positionnement du brassard
1. Retirez l’ensemble des accessoires (montre, bracelet, etc.) de votre poignet. Si votre
médecin a diagnostiqué une mauvaise circulation dans votre poignet, utilisez l’autre
poignet.
2. Roulez ou remontez votre manche pour laisser apparaître la peau.
3. Placez le brassard sur votre poignet avec la paume de la main placée vers le haut.
4. Positionnez l’extrémité du brassard à environ 1-1,5cm.
5. Attachez le brassard autour de votre poignet, en ne laissant aucun espace entre
le brassard et votre peau. Si le brassard est trop lâche, les mesures ne seront pas
précises.
Reposez-vous pendant 5 minutes avant de procéder à la mesure.
Patientez au moins 3 minutes entre chaque mesure. Ceci permet à votre circulation
sanguine de récupérer.
Pour une comparaison cohérente, essayez de procéder aux mesures dans des
conditions similaires. Par exemple, réalisez les mesures quotidiennes à la même
heure, sur le même poignet ou conformément aux instructions d’un médecin.
42
Manuel_BPM_book.indd 42 06/06/2014 10:21:24
Page 43

FR
Appairage du moniteur de pression artérielle à votre appareil
1.Activez le Bluetooth et ouvrez l’application. Assurez-vous que les deux sont allumés
lorsque l’appairage est en cours.
2.Lorsque le moniteur est sur la position OFF, appuyez puis maintenez enfoncée la
touche START pendant 2 secondes an de lancer l’appairage. Les symboles et
En cas de RÉUSSITE, le symbole apparaîtra sur l’écran LCD.
En cas d’ÉCHEC, le symbole apparaîtra sur l’écran LCD.
Le moniteur s’éteindra automatiquement une fois l’appairage terminé.
MESURES
Début des mesures
1. Une fois le brassard correctement positionné, appuyez sur la touche START pour
mettre le moniteur sous tension, ce qui lancera automatiquement le processus de
mesure.
Manuel_BPM_book.indd 43 06/06/2014 10:21:24
alterneront sur l’écran LCD, indiquant ainsi que l’appairage est en cours.
43
Page 44

Réglage sur zéro.
Gonement et mesure.
Achage et enregistrement du résultat de mesure.
2. Cet appareil procèdera automatiquement à la transmission des données à l’issue
des mesures. Le symbole Bluetooth clignote.
44
Manuel_BPM_book.indd 44 06/06/2014 10:21:24
Page 45

FR
3.Si les données sont transmises avec succès, l’écran LCD apparaîtra tel que le montre
l’image de droite.
Si la transmission des données échoue, l’écran LCD indiquera le message «ERROR»
(ERREUR).
4.Appuyez sur la touche STOP (ARRÊT) pour mettre le moniteur hors tension. Dans le
cas contraire, il s’éteindra automatiquement.
Manuel_BPM_book.indd 45 06/06/2014 10:21:24
45
Page 46

GESTION DES DONNÉES
Mémorisation des enregistrements
1.Appuyez sur la touche «MEM» pour accéder à la mémoire.
2. Appuyez sur la touche «MEM/UP» ou sur la touche «SET/DOWN» pour naviguer
dans les enregistrements de l’historique. «MEM/UP» pour avancer; «SET/DOWN»
pour reculer.
L’enregistrement le plus récent (1) est indiqué en premier. Chaque nouvelle mesure est attribuée au
premier (1) enregistrement. Tous les autres enregistrements sont décalés d’un chire (par exemple, 2
devient 3, et ainsi de suite), et le dernier enregistrement (60) est retiré de la liste.
Suppression des enregistrements
Si vous n’avez pas obtenu une mesure précise, vous pouvez supprimer tous les
résultats de mesure en suivant les étapes indiquées ci-dessous.
46
Manuel_BPM_book.indd 46 06/06/2014 10:21:24
AVERTISSEMENT
Page 47

FR
1.En mode Rappel de la mémoire, appuyez puis maintenez enfoncée la touche
«MEM» ainsi que la touche «SET» pendant 3 secondes.
2.L’écran LCD achera «dEL dONE», indiquant ainsi que la suppression de la
mémoire est terminée.
3.Si vous souhaitez abandonner la suppression, appuyez sur la touche «START/
STOP» pour mettre le moniteur hors tension.
4 Lorsque le moniteur ne dispose plus de mémoire, si vous appuyez sur la touche
«MEM» pour consulter
l’historique, l’écran LCD s’achera tel que le montre la gure de droite.
Manuel_BPM_book.indd 47 06/06/2014 10:21:24
47
Page 48

INFORMATIONS DESTINÉES À L’UTILISATEUR
Conseils relatifs aux mesures
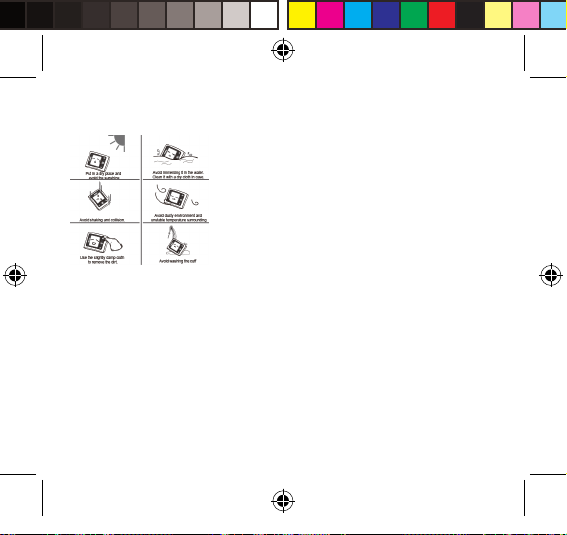
Les résultats pourraient s’avérer imprécis si les mesures sont eectuées dans les
circonstances suivantes.
- 1 heure après le dîner ou l’ingestion d’une boisson
- Mesure prise immédiatement après une consommation de thé, de café ou de
tabac
- 20 minutes après un bain
- En discutant ou en remuant les doigts
- Dans un environnement froid Lorsque vous avez envie d’uriner
Maintenance
An d’obtenir des performances optimales, veuillez respecter les instruc tions
ci-dessous.
- Placez l’appareil dans un lieu sec et évitez les rayons du soleil
- Évitez toute immersion dans de l’eau. Nettoyez l’appareil à l’aide du chiffon
sec fourni dans l’étui.
- Évitez de secouer ou de cogner l’appareil.
- Évitez de le placer dans un environnement poussiéreux et présentant une
température instable
- Utilisez un chiffon légèrement humide pour retirer la poussière.
- Évitez de laver le brassard
Nettoyage: un environnement poussiéreux peut aecter les performances de l’unité.
Veuillez utiliser le chion doux an de retirer la poussière avant toute utilisation.
Veuillez veiller à ce que l’unité fonctionne en toute sécurité et dans de bonnes
conditions de fonctionnement avant toute utilisation.
Veuillez suivre les instructions relatives au remplacement correct des pièces
interchangeables ou détachables identiées par le PERSONNEL DE SERVICE du
FABRICANT comme «remplaçables».
48
Manuel_BPM_book.indd 48 06/06/2014 10:21:24
Page 49

FR
Mise au rebut: des capteurs endommagés ou des électrodes desserrées peuvent
dégrader les performances de l’unité et entraîner d’autres problèmes. Veuillez mettre
au rebut les ACCESSOIRES, les pièces détachables et l’ÉQUIPEMENT ME conformément
aux directives locales.
À PROPOS DE LA PRESSION ARTÉRIELLE
Que sont la pression systolique et la pression diastolique?
Lorsque les ventricules se contractent et pompent le sang hors du cœur, la pression
artérielle atteint sa valeur maximale du cycle, que l’on appelle la pression systolique.
Lorsque les ventricules se relâchent, la pression artérielle atteint sa valeur minimale
au cours du cycle, que l’on appelle quant à elle la pression diastolique.
Manuel_BPM_book.indd 49 06/06/2014 10:21:24
Systolique
sang sortant de
l’artère
Contraction
Diastolique
sang entrant dans
les veines
relâchement
49
Page 50

Quelle est la classication standard de la pression artérielle?
La classication de la pression artérielle publiée en 1999 par l’Organisation mondiale
de la santé (OMS) et par la Société internationale de l’hypertension (ISH) est la
suivante:
AVERTISSEMENT
Seul un médecin est à même de déterminer votre pression artérielle normale. Veuillez contacter un
médecin si vos résultats de mesure se situent en dehors de cette plage.
Veuillez noter que seul un médecin est en mesure de vous indiquer si votre pression artérielle a atteint
une valeur dangereuse.
Pression
artérielle
(mm Hg)
50
Manuel_BPM_book.indd 50 06/06/2014 10:21:24
Niveau
Optimale
SYS 120
DIA 80
Normale
121-
130
81-8586-
G1 G2 G3
131-
141-160
140
91-100 101-110 ≥110
90
Hypertension
161-
180
≥180
Page 51

FR
Détecteur de pouls irrégulier
Ce moniteur de pression artérielle pour poignet est équipé d’une fonction
intelligente appelée Détecteur de pouls irrégulier (IHB). Au cours de chaque mesure,
cet équipement enregistre les intervalles entre chaque pulsation et détermine l’écar ttype. Si les intervalles entre chaque pulsation
sont comparés aux intervalles moyens, l’écart supérieur à 3 dépasse 25% ou si l’écart
supérieur à 5 est de plus de 15%, cet équipement indiquera à l’écran le symbole IHB
lors de l’achage du résultat de mesure.
L’apparition de l’icône IHB indique qu’une irrégularité du pouls accompagnée de pulsations
irrégulières a été détectée pendant la mesure. Ceci ne constitue généralement PAS un fait inquiétant.
Cependant, si le symbole apparaît souvent, nous vous recommandons de demander conseil auprès
d’un médecin. Veuillez noter que l’appareil ne remplace en aucun cas un examen du cœur, mais
permet de détecter des irrégularités du pouls à un stade précoce.
Pourquoi ma pression artérielle varie-t-elle pendant la journée?
1 La pression artérielle individuelle varie tous les jours et est également aectée par
le positionnement de votre brassard et par votre position de mesure; veuillez donc
procéder aux mesures dans des conditions identiques.
2 Les variations de la pression sont supérieures si le patient prend des médicaments.
3 Patientez au moins 3 minutes avant de procéder à une autre mesure.
Manuel_BPM_book.indd 51 06/06/2014 10:21:24
AVERTISSEMENT
51
Page 52

Pourquoi la pression artérielle mesurée à l’hôpital est-elle diérente à la
maison?
La pression artérielle est diérente même à 24 heures d’intervalle, en raison des
conditions climatiques, des émotions, de l’exercice physique, etc., notamment en
raison de la fameuse «blouse blanche» de l’hôpital qui a tendance à produire des
résultats supérieurs à ceux de la maison.
Le résultat est-il identique sur le poignet droit?
Les mesures sont possibles sur les deux poignets, mais les résultats seront diérents
selon les individus; nous vous recommandons donc d’utiliser systématiquement le
même poignet.
Lorsque vous mesurez chez vous votre pression artérielle, vous devez porter une
attention particulière aux aspects suivants:
Le brassard est correctement positionné.
Le brassard est trop serré ou trop lâche.
Le brassard est positionné sur le poignet.
Vous vous sentez anxieux.
Vous devriez respirer profondément à 2-3 reprises avant de commencer.
Conseil: adaptez-vous pendant 4-5 minutes an de vous calmer.
DÉPANNAGE
La présente section comprend une liste de messages d’erreur et des questions
fréquentes concernant les problèmes que vous êtes susceptible de rencontrer avec
votre moniteur de pression artérielle pour poignet. Si le produit ne fonctionne pas
comme il le devrait, consultez cette section avant d’envisager une réparation.
52
Manuel_BPM_book.indd 52 06/06/2014 10:21:24
Page 53

FR
PROBLÈME SYMPTOME À VÉRIFIER SOLUTION
Aucune
alimentation
Batterie faible L’écran présente
Message d’erreur
Manuel_BPM_book.indd 53 06/06/2014 10:21:24
L’écran ne s’allume
pas.
une faible
luminosité ou
indique le symbole
Ache
le symbole
Error 1 (erreur 1) Le gonement
Error 2 (erreur 2) Le brassard est très
Error 3 (erreur 3) La pression du
Les piles sont vides. Remplacez-les par
Les piles ne sont
pas correctement
insérées.
Les piles sont
faibles.
La communication
des données a
échoué
est lent ou le
brassard n’est pas
correctement
positionné.
serré
brassard est
excessive.
de nouvelles piles
Insérez-les
correctement
Remplacez-les par
de nouvelles piles
Vériez si
l’APPLICATION est
allumée, essayez
à nouveau de
procéder à la
transmission des
données.
Positionnez à
nouveau le brassard
puis procédez à une
nouvelle mesure.
Positionnez
à nouveau le
brassard, an qu’il
ne soit ni trop lâche,
ni trop serré, puis
procédez à une
nouvelle mesure.
Positionnez à
nouveau le brassard
puis procédez à une
nouvelle mesure.
53
Page 54

Error 5 (erreur 5) ou Error 6
(erreur 6)
Error 10 (erreur 10) ou Error
11 (erreur 11)
Error 20 (erreur 20) Le processus de mesure ne
Error 21 (erreur 21)
apparaissant à l’écran.
SPÉCIFICATIONS
Alimentation 2*piles AAA
Type d’écran Écran LCD V.A. 36 x 41 mm
Méthode de mesure Méthode d’essai oscillographique
Plage de mesure Pression : 0 kpa-40 kpa (0 mmHg-
Précision Pression :
54
Manuel_BPM_book.indd 54 06/06/2014 10:21:24
Une erreur système est
survenue.
Le moniteur a détecté
un mouvement ou une
discussion ou les pulsations
sont trop faibles pendant
la mesure.
détecte aucun signal de
pulsation.
Le traitement de la mesure
a échoué.
300 mmHg)pulsation :(40-199)
battements/minute
5)-40) avec ± 0,4 kpa (3 mmHg)
0)-45) (en dehors de 5)-40))
avec ± 0,7 kpa (5 mmHg)
pulsations :± 5 %
Procédez à une nouvelle
mesure. Si le problème
persiste, contactez le
détaillant ou notre service
client an d’obtenir de l’aide.
Consultez la garantie an
d’obtenir des informations
de contact et les instructions
de renvoi.
Détendez-vous pendant
quelques instants puis
procédez à une nouvelle
mesure.
Desserrez tout vêtement
porté à proximité du
poignet puis procédez à une
nouvelle mesure.
Détendez-vous pendant
quelques instants puis
procédez à une nouvelle
mesure.
Page 55

FR
Conditions de fonctionnement
normales
Conditions de stockage et de transport Température : -20) à 60)
Périmètre de mesure du poignet Environ 13,5 cm-21,5 cm
Poids net Environ 120 g (hors piles sèches)
Dimensions extérieures Environ 80 × 65 × 22 mm
Matériel supplémentaire 2* piles AAA, manuel de l’utilisateur
Mode de fonctionnement Fonctionnement continu
Degré de protection Pièce appliquée de type BF
Niveau d’étanchéité IP22
Version du logiciel V01
Classication de l’appareil Équipement ME à alimentation interne
AVERTISSEMENT: Aucune modication de cet équipement n’est autorisée.
INFORMATIONS DE CONTACT
Informations de contact
Pour de plus amples informations sur nos produits, veuillez visiter le site www.archos.
com, où vous pourrez obtenir une assistance client, des réponses aux problèmes
fréquents ou télécharger nos documents; ARCHOS vous répondra à tout moment.
Fabriqué par: GUANGDONG TRANSTEK MEDIC AL ELECTRONICS CO., LTD
Entreprise: GUANGDONG TRANSTEK MEDICAL ELECTRONICS CO., LTD
Adresse: Zone A, 5/F., Investment Building, No. 12, Huizhan East Rd., Torch
Development District, Zhongshan, Guangdong, 528437, Chine
Représentant européen autorisé:
Entreprise: MDSS - Medical Device Safety Service GmbH
Adresse: Schigraben 41, 30175 Hannover, Allemagne
Manuel_BPM_book.indd 55 06/06/2014 10:21:24
Température : 5)-40)
Humidité relative ≤ 80 %
Pression atmosphérique : 86 kPa à
106 kPa
Humidité relative : 10 % à 93 % HR
55
Page 56

Liste des normes européennes à respecter
Gestion des risques EN/ISO 14971:2007
Étiquetage EN 15223:2012
Manuel de l’utilisateur EN 1041:2008
Exigences générales
relatives à la sécurité
Sphygmomanomètres
non invasifs
Exigences
générales
Compatibilité
électromagnétique
Durée de vie du logiciel EN 62304:2006/AC:2008
Facilité d’utilisation EN 60601-1-6:2010
56
Manuel_BPM_book.indd 56 06/06/2014 10:21:24
EN 60601-1:2006/AC2010
EN 60601-1-11:2010
EN 1060-1:1995+A2:2009
EN 1060-3:1997+A2:2009
EN 1060-4:2004
EN 60601-1-2:2007/AC:2010
Page 57

DE
Version:1.0
* Vielen Dank für Ihren Kauf eines ARCHOS-Blutdruckmessgeräts.
* Um einen störungsfreien Betrieb dieses Produkts zu gewährleisten, lesen Sie bitte
dieses Benutzerhandbuch sorgfältig durch und bewahren Sie es zum späteren
Nachlesen auf.
Manuel_BPM_book.indd 57 06/06/2014 10:21:24
Benutzerhandbuch
ARCHOS-Blutdruckmessgerät
57
Page 58

KATALOG
EINFÜHRUNG
Sicherheitsinformationen
LCD-Displaysignal
Komponenten des Messgeräts
BEVOR SIE STARTEN
Einlegen und Austausch der Batterien
Einstellung von Datum und Uhrzeit
MESSUNG
Anlegen der Manschette
Messung starten
DATENVERWALTUNG
Abrufen der Aufzeichnungen
Löschen der Aufzeichnungen
INFORMATIONEN FÜR BENUTZER
Tipps für das Messen
Wartung
ÜBER BLUTDRUCK
Was bedeuten systolischer Blutdruck und diastolischer Blutdruck?
Was ist die Standardklassizierung für Blutdruck?
Warum schwankt mein Blutdruck im Tagesverlauf?
Warum unterscheidet sich die Blutdruckmessung im Krankenhaus von der Messung Zuhause?
Erhalte ich bei einer Messung am rechten Handgelenk das gleiche Ergebnis?
FEHLERBEHEBUNG
TECHNISCHE DATEN
KONTAKTINFORMATIONEN
LISTE ENTSPROCHENER EUROPÄISCHER STANDARDS
EMC-LEITFADEN
58
Manuel_BPM_book.indd 58 06/06/2014 10:21:25
Inhaltsverzeichnis
Page 59

DE
EINFÜHRUNG
Allgemeine Beschreibung
Vielen Dank für Ihren Kauf eines ARCHOS-Blutdruckmessgeräts. Das Messgerät bietet
Blutdruckmessung, Pulsfrequenzmessung und eine Ergebnisspeicherung. Das Gerät ist für einen
zuverlässigen Service von 2 Jahren ausgelegt.
Die vom Blutdruckmessgerät gemessenen Werte entsprechen den Wer ten, die eine dafür geschulte
Person anhand von Manschette und Stethoskopabhörung erhalten würde.
Dieses Handbuch enthält wichtige Sicherheits- und Pegeinformationen und stellt Schritt-fürSchritt-Anweisungen für die Verwendung des Produkts bereit.
Vor der Ver wendung des Produkts das Handbuch sorgfältig durchlesen.
MERKMALE:
* Systolischer Blutdruck
* Diastolischer Blutdruck
* Pulsfrequenz
* Speicher: Bis zu 60 Einzelaufzeichnungen
Sicherheitsinformationen
Die folgenden Zeichen können in Benutzerhandbuch, Beschriftungen oder mit
anderen Komponenten vorhanden sein. Sie stellen die Anforderung für Standard und
Verwendung dar.
Manuel_BPM_book.indd 59 06/06/2014 10:21:25
59
Page 60

60
Manuel_BPM_book.indd 60 06/06/2014 10:21:25
Symbol für “DAS
BETRIEBSHANDBUCH MUSS
GELESEN WERDEN”
Symbol für “ENTSPRICHT
DEN MDD93/42/ECCANFORDERUNGEN”
Symbol für “ANGEWENDETER
TEILETYP”
Symbol für “SERIENNUMMER”
Symbol für “GLEICHSTROM”
Symbol für
“HERSTELLUNGSDATUM”
Symbol für “HERSTELLER”
Symbol für “Autorisierter
Vertreter in der Europäischen
Gemeinschaft”
Das BluetoothKombinationszeichen
Symbol für
“UMWELTSCHUTZ–
Elektrische Abfallprodukte
dürfen nicht im Hausmüll
entsorgt werden. Bitte
bei entsprechenden
Einrichtungen entsorgen.
Informationen
über geeignete
Entsorgungsstellen
erhalten Sie über Ihre
Gemeindebehörde oder
Ihren Einzelhändler.
Page 61

DE
Bitte lesen Sie vor der Verwendung dieses Produkts das Benutzerhandbuch sorgfältig durch.
Dieses Gerät ist nur für den Gebrauch durch Erwachsene vorgesehen.
Dieses Gerät ist für nichtinvasive Messungen und die Überwachung des arteriellen Blutdrucks
vorgesehen. Es ist nur für die Verwendung am Handgelenk und für die Blutdruckmessung vorgesehen.
Verwechseln Sie nicht Selbstüberwachung mit Selbstdiagnose. Dieses Gerät ermöglicht die
Überwachung Ihres Blutdrucks.. Bitte beginnen oder beenden Sie eine medizinische Behandlung
ausschließlich auf Ratschlag eines Arztes.
Nehmen Sie Medikamente ein, besprechen Sie mit Ihrem Arzt die geeignete Zeit für Ihre Messung.
Eine verschriebene Medikation darf ohne die Zustimmung Ihres Arztes nicht geändert werden.
Dieses Gerät ist für eine kontinuierliche Überwachung während medizinischer Notfälle oder
Operationen nicht geeignet.
Überschreitet der Druck der Manschette 40 kPa (300 mmHg), wird das Gerät automatisch die Luft
ablassen. Sollte die Luft bei Überschreiten von 40 kPa (300 mmHg) nicht abgelassen werden, die
Manschette vom Handgelenk entfernen und die START/STOP-Taste drücken, um das Aufpumpen zu
beenden.
Das Messgerät nicht in der Nähe starker elektromagnetischer Felder verwenden (z. B. medizinische
Hochfrequenzgeräte), die ein Störsignal oder elektrische Transient-/Burst-Signale ausstrahlen.
Die Maximaltemperatur für das angewendete Teil beträgt 42,5℃ und die Umgebungstemperatur 40℃.
Das Gerät ist nicht nach Kategorie AP bzw. APG zugelassen. Es ist für die Verwendung in Gegenwart
einer brennbaren Betäubungsmittelmischung mit Luft (oder Sauersto, Lachgas) nicht geeignet.
Das Gerät außer Reichweite von Kleinkindern und Kindern aufbewahren, da das Einatmen oder
Verschlucken von Kleinteilen gefährlich und sogar tödlich sein kann. Bitte nur vom HERSTELLER
angegebenes/autorisiertes ZUBEHÖR und abnehmbare Teile verwenden Das Gerät kann sonst
beschädigt werden oder den Benutzer/Patient gefährden.
Der Hersteller wird auf Anfrage Schaltpläne und aufgeführte Bauteile zur Verfügung stellen.
Sensible Menschen, wie beispielsweise schwangere Frauen und diejenigen mit implantierten
elektronischen Instrumenten, sollten dieses Gerät möglichst nicht verwenden. Dieses Gerät ist für eine
kontinuierliche Überwachung während medizinischer Notfälle oder Operationen nicht geeignet.
Bleibt die Manschette längere Zeit aufgepumpt, werden Handgelenk und Finger des Patienten
unbeweglich und es treten Dehnungsschmerzen und Ekchymose auf. Bitte verwenden Sie das Gerät in
der Umgebung, wie im Benutzerhandbuch beschrieben.
Manuel_BPM_book.indd 61 06/06/2014 10:21:25
ACHTUNG
61
Page 62

Leistung und Lebensdauer des Geräts können sonst beeinusst und reduziert werden.
Während der Messung hat der Patient Kontakt mit der Manschette. Das Material der Manschette
wurde getestet und entspricht den Anforderungen der Normen ISO 10993-5:2009 und ISO 1099310:2010. Es verursacht keine potenzielle allergische Reaktion oder Kontaktverletzung. Das Gerät wurde
anhand einer manuellen Manschetten-/Stethoskopabhörung als Referenz klinisch bewertet.
Das Gerät ist für zwei Jahre zuverlässigen Service vorbereitet, eine Kalibrierung ist erst nach diesem
Zeitraum erforderlich.
Wird das Gerät für die Messung bei Patienten mit allgemeinen Herzrhythmusstörungen verwendet,
wie z. B. ventrikuläre Extrasystole oder Vorhoimmern, kann das Testergebnis abweichen. Bitte
besprechen Sie die Ergebnisse mit Ihrem Arzt.
Dieses Gerät ist für schwangere Frauen oder für Frauen, bei denen der Verdacht für eine
Schwangerschaft besteht, kontraindiziert.
Neben ungenauen Messwerten sind die Auswirkungen dieses Geräts auf den Fötus unbekannt.
62
Manuel_BPM_book.indd 62 06/06/2014 10:21:25
Page 63

DE
LCD-Displaysignal
SYMBOL
Manuel_BPM_book.indd 63 06/06/2014 10:21:25
BESCHREIBUNG ERLÄUTERUNG
Systolischer Blutdruck Hoher Blutdruck
Diastolischer Blutdruck Niedriger Blutdruck
Puls Schläge/Minute
Batterie leer
Einheit Maßeinheit des Blutdrucks
Uhrzeit
IHB-Anzeige
Bluetooth
Fehler Fehler
Speicher Abrufen der Aufzeichnungen
Batterie schwach und
Batterien ersetzen.
Stunde: Minute (Monat/
Tag/Jahr)
Erkennung eines
unregelmäßigen Herzschlags
Erfolgreiche BluetoothVerbindung
63
Page 64

Komponenten des Messgeräts
Komponentenliste:
1. PCBA; 2. Luftleitung; 3. Pumpe; 4. Ventil; 5. Manschette.
Liste
1. Blutdruckmessgerät für das Handgelenk 2. Zwei AAA-Batterien 3. Benutzerhandbuch
BEVOR SIE STARTEN
Einlegen und Austausch der Batterien
•.Önen Sie das Batteriefach.
•.Die Batterien entsprechend der Polangaben einlegen. (Immer die autorisierten/
angegebenen Batterien verwenden: Zwei LR03 AAA-Batterien).
•.Schließen Sie das Batteriefach.
64
Manuel_BPM_book.indd 64 06/06/2014 10:21:25
Page 65

DE
Batterielaufzeit: ca. 57 Tage
(Batteriekapazität: 600 mAH. Bei dreimal Messen pro Tag, eine Messung benötigt 30 s
und Überprüfung des Speichers einmal täglich, jede Überprüfung benötigt 60 s. Die
Stromstärke für die Messung beträgt 350 mA, für die Anzeige der Aufzeichnungen 50
mA und die Stromstärke beim Ausschalten 25 uA.)
Das Gerät unter folgenden Bedingungen auaden:
wird auf dem LCD angezeigt
Die LCD-Anzeige wird dunkel.
Beim Einschalten des Messgeräts leuchtet der LCD-Bildschirm nicht auf.
* Wird das Gerät voraussichtlich für längere Zeit nicht verwendet, die Batterien herausnehmen.
* Verschlissene Batterien sind umweltgefährdend. Die Batterien nicht mit dem normalen Hausmüll
entsorgen. * Die alten Batterien unter Einhaltung der Entsorgungsrichtlinien aus dem Gerät
entnehmen. * Die Batterien zur Entsorgung nicht ins Feuer werfen. Die Batterien können explodieren
oder auslaufen.
Manuel_BPM_book.indd 65 06/06/2014 10:21:25
ACHTUNG
65
Page 66

Einstellung von Datum und Uhrzeit
Bitte fahren Sie vor der ersten Verwendung mit der Zeiteinstellung for t, damit
jede Aufzeichnung mit der korrekten Zeit versehen wird. (Jahr Bereich: 2012-2052;
Zeitformat: 12 Stunden)
1. Bei AUSGESCHALTE TEM Messgerät die Taste «SET» 3 drücken und 3 Sekunden
halten, um in den Modus Zeiteinstellung zu gelangen.
2.Wie rechts abgebildet, repräsentiert die blinkende Zahl «6» [HOUR]. Die Taste
«MEM» drücken, um die Zahl zu ändern. Jeder Tastendruck erhöht die Zahl jeweils
um 1 .
3.Drücken Sie die Taste «SE T» erneut, um [HOUR] zu bestätigen. Dann beginnt die
[MINUTE] repräsentierende Zahl zu blinken.
66
Manuel_BPM_book.indd 66 06/06/2014 10:21:25
Page 67

DE
4.Wiederholen Sie Schritt 2 und 3 zur Bestätigung von [MINUTE].
5.Wiederholen Sie Schritt 2 und 3 zur Bestätigung von [MONTH], [DAY] und [YEAR].
6.Nach der Bestätigung von [YEAR], wird auf dem LCD-Bildschirm «DONE» angezeigt
und das Messgerät schaltet sich ab.
Anlegen der Manschette
1. Entfernen Sie vorhandenen Schmuck von Ihrem Handgelenk (Uhr, Armband usw.)
Hat Ihr Arzt an Ihrem Handgelenk eine schlechte Durchblutung diagnostiziert, bitte
das andere Handgelenk verwenden.
2. Den Ärmel nach oben rollen oder ziehen, um so die Haut freizulegen.
Manuel_BPM_book.indd 67 06/06/2014 10:21:25
67
Page 68

3. Mit der Handäche nach oben die Manschette auf Ihr Handgelenk legen.
4. Positionieren Sie den Rand der Manschette etwa 1-1,5 cm.
5. Befestigen Sie die Manschette um das Handgelenk, wobei die Manschette eng
an der Haut anliegen muss. Liegt die Manschette zu locker an, wird die Messung
ungenau.
Vor der Messung 5 Minuten ruhig sitzen bleiben.
Warten Sie mindestens 3 Minuten zwischen den Messungen. So kann sich die
Blutzirkulation wieder erholen.
Für einen aussagekräftigen Vergleich sollten die Messungen unter ähnlichen
Bedingungen erfolgen. Beispielsweise sollte eine täglich stattndende Messung
immer zur gleichen Uhrzeit, am selben Handgelenk oder wie von Ihrem Arzt
angeordnet, durchgeführt werden.
Paarung des Blutdruckmessgeräts mit Ihrem Gerät
1.Bluetooth und App einschalten. Beide müssen während der Paarung
EINGESCHALTET sein.
2.Bei AUSGESCHALTE TEM Messgerät die Taste START drücken und 2 Sekunden halten,
um die Paarung zu starten. Das Symbol und das Symbol werden jeweils im
LCD-Bildschirm angezeigt, was die Durchführung der Paarung anzeigt.
68
Manuel_BPM_book.indd 68 06/06/2014 10:21:25
Page 69

DE
WENN ERFOLGREICH, wird das Symbol im LCD-Bildschirm angezeigt.
WENN NICHT ERFOLGREICH, wird das Symbol im LCD-Bildschirm angezeigt.
Nach der Durchführung des Paarungsvorgangs schaltet sich das Messgerät
automatisch ab.
MESSUNG
Messung starten
1. Nach korrekter Anbringung der Manschette drücken Sie zum Einschalten des
Messgeräts die Taste START und der Messvorgang wird automatisch durchgeführt.
Einstellung auf Null.
Manuel_BPM_book.indd 69 06/06/2014 10:21:25
69
Page 70

Aufpumpen und Messen.
Anzeige und Speicherung des Messergebnisses
2. Dieses Gerät wird nach der Messung automatisch mit der Datenübertragung
fortfahren.
Das Bluetooth-Symbol blinkt.
3.Nachdem die Daten erfolgreich übertragen wurden, sieht die LCD-Anzeige aus, wie
rechts abgebildet.
70
Manuel_BPM_book.indd 70 06/06/2014 10:21:25
Page 71

DE
Schlägt die Datenübertragung fehl, wird im LCD-Display «ERROR» angezeigt.
4.Drücken Sie die Taste STOP, um das Messgerät auszuschalten. Ansonsten wird es
sich automatisch abschalten.
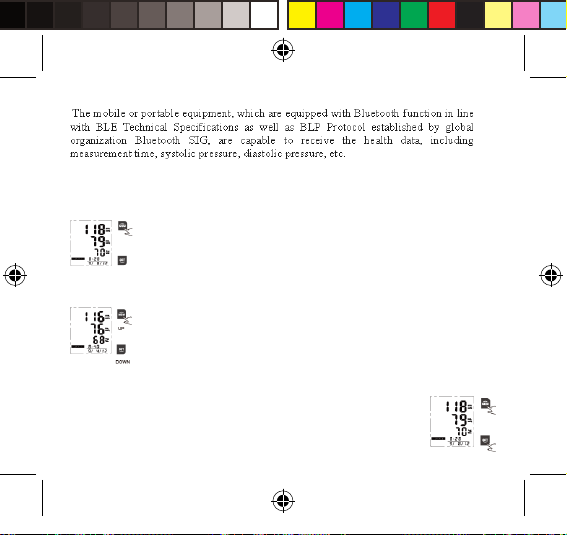
Mobile oder tragbare Geräte, die mit der Bluetooth-Funktion, den technischen
BLE-Spezikationen sowie mit dem von Bluetooth SIG entwickelten BLP-Protokoll
konform sind, sind dazu in der Lage, Gesundheitsdaten zu empfangen, wie z. B.
Messzeitpunkt, systolischer Blutdruck, diastolischer Blutdruck usw.
DATENVERWALTUNG
Abrufen der Aufzeichnungen
1.Die Taste «MEM» drücken, um auf den Speicher zuzugreifen.
Manuel_BPM_book.indd 71 06/06/2014 10:21:25
71
Page 72

2. Drücken Sie die Taste “MEM/UP” oder “SET/DOWN” um in den Aufzeichnungen zu
blättern. Mit «MEM/UP» blättern Sie vorwärts; mit «SET/DOWN» rückwärts.
Die aktuellste Aufzeichnung (1) wird zuerst angezeigt. Jede neue Messung wird der ersten (1)
Aufzeichnung zugewiesen. Alle anderen Aufzeichnungen werden um eine Zier nach hinten versetzt
(d. h. 2 wird zu 3 usw.) und die letzte Aufzeichnung (60) wird aus der Liste entfernt.
Löschen der Aufzeichnungen
Haben Sie keine exakte Messung erhalten, können die Messergebnisse anhand der
folgenden Schritte gelöscht werden.
1.Drücken Sie im Modus Memory Recalling auf und halten Sie die Tasten «MEM» und
«SET» gleichzeitig 3 Sekunden lang gedrückt.
72
Manuel_BPM_book.indd 72 06/06/2014 10:21:25
ACHTUNG
Page 73

DE
2.Im LCD-Display wird jetzt «dEL dONE» angezeigt, was darauf hinweist, dass das
Löschen des Speichers abgeschlossen ist.
3. Soll das Löschen abgebrochen werden, «STAR T/STOP» drücken, um das Messgerät
auszuschalten.
4. Ist im Messgerät nichts gespeichert und Sie drücken die Taste «MEM» für die
Anzeige des Verlaufs, wird die LCD-Anzeige wie rechts abgebildet aussehen.
INFORMATIONEN FÜR BENUTZER
Tipps für das Messen
Unter den folgenden Umständen kann eine Durchführung der Messung zu
ungenauen Ergebnissen führen.
-Innerhalb 1 Stunde nach Abendessen oder Alkohol
-Messung nach Tee, K aee oder Rauchen
-Innerhalb von 20 Minuten nach einem Bad
Manuel_BPM_book.indd 73 06/06/2014 10:21:25
73
Page 74

-Während des Sprechens oder Fingerbewegungen
In einer sehr kalten Umgebung Bei gefüllter Blase
Wartung
Um eine optimale Leistung zu erhalten, bitte unbedingt die folgenden
Anweisungen befolgen.
-Das Gerät an einem trockenen Ort lagern und Sonneneinstrahlung vermeiden
-Eintauchen in Wasser vermeiden. Bei Eintauchen in Wasser mit einem trockenen
Tuch abreiben.
-Schütteln und Stöße vermeiden.
-Staubige und temperaturinstabile Umgebungen vermeiden
-Verschmutzungen mit einem leicht angefeuchteten Tuch entfernen.
-Die Manschette möglichst nicht abwaschen
Reinigung: Eine staubhaltige Umgebung kann sich auf die Leistung des Geräts
auswirken. Vor der Verwendung Verschmutzungen mit einem weichen Tuch
entfernen.
Vor der Verwendung das Gerät auf Sicherheit und eine ordnungsgemäße Funktion
überprüfen.
Bitte folgen Sie den Anweisungen eines korrekten Austauschs für ersetzbare oder
abnehmbare Teile, die vom SERVICEPERSONAL des HERSTELLERS als «Austauschbar»
festgelegt sind.
Entsorgung: Abgenutzte Sensoren oder lose Elektroden können die Leistung des
Geräts reduzieren oder andere Probleme verursachen. Bitte entsorgen Sie ZUBEHÖR,
abnehmbare Teile und das ME-EQUIPMENT entsprechend lokaler Richtlinien.
ÜBER BLUTDRUCK
Was bedeuten systolischer Blutdruck und diastolischer Blutdruck?
74
Manuel_BPM_book.indd 74 06/06/2014 10:21:25
Page 75

DE
Wenn die Herzkammern sich zusammenziehen und Blut aus dem Herzen
herauspumpen, erreicht der Blutdruck in diesem Kreislauf seinen Höhepunkt, der
sogenannte systolische Blutdruck. Sobald die Herzkammern sich entspannen,
erreicht der Blutdruck seinen Minimumwert im Kreislauf, der sogenannte diastolische
Blutdruck.
Systolisch
Blut tritt aus
Was ist die Standardklassizierung für Blutdruck?
Die von der Weltgesundheitsorganisation (WHO) und International Society of
Hypertension (ISH) veröentlichte Blutdruckklassizierung des Jahres 1999 lautet
wie folgt:
Manuel_BPM_book.indd 75 06/06/2014 10:21:25
Arterie
Druck
Diastolisch
Blut gelangt in die
Vene
Entspannung
75
Page 76

Nur ein Arzt kann Ihnen den normal Blutdruckbereich nennen. Bitte wenden Sie sich an einen Arzt,
wenn sich Ihre Messergebnisse außerhalb des Bereichs befinden.
Bitte beachten sie, dass nur ein Arzt feststellen kann, ob Ihr Blutdruck einen gefährlichen Wert erreicht
hat.
BP
(mm
Hg)
Erkennung eines unregelmäßigen Herzschlags
Dieses Blutdruckmessgerät für das Handgelenk ist mit der intelligenten Funktion
Irregular Heartbeat (IHB) Detector ausgestattet. Während einer Messung zeichnet das
Gerät die Herzschlagintervalle auf und berechnet die Standardabweichung. Beträgt
die Abweichung des Herzschlagabstands im Vergleich zum durchschnittlichen
Abstand mehr als 3 und 25 % oder mehr als 5 und über 15 %, leuchtet im Bildschirm
bei der Anzeige des Messergebnisses das IHB-Symbol auf.
Wird das IHB-Symbol angezeigt, bedeutet das, dass während der Messung eine Pulsunregelmäßigkeit
sowie ein unregelmäßiger Herzschlag festgestellt wurden. Das stellt im Allgemeinen KEINEN Grund
zur Sorge dar. Wird das Symbol jedoch öfter angezeigt, empfehlen wir Ihnen, einen Ar zt aufzusuchen.
Bitte beachten Sie, dass dieses Gerät keine Herzuntersuchung ersetzen, aber Pulsunregelmäßigkeiten
76
in einem frühen Stadium erkennen kann.
Manuel_BPM_book.indd 76 06/06/2014 10:21:25
ACHTUNG
Höhe
Optimal
SYS <120
DIA <80
ACHTUNG
Normal
121-
131-
140
141-
130
81-8586-9091-
Bluthochdruck
G1 G2 G3
160
100
161-180 ≥180
101-110 ≥110
Page 77

DE
Warum schwankt mein Blutdruck im Tagesverlauf?
1. Der Blutdruck eines Menschen variiert im Tagesverlauf, er wird außerdem durch die
Art der Befestigung der Manschette und die Messposition beeinusst, die Messung
daher immer unter den selben Bedingungen durchführen.
2. Die Blutdruckabweichungen sind höher, wenn die Person Medikamente einnimmt.
3. Warten Sie mindestens 3 Minuten bis zur nächsten Messung.
Warum unterscheidet sich die Blutdruckmessung im Krankenhaus von der
Messung Zuhause?
Der Blutdruck kann während des Tages auch aufgrund von Wetter, Emotionen, Sport
usw. schwanken, insbesondere die Aufregung vor einem Arztbesuch kann die Werte
in die Höhe treiben.
Erhalte ich bei einer Messung am rechten Handgelenk das gleiche Ergebnis?
Es kann an beiden Handgelenken gemessen werden, es sollte bei einer Messung
jedoch immer das selbe Handgelenk verwendet werden, um so unterschiedliche
Ergebnisse zu vermeiden.
Achten Sie bei einer Blutdruckmessung Zuhause auf Folgendes:
Die Manschette muss fest angebracht sein.
Manuel_BPM_book.indd 77 06/06/2014 10:21:25
77
Page 78

Die Manschette darf nicht zu fest oder zu locker sein.
Die Manschette muss am Handgelenk festgemacht sein.
Löst die Manschette ein Angstgefühl aus?
Atmen Sie vor dem Beginn 2-3 mal tief ein.
Tipp: Vor einer Messung 4-5 Minuten hinsetzen und beruhigen.
FEHLERBEHEBUNG
Dieser Abschnitt enthält eine Liste von Fehlermeldungen und häug gestellter
Fragen bei Problemen, die mit Ihrem Blutdruckmessgerät auftreten können.
Funktioniert das Produkt nicht so, wie es sein sollte, bitte diesen Abschnitt
durchlesen, bevor Sie das Gerät in eine Werkstatt bringen.
PROBLEM SYMPTOM URSACHE LÖSUNG
Kein Strom Display leuchtet
Batterien fast leer Display ist dunkel
78
Manuel_BPM_book.indd 78 06/06/2014 10:21:25
nicht auf.
oder Anzeige von
Batterien sind leer. Durch neue
Batterien wurden
falsch eingelegt.
Batterien sind
fast leer.
Batterien ersetzen
Die Batterien korrekt
einlegen
Durch neue
Batterien ersetzen
Page 79

DE
Fehlermeldung
Manuel_BPM_book.indd 79 06/06/2014 10:21:25
wird
angezeigt
Fehler
1 wird
angezeigt
Fehler
2 wird
angezeigt
Fehler
3 wird
angezeigt
Fehler
5 oder
Fehler
6 wird
angezeigt
Fehler
10 oder
Fehler
11 wird
angezeigt
Fehler
20 wird
angezeigt
Fehler 21
wird im
Display
angezeigt.
Datenkommunikation
fehlgeschlagen
Luftbefüllung
ist niedrig oder
Manschette nicht fest.
Die Manschette liegt
sehr eng an
Der Druck der
Manschette ist zu hoch.
Systemfehler
aufgetreten.
Das Messgerät erkennt
Bewegung, Sprechen
oder der Puls ist
während der Messung
kaum erfassbar.
Das Pulssignal kann
bei der Messung nicht
erkannt werden.
Die Messung ist
fehlgeschlagen.
Überprüfen, ob APP
eingeschaltet ist, und
Datenübertragung neu
probieren.
Manschette neu anlegen und
erneut messen.
Manschette neu anlegen, nicht
zu locker, nicht zu fest und
dann erneut messen.
Manschette neu anlegen und
erneut messen.
Messung wiederholen. Bleibt
das Problem weiter bestehen,
den Einzelhändler oder unsere
Kundendienstabteilung
kontaktieren, um weitere
Unterstützung zu erhalten.
Kontaktinformationen und
Rückgabehinweise nden Sie
in der Gewährleistung.
Für einen Moment entspannen
und erneut messen.
Die Kleidung am Handgelenk
entfernen und erneut messen.
Für einen Moment entspannen
und erneut messen.
79
Page 80

TECHNISCHE DATEN
Stromversorgung 2*AAA-Batterien
Displaymodus Digital LCD V.A.36x41 mm
Messmodus Oszillograscher Testmodus
Messbereich Druck: 0 kpa-40 kpa(0 mmHg-300 mmHg)
Pulswert:(40-199)Schläge/Minute
Genauigkeit Druck:
Normale Betriebsbedingungen Temperatur: 5℃-40℃
Lagerung & Transport Temperatur:-20℃ bis 60℃
Messumfang des Handgelenks ca. 13,5 cm - 21,5 cm
Nettogewicht ca.120 g (ohne Batterien)
Außenmaße ca. 80x65x22 mm
Zubehör 2*AAA-Batterien, Benutzerhandbuch
Betriebsart Dauerbetrieb
Schutzgrad Typ BF
Schutz gegen Wassereintritt IP22
Softwareversion V01
Geräteklassizierung ME-Gerät mit interner Stromversorgung
80
Manuel_BPM_book.indd 80 06/06/2014 10:21:26
5℃-40℃innerhalb±0,4 kpa(3 mmHg)
0℃-45℃(von 5℃-40℃)
innerhalb±0,7 kpa(5 mmHg)
Pulswert:±5%
Relative Luftfeuchtigkeit ≤80%
Atmosphärendruck: 86 kPa bis 106 kPa
Relative Luftfeuchtigkeit: 10 % bis 93 % LF
Page 81

DE
VORSICHT: Eine Modizierung dieses Geräts ist nicht gestattet.
KONTAKTINFORMATIONEN
Kontaktinformationen
Weitere Informationen über unsere Produkte nden Sie unter www.archos.com. Hier
nden Sie Kundenservice, Problemlösungen und Kunden-Downloads - ARCHOS steht
Ihnen zur Seite.
Hergestellt von: GUANGDONG TRANSTEK MEDICAL ELECTRONICS CO., LTD
Firma: GUANGDONG TRANSTEK MEDICAL ELECTRONICS CO., LTD
Adresse: Zone A, 5/F., Investment Building, No. 12, Huizhan East Rd., Torch
Development District, Zhongshan, Guangdong, 528437, China
Autorisierter Repräsentant für Europa:
Firma: MDSS - Medical Device Safety Service GmbH
Adresse: Schigraben 41, 30175 Hannover, Germany
Liste entsprochener europäischer Standards
Risikomanagement EN/ISO 14971:2007
Beschriftung EN 15223:2012
Benutzerhandbuch EN 1041:2008
Allgemeine Sicherheitsanforderungen EN 60601-1:2006/AC2010
Nichtinvasiv
Sphygmomanometer
Allgemeine Anforderungen
Elektromagnetische Kompatibilität EN 60601-1-2:2007/AC:2010
Softwarelaufzeit EN 62304:2006/AC:2008
Benutzbarkeit EN 60601-1-6:2010
Manuel_BPM_book.indd 81 06/06/2014 10:21:26
EN 60601-1-11:2010
EN 1060-1:1995+A2:2009
EN 1060-3:1997+A2:2009
EN 1060-4:2004
81
Page 82

ES
* Muchas gracias por haber escogido el Monitor de Presión Arterial ARCHOS.
* Lea detenidamente el manual de usuario para aprender a utilizar de forma segura
este dispositivo, y guárdelo como referencia en caso de problemas o dudas con el
producto.
Manuel_BPM_book.indd 82 06/06/2014 10:21:26
Versión:1.0
Manual de usuario
Monitor de presión arterial ARCHOS
82
Page 83

ES
SUMARIO
INTRODUCCIÓN
Información de seguridad
Símbolos de la pantalla LCD
Componentes del tensiómetro
ANTES DE EMPEZAR
Colocación y reemplazo de las pilas
Ajuste de fecha y hora
MEDICIÓN
Ajuste del manguito
Inicio de la medición
GESTIÓN DE DATOS
Utilización de la memoria
Borrado de memoria
INFORMACIÓN PARA EL USUARIO
Consejos para la medición
Mantenimiento
SOBRE LA PRESIÓN ARTERIAL
¿Qué son las presiones arteriales sistólica y diastólica?
¿Cuál es la clasicación estándar de la presión arterial?
¿Por qué mi presión arterial uctúa a lo largo del día?
¿Por qué la presión arterial que tengo en consulta no es la misma que la que tengo en casa?
¿El resultado es el mismo si se mide en la misma muñeca?
RESOLUCIÓN DE PROBLEMAS
ESPECIFICACIONES
INFORMACIÓN DE CONTAC TO
CONFORMIDAD CON LA NORMATIVA EUROPEA
COMPATIBILIDAD ELECTROMAGNÉTICA (EMC)
Manuel_BPM_book.indd 83 06/06/2014 10:21:26
Índice
83
Page 84

INTRODUCCIÓN
Descripción general
Gracias por haber escogido el Monitor de Presión Arterial ARCHOS. Este
tensiómetro realiza mediciones de la presión arterial y la frecuencia del pulso,
y guarda los resultados. El producto está pensado para ofrecerle dos años de
funcionamiento able.
Las lecturas realizadas por este tensiómetro son equivalentes a las obtenidas
por un profesional cualicado mediante el método del manguito y la auscultación
estetoscópica.
Este manual contiene información importante sobre su salud y seguridad y le
proporcionará instrucciones paso a paso para utilizar el dispositivo.
Lea el manual atentamente antes de utilizar este producto.
CARACTERÍSTICAS:
* Presión arterial sistólica
* Presión arterial diastólica
* Frecuencia del pulso
* Memoria: hasta 60 registros
Información de seguridad
Los símbolos siguientes pueden aparecer en el manual de usuario, en el etiquetado o
en otros componentes, en aplicación de los requisitos de las normativas de uso.
84
Manuel_BPM_book.indd 84 06/06/2014 10:21:26
Page 85

ES
Símbolo de “LEA EL MANUAL
DE FUNCIONAMIENTO”
Símbolo de “FABRICANTE”
Manuel_BPM_book.indd 85 06/06/2014 10:21:26
Símbolo de “CONFORME
CON LOS REQUISITOS DE
MDD93/42/CEE”
Símbolo de “APARATO
TIPO BF”
Símbolo de “NÚMERO DE
SERIE”
Símbolo de “CORRIENTE
CONTINUA”
Símbolo de “FECHA DE
FABRICACIÓN”
Símbolo de “Representante
Autorizado en la Unión
Europea”
Marca combinada Bluetooth
Símbolo de “PROTECCIÓN
MEDIOAMBIENTAL – No
tirar los aparatos eléctricos
usados a la basura. Utilizar
los puntos de reciclaje
habilitados. Consulte con las
autoridades locales o el lugar
de compra para obtener
información sobre el reciclaje
del producto”
85
Page 86

Lea detenidamente este manual de usuario antes de utilizar el producto.
Este dispositivo está indicado solo para adultos.
Este dispositivo ha sido diseñado para la medición y monitorización no invasivas de la presión arterial,
y no está pensado para ser utilizado en extremidades distintas de la muñeca o para funciones que no
sean la de tomar la tensión arterial.
No confunda la monitorización domiciliaria con el autodiagnóstico. Este dispositivo le permitirá
monitorizar la presión arterial. Básese exclusivamente en los consejos terapéuticos de su médico para
iniciar o interrumpir cualquier tratamiento farmacológico.
Si está tomando medicación, consulte a su médico para determinar el momento más apropiado
para realizar las mediciones. No cambie nunca el medicamento recetado por su facultativo sin la
autorización de este.
Este dispositivo no es apropiado para una monitorización continua durante intervenciones quirúrgicas
o urgencias médicas.
Si la presión del manguito supera los 40 kPa (300 mmHg), el dispositivo se desinará
automáticamente. Si el manguito no se desinara una vez superados los 40 kPa (300 mmHg),
desprenda el manguito de la muñeca y pulse el botón START/STOP para detener el inado.
No utilice el tensiómetro en entornos de campos magnéticos fuertes (ej. equipo médico RF) que
emitan señales de interferencia o transitorios eléctricos rápidos o en ráfagas.
La temperatura máxima que la parte aplicada puede alcanzar es de 42,5 ℃ con una temperatura
ambiente de 40 ℃.
Este dispositivo no es un equipo AP/APG. Su uso no es adecuado en presencia de una mezcla
anestésica inamable con aire (oxígeno u óxido nitroso).
Mantenga el dispositivo fuera del alcance de los niños, ya que la inhalación o la ingestión de las piezas
pequeñas podría ser peligrosa o incluso mortal.
Utilice solo ACCESORIOS y piezas extraíbles especicados/autorizados por el FABRICANTE. De lo
contrario, podría dañar el dispositivo o poner en peligro al usuario/paciente.
El fabricante pondrá a disposición de quien lo solicite los diagramas de circuitos y los componentes
descritos.
Las personas sensibles, como las mujeres embarazadas o las personas que lleven un implante médico
electrónico,
evitarán utilizar este dispositivo en la medida de lo posible.
86
Manuel_BPM_book.indd 86 06/06/2014 10:21:26
PRECAUCIÓN
Page 87

ES
Este dispositivo no es apropiado para una monitorización continua durante intervenciones quirúrgicas
o urgencias médicas.
En tales casos, la muñeca y lo dedos del paciente se hincharían y entumecerían, llegándose a poner
morados debido a la falta de circulación.
Utilice el dispositivo en las condiciones/entornos indicados en el manual de usuario.
De lo contrario, el rendimiento y la vida útil del dispositivo se verían reducidos.
Durante el uso, el paciente estará en contacto con el manguito. Los materiales del manguito han
sido sometidos a pruebas que demuestran su conformidad con los requisitos ISO 10993-5:2009 e
ISO 10993-10:2010. El manguito no presenta ningún potencial de reacción alérgica ni de lesiones de
contacto.
El dispositivo ha sido evaluado en clínica utilizando como referencia el manguito manual y la
auscultación estetoscópica.
El dispositivo ofrece un funcionamiento able durante dos años sin necesidad de calibración.
Cuando el dispositivo se utiliza en pacientes con arritmias frecuentes tales como extrasístoles
auriculares o ventriculares o brilación auricular, pueden aparecer desviaciones en las lecturas. En
dicho caso, consulte a su médico.
Este dispositivo está contraindicado en mujeres embarazadas o que se sospeche que puedan estarlo.
Además de ofrecer lecturas inexactas, se desconocen los efectos de este dispositivo en el feto.
Manuel_BPM_book.indd 87 06/06/2014 10:21:26
87
Page 88

Símbolos de la pantalla LCD
SÍMBOLO
88
Manuel_BPM_book.indd 88 06/06/2014 10:21:26
DESCRIPCIÓN EXPLICACIÓN
Presión arterial sistólica Presión arterial alta
Presión arterial diastólica Presión arterial baja
Pulso latidos/minuto
Batería baja
Tensiómetro
Fecha Hora: Minutos (Mes/Día/Año)
Detector IHB Detector de latidos irregulares
Bluetooth Conexión Bluetooth correcta
Error Error
Memoria
Nivel bajo de batería,
reemplace las pilas
Dispositivo para medir la
presión arterial
Utilización del historial de
lecturas
Page 89

ES
Componentes del tensiómetro
Lista de componentes:
1. PCBA; 2. Tubo de aire; 3. Bomba; 4. Válvula; 5. Manguito.
Lista
1. Monitor de presión arterial de muñeca 2. Dos pilas tamaño AAA 3. Manual de
usuario
Manuel_BPM_book.indd 89 06/06/2014 10:21:26
89
Page 90

ANTES DE EMPEZAR
Colocación y reemplazo de las pilas
•.Abra el compartimento de las pilas.
•.Introduzca las pilas según la polaridad indicada. (Utilice siempre las pilas
autorizadas/especicadas: dos pilas de tipo LR03 AAA).
•.Cierre el compartimento de las pilas.
Duración de las pilas: aprox. 57 días
(Capacidad de las pilas: 600 mAH. Si se realizan 3 mediciones al día, cada medición de
30 s, y se consulta la memoria una vez al día durante 60 s. La corriente de medición
es de 350 mA, y la de visualización de las lecturas de 50 mA, mientras que la corriente
con el dispositivo apagado es de 25 uA).
Cambie las pilas por otras nuevas en los siguientes casos:
aparece en el LCD
La pantalla LCD se oscurece.
Enciende el tensiómetro pero el LCD no se activa.
* Quite las pilas si no tiene pensado utilizar el dispositivo durante un tiempo.
* Las pilas usadas son perjudiciales para el medio ambiente. No tire las pilas a la basura.
* Elimine las pilas usadas del dispositivo respetando la normativa local en materia de reciclaje. * No arroje
las pilas al fuego ya que podrían derramarse o explosionar.
90
Manuel_BPM_book.indd 90 06/06/2014 10:21:26
PRECAUCIÓN
Page 91

ES
Ajuste de fecha y hora
Realice los ajustes horarios antes de utilizar el dispositivo por primera vez para que
cada lectura realizada lleve su hora correspondiente. (Intervalo de años: 2012-2052;
Formato horario: 12 horas)
1. Con el tensiómetro apagado, mantenga pulsado el botón “SET ” durante 3
segundos para entrar en el modo de ajuste horario.
2.Como aparece a la derecha, el número parpadeante “6” representa la [HORA]. Pulse
el botón “MEM” para cambiar de número. Cada pulsación incrementará el número en
una unidad de manera cíclica.
3.Vuelva a pulsar el botón “SE T” para conrmar la [HORA]. Empezará a parpadear
ahora el número que representa los [MINUTOS].
Manuel_BPM_book.indd 91 06/06/2014 10:21:26
91
Page 92

4.Repita los pasos 2 y 3 para conrmar los [MINUTOS].
5.Repita los pasos 2 y 3 para conrmar [MES], [DÍA] y [AÑO].
6.Una vez conrmado el [AÑO], en el LCD aparecerá “DONE” (HECHO) y el tensiómetro
se apagará.
Ajuste del manguito
1. Retire cualquier accesorio de la muñeca (reloj, pulsera, etc.). Si el médico le ha
diagnosticado de mala circulación en la muñeca, utilice la otra muñeca.
92
Manuel_BPM_book.indd 92 06/06/2014 10:21:26
Page 93

ES
2. Remánguese para dejar expuesta la piel.
3. Coloque el manguito en la muñeca con la palma de la mano hacia arriba.
4. El borde del manguito debe quedar a unos 1-1,5 cm por debajo de la muñeca.
5. Coloque el manguito rmemente envuelto alrededor de la muñeca, de modo
que no quede ningún espacio entre el manguito y la piel. Si el manguito estuviera
demasiado suelto, la medición no sería exacta.
Relájese durante 5 minutos antes de la medición.
Espere al menos 3 minutos entre cada medición. Esto permite que la circulación
sanguínea se recupere.
Para una comparación signicativa, realice las mediciones en condiciones similares.
Por ejemplo, haga mediciones diarias a aproximadamente la misma hora, en la
misma muñeca, o como le indique el médico.
Emparejamiento del tensiómetro con un dispositivo móvil
1.Active el Bluetooth y la aplicación. Asegúrese de que ambos estén activados
cuando se realice el emparejamiento.
2.Con el tensiómetro apagado, mantenga pulsado el botón START durante 2
segundos para iniciar el emparejamiento. El símbolo y el símbolo aparecerán
en el LCD de forma alternativa, indicando el proceso de emparejamiento.
Manuel_BPM_book.indd 93 06/06/2014 10:21:26
93
Page 94

Si se realiza correctamente, aparecerá el símbolo en el LCD.
De lo contrario, en el LCD aparecerá el símbolo .
3.El tensiómetro se apagará automáticamente una vez el emparejamiento terminado.
MEDICIÓN
Inicio de la medición
1. Una vez colocado el manguito correctamente, pulse el botón START para encender
el tensiómetro, que procederá a la medición de forma automática.
94
Manuel_BPM_book.indd 94 06/06/2014 10:21:26
Page 95

ES
Ajuste a cero.
Inado y medición.
Visualización y registro del resultado.
2. Tras la medición, el dispositivo transmitirá automáticamente los datos.
El símbolo de Bluetooth parpadea.
Manuel_BPM_book.indd 95 06/06/2014 10:21:27
95
Page 96

3.Si los datos se transmitieron correctamente, el LCD aparecerá como se muestra a
la derecha.
En caso de error en la transmisión, el LCD mostrará la palabra “ERROR”.
4.Pulse el botón STOP para apagar el tensiómetro. De lo contrario, se apagará
automáticamente.
GESTIÓN DE DATOS
Utilización de la memoria
1.Pulse el botón “MEM” para acceder a la memoria.
96
Manuel_BPM_book.indd 96 06/06/2014 10:21:27
Page 97

ES
2. Pulse el botón “MEM/UP” o el botón “SET/DOWN” para visualizar el historial de
lecturas. “MEM/UP” para ir hacia delante; “SET/DOWN” para ir hacia atrás.
La lectura más reciente (1) aparece en primer lugar. Toda nueva medición realizada aparecerá como
primera (1) lectura y las restantes lecturas se desplazarán un dígito (ej. 2 se convierte en 3, y así
sucesivamente). De igual modo, la última lectura (60) desaparecerá de la lista.
Borrado de memoria
Puede decidir borrar todas las lecturas realizadas siguiendo los pasos a continuación
(p. ej. si las mediciones no eran exactas).
1.En el modo de utilización de memoria, mantenga pulsados los botones “MEM” y
“SET” durante 3 segundos.
Manuel_BPM_book.indd 97 06/06/2014 10:21:27
PRECAUCIÓN
97
Page 98

2.En el LCD aparecerá la mención “dEL dONE”, que indica que el borrado de memoria
ha terminado.
3.Si desea interrumpir el borrado, pulse “START/STOP” para apagar el tensiómetro.
4. Si el tensiómetro no guarda ningún dato, al pulsar el botón “MEM” para consultar
el historial,
el LCD mostrará la imagen de la derecha.
INFORMACIÓN PARA EL USUARIO
Consejos para la medición
La medición puede resultar inexacta si se realiza en las siguientes condiciones.
- Si no ha pasado 1 hora después de haber comido o bebido
- Inmediatamente después de fumar o beber té o café
- En los 20 minutos siguientes a un baño
- Si está hablando o moviendo los dedos
- En un entorno muy frío Si tiene ganas de orinar
98
Manuel_BPM_book.indd 98 06/06/2014 10:21:27
Page 99

ES
Mantenimiento
Para conseguir el mejor rendimiento, siga las siguientes instrucciones.
- Guarde el dispositivo en un lugar seco y evite la exposición solar.
- Evite sumergirlo en agua. Límpielo con un paño seco.
- Evite golpes o sacudidas.
- Evite los ambientes con polvo y las temperaturas inestables.
- Utilice un paño ligeramente húmedo para quitar la suciedad.
- No lave el manguito.
Limpieza: El rendimiento de la unidad puede verse afectado en entornos
polvorientos. Utilice el paño suave para quitar la suciedad antes de usarlo.
Compruebe que la unidad está en perfecto estado y funciona de forma segura antes
de usarla.
Siga las instrucciones para la sustitución correcta de las piezas extraíbles o
reemplazables especicadas por el PERSONAL DE ASISTENCIA del FABRICANTE como
Los dispositivos móviles o portátiles equipados con función Bluetooth conforme con
las Especicaciones Técnicas BLE y el Protocolo BLP establecido por la organización
global Bluetooth SIG, pueden recibir datos sanitarios tales como hora de medición,
presión sistólica, presión diastólica, etc.
“Recambiables”.
Eliminación y reciclaje: Los sensores desgastados o unos electrodos sueltos pueden
degradar el rendimiento de la unidad o incluso provocar daños. Proceda a la
eliminación de ACCESORIOS, piezas extraíbles y EQUIPO ME de conformidad con la
normativa local.
Manuel_BPM_book.indd 99 06/06/2014 10:21:27
99
Page 100

SOBRE LA PRESIÓN ARTERIAL
¿Qué son las presiones arteriales sistólica y diastólica?
Cuando el ventrículo se contrae y bombea sangre del corazón, la presión arterial
alcanza su máximo valor en el ciclo, que es la llamada presión sistólica. Cuando el
ventrículo se relaja, la presión arterial alcanza su mínimo valor en el ciclo, que es lo
que que se conoce como presión diastólica.
sale por las arterias
¿Cuál es la clasicación estándar de la presión arterial?
La Organización Mundial de la Salud (OMS) y la Sociedad Internacional de
Hipertensión (SIH) han elaborado en 1999 la siguiente clasicación de los valores de
presión arterial:
100
Manuel_BPM_book.indd 100 06/06/2014 10:21:27
Sistólica
contrae
Diastólica
regresa por las venas
dilata
 Loading...
Loading...