
Technical Note
Performing the EP stray-light test
with potassium chloride on
UV-visible spectrophotometers
Introduction
Measured stray light has two components:
• light coming from the light
source of the instrument that
lies outside of the bandwidth
of the selected wavelength, λ
n
• ambient light that reaches the
detector either directly or by
simple reflections, see figure 1
For instruments with reversed
optics such as the Agilent 8453
UV-visible spectrophotometer
1
ambient light can not reach the
detector, see figure 2. The equation used to calculate transmittance and thereby absorbance is:
T = (I + Is)/(I0+ Is)
Where T is transmittance, I0is
intensity of incident light, I is
intensity of transmitted light and
Isis intensity of stray light. Stray
light has an increasing influence
on spectroscopic measurements at
low levels of intensity, that is, high
absorbances.
The result is that stray light causes a negative bias in instrument
response and eventually becomes
the limiting factor for absorbance
and therefore concentration that
can be measured. The effect of
various levels of stray light on
measured absorbance compared
with actual absorbance is shown
in Figure 3.
This Technical Note examines the
influence of sample composition,
sample temperature, bandwidth
and wavelength accuracy on the
result of the stray-light test using
potassium chloride as described in
the European Pharmacopoeia
(EP).
2
Figure 1
Terminology used when describing stray light
Ambient light
λ
n-1
λ
n
λ
n+1
Sample
Source
Ambient light
Detector
Agilent Technologies
Innovating the HP Way

Measurement of stray light
To measure stray light, a filter is
required that absorbs all light of
the wavelength at which the measurement is to be made and transmits higher and lower wavelengths. Figure 4 shows this ideal
stray light filter. At the measured
wavelength (200 nm) the transmission is 0% whereas at all other
wavelengths it is 100%. Such filters
do not exist in practice, so cut-off
filters are used which transmit all
light above a certain wavelength
and block all light at lower wavelengths.
Salt solutions, for example, potassium chloride (12 g/l), sodium
iodide (10 g/l) and sodium nitrite
(50 g/l) in water, can be used as
standard stray-light filters at 200,
220 and 340 nm respectively (see
figure 5).
The user should keep in mind that
all 3 filters are only approaches to
the ideal test filter. The slope of
the absorption edge shows no infinite value like the ideal filter does.
In addition the used filters block
all light from wavelengths shorter
than the measured one. The contribution of stray light that might
result from these wavelengths is
thus eliminated. This leads to
smaller stray light values than
would be expected from the ideal
filter. The user should be aware of
this systematic deviation as a consequence of using non-ideal stray
light filters.
Source
Ambient light
Detector
Grating
Entrance slit
0.0
0.5
1.0
1.5
2.0
2.5
3.0
3.5
4.0
0.0 0.5 1.0 1.5 2.0 2.5 3.0 3.5 4.0
True absorbance [AU]
Measured absorbance
[AU]
0% Stray light
0.01% Stray light
0.1% Stray light
1% Stray light
Figure 3
The effect of stray light on measured sample absorbance
Figure 2
Simplified schematic of a diode-array spectrophotometer
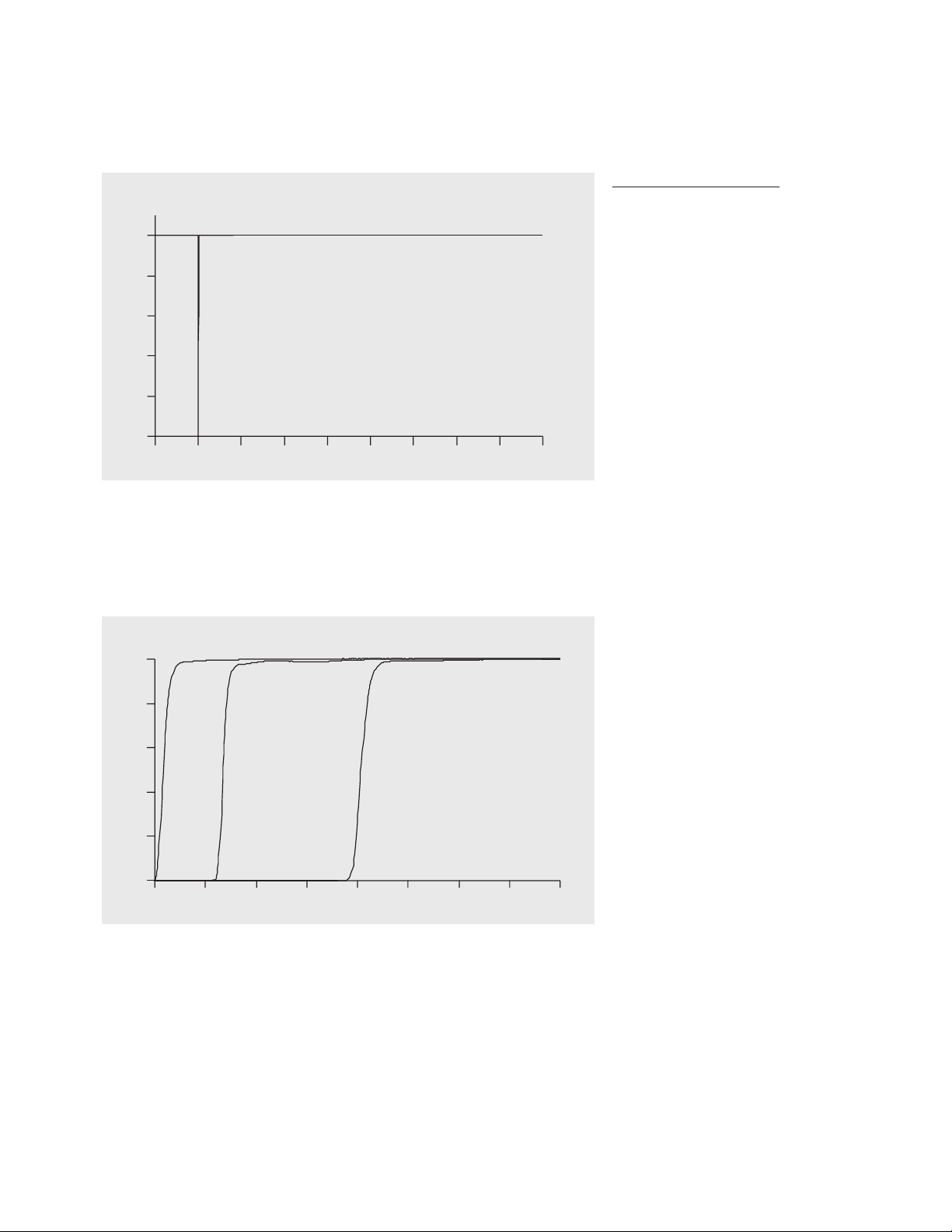
The EP stray-light test
Stray-light measurement with the
Agilent 8453 UV-visible spectrophotometer comprises the
three test described above. The
potassium chloride test is speci-
fied in the EP2:
“…Stray light may be detected at
a given wavelength with suitable
filters or solutions: for example
the absorbance of a 12 g/l solution of potassium chloride in a 1
cm cell should be greater than
two at 200 nm when compared
with water as the compensation
liquid.”
An absorbance of greater than two
means a transmittance of less than
one percent. For a number of reasons the potassium chloride test is
the most critical of the three straylight tests. As shown in figure 5,
this is the only test in which the
measured wavelength is situated
very close to the cut-off slope. As
a consequence the test is extremely sensitive to the wavelength
accuracy of the spectrophotometer. Even small deviations will
result in test failure. For this reason a wavelength recalibration
should be performed before each
stray-light test.
Further, the measurement time is
also relevant. When measuring the
transmittance with the test solution in the light path the intensity
at the detector is very low. The
signal-to-noise ratio can be
improved by increasing the integration time of the spectrophotometer. All three stray-light tests
with the Agilent 8453 spectophotometer were performed using
integration times of 5 s.
0
20
40
60
80
100
100 200 300 400 500 600 700 800 900 1000
Wavelength [nm]
Transmittance
[%]
0
20
40
60
80
100
200 250 300 350 400 450 500 550 600
Wavelength [nm]
Transmittance
[%T]
NaNO
2
NaIKCl
Figure 5
The spectra of potassium chloride (12 g/l), sodium iodide (10 g/l) and
sodium nitrite (50 g/l) in water
Figure 4
The ideal spectrum of a stray-light filter at 200 nm

Another important issue is the
quality of the selected potassium
chloride. The concentration of
bromide plays an important role
on the position of the absorption
edge. The EP allows a maximum
of 0.15 % potassium bromide (0.1
% bromide)
2
. Figure 6 shows spectra of potassium chloride solutions with different bromide percentages measured on the Agilent
8453 spectrophotometer. From left
to right the bromide concentration
is increasing. Spectra 1 to 3 shows
the transmittance of potassium
chloride samples with bromide
contents of <0.005%, 0.05% and
0.10%, respectively.
Increased bromide concentrations
lead to lower percentage transmittance values at 200 nm (see
table1), because the wavelength at
50% transmittance is gradually
shifted from 204.9 to 210.7 nm.
Spectrum 4 shows a non-EP conform sample with bromide content
above the EP limit of 0.1% and
thus resulting in lower transmittance value at 200 nm. Table 1
summarizes the stray light data of
the different samples.
It should be emphasized that problems can arise when using highly
pure potassium chloride samples.
At a very low bromide concentrations the transmittance value at
200 nm may exceed the EP limit
of 1%. It would make no sense to
use ultra-pure potassium chloride
samples for stray light analysis,
although the quality of the chemicals are according to the specifications of the EP.
0
10
20
30
40
50
60
70
80
90
100
190 200 210 220 230 240 250
Wavelength [nm]
Transmittance
[%]
0
1
2
195 200 205
[%]
Wavelength [nm]
195 205
Bromide [%] T <200nm>[%] Wavelength <T=50%> [nm]
<0.005 >0.64139 204.9
0.05 0.25893 208.3
0.10 0.18658 210.7
>0.15 <0.15534 217.6
Table 1
Measured stray light on the Agilent 8453 spectrophotometer
as a function of bromide concentration (blank on water)
Figure 6
Potassium chloride samples measured on the Agilent 8453 spectrophotometer:
Influence of increasing bromide concentration on the shape of the absorption edge
1: < 0.005%
2: 0.05%
3: 0.10%
4: >0.15%
1432

Another factor that has a lasting
effect on the measured transmittance at 200 nm is the spectral
bandwidth (SBW) of the spectrophotometer. Decreasing the
SBW leads to decreasing readings
when the natural bandwidth
(NBW) of the absorbing substance
and SBW have the same order of
magnitude. However, the wavelength at 50% transmittance is virtually independent of the selected
bandwidth. These dependences
are shown in table 2 for the KCl
solution with 0.1% bromide. The
data were aquired with a high performance conventional scanning
spectrophotometer that possesses
a better stray-light specification
than the Agilent 8453 spectrophotometer.
The SBW of the Agilent 8453
spectrophotometer is comparable
to a conventional scanning instrument with a spectral bandwidth of
about 1.5 nm. The bandwidth is
fixed and cannot be changed by
the operator.
The temperature also strongly
affects results of stray-light measurements, see Figure 7. With
increasing temperature the
absorption band is broadend, leading to smaller transmittance values at 200 nm. The EP recommends a temperature of 20°C ±1°C
for the potassium chloride straylight test.
Figure 7
The effect of temperature variation on the transmission of
potassium chloride solution at 200 nm
Slit width <nm> %T <200nm> Wavelength <T=50%> [nm]
0.5 0.0111 210.8
1.0 0.0169 210.7
1.5 0.0286 210.7
Table 2
Stray light data of a conventional scanning spectrophotometer with variable bandwidth.
Sample: potassium chloride (bromide: 0.10%),
Blank: water
0.30
0.40
0.50
0.60
0.70
0.80
15.0 20.0 25.0 30.0
Temperature [˚C]
Transmittance
200 nm [%]

References
1. HP 8453 Spectrophotometer and
Open Sample Area, Agilent
Technologies Technical Note,
1999, publication number
2. European Pharmacopoeia, third
edition, 1997, pages 29 and 1361
3. ASTM E 387-84, Standart Test
Method for Estimating Stray
Radiant Power Ratio of
Spectrophotometers by the
Opaque Filter Method
ASTM stray light tests
3
The sodium nitrite and iodide
tests both have cut-off wavelenghts that are far away (50 and
60 nm) from the measured wavelenths (see figure 5). For this reason the two tests are less sensitive
to wavelength accuracy of the
spectrophotometer. On the other
hand both stray light tests vary
more from the ideal test filter than
the EP test.
Summary
Many factors influence the collected stray-light data. When using liquid filters such as potassium chloride users should be aware that
they are applying non-ideal straylight filters. For the EP stray-light
test, the obtained results depend
not only on several external factors like sample temperature and
wavelength accuracy of the spectrophotometer, but also to a high
degree on the bromide content of
the potasssium chloride used.
The EP allows a maximum of 0.1%
bromide. Independent from the
spectral bandwidth of the spectrophotometer, a bromide concentration of maximum 0.1% corresponds to a wavelength at 50%
transmittance of less than 211 nm.
If the potassium chloride solution
used shows a wavelength at 50%
transmittance that is more than
211 nm, it does not conform to the
requirements of the EP.
The Agilent OQ/PV standards kit
(order number 5063-6503) offers
an easy-to-use, cost-effective and
fully-EP-compliant solution for
stray-light determination of UVvisible spectrophotometers. All
three solutions (KCl, NaI and
NaNO2) are part of this kit and are
provided in sealed ampules for
use with standard 10 mm quartz
cuvettes.


www.agilent.com/chem
The information in this publication is subject to
change withou notice.
Copyright © 2000 Agilent Technologies, Inc.
All Rights Reserved. Reproduction, adaptation
or translation without prior written permission
is prohibited, except as allowed under the
copyright laws.
Printed in Germany 11/2000
Publication Number 5988-0945EN
Agilent Technologies
Innovating the HP Way
 Loading...
Loading...