Page 1

The
Dissolved Oxygen
Handbook
a practical guide to dissolved oxygen measurements
YSI.com/weknowDO
Page 2

CONTENTS
Introduction .......................................................................................................1
Dissolved Oxygen Sensors ................................................................................ 3
Optical Sensors ..............................................................................................5
YSI Optical Dissolved Oxygen Instruments ............................................6
Optical Sensing Element ............................................................................ 7
How an Optical Sensor Measures Dissolved Oxygen ............................. 9
Electrochemical Sensors .............................................................................10
YSI Electrochemical Instruments ........................................................... 10
Electrochemical Membranes ................................................................... 12
How an Electrochemical Sensor Measures Dissolved Oxygen ............ 15
Advancements in Steady-state Electrochemical Sensors ...................... 20
Comparing Steady-state Polarographic and Galvanic Sensors ............ 21
Comparing Optical and Electrochemical Sensing Technologies ................ 25
Measurement Accuracy ..............................................................................25
Approved Methodology .............................................................................. 28
Response Time ............................................................................................. 29
Flow Dependence ........................................................................................32
Warm-Up Time ............................................................................................ 34
Calibration Frequency ................................................................................ 34
Measurement Interferences ........................................................................ 35
Maintenance Requirements ........................................................................ 35
Power Consumption....................................................................................35
Summary .....................................................................................................35
Measuring Dissolved Oxygen with Either Sensor Type ............................... 37
Variables that Affect Dissolved Oxygen Measurements .......................... 38
Temperature .................................................................................................39
Page 3

Salinity .......................................................................................................... 40
Electrode Maintenance ............................................................................67
Correcting for Salinity .............................................................................42
Barometric Pressure .................................................................................... 43
Using Barometric Pressure for DO Calibration ....................................44
Local DO % Measurements ..................................................................... 46
Calibration ........................................................................................................ 46
Calibration Frequency ................................................................................ 46
Calibration Methods ..................................................................................47
Winkler Titration Calibration .................................................................48
Air-saturated Water Calibration ............................................................. 49
Water-saturated Air Calibration ............................................................49
Two Point Calibration .............................................................................. 54
Calibration Musts .......................................................................................55
Calibration Musts for Electrochemical Sensors ....................................55
Calibration Musts for Optical Sensors ..................................................56
Errors During Calibration .......................................................................... 57
Probe Care and Maintenance for Optical Sensors ...................................69
Storage ..........................................................................................................70
Final Words .......................................................................................................70
Appendix A - Oxygen Solubility Table ..........................................................72
Appendix B – Calibration Values for Various Atmospheric Pressures and
Altitudes ............................................................................................................74
References ......................................................................................................... 76
GLP (Good Laboratory Practices) File ...................................................... 58
Taking Measurements ...................................................................................... 59
BOD Measurements .................................................................................... 60
Measurement Precautions and Interferences ...........................................61
Biofouling .................................................................................................. 61
Coating Materials .....................................................................................61
Probe Attacking Liquids .......................................................................... 62
Interfering Gases .....................................................................................63
Membrane and Sensing Element Integrity ............................................ 63
Probe Care and Maintenance .......................................................................... 64
Probe Care and Maintenance for Electrochemical Sensors ....................64
Changing a Membrane ............................................................................. 64
Page 4

THIS PAGE LEFT INTENTIONALLY BLANK
INTRODUCTION
YSI has a long history in developing and manufacturing sensors that
measure dissolved oxygen in aqueous solutions and has had many firsts
over the years including the invention and commercialization of the first
portable dissolved oxygen instrument in 1963. This instrument utilized a
membrane-covered Clark Polarographic sensor, commonly referred to as a
Clark electrode, which was developed in 1956 by Dr. Leland Clark (figure
1), a researcher at Antioch College who was working in collaboration with
YSI scientists. Before the introduction of the Clark electrode, methods for
measuring dissolved oxygen were laborious, time-consuming and highly
susceptible to interference. Today the world continues to benefit from Dr.
Clark’s invention as the Clark electrode is still used by many manufacturers
and in several YSI instruments. In addition to the variety of Clark electrodes
offered, YSI also manufactures optical based dissolved oxygen sensors
for laboratory, spot sampling and long term monitoring applications. See
figure 2 for a brief overview of other YSI milestones in dissolved oxygen
measurement technologies over its 60 year history.
This booklet describes in detail the different types of dissolved oxygen sensing
technologies available. It also covers, in general terms, recommended
calibration methods, regular maintenance procedures that can be performed
by the user and how to take a measurement in order to obtain accurate
data. For instrument specific instructions and recommendations, please
refer to the instrument’s instruction manual.
YSI offers seminars on the topic of dissolved oxygen measurement
technologies which may apply to continuing education units depending on
the certifying agency. If you would like to schedule a seminar for your group
or organization, please contact YSI at environmental@ysi.com, 1-800-8974151 or +1 937-767-7241.
1
Page 5

2
3
Figure 1. Dr. Leland Clark, inventor of the Clark polarographic electrode.
Notable Events in YSI’s History of Developing Sensing
Technologies for Measuring Dissolved Oxygen
1956 – Dr. Leland Clark invents the membrane covered Polarographic
electrode while working with YSI Scientists.
2006 – YSI releases the ROX® optical dissolved oxygen sensor. The
sensor has a dedicated wiper for long term monitoring on multi-parameter
sondes.
2007 – YSI releases a galvanic electrochemical sensor for use on the Pro
Series handheld product family.
2008 – YSI releases the ProODO® optical dissolved oxygen instrument for
spot sampling and laboratory applications.
Figure 2. YSI’s Dissolved Oxygen Time line.
DISSOLVED OXYGEN SENSORS
There are two primary types of dissolved oxygen sensing technologies
available: the optical based sensing method which is commonly referred
to as luminescent and the Clark electrochemical or membrane-covered
electrode. Within these two types of technologies, there are slight variations
available. For example, there are two types of optical sensors. Both types
of optical sensors measure luminescence as it is affected by the presence
of oxygen; however, one sensor measures the lifetime of the luminescence
while the other sensor measures the intensity of the luminescence.
1965 – YSI develops the first biological oxygen monitor. Considered a
breakthrough for modern medicine and surgery, this instrument enabled
physicians to perform open-heart surgery for the first time because immediate
blood oxygen measurements could be taken real-time in the operating room
rather than having a sample drawn and taken to a lab for analysis.
1993 – YSI patents first long-term, in-situ, stirring independent oxygen sensor
(Rapid PulseTM DO) and packages it with multiparameter instruments.
1993 – YSI patents first stirring independent micro-electrode oxygen sensor
(Micro-Electrode Array or MEA) for spot sampling applications.
2002 – YSI releases polyethylene membranes for use on polarographic
dissolved oxygen sensors. This advancement in membrane material
lowered the stirring dependence and quickened the sensor’s response time
over traditional Teflon® membranes.
The two types of Clark electrochemical sensors available are Polarographic
and Galvanic. Additionally, YSI manufacturers two types of Polarographic
sensors: Steady-state and the patented Rapid Pulse sensor. Refer to figure 3
for a diagram of the various sensor types.
Page 6
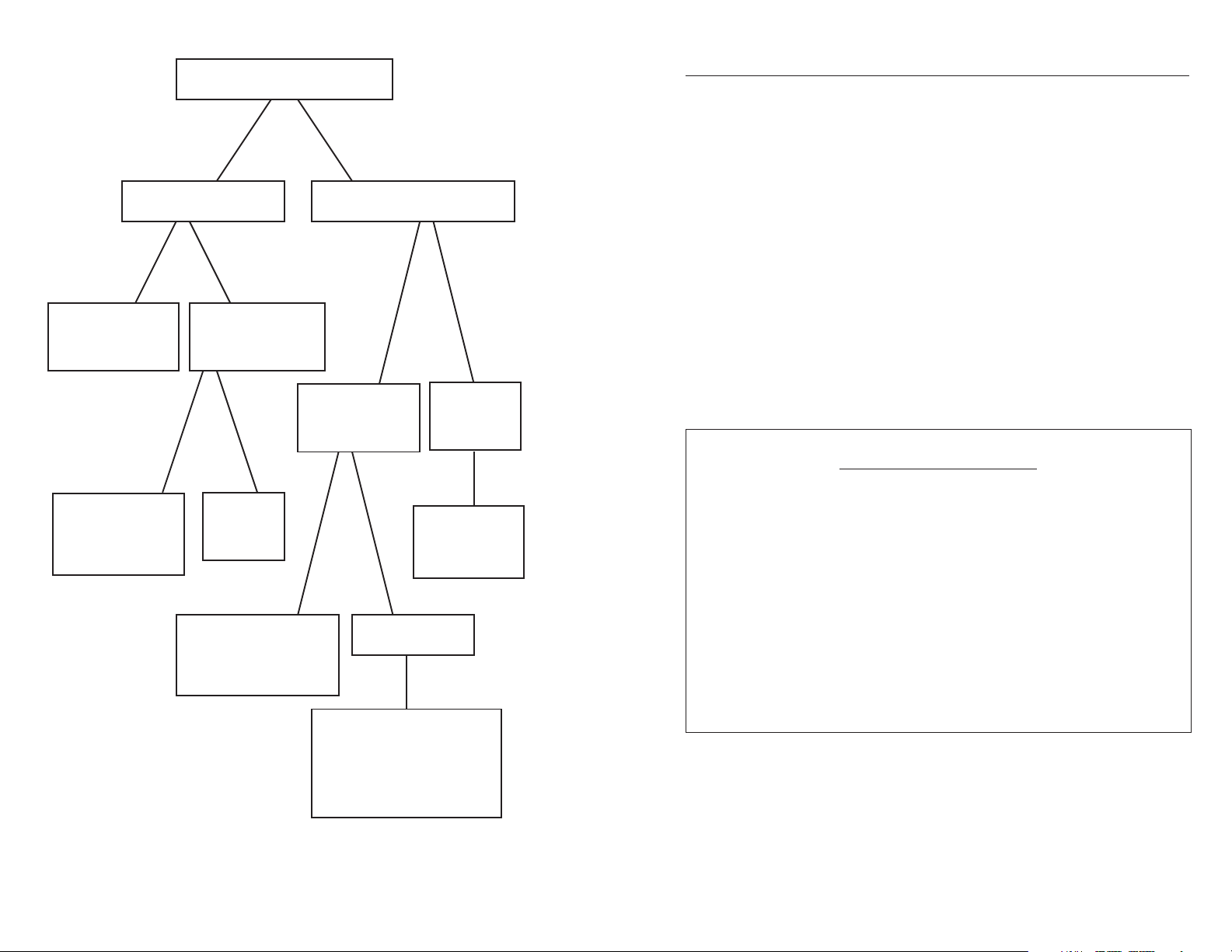
4
5
Dissolved Oxygen Sensors
Optical Sensors
Electrochemical Sensors
Intensity-based
Optical Sensors
Lifetime-based
Optical Sensors
Polarographic
Sensors
Galvanic
Sensors
ROX - available
on most 6-series
sondes
ProODO
Rapid Pulse -
available on some
6-series sondes
Steady-state
Available on
Pro20 and
ProPlus
Available on several
instruments including:
ProPlus, Pro20, 550A,
DO200 and 5100
OPTICAL SENSORS
Lifetime and intensity optical measurement methods detect dissolved oxygen
based on the well documented principle that dissolved oxygen quenches
both the lifetime and intensity of the luminescence associated with carefullychosen chemical dyes. When there is no oxygen present, the lifetime and
intensity of the signal are at their maximum. As oxygen is introduced
to the sensing element, both the lifetime and intensity of the luminescence
become shorter. Therefore, the lifetime and intensity of the luminescence
are inversely proportional to the amount of oxygen present. The relationship
between the oxygen pressure outside the sensor and the lifetime or intensity
of the luminescence in the dye layer of the sensing element can be generally
quantified by the Stern-Volmer equation (figure 4). However, the SternVolmer equation implies an inversely linear relationship which is not strictly
true especially at higher oxygen concentrations; therefore, YSI employs the
use of a 3rd order polynomial to correct for this non-linearity and to obtain
the desired range of dissolved oxygen readings.
Figure 3. Diagram of Dissolved Oxygen Sensors.
The Stern-Volmer Relationship
Io/I = 1 + kqt0 * O2
Where:
Io =Intensity or lifetime of luminescence without the quenching molecule
(O2).
I = Intensity or lifetime of luminescence with the quenching molecule (O2).
kq = Is the quencher rate coefficient.
t0 = Is the luminescence lifetime of the chemical (the dye) to be quenched.
O2 = The concentration of oxygen.
Figure 4. Stern-Volmer equation.
Given that the sensing elements of the two optical sensor types are identical,
the primary advantage of the lifetime method over the intensity method is
that a lifetime sensor will be more stable in the long term. This is because the
Page 7
6
7
degradation of the dye in the sensing element has less effect on the lifetime
based measurement than the intensity based measurement. Therefore, the
intensity method will require more frequent calibrations - particularly at zero
oxygen.
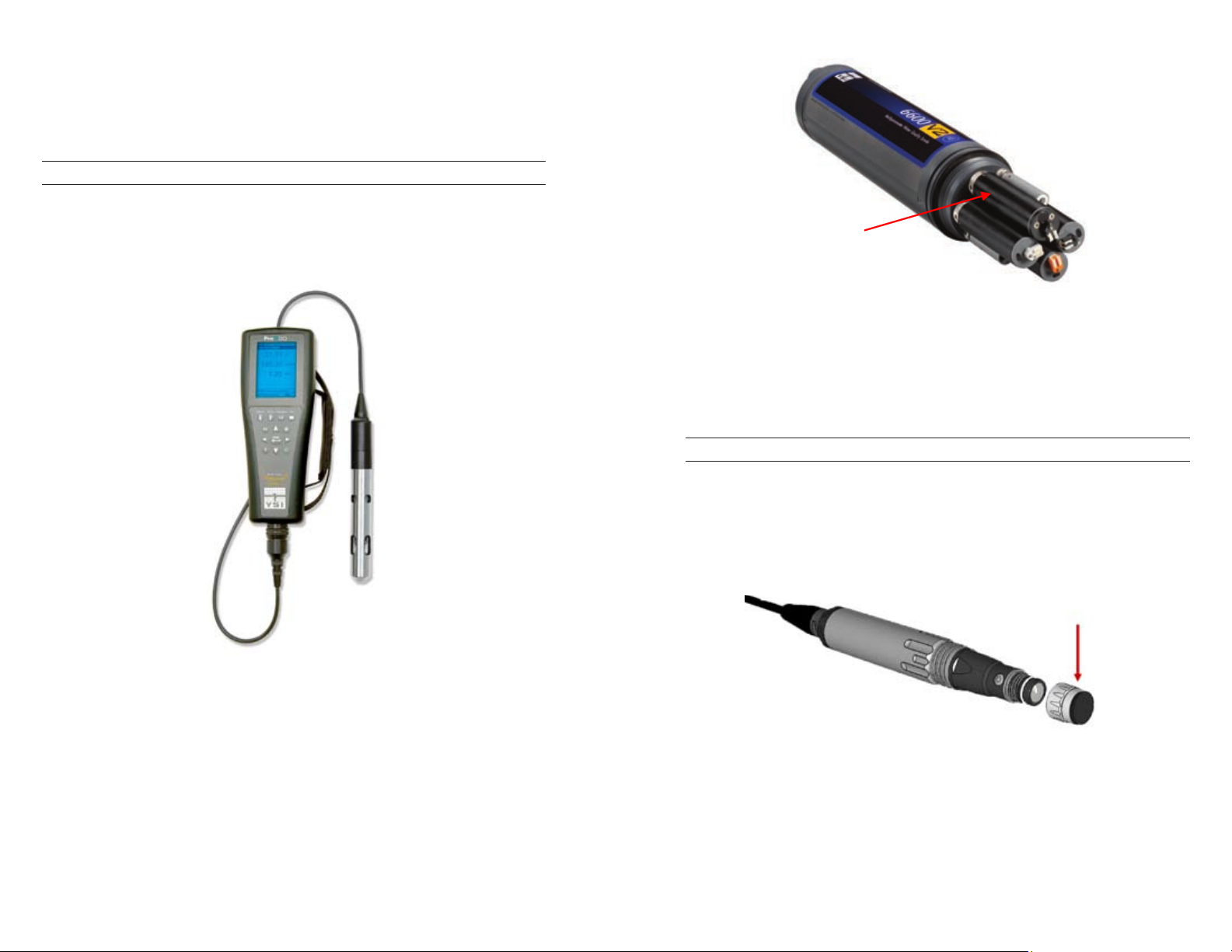
YSI OPTICAL DISSOLVED OXYGEN INSTRUMENTS
YSI offers two lifetime optical sensors: the ProODO (figure 5) sampling
instrument and the 6150 ROX® (figure 6) sensor which can be used on
most 6-series sondes that have an optical port. In addition, there will be an
optical BOD-style sensor available for use on the ProODO in early 2010.
ROX
Sensor
Figure 5. The ProODO is a compact, handheld instrument designed to withstand
the harshest field conditions yet is accurate enough for use in a laboratory.
Figure 6. The ROX sensor’s dedicated wiper and anti-fouling accessories help extend
deployment times while protecting data integrity making it ideal for unattended,
remote and real time monitoring applications.
OPTICAL SENSING ELEMENT
YSI’s two optical dissolved oxygen sensors utilize sensing elements that are
similar in function but slightly different in design. The ProODO’s sensing
element is referred to as a Sensor Cap due to its screw on cap design (figure
7). The ROX’s sensing element is referred to as a ROX Membrane (figure 8)
and is held in place by 3 screws.
Figure 7. ProODO Sensor Cap.
Sensor Cap
Page 8

8
9
Figure 8. ROX membrane.
Oxygen is constantly diffusing
through the paint layer, affecting the
luminescence of the sensing layer.
Each sensing element has two layers. The outer layer is a paint that acts
as an oxygen permeable diffusion layer which allows oxygen molecules
to pass through while protecting the dye layer. The sensing layer is an
immobilized polystyrene dye layer that luminesces when excited with light
of a proper wavelength (figure 9). The degradation of this dye layer over
time is what causes the sensor cap to need replacement and all lifetime
based optical sensors require that this dye layer be replaced periodically.
YSI sensing elements are warranted for 1 year but may last much longer.
The working life of a sensing element may be extended by keeping it clean
and properly stored between uses. See the Probe Care and Maintenance
section of this booklet for more information on cleaning and storage.
The sensing elements are factory calibrated at YSI and a calibration
code specific to each individual sensing element is determined during the
manufacturing process. The calibration code consists of coefficients that
are preloaded into the sensor at the factory for increased measurement
accuracy. Replacement sensing elements are supplied with their unique
calibration codes which can easily be entered into the instrument and probe
without the need to return it to the factory. The unique codes and instructions
for entering them into the instrument can be found on the instruction sheet
provided with the replacement sensing element.
The lifetime of the
luminescence is measured
by the sensor and compared
against a reference.
The amount of oxygen passing through
to the sensing layer is inversely
proportional to the lifetime of the
luminescence in the sensing layer.
Figure 9. Illustration of how a YSI optical sensor measures oxygen.
HOW AN OPTICAL SENSOR MEASURES DISSOLVED OXYGEN
The probe measures dissolved oxygen by emitting a blue light of the proper
wavelength that causes the dye in the sensing element to luminesce or glow
red. Oxygen dissolved in the sample continually passes through the diffusion
layer to the dye layer, affecting the luminescence of the dye both in intensity
and lifetime. The YSI sensor measures the lifetime of the dye’s luminescence
as it is affected by the presence of oxygen with a photodiode (light detector)
in the probe and compares that reading to a reference (figure 9).
To increase the accuracy and stability of the measurement, the sensor also
emits a red light that is reflected by the dye layer back to the photodiode in
the sensor. The sensor measures the reflected light and uses that reading
as the reference value for comparison to the previously measured lifetime
luminescent value. The lifetime of the luminescence from excitation by the
blue light is compared to that of the reference value (red light) and a stable
dissolved oxygen concentration is calculated by the probe.
Although the accuracy of an optical sensor’s measurement is not dependent
on flow, it is dependent on temperature. This temperature dependence
Page 9

10
11
is removed by proprietary algorithms in the system software. As for any
oxygen probe, the mg/L concentration is calculated from the sensor’s %
saturation reading (temperature compensated), temperature, and salinity
after the calibration of the system using barometric pressure. The effects of
these factors on dissolved oxygen readings are described in the Measuring
Dissolved Oxygen with Either Sensor Type section of this booklet.
ELECTROCHEMICAL SENSORS
YSI offers three types of field rugged electrochemical sensors: steady-state
galvanic, steady-state polarographic, and Rapid Pulse polarographic (figure
3). In addition to several different field probes, YSI offers a BOD-style
laboratory polarographic probe with a built in stir bar.
Dr. Leland Clark first invented the Polarographic electrode in 1956 and
variations of this electrode are still used by many manufacturers today. Figure
10 shows a picture of a YSI 5739 polarographic sensor which is currently
offered by YSI and is similar in design to the original Clark electrode.
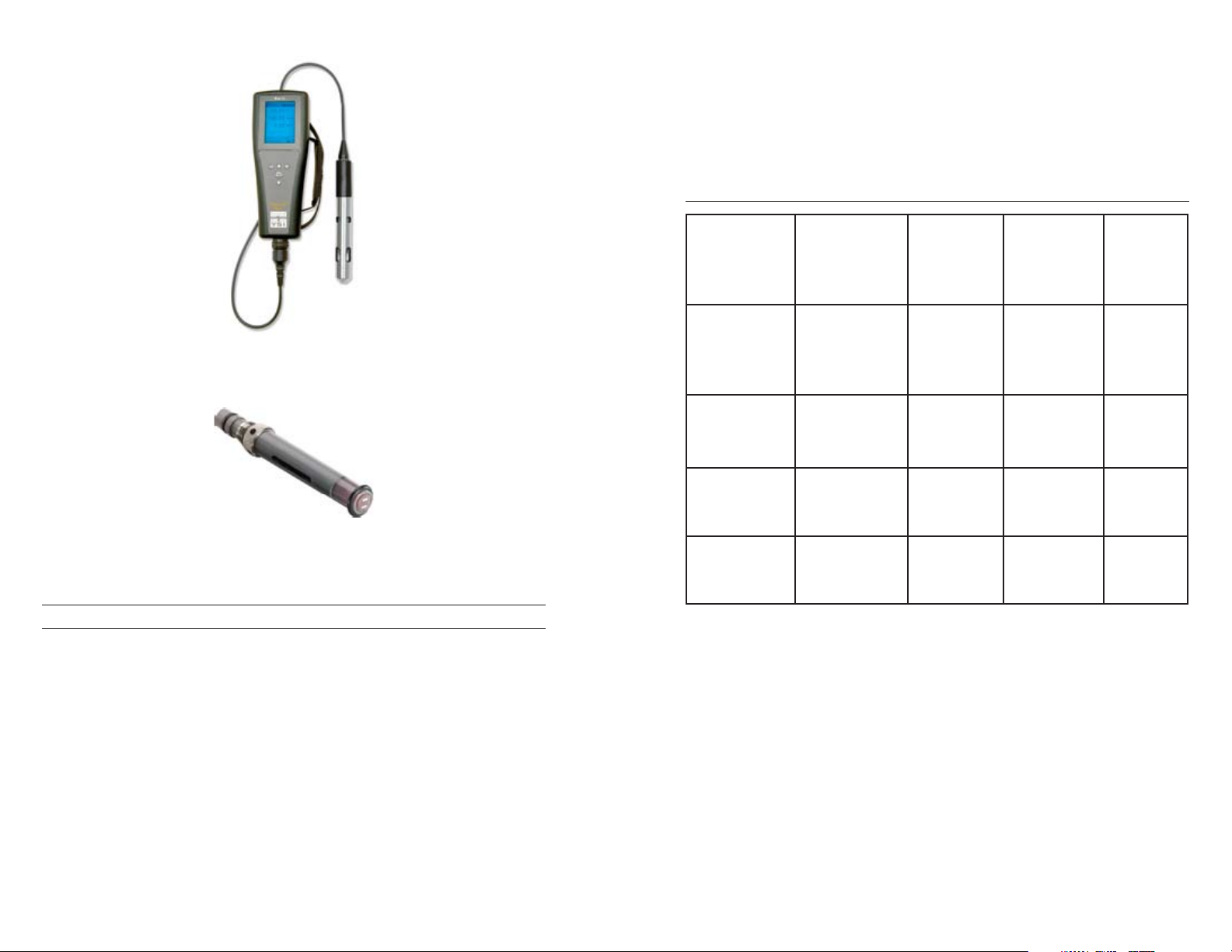
The Professional Plus and Pro20 as well as other YSI instruments can be
equipped with either a field or lab BOD-style sensor (figure 11). This
flexibility allows for the convenience and cost-savings of having one
instrument for both applications. Additionally, these two instruments utilize
a screw-on cap membrane which makes membrane changes simple and
easy to perform. The third electrochemical sensor type, the Rapid Pulse
sensor, can be used on several of the 6-series multiparameter sondes (figure
13).
Figure 10. Model 5739 polarographic sensor.
YSI ELECTROCHEMICAL INSTRUMENTS
YSI offers numerous instruments that utilize an electrochemical sensor. Most
notably are the Professional Plus multiparameter and the Pro20 dissolved
oxygen and temperature instruments (figure 11 and 12). These two models
are the most versatile YSI handheld dissolved oxygen instruments since they
can use either a polarographic or galvanic sensor. Deciding between a
polarographic and galvanic sensor depends on the application and user
preference. See the Comparing Steady-state Polarographic and Galvanic
Sensors section to understand the advantages and disadvantages of using
one type of sensor over the other.
Figure 11. The Professional Plus multiparameter instrument with a BOD-style
sensor. The Professional Plus can be outfitted with several different single and
multi-parameter cables including the Quatro which can measure dissolved oxygen,
temperature, conductivity, and two ISEs at the same time and on the same cable.
Page 10
12
13
thicknesses and materials offered by YSI and their corresponding response
times, flow dependences, and required flow rates. For tips on how to
overcome flow dependence, see the Taking Measurements section. The
topics of flow dependence and response time are discussed further in the
Comparing Optical and Electrochemical Sensing Technologies section.
Membrane Comparison Table
Figure 12. The Pro 20 as well as the Pro Plus can be outfitted with a polarographic
or galvanic sensor.
Figure 13. Rapid Pulse sensor for use on some 6-series sondes.
ELECTROCHEMICAL MEMBRANES
An electrochemical membrane is a thin semi-permeable material, stretched
over the sensor that isolates the electrodes from the environment while
allowing gases to enter. YSI offers several different types of dissolved
oxygen membranes of varying design, thickness and material.
The two types of membrane designs include the traditional, sheet-like stretch
membrane that is held in place by an o-ring and the plastic screw-on cap
membrane that has the membrane material pre-stretched at the factory.
The membrane’s material and thickness affect the sensor’s flow dependence
and response time. The table in figure 14 shows the various membrane
Sensor Membrane
Material
Color of Cap
Optical Diffusion layer
and sensing
element
Response
Time 100 to
0% (T-95)
40
seconds*
Flow
Dependence
after 4
minutes
0 (zero)
Non-
Required
Flow Rate
0 (zero)
consumptive
Steady-state
Galvanic or
Polarographic
Steady-state
Galvanic or
Polarographic
Steady-state
Galvanic or
Polarographic
1.0 mil Teflon
Black Cap
1.25 mil PE
Yellow Cap
2.0 mil PE
Blue Cap
18 seconds 45% 12 inches
per
second
8 seconds 25% 6 inches
per
second
17 seconds 18% 3 inches
per
second
Figure 14. Newer membrane caps are made of polyethylene notated as PE in the
table. The response time in this table is listed as T-95 which is the amount of time
it takes for the sensor to get to 95% of the desired reading when moved from a 100%
saturated sample to a 0 oxygen environment.
*YSI studies have shown that stirring an optical sensor can lower its response
time. For example, using a magnetic stirrer or stir bar could result in an
optical response time of 22 seconds or less for T-95.
The type of membrane that can be used with a particular instrument is
dictated by the sensor type in use and by the instrument’s microprocessor.
Using an incorrect membrane could result in erroneous readings so care
Page 11
14
15
should be taken when ordering replacement membranes. Refer to figure
15 for a guide to ordering the proper membrane for a specific instrument
and sensor. If you have an instrument that can use more then one type of
membrane, refer to figure 14 to determine which option is best for your
application based on the response times and required flow rates of the
membranes available for use on your instrument. For instruments that can
use more than one membrane, it should be noted that the instrument must be
properly configured for the membrane installed in order to obtain accurate
readings.
Membrane Selection Guide
Membrane Model #
(Item #)
5680
(060745)
5775
(098094)
5776
(098095)
5906
(059880)
Type, Thickness, Material Instrument and
probes it is used with
Stretch Membrane,
2.0 mil Teflon
Stretch Membrane,
1.0 mil Teflon
Stretch Membrane,
0.5 mil Teflon
Black Cap Membrane,
1.0 mil Teflon
Probes: 5719, 5739,
and 5750 when used
with a model 58
instrument only.
Instrument: 55
Probes: 5719, 5739,
5750, and Rapid
Pulse
Probes: 5719, 5739,
and 5750 when used
with a model 58
instrument only.
Instrument: 85,
550, 556 MPS,
and Pro Plus with a
Polarographic sensor.
Probes: 5239, 5905
and 5010
Membrane Model #
(Item #)
5909
(059882)
5912
(605912)
5913
(605913)
5914
(605914)
Figure 15. Newer membrane caps are made of polyethylene notated as PE in the
table.
Type, Thickness, Material Instrument and
probes it is used with
Blue Cap Membrane,
2.0 mil Polyethylene (PE)
Black Cap Membrane,
1.0 mil Teflon
Yellow Cap Membrane,
1.25 mil Polyethylene (PE)
Blue Cap Membrane,
2.0 mil Polyethylene (PE)
Instruments: 556
Probes: Pro Series
Polarographic sensors
Pro Series Galvanic
sensor when used
with a Pro Plus
instrument only
Pro Series Galvanic
sensors
Pro Series Galvanic
sensors
HOW AN ELECTROCHEMICAL SENSOR MEASURES DISSOLVED OXYGEN
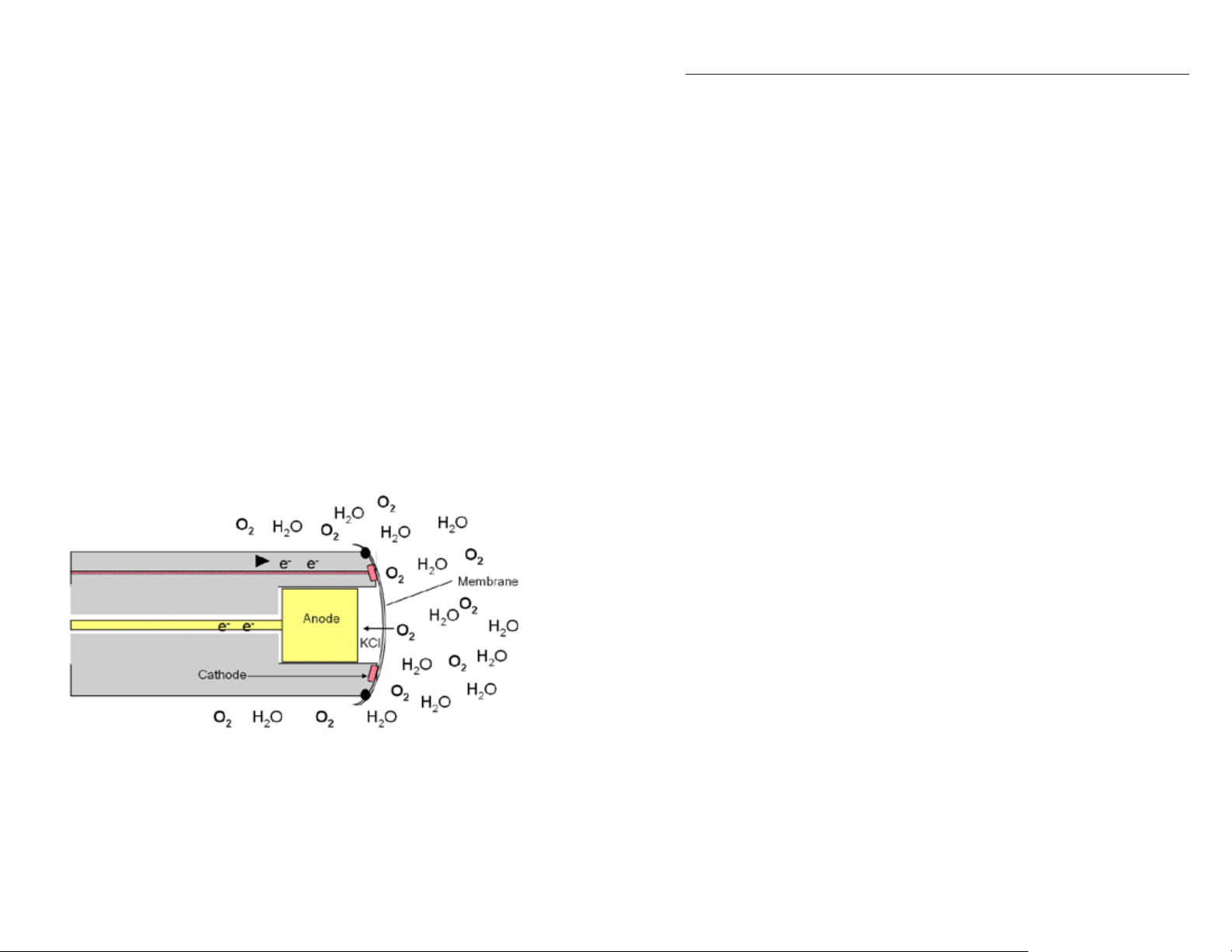
Electrochemical sensors, both polarographic and galvanic, consist of an
anode and a cathode that are confined in electrolyte solution by an oxygen
permeable membrane. Oxygen molecules that are dissolved in the sample
diffuse through the membrane to the sensor at a rate proportional to the
pressure difference across it. The oxygen molecules are then reduced at
the cathode producing an electrical signal that travels from the cathode to
the anode and then to the instrument. Since oxygen is rapidly reduced or
consumed at the cathode, it can be assumed that the oxygen pressure under
the membrane is zero. Therefore, the amount of oxygen diffusing through
the membrane is proportional to the partial pressure of oxygen outside the
membrane.
5908
(059881)
Yellow Cap Membrane,
1.25 mil Polyethylene (PE)
Instruments: DO200,
550A, and 556
Probes: Pro Series
Polarographic sensors
For example, in air or air-saturated water at sea level, the oxygen partial
pressure is approximately 160 mmHg (21% of 760 mmHg), while the
pressure under the membrane is zero. This difference in oxygen pressures
produces the current that is read by the instrument. As the oxygen pressure
varies, so does the oxygen diffusion through the membrane which causes
Page 12
16
17
the probe current to change proportionally. Figure 16 is a dissection of
a Clark electrode and illustrates how an electrochemical sensor, either
polarographic or galvanic, works.
It is important to recognize that oxygen dissolved in the sample is consumed
during the measurement with a steady-state electrochemical sensor. This
results in a measurement that is dependent on flow. It is therefore essential
that the sample be continuously stirred at the sensor tip. If stagnation occurs,
the readings will be artificially low. The flow dependence and therefore the
rate of stirring required for an accurate measurement varies by membrane,
see figure 14. The topics of flow dependence and response time are
discussed further in the Comparing Optical and Electrochemical Sensing
Technologies section. For tips on how to overcome flow dependence, see
the Taking Measurements section.
Electrochemical dissolved oxygen measurements are also affected by
barometric pressure and the temperature and salinity of the sample. These
three factors and how they affect dissolved oxygen readings are described
in detail in Measuring Dissolved Oxygen with Either Sensor Type section.
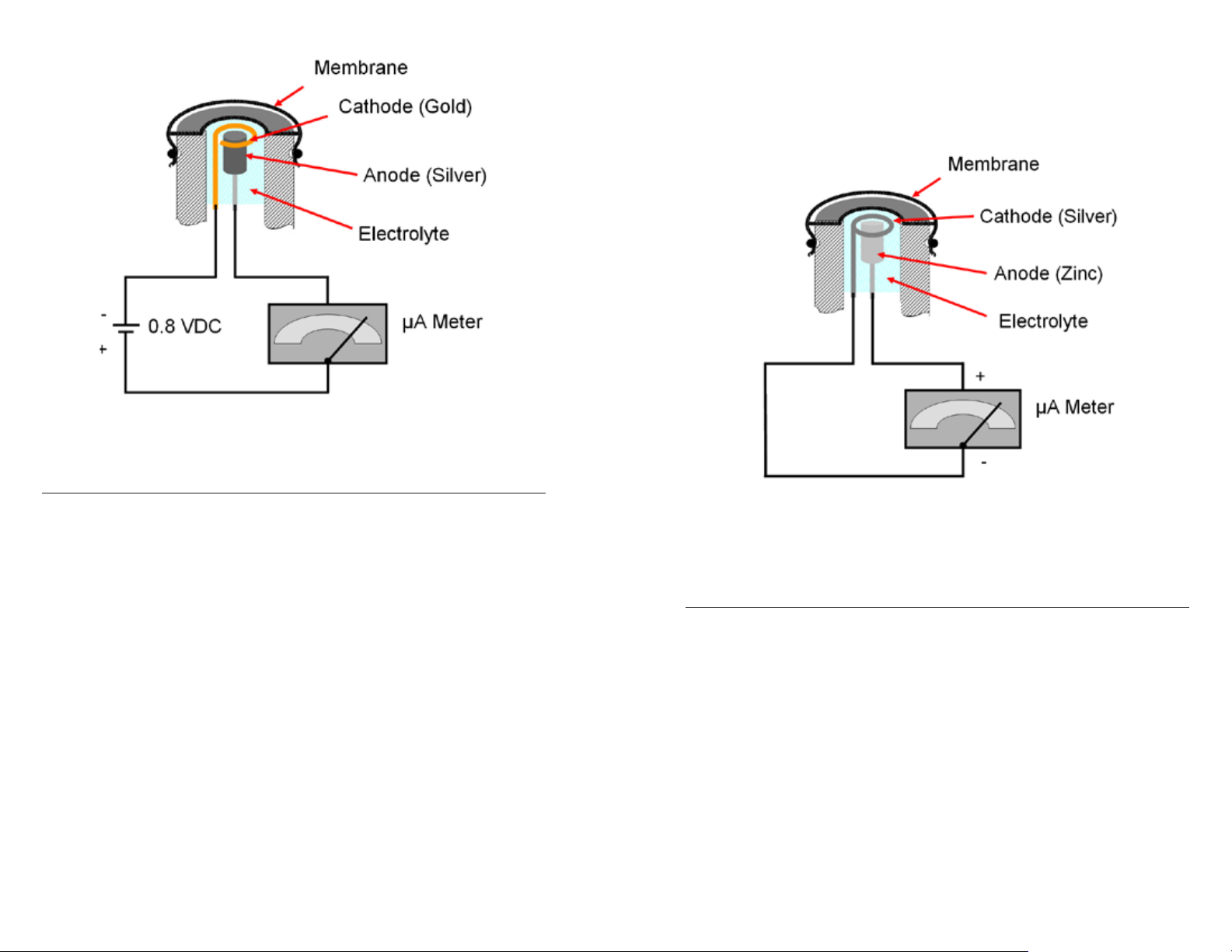
Steady-State Polarographic Sensors
In a polarographic sensor, the cathode is gold and the anode is silver. The
system is completed by a circuit in the instrument that applies a constant
voltage of 0.8 volts to the probe, which polarizes the two electrodes, and a
meter to read the dissolved oxygen response from the sensor.
The electrolyte held under the membrane allows the electrical signal to travel
from the cathode to the anode. The signal continues down to the meter
as shown by the basic circuit diagram in figure 17. The polarographic
sensor operates by detecting a change in this current caused by the variable
pressure of oxygen while the potential is held constant at 0.8 V. The more
oxygen passing through the membrane and being reduced at the cathode,
the greater the electrical signal (current) read by the probe. As oxygen
increases, the signal increases and, conversely, as oxygen decreases, the
signal decreases. Chemically, this is described as the oxidation of the silver
and reduction of oxygen at the gold cathode as follows:
Silver Anode Reaction: 4Ag + 4Cl- 4AgCl + 4e
Gold Cathode Reaction: O2 + 2H2O +4e- 4OH
-
-
Overall reaction: O2 + 2H2O + 4Ag + 4KCl 4AgCl + 4KOH
Figure 16. An illustration of an electrochemical sensor.
Page 13
18
19
Zinc Anode reaction: 2Zn 2Zn
2
+ 4e
-
+
Sliver Cathode reaction: O2 + 2H2O + 4e- 4OH
-
Figure 17. A simplified diagram of a polarographic sensor and circuit.
Steady-State Galvanic Sensors
In the YSI galvanic sensor, the cathode is silver and the anode is zinc. The
anode may be a different material, such as lead, in different manufacturers’
sensors. Figure 18 is an illustration of a galvanic sensor.
Overall reaction: O2 + 2H2O + 2Zn 2Zn(OH)
2
Figure 18. A simplified diagram of a galvanic sensor and circuit.
A circuit completes the measurement, but, unlike the polarographic sensor,
the galvanic sensor does not have or need a constant voltage applied to it.
In the Galvanic sensor, the electrodes are dissimilar enough to self-polarize
and reduce oxygen molecules without an applied voltage. It is similar in
function to a battery.
A galvanic dissolved oxygen system uses a meter to read the electrical signal
coming back from the probe and this signal is proportional to the amount
of oxygen passing through the membrane. Oxygen passing through the
membrane and being reduced at the cathode increases the electrical signal
(current) read by the probe. As oxygen increases, the signal increases and
as oxygen decreases, the signal decreases. Chemically, this is described
as the oxidation of the zinc and reduction of oxygen at the silver cathode
as follows:
Rapid Pulse Polarographic Sensors
The third type of electrochemical sensor offered by YSI is the patented Rapid
Pulse polarographic sensor. This sensor is like the steady-state polarographic
sensor in that it has a gold cathode, silver anode and utilizes electrolyte
solution that is held in place by an oxygen permeable membrane. Also,
similar to the traditional polarographic sensor, a voltage of 1.0 V is applied
to the electrodes and oxygen passing through the membrane is reduced at
the cathode. This reduction causes a change in the electrical signal read by
the instrument. The amount of oxygen passing through the membrane and
being reduced at the cathode is directly proportional to the electrical signal
sent back to the instrument.
Page 14

20
21
The Rapid Pulse sensor differs from a steady-state sensor in that it pulses
on and off during the measurement allowing the oxygen to replenish at
the membrane surface. The voltage is only applied for 40 milliseconds; it
is then turned off for 3960 milliseconds for a total measurement cycle of 4
seconds. This results in a sensor with almost zero flow dependence and
therefore does not require stirring or sample movement in order to obtain
accurate dissolved oxygen readings. It also uses digital electronics for
processing data as opposed to analog electronics like the two steady-state
electrochemical sensors previously discussed.
The Rapid Pulse sensor is also unique in the fact that it uses a third electrode,
see figure 19. The third electrode is a silver reference electrode that is
separate from the silver anode as opposed to the steady-state polarographic
and galvanic sensors where the reference electrodes and anodes are
combined. The three electrode configuration is required to polarize and
de-polarize the electrodes consistently and for a very short period of time,
the feature that makes the Rapid Pulse sensor function as a flow independent
sensor.
1
2
by the user. Most YSI electrochemical sensors manufactured today utilize
this screw-on membrane cap design. Since the cap has a membrane that
is pre-stretched at the factory, there is no need to worry about stretching
the membrane too much or not enough when installing it on a sensor.
Further, the cap is filled with electrolyte and then screwed on to the sensor
as opposed to filling the sensor and then stretching the membrane over the
solution. This design minimizes the chances of an air bubble getting trapped
underneath the membrane which could result in inaccurate readings. See
the Probe Care and Maintenance Section for more information on changing
a membrane.
The next advancement in the steady-state sensor was the release of
polyethylene membranes in 2002. This new membrane material lowered
the stirring dependence and reduced the response time of a sensor when
compared to traditional Teflon membranes. This resulted in a faster, more
accurate dissolved oxygen measurement.
Additionally, the dissolved oxygen sensor and cable are now manufactured
to be detachable so the sensor can be replaced without affecting the cable,
lowering the overall total cost of ownership. Further, no tools are required
to change sensors on YSI’s newest instruments, the Professional Plus and
Pro20, making the sensors truly field replaceable. Newer instruments also
include sensor diagnostics and error messages to help the user determine
when to change a membrane or recondition the electrodes, see the GLP
(Good Laboratory Practices) File section for more information on sensor
diagnostics.
3
Figure 19. Illustrating the 3 electrodes of a Rapid Pulse Sensor.
ADVANCEMENTS IN STEADYSTATE ELECTROCHEMICAL
SENSORS
The design of the electrochemical sensor has undergone many enhancements
over the years. The first YSI advancement in electrochemical sensors was
the redesign of the electrodes and membrane so that the membrane could
be pre-stretched into a plastic cap at the factory for easier replacement
COMPARING STEADYSTATE POLAROGRAPHIC AND GALVANIC
SENSORS
In terms of physical configuration and general performance, YSI galvanic
dissolved oxygen sensors are very similar to the polarographic sensors
which YSI pioneered and has sold for 40 years. The main advantage of
using galvanic sensors is convenience. Galvanic sensors provide an instanton sensor without the need for a warm-up time but this factor also adversely
affects the life of the sensor. Polarographic sensors last longer and have
a longer warranty but require a 5-15 minute warm-up time before use or
calibration. This section describes the similarities and differences between
these two technologies. These points are also summarized in figure 20.
Page 15

22
23
Similarities
First, both the polarographic and galvanic sensors are ‘steady-state’. This
means that when the instrument is turned on in the instance of a polarographic
sensor or, in the case of a galvanic sensor without the instrument being
turned on, the electrodes are continuously polarized or in ‘steady-state’.
Second, both sensor types reduce or consume oxygen at the cathode and
are therefore dependent on flow across the sensor tip. Flow dependence
is caused by the reduction of oxygen at the cathode which depletes
oxygen at the membrane surface in the sample. The oxygen depletion
at the membrane surface results in the need for sample movement across
the membrane in order to get accurate dissolved oxygen measurements.
If enough sample movement is not supplied by the natural flow of the
medium, manual agitation of the sensor by the user, a mechanical stirrer, or
some combination of these factors, then the sensor will continue to deplete
oxygen at the membrane surface which will result in artificially low DO
readings. The flow dependence is dictated by the membrane in use. The
flow dependence and required flow for each type of membrane is listed in
figure 14. The topics of flow dependence and response time are discussed
further in the Comparing Optical and Electrochemical Sensing Technologies
section. Tips on how to overcome flow dependence to obtain accurate
readings can be found in the Taking Measurements section.
Lastly, both sensors utilize electrolyte solution that allows an electrical current
to flow between the cathode and anode and is held in place by an oxygen
permeable membrane.
Differences
A major difference between polarographic and galvanic sensors is that
the galvanic sensor does not require a polarizing voltage to be applied in
order to reduce the oxygen that has passed through the membrane. Rather,
the electrode materials for the galvanic sensor have been chosen so that
their electrode potentials are dissimilar enough to reduce oxygen without
an applied voltage. Thus, the cathode and anode of the galvanic sensor
are made from silver and zinc (a highly reducing metal), respectively, while
the cathode and anode for the polarographic sensor are gold and silver (a
much less reducing metal), respectively.
Given that the galvanic sensor does not require an external voltage for
polarization, it therefore does not require the “warm-up” polarization time
that the polarographic sensor requires. Since a galvanic sensor doesn’t
require a “warm-up” time, the instrument is ready to measure when it is
powered on and, therefore, users are not required to wait to calibrate
or to take readings when using a galvanic sensor. Conversely, when an
instrument with a polarographic sensor is turned on, the polarizing potential
is applied to the sensor and a substantial “warm-up” period is required
before the sensor is stable enough for calibration and measurements. The
polarization warm-up time for a polarographic sensor is between 5-15
minutes depending on the age and condition of the electrodes. Thus, there
is an advantage of galvanic sensors over polarographic sensors in terms of
convenience of use.
Another difference between the two sensor types is in the oxidation of their
anodes. To balance the reduction reaction associated with oxygen at the
cathode, there is a corresponding oxidation reaction at the anode which
converts the zinc to zinc hydroxide (for galvanic sensors) or the silver to silver
chloride (for polarographic sensors). The practical difference between the
two types of anode oxidations is that the silver chloride remains attached
to the anode in a polarographic sensor while much of the zinc hydroxide
detaches from the anode in a galvanic sensor and forms a suspension of
white solid in the electrolyte. Thus, visual inspection of the electrolyte
at the tip of a galvanic sensor is likely to show the presence of a white
solid while the electrolyte for a polarographic sensor will be clear. The
amount of solid which is visible in the electrolyte for a galvanic sensor and
attached to the anode for a polarographic sensor is directly proportional
to the total current from the reduction of oxygen. Eventually, the formation
of oxidized anode material, either on the anode for polarographic sensors
or in the electrolyte for galvanic sensors, will cause degradation of sensor
performance. This degradation usually is indicated by low sensor output
and/or slightly jumpy readings. When these symptoms occur, the user
must remove the old membrane, clean the sensor anode if necessary, and
install a new membrane and electrolyte to restore proper performance. The
presence of the solid in galvanic sensors is likely to require a somewhat
more frequent membrane/electrolyte replacement than for polarographic
sensors due to degradation of overall system performance, giving an
advantage to polarographic sensors in this area. However it should be
Page 16

24
25
noted that (a) YSI has demonstrated 2-3 months of good galvanic sensor
performance even when there is considerable solid present in the electrolyte
and (b) the membrane/electrolyte replacement is very simple and relatively
inexpensive. Thus, the polarographic advantage is likely to be minor. See
Probe Care and Maintenance for more information on routine maintenance
procedures for electrochemical sensors and the recommended intervals for
performing these actions.
The last main difference between the two sensors is their theoretical sensor
life. Because the galvanic sensor’s electrodes are continuously polarized,
it is always ‘on’ even when the instrument is turned off. This results in a
degradation of the consumable anode which could result in a theoretically
shorter sensor life. Since the Polarographic sensors are not self-polarizing,
the sensor is off when the instrument is off and, therefore, the consumable
anode is not being continuously degraded which results in a longer
theoretical sensor life. Therefore, theoretically, the polarographic sensor
may have some advantage over galvanic in overall sensor longevity. In
practical terms, the anode of a YSI galvanic sensor should last for several
years so the advantage is minor; however, it should be noted that a YSI
polarographic sensor is warranted for 1 year while a YSI galvanic sensor
is warranted for 6 months.
Steady-State Polarographic versus Steady-State Galvanic Sensors
Similarities:
Steady-State Polarographic versus Steady-State Galvanic Sensors
Differences:
• Galvanic sensors do not require a warm-up time and are ready for
immediate use after being powered on.
• Polarographic sensors require warm up period before use.
• Galvanic sensors continually consume the anode, even when the
instrument is off. Thus, they have a shorter theoretical sensor life and are
warranted for 6 months
• The consumption of polarographic sensors stops when the instrument
is turned off. Thus, they have a longer theoretical sensor life and are
warranted for 1 year.
Figure 20.
C O M PA R I N G O P T I C A L A N D E L E C T R O C H E M I C A L S E N S I N G TECHNOLOGIES
There are many advantages and disadvantages associated with each
sensing technology and these should be considered when selecting an
instrument. This section describes how the two technologies compare in
accuracy, approval for compliance purposes, and general use.
• Both sensors are Steady-state.
• Both reduce oxygen, thus they require stirring or sample movement for
accurate readings.
• Both use electrolyte that is held in place by an oxygen-permeable
membrane.
MEASUREMENT ACCURACY
Electrochemical and optical dissolved oxygen sensing technologies produce
nearly identical results across a wide measurement range as long as you
provide enough sample movement when using a steady state electrochemical
sensor.
Refer to the graphs in figure 21, the top graph represents dissolved oxygen
measurements from a stirred steady state electrochemical sensor at six
different dissolved oxygen concentrations. The bottom graph represents
measurements of the same samples taken with a lifetime optical sensor.
Each concentration was measured by each sensor seven times. As shown
by the data in the graphs, the two technologies measured dissolved oxygen
equivalently across the wide measurement range.
Page 17

26
27
CON SIST ENCY OF M EM BRANE EL ECT RODE READING S FOR VARIOUS DO
CON CENT RATIO NS
0
1
2
3
4
5
6
7
8
9
0 1 2 3 4 5 6 7 8
BOTTLE #
DO CONCENTRATION, MG/L
CONC 1
CONC 2
CONC 3
CONC 4
CONC 5
CONC 6
CONS IST ENCY O F YS I OPT ICAL READING S FO R VARIOUS DO C ONCENT RATIO NS
0
1
2
3
4
5
6
7
8
9
0 1 2 3 4 5 6 7 8
BOTTLE #
DO CONCENTRATION, MG/L
CONC 1
CONC 2
CONC 3
CONC 4
CONC 5
CONC 6
Figure 22 further illustrates that the two sensor technologies perform
equivalently. In this example, a ROX optical sensor and a Teflon covered
Rapid Pulse polarographic sensor were deployed continuously for 52 days.
Since the Rapid Pulse sensor was used, stirring or sample movement was
not required. Both sensors were equipped with wipers to minimize the
effects of bio-fouling. The data between the two sensors (blue circles for the
Rapid Pulse sensor and red circles for the ROX sensor) tracked consistently
with each other even during a low DO event. A manual spot check of DO
was periodically taken during the test and is represented on the graph with
black triangles. There are a few apparent discrepancies for the manual
readings, which could be the result of insufficient sample movement or
improper calibration of the instrument used to take the manual reading. In
general, the three types of measurements track well.
Figure 21. Studies were performed by YSI Scientists for the Standard Methods
Review Panel.
Figure 22. Source: South Carolina Department of Natural Resources – Waddell
Mariculture Center; Research funded by National Institute of Standards and
Te ch no l og y1.
Even though the two technologies perform equivalently in the field, optical
sensors are slightly more accurate then electrochemical sensors under
controlled conditions in the 0-20 mg/L measurement range as is shown in
YSI’s published specifications, see figure 23.
Page 18

28
29
Optical
% DO Saturation 0-500% 0.1%
mg/L 0-50 mg/L 0.01 mg/L
Electrochemical
% DO Saturation 0-500% 0.1%
Range Resolution Accuracy
Range Resolution Accuracy
0 - 200%: ±1%
of reading or 1%
Saturation, whichever is
greater.
200 - 500%: ±15% of
reading.
0 - 20 mg/L: ±0.1mg/L
or 1% of reading,
whichever is greater.
20 - 50 mg/L: ±15% of
reading.
0 - 200%: ±2%
of reading or 2%
Saturation, whichever is
greater.
Test Protocol) Coordinator for individual approval. Submitting an application
to your Regional ATP Coordinator is typically all that is required but, in some
cases, you may have to submit data in order to get approval to use this
method for compliance reporting. ASTM International has approved the
optical method for measuring dissolved oxygen in water, method D888-05,
but it has not yet been approved by Standard Methods for the Examination
of Water and Wastewater. A Standard Method will likely be adopted in
the near future.
The electrochemical method has been used for decades and is therefore a
proven technology. It is approved for monitoring and compliance reporting
by the US EPA and can be used without contacting a regional US EPA office.
This factor gives it a slight advantage over the optical method.
RESPONSE TIME
The response time of a sensor should be considered when selecting an
instrument since this feature will dictate the amount of time required to
conduct a sampling session. A faster responding sensor could save time
and therefore money if used instead of a slower responding sensor.
200 - 500%: ±6% of
reading.
mg/L 0-50 mg/L 0.01 mg/L
Figure 23.
0 - 20 mg/L: ±0.2
mg/L or 2% of reading,
whichever is greater.
20 - 50 mg/L: ±6% of
reading
APPROVED METHODOLOGY
At the time of publication, the optical sensing method is not nationally
approved by the United States Environmental Protection Agency (US EPA)
for compliance monitoring and reporting. It has gained interim approval in
many EPA regions, but you must contact your Regional EPA ATP (Alternative
The table in figure 24 shows the typical response times of optical and
steady-state electrochemical sensors. The table further illustrates the
response time of an electrochemical sensor when used with various types
of membranes. The response time is expressed as T-95 which is the length
of time it takes to reach 95% of the final (true) reading when a sensor is
transferred from a fully saturated sample to a zero oxygen environment. As
shown, an optical sensor’s response time is about twice as long as a Teflon
covered electrochemical sensor and five times as long as a polyethylene
(PE) covered electrochemical sensor. The varying sensor response times
are also demonstrated in the graph in figure 25 where you can see the
polyethylene covered electrochemical sensor responded much quicker then
the other two sensors.
Although 40 seconds is a relatively short period of time to take one reading,
the table in figure 26 illustrates how much longer it would take to measure
dissolved oxygen in 500 samples with an optical sensor versus a polyethylene
or Teflon covered electrochemical sensor. If using a polyethylene covered
Page 19

30
31
sensor, 500 samples could be measured in about 1 hour where it would
DO Response Time
0
10
20
30
40
50
60
70
80
90
100
1 10 19 28 37 46 55
Time in Seconds
Dissolved Oxygen % Saturation
Teflon Membrane
1.25 PE Membrane
Optical Sensor
take over 5 hours to measure 500 samples with an unstirred optical sensor.
It should be noted that YSI studies have shown that stirring an optical sensor
can lower its response time. For example, using a magnetic stirrer or stir
bar could result in an optical response time of 22 seconds or less for T-95.
Therefore, reading 500 samples with a stirred optical BOD sensor (OBOD)
could take about 3 hours to perform.
Sensor Type Membrane Material Response Time
100 to 0%
(T-95)
Optical Sensing element with
40 seconds**
diffusion layer
Galvanic or
Polarographic
Galvanic or
Polarographic
Galvanic or
Polarographic
1.0 mil Teflon
Black cap
1.25 mil PE
Yellow Cap
2.0 mil PE
Blue Cap
18 seconds
8 seconds
17 seconds
Figure 25. A graph displaying DO measurements over time for three sensors that
were transferred from 100% saturated water to a zero oxygen environment.
Figure 24. A table defining the measurement response time of various sensors and
membranes.
** YSI studies have shown that stirring an optical sensor can lower its
response time. For example, using a magnetic stirrer or stir bar could result
in an optical response time of 22 seconds or less for T-95.
Sensor Type Number of
Polarographic
Samples
500 18 2.5
T-95 Response
in seconds
Total Time in
hours
with 1.0 mil Teflon
membrane
Polarographic
500 8 1.1
with 1.25 mil PE
membrane
Optical 500 40 5.6
Figure 26. The data in the table demonstrates the amount of time it would take to
measure 500 samples with three different sensors.
Page 20

32
33
FLOW DEPENDENCE
As mentioned, steady-state electrochemical sensors consume oxygen during
measurement and therefore require sample movement or the readings will
be artificially low. This is commonly referred to as flow dependence since
the sensor is dependent on flow or water movement across the membrane
in order to obtain accurate readings. Optical sensors, however, use a
non-consumptive method for dissolved oxygen measurements resulting in a
sensing method with zero flow dependence or stirring requirement.
The graphs in figure 27 and 28 illustrate this advantage of the optical sensor.
Figure 27 is a graph of data measured with a steady-state polarographic
sensor in an air-saturated water sample where adequate sample movement
was provided by a mechanical stir bar. When the stirring mechanism was
turned off, the readings began to fall resulting in artificially low dissolved
oxygen measurements. Figure 28 is a graph of data measured with
an optical sensor in the same air-saturated water sample where sample
movement was provided by a stir bar. When the stirring mechanism was
turned off for the optical measurements, the readings remained constant
and accurate proving the optical sensor is not dependent on flow. This
is a considerable advantage of the optical sensor especially for low flow
applications or applications where probe stirring or sample movement is
difficult such as down well.
Figure 28. A graph, created in YSI Data Manager software program, illustrating
the flow independence of an optical sensor.
For steady-state electrochemical sensors, the membrane material and
thickness dictates the degree of the sensor’s flow dependence. For example,
polyethylene membranes, frequently notated as PE, require less movement or
flow then traditional Teflon membranes as illustrated by the graph in figure
29. In this graph, three different sensors were placed in fully aerated water
with a stir bar. Once the stirring was ceased, the steady-state electrochemical
readings began to fall. Notice how the Teflon covered sensor fell further
and more rapidly then the PE covered sensor. The stirring dependence
of each sensor and membrane type, along with the recommended stirring
rates, is listed in figure 14. For tips on how to overcome flow dependence,
see Taking Measurements.
Figure 27. A graph, created in YSI’s Data Manager software program ,illustrating
the flow dependence of a steady state electrochemical sensor.
Page 21

34
35
Figure 29. Flow dependence comparison graph.
60%
100%
Percent
Saturation
Remove Stirring
Steady State Response
Teflon Response
PE Response
Optical
Time in seconds
WARM-UP TIME
A key advantage of the optical sensor for sampling applications is that it
does not have a warm up time since there are no electrodes to polarize.
So, similar to a galvanic sensor, an optical sensor can be calibrated and
used immediately after being turned on. A polarographic sensor, however,
requires 5-15 minutes for the electrodes to polarize before a calibration or
measurement can be performed.
CALIBRATION FREQUENCY
MEASUREMENT INTERFERENCES
Some gases, including hydrogen sulfide, can permeate the membrane of
an electrochemical sensor interfering with the electrodes and the readings.
A list of these gases can be found in the Measurement Precautions and
Interference section. Optical sensors, on the other hand, are unaffected
by the presence of hydrogen sulfide and other gasses that interfere with
the traditional electrochemical method. This gives the optical sensor the
ability to sample in environments with hydrogen sulfide like wastewater,
lake bottoms and wetlands.
MAINTENANCE REQUIREMENTS
The optical sensor requires much less maintenance then traditional
electrochemical sensors. With the optical sensor, the sensing element only
needs to be replaced about once per year where an electrochemical sensor
usually requires a membrane change every 2-8 weeks. Further, there is no
need to clean or resurface electrodes in an optical sensor as is required
when using an electrochemical sensor. For more information on performing
routine maintenance tasks, see the Probe Care and Maintenance section.
POWER CONSUMPTION
Optical sensors have a higher power consumption rate than the
electrochemical sensors. This results in a shorter battery life which could be
a disadvantage for the optical sensor in some sampling applications.
The optical measurement method experiences very little calibration drift
when compared to electrochemical sensors and this advantage results
in less frequent calibrations. In fact, optical sensors are so stable they
are capable of holding their calibration for many months, although YSI
recommends verifying the calibration regularly to ensure the highest data
accuracy. Steady-state electrochemical sensors require a calibration each
day they are used in a spot sampling application. Although this can be
considered a disadvantage of the electrochemical method, it must be noted
that a dissolved oxygen calibration is simple and easy to perform. See the
Calibration section for additional information.
SUMMARY
As you can see, there are several advantages and disadvantages associated
with each sensing method and these factors as well as some additional
points have been summarized in figure 30. It is important to analyze your
specific application needs to help determine which sensor is best for your
specific application. See the figure 31 for a table that outlines the best
suited applications for each sensing method.
Page 22

36
37
Electrochemical Sensors
Advantages Disadvantages
Proven technology. Approved
by the US EPA for compliance
monitoring and reporting.
Faster measurement response time
than an optical sensor.
Lower power consumption than an
optical sensor.
Lower initial acquisition cost than
an optical sensor.
Advantages Disadvantages
Instant on – no warm up time
required.
Exhibits very little calibration drift
and can hold a calibration for
several months.
Not susceptible to interferences like
hydrogen sulfide.
Non consumptive method – no
need to stir or provide sample
movement.
Less maintenance than traditional
electrochemical sensors.
Figure 30. Summary of advantages and disadvantages associated with each sensor
type.
Steady-state sensors are flow
dependent and therefore require
sample movement for accurate
readings.
Requires more frequent calibrations
than an optical sensor.
The measurement is susceptible to
interferences from gases such as
hydrogen sulfide.
The electrodes require periodic
maintenance.
The membrane requires regular
changes.
Optical
Not approved by the US EPA
for compliance monitoring and
reporting.
Higher initial acquisition cost.
Slower measurement response time
than traditional electrochemical
sensors.
Higher power consumption than
traditional electrochemical sensors.
Best Suited Application for each Dissolved Oxygen Sensor
Type
Sensor Type Membrane Material Best Application (in general)
Optical Sensing element with
diffusion layer
Galvanic or
Polarographic
Galvanic or
Polarographic
Figure 31. Best suited application per sensor type.
1.25 mil PE Sampling applications that
2.0 mil PE Sampling applications
Deep water profiling•
Shallow water where •
stirring is difficult
Low flow applications•
Samples that are small in •
volume
Samples with hydrogen •
sulfide
Continuous long-term •
deployments with the
ROX sensor
require the fastest response
time
that require a lower flow
dependence
MEASURING DISSOLVED OXYGEN WITH EITHER SENSOR TYPE
Dissolved oxygen sensors, both electrochemical and optical, do not measure
the concentration of dissolved oxygen in mg/L or ppm (parts per million
which is equivalent to mg/L). Instead, the sensors measure the pressure of
oxygen that is dissolved in the sample. To simplify the readings displayed
by an instrument, the pressure of the dissolved oxygen is expressed as DO
% Saturation. The instrument converts the dissolved oxygen pressure value
from the sensor to % Saturation by dividing the sensor output in mmHg by
160*** (the pressure of oxygen in air at 760 mmHg) and then multiplying
Page 23

38
39
by 100. Thus, a measured oxygen pressure of 150 mmHg would be
displayed by a YSI instrument as 93.8 % Saturation (150/160 * 100).
The fact that the sensor measures oxygen pressure and not dissolved oxygen
concentration is known to be true because a sample of fresh water can
dissolve more oxygen than a sample of sea water at the same temperature
and at the same altitude (or under the same barometric pressure); however,
the sensor’s output signal is identical in both samples since the oxygen
pressure is identical in both media. See figure 32 for an example of this
concept.
***The pressure of oxygen at sea level is 160 mmHg because oxygen is
about 21% of the earth’s atmosphere and 21% of 760 (average sea level
barometric pressure) is about 160 mmHg.
and field use with the use of additional sensors and/or instrument software
settings. The effect of flow dependence has been overcome by advances
in measurement technologies. Newer, optical dissolved oxygen sensors
have no flow dependence. For electrochemical based sensors, users
need to provide sample movement to overcome flow dependence or the
measurements could be artificially low. The stirring dependence and
recommended stirring rates of each sensor and membrane type are listed in
figure 14. For tips on how to overcome flow dependence, see the Taking
Measurements section.
Barometric pressure primarily affects the calibration of dissolved oxygen
sensors as it defines the pressure of oxygen in the calibration environment.
Generally, there is no need to be concerned about changes in barometric
pressure that take place after calibration, except if reporting DO% Local
with a YSI 556 or 650 as explained in the Barometer section.
DO Sensors Measure % Saturation
If two samples, one of fresh water and one of sea water, are fully
saturated with oxygen the dissolved oxygen concentration will be:
Fresh water at 25ºC = 8.26 mg/L
Sea water (36 ppt) at 25ºC = 6.27 mg/L
However, the signal output from either sensor type will be identical in the
two samples. Since both are 100% saturated, % saturation (or oxygen
pressure) is what both sensors are measuring.
Figure 32. DO sensors measure % saturation.
VARIABLES THAT AFFECT DISSOLVED OXYGEN MEASUREMENTS
There are several factors that affect the measurement of dissolved oxygen.
These variables include temperature, salinity, flow or stirring dependence,
and barometric pressure.
Temperature and salinity are compensated for during instrument calibration
TEMPERATURE
The most significant variable for dissolved oxygen measurements is
temperature; therefore, it is important to ensure the temperature sensor on the
instrument is measuring accurately. Temperature affects DO measurements
in two ways.
First, due to the increase or decrease in molecular activity, diffusion of
oxygen through the membrane of an electrochemical probe or sensing
element of an optical probe changes with temperature. The change in
diffusion rate based on temperature can be up to approximately 4% per
degree Celsius depending on the membrane material for steady-state
electrochemical sensors, 1% per degree Celsius for Rapid Pulse sensors, and
is approximately 1.5% per degree Celsius for optical sensors. For example,
if the temperature of a sample changes from 20°C to 15°C, the probe
signal would decrease by varying rates depending on the sensor in use,
giving a lower DO % saturation reading even though the % saturation of the
water has not changed. Therefore, the sensor signal must be compensated
for changes in temperature. This is done by adding a thermistor to the circuit
of older, analog instruments. For newer, digital instruments, the software
compensates for temperature changes with proprietary algorithms that use
the temperature readings from the probe’s thermistor.
Page 24

40
41
The adjustment described so far only compensates for temperature’s effect
on the oxygen diffusion rate through a membrane or sensing element. In
addition to this effect, temperature also affects the ability of water to dissolve
oxygen. It is a scientific fact that the solubility of oxygen in water is directly
proportional to temperature; see Oxygen Solubility Table in Appendix
A. Warmer water cannot dissolve as much oxygen as colder water. For
example, in an oxygen saturated sample of water at sea level (exposed to
760 mmHg of barometric pressure), the % saturation value will be 100%
regardless of the temperature because it is fully saturated. However, the
dissolved oxygen mg/L concentration will change with temperature because
the solubility of oxygen in water changes with temperature. For instance, at
15 ºC water can dissolve 10.08 mg/L while 30 ºC water can only dissolve
7.56 mg/L of oxygen even though the % saturation value is 100% in both
samples. Therefore, we must compensate the mg/L concentration reading
per the temperature of the sample.
Both of these temperature effects are factored into the conversion of the probe
signal to a mg/L concentration. For newer, digital instruments, the software
compensates for both of these temperature-related factors after instrument
calibration and during readings. The temperature compensation for the
% saturation reading is empirically derived, while the conversion from %
saturation, temperature and salinity to a mg/L concentration is automatically
carried out by the instrument’s firmware using formulae available in Standard
Methods for the Examination of Water and Wastewater2. The calculation
for converting % Saturation to mg/L and an example is provided in figure
33.
SALINITY
The second variable that affects DO concentration is the salinity of the water
sample. While the % saturation reading is not a function of the salinity
(or dissolved solids content) of the water, the mg/L concentration changes
significantly with salinity. As the salinity of water increases, its ability to
dissolve oxygen decreases. For example, oxygen saturated freshwater with
0 salinity at 25ºC contains 8.26 mg/L of oxygen while oxygen saturated
sea water (36 ppt) at the same pressure and temperature contains only
6.72 mg/L of dissolved oxygen.
Thus, salinity (along with temperature) must be factored into the instrument’s
calculation of mg/L. This calculation is based on the % saturation reading,
temperature reading, and the measured or entered salinity value using
formulae found in Standard Methods for the Examination of Water and
2
Wastewater
. The calculation for converting % to mg/L and an example is
provided in figure 33.
Determining DO mg/L from % Saturation
The following explains how to convert % Saturation to mg/L (also
referred to as ppm).
In order to perform this conversion, the temperature and salinity of the
sample must be known.
Step one: Determine the % saturation, temperature, and salinity of
the sample.
Step two: Multiply the % saturation reading by the value in
appropriate column (depends on salinity) and row
(depends on temperature) of the Oxygen Solubility Table
in Appendix A.
Example:
Step one: Sample is measured to have: 80% DO saturation
0 salinity
20º C
Step two: Multiply .80 (which is the DO %) by 9.09 (value from
oxygen solubility table at 0 salinity and 20º C) = 7.27
mg/L.
Result: 7.27 is the mg/L value that corresponds to an 80% DO-
Saturation reading of a sample with zero salinity at 20º
C.
Figure 33. Calculating mg/L from % saturation, temperature and salinity.
Page 25

42
43
CORRECTING FOR SALINITY
The salinity value used by the instrument in the calculation of mg/L is
obtained one of two ways, depending on the instrument being used. For
YSI dissolved oxygen instruments that also measure conductivity, the salinity
value measured by the conductivity sensor is used for the mg/L calculation.
Therefore, it is important to ensure the conductivity sensor is calibrated and
reading accurately in order to obtain accurate DO mg/L readings.
dissolved oxygen instrument that also measures conductivity for highest
data accuracy. A dissolved oxygen instrument that also has a conductivity
sensor will use the real-time salinity readings from the conductivity sensor
for every mg/L calculation. This will make sampling easier since it will not
be necessary to manually change the correction factor at each sampling
new site.
BAROMETRIC PRESSURE
For YSI dissolved oxygen instruments that do not have a conductivity sensor,
the salinity value of the sample must be manually entered by the end user.
See the salinity guide in figure 34 for a list of typical salinity values for
various types of water.
Salinity Guide - Average salinity by water type3
Water Type Average Salinity
Fresh water <0.5 ppt****
Brackish water 0.5 to 30 ppt
Sea water 33 to 37 ppt
Saline water 30 to 50 ppt
Brine > 50 ppt
Figure 34. Average salinity of different types of water.
****Salinity is a unitless measurement determined from conductivity and
temperature readings according to the Practical Salinity Scale which can be
found in Standard Methods for the Examination of Water and Wastewater2.
Historically, salinity values determined via the Practical Salinity Scale were
given the designation “ppt” because these values were very close to those
determined by the previously used method where the mass of dissolved salts
in a given mass of water (parts per thousand) were reported. Today, ppt is
commonly replaced by psu (Practical Salinity Units) as the preferred unit to
describe salinity calculated by the Practical Salinity Scale; however, these
values are equivalent since they are determined by the same method.
When sampling water of varying salinity, for example in brackish waters
such as estuaries or coastal wetlands, it is recommended that you use a
The final factor with regard to dissolved oxygen calibration and measurement
is barometric pressure. Barometric pressure affects the pressure of oxygen
in a sample of air or water. For example, the percentage of oxygen in
air is always 21%, but the actual pressure of oxygen varies with changes
in barometric pressure. At sea level, the pressure of oxygen is 160 mmHg
(.21 x 760 mmHg). In a fully aerated sample, under these conditions, the %
saturation measured by a sensor would be 100% (160/160 x 100%, see
Measuring Dissolved Oxygen with Either Sensor Type). If the temperature
of the sample is 25 ºC, the instrument would calculate the dissolved oxygen
concentration as 8.26 mg/L based on the Oxygen Solubility table. As
the sample is moved up in altitude and kept air-saturated, the barometric
pressure would decrease and so would the pressure of oxygen in the
sample. At 1126 ft of elevation, the pressure of oxygen would be 153
mmHg (.21 x 730 mmHg) and the % saturation relative to sea level read by
the probe would be 95.6% (153/160 x 100%) in the fully aerated sample.
If the temperature of the sample is 25 ºC, the instrument would calculate a
dissolved oxygen concentration of 7.92 mg/L or 96% of 8.26 based on the
Oxygen Solubility table.
The effect of barometric pressure is overcome by proper sensor calibration.
Barometric pressure is used in the majority of dissolved oxygen sensor
calibrations as described in the Calibration section since it determines
the absolute pressure of oxygen in a sample of air or water at the time of
calibration and it is this pressure which is measured by all oxygen sensors.
When calibrating oxygen sensors, the sensor’s output is set to this known
pressure of oxygen. If the sensor output changes after calibration, then
the instrument would calculate a % saturation based on a simple linear
regression calculation. Thus, as long as the system does not drift, the
sensor’s output can always be used to define the oxygen pressure in any
Page 26

44
45
medium after performing a proper calibration and the use of the barometric
pressure (or altitude) at the time of calibration is the key factor in setting
the proper calibration coefficient. Therefore, it is not necessary to correct
for changes in barometric pressure after performing a proper calibration in
order to obtain accurate readings in the field.
In summary, as barometric pressure changes due to a change in altitude
or local weather front, the pressure of oxygen changes. However, there is
never any reason to compensate for this change if a proper calibration has
already been performed and the sensor has not drifted.
Note: If DO% Local is being measured with a YSI 6-series sonde or 556
it may be necessary to recalibrate the instrument after extreme changes in
barometric pressure or altitude in order to keep the DO% Local value at
100% in a fully saturated environment. This is not a requirement if only mg/L
values are being recorded since these values will remain accurate without
recalibrating Local DO %. If reporting DO% Local with newer instruments
such as the Professional Plus, ProODO, or Pro20, it is not necessary to
recalibrate after a significant barometric pressure change in order to
report an accurate DO% Local since these instruments have an on-board
barometer that is read by the instrument continuously. See the Local DO %
Measurement section for more information on this reporting option.
When performing a % air saturation calibration on instruments without
an internal barometer, the user inputs either the local “true” barometric
pressure or local altitude into the instrument during the calibration process.
Local altitude is used on some of YSI’s older instruments and barometric
pressure is used on newer instruments. Although entering altitude as
opposed to barometric pressure during calibration is a little less accurate
(up to .1 mg/L), it should provide enough accuracy for most applications.
In either situation, the calibration of the instrument in % saturation is handled
automatically by the system’s software or microprocessor based on either
the internal barometer reading or the value entered by the user at the time
of calibration.
A third method for calibrating DO to barometric pressure is used on
older, box-style instruments that only report mg/L and not % saturation.
In this method, the operator places the probe in either air-saturated water
or water-saturated air and manually corrects for barometric pressure by
calculating the pressure adjusted dissolved oxygen mg/L concentration and
entering this value into the instrument. This value is calculated from the
Oxygen Solubility table and the barometric pressure calibration correction
table found on the back of older instruments or in the instrument’s manual
(Appendix A and B).
“True” Barometric Pressure
USING BAROMETRIC PRESSURE FOR DO CALIBRATION
The method for calibrating dissolved oxygen sensors using the local
barometric pressure depends on the instrument in use and the calibration
technique.
If performing a % air calibration on newer instruments, like the Pro20 and
ProODO, a barometer is built into the instrument and the reading from that
barometer is used to define the oxygen pressure during the calibration.
Because this value determines the accuracy of future DO field readings, it
is recommended to periodically verify the barometer reading’s accuracy
and to recalibrate the barometer to the local “true” barometric pressure
as necessary. See figure 35 for information on obtaining the local “true”
barometric pressure.
When entering the local barometric pressure or calibrating an instrument’s
internal barometer, be sure to use “true” barometric pressure and not
a barometer reading that has been corrected to sea level. Laboratory
barometer readings are usually “true” (uncorrected) values of air pressure
and can be used “as is” for DO % calibrations and for calibrating the
barometer in a YSI instrument. Weather service readings are usually
not “true”, i.e., they are corrected to sea level, and therefore cannot
be used until they are “uncorrected”. An approximate formula for this
“uncorrection” is below:
True BP =
[Corrected BP mmHg] – [2.5*(Local Altitude in ft. above sea level)/100]
Figure 35. Information on “True” Barometric Pressure.
Page 27

46
47
LOCAL DO % MEASUREMENTS
you may reduce calibration frequency.
When calibrating a YSI instrument in DO % saturation, the instrument
compensates for the local barometric pressure or altitude and calibrates
the % reading to a value corresponding to the current barometric pressure
(altitude). Therefore, the calibration value is only 100% when at 760 mmHg
of barometric pressure (sea level). To determine the DO % calibration value
for other barometric pressures/altitudes, refer to the table in Appendix B
or divide the barometer reading by 760 and then multiply that number by
100:
Example: 750 /760 = 0.9868 x100 = 98.68%
Some users may wish to report “Local DO” where the calibration value is
100% regardless of the barometric pressure at the time of calibration. The
100% calibration value for this convention reflects the fact that the calibration
environment is at 100% oxygen pressure for that specific location. There
are several YSI instruments that are capable of reporting Local DO, such as
the Pro Plus, Pro20, and ProODO to name a few. These three instruments
calculate the barometric pressure effect on each Local DO % and mg/L
reading as it is measured in the field or lab.
CALIBR ATION
The most important thing that can be done in order to obtain accurate
dissolved oxygen readings is to perform an accurate calibration. As
a general rule, the data collected is only as accurate as the calibration
performed prior to data collection so care should be taken to ensure that the
calibration is performed correctly. This section describes how to calibrate a
dissolved oxygen sensor in general terms. For instructions specific to your
instrument, consult the instrument’s manual.
CALIBRATION FREQUENCY
Electrochemical sensors require more frequent calibrations than optical
sensors. Steady-state galvanic and polarographic sensors should be
calibrated every day that they are used in a spot sampling application. In
general, it is best to calibrate frequently at first and, as experience dictates,
Optical sensors have greater stability and are less susceptible to drift than
traditional electrochemical sensors. Experience and scientific studies have
shown that optical sensors can hold their calibration for many months.
However, for the highest data accuracy, YSI recommends verifying the
optical sensor’s calibration on a regular basis. To verify the instrument’s
calibration, place the sensor in its calibration environment and check to see
that the instrument’s DO% reading matches the calibration value based on
the barometric pressure. Refer to Appendix B for the DO% calibration values
based on barometric pressure and altitude.
NOTE: It may be useful to check the DO calibration before and after an
unattended monitoring study to ensure high data quality.
When calibrating DO % saturation to barometric pressure/altitude, it is
not necessary to recalibrate the instrument due to a change altitude or
barometric pressure because the DO % saturation and mg/L readings will
remain accurate. However, if Local DO% is being reported with a 556 or
650 it may be desirable to recalibrate after a significant change in altitude
or barometric pressure in order to keep the % saturation value at 100% in the
calibration environment. This is not a requirement if only mg/L values are
being recorded since these values will remain accurate without recalibrating
Local DO %. It is also not required to recalibrate instruments that have an
on-board barometer for accurate Local DO% readings, like the Pro Plus and
ProODO, since the barometer will automatically assure that the Local DO%
value remains at 100% in a fully saturated sample. For more information
on Local DO, refer to the Local DO % Measurements section of this booklet.
CALIBRATION METHODS
In general, calibration consists of exposing the sensor to a sample of known
oxygen content and calibrating the instrument to read that value. There are
three primary methods for calibrating a dissolved oxygen instrument:
Winkler titration•
Air-saturated water •
Water-saturated air•
Page 28

48
49
The Winkler Titration method calibrates the mg/L concentration value while
the air-saturated water and water-saturated air methods both calibrate the
% saturation reading.
The calibration environment in which the sensor is placed for calibration
differs for each method. All three methods are reliable and are described
in this booklet in general terms. For greatest ease and highest accuracy,
YSI recommends the water-saturated air method. However, if conducting
long-term, unattended monitoring with a ROX sensor on a 6-series sonde,
YSI suggests that you calibrate in air-saturated water for greatest accuracy.
Note: It is only necessary to perform one type of calibration. Since
mg/L is calculated from the % saturation reading, calibrating % saturation
automatically calibrates the mg/L reading and vice versa. Therefore, it is
only necessary to calibrate one or the other no matter what you plan to
measure in the field.
WINKLER TITRATION CALIBRATION
If calibrating in mg/L, it is necessary to calibrate to a solution of known
dissolved oxygen. The only way to determine the dissolved oxygen
concentration of a sample without using a sensor is by performing a Winkler
Titration. The Winkler Titration method was developed by Lajos Winkler in
18884. In general, this method is cumbersome and time consuming.
To calibrate against a Winkler Titration, a sample of water should be
divided into four parts. If the instrument being calibrated does not have a
conductivity sensor or salinity correction option, the water sample should
be of the same salinity as the water that will be tested. Titrate three of the
samples. If any one of the samples’ titrated value varies more then +/- 0.5
mg/L, it should be discarded. The remaining results are then averaged.
Next, place the sensor into the fourth sample and allow the temperature and
dissolved oxygen readings to stabilize. Adequate sample movement must
be supplied if using a flow dependent sensor. Next, enter the averaged
mg/L value into the instrument’s calibration menu.
The success of this method depends on how the samples are handled and
is highly subjective to human error. At all stages of the titration, steps must
be taken to ensure that oxygen is neither introduced to nor is lost from the
sample. Furthermore, the water sample must be free of any solutes that
will oxidize iodide ions or reduce iodine. For these reasons, YSI does not
recommend calibrating against a Winkler.
AIRSATURATED WATER CALIBRATION
The second calibration technique is air-saturated water. The air-saturated
water method is the preferred method for calibrating ROX DO sensors for
long-term unattended studies. Like the Winkler technique, this method is
also time consuming and could introduce error if not done properly. To
calibrate an instrument against air-saturated water, use an aquarium air
pump and air stone to aerate a sample of clean tap water for 1 hour. Then
place the sensor in the sample and allow the temperature and dissolved
oxygen readings to stabilize. If using a flow dependent sensor, ensure that
there is adequate sample movement to the sensor. Next, select DO% as the
calibration method and enter the local altitude or “true” barometric pressure
depending on the instrument in use. Refer to figure 35 for information
on how to obtain the local “true” barometric pressure. Then, if using an
instrument without a conductivity sensor, enter the salinity value of the water
that will be tested and press enter to confirm the calibration.
Unless Local DO% is enabled in the instrument, the % calibration value will
only be 100% at sea level or 760 mmHg. To determine the calibration
value based on altitude or barometric pressure, refer to Appendix B or
simply divide the local “true” barometric pressure in mmHg by 760 and
then multiply that number by 100. For example, if the local true barometric
pressure is 700 mmHg, the % calibration value will be 700/760 * 100%
= 92%. For more information on Local DO, refer to the Local DO %
Measurements section of this booklet.
WATERSATURATED AIR CALIBRATION
So far, the two calibration techniques described have some drawbacks.
Winkler Titrations are cumbersome and time consuming. Air-saturated
water requires some preparation time. The third technique, water-saturated
air, overcomes these shortcomings and generally is YSI’s recommended
calibration method. As previously noted, however, if conducting longterm, continuous monitoring with a ROX sensor on a 6-series sonde, YSI
Page 29

50
51
recommends that you calibrate in air-saturated water.
Depending on the sensor and storage method being used, this procedure
should only take a few minutes to perform. First, dry the sensing element
or membrane and temperature sensor by dabbing it with a lint free cloth or
by shaking off excess water droplets. Next, place the sensor into a watersaturated air environment (air with 100% humidity). The probe must be
vented to the atmosphere while in its calibration environment to ensure a
100% water-saturated air environment. Be sure the dissolved oxygen and
temperature sensors are in water-saturated air only and not immersed in
water! The calibration environment can be accomplished several different
ways depending on the style of the sensor. See figure 36 for a table of
calibration environment options.
Examples of Calibration Environments for Water-saturated Air Calibration:
Calibration Environment Description and Tips
Example 1: Clear storage
chamber of older YSI sensors.
Moisten the sponge in the clear •
storage chamber with clean
water.
Chamber is vented to the •
atmosphere without any
additional preparation.
Ensure the sponge is clean •
because bacterial growth may
consume oxygen and affect the
calibration.
Do not allow sunlight to heat •
the calibration chamber since
that could keep the membrane
and the thermistor from reaching
thermal equilibrium.
Calibration Environment Description and Tips
Example 2: Moist sponge in
gray calibration/storage sleeve.
Included with some Pro Series
instruments.
Calibration sleeve is vented to •
the atmosphere without any
additional preparation.
Be sure to use clean water.•
Do not allow sunlight to heat •
the calibration chamber since
that could keep the membrane
and the thermistor from reaching
thermal equilibrium.
Example 3: Clean, moist sponge
in the calibration/storage
chamber that is built into some
handheld instruments.
Built-in storage chambers are •
vented to the atmosphere without
any additional preparation.
Be sure to use clean water.•
Do not allow sunlight to heat •
the calibration chamber since
that could keep the membrane
and the thermistor from reaching
thermal equilibrium.
Example 4: BOD Bottle Place a small amount (1/8 inch •
or 3 mm) of clean water in a
BOD bottle if using a BOD-style
sensor.
This is vented to the atmosphere •
without any additional
preparation.
Page 30

52
53
Calibration Environment Description and Tips
Example 5: Calibration/storage
cup
Place a small amount of clean •
water in the calibration cup and
place this over the sensor.
Be sure to only engage one or •
two threads so the cup is vented
to the atmosphere.
Do not fully thread the calibration •
cup onto the probe!
Do not allow sunlight to heat •
the calibration chamber since
that could keep the membrane
and the thermistor from reaching
thermal equilibrium.
Small amount of water = •
1/8 inch or 3 mm for small •
calibration cups
1 inch or 2.5 cm for larger •
calibration cups
Example 6: Wet towel wrapped
around sensor guard.
Be sure to use clean water to wet •
the towel.
Install the sensor guard and then •
wrap the towel around the sensor
guard.
After placing the probe in the calibration environment, turn the instrument
on and wait 10 minutes for the calibration chamber to become completely
saturated. Next, ensure the temperature and dissolved oxygen readings
have stabilized. Unlike the previous two methods, it is not necessary to
provide sample movement for flow dependent sensors with this method.
Enter the % calibration menu on the instrument and enter or confirm the local
true barometric pressure or altitude. For YSI instruments that calibrate to the
local altitude, enter your local altitude to the nearest 100 feet. Next, if using
an instrument without a conductivity sensor, enter the salinity value of the
water that will be tested and press enter to confirm the calibration.
Unless Local DO% is enabled in the instrument, the % calibration value will
only be 100% at sea level or 760 mmHg. To determine the calibration
value based on altitude or barometric pressure, refer to Appendix B or
simply divide the local true barometric pressure by 760 and then multiply
that number by 100. For example, if the local true barometric pressure
is 700 mmHg, the % calibration value will be 700/760 * 100% = 92%.
For more information on Local DO, refer to the Local DO % Measurements
section of this booklet.
Validity of Water-Saturated Air Calibration
It is a well documented fact that the pressure of oxygen in water-saturated
air is the same as in air-saturated water. For example, air-saturated water
and water-saturated air at sea level both have an oxygen pressure of 160
mmHg, see figure 37. Therefore, we would expect an oxygen sensor to
give the exact same reading in either media. Since it is valid to calibrate
in air-saturated water because it contains a known amount of oxygen, it
must also be valid to calibrate in water-saturated air. In the case of the airsaturated water calibration, the concern was whether or not the water was
completely oxygen-saturated. Since air is, by definition, oxygen saturated,
this uncertainty has been removed.
Figure 36. Examples of water-saturated air calibration environments.
Page 31

54
55
Figure 37. The oxygen pressure in water-saturated air and air-saturated water is
160 mmHg at sea level.
After creating an oxygen-free environment, place the sensor in the zero
medium and allow the readings to stabilize. Stir the sensor if placed in a
liquid and using a stirring dependent sensor
Select the two point calibration method within the instrument’s menu and
enter zero for the first calibration point. After completing the zero calibration
point, thoroughly rinse the sensor of the oxygen-free solution and place it
in a moist environment in order to follow up with a second point at full
saturation.
NOTE: If you have calibrated a ROX sensor in a zero oxygen solution,
remove the wiper from the probe to avoid sensing element contamination in
the saturated environment.
CALIBRATION MUSTS
Regardless of the type of calibration performed, there are several common
requirements for all three options. The lists below summarize the important
points to be mindful of when performing a calibration. As stated before,
your data is only as good as your calibration.
TWO POINT CALIBRATION
Many newer instruments allow the user to perform a two point calibration
with one point at zero and the other at full saturation. YSI recommends
performing a two-point calibration 1.) if you are planning on taking readings
in a low oxygen environment and you are certain that the sensor does not
meet your accuracy requirements at low DO values or 2.) to verify your
instrument’s operation across its entire measurement range.
A zero oxygen solution can be made by dissolving approximately 2
grams of sodium sulfite (Na2SO3) and into 1 liter of tap water or DI water.
Optionally, add a trace of cobalt chloride (CoC2) to the solution to speed
up the reaction. Mix the solution thoroughly and then wait for the solution
to be oxygen free which may take up to 60 minutes. Another method for
making a zero oxygen environment is by dissolving 5-7 grams active dry
yeast in 350 mL of distilled water and allowing the yeast to consume the
oxygen which could take approximately 15 minutes. Or, a third method is
to place the sensor in an inert gas such as nitrogen gas.
CALIBRATION MUSTS FOR ELECTROCHEMICAL SENSORS
First, inspect the membrane surface and probe solution. If there are 1.
air bubbles under the membrane, if the membrane is torn or dirty, or if
the electrolyte solution looks contaminated, replace both the membrane
and solution before calibration.
Second, if calibrating in air, remove water droplets from the membrane 2.
and thermistor by shaking the probe before inserting it into the calibration
environment.
Third, if using a steady-state polarographic sensor, turn the instrument 3.
on and allow it to stabilize for 5-15 minutes before calibrating. This
allows for the polarographic electrodes to polarize. Note that galvanic
electrochemical and optical sensors do not require a warm up period
for polarization.
Next, allow the temperature to stabilize completely in the calibration 4.
environment.
Page 32

56
57
Be sure to provide enough sample movement if calibrating in liquid.5.
ERRORS DURING CALIBRATION
If calibrating in % mode, in either water or air, enter your altitude or 6.
“true” barometric pressure.
Next, input the salinity value of the water you will be testing into the 7.
instrument’s software if using an instrument that does not measure
conductivity.
For electrochemical sensors in spot sampling applications, it is required 8.
to calibrate daily, but not between samples.
CALIBRATION MUSTS FOR OPTICAL SENSORS
First, visually inspect the sensor cap for biofouling or large scratches 1.
in the paint surface. Biofouling could consume or produce oxygen
thereby affecting the calibration. Large scratches in the paint surface
could affect the calibration as well.
Make sure your sensor cap is properly hydrated and has not dried 2.
out.
Ensure that there aren’t any water droplets on the sensing element or 3.
thermistor if calibrating in water-saturated air.
Allow the temperature to stabilize in the calibration environment.4.
If calibrating in % mode, in either water or air, enter your altitude or 5.
“true” barometric pressure.
Input the salinity value of the water you will be testing into the instrument’s 6.
software if using an instrument that does not measure conductivity.
Lastly, optical sensors have shown to hold a calibration for many months. 7.
However, YSI recommends that you verify your calibration regularly.
If an error message such as ‘Out of Range’ or ‘Questionable Results’
appears during the calibration procedure, do not accept or proceed with
the calibration. Instead, cancel or abort the calibration and try to determine
what is causing the error. A list of potential error sources and remedies
are listed in figure 38. For instrument specific recommendations, see the
instrument’s manual.
Source of Error Recommendation
Bad electrochemical membrane Inspect the membrane for
contamination and clean with mild
detergent if necessary. If damaged
or if air bubbles are present under
the membrane, replace it.
Dirty electrodes on steady-state
polarographic and galvanic
sensors
Burn-in not performed on a
Rapid Pulse membrane
High DO charge (a sensor
diagnostic value) for Rapid Pulse
sensor
If a new membrane does not resolve
the error, inspect the electrodes for
tarnishing and, if necessary, perform
a cleaning following the sensor-type
specific recommendations in the
Probe Care and Maintenance section
of this booklet. Tarnishing of the
electrodes is a natural result of the
chemical reaction that takes place
under the membrane and occasional
maintenance of the electrodes is
required to remove this build up.
For Rapid Pulse sensors, perform a
proper burn-in as described in the
6-Series Calibration Tips document.
Resurface the electrodes and install
a new membrane per the instructions
for the Rapid Pulse in the Probe Care
and Maintenance section of this
booklet and in the 6-series manual.
Page 33

58
59
Source of Error Recommendation
Low DO charge (a sensor
diagnostic value) for a Rapid
Pulse sensor
Bad optical sensing element Inspect the sensing element for
Dry optical sensing element If the sensing element has been
Bad Temperature reading Verify the temperature is reading
Bad calibration inputs Ensure the values entered during the
Figure 38.
There may be insufficient electrolyte
under the membrane, an air bubble
under the membrane, or a hole in the
membrane. Replace the membrane
with fresh electrolyte.
contamination and clean with mild
detergent if necessary. If the sensing
element is damaged or past its
expected life (1+ year), replace it.
allowed to dry out, try performing the
rehydration procedure described in
the instrument’s manual.
accurately. If erroneous, contact YSI
Technical Support.
calibration process are accurate,
i.e. “true” barometric pressure, local
altitude, salinity correction value,
DO concentration value of titrated
samples, etc.
value and its optimal range for your particular instrument, see the instrument
specific manual.
TAKING MEASUREMENTS
To take a measurement, place the sensor in the sample to be measured.
If using a steady-state electrochemical sensor, be sure there is enough sample
movement to overcome the sensor’s flow dependency. As mentioned, the
required flow rates vary by membrane type and are listed in figure 14. The
flow requirement can be overcome by the sample’s own movement, stirring
the probe in the sample, a combination of the two, or by using a mechanical
stirrer such as a stir bar or stir plate. A good rule to follow is to first stir the
probe at the rate you think is required. Then, increase that movement. If
the readings go up with the addition of more sample movement then you
were not providing enough sample movement to begin with. If the readings
remain the same, then you were providing enough movement. Continue
with this process until no additional movement results in an increase in the
DO readings. If you are using an optical or Rapid Pulse sensor, you do not
have to stir.
After you are confident that there is sufficient sample movement to overcome
the flow dependence of your sensor, wait for the temperature and DO
readings to stabilize. The response time or time to stabilization varies by
sensor and membrane material as shown in figure 14. It is important to
keep stirring while you wait for probe stabilization. Once the readings
have stabilized, you can record or save the reading.
GLP (GOOD LABORATORY PRACTICES) FILE
Newer YSI instruments contain a GLP (Good Laboratory Practices) file
that records each calibration to the instrument’s non-volatile memory. In
general, the calibration record will contain the date and time the calibration
was performed, the sensor’s value, and whether or not the calibration was
successful. Viewing the sensor’s value in the GLP file can be useful for
verifying the accuracy of the calibration and the condition of the sensor
which can help with sensor diagnostics. For example, a sensor value
that is out of the desired range could indicate that it is time to change the
membrane or recondition the electrodes. For information on the sensor
If taking a measurement in an aeration basin or some other media that has
a large quantity of air bubbles, you can suspend the probe upside down to
allow the bubbles to flow past it as shown in figure 39. Large air bubbles
can be a problem when they burst against the surface of the probe since this
is sensed as 100% saturation. Inverting the probe as shown in figure 39
will keep bubbles from bursting on the membrane surface and help avoid
erroneous measurements.
Page 34

60
61
distinguish between carbonaceous and nitrogenous demand, in which case
a nitrification inhibitor is used. Toxic and chlorinated samples also need
special handling. The operator must be familiar with standard methods and
with the technical literature on the subject.
When properly used, the BOD test provides a reliable characterization of
wastewater. It can be expected to be a standard for regulatory agencies for
many years even though its use as a control tool is limited by the 3 or 5 day
wait required for the test. Various methods (based on short-term monitoring
and extrapolation) of quickly estimating the probable results of the BOD
test on a sample have been devised and the interested reader is advised to
consult the appropriate literature.
MEASUREMENT PRECAUTIONS AND INTERFERENCES
There are a few potential interferences to be aware of when taking readings
in order to get the best data possible.
BIOFOULING
Figure 39. DO sensor inverted to avoid air bubbles bursting on the membrane
surface.
BOD MEASUREMENTS
The BOD (Biochemical Oxygen Demand) test consists of taking an initial
dissolved oxygen reading of a sample and then incubating it in a full, airtight
BOD bottle in the dark at 20°C for either 3 or 5 days depending on the test
protocol. The sample is measured again after the incubation period and the
BOD is calculated from the difference between the two measurements along
with the dilution and seed factors. Since most wastewater contains more
oxygen-demanding matter than the amount of dissolved oxygen available in
air-saturated water, BOD test samples are diluted to appropriately balance
oxygen demand and supply. Because bacterial growth requires such
nutrients as nitrogen, phosphorous and trace metals, these are added as
seed to the dilution water, which is also buffered to ensure a suitable pH.
A variety of strategies are employed to deal with specific sample types.
These include varying dilutions and diluent seeding. It is often desirable to
A few of the potential interferences that should be considered mainly deal
with keeping the sensing area clean. Always keep the sensor free from
biofouling such as bacteria or algae growth. These organisms may not only
prevent oxygen permeation through the membrane, but may also generate
or consume oxygen resulting in erroneous readings for both optical and
electrochemical sensors.
If biofouling is an issue for your application, try rinsing the probe between
readings in order to keep the sensor clean. If deploying an instrument for
continuous monitoring, consider using a mechanical wiper and anti-fouling
protection to reduce the effects of biofouling and extend deployment times.
COATING MATERIALS
Keep the sensor free of oils that could clog the membrane or sensor cap and
prohibit oxygen from diffusing to the sensor. The best advice if measurement
cannot be avoided in these types of environments is to frequently rinse the
probe. If necessary, periodically clean the membrane with a mild detergent
to remove any residue. It may also be necessary to increase the frequency
of membrane changes.
Page 35

62
63
PROBE ATTACKING LIQUIDS
INTERFERING GASES
There are some substances that can attack the probe’s materials and
could potentially cause permanent damage. If using an optical sensor,
avoid exposing the probe to alcohol and other organic solvents. Alcohol
could cause deterioration of the paint layer that protects the dye layer and
other organic solvents may deteriorate the dye layer. A list of solvents
that may damage an optical probe are listed in figure 40. Obviously the
concentration of the substance will also be a factor in whether any damage
to the sensing element will occur.
Optical Probe Attacking Liquids
The following will remove the paint layer of the sensing cap:
Alcohols
The following will remove the paint and dye layer of the sensing cap and
will attack the optical probe housing as well:
Toluene
Benzene
Carbon tetrachloride
Chloroform
Methylene chloride
Acetone
Methyl ethyl ketone,
Other organic solvents
Figure 40. Substances that could damage an optical sensing element.
Certain gases, if dissolved in water, can cause interference for polarographic
and galvanic sensors if they exist in high enough concentrations. An optical
sensor’s reading, however, is generally not affected by the presence of
these gases. The gases that will cause interferences with DO measurements
taken with electrochemical sensors include: hydrogen sulfide, sulfur dioxide,
halogens, neon, nitrous and nitric oxides. Some of these gases and others
have been tested by YSI and the response in apparent oxygen readings are
listed below:
100% Carbon monoxide less than 1%
100% Carbon dioxide around 1%
100% Hydrogen less than 1%
100% Chlorine 2/3 O2 response
100% Helium none
100% Nitrous oxide 1/3 O2 response
100% Ethylene none
100% Nitric oxide 1/3 O2 response
MEMBRANE AND SENSING ELEMENT INTEGRITY
While it is important to look for coating or fouling on the membrane or
sensor cap, it is also important to check the integrity of the membrane or
sensor cap by visually inspecting it. For an electrochemical membrane,
look to make sure the membrane is still stretched properly and that there are
no air bubbles trapped underneath. For an optical sensing element, check
for large scratches. While small scratches in the paint layer will not affect
the reading, large scratches may and you should replace your cap if you
see large scratches and are having problems calibrating. Also, be sure to
keep your membrane or optical sensing element hydrated.
For electrochemical sensors, care should be taken to avoid exposing the DO
probe to strong acids, caustics or solvents. Probe materials that come in
contact with liquid include polyethylene, polyurethane, Teflon, EPR rubber,
epoxy, ABS plastic and stainless steel.
Page 36

64
65
PROBE CARE AND MAINTENANCE
An understanding of dissolved oxygen measurement would not be complete
without discussing probe care and maintenance, much of which can be
performed by the user.
PROBE CARE AND MAINTENANCE FOR ELECTROCHEMICAL SENSORS
This section describes the general care and maintenance recommendations
for electrochemical sensors. For more detailed instructions that are specific
to your instrument, please refer to the instrument’s manual.
CHANGING A MEMBRANE
By far, the most frequent maintenance task with an electrochemical sensor
is replacing the membrane. In general, membranes should be changed
every 2-8 weeks depending on the application. If measuring in a high
fouling environment or in water with high hydrogen sulfide content, it will be
necessary to change the membrane at least every two weeks.
As discussed, YSI probes have one of two membrane styles – either stretch
or cap. Older probes use a sheet-like membrane that is stretched over
the probe and held in place by an o-ring. This is referred to as a stretch
membrane. Figure 41 shows a sequence of drawings that demonstrate how
this type of membrane is changed. After removing the old membrane, rinse
the electrode with distilled or de-ionized water and then dry it. Next, secure
a membrane between your thumb and the probe body. Add electrolyte to the
probe until a large meniscus completely covers the gold cathode. Handle the
membrane material with care, touching it at the ends only. With the thumb
and forefinger of your other hand, grasp the free end of the membrane.
With a continuous motion, stretch the membrane up, over, and down the
other side of the sensor. Stretching forms the membrane to the contour of
the sensor tip. Secure the end of the membrane under your forefinger while
continuing to hold the probe. Roll the o-ring over the end of the probe, being
careful not to touch the membrane surface. There should be no wrinkles in
the membrane or trapped air bubbles under the membrane. Trim off excess
membrane with scissors or a sharp knife. Shake off any excess KCl and then
rinse the probe with clean water.
Figure 41. Sequence of drawings demonstrating a stretch membrane installation.
Newer, cap-style membranes are much easier to change, as shown in the
series of drawings in figure 42. After removing the old membrane, rinse
the electrode with distilled or de-ionized water and then dry it. Next, fill
a new cap a little more than half way full with fresh electrolyte. Then,
with the probe facing down, insert the probe into the membrane cap.
Some electrolyte will spill over; this is normal. Continue with threading the
Page 37

66
67
membrane cap onto the probe. Shake off any excess KCl and then rinse the
probe with clean water. If you would like to see a video of this procedure,
visit YSI’s YouTube channel - www.Youtube.com/YSIInc
NOTE: Always re-calibrate an instrument after changing a membrane.
In between membrane changes, check the membrane regularly to be sure
it is still tightly stretched, not fouled, and that there are no air bubbles
under it. A loose or wrinkled membrane will introduce measurement errors,
make calibration difficult, and cause the electrodes to require more frequent
maintenance.
ELECTRODE MAINTENANCE
Requiring far less attention than the membrane of an electrochemical sensor
are the electrodes, or more specifically the cathode and the anode. It is
important to note that tarnishing of the electrodes is a natural by-product
of the reaction taking place under the membrane due to the presence of
oxygen. In other words, it is unavoidable. If using an electrochemical
sensor, it will eventually require a cleaning. The frequency of the cleaning
depends on use and the type of sensor. In general, electrode maintenance
should only be necessary about once per year for steady-state polarographic
sensors and about once every 3 months for steady-state galvanic sensors.
Do not perform the following maintenance procedures just because a sensor
‘looks’ dirty. Only perform the following procedures if the instrument will not
calibrate or if the readings are unstable and a regular membrane change
did not correct either of these problems.
Steady-state Polarographic Anode Maintenance
A tarnished polarographic silver anode can be cleaned by soaking the probe
in household ammonia cleaner overnight. Alternatively, the electrode may
be soaked in 14% Ammonium Hydroxide for 2 to 3 minutes. After soaking
the probe, rinse it thoroughly with clean water. This will brighten the anode.
If you are using the style of electrode that uses a cap membrane, you may
need to wet sand the anode with 400 grit sand paper after performing the
ammonia soak. This can be done by wrapping the sanding disc around the
anode and twisting as shown in figure 43.
Figure 42. Sequence of drawings demonstrating a cap membrane replacement.
Figure 43.
Page 38

68
69
Always install a new membrane with fresh electrolyte after servicing the
electrodes and allow the probe to stabilize before calibration.
NOTE: Do not perform the ammonia chemical soak on galvanic or Rapid
Pulse sensors.
up in the palm of your hand. Next, touch the cathode to the sanding disc
and twist the probe as shown in figure 44. After sanding, rinse the electrode
with clean water. Always install a new membrane with fresh electrolyte after
servicing the electrodes and allow the probe to stabilize before calibration.
Do not perform the ammonia soak on a galvanic sensor.
Steady-state Polarographic Cathode Maintenance
The cathode on most YSI probes can be restored to its normal polish by
wet sanding the electrode. If there is a heavy build up of deposits, this
procedure works best after the ammonia chemical soak described in the
previous section. To sand the cathode, place a 400 grit wet/dry sanding
disc, face-up in the palm of your hand. Next, touch the cathode to the
sanding disc and twist the probe, see figure 44. After sanding, rinse the
electrode with clean water.
Figure 44.
Always install a new membrane with fresh electrolyte after servicing the
electrodes and allow the probe to stabilize before calibration.
Galvanic Maintenance
To remove the white deposits from the galvanic electrodes, perform a wet
sanding procedure with 400 grit wet/dry sand paper on both the anode
and cathode. After removing the membrane, rinse the electrode with clean
water. Next, wrap the wet sanding disc around the anode and twist as
shown in figure 43. To sand the cathode, place the wet sanding disc, face-
Rapid Pulse Maintenance
The electrodes for the Rapid Pulse sensor can be serviced by performing
a wet sanding procedure with 2400 grit wet/dry sand paper. For this
procedure, place the sanding disc between your thumb and forefinger and
sand parallel to the electrodes 10-15 times like you are striking a match,
see figure 45. Then, rinse the electrodes with clean water and install a new
membrane with fresh electrolyte. Do not perform the ammonia soak or use
400 grit sand paper on a Rapid Pulse sensor.
Figure 45.
PROBE CARE AND MAINTENANCE FOR OPTICAL SENSORS
The maintenance requirements for an optical sensor are less laborious then
electrochemical sensors.
To clean the probe and sensing element, rinse it with clean water and a lint
free cloth. If necessary, use a mild detergent. Do not use alcohols or other
organic solvents that may deteriorate the sensing element.
The sensing element should be replaced about once per year but may last
longer. It should also be replaced if it is cracked or damaged. While
Page 39

70
71
changing the sensing element, rinse the optics with clean water and then
wipe the optics with a lint free cloth or lens tissue. The instruction sheet
that is shipped with the replacement sensing element includes calibration
coefficients specific to the sensing element. For highest accuracy, these
coefficients should be entered by the user into the instrument following
the instructions provided. See the instrument specific manual for detailed
instruction on how to change a sensing element.
STORAGE
For short term storage, electrochemical probes should be stored in a moist
environment to keep the membrane from drying out and needing replaced.
Do not store it directly in water since that could encourage algae growth
on the probe. For long-term storage of the steady-state sensors, remove the
membrane, rinse and dry the electrode and then install a dry membrane to
protect it from dirt and dust during storage.
The Rapid Pulse sensors are a bit different in that they should not be stored
dry as this could shorten their usable life. Instead, keep a membrane
installed on a Rapid Pulse sensor and store in a moist environment for both
short-tem and long-term storage.
method’s EPA approval status and information on how to contact your US
EPA ATP Coordinator, please visit YSI’s We Know DO web page at www.
ysi.com/weknowdo. The We Know DO web page is free and serves as
a valuable resource center for those interested in learning more about
dissolved oxygen.
YSI offers several different dissolved oxygen instruments to meet a wide
variety of application requirements that range from spot sampling to
continuous monitoring, from the field to the lab and from single parameter
to multiparameter. YSI’s knowledgeable Technical Support Staff is available
to assist you in selecting the right instrument for your application. You can
reach a Technical Support staff member by calling 800-897-4151 (+1 937767-7241) or sending an email to environmental@ysi.com. In addition,
YSI has offices located around the world ready assist you with selecting an
instrument, ordering replacement parts or providing repair services. Please
visit www.ysi.com for a list of our global offices.
Optical sensors should not be allowed to dry out either. For storage, keep
the sensing element installed and place the probe in a moist environment
but not directly in water since that could encourage algae growth on the
probe. If it dries out, you must re-hydrate it following the instructions in the
instrument’s manual.
To store the instrument for long periods of time, remove the batteries. Clean
the case and cable with a mild detergent if necessary. Then, store the
instrument in a cool, dry place.
FINAL WORDS
YSI has been manufacturing and distributing dissolved oxygen sensors for
over 40 years and that experience has made the company a leader in
dissolved oxygen measurement technologies. For more information on
the measurement of dissolved oxygen, including the latest on the optical
Page 40

72
73
A P P E N D I X A O X Y G E N
Temp °CChlorinity: 0
5.0 ppt
10.0 ppt
15.0 ppt
20.0 ppt
25.0 ppt
SOLUBILITY TABLE
Solubility of Oxygen in mg/L in water exposed to water-saturated air at
760 mmHg pressure
Temp °CChlorinity: 0
Salinity: 0
0.0 14.621 13.728 12.888 12.097 11.355 10.657
1.0 14.216 13.356 12.545 11.783 11.066 10.392
2.0 13.829 13.000 12.218 11.483 10.790 10.139
3.0 13.460 12.660 11.906 11.195 10.526 9.897
4.0 13.107 12.335 11.607 10.920 10.273 9.664
5.0 12.770 12.024 11.320 10.656 10.031 9.441
6.0 12.447 11.727 11.046 10.404 9.799 9.228
7.0 12.139 11.442 10.783 10.162 9.576 9.023
8.0 11.843 11.169 10.531 9.930 9.362 8.826
9.0 11.559 10.907 10.290 9.707 9.156 8.636
10.0 11.288 10.656 10.058 9.493 8.959 8.454
11.0 10.027 10.415 9.835 9.287 8.769 8.279
12.0 10.777 10.183 9.621 9.089 8.586 8.111
13.0 10.537 9.961 9.416 8.899 8.411 7.949
14.0 10.306 9.747 9.218 8.716 8.242 7.792
15.0 10.084 9.541 9.027 8.540 8.079 7.642
16.0 9.870 9.344 8.844 8.370 7.922 7.496
17.0 9.665 9.153 8.667 8.207 7.770 7.356
18.0 9.467 8.969 8.497 8.049 7.624 7.221
19.0 9.276 8.792 8.333 7.896 7.483 7.090
20.0 9.092 8.621 8.174 7.749 7.346 6.964
21.0 8.915 8.456 8.021 7.607 7.214 6.842
22.0 8.743 8.297 7.873 7.470 7.087 6.723
23.0 8.578 8.143 7.730 7.337 6.963 6.609
24.0 8.418 7.994 7.591 7.208 6.844 6.498
3
.
5.0 ppt
9.0 ppt
10.0 ppt
18.1 ppt
15.0 ppt
27.1 ppt
20.0 ppt
36.1 ppt
25.0 ppt
45.2 ppt
Salinity: 0
9.0 ppt
18.1 ppt
27.1 ppt
36.1 ppt
45.2 ppt
25.0 8.263 7.850 7.457 7.093 6.728 6.390
26.0 8.113 7.711 7.327 6.962 6.615 6.285
27.0 7.968 7.575 7.201 6.845 6.506 6.184
28.0 7.827 7.444 7.079 6.731 6.400 6.085
29.0 7.691 7.317 6.961 6.621 6.297 5.990
30.0 7.559 7.194 6.845 6.513 6.197 5.896
31.0 7.430 7.073 6.733 6.409 6.100 5.806
32.0 7.305 6.957 6.624 6.307 6.005 5.717
33.0 7.183 6.843 6.518 6.208 5.912 5.631
34.0 7.065 6.732 6.415 6.111 5.822 5.546
35.0 6.950 6.624 6.314 6.017 5.734 5.464
36.0 6.837 6.519 6.215 5.925 5.648 5.384
37.0 6.727 6.416 6.119 5.835 5.564 5.305
38.0 6.620 6.316 6.025 5.747 5.481 5.228
39.0 6.515 6.217 5.932 5.660 5.400 5.152
40.0 6.412 6.121 5.842 5.576 5.321 5.078
41.0 6.312 6.026 5.753 5.493 5.243 5.005
42.0 6.213 5.934 5.667 5.411 5.167 4.993
43.0 6.116 5.843 5.581 5.331 5.091 4.861
44.0 6.021 5.753 5.497 5.252 5.017 4.793
45.0 5.927 5.665 5.414 5.174 4.944 4.724
46.0 5.835 5.578 5.333 5.097 4.872 4.656
47.0 5.744 5.493 5.252 5.021 4.801 4.589
48.0 5.654 5.408 5.172 4.947 4.730 4.523
49.0 5.565 5.324 5.094 4.872 4.660 4.457
50.0 5.477 5.242 5.016 4.799 4.591 4.392
Page 41

74
75
A P P E N D I X B C A L I B R A T I O N
VA L U E S F O R VA R I O U S
A T M O S P H E R I C P R E S S U R E S A N D
ALTITUDES
This table is also used to correct mg/L for the local barometric pressure of
older, box style instruments that did not report % Saturation.
Pressure Altitude Calibration
value
Inches Hg mm Hg kPA mbar Feet Meters % Saturation
30.23 768 102.4 1023 -276 -84 101
29.92 760 101.3 1013 0 0 100
29.61 752 100.3 1002 278 85 99
29.33 745 99.3 993 558 170 98
29.02 737 98.3 983 841 256 97
28.74 730 97.3 973 1126 343 96
28.43 722 96.3 963 1413 431 95
28.11 714 95.2 913 1703 519 94
27.83 707 94.2 944 1995 608 93
27.52 699 93.2 932 2290 698 92
27.24 692 92.3 923 2587 789 91
26.93 684 91.2 912 2887 880 90
26.61 676 90.1 901 3190 972 89
26.34 669 89.2 892 3496 1066 88
26.02 661 88.1 881 3804 1106 87
25.75 654 87.2 872 4115 1254 86
25.43 646 86.1 861 4430 1350 85
25.12 638 85.1 851 4747 1447 84
24.84 631 84.1 841 5067 1544 83
24.53 623 83.1 831 5391 1643 82
24.25 616 82.1 821 5717 1743 81
Pressure Altitude Calibration
value
Inches Hg mm Hg kPA mbar Feet Meters % Saturation
23.94 608 81.1 811 6047 1843 80
23.62 600 80.0 800 6381 1945 79
23.35 593 79.1 791 6717 2047 78
23.03 585 78.0 780 7058 2151 77
22.76 578 77.1 771 7401 2256 76
22.44 570 76.0 760 7749 2362 75
22.13 562 74.9 749 8100 2469 74
21.85 555 74.0 740 8455 2577 73
21.54 547 73.0 729 8815 2687 72
21.26 540 72.0 720 9178 2797 71
20.94 532 71.0 709 9545 2909 70
20.63 524 70.0 699 9917 3023 69
20.35 517 69.0 689 10293 3137 68
20.04 509 67.9 679 10673 3371 67
19.76 502 66.9 669 11058 3371 66
Page 42

REFERENCES
(1) South Carolina Department of Natural Resources – Waddell
Mariculture Center; Research funded by National Institute of
Standards and Technology.
(2) Standard Methods for the Examination of Water and Wastewater
(1998), 20th Edition. Published jointly by American Public
Health Association, American Water Works Association, and
Water Environmental Federation, Washington,
D.C.
(3) Wikipedia, The Free Encyclopedia. (2009, September 1).
Wikimedia Foundation, Inc. Retrieved September 17, 2009,
from http://en.wikipedia.org/wiki/Freshwater.
(4) Winkler, L.W. (1888). Die Bestimmung des in Wasser gelösten
Sauerstoffen. Berichte der Deutschen Chemichen Gesellschaft,
21, page 2843.
76
Page 43

The Dissolved Oxygen Handbook
©2009 YSI Incorporated
www.ysi.com/weknowdo
environmental@ysi.com
September 2009
W39 0909
$20.00
 Loading...
Loading...