Welch Allyn Euro-Med Rose Coated Electrosurgical Instrumentation User Manual
Directions for Use & Sterilization
Euro-Med
®
Rose Coated
Electrosurgical
Instrumentation
Part Number # 883421 • Rev. A
Skaneateles Falls, NY 13153 USA
Phone: (315) 685-4560
Toll Free: (800) 535-6663
Fax: (315) 685-3361
Skaneateles Falls, NY 13153 USA
Phone: (315) 685-4560
Toll Free: (800) 535-6663
Fax: (315) 685-3361
NOTICE
To insure proper handling, read the following care and
maintenance instructions before using the Euro-Med
®
Rose Coated Electrosurgical Instrumentation.
ELECTROSURGICAL SPECULA PRODUCT #
Pederson
Medium • 114mm x 22mm 88350
Large • 121mm x 26mm 88352
Graves
Medium • 113mm x 33mm 88346
Large • 125mm x 35mm 88348
Prima Series
®
Medium • 113mm x 33mm 88338
Large • 125mm x 35mm 88340
ELECTROSURGICAL RETRACTORS
Cer-View
™
Lateral Wall Retractors
57mm x 22mm 88342
Working Length: 110mm
70mm x 22mm 88344
Working Length: 110mm
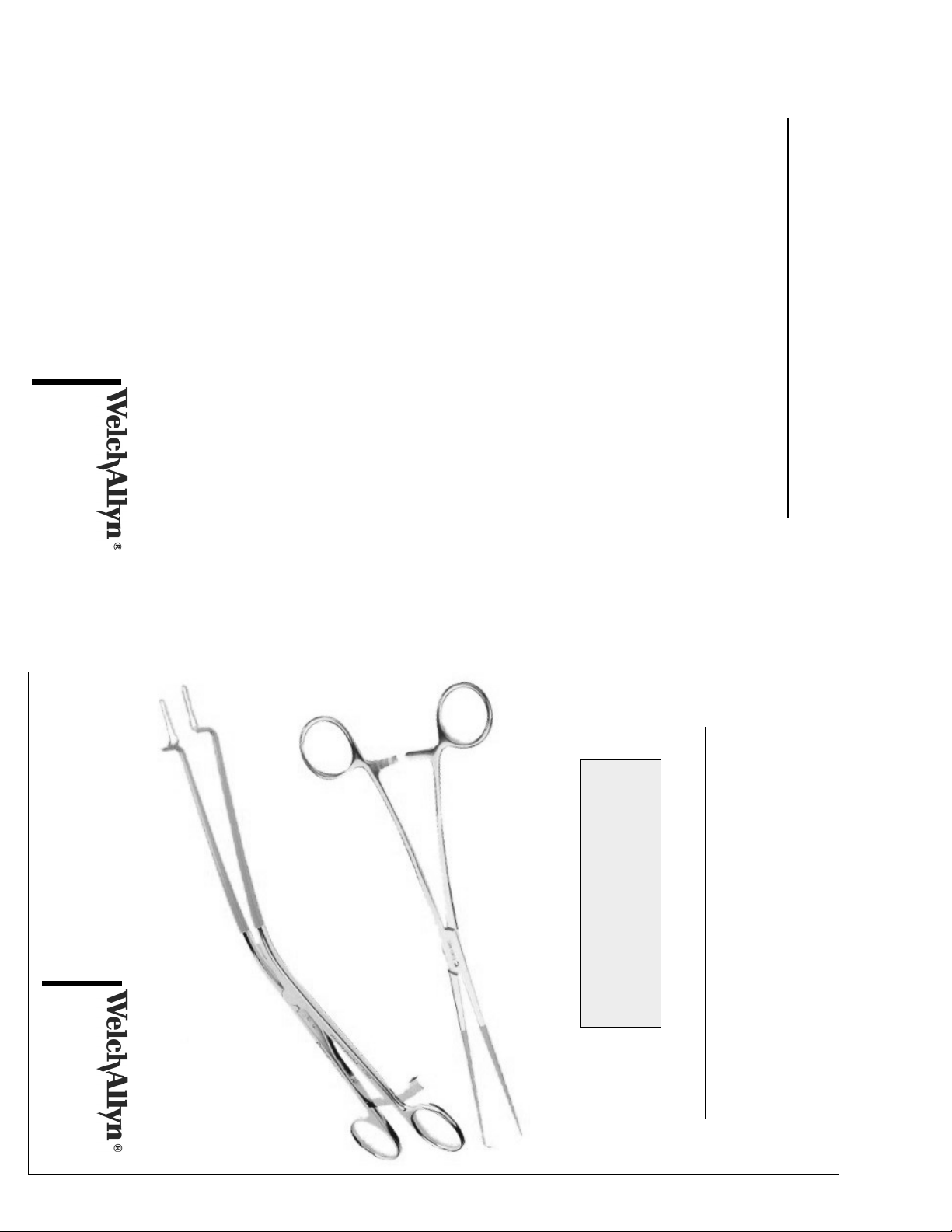
ELECTROSURGICAL INSTRUMENTATION
Campion Forceps 88354
Tissue Forceps 88358
Two-Prong Hook
Length: 260mm 88360
Single Tooth Tenaculum
Length: 240mm 88362
Ring Forceps
Length: 220mm 88356
ENDOCERVICAL SPECULA
Regular
Length: 240mm • Tip size: 6mm 88364
Medium
Length: 240mm • Tip size: 5mm 88366
Complete Product Listing
NOTE • Measurements listed below are Length x Width
Euro-Med
®
Rose Coated Electrosurgical Instrumentation

COLD SOAK STERILIZATION GUIDELINES
1
Thorough cleaning is essential prior to soaking. Failure to properly clean the instrument
prior to soaking may result in protein deposits. These deposits will give the appearance
of corrosion when hardened, and will impair sterilization.
2
Plastic soaking trays are recommended to avoid scratching of the instruments.
Plastic also has better material compatibility with metal instruments when sterilizing.
3
Use only a cold soak solution that has FDA clearance and EPA registration.
Solutions which contain 2% Glutaraldehyde are recommended.
4
Follow the solution manufacturer’s instructions to determine soaking time and
temperature for disinfecting or sterilizing the instrument.
5
Rinse the instruments in sterile water following cold soaking to adequately remove
any solution residue. Cold soak solutions may cause irritation to skin or tissue.
A minimum of two separate rinses with gentle agitation is recommended. A longer
rinse time also reduces the amount of solution residue.
ETO STERILIZATION GUIDELINES
Rose coated electrosurgery instruments are sterilizable using gas following the equipment
manufacturer’s recommendations and AAMI guideline ANSI/AAMI ST-41-1992 for
Good Hospital Practice: Ethylene Oxide Sterilization & Sterility Assurance.
SPECIAL NOTE ON ELECTROSURGICAL HOOKS
Since the coating used to insulate the instruments would significantly dull the
electrosurgical hooks, making them ineffective for their intended use, the tips are not
coated. As long as the exposed hook tip is completely embedded in tissue, no arcing
of the electrical current will occur.
PREPARATION AND PRECAUTIONS
1
Thoroughly examine the integrity of the Rose coating on all instrumentation before
performing every electrosurgical procedure. If any flaws such as cracks,
tears, or chips are seen, DO NOT USE THE INSTRUMENT. Electrode contact with
uncoated sections of instruments used in electrosurgery may result in an electrical shock.
Note: Coating wear at pivot points is considered normal in everyday use. Care must be taken during
the procedure to avoid contact with these areas.
2
NEVER scrub coated instruments with abrasive cleaners or stiff brushes. Use of
these cleaning methods may result in damage to the coating. Only use a soft
material or brush during cleaning procedures.
3
Doctors may use Rose coated electrosurgery instruments for other gynecology procedures.
4
DO NOT use ultrasonic cleaning methods.
5
DO NOT use a glass bead sterilizer.6DO NOT soak in bleach solution.
7
DO NOT use Rose coated electrosurgery instruments for laser procedures.
CLEANING AND STAIN REMOVAL
1
LUGOL’S SOLUTION PERMANENTLY STAINS the Rose coated instruments
unless the instruments are immediately cleaned and treated with StainEnder
™
or a similar solution
after use.
2
Wash the instruments immediately after use with hot soapy water. Rinse with hot water.
To minimize the potential of contamination of the StainEnder solution, use disinfectant
prior to inserting them in the cleaning solution.
3
Completely immerse the instrument in undiluted StainEnder. Allow the instrument to
soak until all stains are removed. Lugol’s not immediately removed from the instrument
may either require additional soaking time or result in permanent staining. Any permanent
Lugol’s stains will appear as a whitish residue after autoclaving.
4
StainEnder is not a disinfectant. The solution may become contaminated if the
instruments are not disinfected prior to immersion. If this is suspected, or if the solution
no longer removes stains, discard the solution following standard bio-hazard disposal
instructions.
AUTOCLAVE STERILIZATION GUIDELINES
All Rose coated electrosurgery instruments are autoclavable.
AAMI guideline SSSA 1988 for
Good Hospital Practice: Steam Sterilization & Sterility
Assurance
is recommended. It is recommended that a maximum temperature cycle
of 275°F be utilized, following the AAMI guidelines.
STERRAD STERILIZATION GUIDELINES
The Sterrad sterilization process is approved for these devices. Accomplish Sterrad
sterilization for electrosurgical coated intruments per the manufacturer’s recommended
procedure.
NOTICE
Over time and use, the coating at the hinge points will wear off due to friction at the contact areas. This will not
affect the safety or efficacy of the instrument. Exposure of base metal in these areas is expected and is not a
cause for rejection or return of the instrument. Operators should notice these points and avoid contact with them
during electrosurgical procedures.
TOP VIEW
SIDE VIEW
BACK VIEW
HINGE
POINT
HINGE
POINT
HINGE
POINT
HINGE
POINT
HINGE
POINT
1
2
STERILE EO
Contact Welch Allyn, Inc. at 1-800-535-6663 for information on instrument repair.
 Loading...
Loading...