Page 1
Disposable Blood Pressure Cuffs with FlexiPort* Technology
Directions for Use
For 30503-series and 30506-series Blood Pressure Cuffs with FlexiPort* Technology and Aneroid
Sphygmomanometers
Standards
The blood pressure cuffs are designed to function within the limits prescribed by:
• American National Standard ANSI/AAMI SP10, 2002, manual, electronic, or automated
sphygmomanometers (except for section 4.2.2a for size 13 disposable cuffs)
• Specification for noninvasive sphygmomanometers - Part 1: General Requirements: EN1060-1:1996
(except for section 9.3a for sizes 6, 7, 8, 9 disposable cuffs)
• Supplementary requirements for mechanical sphygmomanometers: EN1060-2:1996
• Supplementary requirements for electro-mechanical blood pressure measuring systems: EN 1060-3:1997
Introduction
Intended Use
Cardinal Health Blood Pressure Cuffs are noninvasive and are intended for use with manual and automated
noninvasive blood pressure measurement devices.
Contraindications
Cardinal Health Blood Pressure Cuffs are contraindicated for neonate use. Do not use on neonate patients.
Warnings
A warning statement in this manual identifies a condition or practice that, if not corrected or discontinued
immediately, could lead to injury, illness, or death.
Cautions
A caution statement in this manual identifies information within the manual to avoid equipment failure.
CAUTION: U.S. federal law restricts the blood pressure cuffs to sale by or on the order of a
physician or licensed healthcare practitioner. This device should be used by trained
personnel.
WARNINGS: Only use the cuff when visible artery index marker falls within the range markings
indicated on the cuff, otherwise erroneous readings may result.
Do not apply cuff to areas on patient where skin is delicate or damaged. Check cuff site frequently
for irritation.
Allow space for 1 to 2 fingers between patient and cuff.
Do not apply cuff to limbs used for IV infusion.
Minimize cuff movement and limb motion during readings.
Ensure an airtight seal at all connection points prior to use.
Intravenous Systems (IV) - Do not connect cuffs with Luer lock connectors to intravenous fluid
systems or air may enter patient.
CAUTIONS: Do not press cuff with a hot iron.
Do not inflate cuff unless the hook and loop is closed.
Do not allow foreign debris to ingress into tubes or port on cuff.
Do not use steam or heat to sterilize the cuff or tubing.
Do not exceed 250 mmHg with thigh disposable cuffs at or above 30° C (86° F).
Intravenous Systems (IV) - Do not connect cuffs with Luer lock connectors to intravenous fluid
systems or fluid may enter the cuff.
Disposable Blood Pressure Cuffs with FlexiPort* Technology
Directions for Use
For 30503-series and 30506-series Blood Pressure Cuffs with FlexiPort* Technology and Aneroid
Sphygmomanometers
Standards
The blood pressure cuffs are designed to function within the limits prescribed by:
• American National Standard ANSI/AAMI SP10, 2002, manual, electronic, or automated
sphygmomanometers (except for section 4.2.2a for size 13 disposable cuffs)
• Specification for noninvasive sphygmomanometers - Part 1: General Requirements: EN1060-1:1996
(except for section 9.3a for sizes 6, 7, 8, 9 disposable cuffs)
• Supplementary requirements for mechanical sphygmomanometers: EN1060-2:1996
• Supplementary requirements for electro-mechanical blood pressure measuring systems: EN 1060-3:1997
Introduction
Intended Use
Cardinal Health Blood Pressure Cuffs are noninvasive and are intended for use with manual and automated
noninvasive blood pressure measurement devices.
Contraindications
Cardinal Health Blood Pressure Cuffs are contraindicated for neonate use. Do not use on neonate patients.
Warnings
A warning statement in this manual identifies a condition or practice that, if not corrected or discontinued
immediately, could lead to injury, illness, or death.
Cautions
A caution statement in this manual identifies information within the manual to avoid equipment failure.
CAUTION: U.S. federal law restricts the blood pressure cuffs to sale by or on the order of a
physician or licensed healthcare practitioner. This device should be used by trained
personnel.
WARNINGS: Only use the cuff when visible artery index marker falls within the range markings
indicated on the cuff, otherwise erroneous readings may result.
Do not apply cuff to areas on patient where skin is delicate or damaged. Check cuff site frequently
for irritation.
Allow space for 1 to 2 fingers between patient and cuff.
Do not apply cuff to limbs used for IV infusion.
Minimize cuff movement and limb motion during readings.
Ensure an airtight seal at all connection points prior to use.
Intravenous Systems (IV) - Do not connect cuffs with Luer lock connectors to intravenous fluid
systems or air may enter patient.
CAUTIONS: Do not press cuff with a hot iron.
Do not inflate cuff unless the hook and loop is closed.
Do not allow foreign debris to ingress into tubes or port on cuff.
Do not use steam or heat to sterilize the cuff or tubing.
Do not exceed 250 mmHg with thigh disposable cuffs at or above 30° C (86° F).
Intravenous Systems (IV) - Do not connect cuffs with Luer lock connectors to intravenous fluid
systems or fluid may enter the cuff.
Disposable Blood Pressure Cuffs with FlexiPort* Technology
Directions for Use
For 30503-series and 30506-series Blood Pressure Cuffs with FlexiPort* Technology and Aneroid
Sphygmomanometers
Standards
The blood pressure cuffs are designed to function within the limits prescribed by:
• American National Standard ANSI/AAMI SP10, 2002, manual, electronic, or automated
sphygmomanometers (except for section 4.2.2a for size 13 disposable cuffs)
• Specification for noninvasive sphygmomanometers - Part 1: General Requirements: EN1060-1:1996
(except for section 9.3a for sizes 6, 7, 8, 9 disposable cuffs)
• Supplementary requirements for mechanical sphygmomanometers: EN1060-2:1996
• Supplementary requirements for electro-mechanical blood pressure measuring systems: EN 1060-3:1997
Introduction
Intended Use
Cardinal Health Blood Pressure Cuffs are noninvasive and are intended for use with manual and automated
noninvasive blood pressure measurement devices.
Contraindications
Cardinal Health Blood Pressure Cuffs are contraindicated for neonate use. Do not use on neonate patients.
Warnings
A warning statement in this manual identifies a condition or practice that, if not corrected or discontinued
immediately, could lead to injury, illness, or death.
Cautions
A caution statement in this manual identifies information within the manual to avoid equipment failure.
CAUTION: U.S. federal law restricts the blood pressure cuffs to sale by or on the order of a
physician or licensed healthcare practitioner. This device should be used by trained
personnel.
WARNINGS: Only use the cuff when visible artery index marker falls within the range markings
indicated on the cuff, otherwise erroneous readings may result.
Do not apply cuff to areas on patient where skin is delicate or damaged. Check cuff site frequently
for irritation.
Allow space for 1 to 2 fingers between patient and cuff.
Do not apply cuff to limbs used for IV infusion.
Minimize cuff movement and limb motion during readings.
Ensure an airtight seal at all connection points prior to use.
Intravenous Systems (IV) - Do not connect cuffs with Luer lock connectors to intravenous fluid
systems or air may enter patient.
CAUTIONS: Do not press cuff with a hot iron.
Do not inflate cuff unless the hook and loop is closed.
Do not allow foreign debris to ingress into tubes or port on cuff.
Do not use steam or heat to sterilize the cuff or tubing.
Do not exceed 250 mmHg with thigh disposable cuffs at or above 30° C (86° F).
Intravenous Systems (IV) - Do not connect cuffs with Luer lock connectors to intravenous fluid
systems or fluid may enter the cuff.
Disposable Blood Pressure Cuffs with FlexiPort* Technology
Directions for Use
For 30503-series and 30506-series Blood Pressure Cuffs with FlexiPort* Technology and Aneroid
Sphygmomanometers
Standards
The blood pressure cuffs are designed to function within the limits prescribed by:
• American National Standard ANSI/AAMI SP10, 2002, manual, electronic, or automated
sphygmomanometers (except for section 4.2.2a for size 13 disposable cuffs)
• Specification for noninvasive sphygmomanometers - Part 1: General Requirements: EN1060-1:1996
(except for section 9.3a for sizes 6, 7, 8, 9 disposable cuffs)
• Supplementary requirements for mechanical sphygmomanometers: EN1060-2:1996
• Supplementary requirements for electro-mechanical blood pressure measuring systems: EN 1060-3:1997
Introduction
Intended Use
Cardinal Health Blood Pressure Cuffs are noninvasive and are intended for use with manual and automated
noninvasive blood pressure measurement devices.
Contraindications
Cardinal Health Blood Pressure Cuffs are contraindicated for neonate use. Do not use on neonate patients.
Warnings
A warning statement in this manual identifies a condition or practice that, if not corrected or discontinued
immediately, could lead to injury, illness, or death.
Cautions
A caution statement in this manual identifies information within the manual to avoid equipment failure.
CAUTION: U.S. federal law restricts the blood pressure cuffs to sale by or on the order of a
physician or licensed healthcare practitioner. This device should be used by trained
personnel.
WARNINGS: Only use the cuff when visible artery index marker falls within the range markings
indicated on the cuff, otherwise erroneous readings may result.
Do not apply cuff to areas on patient where skin is delicate or damaged. Check cuff site frequently
for irritation.
Allow space for 1 to 2 fingers between patient and cuff.
Do not apply cuff to limbs used for IV infusion.
Minimize cuff movement and limb motion during readings.
Ensure an airtight seal at all connection points prior to use.
Intravenous Systems (IV) - Do not connect cuffs with Luer lock connectors to intravenous fluid
systems or air may enter patient.
CAUTIONS: Do not press cuff with a hot iron.
Do not inflate cuff unless the hook and loop is closed.
Do not allow foreign debris to ingress into tubes or port on cuff.
Do not use steam or heat to sterilize the cuff or tubing.
Do not exceed 250 mmHg with thigh disposable cuffs at or above 30° C (86° F).
Intravenous Systems (IV) - Do not connect cuffs with Luer lock connectors to intravenous fluid
systems or fluid may enter the cuff.
Page 2
Operation
Use the Cardinal Health Blood Pressure Cuff the same as a traditional blood pressure cuff. These cuffs work
with one-tube or two-tube manual and automated sphygmomanometers.
Maintenance
Clean as required.
Cleaning (Tubing and port fittings)
Use one or more of the following methods and allow to air dry:
• Wipe with mild detergent and water solution (1:9 solution). Rinse.
• Wipe with Enzol** cleanser per manufacturer's instructions. Rinse.
• Wipe with 0.5% bleach and water solution. Rinse.
• Wipe with 70% isopropyl alcohol.
Gauge: Clean the manometer with a slightly dampened cloth or alcohol wipe.
FlexiPort* Connections:
*Trademark of Welch Allyn, Inc.
**Registered trademark of Johnson & Johnson Corporation.
CARDINAL HEALTH, and the Cardinal Health LOGO are trademarks or registered trademarks of
Cardinal Health. All other marks are the property of their respective owners.
CAUTION: Examine for physical integrity after cleaning.
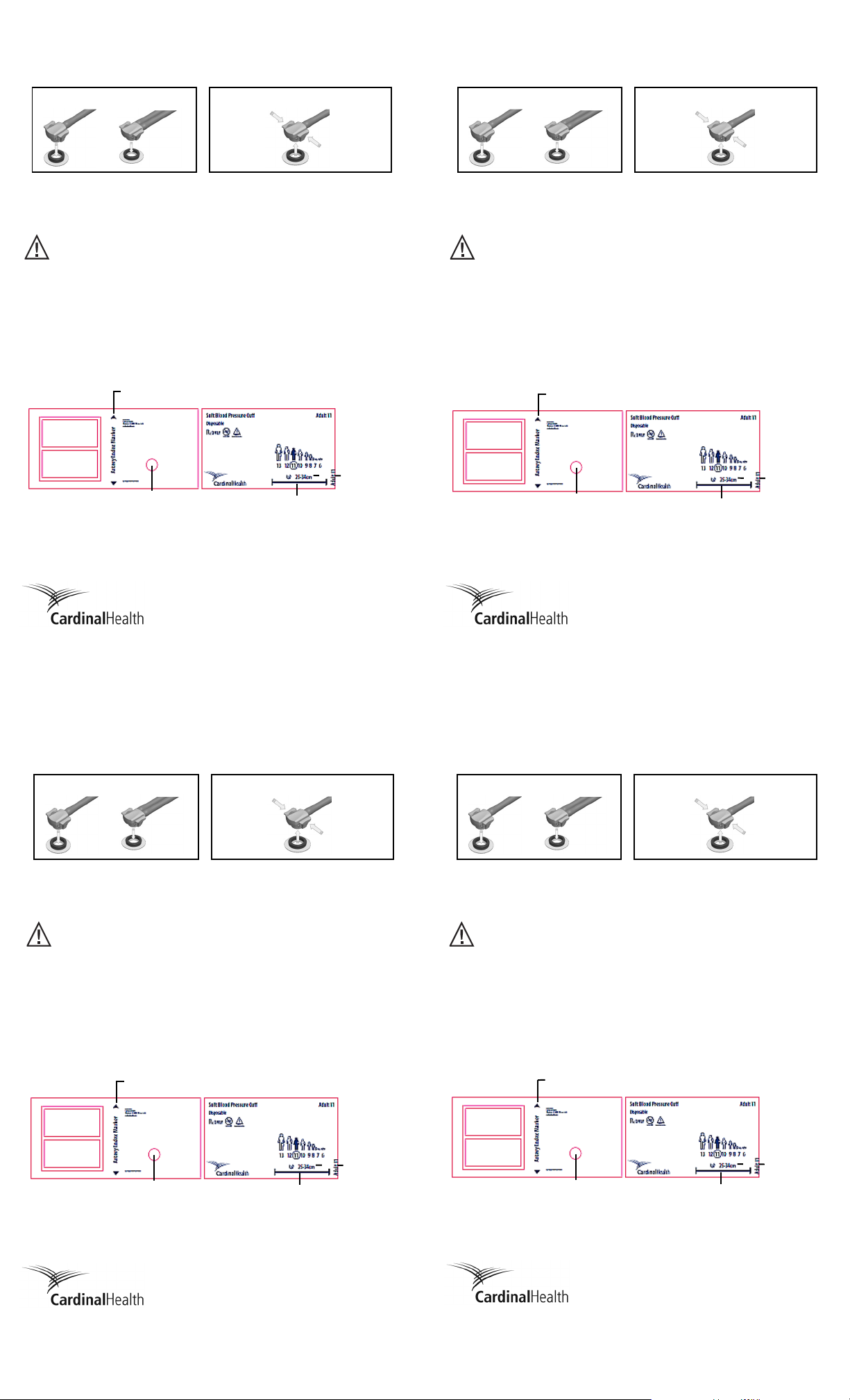
Snap the FlexiPort* fitting into the cuff port. Squeeze the tabs and pull the fitting off the cuff.
Port
Artery range marker
Artery index marker
Limb
circumference
Operation
Use the Cardinal Health Blood Pressure Cuff the same as a traditional blood pressure cuff. These cuffs work
with one-tube or two-tube manual and automated sphygmomanometers.
Maintenance
Clean as required.
Cleaning (Tubing and port fittings)
Use one or more of the following methods and allow to air dry:
• Wipe with mild detergent and water solution (1:9 solution). Rinse.
• Wipe with Enzol** cleanser per manufacturer's instructions. Rinse.
• Wipe with 0.5% bleach and water solution. Rinse.
• Wipe with 70% isopropyl alcohol.
Gauge: Clean the manometer with a slightly dampened cloth or alcohol wipe.
FlexiPort* Connections:
*Trademark of Welch Allyn, Inc.
**Registered trademark of Johnson & Johnson Corporation.
CARDINAL HEALTH, and the Cardinal Health LOGO are trademarks or registered trademarks of
Cardinal Health. All other marks are the property of their respective owners.
CAUTION: Examine for physical integrity after cleaning.
Snap the FlexiPort* fitting into the cuff port. Squeeze the tabs and pull the fitting off the cuff.
Port
Artery range marker
Artery index marker
Limb
circumference
Operation
Use the Cardinal Health Blood Pressure Cuff the same as a traditional blood pressure cuff. These cuffs work
with one-tube or two-tube manual and automated sphygmomanometers.
Maintenance
Clean as required.
Cleaning (Tubing and port fittings)
Use one or more of the following methods and allow to air dry:
• Wipe with mild detergent and water solution (1:9 solution). Rinse.
• Wipe with Enzol** cleanser per manufacturer's instructions. Rinse.
• Wipe with 0.5% bleach and water solution. Rinse.
• Wipe with 70% isopropyl alcohol.
Gauge: Clean the manometer with a slightly dampened cloth or alcohol wipe.
FlexiPort* Connections:
*Trademark of Welch Allyn, Inc.
**Registered trademark of Johnson & Johnson Corporation.
CARDINAL HEALTH, and the Cardinal Health LOGO are trademarks or registered trademarks of
Cardinal Health. All other marks are the property of their respective owners.
CAUTION: Examine for physical integrity after cleaning.
Snap the FlexiPort* fitting into the cuff port. Squeeze the tabs and pull the fitting off the cuff.
Port
Artery range marker
Artery index marker
Limb
circumference
Operation
Use the Cardinal Health Blood Pressure Cuff the same as a traditional blood pressure cuff. These cuffs work
with one-tube or two-tube manual and automated sphygmomanometers.
Maintenance
Clean as required.
Cleaning (Tubing and port fittings)
Use one or more of the following methods and allow to air dry:
• Wipe with mild detergent and water solution (1:9 solution). Rinse.
• Wipe with Enzol** cleanser per manufacturer's instructions. Rinse.
• Wipe with 0.5% bleach and water solution. Rinse.
• Wipe with 70% isopropyl alcohol.
Gauge: Clean the manometer with a slightly dampened cloth or alcohol wipe.
FlexiPort* Connections:
*Trademark of Welch Allyn, Inc.
**Registered trademark of Johnson & Johnson Corporation.
CARDINAL HEALTH, and the Cardinal Health LOGO are trademarks or registered trademarks of
Cardinal Health. All other marks are the property of their respective owners.
CAUTION: Examine for physical integrity after cleaning.
Snap the FlexiPort* fitting into the cuff port. Squeeze the tabs and pull the fitting off the cuff.
Port
Artery range marker
Artery index marker
Limb
circumference
Distributed by:
Cardinal Health
Waukegan, IL 60085 USA Rev. D 6/10
cardinalhealth.com
711444 Ver. B 2011/04
Distributed by:
Cardinal Health
Waukegan, IL 60085 USA Rev. D 6/10
cardinalhealth.com
711444 Ver. B 2011/04
Distributed by:
Cardinal Health
Waukegan, IL 60085 USA Rev. D 6/10
cardinalhealth.com
711444 Ver. B 2011/04
Distributed by:
Cardinal Health
Waukegan, IL 60085 USA Rev. D 6/10
cardinalhealth.com
711444 Ver. B 2011/04
 Loading...
Loading...