Page 1
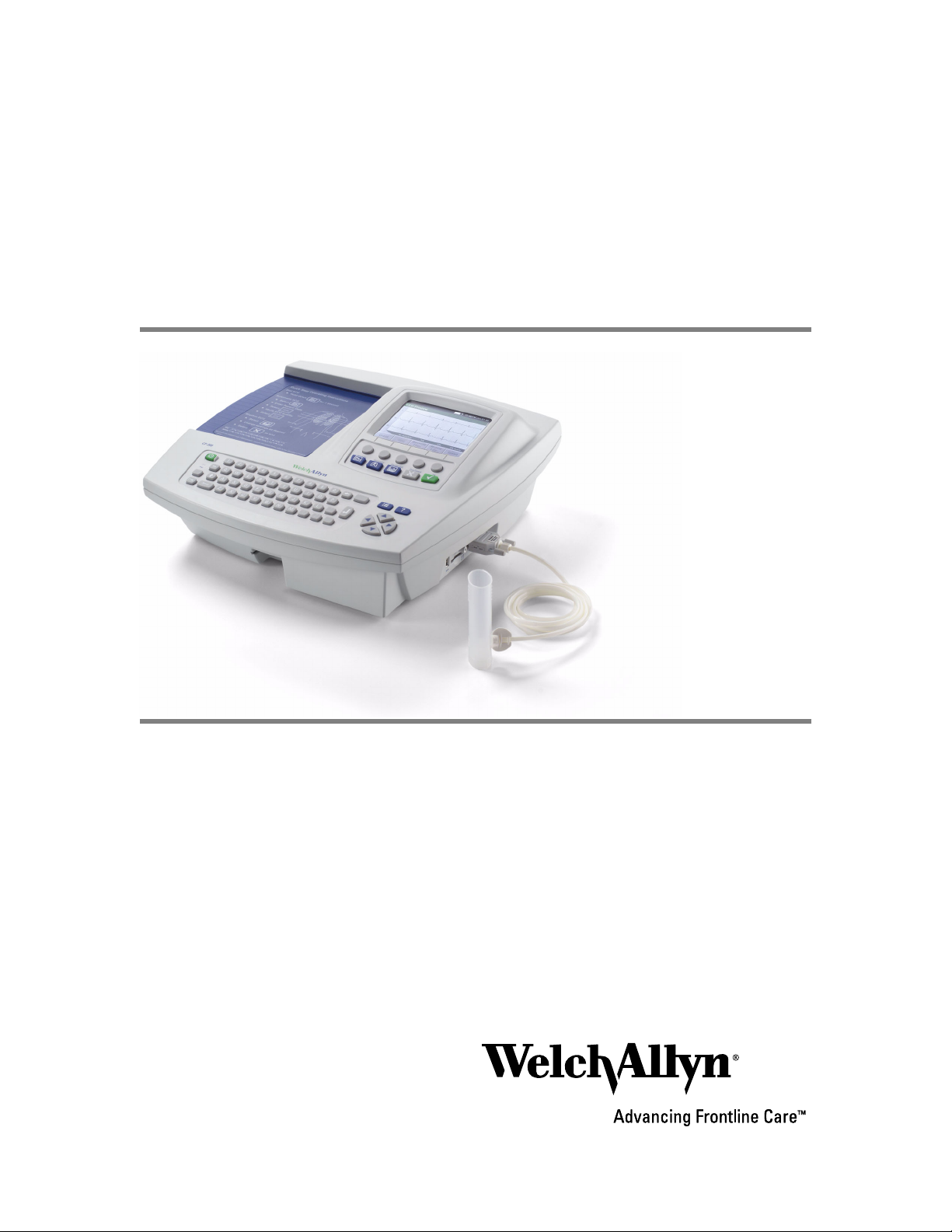
CP 200™ Spirometry Option
Directions for Use
Page 2

ii Welch Allyn CP 200 Spirometry Option
Copyright 2005, Welch Allyn, Inc. All rights are reserved. No one is permitted to reproduce or duplicate, in
any form, this manual or any part thereof without permission from Welch Allyn.
Caution: Federal US law restricts sale of the device identified in this manual to, or on the order of, a
licensed physician.
Welch Allyn assumes no responsibility for any injury, or for any illegal or improper use of the product, that
may result from failure to use this product in accordance with the instructions, cautions, warnings, or
indications for use published in this manual.
Welch Allyn is a registered trademark of Welch Allyn, Inc., and CP 200 and CardioPerfect are trademarks of
Welch Allyn, Inc.
SD is a trademark of Toshiba.
Software in this product is Copyright 2005, Welch Allyn, Inc., or its vendors. All rights are reserved. The
software is protected by United States of America copyright laws and international treaty provisions
applicable worldwide. Under such laws, the licensee is entitled to use the copy of the software
incorporated within this instrument as intended in the operation of the product in which it is embedded.
The software may not be copied, decompiled, reverse-engineered, disassembled or otherwise reduced to
human-perceivable form. This is not a sale of the software or any copy of the software; all right, title and
ownership of the software remains with Welch Allyn or its vendors.
For information about any Welch Allyn product, please call Welch Allyn Technical Support:
USA 1 800 535 6663
+ 1 315 685 4560
Canada 1 800 561 8797 China + 86 216 327 9631
European Call Center + 353 46 906 7790 France + 33 15 569 5849
Germany + 49 747 792 7186 Japan + 81 33 219 0071
Latin America + 1 315 685 2644 Netherlands + 31 15 750 5000
Singapore + 65 6419 8100 South Africa + 27 11 777 7555
United Kingdom + 44 207 365 6780 Sweden + 46 85 853 6551
Australia + 61 29 638 3000
800 074 793
Reorder Number (multi-language CD): 401151
Mat. Number (manual only): 703411, Ver: C
Welch Allyn
4341 State Street Road, PO Box 220
Skaneateles Falls, NY 13153-0220
www.welchallyn.com
Printed in USA
Page 3

Contents
1 - Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1
iii
About This Manual . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
Product Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
Intended Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
Indications for Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
Contraindications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
Features . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
Symbols . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
Using the Spirometer Safely . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
General Warnings. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
General Cautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
Ordering Information for Replacement Parts . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
Getting Help . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10
2 - Reviewing the Spirometry Settings . . . . . . . . . . . . . . . . . . . . . . . . . 11
“Spirometry Settings” Menu Tree . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
Reviewing the Operation Settings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
Reviewing the Calibration Settings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
Reviewing the Spirometry Screen Settings . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16
Reviewing the Spirometry Print Settings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
Reviewing the Patient Data Fields Available . . . . . . . . . . . . . . . . . . . . . . . . . . . . 19
Reviewing the Interpretation List . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20
Reviewing the Auto Send Setting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
3 - Calibrating the Spirometer . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23
About Calibration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 24
Performing a Calibration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 26
Printing Calibration Reports . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 31
4 - Performing Spirometry Tests . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 33
Overview of the Testing Process . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 34
About FVC Efforts. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 35
About SVC Efforts. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 36
About the Spirometry Parameters . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 37
About Pre- and Post-Testing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 38
About Effort Replacement . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 38
Page 4

iv Contents Welch Allyn CP 200 Spirometry Option
Connecting the Spirometer Components . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 39
Preparing the Patient . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 40
Recording a Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 41
Working With a Completed Test. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 45
5 - Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 51
Problem-Solving Suggestions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 52
Limited Warranty . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 53
Service Policy . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 53
A - Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 55
B - Spirometry Protocols . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 57
C - Patient Help Sheets. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 63
D - Predictive Norms, etc. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 67
Glossary . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 77
Index . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 83
Page 5
1
1
Introduction
About This Manual. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
Product Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
Intended Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
Indications for Use. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
Contraindications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
Features. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
Symbols . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
Using the Spirometer Safely . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
Ordering Information for Replacement Parts. . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
Getting Help. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10
Page 6

2 Chapter 1 Introduction Welch Allyn CP 200 Spirometry Option
About This Manual
This manual is written for clinical professionals performing pulmonary function testing.
Users must be familiar with measurements and the clinical significance of basic
spirometry products.
Before using the spirometer, all users and technicians must read and understand this
manual and all other information accompanying the CP 200 spirometry option and the
CP 200 electrocardiograph.
Caregivers need to know how to properly coach patients, to recognize acceptable
waveforms, and to know whether results meet ATS reproducibility criteria.
The hospital's Biomedical/IT support staff shall require primary skills including disciplines
related to maintenance and servicing computer controls/platforms.
It is recommended that users attend a certified spirometry training course. The
instructions given here are only a guide and should not be used to train a technician.
For definitions of specialized terms and abbreviations used in this manual, see “Glossary”
on page 77.
Note
This manual supplements the CP 200 electrocardiograph manual, entitled
CP 200 12-Lead Resting Electrocardiograph Directions for Use.
See the electrocardiograph manual for procedures that are common to both ECG
and spirometry functions, such as how to move through the menus, how to
search for patient data, or how to edit the medication list.
Page 7
Directions for Use Chapter 1 Introduction 3
Product Overview
The CP 200 spirometry option performs FVC and SVC testing, including pre- and postbronchodilator testing. It displays flow/volume and volume/time curves in real time,
depicting both inspiratory and expiratory measurements.
For details, see the following sections:
• “Features” on page 5
• “Ordering Information for Replacement Parts” on page 9
• “Specifications” on page 55
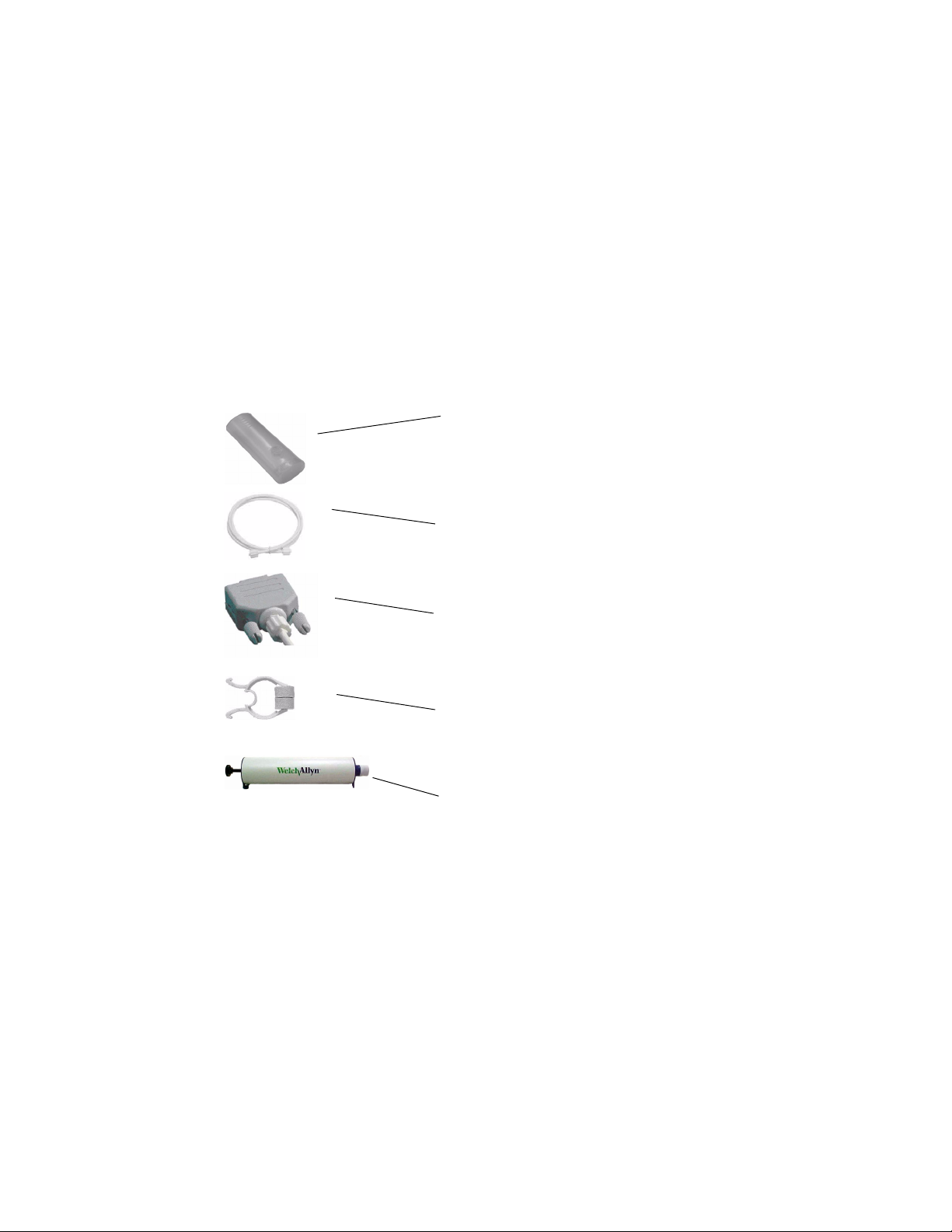
Figure 1. Components of the CP 200 Spirometry Option
Disposable flow transducer
For single patient use. Eliminates the need for disinfecting
procedures, which can be difficult and expensive.
Minimizes the risk of cross-contamination.
Pressure tubing
Connects the flow transducer to the sensor.
Sensor
Connects to the CP 200 electrocardiograph.
Converts pressure to air flow.
Nose clip
Recommended during testing to avoid leaks.
Three-liter calibration syringe
For daily use to calibrate the spirometer for accuracy.
Page 8

4 Chapter 1 Introduction Welch Allyn CP 200 Spirometry Option
Intended Use
The CP 200 spirometry option allows the user to acquire, view, store, and print measures
and waveforms of pulmonary function including, but not limited to, maximal volume and
flow of air that can be moved in and out of a patient's lungs. These measures are used in
the diagnosis and monitoring of lung diseases and interventions for the treatment of
certain lung diseases.
The spirometer may be used with patients who are able to understand the instructions for
performing the test. However, normal values and interpretive results are not calculated
for children under the age of six.
Indications for Use
Spirometry is indicated for use in various common clinical situations:
• Assessing health status before a patient begins strenuous physical activity.
• Evaluating the following symptoms, signs, or abnormal laboratory tests:
Symptoms — dyspnea, wheezing, orthopnea, cough, phlegm production, chest pain
Signs — diminished breath sounds, overinflation, expiratory slowing, cyanosis, chest
deformity, unexplained crackles, shortness of breath
Abnormal laboratory tests — hypoxemia, hypercapnia, polycythemia, abnormal
chest radiographs
Contraindications
The spirometer does not support the MVV effort type.
Page 9

Directions for Use Chapter 1 Introduction 5
Features
• Automatic interpretation and comparison of best pre-bronchodilator effort to best
post-bronchodilator effort
• Real-time flow/volume and volume/time graphs on full-color LCD display
• Incentive graphic for pediatric patient coaching
• Multiple predictive adult norms, including NHANES III, and pediatric norms
• Reduced risk of cross-contamination with Welch Allyn single-use, disposable flow
transducers
• Patient education help sheets
• Instant quality and variability checks for proper test performance
• Customizable report formats
• Independently validated to exceed the 1994 American Thoracic Society spirometry
accuracy standards for both ambient and BTPS humidified air
• Single-flow and multiple-flow calibration protocols with automatic printing
• NIOSH, OSHA, and Social Security operation protocols to create reports that meet
these agency requirements
• PCP (primary care practitioner) protocol that follows NLHEP guidelines
• Meets all industry standards, including ATS, NIOSH, OSHA, and Social Security
• Integrated into the CardioPerfect workstation for easy analysis, reviewing, storing,
printing, and exporting
Page 10
6 Chapter 1 Introduction Welch Allyn CP 200 Spirometry Option
Symbols
The symbols illustrated here may appear on the spirometer components, on the
packaging, on the shipping container, or in this manual.
Documentation Symbols
WARNING Indicates conditions or practices that could lead to illness, injury, or
death.
Caution In the documentation, this symbol indicates conditions or practices
that could damage the equipment or other property.
Caution On the product, this symbol means “Caution — consult
accompanying documentation.”
Operation Symbols
Spirometry key Spirometry port
Stacking limits Do not reuse.
8
Keep away from sunlight. Expiration date
Type BF applied part
200x-xx
Page 11

Directions for Use Chapter 1 Introduction 7
Using the Spirometer Safely
Before using or servicing the spirometer, you must read and understand the following
safety-related information.
General Warnings
The following warning statements apply to spirometer use in general. Warning
statements that apply specifically to particular procedures, such as preparing the patient
for testing, appear in the corresponding sections of the manual.
Warning statements indicate conditions or practices that could lead to illness, injury, or
death.
WARNING Do not perform spirometry tests if any of the following conditions
apply to the patient:
• hemoptysis of unknown origin (forced expiratory maneuver may aggravate the underlying condition)
• pneumothorax
• unstable cardiovascular status (forced expiratory maneuver may worsen angina or cause changes in blood
pressure)
• recent myocardial infarction
• pulmonary embolus
• thoracic, abdominal, or cerebral aneurysms (danger of rupture due to increased thoracic pressure)
• recent eye surgery (for example, cataract)
• presence of an acute disease process that might interfere with test performance (for example, nausea,
vomiting)
• recent surgery of thorax or abdomen
WARNING The spirometer captures and presents data reflecting a patient’s
physiological condition. When reviewed by a trained physician or clinician, this
data can be useful in determining a diagnosis. However, the data should not be
used as a sole means for determining a patient’s diagnosis.
WARNING To minimize chance of a misdiagnosis, it is the physician’s
responsibility to assure that spirometry tests are properly administered,
evaluated, and interpreted.
WARNING To prevent the spread of infection, do not try to clean the flow
transducers and nose clips. Discard these items after a single patient use.
WARNING Read and observe all safety information provided in the flow
transducer instructions.
Page 12

8 Chapter 1 Introduction Welch Allyn CP 200 Spirometry Option
General Cautions
The following caution statements apply to spirometer use in general. Caution statements
that apply specifically to particular procedures appear in the corresponding sections of the
manual.
Caution statements indicate conditions or practices that could damage the equipment or
other property.
Caution Do not clean the spirometer or any of its components. Trapped
moisture in the pressure tubing or sensor could affect their accuracy. Replace
the pressure tubing when it becomes dirty. Replace the sensor when it becomes
faulty.
Caution Do not immerse any part of the spirometer into a cleaning liquid or
sterilize it with hot water, steam, or air.
Caution Do not use aromatic hydrocarbons, rubbing alcohol, or solvents on the
spirometer.
Caution If you choose to clean the calibration syringe, wipe its external
surfaces as needed with a cloth dampened with water only.
Caution Use only parts and accessories supplied with the device and available
through Welch Allyn. The use of accessories other than those specified may
result in degraded performance of this device.
Caution When you put the spirometer away, store its pressure tubing in a
basket or drawer or other place that prevents compression or kinking.
Caution Avoid installing the spirometer in direct sunlight or in a location where
it may be affected by significant changes in humidity, ventilation, or airborne
particles containing dust, salt, or sulfur.
Caution Keep the spirometer away from splashing fluids.
Page 13

Directions for Use Chapter 1 Introduction 9
Ordering Information for Replacement Parts
Replace the following parts as noted:
• flow transducers & nose clips — Replace for each new patient.
• pressure tubing — Replace when dirty.
• sensor — Replace when faulty.
To order parts, call Welch Allyn. For phone numbers, see page ii.
Note
Item Material Numbers Quantity
Disposable flow transducers 703418
Pressure tubing (2 meters) 703415 1
Sensor 703552 1
Nose clip 58550-0000 1
Calibration syringe (3 L) 703480 1
Germicidal Sani-Cloth ® canister 26004-0000 1
Product information
• Spirometry Reference Chart
• Spirometry Effort Acceptability & Reproducibility
• CP 200 Spirometry Option Quick Reference
Discard all spirometry components according to local regulations.
703419
71038-3000 1
(wall poster)
703337 1
(wall poster)
703977 1
(small card)
25
100
• CP 200 Spirometry Option Directions for Use 703411 1
• CP 200 product information multi-language CD 401151
1
Page 14

10 Chapter 1 Introduction Welch Allyn CP 200 Spirometry Option
Getting Help
You can get help with the CP 200 spirometry option in a variety of ways beyond this
manual.
• Press the Help key from the initial spirometry screen for a list of topics
available to print.
• Review the other information that came with the spirometer. For list, see “Product
information” on page 9.
• Contact Welch Allyn. For phone numbers, see page ii.
Page 15
11
2
Reviewing the Spirometry Settings
“Spirometry Settings” Menu Tree. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
Reviewing the Operation Settings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
Reviewing the Calibration Settings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
Reviewing the Spirometry Screen Settings. . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16
Reviewing the Spirometry Print Settings. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
Reviewing the Patient Data Fields Available . . . . . . . . . . . . . . . . . . . . . . . . . . . . 19
Reviewing the Interpretation List . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20
Reviewing the Auto Send Setting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
Note
You can access the spirometry settings only if the spirometer is connected.
See “Connecting the Spirometer Components” on page 39.
Page 16
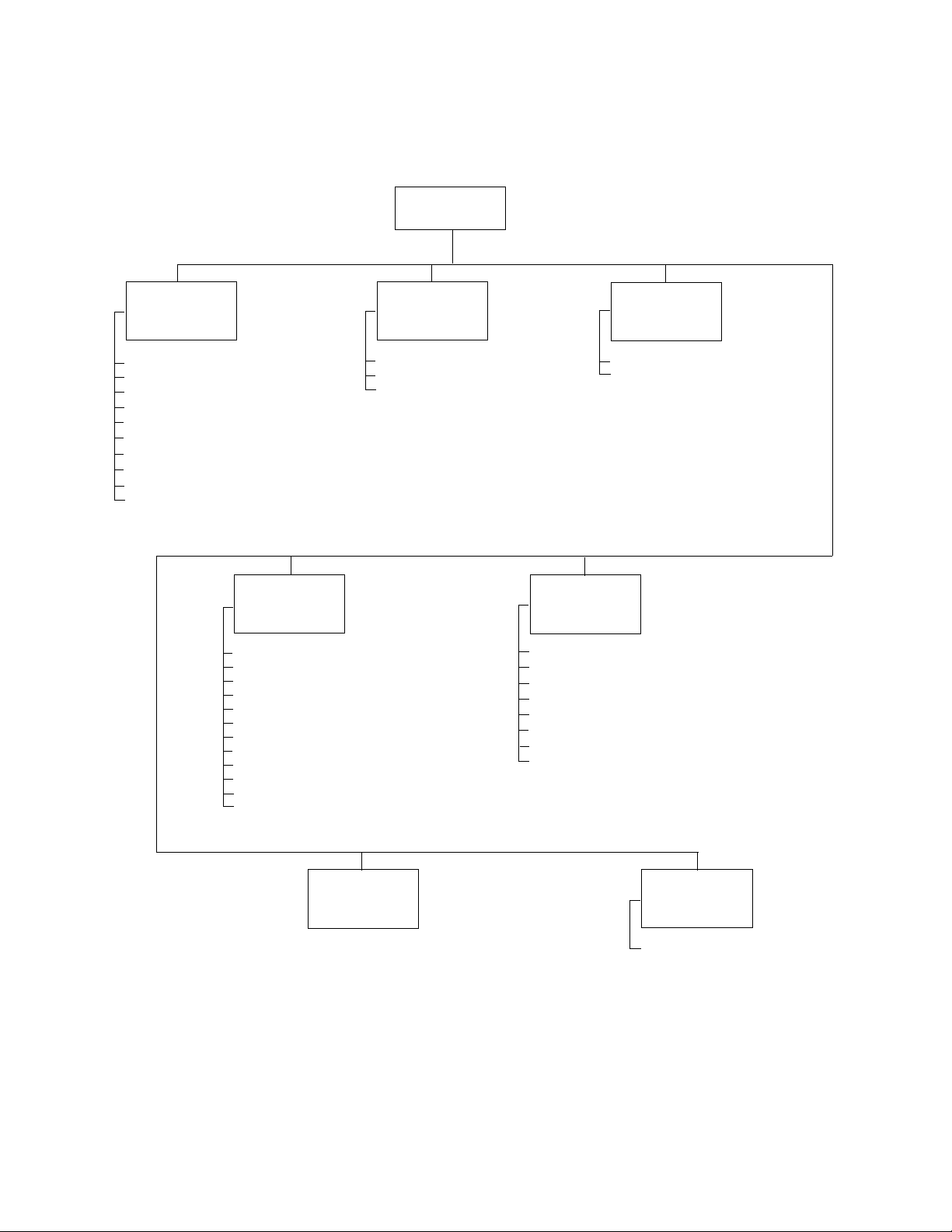
12 Chapter 2 Reviewing the Spirometry Settings Welch Allyn CP 200 Spirometry Option
“Spirometry Settings” Menu Tree
Spirometry
Settings
Operation
Settings
Select Protocol
Select Adult Predictive Norm
Select Ped. Predictive Norm
Select Best Effort Formula
Select FVC Reversibility Formula
Select FEV1% Formula
Enable Predictive Points
Enable Predictive Curve
Enable ATS Interp. Results
Enable Composite Norm Values
Settings
Select Efforts
Select FVC Curves
Select FVC Print Parameters
Select Scale
Print Lung Age
Print “Unconfirmed Report”
Print “Reviewed By”
Print “Patient Cooperation”
Print Quality Grades
Print Patient Education
Auto Print
Print
Calibration
Settings
Calibrate Spirometer
Enable Auto Calibration Report
Print Calibration Report
Screen
Settings
Select Default FVC Curve
Select FVC Display Parameters
Patient Data
Settings
First Name
Second Last Name
Middle Initial
Age/Birth Date
Weight
Medication
History
Comments
Edit
Interpretation
List
Note:
As part of spirometry setup, you can also go to the System Settings > Device Configuration menu
and select the following spirometry-related units of measure.
• Flow: L/sec or L/min (units for the y-axis on flow/volume curves)
• Pressure: mmHg, mbar, inHg, kPa (units for the calibration menu’s atmospheric pressure values)
• Temperature: Fahrenheit or Celsius (units for the calibration menu’s temperature values)
For details, see the electrocardiograph manual.
Communication
Settings
Auto Send
Page 17
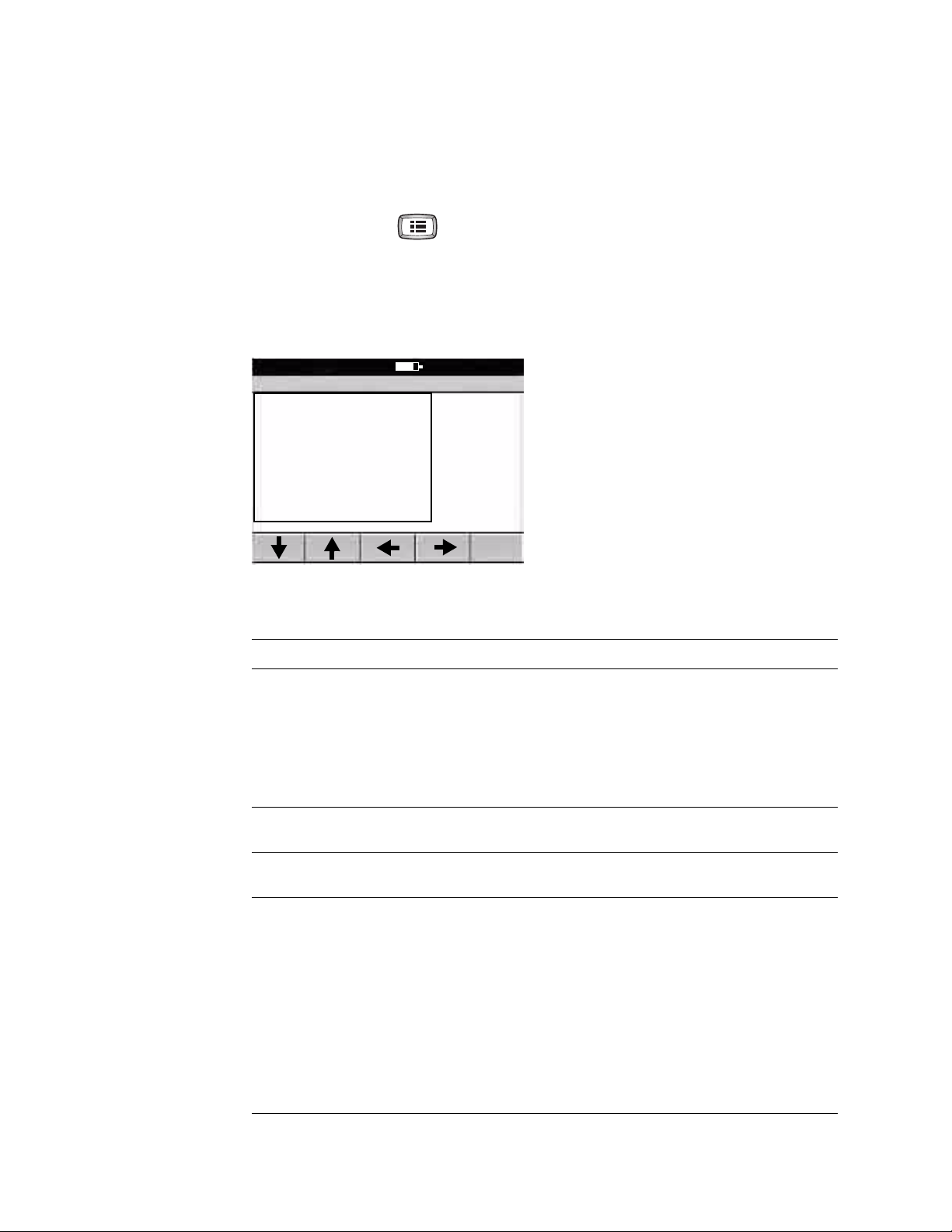
Directions for Use Chapter 2 Reviewing the Spirometry Settings 13
Reviewing the Operation Settings
To review or change the settings that affect the overall operation of the spirometer,
reflected both on screen and in print, follow these steps.
1. Press the Menu key .
2. Choose Spirometry Settings > Operation Settings.
The following screen appears.
Figure 2. “Spirometry Operation Settings” Screen
Spirometry Operation Settings
1 Select Protocol
2 Select Adult Predictive Norm
3 Select Ped. Predictive Norm
4 Select Best Effort Formula
5 Select FVC Reversibility Formula
6 Select FEV1% Formula
7 Enable Predictive Points
8 Enable Predictive Curve
9 Enable ATS Interp. Results
A Enable Composite Norm Values
9:17AM Oct 16 05
3. If desired, change the settings.
Setting Description
Select Protocol The selected protocol determines the way the spirometer operates when testing a patient.
Select Adult
Predictive Norm
Select Ped.
Predictive Norm
Select Best Effort
Formula
Applicable for FVC testing only. For details, see “Spirometry Protocols” on page 57.
•None
• PCP (primary care practitioner)
•NIOSH
• OSHA
• SSD (Social Security & Disability)
The selected adult norm is the primary source of predictive values for adult patients.
For details, see “Norm Profiles” on page 68.
The selected pediatric norm is the primary source of predictive values for pediatric patients.
For details, see “Norm Profiles” on page 68.
A patient’s best effort is a measurement calculated from a set of efforts. To determine the
way in which best effort is calculated, choose from these options:
• Best Measurement —
Defines best effort as the single best effort in a set of efforts (best FVC-pre, best FVCpost, best SVC). This ATS-recommended method uses the effort with the highest sum
of FVC + FEV1, or the effort with the highest SVC value. (For details, see the document
noted in Reference 6 on page 76.)
• Best Composite —
Defines best effort as a composite of the highest parameter values across all selected
efforts (except FVC and FEV1, which are both selected from the highest sum of
FVC + FEV1.)
Page 18
14 Chapter 2 Reviewing the Spirometry Settings Welch Allyn CP 200 Spirometry Option
Setting Description (continued)
Select FVC
Reversibility Formula
Select FEV1%
Formula
Enable Predictive
Points
Enable Predictive
Curve
Enable ATS Interp.
Results
Reversibility is the percentage difference between pre-test and post-test data for FVC
testing. This measurement indicates the effect of medication on lung function. Reversibility
applies to each parameter separately.
To determine the way in which reversibility is calculated, choose from these options:
• ((Post-Pre)/Pre)*100
• (Post/Pre)*100
• ((Post-Pre)/Predictive)*100
The FEV1% formula determines the calculation method for a test’s (not an effort’s) overall
FEV1% value, which affects the automatic interpretation. The variable part of this formula
is the denominator. Both the numerator and the denominator represent best effort values.
To determine the way in which FEV1% is calculated, choose from these options:
• FVC (FEV1% = FEV1/FVC
• FIVC (FEV1% = FEV1/FIVC
• FEV6 (FEV1% = FEV1/FEV6
• Max (FVC, FIVC, SVC) (FEV1% = FEV1/FVC
Yes or no. If yes, predictive points display and print. Predictive points may be enabled with
or without the predictive curve. For details, see “predictive points” on page 80.
Yes or no. If yes, a curve displays and prints along the predictive points. When the curve is
enabled, the points are automatically also enabled.
Yes or no. If yes, ATS interpretative results are included in the test record. For details, see
“ATS interpretive results” on page 77.
)
)
)
or FIVC or SVC, whichever is largest)
Enable Composite
Norm Values
Yes or no. If yes, any parameters that are not supported in the primary (selected) norm are
given predictive values from alternative (composite) norm sources.
If set to no, only the primary norm’s values are used, no composite values. On the screen
and in reports, any unsupported parameters appear without predictive values.
For details, see “About Race Adjustment” on page 70.
Page 19

Directions for Use Chapter 2 Reviewing the Spirometry Settings 15
Reviewing the Calibration Settings
To review or change the settings that affect calibration — or to calibrate the spirometer —
follow these steps.
1. Press the Menu key .
2. Choose Spirometry Settings > Calibration Settings.
The following screen appears.
Figure 3. “Spirometry Calibration Settings” Screen
Spirometry Calibration Settings
1 Calibrate Spirometer
2 Enable Auto Calibration Report
3 Print Calibration Report
0 Previous Menu
9:17AM Oct 16 05
3. Change any desired settings.
Setting Description
Calibrate Spirometer Brings up the Spirometer Calibration screen. See “Calibrating the
Enable Auto Calibration Report Yes or no. If yes, a calibration report prints automatically every time you accept
Print Calibration Report Prints the most recent calibration report.
Spirometer” on page 23.
calibration results.
Page 20
16 Chapter 2 Reviewing the Spirometry Settings Welch Allyn CP 200 Spirometry Option
Reviewing the Spirometry Screen Settings
The spirometry screen is the first screen that displays after you enter patient data.
For example, see Figure 29 on page 43. To review or change the settings for this screen,
follow these steps.
1. Press the Menu key .
2. Choose Spirometry Settings > Screen Settings.
The following screen appears.
Figure 4. “Spirometry Screen Settings” Screen
Spirometry Screen Settings
1 Select Default FVC Curve
2 Select FVC Display Parameters
0 Previous Menu
9:17AM Oct 16 05
3. Change any desired settings.
Setting Description
Select Default FVC Curve Choices: volume/time, flow/volume, tidal volume, incentive. The selected default
curve appears first whenever you begin FVC testing.
Select FVC Display
Parameters
Choose which FVC-test parameters to display during testing.
Choices (up to eight): FVC, FEV1, FEV1%, FEV6, PEF, FEF25-75, FEV0.5, FEV2, FEV3,
FEV5, FEV1/FEV6, FEV0.5%, FEV2%, FEV3%, FEV5%, FEV6%, FEF25, FEF50, FEF75,
FEF0.2-1.2, FEF75-85, FET, FIVC, FIV1, FIV1%, PIF, FIF50, FEF50/FIF50.
Note: For SVC testing, these parameters always display: SVC, ERV, IRV, VT, BF,
Tin /Tex.
Page 21
Directions for Use Chapter 2 Reviewing the Spirometry Settings 17
Reviewing the Spirometry Print Settings
To review or change the settings that affect printed spirometry reports, follow these
steps.
Note
FVC and SVC efforts appear in separate print reports, even when they belong to
the same test.
1. Press the Menu key .
2. Choose Spirometry Settings > Print Settings.
The following screen appears.
Figure 5. “Spirometry Print Settings” Screen
Spirometry Print Settings
1 Select Efforts
2 Select FVC Curves
3 Select FVC Print Parameters
4 Select Scale
5 Print Lung Age
6 Print “Unconfirmed Report”
7 Print “Reviewed By”
8 Print “Patient Cooperation”
9 Print Quality Grades
A Print Patient Education
9:17AM Oct 16 05
3. Change any desired settings.
Setting Description
Select Efforts Choose which efforts are included in printed reports by default. If desired, when printing a
test you can cycle through these choices and change the setting for that one test.
• All efforts
All efforts of each type performed.
• Three best efforts
The three efforts with the highest sum of FVC+FEV1.
• Only best effort
The best effort of each type performed
To learn how to change the definition of best effort, see “Select Best Effort Formula” on
page 13.
Page 22

18 Chapter 2 Reviewing the Spirometry Settings Welch Allyn CP 200 Spirometry Option
Setting (continued) Description (continued)
Select FVC Curves Choose which curve type to print for FVC efforts by default. If desired, you can change the
curve type before you print.
• volume/time
• flow/volume
• tidal volume
• V/T & F/V (both volume/time and flow/volume)
• V/T & display (when auto print is selected, prints both volume/time and the displayed
curve type if flow/volume or tidal volume; incentive screens do not print.)
•no curves
Note: It is not necessary to select a default SVC curve for printing, because SVC curves
are always volume/time.
Select FVC Print
Parameters
Select Scale Choose which type of scaling (graph resizing) to use in printed volume/time curves.
Print Lung Age Yes or no. If yes, the estimated lung age is included in printed reports for patients. For
Print “Unconfirmed
Report”
Print ”Reviewed By” Yes or no. If yes, “Reviewed By ______________” is included in printed reports, giving
Choose which FVC-test parameters to include in printed reports. You may select as many
parameters as you like. If more are selected than fit on a page, the report continues to
another page.
Choices: FVC, FEV1, FEV1%, FEV6, PEF, FEF25-75, FEV0.5, FEV2, FEV3, FEV5, FEV1/FEV6,
FEV0.5%, FEV2%, FEV3%, FEV5%, FEV6%, FEF25, FEF50, FEF75, FEF0.2-1.2, FEF75-85,
FET, FIVC, FIV1, FIV1%, PIF, FIF50, FEF50/FIF50.
Note: It is not necessary to select SVC print parameters, because they all print.
• Auto scale
Graph is scaled to a small size.
•10 mm/s
X axis (time) prints at 10 mm/s. Y axis prints at 10 mm/L.
•20mm/s
X axis (time) prints at 20 mm/s. Y axis prints at 10 mm/L.
details, see “About Lung Age” on page 72.
Yes or no. If yes, “Unconfirmed Report” is included in printed reports.
the clinician a place to sign.
Print “Patient
Cooperation”
Print Quality Grades Yes or no. If yes, a test-quality grade is included in each printed report. See “About Test-
Print Patient Education Yes or no. If yes, the patient help sheets on asthma and adult smoking prints
Auto Print Yes or no. If yes, a report prints automatically when you press the Test Done softkey.
Yes or no. If yes, “Patient Cooperation _______________” is included in printed
reports, giving the clinician a place to comment.
Quality Grades” on page 74.
automatically with every report. For examples of these sheets, see “Patient Help Sheets”
on page 63.
Page 23
Directions for Use Chapter 2 Reviewing the Spirometry Settings 19
Reviewing the Patient Data Fields Available
To review or change the fields that appear during data entry for spirometry patients,
follow these steps.
Note
You choose ECG data-entry fields separately, as described in the CP 200
electrocardiograph manual.
1. Press the Menu key .
2. Choose Spirometry Settings > Patient Data Settings.
The following screen appears.
Figure 6. “Spirometry Patient Data Settings” Screen
Spirometry Patient Data Settings
1 First Name
2 Second Last Name
3 Middle Initial
4 Age/Birth Date
5 Weight
6 Medication
7 History
8 Comments
0 Previous Menu
9:17AM Oct 16 05
Several fields — Patient ID, Last Name, Height,
Gender, Race, and Smoke Years — always appear
on the Enter New Patient screen, as shown in
Figure 28 on page 41. Since these fields cannot be
disabled or edited, they do not appear on this userselectable list.
3. Change any desired settings.
For most of these fields, you have two choices: on (enabled) or off (disabled).
Disabled fields neither display nor print.
You must choose either Age or Birth Date. This field cannot be disabled.
For more details on these settings, see the description of patient data fields in the
electrocardiograph manual.
Page 24

20 Chapter 2 Reviewing the Spirometry Settings Welch Allyn CP 200 Spirometry Option
Reviewing the Interpretation List
To review or change the list of interpretative phrases that you can add to the
interpretation area of the screen and reports, follow these steps.
1. Press the Menu key .
2. Choose Spirometry Settings > Edit Interpretation List.
The following screen appears.
Figure 7. “Spirometry Interpretation List” Screen
Spirometry Interpretation List
ATS Obstruction - May be a physiological variation
ATS Obstruction - Mild
ATS Obstruction - Moderate
ATS Obstruction - Moderately Severe
ATS Obstruction - Normal
ATS Obstruction - Severe
ATS Obstruction - Very Severe
ATS Restriction - Mild
Add Delete Exit
9:17AM Oct 16 05
3. Press the desired softkeys.
Softkey Effect
Add Lets you add statements to the list, up to a total of 50.
Delete Deletes the highlighted statement.
Exit Returns to the Spirometry Settings screen.
Page 25
Directions for Use Chapter 2 Reviewing the Spirometry Settings 21
Reviewing the Auto Send Setting
To review or change the setting for automatically sending all spirometry test records to a
CardioPerfect workstation or to an SD memory card, follow these steps.
1. Press the Menu key .
2. Choose Spirometry Settings > Communication Settings > Auto Send.
The following screen appears.
Figure 8. “Auto Send” Submenu
Spirometry Communication Settings
1 Auto Send
0 Previous Menu
9:17AM Oct 16 05
None
Workstation
Memory Card
3. If desired, change the setting.
For details on these choices, see the CP 200 electrocardiograph manual.
Setting Description
None Test records are not automatically sent.
Workstation All spirometry test records are automatically sent to a CardioPerfect workstation.
Memory Card All spirometry test records are automatically sent to your SD memory card.
Page 26

22 Chapter 2 Reviewing the Spirometry Settings Welch Allyn CP 200 Spirometry Option
Page 27
23
3
Calibrating the Spirometer
About Calibration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 24
Performing a Calibration. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 26
Printing Calibration Reports . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 31
Page 28

24 Chapter 3 Calibrating the Spirometer Welch Allyn CP 200 Spirometry Option
About Calibration
The American Thoracic Society recommends calibrating a spirometer every day before
testing. In addition, each time you open a new package of flow transducers, verify the lot
number on the package label. If this lot number differs from the lot number used during
the most recent calibration, you must recalibrate the spirometer before resuming testing.
There are two types of calibration:
• Single-flow calibration
One inhale/exhale cycle.
• Multiple-flow calibration
Three inhale/exhale cycles at three different rates:
3 L in 1 second (3 L/s)
3 L in 3 seconds (1 L/s)
3 L in 6 seconds (0.5 L/s)
Note
For a diagram illustrating this procedure, see Figure 9 on page 25.
For step-by-step calibration instructions, see “Performing a Calibration” on page 26.
For information on reviewing or changing the settings that affect calibration, see
“Reviewing the Calibration Settings” on page 15.
If you want to add efforts to a saved test, the calibration must stay the same.
Whenever you recalibrate, you lose the ability to add new efforts to tests that
were saved earlier.
Caution For proper performance, the calibration syringe itself must be
recalibrated every year. See its calibration certificate for the most recent
calibration date. When the syringe is due for recalibration, return it to the
manufacturer. For details, see “Service Policy” on page 53.
Page 29

Directions for Use Chapter 3 Calibrating the Spirometer 25
Figure 9. Calibration, Process Diagram
For step-by-step
procedure, see
“Performing a Calibration”
on page 26.
Calibrate
Simulate exhalation
& inhalation.
(3x if multiple flow.)
Accept?
Yes
Spirometer Calibration
Go to
initial screen
Fill in transducer
calibration code, etc.
Verify?
Or calibrate?
No
Verify
Simulate exhalation
& inhalation.
Yes
Retry?
No
Calibration report prints
(if enabled).
Continue previous
procedure.
Page 30

26 Chapter 3 Calibrating the Spirometer Welch Allyn CP 200 Spirometry Option
Performing a Calibration
WARNING To avoid the risk of cross-contamination, always use a new flow
transducer when calibrating the spirometer. Observe all safety information that
came with the flow transducers.
Note
When you open a new package of flow transducers, disregard the calibration CD
that is shipped with them. The CP 200 spirometer does not use the calibration file
on this CD.
1. Go to t h e Spirometer Calibration initial screen (Figure 10).
You can get to this screen in either of two ways:
• At prompt
Press in response to the Calibrate Now? prompt, which appears the first
time you press the Spirometry key each day (as described in Step 3 on page 41).
•Anytime
Press the Menu key , then choose Spirometry Settings > Calibration
Settings > Calibrate Spirometer.
Figure 10. “Spirometer Calibration” Initial Screen
9:17AM Oct 16 05
Spirometer Calibration
Transducer Lot Code: 2
Transducer Cal Code: WKKVDXPB7
Syringe Volume (L): 3.000
Temperature (F): 77.00
Humidity (%): 50.00
Pressure (mmHg): 759.06
Last Calibration: 10/15/2005 3:8:39 PM
Volumes in/ex (L): -3.000/3.000
Enter the current settings, and then press calibrate.
Verif y
Calibration
Calibrate
1Flow
Calibrate
3 Flows
2. Fill in all fields.
• Transducer lot and “cal” codes appear on the transducer package label, as shown
in Figure 11.
• For the syringe volume, see the sticker on the calibration syringe.
• Update the temperature, humidity, and pressure. See your local weather reports.
Note
To learn how to change the pressure units, see page 12.
Page 31

Directions for Use Chapter 3 Calibrating the Spirometer 27
Figure 11. Calibration Code on Flow Transducer Package Label
0050
8
200x-xx
Transducer
REF 703419
Disposable Flow Transducers
“cal” code
CALIBRATION CODE WKKVDXPB7
LOT 2
Lot code
QTY 100
4341 State Street Road
Skaneateles Falls, NY 13153 USA
www. welchallyn.com
Drawing No. 30015257 VER. F
3. Press the desired softkey, as listed here.
• Verify Calibration
To verify the accuracy of the system (without recalibrating).
• Calibrate 1 Flow
To calibrate the system using one inhale/exhale cycle.
• Calibrate 3 Flows
To calibrate the system using three inhale/exhale cycles at three different rates.
The “attach flow transducer” prompt appears, as shown in Figure 12.
Figure 12. “Attach Flow Transducer” Prompt
Spirometer Calibration
9:17AM Oct 16 05
Attach the flow transducer
to the syringe,
Pull the plunger out,
Then select continue
At any time, you can press Back to return to
the initial calibration screen, as shown in
Figure 10 on page 26.
BackContinue
Page 32

28 Chapter 3 Calibrating the Spirometer Welch Allyn CP 200 Spirometry Option
4. Connect a new flow transducer to the pressure tubing. See “Connecting the
Spirometer Components” on page 39.
5. Attach the flow transducer to the syringe’s port, shown here. Push the flow
transducer all the way in for a tight seal.
Figure 13. Calibration Syringe
Plunger
Port
6. Pull the plunger all the way out.
7. P r e s s Continue.
Caution Several things may affect calibration results: movement of the
syringe, movement of the pressure tubing, or blockage of air. Place the
syringe on a hard, level surface with at least 1 cubic meter of open air
surrounding the flow transducer. Place your hand on top of the syringe to
prevent movement.
8. Press Start.
9. When the blue bar begins to move, push the plunger all the way in, then pull it all the
way out, carefully following the bar’s rate. Use a steady motion in both directions.
See Figure 14.
Figure 14. Simulated Exhalation and Inhalation
Spirometer Calibration
Push plunger in following the bar
Tar g e t R a te
9:17AM Oct 16 05
Stop
Spirometer Calibration
9:17AM Oct 16 05
Pull plunger out following the bar
Tar g et Ra t e
Stop
Page 33

Directions for Use Chapter 3 Calibrating the Spirometer 29
If desired, you can press Stop any time. Softkeys will change, as described in
Step 11 on page 30.
Otherwise, when no air has moved for three seconds, the following happens:
• For verifications or single-flow calibrations
The results display.
• For multiple-flow calibrations
Another simulated exhalation screen appears. Repeat from Step 8 twice more.
The results display.
10. Review your results. See the following examples.
Figure 15. Poor Results,
Single-Flow
9:17AM Oct 16 05
Successful Calibration
Vol (L)
Syringe Volume: 3.000 L
Expired Volume: 3.000 L (-0.0%)
Inspired Volume: -3.000 L (-0.0%)
Temperature: 25.0 C
Humidity: 50.0 %
Pressure: 1012.0 mbar
I-Gain: 2.76
E-Gain: 2.71
Legend
Measured Adjusted
Time (s )
AcceptRetry Exit
Large gap between measured and adjusted curves
Figure 17. Good Results,
Multiple-Flow
9:17AM Oct 16 05
Successful Calibration
Vol (L)
Time (s )
Syringe Volume: 3.000 L
Expired Volume
0.5 L/s: 3.03 L (-0.9%)
1.0 L/s: 2.98 L (-0.6%)
3.0 L/s: 2.97 L (-0.9%)
Temperature: 25.0 C
Humidity: 50.0 %
Pressure: 1012.0 mbar
I-Gain: 1.05
E-Gain: 0.97
Legend
Measured Adjusted
Figure 16. Good Results,
Single-Flow
9:17AM Oct 16 05
Successful Calibration
Vol ( L)
Syringe Volume: 3.000 L
Expired Volume: 3.000 L (-0.0%)
Inspired Volume: -3.000 L (-0.0%)
Temperature: 25.0 C
Humidity: 50.0 %
Pressure: 1012.0 mbar
I-Gain: 1.06
E-Gain: 1.07
Legend
Measured Adjusted
Time ( s)
AcceptRetry Exit
Small gap between measured and adjusted curves
Figure 18. Good Results,
Verification
9:17AM Oct 16 05
Calibration Verification Successful
Vol ( L)
Time ( s)
Syringe Volume: 3.000 L
Expired Volume: 2.976 L (-0.8%)
Inspired Volume: -2.989 L (-0.4%)
Temperature: 25.0 C
Humidity: 50.0 %
Pressure: 1012.0 mbar
Legend
Measured Adjusted
AcceptRetry Exit
No gap between measured & adjusted curves
Retry Done
Error < 3%
Page 34

30 Chapter 3 Calibrating the Spirometer Welch Allyn CP 200 Spirometry Option
11. Press the appropriate softkey.
Caution A poor calibration (as shown in Figure 15) indicates that the system
had to make large adjustments to measure the syringe volume accurately.
Do not accept poor calibrations, or your spirometry test results may be
inaccurate.
• Retry
Discards the results. Returns to initial calibration screen. Go to Step 2 on page 26.
• Accept
Saves the results. Resumes your original procedure.
If automatic report printing is enabled, a calibration report prints. To learn how to
enable or disable automatic printing, see “Reviewing the Calibration Settings” on
page 15.
•Exit
Discards the results. Resumes your original procedure.
12. (Optional) Verify the most recent calibration — especially if your calibration results
were questionable.
a. Go back to Step 1 on page 26.
b. Select Verify Calibration in Step 3.
c. On your results screen, check the error percentages for the expired and inspired
volumes. If <3%, your calibration is acceptable. If 3% or over, recalibrate. See
example in Figure 18 on page 29.
Page 35

Directions for Use Chapter 3 Calibrating the Spirometer 31
Printing Calibration Reports
You can set up your system to print a calibration report automatically every time you
accept calibration results. You can also print a report manually any time.
To Turn Automatic Report Printing On or Off
1. Choose Spirometry Settings > Calibration Settings > Enable Auto Calibration
Report.
2. Select Ye s or No.
To Print a Report Manually
Choose Spirometry Settings > Calibration Settings > Print Calibration Report.
Page 36

32 Chapter 3 Calibrating the Spirometer Welch Allyn CP 200 Spirometry Option
Page 37

33
4
Performing Spirometry Tests
Overview of the Testing Process. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 34
Connecting the Spirometer Components . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 39
Preparing the Patient . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 40
Recording a Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 41
Working With a Completed Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 45
Page 38

34 Chapter 4 Performing Spirometry Tests Welch Allyn CP 200 Spirometry Option
Overview of the Testing Process
There are two types of spirometry efforts (also called maneuvers):
• FVC — forceful breathing
• SVC — relaxed breathing
For details, see “About FVC Efforts” on page 35 and “About SVC Efforts” on page 36.
A single test comprises a set of efforts — up to 6 efforts of each type (FVC and SVC) for a
maximum of 12 efforts (6 FVC and 6 SVC). The 6 efforts of a given type can be a mixture
of pre- and post-medication efforts.
For details, see “About Pre- and Post-Testing” on page 38 and “About Effort
Replacement” on page 38.
Figure 19. Spirometry Testing Process Diagram
For step-by-step procedure, see
“Recording a Test” on page 41.
Yes
Enter or search
for patient data.
Choose effort type:
FVC, SVC, FVC-Post, SVC-Post
Perform effort.
Accept effort?
Yes
Another effort?
Yes
Uninterrupted for
< 20 min.?
No
No
No
Test Done
(Optional) Calibrate.
Prompted once daily.
For step-by-step procedure,
see “Working With a
Completed Test” on page 45.
(Optional)
Review test.
Add or edit interpretation.
Send or print test.
Another test?
No
Yes
Page 39

Directions for Use Chapter 4 Performing Spirometry Tests 35
About FVC Efforts
“FVC” stands for forced vital capacity. The goal of an FVC effort is to measure the volume
and flow of air. Patients inhale fully then exhale forcefully. Sometimes they also inhale
forcefully.
When ready to begin an FVC effort, you coach the patient through these steps.
(If preferred, you may reverse the order of inhaling and exhaling.)
1. Inhale fully — calmly fill your lungs as much as you can.
2. Place the flow transducer in your mouth.
3. Exhale forcefully — as fast as you can, as long as you can.
4. (Optional) Inhale forcefully — as fast as you can, as long as you can.
You can view and print FVC data in three types of curves, as shown in the following
figures.
Figure 20. FVC Flow/Volume Curves
Flow (L/s)
Exhaling only
Vol (L )
Figure 21. FVC Tidal Volume Curve
Flow (L/s)
All data from all breaths,
including tidal breathing
(multiple loops)
Vol ( L)
Figure 22. FVC Volume/Time Curves
Vol (L )
Exhaling only
Flow (L/s)
Exhaling and inhaling
(single loop)
Vol (L )
Vol (L )
Exhaling and inhaling
Time (s)
Time (s)
Page 40

36 Chapter 4 Performing Spirometry Tests Welch Allyn CP 200 Spirometry Option
During FVC testing, an animated incentive screen provides an alternative way to view the
data (Figure 23). This screen gives patients, usually children, a fun goal to achieve while
exhaling. (If the selected norm does not provide a valid FVC or PEF predicted value, the
system tries to use the Polgar norm; if Polgar does not fit the patient’s demographics, the
incentive screen is not available.)
Figure 23. FVC Incentive Screen
The more forcefully the patient blows, the more
flames are extinguished.
About SVC Efforts
“SVC” stands for slow (relaxed) vital capacity. Sometimes SVC testing is used when
forced breathing is impossible. The patient inhales and exhales as completely as possible,
as in FVC testing, but the breathing is not forced. The goal of an SVC effort is to measure
the volume of air inhaled and exhaled, not the air flow (speed).
When ready to begin an SVC effort, you coach the patient through these steps.
(If preferred, you may reverse the order of inhaling and exhaling.)
1. Place the flow transducer in your mouth.
2. Breathe normally several times (tidal breathing).
3. Inhale fully — calmly fill your lungs as much as you can.
4. Exhale fully — calmly empty your lungs as much as you can.
The parameters measured during SVC testing are always displayed in a volume/time
curve, as shown in Figure 24.
Figure 24. SVC Curve
Vol ( L)
Time (s)
Tidal breathing
Page 41

Directions for Use Chapter 4 Performing Spirometry Tests 37
About the Spirometry Parameters
During FVC and SVC testing, many parameters are measured and calculated. For
definitions of these parameters, see “Glossary” on page 77.
During FVC testing, the two most important parameters in determining lung problems are
FVC and FEV1. (For a description of how the automatic interpretation software uses these
two measurements to determine the degree of obstruction or restriction, see
“Understanding Your Interpretation Results” on page 75.)
• FVC — forced vital capacity, the maximum volume of air that can be forcibly and
rapidly exhaled
• FEV1 — forced expiratory volume 1, the volume of air that is exhaled at one
second of a forced expiration
The following are important parameters for SVC testing:
• VT — tidal volume
• ERV — expiratory reserve volume
• IC — inspiratory capacity
Page 42

38 Chapter 4 Performing Spirometry Tests Welch Allyn CP 200 Spirometry Option
N
About Pre- and Post-Testing
If desired, a spirometry test may include both pre- and post-efforts (FVC or SVC) to
measure the effectiveness of medication. The “before medication” and “after
medication” efforts may be uninterrupted or interrupted.
• Uninterrupted
If there is no interruption between pre- and post-efforts (that is, no other patient has
been tested and the electrocardiograph has remained on), the same screen continues
to display. You simply continue with the procedure.
• Interrupted
If there is an interruption (that is, another patient has been tested or the
electrocardiograph has been turned off), you need to recall the patient’s test-inprogress before continuing.
Pre- and post-efforts must happen on the same day, with the same calibration.
ote
The next day — or after a recalibration — tests become available for review only;
you can no longer add efforts to them.
About Effort Replacement
You can save up to 6 FVC and 6 SVC efforts per test (maximum total of 12 efforts). After
saving 6 efforts of a given type, the software compares each new effort with the saved
efforts. If the new effort is better than the worst saved effort, the worst effort is deleted
and the new one is saved. If the new effort is worse than all saved efforts, you are asked
whether you want to save it.
If 6 pre-efforts have been saved, the worst pre-effort is deleted when you add a posteffort until you have saved 3 pre- and 3 post-efforts. After that, the “worst” post-effort is
deleted.
Page 43

Directions for Use Chapter 4 Performing Spirometry Tests 39
N
Connecting the Spirometer Components
WARNING To prevent the spread of infection, use a new flow transducer for
each patient. Use rubber gloves when replacing used flow transducers, and wash
hands after touching them. Discard flow transducers after a single patient use.
1. Verify that the sensor and pressure tubing are clean and undamaged. Look for signs
of deterioration, including but not limited to cracks, cuts, discoloration, or oxidation.
If any part exhibits any of these symptoms, replace it. See “Ordering Information for
Replacement Parts” on page 9.
2. Attach a flow transducer to the pressure tubing. See Figure 25.
3. Attach a sensor to the other end of the pressure tubing. See Figure 26.
4. Connect the sensor to the electrocardiograph’s spirometry port. Hand-tighten the
sensor connectors. Do not overtighten the connectors, or they may become stripped.
See Figure 27.
The CP 200 software automatically activates the spirometry functions throughout the
software.
Bacteria filters are unnecessary.
ote
Figure 25. Attaching a Flow Transducer
to the Pressure Tubing
Figure 27. Connecting the Sensor to the Spirometry Port
Figure 26. Attaching the Sensor to the
Pressure Tubing
Page 44

40 Chapter 4 Performing Spirometry Tests Welch Allyn CP 200 Spirometry Option
Preparing the Patient
To prepare patients for any spirometry test, explain the entire procedure for the type of
effort you want them to perform. Remind patients that the test is painless. Demonstrate
at least one effort for the patient.
The accuracy of a spirometry test is highly dependent on the patient's understanding and
cooperation. So, be prepared to coach and encourage the patient with your “body
language” and your words — for example, ”Blow, blow, blow, keep blowing until you
can't blow any more out” — to ensure a good effort with reproducible results.
Instruct patients to do the following:
• Loosen any tight articles of clothing that might constrict lung function, for example, a
tight belt, tie, vest, bra, girdle, or corset.
• Remove any foreign objects from the mouth, including loose dentures.
• Use of a nose clip is optional. Patients may also pinch their noses.
• Place your lips and teeth around a new transducer, sealing their lips tightly around the
transducer. Grip slightly with your teeth in the groove. (If you need to hold the flow
transducer in your hand, keep fingers away from the screen on the back.)
• Keep your tongue away from the flow transducer to avoid blocking it.
• Keep your chin up so as not to restrict the airway.
WARNING Patients may become faint, light-headed, dizzy, or short of breath
during spirometry testing. Watch patients closely. If they choose to stand during
testing, keep a chair immediately behind them. If there is any reason for concern,
stop the test and take proper action.
WARNING Patients should not bite on the flow transducer. Biting could result
in sharp edges, which could injure the mouth.
Page 45

Directions for Use Chapter 4 Performing Spirometry Tests 41
N
Recording a Test
To record a spirometry test, follow these steps.
1. Measure the patient’s standing height to the nearest half inch (or centimeter) in
stocking feet.
Accuracy is important; height greatly influences the predicted values.
If the patient has obvious spinal deformities, measure the arm span from
ote
fingertip to fingertip with arms outstretched against a wall. Enter the arm
span instead of height.
2. If the patient’s demographics do not match the current spirometry norm, select a
more appropriate norm.
To find out how, see “Select Adult Predictive Norm” on page 13 or “Select Ped.
Predictive Norm” on page 13.
3. Press .
The first time this key is pressed each day, the prompt “Calibrate Now?” appears.
4. (Optional) Calibrate. See “Calibrating the Spirometer” on page 23.
The following screen appears.
Figure 28. “Enter New Patient” Screen
Enter New Patient
Patient ID
Last Name
Birth Date
Height
Gender
Race
Smoke Years
Use up and down arrows to change fields
Search Schedule
//
ft. in.
yr.
Clear
9:17AM Oct 16 05
MM / DD / YYYY
Done
To learn how to choose which fields display
here, see “Reviewing the Patient Data Fields
Available” on page 19.
Page 46

42 Chapter 4 Performing Spirometry Tests Welch Allyn CP 200 Spirometry Option
5. Enter or search for patient data.
a. To search for patient data
Press Search or Schedule, and select the patient. (For details, see CP 200
electrocardiograph manual.) Then press the desired softkey, as described here.
Softkey Function Your Next Action
New Test Returns to the “Enter New Patient” screen with some data
filled in.
Continue Test Lets you continue a test-in-progress. This softkey appears
only if it is the same day and the calibration is the same.
Review Test Lets you recall any of that patient’s saved tests and review
its data. You cannot add new efforts, but you can edit the
interpretation, send the test to an SD memory card or
workstation, or print the test.
Go to Step b, below.
Go to Step 6 on page 43.
Go to “Working With a
Completed Test” on page 45.
b. To enter patient data
Fill in the fields. All mandatory fields must be filled in before you can proceed.
Important Fields Description
Patient ID,
Last Name
Age/Birth Date, Height,
Gender, Race
Weight Mandatory only when using Schoenberg or Hedenström norm.
Always mandatory. The patient must be identified.
Always mandatory. This information determines the automatic interpretation.
Smoke Years Not mandatory. If the patient smokes, enter the number of years the patient
has smoked. If this value is 1 or more for an adult patient, and if patient
education is enabled, the smoking help sheet prints after the spirometry test
report. See “Patient Help Sheets” on page 63.
When finished entering data, press the desired softkey:
• Clear — deletes the patient data and returns to the Patient ID field.
• Done — accepts the patient data and goes to the initial spirometry screen.
Page 47

Directions for Use Chapter 4 Performing Spirometry Tests 43
N
6. Press Effort Type as needed to select the type of effort you want the patient to
perform. See Figure 29.
•FVC
• FVC Post*
•SVC
• SVC Post*
*FVC Post and SVC Post are available only if you have already accepted at least one
pre-effort of the same type.)
7. (FVC testing only) Press Curve as needed to select the curve type that you want to
view while testing. See Figure 29.
•Flow/Volume
• Volume/Time
• Tidal Volume
•Incentive
Figure 29. Spirometry Screen, Ready to Start Effort
SVC exampleFVC example (flow/vol curve)
Doe, Jane
FVC #1
Flow (L/s)
5
Effort Type
FVC
Curve
Flow/Vol
NHANES III 1999
ATS: --
FVC 2.09 -- -FEV1 3.06 -- -FEV1% 80.86 -- -FEV6 3.75 -- -PEF 7.14 -- -FEF25-75 3.00 -- -FEV0.5 2.31 -- -FEV2 -- -- --
Vol ( L)
9:17AM Oct 16 05
Pred Value %Pr ed
Start
Te st
Done
Doe, Jane
SVC #1
Vol ( L)
5
Select the desired effort type.Select the desired effort type and curve.
Effort Type
SVC
9:17AM Oct 16 05
SVC -ERV -IRV -VT -BF -Tin/Tex --
5
Time (s)
Star t
Valu e
Te st
Done
To learn how to change the default FVC curve type and parameters, see
ote
“Reviewing the Spirometry Screen Settings” on page 16.
8. When ready, press Start.
9. Coach the patient through the effort. For tips, see “Preparing the Patient” on
page 40.
10. When finished, press Stop.
Page 48

44 Chapter 4 Performing Spirometry Tests Welch Allyn CP 200 Spirometry Option
N
N
Figure 30. “Effort Complete” Screen
SVC exampleFVC example (flow/vol curve)
Doe, Jane
FVC #1
Flow (L/s)
5
NHANES III 1999
ATS : - -
FVC 2.09 3.62 173.5 0
FEV1 3.06 3.08 100.46
FEV1% 80.86 85.01 105.14
FEV6 3.75 0.00 0.00
PEF 7.14 7.18 100.66
FEF25-75 3.00 3.29 109.81
FEV0.5 2.31 2.32 100.3 2
FEV2 -- 3.50 --
Vol ( L)
9:17AM Oct 16 05
Pred Value %Pred
Doe, Jane
SVC #1
Vol ( L)
5
10
5
15 20
9:17AM Oct 16 05
SVC 3.31
ERV 1.35
IRV 1.30
VT 0.66
BF 24.73
Tin/Tex 88.60
Time (s)
Val ue
Curve
Flow/Vol
Accept
Effort
Reject
Effort
Accept
Effort
For FVC efforts, the “% predicted” values display in color as follows:
Red: % predicted values are below LLN.
Black: % predicted values are normal.
Green: % predicted values are at least 100%.
11. Review the data.
For FVC tests, if desired, press Curve to alternate between curve types.
Decide whether to accept the effort. For help deciding, see the Spirometry Effort
Acceptability & Reproducibility poster.
After each effort, a quality message appears on this screen, such as “Don’t
ote
hesitate,” “Blow out longer,” or “Good effort.” For details, see “About
Effort-Quality Messages” on page 73.
12. Press the desired softkey.
• Accept Effort
Saves the effort. See “About Effort Replacement” on page 38.
Reject
Effort
• Reject Effort
Deletes the effort.
In either case, the “ready to start effort” screen reappears (Figure 29 on page 43).
The effort numbers increment with each new effort (FVC #1 becomes FVC
ote
#2, and so on), even if some efforts were deleted, so the test record
indicates the patient’s total number of efforts.
13. Determine your next step.
• If you want to perform another effort, go to Step 6 on page 43.
• If you are finished with this test, press Te s t D o n e .
Page 49

Directions for Use Chapter 4 Performing Spirometry Tests 45
3
2
4
1
Working With a Completed Test
If you are looking at the Test Results main screen, shown here, you arrived here in either
of two ways:
• You pressed Test Done after completing a set of efforts (Step 13 on page 44).
• You pressed Review Test to recall a saved test for review (Step 5 on page 42).
Figure 31. “Test Results” Main Screen
FVC example (vol/time curve)
Doe, Jane
Test Results
Vol ( L)
9:17AM Oct 16 05
Legend
5
Time ( s)
All FVC curves, including any pre- and post-efforts,
are displayed on one graph. Any SVC curves are
3
displayed in a separate graph.
Effort Type
FVC
View
Results
Add/Edit
Interps
Send
Te st
Print
Te st
You are now ready to work with the completed test. Press the desired softkeys:
Softkey Function
Effort Type Alternates between FVC and SVC efforts, if applicable.
View Results See “To View a Test’s Results” on page 46.
Add/Edit Interp See “To Change a Test’s Interpretation Statements” on page 47.
Send Test See “To Send a Test to an SD Memory Card or Workstation” on page 48.
Print Test See “To Print a Test” on page 49.
When finished, determine what to do next.
• Press to start another test for this patient or another patient.
Go to Step 3 on page 41.
• Press to exit spirometry mode.
Page 50

46 Chapter 4 Performing Spirometry Tests Welch Allyn CP 200 Spirometry Option
3
2
4
1
To View a Test’s Results
From the Test Results main screen (Figure 31 on page 45), follow these steps:
1. P r e s s View Results.
The display stays the same. Only the softkeys change, as shown here.
Figure 32. “View Results” Screen
FVC example (vol/time curve)
Doe, Jane
View Results
Vol ( L)
Tes t C o m pl e t e
Effort Type
FVC
Curve
Vol/Ti me
View
Val ues
9:17AM Oct 16 05
Legend
5
Time ( s)
View
Interp
Back
3
2. Press the desired softkeys to view the results in various ways.
Softkey Function Your Next Action
Effort Type Alternates between FVC and SVC efforts, if
applicable.
Curve Alternates between FVC curve types. Press the next desired softkey.
View Values Opens a window containing all of the measured and
calculated parameters across all saved efforts — like
a print preview.
A test-quality grade appears too. For details, see
“About Test-Quality Grades” on page 74.
The best efforts and parameters display according to
the print settings. See “Reviewing the Spirometry
Print Settings” on page 17.
View Interp Opens a window containing the interpretation
statements that have been saved with the test.
A test-quality grade appears too. For details, see
“About Test-Quality Grades” on page 74.
Back Returns to the Test Results main screen, as shown in
Figure 31 on page 45.
Press the next desired softkey.
Press or to close the values
window.
Press the next desired softkey.
Press or to close the
interpretation window.
Press the next desired softkey.
Return to “Working With a Completed
Tes t” on page 45.
Page 51

Directions for Use Chapter 4 Performing Spirometry Tests 47
To Change a Test’s Interpretation Statements
From the Test Results main screen (Figure 31 on page 45), follow these steps:
1. P r e s s Add/Edit Interps.
The following screen appears, displaying any interpretation statements that have
been saved with the test.
Figure 33. “Add/Edit Interpretations” Screen
Doe, Jane
Test Results
Interpretation #1:
Interpretation #2:
Interpretation #3:
Interpretation #4:
Add/Edit Interpretations
Cancel = Save =
9:17AM Oct 16 05
Press a right arrow key to see a list of
interpretation statements that are
available to choose for the highlighted
field.
2. Add or edit interpretation statements as desired.
Each test may include up to four statements — either automatically included, or
manually added, or a combination. If automatic statements appear, you may replace
them with manual statements if you wish.
3. Press to cancel or to save your changes.
The Test Results main screen reappears, as shown in Figure 31 on page 45.
• To learn how to change the statements that are available to choose, see “Reviewing
the Interpretation List” on page 20.
• To learn how to enable automatic interpretation, see “Enable ATS Interp. Results” on
page 14.
• To learn how the automatic interpretation software determines the degree of
obstruction or restriction, see “Understanding Your Interpretation Results” on
page 75.
Page 52

48 Chapter 4 Performing Spirometry Tests Welch Allyn CP 200 Spirometry Option
3
2
4
1
To Send a Test to an SD Memory Card or Workstation
From the Test Results main screen (Figure 31 on page 45), follow these steps:
1. P r e s s Send Test.
The following screen appears.
Figure 34. “Send Test” Screen
Doe, Jane
Send Test
Vol ( L)
9:17AM Oct 16 05
Legend
3
5
Time (s )
Select a destination to begin sending.
Memory
Card
Workstation
Done
2. Select the desired destination.
For details on these choices, see the CP 200 electrocardiograph manual.
• Memory Card
• Workstation
3. Press Done.
The Test Results main screen reappears, as shown in Figure 31 on page 45.
Page 53

Directions for Use Chapter 4 Performing Spirometry Tests 49
3
2
4
1
To Pr in t a Te s t
From the Test Results main screen (Figure 31 on page 45), follow these steps:
1. P r e s s Print Test.
The following screen appears.
Figure 35. “Print Test” Screen
Doe, Jane
Print Test
Vol ( L)
Press Print to generate a report
Efforts
Best Only
Curve
Vol/ Tim e
9:17AM Oct 16 05
Legend
5
Time (s )
Print
Back
3
2. Press the desired softkeys.
Softkey Function Related Information
Efforts Cycles through these print options:
• Best Only
Prints only the best effort of each type
that was saved — best FVC, SVC, FVCpre, FVC-post.
• 3 Best
Prints the three best efforts of each
type that was saved.
To change the default option, see “Reviewing
the Spirometry Print Settings” on page 17.
To change the definition of best, see “Select
Best Effort Formula” on page 13.
• All
Prints all efforts.
Curve Cycles through the curve types that are
available to print:
To change the default curve type for printed
reports, see “Reviewing the Spirometry Print
Settings” on page 17.
•Vol/Time
•Flow/Vol
• Tidal Vol
• V/T and F/V
•None
Print Prints one copy of the test. Press Print again for additional copies.
FVC and SVC efforts print in separate reports.
If “patient education” is enabled in the settings,
one or more patient help sheets automatically
print along with the test. For details, see
“Patient Help Sheets” on page 63.
Back Returns to the Test Results main screen. See Figure 31 on page 45.
Page 54

50 Chapter 4 Performing Spirometry Tests Welch Allyn CP 200 Spirometry Option
Page 55

51
5
Troubleshooting
Problem-Solving Suggestions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 52
Limited Warranty . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 53
Service Policy. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 53
Page 56

52 Chapter 5 Troubleshooting Welch Allyn CP 200 Spirometry Option
Problem-Solving Suggestions
If you try these suggestions and still have problems, contact Welch Allyn. For phone
numbers, see page ii.
Condition Causes Actions
When printing, text prints correctly but
FVC curve does not.
Unable to calibrate. • Poor connection between flow
No sensor detected. Poor connection between the sensor and
Does not print. • Out of paper.
Values are too high (intermittent). • Patient’s fingers obstructed the screen
Values are too high (consistently). Pressure connection is partially obstructed. Remove any foreign substance from the flow
Predictive values are blank. • The selected norm does not support
Print settings Make sure that the desired curve is selected. See “To
Print a Test” on page 49.
transducer and sensor.
• Damage to flow transducer.
• Leak during calibration.
• Uneven calibration strokes.
the electrocardiograph.
• Paper jam.
on the back of the flow transducer,
causing high back pressure and false
reading.
• Patient’s lips were not tightly sealed
around the flow transducer.
• Spirometer was calibrated with the
wrong size syringe.
certain values, and composite norm
values are disabled.
• Check the connection between flow transducer
and sensor.
• Replace the flow transducer if it is damaged.
• Ensure that the connection between the
calibration syringe and flow transducer is tight
with no leaks.
• Use even strokes in calibration.
Disconnect and reconnect the sensor.
• Load paper. See the electrocardiograph manual.
• If paper is jammed, clear it, then reload.
• Retest.
• Recalibrate with a 3-liter syringe. See
“Calibrating the Spirometer” on page 23.
transducer or pressure tubing.
• Re-enter age/birthdate, height, gender, race.
See Step b on page 42.
• Enable composite norm values. See “Reviewing
the Operation Settings” on page 13.
The flow sensor has been dropped. Accident. Recalibrate. See “Calibrating the Spirometer” on
page 23.
Report does not print parameters or
graphs.
Patient test values differ from values
expected by physician.
Improper print settings. Check print settings. See “Reviewing the Spirometry
Print Settings” on page 17.
Various. • If the transducer is contaminated with sputum or
secretions, replace it.
• Verify that proper barometric pressure has been
entered. See “Calibrating the Spirometer” on
page 23.
• Verify the patient data.
• Eliminate any leaks in the pressure tubing.
• Retest using a nose clip.
• Replace the sensor if damaged.
•Recalibrate.
• Replace the transducer and retest.
Add index entries for each error message, verbatim.
Page 57

Directions for Use Chapter 5 Troubleshooting 53
Limited Warranty
For general information on the limited warranty, see electrocardiograph manual.
The following spirometry components have specific warranty periods from date of
shipment to customer:
• Flow transducer — 90 days
• Pressure tubing — 90 days
• Sensor — 12 months
• Calibration syringe — 12 months
Service Policy
For general information on the service policy, see electrocardiograph manual.
The following spirometry components have specific service policies. For disposable
items, see “Ordering Information for Replacement Parts” on page 9.
• Flow transducer — Disposable.
• Pressure tubing — Disposable.
• Sensor — Return to Welch Allyn for replacement if necessary. Replacement is free
within the warranty period.
• Syringe — Return to Welch Allyn for calibration verification if necessary.
Recalibration is free within the warranty period. Beyond the warranty period, return to
the manufacturer:
AM Systems, Inc.
131 Business Park Loop
Carlsborg, WA 98324
(800) 426-1306
Page 58

54 Chapter 5 Troubleshooting Welch Allyn CP 200 Spirometry Option
Page 59

55
A
Specifications
Feature Specification
Dimensions & weights
Flow transducer 2.4 x 2.4 x 2 in. (6 x 6 x 5 cm)
0.4 oz (12 g)
Pressure tubing 2.2 yd (2 m)
0.9 oz (25 g)
Sensor 2.2 x 1.4 x 0.6 in. (5.4 x 3.4 x 1.6 cm)
0.9 oz (25 g)
Tests FVC, SVC, pre- and post-bronchodilator
Flow technology Pneumotach
Power equipment Powered by CP200 electrocardiograph via serial port (no battery)
Power consumption 5 to 15 mA
Accuracy Meets or exceeds 1994 ATS standard
Reproducibility Meets or exceeds 1994 ATS standard
Flow range 0–14 L/s
Predictive norms
Adult Berglund 1963, Crapo 1981, ECCS / Quanjer 1993, Gulsvik 2001, Hedenström 1986, Knudson
Pediatric Berglund 1963, Dockery 1983, Hsu 1979, Knudson 1976, Knudson 1983, Koillinen 1998,
Interpretation 1991 ATS interpretation standards.
Reports
FVC testing Volume/time curve
SVC testing Volume/time curve
1976, Knudson 1983, Kory 1961, Morris 1971, NHANES III 1999, Schoenberg 1978, Viljanen
1981
NHANES III 1999, Polgar 1971, Schoenberg 1978, Solymar 1980, Zapletal 1969
Lung age calculation can be enabled or disabled.
Automatic interpretation can be enabled or disabled.
User-definable interpretation statements are also available to be added manually.
Flow/volume curve
Tidal volume
Both volume/time and displayed curves
No curves
No curve
Page 60

56 Appendix A Specifications Welch Allyn CP 200 Spirometry Option
Feature (continued) Specification (continued)
Parameters
FVC testing FVC, FIVC, FIV1, FIV1%, FEV0.5, FEV1, FEV2, FEV3, FEV5, FEV6, FEV1/FEV6, FEV0.5%, FEV1%,
FEV2%, FEV3%, FEV5%, FEV6%, PEF, FEF25, FEF50, FEF75, FEF0.2-1.2, FEF25-75, FEF75-85,
PIF, FIF50, FEF50/FIF50, FET
SVC testing SVC, ERV, IRV, VT, IC, BF, MV, Tin, Tex, Tin/Tex
Quality checks Effort acceptability and test reproducibility checks.
Effort-quality messages and test-quality grades.
Audio and visual incentive for assistance in coaching patients.
Connectivity Compatible with CardioPerfect workstation.
Protection against ingress
of water, per IEC 60529
(spirometry components)
Protocols PCP (primary care practitioner)
Specifications are subject to change without notice.
IPX0
NIOSH
OSHA
SSD (Social Security & Disability)
None
Page 61

57
B
Spirometry Protocols
About the PCP Protocol. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 58
About the NIOSH Protocol. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 60
About the OSHA / Cotton Dust Protocol . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 61
About the SSD Protocol. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 62
This appendix describes the protocols you can select to change the way the CP 200
spirometer operates when testing a patient. Any features that are not specified in the
protocol use your own settings.
To learn how to review or change the protocol, see “Select Protocol” on page 13.
Page 62

58 Appendix B Spirometry Protocols Welch Allyn CP 200 Spirometry Option
About the PCP Protocol
The PCP (primary care practitioner) protocol is for users who want to make sure that
testing meets the requirement of the National Lung Health Education Program (NLHEP).
When the PCP protocol is selected, the spirometer automatically performs as described
here, regardless of user-defined settings.
For details on PCP requirements, see the document noted in Reference 1 on page 76.
When this protocol is selected, testing and reports are affected as follows:
• Operation Settings
Adult Predictive Norm: NHANES III
Ped. Predictive Norm: NHANES III
Best Effort Formula: Best Measurement
Reversibility Formula: (Post-Pre)/Pre x 100
FEV1% Formula: FEV6
Predictive Points: YES
Predictive Curve: YES
ATS Interpretation Results: NO
Composite Norm Value: NO
Automatic Quality Check: NO
(For details, see “Reviewing the Operation Settings” on page 13.)
• Screen Settings
FVC Display Parameters: FEV1, FEV6, and FEV1/FEV6 only
(For details, see “Reviewing the Spirometry Screen Settings” on page 16.)
• Print Settings
Efforts: Only Best Effort
FVC Curves: V/T & F/V
FVC Print Parameters: FEV1, FEV6, and FEV1/FEV6 only
Scale: 20 mm/s & 10 mm/L
Print Lung Age: YES
Print “Unconfirmed Report”: YES
Print “Reviewed By”: YES
Print “Patient Cooperation”: YES
Print Quality Grades: YES
Print Patient Education: YES
Auto Print: YES
(For details, see “Reviewing the Spirometry Print Settings” on page 17.)
Page 63

Directions for Use Appendix B Spirometry Protocols 59
Post results are compared (%c column) to the pre results only if the test-quality grades
for both pre- and post-test sessions are A, B, or C.
An ATS interpretation is displayed and printed only if the test session pre and post quality
grades are A, B, or C.
If the pre or post quality grades are D or F, interpretation states “results should be
interpreted with caution.”
If the pre or post quality grade is D and the results are within normal limits, the
interpretation states, “normal, but the reported FEV1 and FVC should not be used for
comparisons with previous or subsequent tests.”
Interpretation states “airway obstruction” when the FEV1/FEV6 is below the LLN.
Interpretation states “low vital capacity, perhaps due to restriction of lung volumes” if
FEV1/ FEV6 is above the LLN, but the FEV6 is below the LLN.
Note
When PCP protocol is selected, no inspiration is recorded.
Page 64

60 Appendix B Spirometry Protocols Welch Allyn CP 200 Spirometry Option
About the NIOSH Protocol
The NIOSH (National Institute for Occupational Safety and Health, U.S.) protocol is for
users who want to make sure that occupational testing and reports meet the
requirements of NIOSH. The device automatically performs as described here, regardless
of user-defined settings.
When using this protocol, the spirometer should be calibrated at three different flows
every day before use.
For details on NIOSH requirements, see the document noted in Reference 4 on page 76.
When this protocol is selected, testing and reports are affected as follows:
• Operational Setting
Adult and Pediatric Norm: NHANES III
(For Asian-Americans the reference equations for Caucasians shall be used, but a
correction factor of 0.94 shall be applied to the predicted values.)
Best Effort Formula: Best Measurement
Composite Norm Values: NO
(For details, see “Reviewing the Operation Settings” on page 13.)
• Print Settings
Tests: Three Best Efforts
Scale: 20 mm/s & 10 mm/L
Curves: V/T & F/V
Auto Print: YES
(For details, see “Reviewing the Spirometry Print Settings” on page 17.)
• Calibration Settings
Auto Calibration Report: Yes
(For details, see “Reviewing the Calibration Settings” on page 15.)
Page 65

Directions for Use Appendix B Spirometry Protocols 61
About the OSHA / Cotton Dust Protocol
The OSHA (Occupational Safety & Health Administration, U.S.) Cotton Dust protocol is for
users who want to make sure that occupational testing and reports meet the
requirements of OSHA’s Cotton Dust standard. The device automatically performs as
described here, regardless of user-defined settings.
When using this protocol, the spirometer should be calibrated at three different flows
every day before use.
For details on OSHA / Cotton Dust requirements, see the document noted in Reference 8
on page 76.
When this protocol is selected, testing and reports are affected as follows:
• Operational Settings
Adult and Pediatric Norm: Knudson 1976
(African-American patients shall be adjusted by 0.85. Asian and Hispanic patients
shall be adjusted according to General Norm Value Race Adjustment logic.)
Composite Norm Values: NO
(For details, see “Reviewing the Operation Settings” on page 13.)
• Print Settings
Tests: Three Best Efforts
Scale: 20mm/s & 10mm/L
Curves: V/T & F/V
(For details, see “Reviewing the Spirometry Print Settings” on page 17.)
Page 66

62 Appendix B Spirometry Protocols Welch Allyn CP 200 Spirometry Option
About the SSD Protocol
The SSD (Social Security Disability) protocol is for users who want to make sure that
testing associated with disability determinations meet the requirement of the Social
Security Administration. The device automatically performs as described here, regardless
of user-defined settings.
For details on SSD requirements, see the document noted in Reference 2 on page 76.
When this protocol is selected, testing and reports are affected as follows:
• Calibration Settings
Auto Calibration Report: Yes
(For details, see “Reviewing the Calibration Settings” on page 15.)
• Print Settings
Tests: Three Best Efforts
Scale: 20mm/s & 10mm/L
Curves: V/T & F/V
(For details, see “Reviewing the Spirometry Print Settings” on page 17.)
• Calibrations must be presented in a volume-time format at a speed of at least 20 mm/
sec and a volume excursion of at least 10 mm/L to permit independent evaluation.
• Two of the satisfactory efforts should be reproducible for both pre-bronchodilator
tests and, if indicated, post-bronchodilator tests.
• A test is considered reproducible if the two best efforts’ FVC and FEV1 do not differ
by more than 5 percent or 0.1 L, whichever is greater.
• An effort is satisfactory for measurement of the FEV1 if the expiratory volume at the
back-extrapolated zero time is less than 5 percent of the FVC or 0.1 L, whichever is
greater.
• An effort is satisfactory for measurement of the FVC if maximal expiratory effort
continues for at least 6 seconds.
• The device should accurately measure time and volume, the latter to within +/- 1% of
a 3 L calibrating volume.
• The testing device must have had a recorded calibration performed previously on the
day of the measurement.
• The linearity of the device must be documented by recording volume calibrations at
three different flow rates of approximately 3 L/6 sec, 3 L/3 sec, and 3 L/sec.
• These calibrations may be exhale-only since no inhale parameters are reported.
• Whenever the test report is printed, the calibration report shall also be printed.
• If the calibration accuracy is between 1% and 3%, the electrocardiograph applies
correction factors to the recorded FVC and FEV1.
Page 67

63
C
Patient Help Sheets
About the Patient Help Sheets . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 64
Adult Smokers Help Sheet . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 65
Asthma Symptoms Help Sheet . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 66
Page 68

64 Appendix C Patient Help Sheets Welch Allyn CP 200 Spirometry Option
About the Patient Help Sheets
Two patient help sheets are available to print:
• Adult Smokers
If “patient education” is enabled, the Adult Smokers sheet prints automatically for all
adult smokers whenever you print a test. For example, see “Adult Smokers Help
Sheet” on page 65.
•Asthma Symptoms
If “patient education” is enabled, the Asthma Symptoms sheet prints automatically
for all patients whenever you print a test. For example, see “Asthma Symptoms Help
Sheet” on page 66.
These help sheets print only if “patient education” is enabled in the settings. To learn
how to enable “patient education,” see “Reviewing the Spirometry Print Settings” on
page 17.
The patient's name, FEV1%, and date print automatically on both sheets. If “ATS
Interpretation” is enabled, the appropriate recommendation is also marked. To learn how
to enable “ATS Interpretation,” see “Reviewing the Operation Settings” on page 13.
Note
If no recommendation is marked, the doctor must mark one.
1
1. Both help sheets come from a booklet entitled Simple Office Spirometry for Primary Care Practitioners, by
Thomas L. Petty, MD, and Paul L. Enright, MD. This booklet can be downloaded from the National Lung
Health Education Program (NLHEP) home page: www.nlhep.org/resources.html.
Page 69

Directions for Use Appendix C Patient Help Sheets 65
Adult Smokers Help Sheet
Name _________________________
What Your Lung Function Results Mean For Adult Smokers
You have just performed Spirometry, the basic test of how well your lungs are working.
The results indicate whether you have developed chronic obstructive pulmonary disease
(COPD) due to smoking. COPD occurs in about one of every five smokers after more than
20 years of smoking. COPD slowly “eats away” at the lung's reserves. Affected smokers
are often unaware of lung disease until more than half of their lung function has been lost.
Spirometry testing can detect COPD many years before symptoms occur.
___ Your test result was within the normal range. You do not appear to be developing
COPD. However, as a smoker, you remain at high risk of developing a heart attack,
stroke, and/or lung cancer. Call the number at the bottom of this page for help with
smoking cessation.
___ Your test result shows mild airways obstruction, suggesting that you are a
“susceptible smoker” who already shows signs of early COPD. You are unable to blow
out air as quickly as normal (your FEV1/FVC is low). If you continue smoking, you will
eventually develop disabling lung disease (in about 10-20 years). If you are able to
successfully quit smoking sometime soon, your lung function may return to normal levels
and you will probably never develop symptoms of COPD. Call the number at the bottom
of this page if you would like information about local resources to help you quit smoking.
___ Your test result shows moderate-to-severe airways obstruction. You have COPD. If
you continue smoking, your lung disease will certainly get worse and you will eventually
become short of breath while walking, climbing stairs, or doing other exercise. It is very
important that you seek help to stop smoking. If you are able to successfully quit smoking
sometime soon, you will probably regain a little lung function within three months, and
the abnormally rapid decline in your lung function which you have experienced due to
smoking will be stopped. Call the number at the bottom of this page for information about
local resources to help you quit smoking.
___ Your test shows a low forced vital capacity (FVC). Your FVC is the total amount of air
that you exhaled, in liters (similar to quarts). Values below about 80% are abnormally low
and suggest that you are unable to inhale or exhale as much air as most healthy persons
of your age, height, gender, and race. Obesity may be one of the causes of a mildly
decreased FVC, and pneumonia is another. Consider asking your physician to review this
report at some time during the next couple of months.
Your result: ______________ FEV1 % predicted
For more information contact:
____________________
Date
Page 70

66 Appendix C Patient Help Sheets Welch Allyn CP 200 Spirometry Option
Asthma Symptoms Help Sheet
Name _________________________
What Your Lung Function Results Mean For Those With Symptoms Suggesting
Asthma
You have just performed Spirometry, the basic test of how well your lungs are working.
The results may indicate whether you have asthma and its severity.
___ Your test was within the normal range. If you recently had symptoms such as
episodes of shortness of breath with wheezing, chest tightness, or cough, you may have
asthma, but your lung function is normal today. Consider visiting a physician when you
again have asthma symptoms and then repeat this Spirometry test. If you already know
that you have asthma, it is in good control.
___ Your breathing test shows mild airways obstruction (some narrowing of your
breathing tubes). You are currently unable to blow out air quickly. This result may indicate
asthma that is not well controlled. Discuss with your physician medications to better
control your asthma.
___ Your breathing test shows moderate-to-severe airways obstruction (narrowing of your
breathing tubes). You are currently unable to blow out air quickly. This result usually
indicates asthma that is poorly controlled. Discuss with your physician very soon the use
of medications that will help to better control your asthma and the value of peak flow
monitoring.
___ Your test shows a low forced vital capacity (FVC). Your FVC is the total amount of air
that you exhaled, in liters (similar to quarts). Values below about 80% are abnormally low
and suggest that you are unable to inhale or exhale as much air as most healthy persons
of your age, height, gender, and race. Obesity may be one of the causes of a mildly
decreased FVC, and pneumonia is another. Consider asking a physician to review this
report at some time during the next couple of months.
Your result: ______________ FEV1 % predicted
Your peak flow after inhaling a bronchodilator was ______ L/s (liters per second). You can
compare this value to the peak flow that you measure using your own peak flow meter.
The two numbers should match within 1 L/s. If your asthma is currently in good control,
today's value may be close to your best peak flow reading at home.
_______________________
Date
Page 71

67
D
Predictive Norms, etc.
Norm Profiles. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 68
List of Norm-Related Clinical Studies . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 69
About Norm Extrapolation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 70
About Race Adjustment. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 70
About Composite Norm Values . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 71
About Lung Age. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 72
About Quality Feedback. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 73
Understanding Your Interpretation Results . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 75
References . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 76
Page 72

68 Appendix D Predictive Norms, etc. Welch Allyn CP 200 Spirometry Option
Norm Profiles
Each predictive norm supports a particular subset of parameters and covers a particular
population, as shown here.
Parameters Studied Gender Age Race
Norm Name
(Abbrev.)
Berglund 1963
(be)
FVC
FEV1
FEV1%
FEV0.5
FEV3
FEV3/FVC
FEV6
FEV1/FEV6
PEF
FEF25-75
FEF75
FEF50
FEF25
FEF0.2-1.2
FEV0.5%
Male
Female
Pediatric
XXX X X ≥ 7 ≤ 70 X
Adult
Height (cm)
Weight (kg)
Smoke Years
Caucasian
Black
Hispanic
Asian
Crapo 1981 (cr) X X X X X X X X X No M: 15–91
Dockery 1983
(do)
ECCS/Quanjer
1993 (qu)
Gulsvik 2001
(gu)
Hedenström
1986 (he)
Hsu 1979 (hs) X X X X X X 7–20 No M: 111–200
Knudson 1976
(k)
Knudson 1983
(kn)
Koillinen 1998
(kl)
Kory 1961 (ko) X X X X No No 18–66 X
Morris 1971
(mo)
NHANES III
(nh)
Polgar 1971
(po)
Schoenberg
1978 (sc)
Solymar 1980
(so)
Viljanen 1981
(vi)
Zapletal 1969
(za)
X X X X 6–11 No 110–160 X X
XXX XXXXX X X No 18–70 M: 155–195
X X X X X X X No M: 15–91
X X X X X X X X No No 20–70 160–196 55–
XXX XXX X X ≥ 8 ≤ 90 X
XXX XXX X X ≥ 6M: ≤ 85
XXXX X X X X X 6–16 No X
XX X X X X No 20–84 X
XXX XXXX X X ≥ 8 ≤ 80 XXX
X X X X X X 3–19 No 110–170 X
XXX X XX X X ≥ 7 ≥ 18
XX X XXX X X 7–18 No X
XXX X XX X X No 18–65 X
X X X X X X X X 6–18 No M: 118–181
F: 17–84
F: 17–84
F: ≤ 88
F: 145–180
M: 157–194
F: 146–178
F: 111–180
M: 111.8–195.6
F: 106.7 –182.9
F: 107–173
1095–55
11.7 –
137.2
X
XX X
X
X
XXX
X
XX
X
Page 73

Directions for Use Appendix D Predictive Norms, etc. 69
List of Norm-Related Clinical Studies
Each of the following studies provides expected values for various spirometric
parameters by measuring significant samples of a particular population.
Norm Clinical Study
Berglund 1963 Reference Spirometric Studies in Normal Subjects: Forced Expiratograms in Subjects 7-70 Years of Age, Berglund, et. al.,
Crapo 1981 Reference Spirometric Values using Techniques and Equipment that Meet ATS Recommendations, Crapo, et. al.,
Dockery 1983 Distribution of Forced Vital Capacity and Forced Expiratory Volume in One Second in Children 6-11 Years of Age, Dockery,
ECCS/Quanjer
1993
Gulsvik 2001 Forced Spirometry Reference Values for Norwegian Adults: The Bronchial Obstruction in Nord-Trondelag Study,
Hedenström 1986 Reference Values for Lung Function Tests in Men: Regression Equations With Smoking Variables, Hedenström, et. al.,
Hsu 1979 Ventilatory Functions of Normal Children and Young Adults — Mexican American, White and Black, Katharine HK Hsu, et.
Knudson 1976 The Maximal Expiratory Flow-Volume Curve Normal Standards, Variability, and Effects of Age, Ronald J. Knudson, Ronald
Knudson 1983 Change in the Normal Expiratory Flow Volume Curve With Growth and Aging, Ronald Knudson, et. al., American Review of
Koillinen 1998 Terveiden suomalaislasten spirometrian ja uloshengityksen huippuvirtauksen viitearvot, Hannele Koillinen, Suomen
Kory 1961 The Veterans Administration Army Cooperative Study of Pulmonary Function, Clinical Spirometry in Normal Men, Kory, et.
Acta Medica Scandinavica, volume 173, 1963.
American Review of Respiratory Disease 1981, 123:659-664.
et. al., American Review of Respiratory Disease
Lung Volumes and Forced Ventilatory Flows: Official Statement of the European Respiratory Society, Quanjer, et. al.,
European Respiratory Journal, 1993, supplement 16: 5-40.
Langammer, Gulsvik , et. al., European Respiratory Journal
Upsala Journal of Medicine Science 91:299-310, 1986.
al., Journal of Pediatrics, July 1979, volume 95, 14-23.
C. Slatin, Michael D. Lebowitz, and Benjamin Burrows, et. al., American Review of Respiratory Disease
Respiratory Disease 1983 127, 725-734.
Laakarilehti, et. al., 1998.
al., American Journal of Medicine
, February 1961.
1983, 128:405-412.
2001, 18: 770-779.
, volume 113, 1976.
Morris 1971 Spirometric Standards for Healthy Non-smoking Adults, James F. Morris, et. al., American Review of Respiratory Disease
volume 103, 1971.
NHANES III Spirometric Reference Values from a Sample of the General U.S. Population, John L. Hankinson, John R. Odencrantz, and
Polgar 1971 Pulmonary Function Testing in Children: Techniques and Standards, Polgar and Promadhat.1971.
Schoenberg 1978 Growth and Decay of Pulmonary Function in Healthy Blacks and Whites, Janet B. Schoenberg, Gerald J. Beck, and Arend
Solymar 1980 Nitrogen Single Breath Test, Flow Volume Curves and Spirometry in Healthy Children, 7 -18 Years of Age, L. Solymar, P. H.
Viljanen 1981 Spirometric Studies in Non-smoking, Healthy Adults, Viljanen, et. al., Journal of Clinical Lab Investigation
Zapletal 1969 Maximum Expiratory Flow-Volume Curves and Airway Conductance in Children and Adolescents, Journal of Applied
Kathleen B. Fedan, et. al., Division of Respiratory Disease Studies, National Institute for Occupational Safety and Health,
Centers for Disease Control and Prevention, Morgantown, West Virginia, 1999. The Third National Health And Nutrition
Examination Survey (NHANES III).
Bouhuys, et. al., Respiration Physiology
Aronsson, B. Bake, and J. Bjure.
159, 5-20, 1981.
Physiology, volume 26, number 3, March 1969.
, 1978, 33, 367-393.
, 41 supplement
,
Page 74

70 Appendix D Predictive Norms, etc. Welch Allyn CP 200 Spirometry Option
About Norm Extrapolation
Extrapolation is the practice of applying a norm’s formula to a patient who doesn’t fit that
norm’s demographics. For example, if you were testing an 88-year-old man, and the
primary (selected) norm were based on males 85 or younger, the predicted values would
be extrapolated values.
• When it takes place, extrapolation is indicated in the test record.
• Pediatric norms do not provide any age, weight, or height extrapolation.
• Adult norms allow extrapolation of age up, but not down.
• Adult norms allow extrapolation of height, weight, and smoke years, up and down.
About Race Adjustment
Although expected values for certain parameters vary significantly between ethnic
groups, some norm studies do not include separate regression equations for different
races. For those studies, the following table describes the adjustments made by the
CP 200 software for the FVC and FEV1 predicted values. Where applicable, norm values
are multiplied by the percentages identified in the following table.
Race Choices FVC & FEV1 Recommendation Source
Caucasian No adjustment —
Black 88% ATS
Asian 94% NIOSH
Hispanic No adjustment None found
Note
Race adjustment applies for adults only.
If a race adjustment percentage is used, the same adjustment is applied to the
LLN value.
Page 75

Directions for Use Appendix D Predictive Norms, etc. 71
About Composite Norm Values
When the primary (selected) norm does not support a given parameter — and when
composite norm values are enabled in the operation settings — the missing value is filled
in from one of the alternative (composite) norm sources, listed here. For example, since
the Crapo norm does not support FEV6, this value is filled in from NHANES III.
Composite Norm Source Parameters Filled In When Not Supported in Primary Norm
NHANES III FVC, FEV1, FEV1%, FEV6, FEV1/FEV6, PEF, FEF25-75
Crapo 1981 FEV0.5, FEV3, FEV3/FVC
Morris 1971 FEF0.2-1.2
ECCS/Quanjer 1993 FEF25, FEF50, FEF75
The primary norm takes precedence over the composite source. For example, since the
Crapo norm supports the FVC parameter, this value always comes from Crapo, not from
the composite source.
Composite values are used when the patient does not fit the demographics of either
primary norm (adult or pediatric). For example, if the primary norms are Dockery and
Morris, a 14-year-old patient fits neither norm due to age restrictions. The software would
use values from the appropriate composite norms, for example, NHANES III or ECCS/
Quanjer 1993. It would not use values from Dockery or Morris.
On the screen and in reports, an abbreviation identifies the norm source for each
composite value used. For example, the abbreviation for Polgar is “po.” All norm
abbreviations are listed under “Norm Profiles” on page 68.
Also see “Norm Profiles” on page 68 for a listing of the parameters included in each
norm.
To learn how to enable or disable composite norm values, see “Reviewing the Operation
Settings” on page 13.
Page 76

72 Appendix D Predictive Norms, etc. Welch Allyn CP 200 Spirometry Option
About Lung Age
Lung age is a calculated value based on a patient’s demographics and spirometric
performance that gives a relative indication of the health of the subject's lungs. This value
is used primarily to encourage smoking cessation.
The CP 200 spirometer, calculates lung age values according to the document cited in
Reference 5 on page 76 (Morris 1995). For single-effort tests, lung age is based on the
current effort. Otherwise, it is based on the patient’s “best” effort, as defined in the
settings.
Lung age results less than 20 years are reported as “<20,” and results greater than 84 are
reported as “>84.” This limitation is derived from the subject population on which Morris
based his research.
Lung age, which is expressed in years, is the average of the four formulas in the Morris
article (FVC, FEV1, FEF25-75%, and FEF0.2-1.2). Specifically, lung age is calculated as
follows:
Gender Lung Age Formula
Men
Women
[5.920 (height) – 40.000 (FVC) – 169.640 +
2.870 (height) – 31.250 (FEV1) – 39.375 +
2.319 (height) – 21.277 (FEF200-1200) + 42.766 +
1.044 (height) – 22.222 (FEF25%-75%) + 55.844 ] / 4
[4.792 (height) – 41.667 (FVC) – 118.833 +
3.560 (height) – 40.000 (FEV1) – 77.280 +
4.028 (height) – 27.778 (FEF200-1200) – 70.333 +
2.000 (height) – 33.333 (FEF25%-75%)+18.367 ] / 4
Page 77

Directions for Use Appendix D Predictive Norms, etc. 73
About Quality Feedback
The spirometer provides two kinds of quality feedback: effort-quality messages and testquality grades, as described in the following sections.
About Effort-Quality Messages
One of the following effort-quality messages appears on the screen after each effort is
completed. These messages indicate whether an effort was acceptable and reproducible,
and if not, what the patient needs to do differently.
For an example of the “effort complete” screen where these messages would appear,
see Figure 30 on page 44.
The term “match” here means “variation” or “difference with respect to best test.”
Effort-Quality
Message
Don’t hesitate Back-extrapolated volume > 150 mL or 5%, whichever is greater.
Blast out faster PEF time > 120 ms.
Blow out longer FET < 6.0 seconds, and end-of-test volume > 100 mL (invalid FEV6).
Blast out harder PEF is not reproducible (match > 1.0 L/s).
Deeper breath FEV6 match > 150 mL FVC may be substituted for FEV6.
Good effort Effort meets above criteria.
Good test session Two acceptable efforts match.
Criteria
Page 78

74 Appendix D Predictive Norms, etc. Welch Allyn CP 200 Spirometry Option
About Test-Quality Grades
Another type of feedback is the test-quality grade, as described in the following table.
If Print Quality Grades is enabled in the settings, a grade appears on printed reports and
also displays on screen when you view the values or interpretation of a completed test
(as described under “To View a Test’s Results” on page 46).
To learn how to enable or disable this setting, see “Reviewing the Spirometry Print
Settings” on page 17.
Test-Quality
Grade
A 2 or more Largest two FEV1 values match ≤ 100 mL.
B 2 or more Largest two FEV1 values match > 100 and ≤ 150 mL.
C 2 or more Largest two FEV1 values match > 150 and ≤ 200 mL.
D 1 or more Largest two FEV1 values match > 200 mL.
FNone
Number of Acceptable Efforts Reproducibility
Largest two FVC values match ≤ 100 mL.
Page 79

Directions for Use Appendix D Predictive Norms, etc. 75
Understanding Your Interpretation Results
This diagram shows how the automatic interpretation software uses a patient's FVC and
FEV1 results, in comparison with normal values, to determine the degree of obstruction
or restriction. This diagram follows the American Thoracic Society’s example for
interpretation.
For details on interpretative strategies, see the document noted in Reference 9 on
page 76.
Figure 36. Data Interpretation, Process Diagram
Page 80

76 Appendix D Predictive Norms, etc. Welch Allyn CP 200 Spirometry Option
References
1. Checklist for Compliance with NLHEP Guidelines for Office Spirometers, National
Lung Health Education Program, www.nlhep.org/resources.html#review.
2. Disability Evaluation Under Social Security (the “blue book”), Social Security
Administration SSA publication number 64-039, Office of Disability Programs ICN
468600, January 2003.
See in particular the calibration and reporting sections of this document.
3. Lung Function Testing: Selection of Reference Values and Interpretive Results,
American Thoracic Society, March 1991.
This document describes the methods of selecting the reference values and the
algorithm for interpretative results.
4. National Occupational Respiratory Mortality System, National Institute for
Occupational Safety and Health (NIOSH).
5. Short Report Spirometric “Lung Age” Estimation for Motivating Smoking Cessation,
James F. Morris, M.D., and William Temple, Preventive Medicine
14, 655-662 (1985).
6. Standardization of Spirometry, 1994 Update, American Thoracic Society.
This document describes the methods of acquiring the output parameters and
the required accuracy. For details on ATS acceptability criteria, see these
sections:
• “FVC — Satisfactory Start of Test Criteria,” page 1120
• “FVC — Test Result Reproducibility,” page 1122
7. Standardized Lung Function Testing, European Respiratory Journal
supplement 16, March 1993.
8. U.S. Pulmonary Function Standards for Cotton Dust Standard, 29 CFR 1910.1043,
Appendix D.
9. Lung Function Testing: Selection of reference values and interpretive strategies.
American Thoracic Society, American Review of Respiratory Disease
(1991).
, volume 6,
, 144:1202-1218
Page 81

77
Glossary
adult. Generally, 18 or older. Age limits vary with each norm.
AT S. American Thoracic Society. An organization that provides standards for spirometry common practice
and equipment.
ATS acceptability criteria. Applicable to FVC testing only. (1) Criteria ensuring that an individual effort
started and ended satisfactorily (no leaks or coughs). (2) Criteria ensuring that the patient has made at
least two efforts of the same kind (two FVC-pre or two FVC-post), and that these efforts are
reproducible. For details, see document noted in Reference 6 on page 76.
ATS interpretive results. The software generates interpretive results as described in the document
noted in Reference 3 on page 76.
baseline. See pre-test.
best effort. A measurement calculated from a set of efforts. The formula for calculating best effort is
user-selectable: (1) the single best effort or (2) a composite of best parameter values.
BF. Breathing frequency. See also MV and tidal breathing.
bronchospasm evaluation. See post-test.
BTPS. B
CardioPerfect workstation. A PC using Welch Allyn CardioPerfect software. Stores ECG and spirometry
composite norm value. A value that is filled in from another norm — a “composite norm source” —
ody conditions, normal body temperature (37° C), ambient pressure, saturated with water vapor.
The BTPS correction factor converts ambient conditions — temperature, humidity, and pressure —
to BTPS.
test data. Can communicate with other electronic patient-information systems, such as billing and
medical records.
when the primary (selected) norm does not support a given parameter. Applicable only when
composite norm values are enabled.
COPD. Chronic obstructive pulmonary disease. Characterized by airflow obstruction that is primarily
caused by smoking. Examples include emphysema, chronic bronchitis, and asthmatic bronchitis.
curve. A graphical display of spirometry data. During SVC testing, only one curve type is available: volume/
time. During FVC testing, four curve types are available: volume/time, flow /volume, tidal volume, and
(on screen only) incentive.
Page 82

78 Glossary Welch Allyn CP 200 Spirometry Option
effort. A single spirometry maneuver, for example, one blow. A single test comprises multiple efforts.
See also best effort.
ERS. European Respiratory Society.
ERV. Expiratory reserve volume (in liters). The maximum volume that can be expired from the level of the
functional residual capacity (FRC). See also tidal breathing.
extrapolation. The practice of applying a norm’s formula to a patient who doesn’t fit that norm’s
demographics. For example, if you were testing an 88-year-old man, and the primary (selected) norm
were based on males 85 or younger, the predicted values would be extrapolated values.
FEF50/FIF50. The ratio of these two parameters. See FEF50 and FIF50.
FEF25. Forced expiratory flow (in L/s) at 25% of FVC.
FEF50. Forced expiratory flow (in L/s) at 50% of FVC.
FEF75. Forced expiratory flow (in L/s) at 75% of FVC.
FEF85. Forced expiratory flow (in L/s) at 85% of FVC.
FEF0.2-1.2. Forced expiratory flow average (in L/s) between 0.2 and 1.2 liters of FVC.
FEF25-75. Forced expiratory flow average (in L/s) during the middle half of FVC.
FEF75-85 (“late” FEF). Forced expiratory flow average (in L/s) between 75% and 85% of FVC.
FET. Forced expiratory time (in seconds). The elapsed time from the beginning of expiration until a
specified percentage of FVC.
FEV0.5. Forced expiratory volume (in liters) at 0.5 seconds.
FEV1. Forced expiratory volume (in liters) at 1 second. An important parameter because it reflects the
severity of COPD.
FEV1/FEV6. The ratio of these two parameters. See FEV1 and FEV6.
FEV1/FVC. See FEV1%.
FEV2. Forced expiratory volume (in liters) at 2 seconds.
FEV3. Forced expiratory volume (in liters) at 3 seconds.
FEV5. Forced expiratory volume (in liters) at 5 seconds.
FEV6. Forced expiratory volume (in liters) at 6 seconds.
Page 83

Directions for Use Glossary 79
FEV0.5%. FEV0.5 as % of FVC.
FEV1%. FEV1 as % of FVC. Same as FEV1/FVC. A parameter for a single FVC effort.
FEV1% formula. A user-selectable formula that determines the calculation method for a test’s (not an
effort’s) overall FEV1% value, which affects the automatic interpretation.
FEV2%. FEV2 as % of FVC.
FEV3%. FEV3 as % of FVC.
FEV5%. FEV5 as % of FVC.
FEV6%. FEV6 as % of FVC.
FEVt. Timed forced expiratory volume (in liters). Volume of air exhaled in the specified time during an FVC
effort.
FIF50. Forced inspiratory flow (in L/s) at 50% of FIVC.
FIV1. Forced inspiratory volume (in liters) at one second.
FIV1%. FIV1 as % of FIVC.
FIVC. Forced inspiratory vital capacity (in liters). The maximum volume of air that can be inspired during
forced inspiration starting from full expiration.
FIVt. Timed forced inspiratory volume (in liters). Volume of air inhaled in the specified time (t).
flow. The speed at which air is inhaled or exhaled (in L/s).
flow = f(v). See flow/volume.
flow/volume. Same as flow over volume or flow = f(V). A type of data curve available during FVC testing.
The y axis represents flow (L/s); the x axis represents volume (liters).
flow loop. A flow/volume curve that includes inspiratory data (negative values on the y axis).
FRC. Functional residual capacity (in liters). Volume of air remaining in the lungs and airway at the average
end-expiratory level.
FVC. Forced vital capacity. (1) A type of test in which patients inhale fully and exhale forcefully for as long
as they can. The goal: to measure the volume and flow of air. May or may not include forced inhaling.
When forced inhaling is included, it may be done either before or after exhaling. See also flow loop.
(2) An important parameter (in liters): the maximum volume of air that can be delivered during forced
expiration starting from full inspiration.
IC. Inspiratory capacity (in liters). The maximum volume of air that can be inhaled after a normal —
unforced — exhalation. See also tidal breathing.
Page 84

80 Glossary Welch Allyn CP 200 Spirometry Option
incentive screen. An animated screen that gives patients — usually children — a goal to achieve while
exhaling. This screen is listed as a type of “curve” (data display) available during FVC testing.
IRV. Inspiratory reserve volume (in liters). The maximum volume that can be inspired from the average
end-inspiratory level. See also tidal breathing.
LLN. Lower limits of normal. The lowest expected value for a spirometric parameter. The method of
determining this value varies from norm to norm.
loop. See flow loop.
lung age. A calculated value based on a patient’s demographics and spirometric performance that gives a
relative indication of the health of the subject's lungs. This value is used primarily to encourage
smoking cessation. Lung age is not available for patients under 20 years of age.
maneuver. See effort.
MV. Minute volume (in liters). MV = BF · VT. See also tidal breathing.
NIOSH. National Institute for Occupational Safety and Health (U.S.).
norm. A research-based spirometry data set with a specific profile for race, gender, age, and height. The
software compares each patient’s results with data in the primary (selected) norm, reporting the
results as percentages of the predicted (normal) values.
normal. Consistent with norm data.
OSHA. Occupational Safety & Health Administration (U.S.).
parameter. A commonly defined attribute of a spirometric waveform (FVC, FEV1, and so on).
pediatric. Generally, under 18 years old. Age limits vary with each norm. Also, young children’s lung sizes
vary greatly. Norm values and interpretive results are not available for patients under 3 years of age.
PEF. Peak expiratory flow (in L/s). The largest expiratory flow achieved with a forced effort.
PIF. Peak inspiratory flow (in L/s). The largest inspiratory flow achieved with a forced effort.
post-test. A test that provides data to compare with pre-test data. Sometimes called post-Rx or post-BD
(bronchodilator). A post-test must follow a pre-test within 24 hours. See also reversibility.
predictive curve. A curve that follows a set of predictive points.
predictive points. Key values from the selected norm and from composite norms (if enabled). Applicable
for FVC tests only. For flow/volume curves, predictive values are PEF, FEF25, FEF50, FEF75, and FVC
(all represented as points). For volume/time curves, predictive values are FEV1 (represented as a
point) and FVC (represented as a horizontal line). If predictive points are enabled, all available
predictive values appear on the screen and the printout.
Page 85

Directions for Use Glossary 81
pre-test. A test that provides a baseline for comparison with a post-test taken by the same patient.
Sometimes called pre-Rx or pre-BD (bronchodilator). Pre-tests and post-tests are commonly used to
evaluate the effectiveness of medication. See also reversibility.
reversibility. The percentage difference between pre-test and post-test data. This measurement
indicates the effect of medication on lung function. Reversibility applies to each parameter separately.
The reversibility formula, which determines the way in which reversibility is calculated, is userselectable.
SVC. Slow (relaxed) vital capacity. (1) A type of test in which patients breathe normally several times, then
inhale maximally and exhale maximally, or vice versa. Sometimes SVC testing is used when forced
breathing is impossible. The patient inhales and exhales as completely as possible, as in FVC testing,
but the breathing is not forced. The goal of an SVC effort is to measure the volume of air inhaled and
exhaled, not the air flow (speed). (2) An important parameter (in liters): the maximum volume of air
exhaled from the point of maximum inhalation, or maximum volume of air inhaled from a point of
maximum exhalation.
test. A set of efforts — up to 6 efforts of each type (FVC and SVC) for a maximum of 12 efforts (6 FVC and
6 SVC). The 6 efforts of a given type can be a mixture of pre-medication and post-medication efforts.
Tex. Tidal breathing expiration time (in seconds). See also tidal breathing.
tidal breathing. Multiple breaths, normal breathing. May be used during FVC or SVC testing. After
measuring tidal breathing for several seconds, the following parameters can be extrapolated: MV, VE,
BF, and Tin/Tex. If you combine a VT measurement with a VC measurement, you can also calculate
the ERV, IC, and IRV. For example, COPD patients have a higher ERV and a lower IC and IRV.
tidal volume. See VT.
tidal volume curve. A flow loop that includes all data from all breaths, tidal and forced.
Tin. Tidal breathing inspiration time (in seconds). See also tidal breathing.
Tin/Tex. The ratio of Tin and Tex. See also Tin and Tex .
TV. See VT.
variance. The difference between the best and worst efforts for a parameter (FEV1, FVC, and so on). Pre-
test and post-test variances are reported separately. See also best effort.
VC. Vital capacity. See FVC or SVC.
VE. Ventilation in L/min. See also tidal breathing.
vital capacity. See FVC or SVC.
volume = f(t). See volume/time.
volume/time. Same as volume over time or volume = f(t). A type of data curve available during both FVC
and SVC testing. The y axis represents liters; the x axis represents seconds.
Page 86

82 Glossary Welch Allyn CP 200 Spirometry Option
VT. Tidal volume (in liters). Also called TV, although VT is the preferred abbreviation. The volume of air that
enters the lungs during inspiration and leaves the lungs during expiration in a normal breathing cycle.
One of the most important parameters in SVC testing. See also MV, tidal breathing, and tidal volume
curve.
workstation. See CardioPerfect workstation.
Page 87

Index
83
A
accessories. See parts and accessories
"Add/Edit Interpretations" screen
adult
, 77
Adult Smoker help sheet
, 64, 65
Asthma Symptoms help sheet
atmospheric pressure units, selecting
ATS acceptability criteria
, 47
, 64, 66
, 12
, 76, 77
ATS interpretation. See interpretation
automatic calibration reports. See calibration, report printing
Auto Send to memory card
, 21
B
baseline. See pre-testing
Berglund norm
best effort
BF
, 77
, 68, 69
, 13, 77
bronchospasm evaluation. See post-testing
BTPS
, 77
C
calibration
procedure
report printing
settings
troubleshooting
calibration syringe
cleaning
description & illustration
ordering replacement
warranty period
CardioPerfect workstation
caution symbol defined
CD (product information), ordering replacement
Celsius, selecting
cleaning, why to avoid
communication settings (Auto Send to memory card)
components of spirometer
composite best effort
composite norm values
configuration. See settings
, 24–30
, 15, 30
, 15
, 52
, 8
, 3
, 9
, 53
, 21, 48, 77
, 6
, 9
, 12
, 8
, 21
, 3
, 13
, 13, 71, 77
connection of spirometry components
contact information
contraindications
COPD
, 65, 77
, ii
, 4
, 39
Cotton Dust protocol. See OSHA / Cotton Dust protocol
Crapo norm
curves
, 68, 69, 71
, 77
See also individual curve types
Customer Service
, ii
D
data. See patient data
default FVC curve, selecting
demographics for norms
, 16
, 68
See also patient data
Dockery norm
, 68, 69
E
ECCS/Quanjer norm, 68, 69, 71
"Edit Interpretation List" screen
, 20
effort-quality messages. See quality messages
"Enter New Patient" screen
ERV
, 37, 78
extrapolation of norm values
, 41
, 70, 78
F
Fahrenheit, selecting, 12
features
, 5
FEF parameters
FET
, 78
FEV1%
definition
included in patient help sheets
selecting formula for
FEV parameters
FIF50
, 79
FIV parameters
flow
, 79
flow loop
, 78
, 79
, 64, 65, 66
, 13
, 37, 78–79
, 79
, 35, 79
Page 88

84 Index Welch Allyn spirometer Electrocardiograph
flow transducer
description
ordering replacements
warranty period
, 3, 39, 55
, 9
, 53
flow units (L/sec or L/min), selecting
flow/volume curve
as default
definition
examples
FRC
, 79
FVC curve, selecting default
, 16, 18
, 79
, 35, 44
, 16
See also individual curve types
FVC parameters
, 16, 37, 56, 79
See also individual parameters
FVC test procedure
, 33–44
G
grades. See quality grades
graphs. See curves
Gulsvik norm
, 68, 69
H
Hedenström norm, 42, 68, 69
help, getting
help sheets. See patient help sheets
Hsu norm
, 10
, 68, 69
, 12
L
LLN, 44, 70, 80
loop. See flow loop
L/sec or L/min, selecting
lung age
, 72, 76
, 12
M
mandatory data fields, 42
mbar, selecting
memory card, sending tests to
menu tree, spirometry settings
mmHg, selecting
Morris norm
, 12
, 21, 48
, 12
, 12
, 68, 69, 71
multiple-flow calibration. See calibration
MV
, 80
MVV effort type (not supported)
, 4
N
NHANES III norm, 68, 69, 71
NIOSH protocol
NLHEP compliance, reference
norms
, 13, 67–76
See also composite norm values and individual norms
nose clips
, 13, 60, 76
, 76
, 3, 9
I
IC, 37, 79
incentive screen
indications for use
inHg, selecting
installation
intended use
Internet address
, 16, 36
, 4
, 12
, 8, 39
, 4
, ii
interpretation
adding or editing in a test
editing the phrase list
enabling
, 13
and patient help sheets
process diagram
references
IRV
, 80
, 75
, 76
K
Knudson norms, 68, 69
Koillinen norm
Kory norm
kPa, selecting
, 68, 69
, 68, 69
, 12
, 20
, 64
, 47
O
"Operation Settings" menu, 13
ordering information
OSHA / Cotton Dust protocol
, 9
, 13, 61, 76
P
parameters, 37, 80
See also FVC parameters or SVC parameters
parts and accessories
patient data
enabling and disabling fields
entering or searching for
patient help sheets
PCP protocol
pediatric
PEF
, 80
, 13, 58–59
, 80
phone numbers
PIF
, 80
pneumotach
Polgar norm
, 55
, 36, 68, 69
posters, ordering replacements
post-testing
, 38
power specifications
predictive curve and points
, 8, 9
, 19
, 42
, 18, 63–66
, ii
, 9
, 55
, 13, 80
Page 89

Directions for Use Index 85
predictive norms. See norms
predictive values
preparation of patient
, 44, 52
, 40
pressure tubing
description
ordering replacement
storage
warranty period
pressure units, selecting
pre-testing
print settings
"Print Test" screen
protocols
, 3, 39, 55
, 9
, 8
, 53
, 12
, 38
, 17
, 49
, 13, 57–62
See also individual protocols
Q
quality grades, 17, 46, 74
quality messages
, 44, 73
Quanjer. See ECCS/Quanjer norm
quick reference card, ordering replacement
, 9
R
race adjustment, 70
reference list
replacement of saved efforts
replacement parts and accessories
, 76
, 38
, 9
reports, calibration. See calibration, report printing
reports, spirometry
editing interpretative phrases
printing
, 17, 49
troubleshooting
reproducibility
reversibility
, 52
, 55, 73, 74, 76
, 13, 81
, 20, 47
S
safety, 7–8
Sani-Cloth ® canister, ordering replacement
Schoenberg norm
screen settings
, 42, 68, 69
, 16
SD card. See memory card
"Send Test" screen
, 48
sensor
connecting
description & illustration
ordering replacements
specifications
troubleshooting
warranty period
, 39
, 3
, 9
, 55
, 52
, 53
, 9
settings
Auto Send to memory card
calibration
interpretation list
operation
patient data
print
screen
, 15
, 20
, 13
, 19
, 17
, 16
spirometry (menu tree)
, 21
, 12
single-flow calibration. See calibration
smoke years
, 19, 42, 68
smoking
adult patient help sheet
and lung age
studies on
, 72
, 69
Social Security & Disability (SSD) protocol
Solymar norm
specifications
, 68, 69
, 55–56
"Spirometry Settings" menu tree
, 64, 65
, 13, 62, 76
, 12
SSD. See Social Security & Disability protocol
supplies
, 9
SVC curve examples
SVC parameters
, 36, 44
, 16, 37, 56, 81
See also individual parameters
SVC test procedure
symbols
, 6
, 33–44
syringe. See calibration syringe
T
Technical Support, ii
temperature units (F or C), selecting
, 12
test procedure. See FVC test procedure or SVC test procedure
test quality grades. See quality grades
"Test Results" screen
Tex
, 81
tidal breathing
, 45
, 35, 36, 81
tidal volume (FVC curve)
as default
definition
example
tidal volume (SVC parameter, "VT")
Tin
, 81
Tin/Tex
, 16, 18
, 81
, 35
, 37, 82
, 81
transducer. See flow transducer
troubleshooting
TV
, 81
, 51–52
U
units of measure, selecting, 12
URL
, ii
Page 90

86 Index Welch Allyn spirometer Electrocardiograph
V
VC, 81
VE
, 81
verifying calibration
"View Results" screen
Viljanen norm
, 25, 27, 29, 30
, 46
, 68, 69
vital capacity. See FVC or SVC
volume/time curve
as default
definition
examples
VT
, 37, 82
, 16, 18
, 81
, 35, 45
W
warning symbol defined, 6
warranty
web site
Welch Allyn Technical Support
workstation. See CardioPerfect workstation
, 53
, ii
, ii
Z
Zapletal norm, 68, 69
Page 91

Page 92

4341 State Street Road, PO Box 220, Skaneateles Falls, NY 13153-0220 USA
1 800 535 6663, + 1 315 685 4560 www.welchallyn.com
Reorder Number (multi-language CD): 401151
Mat. Number (manual only): 703411, Ver: C
 Loading...
Loading...