Page 1

CP 200™ 12-Lead Resting
CP200
Electrocardiograph
Directions for Use
Page 2

ii Welch Allyn CP 200 Electrocardiograph
Copyright 2005, Welch Allyn, Inc. All rights are reserved. No one is permitted to reproduce or duplicate, in
any form, this manual or any part thereof without permission from Welch Allyn.
Caution: Federal US law restricts sale of the device identified in this manual to, or on the order of, a
licensed physician.
Welch Allyn assumes no responsibility for any injury, or for any illegal or improper use of the product, that
may result from failure to use this product in accordance with the instructions, cautions, warnings, or
indications for use published in this manual.
Welch Allyn is a registered trademark of Welch Allyn, Inc., and CP 200 and CardioPerfect are trademarks of
Welch Allyn, Inc.
SD is a trademark of Toshiba.
Software in this product is Copyright 2005, Welch Allyn, Inc., or its vendors. All rights are reserved. The
software is protected by United States of America copyright laws and international treaty provisions
applicable worldwide. Under such laws, the licensee is entitled to use the copy of the software
incorporated within this instrument as intended in the operation of the product in which it is embedded.
The software may not be copied, decompiled, reverse-engineered, disassembled or otherwise reduced to
human-perceivable form. This is not a sale of the software or any copy of the software; all right, title and
ownership of the software remains with Welch Allyn or its vendors.
For information about any Welch Allyn product, please call Welch Allyn Technical Support:
USA 1 800 535 6663
+ 1 315 685 4560
Canada 1 800 561 8797 China + 86 216 327 9631
European Call Center + 353 46 906 7790 France + 33 15 569 5849
Germany + 49 747 792 7186 Japan + 81 33 219 0071
Latin America + 1 315 685 2644 Netherlands + 31 15 750 5000
Singapore + 65 6419 8100 South Africa + 27 11 777 7555
United Kingdom + 44 207 365 6780 Sweden + 46 85 853 6551
Australia + 61 29 638 3000
800 074 793
Reorder Number (multi-language CD): 401151
Mat. Number (manual only): 701557, Ver: D
Welch Allyn
4341 State Street Road, PO Box 220
Skaneateles Falls, NY 13153-0220
www.welchallyn.com
Printed in USA
Page 3

Contents
1 - Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1
iii
About This Manual . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
Product Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
Intended Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
Indications for Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
Contraindications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
Standard Features & Benefits. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
Options . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
Accessories . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
Controls, Indicators, and Connectors . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
About the Main Menu. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10
Moving Through the Menus . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
About the Patient Cable and Leads. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
Symbols . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
Using the Electrocardiograph Safely . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
General Warnings. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
General Cautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
Getting Help . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 18
2 - Setting Up the Electrocardiograph . . . . . . . . . . . . . . . . . . . . . . . . . 19
Inspecting the Electrocardiograph . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20
Connecting the Patient Cable . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20
Loading the Thermal Chart Paper . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
Powering the Electrocardiograph . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 22
Verifying Proper Operation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23
3 - Reviewing the System Settings . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25
“System Settings” Menu Tree . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 26
Reviewing the Device Configuration Settings. . . . . . . . . . . . . . . . . . . . . . . . . . . 27
Reviewing the Device Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 29
Reviewing the Medication List . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 30
Reviewing the History List . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 31
4 - Reviewing the ECG Settings. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 33
“ECG Settings” Menu Tree . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 34
Reviewing the Auto Report Settings. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 35
Reviewing the Format Settings for Auto Reports. . . . . . . . . . . . . . . . . . . . . 37
Reviewing the Interpretation and Copy Settings for Auto Reports. . . . . . . . 39
Reviewing the Patient Data Fields Available for Auto Reports . . . . . . . . . . . 40
Page 4

iv Contents Welch Allyn CP 200 Electrocardiograph
Reviewing the Rhythm Report Settings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 42
Reviewing the Miscellaneous ECG Settings. . . . . . . . . . . . . . . . . . . . . . . . . . . . 43
5 - Performing ECG Tests . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 45
Connecting the Leads to the Patient . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 46
Recording an Auto ECG . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 49
Recording a Normal Auto ECG . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 50
Recording a Stat Auto ECG . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 55
Recording a Rhythm ECG . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 56
Searching for Saved Patient Data . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 57
Adjusting the ECG Waveforms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 61
6 - Performing Administrative Tasks. . . . . . . . . . . . . . . . . . . . . . . . . . . 63
Managing Saved Tests . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 64
Managing the Scheduled Patients List . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 68
Managing Data Security . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 69
Working With the User List and User Login. . . . . . . . . . . . . . . . . . . . . . . . . 70
Working With the Audit Trail. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 72
7 - Maintaining the Electrocardiograph . . . . . . . . . . . . . . . . . . . . . . . . 73
Inspecting the Equipment. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 74
Cleaning the Equipment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 74
Testing the Equipment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 75
Recharging a Fully Discharged Battery . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 76
Replacing the Battery . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 77
Replacing the Battery (DC) Fuse. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 79
Replacing the AC Fuses . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 80
Storing the Equipment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 81
Discarding the Equipment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 81
8 - Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 83
Problem-Solving Suggestions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 84
Limited Warranty . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 87
Service Policy . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 88
A - Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 89
B - EMC Guidance and Manufacturer’s Declarations . . . . . . . . . . . . . 91
Glossary . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 95
Index . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 97
Page 5
1
1
Introduction
About This Manual. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
Product Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
Intended Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
Indications for Use. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
Contraindications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
Standard Features & Benefits . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
Options . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
Accessories . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5
Controls, Indicators, and Connectors . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6
About the Main Menu . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10
Moving Through the Menus . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
About the Patient Cable and Leads . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
Symbols . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
Using the Electrocardiograph Safely . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
Getting Help. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 18
Page 6

2 Chapter 1 Introduction Welch Allyn CP 200 Electrocardiograph
About This Manual
This manual is written for clinical professionals with a working knowledge of medical
procedures and terminology as required for monitoring cardiac patients.
Before using the CP 200 electrocardiograph for clinical applications—or before setting up,
configuring, troubleshooting, or servicing the electrocardiograph—you must read and
understand this manual and all other information accompanying the electrocardiograph
and related options or accessories.
Product Overview
The Welch Allyn CP 200 electrocardiograph can display, print, save, and send ECGs
electronically. It features a full alphanumeric keyboard, a color display to preview ECGs
and edit settings, storage for up to 50 ECG and 50 spirometry records, full-size userprogrammable reports, and the ability to operate on either battery or AC power.
For centralized ECG data storage, the CP 200 electrocardiograph can connect to a Welch
Allyn CardioPerfect™ workstation, which in turn can connect with other electronic
patient-information systems, such as billing and medical records.
For details, see the following sections:
• “Standard Features & Benefits” on page 3
• “Options” on page 4
• “Specifications” on page 89
Intended Use
The CP 200 electrocardiograph is specifically intended for acquiring, viewing, storing, and
printing ECG signals from adult and pediatric patients. It will be used in clinical settings by
trained healthcare providers.
The optional interpretation algorithm analyzes these ECG signals to generate
measurements and interpretive statements for adults. The interpretive results are
intended only as guidance for qualified physicians and must not be relied upon as
diagnoses.
The electrocardiograph provides an optional interface to a pulmonary function device.
Communication of ECG and spirometry data with a central data-management system is
optional.
Page 7

Directions for Use Chapter 1 Introduction 3
Indications for Use
The electrocardiograph is one of the tools that clinicians use to evaluate, diagnose, and
monitor patient cardiac function.
The 12-lead interpretive algorithm provides a computer-generated analysis of potential
patient abnormalities which must be used only as guidance for consideration by qualified
physicians, together with other relevant clinical information, for purposes of making
diagnoses.
Contraindications
The 12-lead interpretive algorithm is not intended for use on pediatric patients.
Standard Features & Benefits
Full alphanumeric keypad
Enter or search for patient information quickly and easily.
Color LCD display
View and adjust the ECG waveforms prior to printing, to save time and paper.
With spirometry option, also view patients’ spirometry efforts and results.
Storage for up to 50 ECG and 50 spirometry records
Review, edit, print, or save recent records.
Unlimited storage on SD™ memory cards
Use SD memory cards to save as many ECG or spirometry records as you like. (Cards are
not included.)
Battery operation
Use the electrocardiograph almost anywhere. On battery power, you can print up to 100
ECGs continuously before needing to recharge.
User-definable ECG report formats
Customize one or two formats for efficient reporting.
Removable leads for ECG patient cable
Replace leads individually if needed.
Compatibility with CardioPerfect workstation software
Store and manage data electronically by transferring records to a Welch Allyn
CardioPerfect workstation in one of two ways:
• via an SD memory card (card not included)
• via a hardwired connection (cable not included)
Page 8

4 Chapter 1 Introduction Welch Allyn CP 200 Electrocardiograph
Options
These options are available both for initial purchases and for upgrades.
• Spirometry
With a disposable, single-use flow transducer, the optional spirometer performs FVC
and SVC tests.
• Automatic ECG interpretation
The optional MEANS interpretation algorithm, developed by the University of
Rotterdam in the Netherlands, provides automatic analysis of ECG tests. For more
information, see the MEANS Physicians' Manual on the CD that came with your
electrocardiograph.
•Carts
Two specially designed carts are available for convenient transport and use of the
electrocardiograph, as shown here with the optional cable arm and shelf.
Figure 1. Office Cart Figure 2. Hospital Cart
Cable arm and
shelf (optional)
Free-motion
plastic wheels
Cable arm and
shelf (optional)
Durable, highquality, rubber
wheels with locks
Page 9

Directions for Use Chapter 1 Introduction 5
Accessories
To order accessories, call Welch Allyn. For phone numbers, see page ii.
Item Customer Order Number Quantity
Resting tab electrodes
Resting tab electrode adaptors
Thermal chart paper (1 case = 5 pads, 200 sheets each)
Welch cups
Limb lead clamps, IEC
Limb lead clamps, AHA
Patient cable (Figure 12 on page 12)
•AHA
•IEC
• IEC, vacuum adapter
Lead wires (10 wires per set)
• AHA banana
• IEC banana
• AHA pinch
• IEC pinch
Battery (Figure 47 on page 77)
Dust cover
Carts
• Utility cart
• Office cart (Figure 1 on page 4)
•Hospital cart (Figure 2 on page 4)
• Cable arm & shelf option (page 4)
Connectivity kit to the CardioPerfect workstation
Interpretation upgrade option
45008-0000
58581-0000
94018-0000
RE-ELEC-CUP
RE-ELEC-CLP
401432
400293
400294
401128
401129
401122
401123
401124
100660
401428
08265-0000
401393
401394
401161
100638
100623
1000
10
1 case
6
4
4
1
1
1
1 set
1 set
1 set
1 set
1
1
1
1
1
1
1
1
Spirometry option 100400 1
Product information
• Electrode placement wall poster 71300-0000 1
• CP 200 12-Lead Resting Electrocardiograph Directions
for Use
• CP 200 product information multi-language CD 401151 1
701557 1
Page 10
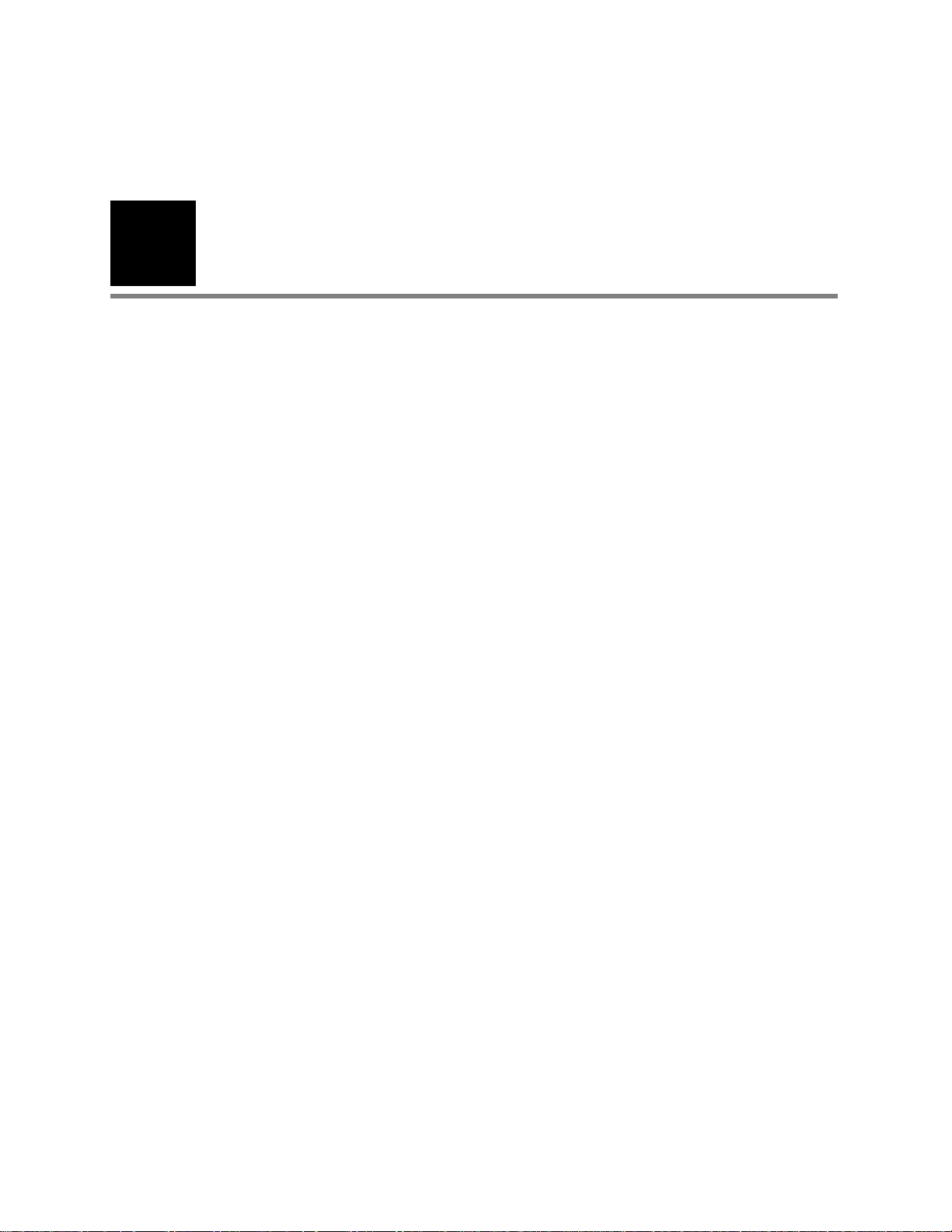
6 Chapter 1 Introduction Welch Allyn CP 200 Electrocardiograph
Controls, Indicators, and Connectors
This section describes the controls, indicators, and connectors that are part of the
electrocardiograph.
Figure 3. Top
Softkeys and functions keys
See Figure 8 on page 9.
Keyboard
See Figure 1 on page 8.
Figure 4. Back
AC power inlet
Equipotential stud
AC fuses
Page 11
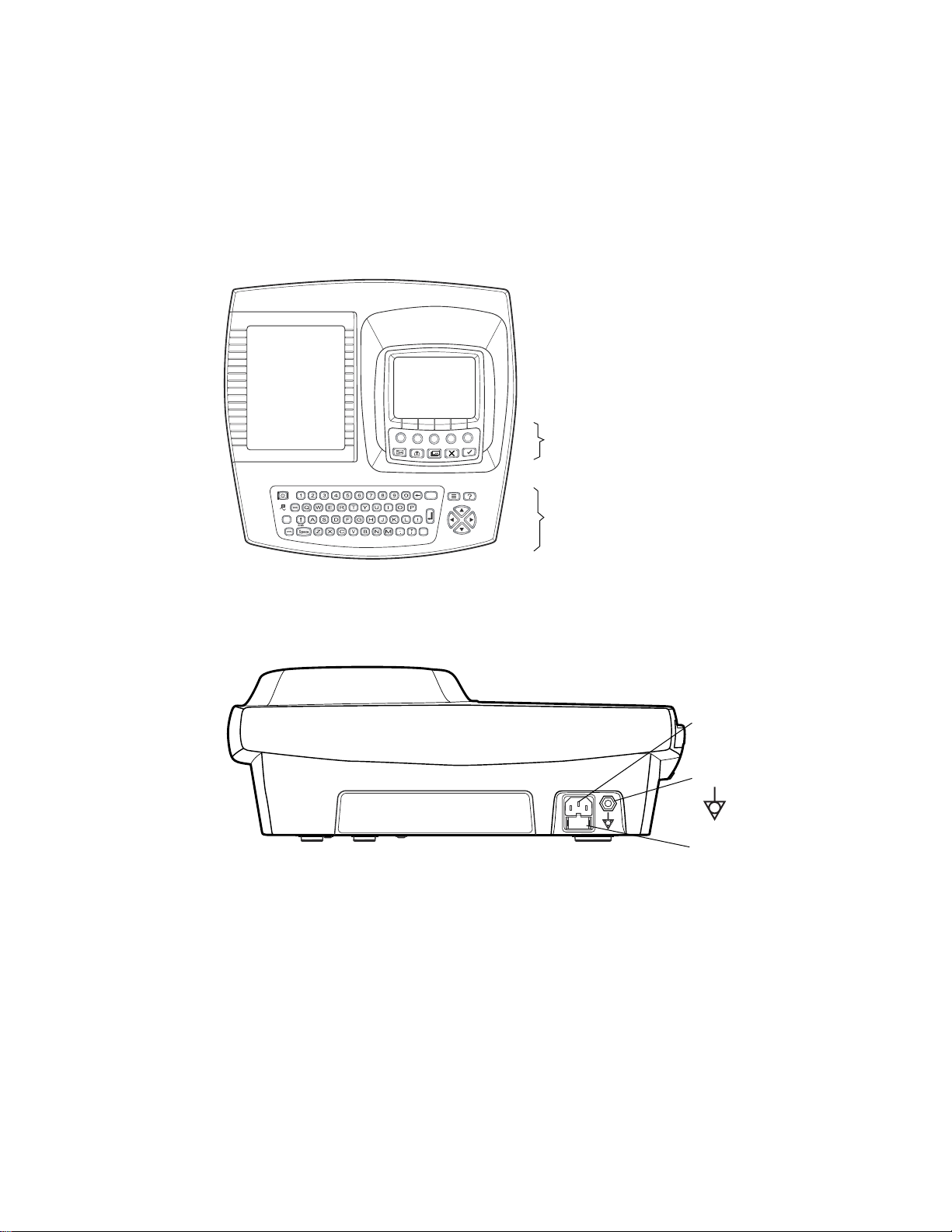
Directions for Use Chapter 1 Introduction 7
Figure 5. Right Side
Com port B
(for USB cable)
Figure 6. Front
SD memory card
slot
Com port A
(for patient cable)
Spirometry port
Figure 7. Left Side
Paper tray latch
Page 12

8 Chapter 1 Introduction Welch Allyn CP 200 Electrocardiograph
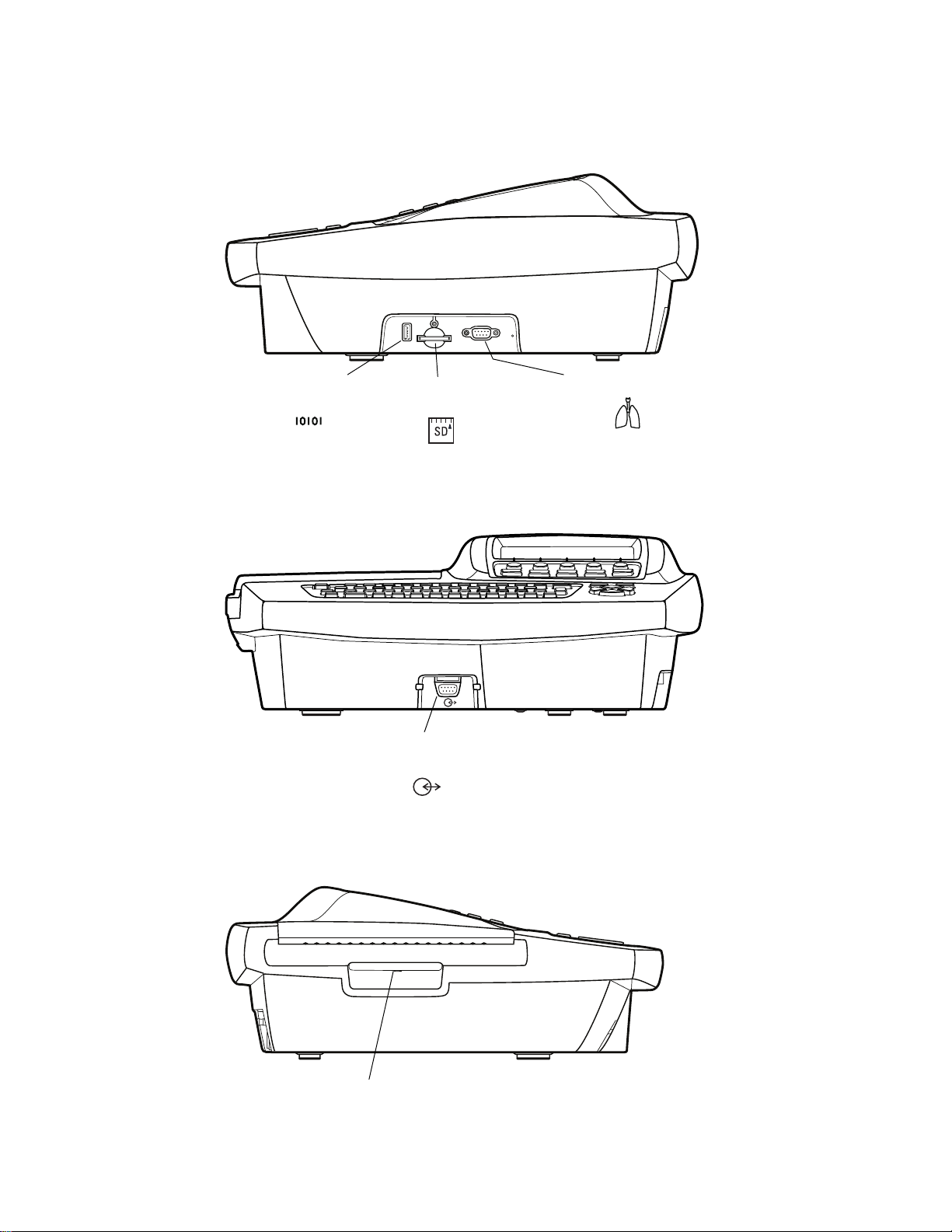
Table 1. Keyboard
A
J
I
H
Key Function
A. On/Off See “Powering the Electrocardiograph” on page 22.
B. Backspace Deletes the character to the left of the cursor.
C. Menu See “About the Main Menu” on page 10.
D. Help See “Getting Help” on page 18.
E. Navigation arrows See “Moving Through the Menus” on page 11.
G
B
F
C
D
E
F. Enter See “Moving Through the Menus” on page 11.
G. Space Enters a space.
H. Shift Capitalizes letters.
I. Tab Moves through the data-entry fields.
J. Green LED Lights up when the electrocardiograph is connected to AC power.
Page 13
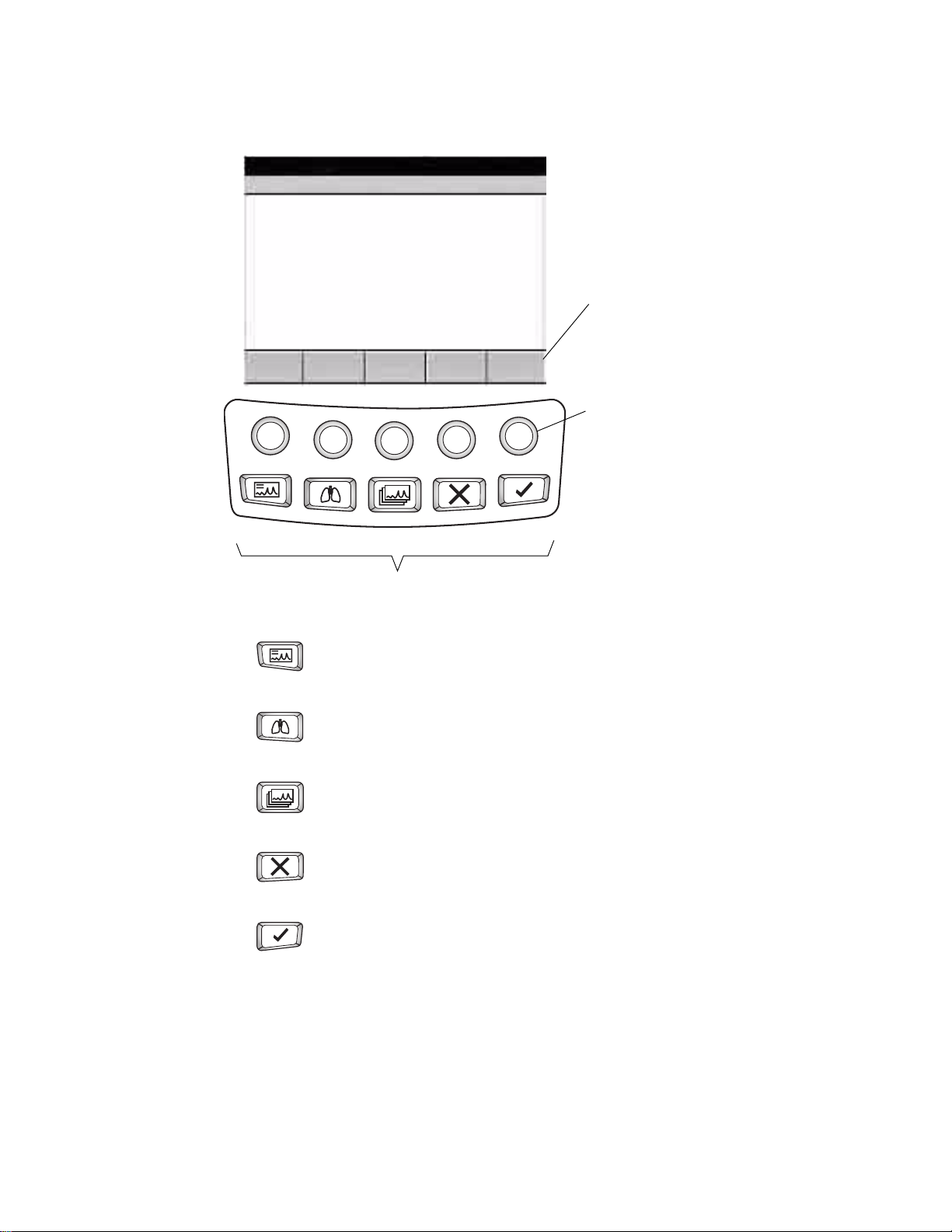
Directions for Use Chapter 1 Introduction 9
Figure 8. Softkeys and Function Keys
Softkeys
These softkeys display text or images that
correspond to the unlabeled buttons below
them. The content changes from screen to
screen.
Softkey buttons
These buttons activate the functions displayed
above them. If a softkey is blank, pressing its
Auto ECG Spirometry Rhythm ECG Stop/Cancel OK
button has no effect.
Function Keys
Auto ECG
Begins Auto ECGs, normal and stat.
See “Recording an Auto ECG” on page 49.
Spirometry
Begins spirometry tests.
See spirometry manual.
Rhythm ECG
Begins Rhythm ECGs.
See “Recording a Rhythm ECG” on page 56.
Stop/Cancel
Stops any current activity.
See “Moving Through the Menus” on page 11.
OK
Accepts data that you have entered, or chooses a highlighted item.
See “Moving Through the Menus” on page 11.
Page 14

10 Chapter 1 Introduction Welch Allyn CP 200 Electrocardiograph
About the Main Menu
The main menu appears when you press the Menu key .
Figure 9. Main Menu
Main Menu
1 Test Directory
2 Scheduled Patients
3 ECG Settings
4 Spirometry Settings
5 System Settings
6 Edit Medication List
7 Edit History List
0 Exit
9:17AM Oct 16 05
Submenu Purpose Procedure
Test Directory View, change, print, or send saved tests. See “Managing Saved Tests” on page 64.
Scheduled Patients View the scheduled patients list, add
patients to the list, or delete patients from
See “Managing the Scheduled Patients List”
on page 68.
the list.
ECG Settings Review or change ECG settings: Auto
Report format, Rhythm Report format, and
See “Reviewing the ECG Settings” on
page 33.
so on.
Spirometry Settings Review or change spirometry settings:
display settings, print settings, and so on.
System Settings Review or change system settings: device
configuration, device info, user setup, and
so on.
Edit Medication List Edit the list of medication choices available
to choose during patient data entry.
Edit History List Edit the list of clinical conditions available to
choose during patient data entry.
See spirometry manual.
See “Reviewing the System Settings” on
page 25.
See “Reviewing the Medication List” on
page 30.
See “Reviewing the History List” on page 31.
Page 15

Directions for Use Chapter 1 Introduction 11
Moving Through the Menus
Figure 10. Standard Menu Figure 11. Parent Menu With Submenu
Edit Auto Report 1
1 Format
2 Interp Settings
3 Patient Data
0 Previous Menu
9:17AM Oct 16 05
Format
1 Lead Arrangement
2 Rhythm Lead 1
3 Rhythm Lead 2
4 Rhythm Lead 3
5 Extended Measurements
6 Average Cycles
0 Previous Menu
Desired Actions Keys to Press
To move up or down a list or (keyboard or softkey arrows)
To open a standard menu (Figure 10)
or or
or item’s number or letter
To move from parent menu to submenu on same
screen (Figure 11)
To perform an action
or
To accept data
To check or uncheck a checkbox
9:17AM Oct 16 05
3x4
3x4 +1R
3x4 +3R
6x2
12x1
6x2 50 mm/s
6x2 Ext.
No Print
To return to parent menu from submenu on same
screen (Figure 11) or
or
(To select the highlighted submenu
item.)
(To make no change.)
To move back through the menus or zero key
To move through data-entry fields
To return to the ECG Preview screen from a
standard menu (Figure 10)
Note Keyboard and softkey arrows work the same way.
Page 16
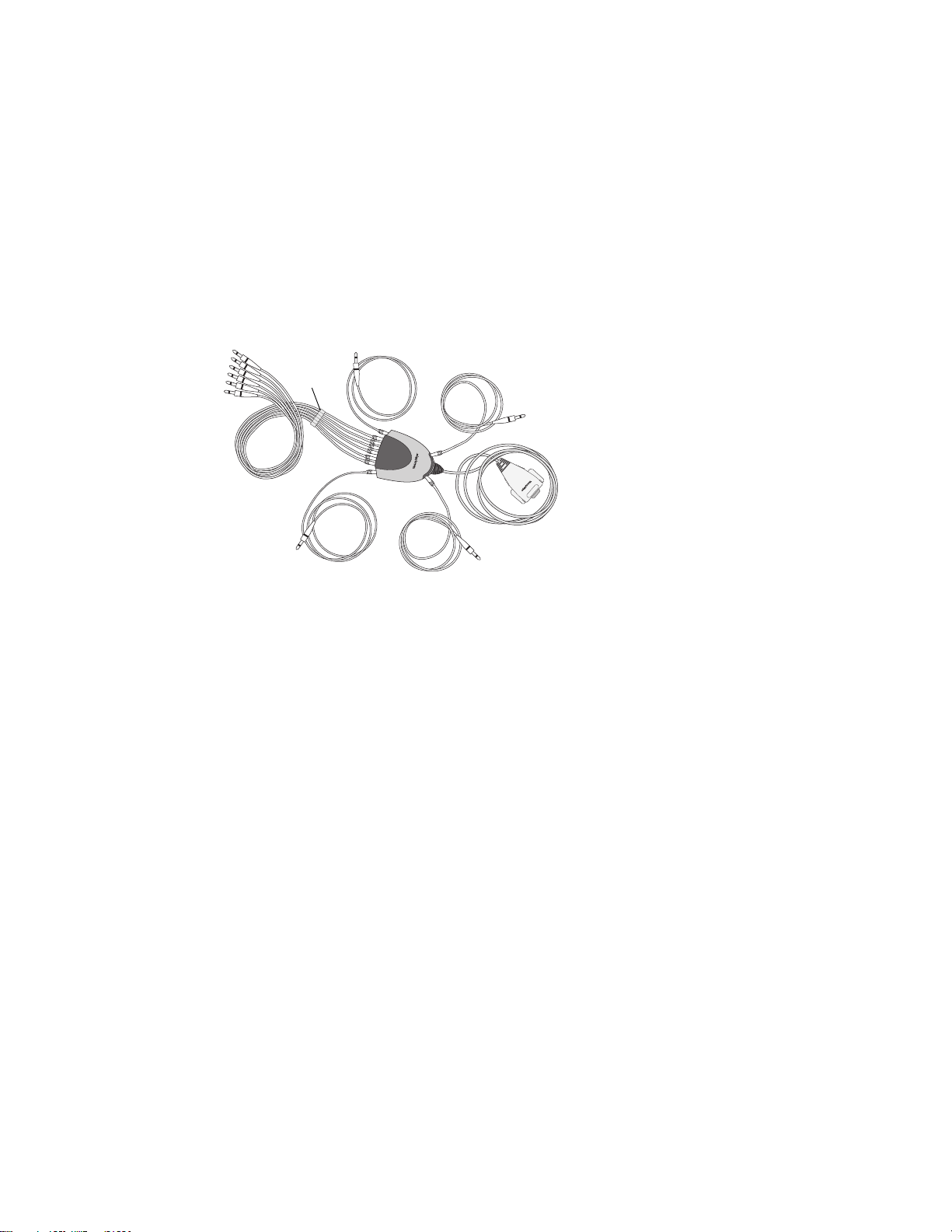
12 Chapter 1 Introduction Welch Allyn CP 200 Electrocardiograph
About the Patient Cable and Leads
The patient cable processes the patient’s ECG data and transmits it to the
electrocardiograph. To make handling convenient, the ten leads are arranged to point
toward the appropriate parts of the body. The cable rake, which slides easily, prevents the
chest leads from tangling.
Figure 12. Patient Cable and Leads
h
Chest leads
Left arm lead
Cable
rake
Right arm lead
Left leg lead
Electrocardiograph
connector
Right leg lead
Page 17
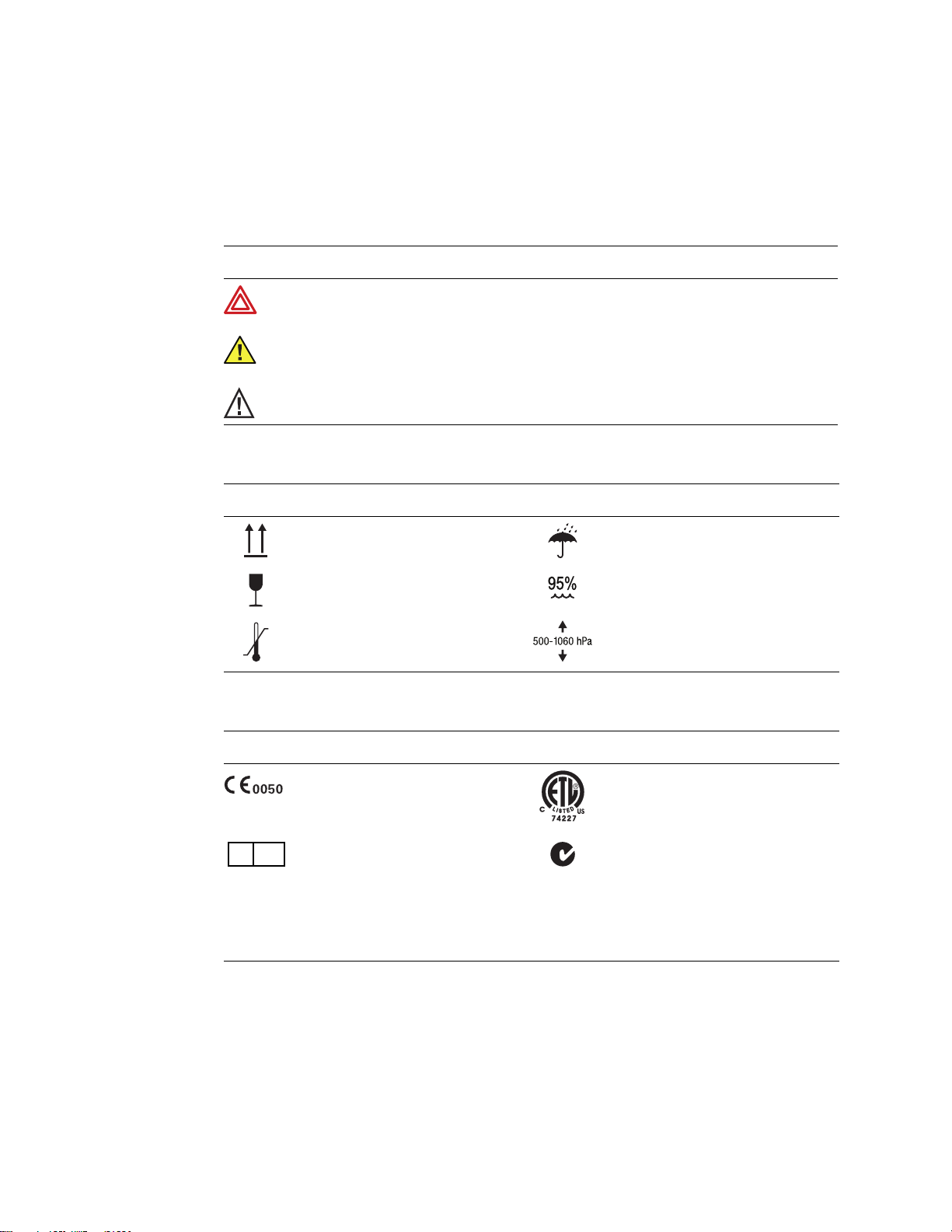
Directions for Use Chapter 1 Introduction 13
Symbols
The symbols illustrated on the following pages may appear on the electrocardiograph, on
the packaging, on the shipping container, or in this manual.
Documentation Symbols
WARNING Indicates conditions or practices that could lead to illness, injury, or
death.
Caution In this manual, indicates conditions or practices that could damage the
equipment or other property.
Caution On the product, means “Consult accompanying documentation.”
Shipping, Storing, and Environment Symbols
-20°C
EC REP
This end up Keep dry
Fragile Relative humidity limit
+49°C
Temperature limits Altitude limits
Certification Symbols
Meets essential requirements of European
Medical Device Directive 93/42/EEC
European Regulatory Manager
Welch Allyn LTD.
Navan Business Park
Dublin Road
Navan, County Meath, Republic of Ireland
Tel.: 353-46-90-67700
Fax: 353-46-90-67756
N344
Complies with applicable U.S. and Canadian
medical safety standards
Australian registered importer
Page 18
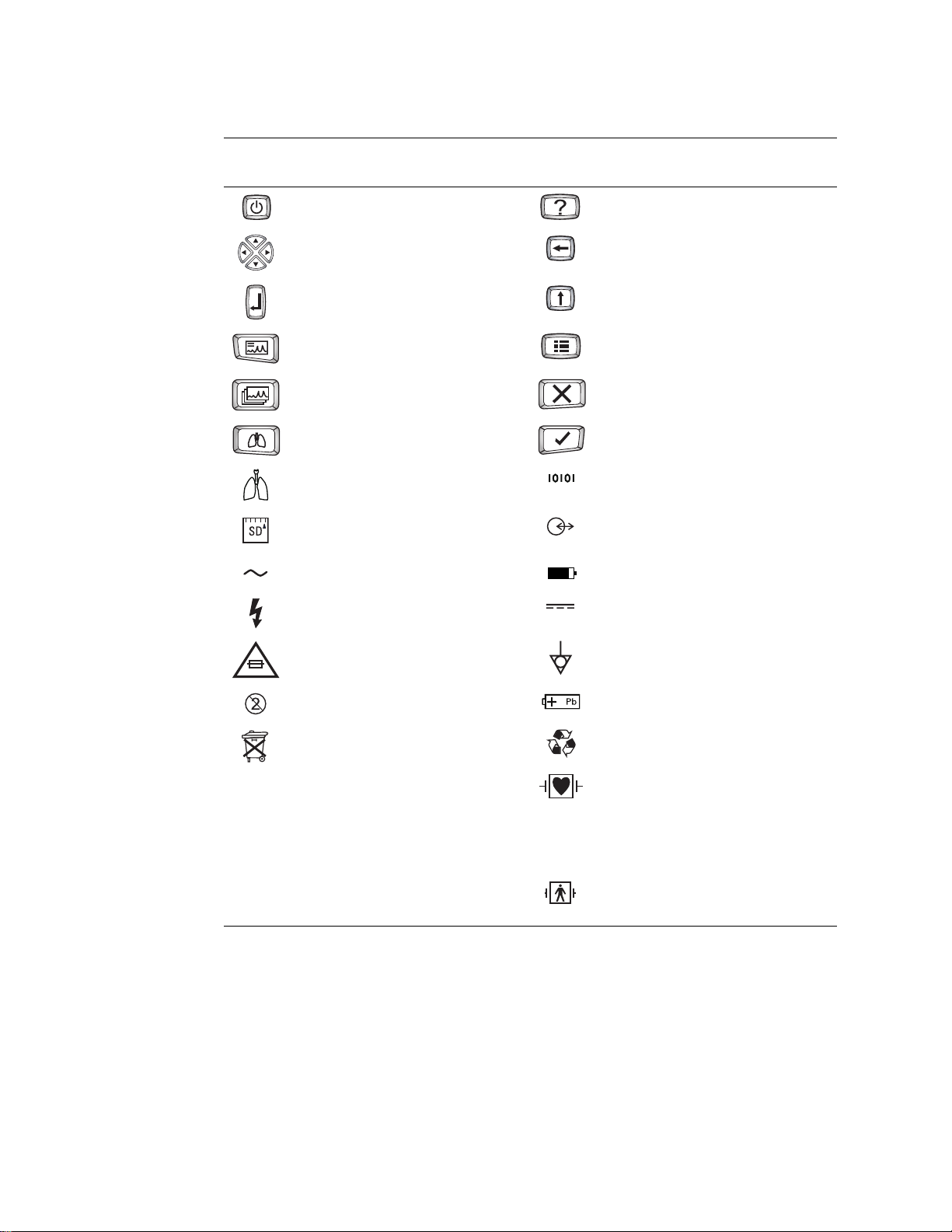
14 Chapter 1 Introduction Welch Allyn CP 200 Electrocardiograph
Operation Symbols
For details on the keys, see Figure 1 on page 8.
On/standby (off) Help
Navigation arrows Backspace
Enter Shift
Auto ECG Menu
Rhythm ECG Stop/Cancel
Spirometry OK
Spirometry port Com port B
(for USB cable)
T2.0A/250V
SD memory card slot Com port A
(for patient cable)
Alternating current Battery charge level
Dangerous voltage Direct current
AC fuse replacement information Ground equipotential
Do not reuse.
Do not dispose of this product as unsorted
Sealed lead-acid battery
Recycle.
municipal waste. Prepare this product for
reuse or separate collection as specified by
Directive 2002/96/EC of the European
Parliament and the Council of the European
Union on Waste Electronic and Electrical
Equipment (WEEE). If this product is
contaminated, this directive does not apply.
For more specific disposal information, see
www.welchallyn.com/weee, or contact
Welch Allyn Customer Service at +44 207
Defibrillation-proof Type CF applied parts.
(While the electrocardiograph is safety-rated
“CF” for direct cardiac contact, it is not
intended to be connected directly to the
patient’s heart. Only surface contact with the
patient’s skin is intended.)
Type BF applied part
365 6780.
Page 19

Directions for Use Chapter 1 Introduction 15
Using the Electrocardiograph Safely
Before using or servicing the electrocardiograph, you must read and understand the
following safety-related information.
General Warnings
The following warning statements apply to electrocardiograph use in general. Warning
statements that apply specifically to particular procedures, such as connecting the patient
cable or performing an ECG test, appear in the corresponding sections of the manual.
Warning statements indicate conditions or practices that could lead to illness, injury, or
death.
Warnings Related to the Environment
WARNING To ensure patient and device safety, leave 5 feet (1.5 meters) of
open area around the patient.
WARNING To avoid a possible explosion, do not use the electrocardiograph in
the presence of flammable anesthetics: mixtures containing air, oxygen, or
nitrous oxide.
WARNING When transporting the electrocardiograph on a cart, tuck the patient
cable away from the wheels so that it does not present a hazard.
Warnings Related to Accessories and Other Equipment
WARNING For operator and patient safety, peripheral equipment and
accessories that can come in direct patient contact must be in compliance with
all appropriate safety, EMC, and regulatory requirements. See “EMC Guidance
and Manufacturer’s Declarations” on page 91.
WARNING All signal input and output (I/O) connectors are intended for
connection of only devices complying with IEC 60601-1, or other IEC standards
(for example, IEC 60950), as appropriate to the device. Connecting additional
devices to the electrocardiograph may increase chassis or patient leakage
currents. To maintain operator and patient safety, consider the requirements of
IEC 60601-1-1. Measure the leakage currents to confirm that no electric shock
hazard exists.
WARNING The electrocardiograph has not been designed for use with highfrequency (HF) surgical equipment and does not protect against hazards to the
patient.
Page 20

16 Chapter 1 Introduction Welch Allyn CP 200 Electrocardiograph
Warnings Related to Using the Electrocardiograph
WARNING This device captures and presents data reflecting a patient’s
physiological condition. When reviewed by a trained physician or clinician, this
data can be useful in determining a diagnosis. However, the data should not be
used as a sole means for determining a patient’s diagnosis.
WARNING To avoid serious injury or death, take these precautions during
patient defibrillation:
• Avoid contact with the electrocardiograph, patient cable, and patient.
• Verify that the patient leads are properly connected. See “Connecting the
Patient Cable” on page 20.
• Place defibrillator paddles properly in relation to electrodes.
• After defibrillation, pull each patient lead out of the patient cable and inspect
the tips for charring (black carbon marks). If there is any charring, the patient
cable and individual leads must be replaced. If there is no charring, fully
reinsert the leads into the patient cable. (Charring can occur only if a lead is
not fully inserted into the patient cable before defibrillation.)
WARNING To prevent the spread of infection, take these precautions:
• Dispose of single-use components (for example, electrodes) after using
them once.
• Regularly clean and disinfect all components that come in contact with
patients. See “Cleaning the Equipment” on page 74.
• Avoid ECG testing for patients with open, infectious sores.
WARNING Avoid positioning any leads or cables so that they could easily trip
someone or become wrapped around a patient’s neck.
WARNING Satisfactory maintenance procedures must be implemented, or
equipment failure and health hazards may result.
WARNING Only qualified service personnel should attempt to repair the
electrocardiograph. In case of a malfunction, call Technical Support and precisely
describe the problem. For phone numbers, see page ii.
Page 21

Directions for Use Chapter 1 Introduction 17
General Cautions
The following caution statements apply to electrocardiograph use in general. Caution
statements that apply specifically to particular procedures, such as connecting the patient
cable or performing an ECG test, appear in the corresponding sections of the manual.
Caution statements indicate conditions or practices that could damage the equipment or
other property.
Caution When removing the electrocardiograph from storage, allow it to
thermally stabilize to surrounding environmental conditions before using it.
Caution To prevent possible damage to the keypad, do not use sharp or hard
objects to press keys. Only use fingertips.
Caution Do not expose the patient cable to strong ultra-violet radiation.
Caution Do not pull or stretch the patient cable. Doing so could result in
mechanical or electrical failures. Form the patient cable into a loose loop before
storing.
Caution Avoid positioning the patient cable where it might get pinched or
stepped on. If the cable’s impedance is altered, measurements might no longer
be accurate, and repair might be necessary.
Caution Using the equipotential terminal for anything but grounding purposes
may contribute to damage of the device.
Caution Use only parts and accessories supplied with the device and available
through Welch Allyn. The use of accessories other than those specified may
result in degraded performance of this device.
Caution Portable and mobile RF communications equipment can affect the
performance of the electrocardiograph.
Caution The electrocardiograph meets the Class A requirements of IEC 606011-2:2000 regarding incidental emission of radio frequency interference. As such it
is suitable for use in commercial grade electrical environments. If the
electrocardiograph is used in residential grade electrical environments and you
experience incidental interference with other equipment that uses radio
frequency signals to operate, minimize the interference as described under
“EMC Guidance and Manufacturer’s Declarations” on page 91.
Caution Other medical equipment—including but not limited to defibrillators,
ultrasound machines, pacemakers, and other stimulators—may be used
simultaneously with the electrocardiograph. However, such devices may disturb
the electrocardiograph signal.
Caution The power cord must be disconnected from AC power before cleaning,
maintaining, or servicing.
Page 22

18 Chapter 1 Introduction Welch Allyn CP 200 Electrocardiograph
Getting Help
You can get help with the electrocardiograph in a variety of ways beyond this manual.
• Press the Help key from the ECG Preview screen or Lead Off screen for a list
of topics available to print.
• Review the other information that came with the electrocardiograph. For list, see
“Product information” on page 5.
• Contact Welch Allyn. For phone numbers, see page ii.
Page 23
19
2
Setting Up the Electrocardiograph
Inspecting the Electrocardiograph . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20
Connecting the Patient Cable . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20
Loading the Thermal Chart Paper . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
Powering the Electrocardiograph. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 22
Verifying Proper Operation. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23
Page 24
20 Chapter 2 Setting Up the Electrocardiograph Welch Allyn CP 200 Electrocardiograph
Inspecting the Electrocardiograph
1. Look for obvious signs of shipping damage. If you find any damage, contact Technical
Support. For phone numbers, see page ii.
2. Verify that you have received all appropriate options and accessories. See “Options”
on page 4 and “Accessories” on page 5.
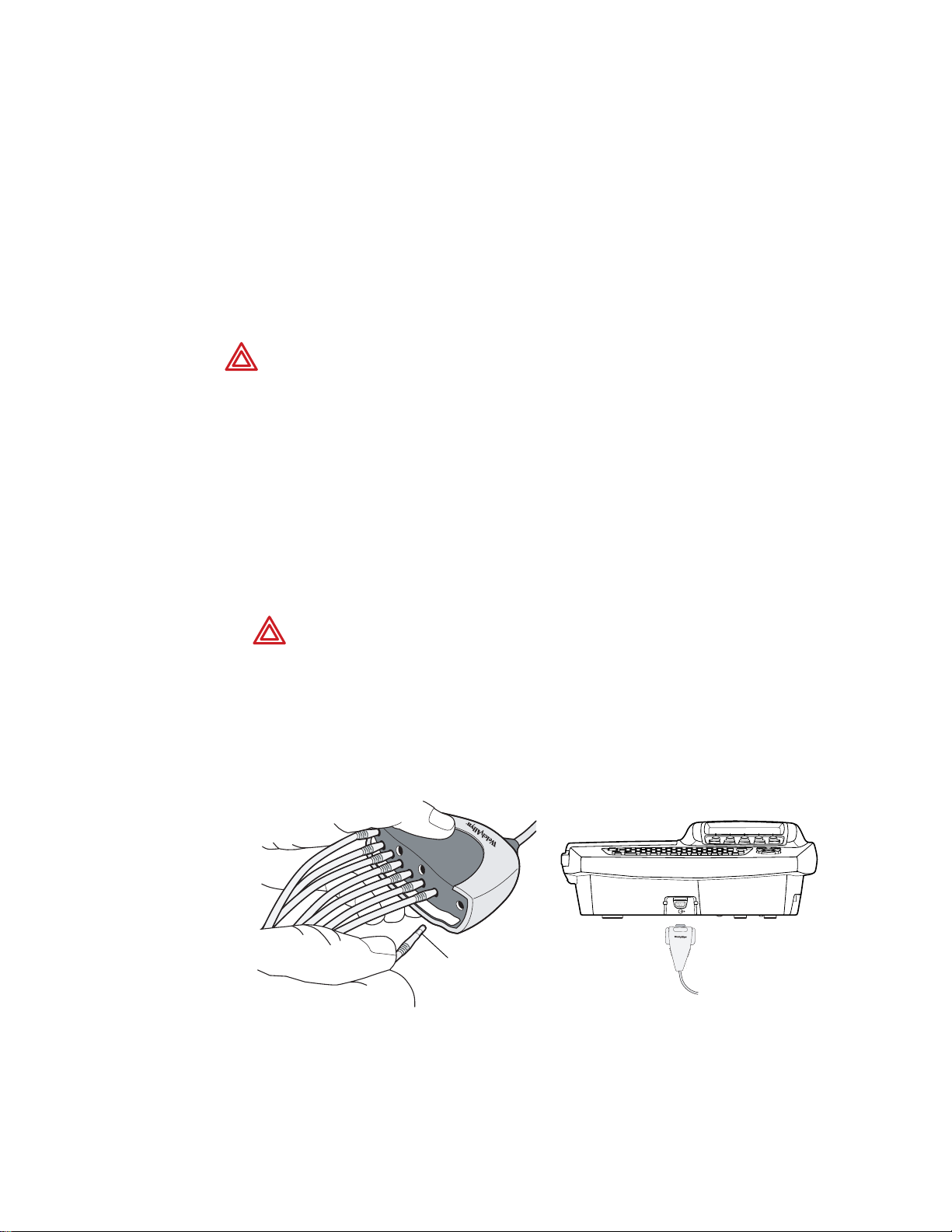
Connecting the Patient Cable
WARNING Conductive parts of the patient cable, electrodes and associated
connections of defibrillation-proof Type CF applied parts, including the neutral
conductor of the patient cable and electrode, should not come into contact with
other conductive parts, including earth ground.
WARNING To avoid injury to the patient or damage to the device, never plug
patient leads into any other device or wall outlet.
1. Insert all leads into their proper positions, as labeled on the connectors.
Insert connectors fully so that no part of the metal ring remains exposed.
For example, see Figure 13. (To see the whole patient cable with all leads inserted,
see Figure 12 on page 12.)
WARNING Failure to insert all connectors fully may result in a loss of energy
being delivered to the patient during defibrillation and damage to the patient
cable itself. For other warnings related to defibrillation, see page 16.
2. Plug the patient cable into the port on the front of the electrocardiograph.
See Figure 14.
Figure 13. Inserting the Leads Figure 14. Plugging in the Connector
Metal ring
Page 25
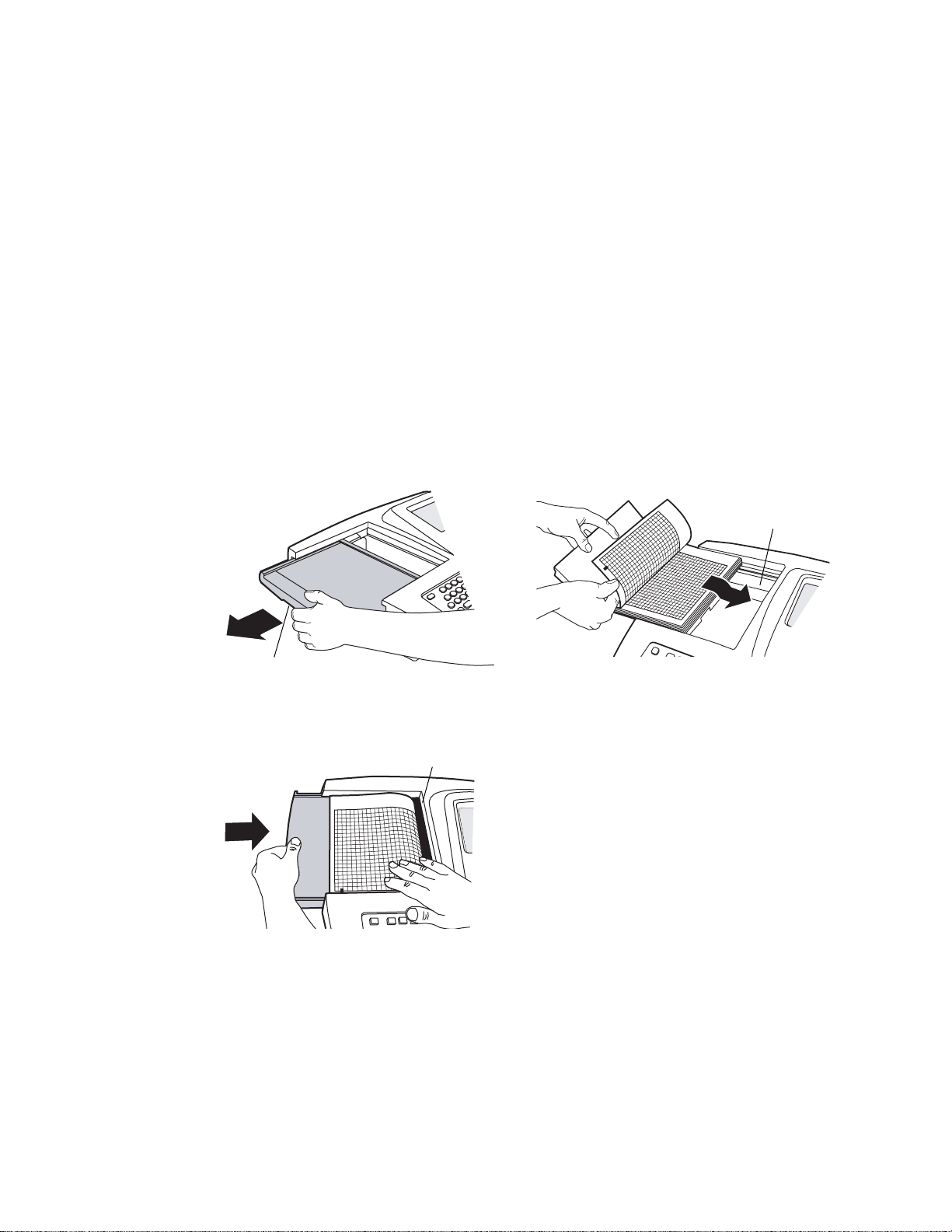
Directions for Use Chapter 2 Setting Up the Electrocardiograph 21
Loading the Thermal Chart Paper
1. Squeeze the latch. Pull the paper door to the left. See Figure 15.
If any paper remains in the tray, remove it.
2. Remove the outer packaging, including the cardboard bottom, from a new pack of
paper. Pull the top sheet back so that the paper’s grid side faces up and the Welch
Allyn name is on the bottom of the paper.
3. Slide the paper into the tray. See Figure 16.
If humidity is high, remove up to 10 sheets so that the paper fits properly.
4. Lay the top sheet over the paper door. Push the door to the right until it clicks.
See Figure 17.
Figure 15. Opening the Paper Door Figure 16. Loading the Paper
Latch
Figure 17. Closing the Paper Door
Tea r bar
Paper tray
Tips for handling thermal paper:
• Store in a cool, dry, dark place.
• Avoid exposure to bright light or UV sources.
• Avoid exposure to solvents, adhesives, or cleaning
fluids.
• Do not store with vinyls, plastics, or shrink wraps.
Page 26
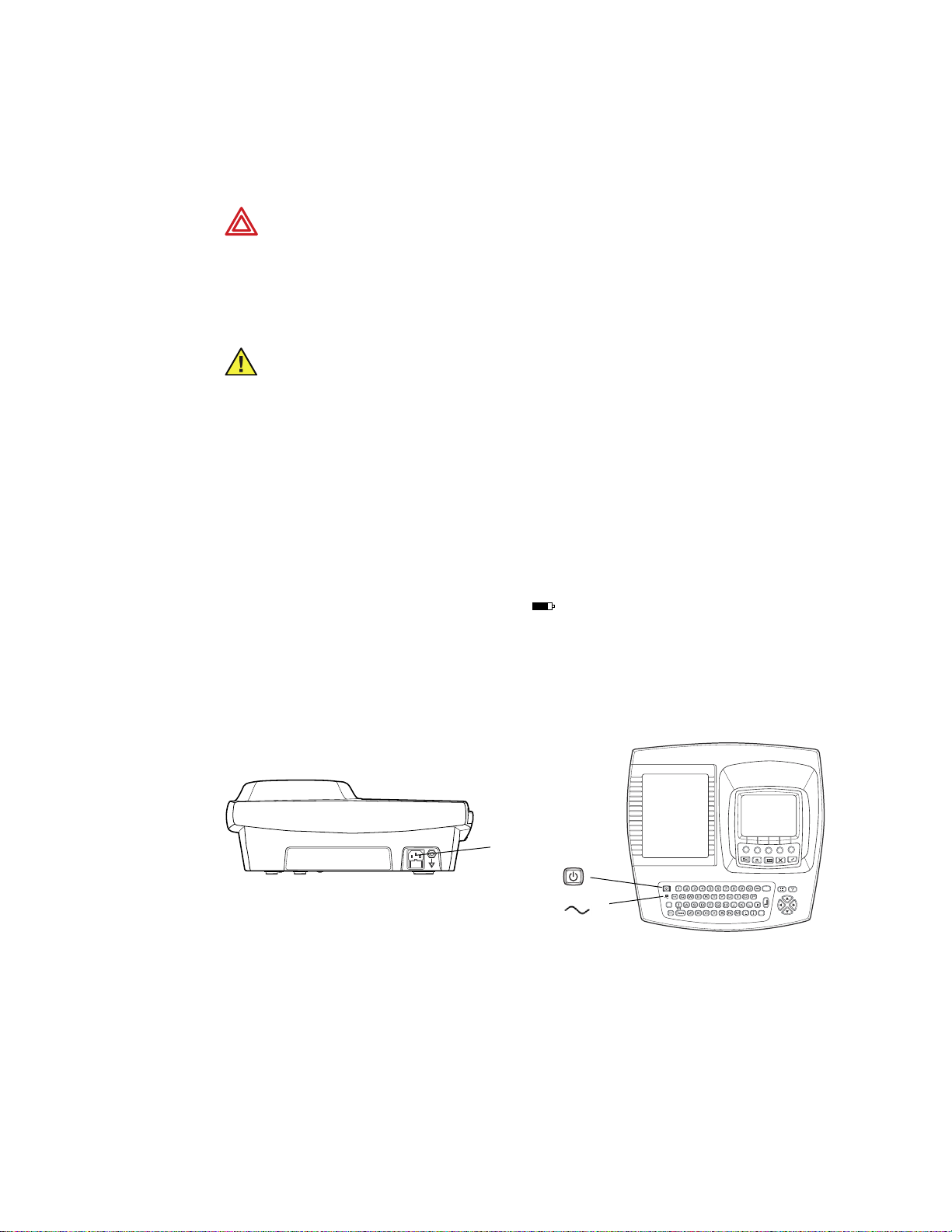
22 Chapter 2 Setting Up the Electrocardiograph Welch Allyn CP 200 Electrocardiograph
Powering the Electrocardiograph
The electrocardiograph can run on AC or battery power.
WARNING To ensure that electrical safety is maintained when using AC power,
the device must be plugged into a hospital-grade outlet.
WARNING Where the integrity of external protective earth conductor
arrangement is in doubt, use battery power.
Caution Medical electrical equipment needs special precautions regarding EMC
and must be installed and used according to the information provided in “EMC
Guidance and Manufacturer’s Declarations” on page 91.
To Connect to AC Power
Plug one end of the power cord into the electrocardiograph’s AC power inlet. Plug the
other end into an AC outlet. The green LED on the keyboard lights up, indicating that
power is connected. See Figure 18.
To Keep the Battery Charged
Leave the electrocardiograph connected to AC power whenever possible. Battery charge
status is indicated on the screen by an icon: . When the charge gets low, the icon
flashes. When the charge gets too low to operate, a warning message appears and the
electrocardiograph beeps every 15 seconds for 1 minute, then it turns off.
For more, see “Recharging a Fully Discharged Battery” on page 76.
Figure 18. AC Power Inlet and Green LED
AC power inlet
On/Off key
Green LED
Page 27
Directions for Use Chapter 2 Setting Up the Electrocardiograph 23
To Turn the Electrocardiograph On
Press .
To Turn the Electrocardiograph Off
Press and hold.
Note
If Power-Save is enabled, the electrocardiograph turns off automatically after
several idle minutes. To learn how to enable or disable Power-Save, see
“Reviewing the Device Configuration Settings” on page 27.
Verifying Proper Operation
Once your electrocardiograph is set up, verify proper operation by using an ECG simulator
to acquire and print a standard 12-lead ECG of known amplitude. See Step 2 on page 75.
Note
As part of your initial set-up, you may want to adjust the display contrast. To learn
how, see “Reviewing the Device Configuration Settings” on page 27.
You may also want to change other software settings, as described in the
following chapters:
• “Reviewing the System Settings” on page 25
• “Reviewing the ECG Settings” on page 33
Page 28

24 Chapter 2 Setting Up the Electrocardiograph Welch Allyn CP 200 Electrocardiograph
Page 29
25
3
Reviewing the System Settings
“System Settings” Menu Tree . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 26
Reviewing the Device Configuration Settings . . . . . . . . . . . . . . . . . . . . . . . . . . . 27
Reviewing the Device Information. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 29
Reviewing the Medication List . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 30
Reviewing the History List. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 31
This chapter documents the system settings, which affect both ECG and spirometry
functions. For information on the following related tasks, see the procedures identified
here:
• Reviewing ECG settings
See “Reviewing the ECG Settings” on page 33.
• Reviewing spirometry settings
Spirometry manual.
• Printing all settings
See “Reviewing the Device Information” on page 29.
Page 30
26 Chapter 3 Reviewing the System Settings Welch Allyn CP 200 Electrocardiograph
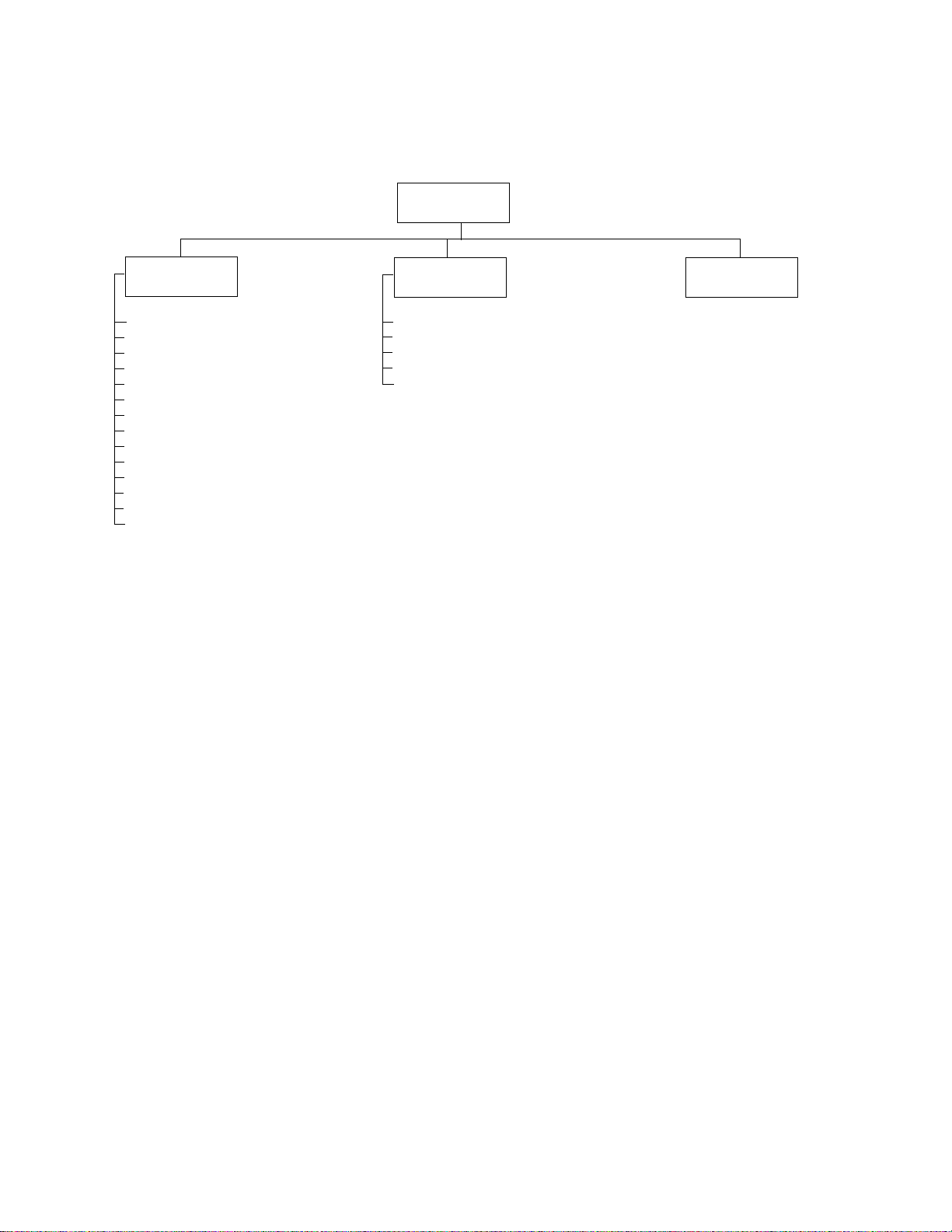
“System Settings” Menu Tree
System Settings
Device
Configuration
Set Date/Time
Language
Date Format
Time F ormat
Weight Unit
Height Unit
Power-Save
Device ID
Audio Beeper
Flow Unit
Pressure Unit
Temperature
Increase Display Contrast
Decrease Display Contrast
*
*
*
*
Applicable for spirometry only.
Device
Info
About
Print Settings
Enable Options
Upgrade Software
Service Info
Device
Administration
See “Managing Data
Security” on page 69.
Page 31

Directions for Use Chapter 3 Reviewing the System Settings 27
Reviewing the Device Configuration Settings
1. Press the Menu key .
2. Choose System Settings > Device Configuration.
The following screen appears.
Figure 19. “Device Configuration” Screen
Device Configuration
1 Set Date/Time
2 Language
3 Date Format
4 Time Format
5 Weight Unit
6 Height Unit
7 Power-Save
8 Device ID
9 Audio Beeper
A Flow Unit
9:17AM Oct 16 05
3. Change any desired settings.
Setting Description
Set Date/Time Current date and time.
Language List of languages available. Changes take effect when the next screen appears.
Date Format MM/DD/YY (month/day/year)
Time Format 24-hour or AM/PM.
Weight Unit Kilograms (kg) or pounds (lb).
Height Unit Centimeters (cm), inches (in), or feet and inches (ft, in).
Power-Save On or off. When on, the electrocardiograph turns itself off after several idle minutes.
Device ID Electrocardiograph identification. Enter up to 20 characters.
Audio Beeper On or off. When on, beeps to indicate errors, such as incorrect input, improper external
Flow Unit L/sec or L/min. For spirometry only. Determines the y-axis units for flow/volume curves.
Pressure Unit mmHg, mbar, inHg, kPa. For spirometry only. Determines the units for the calibration
DD/MM/YY (day/month/year)
connections, or a printer error. Beeps may also indicate a low battery.
menu’s atmospheric pressure values.
Temperature Fahrenheit or Celsius. For spirometry only. Determines the units for the calibration
menu’s temperature values.
Page 32

28 Chapter 3 Reviewing the System Settings Welch Allyn CP 200 Electrocardiograph
Setting (Continued) Description (Continued)
Increase Display
Contrast
Decrease Display
Contrast
Each time you select this choice, the display contrast immediately increases until you
reach maximum contrast.
Each time you select this choice, the display contrast immediately decreases until you
reach minimum contrast.
Page 33

Directions for Use Chapter 3 Reviewing the System Settings 29
Reviewing the Device Information
1. Press the Menu key .
2. Choose System Settings > Device Info.
The following screen appears.
Figure 20. “Device Info” Screen
Device Info
1 About
2 Print Settings
3 Enable Options
4 Upgrade Software
5 Service Info
0 Previous Menu
9:17AM Oct 16 05
3. Select the desired item:
Item Description
About Displays the following information about the electrocardiograph:
• serial number
• modules configured
• version numbers
Print Settings Prints your ECG, spirometry, and system settings as well as medication & history
lists.
Enable Options Contact Technical Support. For phone numbers, see page ii.
Upgrade Software Contact Technical Support. For phone numbers, see page ii.
Service Info Accessible to service support only.
Page 34

30 Chapter 3 Reviewing the System Settings Welch Allyn CP 200 Electrocardiograph
Reviewing the Medication List
The medication list determines which medications are available to choose during patient
data entry.
1. Press the Menu key .
2. Choose Edit Medication List.
The following screen appears.
Figure 21. “Edit Medication List” Screen
Edit Medication List
Medication Name
ACE Inhibitors
Albuterol
Alpha Blockers
Amiodarone
Beclomethasone
Beta Blocker
Bitolterol
Add Delete Exit
3. Press the desired softkeys:
•Add
Lets you add medications, up to a total of 40.
• Delete
Deletes the highlighted medication.
•Exit
Returns to the main menu.
9:17AM Oct 16 05
Page 35

Directions for Use Chapter 3 Reviewing the System Settings 31
Reviewing the History List
The history list determines which clinical conditions are available to choose during patient
data entry.
1. Press the Menu key .
2. Choose Edit History List.
The following screen appears.
Figure 22. “Edit History List” Screen
Edit History List
History Name
Acute Bronchitis
Acute Respiratory Failure
Allergies/Sneezing
Asphyxia
Asthma
Bronchiolitis
Bronchitis
Add Delete Exit
3. Press the desired softkeys:
•Add
Lets you add conditions, up to a total of 40.
•Delete
Deletes the highlighted condition.
•Exit
Returns to the main menu.
9:17AM Oct 16 05
Page 36

32 Chapter 3 Reviewing the System Settings Welch Allyn CP 200 Electrocardiograph
Page 37

33
4
Reviewing the ECG Settings
“ECG Settings” Menu Tree . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 34
Reviewing the Auto Report Settings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 35
Reviewing the Rhythm Report Settings. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 42
Reviewing the Miscellaneous ECG Settings . . . . . . . . . . . . . . . . . . . . . . . . . . . . 43
This chapter documents the ECG settings. For information on the following related tasks,
see the procedures identified here:
• Reviewing system settings (which affect both ECG and spirometry functions)
See “Reviewing the System Settings” on page 25.
• Reviewing spirometry settings
Spirometry manual.
• Printing all settings
See “Reviewing the Device Information” on page 29.
Page 38

34 Chapter 4 Reviewing the ECG Settings Welch Allyn CP 200 Electrocardiograph
“ECG Settings” Menu Tree
ECG Settings
Edit
Auto Report 1
Format
Lead Arrangement
Rhythm Lead 1
Rhythm Lead 2
Rhythm Lead 3
Extended Measurements
Average Cycles
Interp Settings
Print Interpretation?
Copies
Copies With Interp
Reason Statements
Unconfirmed Report
Abnormal ECG
Patient Data
Edit
Auto Report 2
Format
Lead Arrangement
Rhythm Lead 1
Rhythm Lead 2
Rhythm Lead 3
Extended Measurements
Average Cycles
Interp Settings
Print Interpretation?
Copies
Copies With Interp
Reason Statements
Unconfirmed Report
Abnormal ECG
Patient Data
Edit
Rhythm Report
Lead Arrangement
Miscellaneous
Lead Configuration
Electrode Labels
Baseline Centering
Lead Timing
Default Gain Setting
Default Baseline Filter
Default Muscle Filter
Mains Filter
Auto Save
Auto Send
Auto Report 2
First Name
Middle Initial
Age/Birth Date?
Weight
Height
Gender
Race
Medication
History
Blood Pressure
Comments
Custom 1
Custom 1 Label
Custom 2
Custom 2 Label
First Name
Middle Initial
Age/Birth Date?
Weight
Height
Gender
Race
Medication
History
Blood Pressure
Comments
Custom 1
Custom 1 Label
Custom 2
Custom 2 Label
Page 39

Directions for Use Chapter 4 Reviewing the ECG Settings 35
Reviewing the Auto Report Settings
An Auto ECG is a report of ECG data in one of two user-defined formats: Auto Report 1 or
Auto Report 2. For an example, see Figure 23. To learn how to set up or interpret a report,
see the references on page 36.
Note
If you want a second predefined format to be available, enable Auto Report 2.
To learn how, see “Reviewing the Miscellaneous ECG Settings” on page 43.
Figure 23. Auto Report Example — 3x4 +3R Lead Arrangement
A. Patient data B. ECG measurements C. Interpretation (optional)
D. Report status label
E. 3 rows, 4 columns
N. Calibration
pulse
L. Gain
K. Frequency rangeM. Paper speed
J. AC filter
F. Rhythm leads
G. Software version
H. Device ID
I. Date and time
Page 40

36 Chapter 4 Reviewing the ECG Settings Welch Allyn CP 200 Electrocardiograph
Item (in Figure 23 on page 35) Description
A. Patient data See “Reviewing the Patient Data Fields Available for Auto Reports” on page 40.
B. ECG measurements Standard.
C. Interpretation (optional) See “Reviewing the Interpretation and Copy Settings for Auto Reports” on
page 39.
D. Report status label See “Reviewing the Interpretation and Copy Settings for Auto Reports” on
page 39.
E. 3 rows, 4 columns See “Reviewing the Format Settings for Auto Reports” on page 37.
F. Rhythm leads See “Reviewing the Format Settings for Auto Reports” on page 37.
G. Software version See also “Reviewing the Device Information” on page 29.
H. Device ID See “Device ID” on page 27.
I. Date and time See “Set Date/Time” on page 27.
J. AC filter See “Mains Filter” on page 43.
K. Frequency range Lower limit: baseline filter on = 0.5, off = 0.3
L. Gain See “Gain” on page 61.
M. Paper speed See “Speed” on page 61.
N. Calibration pulse Amplitude reference — represents the current height of a one-millivolt signal.
Upper limit: muscle filter on = 35, off = 150
See “Baseline Filter” on page 61 and “Muscle Filter” on page 61.
Is adjusted for the selected gain:
5 mm/mV = 0.5 x
10 mm/mV = 1 x
20 mm/mV = 2 x
Page 41

Directions for Use Chapter 4 Reviewing the ECG Settings 37
Reviewing the Format Settings for Auto Reports
1. Press the Menu key .
2. Choose ECG Settings > Edit Auto Report 1 (or 2) > Format.
The following screen appears.
Figure 24. Auto Report “Format” Screen
Format
1 Lead Arrangement
2 Rhythm Lead 1
3 Rhythm Lead 2
4 Rhythm Lead 3
5 Extended Measurements
6 Average Cycles
0 Previous Menu
9:17AM Oct 16 05
3. Change any desired settings.
For a report example, see Figure 23 on page 35.
Setting Description
Lead Arrangement Arrangement of the leads on the report.
• 3x4 3 rows x 4 columns
• 3x4 +1R 3 rows x 4 columns + 1 rhythm lead
• 3x4 +3R 3 rows x 4 columns + 3 rhythm leads
• 6x2 6 rows x 2 columns
• 12x1 12 rows x 1 column
• 6x2 50 mm/s 6 rows x 2 columns, 50 mm/s
• 6x2 Ext. 6 rows x 2 columns, extended printouts
• No Print No report prints
(two pages, 20 seconds of ECG data)
Rhythm Lead 1 Rhythm lead to print at the bottom of 3x4 +1R and 3x4 +3R reports.
Rhythm Lead 2 Second rhythm lead to print at the bottom of 3x4 +3R reports.
Rhythm Lead 3 Third rhythm lead to print at the bottom of 3x4 +3R reports.
Extended
Measurements
On or off. When on, an additional page prints with the report. Extended measurements
include the values for several common parameters, such as Q, R, and S amplitude and
ST values. The amplitudes are expressed in microvolts. The durations are expressed in
milliseconds. The measurements cannot be edited.
Page 42

38 Chapter 4 Reviewing the ECG Settings Welch Allyn CP 200 Electrocardiograph
Setting (Continued) Description (Continued)
Average Cycles If desired, an additional page prints with the report. Average cycles show the dominant
waveforms for all 12 leads.
• 3x4 50 mm/s + 3R 3 rows x 4 columns + 3 rhythm leads, 50 mm/s
• 6x2 50 mm/s + 6R 6 rows x 2 columns + 6 rhythm leads, 50 mm/s
• No Print Average cycles page does not print.
Page 43

Directions for Use Chapter 4 Reviewing the ECG Settings 39
Reviewing the Interpretation and Copy Settings for Auto Reports
1. Press the Menu key .
2. Choose ECG Settings > Edit Auto Report 1 (or 2) > Interp Settings.
The following screen appears.
Figure 25. “Interpretation Settings” Screen
Interp Settings
1 Print Interpretation?
2 Copies
3 Copies With Interp
4 Reason Statements
5 Unconfirmed Report
6 Abnormal ECG
0 Previous Menu
9:17AM Oct 16 05
3. Change any desired settings.
For a report example, see Figure 23 on page 35.
Setting Description
Print Interpretation? On or off. Determines whether interpretation is printed and saved with reports.
Copies Number of copies to print automatically in addition to the original report:
Copies With Interp On or off. Determines whether interpretation is printed on the automatic copies.
0, 1, 2, 3, 4, or 5.
Reason Statements On or off. Determines whether reasons (criteria) are printed with the interpretation
statements.
Unconfirmed Report On or off. Determines whether the label “Unconfirmed Report” is printed on reports.
Abnormal ECG On or off. Determines whether the label “Abnormal ECG” is printed on reports.
Available only for systems using automatic interpretation.
Page 44

40 Chapter 4 Reviewing the ECG Settings Welch Allyn CP 200 Electrocardiograph
Reviewing the Patient Data Fields Available for Auto Reports
You can determine which fields appear during patient data entry for Auto ECGs.
Note
Spirometry tests use a separate set of data-entry fields, as described in the
spirometry manual.
To Choose the Fields
1. Press the Menu key .
2. Choose ECG Settings > Edit Auto Report 1 (or 2) > Patient Data.
The following screen appears.
Figure 26. “Patient Data” Screen for ECGs
Patient Data
1 First Name
2 Middle Initial
3 Age/Birth Date?
4 Weight
5 Height
6 Gender
7 Race
8 Medication
9 History
A Blood Pressure
9:17AM Oct 16 05
The Patient ID and Last Name fields always
appear on the Enter New Patient screen, as
shown in Figure 33 on page 50. Since these two
fields cannot be disabled, they do not appear
on this user-selectable list.
3. Change any desired settings.
Disabled items (set to off or no) neither display nor print.
Field Description
First Name Yes or no. If yes, this field is enabled.
Middle Initial Yes or no. If yes, this field is enabled.
Age/Birth Date? Birth Date, age, or off. Determines whether and how this data is labeled and entered.
Weight Yes or no. If yes, this field is enabled for entering patients’ weight. For instructions on
Height Yes or no. If yes, this field is enabled for entering patients’ height. For instructions on
Gender Yes or no. If yes, this field is enabled. Data-entry choices: Male, Female, or Unknown.
Race Yes or no. If yes, this field is enabled. Data-entry choices: Blank, Caucasian, Black,
For instructions on changing the date format (MM/DD/YY or DD/MM/YY), see
“Reviewing the Device Configuration Settings” on page 27.
changing the weight units (kg or lb), see “Reviewing the Device Configuration Settings”
on page 27.
changing the height units (cm, in., or ft and in.), see “Reviewing the Device Configuration
Settings” on page 27.
Hispanic, Asian, Unknown.
Page 45

Directions for Use Chapter 4 Reviewing the ECG Settings 41
Field (Continued) Description (Continued)
Medication Yes or no. If yes, this field is enabled. During data entry, choose up to three items from
the list of patient medications. To learn how to edit this list, see “Reviewing the
Medication List” on page 30.
History Yes or no. If yes, this field is enabled. During data entry, choose up to three items from
the list of patient clinical conditions. To learn how to edit this list, see “Reviewing the
History List” on page 31.
Blood Pressure Yes or no. If yes, this field is enabled for entering blood pressure in standard ### / ###
format.
Comments Yes or no. If yes, this field is enabled for entering comments.
Custom 1 Yes or no. If yes, this field is enabled for entering data of your choice.
Custom 1 Label You may define a label for the Custom 1 field if desired.
Custom 2 Yes or no. If yes, this field is enabled for entering data of your choice.
Custom 2 Label You may define a label for the Custom 2 field if desired.
Page 46

42 Chapter 4 Reviewing the ECG Settings Welch Allyn CP 200 Electrocardiograph
Reviewing the Rhythm Report Settings
Rhythm Reports can print either 3, 6, or all 12 leads at a time.
To Change the Number of Leads That Print
1. Press the Menu key .
2. Choose ECG Settings > Edit Rhythm Report > Lead Arrangement.
The following screen appears.
Figure 27. “Lead Arrangement” Submenu
Edit Rhythm Report
1 Lead Arrangement
0 Previous Menu
9:17AM Oct 16 05
3–Lead
6–Lead
12–Lead
3. Choose the number of leads you want to print at a time: 3, 6, or 12.
For instructions on cycling through 3-lead or 6-lead groupings while printing a rhythm
report, see Step 1 on page 56.
Page 47

Directions for Use Chapter 4 Reviewing the ECG Settings 43
Reviewing the Miscellaneous ECG Settings
1. Press the Menu key .
2. Choose ECG Settings > Miscellaneous.
The following screen appears.
Figure 28. “Miscellaneous” Screen for ECG Settings
Miscellaneous
1 Lead Configuration
2 Electrode Labels
3 Baseline Centering
4 Lead Timing
5 Default Gain Setting
6 Default Baseline Filter
7 Default Muscle Filter
8 Mains Filter
9 Auto Save
A Auto Send
9:17AM Oct 16 05
These three default settings—gain, baseline
filter, and muscle filter—determine the
values used every time you begin a new test,
even if these values have been temporarily
changed during ECG testing.
3. Change any desired settings.
Setting Description
Lead Configuration Standard (I II III, aVR aVL aVF, V1 V2 V3, V4 V5 V6) or
Electrode Labels AHA or IEC.
Baseline Centering On or off. When on, aligns the isolectric line of all leads.
Lead Timing Simultaneous or sequential. “Simultaneous” prints ECG data that was captured
Cabrera (aVL I –aVR, II aVF III, V1 V2 V3, V4 V5 V6).
simultaneously for all lead groups. “Sequential” prints ECG data that was captured at
sequential intervals for each lead group in turn.
Default Gain Setting 5 mm/mV, 10 mm/mV, 20 mm/mV, or Auto. (AUTO is available for Auto ECGs only, not
rhythm ECGs. AUTO is usually the best setting, but some waveforms may be easier to
read on other settings.) For details, see “Gain” on page 61.
Default Baseline Filter On or off. For details, see “Baseline Filter” on page 61.
Default Muscle Filter On or off. For details, see “Muscle Filter” on page 61.
Mains Filter Off, 50 Hz, 60 Hz. Use of this filter is recommended. For suggestions on eliminating AC
interference, see page 85.
Auto Save On or off. When on, the electrocardiograph automatically saves all ECGs (except stat
ECGs) to its test directory. When off, every time you print an ECG test you are asked if
you want to save. For description of the test directory, see “Managing Saved Tests”
on page 64.
Page 48

44 Chapter 4 Reviewing the ECG Settings Welch Allyn CP 200 Electrocardiograph
Setting (Continued) Description (Continued)
Auto Send Memory card, workstation, or off. Automatically sends all ECGs (except stat ECGs) to
the option of your choice.
If set to memory card, an SD memory card must be in place during testing. For slot
location, see Figure 5 on page 7.
If set to workstation, a USB cable must connect a CardioPerfect workstation to the
electrocardiograph’s Com port B ( ). For port location, see Figure 5 on page 7.
Auto Report 2 On or off. When on, a second predefined report format is available.
Page 49

45
5
Performing ECG Tests
Connecting the Leads to the Patient . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 46
Recording an Auto ECG . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 49
Recording a Rhythm ECG . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 56
Searching for Saved Patient Data. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 57
Adjusting the ECG Waveforms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 61
Page 50

46 Chapter 5 Performing ECG Tests Welch Allyn CP 200 Electrocardiograph
Connecting the Leads to the Patient
1. Help the patient get comfortable. Patient preparation is important for a successful
ECG.
a. Describe the procedure. If desired, press the Help key and print the page
entitled “What Is An ECG?” for the patient to read.
b. Help the patient get warm and relaxed. Excessive patient movement could
interfere with the operation of the electrocardiograph.
c. Put the patient in a reclining position with the head slightly higher than the heart
and legs.
WARNING ECG electrodes could cause skin irritation. Examine the skin
for signs of irritation or inflammation.
2. Prepare electrode locations. See Figure 29 on page 47.
a. Shave if necessary.
b. Clean with alcohol or acetone.
c. Allow to dry.
3. Attach the electrodes and lead wires securely.
• For reusable electrodes:
Straps must neither slide nor be so tight as to cause discomfort.
The electrode paste, gel, or creme must cover an area the size of the electrode
but no larger, especially on the chest.
• For disposable tab electrodes:
Place the electrode tab between the “jaws” of the electrode adapter, keeping the
tab flat.
Gently tug on the adapter to ensure that it is properly placed on the electrode.
(Each time you remove and reattach an electrode, the conductive gel becomes
weaker and less effective.)
Page 51

Directions for Use Chapter 5 Performing ECG Tests 47
Figure 29. Electrode Placement Locations
B
A
C
D
E
F
G
I
Electrodes
AHA IEC Locations
AV1
red
BV2
yellow
CV3
green
DV4
blue
EV5
orange
FV6
purple
GRA
white
HLA
black
IRL
green
JLL
red
C1
red
C2
yellow
C3
green
C4
brown
C5
black
C6
purple
R
red
L
yellow
N
black
F
green
H
J
Fourth intercostal space at right sternal border.
Fourth intercostal space at left sternal border.
Midway between V2 and V4.
Fifth intercostal space at left of midclavicular line.
Anterior axillary line at same horizontal level as V4.
Mid-axillary line on same horizontal level as V4 and V5.
Just above right wrist on inside of arm.
Just above left wrist on inside of arm.
Just above right ankle.
Just above left ankle.
Page 52

48 Chapter 5 Performing ECG Tests Welch Allyn CP 200 Electrocardiograph
4. If the electrocardiograph’s display is blank, press .
5. If the Lead Off screen appears, as shown here, reattach any leads that are flashing.
Figure 30. “Lead Off” Screen
9:17AM Oct 16 05
Lead off
Check lead
V1
V2
The most common ECG problems are poor electrode contact and loose leads.
When all leads have been connected for three seconds, the following screen
appears.
Figure 31. “ECG Preview” Screen
ECG Preview
I
II
III
AUTO 25 mm/s
Gain
OFF
Baseline
Filter
9:17AM Oct 16 05
OFF
Muscle
Filter
Spee dLeads
6. (Optional) Use the softkeys as desired.
For details, see “Adjusting the ECG Waveforms” on page 61.
7. Go to the procedure for the type of ECG test you want to perform.
• “Recording an Auto ECG” on page 49
• “Recording a Rhythm ECG” on page 56
Page 53

Directions for Use Chapter 5 Performing ECG Tests 49
Recording an Auto ECG
An Auto ECG is a report typically showing a 10-second acquisition of 12 leads of ECG
information combined with patient data, interpretation, and measurements matrix. Two
user-defined formats are available: Auto Report 1 or Auto Report 2. To learn how to set up
the Auto ECG report format, see “Reviewing the Auto Report Settings” on page 35.
As shown in the following diagram, there are the two types of Auto ECG: normal and stat.
For details, see these procedures:
• “Recording a Normal Auto ECG” on page 50
• “Recording a Stat Auto ECG” on page 55
Figure 32. Auto ECG Testing, Process Diagram
Normal
Press quickly.
(Optional)
Select report
format.
Enter, repeat, or
search for patient
data.
(Optional)
Adjust
waveforms.
Press
Print ECG.
Stat
Press and hold.
Auto Report prints.
Auto ECG Post-Print
screen.
Page 54

50 Chapter 5 Performing ECG Tests Welch Allyn CP 200 Electrocardiograph
Recording a Normal Auto ECG
For a normal Auto ECG, you enter patient data and do other optional tasks before printing,
as shown in Figure 32 on page 49.
To Record a Normal Auto ECG
1. Pr es s t he Auto ECG key quickly.
Do not hold it down, or a stat ECG would begin.
2. If prompted, choose Auto Report 1 or Auto Report 2.
3. If asked “Repeat same patient?,” press the desired softkey.
• Ye s — to perform another ECG for the same patient.
The Auto ECG Acquisition screen appears. Go to Step 5 on page 51.
• No — to clear the current patient data.
The following screen appears.
Figure 33. “Enter New Patient” Screen
Enter New Patient
Patient ID
Last Name
First Name
Birth Date
Weight
Height
Gender
Search Schedule
//
lb.
ft. in.
Clear
9:17AM Oct 16 05
MM / DD / YYYY
Done
For details about these data fields—
including how to choose which fields
display and print—see “Reviewing the
Patient Data Fields Available for Auto
Reports” on page 40.
4. Enter or search for the patient data.
• If you want to find a patient whose data has already been entered, go to
“Searching for Saved Patient Data” on page 57.
• If you want to enter the data, fill in the fields.
When finished, press the desired softkey:
Clear deletes the entered data and returns to the Patient ID field.
Done accepts the entered data and goes to the Auto ECG Acquisition
screen. See Figure 34 on page 51.
Page 55

Directions for Use Chapter 5 Performing ECG Tests 51
Figure 34. “Auto ECG Acquisition” Screen
Doe, Jane
Auto ECG Acquisition
I
V
aVR
AUTO ONON
Leads
Gain
Baseline
Filter
9:17AM Oct 16 05
Muscle
Filter
Print
ECG
5. Verify ECG quality on the screen.
WARNING Do not perform ST segment analysis on the ECG screen display,
since these ECG representations are scaled. Make manual measurements of
ECG intervals and magnitudes on printed ECG reports only.
6. (Optional) Adjust the waveforms.
See “Adjusting the ECG Waveforms” on page 61.
7. P r e s s Print ECG.
Softkeys for adjusting the
waveforms or printing
8. If prompted, choose whether to wait for the electrocardiograph to acquire 10 seconds
of filtered, processed data before printing.
If you override the wait time and print the available data immediately, be aware that
the printed data will be insufficient in quality or quantity or both.
The report prints.
Note
If a red stripe appears along the edge of your report, replace the paper.
See “Loading the Thermal Chart Paper” on page 21.
After printing, the Auto ECG Post-Print screen appears. Figure 35 on page 52.
Page 56

52 Chapter 5 Performing ECG Tests Welch Allyn CP 200 Electrocardiograph
Figure 35. “Auto ECG Post-Print” Screen
Doe, Jane
Auto ECG Post-Print
I
V
aVR
9:17AM Oct 16 05
Softkeys for choosing
Print
Copy
Edit
Te st
Manual
Save
Manual
Send
Exit
post-printing options
9. (Optional) If you want to repeat the test, go back to Step 1 on page 50.
10. Press the desired softkey.
Softkey Effect
Print Copy Prints a copy of the test.
To learn how to print multiple copies of all tests automatically, see “Reviewing the
Interpretation and Copy Settings for Auto Reports” on page 39.
Edit Test Brings up the Edit Test – Patient Data screen. From here you can edit or confirm the
patient data and interpretation statements. See “To Edit or Confirm a Test Directly
After Printing” on page 54.
Manual Save Saves the test to the electrocardiograph’s test directory. See “Managing Saved
Tests” on page 64.
This Manual Save softkey appears only when Auto Save is disabled. For more on
Auto Save, see “Reviewing the Miscellaneous ECG Settings” on page 43.
Manual Send Displays two softkey options:
• Memory Card
An SD memory card must be in place. For slot location, see Figure 5 on page 7.
• Workstation
A USB cable must connect the CardioPerfect workstation to the
electrocardiograph’s Com port B ( ). For port location, see Figure 5 on
page 7.
This Manual Send softkey appears only when Auto Send is disabled. For more on
Auto Send, see “Reviewing the Miscellaneous ECG Settings” on page 43.
Exit The ECG Preview screen appears if all leads are connected to the patient.
Page 57

Directions for Use Chapter 5 Performing ECG Tests 53
Caution The requirements of AAMI EC11, Section 3.2.7.2, Frequency and
Impulse Response, for an impulse triangle waveform may be impacted by up
to 5 milliseconds of small amplitude dampened ringing immediately after the
impulse when the muscle filter (35 Hz) is turned on or a small amplitude
offset when the baseline filter (0.5 Hz) is turned on. These requirements are
unaffected by any other combination of filters turned on or off.
Measurements performed by the optional interpretation algorithm are
unaffected by any filter selections.
Page 58

54 Chapter 5 Performing ECG Tests Welch Allyn CP 200 Electrocardiograph
To Edit or Confirm a Test Directly After Printing
Note
A qualified physician must review and confirm all tests before patients are
treated. If changes are needed for any saved test, you can edit two types of data:
• patient data
• interpretation statements
1. From the Auto ECG Post-Print screen (Figure 35 on page 52), press Edit Test.
The Edit Test – Patient Data screen appears.
2. (Optional) Edit the patient data.
3. Press the desired softkey.
Softkey Effect
Interp Saves any changes, and displays the Edit Test – Interpretation screen.
1. (Optional) Edit the interpretation statements to be saved with the test.
2. Press the desired softkey:
• Patient Data saves any changes, and returns to the Edit Test – Patient Data
screen.
• Confirm saves any changes, sets the test status to “confirmed,” and returns to the
Auto ECG Post-Print screen.
• Cancel discards any changes, and returns to the Auto ECG Post-Print screen.
• Done saves any changes, and returns to the Auto ECG Post-Print screen.
For details on interpretation, see “Automatic ECG interpretation” on page 4.
Cancel Discards any changes, and returns to the Auto ECG Post-Print screen.
Done Saves any changes, and returns to the Auto ECG Post-Print screen.
Page 59

Directions for Use Chapter 5 Performing ECG Tests 55
Recording a Stat Auto ECG
A stat Auto ECG is an immediate printout in Auto Report 1 format.
Stat mode bypasses patient data entry, as shown in Figure 32 on page 49. A temporary
ID number is assigned to the patient to identify stat tests. After printing, you may enter
the patient data by editing the test.
In stat mode, Auto Send and Auto Save are always disabled, even if they are enabled in
the ECG settings. If you want to send or save a stat Auto ECG, you may do so manually
after you print.
To Record a Stat Auto ECG
1. Press and hold
The electrocardiograph begins acquiring ECG data. After it has acquired 10 seconds
of quality data, it prints a report.
2. Go to Step 8 on page 51.
Continue the procedure as if you have just pressed the Print ECG softkey.
the Auto ECG key .
Page 60

56 Chapter 5 Performing ECG Tests Welch Allyn CP 200 Electrocardiograph
Recording a Rhythm ECG
A Rhythm ECG is a continuous, real-time printout of a rhythm strip with a user-defined
lead arrangement. For details on reviewing or changing the lead arrangement in your
reports, see “Reviewing the Rhythm Report Settings” on page 42.
Rhythm ECGs are printouts only. They cannot be saved or sent electronically.
Figure 36. Rhythm ECG Testing, Process Diagram
Printing begins.
(Optional) Adjust
waveforms.
or
To Record a Rhythm ECG
1. Pr es s t he Rhythm ECG key .
Printing begins.
The screen displays 3 leads at a time from the leads currently printing. The printout
includes either 3, 6, or 12 leads at a time. To find out how to change this number, see
“Reviewing the Rhythm Report Settings” on page 42.
Figure 37. “Rhythm ECG” Screen
Doe, Jane
Rhythm ECG
I
V
aVR
10 mm/mV
Gain
Baseline
Filter
9:17AM Oct 16 05
25 mm/sONON
Muscle
Filter
SpeedLeads
Softkeys for adjusting
waveforms while printing
1. (Optional) Press the softkeys to adjust the waveforms.
See “Adjusting the ECG Waveforms” on page 61.
2. Press or to stop printing.
The ECG Preview screen appears if all leads are connected to the patient.
(Optional) Adjust the waveforms.
Page 61

Directions for Use Chapter 5 Performing ECG Tests 57
Searching for Saved Patient Data
During an ECG or spirometry test, instead of entering patient data manually you can
search for patient data that has been saved in either of two places:
• In the scheduled patients list
The scheduled patients list identifies up to 40 patients whose data has been entered
into the electrocardiograph’s memory for an ECG or spirometry test that day. For
more, see “Managing the Scheduled Patients List” on page 68.
• In the test directory
The test directory is the collection of tests saved in the electrocardiograph’s memory.
This directory holds up to 50 ECG and 50 spirometry tests. For more, see “Managing
Saved Tests” on page 64.
The following diagram illustrates the procedure for both types of searching. For step-bystep instructions, see these sections:
• “To Search the Test Directory” on page 58
• “To Search the Scheduled Patients List” on page 60
Figure 38. Patient Search, Process Diagram
Go to
Enter New Patient
screen.
Go to
Search for Patient
screen.*
* Password is required if user
login is enabled.
Enter patient’s
last name or ID.
Choose a patient from the
test directory.
Scheduled Patients List
Go to
screen.*
Choose a patient from the
scheduled patients list.
Continue ECG or
spirometry test.
Page 62

58 Chapter 5 Performing ECG Tests Welch Allyn CP 200 Electrocardiograph
To Search the Test Directory
1. From the Enter New Patient screen (Figure 33 on page 50), press Search.
If prompted to log in, enter your user ID and password.
The following screen appears.
Figure 39. “Search for Patient” Screen
Search for Patient
Patient ID:
Last Name:
9:17AM Oct 16 05
Brings up any matching names in
the test directory.
New
Patient
Schedule
Search
2. Enter the patient’s complete identification number or last name (partial or complete).
3. Press Search.
If one or more patients match your entry, their names appear.
Note The other two softkeys exit this search:
• New Patient returns to the Enter New Patient screen. See Figure 33 on
page 50.
• Schedule brings up the Scheduled Patients List screen. Go to “To
Search the Scheduled Patients List” on page 60.
Page 63

Directions for Use Chapter 5 Performing ECG Tests 59
Figure 40. “Patient Search Results” Screen
Patient Search Results
Patient ID
Back
9:17AM Oct 16 05
Patient Name
Select
4. Take the desired action.
• Press Back.
The Search for Patient screen reappears.
• Highlight a patient name, and press Select.
The next screen in your procedure appears. (For ECG tests, go to Step 5 on
page 51. For spirometry tests, see the spirometry manual.)
Your search results (matching
names from test directory).
Page 64

60 Chapter 5 Performing ECG Tests Welch Allyn CP 200 Electrocardiograph
To Search the Scheduled Patients List
1. From the Enter New Patient screen (Figure 33 on page 50), press Schedule.
If prompted to log in, enter your user ID and password.
The following screen appears.
Figure 41. “Scheduled Patient List” Screen (for Searching)
Scheduled Patient List
Patient ID
9:17AM Oct 16 05
Patient Name
Today’s scheduled patients.
New
Patient
Search
Select
2. Highlight the desired name. Press Select.
The next screen in your procedure appears. (For ECG tests, go to Step 5 on page 51.
For spirometry tests, see the spirometry manual.)
Note
The other two softkeys exit this search:
• New Patient returns to the Enter New Patient screen. See Figure 33 on
page 50.
• Search brings up the Search for Patient screen. Go to “To Search the
Test Directory” on page 58.
Page 65

Directions for Use Chapter 5 Performing ECG Tests 61
Adjusting the ECG Waveforms
The following softkeys appear on the Auto ECG Acquisition and Rhythm ECG screens
(Figure 34 on page 51 and Figure 37 on page 56). Use these softkeys before printing an
Auto ECG or while printing a rhythm ECG.
Softkey Effect
Leads Cycles through the leads in groups of three on the screen. For rhythm reports, also cycles through the lead groups that are
printing (if <12). To learn how to change the number of leads per group—3, 6, or 12—on rhythm reports, see “Reviewing
the Rhythm Report Settings” on page 42. To learn how to change between standard and Cabrera lead groupings, see
“Reviewing the Miscellaneous ECG Settings” on page 43.
Gain Cycles through the gain settings in mm/mV (5, 10, 20, AUTO), zooming in and out. (AUTO is available for Auto ECGs only,
not rhythm ECGs. AUTO is usually the best setting, but some waveforms may be easier to read on other settings.) To learn
how to change the the gain’s default setting, see “Reviewing the Miscellaneous ECG Settings” on page 43.
5 mm/mV 20 mm/mV
Baseline Filter Toggles between the two baseline filter settings (on or off). This filter reduces “wandering baseline,” an upward and
downward fluctuation of the waveforms. It is preferable, if possible, to eliminate or reduce wandering baseline by
addressing the causes, as explained on page 84. To learn how to change the the filter’s default setting, see “Reviewing
the Miscellaneous ECG Settings” on page 43. Caution: You cannot perform ST segment analysis on waveforms that were
recorded with the baseline filter turned on. For details, see the caution on page 53.
Muscle Filter Toggles between the two muscle filter settings (on or off). This filter reduces muscle tremor interference: random,
Speed
(available for
Rhythm ECGs only)
irregular voltage superimposed on the waveforms. It is preferable, if possible, to eliminate or reduce muscle tremor by
addressing the causes, as explained on page 84. To learn how to change the the filter’s default setting, see “Reviewing
the Miscellaneous ECG Settings” on page 43. Caution: You cannot perform ST segment analysis on waveforms that were
recorded with the muscle filter turned on. For details, see the caution on page 53.
Cycles through the paper speed settings in mm/sec (10, 25, 50).
10 mm/mV
Wandering baseline
Muscle tremor interference
25 mm/sec 10 mm/sec
50 mm/sec
Page 66

62 Chapter 5 Performing ECG Tests Welch Allyn CP 200 Electrocardiograph
Page 67

63
6
Performing Administrative Tasks
Managing Saved Tests. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 64
Managing the Scheduled Patients List . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 68
Managing Data Security . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 69
Page 68

64 Chapter 6 Performing Administrative Tasks Welch Allyn CP 200 Electrocardiograph
Managing Saved Tests
The test directory is the collection of tests saved in the electrocardiograph’s memory.
This directory holds up to 50 ECG and 50 spirometry tests. When the directory gets full,
the electrocardiograph prompts you to delete the oldest tests before saving new ones.
When performing Auto ECG or spirometry tests, you may select patients from this
directory rather than manually entering their data at the time of the test.
This section explains how to manage—view, edit, print, and send—saved tests. These
tasks are all accomplished through the Test Directory menu, as illustrated here.
Figure 42. ”Test Directory” Menu Tree
Print Directory
Edit
Patient Data
Interpretation
Test Directory*
Search
Search Results
Auto Report 1
Auto Report 2
* Password is required if user
login is enabled.
Print
Send
Memory Card
Workstation
Page 69

Directions for Use Chapter 6 Performing Administrative Tasks 65
To View, Print, or Send Saved Tests
1. Press the Menu key .
2. Choose Test Directory.
If prompted to log in, enter your user ID and password.
The following screen appears.
Figure 43. Test Directory “Search” Screen
Search
9:17AM Oct 16 05
Unprinted Tests
Unconfirmed Tests
Unsent Tests
All Tests
Date From
Date To
Patient ID
Last Name
Print
Directory
//
//
Clear
MM / DD / YYYY
MM / DD / YYYY
Search
Use these criteria to find and perform actions
on a batch
of tests.
Use these criteria to find and perform actions
on a single
test.
3. (Optional) Press Print Directory to print the contents of the test directory.
4. Select or fill in the search criteria, as explained in Figure 43.
To clear all entries, press Clear.
5. Press Search.
If any saved tests match your criteria, the Search Results screen appears.
6. Determine whether you need to select a test.
• If you searched for a batch of tests, all tests are automatically selected.
• If you searched for a particular patient, select the desired test.
7. Press the desired softkey.
• Print — prints the selected test(s).
• Send — sends the selected test(s) to your choice:
Memory Card
An SD memory card must be in place.
Workstation
A USB cable must connect a CardioPerfect workstation to the
electrocardiograph’s Com port B ( ).
Page 70

66 Chapter 6 Performing Administrative Tasks Welch Allyn CP 200 Electrocardiograph
To Edit or Confirm Saved Tests
Note
A qualified physician must review and confirm all tests before patients are
treated. If changes are needed for any saved test, you can edit two types of
information:
• patient data
• interpretation statements
For an example of each type of information as it appears on a report, see
Figure 23 on page 35.
1. Select the desired test(s) from the test directory.
For instructions, see “To View, Print, or Send Saved Tests” on page 65, Step 1
through Step 6.
2. Press Edit.
The Edit Test – Patient Data screen appears.
If you have selected one test, the patient data displays for that test. If you have
selected multiple tests, the patient data displays for the first test.
3. (Optional) Edit the patient data.
4. Press the desired softkey.
Softkey Effect
Interp Saves any changes, and displays the Edit Test – Interpretation screen for the same test.
1. (Optional) Edit the interpretation.
2. Press the desired softkeys:
• Patient Data saves any changes, and returns to the Edit Test – Patient Data
screen for the same test.
• Confirm saves any changes, sets the test status to “confirmed,” and displays patient
data for the next test, if any.
• Previous Test saves any changes, and displays patient data for the previous test.
Appears only when previous tests are available in your search results.
• Next Test saves any changes, and displays patient data for the next test. Appears
only when next tests are available in your search results.
• Done saves any changes, and returns to the Test Directory Search screen.
Page 71

Directions for Use Chapter 6 Performing Administrative Tasks 67
Softkey Effect (Continued)
Previous Test Saves any changes, and displays the previous test. Appears only when previous tests are
available in your search results.
Next Test Saves any changes, and displays the next test. Appears only when next tests are available in
your search results.
Done Saves any changes, and returns to the Test Directory Search screen.
Page 72

68 Chapter 6 Performing Administrative Tasks Welch Allyn CP 200 Electrocardiograph
Managing the Scheduled Patients List
The scheduled patients list identifies up to 40 patients whose data has been entered into
the electrocardiograph’s memory for an ECG or spirometry test that day. At midnight
every night, the list is automatically cleared.
When performing Auto ECG or spirometry tests, you may select patients from this list
rather than manually entering their data at the time of the test.
To View or Edit the Scheduled Patients List
1. Press the Menu key .
2. Choose Scheduled Patients.
If prompted to log in, enter your user ID and password.
The scheduled patients list appears.
Figure 44. “Scheduled Patients” Screen (for Editing)
Scheduled Patients
Patient ID
Patient Name
Add
3. Press the desired softkeys:
•Add
Brings up the Enter New Patient screen, shown in Figure 33 on page 50.
Enter the patient’s data as described on that page.
• Delete
Deletes the highlighted patient name.
•Exit
Returns to the main menu.
9:17AM Oct 16 05
Delete
Today’s scheduled patients.
Exit
Page 73

Directions for Use Chapter 6 Performing Administrative Tasks 69
Managing Data Security
The CP 200 electrocardiograph incorporates security features that can keep patient data
private and confidential. To implement your data security plan, use the Device
Administration menu, which is illustrated here and described in the following pages.
Figure 45. “Device Administration” Menu Tree
System Settings
User Login
(Enable/Disable)
Edit User List
Device
Administration*
* Administrative-level password is
required if user login is enabled.
Audit Trail
(Enable/Disable)
Print Audit Trail
Page 74

70 Chapter 6 Performing Administrative Tasks Welch Allyn CP 200 Electrocardiograph
Working With the User List and User Login
The user list identifies all individuals who are authorized to access patient data.
The user list can hold up to 25 users. After the list reaches 25 users, you can add names
only if some names are set to inactive status. New names replace inactive names. If all
25 names are active, you receive a message that the user list is full.
Two levels of access can be assigned:
•User level
When the user login feature is enabled, all users on the user list have access to the
test directory and the scheduled patient list, including searching and editing
privileges.
• Administrator level
When the user login feature is enabled, only administrator-level users have access to
the administrative functions. For an outline of these functions, see menu tree in
Figure 45 on page 69.
To Enable or Disable User Login
1. Press the Menu key .
2. Choose System Settings > Device Administration.
If prompted to log in, enter an administrator-level user ID and password.
3. Choose User Login.
4. Follow the prompts to enable or disable user login.
To Change a Password
You can change a password in either of two ways:
• At any User Login screen, press Change Password.
Follow the prompts.
• Ask an administrator-level user to change the password.
See “To Review or Edit the User List” on page 71.
Page 75

Directions for Use Chapter 6 Performing Administrative Tasks 71
To Review or Edit the User List
1. Press the Menu key .
2. Choose System Settings > Device Administration.
If prompted to log in, enter an administrator-level user ID and password.
Note
If no administrator-level password has been assigned, the system’s default
user ID and password — available from Welch Allyn — works here.
These defaults exist solely to provide access to the user list in the event that
user login is enabled before an administrator is assigned.
3. Choose User List.
The user list appears.
4. Press the desired softkeys.
Softkey Effect
Add Lets you add new users. For each user, provide the following information:
• User ID (1 to 10 characters)
• Password (5 to 10 characters, displayed as asterisks for security)
• Password Expiration
- Never
- 180 days
•User’s Name
• User Level
- User
- Administrator
• Active?
- Yes (user cannot be replaced)
- No (user can be replaced when list is full)
Edit Lets you edit the information for the highlighted user.
Exit Returns to the Device Administration screen.
Page 76

72 Chapter 6 Performing Administrative Tasks Welch Allyn CP 200 Electrocardiograph
Working With the Audit Trail
An audit trail, a collection of information about user activity, may be helpful or even
required for record-keeping. It tracks who did what when, including these types of
activity:
• Accessing the test directory
• Searching the scheduled patient list
• Editing patient data
• Accessing administrative functions
When the audit trail is enabled, the electrocardiograph collects this type of information in
a file in its memory. When the allotted memory begins to get full, you receive prompts to
print the audit trail. You must print the audit trail to delete (purge) this information and
make room for new information to be collected.
To Enable or Disable the Audit Trail
1. Press the Menu key .
2. Choose System Settings > Device Administration.
If prompted to log in, enter an administrator-level user ID and password.
3. Choose Audit Trail.
4. Follow the prompts to enable or disable the audit trail.
To Pr i n t a n Au d it Tra i l
1. Press the Menu key .
2. Choose System Settings > Device Administration.
If prompted to log in, enter an administrator-level user ID and password.
3. Choose Print Audit Trail.
4. Follow the prompts.
After you verify that printing was successful, the audit information is deleted.
Page 77

73
7
Maintaining the Electrocardiograph
Inspecting the Equipment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 74
Cleaning the Equipment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 74
Testing the Equipment. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 75
Recharging a Fully Discharged Battery. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 76
Replacing the Battery . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 77
Replacing the Battery (DC) Fuse . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 79
Replacing the AC Fuses . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 80
Storing the Equipment. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 81
Discarding the Equipment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 81
Page 78

74 Chapter 7 Maintaining the Electrocardiograph Welch Allyn CP 200 Electrocardiograph
Inspecting the Equipment
WARNING To ensure patient safety and proper operation, perform the
following inspections daily.
• Verify that patient leads are fully inserted. For details, see “Connecting the Patient
Cable” on page 20.
• Check for cracked or broken patient cable, patient leads, power cord,
communications cables, display, and enclosure.
• Check for bent or missing pins on all cables.
• Check all cable and cord connections; reseat if any connectors are loose.
Cleaning the Equipment
WARNING To prevent the spread of infection, the electrocardiograph and
patient cable must be kept clean, especially the components that come in
contact with patients.
Caution Do not let soap or water come into contact with the
electrocardiograph’s internal printer, connectors, or jacks.
Do not attempt to clean the electrocardiograph or the patient cable by
submersing them in liquid, autoclaving, or steam cleaning. Do not pour alcohol
directly on the equipment or soak any components in alcohol. If alcohol or liquids
spill into the electrocardiograph while cleaning, arrange to have it checked before
using it again. For Welch Allyn phone numbers, see page ii.
Monthly, or more often if needed, follow these cleaning instructions:
1. Disconnect the power plug from the AC outlet.
2. Wipe the exterior of the patient cable and electrocardiograph and with a damp cloth
using mild detergent diluted in water.
3. Use 70% isopropyl alcohol to disinfect patient cable, lead wires, and equipment.
4. Dry all components with a clean, soft cloth or paper towel.
5. Wait at least 10 minutes to allow all traces of alcohol to evaporate before turning the
electrocardiograph back on.
Page 79

Directions for Use Chapter 7 Maintaining the Electrocardiograph 75
Testing the Equipment
WARNING Only qualified service personnel should perform leakage current
tests.
Whenever the electrocardiograph is serviced or problems are suspected, Welch Allyn
recommends the following test procedures:
1. Verify continued electrical safety of the device, using IEC 60601-1 or ANSI/AAMI ES1
methods and limits. Test for the following:
• Patient leakage current
• Chassis leakage current
• Earth leakage current
• Dielectric strength (AC and patient circuits)
2. Verify that the electrocardiograph is working properly, using an ECG simulator to
acquire and print a standard 12-lead ECG of known amplitude.
• Printing should be dark and even across the page.
• There should be no evidence of print-head dot failure (no printing breaks forming
horizontal streaks).
• Paper should move smoothly and consistently during printing.
• Waveforms should appear normal, with proper amplitude, and without distortion
or excessive noise.
• Paper should stop with perforations near the tear bar, indicating proper cue-
sensor operation. For tear-bar location, see Figure 17 on page 21.
Page 80

76 Chapter 7 Maintaining the Electrocardiograph Welch Allyn CP 200 Electrocardiograph
Recharging a Fully Discharged Battery
If the electrocardiograph does not turn on when unplugged, the battery may be fully
discharged.
Note
To Recharge the Battery
1. Plug the electrocardiograph into AC power.
2. Verify that the green LED on the keyboard lights up. (See Figure 18 on page 22.)
3. Keep the electrocardiograph plugged in for 12 hours.
The first time you turn the electrocardiograph on after a full battery discharge, you are
prompted to reenter the date and time.
If the electrocardiograph still does not turn on when it is unplugged from AC power, you
may need to replace the battery or battery fuse. See “Replacing the Battery” on page 77
or “Replacing the Battery (DC) Fuse” on page 79.
Regardless of the battery condition, you can use the electrocardiograph
whenever it is plugged in.
If the LED does not light up, go to “Replacing the AC Fuses” on page 80.
Page 81

Directions for Use Chapter 7 Maintaining the Electrocardiograph 77
Replacing the Battery
If you have recharged the battery and the electrocardiograph still does not turn on when
unplugged, or if the battery loses its charge quickly, replace the battery as follows.
(For part number, see “Accessories” on page 5.)
1. Unplug the electrocardiograph from AC power if connected.
2. Turn the electrocardiograph upside-down.
3. Unscrew and remove the battery door. See Figure 46.
4. Lift out the battery. See Figure 47.
Figure 46. Removing the Battery Door Figure 47. Lifting Out the Battery
5. Inspect the fuse. See Figure 48.
• If the “Z” wire is intact, go to Step 6.
• If the “Z” wire is broken or dark, replace the fuse. See “Replacing the Battery
(DC) Fuse” on page 79.
Figure 48. Battery Fuse Plugged In
“Z” wire
Page 82

78 Chapter 7 Maintaining the Electrocardiograph Welch Allyn CP 200 Electrocardiograph
6. Unplug the two battery connectors. See Figure 49.
You may need to pull hard. Use pliers gently if necessary.
Figure 49. Battery Connectors
7. Connect a new battery by matching connector sizes. Do not force connectors to
mismatch.
8. Carefully tuck in all cables. Place the battery into the battery compartment. Fold the
strap so that it will not stick out when you replace the battery door. See Figure 50.
Figure 50. Folding the Battery Strap
Page 83

Directions for Use Chapter 7 Maintaining the Electrocardiograph 79
9. Replace the battery door and screws. Turn the electrocardiograph rightside-up.
It turns on automatically and displays a prompt to enter date and time.
10. Enter the date and time.
The electrocardiograph is ready to use.
11. Discard the old battery appropriately.
• In the USA, call 1-800-SAV-LEAD for instructions on how to recycle it.
• International users, contact your local authorities concerning recycling.
Replacing the Battery (DC) Fuse
If the battery (DC) fuse requires replacing, as described in Step 5 on page 77, follow
these steps. For the fuse value, see “Fuses” on page 89.
1. Remove and discard the fuse. See Figure 51.
You may need to pull hard. Use pliers gently if necessary.
2. Connect a new fuse. It goes in either way.
3. Go to Step 8 on page 78.
Figure 51. Battery Fuse Removed
Page 84

80 Chapter 7 Maintaining the Electrocardiograph Welch Allyn CP 200 Electrocardiograph
Replacing the AC Fuses
If the green LED on the keyboard does not light up when the electrocardiograph is
connected to AC power, you may need to replace one or both of the AC fuses, as follows.
For the fuse value, see “Fuses” on page 89.
1. Unplug the electrocardiograph from AC power if connected.
WARNING Failure to unplug could result in electrocution.
2. Use needle-nosed pliers to remove the fuse case. See Figure 52.
3. Inspect the fuses. If either fuse is dark or has a broken wire, replace the fuse.
See Figure 53.
4. Insert the fuse case. Line it up with the opening; it goes in only one way.
Figure 52. Removing AC Fuse Case Figure 53. AC Fuses Removed
Page 85

Directions for Use Chapter 7 Maintaining the Electrocardiograph 81
Storing the Equipment
When storing the electrocardiograph, cords, and accessories, observe the environmental
storage conditions. See “Specifications” on page 89.
Discarding the Equipment
Discard the old battery appropriately.
• In the USA, call 1-800-SAV-LEAD for instructions on recycling it.
• International users, contact your local authorities concerning recycling.
Discard the electrocardiograph, cords, and accessories according to local laws.
Do not dispose of this product as unsorted municipal waste. Prepare this product for
reuse or separate collection as specified by Directive 2002/96/EC of the European
Parliament and the Council of the European Union on Waste Electronic and Electrical
Equipment (WEEE). If this product is contaminated, this directive does not apply. For
more specific disposal information, see www.welchallyn.com/weee, or contact Welch
Allyn Customer Service at +44 207 365 6780.
Page 86

82 Chapter 7 Maintaining the Electrocardiograph Welch Allyn CP 200 Electrocardiograph
Page 87

83
8
Troubleshooting
Problem-Solving Suggestions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 84
Limited Warranty . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 87
Service Policy. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 88
Page 88

84 Chapter 8 Troubleshooting Welch Allyn CP 200 Electrocardiograph
Problem-Solving Suggestions
This section includes several tables:
• Lead quality problems (Ta bl e 2 on page 84)
• System failure problems (Tabl e 3 on page 85)
• System messages (Tab le 4 on page 86)
If you try these suggestions and still have problems, contact Technical Support.
For phone numbers, see page ii.
Table 2. Lead Quality Problems
Condition Causes Actions
A red dot is flashing on the Lead Off
screen.
OR
Lead-off information is displayed on the
screen.
OR
One or more leads prints as a square
wave:
Wandering baseline (an upward and
downward fluctuation of the waveforms):
Muscle tremor interference (random,
irregular voltage superimposed on the
waveforms). May resemble or coincide
with AC interference:
• Electrode contact may be poor.
• A lead may be loose.
Note: Square waves may indicate that the
electrocardiograph is inoperative, but they
are more likely to indicate loose leads.
• Electrodes that are dirty, corroded,
loose, or positioned on a bony area.
• Insufficient or dried electrode gel.
• Oily skin or body lotions.
• Rising and falling of chest during rapid
or apprehensive breathing.
• Patient is uncomfortable, tense,
nervous.
• Patient is cold and shivering.
• Exam bed is too narrow or short to
comfortably support arms and legs.
• Arm or leg electrode straps are too
tight.
• Reattach the lead.
• Replace the electrode.
• Verify that the electrode area has been properly
prepared: shaved, cleaned with alcohol or
acetone, allowed to dry.
• Verify that electrodes have been properly stored
and handled.
• Clean skin with alcohol or acetone.
• Reposition or replace electrodes.
• Help patient relax.
• If wandering baseline persists, turn the baseline
filter on. See “Baseline Filter” on page 61.
• Help patient get comfortable.
• Check all electrode contacts.
• If interference persists, turn the muscle-tremor
filter on. See “Muscle Filter” on page 61.
• If interference still persists, the problem is
probably electrical in nature. See the following
suggestions for reducing AC interference.
Page 89

Directions for Use Chapter 8 Troubleshooting 85
Table 2. Lead Quality Problems (Continued)
Condition Causes Actions
AC interference (even-peaked, regular
voltage superimposed on the waveforms).
May resemble or coincide with muscletremor interference.
• Electrodes that are dirty, corroded,
loose, or positioned on a bony area.
• Insufficient or dried electrode gel.
• Patient or technician touching an
electrode during recording.
• Patient touching any metal parts of an
exam table or bed.
• Broken lead wire, patient cable, or
power cord.
• Electrical devices in the immediate
area, lighting, concealed wiring in
walls or floors.
• Improperly grounded electrical outlet.
• Incorrect AC filter frequency setting or
AC filter is turned off.
• Check all electrode contacts and lead wires.
• Verify that the patient is not touching any metal.
• Verify that the AC power cable is not touching
• Verify that the proper AC filter is selected. See
• If interference persists, unplug the
• If interference still persists, the noise may be
Table 3. System Failure Problems
Condition Causes Actions
Won't turn on when plugged into AC
power.
Won't turn on when unplugged from AC
power.
• Faulty AC power connection.
• Blown AC fuses.
•No AC power.
• Battery disconnected or incorrectly
connected.
• Battery low, not charging, depleted, or
bad.
• Blown battery fuse.
• Check the AC power source.
• Check the AC fuses. See “Replacing the AC
• Check battery connections. See “Replacing the
• Recharge the battery. See “Recharging a Fully
• Replace battery. See “Replacing the Battery” on
• Replace battery fuse. See “Replacing the Battery
the patient lead cable.
“Mains Filter” on page 43.
electrocardiograph from AC power and run it on
the battery. If this solves the problem, you’ll
know that the noise was introduced through the
power line.
caused by other equipment in the room or by
poorly grounded power lines. Try moving to
another room.
Fuses” on page 80.
Battery” on page 77.
Discharged Battery” on page 76.
page 77.
(DC) Fuse” on page 79.
Shuts down during printing Battery low or bad. • Recharge the battery. See “Recharging a Fully
Prints fewer than 10 reports on a full
battery charge.
Degraded battery. Replace battery. See “Replacing the Battery” on
Discharged Battery” on page 76.
• Replace battery. See “Replacing the Battery” on
page 77.
page 77.
Page 90

86 Chapter 8 Troubleshooting Welch Allyn CP 200 Electrocardiograph
Table 4. System Messages (alphabetical order)
System Message Problems Actions
“A test is being printed that has not been
saved and will be lost. Continue
shutdown?”
“Audit Trail is too large. Please print and
purge.“
“Insufficient battery power to begin.
Please connect AC power and try again.”
“Insufficient space available” Not enough space on memory card. • Delete some tests from the card at a PC.
“Memory card error” Problem writing to memory card. • Verify that the write-protect tab is in the
“Out Of Paper” • Printer is out of paper.
“Paper Error” Paper was loaded incorrectly. Reload the paper. See “Loading the Thermal Chart
“Powering down” Low battery. Recharge the battery. See “Recharging a Fully
was pressed while printing an
Auto ECG.
Audit information is approaching its
maximum allotted storage.
Battery low or bad. • Recharge the battery. See “Recharging a Fully
• Printer door is open.
To shut down without saving the test, press .
To cancel the shutdown, press .
Print and purge the audit trail. See “Working With
the Audit Trail” on page 72.
Discharged Battery” on page 76.
• Replace battery. See “Replacing the Battery” on
page 77.
• Use a different card.
unprotected position.
• Reseat the card in its slot.
• Use a different card.
• Load paper. See “Loading the Thermal Chart
Paper” on page 21.
• Close the printer door. See “Loading the Thermal
Chart Paper” on page 21.
Paper” on page 21.
Discharged Battery” on page 76.
“Problem loading the following settings:
<System>
<ECG>
*
*
*
Using default settings.”
“Shutdown?” was pressed while printing a
“Temperature Error” Printer head temp is too high. Allow to cool, then try again.
“Test has not been saved. Continue
shutdown?”
“Unable to communicate with
workstation“
Problem loading your settings at startup.
May indicate memory problems.
Rhythm ECG.
was pressed after printing an
Auto ECG.
• Configuration not correct.
• Cable not properly connected.
Contact Technical Support. For phone numbers, see
page ii.
To shut down, press .
To cancel the shutdown, press .
To shut down without saving the test, press .
To cancel the shutdown, press .
• Verify communication settings on the PC.
• Check cable connections.
Page 91

Directions for Use Chapter 8 Troubleshooting 87
Limited Warranty
Welch Allyn, Inc., warrants that the Cardiopulmonary line of electrocardiographs,
including the CP 100 and CP 200 models (the Products) meet the labeled specifications of
the Products and will be free from defects in materials and workmanship that occur
within 3 years after the date of purchase, except that accessories used with the Products
are warranted for 90 days after the date of purchase. Such accessories include: lead
wires, cabling, electrodes, and battery.
The date of purchase is: 1) the date specified in our records, if you purchased the Product
directly from us, 2) the date specified in the warranty registration card that we ask you to
send to us, or 3) if you don’t return the warranty registration card, 30 days after the date
on which the Product was sold to the dealer from whom you bought the Product, as
documented in our records.
This warranty does not cover damage caused by: 1) handling during shipping, 2) use or
maintenance contrary to labeled instructions, 3) alteration or repair by anyone not
authorized by Welch Allyn, and 4) accidents.
If a Product or accessory covered by this warranty is determined to be defective because
of defective materials, components, or workmanship, and the warranty claim is made
within the warranty period described above, Welch Allyn will, at its discretion, repair or
replace the defective Product or accessory free of charge. If your Product requires repairs
covered by this warranty, upon your request Welch Allyn will loan to you, at no cost, a
substitute Product for use until your repaired Product is returned.
You must obtain a return authorization from Welch Allyn to return your Product before
you send it to Welch Allyn’s designated service center for repair. Contact Welch Allyn
Technical Support. For phone numbers, see page ii.
THIS WARRANTY IS IN LIEU OF ALL OTHER WARRANTIES, EXPRESS OR IMPLIED,
INCLUDING BUT NOT LIMITED TO THE IMPLIED WARRANTIES OF MERCHANTABILTY
AND FITNESS FOR A PARTICULAR PURPOSE. WELCH ALLYN’S OBLIGATION UNDER
THIS WARRANTY IS LIMITED TO REPAIR OR REPLACEMENT OF PRODUCTS
CONTAINING A DEFECT. WELCH ALLYN IS NOT RESPONSIBLE FOR ANY INDIRECT
OR CONSEQUENTIAL DAMAGES RESULTING FROM A PRODUCT DEFECT COVERED
BY THE WARRANTY.
Page 92

88 Chapter 8 Troubleshooting Welch Allyn CP 200 Electrocardiograph
Service Policy
All repairs on products under warranty must be performed or approved by Welch Allyn.
Unauthorized repairs will void the warranty. In addition, whether or not covered under
warranty, any product repair shall exclusively be performed by Welch Allyn certified
service personnel.
If the product fails to function properly—or if you need assistance, service, or spare
parts—contact the nearest Welch Allyn Technical Support Center. For phone numbers,
see page ii.
Before contacting Welch Allyn, try to duplicate the problem, and check all accessories to
ensure that they are not causing the problem. When calling, please be prepared to
provide:
• Product name and model number and complete description of the problem.
• Serial number of your product (if applicable).
• Complete name, address and phone number of your facility.
• For out-of-warranty repairs or spare parts orders, a purchase order (or credit card)
number.
• For parts orders, the required spare or replacement part numbers.
If your product requires warranty, extended warranty, or non-warranty repair service,
please call first the nearest Welch Allyn Technical Support Center. A representative will
assist you troubleshooting the problem and will make every effort to solve it over the
phone, avoiding potential unnecessary returns.
In case a return cannot be avoided, the representative will record all necessary
information and will provide a Return Material Authorization (RMA) number, as well as the
appropriate return address. An RMA number must be obtained prior to any return.
If you have to return goods for service, follow these recommended packing instructions:
• Remove all hoses, cables, sensors, power cords, and ancillary products (as
appropriate) before packing, unless you suspect they are associated with the
problem.
• Wherever possible use the original shipping carton and packing materials.
• Include a packing list and the Welch Allyn Return Material Authorization (RMA)
number.
It is recommended that all returned goods be insured. Claims for loss or damage to the
product must be initiated by the sender.
Page 93

89
A
Specifications
Item Specification
Dimensions
Weight 11.6 lb (5.3 kg)
Keyboard type Elastomer keypad with complete alphanumeric keys
Paper type 8.25 x 11 inches (21 x 28 cm), Z-fold thermal paper, 200 sheets
Thermal printer (internal) Computer-controlled dot array, 8 dots/mm
Thermal paper speeds 10, 25, 50 mm/s
Gain settings:
Auto ECGs
Rhythm ECGs
16.2 in
(41.1 cm)
6.2 in
(15.7 cm)
15.6 in
(39.7 cm)
Soft-key menu and dedicated function keys
5, 10, 20 mm/mV, AUTO
5, 10, 20 mm/mV
Report print formats:
Auto ECGs
Rhythm ECGs
Average cycles
ECG storage Up to 50 ECGs
Frequency range 0.3 to 150 Hz
Digital sampling rate > 1,000 samples/second/channel
Pacemaker detection ANSI/AAMI EC11
Power requirement Universal AC power supply 100–240 V ~, 50/60 Hz ~, 2.2 A maximum
Fuses
AC
DC (battery fuse)
Lead configurations Standard, Cabrera
Rechargeable battery Lead acid gel, 6 volt, 5 AH
3x4, 3x4+ 1R, 3x4 +3R, 6x2, 12x1, 6x2 50 mm/s, 6x2 Ext.
3, 6, or 12 leads at a time
3x4 50 mm/s + 3R, 6x2 50 mm/s + 6R
Time-delay type, 2 amp 250 V rating, Littlefuse 0215002 or equivalent.
Fast-acting type, 10 amp 32 V rating, Bussman ATC-10 or equivalent.
Prints up to 100 continuous ECGs per charge
12-hour recharging
Page 94

90 Appendix A Specifications Welch Allyn CP 200 Electrocardiograph
Item (Continued) Specification (Continued)
Filters 0.5 Hz high-performance baseline filter
35 Hz muscle-tremor filter
AC-interference filter 50 Hz or 60 Hz
Safety, EMC, and regulatory
compliance
Standard connectivity Com port, SD memory card slot (for use with cards ≥ 64 MB)
Connectivity with electronic medical
records
Electrodes Rigorously tested for conductivity, adhesion, and hypoallergenic qualities, and
Cables and wires Meet or exceed IEC 60601
Environmental operating conditions:
Temperature
Relative humidity
Altitude
Environmental storage conditions:
Temperature
Relative humidity
Altitude
Protection against electric shock Class I, internally powered
ANSI/AAMI EC11* UL60601-1
CAN/CSA C22.2 No. 601.1 IEC/EN 60601-1
CAN/CSA C22.2 No. 601.1.1 IEC/EN 60601-1-1
CAN/CSA C22.2 No. 601.1.2 IEC/EN 60601-1-2
CAN/CSA C22.2 No. 601.1.4 IEC/EN 60601-1-4
CAN/CSA C22.2 No. 601.2.25 IEC/EN 60601-2-25
Supported through the Welch Allyn CardioPerfect workstation software
exceed all AAMI standards
+10° C to +40° C (+50° F to +104° F)
15 – 95% noncondensing
700 – 1060 hPa
-20° C to +49° C (-4° F to +120° F)
15 – 95% noncondensing (30 – 70% for printing)
500 – 1060 hPa
Typ e BF
Protection against ingress of water,
per IEC 60529
Mode of operation Continuous
*Per AAMI EC11:1991/(R)2001 Diagnostic Electrocardiographic Devices, Section 3.1.2.1 Disclosure of cautionary
information/ performance characteristics paragraph c) Accuracy of input signal reproduction, the manufacturer shall
disclose the methods used to establish overall system error and frequency response. Welch Allyn has used methods A & D,
as prescribed in section 3.2.7.2 and 4.2.7.2 of this same standard, to verify overall system error and frequency response.
Because of the sampling characteristics and the asynchronism between sample rate and signal rate, digital ECG systems
such as the CP 100 and CP 200 may produce a noticeable modulating effect from one cycle to the next, particularly in
pediatric recordings. This phenomenon is not physiologic.
Specifications are subject to change without notice.
IPX0
Page 95

EMC Guidance and Manufacturer’s
91
B
Table 5. Electromagnetic Emissions
The CP 200 electrocardiograph is intended for use in the electromagnetic environment specified below. The customer or user of the CP 200
electrocardiograph should assure that it is used in such an environment.
Emissions Test Compliance Electromagnetic Environment - Guidance
RF emissions
CISPR 11
RF emissions
CISPR 11
Harmonic emissions
IEC 61000-3-2
Voltage fluctuations/
flicker emissions
IEC 61000-3-3
Declarations
Group 1 The CP 200 electrocardiograph uses RF energy only for its internal function. Therefore,
its RF emissions are very low and are not likely to cause any interference in nearby
electronic equipment.
Class A The CP 200 electrocardiograph is suitable for use in all establishments other than
domestic and those directly connected to the public low-voltage power supply network
that supplies buildings used for domestic purposes.
Class A
Complies
Page 96

92 Appendix B EMC Guidance and Manufacturer’s Declarations Welch Allyn CP 200 Electrocardiograph
Table 6. Electromagnetic Immunity
The CP 200 electrocardiograph is intended for use in the electromagnetic environment specified below. The customer or user of the CP 200
electrocardiograph should assure that it is used in such an environment.
Immunity Test IEC 60601
Electrostatic discharge
(ESD)
IEC 61000-4-2
Electrical fast transient/
burst
IEC 61000-4-4
Surge
IEC 61000-4-5
Voltage dips, short
interruptions, and
voltage variations on
power supply input
lines.
IEC 61000-4-11
Power frequency
(50/60Hz)
magnetic field
Test Level
± 6 kV contact
± 8 kV air
±2 kV for power supply
lines
±1 kV for input/output
lines
±1 kV differential mode
±2 kV common mode
>95% dip in 0.5 cycle
60% dip in 5 cycles
30% dip for 25 cycles
>95% dip in 5 seconds
3 A/m 3 A/m Power frequency magnetic fields should be at levels
Compliance Level Electromagnetic Environment - Guidance
± 6 kV contact
± 8 kV air
±2 kV for power supply
lines
Not applicable
±1 kV differential mode
±2 kV common mode
>95% dip in 0.5 cycle
60% dip in 5 cycles
30% dip for 25 cycles
>95% dip in 5 seconds
Floors should be wood, concrete, or ceramic tile. If floors are
covered with synthetic material, the relative humidity should
be at least 30%.
Mains power quality should be that of a typical commercial or
hospital environment.
Mains power quality should be that of a typical commercial or
hospital environment.
Mains power quality should be that of a typical commercial or
hospital environment. If the user of the CP 200
electrocardiograph requires continued operation during power
mains interruptions, it is recommended that the CP 200
electrocardiograph be powered from an uninterruptible power
supply or battery.
characteristic of a typical location in a typical commercial or
hospital environment.
IEC 61000-4-8
Page 97

Directions for Use Appendix B EMC Guidance and Manufacturer’s Declarations 93
Table 7. Electromagnetic Immunity
The CP 200 electrocardiograph is intended for use in the electromagnetic environment specified below. The customer or user of the CP 200
electrocardiograph should assure that it is used in such an environment.
Immunity Test IEC 60601
Test Level
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
3 Vrms
150 kHz to 80 MHz
3 V/m
80 MHz to 2.5 GHz
Compliance
Electromagnetic Environment - Guidance
Level
Portable and mobile RF communications equipment should be used no closer
to any part of the CP 200 electrocardiograph, including cables, than the
recommended separation distance calculated from the equation applicable
to the frequency of the transmitter.
Recommended separation distance
3 Vrms d = (1.17)
3 V/m d = (1.17) 80 MHz to 800 MHz
d = (2.33) 800 MHz to 2.5 GHz
P
P
P
where P is the maximum output power rating of the transmitter in watts (W)
according to the transmitter manufacturer and d is the recommended
separation distance in metres (m).
Field strengths from fixed RF transmitters, as determined by an
electromagnetic site survey,
each frequency range.
a
should be less than the compliance level in
b
Interference may occur in the vicinity of equipment marked with the
following symbol:
Note 1: At 80 MHz and 800 MHz, the higher frequency range applies.
Note 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures,
objects, and people.
a
Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios, amateur radio,
AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due
to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field strength in the location in which the CP 200
electrocardiograph is used exceeds the applicable RF compliance level above, the electrocardiograph should be observed to verify normal
operation. If abnormal performance is observed, additional measures may be necessary, such as reorienting or relocating the
electrocardiograph.
b
Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3 V/m.
Page 98

94 Appendix B EMC Guidance and Manufacturer’s Declarations Welch Allyn CP 200 Electrocardiograph
Table 8. Recommended Separation Distances Between Portable and Mobile RF Communications Equipment
and the CP 200 Electrocardiograph
The CP 200 electrocardiograph is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The
customer or user of the CP 200 electrocardiograph can help prevent electromagnetic interference by maintaining a minimum distance between
portable and mobile RF communications equipment (transmitters) and the CP 200 electrocardiograph as recommended below, according to the
maximum output power of the communications equipment.
Separation Distance According to Frequency of Transmitter (m)
Rated Max. Output
Power of Transmitter
(W)
0.01 0.117 0.117 0.233
0.1 0.37 0.37 0.74
1 1.17 1.17 2.33
10 3.70 3.70 7.37
100 11.70 11.70 23.30
For transmitters rated at a maximum output power not listed above, the recommended separation distance d in meters (m) can be estimated
using the equation applicable to the frequency of the transmitter, where P is the maximum output power rating of the transmitter in watts (W)
according to the transmitter manufacturer.
Note 1: At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
Note 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures,
objects, and people.
150 kHz to 80 MHz
d = (1.17)
P P P
80 MHz to 800 MHz
d = (1.17)
800 MHz to 2.5 GHz
d = (2.33)
Page 99

Glossary
AHA. An abbreviation for American Heart Association. The AHA electrode labeling scheme is most
commonly used in the United States.
audit trail. A collection of information about user activity. The audit trail, which can be enabled or
disabled, may be helpful or even required for record-keeping.
Auto ECG. A report typically showing a 10-second acquisition of 12 leads of ECG information combined
with patient data, interpretation, and measurements matrix. Two user-defined formats are available:
Auto Report 1 and Auto Report 2. See also Rhythm ECG.
95
Auto Save. A feature that automatically saves all Auto ECGs (except stat ECGs) to the test directory.
Auto Send. A feature that automatically sends all Auto ECGs (except stat ECGs) to the option of your
choice: SD memory card or CardioPerfect workstation.
average cycles. Dominant waveforms for all 12 leads. Printed on a separate page, if enabled.
CardioPerfect workstation. A PC using Welch Allyn CardioPerfect software. Stores ECG and spirometry
test data. Can communicate with other electronic patient-information systems, such as billing and
medical records.
confirmed, unconfirmed. Status of an Auto ECG report’s interpretation. Indicates whether the report has
been reviewed and accepted by a qualified physician.
electrocardiogram (ECG). A record of the electrical currents associated with heart muscle activity.
Sometimes called EKG for the German term, Elektrokardiogramm. See also Auto ECG; Rhythm ECG.
electrocardiograph. An instrument used to create electrocardiograms (ECGs).
extended measurements. Values for several common parameters, such as Q, R, and S amplitude and
ST values. The amplitudes are expressed in microvolts. The durations are expressed in milliseconds.
The measurements cannot be edited. Printed on a separate page, if enabled.
IEC. An abbreviation for International Electrotechnical Commission. The IEC electrode labeling scheme is
most commonly used in Europe.
lead. (1) An electrocardiograph wire connected to an electrode, which is attached to a patient’s skin.
There are 10 lead wires. (2) A waveform that represents ECG data from a particular view of the heart.
The data from the 10 lead wires is converted into 12 waveforms, also called leads: I, II, III, aVR, etc.
Page 100

96 Glossary Welch Allyn CP 200 Electrocardiograph
MEANS. An acronym for modular ECG analysis system. The optional MEANS interpretation algorithm,
developed by the University of Rotterdam, Netherlands, provides automatic analysis of ECG tests.
patient list. See scheduled patients list.
Power-Save. A user-selectable feature that automatically turns the electrocardiograph off after several
idle minutes.
Rhythm ECG. A continuous, real-time printout of ECG data. See also Auto ECG.
scheduled patients list. A list of patients whose data has been entered into the electrocardiograph’s
memory for an ECG or spirometry test that day. This list can hold up to 40 patients. At midnight every
night, the list is automatically cleared. When performing Auto ECG or spirometry tests, you may
select patients from this list rather than manually entering their data at the time of the test.
test directory. A location in the electrocardiograph’s memory that holds up to 50 ECG and 50 spirometry
tests. When performing Auto ECG or spirometry tests, you may select patients from this directory
rather than manually entering their data at the time of the test.
workstation. See CardioPerfect workstation.
 Loading...
Loading...