Page 1

Scorpio®TS
Single Axis Revision
Knee System
Orthopaedics
Scorpio TS
Trial Cutting Guide Surgical
Protocol
Page 2

Scorpio®TS
Single Axis Revision
Knee System
Orthopaedics
Scorpio TS
Trial Cutting Guide Surgical Protocol
Acknowledgements
Stryker Orthopaedics would like to thank
Dr. Masini for his help in developing the
Scorpio Trial Cutting Guide.
Michael A. Masini, MD
Ann Arbor Bone and Joint Surgery
St. Joseph Mercy Hospital
Ann Arbor, MI
Clinical Instructor in Orthopaedic Surgery
University of Michigan
Ann Arbor, MI
Introduction
This surgical protocol is a supplement
to the Scorpio Total Stabilizer Revision
Knee System Surgical Protocol
(Lit No. LSTS/ST).
The Scorpio Trial Cutting Guide is an
intramedullary based instrumentation
system focused on the restoration of the
joint line and proper flexion-extension
gap assessment.
1
Page 3

Tibial and Femoral Canal Preparation
Scorpio Trial Cutting Guide
Tibial Preparation
Prepare the tibia following the Scorpio
Total Stabilizer Revision Knee System
Surgical Protocol (Lit. No. LSTS/ST, pages
1- 5). If an offset is needed, use the tibial
offset reamer to prepare for the tibial
offset. Insert the assembled trial into the
tibia (Figure 1 and Figure 2).
Note: The Scorpio Trial Cutting
Guide (TCG) is designed for use
with its own mating tibial insert
trial. The trial insert does not have
a post to allow for more accurate
assessment of the ligaments
during surgery.
Femoral Canal Preparation
Prepare the femoral canal to accept a
stem as described in the Scorpio Total
Stabilizer Revision Knee System Surgical
Protocol (Lit. No. LSTS/ST, page 5). If an
offset is needed, use the femoral offset
reamer to prepare for the femoral offset
(Lit. No. LSTS/ST, page 9) (Figure 3).
Figure 1 Tibial Canal Preparation Figure 2 Tibial Trial Assembly and
Insertion
Femoral Offset Reamer
8200-0095
Reamer Depth Stop
8200-0047 80mm stem with offset
8200-0048 155mm stem with offset
2
Figure 3 Femoral Canal Preparation
Tibial Boss/Offset Reamer
8200-0015
Command T-Handle
6266-5-401
IM Reamers
6633-9-408 8mm
6633-9-409 9mm
6633-9-410 10mm
6633-9-411 11mm
6633-9-412 12mm
6633-9-413 13mm
6633-9-414 14mm
6633-9-415 15mm
6633-9-416 16mm
6633-9-417 17mm
6633-9-418 18mm
6633-9-419 19mm
6633-9-420 20mm
6633-9-421 21mm
6633-9-422 22mm
6633-9-423 23mm
Page 4
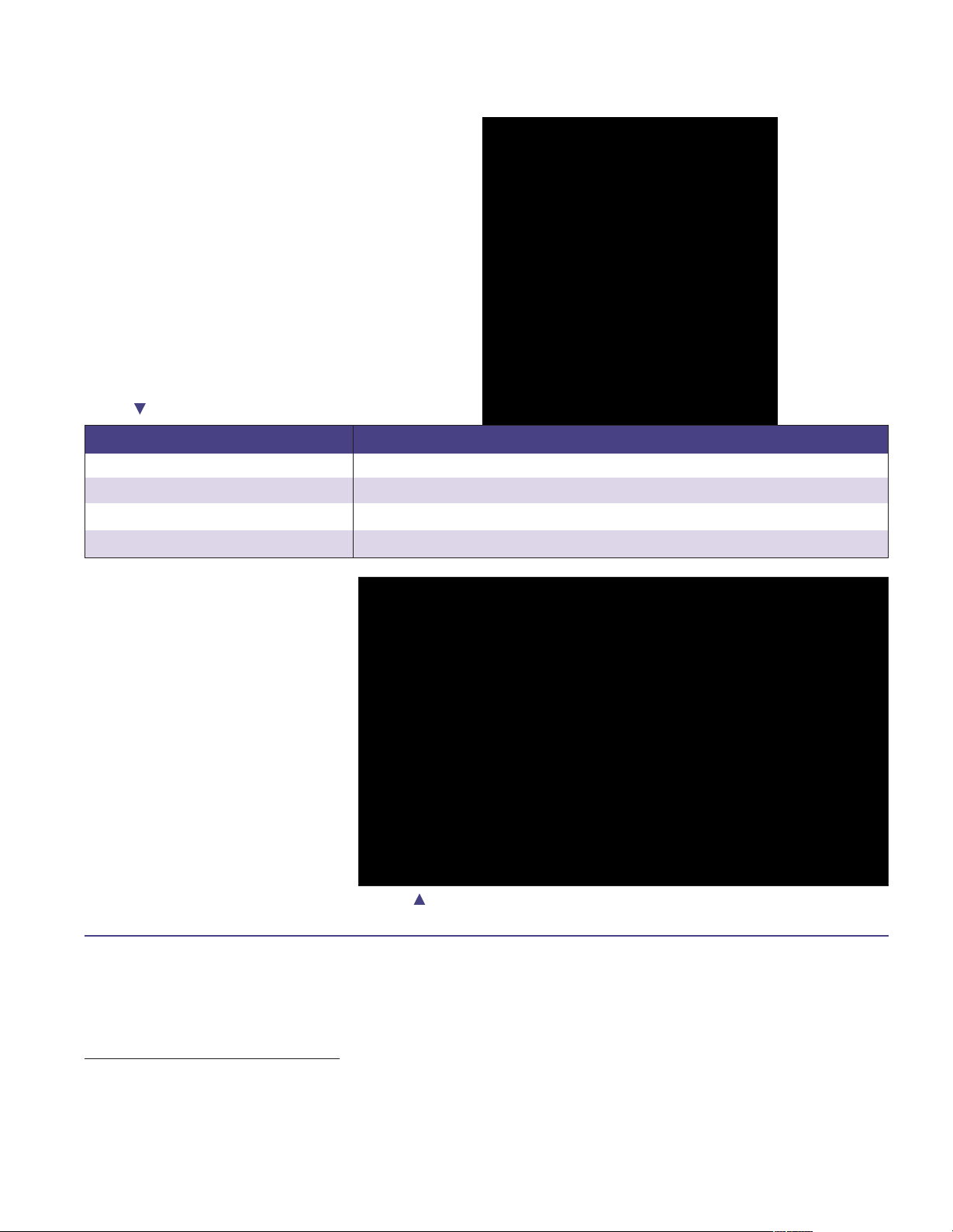
Femoral/Tibial Trial Selection
Scorpio Trial Cutting Guide
Femoral/Tibial Trial Selection
Select the appropriate size femoral trial
cutting guide, offset adapter, and
corresponding tibial insert trial (Table 1).
Appropriate sizing can be achieved
through the use of:
• Previous operative notes
• Size of the original implant removed
• X-ray templates
Femoral
Cutting Guide
Table 1 Trial Cutting Guide Instrument Sizes
Category Sizes
Femoral Cutting Guides 3, 5, 7, 9, 11, 13
Offset Adapter 0mm, 2mm, 4mm, 6mm, 8mm
Tibial Insert Trial Sizes 3, 5, 7, 9, 11, 13
Tibial Insert Trial Thickness 10mm, 12mm, 14mm, 16mm , 18mm, 21mm, 24mm
The Scorpio Trial Cutting Guide (TCG)
can be assembled for either a left or right
knee. Assemble the offset adapter into the
housing of the TCG so the offset adapter
is pointing to the appropriate left or right
orientation (Figure 4).
Figure 4 Trial Cutting Guide in “Right” Orientation
Trial Cutting Guide
8200-5003
8200-5005
8200-5007
8200-5009
8200-5011
8200-5013
3
Page 5
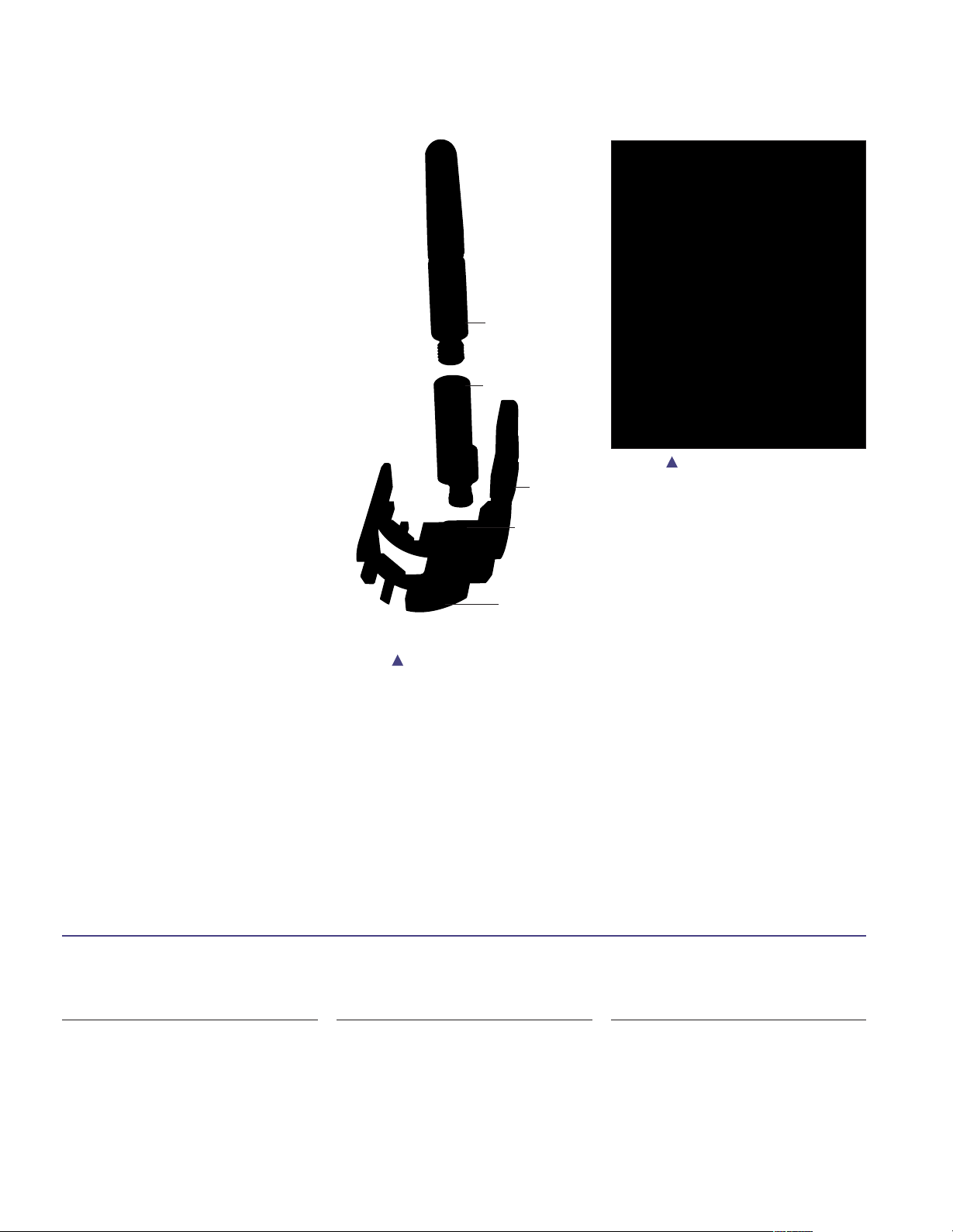
Femoral/Tibial Trial Selection (continued)
Scorpio Trial Cutting Guide
Assembly
Assemble the trial cutting guide, offset
adapter, and appropriate size trial stem
as shown (Figure 5).
A neutral offset adapter may be used
initially to construct the trial cutting
guide assembly until the need for a
femoral offset is determined.
First, assemble the trial stem to the offset
adapter, then assemble the offset adapter
into the Trial Cutting Guide housing and
secure the anterior set screw. Once “finger
tight” pressure is achieved, rotate
counterclockwise 1/2 turn to allow the
offset adapter to rotate freely. The anterior
set screw will be fully secured after the
appropriate offset and rotation is
determined (Figure 6).
Note: Rotating counterclockwise
1/2 turn will allow the offset
adapter to rotate freely without
disassembling from the Trial
Cutting Guide housing. Rotating
counterclockwise more than 1/2
turn will cause the offset adapter
to fall out of the Trial Cutting Guide
housing.
Trial
Stem
Offset
Adapter
Figure 6 Set Screw Assembly
Set
Screw
Trial Cutting
Guide
Housing
Trial
Cutting Guide
Figure 5 Trial Cutting Guide
Assembly
Torx Screwdriver
8200-5110
4
Tibial Insert Trial
T72-7-XXYY
XX = 03, 05, 07, 09, 11
YY = 10, 12, 14, 16, 18, 21, 24
Offset Adapter
8200-5100
8200-5102
8200-5104
8200-5106
8200-5108
Page 6
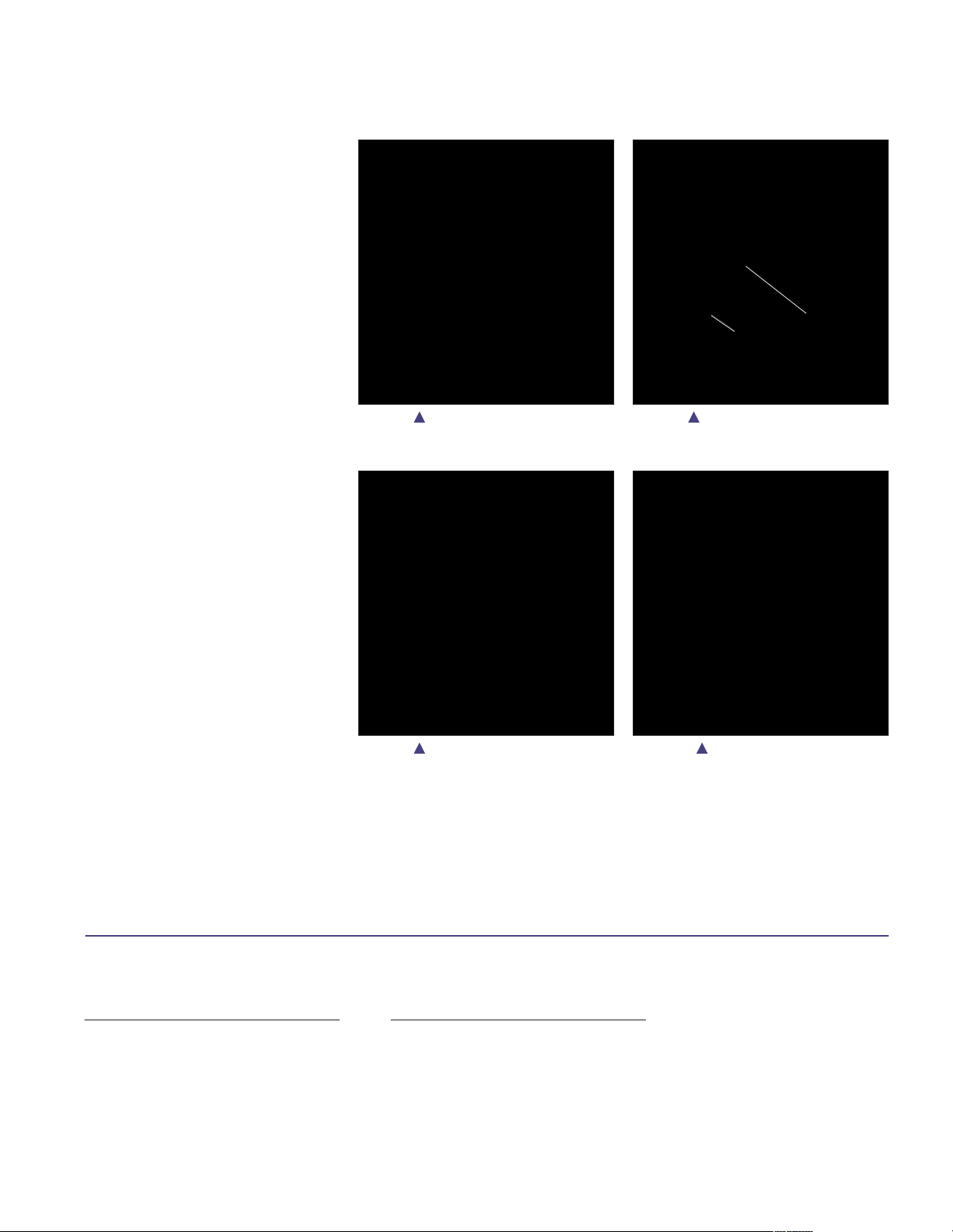
Trial Cutting Guide Orientation
Scorpio Trial Cutting Guide
Note: Femoral chamfer cuts cannot
be made with the TCG. However, if
bone loss is minimal and it is
determined that chamfer cuts are
required, the Scorpio Total
Stabilizer (TS) All-In-One Cutting
Block may be used to make
chamfer cuts (Lit. No. LSTS/ST,
Page 8).
Joint Line Restoration
Insert the TCG into the femoral canal
(Figure 7) and align the TCG medial
epicondyle (ME) scribe line reference mark
with the medial epicondyle (Figure 8). The
ME scribe line is 28mm from the distal
surface of the TCG. When the ME scribe
line is in line with the medial epicondyle,
the distal surface of the TCG will be
approximately located at the joint line.
(The joint line can also be estimated
using pre-operative radiographs and
anatomic landmarks.)
Figure 7 TCG Femoral Canal
Insertion
ME Scribe
Medial
Figure 8 Joint Line Restoration to
Medial Epicondyle
Place an initial fixation pin in the middle
of the medial slot on the TCG (Figure 9
and Figure 10). Pinning the medial slot
will fix the proximal/distal position while
allowing for slight internal and external
rotation of the TCG.
1/8" Headless Pins
7650-1038
Figure 9 Initial Fixation Pin Insertion Figure 10 Initial Fixation Pin –
Extension
Joint Line Scale
8200-0065
5
Page 7

Trial Cutting Guide Orientation (continued)
Scorpio Trial Cutting Guide
Femoral Offsetting
There are several ways to determine the
proper femoral offset required.
1) Start with a neutral offset and measure
or estimate the distance from the
inferior surface of the anterior flange
of the TCG to the anterior femur
(Figure 11) or,
2) Line up the femoral sizing “C” templates
with the trial stem and measure or
estimate the distance from the anterior
femur to the inferior surface of the
anterior flange of the TCG.
Note: A 4mm offset is typical for
many revision scenarios, and if an
A/P offset is not necessary, the
offset adapter may be used to
displace the femur medially or
laterally.
Note: When adjusting the offset of
the TCG, the entire offset adapter
and stem construct will be rotating.
Figure 11 Offset Measuring Figure 12 Trial Cutting Guide
Offsetting
Anterior Set Screw
To adjust the offset, insert the hex driver
into the distal face of the offset adapter
and rotate (Figure 12). After final offset
position has been determined, tighten the
anterior set screw to secure the offset
position on the TCG (Figure 6, page 4).
If an offset is required, record the final
position of the offset by reading the
location of the hash mark on the offset
adapter relative to the clock face on the
TCG. The clock recording will be
required when assembling the implant. If
an offset is not required, use the 0 adapter
(Figure 13).
Note: The numerical clock on the
TCG is a mirror image of the clock
face markings on the femoral trial
and final implant (Figure 14 and
Figure 14a).
Figure 13 Offset Reading Figure 14 Trial Cutting Guide and
Implant/Trial Clock Face
Figure 14A Enlarged View of Clock
Face (0=Posterior Offset)
Femoral Sizing Template
8200-0096 #3/5
8200-0097 #7/9
8200-0098 #11/13
6
Page 8

Trial Cutting Guide Orientation (continued)
Scorpio Trial Cutting Guide
Preliminary Trial Assessment
With the joint line restored and the
appropriate offset determined, a
preliminary trial assessment should be
conducted with the trial tibial
components in place (Figure 15).
Select the appropriate trial tibial insert
and place it onto the trial tibia. Select the
insert that provides varus/valgus stability
in full extension (Figure 16).
Figure 15 Preliminary Trial Assessment
Figure 16 Varus/Valgus Stability Check at Full Extension
7
Page 9

Trial Cutting Guide Orientation (continued)
Scorpio Trial Cutting Guide
Rotation
With the knee flexed at 90°, appropriate
external rotation can be set by
positioning the TCG on the tibial trial
insert so that it is seated with no
varus/valgus tilt. The transepicondylar
axis or Whiteside’s axis can be used to
estimate rotation as well.
A second headless fixation pin should
then be placed in the TCG’s anterior pin
hole to fix the TCG position once
rotation has been established (Figure 17).
Place as many pins as necessary to
securely fix the TCG on the distal femur.
Note: Headless pins may now be
replaced with short headed fixation
pins to facilitate joint reduction.
Note: The TCG trial inserts do not
have a post. This allows for a more
accurate assessment of the
ligaments during trialing.
Figure 17 Second Fixation Pin – Rotation
Headed Fixation Pin
7650-1136 1” Headed Pin
7650-1137 3” Headed Pin
8
Page 10

Trial Cutting Guide Orientation (continued)
Scorpio Trial Cutting Guide
Trial Assessment
Once the TCG is fixed to the femur, a
trial reduction may be conducted. Reduce
the extensor mechanism and patella. The
inferior pole of the patella should rest
approximately 14mm above the joint line
with the knee in 90° of flexion unless
patella baja or patella alta was present
pre-operatively. Tracking of the patella
can then be assessed (Figure 18).
Note: A suture or towel clip may be
used to facilitate reduction and
trial assessment (Figure 19).
It should be noted that the flexion gap
often feels “too loose” in the revision
situation even when the appropriately
sized femoral implant is positioned at the
joint line. Using the TCG gives the
surgeon the unique opportunity to upsize
the femoral component and offset the
next size femur to selectively fill the
flexion gap that feels “too loose.”
However, if upsizing results in poor
tracking and “overstuffing of the joint”,
the surgeon need only return to the
previous size TCG and offset.
Note: A full evaluation of stability
and range of motion can be
performed before making any
resections on the distal femur.
Adjustment of the implant position
and size is possible before making
any femoral bone cuts.
Figure 18 Preliminary Trial Reduction
Suture
Figure 19 Preliminary Trial Reduction With Suture
9
Page 11

Femoral bone Cuts
Scorpio Trial Cutting Guide
Note: A Stryker 152 saw blade
(narrow-thick) or a reciprocating
saw blade are recommended for
augment cuts and the box cut.
Augment Cuts
With the TCG properly positioned,
visually determine the appropriate
posterior and distal resections required
(Figure 20 and Figure 21). A blade
runner may be used to assess the level of
resection if necessary. The appropriate cut
is selected by resting the blade on the
surface of the TCG apertures that will
provide a clean up cut.
Note: If an augment cannot
effectively “fill the gap,” i.e.,
deficiencies greater than 15mm
distally or 10mm posteriorly, a
bone graft may be required
(Figure 22).
Figure 20 Posterior Femoral Cuts Figure 21 Distal Femoral Cuts
Box Cut
When making the box cut, cut along the
outer sides of the box guide and cut
completely through the femur (Figure 23
and Figure 24). The posterior portion of
the proximal box cannot be completed
due to the presence of the stem. Complete
the proximal posterior box cut after the
TCG has been removed using the initial
resection as the guide.
Note: The sides of the box may be
completed with a distal approach
(Figure 25) in addition to the
anterior approach.
Figure 22 Bone Graft Figure 23 Box Cut – Side Walls
10
Figure 25 Box Cut – Distal ApproachFigure 24 Box Cut – Proximal Wall
Page 12

Final Trial Assessment
Scorpio Trial Cutting Guide
A final trial assessment should be
conducted with the Scorpio TS trial
femoral components to verify the
accuracy of the cuts and that the offset
has been properly recreated (Figure 26).
See the Scorpio Total Revision Knee
System Surgical Protocol (Lit. No.
LSTS/ST, page 10) for details on trial
assembly and trial reduction.
Figure 26 Final Trial Assessment
11
Page 13

Quick Pictorial Surgical Technique Reference
Scorpio Trial Cutting Guide
1.
Tibial
Canal
Preparation
2.
Femoral
Canal
Preparation
3.
Trial
Cutting
Guide
Assembly
Trial
Stem
Offset
Adapter
Set
Screw
Trial
Cutting
Guide
4.
TCG
Femoral
Canal
Insertion
5.
Joint Line
Restoration
to Medial
Epicondyle
6.
Initial
Fixation Pin
Insertion
12
Page 14

8.
Preliminary
Trial
Assessment
9.
2nd
Fixation Pin –
External
Rotation
Alignment
10.
Preliminary
Trial
Reduction
7.
Trial
Cutting
Guide
Offsetting
Page 15

Quick Pictorial Surgical Technique Reference
11.
Posterior
Femoral Cuts
Scorpio
®
Trial Cutting Guide
12.
Distal
Femoral Cuts
14.
Final Trial
Assessment
13.
Box Cut
1413
Page 16

Sizing Information
Scorpio Trial Cutting Guide
Scorpio TS Femoral Component Specifications
Size A/P-Med A/P-Lat M/L A/P Height Box Width Width Height*
(mm) (mm) (mm) (mm) (mm) (mm) (mm) (mm)
#3 52 54 56 35 20 17 20 42
#5 56 58 61 39 20 17 22 42
#7 60 62 66 44 23 19 24 46
#9 66 68 71 49 23 19 26 46
#11 72 72 76 53 25 21 28 48
#13 74 76 81 58 25 21 30 48
*With end cap
Resected Box Intercondylar Condylar Stem
77-4003 77-4005 77-4007 77-4009 77-4011
76-4103
76-4105
72-4-03xx 72-4-05xx
72-4-03xx 72-4-05xx
76-4107
Femoral
76-4109
Components
76-4111
76-4113
**Scorpio®PS Inserts may be used.
Scorpio TS Tibial Tray Specifications
A/P M/L Stem Boss
Size
(mm) (mm) Length* (mm)
3 40 61 35
5 44 66 35
7 47 71 37
9 51 77 37
11 54 82 43
13 58 88 43
*With end cap
Tibial Baseplates**
72-4-57xx
Crossover
72-4-75xx
Crossover
72-4-07xx 72-4-09xx
72-4-07xx 72-4-09xx
Scorpio TS Tibial Insert Specifications
Size
3 40 61 23 16
5 44 66 23 16
7F/5T 44 66 27 18
5F/7T 47 71 23 16
7 47 71 27 18
9 51 77 27 18
11F/9T 51 77 29 20
9F/11T 54 82 27 18
11 54 82 29 20
72-4-91xx
Crossover
72-4-19xx
Crossover
A/P M/L Height Width
(mm) (mm) (mm) (mm)
72-4-11xx
72-4-11xx
Post Post
Page 17

A surgeon must always rely on his or her own professional clinical judgment when deciding whether to use a particular
product when treating a particular patient. Stryker does not dispense medical advice and recommends that surgeons be
trained in the use of any particular product before using it in surgery.
The information presented is intended to demonstrate the breadth of Stryker product offerings. A surgeon must always
refer to the package insert, product label and/or instructions for use before using any Stryker product. Products may not
be available in all markets because product availability is subject to the regulatory and/or medical practices in individual
markets. Please contact your Stryker representative if you have questions about the availability of Stryker products in
your area.
Stryker Corporation or its divisions or other corporate affiliated entities own, use or have applied for the following
trademarks or service marks: Scorpio, Stryker. All other trademarks are trademarks of their respective owners or holders.
Literature Number: LSPK34 Rev. 2
MS/GS 06/11
Copyright © Stryker 2011
Printed in USA.
325 Corporate Drive
Mahwah, NJ 07430
t: 201 831 5000
www.stryker.com
 Loading...
Loading...