Page 1

®
Ultraview 1600 Monitor
90364
Technical Reference
070-0784-00 Rev. D
more time to care
Page 2

© 2004 Spacelabs Medical, Inc.
All rights reserved. Contents of this publication may not be reproduced in any form without the written permission of Spacelabs
Medical. Products of Spacelabs Medical are covered by U.S. and foreign patents and/or pending patents. Printed in U.S.A.
Specifications and price change privileges are reserved.
Spacelabs Medical considers itself responsible for the effects on safety, reliability and performance of the equipment only if:
• assembly operations, re-adjustments, modifications or repairs are carried out by persons authorized by Spacelabs
Medical, and
• the electrical installation of the relevant room complies with the requirements of the standard in force, and
• the equipment is used in accordance with the operations manual.
Spacelabs Medical will make available, on request, such circuit diagrams, component part lists, descriptions, calibration instructions
or other information which will assist appropriately qualified technical personnel to repair those parts of the equipment which are
classified by Spacelabs Medical as field repairable.
Spacelabs Medical is committed to providing comprehensive customer support beginning with your initial inquiry through purchase,
training, and service for the life of your Spacelabs Medical equipment.
CORPORATE OFFICES
U.S.A.
Spacelabs Medical
5150 220th Ave SE
Issaquah, WA 98029
Telephone: 425-657-7200
Telephone: 800-522-7025
Fax: 425-657-7212
Authorized EC Representative
UNITED KINGDOM
Spacelabs Limited
71 Great North Road, Hatfield
Herts AL9 5EN
Telephone: 44-1707-263-570
Fax: 44-1707-260-065
BirthNet, Data Shuttle, Flexport, Intesys Clinical Suite, Maternal Obstetrical Monitor, MOM, Mermaid, Multiview, PCIS, PCMS,
PrintMaster, Quicknet, Sensorwatch, TRU-CAP, TRU-CUFF, TRU-LINK, UCW, Ultralite, Ultraview, Ultraview Clinical Messenger,
Ultraview SL, Uni-Pouch, Universal Flexport, Varitrend and WinDNA are trademarks of Spacelabs Medical, Inc.
Other brands and product names are trademarks of their respective owners.
CAUTION:
US Federal law restricts the devices documented herein to sale by, or on the order
Rx
Only
of, a physician.
Page 3

Table of Contents
Chapter Page
Drawings
Schematics . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-1
Symbols
i
Page 4

Page 5
Drawings
Contents
Schematics . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1
Schematics
Title Part Number Drawing
90364 PCB Assembly, I/O Board 670-0878-02
90364 PCB Assembly, 860 CPU 670-0888-02
90364 PCB Assembly, Mother Board 670-0914-01
90364 PCB Assembly, Backplane 670-0915-01
CAUTION:
• Printed circuit boards in this equipment contain static sensitive devices;
only handle at a static-safe workstation.
1-1
(9 sheets)
2-1
(42 sheets)
3-1
(1 sheet)
4-1
(5 sheets)
1-1
Page 6

Page 7

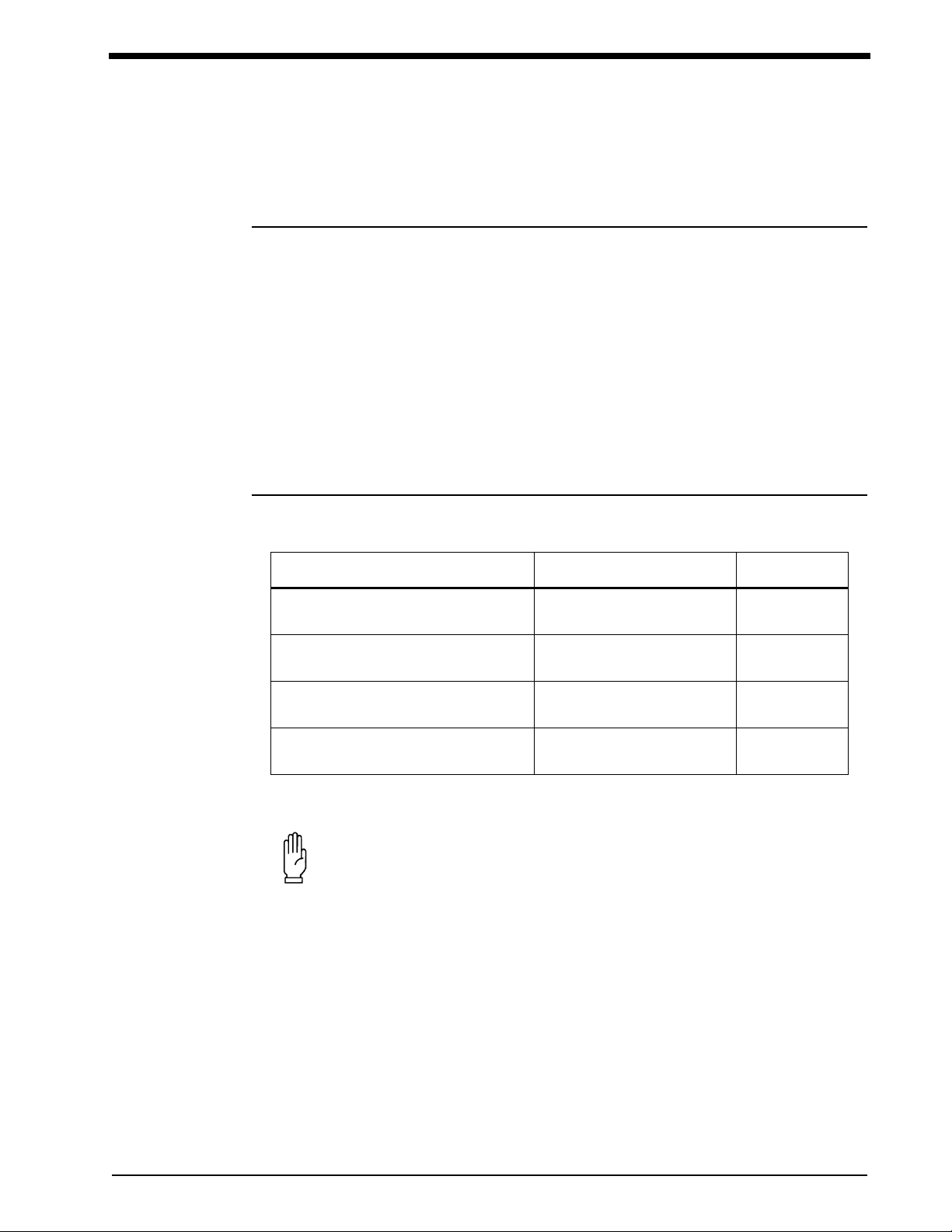
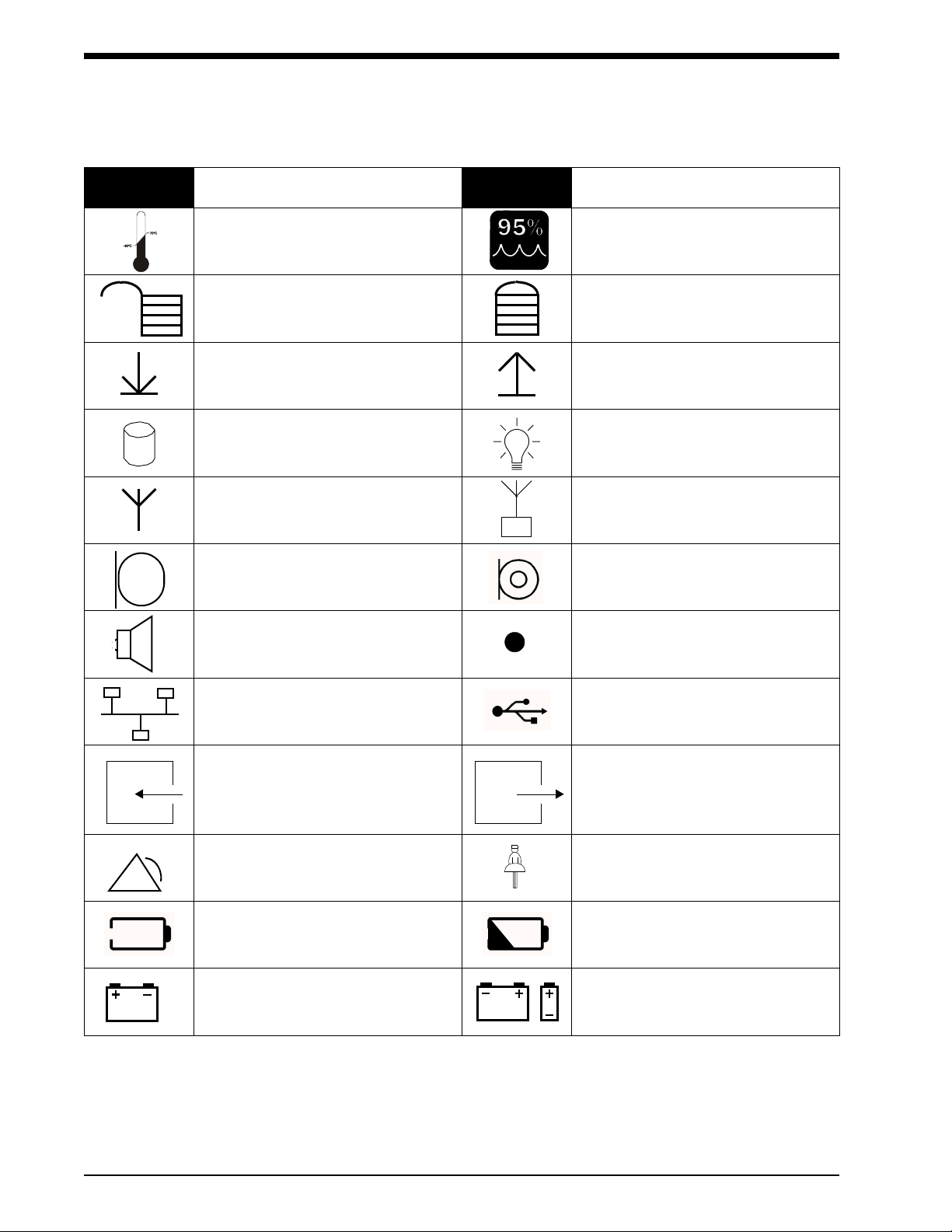
Symbols
The following list of international and safety symbols describes all symbols used on Spacelabs Medical products. No one
product contains every symbol.
Symbol Description Symbol Description
HELP Key Keyboard Connection
SPECIAL FUNCTIONS Key Mouse connection
RECORD Key START/STOP Key
NORMAL SCREEN Key START/STOP
MONITOR SETUP Key STOP or CANCEL Key
ALARMS Key CONTINUE Key
PREVIOUS MENU Key ENTER Key
ON — Power Connection to Mains
ON Position for Push Button Power
Switch
On Direction ON/OFF
Television; Video Display Video Output
OFF — Power Disconnection from
Mains
OFF Position for Push Button Power
Switch
ON — Part of the Instrument Only OFF — Part of the Instrument Only
2-1
Page 8

Ultraview 1600 Monitor
Symbol Description Symbol Description
Stand-by STAND-BY Key
PAUSE or INTERRUPT Slow Run
Reset Power Indicator LED
?
Alarm
Temporary Shut Off of Alarm Tone or
Screen Indicators
Indicator — Remote Control Indicator — Local Control
PRINT REPORT Key Indicator — Out of Paper
Partial ON/OFF Recorder Paper
1
2
1
3
Normal Screen Return to Prior Menu
2
3
Clock/Time Setting Key TREND/TIMER Key
HELP (Explain Prior Screen) Key Keypad
2-2
Activate Recorder for Graphics Indoor Use Only
START (NIBP) Key Auto Mode (NIBP)
Output No Output (Terminated)
Page 9

Symbol Description Symbol Description
Data Input/Output Input/Output
Input Reset
Menu Keys Waveform/Parameter Keys
Symbols
1
2
3
1
2
3
Monitor Setup
Select Program Options
B
Access Special Function Menu Return Unit to Monitor Mode
1
Serial Port 1 Serial Port 2
External marker push button connection SDLC Port
Arterial Pulse
1
2
3
1
2
3
A
Set Initial Conditions Menu
2
SDLC
Electrocardiograph or
Defibrillator Synchronization
Gas Exhaust Foot Switch
Enlarge, Zoom Delete
x
12,200 m
PCMCIA Card Event
Keep Dry Fragile; handle with care
Environmental Shipping/Storage Altitude
Limitations
This Way Up
2-3
Page 10
Ultraview 1600 Monitor
Symbol Description Symbol Description
Environmental Shipping/Storage
Temperature Limitations
Open Padlock Closed Padlock
Down Arrow Up Arrow
Hard Drive Power Indicator LED
Antenna Mermaid Connector
Microphone Omnidirectional Microphone
Audio Output, Speaker Activate Telemetry Recorder
Environmental Shipping/Storage
Humidity Limitations
Network Connection Universal Serial Bus
Gas Sampling Port Gas Return Port
Remote Alarm; Nurse Alert Nurse Call
Battery Status Low Battery
Battery
Replace only with the appropriate
battery.
Replace only with the appropriate
battery.
(+ / - signs may be reversed)
2-4
Page 11

Symbol Description Symbol Description
All batteries should be disposed of
properly to protect the environment.
Lithium batteries should be fully
discharged before disposal. Batteries
such as lead-acid (Pb) and nickelcadmium (Ni-Cd) must be recycled.
Please follow your internal procedures
and or local (provincial) laws regarding
disposal or recycling.
Protective Earth Ground Functional Earth Ground
Replace Fuse Only as Marked Fuse
Caution - hazardous voltages. To reduce
risk of electric shock, do not remove the
cover or back. Refer servicing to a
qualified service personnel (U.S.A.).
DANGER - High Voltage (International)
Symbols
Power supply jack polarity.
(+ / - signs may be reversed)
Alternating Current Direct Current
Both Direct and Alternating Current AC/DC Input
AHz
VW
Amperes Hertz
Volts Watts
IEC 601-1 Type B equipment. The unit
displaying this symbol contains an
adequate degree of protection against
electric shock.
IEC 601-1 Type BF equipment which is
defibrillator-proof. The unit displaying
this symbol contains an F-type isolated
(floating) patient-applied part which
contains an adequate degree of
protection against electric shock, and is
defibrillator-proof.
Equipotentiality Terminal
Class II Equipment
IEC 601-1 Type BF equipment. The unit
displaying this symbol contains an Ftype isolated (floating) patient-applied
part providing an adequate degree of
protection against electric shock.
2-5
Page 12

Ultraview 1600 Monitor
S
Symbol Description Symbol Description
Note
WARNING
IEC 601-1 Type CF equipment. The unit
displaying this symbol contains an Ftype isolated (floating) patient-applied
part providing a high degree of
protection against electric shock, and is
defibrillator-proof.
Loop Filter Adult NIBP
ETL Laboratory Approved
®
IEC 601-1 Type CF equipment. The unit
displaying this symbol contains an Ftype isolated (floating) patient-applied
part providing a high degree of
protection against electric shock.
Canadian Standards Association
Approved
U
Risk of Explosion if Used in the
Presence of Flammable Anesthetics
Note
Warning About Potential Danger to
Human Beings
!
!
CAUTION
Operates on Non-Harmonized Radio
Frequencies in Europe
Attention - Consult Operations or
Service Manual for Description
Caution About Potential Danger to a
Device
Noninvasive Blood Pressure (NIBP),
Neonate
Fetal Monitor Connection
RS232 (Digital)
Happy Face Sad Face
Magnifying Glass Compression
File Cabinet List of Rooms
Arrows Printer
Recycle Service Message
Fetal Monitor Connection (Analog)
Physiological Monitor Connection
RS232 (Digital)
2-6
Page 13
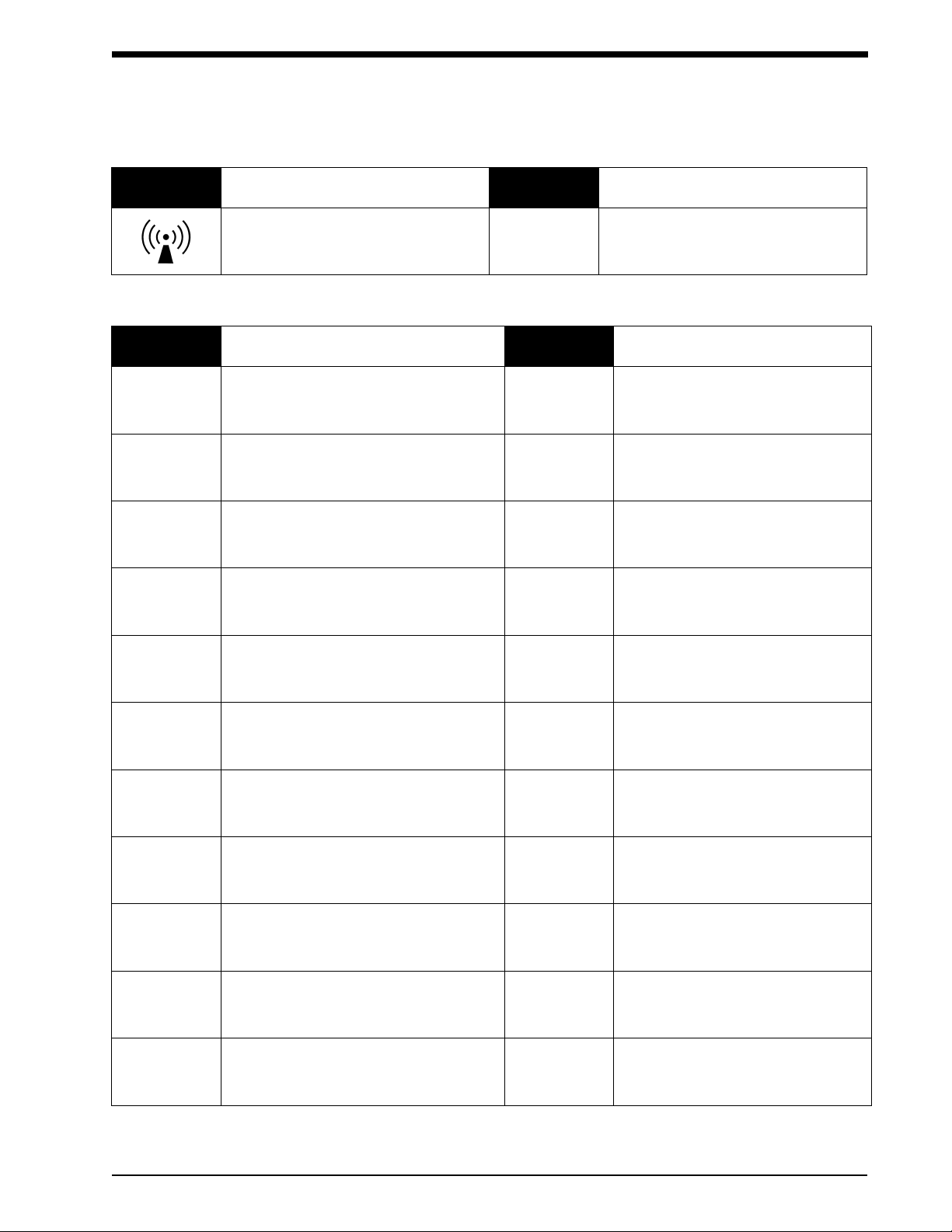
Symbol Description Symbol Description
Radio transmitting device; elevated
levels of non-ionizing radiation
Abbreviations used as symbols are shown below.
Symbol Description Symbol Description
Symbols
1 - 32
ANT 1
ANT 2
CH
ch
C.O.
CO
co
ECG
ecg
EMG
emg
EXT External FECG Fetal Electrocardiogram
Access Codes
1 Through 32
Diversity Antenna System 1
Diversity Antenna System 2
EEG, EMG, or ECG Channel
EEG Channels - CH1, CH2, CH3, CH4
EMG Channel - CH5
Cardiac Output
Electrocardiogram
Electromyogram ESIS
AIR Air
Arr1
ArrNet2
cmH
DIA
dia
EEG
eeg
Arrhythmia Net 1
Arrhythmia Net 2
O Centimeters of Water
2
Diastolic
Electroencephalogram
Electrosurgical Interference
Suppression
FHR1
FHR2
HLO
hlo
NIBP
nibp
O
2
Fetal Heart Rate, Channel 1
Fetal Heart Rate, Channel 2
High-Level Output Multiview Multi-Lead Electrocardiogram
Noninvasive Blood Pressure N
Oxygen
GND
gnd
O Nitrous Oxide
2
PRESS
press
PRS
Patient Isolated Ground
Pressure
2-7
Page 14

Ultraview 1600 Monitor
Symbol Description Symbol Description
RESP
resp
SPO2
SpO2
SpO
2
SaO
2
SYS
sys
TEMP
temp
VAC Vacuum Connection
Respiration SDLC Synchronous Data Link Control
Arterial Oxygen Saturation
as Measured by Pulse Oximetry
Systolic
Temperature UA Uterine Activity or Umbilical Artery
SVO2
SvO2
O
Sv
T1
T2
T3
T4
Mixed Venous Oxygen Saturation
2
Temperature 1
Temperature 2
Temperature 3
Temperature 4
2-8
 Loading...
Loading...