Samsung UGEO H60 User manual Vol.1


WARRANTY
Samsung Medison provides the following warranty to the purchaser of this unit. This warranty is valid for
a period of one year from the date of installation and covers all problems caused by faulty workmanship
or faulty material. Samsung Medison will, as sole and exclusive remedy and at no charge, replace any such
defective unit returned to Samsung Medison within the designated warranty period.
The warranty does not cover damages and loss caused by outside factors including, but not limited to, re,
ood, storm, tidal wave, lightning, earthquake, theft, abnormal conditions of operation, and intentional
destruction of the equipment. Damage caused by equipment relocation is not covered.
The warranty is void in cases where the equipment has been damaged as a result of an accident, misuse,
abuse, dropping, or when attempts to modify or alter any part or assembly of the equipment have taken
place.
Parts with cosmetic defects or deterioration will not be replaced. Replacement of batteries, training
materials, and supplies are not covered.
Samsung Medison will not be responsible for incidental or consequential damages of any kind arising
from or connected with the use of the equipment.
Samsung Medison will not be responsible for any loss, damage, or injury resulting from a delay in services
rendered under the warranty
This limited warranty is in lieu of all other warranties expressed or implied, including warranties of
merchant ability or tness for any particular use. No representative or other person is authorized to
represent or assume for Samsung Medison any warranty liability beyond that set forth herein.
Defective equipment shipped from you to Samsung Medison must be packed in the replacement
cartons. Shipping and insurance costs are the responsibility of the customer. To return defective material
to Samsung Medison contact the Samsung Medison Customer Service Department.
Samsung Medison or a local distributor will make available, upon request, circuit diagrams, a component
parts list, descriptions, calibration instructions and other information which will assist your appropriately
quali ed technical personnel to repair those parts of the equipment which are designed by Samsung
Medison as repairable.
CAUTION: United State federal law restricts this device to sale by or on the order of physicians.
MANUFACTURER : SAMSUNG MEDISON CO., LTD.
42, Teheran-ro 108-gil, Gangnam-gu, Seoul, Korea
Customer Service Department : SAMSUNG MEDISON CO., LTD.
EC Representative : SAMSUNG ELECTRONICS (UK) LTD.
TEL : 82-2-2194-1234 FAX : 82-2-2194-1071
Website: www.samsungmedison.com
Blackbushe Business Park, Saxony Way,
Yateley, Hampshire, GU46 6GG, UK


Diagnostic Ultrasound System
Operation Manual
Version 1.00.00
English
MI68-02334A


PROPRIETRAY INFORMATION AND SOFTWARE LICENSE
The Customer shall keep confidential all proprietary information furnished or disclosed to the Customer
by Samsung Medison, unless such information has become part of the public domain through no fault of
the Customer. The Customer shall not use such proprietary information, without the prior written consent
of Samsung Medison, for any purpose other than the maintenance, repair or operation of the goods.
Samsung Medison’s systems contain Samsung Medison’s proprietary software in machine-readable
form. Samsung Medison retains all its rights, title and interest in the software except that purchase of this
product includes a license to use the machine-readable software contained in it. The Customer shall not
copy, trace, disassemble or modify the software. Transfer of this product by the Customer shall constitute
a transfer of this license that shall not be otherwise transferable. Upon cancellation or termination of this
contract or return of the goods for reasons other than repair or modification, the Customer shall return to
Samsung Medison all such proprietary information.


Safety Requirements
Classifications:
X
Type of protection against electrical shock: Class I
X
Degree of protection against electrical shock (Patient connection): Type BF equipment
X
Degree of protection against harmful ingress of water: Ordinary equipment
X
Degree of safety of application in the presence of a flammable anesthetic material with air or
with oxygen or nitrous oxide: Equipment not suitable for use in the presence of a flammable
anesthetic mixture with air or with oxygen or nitrous oxide.
X
Mode of operation: Continuous operation
Electromechanical safety standards met:
X Medical Electrical Equipment, Part 1: General Requirements for Basic Safety and Essential
Performance IEC 60601-1:2005
X Medical Electrical Equipment, Part 1-2: General Requirements for Basic Safety and Essential
Performance- Collateral Standard: Electromagnetic Compatibility - Requirements and Tests IEC
60601-1-2:2007
X Medical Electrical Equipment, Part 1-6: General Requirements for Basic Safety and Essential
Performance- Collateral Standard: Usability IEC 60601-1-6:2006
X Medical Electrical Equipment, Part 2-37: Particular Requirements for the Basic Safety and Essential
Performance of Ultrasonic Medical Diagnostic and Monitoring Equipment IEC60601-2-37:2007
X Medical Electrical Equipment, Part 1: General Requirements for Safety IEC 60601-1:1988 with
A1:1991 and A2:1995
X Medical Electrical Equipment, Part 1: General Requirements for Safety - 1 Collateral Standard: Safety
Requirement for Medical Electrical Systems IEC 60601-1-1:2000
X Medical Electrical Equipment, Part 1: General Requirements for Safety - 2 Collateral Standard:
Electromagnetic Compatibility - Requirements and Test IEC 60601-1-2:2001, A1:2004
X Medical Electrical Equipment, Part 1: General Requirements for Safety - 4 Collateral Standard:
Programmable Electrical Medical Systems IEC 60601-1-4: 1996, A1:1999

X Medical Electrical Equipment, Part 2: Particular Requirements for Safety - 37 Ultrasonic Medical
Diagnostic and Monitoring Equipment IEC60601-2-37: 2001 with A1:2004, A2:2005
X Medical Devices - Application of Risk Management to Medical Devices ISO 14971:2007
X Medical Electrical Equipment, Part 1: General Requirements for Safety UL60601-1:2003
X Medical Electrical Equipment - Part 1: General Requirements for Safety CAN/CSA 22.2
No.601.1-M90:1990, with R2003, with R2005
X Biological Evaluation of Medical Devices ISO10993:2009
X Standard Means for the Reporting of the Acoustic Output of Medical Diagnostic Ultrasonic Equipment
IEC61157:2007
Declarations
This is the CSA symbol for Canada and United States of America.
This is the manufacturer’s declaration of product compliance with applicable EEC
directive(s) and the European notified body.
This is the manufacturer’s declaration of product compliance with applicable EEC
directive(s).
This is the GMP symbol that shows that the product complies with the Korean Good
Manufacturing Practice quality regulation system.
Precautions For Use
Be sure to read this manual thoroughly to familiarize yourself with the operation of the product and the
relevant safety information before attempting to use the product.
Keep this manual near the product and refer to it when using the product.
‘Chapter 1. Safety‘ and ‘Chapter 4. Maintenance’, in particular, contain important safety information and
must be thoroughly understood.
This manual does not include diagnosis results or opinions. Also, check the reference information for
the measured area of the body before using the application’s measurement results in any diagnosis.
This product is an ultrasound diagnosis device and cannot be used with your PC. The manufacturer is
not responsible for any problems that may be caused by such attempts.
This product must only be used by persons who are trained and/or certified to operate clinical
diagnostic devices. Unqualified persons must not use this product.
The manufacturer is not responsible for any damage to this product caused by user carelessness and/or
neglect.
The contents and specifications described in this manual may be changed without notice.
Products that are not manufactured by Samsung Medison are indicated with the trademarks of their
respective owners.
The following terms are used to highlight safety precautions that the user must be aware of:
DANGER: Disregarding this instruction may result in death, serious injury, or other dangerous
situations.
WARNING: Follow these instructions to prevent a serious accident or damage to property.
CAUTION: Follow these instructions to prevent a minor accident or damage to property.
NOTE: The accompanying information covers an installation, operation, or maintenance
procedure that requires careful attention from the user, but has little chance of leading directly to
a dangerous situation.

If You Need Assistance
If you need any assistance with the equipment, or the service manual, please contact Samsung Medison‘s
Customer Service Department or one of their worldwide customer service representatives immediately.
Revision History
The revision history of this manual is as follows.
VERSION DAT E NOTE
v1.00.00-00 2012-04-02 Initial Release
Product Upgrade and Manual Update
Upgrades to this product can include upgrades to its hardware or software components. Revised versions
of this manual will be published to reflect any upgrades to the product.
Please make sure that your operation manual is appropriate for your product version. If not, please contact
Samsung Medison's Customer Service Department.


Table of Contents
Table of Contents – Volume 1
Chapter 1 Safety
Indication for Use ....................................................................................................................... 1-3
Safety Information ..................................................................................................................... 1-4
Safety Symbols .................................................................................................................................................................... 1-4
Labels ...................................................................................................................................................................................... 1-6
Electrical Safety .......................................................................................................................... 1-7
Prevention of Electric Shocks ........................................................................................................................................ 1-7
ESD ........................................................................................................................................................................................... 1-8
EMI ........................................................................................................................................................................................... 1-9
EMC ........................................................................................................................................................................................ 1-9
Mechanical Safety .................................................................................................................... 1-17
Moving the Equipment ..................................................................................................................................................1-17
Precautions for Use ..........................................................................................................................................................1-18
Biological Safety ......................................................................................................................1-20
The ALARA Principle .......................................................................................................................................................1-20
Protecting the Environment .................................................................................................. 1-35
Waste Electrical and Electronic Equipment ..........................................................................................................1-35
Chapter 2 Introduction
Product Specifications .............................................................................................................. 2-3
Product Configuration .............................................................................................................. 2-5
The Monitor .......................................................................................................................................................................... 2-6
The Control Panel ............................................................................................................................................................... 2-8
The Console .......................................................................................................................................................................2-15
Peripheral Devices ...........................................................................................................................................................2-17
Probes ...................................................................................................................................................................................2-20
Accessories .........................................................................................................................................................................2-21
Optional Functions ..........................................................................................................................................................2-22
15

Operation Manual
Chapter 3 Utilities
System Settings .......................................................................................................................... 3-3
General System Settings ................................................................................................................................................. 3-4
General ................................................................................................................................................................................... 3-4
Patient ....................................................................................................................................................................................3-7
Screen Display Settings (Imaging)............................................................................................................................... 3-8
Display .................................................................................................................................................................................... 3-8
Measurement Settings ...................................................................................................................................................3-11
Report ...................................................................................................................................................................................3-25
Anatomy ..............................................................................................................................................................................3-25
Comments ..........................................................................................................................................................................3-26
Annotation .........................................................................................................................................................................3-27
Body Marker .......................................................................................................................................................................3-31
Application .........................................................................................................................................................................3-35
Peripheral Device Settings ...........................................................................................................................................3-37
User Defined Keys (Customize Keys) ........................................................................................................................3-39
Customize Touch Menu .................................................................................................................................................3-42
Device ...................................................................................................................................................................................3-43
Connectivity Settings ....................................................................................................................................................3-44
DICOM Settings ................................................................................................................................................................3-44
Network Settings ...........................................................................................................................................................3-55
Service ..................................................................................................................................................................................3-56
Help .......................................................................................................................................................................................3-56
Chapter 4 Maintenance and Storage
Operational Environment ........................................................................................................ 4-3
Product Maintenance................................................................................................................ 4-4
Cleaning and disinfecting ............................................................................................................................................... 4-4
Fuse Replacement .............................................................................................................................................................4-6
Cleaning Air Filters ............................................................................................................................................................. 4-7
Accuracy Checks ................................................................................................................................................................. 4-7
Information Maintenance ........................................................................................................ 4-8
User Settings Backup ........................................................................................................................................................ 4-8
Backing Up Patient Information ................................................................................................................................... 4-8
Software................................................................................................................................................................................. 4-8
16

Table of Contents
Chapter 5 Probes
Probes .......................................................................................................................................... 5-3
Ultrasound Transmission Gel ......................................................................................................................................... 5-8
Sheaths................................................................................................................................................................................... 5-9
Probe Safety Precautions ..............................................................................................................................................5-10
Cleaning and Disinfecting the Probe .......................................................................................................................5-12
Biopsy ......................................................................................................................................... 5-20
Biopsy Kit Components .................................................................................................................................................5-20
Using the Biopsy Kit ........................................................................................................................................................5-21
Cleaning and Disinfecting the Biopsy Kit ...............................................................................................................5-23
Assembling the Biopsy Kit ............................................................................................................................................5-25
**Reference Manual
A Reference Manual (English) is supplied with this product.
17

Chapter 1
Safety
Indication for Use .............................................1-3
Safety Information ..........................................1-4
Safety Symbols ......................................................................... 1-4
Labels ........................................................................................... 1-6
Electrical Safety ................................................1-7
Prevention of Electric Shocks .............................................. 1-7
ESD ................................................................................................ 1-8
EMI .................................................................................................1-9
EMC .............................................................................................. 1-9
Mechanical Safety ........................................ 1-17
Moving the Equipment .......................................................1-17
Precautions for Use ...............................................................1-18
Biological Safety ........................................... 1-20
The ALARA Principle .............................................................1-20
Protecting the Environment ........................ 1-35
Waste Electrical and Electronic Equipment ................1-35


Chapter 1 Safety
Indication for Use
The UGEO H60 Diagnostic Ultrasound System and transducers are intended for diagnostic ultrasound
imaging and fluid analysis of the human body.
The clinical applications include: Fetal, Abdominal, Pediatric, Small Organ, Neonatal Cephalic, Transrectal, Trans-vaginal, Muscular-Skeletal (Conventional, Superficial), Peripheral vessel.
Contraindications
The UGEO H60 system is not intended for ophthalmic use or any use causing the acoustic beam to
pass through the eye.
CAUTION:
X
Federal law restricts this device to sale by or on the order of a physician.
X
The method of application or use of the device is described in the manual 'Chapter 6. Starting
Diagnosis' and 'Chapter 7. Diagnosis Modes'.
1-3
Operation Manual
Safety Information
Please read the following safety information before using this product. It is relevant to the ultrasound
system, the probes, the recording devices, and any of the optional equipment.
The product is intended for use by, or by the order of, and under the supervision of, a licensed physician
who is qualified for direct use of the medical device.
This equipment should not be used by any healthcare professional or individual who is not properly
qualified to operate it. Prolonged use of three-dimensional ultrasound (3D, 4D) by an unqualified
individual, such as to produce a commemorative photograph or video of the fetus, may have an adverse
effect on the fetus.
Please use the 3D ultrasound diagnostic imaging system for appropriate purposes only, since using it for
non-diagnostic purposes such as recording videos of the fetus may adversely affect the fetus.
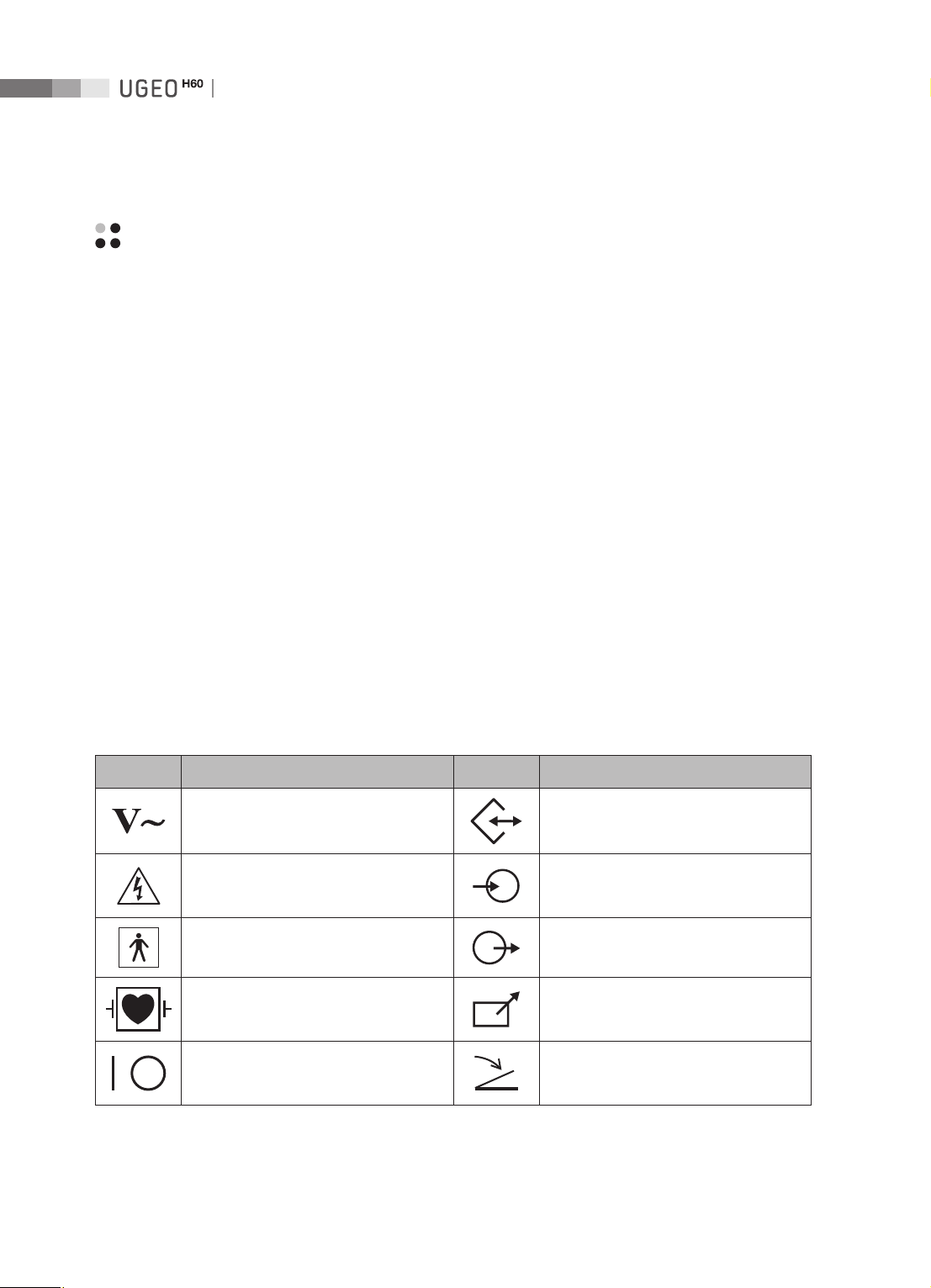
Safety Symbols
The International Electro Technical Commission (IEC) has established a set of symbols for medical
electronic equipment, which classify a connection or warn of potential hazards. The classifications and
symbols are shown below.
Symbols Description Symbols Description
AC (alternating current) voltage source Data Input/Output port
Electric shock warning Left and right Audio / Video input
Isolated patient connection (Type BF
applied part).
Isolated patient connection (Type CF
applied part).
Power switch (Supplies/cuts the power
for product)
Left and right Audio / Video output
Remote print output
Foot switch connector
1-4
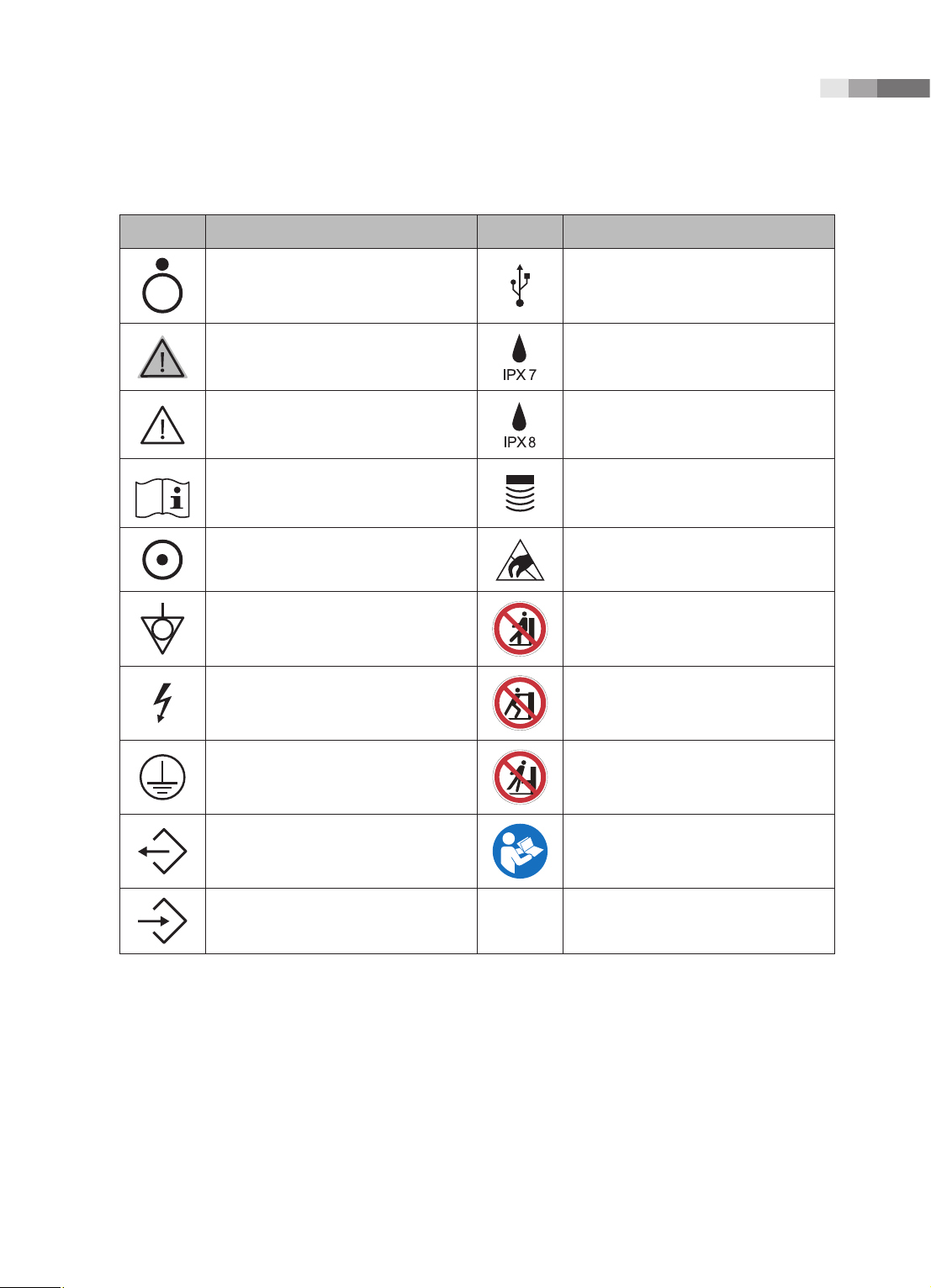
Chapter 1 Safety
Symbols Description Symbols Description
OFF (Cuts the power to a part of the
product)
WARNING: Follow this information to
prevent a serious accident or damage to
property.
CAUTION: Hazards or unsafe practices
that may result in minor personal injury
or property damage.
Refer to the operation manual Probe connector
ON (Supplies power to a part of the
product)
Identifies an equipotential ground. Do not sit on control panel
Indicates dangerous voltages over
1000V AC or over 1500V DC.
Protective earth connected to
conductive parts of Class I equipment
for safety purposes.
USB connector
Protection against the effects of
immersion
Protection against long periods of
immersion under pressure
ESD (Electrostatic discharge) caution
symbol
Do not push the product
Do not lean against the product
Data Output port Follow the operation manual
Data Input port
1-5

Operation Manual
Labels
Warning and caution labels that contain information and instructions concerning the protection of the
product can be found on the exterior of the product.
[Label 1. ID label]
1-6
[Label 2. Cautions on ‘TIP-OVER’]
[Label 3. Probe ID Label]
[Label 4. Probe label]

Chapter 1 Safety
Electrical Safety
This equipment has been verified as a Class I device with Type BF applied parts.
Prevention of Electric Shocks
Additional equipment connected to medical electrical equipment must comply with the respective IEC
standards (e.g. IEC60950/EN60950 for data processing equipment, IEC60601-1/EN60601-1 for medical
devices). Furthermore, all configurations shall comply with the requirements for medical electrical
systems (see IEC60601-1-1/EN60601-1-1). Anybody connecting additional equipment to signal input
and output ports of medical electrical equipment should make sure that the equipment complies with
IEC60601-1-1/EN60601-1-1.
WARNING:
X
Electric shock may result if this system, including all of its externally mounted recording and
monitoring devices, is not properly grounded.
X
Never open the cover of the product. Hazardous voltages are present inside All internal
adjustments and replacements must be made by qualified Samsung Medison Customer
Support Department personnel.
X
Always check the product’s housing, cables, cords, and plugs before using the product
Disconnect the power source and do not use the equipment if the housing is damaged such as
cracked, and chipped, or if the cable is worn.
X
Always disconnect the system from the wall outlet prior to cleaning the system.
X
All patient contact devices, such as probes and ECG leads, must be removed from the patient
prior to application of a high voltage defibrillation pulse.
X
The use of flammable anesthetic gas or oxidizing gases (N2O) should be avoided. There is a risk
of explosion.
X
Avoid installing the system in such a way that it is difficult for the operator to disconnect it from
the power source.
X
Do not use HF surgical equipment with the system. Any malfunctions in the HF surgical
equipment may result in burns to the patient.
X
The System must only be connected to a supply mains with protective earth to avoid risk of
electric shock.
1-7

Operation Manual
CAUTION:
X
The system has been designed for 100-240VAC; you should select the input voltage of any
connected printer and VCR. Prior to connecting a peripheral power cord, verify that the voltage
indicated on the power cord matches the voltage rating of the peripheral device.
X
An isolation transformer protects the system from power surges. The isolation transformer
continues to operate when the system is in standby.
X
Do not immerse the cable in liquids. Cables are not waterproof.
X
The auxiliary socket outlets installed on this system are rated 100-240VAC with maximum total
load of 150VA. Use these outlets only for supplying power to equipment that is intended to be
part of the ultrasound system. Do not connect additional multiple-socket outlets or extension
cords to the system.
X
Do not connect any peripheral devices that are not listed in this manual to the auxiliary socket
outlet of the system. It may cause an electrical hazard.
X
Do not touch SIP/SOP and the patient simultaneously. There is a risk of electric shock from
leakage current.
ESD
Electrostatic discharge (ESD), commonly referred to as a static shock, is a naturally occurring
phenomenon. ESD is most prevalent during conditions of low humidity, which can be caused by
heating or air conditioning. The static shock or ESD is a discharge of the electrical energy build-up
from a charged individual to a less or non-charged individual or object. An ESD occurs when an
individual with an electrical energy build-up comes into contact with conductive objects such as metal
doorknobs, file cabinets, computer equipment, and even other individuals.
CAUTION:
X
The level of electrical energy discharged from a system user or patient to an ultrasound system
can be significant enough to cause damage to the system or probes.
X
Always perform the pre-ESD preventive procedures before using connectors marked with the
ESD warning label.
− Apply anti-static spray to carpets or linoleum.
− Use anti-static mats.
− Ground the product to the patient table or bed.
X
It is highly recommended that the user be given training on ESD-related warning symbols and
preventive procedures.
1-8
Chapter 1 Safety
EMI
Although this system has been manufactured in compliance with existing EMI (ElectroMagnetic Interface)
requirements, use of this system in the presence of an electromagnetic field can cause degradation of the
ultrasound image or product damage.
If this occurs often, Samsung Medison suggests a review of the environment in which the system is being
used, to identify possible sources of radiated emissions. These emissions could be from other electrical
devices used within the same room or an adjacent room. Communication devices such as cellular phones
and pagers can cause these emissions. The existence of radios, TVs, or microwave transmission equipment
nearby can also cause interference.
CAUTION: In cases where EMI is causing disturbances, it may be necessary to relocate this system.
EMC
The testing for EMC(Electromagnetic Compatibility) of this system has been performed according to the
international standard for EMC with medical devices (IEC60601-1-2). This IEC standard was adopted in
Europe as the European norm (EN60601-1-2).
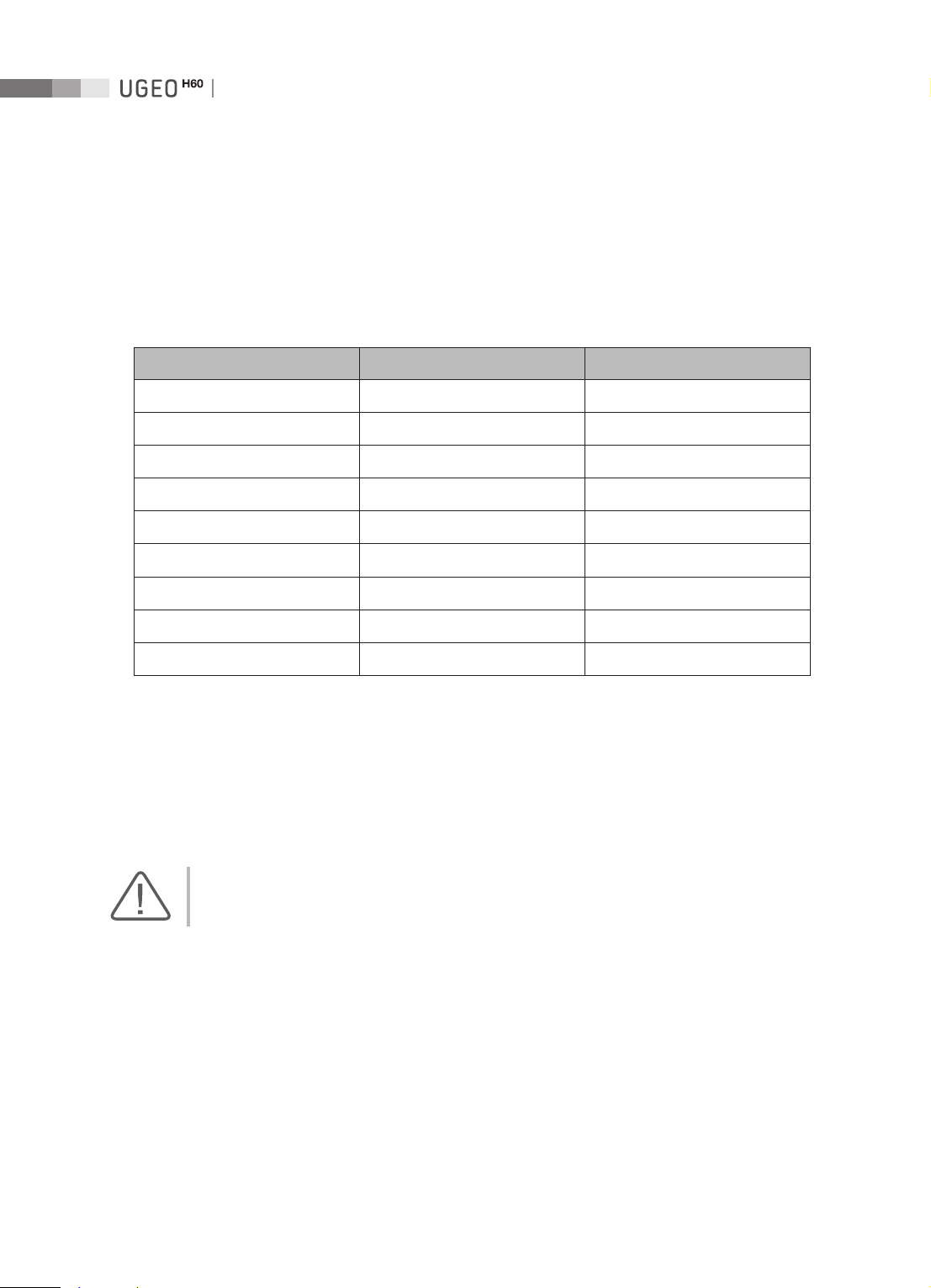
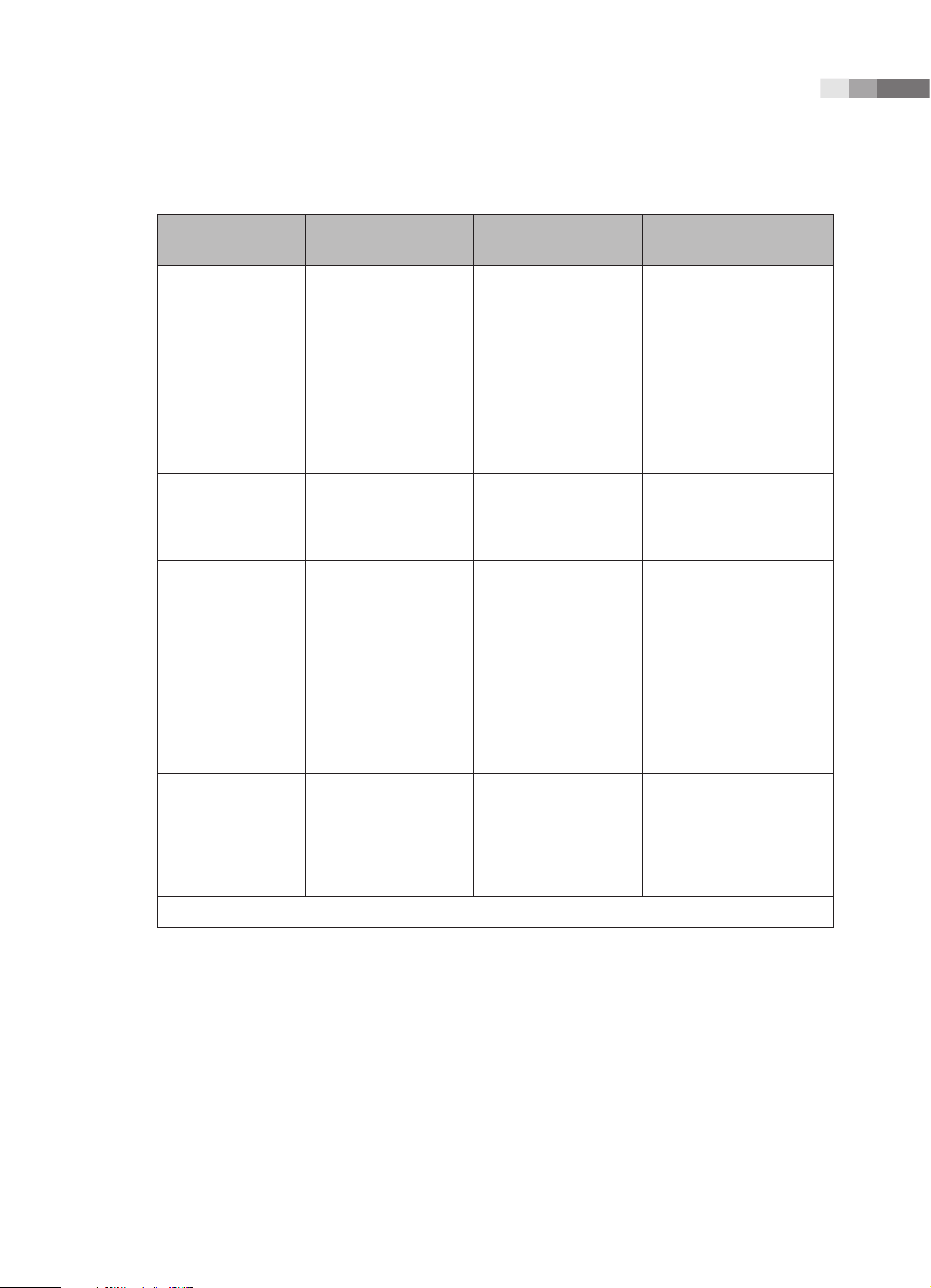
Guidance and Manufacturer’s Declaration - Electromagnetic Emission
This product is intended for use in the electromagnetic environment specified below. The customer or
the user of this product should ensure that it is used in such an environment.
Emission test Compliance Electromagnetic environment - guidance
RF Emission
CISPR 11
RF Emission
CISPR 11
Harmonic Emission
IEC 61000-3-2
Flicker Emission
IEC 61000-3-3
Group 1
Class B
Class A
Complies
The Ultrasound System uses RF energy only for its internal
functions. Therefore, its RF emissions are very low and are not
likely to cause any interference in nearby electronic equipment.
The Ultrasound System is suitable for use in all establishments,
including domestic establishments and those directly
connected to the public low-voltage power supply network
that supplies building used for domestic purposes.
1-9
Operation Manual
Approved Cables, Transducers and Accessories for EMC
Approved Cable for Electromagnetic Compliance
Cables connected to this product may affect its emissions; use only the cable types and lengths
listed in the table below.
Cable Type Length
VGA Shielded Normal
USB Shielded Normal
LAN(RJ45) Twisted pair Any
S-Video Shielded Normal
Foot Switch Shielded < 3m
e-Motion Marker Shielded < 3m
Audio R.L Shielded Normal
Parallel Shielded Normal
HDMI Shielded Normal
Probes
Probes listed in ‘Chapter 5. Probes’ meet the Group 1 Class B requirements of the CISPR 11 standard.
Approved Accessories for Electromagnetic Compliance
Accessories used with this product may affect its emissions.
CAUTION: When connecting a VCR or other customer-supplied accessory to the system, it is the
user’s responsibility to ensure the electromagnetic compatibility of the system. Only use devices
that are compliant with CISPR 11 or CISPR 22, CLASS B.
1-10
Chapter 1 Safety
Immunity test IEC 60601 Test level Compliance level
Electrotatic
discharge (ESD)
IEC 61000-4-2
Electrical fast
transient/burst
IEC 61000-4-4
Surge
IEC 61000-4-5
Voltage dips, short
interruptions and
voltage variations
on power supply
input lines
IEC 61000-4-11
±6KV Contact
±8KV air
±2KV
for power supply lines
±1KV
for input/output lines
±1KV differential mode
±2KV common mode
<5% Uт for 0.5 cycles
(>95% dip in Uт)
40% Uт for 5 cycles
(60% dip in Uт)
70% Uт for 25 cycles
(30% dip in Uт)
<5% Uт for 5 s
(<95% dip in Uт)
±6KV Contact
±8KV air
±2KV
for power supply lines
±1KV
for input/output lines
±1KV differential mode
±2KV common mode
<5% Uт for 0.5 cycles
(>95% dip in Uт)
40% Uт for 5 cycles
(60% dip in Uт)
70% Uт for 25 cycles
(30% dip in Uт)
<5% Uт for 5 s
(<95% dip in Uт)
Electromagnetic
environment - guidance
Floors should be wood,
concrete or ceramic tile.
If floors are covered with
synthetic material, the
relative humidity should be
at least 30%.
Mains power quality
should be that of a typical
commercial or hospital
environment.
Mains power quality
should be that of a typical
commercial or hospital
environment.
Mains power quality
should be that of a typical
commercial or hospital
environment. If the user
of this product requires
continued operation during
power mains interruptions,
it is recommended that this
product be powered from
an uninterruptible power
supply or a battery.
Power frequency
(50/60Hz) magnetic
field
IEC 61000-4-8
NOTE: Uт is the A.C. mains voltage prior to application of the test level.
3 A/m 3 A/m Power frequency magnetic
fields should be at levels
characteristic of a typical
location in a typical
commercial or hospital
environment.
1-11
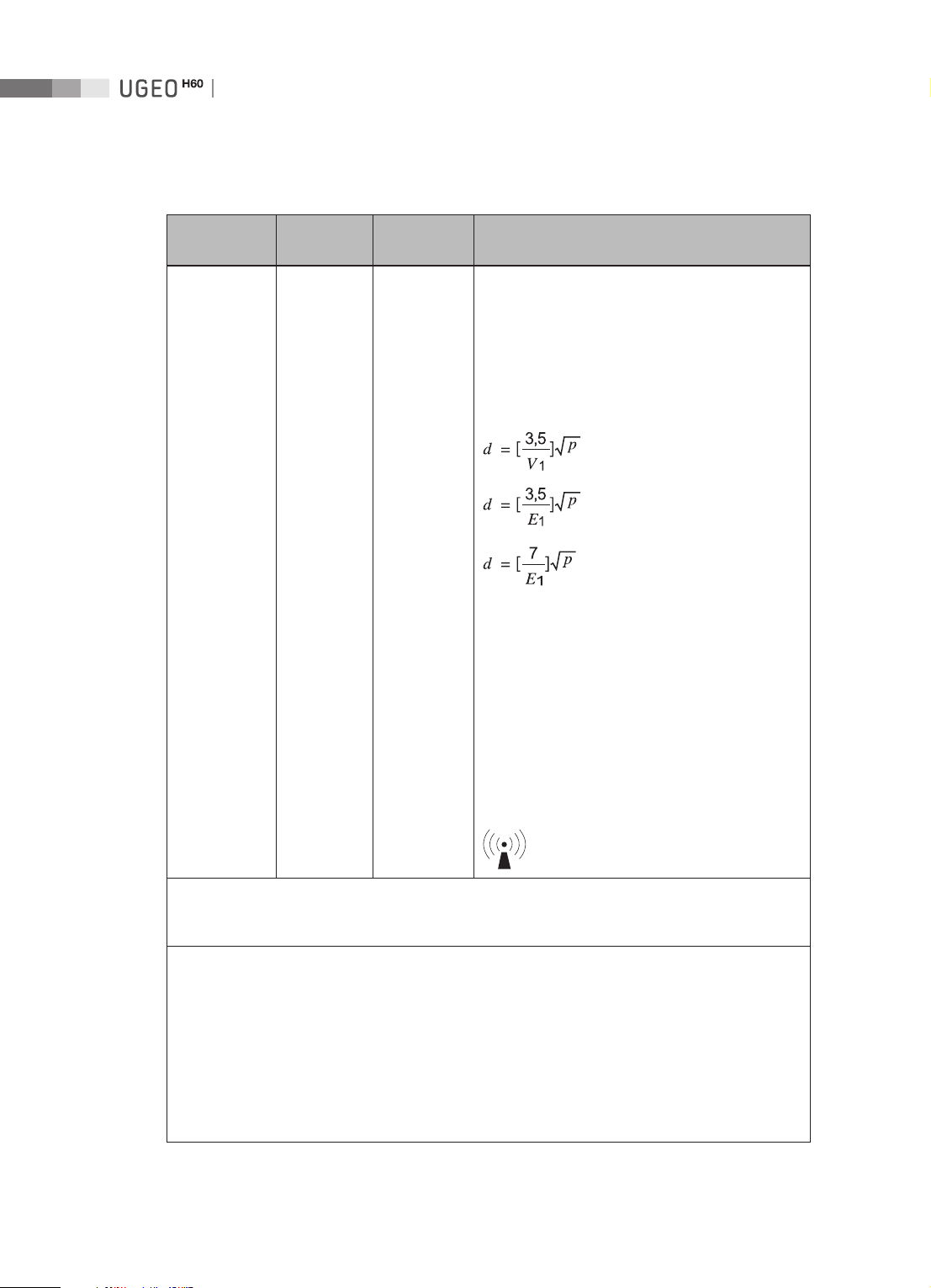
Operation Manual
Immunity test
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
IEC 60601
test level
3 Vrms
150 kHz
to 80 MHz
3 V/m
80 MHz
to 2.5 GHz
Compliance
level
Electromagnetic
environment - guidance
0.01 V Portable and mobile RF communications
equipment should be used no closer to any part of
the Ultrasound System, including cables, than the
recommended separation distance calculated from
the equation applicable to the frequency of the
transmitter.
Recommended separation distance
80MHz to 800MHz
800MHz to 2.5GHz
3 V/m Where P is the maximum output power rating
of the transmitter in watts (W) according to
the transmitter manufacturer and d is the
recommended separation distance in meters (m).
Field strengths from fixed RF transmitters, as
determined by an electromagnetic site survey,
should be less than the compliance level in each
frequency range.
b
a
1-12
Interference may occur in the vicinity of
equipment marked with the following symbol :
NOTE 1: At 80MHz and 800MHz, the higher frequency range applies.
NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by
absorption and reflection from structures, objects and people.
a
Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones
and land mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be
predicted theoretically with accuracy. To assess the electromagnetic environment due to fixed RF
transmitters, an electromagnetic site survey should be considered. If the measured field strength
in the location in which the Ultrasound System is used exceeds the applicable RF compliance
level above, the Ultrasound System should be observed to verify normal operation. If abnormal
performance is observed, additional measures may be necessary, such as re-orienting or relocating
the Ultrasound System, or using a shielded location with a higher RF shielding effectiveness and
filter attenuation.
b
Over the frequency range 150kHz to 80MHz, field strengths should be less than V1 V/m.
 Loading...
Loading...