
Philips M1165/66/67/75/76/77A
CMS Patient Monitoring System
and
Philips M1205A
V24 and V26 Patient Monit or
User’s Reference Manual
Volume 1
System Information
PHI
Part Number M1046-9220L
Printed 02/2003
Notice
This document contains proprietary information which is protected by copyright. All Rights
Reserved. Reproduction, adaptation, or translation without prior written permission is
prohibited, except as allowed under the copyright laws.
Philips Medical Systems
Cardiac and Monitoring Systems
3000 Minuteman Road
Andover, MA 01810
Publication number
M1046-9220L
Warranty
The information contained in this document is subject to change without notice.
Philips Medical Systems makes no warranty of any kind with regard to this material,
including, but not limited to, the implied warranties or merchantability and fitness for a
particular purpose.
Philips Medical Systems shall not be liable for errors contained herein or for incidental or
consequential damages in connection with the furnishing, performance, or use of this
material.
© 2003 Philips Medizin Systeme Böblingen GmbH
All rights are reserved.
Reproduction in whole or in part is prohibited without the prior written consent of the
copyright holder.
Important
United States federal law restricts these devices to sale by or on the order of a physician.
ii
The M1165/66/75/76A Systems comply with UL544, CSA 22.2-125, IEC 601-1, EN 60601-1, and
EN 60601-1-2 and carries Marking to Council Directive 93/42/EEC, European Medical
0366
Device Directive (MDD).
The M1167/77A Systems comply with UL2601-1, CSA 22.2 No. 601.1-M90, IEC 601-1,
EN 60601-1, and EN 60601-1-2 and carries Marking to Council Directive 93/42/EEC,
0366
European Medical Device Directive (MDD).
The M1205A Systems comply with UL2601, IEC 601-1, CSA C22.2 no. 601-1, EN60601-1, and
EN60601-1-2 and carries Marking to Council Directive 93/42/EEC, European Medical
0123
Device Directive (MDD).
iii

Electromagnetic Interference
Anomalies due to electromagnetic interference are not unique to the M1165/66/67/75/76/77A
or the M1205A but are characteristic of patient monitors in use today. This performance is
due to the very sensitive high gain front end amplifiers used to display the physiological
signals. Among the many similarly performing patient monitors already in use by customers,
interference from electromagnetic sources is rarely a problem in actual use.
Avoiding Electromagnetic Interference
When electromagnetic interference (EMI) is encountered there are a number of actions that
can be taken to mitigate the problem.
• Eliminate the source. Possible sources of EMI can be turned off or moved away to
reduce their strength.
• Attenuate the coupling. If the coupling path is through the patient leads, the
interference may be reduced by moving and/or rearranging the leads. If the coupling is
through the power cord, plugging the patient monitor into a different circuit may help.
• Reduce the sensitivity of the system. In all of the EMC testing the patient monitor was
adjusted to maximum sensitivity. For the ECG amplifier the gain was four times what is
normally required. By reducing the gain of the system receiving the EMI, the
interference can often be eliminated.
• Add external attenuators. If EMI becomes an unusually difficult problem external
devices such as an isolation transformer or a transient suppressor may be of help. A
Philips Customer Engineer can be of help in determining the need for external devices.
Electromagnetic Compatibility (M1205A Only)
The electromagnetic compatibility (EMC) validation of the M1205A included testing
performed according to international standards for EMC with medical devices. See the
Manufacturer's Declaration for details.
EMC Testing. During the test program the M1205A was subjected to many EMC tests,
both international standard and Philips proprietary tests. There were no anomalies observed
during this testing.
iv

Intended Use
Intended Use
Description
The Philips M1165/66/67/75/76/77A CMS Patient Monitoring System and the Philips M1205A
V24 and V26 Patient Monitors are network connectable bedside patient monitoring devices.
The Philips M1205A Models V24CT and V26CT may powered by either AC line power or by
battery power.
Purpose
The Philips M1165/66/67/75/76/77A CMS Patient Monitoring System and the Philips M1205A
V24 and V26 Patient Monitors measure and display multiple physiological parameters and
waves, and generate alarms and recordings. They exchange information with compatible
devices. The Philips M1165/66/67/75/76/77A CMS Patient Monitoring System and the Philips
M1205A V24 and V26 Patient Monitors are not therapeutic devices.
Environment
The Philips M1165/66/67/75/76/77A CMS Patient Monitoring System and the Philips M1205A
V24 and V26 Patient Monitors are intended to be used in a clinical environment by trained
healthcare professionals. They are not intended for home use.
They communicate with devices such as a central station through network interface ports and
a serial I/O port.
The Philips M1165/66/67/75/76/77A CMS Patient Monitoring System and the Philips M1205A
V24 and V26 Patient Monitors are prescription devices and will carry the following label,
“United States Federal law restricts this device to sale by or on the order of a physician.”
v

Indications for Use
Indications for Use
Condition
The Philips M1165/66/67/75/76/77A CMS Patient Monitoring System and the Philips M1205A
V24 and V26 Patient Monitors are generally indicated when the clinician decides there is a
need to measure and display multiple physiological parameters and waves, to generate
alarms and recordings of adult, pediatric, or neonatal patients.
Part of Body or Type of Tissue with Which the Device Interacts
The Philips M1165/66/67/75/76/77A CMS Patient Monitoring System and the Philips M1205A
V24 and V26 Patient Monitors do not contact the body or tissue of the patient. Signals are
obtained from accessory electrode, transducer, and sensor devices.
Frequency of Use
The Philips M1165/66/67/75/76/77A CMS Patient Monitoring System and the Philips M1205A
V24 and V26 Patient Monitors are indicated for use when prescribed by a clinician.
Physiological Purpose
The Philips M1165/66/67/75/76/77A CMS Patient Monitoring System and the Philips M1205A
V24 and V26 Patient Monitors are indicated when the purpose is to gain information for
treatment, to assess adequacy of treatment, or to rule out causes of symptoms. The Philips
M1165/66/67/75/76/77A CMS Patient Monitoring System and the Philips M1205A V24 and V26
Patient Monitors are well suited for patient monitoring.
Patient Population
Adult, pediatric, and neonatal non-ambulatory patients.
vi

Indications for Use
Prescription Versus Over-the-Counter
The Philips M1165/66/67/75/76/77A CMS Patient Monitoring System and the Philips M1205A
V24 and V26 Patient Monitors are prescription devices.
vii
Indications for Use
Warnings, Cautions, and Notes
Warnings, cautions, and notes are used throughout this User's Manual to give you additional
information about the Philips M1165/66/67/75/76/77A CMS Patient Monitoring System and the
Philips M1205A V24 and V26 Patient Monitors. The warnings and cautions included in this
safety section refer to the equipment in general.
WarningWarning
A “warning” calls attention to the user of imminent hazard to people if proper
procedures are not followed.
• For continued safe use of this equipment, it is necessary that the listed instructions are
followed. Instructions in this manual in no way supersede established medical
procedures.
• Explosion Hazard- Do not use this equipment in the presence of flammable anesthetics.
• Alarms - Do not rely exclusively on the audible alarm system for patient monitoring.
Adjustment of alarm volume to a low level or off during patient monitoring may result
in patient jeopardy. Remember that the most reliable method of patient monitoring
combines close personal surveillance with correct operation of monitoring equipment.
• This equipment is only intended for use in healthcare facilities by trained healthcare
professionals.
• The product is not intended for outside hospital use such as a helicopters or
ambulances.
• This product is not intended for home use.
• To reduce the risk of electrical shock, do NOT remove any cover. Refer servicing to
qualified personnel.
• This equipment may interfere with ultrasound imaging equipment by causing
interference on the ultrasound display. Try to keep the instruments as far apart as
possible.
viii

Indications for Use
• Exposure of electrical contacts or connections to saline or other liquids and gels is
dangerous. Electrical contacts and connections such as cable connectors, power
supplies, parameter module plug-in connections and rack connections must be kept
clean and dry. Thoroughly dry any electrical connections that become contaminated
with liquids. If additional decontamination is required please contact your biomedical
department or Philips Medical Systems Response Center.
• Although this equipment is shielded against Electromagnetic Interference (EMI), it is
recommended to avoid the use of electrically radiating devices in close proximity to this
equipment.
• Connecting the Philips monitoring network (SDN) cable when the product is powered on
is not supported. Error codes and Philips monitoring network (SDN) interface lock-up
may occur. Power cycling the product will recover the product. No permanent damage
will result. To prevent unintentional disruption in monitoring, be sure the SDN interface
cable is properly secured at both ends when connecting to the Philips monitoring
network (SDN).
• Do not connect a second rack by a cable when using a module rack docked to the back
of the V24CT or V26CT. Using a second rack connected by a cable may disrupt module
communication.
Caution
A “caution” calls attention to a condition or possible situation that could cause injury to the
user.
• Ventilation Requirements - Failure to meet ventilation requirements may cause
equipment failure and, in turn, jeopardize the functions of automated monitoring. Do not
locate equipment in an enclosed area which could restrict heat dissipation.
• Maintenance - Failure on the part of the responsible individual, hospital, or institution
employing the use of this equipment to implement a satisfactory maintenance schedule
may cause undue equipment failure and possible health hazards.
• Do not spray cleaning solutions directly onto the patient monitor. Moisture droplets may
enter the internal components and cause equipment malfunction or failure. Cleaning
solutions should be applied to a cloth and the cloth used to wipe the monitor clean. The
monitor should be turned off during cleaning.
ix
Indications for Use
• Replacement Parts - It is highly recommended that only Philips Medical Systems
recommended parts and accessories be used with this equipment. Failure to do so may
result in the degradation of performance. Accessories and parts for individual modules
and components are listed at the back of the appropriate section in this manual.
Note—A note gives special instructions to highlight an operating procedure or practice. Notes
may precede or follow the applicable text.
At this time, Philips Medical Systems will make available on request, and in English only,
such circuit diagrams, component part lists, descriptions, calibration instructions, or other
information which will assist the user's appropriate qualified technical personnel to repair
those parts of the equipment which are classified by Philips Medical Systems to be
repairable. A list of Philips Sales and Support Offices is provided at the end of this manual.
Notice to the User
Although there may be products in your area that look similar to the Philips M1165/66/67/75/
76/77A CMS Patient Monitoring System and the Philips M1205A V24 and V26 Patient
Monitors, their functionality may not be the same. This User's Reference Manual is intended
to be used with the Philips M1165/66/67/75/76/77A CMS Patient Monitoring System, the
M1026A Anesthetic Gas Module and the Philips M1205A V24 and V26 Patient Monitors only.
This Manual is only applicable for Release C.1 versions of the CMS monitors and for Release
D.0 of the V24 and V26 monitors. A Release C.1 or Release D.0 monitor can be identified by:
a. the Release C.1 or D.0 label on the monitor, or
b. the suffix of the EPROM pack part number. To view this number, press
Monitor Setup
→ → .
Monitor Revision
Show SW Rev
The suffix of the EPROM pack part number on a Release C.1 CMS is E.
The Software Revision of a Release D.0 V24 or V26 monitor is M.00.03
x

Responsibility of the Manufacturer
Responsibility of the Manufacturer
Philips Medical Systems only considers itself responsible for any effects on safety, reliability
and performance of the equipment if:
assembly operations, extensions, re-adjustments, modifications or repairs are carried out
by persons authorized by Philips, and
the electrical installation of the relevant room complies with national standards, and
the instrument is used in accordance with the instructions for use.
To ensure optimum usage, we recommend that Philips parts and accessories are used in
conjunction with the Philips M1165/66/67/75/76/77A CMS Patient Monitoring System, the
Philips M1026A Anesthetic Gas Module and the Philips M1205A V24 and V26 Patient Monitors,
wherever available. If non-Philips parts are used, Philips Medical Systems is not liable for any
damage that these parts may cause to the Philips equipment.
Manufacturer´s Address
For South America, North America and Canada:
Philips Medical Systems, Inc.
3000 Minuteman Road
Andover
MA 01810-1099
For all other countries:
Philips Medical Systems GmbH
Hewlett-Packard Str. 2
71034 Böblingen
Germany
xi

Responsibility of the Manufacturer
xii

Contents
This book is divided into three volumes.
This volume contains chapters 1 to 13 (see the following table of contents for more details):
1. The CM S and V24 and V26 Patient Monitors
2. Getting Started
3. Setting up your Monitor
4. Other Patients
5. Alarm Functions
6. Recording Functions
7. Admit/Discharge/End Case
8. Trends and Calculations
9. Neonatal Event Review
10. Data Transfer
11. Monitor Installation and Patient Safety
12. Battery Information (V24CT and V26CT only)
13. Maintenance
Volume 2 contains chapters 12 to 22 (see the table of contents of Volume 2 for more details):
14. ECG and ECG/Respiration Module Section
15. Noninvasive Blood Pressure Module Section
16. SpO2/PLETH Module Section
17. Temperature Module Section
18. CO2 M odule and Sidestream Module S ection
19. FIO2 Module Section (CMS on ly)
20. Pressure Module Section
21. Cardiac Output Module Section
22. V ueLink Modul e Section
Volume 3 contains chapters 23 to 29 and appendices A to E (see the table of contents of Volume 3 for more
details):
23. SvO2 Module Section (CMS only))
24. tcpO2/tcpCO2 Module Section
25. Ventilator Interfaces and Respiratory Loops (CMS only)
26. Anesthetic Gas Module Section (Option #A05, #C03)
27. Blood Analysis
28. EEG Module Section (CMS only)
29. BIS“ Module Section
A. Summary of Formulas Used in Calculations
B. Analog Output Section (CMS Only)
C. Calibrating the Pressure System
D. SpO2 Transducer Information
E. Main Sales and Support Offices
Contents-1

Intended Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-v
Description. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-v
Purpose. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-v
Environment. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-v
Indications for Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-vi
Condition . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-vi
Part of Body or Type of Tissue with Which the Device Interacts. . . . . . . . . . . . . . . . . . . . 1-vi
Frequency of Use. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-vi
Physiological Purpose . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-vi
Patient Population . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-vi
Prescription Versus Over-the-Counter. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-vii
Responsibility of the Manufacturer . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-xi
Manufacturer´s Address. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-xi
The CMS and V24 and V26 Patient Monitors 1-1
Introduction. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-2
CMS Patient Monitoring System. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-2
V24 and V26 Patient Monitor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-7
Control Panel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-8
The Handheld Keypad . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-10
External Alarm Device. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-11
Hardkey Functions. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-12
V26CT/V24CT Power Supply . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-14
Battery Power Supply . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-14
Parameter Modules . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-16
Symbols to Indicate Key Functions. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-17
Operating Levels. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-20
Main Screen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-21
Selection Window . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-23
Task Window. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-24
Getting into the Operating Levels . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-25
Touch or Mouse/Trackball Operation. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-27
Main Screen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-28
Control Panel Task Window . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-28
General Touch/Mouse/Trackball Operation (CMS only) . . . . . . . . . . . . . . . . . . . . . . . . . . 1-29
Disabling Touch/Mouse/Trackball Operation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1 -32
The CMS Computer Modules. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-33
M1046A Computer Module. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-33
M1046B Computer Module. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-34
Contents-2

ECG Output and Defibrillator Marker Input . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-34
The V24 and V26 Parameter Module Rack. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-35
Operating Rules to Remember. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-36
Performance Specifications of the Philips Displays . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-37
M1095A Flatscreen Display. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-37
M1094A/B and M1092A CRT Display . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-37
M1097A #A02 XGA Flatscreen Display . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-37
Using an ITE Display. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-38
Safety . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-38
EMC . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-39
Performance Requirements. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-39
Getting Started 2-1
Setting up the Monitor (V24 and V26 only) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-2
Setting up the Parameter Modules. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-5
Attaching the Patient . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-6
Adjusting Screen Contrast . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-7
Starting Monitoring. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-8
Screen Messages. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-8
Reserving a Channel. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-9
Power Failure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-10
Patient Information Center . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-10
Monitor Standby. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-10
Setting up your Monitor 3-1
Changing Display Screens. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-2
Selecting a Screen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-3
Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-3
Freezing Waves (CMS only) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-4
What you Can Configure. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-5
Changes to the Configuration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-6
Making Changes to the Main Display . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-7
Assigning Waves to Screen Channels . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-8
Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-9
Selecting a Screen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-10
Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-10
Selecting Screen Labels for Realtime Display Screens. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-11
Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-11
Contents-3

Selecting the Number of Waves . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-13
Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-13
Changing the Wave Overlap. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-14
Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-14
Selecting Realtime Wave Speeds . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-15
Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-15
Numerics On/Off . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-17
Additional Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-19
Selecting an Application Window. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-20
Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-20
Displaying Split Screen Trends. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-22
oxyCRG Display. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-25
Notes on oxyCRG . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-26
CSA Display (CMS only) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-28
Notes on CSA. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-29
Wave Replace . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-30
Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-30
Trace Mode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-31
Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-31
Configuring a Second Independent Display (CMS only) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-32
Other Functions You Can Configure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-33
Adjusting the Volume Control . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-35
Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-35
Adjusting the Date and Time . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-37
Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-37
Selecting Waves for Central Recorders . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-39
Configuring Module, Bedside and Central Recordings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-40
Other Patients Controls . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-41
The Status Log Function. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-42
The Monitor Revision Function . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-43
Changing Default Settings and Patient Category . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-44
Changing the Patient Category . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-45
NBP . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-47
NBP Examples. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-47
ECG . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-49
Heart Rate (HR) / Pulse . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-51
RESP . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-51
Pressure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-51
SpO2. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-51
Contents-4

Changing the Configuration Set. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-53
Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-54
Changing Operating Modes. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-55
Returning to Monitoring Mode. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-56
Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-57
The Test Signals Function . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-58
Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-59
Analog Output (CMS only) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-59
Parameter Settings Transfer. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-61
Parameter Settings Transfer Messages . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-63
Other Patients 4-1
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-2
Philips Patient Care System. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-3
The Other Patients Selection Window . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-3
Automatic Alarm Other Patients. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-4
Configuring the Other Patients Controls . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-7
Using Philips Patient Care System with an Arrhythmia Computer. . . . . . . . . . . . . . . . . . . . . . . .4-8
Extended Overview (CMS only) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-15
To View an Extended Other Patients Bed . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-16
Alert Notification . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-17
Alarm Functions 5-1
Alarm Display . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-2
Alarm Functions on the Control Panel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-2
Suspending Alarms. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-3
Silencing and Resetting Alarms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-4
Alarm Priorities. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-8
Individual Parameter Alarms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-10
When an Alarm Occurs. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-11
Alarm Setup . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-12
Getting into the Alarms Selection Window . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-12
Changing the Alarm Limits . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-13
Setting the Volume Control . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-14
The Nurse Call Relay . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-15
Recording Functions 6-1
General Recorder Information. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-2
Contents-5

Recorders . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-2
Controls and Indicators on the Plug-In Recorder. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-3
Controls and Indicators on the 4-Channel Recorder (CMS only) . . . . . . . . . . . . . . . . . . . . .6-4
Recorder Capabilities. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-5
Types of Recordings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-7
Delayed Recording . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-8
Definitions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-8
Configuring Delayed Recordings. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-9
Making Delayed Recordings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-11
Alarm Recording. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-12
Configuring Alarm Recordings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-13
Alarm Recording Priorities . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-14
Procedure Recordings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6 -16
Configuring Procedure Recordings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-16
Making Procedure Recordings. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-17
ST Recordings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-18
Realtime Wave Recordings. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-20
Definitions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-20
Configuring Preset Recording Modes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-21
Making Preset Recordings. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-22
Making Non-Preset Recordings. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-22
Making Calibrated ECG Recordings. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-23
If the Recorder is Busy. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-23
Realtime Vital Signs / Blood Recordings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-25
Definitions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-25
Making a Single Vital Signs/Blood Recording . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-27
Making Timed Sequences of Vital Signs/Blood Recordings. . . . . . . . . . . . . . . . . . . . . . . .6 -27
Trended Vital Signs Recordings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-29
Header Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-29
Trend Data . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-30
Making Trended Vital Signs Recordings. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-32
Neonatal Event Review Recordings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-33
Tabular Neonatal Event Recordings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-33
oxyCRG Episode Recordings for Neonatal Events . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-34
oxyCRG Recordings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6 -36
oxyCRG Alarm Recording. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-38
Additional Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-39
Annotations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-39
Changing the Recording Length . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-42
Contents-6

Changing the Recorder Speed. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-43
Changing the Recorder . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-43
Continuing a Timed Recording. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-43
Inserting a Calibration Signal. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-44
Recording Layouts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-44
Recording Status Messages . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-46
Accessories and Ordering Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-48
Loading Paper . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-49
Central Recorders . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-49
Loading Paper into the Plug-In Recorder. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-49
To Replace Paper in the Plug-In Recorder. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-50
Cleaning the Print head in the Plug-In Recorder . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-52
Loading Paper into the Four Channel (M1117A) Recorder (CMS only). . . . . . . . . . . . . . .6-53
Cleaning the Roller on the Four Channel (M1117A) Recorder . . . . . . . . . . . . . . . . . . . . . .6-55
Admit/Discharge/End Case 7-1
Admitting a Patient . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7-2
Changing Patient Information. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7-5
Discharging a Patient/Ending a Case . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7-9
Trends and Calculations 8-1
Introduction to Trends & Calculations. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8-2
Viewing Patient Data . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8-3
Trending Priority. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8-5
Viewing Blood Measurements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8-5
Viewing Vital Signs . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8-9
Selecting Parameters for Graph Trends . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8-12
Viewing Graph Trends . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8-13
Performing and Reviewing Calculations. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8-20
Performing Calculations. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8-21
Changing or Entering an Input Value. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8-25
Reviewing Calculations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8-25
Printing Reports . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8-26
Printing Task Window Reports. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8-26
Printing Scheduled Reports . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8-26
What to Do If Your Report Does Not Print . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8-30
Drug Calculator . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8-32
Contents-7

Neonatal Event Review 9-1
Introduction to Neonatal Event Review. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .9-2
Viewing Neonatal Events . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .9-3
Manual Event Storage . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .9-4
Graphical Details . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-5
Operating Controls. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .9-12
Viewing oxyCRG Episodes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .9-14
Operating Controls. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .9-16
Adjusting Neonatal Event Review Settings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .9-18
Event Criteria. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .9-19
Operating Controls. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .9-21
Data Transfer 10-1
Data Transfer Module . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-2
Symbols to Indicate Key Functions. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .10-5
What is Transferred . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .10-5
Types of Transfer . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .10-7
To Module . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .10-7
To Monitor. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .10-10
Transferring Blood Analysis Data. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .10-11
Combining Data . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-14
Time Conversion . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .10-14
Database Conversion . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .10-14
Vital Signs, Blood Review and Graphs. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .10-17
Time Stamp . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .10-17
Reports. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .10-18
Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-19
Performance Specifications. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-20
Data Transfer Module . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-20
Monitor Installation and Patient Safety 11-1
Introduction. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11-2
Installation Information. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11-5
Power Source Requirements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11-5
Grounding the System . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11-5
Combining Equipment. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11-8
Environment. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11-9
Condensation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11-11
Contents-8

Explanation of Symbols used . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11-12
Maintenance Checks . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11-14
Patient Cables and Leads . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11-16
Controls and Connectors . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11-17
The Front Panel of the M1046A Computer Module. . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11-17
The Front Panel of the M1046B Computer Module. . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11-19
The Rear Panel of the M1046A/B Computer Modules . . . . . . . . . . . . . . . . . . . . . . . . . . .11-21
The Rear Panel of the Display Modules. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11-23
The Rear Panel of the M1109A External Alarm Device . . . . . . . . . . . . . . . . . . . . . . . . . .11-29
The Rear Panel of the M1026A Anesthetic Gas Module. . . . . . . . . . . . . . . . . . . . . . . . . .11-30
Assembling the System. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11-31
The V24 and V26 Connectors . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11-34
Assembling the V24 and V26. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11-36
Accessories and Ordering Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11-37
Battery Information (V24CT and V26CT only) 12-1
AC and DC (Battery) Operation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .12-2
Operating Instructions. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .12-3
Battery Indicator and Messages. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .12-6
External Battery Charger . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .12-8
Battery Care and Maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .12-9
Storage . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .12-9
Care and Handling . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .12-10
Accessories and Ordering Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .12-11
Maintenance 13-1
General cleaning of the System . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .13-2
General Disinfecting of the System. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .13-4
Monitor Maintenance. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .13-6
Inspect the System . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .13-8
Perform a Start-up Sequence Test of the System . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .13-9
Verify the Integrity of the Display . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .13-9
Perform a System Self-Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .13-10
Performance Assurance Checks. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .13-11
Performance Assurance Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .13-11
Functional Testing Procedures . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .13-13
Performing the ECG Module and ECG/RESP Self-Test . . . . . . . . . . . . . . . . . . . . . . . . . .13-15
Performing the Invasive . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .13-16
Contents-9

Pressure Module Self-Test. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13-16
Performing the NBP Module Self-Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13-17
Performing the SpO2/Pleth Module Self-Test. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .13-17
Performing the Cardiac Output Module Self-Test. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13-17
Performing the tcpO2/tcpCO2 Module Self-Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13-18
Performing the CO2 Module Self-Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .13-19
Performing the Temperature Module Self-Test. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .13-19
Performing the Blood Analysis Module Self-Test. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13-20
Performing the Recorder Module Self-Test. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .13-20
Performing the Data Management Database Self-Test . . . . . . . . . . . . . . . . . . . . . . . . . . . 13-20
Tests for VueLink Module and Anesthetic Gas Module . . . . . . . . . . . . . . . . . . . . . . . . . . 13-21
Contents-10

1
The CMS and V24 and V26 Patient
Monitors
This chapter provides an overview of the CMS Patient
Monitoring Systems and V24 and V26 Patient Monitor
includes the following sections:
• Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-2
• Parameter Modules . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-16
• V26CT/V24CT Power Supply . . . . . . . . . . . . . . . . . . . . . . . 1-14
• Operating Levels . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-20
• Touch or Mouse/Trackball Operation. . . . . . . . . . . . . . . . 1-27
• The CMS Computer Modules . . . . . . . . . . . . . . . . . . . . . . . 1-33
• The V24 and V26 Parameter Module Rack. . . . . . . . . . . . 1-35
• Operating Rules to Remember. . . . . . . . . . . . . . . . . . . . . . 1-36
s. It
The CMS and V24 and V26 Patient Monitors 1-1

Introduction
Introduction
V26 Patient Monitors
The CMS and V24 and
The Philips M1165/66/67/75/76/77 CMS Patient Monitoring System and
the Philips M1205A V24 and V26 Patient Monitors, hereafter referred to
as the “patient monitor”, are modular patient monitors with networking
and data management capabilities. All the systems can have modules
added or removed at a later time as needed, or you can interchange the
modules between systems in your unit.
Note—Some features explained in this manual are not available for both
the CMS and for the V24 and V26 Monitors. The respective sections are
marked throughout the manual with either “CMS only” or “V24 and V26
only”.
The following system types are available:
CMS Patient Monitoring System
1-2 The CMS and V24 and V26 Patient Monitors
The CMS Patient Monitoring System is available as a choice of three
system types. Each system consists of three individual parts; a display
module, a computer module and parameter modules:
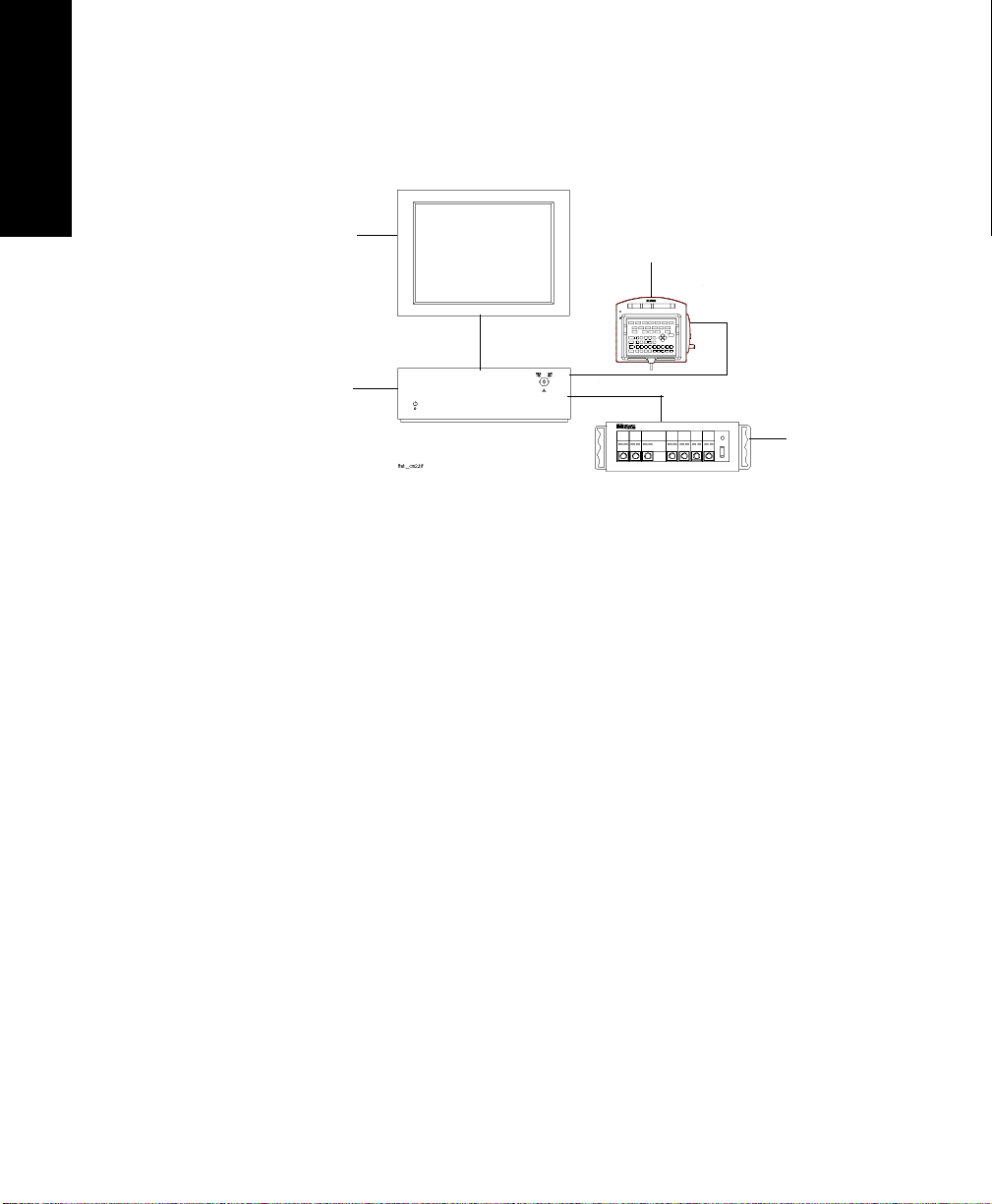
1. M1167/77A Color Flatscreen Display and Computer Module with
Satellite Module Rack
Note—This system is also available with an External Alarm Device and
an XGA compatible display controller to drive commercially available
ITE (Information Technology Equipment) displays (XGA Type).
2. M1165/75A Monochrome CRT Display and Computer Module with
Integral Module Rack
3. M1166/76A color CRT Display and Computer Module with Integral
Module Rack

M1167/77A System
Display Module M1095A 10.4” Flatscreen Display
Computer Module M1046B Computer Module
Parameter Modules Satellite Rack
Introduction
The CMS and V24 and
V26 Patient Monitors
The CMS and V24 and V26 Patient Monitors 1-3
The CMS and V24 and
Introduction
V26 Patient Monitors
M1167/77A System with External Alarm Device
XGA Display
External Alarm Device
Computer
Module
Parameter
Modules
Display Module ITE Display of choicea
Computer Module M1046B Computer Module
Parameter Modules Satellite Rack
a. Philips offers the M1167/77A #H05 and #H07 (XGA Touchscreen
display configuration).
b. A 15” flat touchscreen display is also available separately under the
order number M1097A #A02. A 17” CRT touchscreen display is also
available separately under the order number M1098A
1-4 The CMS and V24 and V26 Patient Monitors
b
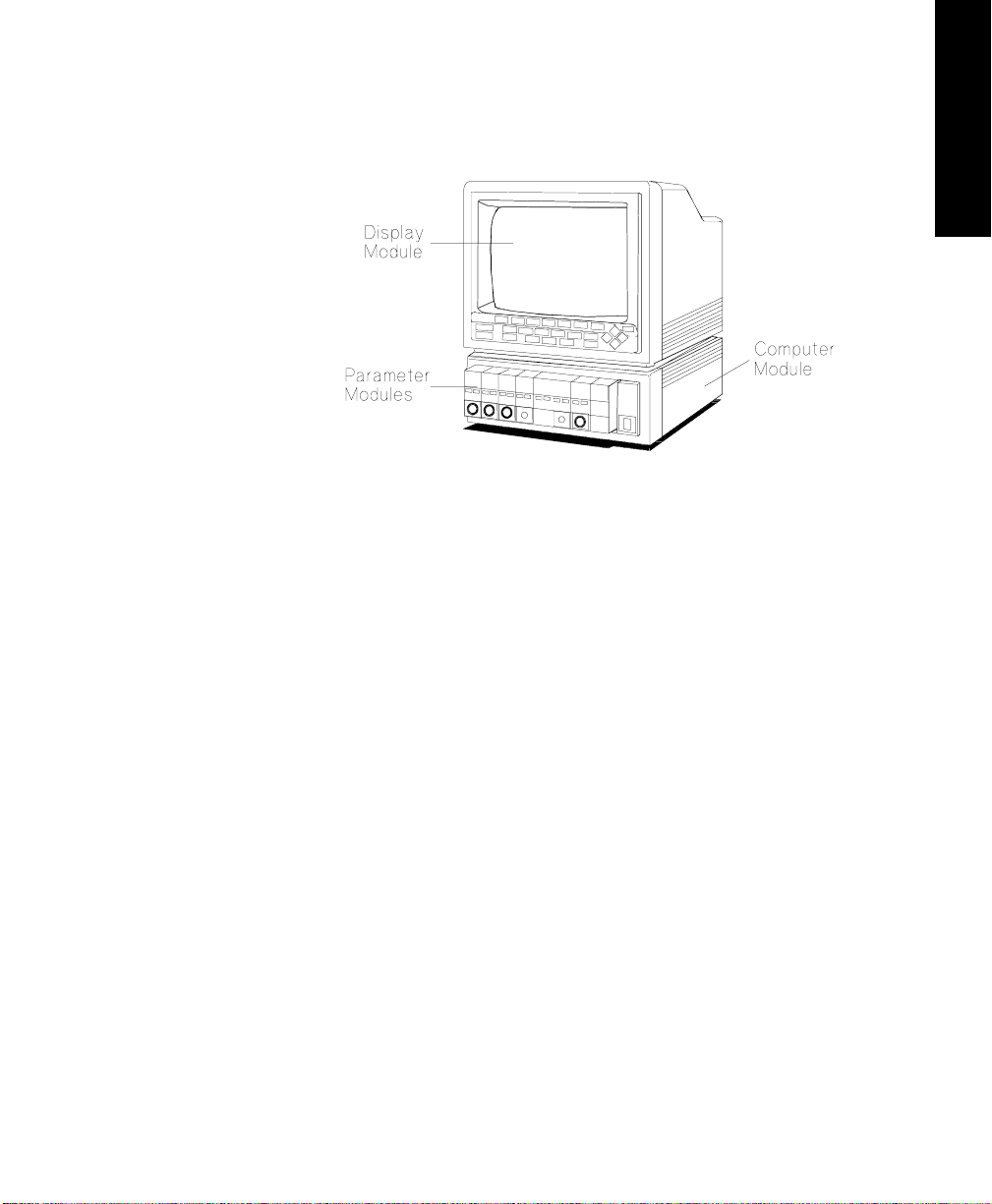
M1165/75A and M1166/76A System
Display Module M1094A/B/92A 14” CRT Display
Computer Module M1046A Computer Module
Parameter Modules Integral and/or Satellite Rack
Introduction
The CMS and V24 and
V26 Patient Monitors
Model Types All system types are also available as a choice of three different model
types:
Full Title Abbreviation
The Philips CMS Patient Monitoring System CMS
The Philips CMS Patient Monitoring System for
ACMS
Anesthesia Care
The Philips CMS Patient Monitoring System for
NCMS
Neonatal Care
Note—In this manual, the system will be referred to as the CMS, the ACMS
and the NCMS.
The CMS and V24 and V26 Patient Monitors 1-5
Introduction
The CMS and V24 and
Display
Modules
Below are labeled diagrams of the display modules provided by Philips
Medical Systems. The control panel is described in more detail in the
following sections
V26 Patient Monitors
.
M1095A Flatscreen Display Module
M1092A / M1094B CRT Display Module
1-6 The CMS and V24 and V26 Patient Monitors
Introduction
V24 and V26 Patient Monitor
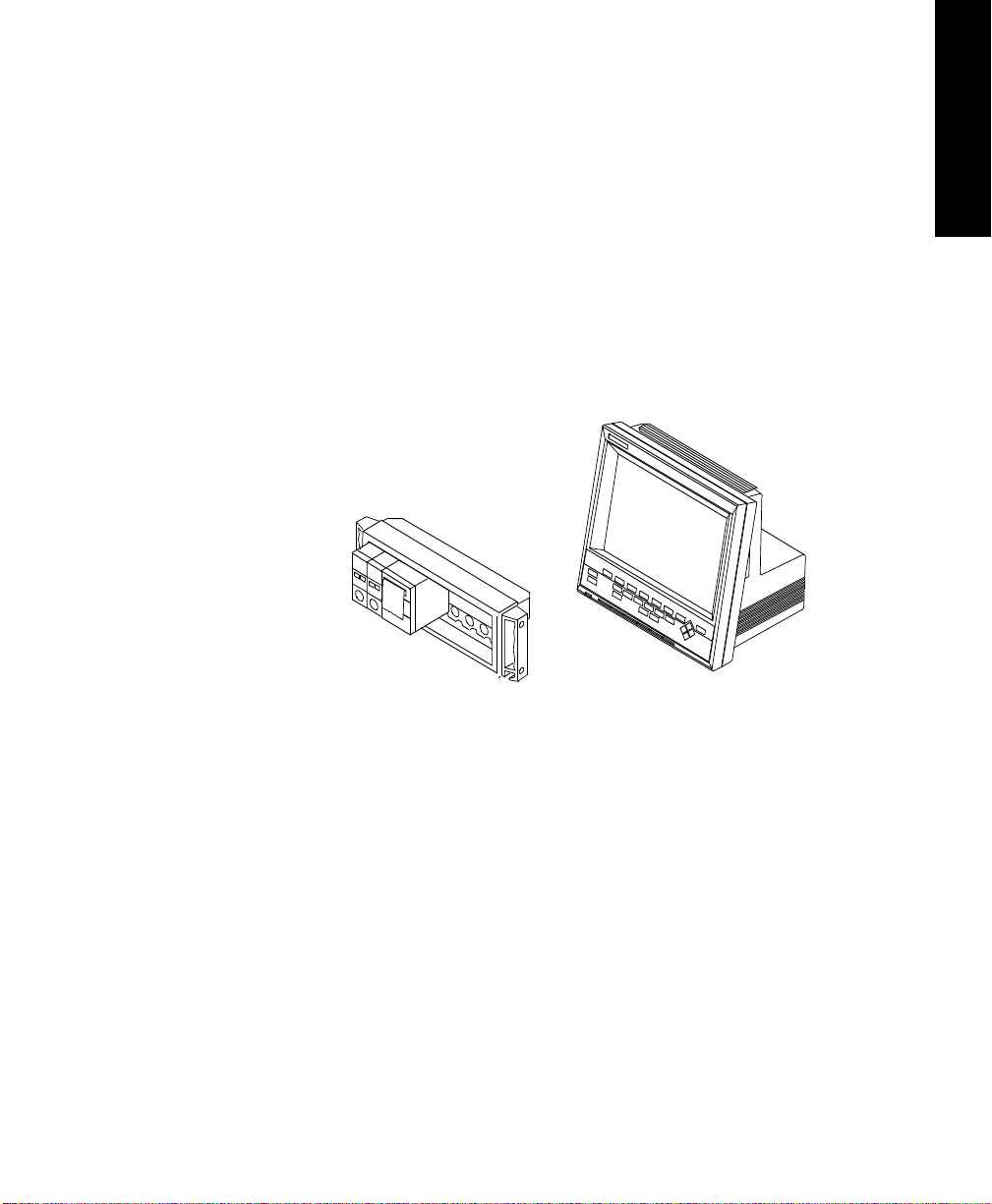
Each V24 and V26 Patient Monitor consists of two individual parts.
1. One of two types of Display Modules, depending on the particular
model monitor you have purchased—either:
a. A monochrome display with control panel supporting the V24,
or
b. A color flat panel display with control panel supporting the
V24C, the V24CT, the V26C and the V26CT.
2. The Rack with Parameter Modules
The CMS and V24 and
V26 Patient Monitors
The V24, V24C and V26C are powered by connection to an AC power
supply. The V24CT and V26CT can be powered by rechargeable batteries
or by connection to an AC power supply. See “V26CT/V24CT Power
Supply” on page 1-14.
The CMS and V24 and V26 Patient Monitors 1-7
Introduction
The CMS and V24 and
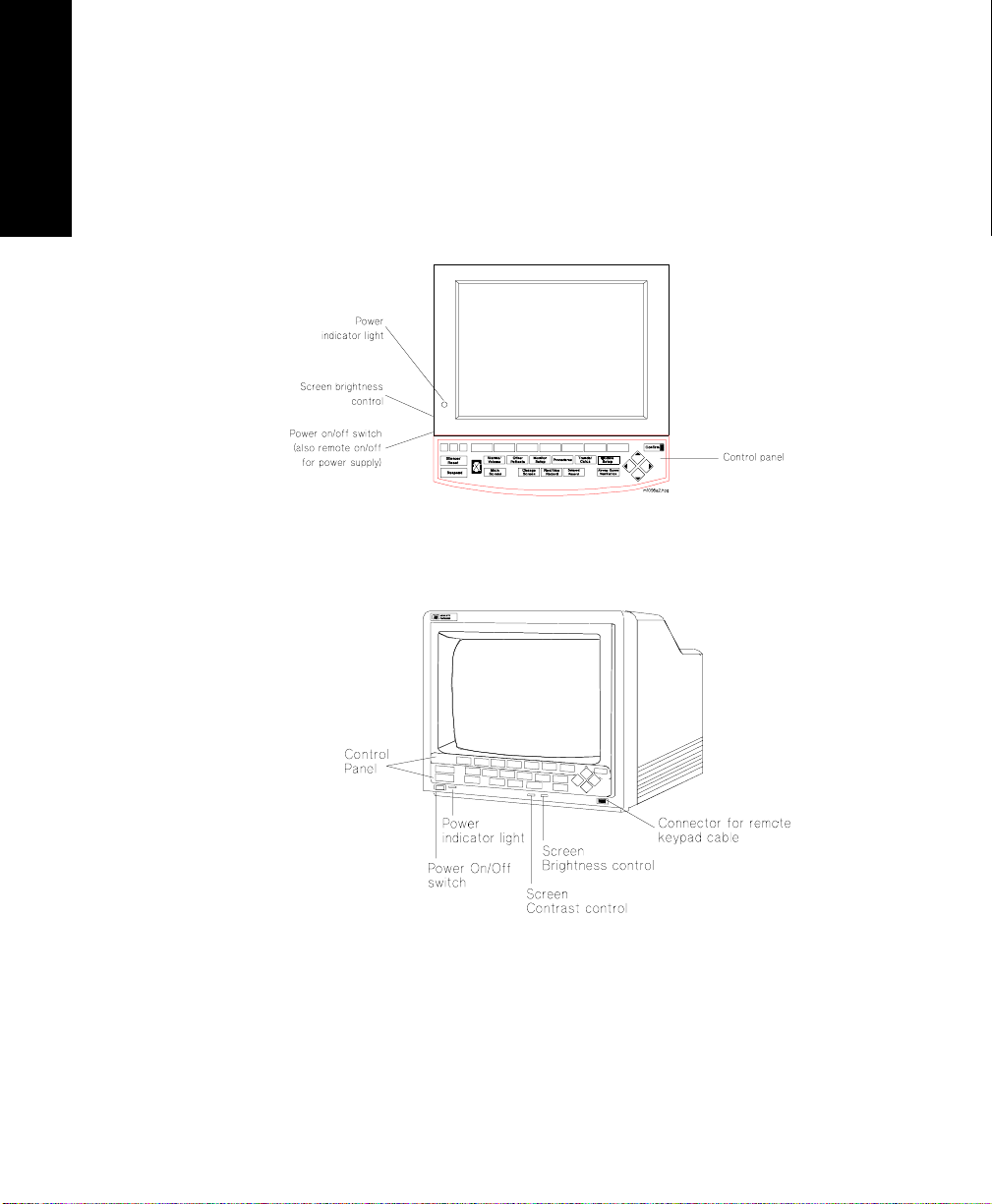
Control Panel
V26 Patient Monitors
The control panel consists of softkeys, hardkeys and alarm lamps.
Softkeys The softkeys perform multiple functions. Their functions correspond to
the labels displayed at the bottom of the screen. When no softkey labels
are on the screen, the softkeys do not function.
Hardkeys The hardkeys have only one function defined by the label on the key.
The hardkeys are labeled in blue. Each one of these keys gets you into a
level where adjustments and changes can be made or performs an
immediate action. The keys are labeled according to their function, for
example, key allows you to start a recording of a
waveform.
Note—If you are using the M1167/77A system with the External Alarm
Device, the handheld keypad can be used to operate the system and to
enter data. It contains all the hardkeys and softkeys available on the
control panel of the other systems.
Realtime Record
1-8 The CMS and V24 and V26 Patient Monitors
 Loading...
Loading...