Page 1

HeartStart OnSite Defibrillator
OWNER’S MANUAL
Guide to Set-Up, Operation, Maintenance, and Accessories
M5066A
Edition 7
Page 2
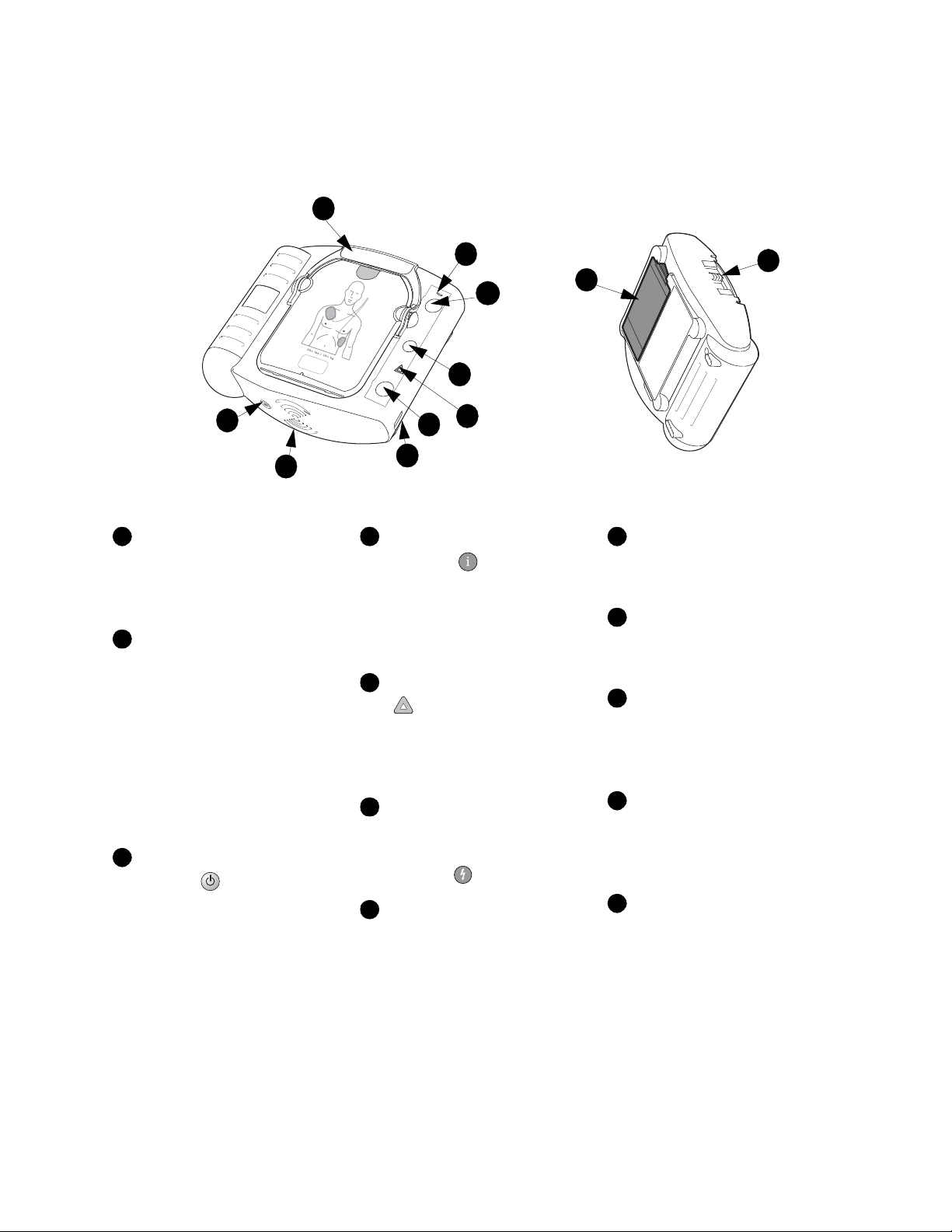
The HeartStart OnSite Defibrillator
D
F
I
top
top
C
B
D
E
G
F
H
A
front view
I
B
A
back view
K
L
K
L
A
Pads Cartridge Handle. Pull
the handle to turn on the
HeartStart and remove the
cartridge’s hard cover.
B
Ready Light. This green light
tells you the readiness of the
HeartStart.
Blinking: standby mode
(ready for use)
Solid: in use
Off: needs attention
(HeartStart
“chirps” and
i-button flashes)
C
On/off Button. Press this
green button to turn on the
HeartStart. To turn off the
HeartStart, press the green button
again and hold it down for one (1)
second.
Information-Button. This
blue “i-button” flashes when it
has information you can access by
pressing it. It also flashes at the
beginning of a patient care pause
when CPR Coaching is enabled.
E
Caution Light. This triangular
light flashes during rhythm
analysis and is on when a shock is
advised, as a reminder that no one
should be touching the patient.
Shock Button. When
instructed by the HeartStart to
deliver a shock, press this flashing
orange button .
G
Infrared (IR)
Communications Port.
special lens, or “eye,” is used to
transfer HeartStart data directly to
or from a computer.
This
H
Speaker. When the device is
being used, its voice instructions
come from this speaker.
Beeper. The HeartStart
“chirps” through this beeper to
alert you when it needs attention.
J
SMART Pads Cartridge. This
disposable cartridge contains selfadhesive pads with attached cable.
Shown with adult pads cartridge.
K
SMART Pads Cartridge
Slide the latch to the right
Latch.
to release the pads cartridge for
replacement.
L
Battery. The disposable
battery is inserted in a recess on
the back of the HeartStart.
Philips Medical Systems
Page 3
HeartStart OnSite Defibrillator
QUICK REFERENCE
Philips Medical Systems
Page 4

Intentionally blank.
Philips Medical Systems
Page 5

HeartStart OnSite
M5066A
Automated External Defibrillator
OWNER’S MANUAL
Edition 7
IMPORTANT NOTE:
It is important to understand that survival rates for sudden cardiac arrest are
directly related to how soon victims receive treatment. For every minute of
delay, the chance of survival declines by 7% to 10%.
Defibrillation cannot assure survival. In some victims, the underlying problem
causing the cardiac arrest is simply not survivable despite any available care.
Philips Medical Systems
Page 6

About this edition
The information in this guide applies to the model
M5066A HeartStart OnSite Defibrillator. Its
technical contents apply to all models in the
HeartStart HS1 family of defibrillators. This
information is subject to change. Please contact
Philips at www.medical.philips.com/heartstart or
1.800.263.3342 for information on revisions.
Edition history
Edition 7
Publication date: January 2007
Publication #: M5066-91900
Assembly #: 011666-0007
Printed in the U.S.A.
Copyright
© 2007 Philips Electronics North America Corp.
No part of this publication may be reproduced,
transmitted, transcribed, stored in a retrieval
system or translated into any human or computer
language in any form by any means without the
consent of the copyright holder.
Unauthorized copying of this publication may not
only infringe copyright but also reduce the ability
of Philips Medical Systems to provide accurate and
up-to-date information to users and operators
alike.
Authorized EU representative
Philips Medizin Systeme Boeblingen GmbH
Hewlett-Packard Strasse 2
71034 Boeblingen, Germany
(+49) 7031 463-2254
Caution
The Philips HeartStart OnSite Defibrillator is
designed to be used only with Philips-approved
accessories. The HeartStart may perform
improperly if non-approved accessories are used.
Device tracking
In the U.S.A., this device is subject to tracking
requirements by the manufacturer and distributors.
If the defibrillator has been sold, donated, lost,
stolen, exported, or destroyed, notify Philips Medical
Systems or your distributor.
Device manufacturer
The HeartStart OnSite Defibrillator is manufactured
by Philips Medical Systems, Seattle, Washington,
USA.
Patents
This product is manufactured and sold under one
or more of the following United States patents:
US6047212, US6317635, US5892046, US5891049,
US6356785, US5650750, US6553257, US5902249,
US6287328, US6662056, US5617853, US5951598,
US6272385, US6234816, US6346014, US6230054,
US6299574, US5607454, US5803927, US5735879,
US5749905, US5601612, US6441582, US5889388,
US5773961, US6016059, US6075369, US5904707,
US5868792, US5899926, US5879374, US5632280,
US5800460, US6185458, US5611815, US6556864,
US5607454, and other patents pending.
Philips Medical Systems
HEARTSTART ONSITE M5066A
Page 7

Philips Medical Systems
CONTENTS
1 Introduction to the HeartStart OnSite
Description ........................................................................................................... 1-1
Sudden Cardiac Arrest ....................................................................................... 1-1
Indications for Use .............................................................................................. 1-2
Training and practice .......................................................................................... 1-2
State and local requirements ............................................................................ 1-2
For more information ......................................................................................... 1-3
2 Setting up the HeartStart OnSite
Package contents ................................................................................................. 2-1
Setting up the OnSite ......................................................................................... 2-1
Recommended accessories ............................................................................... 2-4
3 Using the HeartStart OnSite
Overview ............................................................................................................... 3-1
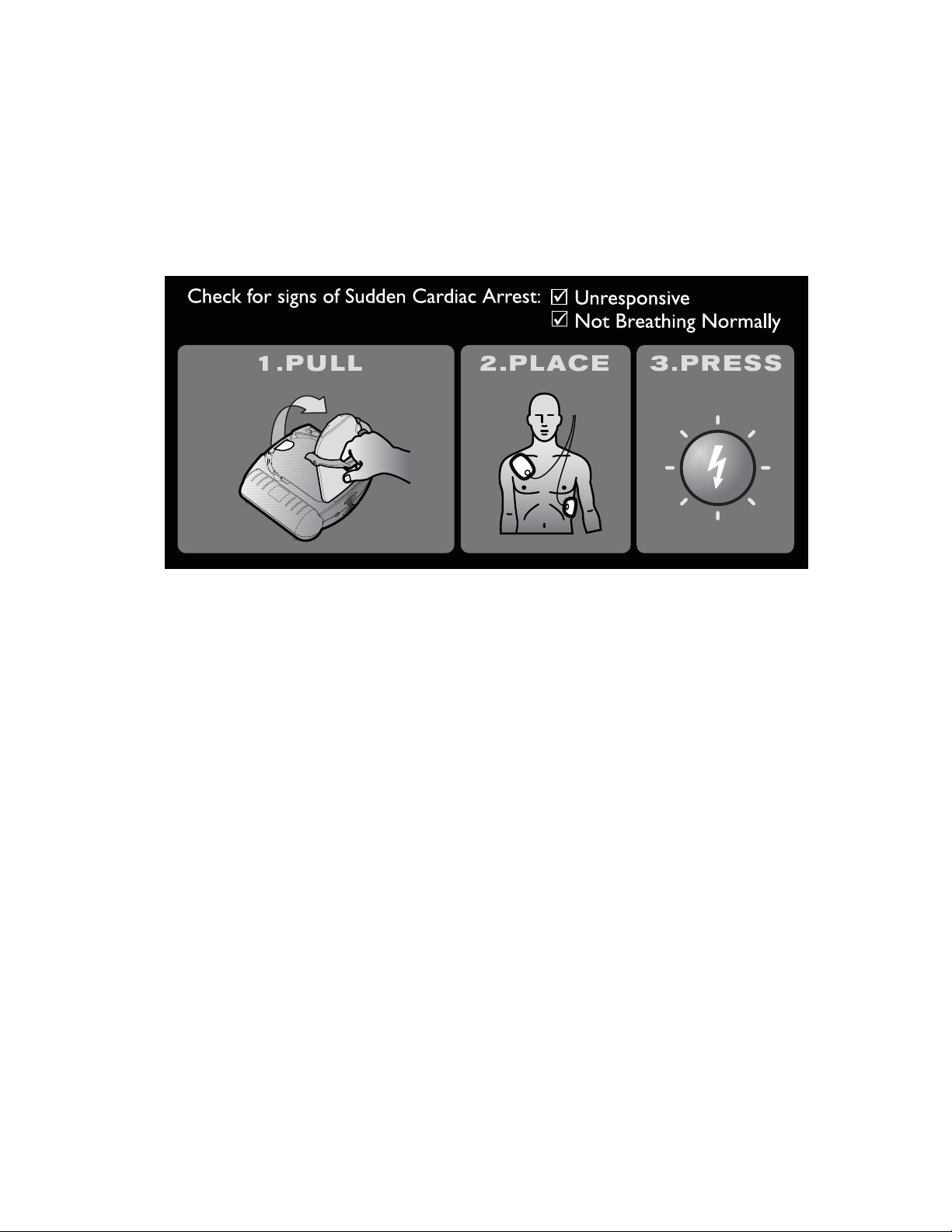
STEP 1: PULL the green handle ........................................................................ 3-2
STEP 2: PLACE the pads .................................................................................... 3-3
STEP 3: PRESS the Shock button ..................................................................... 3-4
Treating infants and children ............................................................................ 3-5
When emergency medical services arrive ..................................................... 3-6
4 After using the HeartStart OnSite
After each use ...................................................................................................... 4-1
OnSite data storage ............................................................................................ 4-2
5 Maintaining the HeartStart OnSite
Routine Maintenance .......................................................................................... 5-1
Periodic checks .................................................................................................... 5-1
Cleaning the OnSite ............................................................................................ 5-2
Disposing of the OnSite ..................................................................................... 5-2
Troubleshooting tips ........................................................................................... 5-2
i
Page 8

APPENDICES
A Accessories for the HeartStart OnSite
B Glossary of terms
C glossary of symbols/controls
D Warnings and precautions
E Technical information
F Configuration
G Testing and troubleshooting
H Additional technical information required for European conformity
ii
Philips Medical Systems
HEARTSTART ONSITE M5066A
Page 9

1
1 Introduction to the HeartStart OnSite
Description
The Philips HeartStart OnSite Defibrillator M5066A (“OnSite”) is
part of the Philips HeartStart HS1 family of defibrillators. Small,
lightweight, and battery powered, it is designed for simple and
reliable operation.
Sudden Cardiac Arrest
The OnSite is used to treat the most common causes of sudden
cardiac arrest (SCA), including ventricular fibrillation (VF). SCA is a
condition that occurs when the heart unexpectedly stops pumping.
SCA can occur to anyone – infant, child, adult, male or female –
anywhere, at any time. Many victims of SCA do not have warning
Philips Medical Systems
signs or symptoms.
VF is a chaotic quivering of the heart muscle that prevents it
from pumping blood. The only effective treatment for VF is
defibrillation.The OnSite treats VF by sending a shock across the
heart, so it can start beating regularly again. Unless this is successful
within the first few minutes after the heart stops beating, the victim
is not likely to survive.
1-1
Page 10

1-2
Indications for Use
The OnSite should be used to treat someone you think may be a
victim of SCA. A person in SCA:
• does not respond when shaken, and
• is not breathing normally.
If in doubt, apply the pads. Follow the voice instructions for each
step in using the defibrillator.
Training and practice
The OnSite is one part of a well-designed emergency response plan.
Any emergency response plan should be under the oversight of a
physician and should include training in cardiopulmonary
resuscitation (CPR). Philips recommends that you train on the
device you will be using.
Several national and local organizations offer combined CPR/
defibrillator training. Contact your Philips representative, or visit us
on-line at www.medical.philips.com, for information about training
programs in your area.
Philips Medical Systems
HEARTSTART ONSITE M5066A
NOTE: Training accessories are available from Philips for
practicing use of the OnSite. See Appendix A for information on
ordering accessories.
State and local requirements
Check with your state health department to see if there are any
local or state requirements about owning and using a defibrillator.
Page 11

1-3
For more information
Contact your local Philips representative for additional information
about the OnSite. They will be happy to answer any questions you
may have and to provide you with copies of the clinical summaries of
several key studies using Philips automated external defibrillators.
You can also find the clinical summaries online at www.medical.
philips.com/heartstart. Technical information about all Philips
HeartStart automated external defibrillators is also available online,
in the Technical Reference Guide for HeartStart Defibrillators.
*
1
Philips Medical Systems
* Clinical summaries also include Heartstream ForeRunner and FR2
Defibrillators.
Page 12

Notes
Philips Medical Systems
HEARTSTART ONSITE M5066A
Page 13

2 Setting up the HeartStart OnSite
Package contents
2
Check the contents of the HeartStart OnSite Defibrillator M5066A
box to be sure it contains:
• 1 HeartStart OnSite Defibrillator
• 1 battery M5070A
• 1 Adult SMART Pads Cartridge M5071A, containing one set of
adhesive defibrillation pads
• 1 Owner’s Manual
• 1 Quick Reference Guide
• 1 Quick Start poster
Training materials and optional accessories for the HeartStart
OnSite are also available from Philips. See Appendix A for a
Philips Medical Systems
description of these items.
Setting up the OnSite
Setting-up the OnSite is simple and quick.
1. Remove the OnSite from its packaging.
2. Remove a new SMART Pads Cartridge from its package.
* To replace a used cartridge or insert a
different cartridge, first locate the latch at
the top edge of the OnSite, and slide it to
the side. The pads cartridge will be released.
Lift out the cartridge and replace as described
in steps 2 and 3.
*
2-1
Page 14

2-2
55+
l
b
s
/
25
+ k
g
55
+
lbs
/
25+ k
g
3. Insert the cartridge into the cartridge well on the front of the
OnSite. It should click into place when properly seated. The
green PULL handle should be all the way down.
NOTE: To prevent the pads’ adhesive gel from drying out, do not
open the hard cover or film seal of the cartridge until you need to
use the pads.
4. Remove the battery from its packaging. Install it in the battery
compartment on the back of the OnSite.
5. The OnSite will automatically run a self-test when the battery is
inserted. Press the Shock button when instructed. When the
self-test is over, the OnSite will report the result, and tell you to
push the green On/Off button in case of an emergency. (Do not
push the green button unless this is an actual emergency.) Then the
OnSite will turn off and go to standby mode. The green Ready
light will be blinking to show the OnSite is ready for use.
* As long as a battery is installed, turning the OnSite “off” puts it into standby
mode, which means that it is ready for use.
*
Philips Medical Systems
HEARTSTART ONSITE M5066A
Page 15

2-3
NOTE: Always store the OnSite with a pads cartridge and a
battery installed, so it will be ready to use and can perform daily
self-tests.
6. Place the OnSite in the carry case, pressing it firmly into place.
Insert the Quick Reference Guide,
window on the inside of the case.
*
face up, in the clear plastic
†
If you purchased a spare
2
SMART Pads Cartridge or an Infant/Child Pads Cartridge, place it
in the storage area in the case.
NOTE: Do not store anything in the defibrillator carry case that it
is not designed to accommodate. Store all objects in their
intended location in the case.
7. Store the OnSite in accordance with your site’s emergency
response protocol. Typically, this will be in a high-traffic area that
is easy to access, convenient for checking the Ready light
periodically, and easy to hear the alarm chirp if the battery
power gets low or the OnSite needs attention. Ideally, the
Philips Medical Systems
OnSite should be stored near a telephone, so the Emergency
Response Team or Emergency Medical Services can be alerted as
fast as possible in the event of a possible SCA. If possible, keep
the spare SMART Pads Cartridge and other accessories with the
defibrillator - in the carry case if one is used - for quick access
when needed. In general, treat the OnSite as you would any
piece of electronic equipment, such as a computer. Be sure to
store the OnSite according to its specifications. See Appendix E
* The illustration on the cover of the Quick Reference Guide is a 3-step guide to
using the HeartStart. Detailed illustrated directions are inside, for reference in
an emergency, or if you are hearing impaired or using the HeartStart where it is
hard to hear the voice instructions.
† If you do not have a carry case, put the Quick Reference Guide in the separate
clear plastic sleeve. Press the hook-and-loop strip on the sleeve onto the strip
on the back of the OnSite. Make sure the “Call 911” sticker is easy to see if
someone needs to use the OnSite in an emergency. If your Emergency Medical
Services number is not 911, use the blank sticker with the telephone symbol to
write the correct number. Then put the sticker over the “Call 911” sticker on
the front of the sleeve.
Page 16

2-4
for details. As long as a battery and a pads cartridge are installed,
the green Ready light should be blinking to show that the
HeartStart has passed its most recent self-test and is therefore
ready to use.
NOTE: If you have a training pads cartridge, it is recommended
that you store it separately from the OnSite, so the training pads
cannot be confused with the regular pads in an emergency.
Recommended accessories
It is always a good idea to have a spare battery and a spare pads set.
Other things that are useful to keep with the OnSite include:
• scissors — for cutting the victim’s clothes if needed
• disposable gloves — to protect the user
• a disposable razor — to shave the chest if hair prevents good
pads contact
• a pocket mask or face shield — to protect the user
• a towel or absorbent wipes — to dry the victim's skin for good
pads contact
Philips Medical Systems
HEARTSTART ONSITE M5066A
Philips has a Fast Response Kit with all these items. See Appendix A
for details.
If you may need to defibrillate an infant or a child under 55 pounds (25
kg) or 8 years old, it is recommended that you order the Infant/Child
SMART Pads Cartridge, available separately by prescription. When
the Infant/Child Pads Cartridge is installed in the OnSite, the OnSite
automatically reduces the defibrillation energy to an energy level
more appropriate for infants and children. In addition, if optional
CPR Coaching is selected, the OnSite provides coaching appropriate
for infants and children when the Infant/Child SMART Pads Cartridge
is installed. Directions for using the Infant/Child SMART Pads
Cartridge are provided in Chapter 3, “Using the HeartStart OnSite.”
 Loading...
Loading...