Page 1

HeartStart Defibrillator
OWNER’S MANUAL
Guide to Set Up, Operation, Maintenance, and Accessories
M5066A
Edition 7
Page 2

Intentionally blank.
Philips Medical Systems
Page 3
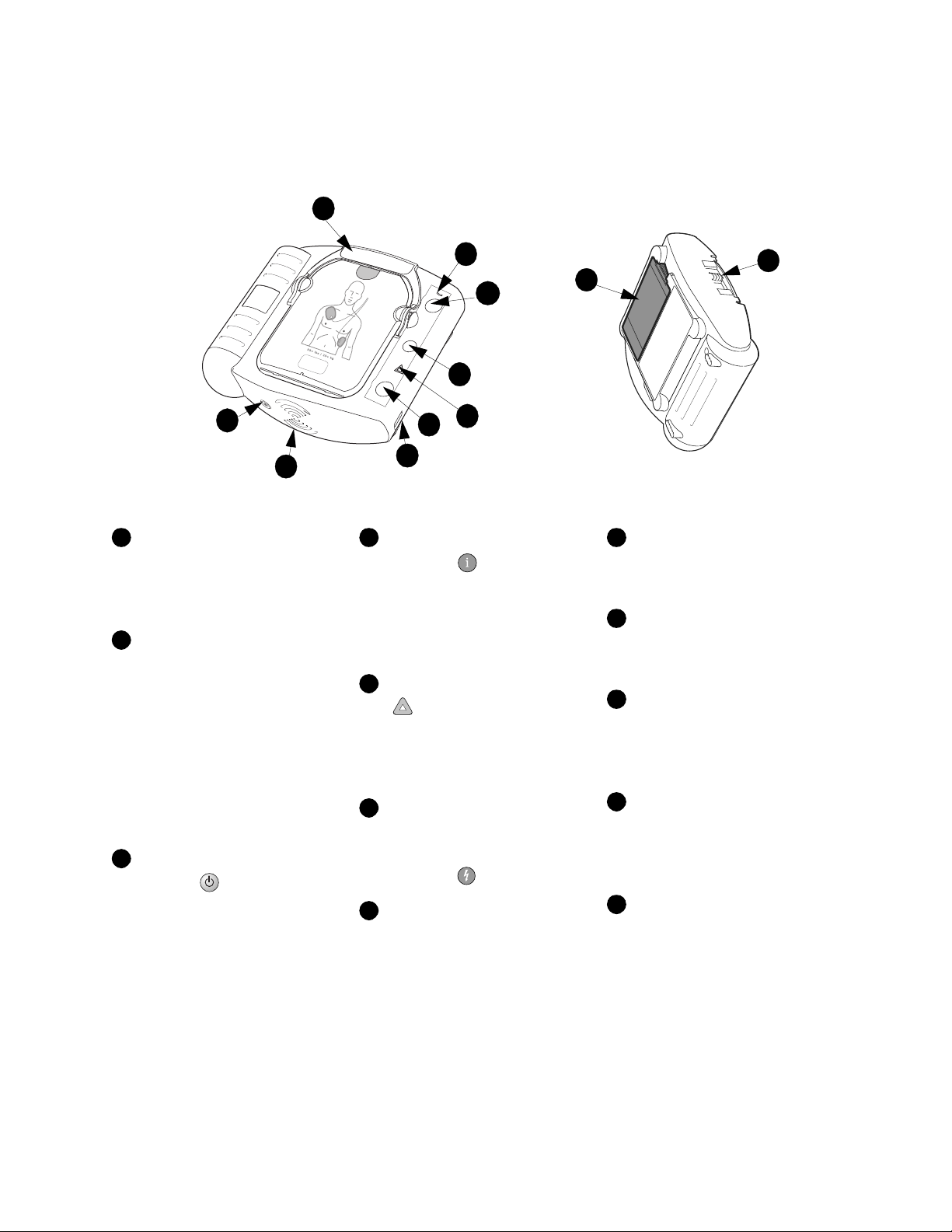
The Hear tStart Defibrillator M5066A
A
BCDEFGH
I
JKL
top
top
C
B
D
E
G
F
H
A
front view
I
B
A
back view
K
L
K
L
Pads Cartridge Handle.
Pull the handle to turn on the
HeartStart and remove the
cartridge’s hard cover.
Ready Light. This green light
Philips Medical Systems
tells you the readiness of the
HeartStart.
Blinking: standby mode
(ready for use)
Solid: in use
Off: needs attention
(HeartStart
“chirps” and
i-button flashes)
On/Off Button. Press this
green button to turn on the
HeartStart. To turn off the
HeartStart, press the green button
again and hold it down for one (1)
second.
Information Button. This
blue “i-button” flashes when it
has information you can access by
pressing it. It also flashes at the
beginning of a patient care pause
when CPR coaching is enabled.
Caution Light. This triangular
light flashes during rhythm
analysis and is on when a shock is
advised, as a reminder that no one
should be touching the patient.
Shock Button. When
instructed by the HeartStart to
deliver a shock, press this flashing
orange button .
Infrared (IR)
Communications Port.
special lens, or “eye,” is used to
transfer HeartStart data directly to
or from a computer.
This
Speaker. When the device is
being used, its voice instructions
come from this speaker.
Beeper. The HeartStart
“chirps” through this beeper to
alert you when it needs attention.
SMART Pads Cartridge. This
disposable cartridge contains selfadhesive pads with attached cable.
Shown with adult pads cartridge.
SMART Pads Cartridge
Slide the latch to the right
Latch.
to release the pads cartridge for
replacement.
Battery. The non-
rechargeable battery is inserted in
a recess on the back of the
HeartStart.
Page 4

Intentionally blank.
Philips Medical Systems
Page 5

HeartStart Defibrillator M5066A
QUICK REFERENCE
Philips Medical Systems
Page 6

Intentionally blank.
Philips Medical Systems
Page 7

HeartStart
M5066A
Automated External Defibrillator
OWNER’S MANUAL
Edition 7
IMPORTANT NOTE:
It is important to understand that survival rates for sudden cardiac arrest
are directly related to how soon victims receive treatment. For every
minute of delay, the chance of survival declines by 7% to 10%.
Defibrillation cannot assure survival. In some victims, the underlying problem
causing the cardiac arrest is simply not survivable despite any available care.
Philips Medical Systems
Page 8

Intentionally blank.
Philips Medical Systems
Page 9

About this edition
The information in this guide applies to
the model M5066A HeartStart Defibrillator. Its
technical contents apply to all models
in the HeartStart HS1 family of defibrillators,
including the HeartStart, the HeartStart OnSite,
and the HeartStart First Aid Defibrillator. This
information is subject to change. Please contact
Philips at www.medical.philips.com/heartstart or
contact your local Philips representative for
information on revisions.
Edition history
Authorized EU representative
Philips Medizin Systeme Boeblingen GmbH
Hewlett-Packard Strasse 2
71034 Boeblingen, Germany
(+49) 7031 463-2254
CAUTION: Federal law (USA) restricts this
device to sale by or on the order of a physician.
The Philips HeartStart Defibrillator is designed to
be used only with Philips-approved accessories.
The HeartStart may perform improperly if nonapproved accessories are used.
Edition 7
Publication date: July 2007
Publication #: M5066-91901
Assembly #: 011872-0007
Printed in the U.S.A.
Copyright
© 2007 Philips Electronics North America Corp.
No part of this publication may be reproduced,
transmitted, transcribed, stored in a retrieval
system or translated into any human or
computer language in any form by any means
without the consent of the copyright holder.
Unauthorized copying of this publication may
not only infringe copyright but also reduce the
ability of Philips Medical Systems to provide
accurate and up-to-date information to users
and operators alike.
Device tracking
In the U.S.A., this device is subject to tracking
requirements by the manufacturer and distributors.
If the defibrillator has been sold, donated, lost,
stolen, exported, or destroyed, notify Philips
Medical Systems or your distributor.
Device manufacturer
The HeartStart Defibrillator is manufactured by
Philips Medical Systems, Seattle, Washington, USA.
Patents
This product is manufactured and sold under one
or more of the following United States patents:
US6047212, US6317635, US5892046, US5891049,
US6356785, US5650750, US6553257, US5902249,
US6287328, US6662056, US5617853, US5951598,
US6272385, US6234816, US6346014, US6230054,
US6299574, US5607454, US5803927, US5735879,
US5749905, US5601612, US6441582, US5889388,
US5773961, US6016059, US6075369, US5904707,
US5868792, US5899926, US5879374, US5632280,
US5800460, US6185458, US5611815, US6556864,
US5607454, and other patents pending.
Philips Medical Systems
HEARTSTART M5066A
Page 10

Intentionally blank.
Philips Medical Systems
Page 11

Philips Medical Systems
CONTENTS
1 Introduction to the HeartStart
Description ........................................................................................................... 1-1
Sudden Cardiac Arrest ....................................................................................... 1-1
Indications for Use .............................................................................................. 1-1
Training and practice .......................................................................................... 1-2
National and local requirements ...................................................................... 1-2
For more information ......................................................................................... 1-2
2 Setting up the HeartStart
Package contents ................................................................................................. 2-1
Setting up the HeartStart ................................................................................... 2-1
Recommended accessories ............................................................................... 2-3
3 Using the HeartStart
Overview ............................................................................................................... 3-1
STEP 1: PULL the green handle ........................................................................ 3-2
STEP 2: PLACE the pads .................................................................................... 3-3
STEP 3: PRESS the Shock button ..................................................................... 3-3
Treating infants and children ............................................................................ 3-4
When emergency medical services arrive ..................................................... 3-6
4 After using the HeartStart
After each use ...................................................................................................... 4-1
HeartStart data storage ..................................................................................... 4-1
5 Maintaining the HeartStart
Routine Maintenance .......................................................................................... 5-1
Periodic checks .................................................................................................... 5-1
Cleaning the HeartStart ..................................................................................... 5-2
Disposing of the HeartStart .............................................................................. 5-2
Troubleshooting tips ........................................................................................... 5-2
i
Page 12

APPENDICES
A Accessories for the HeartStart
B Glossary of terms
C Glossary of symbols/controls
D Warnings and precautions
E Technical information
F Configuration
G Testing and troubleshooting
H Additional technical information required for European conformity
ii
Philips Medical System s
HEARTSTART M5066A
Page 13
1
1 Introduction to the Hear tStart
Description
The HeartStart Defibrillator M5066A is part of the Philips HeartStart HS1 family
of defibrillators. Small, lightweight, and battery powered, it is designed for simple
and reliable operation.
Sudden Cardiac Arrest
The HeartStart is used to treat the most common causes of sudden cardiac arrest
(SCA), including ventricular fibrillation (VF). SCA is a condition that occurs when
the heart unexpectedly stops pumping. SCA can occur to anyone – infant, child,
adult, male or female – anywhere, at any time. Many victims of SCA do not have
warning signs or symptoms.
VF is a chaotic quivering of the heart muscle that prevents it from pumping blood.
The only effective treatment for VF is defibrillation. The HeartStart treats VF by
sending a shock across the heart, so it can start beating regularly again. Unless this
Philips Medical Systems
is successful within the first few minutes after the heart stops beating, the victim is
not likely to survive.
Indications for Use
The HeartStart should be used to treat someone you think may be a victim of
SCA. A person in SCA:
• does not respond when shaken, and
• is not breathing normally.
If in doubt, apply the pads. Follow the voice instructions for each step in using the
defibrillator.
1-1
Page 14
1-2
Training and practice
The HeartStart is one part of a well-designed emergency response plan. Any
emergency response plan should be under the oversight of a physician and should
include training in cardiopulmonary resuscitation (CPR). Philips recommends that
you train on the device you will be using.
Several national and local organizations offer combined CPR/defibrillator training.
Contact your Philips representative, or visit us on-line at
www.medical.philips.com, for information about training programs in your area.
NOTE: Training accessories are available from Philips for practicing use of the
HeartStart. See Appendix A for information on ordering accessories.
National and local requirements
Check with your local health department to see if there are any national or local
requirements about owning and using a defibrillator.
For more information
Contact your local Philips representative for additional information about the
HeartStart. They will be happy to answer any questions you may have and to
provide you with copies of the clinical summaries of several key studies using
Philips automated external defibrillators.
*
Philips Medical Systems
HEARTSTART M5066A
You can also find the clinical summaries online at www.medical.philips.com/
heartstart. Technical information about all Philips HeartStart automated external
defibrillators is also available online, in the Technical Reference Guide for HeartStart
Defibrillators.
* Clinical summaries also include Heartstream ForeRunner and FR2 Defibrillators.
Page 15
2 Setting up the Hear tStart
Package contents
Check the contents of the HeartStart Defibrillator M5066A box to be sure it
contains:
• 1 HeartStart Defibrillator
• 1 battery M5070A
• 1 Adult SMART Pads Cartridge M5071A,
containing one set of adhesive defibrillation pads
• 1 Owner’s Manual
• 1 Quick Reference Guide
Training materials and optional accessories for the HeartStart are also available
from Philips. See Appendix A for a description of these items.
2
Philips Medical Systems
Setting up the HeartStart
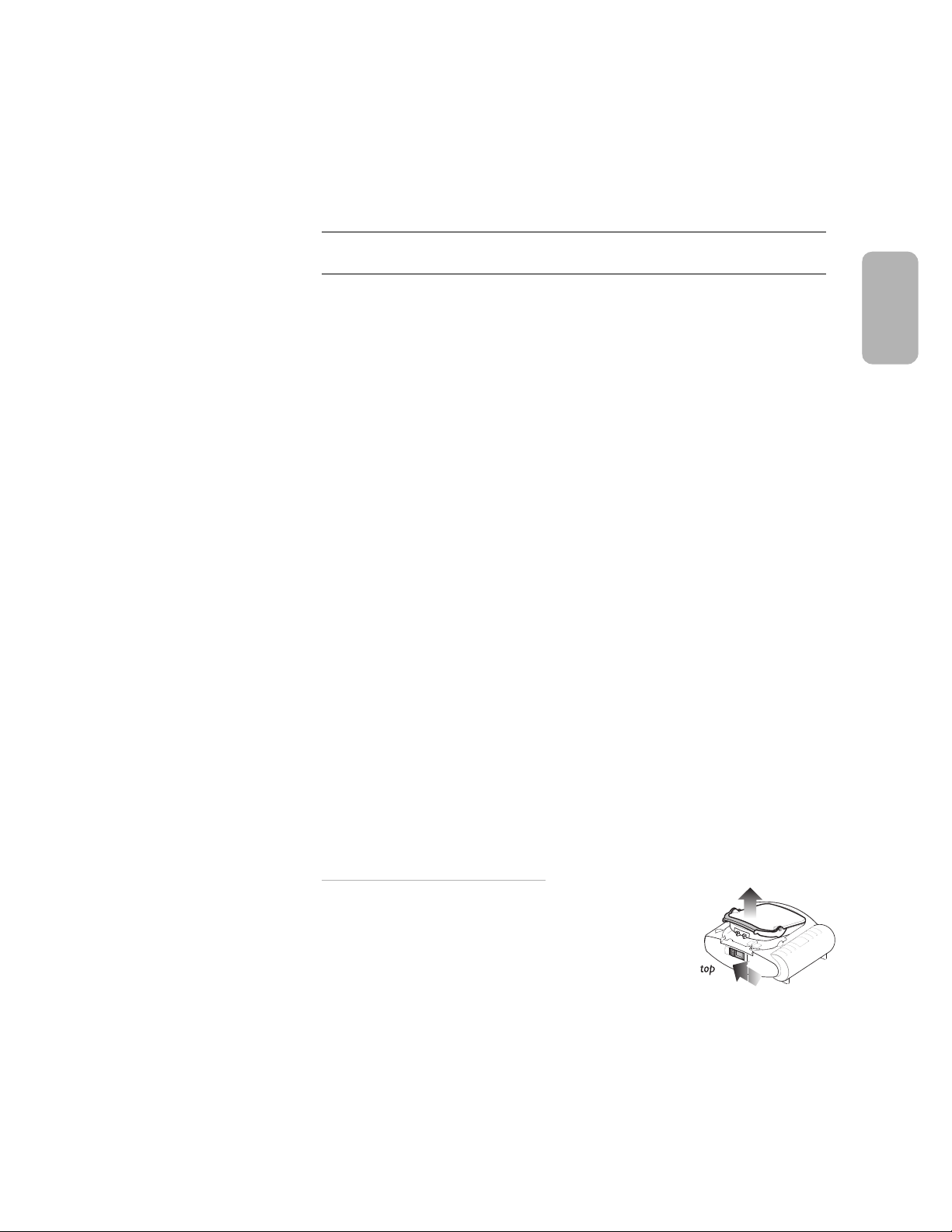
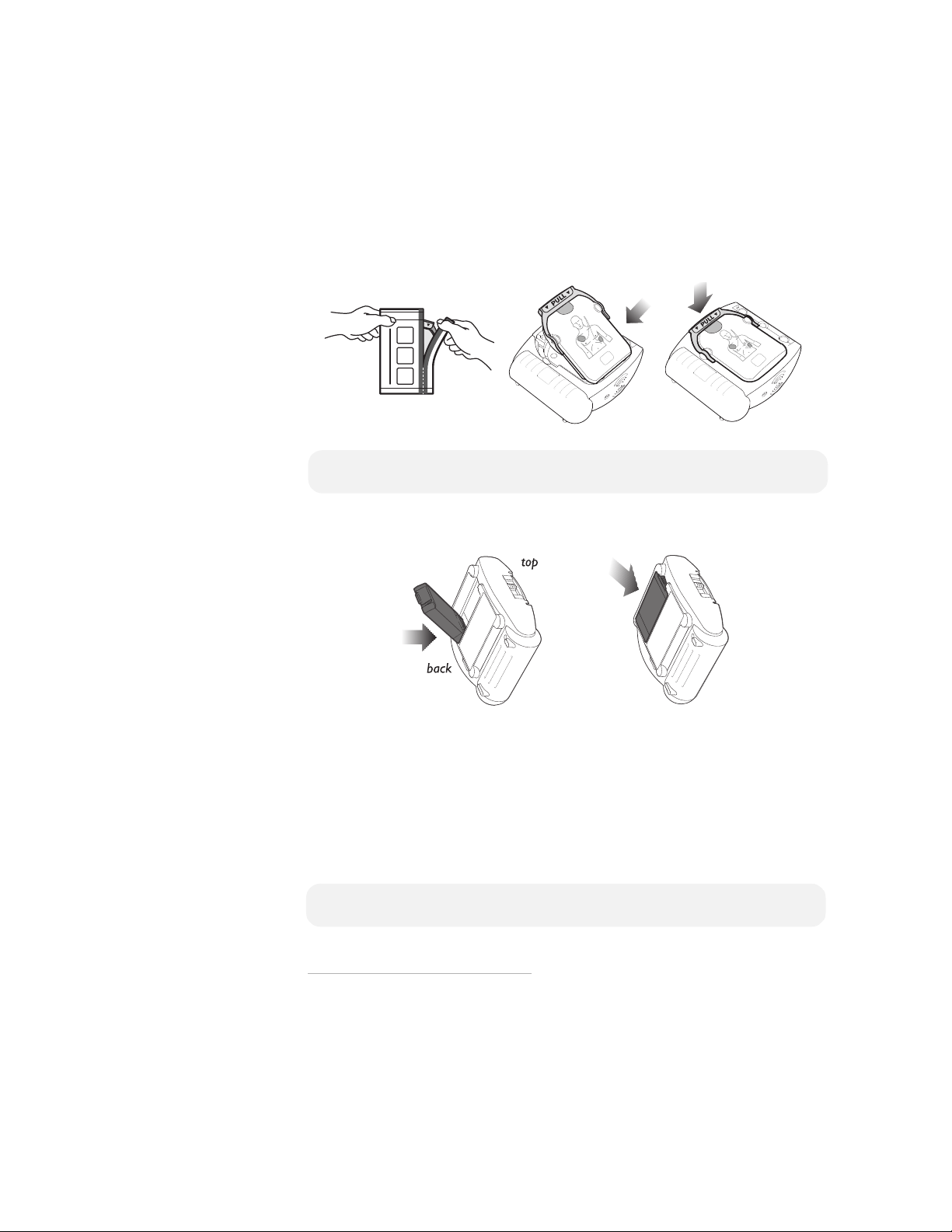
Setting-up the HeartStart Defibrillator is simple and quick.
1. Remove the HeartStart from its packaging.
2. Remove a new SMART Pads Cartridge from its package.
* To replace a used cartridge or insert a different cartridge,
first locate the latch at the top edge of the defibrillator, and
slide it to the side. The pads cartridge will be released. Lift
out the cartridge and replace as described in steps 2 and 3.
*
2-1
Page 16
2-2
55
+
l
b
s
/
25
+ kg
55+
l
b
s
/
25+ kg
3. Insert the cartridge into the cartridge well on the front of the defibrillator. It
should click into place when properly seated. The green PULL handle should
be all the way down.
NOTE: To prevent the pads’ adhesive gel from drying out, do not open the hard
cover or film seal of the cartridge until you need to use the pads.
4. Remove the battery from its packaging. Install it in the battery compartment
on the back of the defibrillator.
Philips Medical Systems
HEARTSTART M5066A
5. The HeartStart will automatically run a self-test when the battery is inserted.
Press the Shock button when instructed. When the self-test is over, the
defibrillator will report the result, and tell you to push the green On/Off
button in case of an emergency. (Do not push the green button unless this is an
actual emergency.) Then the defibrillator will turn off and go to standby mode.
The green Ready light will be blinking to show the HeartStart is ready
for use.
*
NOTE: Always store the HeartStart with a pads cartridge and a battery installed,
so it will be ready to use and can perform daily self-tests.
* As long as a battery is installed, turning the HeartStart “off” puts it into standby mode,
which means that it is ready for use.
Page 17

2-3
6. Place the defibrillator in the carry case, pressing it firmly into place. Insert the
*
Quick Reference Guide,
face up, in the clear plastic window on the inside of
the case. If you purchased a spare SMART Pads Cartridge or an Infant/Child
Pads Cartridge, place it in the storage area in the case.
2
NOTE: Do not store anything in the defibrillator carry case that it is not
designed to accommodate. Store all objects in their intended location in the
case.
7. Store the HeartStart in accordance with your site’s emergency response
protocol. Typically, this will be in a high-traffic area that is easy to access,
convenient for checking the Ready light periodically, and easy to hear the
alarm chirp if the battery power gets low or the defibrillator needs attention.
Ideally, the HeartStart should be stored near a telephone, so the Emergency
Response Team or Emergency Medical Services can be alerted as fast as
possible in the event of a possible SCA. If possible, keep the spare SMART
Pads Cartridge and other accessories with the defibrillator
– in the carry case
if one is used – for quick access when needed. In general, treat the HeartStart
as you would any piece of electronic equipment, such as a computer. Be sure
to store the defibrillator according to its specifications. See Appendix E for
details. As long as a battery and a pads cartridge are installed, the green Ready
Philips Medical Systems
light should be blinking to show that the HeartStart has passed its most
recent self-test and is therefore ready to use.
NOTE: If you have a training pads cartridge, it is recommended that you
store it separately from the HeartStart, so the training pads cannot be confused
with the regular pads in an emergency.
* The illustration on the cover of the Quick Reference Guide is a 3-step guide to using the
HeartStart. Detailed illustrated directions are inside, for reference in an emergency, or if
you are hearing impaired or using the HeartStart where it is hard to hear the voice
instructions.
Page 18

2-4
Recommended accessories
It is always a good idea to have a spare battery and a spare pads set. Other things
that are useful to keep with the HeartStart include:
• scissors — for cutting the victim’s clothes if needed
• disposable gloves — to protect the user
• a disposable razor — to shave the chest if hair prevents good pads
contact
• a pocket mask or face shield — to protect the user
• a towel or absorbent wipes — to dry the victim's skin for good pads
contact
Philips has a Fast Response Kit with all these items. See Appendix A for details.
If you may need to defibrillate an infant or a child under 25 kg (55 pounds) or 8 years
old, it is recommended that you order the Infant/Child SMART Pads Cartridge,
available separately. When the Infant/Child Pads Cartridge is installed in the
HeartStart, the HeartStart automatically reduces the defibrillation energy to an
energy level more appropriate for infants and children. In addition, if optional CPR
coaching is selected, the HeartStart provides coaching appropriate for infants and
children. Directions for using the Infant/Child SMART Pads are provided in
Chapter 3, “Using the HeartStart.”
Philips Medical Systems
HEARTSTART M5066A
Page 19
3 Using the Hear tStar t
IMPORTANT NOTE: Be sure to read the Reminders section at the end of this
chapter as well as the warnings and precautions in Appendix D.
Overview
If you think someone is in SCA, act quickly and calmly. If someone else is available,
ask him or her to call for emergency medical assistance while you get the
HeartStart. If you are alone, follow these steps:
• Call your emergency services provider.
• Quickly get the HeartStart and bring it to the victim’s side. If there is any
delay in getting the defibrillator, check the patient and perform
cardiopulmonary resuscitation (CPR) if needed until the HeartStart is
available.
• If the patient is an infant or child, first perform CPR, then call for
emergency medical services (EMS) before you apply the HeartStart. See
Philips Medical Systems
There are three basic steps to using the defibrillator to treat someone who may be
in sudden cardiac arrest:
special section on treating infants and children on page 3-5.
• Check the immediate environment for flammable gases. Do not use the
HeartStart in the presence of flammable gases, such as an oxygen tent.
However, it is safe to use the HeartStart on someone wearing an oxygen
mask.
1. PULL up the handle on the SMART Pads Cartridge.
2. PLACE the pads on the patient’s bare skin.
3. PRESS the flashing Shock button if instructed.
3
The following pages provide details about each step.
3-1
Page 20
3-2
55+
l
b
s
/
25+
k
g
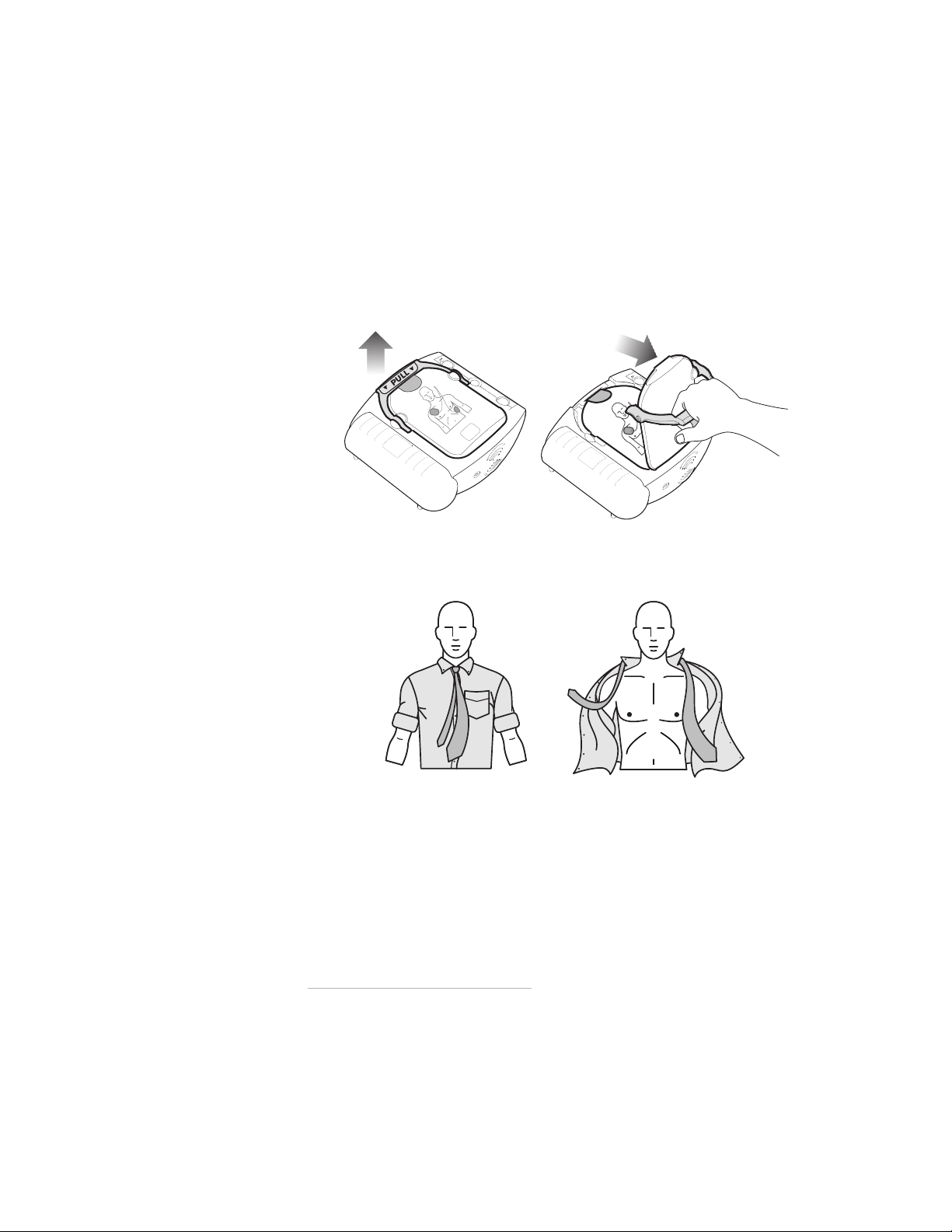
STEP 1: PULL the green handle
Turn on the HeartStart by pulling the SMART Pads Cartridge’s green handle.*
Remove the hard cover from the pads cartridge and set it aside. Remain calm and
follow the HeartStart’s instructions.
The HeartStart starts by directing you to remove all clothes from the patient’s
chest. If necessary, rip or cut off the clothing to bare the person’s chest
* You can also turn on the HeartStart Defibrillator by pressing the green On/Off button.
Philips Medical Systems
HEARTSTART M5066A
Page 21
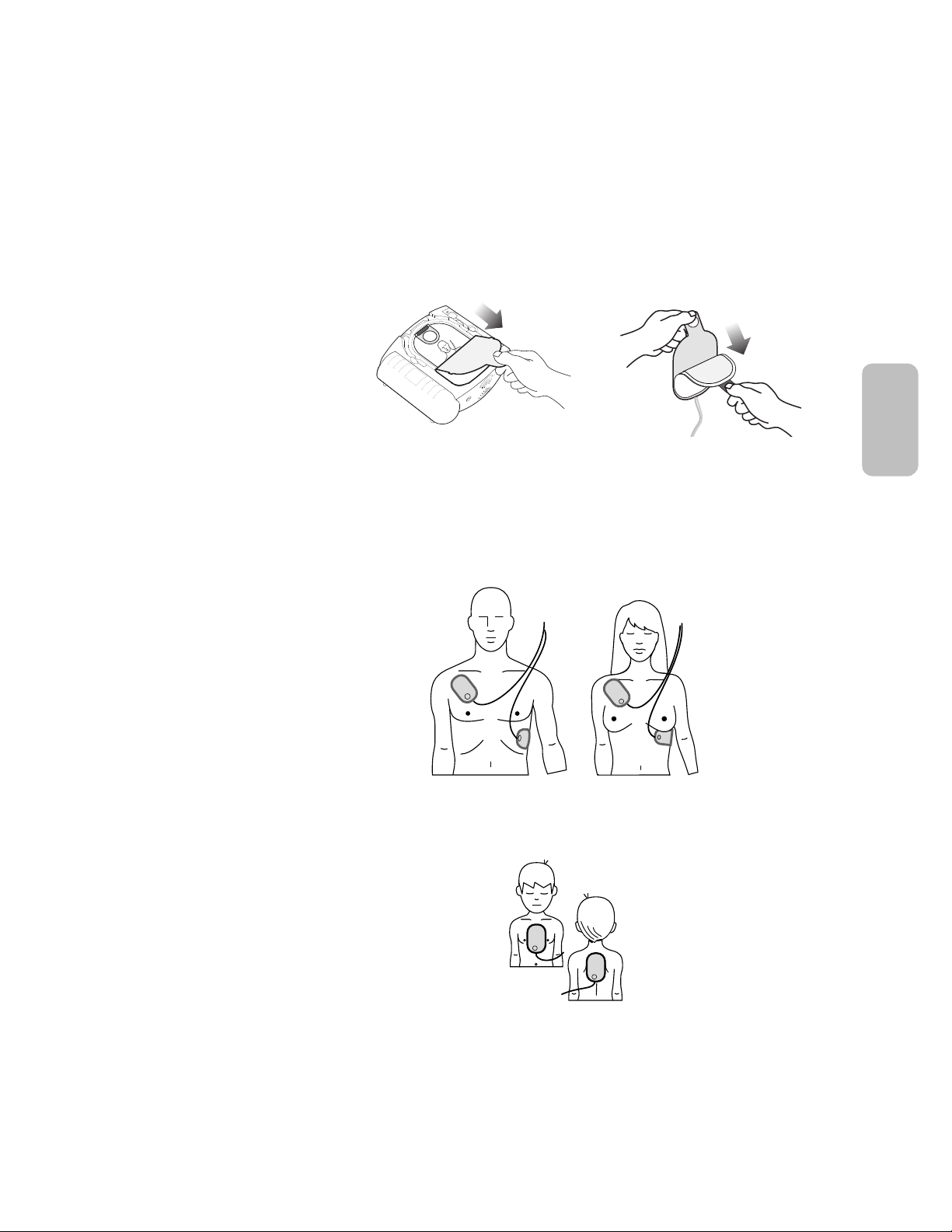
3-3
Where to place pads on adults and children over
25 kg/55 pounds or 8 years old (anterior-anterior).
Where to place pads on infants or children under
25 kg/55 pounds or 8 years old (anterior-posterior).
STEP 2: PLACE the pads
Pull the tab at the top of the pads cartridge to peel off the film seal. Inside are two
adhesive pads on a plastic liner. Remove the pads from the cartridge.
Peel one pad off the liner. Place the pad on the patient’s bare skin, exactly as shown
in the picture on the pad. Press the pad down firmly. Then repeat this with the other
pad. Be sure the pads have been removed from the liner before placing them.
3
Philips Medical Systems
Page 22

3-4
STEP 3: PRESS the Shock button
As soon as the HeartStart detects that the pads are attached to the patient, it
begins analyzing the patient’s heart rhythm. It tells you that no one should be
touching the patient, and the Caution light begins flashing as a reminder.
If a shock is needed:
The Caution light goes from flashing to solid, the orange Shock button
starts flashing, and the defibrillator tells you to press the flashing orange button.
Before you press the button, make sure no one is touching the patient. When you
press the Shock button, the defibrillator tells you that the shock has been
delivered. Then the HeartStart tells you it is safe to touch the patient, instructs
you to begin CPR, and invites you to press the flashing blue i-button for CPR
Coaching if desired.
If a shock is not needed:
The HeartStart tells you it is safe to touch the patient and instructs you to
perform CPR if needed. (If CPR is not needed – for example, if the patient is
moving or regaining consciousness – follow your local protocol until emergency
medical personnel arrive.) Then the HeartStart invites you to press the flashing
blue i-button for CPR Coaching, if desired.
Philips Medical Systems
HEARTSTART M5066A
For CPR Coaching:
Press the flashing blue i-button
pause to activate CPR Coaching.
during the first 30 seconds of the patient care
*
(If the Infant/Child SMART Pads Cartridge is
inserted, CPR Coaching will provide coaching for infant/child CPR.) When the
pause is over, the defibrillator tells you to stop CPR, so it can analyze the patient’s
heart rhythm. The motion caused by CPR can interfere with analysis, so be sure to
stop all motion when instructed.
* The default configuration for the HeartStart provides CPR Coaching when you press the
i-button in this situation; however, the default setting can be revised by your Medical
Director using Philips software available separately. See Appendix F for more
information.
Page 23

3-5
Treating infants and children
WARNING: Most cardiac arrests in children are not caused by heart problems.
When responding to cardiac arrest in an infant or child:
• Provide infant/child CPR while a bystander calls EMS and brings the
HeartStart.
• If no bystander is available, provide 1-2 minutes of CPR before calling EMS
and retrieving the HeartStart.
• If you witnessed the child's collapse, call EMS immediately and then get the
HeartStart.
Alternatively, follow your local protocol.
If the patient is under 55 pounds or 8 years old, and you have an Infant/Child Pads
Cartridge:
• Remove the Infant/Child Pads Cartridge from its package.
*
• Locate the latch at the top edge of the defibrillator, and slide it to the
side. The pads cartridge will be released. Remove the old cartridge.
• Install the new cartridge: slide the bottom end of the cartridge into the
recess, then press in the cartridge until the latch clicks into place. Be sure
the green handle is pressed down firmly. The HeartStart will tell you that
Philips Medical Systems
Infant/Child pads have been inserted, then it will turn off to be ready for
use.
• Pull the green handle to start the rescue.
3
• Remove all clothing from the upper body, to bare both the chest and the
back. Place one pad in the center of the chest between the nipples, and
the other in the center of the back (anterior-posterior).
With the Infant/Child Pads Cartridge inserted, the HeartStart automatically
†
reduces the defibrillation energy from the adult dose of 150 joules to 50 Joules
and provides optional infant/child CPR Coaching. Place the pads exactly as shown
on the illustration on the pads.
* Philips recommends that the HeartStart be stored with an adult pads cartridge installed,
as pediatric cardiac arrest is not common.
† This lower energy level may not be effective for treating an adult.
Page 24

3-6
If the patient is under 55 pounds or 8 years old, but you do NOT have an Infant/Child
Pads Cartridge:
• DO NOT DELAY TREATMENT.
• Remove all clothing from the torso, to bare both the chest and the back.
• Apply the HeartStart using the adult pads cartridge, but place one pad in the
center of the chest between the nipples, and the other in the center of the
back (anterior-posterior).
If the patient is over 55 pounds or 8 years old, or if you are not sure of the exact weight
or age:
• DO NOT DELAY TREATMENT.
• Remove all clothing from the chest.
• Apply the HeartStart using the adult pads cartridge, and place the pads as
illustrated on the pads (anterior-anterior). Make sure the pads do not overlap
or touch each other.
When emergency medical services arrive
When Emergency Medical Services (EMS) personnel arrive to care for the patient,
they may decide to apply another defibrillator to allow monitoring of the patient.
The SMART Pads should be removed from the patient prior to using another
defibrillator. EMS personnel may want a summary of the last-use data
the HeartStart. To hear the summary data, hold down the i-button until the
HeartStart beeps.
NOTE: After the EMS team removes the SMART Pads from the patient,
remove the used pads cartridge, and insert a new pads cartridge before
returning the HeartStart to service, to be sure it is ready for use.
* See Chapter 4, “After using the HeartStart” for details about data storage.
*
stored in
Philips Medical Systems
HEARTSTART M5066A
Page 25

Reminders • Remove any medicine patches and residual adhesive from the patient’s chest
before applying the pads.
• Do not allow the pads to contact other electrodes or metal parts that are in
contact with the patient.
• Avoid placing the pads directly over an implanted pacemaker or defibrillator.
A noticeable lump with a surgical scar should indicate the position of an
implanted device.
3-7
• If the pads do not stick well, check that the pads adhesive has not dried out.
Each pad has a layer of adhesive gel. If the gel is not sticky to the touch,
replace the pads with a new set.
3
• Keep the patient still and keep any movement around the patient to a
minimum during rhythm analysis. Do not touch the patient or the pads while
the Caution light is on solid or flashing. If the HeartStart is unable to analyze
due to electrical “noise” (artifact), it will tell you to stop all movement and
remind you not to touch the patient. If the artifact continues for more than
30 seconds, the HeartStart will pause briefly to allow you to deal with the
source of the noise, then resume analysis.
• The HeartStart will not deliver a shock unless you press the flashing orange
Philips Medical Systems
Shock button. If you do not press the Shock button within 30 seconds after
the defibrillator tells you to, it will disarm itself, and (for the first CPR
interval) give a reminder to make sure emergency medical services have been
called, then begin a CPR interval. This is designed to minimize interruption of
CPR and help ensure ongoing patient support.
• While waiting for you to press the Shock button, the HeartStart will
continue to analyze the heart rhythm. If the patient’s rhythm changes before
you press the Shock button, and a shock is no longer needed, the
defibrillator will disarm and tell you a shock is not advised.
• If for any reason you want to turn off the defibrillator during a use, you can
press the On/Off button – holding it down for at least one second – to
return the device to standby mode.
Page 26

Notes
Philips Medical Systems
HEARTSTART M5066A
Page 27

4 After using the HeartStart
After each use
1. Check the outside of the HeartStart for signs of damage, dirt, or
contamination. If you see signs of damage, contact Philips for technical
support. If the defibrillator is dirty or contaminated, clean it according to the
guidelines in Chapter 5, “Maintaining the HeartStart.”
2. Insert a new SMART Pads cartridge into the HeartStart. Check supplies and
accessories for damage and expiration dates. Replace any used, damaged or
expired items. For directions on changing the pads and replacing the battery,
please see Chapter 2, “Setting up the HeartStart.” The single-use pads must
be replaced after being used.
3. Unless your protocol requires that the battery remain installed, remove the
battery for five seconds, then reinstall it to run the battery insertion self-test
to check the operation of the defibrillator.
that the green Ready light is blinking.
4. Return the HeartStart to its storage location so it will be ready for use when
Philips Medical Systems
needed.
*
When the test is complete, check
4
HeartStart data storage
The HeartStart automatically stores data about its last clinical use in its internal
memory. The stored data can be conveniently transferred to a personal computer
or a handheld computer running the appropriate application in the Philips
HeartStart Event Review data management software suite. Event Review software
is for use by trained personnel only. Information about HeartStart Event Review is
available online at www.medical.philips.com/goto/eventreview.
* If you leave the battery in the HeartStart after using the defibrillator, then transfer the
last-use data to a computer running HeartStart Event Review software, the software will
calculate the local date and time of the device use. However, if you remove the battery
prior to transferring the data, the software will only show elapsed time.
4-1
Page 28

4-2
Follow your local protocol with regard to prompt data transfer for medical review
*
after using the HeartStart.
Details about data transfer and timing are provided in
Event Review documentation.
The information automatically stored by the HeartStart includes a summary of
last-use data and detailed data about its last clinical use. You can get a voice
summary of information about the last use of the defibrillator by holding the ibutton down until it beeps once. The HeartStart will tell you how many shocks
were delivered and how long it has been since it was turned on. Summary data are
available anytime the defibrillator is ready for use (the battery and pads are
installed, and the defibrillator is not turned on) or while it is actually in use.
Removing the battery erases the summary data for the last use.
Last-use data stored in internal memory include:
†
• ECG recordings (a maximum of 15 minutes following pads application
)
• the HeartStart’s status (entire incident)
• the HeartStart’s rhythm analysis decisions (entire incident)
• the elapsed time associated with stored events (entire incident)
Philips Medical Systems
HEARTSTART M5066A
* The HeartStart automatically stores information about its last clinical use in its internal
memory for at least 30 days, so the data can be downloaded to a computer running
appropriate Event Review software. (If the battery is removed during this period, the
defibrillator retains the files. When the battery is reinstalled, the last-use ECG recording
will be kept in defibrillator memory for an additional 30 days.) After this time, the lastuse ECG recordings will automatically be erased to prepare for a future use.
† If ECG recordings from a previous use have not been erased, the maximum time for new
ECG recordings may be less.
Page 29

5 Maintaining the HeartStart
Routine Maintenance
The HeartStart is very simple to maintain. The defibrillator performs a self-test
every day. In addition, a battery insertion self-test is run whenever a battery is
installed in the device. The defibrillator’s extensive automatic self-test features
eliminate the need for any manual calibration. The HeartStart has no userserviceable parts.
WARNING: Electrical shock hazard. Do not open the HeartStart, remove its
covers, or attempt repair. There are no user-serviceable components in the
HeartStart. If repair is required, return the HeartStart to Philips for service.
Reminders:
• Do not leave the HeartStart without a pads cartridge installed; the
defibrillator will start chirping and the i-button will start flashing. For
directions on changing the pads cartridge, see Chapter 2, “Setting up the
HeartStart.”
Philips Medical Systems
• The HeartStart runs daily self-tests. As long as the green Ready light is
blinking, it is not necessary to test the defibrillator by initiating a battery
insertion self-test. This uses battery power and risks draining the battery
prematurely.
5
Periodic checks
Other than the checks recommended after each use of the HeartStart,
maintenance is limited to periodically checking the following:
• Check the green Ready light. If the green Ready light is not blinking, see
Troubleshooting Tips, below.
• Replace any used, damaged or expired supplies and accessories
• Check the outside of the defibrillator. If you see cracks or other signs of
damage, contact Philips for technical support.
5-1
Page 30

5-2
Cleaning the HeartStart
The outside of the HeartStart and its carry case can be cleaned with a soft cloth
dampened in soapy water, chlorine bleach (2 tablespoons per quart or liter of
water), or ammonia-based cleaners.
Reminders:
• Do not use isopropyl (rubbing) alcohol, strong solvents such as acetone or
acetone-based cleaners, abrasive materials, or enzymatic cleaners to clean
your HeartStart.
• Do not immerse the HeartStart in fluids or allow fluids to spill onto it.
Do not sterilize the defibrillator or its accessories.
Disposing of the HeartStart
The HeartStart and its accessories should be disposed of in accordance with local
regulations.
Troubleshooting tips
The HeartStart’s green Ready light is your guide to knowing if the defibrillator is
ready for use.
Philips Medical Systems
HEARTSTART M5066A
• If the Ready light is blinking: The HeartStart has passed the battery
insertion self-test and the last periodic self-test and is therefore ready
for use.
• If the Ready light is solid: The HeartStart is in use or running a self-test.
• If the Ready light is off, the HeartStart is chirping, and the i-button is
flashing: A self-test error has occurred, there is a problem with the pads
or the battery power is low. Press the i-button for instructions.
• If the Ready light is off but the HeartStart is not chirping and the i-button
is not flashing: there is no battery inserted, the battery is depleted, or the
defibrillator needs repair. Insert/replace battery and run the self-test. As
long as the HeartStart passes the self-test, you can be assured it is ready
for use.
More detailed testing and troubleshooting information is available in
Appendix G.
Page 31

A
A Accessories for the Hear tStar t
Accessories* for the HeartStart Defibrillator available separately from your Philips
representative or on-line at www.medical.philips.com/heartstart include:
• Battery (spare recommended) [REF: M5070A]
•Pads
• Adult SMART Pads Cartridge (spare recommended) [REF: M5071A]
• Infant/Child SMART Pads Cartridge [REF: M5072A]
• Carry Cases
• Standard carry case, with paramedic’s scissors and room for spare pad
cartridge and battery [REF: M5075A]
• Slim carry case, with paramedic’s scissors [REF: M5076A]
• Plastic waterproof hardshell carry case [REF: YC]
• Fast Response Kit (pouch containing a pocket mask, a disposable razor, 2 pairs
of gloves, a pair of paramedic’s scissors, and an absorbent wipe)
[REF: 68-PCHAT]
• Data Management Software
Philips Medical Systems
• HeartStart Configure PDA software [REF: 989803143041]
• HeartStart CaseCapture PDA software [REF: 989803143051]
• HeartStart Review Express Connect [REF: 861311 option A01]
• HeartStart Event Review, single PC license [REF: M3834A]
• HeartStart Event Review, organization-wide license [REF: 989803141811]
• HeartStart Event Review Pro, single PC license [REF: 861276 option A01]
• HeartStart Event Review Pro, three-PC license [REF: 861276 option A02]
• HeartStart Event Review Pro, organization-wide license
[REF: 861276 option A03]
• Infrared cable for use with HeartStart Event Review software [REF: ACT-IR]
• HeartStart Defibrillator Quick Reference [REF: M5066-97800]
* Certain accessories require a prescription in the United States.
A-1
Page 32

A-2
• Training
• Adult Training Pads Cartridge [REF: M5073A]
• Adult Training Replacement Pads [REF: M5093A]
• Adult Pads Placement Guide [REF: M5090A]
• Infant/Child Training Pads Cartridge [REF: M5074A]
• Infant/Child Training Replacement Pads [REF: M5094A]
• Infant/Child Pads Placement Guide [REF: 989803139281]
• HeartStart HS1 and FR2+ Instructor's Training Toolkit, NTSC
[REF: M5066-89100] or PAL [REF: M5066-89101]
• HeartStart Trainer [REF: M5085A]
• Internal Manikin Adapter [REF: M5088A]
• External Manikin Adapter, 5 pack [REF: M5089A]
Philips Medical Systems
HEARTSTART M5066A
Page 33

BGlossary of terms
The terms listed in this Glossary are defined in the context of the Philips
HeartStart Defibrillator and its use.
AED Automated external defibrillator (a semi-automatic defibrillator).
AED mode The standard treatment mode for the HeartStart Defibrillator. It provides voice
instructions guiding the rescuer through applying the adhesive pads, waiting for
rhythm analysis, and delivering a shock if needed.
analysis See “SMART analysis.”
arrhythmia An unhealthy, often irregular, beating of the heart.
artifact Electrical “noise” caused by sources such as muscle movements, CPR, patient
transport, or static electricity that may interfere with rhythm analysis.
battery The sealed lithium manganese dioxide battery used to power the HeartStart
Defibrillator. It is provided in a pack that fits into a compartment on the back of
the defibrillator.
Caution light A triangular light on the front of the HeartStart Defibrillator that flashes during
rhythm analysis and is on solid when a shock is advised, as a reminder not to touch
Philips Medical Systems
configuration The settings for all operating options of the HeartStart Defibrillator, including
CPR Cardiopulmonary resuscitation. A technique for providing artificial respiration and
the patient.
treatment protocol. The factory default configuration can be modified by
authorized personnel using HeartStart Event Review software.
heart compressions.
B
CPR Coaching Basic verbal instructions for performing cardiopulmonary resuscitation, including
hand placement, rescue breathing, compression depth and timing, provided by the
HeartStart when the flashing blue i-button is pressed during the first 30 seconds of
a patient care pause.
defibrillation Termination of cardiac fibrillation by applying electrical energy.
ECG Electrocardiogram, a record of the electrical rhythm of the heart as detected
through defibrillation pads.
B-1
Page 34

B-2
fibrillation A disturbance of the normal heart rhythm that results in chaotic, disorganized
activity that cannot effectively pump blood. Ventricular fibrillation (fibrillation in
the lower chambers of the heart) is associated with sudden cardiac arrest.
HeartStart Event Review A suite of data management software applications for use by trained personnel to
review and analyze HeartStart Defibrillator patient use and by authorized
personnel to alter HeartStart configuration. Information is available from Philips
Medical Systems on the internet at http://www.medical.philips.com/goto/
eventreview.
i-button A blue “information” button on the front of the HeartStart Defibrillator. If the
i-button is pressed during the 30 seconds it flashes during a patient care pause, the
HeartStart provides CPR Coaching;
*
if the i-button is pressed when it is flashing
and the HeartStart is chirping, the HeartStart provides troubleshooting guidance.
At other times, if the i-button is pressed and held until it beeps once, the
HeartStart provides summary information about its last clinical use and device
status. When the i-button is on solid (not flashing), it indicates the user may safely
touch the patient.
infrared
communications
A method of sending information using a special part of the light spectrum. It is
used to transmit information between the HeartStart Defibrillator and a computer
running HeartStart Event Review software.
NSA “No Shock Advised,” a decision made by the HeartStart Defibrillator that a shock
is not needed, based on analysis of the patient’s heart rhythm.
NSA pause A pause provided by the HeartStart Defibrillator following an NSA decision. The
pause can be configured to a “standard” NSA pause or a “SMART” NSA pause.
During a standard NSA pause the defibrillator performs no background
monitoring of patient rhythm. During a SMART NSA pause, the defibrillator
conducts background monitoring and, if it detects an artifact-free shockable
rhythm, will exit the pause and begin rhythm analysis. If the HeartStart detects
artifact such as that created by CPR, or if the user presses the i-button for CPR
Coaching during a SMART NSA pause, the defibrillator will not exit the pause for
rhythm analysis in order to allow CPR to be completed uninterrupted.
non-shockable rhythm A heart rhythm that the HeartStart Defibrillator determines is not appropriate for
defibrillation.
On/Off button A green button located on the front of the HeartStart Defibrillator. Pressing the
On/Off button when the defibrillator is in standby mode turns the defibrillator on;
pressing and holding the On/Off button for one second when the defibrillator is on
turns the defibrillator off and disarms the defibrillator. In addition, pressing the
* Pressing the i-button for CPR Coaching during a SMART NSA pause turns off
background monitoring.
HEARTSTART M5066A
Philips Medical Systems
Page 35

On/Off button stops the battery insertion self-test that automatically runs when a
battery is inserted.
pads See “SMART pads.”
B-3
patient care pause A defined pause to allow patient assessment, treatment, and/or CPR. See
“NSA pause” and “protocol pause.”
periodic self-tests Daily, weekly, and monthly tests automatically conducted by the HeartStart
Defibrillator when it is in its standby mode. The tests monitor many key functions
and parameters of the defibrillator, including battery capacity, pads cartridge
readiness, and the state of its internal circuitry.
protocol A sequence of operations performed by the HeartStart Defibrillator to direct
patient care in the AED mode.
protocol pause A pause provided by the HeartStart Defibrillator after a shock series, during which
the responder can administer CPR. The defibrillator does not conduct background
monitoring of the patient’s heart rhythm during this pause.
Ready light A green LED showing the readiness for use of the HeartStart Defibrillator. A
blinking Ready light means the defibrillator is ready for use; a solid Ready light
means the defibrillator is being used.
rhythm analysis See “SMART analysis.”
Shock button An orange button with a lightning bolt symbol on it, located on the front of the
Philips Medical Systems
shockable rhythm A heart rhythm that the HeartStart Defibrillator determines is appropriate for
HeartStart Defibrillator. The Shock button flashes when a shock is advised. You
must press the button for the shock to be delivered.
defibrillation, such as ventricular fibrillation and some ventricular tachycardias
associated with sudden cardiac arrest.
B
shock series interval A configurable interval between shocks, used by the HeartStart Defibrillator to
decide if the shocks are part of the same shock series.
SMART analysis The proprietary algorithm used by the HeartStart Defibrillator to analyze the
patient’s heart rhythm and determine whether the rhythm is shockable.
SMART biphasic
waveform
SMART NSA pause See “NSA pause.”
SMART Pads The adhesive pads, supplied in a cartridge, used with the HeartStart Defibrillator.
The patented, low-energy defibrillation shock waveform used by the HeartStart
Defibrillator. It is an impedance-compensated biphasic waveform. Used with the
Adult SMART Pads, it delivers 150 Joules, nominal, into a 50 ohm load; used with
the Infant/Child SMART Pads, it delivers 50 Joules, nominal, into a 50 ohm load.
Pulling the handle on the cartridge turns on the defibrillator and opens the
Page 36

B-4
cartridge. The pads are applied to the patient’s bare skin and used to detect the
patient’s heart rhythm and to transfer the defibrillation shock. Only HeartStart
SMART Pads can be used with the HeartStart Defibrillator.
standby mode The operating mode of the HeartStart Defibrillator when a battery has been
installed, and the unit is turned off and ready for use when needed. Shown by
blinking green READY light.
standard NSA pause See “NSA pause.”
sudden cardiac arrest
(SCA)
waveform See “SMART biphasic waveform.”
The sudden stopping of the heart’s pumping rhythm, accompanied by loss of
consciousness, absence of respiration, and lack of a pulse.
Philips Medical Systems
HEARTSTART M5066A
Page 37

C Glossary of symbols/controls
PULL
symbol description
Pads cartridge handle. Green. Pulling the handle turns on
the defibrillator and opens pads cartridge for use.
C
Refer to operating instructions.
On/Off button. Green. Pressing the On/Off button when
the defibrillator is in standby mode turns the defibrillator
on; pressing and holding the On/Off button for one
second when the defibrillator is on turns the defibrillator
off and disarms the defibrillator. In addition, pressing the
On/Off button stops the battery insertion self-test that
automatically runs when a battery is inserted.
Information button (i-button). Blue. Pressing the i-button while it is
flashing during a patient care pause provides CPR Coaching; pressing it
while it is flashing and the defibrillator is chirping provides
troubleshooting guidance. Pressing it until it beeps at other times
Philips Medical Systems
provides summary information about the defibrillator’s last clinical use
and device status.
Caution light. Flashes during rhythm analysis, and is on
but not flashing when a shock is advised, as a reminder
not to touch the patient.
Shock button. Orange. Flashes when the defibrillator is
charged. If a shock is needed, the defibrillator directs the
user to press the Shock button to deliver a shock to the
patient.
Defibrillation protection. Defibrillation protected, type
BF patient connection.
Meets the requirements of the European medical device directives 93/
42/EEC.
Indicates that this device is optimized for Guidelines 2005.
C-1
Page 38

C-2
symbol description
Certified by the Canadian Standards Association.
Reference order number.
Expiration date.
Lithium manganese dioxide battery.
One battery in package.
Do not crush the battery.
Do not expose the battery to high heat or open flames.
Do not incinerate the battery.
Do not mutilate the battery or open the battery case.
Class 9 miscellaneous dangerous goods. (Symbol
required on outer packaging by freight carrier regulations
to identify shipments containing lithium batteries.)
Install the battery in the defibrillator before the date
(MM-YYYY) shown on the associated label.
Do not expose to moisture.
Handle with care.
Philips Medical Systems
HEARTSTART M5066A
Page 39

C-3
NO N-
ST ER IL E
LA TE X
symbol description
This side up.
Transportation requirements (refer to associated thermometer
symbol).
Storage requirements (refer to associated thermometer symbol).
C
Environmental (temperature and relative humidity) requirements.
These pads are disposable and are for single patient use
only.
Cartridge contents: one set of two defibrillation pads.
Store the pads at temperatures between 0° and 43° C
Philips Medical Systems
(32° and 110° F).
This product is not sterile.
This product does not contain natural rubber latex.
Meets the requirements of the European electromagnetic compatibility
directive 89/336/EEC.
Pads intended for use on infant or child under 8 years or 55 pounds
(25 Kg).
Expiration (see associated date code).
Page 40

C-4
symbol description
Serial number.
Lot number.
Federal law (USA) restricts this device to sale by or on the order of a
physician.
Dispose of in accordance with your country's requirements.
Printed on recycled paper.
Philips Medical Systems
HEARTSTART M5066A
Page 41

D Warnings and precautions
It is important to understand how to use your HeartStart Defibrillator safely.
Please read these warnings and precautions carefully.
A warning describes something that could cause serious personal injury or death.
A precaution describes something that could cause minor personal injury,
damage to the HeartStart, loss of data stored in the HeartStart, or less chance of
successful defibrillation.
NOTE: The HeartStart Defibrillator is designed to be used only with Philipsapproved accessories. The HeartStart may perform improperly if nonapproved accessories are used.
Warnings
flammable gases If the HeartStart is used to give a shock in the presence of flammable gases such as
in an oxygen tent, there is a risk of explosion. Move supplemental oxygen and
oxygen delivery devices away from the defibrillation pads. (However, it is safe to
use the HeartStart on someone wearing an oxygen mask.)
Philips Medical Systems
patient handling Performing CPR or otherwise handling or moving the patient while the HeartStart
battery The HeartStart M5070A battery is not rechargeable. Do not try to recharge,
open, crush, or burn the battery, or it may explode or catch fire.
fluids Do not let fluids get into the HeartStart. Avoid spilling any fluids on the HeartStart
or its accessories. Spilling fluids into the HeartStart may damage it or cause a fire
or shock hazard. Do not sterilize the HeartStart or its accessories.
accessories Using damaged or expired equipment or accessories may cause the HeartStart
Defibrillator to perform improperly, and/or injure the patient or the user.
is analyzing heart rhythm can cause an incorrect or delayed analysis. If the
HeartStart tells you a shock is advised while you are handling or moving the
patient, stop the vehicle or CPR and keep the patient as still as possible for at least
15 seconds. This will give the HeartStart time to reconfirm the analysis before
telling you to press the Shock button.
cell phones The HeartStart can work correctly when it is fairly close to equipment like
emergency two-way radios and cell phones. Normally, using a cell phone near the
patient should not cause a problem for the HeartStart. However, it is best to keep
such equipment only as close as necessary to the patient and the HeartStart.
D
D-1
Page 42

D-2
pads Do not allow the pads to contact other electrodes or metal parts that are in
contact with the patient.
Precautions
device handling The HeartStart was designed to be sturdy and reliable for many different use
conditions. However, handling the HeartStart too roughly can damage it or its
accessories and will invalidate the warranty. Check the HeartStart and accessories
regularly for damage, according to directions.
maintenance Improper maintenance may damage the HeartStart or cause it to function
improperly. Maintain the HeartStart according to directions.
skin burns Do not let the pads touch each other or other electrodes, lead wires, dressings,
medicine patches, etc. Such contact can cause electrical arcing and skin burns
during a shock and may also divert the electrical current away from the patient’s
heart. During a shock, air pockets between the skin and pads can cause skin burns.
To help prevent air pockets, make sure pads stick well to the skin. Do not use
dried out pads because they will not provide good contact with the skin.
patient handling Before delivering a shock, it is important to disconnect the patient from other
medical electrical equipment, such as blood-flow meters, that may not incorporate
defibrillation protection. In addition, make sure the pads are not in contact with
metal objects such as a bed frame or stretcher.
Philips Medical Systems
HEARTSTART M5066A
Page 43

E Technical information
HeartStart Defibrillator specifications
The specifications provided in the following tables are nominal values. Additional
information can be found in the Technical Reference Manual for HeartStart
Automated External Defibrillators, located online at www.medical.philips.com.
Physical
category specifications
size 2.80” H x 7.40” D x 8.30” W (7.1cm H x 19cm D x 21cm W).
weight Approximately 3.3 lbs (1.5 kg) with battery and pads cartridge installed.
Environmental
category specifications
E
Philips Medical Systems
temperature and
relative humidity
Operating (battery and pads cartridge installed):
32° to 122° F (0° to 50° C)
0% to 95% RH (non-condensing).
Standby (battery and pads cartridge installed):
50° to 109° F (10° to 43° C)
10% to 75% RH (non-condensing).
Storage/shipping (with battery and pads cartridge):
-4° to 140° F (-20° to 60° C) for up to 2 days
0% to 85% RH (non-condensing)
altitude Operates at 0 to 15,000 feet; can be stored at up to 8,500 feet, in standby
shock/drop abuse
tolerance
vibration Operating: meets EN1789 random, road ambulance.
mode.
Withstands 1 meter drop to any edge, corner, or surface.
Standby: meets EN1789 swept sine, road ambulance.
E-1
Page 44

E-2
category specifications
sealing Drip proof per EN60529 class IPx1.
Solid Objects per EN60529 class IP2x.
ESD/EMI (radiated and
immunity)
category specifications
controls Green SMART Pads cartridge handle
indicators Ready light: green; blinks when the defibrillator is in standby mode (ready for
audio speaker Provides voice prompts and warning tones during normal use.
beeper Provides chirps when troubleshooting is needed.
See Electromagnetic Conformity tables.
Controls and indicators
Green On/Off button
Blue i-button
Orange Shock button
use); solid when the defibrillator is being used.
i-button: blue, flashes when information is available, on solid during patient care
pause.
Caution light: flashes when the defibrillator is analyzing, comes on solid when
the defibrillator is ready to deliver a shock.
Shock button: orange, flashes when the defibrillator is charged and ready to
deliver a shock.
Philips Medical Systems
HEARTSTART M5066A
Page 45

Defibrillation Waveform
category specifications
E-3
waveform parameters Biphasic truncated exponential. Waveform parameters are automatically
Philips Medical Systems
adjusted as a function of patient defibrillation impedance. In the diagram at left,
D is the duration of phase 1 and E is the duration of phase 2 of the waveform, F
is the interphase delay (500 µs), and Ip is the peak current.
The HeartStart delivers shocks to load impedances from 25 to 180 ohms. The
duration of each phase of the waveform is dynamically adjusted based on
delivered charge, in order to compensate for patient impedance variations, as
shown below:
adult defibrillation
load phase 1 phase 2 peak delivered
resistance (Ω) duration (ms) duration (ms) current (A) energy (J)
25 2.8 2.8 65 128
50 4.5 4.5 40 150
75 6.25 5.0 30 155
100 8.0 5.3 24 157
125 9.65 6.4 21 159
150 11.5 7.7 18 160
175 12.0 8.0 16 158
pediatric defibrillation
(using M5072A infant/child reduced-energy defibrillator pads)
load phase 1 phase 2 peak delivered
resistance (Ω) duration (ms) duration (ms) current (A) energy (J)
25 4.1 2.8 28 35
50 5.1 3.4 20 46
75 6.2 4.1 15 52
100 7.2 4.8 12 54
125 8.3 5.5 10 56
150 9.0 6.0 9 57
175 9.0 6.0 8 55
E
Page 46

E-4
category specifications
energy*
(pediatric doses indicated
are based on CDC
growth charts for the
50th percentile weights
for boys.)
Using HeartStart Adult SMART Pads: 150 J nominal (
Using HeartStart Infant/Child SMART Pads: 50 J nominal (
load. Sample pediatric energy doses:
age energy dose
newborn 14 J/kg
1 year 5 J/kg
2 − 3 years 4 J/kg
4 − 5 years 3 J/kg
6 − 8 years 2 J/kg
* National Center for Health Statistics in collaboration with the National Center for Chronic
Disease Prevention and Health Promotion. CDC growth charts: weight-for-age percentiles, revised
and corrected November 28, 2000. Atlanta, GA: Centers for Disease Control and Prevention ©
2000.
charge control Controlled by Patient Analysis System for automated operation.
“charge complete”
Shock button flashes, audio tone sounds.
indicator
shock-to-shock cycle time <20 seconds, typical, including analysis.
patient care
pause-to-shock time
Quick Shock. 8 seconds, typical, from end of patient care pause to shock
delivery.
disarm (AED mode) Once charged, the defibrillator will disarm if:
• the patient’s heart rhythm changes to non-shockable rhythm,
• a shock is not delivered within 30 seconds after the defibrillator has charged
for shock delivery,
• the On/Off button is pressed and held down for at least one (1) second to
turn off the defibrillator,
• the adhesive pads are removed from the patient or the pads cartridge is
disconnected from the defibrillator,
• the battery is removed or is completely depleted or
• the impedance between pads is out of range.
±15%) into a 50 ohm load.
±15%) into a 50 ohm
Philips Medical Systems
adult shock
delivery vector
infant/child shock
delivery vector
HEARTSTART M5066A
Via adhesive pads placed in the anterior-anterior (Lead II) position.
Via adhesive pads typically placed in the anterior-posterior position.
Page 47

ECG analysis system
category specifications
E-5
function Evaluates impedance of adhesive pads for proper contact with the patient’s skin,
shockable rhythms Ventricular fibrillation (VF) and some ventricular tachycardias associated with a
non-shockable rhythms SMART Analysis is designed to detect non-shockable rhythms as defined by
pacemaker detection Pacemaker artifact is removed from the signal for rhythm analysis.
artifact detection If electrical “noise” (artifact) is detected which interferes with accurate rhythm
analysis protocol Depending on results of analysis, either prepares for shock delivery or provides
Philips Medical Systems
and evaluates the ECG rhythm and signal quality to determine if a shock is
appropriate.
lack of circulation, including ventricular flutter and polymorphic ventricular
tachycardia (VT). The HeartStart uses multiple parameters to determine if a
rhythm is shockable.
NOTE: For patient safety reasons, some very low-amplitude or low-frequency rhythms
may not be interpreted as shockable VF rhythms. Also, some VT rhythms usually
associated with circulation will not be interpreted as shockable rhythms.
AHA/AAMI DF-80. See following table. On detection of any non-shockable
rhythm, the HeartStart prompts user to perform CPR if needed.
analysis, analysis will be delayed until the ECG signal is clean.
E
a pause. For details of protocol, see Appendix F, “Configuration.”
Page 48

E-6
ECG analysis performance
rhythm class
shockable rhythm —
ventricular fibrillation
shockable rhythm —
ventricular tachycardia
non-shockable rhythm —
normal sinus rhythm
non-shockable rhythm —
asystole
non-shockable rhythm —
all other non-shockable
rhythms
a. From Philips Medical Systems Heartstream ECG rhythm databases.
b. American Heart Association (AHA) AED Task Force, Subcommittee on AED Safety & Efficacy. Automatic External Defibrillators for Public
Access Use: Recommendations for Specifying and Reporting Arrhythmia Analysis Algorithm Performance, Incorporation of New
Waveforms, and Enhancing Safety. Circulation 1997;95:1677-1682.
c. Supraventricular tachycardia (SVT) is specifically included in the non-shockable rhythm class, in accordance with AHA recommendations
and the AAMI standard DF80.
c
ECG test
sample
a
size
observed 90% one-sided
performance lower confidence limit
300 sensitivity >90%
(meets AAMI DF80 requirement)
100 sensitivity >75%
(meets AAMI DF80 requirement)
300 specificity >99%
(meets AAMI DF80 requirement)
100 specificity >95%
(meets AAMI DF80 requirement)
450 specificity >95%
(meets AAMI DF80 requirement)
(87%)
(67%)
(97%)
(92%)
(88%)
b
meets AHA recommendationsb for adult defibrillation
Philips Medical Systems
HEARTSTART M5066A
Page 49

Accessories specifications
Battery M5070A
category specifications
battery type 9 VDC, 4.2 Ah, lithium manganese dioxide. Disposable, long-life primary cell.
E-7
capacity When new, a minimum of 200 shocks or 4 hours of operating time at 77° F
shelf life
(prior to insertion)
standby life
(after insertion)
training life Supports 10 hours of use in training mode.
category specifications
(25° C). (IEC 60601-2-4 2002)
A minimum of 5 years from date of manufacture when stored and maintained
according to directions provided in this Owner’s Manual.
Typically, 4 years when stored and maintained according to directions provided
in this Owner’s Manual.
HeartStart
Infant/Child SMART Pads M5072A
Adult SMART Pads M5071A and
E
Philips Medical Systems
adult pads Disposable, adhesive defibrillation pads with a nominal active surface area of 85
infant/child pads Disposable, adhesive defibrillation pads with a nominal active surface area of 85
defibrillation pad
requirements
2
each, provided in a snap-in cartridge with an integrated 54” (137.1 cm),
cm
typical, cable.
2
each, provided in a snap-in cartridge with an integrated 40 inch (101.6 cm),
cm
typical, cable. Cartridge incorporates teddy bear icon on cover of seal for ready
identification.
Use only HeartStart Adult SMART Pads M5071A or Infant/Child SMART Pads
M5072A with the HeartStart Defibrillator.
Page 50

E-8
Environmental considerations
By complying with your national regulations regarding disposal of electric,
electronic, and battery waste, you can make a positive contribution to our shared
environment. Such waste can introduce harmful elements into the environment as
a whole and may also endanger human health
product information
.
defibrillator The defibrillator contains electronic components. Do not dispose of it as
unsorted municipal waste. Collect such electronic waste separately and dispose
of it at an appropriate recycling facility according to your country's regulations.
battery The battery cells contain chemicals. The chemistry used in each battery is
identified by a symbol on the label; symbols are defined in the defibrillator
User's Guide/Instructions for Use/Owner's Manual. Recycle the battery at an
appropriate recycling facility.
pads The used pads may be contaminated with body tissue, fluid, or blood. Cut them
off and dispose of them as infectious waste. Recycle the remaining cartridge
components at an appropriate recycling facility in accordance with local
regulations.
Philips Medical Systems
HEARTSTART M5066A
Page 51

FConfiguration
Overview
The Philips HeartStart Defibrillator comes with a factory default configuration
designed to meet the needs of most users. This configuration can only be
changed by an authorized person using HeartStart Configure PDA software or
Event Review software. This software is for use by trained personnel.
Information about HeartStart data management products is available online at
http://www.medical.philips.com/goto/eventreview.
Device options
The following table includes the features of HeartStart Defibrillator operation that
are not related to patient treatment.
parameter settings default default description
Philips Medical Systems
speaker volume 1, 2, 3, 4,
5, 6, 7, 8
auto send periodic
self-test (PST) data
ECG out data On, Off On Enables the ECG data to be broadcast
On, Off On Enables the periodic self-test data to be
8 The volume of the HeartStart’s speaker is set
to 8, highest.
broadcast through the device's infrared data
port.
F
through the device's infrared data port.
F-1
Page 52

F-2
Patient treatment protocol options
parameter settings default default description
“call EMS” voice
reminder timing
shock series 1, 2, 3, 4 1 The automatic protocol pause for CPR
shock series interval
(minutes)
• At power on (when
the user turns on
the HeartStart)
• At power on and at
the start of the first
patient care pause
• At the start of the
first patient care
pause
•No reminder
1.0, 2.0,
∞ (infinity)
At the start of
the first
patient care
pause
Provides a voice reminder to make
sure emergency medical services have
been called, at the start of the first
patient care pause.
is activated each time a shock is
delivered.
*
During the protocol pause, the
HeartStart does not perform rhythm
analysis.
The length of the protocol pause after
a shock series is completed is
determined by the protocol pause
timer setting.
1.0 A delivered shock must occur within 1
minute of the previous shock to be
counted as part of the current shock
series.
NOTE: This parameter is only applicable
when the shock series is not configured to
the default 1 shock.
Philips Medical Systems
* A shock series begins when a shock is delivered after the HeartStart is turned on. A new shock series begins after a
protocol pause. If shock series is configured for 2 or more, a new shock series also begins if the time since the previous
shock exceeds the shock series interval setting.
HEARTSTART M5066A
Page 53

parameter settings default default description
F-3
protocol pause timer
(minutes)
NSA pause type • Standard NSA
Philips Medical Systems
0.5, 1.0, 1.5,
2.0, 2.5, 3.0
pause: HeartStart
does not perform
rhythm analysis
during the NSA
pause.
• SMART NSA pause:
HeartStart conducts background
monitoring during
the SMART NSA
pause. If a potentially shockable
rhythm is detected,
HeartStart terminates the SMART
NSA pause and
resumes rhythm
analysis.
2.0 A 2-minute protocol pause for CPR
automatically starts after voice
instruction is given when a shock
series is completed. After the protocol
pause, the defibrillator returns to
rhythm analysis.
If the user presses the i-button for
optional CPR coaching, the HeartStart
provides coaching for 5 cycles of CPR,
starting and ending with compressions,
when the CPR Coaching parameters
are also set to their default values. The
number of CPR cycles varies for other
protocol pause timer and CPR
Coaching parameter settings.
Note: Because the protocol pause ends
upon completion of a CPR cycle in order
to maximize the benefits of CPR, the
actual duration of the pause may differ
slightly from the timer setting.
SMART NSA
pause
During a SMART NSA pause, the
defibrillator conducts background
monitoring. If a potentially shockable
rhythm is detected in a motionless
patient, the defibrillator terminates the
SMART NSA pause and resumes
rhythm analysis.
NOTE: If the HeartStart detects CPR in
progress or if the responder has pressed
the i-button for CPR Coaching, the SMART
NSA pause will be converted to a
standard NSA pause. During the standard
NSA pause, the defibrillator does not
perform rhythm analysis.
F
Page 54

F-4
parameter settings default default description
NSA pause timer
(minutes)
0.5, 1.0, 1.5,
2.0, 2.5, 3.0
CPR prompt • CPR1: Instructs the
user to begin CPR.
•CPR2: Instructs the
user that it is safe
to touch the
patient and to begin
CPR.
•CPR3: Instructs the
user to begin CPR
and to press the
i-button for CPR
Coaching.
•CPR4: Instructs the
user that it is safe
to touch the
patient, to begin
CPR, and to press
the i-button for
CPR Coaching.
2.0 A 2-minute NSA pause for CPR
automatically starts after voice
instruction is given when no shock is
advised (NSA).
*
If the user presses the i-button for
optional CPR coaching, the HeartStart
provides coaching for 5 cycles of CPR,
starting and ending with compressions,
when the CPR Coaching parameters
are also set to their default values. The
number of CPR cycles varies for other
NSA pause timer and CPR Coaching
parameter settings.
Note: Because the NSA pause ends upon
completion of a CPR cycle in order to
maximize the benefits of CPR, the actual
duration of the pause may differ slightly
from the timer setting.
CPR4:
Instructs the
user that it is
safe to touch
the patient, to
begin CPR, and
to press the
i-button for
CPR Coaching.
The CPR reminder voice instructions
provided at the beginning of a pause
interval assures the user that it is safe
to touch the patient, instructs the user
to begin CPR, and invites the user to
press the i-button for guidance in the
basic steps of CPR.
Note: CPR Coaching is available only with
the CPR3 and CPR4 settings.
Philips Medical Systems
* If the shock series is configured to 2 or more, and a shock has been delivered as part of a series, the length of the first
NSA pause within that shock series is determined by the protocol pause timer setting. Otherwise, the length of an NSA
pause is determined by the NSA pause timer setting.
HEARTSTART M5066A
Page 55

parameter settings default default description
F-5
CPR Coaching
adult ventilation
instruction
infant/child ventilation
compression:ventilation
Philips Medical Systems
CPR Coaching
instruction
CPR Coaching
ratio
Yes, No Yes Optional CPR Coaching includes
rescue breaths at the rate determined
by the CPR Coaching
compression:ventilation ratio for
adults when an adult pads cartridge is
installed.
NOTE: if this parameter is configured to
NO, CPR Coaching will always be
compressions-only when an adult pads
cartridge is installed.
Yes, No Yes Optional CPR Coaching includes
• 30:2 adult and
30:2 infant/child
• 30:2 adult and
15:2 infant/child
• 15:2 adult and
15:2 infant/child
30:2 adult and
30:2 infant/
child
rescue breaths at the rate determined
by the CPR Coaching
compression:ventilation ratio for
infants and children when an infant/
child pads cartridge is installed.
NOTE: if this parameter is configured to
NO, CPR Coaching will always be
compressions-only when an infant/child
pads cartridge is installed.
If the user presses the i-button for
optional CPR Coaching during a
protocol pause or NSA pause, the
HeartStart provides coaching in basic
CPR for cycles of 30 compressions and
2 ventilations for adults, children, and
infants. Pauses begin and end with
compressions.
F
Page 56

Notes
Philips Medical Systems
HEARTSTART M5066A
Page 57

G Testing and troubleshooting
Te s t i n g
As long as a battery is installed, the HeartStart Defibrillator automatically tests
itself every day and alerts you if it finds a problem. The self-test includes pads
readiness testing. In addition, it runs a pads self-test each time a pads cartridge is
inserted. It alerts you if it finds a problem. See the Technical Reference Manual,
available online at www.medical.philips.com, for a detailed discussion of the selftests.
You can also test the defibrillator at any time by removing the battery for five
seconds then reinstalling it. This test takes about one minute. Because the battery
insertion self-test is very detailed and uses battery power, running it more often
than necessary will drain the battery prematurely. It is recommended that you run
the battery insertion self-test only:
• when the defibrillator is first put into service.
• after each time the defibrillator is used to treat a patient.
• when the battery is replaced.
Philips Medical Systems
• when the defibrillator may have been damaged.
If you need to use the defibrillator in an emergency while you are running a
battery self-test, pull the SMART Pads cartridge handle to stop the test and to turn
on the HeartStart for use.
Troubleshooting
The HeartStart’s green Ready light is the signal that tells you if the defibrillator is
ready for use. The defibrillator also uses chirps and the i-button flashes to alert
you to a problem.
Recommended action during an emergency
If for any reason the HeartStart does not turn on when you pull the SMART Pads
cartridge handle, press the On/Off button.
If that does not turn on the defibrillator, remove the battery and replace it with a
new battery if available and press the On/Off button to turn on the defibrillator. If
G-1
G
Page 58

G-2
no spare battery is available, remove the installed battery for five seconds, then
reinsert it and run a battery insertion self-test.
If the problem continues, do not use the HeartStart. Attend to the patient,
providing CPR if needed, until Emergency Medical Services Personnel arrive.
Troubleshooting while the HeartStart is in use
(green Ready light is solid)
HeartStart tells you: possible cause recommended action
... to replace the battery
immediately
... there is no cartridge
installed, and
... to insert a pads cartridge
... to press the pads firmly to
the skin
... to make sure the pads
have been removed from
the liner
... the pads should not be
touching the patient’s
clothing.
... to insert new
pads cartridge
The battery is nearly depleted. The
defibrillator will turn off if a new
battery is not inserted.
• The pads cartridge has been
removed.
• The pads cartridge has been
damaged.
• The pads are not properly applied
to the patient.
• The pads are not making good
contact with the patient's bare
chest because of moisture or
excessive hair.
• The pads are touching each other.
• The pads may not have been
removed from the liner or may be
on the patient’s clothing.
The pads cartridge has been opened
and the pads peeled off the liner, but
the pads have not been successfully
attached to the patient. There may
be a problem with the pads cartridge.
Replace the battery with a new battery
immediately.
Insert a new pads cartridge.
Philips Medical Systems
• Make sure that the pads are sticking
completely to the patient’s skin.
• If the pads are not sticking, dry the
patient's chest and shave or clip any
excessive chest hair.
• Reposition the pads.
• Make sure the pads are not on the
liner or the patient’s clothing.
If the voice instruction continues after
you do these things, insert another
pads cartridge.
Replace the damaged pads cartridge.
Pull up the handle on the cartridge
cover, and replace pads on patient
with new pads to continue with the
rescue.
HEARTSTART M5066A
Page 59

HeartStart tells you: possible cause recommended action
G-3
... to stop all motion • The patient is being moved or
jostled.
• The environment is dry and
movement around the patient is
causing static electricity to
interfere with ECG analysis.
• Radio or electrical sources are
interfering with ECG analysis.
... the shock was not
delivered
... the shock button was not
pressed
Philips Medical Systems
• The pads may not be making good
contact with the patient’s skin.
• The pads may be touching each
other.
• The pads may be damaged.
Shock has been advised but the shock
button has not been pressed within
30 seconds.
• Stop CPR; do not touch the patient.
Minimize patient motion. If the
patient is being transported, stop
the vehicle.
• Responders and bystanders should
minimize motion, particularly in dry
environments that can generate
static electricity.
• Check for possible causes of radio
and electrical interference and turn
them off or remove them from the
area.
• Press the pads firmly to the patient's
chest.
• Make sure the adhesive pads are
correctly positioned on the patient.
• Replace the pads if necessary.
When next prompted, press the Shock
button to deliver shock.
G
Page 60

G-4
Troubleshooting while the HeartStart is not in use
(green Ready light is not on)
behavior possible cause recommended action
chirps or
i-button flashes
no chirping and/or
i-button does not flash
• The battery power is low or the
SMART Pads cartridge needs to be
replaced.
• The defibrillator may have been
turned off without a pads cartridge
installed, or the installed pads
cartridge may not have its hard
cover in place.
• The training pads cartridge has been
left in the defibrillator.
• The defibrillator has been stored
outside the recommended
temperature range.
• The defibrillator has detected an
error during a self-test or cannot
perform a self-test, or the Shock
button is damaged.
• The battery is missing or completely
depleted.
• The defibrillator may have been
physically damaged.
• Press the blue i-button. Replace the
battery or pads cartridge if
instructed.
• Make sure the pads cartridge is
properly installed with the hard
cover in place. (See Chapter 5,
“Maintaining the HeartStart,” for
directions on installing the pads
cartridge.)
• Remove the training pads cartridge
and replace it with an Adult or
Infant/Child Pads Cartridge.
• Remove the battery for five
seconds then reinstall it to start
the battery insertion self-test. If it
fails, insert a new battery to repeat
the test. If it fails again, do not use
the defibrillator. If it passes, store
the defibrillator within the
recommended temperature range.
• Contact Philips for service if
needed.
Remove the battery for five seconds
then reinstall it to start the battery
insertion self-test. If it fails, insert a
new battery and repeat the test. If it
fails again, do not use the defibrillator.
Contact Philips for service.
Philips Medical Systems
HEARTSTART M5066A
Page 61

H Additional technical information required for European
conformity
Electromagnetic conformity
Guidance and manufacturer’s declaration: The HeartStart is intended for use in the
electromagnetic environment specified in the tables below. The customer or user
of the HeartStart should assure that it is used in such an environment.
Electromagnetic emissions
emissions test compliance electromagnetic environment – guidance
RF
CISPR 11
Philips Medical Systems
Group 1
Class B
The HeartStart uses RF energy only for its internal function.
Therefore, its RF emissions are very low and are not likely to
cause any interference in nearby electronic equipment.
The HeartStart is suitable for use in all establishments,
including domestic establishments and those directly
connected to the public low-voltage power supply network
that supplies buildings used for domestic purposes.
H
H-1
Page 62

H-2
Electromagnetic immunity
immunity test
electrostatic discharge
(ESD)
IEC 61000-4-2
power frequency
(50/60 Hz) magnetic field
IEC 61000-4-8
IEC 60601
test level
± 6 kV contact
± 8 kV air
compliance level
± 6 kV contact
± 8 kV air
3 A/m 3 A/m Power frequency magnetic fields
electromagnetic environment -
guidance
There are no special requirements
with respect to electrostatic
discharge.
a
should be at levels characteristic of a
typical location in a typical
commercial/hospital environment.
There are no special requirements for
non-commercial/non-hospital
environments.
radiated RF
IEC 61000-4-3
10 V/m
80 MHz to 2.5
GHz
20 V/m Portable and mobile RF
communications equipment should be
used no closer to any part of the
HeartStart, including cables, than is
absolutely necessary.
b,c
The
recommended separation distances
for various transmitters and the AED
are shown in the following table.
Interference may occur in the
vicinity of equipment marked
with the following symbol:
NOTE 1. At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2. These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and
reflection from structures, objects and people.
Philips Medical Systems
a. Generally, AEDs are sometimes susceptible to interference generated by patient and/or responder motion in environments in which a high
static electric field is present (e.g., low humidity, synthetic carpets, etc.). As a safety measure, Philips AEDs incorporate a patented method
to sense possible corruption of the ECG signal by such interference and to respond by directing the user to stop all motion. In these cases,
it is important to minimize movement in the vicinity of the patient during rhythm analysis in order to ensure that the signal being analyzed
accurately reflects the patient’s underlying heart rhythm.
b. The ISM (industrial, scientific and medial) bands between 150 kHz and 80 MHz are 6,765 MHz to 6,795 MHz; 13,553 MHz to 13,567 MHz;
26,957 MHz to 27,283 MHz; and 40,66 MHz to 40,70 MHz.
c. Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios, amateur radio,
AM and FM radio broadcast, and TV broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic enviro nment
due to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field strength in the location in which the
HeartStart is used exceeds the applicable RF compliance level above, the HeartStart should be observed to verify normal operation. If
abnormal performance is observed, additional measures may be necessary, such as re-orienting or relocating the HeartStart.
HEARTSTART M5066A
Page 63

Recommended separation distances between portable and mobile RF
communications equipment and the HeartStart
The HeartStart is intended for use in an electromagnetic environment in which
radiated RF disturbances are controlled. The customer or the user of the
HeartStart can help prevent electromagnetic interference by maintaining a
minimum distance between portable and mobile RF communications equipment
(transmitters) and the HeartStart as recommended below, according to the
maximum output power of the communications equipment.
separation distance according to frequency of transmitter (m)
H-3
rated maximum output
power of transmitter (W)
80 MHz to
800 MHz
d = 0.6√ P
800 MHz to
2.5 GHz
d = 1.15√ P
0.01 0.06 0.115
0.1 0.19 0.36
1 0.6 1.15
10 1.9 3.64
Philips Medical Systems
100 6.0 11.5
For transmitters rated at a maximum output power not listed above, the recommended separation distance d
in meters (m) can be determined using the equation applicable to the frequency of the transmitter, where P is
the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer.
NOTE 1. At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
NOTE 2. The ISM (industrial, scientific and medial) bands between 150 kHz and 80 MHz are 6,765 MHz to 6,795 MHz;
13,553 MHz to 13,567 MHz; 26,957 MHz to 27,283 MHz; and 40,66 MHz to 40,70 MHz.
NOTE 3. An additional factor of 10/3 is used in calculating the recommended separation distance for transmitters in the
ISM frequency bands between 150 kHz and 80 MHz and in the frequency range 80 MHz to 2.5 GHz to decrease the
likelihood that mobile/portable communications equipment could cause interference if it is inadvertently brought into
patient areas.
NOTE 4. These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and
reflection from structures, objects and people.
H
Page 64

H-4
Important warnings and reminders
• Do not allow the pads to contact other electrodes or metal parts that are in
contact with the patient.
• Before delivering a shock, it is important to disconnect the patient from other
medical electrical equipment, such as blood-flow meters, that may not
incorporate defibrillation protections. In addition, make sure the pads are not
in contact with metal objects such as a bedframe or stretcher.
• Check supplies, accessories, packaging, and spares for damage and expiration
dating.
Environmental considerations
• The defibrillator contains electronic components. Dispose of it at an
appropriate recycling facility.
• The battery cells contain chemicals. Recycle the battery at an appropriate
recycling facility.
• The used pads may be contaminated. Cut them off and dispose of them
properly. Recycle the remaining cartridge components at an appropriate
recycling facility.
Shock cycle timing
Philips Medical Systems
HEARTSTART M5066A
The HeartStart Quick Shock feature allows it to deliver a shock within 8 seconds,
typical, following the prompt ending a CPR Interval. From shock to shock, the
HeartStart takes <20 seconds, typical, including analysis. After 15 shocks, the
HeartStart takes <30 seconds from analyzing to ready-to-shock. After 200 shocks,
the HeartStart takes <40 seconds from initial power-on to ready-to-shock.
Page 65

Intentionally blank.
Philips Medical Systems
Page 66

Intentionally blank.
Philips Medical Systems
Page 67

Intentionally blank.
Philips Medical Systems
Page 68

Intentionally blank.
Philips Medical Systems
Page 69

Intentionally blank.
Philips Medical Systems
Page 70

POWER TO SAVE A LIFE
Philips Medical Systems is part of
Royal Philips Electronics
Philips Medical Systems
United States
Philips Medical Systems
2301 Fifth Avenue, Suite 200
Seattle, WA, USA 98121
(800) 263-3342
Canada
Philips Medical Systems
281 Hillmount Road
Markham, Ontario
L6C 2S3
(800) 291-6743
Europe, Middle East, and Africa
Philips Medizin Systeme Boeblingen GmbH
Cardiac and Monitoring Systems
Hewlett-Packard Strasse 2
71034 Boeblingen, Germany
+49 7031 463 2254
Latin America
Philips Medical Systems
1550 Sawgrass Corporate Parkway, Suite 300
Sunrise, FL 33323, USA
(954) 835-2660
REF: M5066-91901
Asia Pacific
Philips Electronics Hong Kong Ltd.
30th Floor, Hopewell Centre
17, Kennedy Road, Wanchai
Hong Kong
(852) 2821 5888
 Loading...
Loading...