Page 1
Page 2

G5 Solution
Wearable biosensor
Instructions
for use
989803199491
Page 3

2
Introduction 3
Intended use 3
Product description 4
General warnings
and precautions 8
Warnings 8
Precautions 9
Use environment 9
Radiofrequency
interference 10
Security and privacy
recommendations 10
Product operation 12
Product setup 12
Preparing skin and
applying biosensor 12
Connecting to the
G5 application 14
Disconnecting the biosensor
and saving patient data 16
Biosensor interface 18
G5 application interface 19
Heart rate transmission
conguration 19
Notications 19
Reviewing data 22
Maintenance 23
Cleaning and disinfection 23
Storage 23
Troubleshooting 24
Specications 26
Symbols 26
Manufacturer’s
information 27
Open source software 27
Regulatory and safety
specications 27
EMC and radio regulatory
compliance 28
FCC compliance statement 29
Safety and
performance tests 30
Biosensor specications 34
Software application
specications 35
Contents
Page 4

3
Introduction
Intended use
Philips wearable biosensor-G5 solution is a single-location, chest-
worn heart rate monitor. The wearable biosensor-G5 solution includes
a wearable biosensor-G5 and software application. The wearable
biosensor-G5 is a single-use device measuring heart rate by continuously
acquiring surface electrical waveforms related to cardiac excitations
and measuring beat-to-beat intervals when a patient is stationary or
ambulatory. The biosensor functions by capturing and then sending
physiological data wirelessly to the software application. The biosensor’s
frequency of data collection and transmission is congurable.
The software application is a single-patient use device, intended as
an accessory to the biosensor to display and store physiological and
operational data. The software application receives and displays data
from the biosensor providing a user interface and exportable le for
retrospective review and analysis. The application allows conguring the
biosensor frequency of data collection and transmission.
Indications for use
The Philips wearable biosensor-G5 is indicated for single patient use
whenever heart rate measurement is needed in non-critical hospital
settings. The Philips wearable biosensor-G5 solution is used as a higher
resolution heart rate log by nurses or physicians retroactively as an aid
in making non-critical or non-life threatening therapeutic decisions. The
biosensor is intended for patients who are 18 years of age or older.
Note
Before using this product to obtain heart rate, carefully read the instructions
for use and the quick start guide on the G5 biosensor package.
Page 5
4
Product description
Biosensor
Philips wearable biosensor-G5 solution is a patient heart rate sensing
system – comprised of a Philips wearable biosensor-G5 and a software
application G5 application – which gathers, stores and displays a
patient’s heart rate. The biosensor G5 is designed to connect with the G5
application to let clinicians review and export patient heart rate. Heart
rate measurements are sent to a compatible device using a USB cable for
oine review and analysis.
Philips wearable biosensor-G5 is a wireless, single-use, single-location
chest-worn device that acquires surface electrical waveforms related to
cardiac excitations, and measures beat-to-beat intervals. The biosensor
calculates patient heart rate based upon a combination of patient’s
single-vector ECG and their motion data. The biosensor has two days of
wear life, after which it turns o automatically.
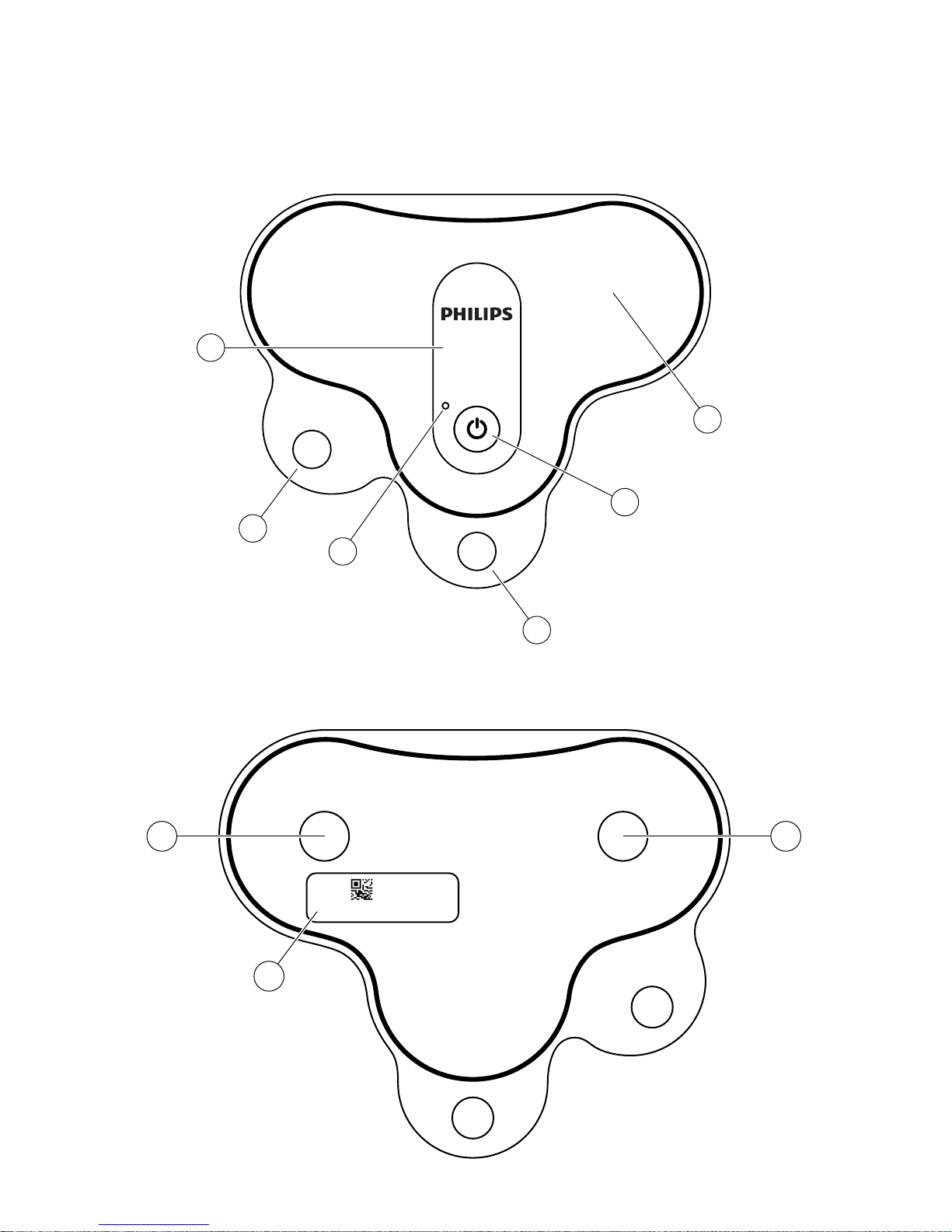
Biosensor top cover
Biosensor label
LED light
ON button
Release liner 1
Release liner 2
Biosensor ID on the back
of the release liner
Electrodes
Page 6
5
Front of biosensor
2
1
2
4
3
5
6
1
2
1
0001AB50012
88
7
Back of biosensor
Page 7
6
Product description
Application
G5 software application is an Android-based application for mobile
devices. The app receives patient’s heart rate data, exports data into a
password-protected le and displays the following:
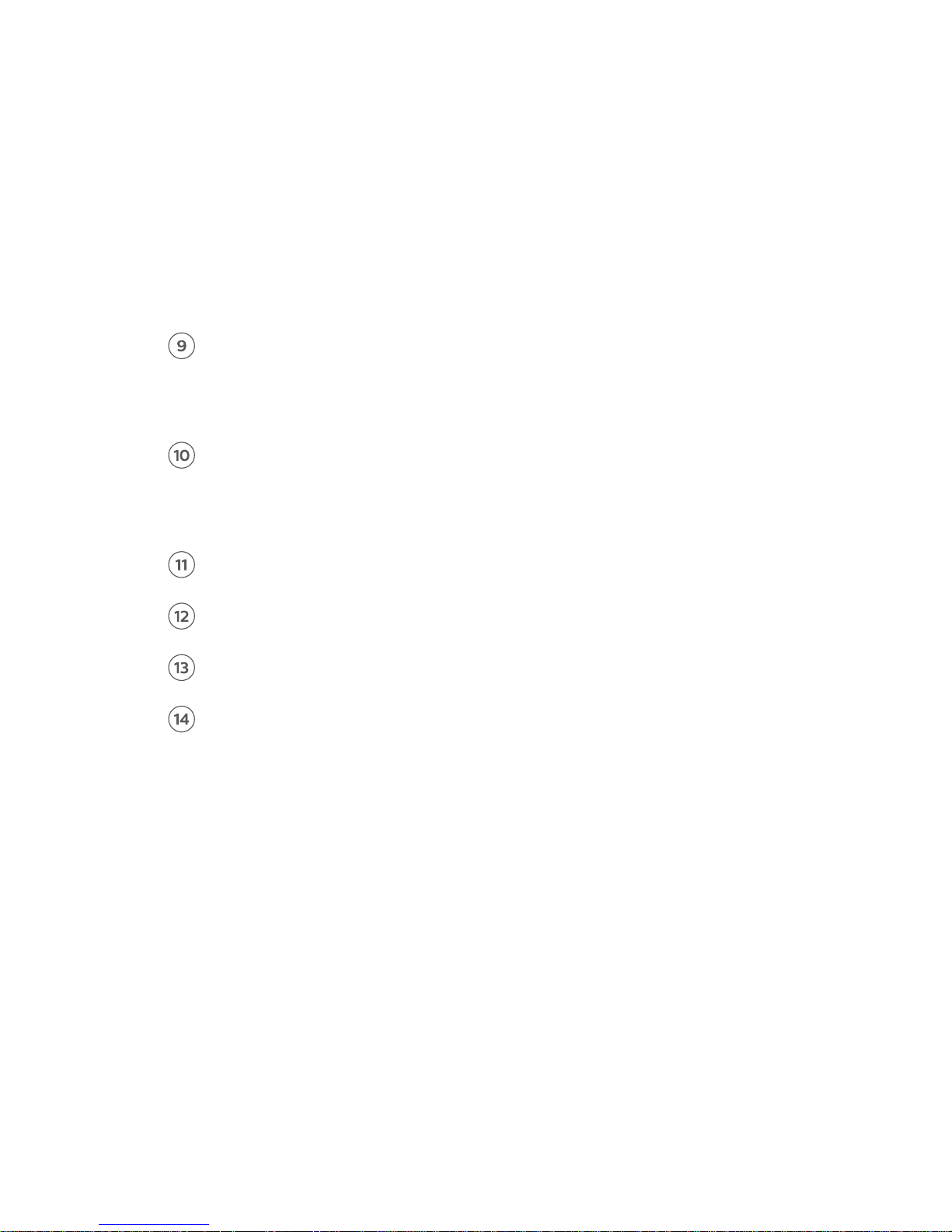
Menu
• Disconnect biosensor and exit
• Clear all data and exit
Heart rate (instantaneous)
Instantaneous heart rate is measured every beat over the reporting
interval, which is user-congurable between 1 to 30 minutes
G5 biosensor battery level
Date and time
Reporting interval
Notications log
Page 8
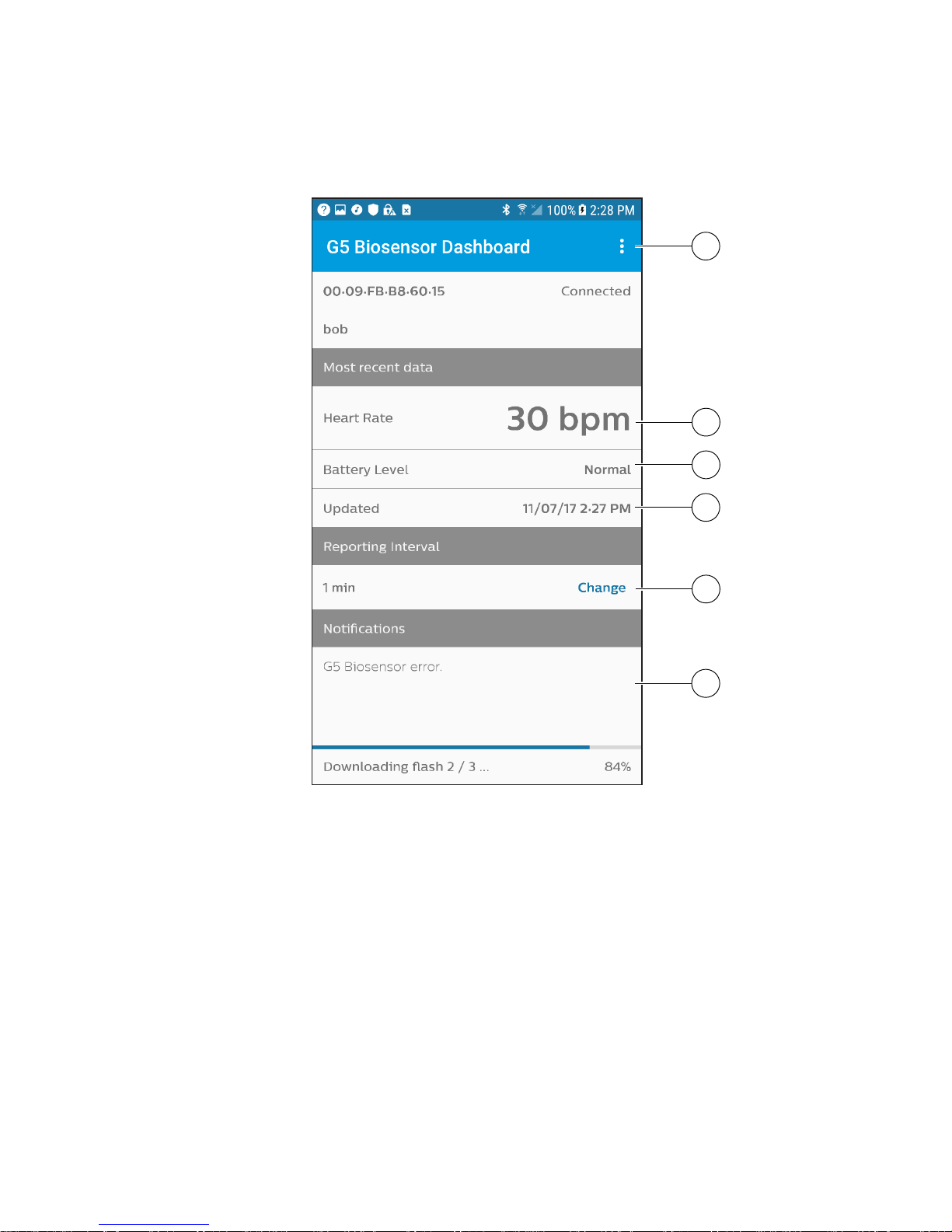
7
Application dashboard
12
9
10
11
13
14
Page 9

8
Warnings
• Do not use the
biosensor during
MRI procedures.
The biosensor is MRI unsafe.
• Do not use the biosensor
during X-ray. The biosensor
will obstruct the view of an
X-ray of the chest.
• The biosensor should only be
used under direct supervision
of a licensed physician or
healthcare provider, according
to the hospital standard of care.
• Do not use for more than 48
hours. Replace biosensor if
it no longer sticks rmly to
the skin. The biosensor must
be properly adhered to the
patient to obtain signal from
the body.
• Do not use if hydrogel is dry.
Keep biosensor in sealed
package. Only open
immediately before use to
prevent hydrogel from drying.
• Biosensor is for single use
only. Do not reuse due to
risk of cross-infection,
degradation of adhesive or
electrical performance.
• Do not apply over open
wounds, lesions, infected,
irritated, scarred or inamed
areas. The biosensor should
only be applied to intact skin.
General warnings and precautions
• Do not apply to patients
with a history of known
tape or adhesive allergy.
The biosensor contains an
adhesive which adheres
to the skin.
• Warning pacemaker patients:
Biosensor can detect a
patient’s pacemaker pulses.
Biosensor may continue to
count the pacemaker rate
during occurrences of cardiac
arrest or some arrhythmias.
Do not rely entirely upon
biosensor.
Keep pacemaker patients
under close surveillance. See
the G5 application interface
section of this manual for
disclosure of the pacemaker
pulse detection capability of
the biosensor.
• Warning pacemaker patients:
Biosensor can detect a
patient’s pacemaker pulses.
Do not use the biosensor for
rejecting pace pulses. A ‘pacepulse detected’ notication
will appear whenever
interrupted measurements
occur. The heart rate value will
not display during a detected
pacing event.
• Do not use the biosensor
simultaneously with the
cardiac monitors or cardiac
Page 10

9
telemetry devices. Cardiac
monitors or cardiac telemetry
devices in direct contact with
the thorax may degrade the
biosensor signal quality or
produce erroneous results.
This potential interaction has
not been evaluated.
• Do not use the device for
diagnosis. Whenever patient
condition does not match
values, take a conrmatory,
independent check of vitals
(including 12-lead ECG).
• Do not use the device to
discern abnormal rhythm
patterns or for alarms.
• Do not use the biosensor
with high frequency surgical
equipment.
• No modication of this
equipment is allowed.
Precautions
• Only apply the biosensor over
clean and dry skin. Do not
apply the biosensor over body
hair. Remove any oil, lotion,
residue, or debris from skin
before application.
• Use caution when removing
biosensor to prevent skin
irritation. Gently swab area
under the biosensor with water
as you are removing the sensor.
• To frequently collect
instantaneous heart rate,
assess the biosensor
connectivity to the G5
application every 8 hours.
If connectivity is lost, the
biosensor will store up to
4 hours of data.
• Keep your mobile device
charged at all times.
• The biosensor is debrillation
proof. Remove the biosensor
from the chest if the biosensor
is located on the area where
debrillation pads need to be
applied.
• Heart rate value may be lower
or higher with patients with
arrhythmia.
• Heart rate value may be
higher when pacemaker
pulses are present outside
of the disclosed pacemaker
pulse range.
Use environment
• The G5 solution is intended
for non-critical care hospital
environments.
• Maintain a minimum separation
distance as described in the
EMC section, between portable
radiofrequency communications
equipment and biosensor to
avoid potential performance
degradation.
Page 11

10
General warnings and precautions
Use
environment
continued
• Keep biosensor pouch sealed
until the biosensor is ready
for use.
• The biosensor should not
be exposed to more than
three showers. Each shower
should not be longer than 10
minutes long, and the water
temperature should not be
higher than 105°F.
• The biosensor should not
be worn during baths or
submerged under water.
• The biosensor’s adhesive
should not be handled directly
with the ngers once the liners
have been removed.
• The biosensor should not
be subjected to aggressive
mechanical handling
(e.g. twisting, pulling, etc.)
during setup.
• The biosensor should
be disposed according
to hospital’s disposable
electronic devices guidelines
after its use.
Radiofrequency
interference
If interference problems occur,
try moving biosensor away from
the source of the interference.
You can also move the electronic
device or its antenna to another
location to solve the problem.
These guidelines help ensure
that the biosensor will not aect
the operation of other nearby
electronic devices. Additionally,
other electronic devices should
not aect the use of the biosensor.
Security and privacy
recommendations
Customer’s role in the product
security partnership
Security of Philips products
is an important part of each
facility’s overall security strategy.
However, these benets can only
be realized in combination with
a comprehensive, multi-layered
strategy that includes policies,
procedures, and technologies to
protect information and systems
from external and internal threats.
In accordance with security and
industry best practices, security
strategies should address:
Data encryption
• Heart rate data is encrypted
on the biosensor using 128-bit
AES and sent via Short Range
Page 12

11
Wireless to the compatible
mobile device.
Customer network security and
importance of security policy
• Ensure there are sucient
intrusion prevention and
detection measures as part of
the IT security policies.
• The mobile device should
not be connected to the
hospital network.
• It is recommended to have anti-
virus or malware on the mobile
device.
• Philips recommends operating
Biosensor in secure network by
turning o the Wi-Fi connectivity
on the mobile device running
the G5 App. Connectivity of G5
app with biosensor shall only
be established on Bluetooth
protocol (Version 4.2).
• If Wi-Fi cannot be disabled on
the mobile device, it is advised
to connect on a highly secure
wireless network (e.g. WPA2)
with strong password enabled.
User account maintenance
• Do not share the G5 App
password with any unauthorized
personnel.
• Use physical security; for
example, locks, cameras,
keycards, sensors, and so on,
to restrict unauthorized access
of mobile device.
• Use procedural security; for
example, unattended mobile
device locking, no sharing of
access credentials, termination
checklists, risk management (that
is, performing risk assessments
and mitigating identied risks),
and so on.
• Operational security; for
example, access/authorization
controls, change management,
and network segmentation
based on data classication.
Auto-lock settings
• Philips recommends that you
congure the mobile device
auto-lock time to match the
security policies (Permissible is
between 1-9 minutes).
Application
• Philips recommend that G5
application should only be used
with a compatible mobile device.
– Any unnecessary applications
should be removed from the
compatible device.
• For optimal performance, only
the G5 app should be running on
the device
Page 13
12
Product operation
Product setup
1 Gather required materials
a Materials needed to prep the skin and trim excessive body hair.
b G5 biosensor.
c Mobile device with G5 software application (app) installed.
Preparing skin and applying biosensor
Warning
• Do not use the biosensor during MRI procedures. The biosensor
is MRI unsafe.
• Do not use alcohol for cleaning purposes because it dries the
skin and may diminish electrical ow. Alcohol may also increase
likelihood of skin irritation.
• Do not apply the biosensor to patients with skin integrity issues.
Only apply the biosensor to intact skin.
• Do not apply the biosensor over visible scars.
• Do not apply the biosensor to patients with known allergies
to tape or adhesives.
Page 14

13
2 Prepare patient’s skin
a Locate the upper left chest area (over the heart) for biosensor
application.
b Shave or cut hair from electrode sites since excessive hair prevents
good electrode contact.
c Clean each site thoroughly with soap and water or use an alcohol-
free wipe to improve electrical ow.
d Let skin dry.
Note: Thorough cleaning and drying of the skin can improve
biosensor adhesion.
Note: Skin prep solution may be applied to patients with delicate skin
to make removal easier later.
3 Prepare biosensor
a To open the package, pull the two silver layers apart.
b Carefully remove the biosensor from the package.
c Examine biosensor for physical damage and liner integrity
before setup.
Note: Discard the biosensor and use a new one if the biosensor foam is
not intact, if the ON button appears damaged, or if a liner is missing.
d Turn on the biosensor by pressing the ON button .
e A green light will ash, indicating the biosensor is ready for
placement on the patient.
Note: If the light does not turn on, use a new biosensor and repeat the
previous steps.
Page 15

14
Product operation
4 Apply biosensor
a Without touching the adhesive, remove release liner (1).
b Apply the biosensor to the patient’s upper left chest, over the heart.
c Apply pressure on the biosensor evenly across the applied side.
d Without touching the adhesive, remove release liner (2).
e Press rmly over the entire biosensor to ensure it is fully adhered to
the skin.
Note: If the biosensor does not adhere properly or if it falls o after
application, remove it and replace with a new one.
Note: A quick start guide with instructions for correct biosensor placement
is located on the outside of the package.
Connecting to the G5 application
5 Connect G5 biosensor to G5 software application
a Open the app on the mobile
device by tapping the icon
for the G5 software app.
Note: G5 app will be loaded on the
customer supplied mobile device
by Philips Field Service.
Note: The app will prompt you to
enter your password after logging
in for the first time.
b Enter the new password in the New Password and Conrm New
Password elds. During login, the app will oer 4 opportunities to
enter the correct password before the app terminates itself.
Note: Do not forget your password, as without it any data collected with
the app will not be recoverable.
c Enter patient ID (required) and patient assignment information
(optional). Select Save and Connect to G5 Biosensor.
Note: Do not enter patient’s medical record number (MRN) or patient’s
name as patient ID.
Page 16

15
Note: The alphanumeric Biosensor ID can be found on the
biosensor package, underneath the square QR code labeled
Biosensor ID.
d Select the patient’s Biosensor ID from the list of biosensors
displayed. Scroll down, if applicable, to ensure all biosensors in the
range are visible. If the biosensor does not appear, proceed to the
troubleshooting steps (page 24).
e Tap Connect if G5 biosensor ID and patient ID are correct. If not,
tap Cancel.
Note: If the patient ID or Biosensor ID is incorrect, select Cancel to
return to the biosensors in the range screen.
Note: The biosensor pairing process is not instantaneous. It may take
a little time.
Note: If the app could not connect to the selected biosensor, verify whether
the correct biosensor was selected. If not, select the correct biosensor.
f Once the biosensor has successfully connected with the app, the
app dashboard will be displayed. The green light on the biosensor
will become steady and will stay on for 20 seconds, and then turn
o automatically.
g Visually conrm that the heart rate measurement is displayed on
the app. Now the biosensor is properly set up.
h The default reporting interval is heart rate every 1 minute. Change
the reporting interval by clicking Change.
Note: If the app doesn’t display heart rate, review the troubleshooting steps.
Note: In the event the biosensor is out of range of the app, it will
automatically reconnect with the app when it is in range again.
Page 17

16
Product operation
Disconnecting biosensor and saving patient data
Note: Use caution when removing biosensor to prevent skin irritation.
Take care not to pull the hair or skin. An adhesive tape remover may help
with removal.
Note: Dispose of the biosensor according to local laws for battery
operated electronics and hospital guidelines.
1 Disconnect biosensor and begin exporting patient data
a Tap menu on top right of
the app dashboard screen.
Select Disconnect, Save and
Exit.
b Using a USB cable, plug
the mobile device into the
computer where patient data
will be stored. Click Next to
proceed.
c Tap Disconnect and Export
Data on app screen. This will
disconnect the G5 biosensor,
if one is paired, and begin
the process of exporting the
patient’s data.
Note: You must continue with step 2
in order to save the patient’s data.
2 Save patient data
on computer
• On the computer, click on the
folder icon in the toolbar.
• On the left hand side of the
window, click on “Samsung”
• Navigate through the folders:
Android > Data > com.philips.
cs.g5.android > Files > Export
Page 18
17
• If this folder is empty, wait until the le export has completed.
• Click on the zip le. This will open a new window.
• Click on the ‘export’ folder.
• This will display a few Excel les. Highlight the Excel le that starts
with “G5_RAW”
• Click “extract to”
• Click “OK”
• Enter the password that you created for the app
The les are now saved to the computer’s desktop for future review.
Note: Use the app login password to open the .zip files for viewing on the
computer. See the ‘Reviewing data’ section on page 22 for additional
instructions for viewing patient data.
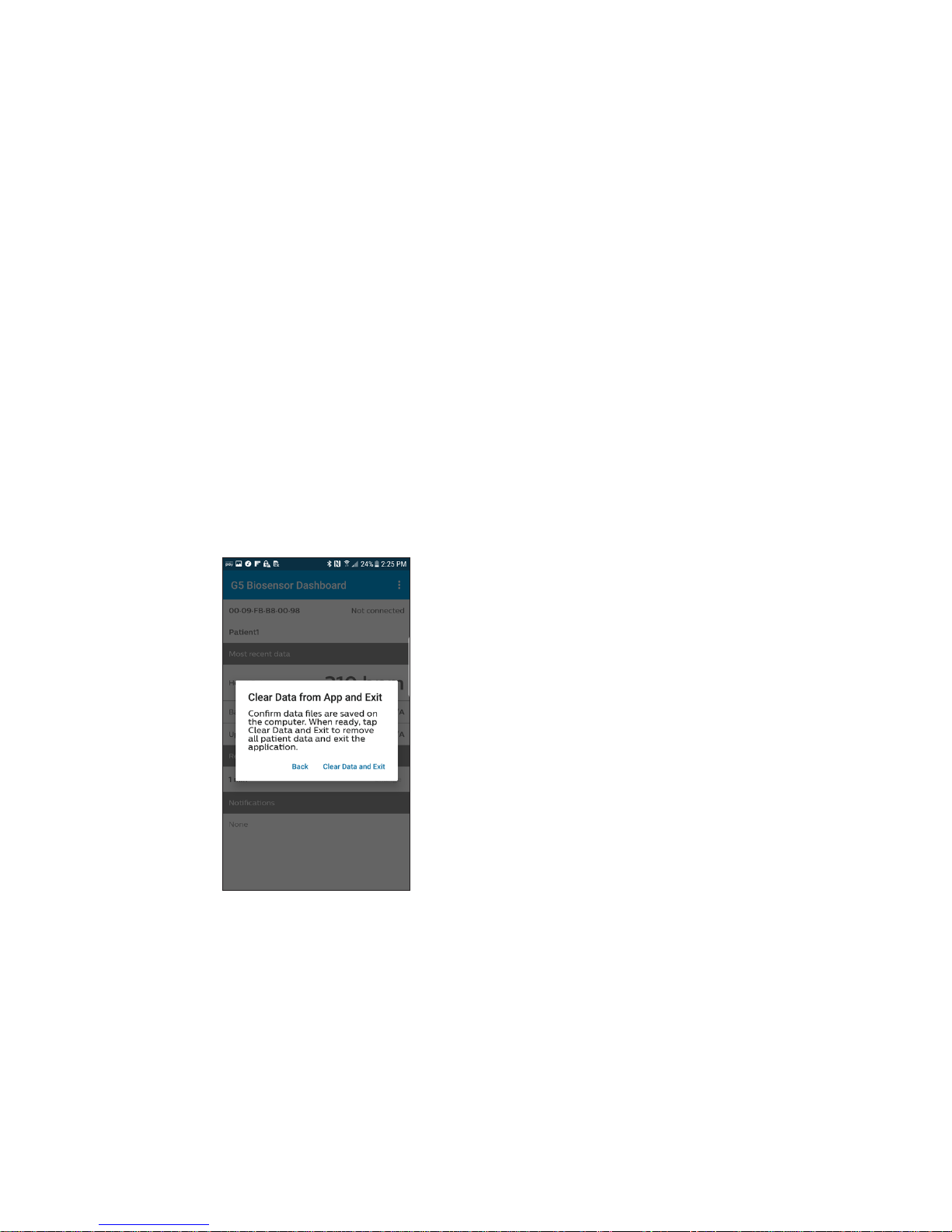
3 Clear data from app
and exit
Note: Be sure to exit the app to
delete the patient’s files on the
mobile device.
a Tap Clear Data and Exit. This
will clear out all patient data
from the app and exit the
application.
4 Remove biosensor from patient
a Gently peel each side of the biosensor one at a time, leaving the
center adhered. Gently swab with water while removing the device.
b Gently peel the center of the biosensor from top to bottom, until the
entire surface becomes loose and comes o.
c Use adhesive tape remover if necessary.
Note: If the same patient requires another biosensor, repeat steps on
page 12 for setup and connection of new biosensor.
Page 19
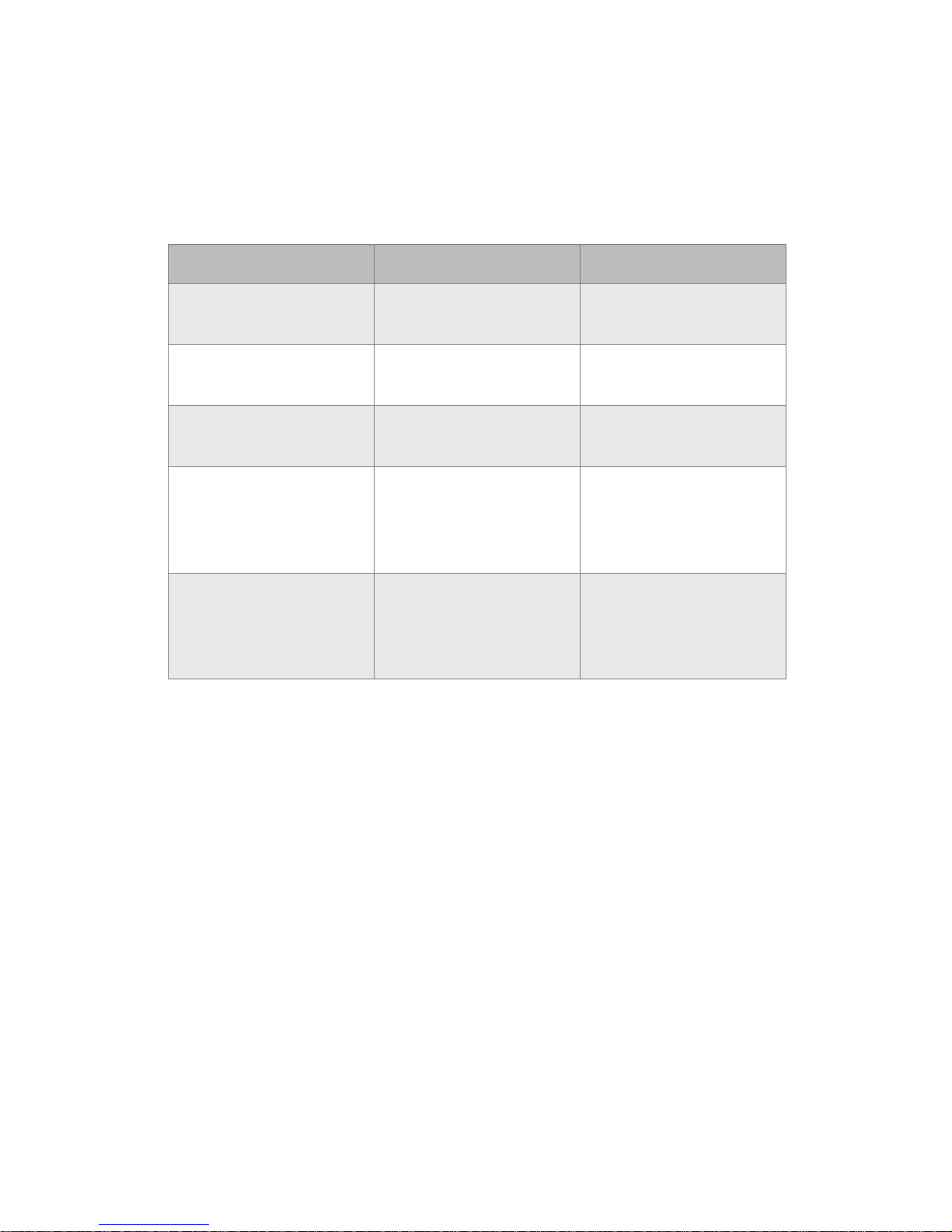
18
Biosensor interface
The biosensor interface consists of a one-time ON button and LED light.
The LED light indicates the status of the device.
LED Behavior Meaning Action Required
Green ashing Biosensor ON/Setup
in process
Continue with setup
Steady green for 20
seconds
Setup complete None. Biosensor is
operating correctly
Flashing red Error Remove and replace
biosensor
No light (biosensor
broadcasting
to app)
Biosensor is
operational
None
No light (biosensor
not broadcasting
to app)
Biosensor is not
functional
Remove and replace
biosensor
If the light on the biosensor ashes red at any time during use, remove
the biosensor (see Remove biosensor from patient) and replace it with
a new one.
Page 20
19
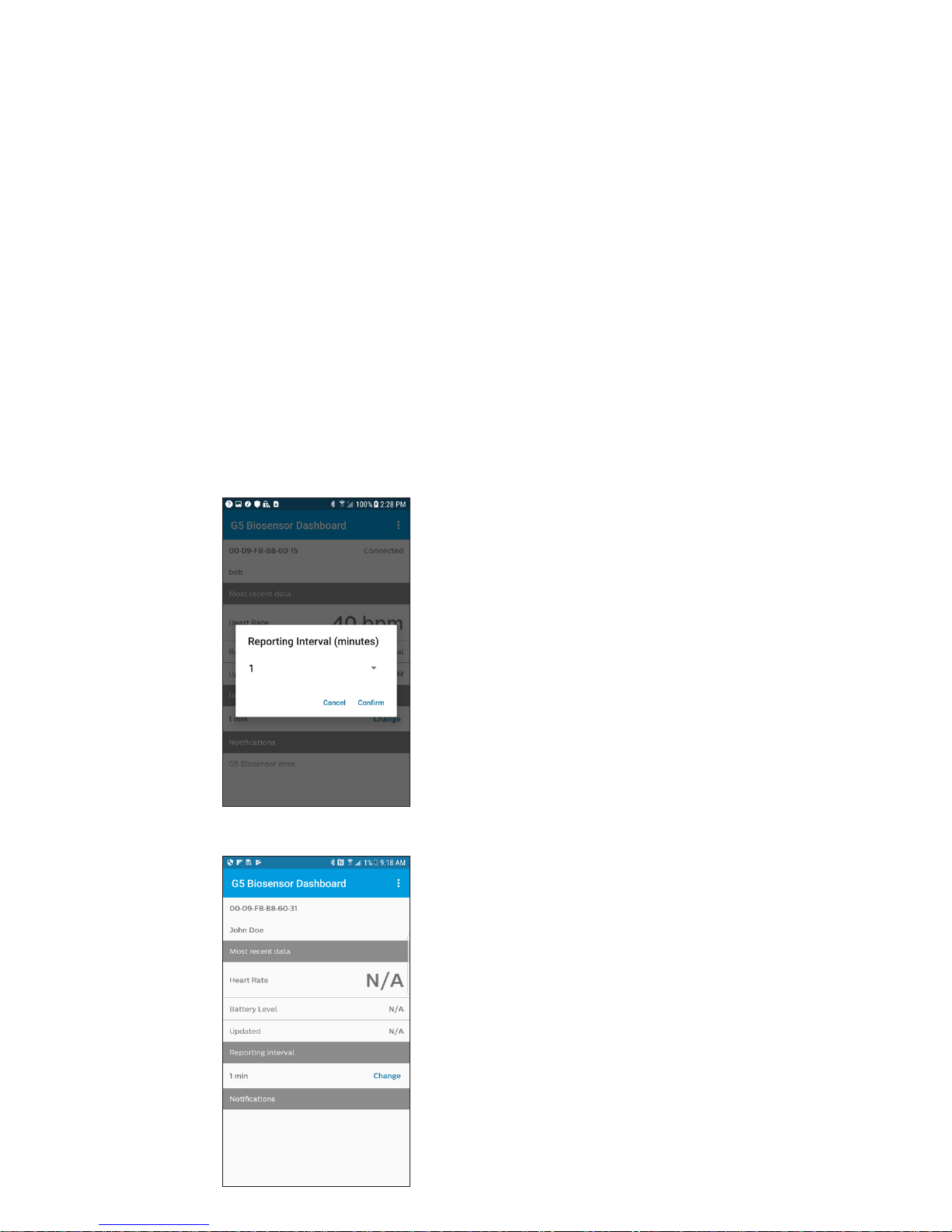
G5 application interface
The app dashboard will display the following in addition to the heart rate:
• Biosensor ID of connected G5 biosensor
• Patient ID of assigned patient
• (Optional) patient assignment information of assigned patient
• Date and time of displayed heart rate
• Biosensor battery status
• Notications
Heart rate transmission
conguration
The frequency of data collection
and transmission is clinician-
congurable. Transmission
intermittence period can be
adjusted from 1 minute to 30
minutes. The biosensor will store
data locally up to 4 hours.
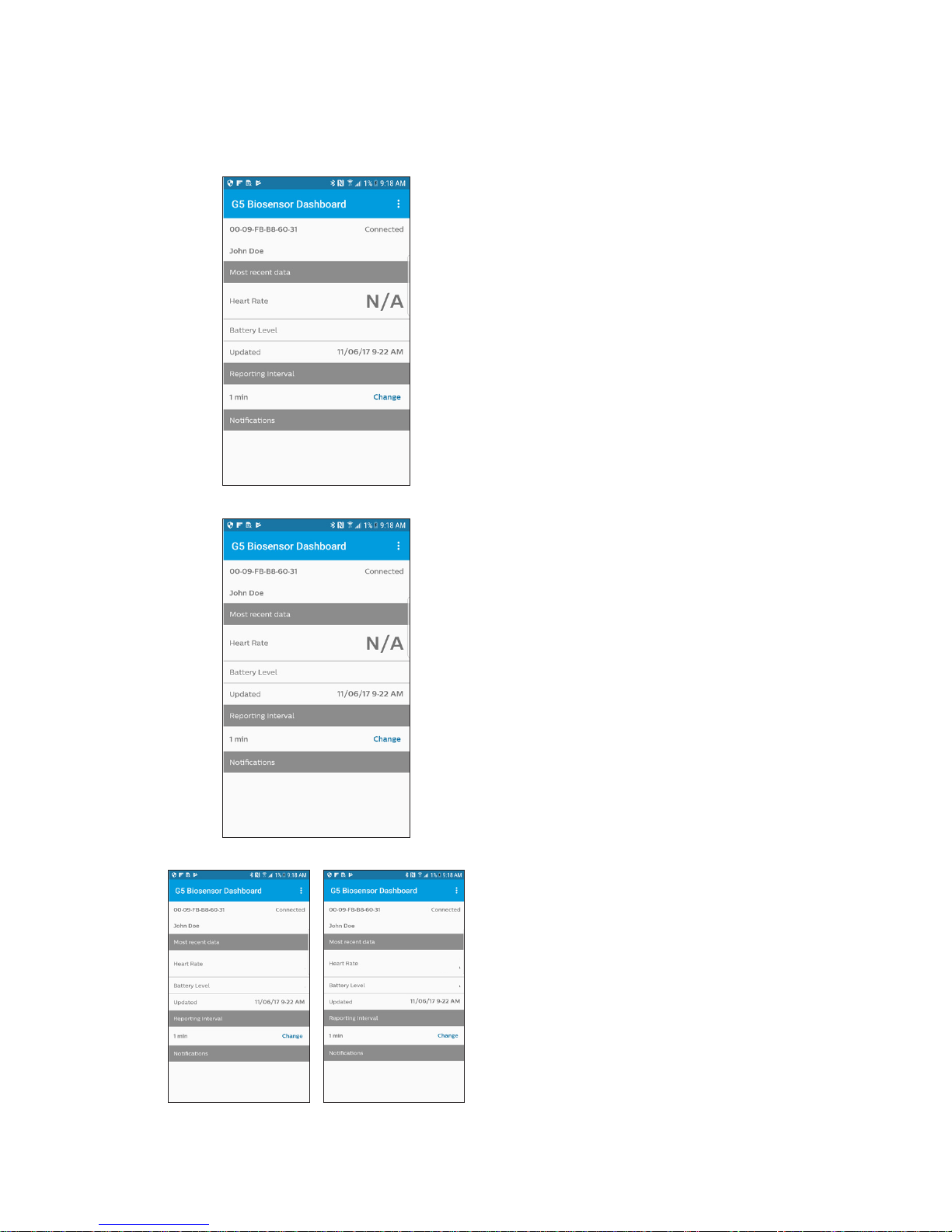
Notications
‘No connection’
No Connection notication will be
displayed whenever biosensor is
not connected with the app.
NoConne ction: The biosensor isnot
connect ed tot heapp. Ensure t he biosensor
and mobile device a rene are achot her.Ift hat
doesnot w ork, see theTrouble shooting
section of the Instr uctions forUse.
Noc onnect ion
Page 21
20
Notications continued
‘Pace pulse detected’
The Philips biosensor-G5 can
detect a patient’s pacemaker
pulses. Pace pulse detection is
used to ag, not reject, pacing
events. Pacemaker pulses are
detected when amplitudes from
±2mV to ±700mV; pulse widths
from 0.1ms to 2ms; and rise time
of 10% of the pulse width, but not
greater than 100μs are present.
‘Leads o’
Leads o notication is displayed
on the app when the biosensor
does not have appropriate
electrical contact with the skin.
Press down rmly on the device to
see if the notication is resolved.
If the notication continues,
remove and replace the biosensor
following the instructions in the
Basic operation section.
‘Heart rate out of range’
The biosensor can detect heart
rate from 30-220 bpm (beats per
minute). Whenever the heart rate
value calculated outside of this
range, a notication will indicate
Out of Range.
G5 application interface
Normal
HROut -of-Range
++
HROut ofR ange: Thebiosensor c andete ct
heartratefrom30-220bpm. Theca lculat ed
heart rate valueisout side oft hisra nge. Takea
confirmat ory,in dependent c heckof vitals.
Normal
HROut ofR ange: Thebiosensor c andete ct
heartratefrom30-220bpm. Theca lculat ed
heart rate valueisout side oft hisra nge. Takea
confirmat ory,i ndependent c heckof vitals.
--
Norma l
PacePulseDetected:Thebiosensor candetect
apat ient’s pac emake rpulses. Pa cepulse
detec tion is usedt oflag ,not reje ct, pac ing
event s. No act ioni sne cessa ry.
Norma l
Leads Off:The biosensor does nothave
sufficient electricalc ontactwith the
patie nt’sskin. Pr essdown firml yon the
device. Ifthis notifica tion continues,
remove andre place the biosensor.
Page 22

21
‘Heart rate invalid’
The biosensor may detect a noise
in the signal or another error which
may cause the heart rate to be
invalid. This may occur when a
patient is moving, and the activity
could impact the heart rate value.
‘Low battery’
The biosensor will send low battery
notication in the event the battery
level falls below the normal level.
‘Biosensor error’
The biosensor has detected
system error that will be displayed
under notication log. Refer to
biosensor troubleshooting section
for next steps.
Norma l
Biosensor Error :T hebiosensor has detec ted
asystemerror.RefertotheTroubleshooting
section of the Instr uctions forUse.
92
Low
LowB att ery: Thebiosensor ba tte ryhas
fallenbelowthenormallevel.Remove
and repla cet hebiosensor, ifne cessar y.
Page 23

22
Reviewing data
The G5 software application provides the ability to export a le for
retrospective review and analysis on a computer.
The le must be unlocked (using the same password that was used on
the G5 biosensor app) to review and analyze the data. Time-stamped
heart rate data and notifications are listed in the file. Files are named
using patient ID and date. Use any data analysis software program to
sort the data by date, as needed.
Page 24

23
Maintenance
Cleaning and disinfection
The biosensor is a disposable, single use device. Do not reuse the
biosensor. After use, the biosensor is considered non-biohazardous waste
and should be discarded according to hospital guidelines and local laws
for battery operated electronics.
Refer to the instructions for use for the mobile device for cleaning and
disinfection procedures.
Storage
Biosensors must be stored in their sealed pouch. The pouch cannot be
resealed after opening. The biosensor should be used immediately after
opening the pouch to prevent the hydrogel from drying.
Biosensors should be stored at:
• Temperature between 15 and 35 °C (59 and 95°F)
• Humidity between 5 and 95%
Biosensors should not be stored in direct sunlight.
Page 25

24
Troubleshooting
The patient’s heart rate is not displayed in the software app
• Press the ON button on the biosensor. If the green light ashes, the
biosensor was not previously turned on.
• If no light ashes,
• Ensure the biosensor and the mobile device are next to each other.
• Close the app, turn the mobile device Short Range Wireless o
and on and re-launch the app and proceed to connect biosensor
to the app.
• If this does not resolve the issue, close the app, restart the mobile
device and attempt to connect to the biosensor.
• If this does not resolve the issue, remove the biosensor and replace
with a new one.
The biosensor won’t properly adhere to the patient’s chest
• If you touched a large area of the adhesive, you may need to dispose of
the biosensor and apply a new one.
• Before applying a new biosensor to the patient’s chest, ensure the skin
is clean of any oil, lotion, debris or residue and the area is completely dry.
• Press rmly to adhere the sensor to the patient’s skin.
The patient’s heart rate does not seem to be updating
• Ensure the biosensor and the mobile device are next to each other.
The Biosensor ID is not found
• Press the ON button on the biosensor. If the green light ashes, the
biosensor was not previously turned on.
• If no light ashes,
• Ensure the biosensor and the mobile device are next to each other.
• Close the app, turn the mobile device Short Range Wireless o
and on and re-launch the app and proceed to connecting biosensor
to the app.
Page 26

25
• If this does not resolve the issue, close the app, restart the mobile
device and attempt to connect to the biosensor.
• If this does not resolve the issue, remove the biosensor and replace
with a new one.
The red light is ashing on the biosensor
• The biosensor has a low battery or has an error.
• Remove and discard the biosensor and replace it with a new one.
The biosensor appears partially adhered to the patient
• Check to see if the biosensor has a red ashing light.
• If the red light is ashing, remove and replace the biosensor.
• If there is no red light ashing, press down rmly on the biosensor
to adhere.
• If the biosensor still does not adhere, remove and replace
the biosensor.
The G5 app shows “Could not connect to G5
biosensor” message
• Ta p Cancel to return to the biosensors in the range screen.
• Enter the patient information and select correct Biosensor ID.
The biosensor is causing skin irritation
• Gently remove the biosensor and assess the skin irritation. Treat the
area per clinical practice, if needed.
• Replace the biosensor with a new one, selecting a dierent area of the
upper left chest to avoid further irritating the patient’s skin.
• If skin irritation persists, discontinue use.
Page 27

26
Symbols
Do not reuse
Non-ionizing radiation
Manufacturer
Use by date
Batch code
Caution
MR unsafe
Debrillation Proof
Type CF Applied Part
(Entire G5 biosensor
is an applied part)
Read instructions for use
Do not use if package is
damaged
Prescription use only
Storage humidity
range limits
Storage temperature
range limits
Box of 5
Protected against access
to hazardous parts and
the ingress of solid foreign
objects greater than
12.5mm (0.5 inch); and,
protected against eects
of temporary immersion.
Catalogue number
Specications
Storage ambient
pressure range limits
Page 28

27
Manufacturer’s information
Connected Sensing – Division of Philips Medical Systems
50 Milk Street
Boston, MA 02109, USA
(800) 225-0230
For more information or to reorder, go to
www.philips.com/healthcarestore
Locate your local Philips sales oce at
www.healthcare.philips.com
Open source software
Following is a list of software used for the development of G5 Application.
Title Description Version Vendor
Zip4j Password protect the
exported data
1.2.4 net.lingala
OpenCSV Library to create CSV les 4.0 OpenCSV
Android
SDK
Platform to build and run
Android apps
API25 Google
Butterknife Annotation processing 8.8.1 Square
Regulatory and safety specications
This Philips product has been tested in a typical conguration as
described in this Instructions for Use, and are fully compliant with the
standards listed below.
• EN IEC 60601-1:2006, EN IEC 60601-1:2006/A1:2013, General
requirements for basic safety and essential performance.
• EN 60601-1-2:2015, IEC 60601-1-2:2014, General requirements for basic
safety and essential performance.
• Collateral standards: Electromagnetic Compatibility requirements
and test.
• EN IEC 60601-1-6:2010, General requirements for basic safety and
essential performance.
• Collateral standards: usability.
Page 29

28
Specications
Regulatory and safety specications continued
• EN ISO 10993-1:2009, EN ISO 10993-1:2009/AC:2010 ISO 10993-1 and
Biological Evaluation of Medical Devices
• EN ISO 10993-5:2009 Biological Information of Medical Devices-Part 5:
Test for cytotoxicity
• ISO 10993-10:2010 Biological Information of Medical Devices-Part 10:
Test for irritation and skin sensitization
• ANSI/AAMI/IEC 60601-2-47:2012, EN 60601-2-47:2001 Particular
requirements for the basic safety and essential performance and
ambulatory electrocardiographs system.
• ANSI/AAMI/IEC 60601-2-27:2011, IEC 60601-2-27 Ed 3.0 2011-03
Particular requirements for the basic safety and essential performance
of Electrocardiographic Monitoring Equipment
• ANSI/AAMI/ISO EC57:1998(R)2008, Testing and reporting performance
results of cardiac rhythm and st-segment measurement algorithm.
• ANSI/AAMI/ISO EC12:2000/®2010 Disposable electrodes
EMC and radio regulatory compliance
This Philips product complies with relevant international and national law
and standards on EMC (electromagnetic compatibility) for this type of
product when used as intended. Such laws and standards dene both the
permissible electromagnetic emission levels from product and its required
immunity to electromagnetic interference from external sources.
Other electronic products exceeding the limits dened in such EMC
standards could, under unusual circumstances, aect the operation of the
product.
• Medical electrical products needs special precautions regarding
EMC, and needs to be installed and put into service according to EMC
information provided in this Instructions for use.
• The use of accessories and cables other than those specied, may
result in increased emission or decreased immunity levels.
• The product should not be used adjacent to or stacked with other
products and that if adjacent or stacked use is necessary, it should be
observed to verify normal operation.
Page 30

29
FCC compliance statement
Caution: Changes or modications not expressly approved could void your
authority to use this equipment.
This device complies with Part 15 of the FCC Rules. Operation is subject to
the following two conditions:
1 this device may not cause harmful interference and
2 this device must accept any interference received, including
interference that may cause undesired operation.
Changes or modications not expressly approved by the party responsible
for compliance could void the user’s authority to operate the equipment.
This equipment has been tested and found to comply with the limits for a
class B digital device, pursuant to part 15 of the FCC Rules. These limits are
designed to provide reasonable protection against harmful interference in
a residential installation. This equipment generates, uses and can radiate
radio frequency energy and if not installed and used in accordance with
the instructions, may cause harmful interference to radio communications.
However, there is no guarantee that interference will not occur in a
particular installation. If this equipment does cause harmful interference
to radio or television reception, which can be determined by moving the
equipment away and back, the user is encouraged to try to correct the
interference by one or more of the following measures
• Reorient or relocate the receiving antenna
• Increase the separation between the equipment and receiver
• Consult the dealer or an experienced radio/TV technician for help
Page 31

30
Equipment classication (according to IEC 60601-1)
According to the type of
protection against electrical
shock:
Internally powered ME
equipment
According to the degree of
protection against electrical
shock:
Debrillation Proof Applied Part
TYPE BF
According to the degree of ingress
protection:
IP27, Protected against access
to hazardous parts and the
ingress of solid foreign objects
greater than 12.5mm (0.5 inch);
and, protected against eects of
temporary immersion.
According to the mode of
operation:
Continuous operation
ME equipment Type Body-worn
The device is intended for use in the electromagnetic environment
specified below. Given the device’s electromagnetic emissions and
immunity characteristics, the customer or user should assure that the
device is used within such an environment. The following information
is mandated by IEC 60601-1-2, the international standard for the
electromagnetic compatibility (EMC) of medical electrical equipment.
Specications
Page 32
31
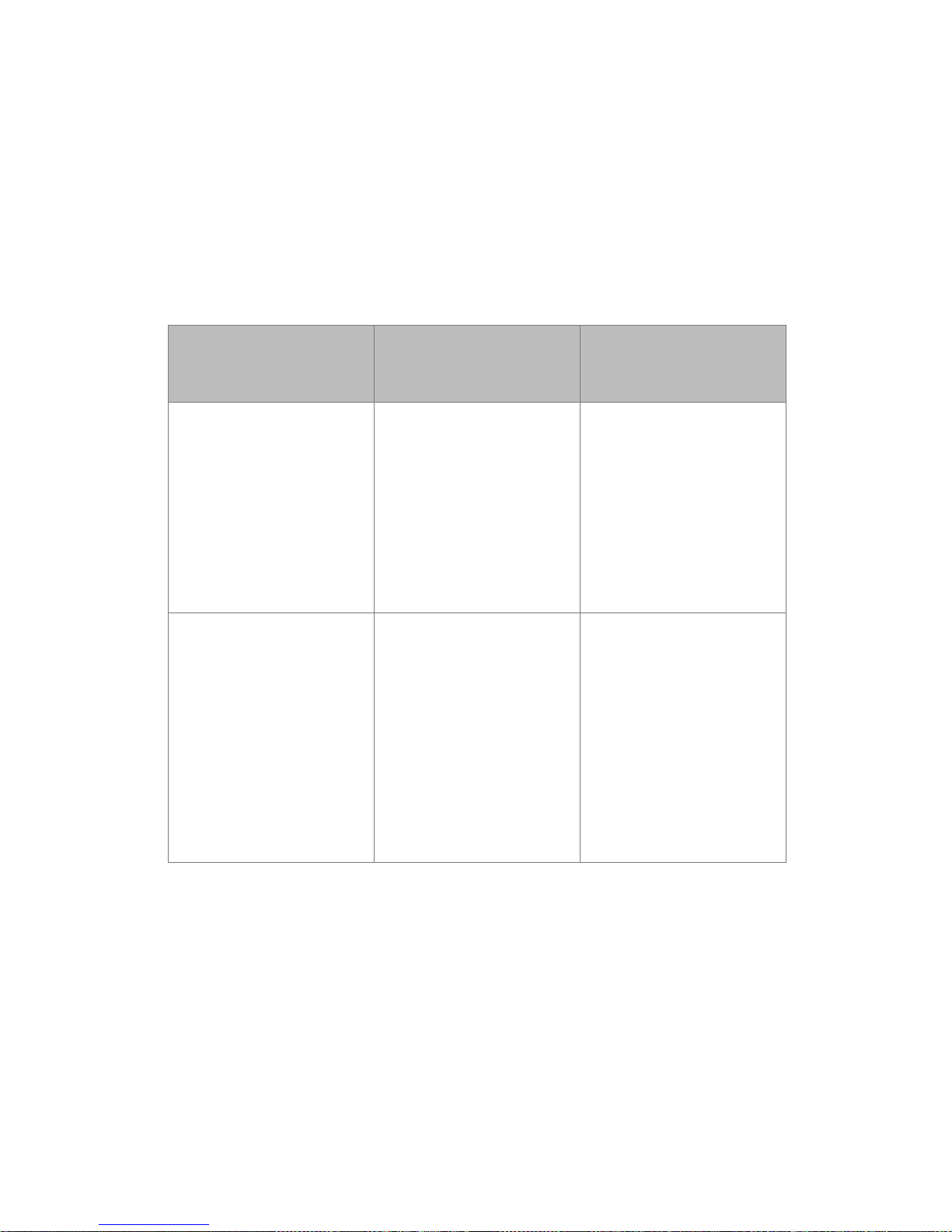
Guidance and manufacturer’s declarationelectromagnetic emissions
The Philips wearable biosensor-G5 Solution is intended for use in the
electromagnetic environment specied below, and the customer or the
user should assure that it is used in such an environment.
Emission Test Compliance Electromagnetic
Environment
Guidance
RF Emissions, CISPR 11 Group 1 The Philips wearable
biosensor-G5 Solution
uses RF energy only for
its internal functions.
Therefore, its RF
emissions are very low
and are not likely to
cause any interference
in nearby electronic
equipment.
RF Emissions, CISPR 11 Class A The Philips wearable
biosensor-G5 Solution
is suitable for use in
all establishments,
other than domestic
establishments
and those directly
connected to the public
low voltage power
supply network that
supplies buildings used
for domestic purposes.
Page 33
32
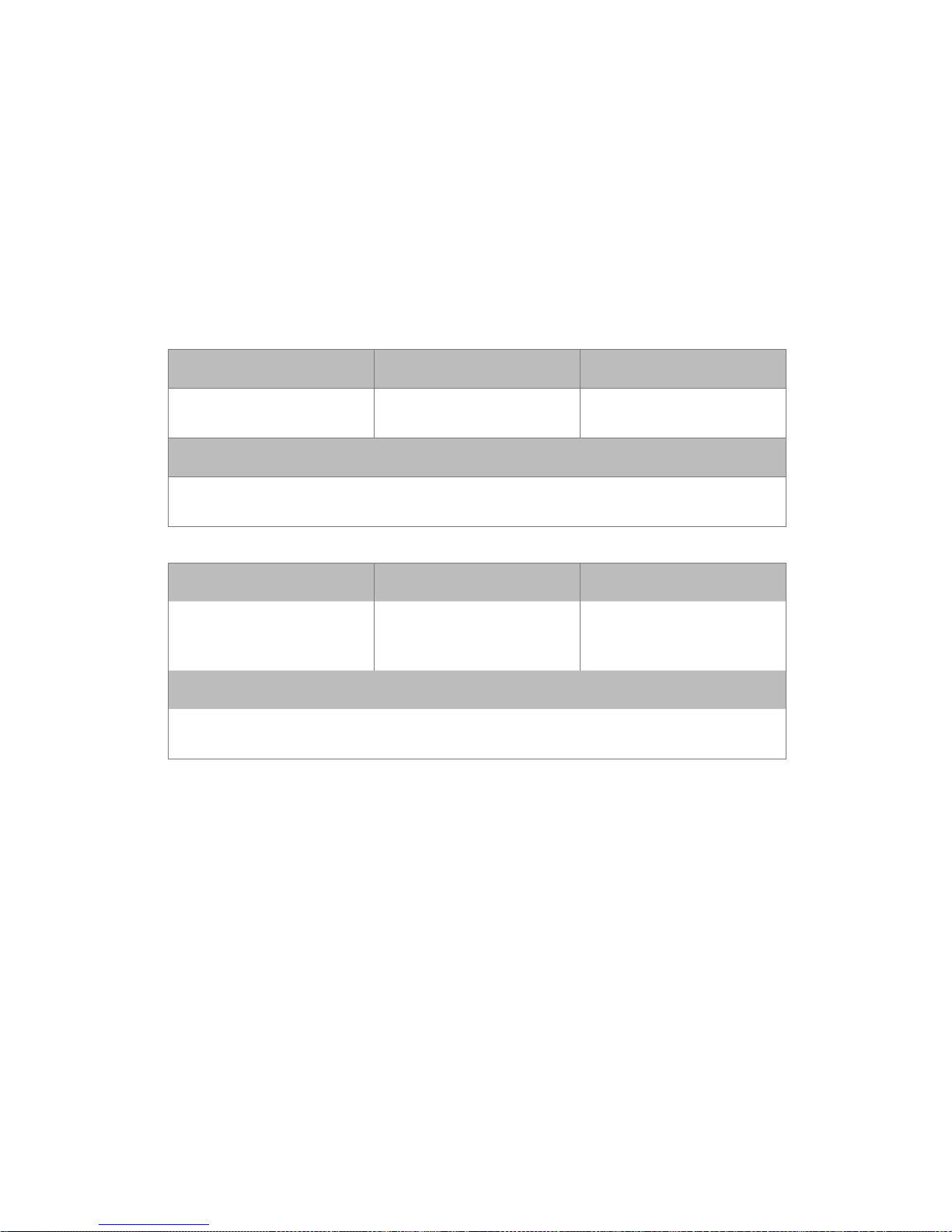
Guidance and manufacturer’s declarationelectromagnetic immunity
The Philips wearable biosensor-G5 Solution is intended for use in the
electromagnetic environment specied below. The customer or the user
of the Philips wearable biosensor-G5 Solution should assure that it is
used in such an environment.
Immunity Test IEC 60601 Test Level Compliance Level
Electrostatic discharge
(ESD) IEC 61000-4-2
± 8kV contact
± 15kV air
± 8kV contact
± 15kV air
Electromagnetic Environment Guidance
Floors should be wood, concrete or ceramic tile. If oors are covered with
synthetic material, the relative humidity should be at least 30%.
Immunity Test IEC 60601 Test Level Compliance Level
Power frequency (50/60
Hz) magnetic eld IEC
61000-4-8
30 A /m 30 A /m
Electromagnetic Environment Guidance
Power frequency magnetic elds should be at levels characteristic of a typical
location in a typical commercial or hospital environment.
Specications
Page 34

33
Immunity Test IEC 60601 Test Level Compliance Level
Radiated RF
IEC 61000-4-3
3 V/m 80-2700 MHz
plus intentional radiator
requirement Table 9 from
60601-1-2: 2014
3 V/m 80-2700 MHz
plus intentional radiator
requirement Table 9 from
60601-1-2: 2014
Electromagnetic Environment Guidance
Portable and mobile RF communications equipment should not be used no
closer to any part of the Philips wearable biosensor G5-Solution, including
cables, than the recommended separation distance calculated from the equation
applicable to the frequency of the transmitter. Recommended separation
distance is 30cm. Field strengths from xed RF transmitters, as determined by
an electromagnetic site survey,a should be less than the compliance level in each
frequency range. Interference may occur in the vicinity of equipment marked with
the symbol.
• These guidelines may not apply in all situations. Electromagnetic propagation
is aected by absorption and reection from structures, objects and people.
a Field strengths from fixed transmitters, such as base stations for radio (cellular/
cordless) telephones and land mobile radios, amateur radio, AM and FM radio
broadcast and TV broadcast cannot be predicted theoretically with accuracy.
To assess the electromagnetic environment due to fixed RF transmitters,
an electromagnetic site survey should be considered. If the measured field
strength in the location in which the Philips wearable biosensor-G5 Solution is
used exceeds the applicable RF compliance level above, the Philips wearable
biosensor-G5 Solution should be observed to ensure normal operation. If
abnormal performance is observed, additional measures may be necessary,
such as re-orienting or relocating the Philips wearable biosensor-G5 Solution.
Page 35

34
Specications
Biosensor specications
Hardware
Size (W x H x D) 100mm x 69mm x 6.2mm ±5% (without
the release liners)
Weight 12 g ±10%
Battery CR2032, 3V primary cell
Memory 1MB non-volatile ash
Robustness Survives shock, vibration, free fall,
and bump
Ingress Protection IP27
Manufactured with Latex No
Use
MRI Safe No
Singe Use Yes
Disposable Yes
Serviceable No
Performance
Heart Rate Measurement Range 30-220 bpm (beats per minute)
Heart Rate Accuracy 10% or ±5bpm (whichever is greater)
Heart Rate Resolution 1 bpm
Heart Rate Calculation Heart Rate is calculated:
• Taking into account last 10 beat-tobeat intervals
• Excluding the minimum and the
maximum intervals
• Averaging the remaining eight
intervals to compute “mean_interval”
and
• Compute 60/mean_interval (in
seconds) to convert to bpm
Heart Rate Sampling Rate 250 samples per second
Heart Rate Meter Accuracy and
Response to Irregular Rhythm
Provides correct heart rates (60, 80,
90, 120 bpm) using test waveforms
as indicated in ANSI/AAMI EC13 Sec.
4.1.2.1(e). All QRS are counted with test
waveforms within HR accuracy dened
above
Response time of heart rate meter to
change in heart rate
Inside a reporting interval,
instantaneous heart rate change from
80 bpm to 120 bpm shall be captured
within 8 seconds (margin of +2 sec)
Page 36

35
Debrillator-Proof Debrillator has no adverse eects on
biosensor
Applied Current 29.1 μA (max), 32 kHz current pulse is
applied to the patient
Tall T Wave Rejection Up to 1mV peak to peak will be rejected
Wireless
Radio Bluetooth Low Energy (4.2)
Transmission 1-30 minutes (programmable)
Local Storage 4 hours
Battery Life 4 days
Frequency Band 2402-2480 MHz
RF Radiate Power Output Transmit Power 0dBm(1mW)
Maximum power 8dBm (6.31mW)
Operating Range 10 meters, Line of Sight
Environmental
Operating Temperature Range 15-35 °C
Operating Humidity Range 20-85 %
Operating Atmospheric Pressure Range 10-106 kPa
Storage Temperature Range 15-35 ° C
Storage Humidity Range 5-95%
Storage Ambient Pressure Range 50-106 kPa
Shelf Life 3 Months
Software application specications
Mobile device
Operating System Android OS 7.0 or higher
Compatible Device Smartphone with Bluetooth Low
Energy (4.2)
8 GB Storage
1GB RAM
Dashboard screen
Heart Rate (Instantaneous) bpm (beats per minute)
Reporting Interval 1, 2, 3, 4, 5, 10, 15, 30 minutes
Page 37

www.philips.com
© 2018 Koninklijke Philips N.V.
All rights reserved. Printed in the United States
of America. First printing: January 2018
Part Number: 453564741671
Artwork: A-453564741671-2 Rev B
 Loading...
Loading...