Penlon Sigma Delta User manual
Sigma Delta Vaporizer
User Instruction Manual
Quality and Assurance in Anaesthesia
This manual contains
calibration and service
records for Sigma Delta
Vaporizer, Serial No.
. . . . . . . . . . . . . . . . . . . .
Keep this manual with
the vaporizer at all times

WARNING
Anaesthetic systems have the
capability to deliver mixtures of
gases and vapours to the patient
which could cause injury or
death unless controlled by a
qualified anaesthetist.
There can be considerable
variation in the effect of
anaesthetic drugs on individual
patients so that the setting and
observation of control levels on
the anaesthesia systems does
not in itself ensure total patient
safety.
Anaesthesia system monitors
and patient monitors are very
desirable aids for the
anaesthetist but are not true
clinical monitors as the
condition of the patient is also
dependent on his respiration
and the functioning of his
cardio-vascular system.
IT IS ESSENTIAL THAT THESE
ELEMENTS ARE MONITORED
FREQUENTL Y AND REGULARLY
AND THA T ANY OBSERVATIONS
ARE GIVEN PRECEDENCE
OVER MACHINE CONTROL
PARAMETERS IN JUDGING THE
STATE OF A CLINICAL
PROCEDURE.

Servicing and Repairs
In order to ensure the full
operational life of the Sigma Delta
vaporizer, we recommend that a
periodic service check should be
performed by a Penlon trained
engineer. This check comprises a
vaporizer CALIBRATION CHECK
and LEAK CHECK.
Note:
(a) The calibration check must be
performed using a suitable agent
analyser, e.g. a Riken
refractometer or infrared analyser.
(b) The service check is part of the
recommended pre-use check for
your Anaesthesia System.
Should the calibration check show
the unit to be outside the specified
performance requirement (see
section 11) then a service must be
performed.
This may be done on site by:
(a) A trained user.
(b) An authorised Penlon
agent.
(c) A Penlon service engineer.
A calibration and service record
section is provided to maintain a
record of the vaporizer's
performance.
For any enquiry regarding the
service or repair of this vaporizer,
contact the nearest accredited
Penlon agent* or contact the
Service Department at Penlon
Limited.
*Agent's name and address:
Service and Repair Department
Penlon Ltd
Abingdon
OX14 3PH
UK
Tel: +44 (0) 1235 547063
Fax: +44 (0) 1235 547062
E-mail: service@penlon.co.uk
Always give as much of the
following information as possible:
1. Type of equipment
2. Product name
3. Serial number
4. Approximate date of
purchase
5. Apparent fault
(i)
IMPORTANT

This manual has been produced to
provide authorised personnel with
information on the function, routine
performance and maintenance
checks, applicable to the Penlon
Sigma Delta vaporizer.
Information contained in the
manual is correct at the date of
publication. The policy of Penlon
Limited is one of continued
improvement to its products.
Because of this policy Penlon
Limited reserves the right to make
any changes, which may affect
instructions in this manual, without
giving prior notice.
Personnel must make themselves
familiar with the contents of this
manual before using the vaporizer.
Terminology
This manual complies with ISO
4135, Anaesthetic Apparatus
Terminology.
The following additional definitions
should be noted:
Vol.% - shortened form of
volumetric percentage.
The commonly used method of
expressing vapour concentrations
so that they can be compared with
concentrations of true gases.
100 Vol.% is equivalent to 100%
partial pressure in a mixture.
Copyright © Penlon Ltd, 2002.
All rights reserved.
(ii)
FOREWORD

USER RESPONSIBILITY 1
1. WARNINGS AND CAUTIONS 2
2. PURPOSE 7
3. DESCRIPTION
3.1 Operating Principles 8
3.2 Controls 8
4. SPECIFICATION
4.1 Physical Dimensions 10
4.2 Weight 10
4.3 Capacity 10
4.4 Filling System 10
4.5 Concentration Control Dial Scale 11
4.6 Patents 11
4.7 Temperature Range 11
4.8 Flow Range 11
4.9 Pressure Range 11
5. FILLING AND DRAINING
5.1 Key Filler System 12
5.2 Screw Cap (Pour Fill) System 15
5.3 Quik-Fil Filling System 18
6. INSTALLATION
6.1 Gas Port Transit Seals 21
6.2 Selectatec Compatible Models (with interlock) 21
6.3 Cagemount (23 mm) Taper Models 23
6.4 Penlon Off-line System (Mk.2 and Mk.3) 24
6.5 Drager 'Plug-in' Compatible 25
6.6 North American Drager Compatible 26
6.7 Pre-Use Checklist - All Models 27
7. PERFORMANCE CHARACTERISTICS
7.1 Performance Graphs 28
7.1.1 Halothane Models 29
7.1.2 Enflurane Models 30
7.1.3 Isoflurane Models 31
7.1.4 Sevoflurane Models 32
7.2 Temperature Compensation 33
CONTENTS
(iii)

7.3 Pressure Effects 33
7.3.1 Ambient Pressure 33
7.3.2 Back Pressure 33
7.3.3 Intermittent Back Pressure 33
7.4 Summary of Performance Specifications 34
7.4.1 Factors Affecting Output Accuracy 34
7.4.2 Resistance to Gas Flow 35
7.5 Effect of IPPV on Output 35
7.6 Effect of Gas Composition on Output 35
7.7 Output when Control is at 0 (Zero) 35
7.8 Effect of Flush Valve Operation 36
8. USER MAINTENANCE
8.1 Servicing 37
8.2 Cleaning 38
8.3 Draining - Halothane Models 38
8.4 Checking Vaporizer Output 38
8.5 Training Course 39
8.6 Returning the Vaporizer for Service or Repair 39
9. REFERENCES 40
10. ORDERING INFORMATION 41
11. SERVICE RECORDS
11.1 Service Policy 42
11.2 Calibration Procedure using the Riken Analyser 43
11.3 Servicing and Repair Details 46
11.4 Calibration Check 50
CONTENTS
(iv)

This vaporizer has been built to
conform with the specification and
operating procedures stated in this
manual and/or accompanying
labels and notices when checked.
assembled, operated, maintained
and serviced in accordance with
these instructions provided.
To ensure the safety of this
vaporizer it must be checked and
serviced to at least the minimum
standards laid out in this manual.
A defective or suspected defective,
product must not, under any
circumstances be used.
The user must accept responsibility
for any malfunction which results
from non-compliance with the
servicing requirements detailed in
section 8.1.
Worn, broken, distorted,
contaminated or missing
components must be replaced
immediately. Should such a repair
become necessary it is
recommended that a request for
service advice is made to the
nearest Penlon service centre.
This vaporizer and any of its
constituent parts must be repaired
only in accordance with written
instructions issued by Penlon
Limited, and must not be altered or
modified in any way without the
written approval of Penlon Limited.
The user of this equipment shall
have the responsibility for any
malfunction which results from
improper use, maintenance, repair,
damage or alteration by anyone
other than Penlon Limited or its
appointed agents.
This vaporizer must only be
supplied to, and used by, suitably
qualified medical practitioners.
In the USA and Canada:
Caution: Federal Law restricts this
device to sale by or on the order of
a physician.
Statements in this manual
preceded by the following words
are of special significance.
WARNING - means there is a
possibility of personal injury to
yourself or others.
CAUTION - means there is a
possibility of damage to the
instrument or other property.
NOTE - indicates points of
particular interest for more efficient
and convenient operation.
The reader must take particular
notice of the warnings, cautions.
and notes printed throughout the
manual.
USER RESPONSIBILITY
1

The following Warnings and
Cautions must be read and
understood before using this
vaporizer
WARNINGS
General Information
1. The user must read and
be familiar with the
contents of this
instruction manual before
using the vaporizer
2. The vaporizer is designed
for use only with the
specific anaesthetic agent
named on the filler block
(and further indicated by
colour coded labelling).
Misdosage may occur if
the vaporizer is filled with
the wrong drug.
Agent specific (keyed)
filler devices are provided
on certain models to meet
national and international
standards.
(See section 9 for
standards).
3. The pharmacopoeia name
of the drug is used on the
label according to BP,
USP, or Ph EUR.
The user is responsible
for confirming that any
trade name of a drug is
equivalent to the
registered name.
4 This vaporizer must not
be modified or
disassembled by an
unauthorised person. It
should be regularly
serviced by a Penlonauthorised service agent,
trained technician or
engineer and by no other
person.
(see section 8)
5. Vaporizers may
malfunction if exposed to
excessively high
temperatures, e.g. by
storage above a radiator.
This may affect the
calibration.
Maximum storage
temperature: 50oC (122oF)
Minimum storage
temperature: -20oC (-5oF)
Operating temperature
range: 15 to 35oC
(58 to 95oF)
Before use, function test
any vaporizer that has
been subjected to
temperatures near the
upper/lower limits given
above.
1. WARNINGS AND CAUTIONS
2

Filling and draining the
vaporizer
6. The filler system must be
maintained in accordance
with the instructions
given in the User
Maintenance section.
7. The vaporizer must be
filled only by suitably
skilled and trained
personnel.
8. Anaesthetic drugs are
poisonous and there is
evidence that there is a
health hazard to
personnel due to
prolonged inhalation of
trace concentration in the
atmosphere. Care must
be taken to avoid spillage
of anaesthetic drugs
when filling or draining
vaporizers.
9. The vaporizer control
must be in the 0 (zero)
position during the filling
or draining process.
Overfilling and/or spilling
may occur if the control is
not in the 0 (zero)
position.
Provided the control is in
the 0 (zero) position, gas
may continue to be
delivered from the
anaesthetic machine to
the patient during the
filling procedure.
10. The vaporizer must be
upright during filling to
minimise the risk of
overfilling.
11. Do not use the
anaesthetic agent bottle
to fill the vaporizer if the
bottle is cracked or the
filler connector is loose or
broken.
This may result in
overfilling or
contaminated agent
entering the vaporizer.
12. If a new bottle of
anaesthetic agent is to be
used, check that the
tamper-evident shrink
band is undamaged.
13. Ensure that the drain plug
screw, located on the
lower front of the
vaporizer, is correctly
tightened to prevent loss
of liquid agent.
14. Do not tamper with the
filling system valve. This
may cause a vapour and
fresh gas leak.
Anaesthesia system
monitors and patient
monitors are very
desirable aids for the
WARNINGS AND CAUTIONS
3

anaesthetist but are not
true clinical monitors as
the condition of the
patient is also dependent
on his respiration and the
functioning of his
cardiovascular system.
15. Do not overfill.
A vaporizer that has been
overfilled must be
withdrawn from use.
Contact the Service
Department at Penlon for
advice.
16. Anaesthetic drugs must
be treated as a
pharmaceutical product.
Liquid should never be
drained from a vaporizer
into an open container
and then reused.
Contamination is likely.
Always dispose of such
drained liquid as a
hazardous chemical.
17. After filling or draining:
Pour Fill (Screw cap) filler
- always refit and
retighten the filler cap.
Key (agent specific) filler
models - always tighten
the filler control.
In addition, on key filler
systems, always refit the
key filler plug and tighten
the clamp screw before
using the vaporizer. The
vaporizer will leak if this
is not done.
Quik-Fil models - remove
the bottle and refit the
filler block cap before
using the vaporizer.
Delivered concentrations
are inaccurate while the
filler port is open.
Before using the vaporizer
18. Do not use the vaporizer if
the agent level is not
visible in the sight glass
or the level is outside of
the Max - Min indicator.
19. If a vaporizer is
transported when filled
with liquid drug the
control must be in the 0
(zero) position during
transport and a period of
at least ten minutes in a
secured upright position
must elapse before
connection to an
anaesthetic breathing
system.
Movement during
transport can result in
over-dosage unless time
is allowed for drainage of
liquid to the normal
WARNINGS AND CAUTIONS
4

position.
If a vaporizer has been
transported with the
control in the open
position it must be
flushed at 5 L/min for ten
minutes before clinical
use on a patient
20. The vaporizer must not be
tipped over or inverted.
If the vaporizer has been
tipped over or inverted it
must be set to maximum
output and flushed at 5 L/
min for ten minutes
before clinical use on a
patient.
21. The vaporizer must be
securely fixed and in an
upright position before
connection to a patient.
There is a danger of
overdosage if sudden
inadvertent movement
occurs during use.
22. Anaesthetic machine
designs are constantly
evolving, and new models
may differ dimensionally
from existing equipment.
It is the user's
responsibility to ensure
that the configuration of
the anaesthetic machine
allows correct installation
of the vaporizer.
There must be sufficient
clearance between the
selectatec manifold and
the rear frame panelling
of the machine to allow
the vaporizer connector
block to seal correctly on
the manifold.
23. Before use test all joints
for gas tightness, and
perform back bar function
tests as detailed in the
anaesthetic machine user
manual.
Using the vaporizer
24. Check the liquid level
frequently when using the
vaporizer and maintain
the level between the min.
and max. marks.
The vaporizer control
must be in the zero (0)
position during the filling
process (see warning 7).
25. Vaporizer outputs are
sensitive to barometric
pressure.
A correction factor may
be necessary when
assessing the output
using an analyser, for
example at high altitude.
Barometric pressure
effects are not usually of
clinical importance. (see
section 7.3).
WARNINGS AND CAUTIONS
5

26. The vaporizer is a flow
direction-sensitive apparatus
and the direction of gas flow
towards the patient must be
as indicated by the arrow on
the top label.
Reversal of flow may cause
inaccuracies of delivered
concentration.
27. The vaporizer must not be
used downstream of the
common gas outlet.
28. As stated in section 2, the
vaporizer is of relatively high
resistance and must not be
incorporated within a
breathing system.
29. Expired anaesthetic vapours
should be extracted from the
theatre by an anaesthetic
gas scavenging system.
(see section 9 for
standards.)
30. Do not use the vaporizer
with flammable anaesthetics.
User Maintenance
31. Do not pour water, or any
cleaning solutions into the
vaporizer.
CAUTIONS
1 Anaesthetic machine and
workstation standards
require that means be
provided to ensure that gas
cannot pass through more
than one vaporizer
chamber.
Vaporizers without interlock
devices or systems must
only be used on machines
which only have one
vaporizer mounting station.
WARNINGS AND CAUTIONS
6

The Sigma Delta vaporizer is
designed for incorporation in the
fresh gas supply system of
continuous flow anaesthetic
machines, directly connected
between the flowmeter unit and the
common gas outlet of the machine.
The vaporizer is unsuitable for use
within a breathing system 'in circuit'
because of the relatively high
internal resistance.
Its purpose is the provision of
accurate concentrations of
anaesthetic drugs in the fresh gas
supply, in accordance with the
setting of the control dial, when the
fresh gas supply flow is between
0.2 and 15 litres/min.
Refer to section 7 (Performance
Characteristics), which shows the
extent of modifications to the
control calibration.
2. PURPOSE
7
3.1 Operating
Principles
Each model is uniquely designed
and tested for use only with the
drug specified on the filler block.
The vaporizer contains a chamber,
the base of which holds the anaesthetic agent in liquid form. A wick
ensures that the upper part of the
chamber is filled with the saturated
vapour of the agent. The wick has
a patented construction.
The concentration of saturated
vapour is many times higher than
those used clinically and the function of the concentration control is
to proportion the flow of the carrier
gas through a bypass passage and
through the vapour chamber so
that the desired dilution is produced.
In the zero position the bypass
remains open but the vaporizing
chamber is shut off completely
from the gas flow to the patient.
A temperature-compensating valve
is situated in the bypass, arranged
to operate so that as the vapour
pressure varies with temperature.
the dilution ratio produced by the
control valve is varied to compensate. and maintain a constant output concentration.
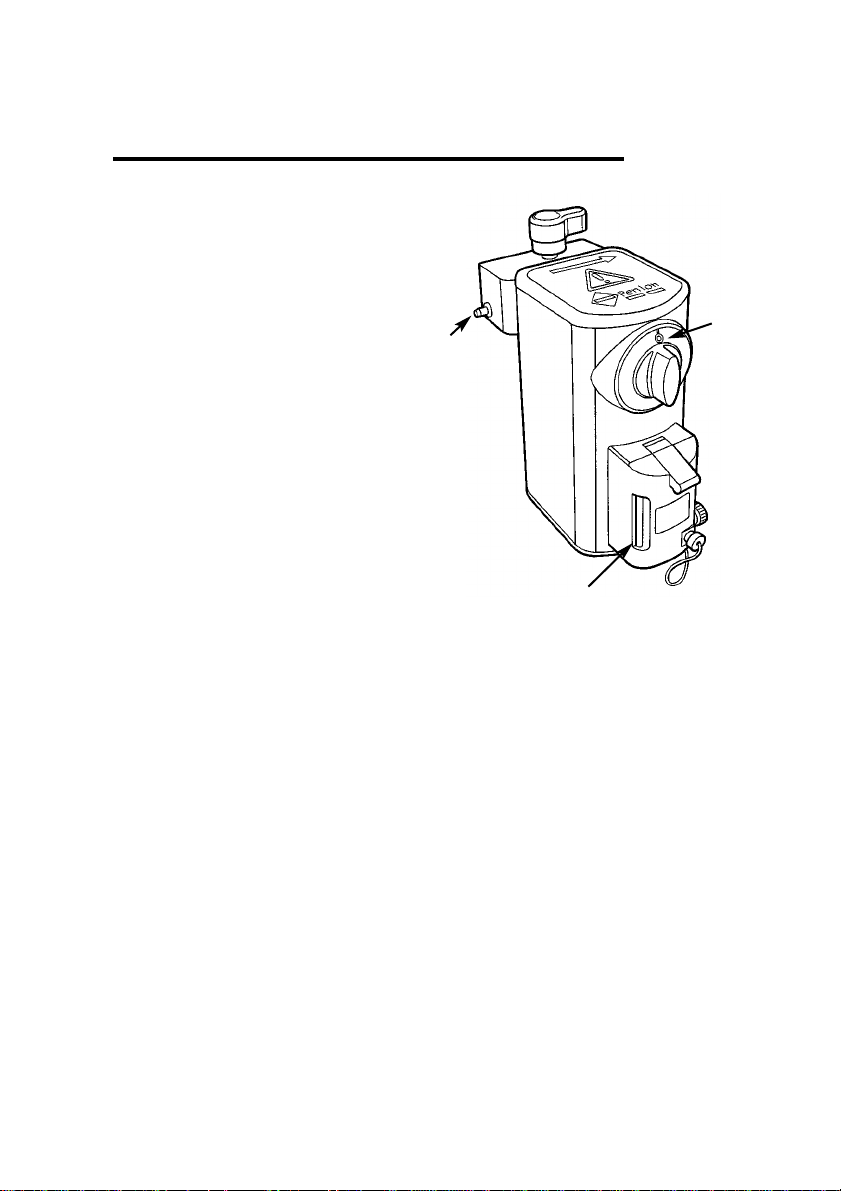
The vaporizer has a liquid level
indicator, with maximum and minimum level marks.
1. Liquid level indicator
2. Control dial 0 (zero) position
3. Interlock bolt
3.2 Controls
The vaporizer has a single, forward
facing calibrated control to regulate
the vapour concentration delivered.
The dial is locked at zero when not
in use. To set a concentration level,
push the dial assembly in and
rotate anti-clockwise.
Align the required concentration
graduation with the mark at the top
of the bezel.
On returning the dial to zero, the
dial assembly will automatically
spring outwards into the locked 'off'
position.
3. DESCRIPTION
2
3
1
8

Interlock Models
When the vaporizer is mounted on
the anaesthetic machine back bar
with other interlock vaporizers, initial operation of the concentration
control dial activates the interlock
system ensuring that only one of
the vaporizers con be in use at any
time.
The interlock deactivates as soon
as the control dial is returned to the
locked out zero position.
NOTE
The Sigma Delta Selectatec
Compatible Vaporizer with
Interlock can be used on a
Selectatec Universal Series
Manifold back bar in conjunction
with other types of Selectatec compatible vaporizers (i.e. from other
manufacturers) fitted with the interlock function.
WARNING
The Drager compatible model
with interlock must only be used
with other Drager-compatible
interlock vaporizers, to maintain
the integrity of the interlock system.
9

4.1 Physical Dimensions
Width Height Depth
Cagemount 133 219 158
Selectatec Compatible with Interlock 120 242 190
Drager 'plug in' Compatible 100 242 190
Dimensions given above are in millimetres
NOTE
For Screw Cap Filler models depth, subtract 11 mm from the depth dimensions given above.
4.2 Weight
Approximate weight: 4.8 kg.
4.3 Capacity
Volume at MAX mark 250 ml (nominal)
Volume at MIN mark 35 ml (nominal)
NOTE
After draining, approximately 60 ± 10 mI of liquid is retained by the wick.
4.4 Filling System
Key Filler (Agent Specific)
Used with corresponding agent specific filler adaptor, see section 10,
Ordering Information.
Pour Fill (Screw Top)
Quik Fil - Sevoflurane only
Use with corresponding agent specific bottle.
4. SPECIFICATION
10

4.5 Control Dial Scale
The control dial is marked as follows:
From 0 to 2% vol, by intervals of 0.2% vol
From 2 to maximum, by intervals of 0.5% vol
The control dial is marked '0' at zero
4.6 Patents
The Sigma Delta is protected by UK and foreign patents.
4.7 Temperature Range
Operating Temperature Range 15 to 35oC (58 to 95oF)
Storage Temperature Range -20 to 50oC (-5 to 122oF)
Storage in Transit (up to 7 days) -40 to 60oC (-40 to 149oF)
4.8 Flow Range
Operating Flow Range 0.2 to 15 litres/min.
See section 7.4.1 for output accuracies at extreme conditions.
4.9 Pressure Range
Operating Pressure Range 0 to 5 kPa (0 to 0.7 psi)
Maximum Manifold Pressure 38 kPa (5.5 psi)
Maximum Test Pressure 38 kPa (5.5 psi)
SPECIFICATION
11
5.1 Key Filler System
WARNING
The vaporizer must be either
secured to the anaesthetic
machine or free standing on a
level table so that in either case
it is upright during the filling
process.
Overfilling may occur if the
vaporizer is tipped during the
filling process.
WARNING
The vaporizer concentration
control must be in the 0 (zero)
position during the filling
process. Provided this is done,
gas may continue to be delivered from the anaesthetic
machine to a patient during the
filling process.
WARNING
Check that the drug name on the
vaporizer and the supply bottle
are the same before commencing the filling process, and
ensure that the bottle is fitted
with a keyed collar.
Filling the Vaporizer
This system is manufactured in
compliance with ISO 5360.
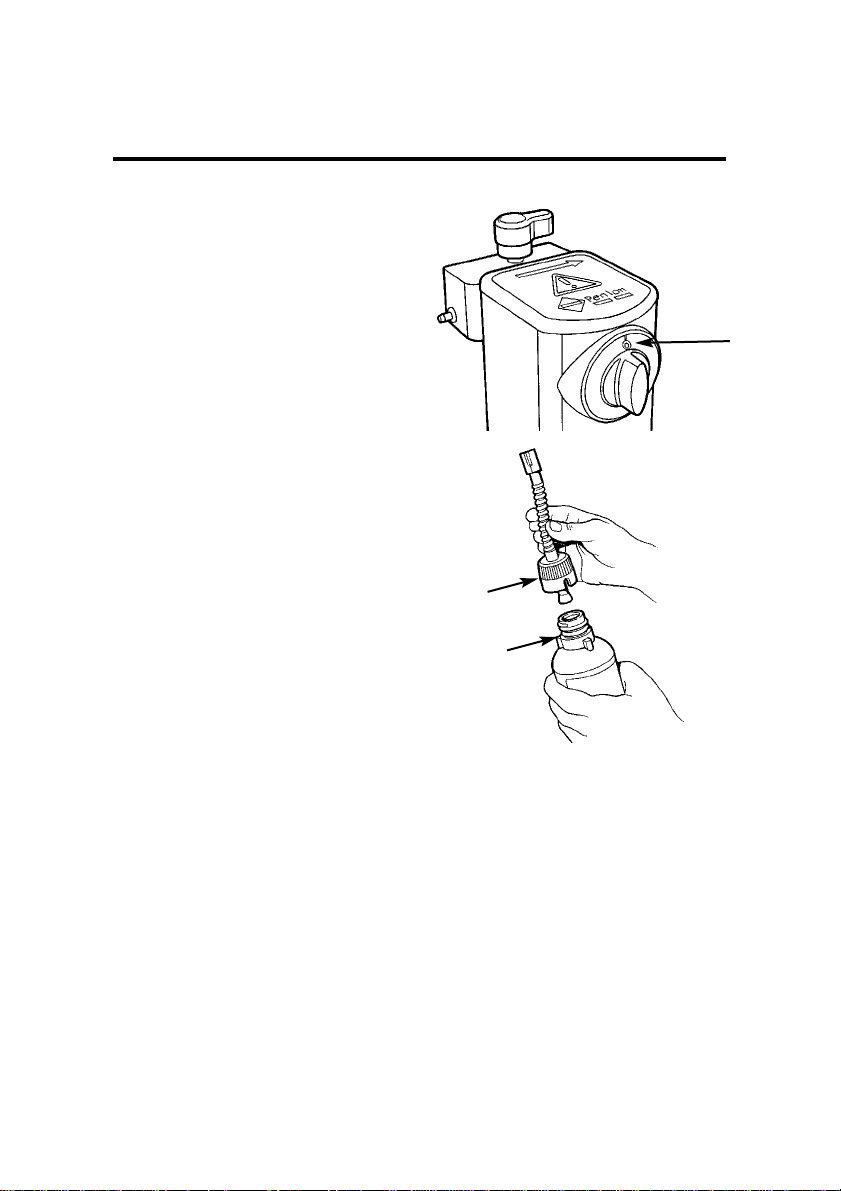
1. Check that the vaporizer
concentration control (1) is
in the 0 (zero) position as
illustrated.
2. Attach the keyed filler adaptor (2) to the bottle (3).
NOTE
Penlon supply a complete range of
agent specific filler adaptors, see
section 10.
3. Tighten the adaptor to
ensure an airtight joint,
which must be maintained
throughout the filling operation.
WARNING
Failure to observe this instruction may result in overfilling.
5. FILLING AND DRAINING
1
2
3
12
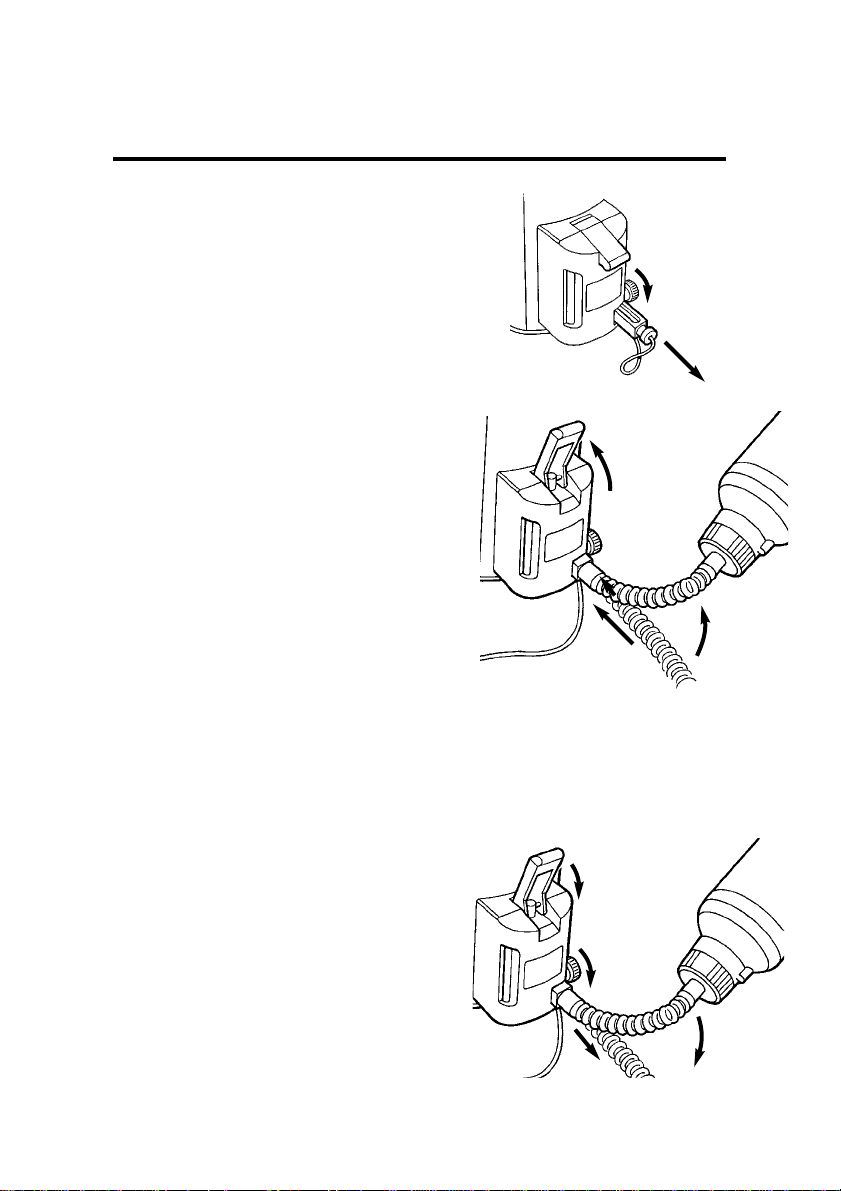
4. Loosen the clamp screw (4).
Remove the plug (5).
5. Insert the keyed end of the bottle
adaptor (2) fully into the vaporizer
receiver.
Only the correct keyed-adaptor can
enter the receiver.
Tighten the clamp screw (4) to
secure the adaptor.
6. Raise the bottle above the filler
(see arrow on the illustration).
7. Open the filler control (6) - lift
upwards.
Allow the liquid to flow into the
vaporizer until the upper mark is
reached on the filler block (7).
WARNING
DO NOT OVERFILL.
A vaporizer that has been overfilled
must be withdrawn from use.
If the vaporizer has been inadvertently
overfilled, excess liquid agent will spill
from the drain hole in the keyed slot in the
filler block. DO NOT REUSE THIS AGENT
Allow all the excess liquid to drain from the
vaporizer before inserting the plug (5).
8. Close the filler control (6).
9. Lower the bottle below the level of
the filler and allow the liquid in the
bottle adaptor to flow back into the
bottle.
Loosen the clamp screw (4),
remove the bottle adaptor from the
receiver.
NOTE
A small amount of liquid is always likely to
spill when the bottle adaptor is removed
from the receiver.
FILLING AND DRAINING
5
2
7
4
6
4
6
13
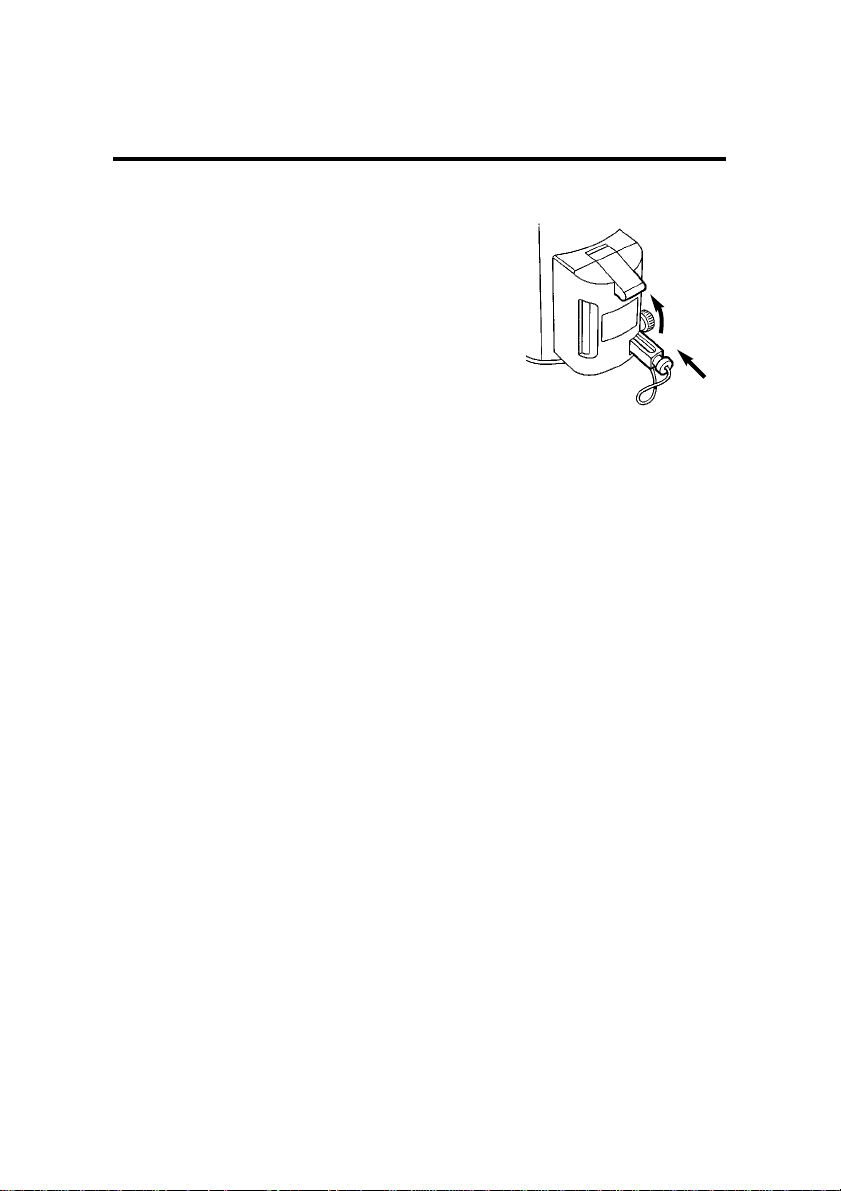
10. Insert the plug (5) and tighten
the clamp screw (4).
WARNING
For the vaporizer to function correctly it is important to insert the
sealing plug (5) fully, until it stops,
before clamping it into position with
the clamp screw (4) after filling is
completed.
If this is not done, the possibility
exists that agent may leak from the
vaporizer or the vaporizer may not
pressurise properly, giving reduced
concentration output and gas flow
to the patient.
FILLING AND DRAINING
5
4
14
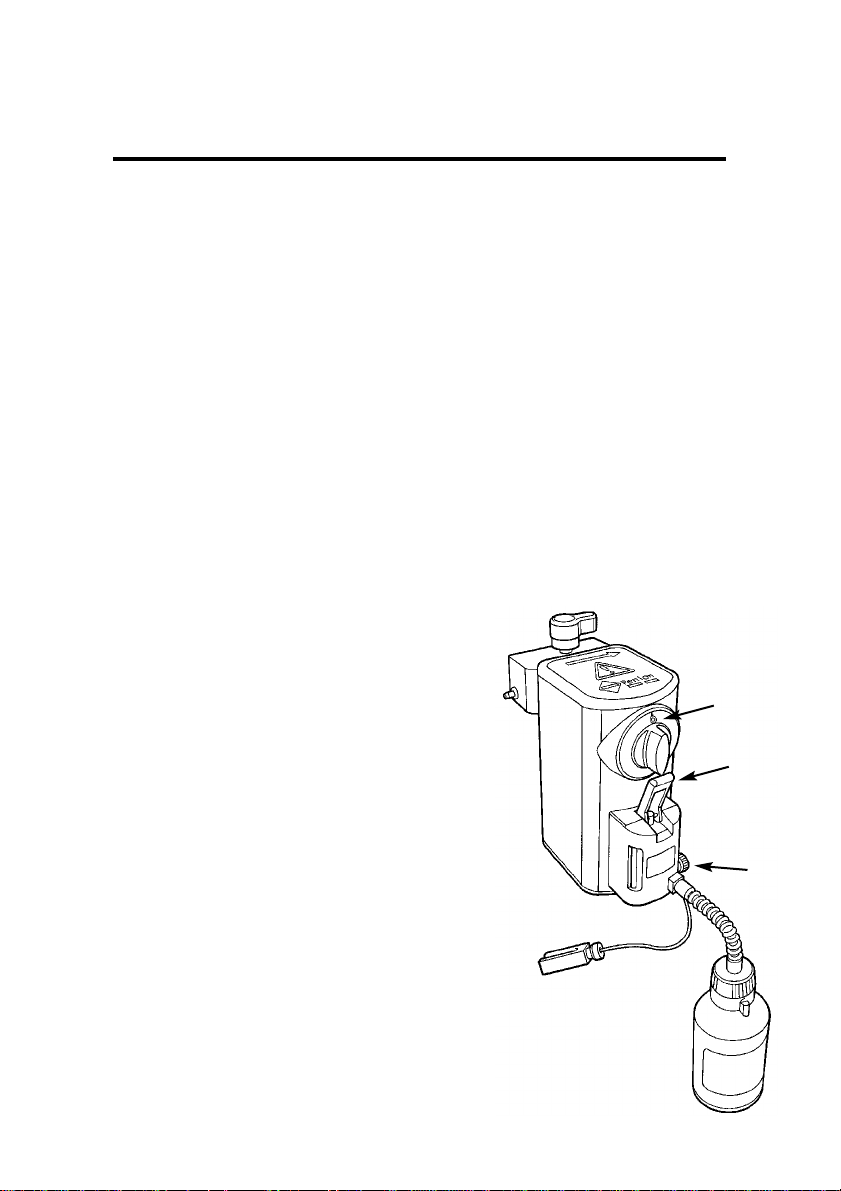
Draining the Vaporizer
CAUTION
To reduce atmospheric pollution in
the operating room, it is recommended that vaporizer drainage
should be performed in a fume
cupboard or under an extractor
hood.
WARNING
The vaporizer must be either
secured to the anaesthetic
machine or free standing on a
level table so that in either case
it is upright during the draining
process.
WARNING
The vaporizer concentration
control must be in the 0 (zero)
position during the draining
process
1. Check that the vaporizer
concentration control (1) is
in the 0 (zero) position.
2. Follow steps 2 to 5 of the
procedure for filling the
vaporizer (see above), but
keep the bottle below the
filler.
3. Raise the the filler control
(2) and allow the liquid to
run into the bottle until the
flow ceases.
4. Close the filler control (2),
loosen the clamp screw (3),
and reinsert the plug (4).
Tighten the clamp screw
(3).
FILLING AND DRAINING
2
3
4
1
WARNING
Anaesthetic drugs must be treated as a pharmaceutical product.
Liquid should never be drained
from a vaporizer into an open
container and reused.
Contamination is likely. Always
dispose of such drained liquid
as a hazardous chemical.
15
 Loading...
Loading...